Abstract
COVID‐19 (severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2]) is associated with coagulopathy through numerous mechanisms. The reported incidence of venous thromboembolism (VTE) in hospitalized patients with COVID‐19 has varied widely, and several meta‐analyses have been performed to assess the overall prevalence of VTE. The novelty of this coronavirus strain along with its unique mechanisms for microvascular and macrovascular thrombosis has led to uncertainty as to how to diagnose, prevent, and treat thrombosis in patients affected by this virus. This review discusses the epidemiology and pathophysiology of thrombosis in the setting of SARS‐CoV‐2 infection along with an updated review on the preventative and treatment strategies for VTE associated with SARS‐CoV‐2 infection.
Keywords: COVID‐19, incidence, review, therapeutics, venous thromboembolism
Essentials.
Covid‐19 (severe acute respiratory syndrome coronavirus 2) is associated with an increased risk of blood clots.
The cause of blood clots in the setting of COVID‐19 infection is complex.
Screening and workup for blood clots largely remains up to treating physicians.
Data regarding the optimal prevention and treatment of blood clots is evolving.
1. INTRODUCTION
The novel coronavirus COVID‐19 (severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2]) has led to a global pandemic, with over 272 598 201 cases and 5 334 221 deaths as of December 16, 2021 (https://coronavirus.jhu.edu/map.html; accessed December 16, 2021). In addition to respiratory complications, early reports discussed higher rates of venous thromboembolism (VTE) in patients with severe COVID‐19 disease compared to data from similar patients not affected by SARS‐CoV‐2. 1 , 2 , 3 Coagulation abnormalities are common in patients with COVID‐19, and those with severe illness frequently have elevated coagulation markers, such as D‐dimer and fibrinogen degradation product, with several proposed mechanisms of hypercoagulability. 4 , 5 As such, preventing and treating VTE in patients with COVID‐19, particularly in the inpatient setting, is of paramount importance.
2. RISK OF VTE
The increased risk of VTE in severe SARS‐CoV‐2 infection was reported early in the pandemic, although there has been a high variability of reported rates. In one of the first reports, Cui et al 1 retrospectively evaluated 81 patients with severe COVID‐19 hospitalized in a single institution in China and reported 25% (20/81) of intensive care unit (ICU) patients developed VTE. In this study, no preventative anticoagulant was administered. Another early report from the Netherlands described a similar VTE incidence of 27% in patients with severe COVID‐19 admitted to the ICU, despite the use of pharmacologic VTE prophylaxis. 6 Other institutions have reported a smaller incidence of VTE. For example, data from the Brigham and Women’s Hospital reported a 14‐day cumulative incidence of symptomatic VTE of 9.3% in patients with COVID‐19 who required an ICU level of care. 7 The variability in reporting is likely due to several confounders including individual institutions’ VTE prophylaxis strategy, length of study, deep vein thrombosis (DVT) screening procedures, patient selection, reporting bias, and outcome definitions. Several meta‐analyses and pooled aggregates have been published in an attempt to describe a more accurate depiction of the prevalence of VTE in patients with COVID‐19.
A meta‐analysis of 21 studies that included nearly 2000 patients with COVID‐19 reported that the weighted mean prevalence (WMP) of VTE was 31.3%, with similar results seen in ICU patients (WMP, 32.7%) and in those who received standard VTE prophylaxis (WMP, 23.9%). The WMP of VTE was 37.1% in studies that employed routine DVT screening, whereas the WMP of VTE was 29.4% in studies that performed diagnostic imaging solely based on clinical suspicion. 8 Similarly, a meta‐analysis reported by Hasan et al 9 reported a VTE prevalence of 31% in patients with COVID‐19 requiring an ICU level of care, despite the use of prophylactic or therapeutic anticoagulation. When compared to non–COVID‐19 medical inpatients, Li et al 10 reported a COVID‐19–associated VTE odds ratio (OR) of 2.79 and 5.94 for hospitalized patients with nonsevere and severe COVID‐19, respectively. Nopp et al 11 performed a meta‐analysis with subgroup analysis based on an ICU versus a non‐ICU setting and DVT screening versus no screening. The overall VTE prevalence was 14.1%, with higher rates found in patients with ultrasound screening versus no screening (40.3% and 9.5%, respectively). VTE prevalence was lower (7.9%) in non‐ICU patients compared to those who required an ICU level of care (22.7%).
The reported rates of venous thrombosis in the published randomized control trials that aimed to assess clinical outcomes using different doses of anticoagulation (standard prophylactic dose vs higher‐than‐standard dose anticoagulation) are noted in Table 1. Aside from the HEP‐COVID trial, 12 which reported thromboembolism in 29% in a standard anticoagulation dose group versus 10.9% in a therapeutic anticoagulation dose group, these trials reported much lower rates of VTE compared to rates noted in the observational studies. The difference in these rates may reflect early reporting bias in addition to current early diagnostic and treatment strategies (ie, antiviral/corticosteroids that have since become standard of care). It is important to note, however, that these trials were not powered for venous thrombosis as a primary end point. However, the true incidence of VTE may be even higher than reported in these studies, as pulmonary embolism (PE) may be the cause of sudden respiratory decompensation in severely ill patients with COVID‐19. A German autopsy study of patients who died of COVID‐19 revealed venous thrombosis in 58% of patients, in whom VTE was not suspected before death. In this study, PE was the cause of death in 4 of 12 autopsy specimens. 13 Another autopsy study described thrombosis of small and midsized pulmonary arteries in all 11 patients examined. 14
TABLE 1.
Published randomized controlled trials evaluating VTE prophylactic strategies in hospitalized patients with COVID‐19
| Clinical trial | Patient population | Interventions | Primary efficacy outcome | Total participants | Follow up | Efficacy results | Thrombotic event rate | Safety results |
|---|---|---|---|---|---|---|---|---|
| INSPIRATION 48 | ICU | Enoxaparin, 1 mg/kg daily vs enoxaparin, 40 mg daily a | Composite of venous or arterial thrombosis, treatment with ECMO, or mortality within 30 days | 600 | 30 days | Primary outcome occurred in 45.7% (n = 126) of patients who received intermediate‐dose anticoagulation and in 44.1% (n = 126) of those who received standard‐dose prophylaxis (odds ratio, 1.06; 95% CI, 0.76‐1.48; P = .70) | VTE occurred in 3.3% (n = 9) in intermediate dose vs 3.5% (n = 10) in standard dose (odds ratio, 0.93; 95% CI, 0.37‐2.32; P = .87) | Major bleed b occurred in 2.5% (n = 7) in the intermediate‐dose group and in 1.4% (n = 4) in those who received standard prophylaxis (odds ratio, 1.83; 1‐sided 97.5% CI, 0.00‐5.93, not meeting the noninferiority criteria; P for noninferiority 0.99) |
| Zed 49 | Hospitalized with severe COVID defined as ICU admission and/or modified ISTH Overt DIC score ≥3 | Enoxaparin, 1 mg/kg daily vs enoxaparin, 40 mg daily c | All‐cause mortality | 176 | 30 days | Primary outcome occurred in 21% (n = 18) of those who received standard dose prophylaxis and in 15% (n = 13) of those who received intermediate dose (odds ratio, 0.66; 95% CI, 0.30‐1.45; P = .31 | VTE occurred in 7% (n = 6) in standard dose vs. 8% (n = 7) in intermediate dose (odds ratio, 1.79; 95% CI, 0.51‐6.25; P > .95) | Major bleed d 2% (n = 2) in both arms; minor bleed 7% (n = 6) in both arms; P > .99 for both major and minor bleeding |
| ATTACC, ACTIV‐4a, and REMAP‐CAP e , 50 | ICU‐level respiratory or cardiovascular organ support | Therapeutic‐dose anticoagulation with heparin or LMWH vs pharmacologic thromboprophylaxis in accordance with local usual care | Organ support–free days | 1207 | 21 days | Therapeutic‐dose anticoagulation group’s median value for organ support–free days was 1 (interquartile range, −1 to 16). Those assigned to usual care prophylaxis the median value was 4 (interquartile range, −1 to 16) | Arterial or VTE occurred in 7.2% (n = 38) in therapeutic dose vs 11.1% (n = 62) in usual care prophylaxis | Major bleed d occurred in 3.8% (n = 20) in the therapeutic dose anticoagulation arm vs 2.3% (n = 13) in usual care arm (adjusted odds ratio, 1.48; 95% CI, 0.75‐3.04) |
| ATTACC, ACTIV‐4a, and REMAP‐CAP 52 | Non–critically ill at enrollment | Therapeutic‐dose anticoagulation with heparin or LMWH vspharmacologic thromboprophylaxis in accordance with local usual care | Organ support–free days | 2219 | 21 days | Probability that therapeutic‐dose anticoagulation increased organ support–free days as compared with usual care thromboprophylaxis was 98.6% (adjusted odds ratio, 1.27; 95% credible interval, 1.03‐1.58) | Arterial or VTE occurred in 1.1% (n = 13) in therapeutic dose vs 2.1% (n = 22) in usual care prophylaxis | Major bleeding d occurred in 1.9% (n = 22) of the patients receiving therapeutic‐dose anticoagulation and in 0.9% (n = 9) of those receiving thromboprophylaxis |
| ACTION 53 | Hospitalized with elevated D‐dimer | Therapeutic (rivaroxaban if clinically stable or enoxaparin if clinically unstable) vs prophylactic anticoagulation (UFH or LMWH) | Time to death, duration of hospitalization, or duration of supplemental oxygen | 615 | 30 days | Composite thrombotic outcome and all‐cause death occurred in 15% (n = 46) in the therapeutic anticoagulation group and in 14% (n = 44) in the prophylactic anticoagulation group (RR, 1.04; 95% CI, 0.70‐1.50; P = .91) | VTE occurred in 4% (n = 11) in therapeutic anticoagulation group vs. 6% (n = 18) in prophylactic anticoagulation group (RR, 0.60; 95% CI, 0.29‐1.25; P = .19) | Major bleed or CRNMB d occurred in 8% (n = 26) in the therapeutic anticoagulation group and in 2% (n = 7) in the prophylactic anticoagulation group (RR, 3.64; 95% CI, 1.61‐8.27; P = .001) |
| HEP‐COVID 12 | Hospitalized adult patients with COVID‐19 with D‐dimer levels >4 times the upper limit of normal or sepsis‐induced coagulopathy score of ≥4 | Standard prophylactic or intermediate‐dose LMWH or unfractionated heparin vs therapeutic‐dose enoxaparin | Venous thromboembolism, arterial thromboembolism, or death from any cause | 257 | 30 ± 2 days | Primary outcome occurred in 41.9% in the standard‐dose group vs 28.7% in the therapeutic‐dose group. RR, 0.68; 95% CI, 0.49‐0.96); P = .03 | thromboembolism (29.0% in standard –dose group vs 10.9% in therapeutic dose group. RR, 0.37 95% CI, 0.21–0.66; p < 0.001) | 2 major bleeds (1.6%) in the standard‐dose vs 6 major bleeds (4.7%) in the therapeutic‐dose groups (RR, 2.88; 95% CI, 0.59–14.02; p = 0.17) |
| RAPID 54 | Adults admitted to hospital wards with COVID‐19 and increased D‐dimer levels | Therapeutic‐dose or prophylactic‐dose heparin (low‐molecular‐weight or unfractionated heparin) | Composite of death, invasive mechanical ventilation, noninvasive mechanical ventilation, or admission to an ICU | 465 | 28 days | Primary outcome occurred in 16.2% assigned to therapeutic heparin and in 21.9% assigned to prophylactic heparin (odds ratio, 0.69; 95% CI, 0.43‐1.10; P = .12) | Venous thromboembolism occurred in two patients (0.9%) assigned to therapeutic heparin and six (2.5%) assigned to prophylactic heparin (odds ratio, 0.34; 95% CI, 0.07‐1.71; P = .19) | Major bleeding occurred in two patients (0.9%) assigned to therapeutic heparin and four (1.7%) assigned to prophylactic heparin (odds ratio, 0.52; 95% CI, 0.09‐2.85; P = .69) |
Abbreviations: CI, confidence interval; CRNBM, clinically relevant nonmajor bleeding; DIC, disseminated intravascular coagulation; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; LMWH, low‐molecular‐weight heparin; RR, relative risk; UFH, unfractionated heparin; VTE, venous thromboembolism.
With modification according to body weight and creatinine clearance.
According to the Bleeding Academic Research Consortium.
Adjusted for obesity.
According to ISTH criteria.
Trial was stopped when the prespecified criterion for futility was met for therapeutic‐dose anticoagulation.
3. CLINICAL CHARACTERISTICS
The RIETE registry is an ongoing, international, multicenter prospective registry of patients with acute VTE. This group analyzed clinical features and outcomes of 455 patients with COVID‐19 who had a VTE during their hospital admission. In this registry, men comprised 71% of the population, and the median age was 65 years. The vast majority of events were PEs (83%), while 17% had isolated DVT. At the time of VTE diagnosis, 88% were receiving pharmacologic VTE prophylaxis. The mortality rate was 12% within 10 days, and 2.9% of patients had a major bleeding event. 15
Hematologic and inflammatory laboratory abnormalities have been found to correlate with disease severity in patients with COVID‐19. Specifically, elevations in fibrinogen, fibrinogen degradation product, C‐reactive protein (CRP), interleukin‐6 (IL‐6), erythrocyte sedimentation rate (ESR), and D‐dimer have been found to be associated with increased severity of disease. 16 , 17 , 18 Severe thrombocytopenia and lymphopenia have also been associated with poorer outcomes, both independently and in the setting of disseminated intravascular coagulation (DIC). 19 , 20 Obtaining these clinical parameters may provide further information for the prediction of VTE (as discussed below) as well as overall prognosis.
4. PATHOPHYSIOLOGY
Hospitalized patients with COVID‐19 share many risk factors for VTE as traditional inpatients including older age, obesity, ICU level of care, and immobility. However, in addition to these well‐established VTE risk factors, severe SARS‐CoV‐2 infection is associated with coagulopathy and an inherent increased risk of thromboembolic complications. 21
Early reports from China described the risk of mortality from severe SARS‐CoV‐2 infection was associated with older age in addition to an abnormal coagulation profile similar to DIC. 22 In this study, 71% of nonsurviving patients with COVID‐19 meet criteria for DIC using the ISTH criteria. 23 There are, however, some differences between traditional DIC seen in sepsis and the coagulopathy seen in patients with severe COVID‐19. For example, DIC due to sepsis usually results in a more profound consumptive coagulopathy and thrombocytopenia compared to the coagulopathy seen in patients with COVID‐19. 24 It is proposed that the relative lack of consumptive coagulopathy may be why patients with COVID‐19 are more prothrombotic rather than disease evolution into a bleeding propensity due to hyperfibrinolysis. Also in SARS‐CoV‐2 infection, there is a predilection for thrombotic microangiopathy to affect the lung vasculature, rather than widespread systemic organ damage from microthrombosis. 25 Several studies have reported widespread microangiopathy and thrombosis within the pulmonary vasculature of patients with COVID‐19. 14 , 26 , 27 Localized pulmonary thrombi may be one mechanism for the predilection of PE over DVT in patients with COVID‐19. It has been postulated that localized pulmonary thrombi (as opposed to PE) may develop as a consequence of pulmonary vascular damage and severe localized inflammation. 27
COVID‐19 is thought to promote coagulation by several mechanisms. The virus interacts with the angiotensin‐converting enzyme 2 receptor on endothelial cells, which can cause severe endothelial inflammation with a resultant shift toward a procoagulant state with microvascular coagulopathy. 28 The robust inflammatory response is thought to play a primary role in COVID‐19–induced coagulopathy by several mechanisms. Microorganisms accumulate polyphosphates, which activate the contact pathway of coagulation. 29 Complement activation, endothelial injury, platelet activation, and cytokines such as IL‐6 also play notable roles in thrombogenesis. 30 , 31 There have also been several reports of positive antiphospholipid antibodies in critically ill patients with COVID‐19. 32 , 33 , 34 , 35 However, it is not clear whether these antibodies are reactive (as often seen in critical illness), or whether they contribute to a direct causative role of developing thrombosis. Stringent design of data collection and interpretation is needed to understand the role of antiphospholipid antibodies in COVID‐19 coagulopathy. Overall, the coagulopathy of COVID‐19 likely results from a mixture of inflammation with endothelial dysfunction, low grade DIC, and microvascular thrombosis (Figure 1).
FIGURE 1.
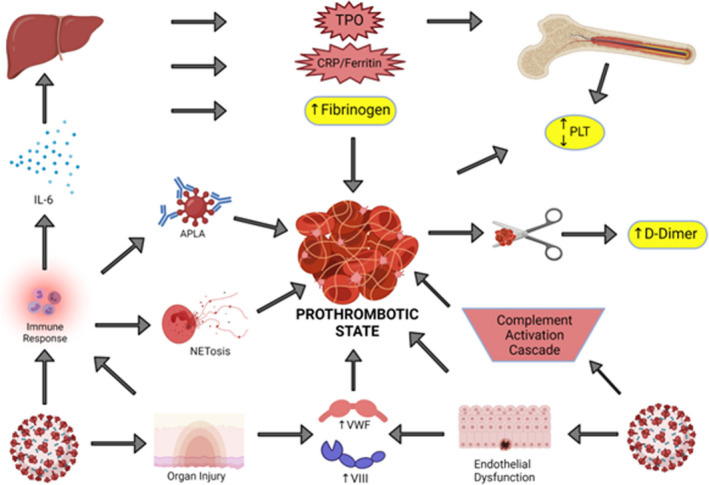
Prothrombotic state of COVID‐19 infection. The pathogenesis of the hypercoagulable state of COVID‐19 infection is depicted above. Bottom left: COVID‐19 infection can lead to a robust immune response with resultant secretion of cytokines (such as interleukin‐6 [IL‐6]), antiphospholipid antibodies (APLA), and neutrophil extracellular traps (NETosis). Bottom right: COVID‐19 infection also leads to complement activation in addition to endothelial dysfunction and organ injury which increases procoagulant molecules such as von Willebrand factor and factor VIII. Top left: Liver injury can occur due to endotheliopathy, which leads to an overall increase in inflammatory markers such as fibrinogen, CRP (C Reactive Protein) and thrombopoietin (TPO). Top right: Acute infection can have a variable effect on the platelet (PLT) count and the D‐dimer is elevated in the setting of fibrinolysis of micro‐ or macrovascular thrombosis
5. PREDICTIVE BIOMARKERS FOR VTE
With the known risks of micro‐ and macrovascular thromboses, there have been numerous attempts to identify predictive biomarkers for VTE in patients with COVID‐19. The previously mentioned early report from Cui et al 1 reported that VTE was associated with a lower lymphocyte count, longer activated partial thromboplastin time (aPTT), and higher D‐dimer quantification. Quantitative D‐dimer was one of the first biomarkers studied in patients with COVID‐19. A multicenter retrospective study reported by Al‐Samkari et al 36 quantified that D‐dimer >2500 had an adjusted OR of 6.79 for developing thrombosis. Additional biomarkers predictive of VTE in this study included platelet count >450 × 109/L (adjusted OR, 3.56), CRP > 100 mg/L (adjusted OR, 2.71), and ESR >40 mm/h (adjusted OR, 2.64). However, this study also reported that D‐dimer was associated with increased bleeding (adjusted OR, 3.56). Another study found that male sex, elevated admission CRP, and elevated admission platelet count were associated with VTE risk in a univariate analysis, although only male sex continued to show predictions in the multivariate analysis. 7 It is known that men are at increased risk for recurrent VTE compared to women, but the risk of a first VTE is similar among both sexes. It is postulated that men are more at risk than women when hospitalized for COVID‐19 because those hospitalized in the initial waves of the pandemic were typically older adults, which removes the traditional VTE risk factors in women such as oral contraception and pregnancy.
Dujardin et al 37 retrospectively evaluated several clinical variables including positive end‐expiratory pressure, ratio of arterial oxygen partial pressure to fractional inspired oxygen, platelet count, international normalized ratio (INR), aPTT, fibrinogen, antithrombin, D‐dimer, and CRP in an effort to predict VTE in critically ill patients with COVID‐19. In this study, elevated CRP and D‐dimer had the highest positive predictive value with an area under the curve (AUC) of 0.75 and 0.64, respectively. Similarly, in non‐ICU patients with COVID‐19, a prospective study evaluating patients for asymptomatic DVT reported an elevated D‐dimer (defined as >1000 ng/mL) had an AUC of 0.72. 38 The timing and type of D‐dimer assays are important considerations when applying these findings to clinical practice.
One study demonstrated that an elevated prothrombin fragment 1.2 was potentially more discriminant than D‐dimer for identifying thrombotic manifestations in hospitalized patients with COVID‐19. 39 While this could provide a helpful biomarker tool, this study was small, with only 115 patients, and thus, more research is required. Other laboratory values such as prothrombin time, aPTT, ferritin, procalcitonin, lactate dehydrogenase, and troponin have also been studied, but there is no clear correlation with VTE in patients with COVID‐19. However, to date, there are no specific and reliable laboratory values to predict for VTE in patients with COVID‐19. Given this challenge, clinicians should be diligent in assessing their patients for potential thromboses and, if symptoms occur, imaging evaluations should be obtained to confirm thrombosis and guide anticoagulation strategies.
6. VTE PROPHYLACTIC STRATEGIES
In addition to differing protocols for obtaining imaging studies (symptomatic versus screening), another contributing factor for the disparate rates of VTE reported across institutions may be due to the varying practices surrounding the use of prophylactic anticoagulation. Because COVID‐19 has been associated with thrombotic complications, there has been an intense debate surrounding the optimal prophylactic anticoagulation management for these patients. Several studies early in the pandemic demonstrated improved survival and lower VTE rates with the use of pharmacologic VTE prophylaxis. 40 , 41 , 42 , 43 However, there is an ongoing debate about using higher‐than‐standard prophylactic anticoagulation (intermediate or therapeutic doses of anticoagulation) in inpatients with COVID‐19. A recent review comparing and contrasting major societal guidelines found that the most common theme was to take an individualized approach to patient management and that randomized controlled trials (RCTs) to address these important anticoagulation issues are much needed. 44 Given the observation of increased thrombotic events, especially in patients with more severe disease, higher‐than‐prophylactic doses of anticoagulation were used during the early phase of the pandemic. However, the retrospective observational data for such intermediate or therapeutic dosing has been mixed; some studies showed a potential improvement in outcomes with higher doses of anticoagulation in some, but no difference or worse outcomes in others. 45 , 46 , 47
There are now emerging data from prospective randomized trials to address the question of optimal thromboembolism prophylaxis. Of note, these trial outcomes were composite outcomes of thromboembolism or clinical deterioration that might be related to immune‐mediated inflammatory microthrombosis. A RCT of 600 critically ill patients positive for COVID‐19, the INSPIRATION study, comparing standard prophylactic dosing of primarily enoxaparin (40 mg daily) with intermediate dose enoxaparin (1 mg/kg daily for most patients), found that there was no difference in the rates of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days (OR, 1.06; 95% CI, 0.76‐1.48; P = .70). 48 The Zed trial, a multicenter, open‐label, randomized study, compared standard‐ versus intermediate‐dose enoxaparin in 176 patients with COVID‐19 requiring an ICU level of care and in those with a modified ISTH Overt DIC score of ≥3. Similar to what was seen in the INSPIRATION trial, there was no difference in overall mortality, thrombosis, or bleeding between the two arms (OR for primary efficacy outcome was 0.66; 95% CI, 0.30‐1.45; P = .31). 49 A conglomerate of three open‐label, harmonized, adaptive international multicenter RCTs (ATTACC, ACTIV‐4a, and REMAP‐CAP) evaluated therapeutic dose anticoagulation (≈90% low‐molecular‐weight heparin [LMWH]) versus usual care prophylaxis (composed of standard‐ or intermediate‐dose anticoagulation) in hospitalized patients with COVID‐19. The authors report that in critically ill patients (defined as presence of critical care support at enrollment), therapeutic dose anticoagulation with heparin or LMWH did not lead to improved probability of survival to hospital discharge nor did it lessen the days requiring organ support. 50 Interestingly, the same trial showed therapeutic anticoagulation (compared to usual care dosing) led to fewer days requiring organ support in non–critically ill patients with COVID. 51 , 52 The ACTION trial randomized 615 hospitalized patients with COVID‐19 with elevated D‐dimer and compared therapeutic (rivaroxaban 20 mg daily for most patients) for 30 days to standard prophylactic anticoagulation. Treatment with therapeutic anticoagulation did not improve mortality, duration of hospitalization, or duration of oxygen use. 53 The most recent published trial, the HEP‐COVID trial, evaluated therapeutic LMWH versus standard or intermediate dose thromboprophylaxis in high‐risk hospitalized patients with a D‐dimer level greater than 4× the upper limit of or a sepsis‐induced coagulopathy score of ≥4. These results mirrored the results of the ATTACC/ACTIV‐4a/REMAP‐CAP outcomes and found that therapeutic‐dose LMWH reduced thromboembolism and death compared to lower‐dose anticoagulation among high‐risk hospitalized patients, but the treatment effect was not seen in the critically ill ICU patients. 12 The RAPID trial randomized non‐ICU patients with elevated D‐dimer to therapeutic‐ versus prophylactic‐dose heparin with a primary composite outcome of death, mechanical ventilation, or ICU admission and did not show statistical difference among the two arms (OR, 0.69; 95% CI, 0.43‐1.10; P = .12). However, there was a decreased rate of death at 28 days in the therapeutic arm (OR, 0.22; 95% CI, 0.07‐0.65; P = .006) as well as a decrease in the number of VTE events in those who received therapeutic anticoagulation. 54 Table 1 provides a summary of published RCTs regarding VTE prophylactic strategy in the hospitalized patients with COVID‐19.
It is accepted that higher doses of anticoagulation generally correlate with increased bleeding risk. Although early reports on COVID‐19 coagulopathy were focused on thrombotic risks, there have been several reports on the risk of bleeding in this hospitalized population. 36 , 55 , 56 The risk of bleeding in patients with COVID‐19 receiving higher‐than‐prophylactic anticoagulation was recently evaluated using the prospective RIETE‐BLEEDING registry, which enrolled hospitalized patients with COVID‐19 who received intermediate or therapeutic anticoagulation for VTE prophylaxis. Over a short median duration (12 days), 5.7% of patients developed a major bleed, and 6.7% developed nonmajor bleeding. Major bleed was associated D‐dimer >10 times the upper limit of normal, elevated ferritin, ICU stay, and therapeutic‐level anticoagulation and correlated with a twofold higher risk of death. 57 This study, however, did not compare bleeding outcomes to those on standard doses of anticoagulation, and it was a noninterventional descriptive study. In the previously mentioned INSPIRATION trial, critically ill patients who received an intermediate dose of anticoagulation had a major bleeding incidence of 2.5% compared to 1.4% in the standard‐dose prophylactic group. 48 In the critically ill population, The REMAP‐CAP, ACTIV‐4a, and ATTACC Investigators report that major bleeding occurred in 3.8% of patients who received therapeutic anticoagulation compared to 2.3% of patients who received usual care pharmacologic prophylaxis. 50 In the Hep‐COVID trial, major bleeding occurred in 1.6% in the standard‐dose group versus 4.7% in the therapeutic‐dose group (relative risk, RR, 2.88; 95% CI, 0.59‐14.02; P = .17). 12 Additional bleeding rates from published trials are listed in Table 1. Bleeding in this population may be related to thrombocytopenia, hyperfibrinolysis, and coagulation abnormalities along with therapeutic interventions including invasive procedures and anticoagulation itself. These data highlight the importance of balancing the risk of bleeding when considering thromboprophylaxis in this population.
Given these data, the ideal dose for thromboprophylaxis is evolving in hospitalized patients with COVID‐19. Current data outline that standard dosing of thromboprophylaxis in severely ill patients with COVID‐19 requiring organ support in critical care units is appropriate. Paradoxically however, patients with less severe illness may benefit from higher anticoagulation doses. Hypotheses for this finding include (i) critically ill patients may have too much microthrombosis, and it is too late for higher doses of anticoagulation to have an effect; or (ii) micro pulmonary hemorrhage may occur later in the disease course. The results of ongoing trials will provide further data on the role of prophylactic anticoagulation versus full‐dose anticoagulation in hospitalized non–critically ill patients.
As over 50% of the burden of hospital‐associated VTE in general medical patients occurs after discharge, 58 there has been an increased interest in strategies around thromboprophylaxis after hospitalization for patients with COVID‐19. Several factors including the hypercoagulability of the disease itself, but also burgeoning caseloads during waves of the pandemic leading to earlier discharges when patients are still relatively sick, could in theory be associated with increased risk of postdischarge thrombotic events. However, data from observational studies are mixed. A single‐center retrospective study demonstrated that the rates of postdischarge VTE in patients with COVID‐19 were relatively low, at 2.5%. 59 A study from the United Kingdom compared rates of postdischarge VTE among patients with COVID‐19 against hospitalized medical patients from the year prior, and did not find a significantly higher rate in patients who were admitted with COVID‐19 (OR, 1.6; 95% CI, 0.77‐3.1). 60 On the other hand, a recently published prospective registry study from a US health care system found that the 90‐day venous thrombotic event rate was 1.55%, and that anticoagulation at discharge was associated with a significant reduction in the combined outcome of venous/arterial thromboembolism and all‐cause mortality (OR, 0.54; 95% CI, 0.47‐0.81). 61 Several studies are currently enrolling patients, which will provide evidence to guide clinicians on this topic. 62 One such trial, the MICHELLE trial, was recently presented in abstract form by Dr Eduardo Ramacciotti at the European Society of Cardiology Virtual Congress in August 2021. This study evaluated rivaroxaban 10 mg daily versus placebo in patients with COVID‐19 discharged from the hospital. The composite primary outcome of symptomatic VTE, VTE‐related death, bilateral VTE, symptomatic arterial thromboembolism, myocardial infarction, nonhemorrhagic stroke, major adverse limb event, or cardiovascular death at 35 days was 3.14% in the rivaroxaban group compared with 9.43% in the control group (P = .03). While we await final publication and further data, clinicians may individualize decisions around postdischarge anticoagulation based on known prothrombotic risk factors (eg, severe immobility, personal history of VTE, known thrombophilia, cancer).
The outpatient setting is another arena in which the use of prophylactic anticoagulation has been investigated. The randomized double‐blind placebo‐controlled, National Institutes of Health ACTIV‐4B trial was developed to determine if symptomatic outpatients with COVID‐19 would benefit from prophylactic anticoagulant or antiplatelet agents. Patients were randomized to a 45‐day course of prophylactic dose apixaban (2.5 mg orally twice daily), therapeutic‐dose apixaban (5.0 mg orally twice daily), aspirin (81 mg orally twice daily), or placebo (orally twice daily). The trial was stopped early by the study’s Data and Safety Monitoring Board when investigators found that for mildly symptomatic outpatients with COVID‐19 who were sick at home for at least a week and who remained clinically stable and had no risk factors for thrombotic events, rates of major cardiopulmonary complications did not justify antithrombotic therapy. 63
7. TREATMENT OF VTE IN COVID‐19
Patients with COVID‐19 with confirmed VTE or high suspicion of VTE should be treated with full‐dose anticoagulation. Currently, there are no randomized trials exploring the therapeutic efficacy of different agents, dosing, duration, safety, or bleeding risks in these patients. Current treatment protocols for managing VTE in patients with COVID‐19 are primarily extrapolated from preexisting evidence‐based management of VTE in patients without COVID. Therapeutic anticoagulation with unfractionated heparin (UFH), LMWH, direct oral anticoagulants (DOACs) or vitamin K antagonists (eg, warfarin) remain the mainstay of treatment. 64 , 65 , 66 , 67 Since the coexistence of COVID‐19 and PE, two life‐threatening illnesses, in the same patient presents a unique challenge, the National Pulmonary Embolism Response Team recently released a position paper that specifically addresses issues related to the diagnosis and management of PE in patients with COVID‐19. 68
7.1. Choice of anticoagulant during hospital admission
Clinical practice guidelines for treatment of VTE in patients without COVID‐19 recommend use of DOACs for most patients. 69 , 70 , 71 However, the risk of rapid clinical decompensation in patients with COVID‐19, alterations in renal function, and drug interactions with various investigational therapies (including dexamethasone) can alter pharmacodynamics of DOACs. Therefore, current guidelines recommend using parenteral agents for hospitalized patients with COVID‐19–related VTE. 64 , 65 , 66 , 67 For acutely ill patients who are admitted to the hospital, initiation of parenteral anticoagulation with UFH or LMWH should be preferred. Parenteral anticoagulation offers numerous advantages and have been extensively studied and used over the years for treatment of VTE with a good efficacy and safety profile. They are more easily reversible compared to fondaparinux or DOACs.
The initial choice between UFH and LMWH should be determined on the basis of the patient’s clinical parameters like hemodynamic stability, renal function, and the potential need for invasive procedures. In non–critically ill inpatients, LMWH is the preferred first‐line agent for treatment of VTE because it does not require laboratory monitoring and minimizes exposure and personal protective equipment use. For patients with contraindications to LMWH, UFH should be used and provides the advantage of prompt reversal of the anticoagulant effect with discontinuation of the infusion and protamine sulfate. Monitoring UFH using aPTT can be unreliable in the setting of baseline abnormalities in coagulation tests, 64 and patients with COVID‐19 have been reported to have a prolonged aPTT. 22 , 72 Therefore, it is important to obtain baseline aPTT before starting heparin infusion. In patients with a prolonged aPTT at baseline, anti‐Xa assays should be preferred for monitoring the therapeutic range of UFH. 64
Another potential issue with the use of UFH reported in some patients with COVID‐19 is heparin resistance, which is defined as the need for >35 000 units of heparin in 24 hours as measured by partial thromboplastin time. 73 , 74 White et al 73 reported heparin resistance in 8 of 10 ICU patients on UFH, and Beun et al 74 reported very high doses of UFH to achieve appropriate aPTT. They noted that factor VIII and fibrinogen levels were extremely high in patients with COVID‐19 and was likely responsible for decreasing aPTT in in vitro assays but less likely to affect anti‐Xa levels. Therefore, monitoring of antithrombotic activity by measuring anti‐Xa levels may be more appropriate. 64 , 65
Use of DOACs in hospitalized patients with COVID‐19 requires caution because of the potential for significant drug‐drug interactions with investigational antiviral therapies since both use cytochrome P450 isozymes (CYP3A4) and P‐glycoprotein (P‐gp) drug transporter pathways. 75 , 76 Inhibition of these pathways can result in increased levels of DOACs, while induction can result in lower levels. Testa et al 75 , 76 reported that patients with COVID‐19 treated with the antiviral drugs lopinavir, ritonavir, or darunavir, which are inhibitors of CYP3A4/P‐gp pathways, resulted in significantly elevated DOAC levels. The C‐trough levels for DOACs were more than six times higher during hospitalization compared to prehospitalization levels.
7.2. Choice of anticoagulant at discharge
At the time of discharge, clinicians should reassess the choice of anticoagulant to be prescribed for outpatient treatment. The available options are LMWH, DOACs, and vitamin K antagonists. DOACs are the guideline‐based preferred anticoagulant for the treatment of VTE and result in less bleeding compared to VKAs. 69 , 70 , 71 Selection of a specific DOAC agent needs to be based on individual patient‐specific factors including renal function, hepatic function, and insurance coverage. Of note, dabigatran and endoxaban are approved after an initial parenteral lead‐in. It is also important to screen for drug‐drug interactions as above. A useful online resource for assessing interactions is available at www.covid19‐druginteractions.org.
For patients with contraindications to DOACs, LMWH, fondaparinux, or VKAs should be considered. LMWH or fondaparinux offers the advantage of avoiding INR checks and minimizing contact with health care settings. Patients reluctant to self‐administer injections, or having contraindications, will need a VKA. It is imperative for hospitals and anticoagulation clinics to set up protocols to ensure safe monitoring of INRs for outpatients.
7.3. Use of thrombolytic agents in patients with COVID‐19
Wang et al 77 reported three cases involving administration of tissue‐type plasminogen activator (t‐PA) in patients with COVID‐19 having acute respiratory distress syndrome and all three cases showed limited initial evidence of decreased oxygen requirements and ventilatory support. Barrett et al 78 reported a case series of 5 patients with respiratory failure treated with systemic t‐PA, some of which resulted in improved but transient respiratory status. Overall, use of these fibrinolytic therapies should be reserved for current established indications as in patients without COVID. 64 , 65 , 67
7.4. Duration of anticoagulation
VTE associated with COVID‐19 should be treated for at least 3 months. 64 , 65 , 66 If there are no ongoing risk factors, it seems reasonable to classify this type of VTE as provoked by a transient strong risk factor, and stopping therapy at 3 months, in line with prepandemic guidelines. 69 , 70 , 71
8. UNANSWERED QUESTIONS/FUTURE DIRECTIONS
Several questions in the field of COVID‐19–related VTE remain unanswered, and there is an urgent need for high‐quality data. Key questions that remain unanswered include:
What are the appropriate risk assessment models to estimate VTE and bleeding risk in hospitalized patients with COVID‐19?
Should providers use higher doses of prophylactic anticoagulation in certain patients with COVID‐19?
What is the optimal approach to patients on full‐dose anticoagulation who are admitted to the ICU during their hospitalization?
What is the role for extended VTE prophylaxis?
What is the best approach to manage arterial thrombosis in the setting of SARS‐CoV‐2 infection?
Ongoing registries such as CORONA VTE NET, CORE‐THROMBOSIS, VVIRTUOSO, CORE 19, and other multicenter cohort studies have been developed to study the epidemiology, risk factors, prevention, management, and thromboembolic outcomes in patients with COVID‐19. Along with these registries, there has been an intense interest and explosion of randomized trials to answer some of these important and still unanswered clinical questions. Table 2 includes several randomized studies near recruiting or actively recruiting patients with COVID‐19 to study the optimal approach for VTE prevention. Figure 2 highlights some emerging data that will help answer these important clinical questions.
TABLE 2.
Clinical studies evaluating mitigation strategies of venous thromboembolism in inpatients with COVID‐19 listed on the clinicaltrials.gov website on December 16, 2021
| ClinicalTrials.gov identifier | Title of study | Question/outcome(s) of interest | Comparator arms | Status (12/16/2021) |
|---|---|---|---|---|
| Outpatient setting (prehospital) | ||||
| NCT04508023 | A Study of Rivaroxaban to Reduce the Risk of Major Venous and Arterial Thrombotic Events, Hospitalization and Death in Medically Ill Outpatients With Acute, Symptomatic Coronavirus Disease 2019 (COVID‐19) Infection (PREVENT‐HD) | Evaluate the safety and efficacy of prophylactic dose of rivaroxaban to reduce thrombotic events, hospitalization, and death in outpatients with symptomatic SARS‐CoV‐2 infection | Prophylactic rivaroxaban (10 mg daily) vs placebo | Recruiting |
| NCT04400799 | Enoxaparin for Primary Thromboprophylaxis in Ambulatory Patients With COVID‐19 | Age ≥50 y; primary outcome of hospitalization and all‐cause death | Enoxaparin 40 mg daily vs no treatment | Recruiting |
| Moderate‐severe hospitalized patients | ||||
| NCT04416048 | Effect of Anticoagulation Therapy on Clinical Outcomes in COVID‐19 (COVID‐PREVENT) | Rivaroxaban for the prevention of thrombotic events and all‐cause mortality in patients with moderate to severe COVID‐19 | Rivaroxaban 20 mg daily × 7 days or hospital discharge followed by rivaroxaban 10 mg daily for 28 days vs standard of care thromboprophylaxis | Recruiting |
| NCT04505774 | Accelerating COVID‐19 Therapeutic Interventions and Vaccines 4 ACUTE (ACTIV‐4A) | 21‐day organ support–free days. Secondary outcomes include thrombotic events and all‐cause mortality | Therapeutic‐dose anticoagulation vs prophylactic‐dose anticoagulation, vs therapeutic anticoagulation +P2Y12 inhibitor vs prophylactic anticoagulation +P2Y12 inhibitor | Recruiting |
| NCT04373707 | Effectiveness of Weight‐Adjusted Prophylactic Low Molecular Weight Heparin Doses Compared With Lower Fixed Prophylactic Doses to Prevent Venous Thromboembolism in COVID‐2019 (The Multicenter Randomized Controlled Open‐label Trial COVI‐DOSE) | Risk of DVT or PE or VTE‐related death | Standard prophylactic dose LMWH vs weight‐adjusted prophylactic dose LMWH | Recruiting |
| NCT04730856 | Standard vs High Prophylactic Doses or Anticoagulation in Patients With High Risk of Thrombosis Admitted With COVID‐19 Pneumonia (PROTHROMCOVID) | Risk of thrombotic events, use of mechanical ventilation, length of hospitalization, length of ICU stay, overall survival | Tinzaparin 4500 UI/day vs tinzaparin 100 UI/kg/day vs. tinzaparin 175 UI/kg/day | Recruiting |
| NCT04646655 | Enoxaparin at Prophylactic or Therapeutic Doses With Monitoring of Outcomes in Subjects Infected With COVID‐19: a Pilot Study on 300 Cases Enrolled at ASST‐FBF‐Sacco | Mortality rate, respiratory failure, major bleeding; secondary outcome measures include DVT | Enoxaparin prophylactic dose vs enoxaparin therapeutic dose | Recruiting |
| NCT04409834 | A Multicenter, Randomized‐Controlled Trial to Evaluate the Efficacy and Safety of Antithrombotic Therapy for Prevention of Arterial and Venous Thrombotic Complications in Critically Ill COVID‐19 Patients | Prevention of thrombotic events | Full‐dose anticoagulation+antiplatelet vs full‐dose anticoagulation without antiplatelet vs prophylactic anticoagulation +antiplatelet vs prophylactic anticoagulation without antiplatelet | Recruiting |
| NCT04483960 | Australasian COVID‐19 Trial (ASCOT) ADAptive Platform Trial (ASCOT ADAPT) | All‐cause mortality or new intensive respiratory support or vasopressor/ionotropic support | Standard‐dose thromoboprophylaxis vs intermediate dose thromboprophylaxis vs therapeutic anticoagulation | Recruiting |
| NCT04345848 | Preventing COVID‐19‐associated Thrombosis, Coagulopathy and Mortality With Low‐ and High‐dose Anticoagulation: a Multicentric Randomized, Open‐label Clinical Trial | Thrombosis, DIC, and all‐cause mortality | Therapeutic LMWH or UFH vs Prophylactic LWMH or UFH | Terminated (low recruitment) |
| NCT04344756 | Cohort Multiple Randomized Controlled Trials Open‐label of Immune Modulatory Drugs and Other Treatments in COVID‐19 Patients CORIMUNO‐COAG Trial | Survival without ventilation and ventilator‐free survival. Secondary outcomes include thrombotic complications | Therapeutic anticoagulation with tinzaparin or UFH vs prophylactic anticoagulation | Not yet recruiting |
| NCT04367831 | Intermediate or Prophylactic‐Dose Anticoagulation for Venous or Arterial Thromboembolism in Severe COVID‐19: A Cluster Based Randomized Selection Trial (IMPROVE‐COVID) | Clinically relevant thrombotic events | Prophylactic enoxaparin or heparin vs intermediate dose enoxaparin or heparin | Recruitment completed |
| NCT04377997 | A Randomized, Open‐Label Trial of Therapeutic Anticoagulation in COVID‐19 Patients With an Elevated D‐Dimer | Death, cardiac arrest, thrombotic event or hemodynamic shock | Therapeutic anticoagulation vs prophylactic anticoagulation | Not yet recruiting |
| NCT04512079 | FREEDOM COVID Anticoagulation Strategy Randomized Trial | All‐cause mortality, intubation, systemic VTE or ischemic stroke | Prophylactic enoxaparin vs full‐dose enoxaparin vs apixaban 5 mg every 12 h | Recruiting |
| NCT04366960 | Comparison of Two Doses of Enoxaparin for Thromboprophylaxis in Hospitalized COVID‐19 Patients (X‐Covid 19) | Incidence of VTE | Enoxaparin 40 mg twice daily vs enoxaparin 40 mg daily | Recruitment completed |
| NCT04406389 | Anticoagulation in Critically Ill Patients With COVID‐19 (The IMPACT Trial) | 30‐day mortality | Therapeutic‐dose anticoagulation vs intermediate‐dose prophylaxis | Recruiting |
| NCT04408235 | High Versus Low LMWH Dosages in Hospitalized Patients With Severe COVID‐19 Pneumonia and Coagulopathy (COVID‐19 HD) | Clinical worsening defined by death, acute MI, symptomatic arterial or venous thromboembolism, need for advanced respiratory support. | Low‐Dose LMWH group (4000 IU daily) vs. High‐Dose LMWH (70 IU/kg every 12 h) | Not yet recruiting |
| NCT04360824 | COVID‐19‐associated Coagulopathy: Safety and Efficacy of Prophylactic Anticoagulation Therapy in Hospitalized Adults With COVID‐19 | All‐cause mortality | Prophylactic‐dose enoxaparin vs intermediate‐dose enoxaparin | Recruiting |
| NCT04351724 | Austrian CoronaVirus Adaptive Clinical Trial (COVID‐19) (ACOVACT) Substudy A | Sustained improvement (>48 h) of one point on the World Health Organization Scale | Rivaroxaban 5 mg twice daily vs local standard thromboprophylaxis | Recruiting |
| NCT04829552 | Prophylactic vs Therapeutic Dose Anticoagulation in COVID‐19 Infection at the Time of Admission to Critical Care Units | All‐cause mortality | LMWH 40 mg daily or UFH 5000 IU two or three times daily vs LMWH 1 mg/kg twice or 1.5 mg/kg/d or continuous infusion of UFH | Recruitment complete |
| NCT04508439 | Effect of the Use of Anticoagulant Therapy During Hospitalization and Discharge in Patients With COVID‐19 Infection | Ventilatory support time, length of hospital stay, mortality rate | Prophylactic vs therapeutic enoxaparin | Recruiting |
| NCT04542408 | Hamburg Edoxaban for Anticoagulation in COVID‐19 Study (HERO‐19) | All‐cause mortality and/ or VTE and/or arterial thromboembolism | Prophylactic vs therapeutic enoxaparin | Recruiting |
| NCT04600141 | Clinical Efficacy of Heparin and Tocilizumab in Patients With Severe COVID‐19 Infection (HEPMAB) | Clinical improvement within 30 days, defined by hospital discharge or clinical status | Prophylactic vs therapeutic anticoagulation (UFH or LMWH in each group) | Recruiting |
| NCT04604327 | Comparison of Two Different Doses of Bemiparin in COVID‐19 (BEMICOP) | Death, ICU admission, mechanical ventilator support, progression to ARDS, arterial or venous thrombosis | Prophylactic bemiparin vs therapeutic bemiparin | Recruiting |
| NCT04420299 | Clinical Trial on the Efficacy and Safety of Bemiparin in Patients Hospitalized Because of COVID‐19 | Death, ICU admission, mechanical ventilator support, progression to ARDS, arterial or venous thrombosis | Prophylactic bemiparin vs therapeutic bemiparin | Recruiting |
| Postdischarge thromboprophylaxis | ||||
| NCT04662684 | Medically Ill Hospitalized Patients for COVID‐19 Thrombosis Extended Prophylaxis With Rivaroxaban Therapy: The MICHELLE Trial | VTE and VTE‐related death | Rivaroxaban 10 mg daily vs no intervention | Abstract available |
| NCT04650087 | COVID‐19 Post‐hospital Thrombosis Prevention Trial: An Adaptive, Multicenter, Prospective, Randomized Platform Trial Evaluating the Efficacy and Safety of Antithrombotic Strategies in Patients With COVID‐19 Following Hospital Discharge | Thrombotic events and all‐cause mortality | Apixaban 2.5 mg twice daily vs placebo | Recruiting |
| NCT04508439 | Effect of the Use of Anticoagulant Therapy During Hospitalization and Discharge in Patients With COVID‐19 Infection | Thrombotic complications | Rivaroxaban 10 mg PO daily vs only clinical follow‐up | Recruiting |
| NCT04542408 | Hamburg Edoxaban for Anticoagulation in COVID‐19 Study (HERO‐19) | All‐cause mortality and/ or VTE and/or arterial thromboembolism | Edoxaban 60 mg daily vs placebo | Recruiting |
Abbreviations: ARDS, acute respiratory distress syndrome; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; ICU, intensive care unit; LMWH, low‐molecular‐weight heparin; MI, myocardial infarction; PE, pulmonary embolism; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; UFH, unfractionated heparin; VTE, venous thromboembolism.
FIGURE 2.
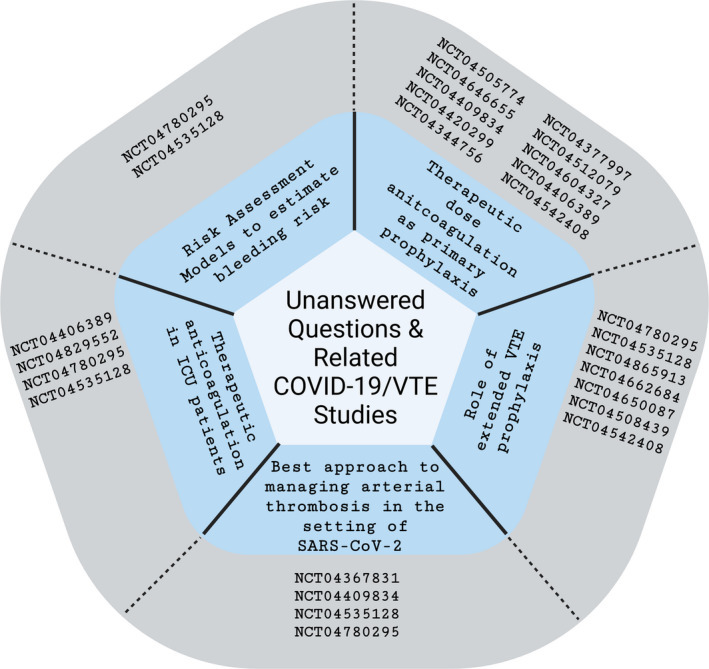
Emerging data to answer clinical queries surrounding COVID‐19 infection and risk of venous thrombosis. NCT04780295: COVID‐19 Registry on Thrombosis Complications (CORE‐THROMB). NCT04535128: COVID‐19 Registry to Assess Frequency, Risk Factors, Management, and Outcomes of Arterial and Venous Thromboembolic Complications (CORONA‐VTE‐NET). NCT04505774: Accelerating COVID‐19 Therapeutic Interventions and Vaccines 4 ACUTE (ACTIV‐4A). NCT04646655: Enoxaparin at Prophylactic or Therapeutic Doses in COVID‐19 (EMOS‐COVID). NCT04409834: Prevention of Ateriovenous Thrombotic Events in Critically Ill COVID‐19 Patients Trial (COVID‐PACT). NCT04344756: Trial Evaluation Efficacy and Safety of Anticoagulation in Patients with COVID‐19 Infection, Nested in the Corimmuno‐19 Cohort (CORIMMUNO‐COAG). NCT04377997: Safety and Efficacy of Therapeutic Anticoagulation on Clinical Outcomes in Hospitalized Patients with COVID‐19. NCT04512079: FREEDOM COVID‐19 Anticoagulation Strategy (FREEDOM COVID). NCT04406389: Anticoagulation in Critically Ill Patients with COVID‐19 (The IMPACT Trial). NCT04865913: Venous Thrombosis Virtual Surveillance in COVID (VVIRTUOSO). NCT04662684: Medically Ill Hospitalized Patients for COVID‐19 Thrombosis Extended Prophylaxis with Rivaroxaban Therapy: The MICHELLE Trial. NCT04650087: COVID‐19 Thrombosis Prevention Trials: Post‐hospital Thromboprophylaxis. NCT04508439: Effect of the Use of Anticoagulant Therapy During Hospitalization and Discharge in Patients With COVID‐19 Infection. NCT04542408: Hamburg Edoxaban for Anticoagulation in COVID‐19 Study (HERO‐19). NCT04367831: Intermediate or Prophylactic‐Dose Anticoagulation for Venous or Arterial Thromboembolism in Severe COVID‐19: A Cluster Based Randomized Selection Trial (IMPROVE‐COVID). NCT04409834: Prevention of Arteriovenous Thrombotic Events in Critically‐Ill COVID‐19 Patients Trial (COVID‐PACT). NCT04829552: Prophylactic vs Therapeutic Dose Anticoagulation in COVID‐19 Infection at the Time of Admission to Critical Care Units. Please note this list is not meant to be exhaustive, but rather illustrate the vast number of studies occurring in each of the areas of interest. ICU, intensive care unit; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; VTE, venous thromboembolism
9. CONCLUSION
The COVID‐19 pandemic has affected hundreds of millions of people worldwide, and it is known that SARS‐CoV‐2 infection is associated with coagulopathy and an increased risk of VTE. The pathophysiology of thrombosis in severely ill patients with COVID‐19 is likely multifactorial due to an intense immune‐inflammatory response, endothelial injury, and microvascular thrombosis. Worldwide, the medical community has worked tirelessly to improve prediction, diagnostic approach, prevention, and treatment of VTE in these patients. Despite these efforts, the optimal VTE prediction tools, thromboprophylaxis, and treatment strategies are still not clear. Many well‐designed prospective studies are under way to optimize our clinical approach to these patients. Given the recent resurgence of COVID‐19 cases, the medical community will continue to press forward in effort to provide high‐quality data to help answer these important questions.
RELATIONSHIP DISCLOSURE
SK reports Grants or contracts from Janssen, Bristol‐Myers Squibb, and Osmosis Research; and consulting fees from Janssen, Pfizer, Portola/Alexion, Bristol‐Myers Squibb, Novartis, CSL Behring, and Gilead. RZ reports consulting fees from Amagma Therapeutics and is a stockholder for Amagma Therapeutics. RR reports grants or contracts from Bristol‐Myers Squibb and Janssen; and consulting fees from Bristol‐Myers Squibb, Janssen, Dova, and Inari. She is vice president and serves on the Executive Committee and Board of Directors of the Pulmonary Embolism Response Team. AK reports consulting fees from Janssen, Bayer, Sanofi, Pfizer, and Anthos; payment for expert testimony; participation on DSMB for Jannsen, Bayer, Pfizer, and Sanofi; chair of the National Blood Clot Alliance Medical and Scientific Advisory Board. DA. SP, RP, PE, and WR report no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to the organization of the work and contributed to writing and revising the manuscript critically for accuracy and completeness.
ACKNOWLEDGMENTS
This work was authored by members of a steering committee at the National Blood Clot Alliance and supported by subcontract (02‐21‐8813) from the Association of University Centers on Disabilities (AUCD), under a cooperative agreement between AUCD and the Centers for Disease Control and Prevention (CDC) (6 NU38OT000280‐02‐02). The findings and conclusions of this review are those of the authors and do not necessarily represent the official positions of the CDC or the AUCD.
Angelini DE, Kaatz S, Rosovsky RP, et al. COVID‐19 and venous thromboembolism: A narrative review. Res Pract Thromb Haemost. 2022;6:e12666. doi: 10.1002/rth2.12666
Handling Editor: Dr Cihan Ay
Contributor Information
Dana E. Angelini, Email: angelid@ccf.org, @dangeliniMD.
Rachel P. Rosovsky, @RosovskyRachel.
Rebecca L. Zon, @beckyzon.
William E. Robertson, @medikprof.
Rushad Patell, @rushadpatell.
REFERENCES
- 1. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421‐1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Minet C, Potton L, Bonadona A, et al. Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Critical Care (London, England). 2015;19(1):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients with COVID‐19: awareness of an increased prevalence. Circulation. 2020;142(2):184‐186. [DOI] [PubMed] [Google Scholar]
- 4. Asakura H, Ogawa H. COVID‐19‐associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021;113(1):45‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID‐19. Lancet Haematol. 2020;7(6):e438‐e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moll M, Zon RL, Sylvester KW, et al. VTE in ICU patients with COVID‐19. Chest. 2020;158(5):2130‐2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Minno A, Ambrosino P, Calcaterra I, Di Minno MND. COVID‐19 and venous thromboembolism: a meta‐analysis of literature studies. Semin Thromb Hemost. 2020;46(7):763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hasan SS, Radford S, Kow CS, Zaidi STR. Venous thromboembolism in critically ill COVID‐19 patients receiving prophylactic or therapeutic anticoagulation: a systematic review and meta‐analysis. J Thromb Thrombolysis. 2020;50(4):814‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li JY, Wang HF, Yin P, et al. Clinical characteristics and risk factors for symptomatic venous thromboembolism in hospitalized COVID‐19 patients: a multicenter retrospective study. J Thromb Haemost. 2021;19(4):1038‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID‐19: a systematic review and meta‐analysis. Res Pract Thromb Haemost. 2020;4(7):1178‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and safety of therapeutic‐dose heparin vs standard prophylactic or intermediate‐dose heparins for thromboprophylaxis in high‐risk hospitalized patients with COVID‐19: the HEP‐COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020;173(4):268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID‐19 with fatal outcome : results from a prospective, single‐center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernández‐Capitán C, Barba R, Díaz‐Pedroche MDC, et al. Presenting characteristics, treatment patterns, and outcomes among patients with venous thromboembolism during hospitalization for COVID‐19. Semin Thromb Hemost. 2020;47(04):351‐361. [DOI] [PubMed] [Google Scholar]
- 16. Berger JS, Kunichoff D, Adhikari S, et al. Prevalence and outcomes of D‐dimer elevation in hospitalized patients with COVID‐19. Arterioscler Thromb Vasc Biol. 2020;40(10):2539‐2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nugroho J, Wardhana A, Mulia EP, et al. Elevated fibrinogen and fibrin degradation product are associated with poor outcome in COVID‐19 patients: a meta‐analysis. Clin Hemorheol Microcirc. 2021;77(2):221‐231. [DOI] [PubMed] [Google Scholar]
- 18. Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID‐19: a meta‐analysis. Int J Infect Dis. 2020;96:467‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID‐19. J Thromb Haemost. 2020;18(6):1469‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bertoletti L, Couturaud F, Montani D, Parent F, Sanchez O. Venous thromboembolism and COVID‐19. Resp Med Res. 2020;78:100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. Br J Haematol. 2009;145(1):24‐33. [DOI] [PubMed] [Google Scholar]
- 24. Hoechter DJ, Becker‐Pennrich A, Langrehr J, et al. Higher procoagulatory potential but lower DIC score in COVID‐19 ARDS patients compared to non‐COVID‐19 ARDS patients. Thromb Res. 2020;196:186‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID‐19: an autopsy series from New Orleans. Lancet Resp Med. 2020;8(7):681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383(2):120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cattaneo M, Bertinato EM, Birocchi S, et al. Pulmonary embolism or pulmonary thrombosis in COVID‐19? Is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120(8):1230‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet (London, England). 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baker CJ, Smith SA, Morrissey JH. Polyphosphate in thrombosis, hemostasis, and inflammation. Res Pract Thromb Haemost. 2019;3(1):18‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Subramaniam S, Jurk K, Hobohm L, et al. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood. 2017;129(16):2291‐2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Libby P, Simon DI. Inflammation and thrombosis: the clot thickens. Circulation. 2001;103(13):1718‐1720. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with COVID‐19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harzallah I, Debliquis A, Drénou B. Lupus anticoagulant is frequent in patients with Covid‐19. J Thromb Haemost. 2020;18(8):2064‐2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Devreese KMJ, Linskens EA, Benoit D, Peperstraete H. Antiphospholipid antibodies in patients with COVID‐19: a relevant observation? J Thromb Haemost. 2020;18(9):2191‐2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xiao M, Zhang Y, Zhang S, et al. Antiphospholipid antibodies in critically ill patients with COVID‐19. Arthritis Rheumatol (Hoboken, NJ). 2020;72(12):1998‐2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al‐Samkari H, Karp Leaf RS, Dzik WH, et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood. 2020;136(4):489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dujardin RWG, Hilderink BN, Haksteen WE, et al. Biomarkers for the prediction of venous thromboembolism in critically ill COVID‐19 patients. Thromb Res. 2020;196:308‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Demelo‐Rodríguez P, Cervilla‐Muñoz E, Ordieres‐Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID‐19 pneumonia and elevated D‐dimer levels. Thromb Res. 2020;192:23‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Al‐Samkari H, Song F, Van Cott EM, Kuter DJ, Rosovsky R. Evaluation of the prothrombin fragment 1.2 in patients with coronavirus disease 2019 (COVID‐19). Am J Hematol. 2020;95(12):1479‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID‐19. J Am Coll Cardiol. 2020;76(16):1815‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372:n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patell R, Chiasakul T, Bauer E, Zwicker JI. Pharmacologic thromboprophylaxis and thrombosis in hospitalized patients with COVID‐19: a pooled analysis. Thromb Haemost. 2021;121(1):76‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Flaczyk A, Rosovsky RP, Reed CT, Bankhead‐Kendall BK, Bittner EA, Chang MG. Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: implications for clinical practice and future investigations. Crit Care. 2020;24(1):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meizlish ML, Goshua G, Liu Y, et al. Intermediate‐dose anticoagulation, aspirin, and in‐hospital mortality in COVID‐19: a propensity score‐matched analysis. Am J Hematol. 2021;96(4):471‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Motta JK, Ogunnaike RO, Shah R, et al. Clinical outcomes with the use of prophylactic versus therapeutic anticoagulation in coronavirus disease 2019. Crit Care Explor. 2020;2(12):e0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Al‐Samkari H, Gupta S, Leaf RK, et al. Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with COVID‐19. Ann Intern Med. 2021;174(5):622‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of intermediate‐dose vs standard‐dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID‐19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620‐1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perepu US, Chambers I, Wahab A, et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID‐19: a multi‐center, open‐label, randomized controlled trial. J Thromb Haemost. 2021;19(9):2225‐2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically ill patients with COVID‐19. N Engl J Med. 2021;385(9):777‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation in non‐critically ill patients with COVID‐19. medRxiv. 2021:2021.05.13.21256846. [Google Scholar]
- 52. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID‐19. N Engl J Med. 2021;385(9):790‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lopes RD, de Barros ESPGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID‐19 and elevated D‐dimer concentration (ACTION): an open‐label, multicentre, randomised, controlled trial. Lancet (London, England). 2021;397:2253‐2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sholzberg M, Tang GH, Rahhal H, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid‐19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Daughety MM, Morgan A, Frost E, et al. COVID‐19 associated coagulopathy: thrombosis, hemorrhage and mortality rates with an escalated‐dose thromboprophylaxis strategy. Thromb Res. 2020;196:483‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jiménez D, García‐Sanchez A, Rali P, et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta‐analysis. Chest. 2021;159(3):1182‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Demelo‐Rodriguez P, Farfán‐Sedano AI, Pedrajas JM, et al. Bleeding risk in hospitalized patients with COVID‐19 receiving intermediate‐ or therapeutic doses of thromboprophylaxis. J Thromb Haemost. 2021;19(8):1981‐1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Patell R, Bogue T, Koshy A, et al. Postdischarge thrombosis and hemorrhage in patients with COVID‐19. Blood. 2020;136(11):1342‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roberts LN, Whyte MB, Georgiou L, et al. Postdischarge venous thromboembolism following hospital admission with COVID‐19. Blood. 2020;136(11):1347‐1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Giannis D, Allen S, Tsang J, et al. Post‐discharge thromboembolic outcomes and mortality of hospitalized COVID‐19 patients: the CORE‐19 registry. Blood. 2021;137(20):2838‐2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Talasaz AH, Sadeghipour P, Kakavand H, et al. Recent randomized trials of antithrombotic therapy for patients with COVID‐19: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;77(15):1903‐1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Connors JM, Brooks MM, Sciurba FC, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID‐19: the ACTIV‐4B randomized clinical trial. JAMA. 2021;326(17):1703‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID‐19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1859‐1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75(23):2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rosovsky RP, Grodzin C, Channick R, et al. Diagnosis and treatment of pulmonary embolism during the coronavirus disease 2019 pandemic: a position paper from the national PERT consortium. Chest. 2020;158(6):2590‐2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315‐352. [DOI] [PubMed] [Google Scholar]
- 70. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Resp J. 2019;54(3):1901647. [DOI] [PubMed] [Google Scholar]
- 71. Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693‐4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Arachchillage DRJ, Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(5):1233‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. White D, MacDonald S, Bull T, et al. Heparin resistance in COVID‐19 patients in the intensive care unit. J Thromb Thrombolysis. 2020;50(2):287‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Beun R, Kusadasi N, Sikma M, Westerink J, Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS‐CoV‐2. Int J Lab Hemat. 2020;42(S1):19‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Foerster KI, Hermann S, Mikus G, Haefeli WE. Drug‐drug interactions with direct oral anticoagulants. Clin Pharmacokinet. 2020;59(8):967‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Testa S, Prandoni P, Paoletti O, et al. Direct oral anticoagulant plasma levels’ striking increase in severe COVID‐19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J Thromb Haemost. 2020;18(6):1320‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang J, Hajizadeh N, Moore EE, et al. Tissue plasminogen activator (tPA) treatment for COVID‐19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18(7):1752‐1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Barrett CD, Oren‐Grinberg A, Chao E, et al. Rescue therapy for severe COVID‐19‐associated acute respiratory distress syndrome with tissue plasminogen activator: a case series. J Trauma Acute Care Surg. 2020;89(3):453‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]


