Abstract
We did this study to clarify the association between carbohydrate intake and the risk of type 2 diabetes (T2D) and potential effect modification by geographical location. PubMed, Scopus and Web of Science were searched to find prospective cohort studies of dietary carbohydrate intake and T2D risk. A random-effects dose–response meta-analysis was performed to calculate the summary hazard ratios (HRs) and 95%CIs. The quality of cohort studies and the certainty of evidence was rated using the Newcastle–Ottawa Scale and GRADE tool, respectively. Eighteen prospective cohort studies with 29,229 cases among 607,882 participants were included. Thirteen studies were rated to have high quality, and five as moderate quality. The HR for the highest compared with the lowest category of carbohydrate intake was 1.02 (95%CI: 0.91, 1.15; I2 = 67%, GRADE = low certainty). The HRs were 0.93 (95%CI: 0.82, 1.05; I2 = 58%, n = 7) and 1.26 (95%CI: 1.11, 1.44; I2 = 6%, n = 6) in Western and Asian countries, respectively. Dose–response analysis indicated a J shaped association, with the lowest risk at 50% carbohydrate intake (HR50%: 0.95, 95%CI: 0.90, 0.99) and with risk increasing significantly at 70% carbohydrate intake (HR70%: 1.18, 95%CI: 1.03, 1.35). There was no association between low carbohydrate diet score and the risk of T2D (HR: 1.14, 95%CI: 0.89, 1.47; I2 = 90%, n = 5). Carbohydrate intake within the recommended 45–65% of calorie intake was not associated with an increased risk of T2D. Carbohydrate intake more than 70% calorie intake might be associated with a higher risk.
Subject terms: Diseases, Medical research
Introduction
Type 2 diabetes (T2D) is a serious non-communicable chronic disease described by impaired insulin action or secretion or impaired response of body cells to insulin, followed by the endocrine pancreas' incapability to compensate for this weakened response1,2. It is estimated that at least 514 million people are affected by T2D all over the world1. The Middle East and North Africa is a region with the highest prevalence of T2D across the globe2,3. T2D is a chronic progressive disease, and therefore, lifestyle modifications such as dietary interventions, physical activity, and weight reduction are the core part of first-line interventions for the prevention of T2D4–6.
Dietary factors might have a role in the development of T2D7. Of note, dietary carbohydrates have received specific attention because of their effect on blood glucose level8. Dietary carbohydrates are the major dietary energy source9,10 and have the greatest impact on postprandial blood glucose levels11. Studies have shown that glycemic properties of the diet including glycemic index and load might be associated with the risk of developing T2D and other chronic diseases11.
With regard to T2D, three meta-analyses of cohort studies have been undertaken of the association between dietary carbohydrate and the risk of T2D, but the results have been inconsistent11–13. However, almost all studies included in the published meta-analyses have been conducted in Western countries, where the intake of carbohydrates was lower than that of Asian countries14,15. A recent publication from the PURE study in 21 countries across the world indicated that higher rice consumption was associated with a greater risk of developing T2D, with the strongest association in South Asia and a modest, nonsignificant association in other regions16.
A number of population-based prospective cohort studies in Asian countries have recently been published that reported significant positive associations between dietary carbohydrates and the risk of T2D17–19. In the current study, we therefore aimed to update the evidence from prospective cohort studies of the association between dietary carbohydrate intake and low-carbohydrate diet score (LCDS) with the risk of T2D in the general population. Our secondary outcome was to assess this association separately in Asian and Western countries.
Martials and methods
The Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines have been used for reporting this meta-analysis20. The protocol of the study was registered at Open Science Framework (https://osf.io/tvam2; registered form: osf.io/4vu5s; registration https://doi.org/10.17605/OSF.IO/TVAM2).
Search strategy
We performed a comprehensive systematic search on all literature issued earlier than April 2021 in online databases including PubMed/Medline, Scopus and ISI Web of Science. We did not exert any limitation in term of language or time of publication. We used search terms relevant to type 2 diabetes, carbohydrate, and study design to find potential eligible cohort studies (Supplementary Table 1). Reference lists of retrieved articles and relevant reviews were also manually searched. Unpublished data was not included.
Inclusion and exclusion criteria
Relevant articles with all of the following inclusion criteria were included: (1) published prospective cohort studies conducted in the general population; (2) reported carbohydrate consumption (as either g/d or percentage energy) and LCDS as exposure; (3) considered T2D incidence as the outcomes of interest; (4) provided estimates of the effect size in the form of relative risk, hazard ratio (HR) or rate ratio with corresponding 95% confidence intervals (CIs) for ≥ 2 quantitative categories of carbohydrate consumption or LCDS; and (5) provided the numbers of cases and non-cases or person-years in each category of dietary carbohydrate or LCDS. Studies that reported continuous estimation from the associations were also included. For duplicate publications form the same cohort, the one with the greater number of cases was included in our meta-analysis. We excluded letters, comments, reviews and meta-analyses, and ecologic studies. We also did not include studies that were performed on children or adolescences or those that were conducted among patients with type one diabetes. All outcomes were classified based on the World Health Organization’s international classification of disease criteria.
Data extraction
Data extraction process was executed by two reviewers in duplicate (FH and AJ), and any divergences were resolved by consultation the principal investigator (SS-B). We extracted the following information from the publications identified: name of the first author, publication year, country, age, sex, study participants, number of cases, duration of follow-up, method of assessment of carbohydrate consumption and LCDS, the fully-adjusted estimates and their 95%CI and list of potential confounders entered into the multivariable statistical model. Gender-specific estimates were combined a by fixed-effects model to include each cohort once in the main analysis. We used web plot digitizer (http://plotdigitizer.sourceforge.net/) to extract numerical estimates from graphs.
Data synthesis and analysis
We considered the HR and its 95%CI as the effect size for the present study. Relative risks were considered equal to HR21. We first performed a pairwise meta-analysis by combining the reported effect sizes for the highest compared with the lowest category of dietary carbohydrate or LCDS in each study. Study-specific results were combined with a random-effects model22. The Cochran Q23 and I2 statistic24 were used to test for presence of heterogeneity.
Subgroup analyses of dietary carbohydrates were performed based on sex, geographic location, number of cases, duration of follow-up and adjustments for main confounders including body mass index (BMI), smoking status, alcohol drinking, and energy and fiber intakes. P value for subgroup difference was generated using meta-regression analysis. Subgroup analyses of LCDS were performed based on sex, study location, and duration of follow-up. Publication bias was assessed by visual inspection of funnel plot23 and Egger’s25 and Begg’s26 tests, when at least 10 studies were available. To determine whether the pooled effect size was influenced heavily by a single cohort, sensitivity analysis was done by step-by-step omission of each cohort at a time.
We used the method introduced by Greenland27 and Orsini28 for dose–response meta-analysis. We calculated the HRs for a 10% increment in carbohydrate intake or a 10-point increment in LCDS in each study. Study-specific HRs were combined by a random-effects model. For this purpose, each cohort study must report the number of cases and person-years and median or range of dietary carbohydrate or LCDS across categories of exposures. For studies that reported dietary carbohydrate as g/d, we converted g/d to percentage calorie from carbohydrate by using the average daily energy intake of the study participants. For studies that reported the results per unit increment in dietary carbohydrate (i.e., per 200 g/d increment), we first converted g/d to percentage energy from carbohydrate and then translated it to a 10% increment in energy intake from carbohydrate. For studies that used different units (for example, 5% increase in carbohydrate intake), we calculated the logarithm of the HR and its 95%CI, multiplied by the corresponding unit, and then exponentiated the results. For studies that reported carbohydrate intake as a range in each category, we used the midpoint of lower bounds as a proxy of the median. The widths of the open-ended categories were considered equal to the closest categories.
Finally, we performed a one-stage weighted mixed-effects meta-analysis to model dose–response associations29. This method estimates the study-specific slope lines and combines them to obtain an overall average slope in a single stage. We included all studies in the main analysis. However, due to substantial difference in carbohydrate consumption in Asian and Western countries, we performed separate nonlinear dose–response analyses in Asian and Western countries. Statistical analyses were conducted using STATA software, version 15.0. P < 0.05 was considered statistically significant.
Quality assessments and grading the evidence
The quality of the original studies included in the present meta-analysis was evaluated using a 9-point Newcastle–Ottawa Scale by two independent investigators (FH and AJ)30. Accordingly, studies with 1–3, 4–6, and 7–9 points were rated as poor, fair, and high quality, respectively. The certainty in the estimates was rated by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. GRADE tool is a metric to assess the certainty of the evidence31. This tool grades observational studies as low with downgrades for study limitations, inconsistency, indirectness, imprecision, and publication bias, and upgrades for large effect size, dose–response gradient, and attenuation by plausible confounding.
Results
Literature search
We totally identified 3903 articles in our initial search. We excluded 753 duplicates and additional 3125 articles by reviewing the title and abstract. A total of 25 full text were completely reviewed for eligibility. After full-text reviewing, we excluded six articles that were duplicate publications from the same studies32–37 and one study with insufficient data38. Finally, 18 prospective cohort studies were included17–19,39–53 (Supplementary Fig. 1).
Characteristics of included studies
Characteristics of the included studies are provided in Supplementary Table 2. In total, 607,882 participants with an age range between 19 and 79 years were included. The length of the follow-up periods ranged from 3 to 24 years. Six studies were conducted in women41,44,45,49–51, three in men19,39,47, and the rest were in mixed. Six studies were conducted in the United States39,41,44,45,50,51, and 12 in other countries including, UK40, Australia42,43, Korea17,18, Japan19,52, Germany46, Finland47, China49 and Netherlands48. To assess dietary carbohydrate intake and LCDS, all studies used a food frequency questionnaire, except two studies that used a diet history questionnaire19 and a 7-day food diary40. Most studies controlled for important conventional confounders including physical activity (n = 18), smoking status (n = 17), energy intake (n = 17), BMI (n = 16), and alcohol consumption (n = 15). Only a few studies included in this meta-analysis did not adjust for energy intake42 and BMI42,53. Ten studies did not adjust for fiber intake17,39,40,43,44,47,49,50,52,53. Looking at the variation of NOS score, 13 studies out of 18 studies were rated high quality (NOS score of ≥ 7)17,18,39–41,44–51, and the others were rated to have moderate quality (NOS score of ≤ 7)19,42,43,52,53 (Supplementary Table 3) with none rated as low quality. Characteristics of primary cohort studies are presented in Supplementary Table 2 and reported effect sizes of type 2 diabetes across categories of dietary carbohydrate intake and LCDS are indicated in Supplementary Tables 4 and 5, respectively.
Dietary carbohydrate and type 2 diabetes
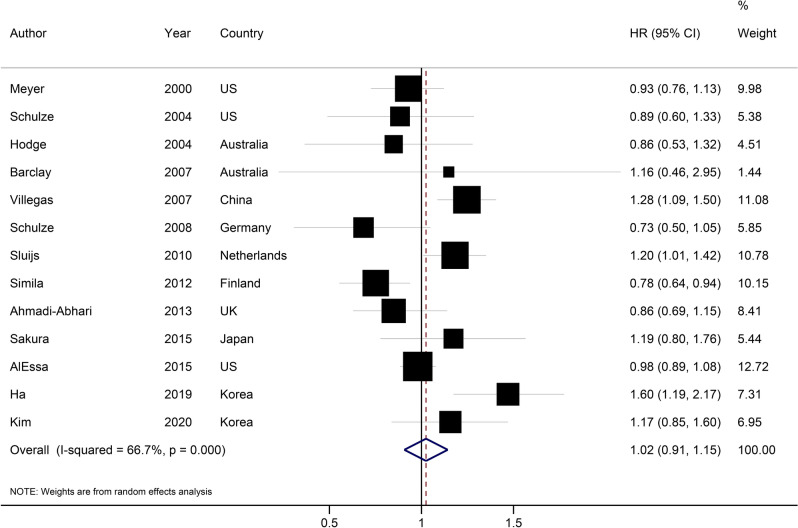
Thirteen prospective cohort studies investigated the association between intake of carbohydrates from diet and T2D17–19,40–49. These studies included 403,883 participants, among whom 19,833 cases of T2D were found. In the main analysis, the highest compared with the lowest category of dietary carbohydrate intake was not associated with the risk of T2D (HR: 1.02, 95%CI: 0.91, 1.15; Fig. 1), with substantial heterogeneity (I2 = 67%, Phet < 0.001).
Figure 1.
Hazard ratio of type 2 diabetes for the highest compared with the lowest category of type 2 diabetes. HR Hazard ratio.
The association did not reach statistical significance by the stepwise exclusion of each primary study at a time (HR range: 0.99 to 1.05). In the subgroup analyses, there was no significant association across subgroups except for studies conducted in Asia (HR: 1.26, 95%CI: 1.11, 1.44; I2 = 6%, n = 6; Table 1). Geographical location, number of cases, and adjustment for dietary fiber intake were potential sources of heterogeneity. There was no evidence of small-study effect such as publication bias with Egger’s test (P = 0.99) and Begg’s test (P = 0.95) (Supplementary Fig. 2).
Table 1.
Subgroup analyses of dietary carbohydrate and the risk of type 2 diabetes (highest versus lowest category meta-analysis).
| n | HR (95%CI) | I2, Pheterogeneity | Chi-squared | P subgroup difference | |
|---|---|---|---|---|---|
| All studies | 13 | 1.02 (0.91, 1.15) | 67%, < 0.001 | 36.05 | – |
| Sex | 0.82 | ||||
| Men | 4 | 1.03 (0.76, 1.39) | 72%, 0.01 | 10.79 | |
| Women | 6 | 1.06 (0.90, 1.24) | 66%, 0.01 | 14.86 | |
| Both | 5 | 1.02 (0.83, 1.25) | 52%, 0.08 | 8.34 | |
| Geographical region | 0.02 | ||||
| US + Europe | 7 | 0.93 (0.82, 1.05) | 58%, 0.27 | 14.28 | |
| Asia | 6 | 1.26 (1.11, 1.44) | 6%, 0.38 | 5.48 | |
| Number of cases | 0.64 | ||||
| < 1000 | 8 | 1.00 (0.86, 1.16) | 34%, 0.16 | 10.62 | |
| > 1000 | 5 | 1.06 (0.87, 1.29) | 84%, < 0.001 | 25.37 | |
| Follow-up duration | 0.67 | ||||
| < 10 years | 6 | 0.99 (0.82, 1.20) | 61%, 0.03 | 12.84 | |
| > 10 years | 7 | 1.05 (0.89, 1.24) | 73%, 0.001 | 22.53 | |
| Adjustments | |||||
| Smoking status | 0.56 | ||||
| Yes | 10 | 1.03 (0.91, 1.17) | 69%, < 0.001 | 35.48 | |
| No | 1 | 0.84 (0.51, 1.15) | – | 0.00 | |
| Energy intake | 0.81 | ||||
| Yes | 10 | 1.02 (0.91, 1.15) | 70%, < 0.001 | 35.98 | |
| No | 1 | 1.14 (0.43, 3.01) | – | 0.00 | |
| Body mass index | 0.81 | ||||
| Yes | 10 | 1.02 (0.91, 1.15) | 70%, < 0.001 | 35.98 | |
| No | 1 | 1.14 (0.43, 3.01) | – | 0.00 | |
| Alcohol drinking | 0.20 | ||||
| Yes | 9 | 1.06 (0.94, 1.19) | 64%, 0.002 | 27.48 | |
| No | 2 | 0.79 (0.66, 0.96) | 0%, 0.45 | 0.67 | |
| Fiber intake | 0.94 | ||||
| Yes | 7 | 1.04 (0.92, 1.17) | 34%, 0.17 | 9.10 | |
| No | 6 | 1.02 (0.81, 1.27) | 82%, < 0.001 | 26.94 | |
All studies but one18 reported sufficient data for dose–response analysis. A 10% increment in energy intake form carbohydrate was not associated with the risk of T2D (HR: 1.02, 95%CI: 0.95, 1.09; I2 = 70%, Supplementary Fig. 3). Dose–response analysis indicated a J-shaped association between percentage energy from carbohydrate and the risk of T2D (Pnonlinearity < 0.001, Pdose-response < 0.001; Fig. 2), with the lowest risk at 50% energy from carbohydrate (HR50%: 0.95, 95%CI: 0.90, 0.99) and higher risk as carbohydrate intake increased. The HRs for 60%, 70%, and 80% calorie intake from carbohydrate were, respectively, 1.01 (95%CI: 0.93, 1.09), 1.18 (95%CI: 1.03, 1.35), and 1.41 (95%CI: 1.15, 1.73).
Figure 2.
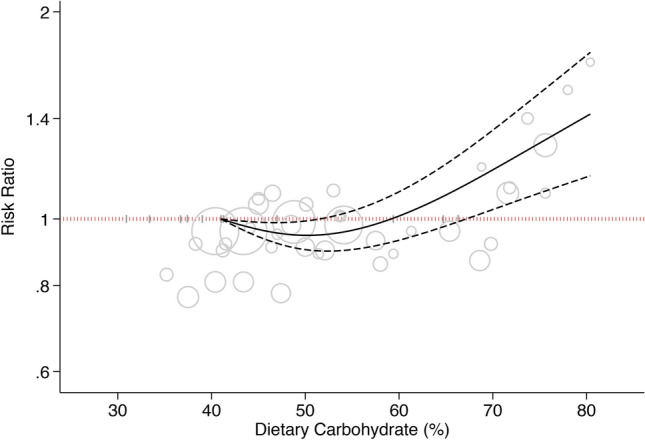
Dose–response association between carbohydrate intake and risk of type 2 diabetes. Solid line represents non-linear dose response and dotted lines represent 95% confidence interval. Circles represent hazard ratio point estimates for carbohydrate intake categories from each study with circle size proportional to inverse of standard error. Small vertical grey lines are baseline carbohydrate intake categories in each study.
Restricting dose–response analyses to studies from Western countries only indicated that the risk of T2D did not change remarkably with increasing carbohydrate intake from 37 to 60% of total calorie (Pnonlinearity = 0.43, Pdose-response = 0.01.; n = 7, Fig. 3A). The HRs for 40%, 50%, and 60% calorie intake from carbohydrate in Western countries were 0.98 (95%CI: 0.95, 1.00), 0.93 (95%CI: 0.86, 0.99), and 0.90 (95%CI: 0.80, 1.02), respectively.
Figure 3.
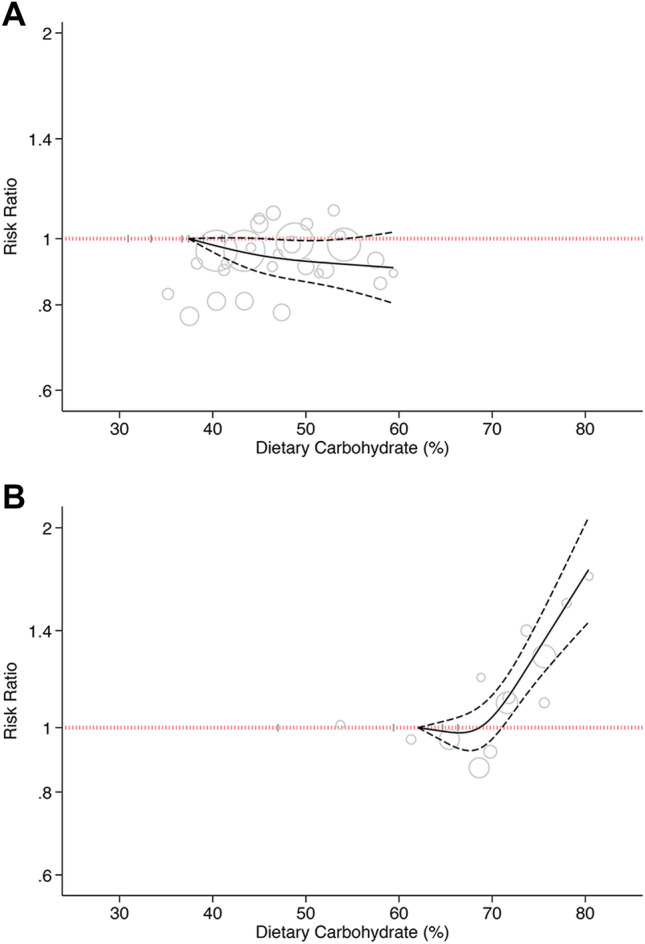
Dose–response association between carbohydrate intake and risk of type 2 diabetes. (A) Western countries. (B) Asian countries. Solid line represents non-linear dose response and dotted lines represent 95% confidence interval. Circles represent hazard ratio point estimates for carbohydrate intake categories from each study with circle size proportional to inverse of standard error. Small vertical grey lines are baseline carbohydrate intake categories in each study.
The analysis of Asian studies indicated that the risk of T2D did not change remarkably with increasing carbohydrate intake from 63 to 70% of total calorie (HR70%: 1.04, 95%CI: 0.96, 1.12), followed by a sharp and linear increment in risk (Pnonlinearity < 0.001, Pdose-response < 0.001; n = 5, Fig. 3B). The HR of T2D for carbohydrate intake of 80% total calorie was 1.70 (95%CI: 1.42, 2.02).
Low-carbohydrate diet score and type 2 diabetes
Five studies investigated the association between LCDS and T2D39,50–53. These studies included 198,172 participants, among whom 9395 cases of T2D were found. There was no association between LCDS and the risk of T2D, either in the highest versus lowest category meta-analysis (HR: 1.14, 95%CI: 0.89, 1.47; I2 = 86%, n = 5; Supplementary Fig. 4), or in dose–response meta-analysis (HRper 10-unit increase: 1.06, 0.95%CI: 0.92, 1.21; I2 = 90%, n = 5; Supplementary Fig. 5). A non-significant association persisted in the subgroups defined by geographical location and follow-up duration (Supplementary Table 6).
Grading the evidence
The certainty in the estimates was rated by the GRADE approach. The certainty of the evidence was rated low for dietary carbohydrate, with a downgrade for imprecision and an upgrade for dose–response gradient (Supplementary Table 7). The certainty in the estimates was rated very low for LCDS, with downgrades for imprecision and inconsistency.
Discussion
This is the most recent and up-to-date meta-analysis of prospective cohort studies that examined the association between carbohydrate intake from diet and risk of T2D. Since the release of the three earlier meta-analyses11–13, some prospective cohort studies, especially those conducted in Asian countries, have been published that highlighted a need to present updated evidence for this association. We found evidence of a J-shaped relationship between carbohydrate intake and T2D in the non-linear dose–response, with the lowest risk at carbohydrate intake of 50% total calorie and with risk increasing significantly at 70% of total calorie. There appeared to be a marked difference in the association between carbohydrate intake and T2D between Asian and Western countries. Low carbohydrate diet score was not associated with the risk of T2D.
In line with ours, a previous meta-analysis on eight prospective studies in 2013 revealed that total carbohydrate intake was not associated with the risk of T2D in the linear dose–response analysis12. In addition, some earlier studies, mostly conducted in Western countries, did not find an association between carbohydrate intake from diet and the risk of T2D32,42,44,45.
Another recent meta-analysis of cohort studies showed a non-significant association between carbohydrate intake and the risk of T2D in Western countries and in contrast, found a significant positive association in one Asian study11. We updated the evidence and included additional recent studies conducted in Asian countries which showed that carbohydrate intake, within the recommended daily intake of 45–65% of total calorie, as reported in Western countries, was not associated with an increased risk of type 2 diabetes, and even was associated with a modest lower risk at 50% carbohydrate intake. However, the nonlinear dose–response meta-analysis of five Asian studies suggested that carbohydrate intake higher than 70% of total calorie was strongly associated with a higher risk of T2D.
A recent meta-analysis of prospective cohort studies found a similar U-shaped association between carbohydrate intake and total mortality, with the lowest risk being found at 50–55% of carbohydrate intake, and an increased risk at an intake of more than 70% carbohydrate intake54. Evidence from earlier prospective cohort studies evaluating the association between the quality and quantity of dietary carbohydrates, reflected by dietary glycemic index and load, suggests that both quality and quantity of dietary carbohydrates are associated with the risk of T2D18,43,55,56. In addition, there was also evidence of a causal association between dietary glycemic index and load and the risk of T2D56,57.
Studies have suggested some mechanisms relating dietary carbohydrates to the risk of T2D. The long-term exposure to dietary carbohydrates may provide a continuous signal to the pancreatic β-cell to secret insulin to reduce blood glucose levels. Consequently, β-cell exhaustion can result in glucose intolerance58. Furthermore, excessive carbohydrates intake produces a large amount of acetyl CoA in the metabolic pathways, thus releasing lots of free radical and thereby exacerbating insulin resistance58,59.
There are also several explanations for the observed geographical difference found in the present study. First and most importantly, carbohydrate intake is substantially higher in Asian countries (generally > 60%) than in Western countries (generally < 50%)54. We found a relatively J-shaped association, wherein the US and European countries mainly represented the left side of the curve and in contrast, Asian countries represented the right side of the curve54. Higher carbohydrate intake increases demand for insulin secretion, leading to β-cell exhaustion. Second, Asian populations have a lower capacity of insulin secretion than that of their Western counterparts60–62. In addition, type of carbohydrate consumed, especially proportion of whole and refined grains, may be different across the globe and this may create a difference in the association between dietary carbohydrates with the risk of T2D. The main source of carbohydrates in most Asian countries is refined carbohydrates such as white rice and bread, reflecting low diet quality63,64. White rice, a high glycemic index food, was associated with an increased risk of T2D, especially in Asian societies65,66. More recently, a prospective cohort study conducted in 21 countries across the globe indicated that higher rice consumption was associated with an increased risk of type 2 diabetes in South Asian countries, and a modest non-significant association in other regions16.
Strength and limitations
We updated previous meta-analyses and included the most recent studies, especially those conducted in Asia. We included new Asian articles and looked at them separately by subgrouping them because of the difference in their diet. Here we showed that higher carbohydrate intake more than the recommended daily intake of 45–65% was strongly associated with the risk of T2D. We applied a newly-developed one-stage linear mixed effects meta-analysis that creates more efficient and flexible plots than the conventional two-stage model.
Some limitations should be noted in the context of our findings. Due to the observational nature of the studies included, our resulting associations cannot establish causality. According to the GRADE, the certainty of the evidence was rated low for dietary carbohydrate and very low for LCDS. In addition, we had insufficient data for the analysis of LCDS. We used total carbohydrate intake as exposure which represents a large diverse group of foods such as whole and refined grains. The potential difference in foods constituting total carbohydrate intake in Asian and Western countries might confound the association between total carbohydrate intake and T2D.
Conclusion
The results of this updated meta-analysis of 18 cohort studies (607,882 participants with 29,228 cases) showed that carbohydrate intake within the recommended dietary intake of 45% to 65% of total calorie was not associated with a higher risk of T2D and even was associated with a modest lower risk at 50% carbohydrate intake. Carbohydrate intake more than 70% of total calorie, as found in Asian countries, was associated with substantial higher risk of T2D. However, these findings were obtained from observational studies and thus, could not prove causality. More research, especially in Asian countries, is needed to investigate the association between carbohydrate intakes higher than recommended dietary intake with the risk of T2D.
Supplementary Information
Abbreviations
- GRADE
Grading of recommendations assessment, development, and evaluation (GRADE) approach
- LCDS
Low carbohydrate diet score
Author contributions
F.H. contributed to the literature search, data extraction, and manuscript drafting. A.J. contributed to the study conception, literature search, data extraction, data analysis, and manuscript drafting. S.S.B. contributed to study conception and data analysis. T.A.K. contributed to data analysis. S.S.-B. and T.A.K. critically revised the manuscript. All authors acknowledge the full responsibility for the analyses and interpretation of the report.
Data availability
The data, codes, analytical syntax, and other additional data used for the present meta-analysis are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06212-9.
References
- 1.Federation I. IDF diabetes atlas eighth edition 2017. (2017).
- 2.Guariguata L, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Aguiree FT, et al. IDF diabetes atlas. 6. International Diabetes Federation; 2013. [PubMed] [Google Scholar]
- 4.Eriksson K-F, Lindgärde F. Prevention of Type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise The 6-year Malmö feasibility study. Diabetologia. 1991;34:891–898. doi: 10.1007/BF00400196. [DOI] [PubMed] [Google Scholar]
- 5.Armato JP, DeFronzo RA, Abdul-Ghani M, Ruby RJ. Successful treatment of prediabetes in clinical practice using physiological assessment (STOP DIABETES) Lancet Diabetes Endocrinol. 2018;6:781–789. doi: 10.1016/S2213-8587(18)30234-1. [DOI] [PubMed] [Google Scholar]
- 6.Group DPPR Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherwin RS, Anderson RM, Buse JB, Chin MH. The prevention or delay of type 2 diabetes. Diabetes Care. 2002;25:742. doi: 10.2337/diacare.26.2007.s62. [DOI] [PubMed] [Google Scholar]
- 8.Hu FB, Van Dam R, Liu S. Diet and risk of type II diabetes: The role of types of fat and carbohydrate. Diabetologia. 2001;44:805–817. doi: 10.1007/s001250100547. [DOI] [PubMed] [Google Scholar]
- 9.Jequier E. Carbohydrates as a source of energy. Am. J. Clin. Nutr. 1994;59:682S–685S. doi: 10.1093/ajcn/59.3.682S. [DOI] [PubMed] [Google Scholar]
- 10.Brand-Miller JC. Postprandial Glycemia, Glycemic Index, and the Prevention of Type 2 Diabetes. Oxford University Press; 2004. [DOI] [PubMed] [Google Scholar]
- 11.Hardy DS, Garvin JT, Xu H. Carbohydrate quality, glycemic index, glycemic load and cardiometabolic risks in the US, Europe and Asia: A dose-response meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2020;30(6):853–871. doi: 10.1016/j.numecd.2019.12.050. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood DC, et al. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: Systematic review and dose–response meta-analysis of prospective studies. Diabetes Care. 2013;36:4166–4171. doi: 10.2337/dc13-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhazmi A, Stojanovski E, McEvoy M, Garg ML. Macronutrient intakes and development of type 2 diabetes: A systematic review and meta-analysis of cohort studies. J. Am. Coll. Nutr. 2012;31:243–258. doi: 10.1080/07315724.2012.10720425. [DOI] [PubMed] [Google Scholar]
- 14.Zhou B, et al. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: The INTERMAP study. J. Hum. Hypertens. 2003;17:623–630. doi: 10.1038/sj.jhh.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown IJ, et al. Dietary starch intake of individuals and their blood pressure: The INTERMAP study. J. Hypertens. 2009;27:231. doi: 10.1097/HJH.0b013e32831a7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhavadharini B, et al. White rice intake and incident diabetes: a study of 132,373 participants in 21 countries. Diabetes Care. 2020;43:2643–2650. doi: 10.2337/dc19-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha K, Joung H, Song Y. Inadequate fat or carbohydrate intake was associated with an increased incidence of type 2 diabetes mellitus in Korean adults: A 12-year community-based prospective cohort study. Diabetes Res. Clin. Pract. 2019;148:254–261. doi: 10.1016/j.diabres.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Kim SY, et al. Association of dietary glycaemic index, glycaemic load, and total carbohydrates with incidence of type-2 diabetes in adults aged≥ 40 years: The Multi-Rural Communities Cohort (MRCohort) Diabetes Res. Clin. Pract. 2020;160:108007. doi: 10.1016/j.diabres.2020.108007. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai M, et al. Dietary carbohydrate intake, presence of obesity and the incident risk of type 2 diabetes in Japanese men. J. Diabetes Investig. 2016;7:343–351. doi: 10.1111/jdi.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Symons M, Moore D. Hazard rate ratio and prospective epidemiological studies. J. Clin. Epidemiol. 2002;55:893–899. doi: 10.1016/s0895-4356(02)00443-2. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, et al. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2019. [Google Scholar]
- 24.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1088–1101 (1994). [PubMed]
- 27.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 28.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stand. Genom. Sci. 2006;6:40–57. [Google Scholar]
- 29.Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose–response meta-analysis for aggregated data. Stat. Methods Med. Res. 2019;28:1579–1596. doi: 10.1177/0962280218773122. [DOI] [PubMed] [Google Scholar]
- 30.Wells, G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (2004).
- 31.Guyatt GH, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salmeron J, et al. Dietary fiber, glycemic load, and risk of non—insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 33.Eslamian, G. et al. Low carbohydrate diet score does not predict metabolic syndrome in children and adolescents: Tehran Lipid and Glucose Study. Arch. Iran. Med.17 (2014). [PubMed]
- 34.Mekary RA, et al. Joint association of glycemic load and alcohol intake with type 2 diabetes incidence in women. Am. J. Clin. Nutr. 2011;94:1525–1532. doi: 10.3945/ajcn.111.023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherly, X. L. et al. Interplay between genetic predisposition, macronutrient intake and type 2 diabetes incidence: Analysis within EPIC-InterAct across eight European countries. (2018). [DOI] [PMC free article] [PubMed]
- 36.Lee KW, Lyu J, Park JK, Jo C, Kim SS. Dietary carbohydrate quality and quantity in relation to the incidence of type 2 diabetes: A prospective cohort study of middle-aged and older Korean adults. Nutrition. 2019;57:245–251. doi: 10.1016/j.nut.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Colditz GA, et al. Diet and risk of clinical diabetes in women. Am. J. Clin. Nutr. 2019;55(5):1018–1023. doi: 10.1093/ajcn/55.5.1018. [DOI] [PubMed] [Google Scholar]
- 38.Sonestedt E, et al. Genetic variation in the glucose-dependent insulinotropic polypeptide receptor modifies the association between carbohydrate and fat intake and risk of type 2 Diabetes in the Malmö Diet and Cancer Cohort. J. Clin. Endocrinol. 2012;97:E810–E818. doi: 10.1210/jc.2011-2444. [DOI] [PubMed] [Google Scholar]
- 39.de Koning L, et al. Low-carbohydrate diet scores and risk of type 2 diabetes in men. Am. J. Clin. Nutr. 2011;93:844–850. doi: 10.3945/ajcn.110.004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmadi-Abhari S, et al. Dietary intake of carbohydrates and risk of type 2 diabetes: The European Prospective Investigation into Cancer-Norfolk study. Br. J. Nutr. 2014;111:342–352. doi: 10.1017/S0007114513002298. [DOI] [PubMed] [Google Scholar]
- 41.AlEssa HB, et al. Carbohydrate quality and quantity and risk of type 2 diabetes in US women. Am. J. Clin. Nutr. 2015;102:1543–1553. doi: 10.3945/ajcn.115.116558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barclay AW, Flood VM, Rochtchina E, Mitchell P, Brand-Miller JC. Glycemic index, dietary fiber, and risk of type 2 diabetes in a cohort of older Australians. Diabetes Care. 2007;30:2811–2813. doi: 10.2337/dc07-0784. [DOI] [PubMed] [Google Scholar]
- 43.Hodge AM, English DR, O’Dea K, Giles GG. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27:2701–2706. doi: 10.2337/diacare.27.11.2701. [DOI] [PubMed] [Google Scholar]
- 44.Meyer KA, et al. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000;71:921–930. doi: 10.1093/ajcn/71.4.921. [DOI] [PubMed] [Google Scholar]
- 45.Schulze MB, et al. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am. J. Clin. Nutr. 2004;80:348–356. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 46.Schulze MB, et al. Carbohydrate intake and incidence of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Br. J. Nutr. 2008;99:1107–1116. doi: 10.1017/S0007114507853360. [DOI] [PubMed] [Google Scholar]
- 47.Similä M, et al. Carbohydrate substitution for fat or protein and risk of type 2 diabetes in male smokers. Eur. J. Clin. Nutr. 2012;66:716–721. doi: 10.1038/ejcn.2012.24. [DOI] [PubMed] [Google Scholar]
- 48.Sluijs I, et al. Carbohydrate quantity and quality and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition-Netherlands (EPIC-NL) study. Am. J. Clin. Nutr. 2010;92:905–911. doi: 10.3945/ajcn.2010.29620. [DOI] [PubMed] [Google Scholar]
- 49.Villegas R, et al. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch. Intern. Med. 2007;167:2310–2316. doi: 10.1001/archinte.167.21.2310. [DOI] [PubMed] [Google Scholar]
- 50.Bao W, et al. Low carbohydrate–diet scores and long-term risk of type 2 diabetes among women with a history of gestational diabetes mellitus: a prospective cohort study. Diabetes Care. 2016;39:43–49. doi: 10.2337/dc15-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halton TL, Liu S, Manson JE, Hu FB. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2008;87:339–346. doi: 10.1093/ajcn/87.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nanri A, et al. Low-carbohydrate diet and type 2 diabetes risk in Japanese men and women: the Japan Public Health Center-Based Prospective Study. PLoS ONE. 2015;10:e0118377. doi: 10.1371/journal.pone.0118377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sali S, et al. Animal based low carbohydrate diet is associated with increased risk of type 2 diabetes in Tehranian adults. Diabetol. Metab. Syndr. 2020;12:1–10. doi: 10.1186/s13098-020-00596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seidelmann SB, et al. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health. 2018;3:e419–e428. doi: 10.1016/S2468-2667(18)30135-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jayedi, A., Soltani, S., Jenkins, D., Sievenpiper, J. & Shab-Bidar, S. Dietary glycemic index, glycemic load, and chronic disease: an umbrella review of meta-analyses of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 1–10. 10.1080/10408398.2020.1854168 (2020). [DOI] [PubMed]
- 56.Livesey G, et al. Dietary glycemic index and load and the risk of type 2 diabetes: A systematic review and updated meta-analyses of prospective cohort studies. Nutrients. 2019;11:1280. doi: 10.3390/nu11061280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Livesey G, et al. Dietary glycemic index and load and the risk of type 2 diabetes: Assessment of causal relations. Nutrients. 2019;11:1436. doi: 10.3390/nu11061436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am. J. Clin. Nutr. 2002;76:274S–280S. doi: 10.1093/ajcn/76/1.274S. [DOI] [PubMed] [Google Scholar]
- 59.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 60.Chen K-W, et al. Earlier appearance of impaired insulin secretion than of visceral adiposity in the pathogenesis of NIDDM: 5-year follow-up of initially nondiabetic Japanese-American men. Diabetes Care. 1995;18:747–753. doi: 10.2337/diacare.18.6.747. [DOI] [PubMed] [Google Scholar]
- 61.Matsumoto K, et al. Glucose tolerance, insulin secretion, and insulin sensitivity in nonobese and obese Japanese subjects. Diabetes Care. 1997;20:1562–1568. doi: 10.2337/diacare.20.10.1562. [DOI] [PubMed] [Google Scholar]
- 62.Sakurai M, et al. J-shaped relationship between waist circumference and subsequent risk for Type 2 diabetes: An 8-year follow-up of relatively lean Japanese individuals. Diabet. Med. 2009;26:753–759. doi: 10.1111/j.1464-5491.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- 63.Dehghan M, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet. 2017;390:2050–2062. doi: 10.1016/S0140-6736(17)32252-3. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura Y, et al. Low-carbohydrate diets and cardiovascular and total mortality in Japanese: A 29-year follow-up of NIPPON DATA80. Br. J. Nutr. 2014;112:916–924. doi: 10.1017/S0007114514001627. [DOI] [PubMed] [Google Scholar]
- 65.Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ. 2012;344:e1454. doi: 10.1136/bmj.e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012;142:1304–1313. doi: 10.3945/jn.111.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, codes, analytical syntax, and other additional data used for the present meta-analysis are available from the corresponding author on reasonable request.