Abstract
The foraging gene in Drosophila melanogaster, which encodes a cGMP-dependent protein kinase, is a highly conserved, complex gene with multiple pleiotropic behavioral and physiological functions in both the larval and adult fly. Adult foraging expression is less well characterized than in the larva. We characterized foraging expression in the brain, gastric system, and reproductive systems using a T2A-Gal4 gene-trap allele. In the brain, foraging expression appears to be restricted to multiple sub-types of glia. This glial-specific cellular localization of foraging was supported by single-cell transcriptomic atlases of the adult brain. foraging is extensively expressed in most cell types in the gastric and reproductive systems. We then mapped multiple cis-regulatory elements responsible for parts of the observed expression patterns by a nested cloned promoter-Gal4 analysis. The mapped cis-regulatory elements were consistently modular when comparing the larval and adult expression patterns. These new data using the T2A-Gal4 gene-trap and cloned foraging promoter fusion GAL4’s are discussed with respect to previous work using an anti-FOR antibody, which we show here to be non-specific. Future studies of foraging’s function will consider roles for glial subtypes and peripheral tissues (gastric and reproductive systems) in foraging’s pleiotropic behavioral and physiological effects.
Keywords: Drosophila melanogaster, foraging gene, expression, promoter analysis, cis-regulatory element, pleiotropy
Introduction
Genes that influence behavior are often pleiotropic, expressed throughout development and in many tissues (Hall, 1994). Investigating the temporal and spatial expression of pleiotropic genes can aid in the interpretation of their many functions. The Drosophila melanogaster foraging (for) gene, which codes for a cGMP-dependent protein kinase (PKG), has long been a model system for studies of gene–environment interaction and pleiotropy in the field of behavior genetics (Allen, Anreiter, Neville, & Sokolowski, 2017; de Belle, Hilliker, & Sokolowski, 1989; Osborne et al., 1997; Sokolowski, 1980; reviewed in Anreiter & Sokolowski, 2019). foraging is a complex gene with a modular structure that includes four promoters (forpr1-4), which produce approximately 20 RNA transcripts that code for nine distinct protein isoforms (Allen et al., 2017). The different isoforms of foraging have varied expression levels depending on the developmental stage and tissue being assayed (Allen et al., 2017; Anreiter, Kramer, & Sokolowski, 2017; Brown et al., 2014; Dason et al., 2020; Leader, Krause, Pandit, Davies, & Dow, 2018), but little is known about the cell-specific expression of foraging.
Some indication of the tissue-specific requirement for a suite of feeding-related and physiological phenotypes has been described in the larva (Allen, Anreiter, Vesterberg, Douglas, & Sokolowski, 2018; Dason, Allen, Vasquez, & Sokolowski, 2019, Dason & Sokolowski, 2021). We previously generated a full genetic deletion of the foraging gene, for0, and found that these foraging null larvae had reduced locomotion on food, reduced food intake, and increased triglyceride content (Allen et al., 2017). We were then able to rescue these attributes using different cloned foraging regulatory regions, which drove expression in discrete and restricted patterns in third instar larvae (Allen et al., 2018). Specifically, the decrease in larval locomotion on food of the for0 mutant was rescued by forpr1-Gal4 when driving UAS-forcDNA. forpr1-Gal4 expressed in neurons of the CNS and enteroendocrine cells (EE cells) of the gut. Triglyceride levels were rescued by forpr3-Gal4, which expressed in many cell types such as perineurial glia, visceral muscle, trachea, and fat cells (among others). Finally, food intake was rescued by forpr4-Gal4, which expressed in the developing optic lobes, hindgut, and spiracles (Allen et al., 2018). Similar tissue-specific requirements of foraging were found at the larval neuromuscular junction where the for0 null mutant phenotype of nerve terminal overgrowth was rescued by glial expression, and increased neurotransmission was rescued by neuronal expression (Dason et al., 2019). Hence, these discrete and restricted expression patterns were successfully used to dissect some of the pleiotropic behavioral and physiological functions of foraging during the larval stage.
In addition to its larval phenotypes, the foraging gene has been implicated in many adult behavioral phenotypes. These include post-feeding locomotion (Pereira & Sokolowski, 1993), sucrose responsiveness (Belay et al., 2007; Scheiner, Sokolowski, & Erber, 2004), learning and memory (Kaun et al., 2007; Kohn et al., 2013; Kuntz, Poeck, Sokolowski, & Strauss, 2012; Mery, Belay, So, Sokolowski, & Kawecki, 2007; Reaume, Sokolowski, & Mery, 2011; Wang et al., 2008), habituation (Eddison, Belay, Sokolowski, & Heberlein, 2012; Engel, Xie, Sokolowski, & Wu, 2000; Scheiner et al., 2004), social behavior (Donlea et al., 2012; Foucaud et al., 2013; Alwash et al., 2021), sleep (Donlea et al., 2012), starvation resistance (Anreiter et al., 2017; Donlea et al., 2012), aggression (Wang & Sokolowski, 2017), and stress tolerance (Dawson-Scully, Armstrong, Kent, Robertson, & Sokolowski, 2007; Dawson-Scully et al., 2010). Many of these phenotypes show parallels between the larva and the adult developmental stages. For instance, foraging affects food search behavior in both larvae and adults (Anreiter et al., 2017; Hughson et al., 2018; Kent, Daskalchuk, Cook, Sokolowski, & Greenspan, 2009; Pereira & Sokolowski, 1993; Sokolowski & Riedl, 1999). However, the behavioral patterns seen for food intake are reversed when comparing larvae and adults. Larvae carrying the for0 null allele show decreased food intake relative to controls, and this can be rescued by a full transgenic complement of the locus as well with a tissue-specific expression of a foraging cDNA (Allen et al., 2017, 2018). In contrast, food deprived adults with forpr4 foraging transcripts ubiquitously knocked-down with RNAi consume more sucrose drops than controls (Anreiter et al., 2017). This suggests the possibility of differential regulation of the foraging gene for specific phenotypes at the larval and adult developmental stages. In the larva, foraging’s pleiotropy is in part due to foraging’s four promoters driving distinct expression patterns of subsets of transcripts, each associated with different phenotypes. We wondered whether foraging exhibited promoter-specific expression patterns in the adult and if they paralleled those observed in larvae.
Here we characterize the adult expression of foraging in the brain, gastric system, and reproductive systems, using: a) a transcriptional and translational trap T2A-Gal4 allele (Lee et al., 2018), b) published data from single-cell transcriptomic analyses (Davie et al., 2018; Hung et al., 2020), c) foraging promoter Gal4 fusions, and d) by revisiting a previously published anti-FOR antibody. We further refine and map regions containing cis-regulator elements using a series of nested promoter Gal4 fusions and compare the modular patterns of expression of the larval and adult fly.
Materials and methods
Fly strains and rearing
Flies were reared in 40 ml vials with 10 ml of food with a 12 L:12D photocycle at 25 ± 1 °C. The food recipe has previously been described (Allen et al., 2017). The following fly strains were used in this study: for0/CyO, {Act-GFP} (Allen et al., 2018), {forpr1-Gal4}attP2 (Allen et al., 2018), {forpr2-Gal4}attP2 (Allen et al., 2018), {forpr3-Gal4}attP2 (Allen et al., 2018), {forpr4-Gal4}attP2 (Allen et al., 2018), y1,w*; TI{GFP[3xP3.cLa]=CRIMIC.TG4.2}forCR00867-TG4.2 (Bloomington Drosophila Stock Center # 79329; Lee et al., 2018), w*; P{UAS-mCD8::GFP}/CyO (denoted as mCD8::GFP), w1118; P{UAS-Stinger}2 (Bloomington Drosophila Stock Center # 84277, denoted as nls::GFP; Barolo, Carver, & Posakony, 2000), w*; UAS-myr-GFP-V5-P2A-H2B-mCherry-HA/TM3, Ser (denoted as UAS-Watermelon; Chang, Keegan, Prazak, & Dubnau, 2019).
Construction of nested Gal4s
Construction of the forpr-Gal4s was previously described (Allen et al., 2018). A similar strategy was used to generate the nested forprΔ-Gal4s constructs. The NotI fragment, containing the Gal4 sequence, of pMARTINI-Gal4 (Billeter & Goodwin, 2004) was cloned into the NotI digested pStinger-attB vector, replacing the GFP. This insulated Gal4 vector with attB was then digested with KpnI and end filled with Klenow (cat # M0210S, New England Biolabs). Nested regions of foraging were amplified by PCR from the larger forpr-Gal4 vectors and cloned into the end filled insulated Gal4 vector. All forprΔ-Gal4s constructs were injected into the P{CaryP}attP2 landing site (Groth, Fish, Nusse, & Calos, 2004) by Genetic Services Inc. (Cambridge, MA, USA). Successful integration was confirmed with PCR.
The primers used for the nested forprΔ-Gal4s are as follows:
forpr1Δ1-F: 5′-TCGCAAAAACCAACCCTTAC-3′,
forpr1Δ2-F: 5′-CGACGAACATTATTTGGCTCT-3′,
forpr1Δ3-F: 5′-CCTTTCTCCCAGCTGCTATCT-3′,
forpr1Δ4-F: 5′-CAAAGTTAATCCTGCATTGGC-3′,
forpr1-R: 5′-ACAAGTCGATGAAAAACCGCC-3′,
forpr2Δ1-F: 5′-CTAAACGTTTTCCGCAGCA-3′,
forpr2Δ2-F: 5′-ACAAACGAATGGAACGGAAC-3′,
forpr2-R: 5′-CCAAAACCAAGTGTAACACAC-3′,
forpr3Δ1-F: 5′-ATACCCTCCATCCAAAGCG-3′,
forpr3Δ2-F: 5′-TCCAAACGGATCTTTGTCTTTT-3′,
forpr3Δ3-F: 5′-CAGGGGAAATGATAACCGAA-3′,
forpr3Δ4-F: 5′-GCACATAGAACCCGTAGAGGA-3′,
forpr3-R: 5′-GGGATCCTGGTTCAATTGCTG-3′,
forpr4Δ1-F: 5′-CCCTACTCATAAAACTGCCCC-3′,
forpr4Δ2-F: 5′-AGTTCGCCGGTTTGGTACT-3′,
forpr4Δ3-F: 5′-TTTTCGCTCTCCCAGACACAC-3′,
forpr4-R: 5′-CGAATTGAAAATCACGATACG-3′.
Recombineering BAC{forIRES-Gal4} allele
We generated a transgenic copy of the entire foraging locus, replacing the common coding region with a Gal4 coding sequence, using recombineering. We used the BAC containing the entire 35 kb foraging locus (previously described in Allen et al., 2017) and replaced the common coding region of foraging with a premature stop codon, followed by an Internal-Ribosomal-Entry-Site (IRES) and codon optimized Gal4 coding sequence. The Gal4 coding sequence replaced the foraging common coding sequence, but still utilized the endogenous foraging 3’UTR.
To generate this BAC{forIRES-Gal4} allele we needed to clone a donor construct with Gal4 sequence flanked with homology arms to foraging. Restriction sites added to primers are in parentheses and their sequences are italicized and separated by a hyphen. A D. melanogaster codon optimized Gal4 sequence was PCR amplified, with primers dmGal4-F (AscI) 5′-GGCGCGCC-ATGAAGCTGCTGAGTAGTATTG-3′ and dmGal4-R (SbfI) 5′-CCTGCAGG-CTACTCCTTCTTTGGGTTCGG-3′, from the pBPGAL4.1Uw vector (Pfeiffer et al., 2010; addgene # 26226) and cloned into the pSC-A-amp/kan vector from the StrataClone PCR Cloning Kit (Agilent, cat # 240205). The SpeI–NotI fragment containing an FRT-kan-FRT cassette from the pIGCN21 vector (Lee et al., 2001) was cloned into the SpeI and NotI sites of the pSC-dmGAl4 vector. An Internal-Ribosomal-Entry-Site (IRES) was PCR amplified from the Ubx locus (as in Halfon et al., 2002) with the primers Stop-IRES-F (HindIII) 5′-AAGCTT-CTAGACTAG-TCTAGCAGCAAAGTGCAATTGGCTAAAAACC-3′ and Stop-IRES-R (AscI) 5′-GGCGCGCC-GATTCTTACCGCCAGCAGCGC-3′ and cloned into the HindIII and AscI sties of the pSC-dmGAl4-FRT-kan-FRT vector. An all 6 frame stop codon cassette added to the forward primer is underlined and separated by a hyphen. A left homology arm corresponding to foraging specific sequence was PCR amplified with L-comGal4-F (KpnI) 5′-GGTACC-GCTCCGCCACCCAGAGAACC-3′ and L-comGal4-R (HindIII) 5′-AAGCTT-CCTCGCGGGAAACCTCCACG-3′ and cloned into the KpnI and HindIII sites of the pSC-IRES-dmGAl4-FRT-kan-FRT vector. A right homology arm corresponding to foraging specific sequence was PCR amplified with R-comGal4-F (BglII) 5′-AGATCT-GGAGAATCAGAACCCGTTTC-3′ and R-comGal4-R (NotI) 5′-GCGGCCGC-GCATACAAATCGGGTTGCCTT-3′ and cloned in the BglII and NotI sites of the pSC-LHA-IRES-dmGAl4-FRT-kan-FRT vector.
The KpnI–NotI fragment from the pSC-LHA-IRES-dmGAl4-FRT-kan-FRT-RHA vector was transformed into EL250 E. coli strain (Lee et al., 2001) which already contained a bacterial artificial chromosome (BAC) containing the 35 kb foraging locus (previously described in Allen et al., 2017). Recombineered BACs were selected by kanamycin resistance. The FRT-kan-FRT was then removed by arabinose induction. Proper integration Gal4 sequence and replacement of the foraging common coding region was verified with PCR, restriction digest, and Sanger sequencing. φC31 integration was used to integrate the BAC into the VK00013 landing site on the third chromosome (Venken, He, Hoskins, & Bellen, 2006). Transgenesis was performed by BestGene Inc. Primer design, in-slico cloning, and analysis of Sanger sequencing reactions were all performed in the Geneious 8 software package (Kearse et al., 2012).
Immunohistochemistry
Adult and larval samples were dissected in 1 × PBS and then fixed in 4% paraformaldehyde in 1× PBS for 40 min. The tissues were then rinsed twice and then washed 4× for 45 min each in 0.3% Triton X in 1× PBS (PBT). The tissues were blocked in 10% normal goat serum (NGS, Jackson ImmunoResearch Laboratories) in PBT for 18–36 h at 4 °C. Primary antibody incubations were conducted in blocking solution and incubated for 36–48 h at 4 °C. The tissues were then rinsed twice and washed 4× for 45 min. each in PBT. Secondary antibody, in blocking solution, were incubated for 36–48 h at 4 °C. Tissues were washed as described above for the primary antibody. Tissues were then cleared in 70% glycerol in PBS for 18–24 h at 4 °C. Tissues were mounted on slides in Vectashield (cat # H-1000–10, Vector Laboratories). Tissues were imaged using a Zeiss Axioscope epifluorescence microscope as well as a Zeiss LSM 510 and Leica SP5 confocal microscopes. Images were analysed using Fiji (Schindelin et al., 2012). The following antibodies were used at the following concentrations: guinea pig anti-FOR (1:200, Belay et al., 2007), mouse anti-Brp (1:50, Developmental Studies Hybridoma Bank), chicken anti-GFP (1:400, cat # A10262, Thermo Fisher Scientific), rabbit anti-GFP (1:200, cat # A-11122, Thermo Fisher Scientific), rat anti-mCherry (1:400, cat # M11217, Thermo Fisher Scientific), Alexa Fluor 546 Phalloidin (1:400, cat # A22283, Thermo Fisher Scientific), goat antichicken Alexa Fluor 488 (1:400, cat # A-11039, Thermo Fisher Scientific), goat antirabbit Alexa Fluor 488 (1:400, cat # A-11008, Thermo Fisher Scientific), goat antimouse Alexa Fluor 488 (1:400, cat # A-11001, Thermo Fisher Scientific), goat antimouse Alexa Fluor 633 (1:400, cat # A-21052, Thermo Fisher Scientific), goat anti-rat Alexa Fluor 546 (1:400, cat #A-11081, Thermo Fisher Scientific), goat antirat Alexa Fluor 633 (1:400, cat # A-21094, Thermo Fisher Scientific), goat antiguinea pig Alexa Fluor 633 (1:400, cat # A-21105, Thermo Fisher Scientific).
Single-cell RNA-Seq data and analysis
The single-cell transcriptomic atlas of the adult brain was previously published (Davie et al., 2018) and the data were acquired from Scope (http://scope.aertslab.org/#/e0816194-aea3-48d8-af80-569baf58be35/Aerts_Fly_AdultBrain_Filtered_57k.loom/gene). These cells were reprocessed as previously described (Allen et al., 2020). Briefly, the data were processed in R (R Core Team, 2020) with Seurat v2.3.4 (Satija, Farrell, Gennert, Schier, & Regev, 2015). Data were normalized and scaled with ‘NormalizData’ and ‘ScaleData’ functions. A principal component analysis was run, with the ‘RunPCA’ function, on the variably expressed genes (as deduced by ‘FindVariableGenes’). A 2-dimensional t-distributed stochastic neighbor embedding (t-SNE; Van Der Maaten, Courville, Fergus, & Manning, 2014) was performed, using ‘RunTSNE’, on the first 82 principal components for visualization. Groups of cells with similar expression patterns were deduced with the ‘FindClusters’ function. The expression patterns of repo and foraging were visualized with the ‘FeaturePlot’ function. The per cluster average scaled expression was calculated with the ‘AverageExpression’ function and plotted using the pheatmap package (Kolde, 2019).
The single-cell transcriptomic atlas data of the adult midgut were previously published (Hung et al., 2020) and the data were acquired from Gene Expression Omnibus (accession no. GSE120537). The data were processed with Seurat v3.2.2 (Stuart et al., 2019) as described in the original publication (Hung et al., 2020). Briefly, the data were normalized and scaled with ‘NormalizData’ and ‘ScaleData’ functions. Variably expressed genes were identified with the ‘FindVariableGenes’ function. The different replicates were integrated with the ‘FindIntegrationAnchors’ and ‘IntegrateData’ functions. A principal component analysis was run, with the ‘RunPCA’ function. A 2-dimensional Uniform Manifold Approximation and Projection (UMAP; McInnes, Healy, & Melville, 2018) was performed, using ‘RunUMAP’, for the first 30 principal components for visualization. Groups of cells with similar expression patterns were deduced with the ‘FindClusters’ function. The per cluster average scaled expression was calculated with the ‘AverageExpression’ function and plotted using the pheatmap package (Kolde, 2019).
Results
Foraging expression in the adult brain
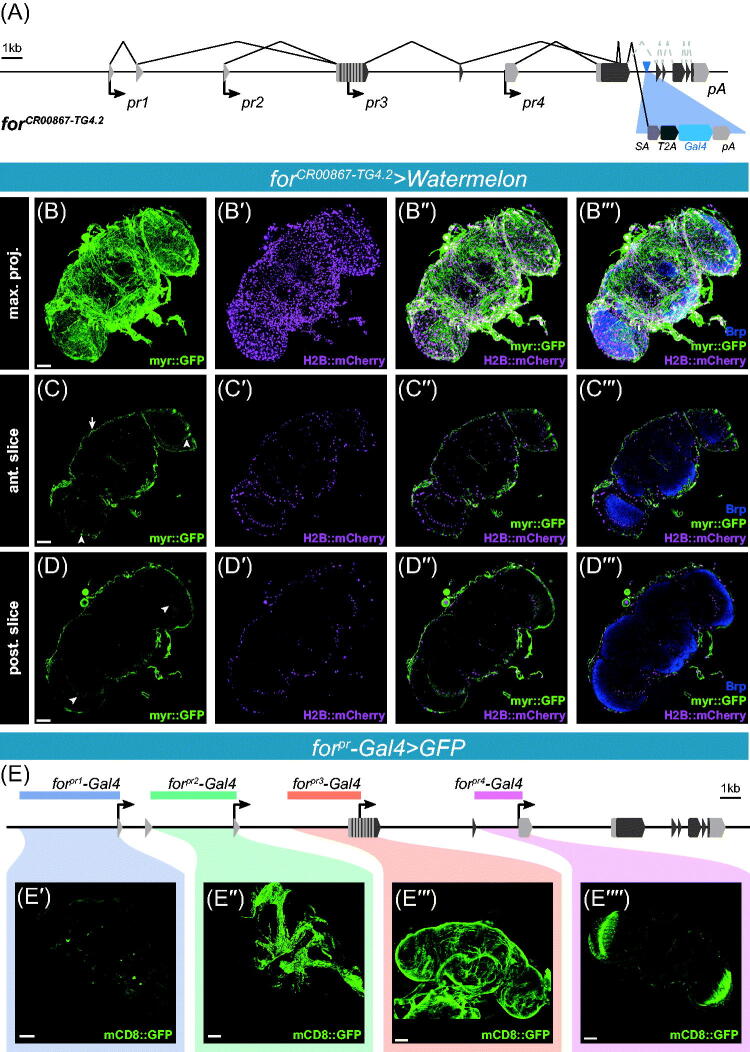
To infer the expression patterns and regulation of foraging in the adult, we took advantage of a novel T2A-based Gal4 line and cloned promoter Gal4 fusion lines from multiple regions of the locus. T2A-based Gal4 alleles are designed to reliably capture endogenous gene expression due to being a transcriptional trap, as well as a translational trap. In the present study, we used the previously generated forCR00867-TG4.2 CRIMIC allele (Lee et al., 2018), which is integrated downstream of the first common coding exon (Figure 1(A)), allowing the splice acceptor sequence to capture the endogenous transcription from foraging. forCR00867-TG4.2 is a homozygous lethal allele of foraging, dying as a pharate adult late in pupal development and does not complement the pupal lethality of the for0 null allele (Allen et al., 2017; Anreiter et al., 2021). Heterozygous forCR00867-TG4.2 driven UAS-forRNAi induces a consistent late pupal lethality (Anreiter et al., 2021). We found that forCR00867-TG4.2 drove expression in many cells with morphology consistent with multiple glial subtypes in the adult brain (Figure 1(B–D)). Most prominently, forCR00867-TG4.2 expressed in the surface glia (Figure 1(C), arrow). Although we did not perform co-localization with a perineural marker, the surface glial pattern observed using the forCR00867-TG4.2 was consistent with perineurial glia as evident from the number of cells (Figure 1(C′); Awasaki, Lai, Ito, & Lee, 2008). Previous studies found that foraging was among the top 50 most enriched genes when comparing surface glia transcriptomes to neuronal transcriptomes, and a protein trap allele P{PTT-GB}forCB02956 also expressed in perineurial glia (DeSalvo et al., 2014). Surface glia function as the blood–brain barrier in the larval and adult fly and regulate the transport of hormones, nutrients, and metabolites between the hemolymph and the brain (Bainton et al., 2005; Laughlin, De Ruyter Van Steveninck, & Anderson, 1998; Limmer, Weiler, Volkenhoff, Babatz, & Klämbt, 2014; Schwabe, Bainton, Fetter, Heberlein, & Gaul, 2005; Stork et al., 2008). Functions for glial subtypes and neuron–glia interactions in behavioral phenotypes have received relatively little attention (Artiushin & Sehgal, 2020; Bittern et al., 2020).
Figure 1.
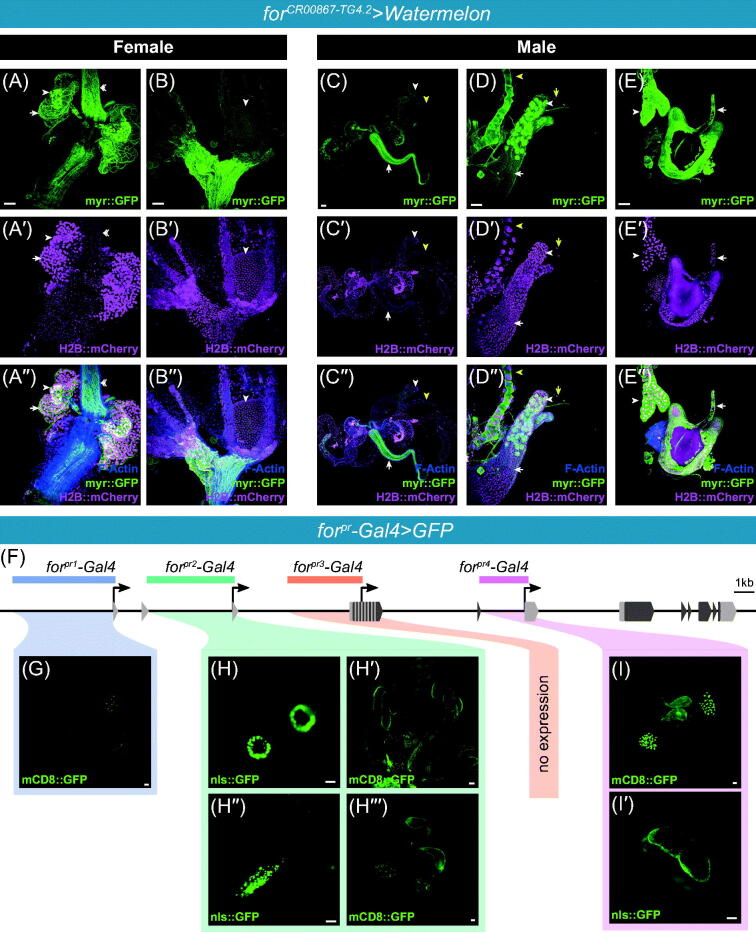
(A) Schematic of the forCR00867-TG4.2 CRIMIC allele in the foraging locus. UTR regions are depicted with grey boxes and coding sequences are depicted with black boxes. Splicing patterns are depicted above the locus. The four transcription start sites are depicted below the locus, pr1–4. The CRIMIC element is inserted in the first intron after the first common coding exon (blue triangle). The splice acceptor sequence (SA) is designed to trap the endogenous transcription of foraging. The self-cleaving T2A sequence then allows for the translation of the Gal4 coding sequence into a separate peptide. (pA – poly adenylation site). (B–B′′′) Maximal projections of the forCR00867-TG4.2 CRIMIC allele driving UAS-Watermelon in the adult brain. Membrane bound GFP in green (B), nuclear mCherry in magenta (B′), membrane and nuclear merged (B′′), membrane and nuclear merged with Bruchpilot (nc82) in blue (B′′′). (C–C′′′) A single section in the anterior of the adult brain of the forCR00867-TG4.2 CRIMIC allele driving UAS-Watermelon. Arrow denotes surface glia expression. Arrow heads in the optic lobes depict the cells with morphology consistent with outer chiasm glia. (D–D′′′) A single section in the posterior of the adult brain of the forCR00867-TG4.2 CRIMIC allele driving UAS-Watermelon. Arrowheads in the optic lobes depict the cells with morphology consistent with inner chiasm glia. (E–E′′′′) Schematic of the foraging locus depicting regions of cloned forpr-Gal4s (E). forpr1-Gal4 driven GFP expression in neurons innervating the optic lobe (E′). forpr2-Gal4 driven expression in the trachea and air sacs (E′′). forpr3-Gal4 driven expression in the perineurial surface glia (E′′′). forpr4-Gal4 driven expression in the outer optic chiasm glia (E′′′′). Scale bars = 50 µm. [Please refer to the online version for colors.]
Multiple distinct glial subtypes are found in the fly brain, all of which play distinct roles (Kremer, Jung, Batelli, Rubin, & Gaul, 2017; Yildirim, Petri, Kottmeier, & Klämbt, 2019). forCR00867-TG4.2 drove expression in many of these subtypes. The next strongest expression in glial subtypes was found in the outer optic chiasm glia (Figure 1(C), arrowhead) and the inner optic chiasm glia (Figure 1(D), arrowhead). The outer optic chiasm glia have a fine, wispy structure found between the lamina and medulla (Kremer et al., 2017). The inner chiasm glia fill the space between the medulla and the lobula and lobula plate (Kremer et al., 2017). Weaker expression was seen in the neuropil- and tract-ensheathing glia (Figure S1(A,B)) and in the cortex glia (Figure S1(C–C′′)).
We also observed expression in the tracheal system surrounding and innervating the brain (data not shown). The forCR00867-TG4.2 allele drove expression in both the large air sacs and the fine branching trachea in the brain. foraging expression in larval trachea has previously been found (Leader et al., 2018).
To map the regulatory elements responsible for the observed expression, we next examined expression from the forpr-Gal4 constructs (Figure 1(E)). The cloned regions encompassed 2–5kb upstream of each transcription start site (TSS) to 200 bp downstream of each TSS. These regions were cloned into a Gal4 vector and inserted into the attP2 landing site (described in Allen et al., 2018). The forpr-Gal4 regulatory regions together cover 15 kb of the 35 kb foraging locus. As was previously characterized in the larval CNS (Allen et al., 2018), each forpr-Gal4 line showed different expression patterns in the adult brain (Figure 1(E′–E′′′′)). In adults, forpr1-Gal4 drove expression in only a few neurons in the brain (Figure 1(E′)). We saw the most robust expression in a pair of neurons with arbors connecting the suboesophageal zone to the medulla. None of these neurons were evident in the expression patterns observed with the forCR00867-TG4.2 allele. forpr2-Gal4 expressed in the trachea and air sacs surrounding and innervating into the adult brain, but there was no observable neuronal or glial expression (Figure 1(E′′)). forpr3-Gal4 drove expression in the surface glia (Figure 1(E′′′), Figure S1(D)). This expression is consistent with perineurial glia as was seen in forCR00867-TG4.2. forpr4-Gal4 expressed in the outer and inner chiasm glia of the optic lobes (Figure 1(E′′′′), Figure S1F).
The forpr-Gal4s do not express in all of the cells observed in forCR00867-TG4.2 but mostly comprise a subset of the brain expression pattern observed in forCR00867-TG4.2. As less than half of the foraging locus is covered by these forpr–Gal4s lines, the necessary CREs for the remaining expression may lie in the un-cloned regions. Nevertheless, we found parallels between the expression patterns observed using the forCR00867-TG4.2 gene trap and cloned forpr–Gal4s.
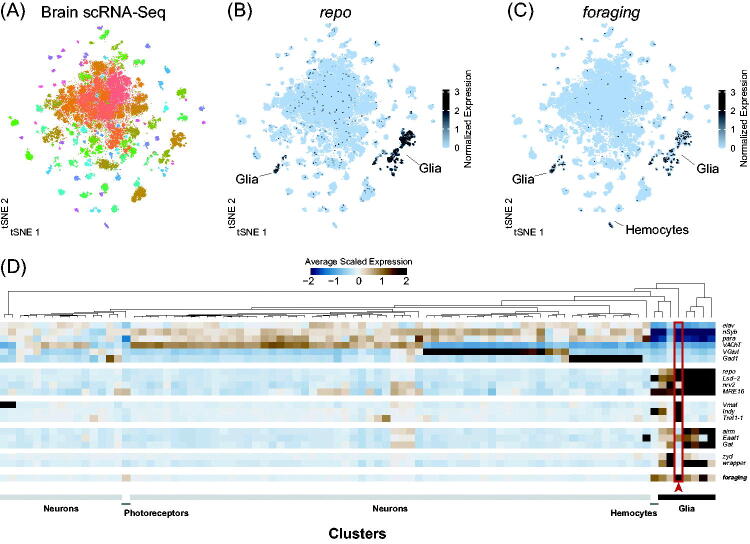
In recent years, the advent of massively parallel single-cell transcriptomics has led to the unprecedented characterization of gene expression of individual cell types. Multiple data sets characterize the transcriptomic profiles of different subsets of the adult CNS (Allen et al., 2020; Croset, Treiber, & Waddell, 2018; Davie et al., 2018; Gao et al., 2015; Konstantinides et al., 2018; Li et al., 2017). We took advantage of a whole brain data set (Davie et al., 2018) to explore foraging expression at the single-cell resolution. Individual cells are grouped based on the similarity of their gene expression profiles, and different colors represent distinct cell types (see “Methods”, Figure 2(A)). Using the established glial marker gene repo, we can identify the glial clusters (Figure 2(B)). foraging expression was for the most part restricted to repo positive clusters, but expression was also seen in hemocytes and photoreceptors (Figure 2(C,D)). Looking at the average scaled expression for multiple neuronal, glial, and glial sub-type markers across each cluster of cells (colors in Figure 2(A)), it is clear that foraging is specifically enriched in glia with highest enrichment in perineurial glia (Figure 2(D), red arrow).
Figure 2.
(A) t-SNE plot of single-cell RNA sequencing from the adult brain (data from Davie et al., 2018). Each point represents the transcriptome of a single cell. Cells are clustered based on similarity of gene expression. Distinct cell types are represented by different colors. (B) Expression of the glial specific transcription factor repo in the single-cell brain atlas. repo expression is restricted to only a few clusters of cells. Cells are color coded according to the level of normalized expression. (C) Expression of foraging in the single cell brain atlas. foraging expression is restricted to a few clusters, most of which were also repo positive, and one was Hml positive. (D) Heatmap showing the average scaled expression of neuronal and glial marker genes across each cell cluster (colors in A). Neurons are marked by the pan-neuronal markers elav, nSyb, para, as well as the neurotransmitter specific genes VAChT (Acetylcholine), VGlut (Glutamate), and Gad1 (GABA). Glia are marked by repo, MRE16, nrv2, Lsd-2. The genes Vmat, Indy, and Tret1-1 label perineurial glia (among other things). alrm, Gat, and Eaat1 label astrocyte-like glia. zyd labels cortex glia and ensheathing glia, and wapper labels tract cortex glia. foraging is enriched in all the glia clusters, as well as the Hml expressing hemocyte cluster. [Please refer to the online version for colors.]
The forCR00867-TG4.2 allele (Figure 1(B–D)) and the single-cell transcriptomics data (Figure 2(A–D)) found no detectable neuronal expression in the adult fly brain. We were surprised not to detect neuronal expression in the adult fly brain because functional studies that manipulate foraging levels in neurons are known to alter several behavioral phenotypes (reviewed in Anreiter and Sokolowski 2019 and discussed further below). Consequently, we used two more Gal4 alleles, the forMI01791-TG4.1 (Diao et al., 2015; Lee et al., 2018) and BAC{forIRES-Gal4} to investigate foraging expression in the adult brain. The forMI01791-TG4.1 allele also drove expression in the surface glia, and similar to the CRIMIC allele did not have any detectable neuronal expression (Figure S1(G–G′′)). Both the forMI01791-TG4.1 and forCR00867-TG4.2 alleles rely on a splice acceptor and T2A sequence to trap the endogenous transcription and translation of foraging. We also implemented an independent strategy to generate a recombineered bacterial artificial chromosome (BAC), containing the entire foraging locus, with the coding sequence common to all isoforms replaced with an Internal-Ribosomal-Entry-Sequence and Gal4 coding sequence. An un-mutated copy of this BAC was previously shown to have similar expression, as measured by RT-qPCR and western blot, to that of wild-type flies, and was sufficient to rescue the for0 null mutant in many of foraging’s larval associated phenotypes (Allen et al., 2017; Dason et al., 2019). This BAC{forIRES-Gal4} allele drove expression in the surface glia and again had no detectable neuronal expression (Figure S1(H–H′′)). We provide a more detailed expression analysis using the forCR00867-TG4.2 allele as its expression was stronger than the forMI01791-TG4.1 and BAC{forIRES-Gal4} alleles.
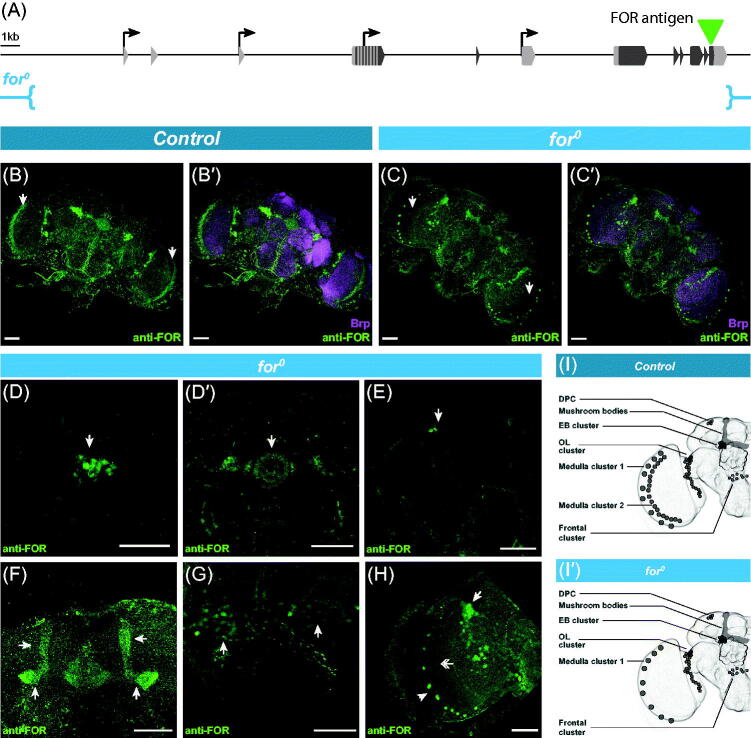
Previous studies characterized the expression of foraging proteins in the adult brain using an anti-FOR antibody (Belay et al., 2007; Mery et al., 2007). A 40 amino acid sequence from the C-terminus shared by all predicted FOR isoforms was used to generate the polyclonal antibody used in this study (Figure 3(A)). Alignments showed that the full C-terminal segment used to make this antibody was not encoded by any other sequences in the D. melanogaster genome (Belay et al., 2007). At the time of publishing Belay et al. (2007), we did not possess a complete genetic deletion of foraging. Consequently, we used a partial deletion of foraging called Df(2L)ED243 to confirm the effectiveness of this anti-FOR antibody. This deletion removed 2 of the 4 TSSs of foraging spanning from 50 bp downstream of forpr1 to 65 bp upstream of forpr4 (Ryner et al., 1996). In Belay et al. (2007), the specificity of the anti-FOR antibody in immunohistochemical analyses was evaluated using the larval proventriculus instead of adult tissue because homozygous Df(2L)ED243 do not survive to adulthood. Immunoreactivity in the larval proventriculus was absent in Df(2L)ED243 homozygous larvae compared to the control. Localized signal in the adult brain was also missing when tissues were incubated with the preabsorbed antibody. Belay et al. (2007) concluded that the anti-FOR antiserum detects endogenous FOR.
Figure 3.
(A) Schematic of the foraging gene (as in Figure 1(A)). The 120bp (40aa) antigenic region used to generate the polyclonal anti-FOR antibody is depicted with a green arrowhead (described in Belay et al., 2007). The for0 genetic deletion (described in Allen et al., 2017) and its break points (in blue) are depicted below. (B) Maximal projection of anti-FOR antibody staining in control animals of late pupal brains (4 days post-puparium formation). Arrows in optic lobes indicating cells with morphology consistent with outer optic chiasm glia. Neuropil visualised with anti-Brp (B′). (C) Maximal projection of anti-FOR antibody staining in for0 null mutant (described in Allen et al., 2017) animals of late pupal brains (4 days post-puparium formation). Arrows in optic lobes indicating lack of expression in cells with morphology consistent with outer optic chiasm glia. Neuropil visualized with anti-Brp (C′). (D–H) Magnification of anti-FOR positive clusters in the for0 null mutant. Immunoreactivity in the ellipsoid body cluster, EB cluster (arrow, D) and its projections into the ellipsoid body (arrow, D′). Staining was also seen in the 4 cells of the dorsal posterior cluster, DPC (arrow, E). Staining in the mushroom bodies (arrow, F). Staining in the frontal cluster (arrow, G). Staining in the optic lobe cluster, OL cluster (arrow, H), medulla cluster 1 (arrowhead, H), and absent in medulla cluster 2 (double arrow, H). (I–I′) Schematics of anti-FOR expression patterns in control and for0 null mutant animals. Expression in the for0 null mutant was the same as control except for the lack of medulla cluster 2. Scale bars = 50 µm. [Please refer to the online version for colors.]
However, we recently published a full 35 kb genetic null of the foraging gene, for0 (Allen et al., 2017). The stage of lethality of the for0 null mutant is late pupal lethal (Allen et al., 2017; Anreiter et al., 2021). This new allele allowed us to re-examine the efficacy of the anti-FOR staining using late stage for0 null mutant pupal brains (4 days post-pupariation). Anti-FOR antibody staining in control pupae showed the same expression patterns previously reported in adult heads (Figure 3(B); Belay et al., 2007; Mery et al., 2007). All of the previously reported primary anti-FOR clusters were seen in the pupal brain, optic lobe cluster, medulla cluster, ellipsoid body cluster, frontal cluster, dorsal posterior cluster, and the mushroom bodies. However, when we examined the for0 pupal brains, all of these clusters remained (Figure 3(C)), except for part of the medulla cluster (Figure 3(B,C), arrows). Specifically, immunoreactivity in the for0 null brains was seen in the ellipsoid body cluster (Figure 3(D–D′)), the dorsal posterior cluster (Figure 3(E)), the mushroom bodies (Figure 3(F)), the frontal cluster (Figure 3(G)), the optic lobe cluster (Figure 3(H), arrow), and medulla cluster 1 (Figure 3(H), arrowhead), but was lacking from medulla cluster 2 (Figure 3(H), double-headed arrow). The medulla cluster 2 was previously reported to be ELAV negative (Belay et al., 2007), has a similar characteristic structure to that of the outer optic chiasm glia (Kremer et al., 2017), and is consistent with that seen in the forCR00867-TG4.2 and forpr4-Gal4 expression patterns (Figure 1(C,E′′′′)). This finding suggests that in the adult brain, the anti-FOR antibody labels bone fide FOR expression in the outer optic chiasm glia but also has significant non-specific immunoreactivity suggesting that the majority of the previously described primary expression patterns in the adult brain is not FOR. Previous functional studies that showed significant effects on adult behaviors when manipulating foraging expression in the mushroom bodies and ellipsoid body are discussed below.
It is notable that the anti-FOR antibody displays high specificity to FOR proteins in western blot analyses of control and for0 mutant larvae and that the for0 null mutants did not display any immunoreactivity (Allen et al., 2017; Dason et al., 2019). We confirmed that there were no strong matches other than FOR along the whole 40 amino acid sequence (Belay et al., 2007), with a BLASTp search. However, low-affinity targets may be of concern when non-specificity of an antibody is found (Fritschy, 2008), and we did find smaller stretches of significant similarity with Protein kinase A catalytic subunits Pka-C1 and Pka-C3 (data not shown). Both Pka-C1 and Pka-C3 have been shown to be expressed in the Kenyon cells of mushroom bodies (Croset et al., 2018; Davie et al., 2018; Davis et al., 2020; Skoulakis, Kalderon, & Davis, 1993). Further purification of this antibody may ameliorate its performance in the context of whole mount immunohistochemistry, for instance, pre-absorbing the antibody with protein extracted from for0 mutants. The anti-FOR antibody specificity and utility works well to detect FOR proteins on westerns but not in immunohistochemical analysis. The context specificity of the utility of antibodies is a common problem (Baker, 2015).
Foraging expression in the adult gastric system
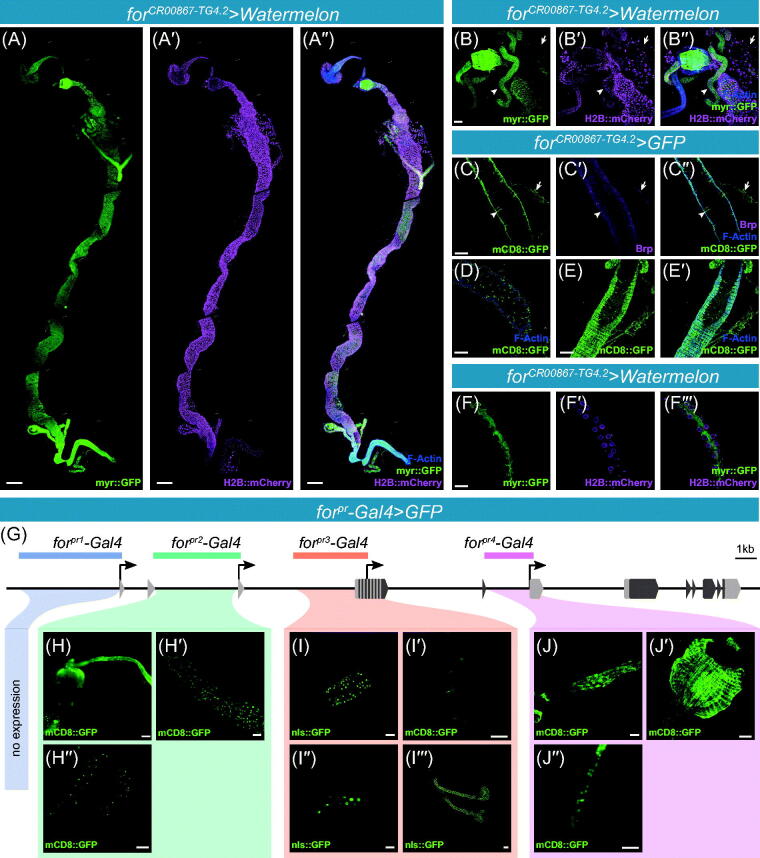
The gastric system plays a crucial and central role in feeding-related phenotypes. Not only is it essential for the digestion of food, absorption of nutrients, and excretion of waste, but also it provides feedback to the brain via hormone signaling that can affect behavior (reviewed in Miguel-Aliaga, Jasper, & Lemaitre, 2018). The foraging gene influences many feeding-related phenotypes suggesting that foraging may have functions in the adult gastric system. We found that the forCR00867-TG4.2 allele expressed throughout the gastric system (Figure 4(A–F)). Expression was evident in all major compartments (foregut and crop, cardia, midgut, hindgut, and Malpighian tubules) of the gastric system and all major cell types.
Figure 4.
(-A″) Maximal projections of the forCR00867-TG4.2 CRIMIC allele driving UAS-Watermelon in the adult gut. Membrane bound GFP in green (A), nuclear mCherry in magenta (A′), membrane and nuclear merged with F-actin (A′′). Scale bar = 200 µm. (B–B′′) Maximal projections of forCR00867-TG4.2 driving UAS-Watermelon in the cardia and other tissues. Membrane bound GFP in green (B), nuclear mCherry in magenta (B′), membrane and nuclear merged with F-actin in blue (B′′). White arrow heads indicating the salivary gland. White arrows indicating the Malpighian tubules. (C–C′′′) Single section of forCR00867-TG4.2 driving UAS-mCD8::GFP in the anterior midgut. GFP expression can be seen in the enteroendocrine (EE) cells and the muscle (C). EE cells can be identified by the expression of Brp (C′), and muscles with F-actin (C′′). White arrowhead indicating an example of co-expression of GFP and Brp (C′′′). (D) Single section of forCR00867-TG4.2 driving UAS-mCD8::GFP in the intestinal stem cells of the midgut. (E–E′) Subsection of forCR00867-TG4.2 driving UAS-mCD8::GFP in the visceral muscle of the midgut. (F–F′′′) Maximal projections of forCR00867-TG4.2 driving UAS-Watermelon in the Malpighian tubules. (G) Schematic of the of the foraging locus depicting regions of cloned forpr-Gal4s. (H–H′′) forpr2-Gal4 driven expression in the gastric system. Expression was seen in the foregut and cardia (G), as well as the midgut intestinal stem cells (G′), and the Malpighian stem cells in the ureter (G′′). I–I′′′. forpr3-Gal4 driven expression in the gastric system. Expression was seen in middle midgut enterocytes (H), anterior EE cells (H′), distal segment of the Malpighian tubules (H′′), and the salivary glands (H′′′). J–J′. forpr4-Gal4 driven expression in the gastric system. Expression was seen in epithelia of the hindgut (I), the ampulla (I′), and the salivary duct (I′′). B–J. Scale bars = 50 µm. [Please refer to the online version for colors.]
Expression was found in the esophagus, foregut (crop duct), and crop (Figure 4(A,B)). The crop provides a central role in nutrient sensing and digestion in the adult fly (Hadjieconomou et al., 2020). forCR00867-TG4.2 expressed in the enterocytes (ECs; Figure 4(A,B)) and enteroendocrine cells (EE cells; Figure 4(C)) of the midgut. The EE cells can be identified by their morphology and by co-expression with Brp (Figure 4(C′); Zeng, Lin, & Hou, 2013). The ECs and EEs are important for absorption and digestion of ingested nutrients. Moving from the anterior to the posterior, digestion shifts from larger macromolecules to smaller monosaccharides. We also observed expression in the intestinal stem cells of the midgut (Figure 4(D)). The ISCs are important for midgut homeostasis (Micchelli & Perrimon, 2006; Ohlstein & Spradling, 2006; reviewed in Miguel-Aliaga et al., 2018).
Expression was found in the visceral muscle throughout the entire length of the gut, from the foregut to the hindgut (Figure 4(E)). The visceral muscle is required to push these nutrients along via peristalsis. forCR00867-TG4.2 was expressed throughout the Malpighian tubules (Figure 4(B), arrow; Figure 4(F)). The tubules are vital for ion balance in the fly's hemolymph. There was strong expression in the hindgut proliferation zone of the pylorus, and in the epithelial cells throughout the rest of the hindgut and ampulla (Figure 4(A)). Much of the hindgut is important for ion absorption, and the ampulla is crucial for water balance (Lemaitre & Miguel-Aliaga, 2013). forCR00867-TG4.2 also drove expression in the salivary glands (Figure 4(B), arrowhead) and the trachea innervating the gut (Figure 4(C), arrow).
We next turned to our forpr-Gal4 driver lines to parse this expression pattern and map its regulatory elements (Figure 4(G)). As for the larva, we found a modular pattern of expression in the adult. We found no detectable expression from forpr1-Gal4 in the gastric system. forpr2-Gal4 expression was found in the epithelia of the foregut, crop, and cardia (Figure 4(H)). forpr2-Gal4 expression was also seen in the midgut ISCs (Figure 4(H′)) as typified by co-expression with DELTA (Figure S2(A); Micchelli & Perrimon, 2006; Ohlstein & Spradling, 2006 ) and in the EE precursor cells (pre-EE) as typified by co-expression with PROSPERO (Figure S2(B); Zeng & Hou, 2015). forpr2-Gal4 expression was found in the stem cell zone of the ureter and lower Malpighian tubule (Figure 4(H′′); Singh & Hou, 2009; Sözen, Armstrong, Yang, Kaiser, & Dow, 1997; Wang & Spradling, 2020). The forpr3-Gal4 drove expression in a narrow band of ECs in the mid-region of the midgut, a few anterior EEs, visceral gut muscle, and the principal cells of the transitional segment of the Malpighian tubules (Figure 4(I–I′′)). forpr4-Gal4 drove expression in the epithelia of the hindgut and in the rectal ampulla (Figure 4(J–J′)). forpr3-Gal4 drove expression throughout the salivary glands (Figure 4(I′′′)), and forpr4-Gal4 was restricted to the salivary duct of the salivary gland (Figure 4(J′′)). Overall, the expression seen in the forpr-Gal4 lines is a subset of the expression found using the forCR00867-TG4.2 allele.
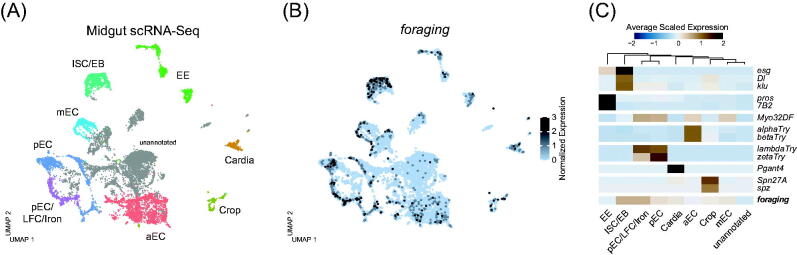
foraging transcripts have previously been detected throughout the gastric system in dissected bulk RNA-Seq experiments (Buchon et al., 2013; Leader et al., 2018), and sorted cell types from the midgut (Dutta et al., 2015). foraging transcripts were detected in all major cell types (ISC, EB, EE, EC, and VM) in all major segments (cardia and R1, R2, R3, R4, and R5) of the midgut (Dutta et al., 2015). To further explore and validate foraging's expression in the gastric system, we turned to the adult midgut's single-cell transcriptomic atlas (Figure 5(A); Hung et al., 2020). Cell types were identified using known and established markers (as described in Hung et al., 2020). foraging expression was seen in all the midgut cell types (Figure 5(B)), with its expression most enriched in the ISC/EB cluster (Figure 5(C)).
Figure 5.
(A) UMAP plot of single-cell RNA sequencing from the adult midgut (data from Hung et al., 2020). Each point represents the transcriptome of a single cell. Cells are clustered based on similarity of gene expression. Distinct cell types are represented by different colors. EE: enteroendocrine cells; ISC: intestinal stem cells; EB: enteroblasts; LFC: large flat cells; Iron: iron cells; aEC: anterior enterocytes; mEC: middle enterocytes; pEC: posterion enterocytes. (B) Expression of foraging in the single-cell midgut Atlas. Cells are color coded according to the level of normalized expression. (C) Heatmap showing the average scaled expression of cell type marker genes across each cell cluster. Initialisms are defined in A. foraging is most enriched in the ISC/EB and pEC/LFC/Iron clusters. EE cells are marked by pros and 7B2. ISC/EB are marked by esg, Dl, and klu. All ECs are marked by Myo32DF, and the anterior to posterior access is delineated by a series of trypsin coding genes (alphaTry, betaTry, lambdaTry, zetaTry, among others). The cardia is marked by Pgant4, and the crop by Spn27A and spz. [Please refer to the online version for colors.]
Foraging expression in the adult reproductive systems
forCR00867-TG4.2 was expressed in most cell types throughout the female and male reproductive system. Expression was seen in the epithelia of the uterus, common oviduct, lateral oviducts (Figure 6(A,B)). Expression was observed in the ovarioles and epithelial sheath surrounding the ovarioles (Figure 6(B)). The most robust expression was seen in the spermatheca (Figure 6(A), arrowhead; Figure S2(C)) and fat cells associated with the spermatheca (Figure 6(A), arrow). foraging is also known to have high levels of expression in the spermatheca, from microarray and RNA-Seq experiments on dissected tissue (Chintapalli, Wang, & Dow, 2007; Leader et al., 2018). forCR00867-TG4.2 is also expressed in the follicle cells of the developing eggs (Figure 6(B), arrowhead). The maternal loading of foraging in the developing eggs and the early embryo has been well characterized (Graveley et al., 2011; Jambor et al., 2015; Koenecke et al., 2016; Tomancak et al., 2002).
Figure 6.
(A–E′′′) forCR00867-TG4.2 CRIMIC allele driving UAS-Watermelon in female and male reproductive systems with membrane bound GFP in green (A–E), nuclear mCherry in magenta (A′–E′), membrane and nuclear merged with F-actin in blue (A′′–E′′). (A–A′′) Maximal projections of expression in the female reproductive system; uterus, spermatheca (white arrowhead), and common oviduct (white double arrowhead) of the female reproductive system. Expression was also seen in the spermatheca associated fat (white arrow) and smooth muscle. (B–B′′) Maximal projections of expression in the female reproductive system; ovarioles and lateral oviducts. Expression was seen in the common and lateral oviducts, epithelial sheath surrounding the ovariole, and follicle cells (arrowhead). (C–C′′) Maximal projections of expression in the male reproductive system. Expression was seen throughout; ejaculatory duct (white arrow), seminal vesicles, testis, and accessory glands (white arrowhead). Other tissues are also present (Malpighian tubules – yellow arrowhead). (D–D′′) Magnification of the accessory gland. Primary cells indicated with white arrow, and secondary cells indicated with white arrowhead. Other tissues are also present (Malpighian tubules – yellow arrowhead, trachea – yellow arrow). (E–E′′) Magnification of the ejaculatory bulb. Lower ejaculatory duct (white arrow) and fat tissue (white arrowhead) are indicated. (F) Schematic of the foraging locus depicting regions of cloned forpr-Gal4s. G. forpr1-Gal4 driven GFP expression in the male reproductive system. (H) forpr2-Gal4 driven expression in the spermatheca. (H′) forpr2-Gal4 driven expression in the ovaries. H′′. forpr2-Gal4 driven expression in the oviduct. (H′′′) forpr2-Gal4 driven expression in the male reproductive system. (I) forpr4-Gal4 driven expression in the male reproductive system. (I′) forpr4-Gal4 driven expression in the ejaculatory bulb. Scale bars = 50 µm. [Please refer to the online version for colors.]
forCR00867-TG4.2 is expressed throughout the male reproductive system, with strongest expression in the anterior ejaculatory duct (Figure 6(C), white arrow), secondary cells of the accessory gland (Figure 6(C,D), white arrowhead), and ejaculatory bulb (Figure 6(E)). The male accessory glands produce many proteins required for fertility, and their secretions have been shown to have substantial effects on female post-mating behaviors (Wolfner, 1997). The ejaculatory bulb functions to pump the ejaculate and contributes to glandular secretions. Extensive expression was seen in the rest of the reproductive system, including the testes, seminal vesicle, and primary cells of the accessory gland (Figure 6(C,D)). The seminal vesicles are primarily a storage organ for mature sperm prior to copulation, and they also produce glandular secretions for the seminal fluid (Ram & Wolfner, 2007). Expression was also seen in the smooth muscle throughout the female and male reproductive systems.
Once again, we return to the forpr-Gal4 lines to parse these expression patterns and map its regulatory elements. We saw expression of forpr2-Gal4, but not forpr1-, forpr3-, and forpr4-Gal4, in the female reproductive system. forpr2-Gal4 expressed in the spermatheca (Figure 6(H)) and the follicle cells of the developing eggs (Figure 6(H′)). forpr2-Gal4 also expressed in a small segment of the common oviduct (Figure 6(H′′)).
forpr1-, forpr2-, and forpr4-Gal4 all drove expression in the seminal vesicle and secondary cells of the accessory glands, albeit to varying extents (Figures 6(H′′′, I, G), respectively). forpr2-Gal4 had the added inclusion of a small ring of cells at the base of the vas deferens where it joins with the ejaculatory duct (data not shown). forpr4-Gal4 drove expression in the ejaculatory bulb of the male reproductive system (Figure 6(I′)). forpr3-Gal4 had no observed expression in the male reproductive system.
Mapping foraging’s cis-regulatory elements (CREs)
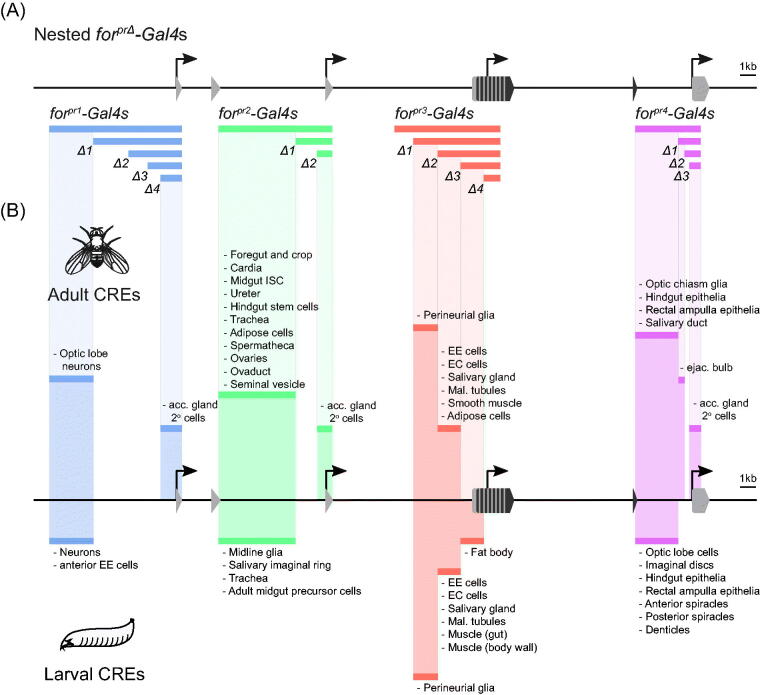
To further map and refine the CREs within the foraging gene, we generated a series of 13 nested forprΔ-Gal4 driver lines covering regions within the four original forpr-Gal4s (Figure 7(A), bars below locus). These regions were cloned into the same insulated vector used for the original forpr-Gal4s and were integrated into attP2 with φC31 integration (see “Methods”). By comparing the expression patterns seen between these different lines, we could narrow the putative CREs in the foraging locus (Figure 7(B), above the locus). For instance, in the adult fly, forpr3-Gal4 and forpr3Δ1-Gal4 did not differ in their expression patterns, so we did not map any element to the first forpr3 region. In contrast, forpr3Δ2-Gal4 had all the expression of forpr3Δ1-Gal4, except for the perineurial glia expression. This allowed us to map the perineurial CRE to this second segment of the forpr3 region. All nested forprΔ-Gal4s exhibited either the same or a subset of the expression patterns seen in their respective larger constructs.
Figure 7.
(A). Schematic of cloned regions for the nested forpr-Gal4s. The 5′-end of the foraging locus is depicted with regions corresponding to the 4 original forpr-Gal4s, and the 13 newly generated nested forprΔ-Gal4s are below the locus, each numbered according to ranked size (e.g. forpr1Δ1-Gal4, forpr1Δ2-Gal4, etc.). These regions were cloned into a gypsy insulated Gal4 vector and inserted into attP2 landing site with φC31 integration. (B) Mapped cis-regulatory elements (CREs) in the adult (depicted above the locus) and larval (depicted below the locus) D. melanogaster. CREs were mapped along the locus by comparing the expression patterns of the 17 nested cloned forpr-Gal4s.
Many genes have highly conserved promoter structure and show consistent expression across development (Graveley et al., 2011; Hoskins et al., 2011). Is this trend also true for foraging? The forpr1-4-Gal4 lines were previously characterized for their expression in the larval CNS and gastric system (Allen et al., 2018). Examining the expression of the newly generated nested lines allows us to compare the regulation between the larva and adult fly. As was seen in the adult, the expression in the larvae of the nested Gal4 lines were either the same or a subset of the expression seen in the larger forpr-Gal4 lines. A number of these refined CREs drove comparable expression in the adult (Figure 7(B), above the locus) and the larvae (Figure 7(B), below the locus). For example, the first region from forpr1-Gal4 drove expression in neurons. The first region from forpr2-Gal4 drove expression in the larval and adult intestinal stem cells (“Adult midgut precursor cells” and “Midgut ISC”, respectively). The perineurial CRE and the CRE driving expression in the salivary gland, EE cells, ECs, and Malpighian tubules mapped in the forpr3-Gal4s also drove consistent expression in the larva and adult. The CRE driving expression in the epithelia of the hindgut and rectal ampulla from forpr4-Gal4s was also shared. These data provide evidence that foraging has conserved promoter structure exhibiting consistencies in expression between the larval and adult stage of development.
Discussion
Relatively little is known about the precise spatial- and cell-specific expression of foraging, a highly conserved gene with a complex gene structure (reviewed in Anreiter & Sokolowski, 2019). Here we set out to characterize foraging gene expression in the adult brain, gastric system, and reproductive systems of D. melanogaster. We employed a multimodal approach by combining foraging gene-trap T2A-Gal4, promoter Gal4 fusions, and the mining of existing single-cell transcriptomic data sets. The advantages and limitations of these techniques are described below.
Trojan/CRIMIC style insertion alleles are unparalleled in their expression fidelity due to their function as both a transcriptional and translational trap of the locus (Diao et al., 2015; Lee et al., 2018). Unlike insertion alleles made with older technologies, Trojan/CRIMIC insertion alleles significantly limit offsite effects on the readout of a gene’s expression. Consequently, it is more likely that the forCR00867-TG4.2 allele, inserted into the foraging locus, captures the expression of all known foraging isoforms and therefore, should represent the full foraging expression pattern (Figure 1(A)).
Promoter fusion reporters have been widely used to characterize the spatial expression pattern of genes in D. melanogaster. However, the position where they are integrated into the fly’s genome can significantly alter the level and patterns of expression that are seen (Kellum & Schedl, 1991; Markstein, Pitsouli, Villalta, Celniker, & Perrimon, 2008; Spradling & Rubin, 1983). Problems with position effects can be reduced using gypsy insulators (Barolo et al., 2000; Billeter & Goodwin, 2004; Gdula, Gerasimova, & Corces, 1996; Markstein et al., 2008) and φC31 site-specific integration (Groth et al., 2004; Markstein et al., 2008). We employed both these strategies when constructing our forpr-Gal4 transgenic constructs. The lack of shared expression between the largest of each forpr-Gal4 constructs suggests a lack of position effects from our forpr-Gal4 insertions or expression driven by the empty Gal4 vector itself. An enhancer’s ability to induce expression can also be dependent on the core promoter element it is paired with (Lehman et al., 1999; Merli, Bergstrom, Cygan, & Blackman, 1996; Ohtsuki, Levine, & Cai, 1998). Our lines exploited foraging’s native core promoters and TSS from the locus. Together, these strategies should increase the likelihood that the expression we found in our forpr-Gal4 experiments faithfully recapitulates foraging expression.
Massively parallel single-cell transcriptomic experiments provide unprecedented cellular resolution of gene expression but are not without their caveats. For instance, droplet-based methods suffer from a number of factors, such as drop-out events where only a subset of the transcriptome from a cell is captured (Kim, Zhou, & Chen, 2020; Qiu, 2020), doublet and multiple formation where multiple cells are co-encapsulated (Bernstein et al., 2020; McGinnis, Murrow, & Gartner, 2019; Wolock, Lopez, & Klein, 2019), and contamination of ambient RNA generated during the dissection and dissociation (Yang et al., 2020; Young & Behjati, 2018). Sufficient sampling of each given cell type along with the above cited bioinformatic approaches can reduce these issues.
Overall, we found foraging expressed in multiple glia sub-types in the brain with the strongest expression seen in the perineurial glia. This was supported by the T2A-Gal4 alleles forCR00867-TG4.2 (Figure 1(B–D)) and forMI01791-TG4.1 (Figure S1(G)), the BAC{forIRES-Gal4} recombineered allele (Figure S1(H)), the forpr3-Gal4 cloned promoter Gal4 transgene (Figure 1(E′′′); Figure S1(D–E)), single-cell RNA-Seq data sets (Figure 2(A–D); Davie et al., 2018; Croset et al., 2018), and bulk RNA-Seq data on sorted cells (DeSalvo et al., 2014). No clear neuronal expression was seen in the brain in either gene-trap allele, or the single-cell transcriptomic data, however, a few neurons were labelled with forpr1-Gal4 (Figure 1(E′)). We also show that the previously characterized anti-FOR immunoreactivity in adult brain neurons (Belay et al., 2007; Mery et al., 2007) is non-specific and does not reflect bone fide FOR expression (Figure 3). Finally, we also found that the foraging gene expressed extensively throughout the gastric and reproductive systems (Figures 4–6) and the expression in these tissues and cells was corroborated with data from microarray, bulk RNA-Seq, and single-cell RNA-Seq based transcriptomic studies (Brown et al., 2014; Buchon et al., 2013; Chintapalli et al., 2007; Dutta et al., 2015; Hung et al., 2020; Leader et al., 2018). Each of these results are discussed in more detail below.
Foraging in the adult brain
Glia are intwined with neuronal physiology and behavior. Glia influence the development and function of the synapse, neurotransmitter release, ion homeostasis and immune function throughout an organism’s life (reviewed in Artiushin & Sehgal, 2020). The number of studies of the role of glia in behavior is growing rapidly but is still much more limited than their neuronal counterparts. Glia serve important roles in behavior (reviewed in Freeman, 2015; Zwarts, Van Eijs, & Callaerts, 2015). For example, in D. melanogaster, glia function in embryonic motility and adult locomotion (Lehmann & Cierotzki, 2010; Pereanu et al., 2007) and in male courtship behavior (Grosjean, Grillet, Augustin, Ferveur, & Featherstone, 2008). A subset of glia called astrocytes function in sleep and circadian rhythms of locomotion and their cellular correlates (Artiushin & Sehgal, 2020).
At the larval neuromuscular junction (NMJ) glial-specific expression of foraging decreases neurotransmitter release by negatively regulating nerve terminal growth (Dason et al., 2019; Dason & Sokolowski, 2021). The for0 null mutant has increased nerve terminal growth which can be rescued by glial-specific, and not neuronal-specific (pre-synaptic) or muscle-specific (post-synaptic) expression of foraging (Dason et al., 2019). Furthermore, glial-specific knockdown of foraging phenocopies the nerve terminal over-growth and increased neurotransmitter release phenotypes seen in the for0 null mutant (Dason & Sokolowski, 2021). Knockdown of foraging in presynaptic neurons of the NMJ impairs synaptic vesicle endocytosis, whereas knockdown of foraging in glia does not. Overall, D. melanogaster foraging can alter neurotransmitter release at the NMJ by regulating both synaptic structure and function. Similar functions are likely also at play in the adult nervous system. In mammalian systems, PKG has been implicated in Ca2+ mobilization and influx in glia (Willmott, Wong, & Strong, 2000), and in translocation to the nucleus to regulate gene expression in glia (Gudi, Hong, Vaandrager, Lohmann, & Pilz, 1999). In D. melanogaster, the perineurial and sub-perineurial glia function as the blood–brain barrier and are important for regulating the transport and exchange of nutrients, metabolites, and hormones between the brain and the hemolymph (Stork et al., 2008). foraging may affect behaviors, such as learning and memory, in the fly by regulating the Ca2+ environment surrounding neuronal cell bodies via its cortex glial expression. Cortex glia, in the fly, have been shown to have Ca2+ oscillations which when disrupted can alter behavior (Melom & Littleton, 2013). Alternatively, foraging may alter the metabolic environment of the brain by mediating nutrient and waste transport via its perineurial glia expression. Further experiments are required to test this hypothesis.
Although we found strong evidence that foraging is expressed in glia of the adult brain, we found no detectable neuronal expression in the adult brain using the forCR00867-TG4.2 allele (Figure 1(B–D)) and the single-cell transcriptomics data (Figure 2(A–D)). In contrast, foraging is expressed in some peripheral neurons and central neurons at earlier stages of development. foraging was detected in adult photoreceptor neurons (Davie et al., 2018; Davis et al., 2020; Figure 2(D)), the 3rd instar larval neuromuscular junction (Dason et al., 2019), and in the VNC along the midline of late-stage embryos (Peng et al., 2016). Many studies have demonstrated a role for PKG in mammalian nervous systems (reviewed in Feil, Hofmann, & Kleppisch, 2005; Hofmann, Feil, Kleppisch, & Schlossmann, 2006). For example, cerebellum specific conditional knockout of PKG in the mouse impairs long-term depression affecting motor learning (Feil et al., 2003), and a nociceptor specific knockout in the dorsal root ganglia abolishes long-term potentiation affecting pain sensitivity (Luo et al., 2012). Prkg1, foraging’s mouse homologue, is readily detected in many subtypes of neurons and glia in the brain of both the developing mouse embryo and adolescent mouse, using single-cell RNA-Seq (La Manno et al., 2020; Zeisel et al., 2018). The readily detectable neuronal expression in the mouse may suggest some differences in regulation between the fly and the mouse. The expression was strongest in the neuroblasts of the developing embryo (La Manno et al., 2020) and the enteric and sensory neurons of the peripheral nervous system in the adolescent mouse (Zeisel et al., 2018). Taken together, it is possible that foraging, in D. melanogaster, may play a stronger role in the developing nervous system and the developed peripheral nervous system then it does in the adult central brain.
We also found that our previously published anti-FOR antibody (Belay et al., 2007) is not an effective tool for immunohistochemical analyses of FOR in the adult brain. Problems with the unreliability of antibodies have been well discussed in the literature (Egelhofer et al., 2011; Fritschy, 2008; Michel, Wieland, & Tsujimoto, 2009; Rhodes & Trimmer, 2006; Saper & Sawchenko, 2003). We re-evaluated the efficacy of immunohistochemistry of a previously published (Belay et al., 2007) anti-FOR antibody using a complete null allele of foraging (for0; Allen et al., 2017). We found that the anti-FOR antibody labels bone fide FOR expression in cells with morphology consistent with the outer optic chiasm glia. However, the anti-FOR antibody had extensive nonspecific immunoreactivity in neurons, including mushroom and ellipsoid bodies. We concluded that the anti-FOR antibody known to be a valuable tool on westerns (Allen et al., 2017; Dason et al., 2019) is not effective for immunohistochemical analyses of FOR in the adult brain. Data from single-cell RNA-Seq supported the findings of glial expression, but no neuronal expression, seen in the forCR00867-TG4.2 allele. We cannot, however, distinguish between a very low expression of foraging or no expression of foraging outside of glia in the adult brain. This is discussed further, below.
Previous studies suggest that foraging functions in neurons to affect adult behaviors, including various forms of adult learning and memory (Kuntz et al., 2012; Mery et al., 2007; Wang et al., 2008), olfactory startle habituation (Eddison et al., 2012), movement in an open field (Burns et al., 2012) and sleep (Donlea et al., 2012). Many of these studies relied on targeting a UAS-forcDNA pan-neuronally, or to specific subsets of neurons from multiple regions of the brain, including antennal lobe, mushroom bodies, and central complex. These studies, which use a UAS-forcDNA, clearly show that FOR protein can alter multiple behaviors when expressed in these neuronal populations. What is not clear is whether foraging is naturally expressed in the cells in these above studies. Two of the above studies, however, took advantage of UAS-forRNAi (Donlea et al., 2012; Kuntz et al., 2012). Donlea et al. (2012) showed that driving UAS-forRNAi in the α/β Kenyon cells, with the 30Y enhancer trap, altered short term memory when compared with controls. Kuntz et al. (2012) altered visual orientation memory by driving UAS-forRNAi in Kenyon cells and ring neurons, with the lilli189Y enhancer trap. This strengthens the case for bone fide foraging expression in these respective cells, although, RNAi can have off-target effects (Ma et al., 2006; Moffat et al., 2007).
Many of these studies relied on the previously published anti-FOR pattern in the adult brain (Belay et al., 2007; Mery et al., 2007) which we show in this study to be non-specific (Figure 3). It is important to note that non-specific binding of the anti-FOR antibody does not necessarily mean that foraging is not expressed in neurons such as the Kenyon cells of the mushroom bodies or the ring neurons of the central complex. One of the above studies showed that driving a UAS-forcDNA in the R3 ellipsoid body ring neurons affects working memory in a visual orientation paradigm (Kuntz et al., 2012). In this case, a number of Gal4 enhancer trap lines were used, targeting subsets of the ellipsoid body ring neurons, and mushroom body Kenyon cells. One such enhancer trap was lilli189Y (previously referred to as for189Y) that was initially thought to be inserted in foraging (Osborne et al., 1997) but was subsequently shown to be inserted in lilliputian (Sokolowski 2007 FlyBase update FBrf0198702; Wang et al., 2008). Since lilli189Y is not inserted in foraging, we cannot assume that it would necessarily recapitulate foraging expression.
Another of the above studies used enhancer traps in foraging to infer the expression of foraging in the adult brain (Eddison et al., 2012). Eddison et al. (2012) used two enhancer trap lines, for11.247 and for2614, which were inserted in the foraging gene at its most 5′ end near the forpr1 TSS. At the time, parts of the expression of for11.247 were validated with the anti-FOR antibody. The enhancer traps in foraging and the promoter fusions from foraging, used in the present study, are equally likely to reflect actual foraging expression and may represent distinct subsets of expression in the adult CNS. However, it is also possible that these 5′ insertions may trap enhancers of the neighbouring gene, Drgx, which has been shown to contain regulatory elements that drive expression in the antennal lobe, mushroom bodies, as well as the ellipsoid body (Jenett et al., 2012). The possibility of crosstalk with Drgx enhancers may also exist for our forpr1-Gal4 as this cloned region lies between foraging and Drgx.
There is an apparent discrepancy between the lack of detectable foraging expression in adult brain neurons found in the present study and published papers showing that transgenically expressing foraging, with a UAS-forcDNA, in adult brain neurons alters behavioral phenotypes. Some possible reasons for this are as follows: 1) foraging is expressed in these tissues, but at levels too low to be detected with the methods used in the present study. 2) foraging is only conditionally expressed in these neuronal populations depending on certain environmental conditions. 3) foraging is expressed in these neurons, but only during development. 4) These previous studies using UAS-forcDNA represent ectopic expression, driving FOR protein where it is not naturally expressed. We discuss these possibilities below.
To attempt to address the sensitivity issue of single-cell RNA-Seq, we looked for foraging expression in bulk RNA-Seq studies, which are more sensitive, on sorted neuronal cell populations. Three separate studies, in which multiple sub-populations of Kenyon cells were assayed, failed to detect foraging in Kenyon cells at levels higher than that of repo, or other glial-specific or non-neuronal markers (Crocker, Guan, Murphy, & Murthy, 2016; Davis et al., 2020; Shih, Davis, Henry, & Dubnau, 2019). Davis et al. (2020) did however find foraging expression in photoreceptor neurons in the eye of the fly, which was also seen in single-cell transcriptomic data (Davie et al., 2018; Figure 2(D)). Mammalian PKG has previously been shown to be involved in neurite plasticity in rod and cone cells (Zhang, Beuve, & Townes-Anderson, 2005).
We currently do not know whether foraging is conditionally expressed in adult brain neurons in different environmental contexts, such as different nutritional states or different learning paradigms. foraging expression has previously been shown to be up regulated by food deprivation in whole adult heads (Kent et al., 2009). Although, the head is more than just neurons, and so fat cells, trachea, and glia in the head capsule are also candidate cell types for this observed effect. Further experiments specifically assaying adult brain neurons in differing environmental conditions are required to explicitly test any potential condition expression of foraging.
We currently do not have any direct measures of foraging expression in neurons during different developmental stages. However, two of the above-mentioned studies (Kuntz et al., 2012; Wang et al., 2008) did employ the temperature sensitive GAL4 repressor GAL80ts (McGuire et al., 2003) to restrict expression of the UAS-forcDNA to post-eclosion adults. In both cases, the authors found that adult restricted expression was sufficient to modulate their respective phenotypes, working memory in a visual orientation paradigm (Kuntz et al., 2012) and visual pattern memory (Wang et al., 2008). In these cases, the developmental possibility is, at least in part, ruled out. Again, further experiments are required to assess the potential role for foraging in neurons of developing larvae and pupae.
Finally, if in fact, foraging is not naturally expressed in the adult brain neurons where UAS-forcDNA was expressed in previous studies, then the UAS-forcDNA manipulations constitute ectopic expression. In this case, FOR protein may still be interacting with bone fide PKG targets, which may typically co-express in other cell types. The observed effects may then represent neomorphic-like phenotypes. Alternatively, at the biochemical level there can be crosstalk between the cAMP and cGMP systems, whether by PKG activation by cAMP (Lin, Liu, Chow, & Lue, 2002; Lin, Liu, Tu, Chow, & Lue, 2001; Lincoln & Cornwell, 1991; Ruiz-Velasco, Zhong, Hume, & Keef, 1998; White, Kryman, El-Mowafy, Han, & Carrier, 2000), or PKG phosphorylating a PKA target due to similar phosphorylation target recognition sites (Douglass et al., 2012), or even by PKG phosphorylating shared target proteins (Döppler & Storz, 2013; Huang, Tsai, Chen, Wu, & Chen, 2007). More research is needed into the precise cellular localization and expression of foraging to decipher how its expression influences each of the gene’s pleiotropic phenotypes.
Foraging in the gastric and reproductive systems
There are many potential roles for foraging in the gastric and reproductive systems. The visceral muscle of the gut is required to push nutrients along via peristalsis. Mice with foraging's orthologue knocked out in smooth muscle show increased gut passage times relative to controls (Weber et al., 2007). foraging has been implicated fecal excretion rate in adults (Urquhart-Cronish & Sokolowski, 2014) and the visceral muscle is a candidate tissue to mediating this function. foraging has also been implicated in the rate of glucose absorption in larvae (Kaun et al., 2007). Simple sugars are absorbed by the enterocytes of the midgut (reviewed in Miguel-Aliaga et al., 2018) and so are strong candidate cells for foraging’s glucose absorption phenotype. foraging has also previously been shown to affect adult Malpighian tubule secretion rate (MacPherson, Broderick, et al., 2004; MacPherson, Lohmann, & Davies, 2004) and the expression in the principal cells is consistent with this phenotype.
As for the reproductive systems, differences in the number of eggs laid by flies reared in differing nutritional conditions during development (Burns et al., 2012), as well as oviposition site selection (McConnell & Fitzpatrick, 2017) has previously been associated with foraging. The expression seen in the spermatheca and visceral muscle of the reproductive system represent candidate cells for mediating this difference. The spermathecae are essential for long term storage of sperm (Gilbert, 1981), and perturbations in these cells can cause a decrease in egg-laying over time (Schnakenberg, Matias, & Siegal, 2011). foraging has also been shown to play a role in developmental death and cell clearance in the epithelial follicle cells of the D. melanogaster ovary under starvation conditions (Lebo et al., 2021). The maternal loading of foraging in the developing eggs and the early embryo is also well known (Graveley et al., 2011; Jambor et al., 2015; Koenecke et al., 2016; Tomancak et al., 2002), suggesting a role for foraging in very early development.
Foraging’s complexity and the mapping of CREs
Three genes, foraging, Pkg21D, and CG4839 code for a cGMP-dependent protein kinase (PKG) in D. melanogaster (Thurmond et al., 2019). foraging is by far the most complex locus. Pkg21D is less than 5 kb with 1 TSS, 1 transcript and 1 protein isoform, while CG4839 is less than 12 kb with 1 TSS, 2 transcripts, and 1 protein isoform (Thurmond et al., 2019). In contrast, foraging is a 35 kb locus with 4 TSSs, more than 20 transcripts, and potentially more than 9 protein isoforms (Allen et al., 2017). The spatial and temporal expression of the Pkg21D and CG4839 is much more restricted than that of foraging, mirroring their relative complexities. Pkg21D is primarily expressed in hindgut and Malpighian tubules, and CG4839 is primarily expressed in the midgut, salivary gland, and reproductive tissues (Leader et al., 2018). Neither appear to be detected in the adult brain (Davie et al., 2018; Leader et al., 2018). foraging orthologues play multiple roles in behavioral and physiological phenotypes in multiple taxa (reviewed in Anreiter & Sokolowski, 2019; reviewed in Reaume & Sokolowski, 2009).
A clue to foraging’s complexity may reside in the structure of foraging’s promoters and the mapped CREs. Core promoters are described by two different shapes; “narrow” (a.k.a. “peaked”, “focused”, “sharp”) promoters with transcription always initiating at a within a narrow range of a few bases, and “broad” (a.k.a. “dispersed”) promoters with transcription initiating over a larger range of bases (Haberle & Stark, 2018). Narrow promoters tend to have more spatially and temporally restricted expression patterns, whereas broad promoters tend to be more ubiquitous (Bhardwaj, Semplicio, Erdogdu, Manke, & Akhtar, 2019; Hoskins et al., 2011; Schor et al., 2017). Three of foraging’s promoters, forpr1, forpr2, and forpr4, are narrow promoters and one, forpr3, is broad (Allen et al., 2017; Hoskins et al., 2011). Based on the forpr-Gal4 expression patterns reported here, the CRE driving the secondary cells of the male accessory glands mapped very close to the TSS of forpr1, forpr2, and forpr4. This closely linked accessory gland CRE and the fact that forpr1, forpr2, and forpr4 are all narrow promoters may suggest a common evolutionary origin of these TSSs.
Promoter analyses has been successfully used previously to identify CREs and deduce isoform-specific expression and function in many genes (Arredondo et al., 2001; Billeter & Goodwin, 2004; Brenner, Thomas, Becker, & Atkinson, 1996; Lehman et al., 1999; Okada et al., 2001; Park et al., 2000). Consequently, we used a promoter analysis strategy to map multiple CREs along the foraging locus responsible for a subset of the expression seen in the T2A-Gal4 gene-trap allele. The expression seen in the forpr-Gal4s may be only a subset of that seen in the forCR00867-TG4.2 allele because the regions cloned into the forpr-Gal4s encompass only 15 kb of the 35 kb locus, suggesting that some CREs were missed and/or because each forpr-Gal4 was inserted outside the context of the foraging locus at a common site on the third chromosomes (see “Methods”).
Conserved expression and regulation across development
We can draw parallels between the regulation, expression, and function of genes across development from larva to adult despite the differences in life-history traits of these developmental stages. Many genes show consistent expression across development (Graveley et al., 2011). Additionally, promoter structure can be highly conserved between developmental stages (Hoskins et al., 2011). Like foraging, the CREs of slowpoke, pigment-dispersing factor, and paramyosin drive consistent expression across development (Arredondo et al., 2001; Brenner & Atkinson, 1996; Park et al., 2000). The expression levels and spatial distribution of foraging are similar between tissues of the third instar larva and adult fly, as deduced from microarray and RNA-Seq on dissected tissues (Chintapalli et al., 2007; Leader et al., 2018). Here we show that many but not all the CREs within the foraging locus also drive consistent expression between these two stages. The parallels in the expression of foraging at these two developmental stages mirror many of the parallels seen in the larval and adult phenotypic traits.
Conclusion
Our characterized expression patterns of foraging provide novel candidate tissues and cell types to explore foraging’s pleiotropic influences on physiology and behavior. We provide exciting new data on foraging expression in multiple subsets of glia in the adult brain, paving the way towards functional studies that address the importance of glial subtypes in each of foraging’s suite of pleiotropic behavioral phenotypes.
Supplementary Material
Acknowledgments
The authors thank the past and present members of the Sokolowski Lab for years of helpful discussions. The authors thank Andreas Prokop for the adult Drosophila illustration and Josh Dubnau for the UAS-Watermelon line. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. The authors thank Stephen F. Goodwin for comments on the manuscript and support for the work conducted while at the Centre for Neural Circuits and Behaviour. The authors thank the two anonymous reviewers for their comments and review of the manuscript.
Funding Statement
This research was supported by grants from the Natural Sciences and Engineering Council of Canada (NSERC), the Canadian Institutes for Health Research (CIHR) and NIDDK grant 5R01DK70141-2 to M.B.S. A.M.A. was also supported by a Wellcome Investigator Award [106189/Z/14/Z] to Stephen F. Goodwin.
Disclosure statement
The authors report no conflict of interest.
References
- Allen, A. M., Anreiter, I., Neville, M. C., & Sokolowski, M. B. (2017). Feeding-related traits are affected by dosage of the foraging gene in Drosophila melanogaster. Genetics, 205(2), 761–773. doi: 10.1534/genetics.116.197939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, A. M., Anreiter, I., Vesterberg, A., Douglas, S. J., & Sokolowski, M. B. (2018). Pleiotropy of the Drosophila melanogaster foraging gene on larval feeding-related traits. Journal of Neurogenetics, 32(3), 1–11. doi: 10.1080/01677063.2018.1500572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, A. M., Neville, M. C., Birtles, S., Croset, V., Treiber, C. D., Waddell, S., & Goodwin, S. F. (2020). A single-cell transcriptomic atlas of the adult Drosophila ventral nerve cord. eLife, 9. doi: 10.7554/eLife.54074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwash, N., Allen, A. M., Sokolowski, M. B., & Levine, J. D. (2021). The Drosophila melanogaster foraging gene affects social networks. Journal of Neurogenetics. 10.1080/01677063.2021.1936517 [DOI] [PubMed] [Google Scholar]
- Anreiter, I., & Sokolowski, M. B. (2019). The foraging gene and its behavioral effects: Pleiotropy and plasticity. Annual Review of Genetics, 53(1), 373–392. doi: 10.1146/annurev-genet-112618-043536 [DOI] [PubMed] [Google Scholar]
- Anreiter, I., Allen, A. M., Vasquez, O. E., To, L., Douglas, S. J., Alvarez, J. V., … Sokolowski, M. B. (2021). The Drosophila foraging gene plays a vital role at the start of metamorphosis for subsequent adult emergence. Journal of Neurogenetics, doi: 10.1080/01677063.2021.1914608 [DOI] [PubMed] [Google Scholar]
- Anreiter, I., Kramer, J. M., & Sokolowski, M. B. (2017). Epigenetic mechanisms modulate differences in Drosophila foraging behavior. Proceedings of the National Academy of Sciences of the United States of America, 114(47), 12518–12523. doi: 10.1073/pnas.1710770114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo, J. J., Ferreres, R. M., Maroto, M., Cripps, R. M., Marco, R., Bernstein, S. I., & Cervera, M. (2001). Control of Drosophila paramyosin/miniparamyosin gene expression. Journal of Biological Chemistry, 276(11), 8278–8287. doi: 10.1074/jbc.M009302200 [DOI] [PubMed] [Google Scholar]
- Artiushin, G., & Sehgal, A. (2020). The glial perspective on sleep and circadian rhythms. Annual Review of Neuroscience, 43, 119–140. doi: 10.1146/annurev-neuro-091819-094557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki, T., Lai, S. -L., Ito, K., & Lee, T. (2008). Organization and postembryonic development of glial cells in the adult central brain of Drosophila. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(51), 13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton, R. J., Tsai, L. T. Y., Schwabe, T., DeSalvo, M., Gaul, U., & Heberlein, U. (2005). Moody encodes two GPCRs that regulate cocaine behaviors and blood–brain barrier permeability in Drosophila. Cell, 123(1), 145–156. doi: 10.1016/j.cell.2005.07.029 [DOI] [PubMed] [Google Scholar]
- Baker, M. (2015). Reproducibility crisis: Blame it on the antibodies. Nature, 521(7552), 274–276. doi: 10.1038/521274a [DOI] [PubMed] [Google Scholar]
- Barolo, S., Carver, L. A., & Posakony, J. W. (2000). GFP and β-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. BioTechniques 294, 726–732. doi: 10.2144/00294bm10 [DOI] [PubMed] [Google Scholar]
- Belay, A. T., Scheiner, R., So, A. K.-C., Douglas, S.J., Chakaborty-Chatterjee, M., Levine, J. D., & Sokolowski, M. B. (2007). The foraging gene of Drosophila melanogaster: Spatial-expression analysis and sucrose responsiveness. The Journal of Comparative Neurology, 504(5), 570–582. doi: 10.1002/cne.21466 [DOI] [PubMed] [Google Scholar]
- Bernstein, N. J., Fong, N. L., Lam, I., Roy, M. A., Hendrickson, D. G., & Kelley, D. R. (2020). Solo: Doublet identification in single-cell RNA-seq via semi-supervised deep learning. Cell Systems, 11 (1), 95–101.e5. doi: 10.1016/j.cels.2020.05.010 [DOI] [PubMed] [Google Scholar]
- Bhardwaj, V., Semplicio, G., Erdogdu, N. U., Manke, T., & Akhtar, A. (2019). MAPCap allows high-resolution detection and differential expression analysis of transcription start sites. Nature Communications, 10(1). Article number: 3219. doi: 10.1038/s41467-019-11115-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter, J. C., & Goodwin, S. F. (2004). Characterization of Drosophila fruitless-gal4 transgenes reveals expression in male-specific fruitless neurons and innervation of male reproductive structures. The Journal of Comparative Neurology, 475(2), 270–287. doi: 10.1002/cne.20177 [DOI] [PubMed] [Google Scholar]
- Bittern, J., Pogodalla, N., Ohm, H., Brüser, L., Kottmeier, R., Schirmeier, S., & Klämbt, C. (2020). Neuron–glia interaction in the Drosophila nervous system. In Developmental neurobiology. New York: Wiley. doi: 10.1002/dneu.22737 [DOI] [PubMed] [Google Scholar]
- Brenner, R., & Atkinson, N. (1996). Developmental- and eye-specific transcriptional control elements in an intronic region of a Ca2+-activated K + channel gene. Developmental Biology, 177(2), 536–543. doi: 10.1006/dbio.1996.0183 [DOI] [PubMed] [Google Scholar]
- Brenner, R., Thomas, T. O., Becker, M. N., & Atkinson, N. S. (1996). Tissue-specific expression of a Ca(2+)-activated K + channel is controlled by multiple upstream regulatory elements. The Journal of Neuroscience, 16(5), 1827–1835. doi: 10.1523/JNEUROSCI.16-05-01827.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. B., Boley, N., Eisman, R., May, G. E., Stoiber, M. H., Duff, M. O., … Celniker, S. E. (2014). Diversity and dynamics of the Drosophila transcriptome. Nature, 512(7515), 393–399. doi: 10.1038/nature12962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon, N., Osman, D., David, F. P. A., Yu Fang, H., Boquete, J. P., Deplancke, B., & Lemaitre, B. (2013). Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Reports, 3(5), 1725–1738. doi: 10.1016/j.celrep.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Burns, J. G., Svetec, N., Rowe, L., Mery, F., Dolan, M. J., Boyce, W. T., & Sokolowski, M. B. (2012). Gene–environment interplay in Drosophila melanogaster: Chronic food deprivation in early life affects adult exploratory and fitness traits. Proceedings of the National Academy of Sciences of Sciences, 109 (Supplement_2), 17239–17244. doi: 10.1073/pnas.1121265109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Y. -H., Keegan, R. M., Prazak, L., & Dubnau, J. (2019). Cellular labeling of endogenous retrovirus replication (CLEVR) reveals de novo insertions of the gypsy retrotransposable element in cell culture and in both neurons and glial cells of aging fruit flies. PLoS Biology, 17(5), e3000278. doi: 10.1371/journal.pbio.3000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli, V. R., Wang, J., & Dow, J. a T. (2007). Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nature Genetics, 39(6), 715–720. doi: 10.1038/ng2049 [DOI] [PubMed] [Google Scholar]
- Crocker, A., Guan, X. J., Murphy, C. T., & Murthy, M. (2016). Cell-type-specific transcriptome analysis in the Drosophila mushroom body reveals memory-related changes in gene expression. Cell Reports, 15(7), 1580–1596. doi: 10.1016/j.celrep.2016.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croset, V., Treiber, C. D., & Waddell, S. (2018). Cellular diversity in the Drosophila midbrain revealed by single-cell transcriptomics. eLife, 7. . doi: 10.7554/eLife.34550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dason, J. S., & Sokolowski, M. B. (2021). A cGMP-dependent protein kinase, encoded by the Drosophila foraging gene, regulates neurotransmission through changes in synaptic structure and function. Journal of Neurogenetics (this issue). DOI: 10.1080/01677063.2021.1905639 [DOI] [PubMed] [Google Scholar]
- Dason, J. S., Allen, A. M., Vasquez, O. E., & Sokolowski, M. B. (2019). Distinct functions of a cGMP-dependent protein kinase in nerve terminal growth and synaptic vesicle cycling. Journal of Cell Science, 132(7), jcs227165. doi: 10.1242/jcs.227165 [DOI] [PubMed] [Google Scholar]
- Dason, J. S., Cheung, A., Anreiter, I., Montemurri, V.A., Allen, A. M., & Sokolowski, M. B. (2020). Drosophila melanogaster foraging regulates a nociceptive-like escape behavior through a developmentally plastic sensory circuit. Proceedings of the National Academy of Sciences of the United States of America, 201820840. doi: 10.1073/PNAS.1820840116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie, K., Janssens, J., Koldere, D., De Waegeneer, M., Pech, U., Kreft, Ł., … Aerts, S. (2018). A single-cell transcriptome atlas of the aging Drosophila brain. Cell, 174(4), 982–998.e20. doi: 10.1016/j.cell.2018.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, F. P., Nern, A., Picard, S., Reiser, M. B., Rubin, G. M., Eddy, S. R., & Henry, G. L. (2020). A genetic, genomic, and computational resource for exploring neural circuit function. eLife, 9. doi: 10.7554/eLife.50901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Scully, K., Armstrong, G. A. B., Kent, C., Robertson, R. M., & Sokolowski, M. B. (2007). Natural variation in the thermotolerance of neural function and behavior due to a cGMP-dependent protein kinase. PloS One, 2(8), e773. doi: 10.1371/journal.pone.0000773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Scully, K., Bukvic, D., Chakaborty-Chatterjee, M., Ferreira, R., Milton, S. L., & Sokolowski, M. B. (2010). Controlling anoxic tolerance in adult Drosophila via the cGMP-PKG pathway. Journal Experimental Biology, 213(Pt 14), 2410–2416. doi: 10.1242/jeb.041319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Belle, J. S., Hilliker, A. J., & Sokolowski, M. B. (1989). Genetic localization of foraging (for): A major gene for larval behavior in Drosophila melanogaster. Genetics, 123(1), 157–163. doi: 10.1093/genetics/123.1.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalvo, M. K., Hindle, S. J., Rusan, Z. M., Orng, S., Eddison, M., Halliwill, K., & Bainton, R. J. (2014). The Drosophila surface glia transcriptome: Evolutionary conserved blood–brain barrier processes. Frontiers in Neuroscience, 8(OCT), 346. doi: 10.3389/fnins.2014.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao, F., Ironfield, H., Luan, H., Diao, F., Shropshire, W. C., Ewer, J., … White, B. H. (2015). Plug-and-play genetic access to Drosophila cell types using exchangeable exon cassettes. Cell Reports, 10(8), 1410–1421. doi: 10.1016/j.celrep.2015.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea, J., Leahy, A., Thimgan, M. S., Suzuki, Y., Hughson, B. N., Sokolowski, M. B., & Shaw, P. J. (2012). foraging alters resilience/vulnerability to sleep disruption and starvation in Drosophila. Proceedings of the National Academy of Sciences of the United States of America, 109(7), 2613–2618. doi: 10.1073/pnas.1112623109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döppler, H., & Storz, P. (2013). Regulation of VASP by phosphorylation Consequences for cell migration. Cell Adhesion and Migration, 7(6), 482–486. 10.4161/cam.27351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass, J., Gunaratne, R., Bradford, D., Saeed, F., Hoffert, J. D., Steinbach, P. J., … Pisitkun, T. (2012). Identifying protein kinase target preferences using mass spectrometry. American Journal of Physiology: Cell Physiology, 303(7), C715–C727. doi: 10.1152/ajpcell.00166.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, D., Dobson, A. J. J., Houtz, P. L. L., Gläßer, C., Revah, J., Korzelius, J., … Buchon, N. (2015). Regional cell-specific transcriptome mapping reveals regulatory complexity in the adult Drosophila midgut. Cell Reports, 12(2), 346–358. doi: 10.1016/j.celrep.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Eddison, M., Belay, A. T., Sokolowski, M. B., & Heberlein, U. (2012). A Genetic Screen for Olfactory habituation mutations in Drosophila: Analysis of novel foraging alleles and an underlying neural circuit. PLoS One, 7(12), e51684. doi: 10.1371/journal.pone.0051684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhofer, T. A., Minoda, A., Klugman, S., Lee, K., Kolasinska-Zwierz, P., Alekseyenko, A. A., … Lieb, J. D. (2011). An assessment of histone-modification antibody quality. Nature Structural & Molecular Biology, 18(1), 91–94. doi: 10.1038/nsmb.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, J. E., Xie, X. J., Sokolowski, M. B., & Wu, C. F. (2000). A cGMP-dependent protein kinase gene, foraging, modifies habituation-like response decrement of the giant fiber escape circuit in Drosophila. Learning & Memory (Cold Spring Harbor, NY), 7(5), 341–352. doi: 10.1101/lm.31600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil, R., Hartmann, J., Luo, C., Wolfsgruber, W., Schilling, K., Feil, S., … Hofmann, F. (2003). Impairment of LTD and cerebellar learning by Purkinje cell-specific ablation of cGMP-dependent protein kinase I. Journal of Cell Biology, 163(2), 295–302. doi: 10.1083/jcb.200306148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil, R., Hofmann, F., & Kleppisch, T. (2005). Function of cGMP-dependent protein kinases in the nervous system. In Reviews in the Neurosciences. (Vol. 16, Issue 1, pp. 23–41). Freund Publishing House Ltd. doi: 10.1515/REVNEURO.2005.16.1.23 [DOI] [PubMed] [Google Scholar]
- Foucaud, J., Philippe, A. -S., Moreno, C., & Mery, F. (2013). A genetic polymorphism affecting reliance on personal versus public information in a spatial learning task in Drosophila melanogaster. Proceedings of the Royal Society B: Biological Sciences, 280(1760), 20130588. doi: 10.1098/rspb.2013.0588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, M. R. (2015). Drosophila central nervous system glia. Cold Spring Harbor Perspectives in Biology, 7(11), a020552. doi: 10.1101/cshperspect.a020552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy, J. M. (2008). Is my antibody-staining specific? How to deal with pitfalls of immunohistochemistry. European Journal of Neuroscience, 28(12), 2365–2370. doi: 10.1111/j.1460-9568.2008.06552.x [DOI] [PubMed] [Google Scholar]
- Gao, X. J., Riabinina, O., Li, J., Potter, C. J., Clandinin, T. R., & Luo, L. (2015). A transcriptional reporter of intracellular Ca2+ in Drosophila. Nature Neuroscience, 18(6), 917–925. doi: 10.1038/nn.4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdula, D. A., Gerasimova, T. I., & Corces, V. G. (1996). Genetic and molecular analysis of the gypsy chromatin insulator of Drosophila. Proceedings of the National Academy of Sciences of the United States of America, 93(18), 9378–9383. doi: 10.1073/pnas.93.18.9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, D. G. (1981). Ejaculate esterase 6 and initial sperm use by female Drosophila melanogaster. Journal of Insect Physiology, 27(9), 641–650. doi: 10.1016/0022-1910(81)90112-8 [DOI] [Google Scholar]
- Graveley, B. R., Brooks, A. N., Carlson, J. W., Duff, M. O., Landolin, J. M., Yang, L., … Celniker, S. E. (2011). The developmental transcriptome of Drosophila melanogaster. Nature, 471(7339), 473–479. doi: 10.1038/nature09715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean, Y., Grillet, M., Augustin, H., Ferveur, J. F., & Featherstone, D. E. (2008). A glial amino-acid transporter controls synapse strength and courtship in Drosophila. Nature Neuroscience, 11(1), 54–61. doi: 10.1038/nn2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth, A. C., Fish, M., Nusse, R., & Calos, M. P. (2004). Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics, 166(4), 1775–1782. doi: 10.1534/genetics.166.4.1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi, T., Hong, G. K. ‐P., Vaandrager, A. B., Lohmann, S. M., & Pilz, R. B. (1999). Nitric oxide and cGMP regulate gene expression in neuronal and glial cells by activating type II cGMP‐dependent protein kinase. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 13(15), 2143–2152. doi: 10.1096/fasebj.13.15.2143 [DOI] [PubMed] [Google Scholar]
- Haberle, V., & Stark, A. (2018). Eukaryotic core promoters and the functional basis of transcription initiation. Nature Reviews Molecular Cell Biology, 19(10), 621–637. doi: 10.1038/s41580-018-0028-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjieconomou, D., King, G., Gaspar, P., Mineo, A., Blackie, L., Ameku, T., … Miguel-Aliaga, I. (2020). Enteric neurons increase maternal food intake during reproduction. Nature, 587(7834), 455–459. doi: 10.1038/s41586-020-2866-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon, M. S., Gisselbrecht, S., Lu, J., Estrada, B., Keshishian, H., & Michelson, A. M. (2002). New fluorescent protein reporters for use with the Drosophila gal4 expression system and for vital detection of balancer chromosomes. Genesis (New York, NY: 2000), 34(1–2), 135–138. doi: 10.1002/gene.10136 [DOI] [PubMed] [Google Scholar]
- Hall, J. (1994). The mating of a fly. Science (New York, NY), 264(5166), 1702–1714. doi: 10.1126/science.8209251 [DOI] [PubMed] [Google Scholar]
- Hofmann, F., Feil, R., Kleppisch, T., & Schlossmann, J. (2006). Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiological Reviews, 86(1), 1–23. doi: 10.1152/physrev.00015.2005 [DOI] [PubMed] [Google Scholar]
- Hoskins, R. A., Carlson, J. W., Wan, K. H., Park, S., Mendez, I., Galle, S. E., … Celniker, S. E. (2015). The Release 6 reference sequence of the Drosophila melanogaster genome. Genome Research, 25(3), 445–458. doi: 10.1101/gr.185579.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins, R. A., Landolin, J. M., Brown, J. B., Sandler, J. E., Takahashi, H., Lassmann, T., … Celniker, S. E. (2011). Genome-wide analysis of promoter architecture in Drosophila melanogaster. Genome Research, 21(2), 182–192. doi: 10.1101/gr.112466.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. Y., Tsai, M. L., Chen, G. Y., Wu, C. J., & Chen, S. H. (2007). A systematic MS-based approach for identifying in vitro substrates of PKA and PKG in rat uteri. Journal of Proteome Research, 6(7), 2674–2684. doi: 10.1021/pr070134c [DOI] [PubMed] [Google Scholar]
- Hughes, A. L. (1994). The evolution of functionally novel proteins after gene duplication. Proceedings of the Royal Society B: Biological Sciences, 256(1346), 119–124. doi: 10.1098/rspb.1994.0058 [DOI] [PubMed] [Google Scholar]
- Hughson, B. N., Anreiter, I., Jackson Chornenki, N. L., Murphy, K. R., Ja, W. W., Huber, R., & Sokolowski, M. B. (2018). The adult foraging assay (AFA) detects strain and food-deprivation effects in feeding-related traits of Drosophila melanogaster. Journal of Insect Physiology, 106(Pt 1), 20–29. doi: 10.1016/j.jinsphys.2017.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, R. -J., Hu, Y., Kirchner, R., Liu, Y., Xu, C., Comjean, A., … Perrimon, N. (2020). A cell atlas of the adult Drosophila midgut. Proceedings of the National Academy of Sciences of the United States of America, 201916820. doi: 10.1073/pnas.1916820117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innan, H., & Kondrashov, F. (2010). The evolution of gene duplications: Classifying and distinguishing between models. In: Nature reviews. Genetics (Vol. 11 Issue (2), pp. 97–108). doi: 10.1038/nrg2689 [DOI] [PubMed] [Google Scholar]
- Jambor, H., Surendranath, V., Kalinka, A. T., Mejstrik, P., Saalfeld, S., & Tomancak, P. (2015). Systematic imaging reveals features and changing localization of mRNAs in Drosophila development. ELife, 2015(4). doi: 10.7554/eLife.05003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenett, A., Rubin, G. M., Ngo, T. T. B., Shepherd, D., Murphy, C., Dionne, H., … Zugates, C. T. (2012). A GAL4-driver line resource for Drosophila neurobiology. Cell Reports, 2(4), 991–1001. doi: 10.1016/j.celrep.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun, K. R., Riedl, C. A. L., Chakaborty-Chatterjee, M., Belay, A. T., Douglas, S. J., Gibbs, A. G., & Sokolowski, M. B. (2007). Natural variation in food acquisition mediated via a Drosophila cGMP-dependent protein kinase. Journal of Experimental Biology, 210(20), 3547–3558. doi: 10.1242/jeb.006924 [DOI] [PubMed] [Google Scholar]
- Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., … Drummond, A. (2012). Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics (Oxford, England), 28(12), 1647–1649. doi: 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum, R., & Schedl, P. (1991). A position-effect assay for boundaries of higher order chromosomal domains. Cell, 64(5), 941–950. doi: 10.1016/0092-8674(91)90318-s [DOI] [PubMed] [Google Scholar]
- Kent, C. F., Daskalchuk, T., Cook, L., Sokolowski, M. B., & Greenspan, R. J. (2009). The Drosophila foraging gene mediates adult plasticity and gene–environment interactions in behaviour, metabolites, and gene expression in response to food deprivation. PLoS Genetics, 5(8), e1000609. doi: 10.1371/journal.pgen.1000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T. H., Zhou, X., & Chen, M. (2020). Demystifying “drop-outs” in single-cell UMI data. Genome Biology, 21(1), 196. doi: 10.1186/s13059-020-02096-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenecke, N., Johnston, J., Gaertner, B., Natarajan, M., Zeitlinger, J., Bier, E., … Darnell, J. (2016). Genome-wide identification of Drosophila dorso-ventral enhancers by differential histone acetylation analysis. Genome Biology, 17(1), 196. doi: 10.1186/s13059-016-1057-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn, N. R., Reaume, C. J., Moreno, C., Burns, J. G., Sokolowski, M. B., & Mery, F. (2013). Social environment influences performance in a cognitive task in natural variants of the foraging gene. PLoS One, 8(12), e81272. doi: 10.1371/journal.pone.0081272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolde, R. (2019). Pheatmap: Pretty heatmaps. https://cran.r-project.org/package=pheatmap
- Konstantinides, N., Kapuralin, K., Fadil, C., Barboza, L., Satija, R., & Desplan, C. (2018). Phenotypic convergence: Distinct transcription factors regulate common terminal features. Cell, 174(3), 622–635.e13. doi: 10.1016/j.cell.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer, M. C., Jung, C., Batelli, S., Rubin, G. M., & Gaul, U. (2017). The glia of the adult Drosophila nervous system. Glia, 65(4), 606–638. doi: 10.1002/glia.23115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz, S., Poeck, B., Sokolowski, M. B., & Strauss, R. (2012). The visual orientation memory of Drosophila requires Foraging (PKG) upstream of Ignorant (RSK2) in ring neurons of the central complex. Learning & Memory (Cold Spring Harbor, NY), 19(8), 337–340. doi: 10.1101/lm.026369.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Manno, G., Siletti, K., Furlan, A., Gyllborg, D., Vinsland, E., Langseth, C. M., … Linnarsson, S. (2020). Molecular architecture of the developing mouse brain. doi: 10.1101/2020.07.02.184051 [DOI] [PubMed] [Google Scholar]
- Laughlin, S. B., De Ruyter Van Steveninck, R. R., & Anderson, J. C. (1998). The metabolic cost of neural information. Nature Neuroscience, 1(1), 36–41. doi: 10.1038/236 [DOI] [PubMed] [Google Scholar]
- Leader, D. P., Krause, S. A., Pandit, A., Davies, S. A., & Dow, J. A. T. (2018). FlyAtlas 2: A new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Research, 46(D1), D809–D815. doi: 10.1093/nar/gkx976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebo, D. P. V., Chirn, A., Taylor, J. D., Levan, A., Torres, V. D., Agreda, E., … McCall, K. (2021). An RNAi screen of the kinome in epithelial follicle cells of the Drosophila melanogaster ovary reveals genes required for proper germline death and clearance. G3: Genes, Genomes, Genetics 11 (2). doi: 10.1093/g3journal/jkaa066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, E. C., Yu, D., Martinez De Velasco, J., Tessarollo, L., Swing, D. A., Court, D. L., … Copeland, N. G. (2001). A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics, 73(1), 56–65. doi: 10.1006/geno.2000.6451 [DOI] [PubMed] [Google Scholar]
- Lee, P. T., Zirin, J., Kanca, O., Lin, W. W., Schulze, K. L., Li-Kroeger, D., … Bellen, H. J. (2018). A gene-specific T2A-GAL4 library for Drosophila. eLife, 7. . doi: 10.7554/eLife.35574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman, D. A., Patterson, B., Johnston, L. A., Balzer, T., Britton, J. S., Saint, R., & Edgar, B. A. (1999). Cis-regulatory elements of the mitotic regulator, string/Cdc25. Development (Cambridge, England), 126(9), 1793–1803. doi: 10.1242/dev.126.9.1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, F. O., & Cierotzki, V. (2010). Locomotor performance in the Drosophila brain mutant drop-dead. Comparative Biochemistry & Physiology. Part A, Molecular & Integrative Physiology, 156(3), 337–343. doi: 10.1016/j.cbpa.2009.12.019 [DOI] [PubMed] [Google Scholar]
- Lemaitre, B., & Miguel-Aliaga, I. (2013). The digestive tract of Drosophila melanogaster. Annual Review of Genetics, 47, 377–404. doi: 10.1146/annurev-genet-111212-133343 [DOI] [PubMed] [Google Scholar]
- Li, H., Horns, F., Wu, B., Xie, Q., Li, J., Li, T., … Luo, L. (2017). Classifying Drosophila olfactory projection neuron subtypes by single-cell RNA sequencing. Cell, 171(5), 1206–1220.e22. doi: 10.1016/j.cell.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limmer, S., Weiler, A., Volkenhoff, A., Babatz, F., & Klämbt, C. (2014). The Drosophila blood–brain barrier: Development and function of a glial endothelium. Frontiers Neurosciences, 8, 365 . doi: 10.3389/fnins.2014.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.-S., Liu, X., Chow, S., & Lue, T. F. (2002). Cyclic AMP and cyclic GMP activate protein kinase G in cavernosal smooth muscle cells: Old age is a negative factor. BJU International, 89(6), 576–582. doi: 10.1046/j.1464-410x.2002.02643.x [DOI] [PubMed] [Google Scholar]
- Lin, C. S., Liu, X., Tu, R., Chow, S., & Lue, T. F. (2001). Age-related decrease of protein kinase G activation in vascular smooth muscle cells. Biochemical & Biophysical Research Communications, 287(1), 244–248. doi: 10.1006/bbrc.2001.5567 [DOI] [PubMed] [Google Scholar]
- Lincoln, T. M., & Cornwell, T. L. (1991). Towards an understanding of the mechanism of action of cyclic AMP and cyclic GMP in smooth muscle relaxation. Blood Vessels, 28(1–3), 129–137. doi: 10.1159/000158852 [DOI] [PubMed] [Google Scholar]
- Luo, C., Gangadharan, V., Bali, K. K., Xie, R. G., Agarwal, N., Kurejova, M., … Kuner, R. (2012). Presynaptically localized cyclic GMP-dependent protein kinase 1 is a key determinant of spinal synaptic potentiation and pain hypersensitivity. PLoS Biology, 10(3), e1001283. doi: 10.1371/journal.pbio.1001283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y., Creanga, A., Lum, L., & Beachy, P. A. (2006). Prevalence of off-target effects in Drosophila RNA interference screens. Nature, 443(7109), 359–363. 10.1038/nature05179 [DOI] [PubMed] [Google Scholar]
- MacPherson, M. R., Broderick, K. E., Graham, S., Day, J. P., Houslay, M. D., Dow, J. A. T., & Davies, S. A. (2004). The dg2 (for) gene confers a renal phenotype in Drosophila by modulation of cGMP-specific phosphodiesterase. Journal of Experimental Biology, 207(Pt 16), 2769–2776. doi: 10.1242/jeb.01086 [DOI] [PubMed] [Google Scholar]
- MacPherson, M. R., Lohmann, S. M., & Davies, S. A. (2004). Analysis of Drosophila cGMP-dependent protein kinases and assessment of their in vivo roles by targeted expression in a renal transporting epithelium. The Journal of Biological Chemistry, 279(38), 40026–40034. doi: 10.1074/jbc.M405619200 [DOI] [PubMed] [Google Scholar]
- Markstein, M., Pitsouli, C., Villalta, C., Celniker, S. E., & Perrimon, N. (2008). Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nature Genetics, 40(4), 476–483. doi: 10.1038/ng.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire, S. E., Le, P. T., Osborn, A. J., Matsumoto, K., & Davis, R. L. (2003). Spatiotemporal rescue of memory dysfunction in Drosophila. Science (New York, N.Y.), 302(5651), 1765–1768. 10.1126/science.1089035 [DOI] [PubMed] [Google Scholar]
- McConnell, M. W., & Fitzpatrick, M. J. (2017). 'Foraging' for a place to lay eggs: A genetic link between foraging behaviour and oviposition preferences”. PLoS One, 12(6), e0179362. doi: 10.1371/journal.pone.0179362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis, C. S., Murrow, L. M., & Gartner, Z. J. (2019). DoubletFinder: Doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Systems, 8(4), 329–337.e4. doi: 10.1016/j.cels.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes, L., Healy, J., & Melville, J. (2018). UMAP: Uniform manifold approximation and projection for dimension reduction. ArXiv. http://arxiv.org/abs/1802.03426
- Melom, J. E., & Littleton, J. T. (2013). Mutation of a NCKX eliminates glial microdomain calcium oscillations and enhances seizure susceptibility. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(3), 1169–1178. doi: 10.1523/JNEUROSCI.3920-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merli, C., Bergstrom, D. E., Cygan, J. A., & Blackman, R. K. (1996). Promoter specificity mediates the independent regulation of neighboring genes. Genes & Development, 10(10), 1260–1270. doi: 10.1101/gad.10.10.1260 [DOI] [PubMed] [Google Scholar]
- Mery, F., Belay, A. T., So, A. K.-C., Sokolowski, M. B., & Kawecki, T. J. (2007). Natural polymorphism affecting learning and memory in Drosophila. Proceedings of the National Academy of Sciences of the United States of America, 104(32), 13051–13055. doi: 10.1073/pnas.0702923104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli, C. A., & Perrimon, N. (2006). Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature, 439(7075), 475–479. doi: 10.1038/nature04371 [DOI] [PubMed] [Google Scholar]
- Michel, M. C., Wieland, T., & Tsujimoto, G. (2009). How reliable are G-protein-coupled receptor antibodies? Naunyn-Schmiedeberg's Archives of Pharmacology, 379(4), 385–388. doi: 10.1007/s00210-009-0395-y [DOI] [PubMed] [Google Scholar]
- Miguel-Aliaga, I., Jasper, H., & Lemaitre, B. (2018). Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics, 210(2), 357–396. doi: 10.1534/genetics.118.300224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat, J., Reiling, J. H., & Sabatini, D. M. (2007). Off-target effects associated with long dsRNAs in Drosophila RNAi screens. In Trends in Pharmacological Sciences (Vol. 28, Issue 4, pp. 149–151). Elsevier Current Trends. 10.1016/j.tips.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Ohlstein, B., & Spradling, A. (2006). The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature, 439(7075), 470–474. doi: 10.1038/nature04333 [DOI] [PubMed] [Google Scholar]
- Ohtsuki, S., Levine, M., & Cai, H. N. (1998). Different core promoters possess distinct regulatory activities in the Drosophila embryo. Genes & Development, 12(4), 547–556. doi: 10.1101/gad.12.4.547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, T., Sakai, T., Murata, T., Kako, K., Sakamoto, K., Ohtomi, M., … Ishida, N. (2001). Promoter analysis for daily expression of Drosophila timeless gene. Biochemical & Biophysical Research Communications, 283(3), 577–582. doi: 10.1006/bbrc.2001.4793 [DOI] [PubMed] [Google Scholar]
- Osborne, K. A., Robichon, A., Burgess, E., Butland, S., Shaw, R. A., Coulthard, A., … Sokolowski, M. B. (1997). Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science (New York, NY), 277(5327), 834–836. doi: 10.1126/science.277.5327.834 [DOI] [PubMed] [Google Scholar]
- Park, J. H., Helfrich-Förster, C., Lee, G., Liu, L., Rosbash, M., & Hall, J. C. (2000). Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proceedings of the National Academy of Sciences of the United States of America, 97(7), 3608–3613. doi: 10.1073/pnas.97.7.3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Q., Wang, Y., Li, M., Yuan, D., Xu, M., Li, C., … Liu, L. (2016). cGMP-Dependent protein kinase encoded by foraging regulates motor axon guidance in Drosophila by suppressing lola function. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 36(16), 4635–4646. doi: 10.1523/JNEUROSCI.3726-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereanu, W., Spindler, S., Im, E., Buu, N., & Hartenstein, V. (2007). The emergence of patterned movement during late embryogenesis of Drosophila. Developmental Neurobiology, 67(12), 1669–1685. doi: 10.1002/dneu.20538 [DOI] [PubMed] [Google Scholar]
- Pereira, H. S., & Sokolowski, M. B. (1993). Mutations in the larval foraging gene affect adult locomotory behavior after feeding in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America, 90(11), 5044–5046. doi: 10.1073/pnas.90.11.5044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer, B. D., Ngo, T. T. B., Hibbard, K. L., Murphy, C., Jenett, A., Truman, J. W., & Rubin, G. M. (2010). Refinement of tools for targeted gene expression in Drosophila. Genetics, 186(2), 735–755. doi: 10.1534/genetics.110.119917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, P. (2020). Embracing the dropouts in single-cell RNA-seq analysis. Nature Communications, 11(1), 1–9. doi: 10.1038/s41467-020-14976-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2020). R: A language and environment for statistical computing. https://www.r-project.org/
- Ram, K. R., & Wolfner, M. F. (2007). Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integrative & Comparative Biology, 47(3), 427–445. doi: 10.1093/icb/icm046 [DOI] [PubMed] [Google Scholar]
- Reaume, C. J., & Sokolowski, M. B. (2009). cGMP-dependent protein kinase as a modifier of behaviour. Handbook of Experimental Pharmacology, 191(191), 423–443. doi: 10.1007/978-3-540-68964-5_18 [DOI] [PubMed] [Google Scholar]
- Reaume, C. J., Sokolowski, M. B., & Mery, F. (2011). A natural genetic polymorphism affects retroactive interference in Drosophila melanogaster. Proceedings: Biological Sciences, 278(1702), 91–98. doi: 10.1098/rspb.2010.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes, K. J., & Trimmer, J. S. (2006). Antibodies as valuable neuroscience research tools versus reagents of mass distraction. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(31), 8017–8020. doi: 10.1523/JNEUROSCI.2728-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Velasco, V., Zhong, J., Hume, J. R., & Keef, K. D. (1998). Modulation of Ca2+ channels by cyclic nucleotide cross activation of opposing protein kinases in rabbit portal vein. Circulation Research, 82(5), 557–565. doi: 10.1161/01.res.82.5.557 [DOI] [PubMed] [Google Scholar]
- Ryner, L. C., Goodwin, S. F., Castrillon, D. H., Anand, A., Villella, A., Baker, B. S., … Wasserman, S.A. (1996). Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell, 87(6), 1079–1089. doi: 10.1016/S0092-8674(00)81802-4 [DOI] [PubMed] [Google Scholar]
- Saper, C. B., & Sawchenko, P. E. (2003). Magic peptides, magic antibodies: Guidelines for appropriate controls for immunohistochemistry. The Journal of Comparative Neurology, 465(2), 161–163. doi: 10.1002/cne.10858 [DOI] [PubMed] [Google Scholar]
- Satija, R., Farrell, J.A., Gennert, D., Schier, A. F., & Regev, A. (2015). Spatial reconstruction of single-cell gene expression data. Nature Biotechnology, 33(5), 495–502. doi: 10.1038/nbt.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner, R., Sokolowski, M. B., & Erber, J. (2004). Activity of cGMP-dependent protein kinase (PKG) affects sucrose responsiveness and habituation in Drosophila melanogaster. Learning & Memory (Cold Spring Harbor, NY), 11(3), 303–311. doi: 10.1101/lm.71604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., … Cardona, A. (2012). Fiji: An open-source platform for biological-image analysis. Nature Methods, 9(7), 676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnakenberg, S. L., Matias, W. R., & Siegal, M. L. (2011). Sperm-storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biology, 9(11), e1001192. doi: 10.1371/journal.pbio.1001192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor, I. E., Degner, J. F., Harnett, D., Cannavò, E., Casale, F. P., Shim, H., … Furlong, E. E. M. M. (2017). Promoter shape varies across populations and affects promoter evolution and expression noise. Nature Genetics, 49(4), 550–558. doi: 10.1038/ng.3791 [DOI] [PubMed] [Google Scholar]
- Schwabe, T., Bainton, R. J., Fetter, R. D., Heberlein, U., & Gaul, U. (2005). GPCR signaling is required for blood–brain barrier formation in Drosophila. Cell, 123(1), 133–144. doi: 10.1016/j.cell.2005.08.037 [DOI] [PubMed] [Google Scholar]
- Shih, M. F. M., Davis, F. P., Henry, G. L., & Dubnau, J. (2019). Nuclear transcriptomes of the seven neuronal cell types that constitute the drosophila mushroom bodies. G3. G3 (Bethesda, MD), 9(1), 81–94. doi: 10.1534/g3.118.200726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S. R., & Hou, S. X. (2009). Multipotent stem cells in the Malpighian tubules of adult Drosophila melanogaster. The Journal of Experimental Biology, 212(Pt 3), 413–423. doi: 10.1242/jeb.024216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoulakis, E. M. C., Kalderon, D., & Davis, R. L. (1993). Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron, 11(2), 197–208. doi: 10.1016/0896-6273(93)90178-T [DOI] [PubMed] [Google Scholar]
- Sokolowski, M. B. (1980). Foraging strategies of Drosophila melanogaster: A chromosomal analysis. Behavior Genetics, 10(3), 291–302. doi: 10.1007/BF01067774 [DOI] [PubMed] [Google Scholar]
- Sokolowski, M. B., & Riedl, C. A. L. (1999). Chapter 3.3.2 Behavior-genetic and molecular analysis of naturally occurring variation in Drosophila larval foraging behavior. Techniques in the Behavioral & Neural Sciences, 13(C), 496–511. doi: 10.1016/S0921-0709(99)80041-8 [DOI] [Google Scholar]
- Sözen, M. A., Armstrong, J. D., Yang, M., Kaiser, K., & Dow, J. A. T. (1997). Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proceedings of the National Academy of Sciences of the United States of America, 94(10), 5207–5212. doi: 10.1073/pnas.94.10.5207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling, A. C., & Rubin, G. M. (1983). The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell, 34(1), 47–57. doi: 10.1016/0092-8674(83)90135-6 [DOI] [PubMed] [Google Scholar]
- Stork, T., Engelen, D., Krudewig, A., Silies, M., Bainton, R. J., & Klambt, C. (2008). Organization and function of the blood–brain barrier in Drosophila. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(3), 587–597. doi: 10.1523/JNEUROSCI.4367-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart, T., Butler, A., Hoffman, P., Hafemeister, C., Papalexi, E., Mauck, W. M., … Satija, R. (2019). Comprehensive integration of single-cell data. Cell, 177(7), 1888–1902.e21. doi: 10.1016/j.cell.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond, J., Goodman, J. L., Strelets, V. B., Attrill, H., Gramates, L. S., Marygold, S. J., … Baker, P, FlyBase Consortium (2019). FlyBase 2.0: The next generation. Nucleic Acids Research, 47(D1), D759–D765. doi: 10.1093/nar/gky1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomancak, P., Beaton, A., Weiszmann, R., Kwan, E., Shu, S., Lewis, S. E., … Rubin, G. M. (2002). Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biology, 3(12). doi: 10.1186/gb-2002-3-12-research0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart-Cronish, M., & Sokolowski, M. B. (2014). Gene–environment interplay in Drosophila melanogaster: Chronic nutritional deprivation in larval life affects adult fecal output. Journal of Insect Physiology, 69, 95–100. doi: 10.1016/j.jinsphys.2014.06.001 [DOI] [PubMed] [Google Scholar]
- Van Der Maaten, L., Courville, A., Fergus, R., & Manning, C. (2014). Accelerating t-SNE using tree-based algorithms. Journal of Machine Learning Research, 15(Issue 93), 3221–3245. http://homepage.tudelft.nl/19j49/tsne [Google Scholar]
- Venken, K. J. T., He, Y., Hoskins, R. A., & Bellen, H. J. (2006). P[acman]: A BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science (New York, NY), 314(5806), 1747–1751. doi: 10.1126/science.1134426 [DOI] [PubMed] [Google Scholar]
- Wang, C., & Spradling, A. C. (2020). An abundant quiescent stem cell population in Drosophila Malpighian tubules protects principal cells from kidney stones. eLife, 9. . doi: 10.7554/eLife.54096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., & Sokolowski, M. B. (2017). Aggressive behaviours, food deprivation and the foraging gene. Royal Society Open Science, 4(4), 170042. doi: 10.1098/rsos.170042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., Pan, Y., Li, W., Jiang, H., Chatzimanolis, L., Chang, J., … Liu, L. (2008). Visual pattern memory requires foraging function in the central complex of Drosophila. Learn Memory, 15(3), 133–142. doi: 10.1101/lm.873008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, S., Bernhard, D., Lukowski, R., Weinmeister, P., Wörner, R., Wegener, J. W., … Feil, R. (2007). Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circulation Research, 101(11), 1096–1103. doi: 10.1161/CIRCRESAHA.107.154351 [DOI] [PubMed] [Google Scholar]
- White, R. E., Kryman, J. P., El-Mowafy, A. M., Han, G., & Carrier, G. O. (2000). cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate Bk(Ca) channel activity in coronary artery smooth muscle cells. Circulation Research, 86(8), 897–905. doi: 10.1161/01.res.86.8.897 [DOI] [PubMed] [Google Scholar]
- Willmott, N. J., Wong, K., & Strong, A. J. (2000). A fundamental role for the nitric oxide-G-kinase signaling pathway in mediating intercellular Ca2+ waves in glia. The Journal of Neuroscience, 20(5), 1767–1779. doi: 10.1523/JNEUROSCI.20-05-01767.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner, M. F. (1997). Tokens of love: Functions and regulation of Drosophila male accessory gland products. Insect Biochemistry & Molecular Biology, 27(3), 179–192 (Vol. Issue Elsevier Ltd.). doi: 10.1016/S0965-1748(96)00084-7 [DOI] [PubMed] [Google Scholar]
- Wolock, S. L., Lopez, R., & Klein, A. M. (2019). Scrublet: Computational identification of cell doublets in single-cell transcriptomic data. Cell Systems, 8(4), 281–291.e9. doi: 10.1016/j.cels.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S., Corbett, S. E., Koga, Y., Wang, Z., Johnson, W. E., Yajima, M., & Campbell, J. D. (2020). Decontamination of ambient RNA in single-cell RNA-seq with DecontX. Genome Biology, 21(1), 57. doi: 10.1186/s13059-020-1950-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim, K., Petri, J., Kottmeier, R., & Klämbt, C. (2019). Drosophila glia: Few cell types and many conserved functions. Glia, 67(1), 5–26. doi: 10.1002/glia.23459 [DOI] [PubMed] [Google Scholar]
- Young, M. D., & Behjati, S. (2018). SoupX removes ambient RNA contamination from droplet based single-cell RNA sequencing data. In bioRxiv (Vol. 2020). bioRxiv. doi: 10.1101/303727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zars, T., Fischer, M., Schulz, R., & Heisenberg, M. (2000). Localization of a short-term memory in Drosophila. Science (New York, NY), 288(5466), 672–675. doi: 10.1126/science.288.5466.672 [DOI] [PubMed] [Google Scholar]
- Zeisel, A., Hochgerner, H., Lönnerberg, P., Johnsson, A., Memic, F., van der Zwan, J., … Linnarsson, S. (2018). Molecular architecture of the mouse nervous system. Cell, 174(4), 999–1014.e22. doi: 10.1016/j.cell.2018.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, X., & Hou, S. X. (2015). Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult Drosophila posterior midgut. Development (Cambridge, England), 142(4), 644–653. doi: 10.1242/dev.113357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, X., Lin, X., & Hou, S. X. (2013). The Osa-containing SWI/SNF chromatin–remodeling complex regulates stem cell commitment in the adult Drosophila intestine. Development (Cambridge, England), 140(17), 3532–3540. doi: 10.1242/dev.096891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N., Beuve, A., & Townes-Anderson, E. (2005). The nitric oxide-cGMP signaling pathway differentially regulates presynaptic structural plasticity in cone and rod cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(10), 2761–2770. doi: 10.1523/JNEUROSCI.3195-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwarts, L., Van Eijs, F., & Callaerts, P. (2015). Glia in Drosophila behavior. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, & Behavioral Physiology, 201(9), 879–893. doi: 10.1007/s00359-014-0952-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.