Abstract
Adolescence is marked by changes in decision-making and perspective-taking abilities. Although adolescents make more adaptive decisions with age, little is understood about how adolescents take adaptive risks that impact others and how this behavior changes developmentally. Functional coupling between reward [e.g., ventral striatum (VS)] and ‘social brain’ [e.g. temporal parietal junction (TPJ)/ posterior superior temporal sulcus (pSTS), medial prefrontal cortex (mPFC)] systems may be differentially shape adaptive risks for the self and other. A total of 173 participants completed between one and three sessions across three waves [a total of 433 behavioral and 403 functional magnetic resonance imaging (fMRI) data points]. During an fMRI scan, adolescents completed a risky decision-making task where they made risky decisions to win money for themselves and their parent. The risky decisions varied in their expected value (EV) of potential reward. Results show that from the 6th through 9th grades, adolescents took increasingly more adaptive risks for themselves than for their parent. Additionally, greater VS–TPJ/pSTS and VS–mPFC connectivity that tracks EV when making risky decisions for themselves in 6th grade, but a lower VS–mPFC connectivity in 9th grade, predicted greater adaptive risk-taking for their parent. This study contributes to our understanding of the self as a neural proxy for promoting adaptive social behaviors in youth.
Keywords: adaptive risk-taking, adolescence, fMRI, longitudinal, social context
Contrary to the popular belief that adolescents generally make maladaptive decisions, adolescents also make rational, adaptive risky decisions. For instance, adolescents make adaptive risky decisions in economic tasks similarly as adults (Somerville et al., 2019) or even more so than adults (Barkley-Levenson and Galván, 2014), suggesting that adolescents are capable of making risk decisions that are intentional and deliberate. Adolescence is also a time of social-cognitive development that promotes adolescents’ ability to optimally engage with others (Crone and Fuligni, 2020). In the current study, we sought to examine how adolescents take adaptive risks for the self and others and how the neurocognitive processes of these behaviors change across adolescence. We also examined how neural sensitivity to the self ultimately fosters adaptive risky decisions for others, with a particular focus on the family.
Risk-taking in adolescence is thought to be highly adaptive for survival (Ellis et al., 2012). Although risky decisions can lead to health-compromising outcomes, they also allow adolescents to adjust to new environments and navigate their complex social world. One way to assess the rationality of risky decisions is by using economic models, whereby the expected value (EV) of the potential reward determines whether or not the risky decision is relatively more optimal than the safe decision. Adolescents tend to be more sensitive to the EV of potential rewards than are adults, as evidenced by taking more advantageous risks but avoiding more disadvantageous risks (Barkley-Levenson and Galván, 2014). The ability to evaluate components of risk (e.g. the magnitude and the probability of the potential reward) develops across adolescence (van Duijvenvoorde et al., 2015), making adolescence an important developmental window for heightened sensitivity to EV and efficient switching between safe and risky decisions based on the economic nature of risk and thus adaptive risk-taking.
Decision-making in adolescence not only affects the self but also impacts others (Do et al., 2017). Indeed, more so than children, adolescents are able to take others’ perspectives into account and understand how their own decisions affect others (Crone et al., 2008). Although other-oriented risks can be negative, they can also be adaptive as adolescents appropriately switch between safe and risky decisions, depending on the context (e.g. EV of the potential reward). To date, cross-sectional work has shown that early adolescents in middle school make risky decisions similarly for themselves and their parent (Guassi Moreira and Telzer, 2018a), but they tend to win money for their parent at the expense of incurring a cost for their friend by late adolescence (Guassi Moreira et al., 2020). This suggests a developmental shift in risk-taking, depending on who the behavior targets. Additionally, longitudinal work has shown that the subjective feeling of pleasure when winning money for one’s parent, relative to oneself, increases across adolescence (Braams and Crone, 2017). Together, this initial work indicates that other-oriented rewards become increasingly appetitive across adolescence and may differentially modulate motivations for other-oriented adaptive risk decisions over time.
Social-cognitive skills, such as perspective-taking and considering others’ needs, improve from middle to high school (Crone and Fuligni, 2020), and may support evaluating EV for others. Key neural regions involved in social-cognitive processes include the medial prefrontal cortex (mPFC), the temporal parietal junction (TPJ) and the posterior superior temporal sulcus (pSTS), which undergo rapid functional shifts during adolescence (Blakemore, 2012) and are also consistently active when adolescents make risky decisions in a social context (van Hoorn et al., 2019). The mPFC is involved in self-perception such as comparing self-referential and other-referential thoughts (Pfeifer and Peake, 2012), describing the self from the perspective of close others (Pfeifer et al., 2009) and evaluating the overlap between self and parent (van der Cruijsen et al., 2019). The TPJ and pSTS play a key role in social perception such as mentalizing about others and evaluating the emotional states of others (van Overwalle, 2009; van der Cruijsen et al., 2019). Further, the dynamic interaction between social-cognitive and reward-valuation regions (e.g. VS) may help adolescents differentiate the salience and reward associated with adaptive risks when decisions affect themselves and their close others. Stronger functional connectivity between the VS and mPFC and between the VS and pSTS is associated with social-emotional processing (Burnett and Blakemore, 2009) and more prosocial decisions (Do and Telzer, 2019). In sum, longitudinal changes in connectivity between the VS and social-cognitive brain regions may differentiate how adolescents make adaptive risks for close others and themselves across time.
The present study tested how the longitudinal changes in VS–TPJ/pSTS and VS–mPFC functional connectivity that tracks EV are associated with adaptive risk decisions for the self and other. In a three-wave longitudinal study, adolescent participants completed a risk-taking task during a functional magnetic resonance imaging (fMRI) scan where they made risky decisions that varied in their EV to win money for themselves and their parent. Adaptive risk-taking refers to an individual’s sensitivity to EV and was thereby defined as taking more risks as the EV increases.
First, we probed the ‘differentiation’ between the self and other by examining (i) longitudinal differences in behavioral trajectories in adaptive risk-taking for themselves and their parent and (ii) longitudinal differences in neural trajectories in functional connectivity between the VS and social brain regions (i.e. mPFC and TPJ/pSTS) that track the EV of potential rewards for themselves and their parent. We did not have specific hypotheses regarding the direction of the difference in trajectories. For instance, adaptive risk-taking and neural connectivity patterns for the parent could become increasingly greater than those for the self, as other-oriented processing improves over adolescence (e.g. Braams and Crone, 2017). In contrast, adaptive risk-taking and neural connectivity patterns for the self could become increasingly greater than those for the parent, as adolescents become more autonomous and differentiate from their family (e.g. McElhaney et al., 2009).
Second, we sought to capture the ‘relationship’ between the self and other by testing whether neural development of the self (i.e. neural tracking of EV for the self) promotes other-oriented adaptive risk decisions and whether neural development of the parent promotes self-oriented adaptive risk decisions. For both, we tested whether this relationship changes across adolescence. We hypothesized that the neural connectivity patterns for the self will increasingly promote adaptive risk decisions for the parent, as understanding oneself and thus the decisions one makes for oneself serves as a precursor to understanding and making decisions for others (Dimaggio et al., 2008). Thus, self-oriented neural processes in the context of adaptive risk-taking—particularly in brain regions implicated in the processing of the self and the other—may enhance other-oriented adaptive risk-taking.
Methods
Participants
Adolescent participants were recruited as part of a larger study of 873 6th- and 7th-grade students from three public middle schools to participate in a longitudinal fMRI study. A total of 173 participants completed between one and three sessions annually across three waves (433 total behavioral data points and 403 total fMRI data points). See Table 1 for demographic information about adolescent and parent participants. Participants were compensated for completing the session. All participants provided informed consent/assent, and the University’s Institutional Review Board approved all aspects of the study.
Table 1.
Demographic information of adolescent and parent participants
| Percentage | |
|---|---|
| Adolescent participant | |
| Biological sex | |
| Female | 52.6 |
| Male | 47.4 |
| Race | |
| White | 29.5 |
| Black | 23.1 |
| Hispanic/Latinx | 34.7 |
| Mixed | 9.3 |
| Other | 3.5 |
| Parent participant | |
| Relationship with adolescent participant | |
| Biological mother | 82.7 |
| Biological father | 9.8 |
| Other guardians | 8.1 |
| Education | |
| Less than middle school completion | 10.4 |
| Middle school completion | 3.5 |
| Some high school | 11 |
| High school diploma | 14.5 |
| Some college | 30.1 |
| Associate’s or bachelor’s degree | 23.1 |
| Some graduate school | 2.3 |
| Graduate or professional degree | 5.2 |
Note: For families who participated in more than one wave of data collection, 7.4% of participating parents changed at least once across their years of participation.
In order to reach our target sample size of 150 participants after accounting for attrition and for excluded participants between waves of data collection, we recruited two cohorts of participants across 2 years of the study (e.g. Herd et al., 2020 for a similar study design). We recruited 148 participants at wave 1 of the study (cohort 1) and 30 additional participants at wave 2 (cohort 2). Adolescents who began the study at wave 1 had up to three waves of data, and those who began the study at wave 2 had up to two waves of data. Across the three waves, 25 participants had 1 time point of behavioral data (34 for fMRI data), 36 had 2 (39 for fMRI data) and 112 had 3 (97 had fMRI data).
At wave 1, five participants were excluded due to exclusionary criteria being met after recruitment (e.g. major claustrophobia during the fMRI session). These participants were not invited back for subsequent study participation. Out of the remaining 143 participants at wave 1 (Mage = 12.8, s.d.age = 0.52; N = 73 female), 1 additional participant was excluded for acute anxiety, and 3 and 14 additional participants were excluded from behavioral and neural analyses, respectively, due to task-specific exclusionary criteria. The final wave 1 sample size with behavioral data included 139 adolescents and with fMRI data included 125 adolescents. At wave 2, 116 participants from cohort 1 and 30 new participants from cohort 2 participated (Mage = 13.7; s.d.age = 0.58; N = 78 female). The final wave 2 sample size with behavioral data included 143 adolescents and with fMRI data included 120 adolescents. At wave 3, 119 participants from cohort 1 and 26 participants from cohort 2 participated (Mage = 14.7; s.d.age = 0.58; N = 74 female). The final wave 3 sample size with behavioral data included 144 adolescents and with fMRI data included 104 adolescents. Retention from wave 1 to wave 2 for cohort 1 participants was 81.1% and from waves 2 to 3 across cohorts was 85.3%. See supplementary material for reasons for exclusion for behavioral and neural analyses and reasons for attrition between waves.
Risky decision-making task
Adolescents completed a modified Cups Task (modified Levin and Hart, 2003), which has previously been utilized to examine risky decision-making for self and others in developmental samples (e.g. Guassi Moreira and Telzer, 2018a). Participants completed 3 rounds of the Cups Task: one in which they made decisions for the self, one for parent and one for best friend. The order in which participants completed each round was counterbalanced. Note that the best friend round is part of another, preregistered study.
Each round consisted of 45 trials. On each trial, participants were presented with two scenarios of cups: the left side always had one cup with a guaranteed 15 cents hidden under the cup (Figure 1). On the right side, the number of cups (either two, three or five cups) as well as the amount of money hidden (either 30, 45 or 75 cents) varied. The right side always had a value >15 cents; however, the money was hidden under only one of the overturned cups. Participants were told that if they chose the right side, then the computer would randomly select one of the cups and they may earn the higher amount or 0 cent, whereas if they chose the left side, then they were guaranteed to earn 15 cents. Picking the left side equates to making a safe decision since it is always associated with a known outcome of 15 cents. Picking the right side equates to making a risky decision since the outcome may be the higher amount or it may be zero (in which case, the participant does not gain any money for that trial), and thus the outcome is unknown. After each decision, participants were shown the outcome of their decision. In the event that participants did not make a decision within the given time, participants were told that they were ‘too late’ and there was no change in the total points. Outcomes of each decision were added to the running total for that round, which was shown to the participant at the end of each round.
Fig. 1.
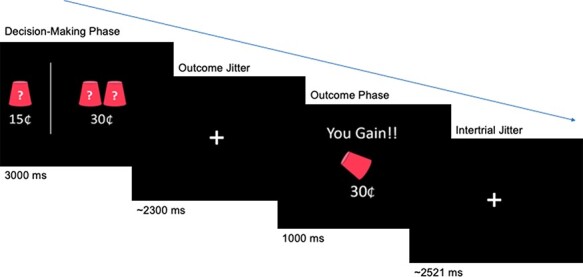
Example trial of the modified Cups Task. In this example, participants chose the risky option and subsequently gained a reward of 30 cents.
On each trial, the cups were shown for 3000 ms, within which participants made their decision. Next, a fixation cross was jittered around an average of 2300 ms (range: 526.68–4017.12), which was followed by the outcome for 1000 ms. Finally, there was an intertrial fixation cross that was jittered around an average of 2521.39 ms (range: 521.14–3913.31). At the end of each session, adolescents received the money they had earned for themselves, their parent was given the money their child had earned for them and the best friend was provided with their earnings in cash. The participating parent and best friend did not know the adolescent was winning money for them until they received the award. See supplementary material for the full study procedure.
Adaptive risk-taking
To operationalize adaptive risk-taking, we assessed how adolescents made risky decisions as a function of the EV of the potential reward. Consistent with prior work, EV was comprised of two factors: magnitude of reward and probability of reward, both of which contribute to making risky decisions when rewards are at stake (van Duijvenvoorde et al., 2015; Guassi Moreira and Telzer, 2018a). EV was calculated by dividing the amount of money under the cup (i.e. magnitude of reward) by the number of cups (i.e. probability of reward) for that trial. For instance, on a trial with two cups with 30 cents hidden under one of them, the EV is 30/2 = 15. Given the parameters of the magnitudes and probabilities of reward, the EVs for risky decisions are 6, 9, 10, 15, 22.5, 25 and 37.5. The EV of safe decision is always 15, since making a safe decision always guarantees a gain of 15 cents. In this task, it is advantageous to make a risky decision when the EV is >15, whereas it is disadvantageous to make a risky decision when the EV is <15. If the EV is 15, then there is an equal EV between the safe and risky decisions. It is thus adaptive to make risky decisions as the EV increases.
Mathematical EV model
In order to model how adolescents made adaptive risks, we calculated their frequency of risky decisions as a function of EV. These behavioral analyses were constructed at the trial level, whereby we tested whether participants made a risky decision as a function of the EV of that trial. We used hierarchical linear modeling (HLM for Windows, version 6.06; Raudenbush and Bryk, 2002; Raudenbush, 2004) to model how EV affects risky decisions. We extracted the predicted estimated slope (i.e. Empirical Bayes estimate), which represents the change in risky decision with respect to the change in EV and therefore the measure of adaptive risk-taking. Higher mean scores indicate more adaptive risk-taking, whereby the participant makes risky decisions when the EV is high but makes safe decisions when the EV is low. Lower mean scores indicate less adaptive risk-taking, whereby the participant does not incorporate EV information into their decision-making process. This index was used in subsequent models to test for changes in adaptive risk-taking across development. See supplementary material for a description of the two-level model that we estimated.
fMRI data acquisition and analysis
See supplementary material for fMRI scan parameters and preprocessing procedures.
The task was modeled using an event-related design within the Statistical Parametric Mapping software package (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Individual-level fixed-effects models were created for each participant using the general linear model in SPM with regressors for the following five conditions: trials for each decision (safe and risky) and trials for each outcome (15 cents, 0 cent or >15 cents). Trials in which participants did not respond, the final outcome trial and volumes containing motion in excess of 2 mm were included as separate regressors of no interest. Each trial was modeled using the onset of the cups (or outcome) and a duration equal to zero. Each of the five conditions was modeled separately for each context (i.e. self, parent and peer), totaling 15 conditions of interest. Jittered intertrial periods (e.g. fixation cross) were not explicitly modeled and therefore served as the implicit baseline for task conditions. A parametric modulator (PM) was included for each risky decision, which modeled the EV of the risky decision of each trial. Similar to the behavioral analyses, we tested whether participants show increases in functional connectivity as a function of the EV of that trial. The PM served to examine the neural activity that tracks the EV of adolescents’ decisions. Each participant completed three rounds of the task (self, parent and best friend), but for the purposes of this study, we focused only on the self and the parent rounds.
Seed-to-seed functional connectivity
To examine neural connectivity, we conducted psychophysiological interaction (PPI) analyses using a generalized form of the context-dependent PPI from the automated generalized PPI (gPPI) toolbox in SPM (McLaren et al., 2012). We utilized the VS as our seed region, which was defined using the Harvard-Oxford Atlas (Harvard Center for Morphometric Analysis). Time series were extracted from the VS and served as the physiological variable. Trials were then convolved with the canonical HRF to create the psychological regressor. Finally, the physiological and psychological variables were multiplied in order to create the PPI term. Each participant’s individual gPPI model included a deconvolved BOLD signal alongside the psychological and interaction term for each event type.
In order to assess VS coupling with TPJ/pSTS and mPFC, we extracted parameter estimates from the conditions of interests using TPJ/pSTS and mPFC regions of interest (ROIs). Our conditions of interests were risky decision-making with PM (i.e. representing EV), separately for the parent and the self. The ROIs were obtained from the Saxe Lab social brain ROIs (TPJ; Dufour et al., 2013) and the Mills Lab social brain ROIs (pSTS, mPFC; Mills et al., 2014). For the TPJ and pSTS, we combined the two masks (i.e. TPJ/pSTS), given the proximity of the two regions as well as the overlapping functions in social processing during adolescence. Further, for the mPFC, we subtracted voxels that were within the vmPFC (defined using the Harvard-Oxford Atlas) from the mPFC in order to ensure that the mPFC mask represents the medial portion only. All masks were bilateral (Figure 2). We extracted parameter estimates from these ROIs for the self and the parent separately for each time point and for each participant.
Fig. 2.
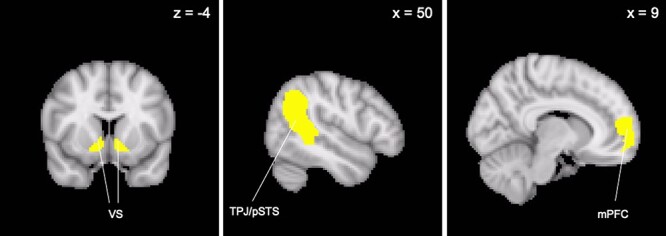
Regions of interest used for gPPI analyses.
Note: All masks are bilaterally defined.
Analysis plan
Differentiation between the self and other.
We first determined the best-fitting trajectory (i.e. linear or quadratic) of behavioral adaptive risk-taking and of neural tracking of EV, separately for the self and the parent, by using an unconditional growth model (conducted using SPSS 26). For the behavioral model, adaptive risk-taking was the dependent variable (DV). For neural tracking of EV, we estimated separate models for each of the two ROIs (VS–TPJ/pSTS connectivity and VS–mPFC connectivity) as the DV. Time points were nested within individuals in the series of two-level models. Higher connectivity estimates indicate steeper ‘increasing’ functional connectivity with increasing EV over time. Lower connectivity estimates indicate steeper ‘decreasing’ functional connectivity with increasing EV over time. See supplementary material for the full formal model-building procedure.
Next, we compared whether the trajectories of adaptive risk-taking and neural tracking of EV differ between the self and the parent. We evaluated the same two-level model as above, but now in this model, the DV was the difference between the parent and the self in adaptive risk-taking, as well as in neural tracking of EV. Using the difference scores as the DVs tests how the relative effect of the parent and the self changes across time (e.g. Braams and Crone, 2017).
Relationship between the self and other.
In order to test how neural development of one context (e.g. neural tracking of EV for the self) promotes adaptive risk decisions for another (e.g. those for the parent), we followed a conditional growth model with parameter estimates of neural connectivity added as a time-varying covariate to predict adaptive risk-taking. At level 1, we added person-mean centered neural connectivity at each time point, and at level 2, we added group-mean centered neural connectivity on the intercept in order to account for within- and between-person changes in neural connectivity across time, respectively (Curran and Bauer, 2011). In other words, person-mean centered neural connectivity captures how each person’s functional connectivity at each time point compares to his/her own average functional connectivity across time points (i.e. within-person effects), and group-mean centered neural connectivity captures how each person’s average functional connectivity compares to the group’s average neural connectivity (i.e. between-person effects). This allows for person-specific intercepts and for unbiased estimates of within- and between-person effects (Curran and Bauer, 2011). We included linear grade and a grade × connectivity interaction at level 1 to test whether neural tracking of EV for the self and the parent predicts adaptive risk-taking for the parent and the self, respectively. We also tested whether this cross-context relationship changes across grade.
Results
Differentiation between the self and other
Behavioral results.
Table 2 shows descriptive statistics of adaptive risk-taking for the self and the parent at each grade. In order to examine developmental changes in adaptive risk-taking for the self and the parent, we first used an unconditional growth model and probed the shape of behavioral trajectory for each context. Results suggest that linear trajectories best fit developmental changes in adaptive risk-taking for both the self and the parent. There was a trend in increases in adaptive risks for the self across time (β = 0.009, P = 0.07) and no longitudinal changes in adaptive risks for the parent (β = −0.0001, P = 0.98). We next tested whether the difference between the parent and the self in adaptive risk-taking changed across time. Results suggest that the linear trajectory of adaptive risk-taking for the self increased faster than for the parent, as shown by a significant difference in the slope (β = −0.008, P = 0.02). As shown in Figure 3, in 6th grade, adolescents took adaptive risks similarly for the self and the parent (β = 0.002, P = 0.77), but the two trajectories diverged across time such that by 9th grade, adolescents took significantly more adaptive risks for the self than for the parent (β = −0.023, P = 0.001; Table 3). See supplementary material for secondary analyses using reaction time.
Table 2.
Descriptive statistics of behavioral adaptive risk-taking and neural tracking of EV for the self and the parent at each grade
| Grade | ||||||||
|---|---|---|---|---|---|---|---|---|
| 6 | 7 | 8 | 9 | |||||
| M | SE | M | SE | M | SE | M | SE | |
| Adaptive risk-taking | ||||||||
| Parent | 0.088 | 0.009 | 0.099 | 0.007 | 0.092 | 0.010 | 0.108 | 0.016 |
| Self | 0.089 | 0.009 | 0.104 | 0.007 | 0.108 | 0.010 | 0.129 | 0.016 |
| VS–TPJ/pSTS | ||||||||
| Parent | −0.008 | 0.015 | −0.009 | 0.009 | −0.004 | 0.008 | −0.023 | 0.015 |
| Self | −0.002 | 0.018 | 0.011 | 0.009 | −0.006 | 0.009 | −0.028 | 0.009 |
| VS–mPFC | ||||||||
| Parent | −0.003 | 0.017 | 0.0001 | 0.013 | −0.013 | 0.009 | −0.004 | 0.015 |
| Self | −0.019 | 0.014 | −0.008 | 0.010 | 0.012 | 0.011 | −0.023 | 0.012 |
Fig. 3.
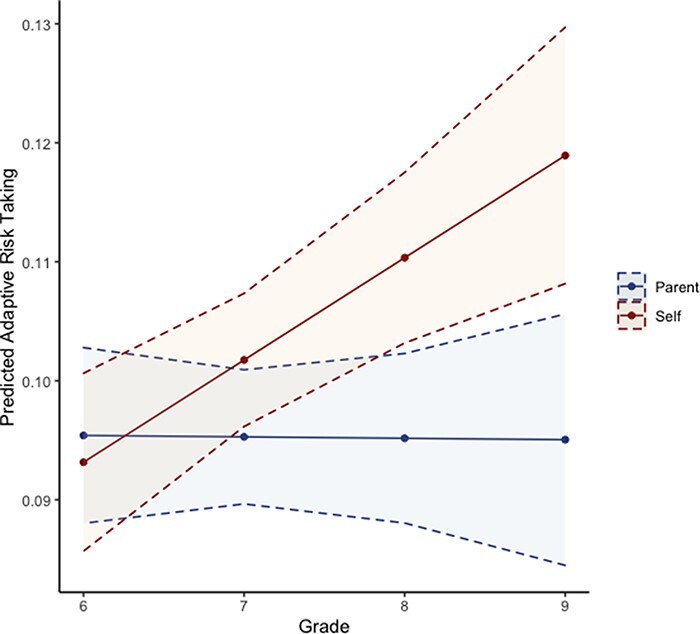
Longitudinal changes in adaptive risk-taking for the self and the parent. Adolescents took significantly more adaptive risks for themselves than for their parent across time.
Table 3.
Best-fitting models for behavioral and neural trajectories for each context (parent, self) and differences between context (parent–self)
| 95% confidence interval | |||||
|---|---|---|---|---|---|
| Estimate | SE | P-value | Lower | Upper | |
| Adaptive risk-taking | |||||
| Parent | |||||
| Intercept | 0.095 | 0.007 | <0.001 | 0.081 | 0.110 |
| Linear grade | −0.0001 | 0.005 | 0.98 | −0.0.009 | 0.009 |
| Self | |||||
| Intercept | 0.093 | 0.007 | <0.001 | 0.078 | 0.108 |
| Linear grade | 0.009 | 0.005 | 0.07 | −0.001 | 0.018 |
| Parent–self | |||||
| Intercept | 0.002 | 0.005 | 0.77 | −0.009 | 0.012 |
| Linear grade | −0.008 | 0.003 | 0.02 | −0.015 | −0.002 |
| VS–TPJ/pSTS | |||||
| Parent | |||||
| Intercept | −0.005 | 0.012 | 0.65 | −0.029 | 0.018 |
| Linear grade | −0.002 | 0.007 | 0.75 | −0.016 | 0.011 |
| Self | |||||
| Intercept | 0.020 | 0.012 | 0.09 | −0.003 | 0.042 |
| Linear grade | −0.015 | 0.006 | 0.01 | −0.026 | −0.003 |
| Parent–self | |||||
| Intercept | −0.020 | 0.017 | 0.24 | −0.053 | 0.013 |
| Linear grade | 0.009 | 0.009 | 0.36 | −0.010 | 0.027 |
| VS–mPFC | |||||
| Parent | |||||
| Intercept | −0.003 | 0.014 | 0.85 | −0.030 | 0.025 |
| Linear grade | −0.003 | 0.007 | 0.67 | −0.018 | 0.012 |
| Self | |||||
| Intercept | −0.007 | 0.011 | 0.53 | −0.030 | 0.015 |
| Linear grade | −0.0003 | 0.006 | 0.96 | −0.013 | 0.012 |
| Parent–self | |||||
| Intercept | 0.005 | 0.018 | 0.80 | −0.032 | 0.041 |
| Linear grade | −0.004 | 0.010 | 0.70 | −0.024 | 0.016 |
fMRI results.
Table 2 shows descriptive statistics of parameter estimates of VS–TPJ/pSTS and VS–mPFC connectivity for the self and the parent at each grade. In order to examine developmental changes in neural connectivity when adolescents make risky decisions for the self and the parent with respect to changes in EV, we first used an unconditional growth model to probe the shape of neural trajectories for each context.
For VS–TPJ/pSTS functional connectivity, linear trajectories best fit developmental changes in VS-TPJ/pSTS connectivity when adolescents made risky decisions for the self and the parent (Table 3). VS–TPJ/pSTS connectivity that tracks EV significantly decreased over time for the self (β = −0.015, P = 0.01) but did not significantly change across time for the parent (β = −0.002, P = 0.75). Yet, VS–TPJ/pSTS functional connectivity did not differ between the parent and the self in intercepts (i.e. starting points; P = 0.24) or in slopes (i.e. trajectories; P = 0.36). For descriptive purposes, we plotted the trajectory of VS–TPJ/pSTS functional connectivity for the self (Figure 4). Along the y-axis, positive values indicate higher VS–TPJ/pSTS connectivity that tracks increases in EV (i.e. increasing connectivity with increasing EV), whereas negative values indicate lower VS–TPJ/pSTS connectivity that tracks increases in EV (i.e. decreasing connectivity with increasing EV). As shown in Figure 4, greater tracking of EV for the self is supported by higher VS–TPJ/pSTS connectivity in early adolescence but by lower connectivity in later adolescence.
Fig. 4.
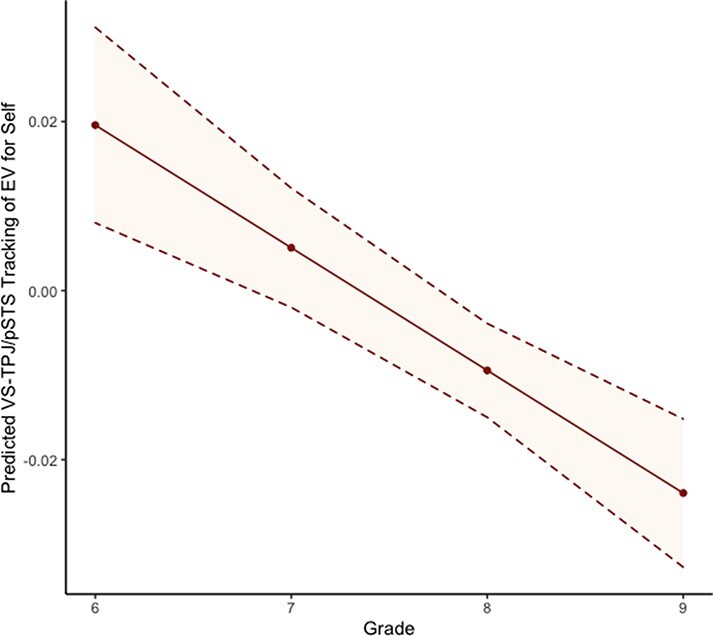
VS–TPJ/pSTS connectivity that tracks EV of potential reward when adolescents make risky decisions for themselves significantly changed across time.
For VS–mPFC functional connectivity, linear trajectories best fit developmental changes in VS–mPFC connectivity when adolescents made risky decisions for the self and the parent (Table 3). However, VS–mPFC connectivity that tracks EV for neither the self (β = −0.0003, P = 0.96) nor the parent (β = −0.003, P = 0.67) significantly changed over time. Moreover, VS–mPFC functional connectivity that tracks EV did not differ between the parent and the self in intercepts (i.e. starting points; P = 0.80) or slopes (i.e. trajectories; P = 0.70).
Relationship between the self and other.
Next, we investigated whether there is a relationship between the self and the parent and whether this association varies across time. We first tested whether VS–TPJ/pSTS and VS–mPFC neural tracking of EV for the self longitudinally predict more adaptive risk-taking for the parent (Table 4).
Table 4.
Self- and other-oriented neural connectivity predicting other- and self-oriented adaptive risk taking, respectively
| β | SE | P-value | |
|---|---|---|---|
| Self predicting parent | |||
| VS–TPJ/pSTS | 0.119 | 0.057 | 0.04 |
| VS–TPJ/pSTS × grade | −0.095 | 0.046 | 0.04 |
| VS–mPFC | 0.133 | 0.061 | 0.03 |
| VS–mPFC × grade | −0.101 | 0.043 | 0.02 |
| Parent predicting self | |||
| VS–TPJ/pSTS | −0.064 | 0.057 | 0.27 |
| VS–TPJ/pSTS × gade | 0.009 | 0.041 | 0.82 |
| VS–mPFC | −0.074 | 0.049 | 0.14 |
| VS–mPFC × grade | 0.062 | 0.037 | 0.10 |
VS–TPJ/pSTS connectivity that tracks EV when making risky decisions for the self predicted greater adaptive risk-taking for the parent in 6th grade (β = 0.119, P = 0.04), and this association changed across grade (β = −0.095, P = 0.04). To probe this interaction, we queried this association at each grade by re-centering grade and examining statistical significance at the intercepts. As shown in Figure 5A, the relationship between VS–TPJ/pSTS connectivity for the self and adaptive risk-taking for the parent became weaker over time, such that by 9th grade, there was a marginal relationship between VS–TPJ/pSTS connectivity that tracks EV for the self and adaptive risk-taking for the parent (β = −0.166, P = 0.09).
Fig. 5.
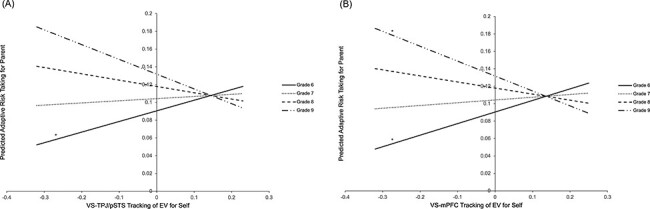
Longitudinal changes in the relationship between self-oriented neural tracking of EV and other-oriented adaptive risk-taking in the (A) VS–TPJ/pSTS and the (B) VS–mPFC.
*Above each line indicates P < 0.05.
A similar pattern was observed with VS–mPFC connectivity such that greater VS–mPFC connectivity that tracks the EV when making risky decisions for the self predicted greater adaptive risk-taking for the parent in 6th grade (β = 0.133, P = 0.03), and this association changed across grade (β = −0.101, P = 0.02). To probe this interaction, we queried this association at each grade. As shown in Figure 5B, the relationship between VS–mPFC connectivity for the self and adaptive risk-taking for the parent became weaker over time and flipped such that by 9th grade, there was a negative relationship between VS–mPFC connectivity that tracks EV for the self and adaptive risk-taking for the parent (β = −0.171, P = 0.05). Therefore, for younger adolescents, increased VS–mPFC connectivity that tracks EV for the self predicts greater adaptive risk-taking for the parent, whereas for older adolescents, decreased VS–mPFC connectivity that tracks EV for the self predicts greater adaptive risk-taking for the parent. See supplementary material for secondary analyses that further probed grade-related differences in other-oriented adaptive risk-taking as a function of self-oriented neural connectivity.
There were no significant relationships between neural connectivity for the parent and adaptive risk-taking for the self (Ps > 0.14), nor any interactions with grade (Ps > 0.10; Table 4). See supplementary material for within-context associations (i.e. how neural connectivity for the self and the parent predict adaptive risk-taking for the self and the parent, respectively).
Discussion
The goal of the current study was to investigate the development of self-other overlap in the context of decision-making by probing the differentiation and the relationship between the self and other in adaptive risk-taking in adolescence. For ‘differentiation’, we examined (i) how adolescents make adaptive risk decisions for themselves and their parent, and how this differentiation changes across time and (ii) how the VS–TPJ/pSTS and the VS–mPFC functional connectivity track EV of potential reward for themselves and their parent, and how this differentiation changes across time. For ‘relationship’, we examined how functional connectivity that tracks EV when adolescents make risky decisions for themselves and their parent predict adaptive risk decisions for their parent and themselves, respectively, and how this self-other relationship changes across time.
Differentiation between the self and other
At the behavioral level, adolescents took more adaptive risks for themselves than for their parent over time. This behavioral pattern aligns with adolescents’ increasing individuation and shifts away from their family (McElhaney et al., 2009). Greater independence in later adolescence parallels more adaptive risks for themselves relative to their parent. This may suggest that older adolescents are especially sensitive to the EV of potential rewards when they themselves are at stake during risk-taking. Further, greater self-oriented than other-oriented adaptive risks might be especially important as adolescents transition from middle to high school. Relative to other-oriented adaptive risks, greater self-oriented adaptive risks could allow adolescents to prioritize their new social needs and be more sensitive about being safe in some risky situations (e.g. abstaining from substance use) while being risky in others (e.g. joining a new extracurricular group). In tandem, relatively stable adaptive risk-taking for the parent may be indicative of a consistent other-oriented adaptive risk-taking, whereby this behavior does not necessarily improve nor worsen over time and is thus in place by early adolescence. Together, being able to make more adaptive risks for themselves than for their parent could allow older adolescents to strategically navigate and succeed independently in their new social environment.
At the neural level, VS–TPJ/pSTS and VS–mPFC connectivity did not differ between the self and the parent across time. Although unexpected, this could indicate that while VS–TPJ/pSTS and VS–mPFC are involved in social contextual decision-making (e.g. Do and Telzer, 2019), such connectivity may similarly track reward value for themselves and their parents. Nonetheless, we did find longitudinal decreases in VS–TPJ/pSTS that tracks EV when adolescents made risky decisions for themselves, albeit this trajectory did not significantly differ from parent. Such decreasing neural tracking of EV may indicate that VS–TPJ/pSTS in early adolescence may be more sensitive to potential gain of reward than to potential loss when making risky decisions for the self (i.e. stronger coupling as EV increases). This pattern reverses in later adolescence such that the same connectivity may be more sensitive to potential loss of reward than to potential gain for the self (i.e. stronger coupling as EV decreases). Indeed, neural processing of gain and loss differentially develops during adolescence (Insel and Somerville, 2018), corroborating that sensitivity to expected reward value differs across adolescence. Findings suggest that shifting VS–TPJ/pSTS connectivity may underlie changing sensitivity to reward in the context of decision-making for the self and underscores the importance of specific social brain regions in modulating self-related behaviors during adolescence (Crone and Fuligni, 2020). Although both the TPJ/pSTS and the mPFC are parts of the ‘social brain network’, they are implicated in different processes of perspective-taking such that the TPJ is more involved in cognitive perspective-taking such as understanding others’ goal-directed behaviors, while the mPFC is more involved in affective perspective-taking such as understanding others’ traits and emotions (Ma et al., 2012; Koster-Hale et al., 2017). Developmental changes in the VS–TPJ/pSTS connectivity that subserves self-oriented adaptive risk-taking demonstrate that youth are differentially thinking about others’ goals and intentions to guide their own decision-making process, and so taking adaptive risks even in the absence of social contextual cues may be highly socially sensitive.
Relationship between the self and other
We also examined how closely related the self and other are by investigating whether neural tracking of EV when adolescents make risky decisions for themselves is linked to adaptive risks for their parent. We found that greater VS–TPJ/pSTS and VS–mPFC that track EV for the self are differentially associated with adaptive risks for their parent across adolescence. In 6th grade, greater connectivity when making risky decisions for the self was significantly associated with taking more adaptive risks for the parent, suggesting that greater coupling between brain regions that encode reward and social information is particularly crucial in early adolescence for promoting other-oriented adaptive risks. When early adolescents make adaptive risky decisions that involve themselves, they may recruit the VS to gauge the expected reward, which in turn may recruit TPJ/pSTS and mPFC to mentalize about the self and others (e.g. ‘Will I win money if I take a risk now?’ and ‘Will my parent be proud of me?’). Taken together, greater mentalizing about the self and others in one’s own decision-making may serve as a leverage point for taking adaptive risks for others.
Interestingly, the relationship between neural tracking of EV for themselves and adaptive risks for their parent shifts across development, such that by 9th grade, older adolescents with lower VS–mPFC (but not VS–TPJ/pSTS) connectivity that tracks EV for themselves take more adaptive risks for their parent. Other-oriented adaptive risk decisions may become more automatic with development, such that older adolescents mentalize about others more effortlessly during this process and therefore do not recruit VS–mPFC connectivity as much as younger adolescents. Indeed, lower VS–mPFC coupling during risk-taking in a social context in a similar age group represents a mature neural phenotype (Guassi Moreira and Telzer, 2018b), demonstrating that our finding of decreasing VS–mPFC connectivity in 9th grade may be developmentally normative and contributes to adaptive social behaviors. In sum, these findings show a developmental shift in self-oriented reward valuation that supports other-oriented adaptive risks, such that different patterns of self-oriented neural tracking enhance other-oriented adaptive behaviors at different developmental stages.
Although some studies have shown that greater theory of mind in youth predicts a better understanding of the self (Białecka-Pikul et al., 2020), our results demonstrate that other-oriented neural tracking does not predict self-oriented adaptive risks. This may indicate that self-oriented neural circuitry may develop prior to other-oriented neural circuitry. Indeed, we observed developmental changes in VS–TPJ/pSTS connectivity that tracks self-oriented rewards but not other-oriented rewards, suggesting that other-oriented neural tracking of EV may occur developmentally later or may be attenuated across development. This corroborates previous research that evince neurodevelopmental changes in the TPJ that underlie a shift from the self to the other (van den Bos et al., 2011). Neural markers associated with the self therefore informs how adolescents interact with their close others and community at large (Crone and Fuligni, 2020), which aligns with prior research showing that neural sensitivity associated with the self serves as a proxy for understanding others in adults (e.g. Waytz and Mitchell, 2011). Taken together, neural indices associated with the self in early adolescence can already be used as a basis for thinking about and understanding close others, which have implications on youth’s social functioning and how they engage with others in positive and adaptive ways.
Limitations
The current study uses novel methods to examine the neurodevelopment of adaptive risks for self and others across adolescence. Using a large, longitudinal sample provided multiple strengths such as accounting for within-person changes in brain and behavior and identifying neural predictors that promote adaptive behaviors. Nonetheless, this study has limitations. First, distinct patterns may arise if positive and negative EVs were tested separately. Although the goal of our study was to understand the linear tracking of EV, some studies have shown that trajectories of decision-making differ between positive and negative EV such that affinity for advantageous trials peaked in mid-adolescence while that for disadvantageous trials decreased linearly across time (Cauffman et al., 2010). Given the developmental differences in approach and avoidant behaviors, future investigations should tease apart the two types of EVs in order to test whether or not there are social contextual differences between the two. Second, the current study utilized an ROI approach to examine functional connectivity. Since we did not identify developmental differences in functional connectivity by the self and the parent in VS–mPFC or VS–pSTS/TPJ, it is possible that regions beyond those identified by our ROIs (i.e. precuneus) are differentially connected across adolescence as a function of context. Future investigations should use longitudinal whole-brain methods in order to expand these research questions and enrich our understanding beyond the a priori regions identified in this study. Third, we utilized adolescents’ grade to examine developmental effects since youth’s risk-taking behaviors, particularly in social contexts, may be contingent on their social experiences that are linked to their grade levels in school. Our study examined adolescents from the start of middle school to the start of high school; however, school systems vastly vary across countries and so our results pertain primarily to US adolescents. Future investigations should query how our findings extend to non-US samples.
Conclusion
In conclusion, the current longitudinal fMRI study extends our understanding of self–other overlap during adolescence. In particular, our study highlights that adolescents take increasingly more adaptive risks for themselves than for their parent. Further, self-oriented neural development supports other-oriented adaptive behaviors, underscoring the central role of the self in understanding and engaging with others. In particular, our results highlight that developmental shifts in self-oriented neural connectivity patterns predict other-oriented adaptive risk decisions, which suggests that changing interactions between reward and social brain systems have implications on youth’s changing social behaviors.
Supplementary Material
Acknowledgements
We greatly appreciate the assistance of the Biomedical Research Imaging Center at the University of North Carolina at Chapel Hill, as well as Carina Fowler, Susannah Ivory, Amanda Benjamin, Virnaliz Jimenez, Emily Watlington, Emily Bibby, Rosario Villa, Melissa Burroughs, Kathy Do, Ethan McCormick, Paul Sharp, Lynda Lin, Nathan Jorgensen, Christina Rogers, Jorien van Hoorn and Tae-Ho Lee for assistance with study design, data collection and analysis.
Contributor Information
Seh-Joo Kwon, Department of Psychology and Neuroscience, University of North Carolina at Chapel Hill, Chapel Hill, NC 27514, USA.
Caitlin C Turpyn, Department of Psychology and Neuroscience, University of North Carolina at Chapel Hill, Chapel Hill, NC 27514, USA.
Mitchell J Prinstein, Department of Psychology and Neuroscience, University of North Carolina at Chapel Hill, Chapel Hill, NC 27514, USA.
Kristen A Lindquist, Department of Psychology and Neuroscience, University of North Carolina at Chapel Hill, Chapel Hill, NC 27514, USA.
Eva H Telzer, Department of Psychology and Neuroscience, University of North Carolina at Chapel Hill, Chapel Hill, NC 27514, USA.
Funding
This research was supported by the National Institutes of Health (R01DA039923 to E.H.T. and F32DA04946 to C.C.T) and the National Science Foundation (SES 1459719 to E.H.T.).
Conflict of interest
None declared.
Supplementary data
Supplementary data are available at SCAN online.
References
- Barkley-Levenson E., Galván A. (2014). Neural representation of expected value in the adolescent brain. Proceedings of the National Academy of Sciences of the United States of America, 111(4), 1646–51. doi: 10.1073/pnas.1319762111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Białecka-Pikul M., Szpak M., Zubek J., Bosacki S., Kołodziejczyk A. (2020). The psychological self and advanced theory of mind in adolescence. Self and Identity, 19(1), 85–104. doi: 10.1080/15298868.2018.1538900 [DOI] [Google Scholar]
- Blakemore S.-J. (2012). Development of the social brain in adolescence. Journal of the Royal Society of Medicine, 105(3), 111–6. doi: 10.1258/jrsm.2011.110221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., Crone E.A. (2017). Longitudinal changes in social brain development: processing outcomes for friend and self. Child Development, 88(6), 1952–65. doi: 10.1111/cdev.12665 [DOI] [PubMed] [Google Scholar]
- Burnett S., Blakemore S.J. (2009). Functional connectivity during a social emotion task in adolescents and in adults. European Journal of Neuroscience, 29(6), 1294–301. doi: 10.1111/j.1460-9568.2009.06674.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman E., Shulman E.P., Steinberg L., et al. (2010). Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychology, 46(1), 193–207. doi: 10.1037/a0016128 [DOI] [PubMed] [Google Scholar]
- Crone E.A., Bullens L., van der Plas E.A.A., Kijkuit E.J., Zelazo P.D. (2008). Developmental changes and individual differences in risk and perspective taking in adolescence. Development and Psychopathology, 20(4), 1213–29. doi: 10.1017/S0954579408000588 [DOI] [PubMed] [Google Scholar]
- Crone E.A., Fuligni A.J. (2020). Self and others in adolescence. Annual Review of Psychology, 71(1), 447–69. doi: 10.1146/annurev-psych-010419-050937 [DOI] [PubMed] [Google Scholar]
- Curran P.J., Bauer D.J. (2011). The disaggregation of within-person and between-person effects in longitudinal models of change. Annual Review of Psychology, 62(1), 583–619. doi: 10.1146/annurev.psych.093008.100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimaggio G., Lysaker P.H., Carcione A., Nicolò G., Semerari A. (2008). Know yourself and you shall know the other to a certain extent: multiple paths of influence of self-reflection on mindreading. Consciousness and Cognition, 17(3), 778–89. doi: 10.1016/j.concog.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Do K.T., Guassi Moreira J.F., Telzer E.H. (2017). But is helping you worth the risk? Defining prosocial risk taking in adolescence. Developmental Cognitive Neuroscience, 25, 260–71. doi: 10.1016/j.dcn.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do K.T., Telzer E.H. (2019). Corticostriatal connectivity is associated with the reduction of intergroup bias and greater impartial giving in youth. Developmental Cognitive Neuroscience, 37, 1–7. doi: 10.1016/j.dcn.2019.100628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour N., Redcay, E., Young, L., et al. (2013). Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLoS One, 8(9), 75468. doi: 10.1371/journal.pone.0075468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B.J., Del Giudice, M., Dishion, T.J., et al. (2012). The evolutionary basis of risky adolescent behavior: implications for science, policy, and practice. Developmental Psychology, 48(3), 598–623. doi: 10.1037/a0026220 [DOI] [PubMed] [Google Scholar]
- Guassi Moreira J.F., Tashjian S.M., Galván A., Silvers J.A. (2020). Is social decision making for close others consistent across domains and within individuals? Journal of Experimental Psychology, 149(8), 1509–26. doi: 10.1037/xge0000719 [DOI] [PubMed] [Google Scholar]
- Guassi Moreira J.F., Telzer E.H. (2018a). Family conflict shapes how adolescents take risks when their family is affected. Developmental Science, 21, e12611. doi: 10.1111/desc.12611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guassi Moreira J.F., Telzer E.H. (2018b). Mother still knows best: maternal influence uniquely modulates adolescent reward sensitivity during risk taking. Developmental Science, 21(1), 1–11. doi: 10.1111/desc.12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd T., King-Casas B., Kim-Soon J. (2020). Developmental changes in emotion regulation during adolescence: associations with socioeconomic risk and family emotional context. Journal of Youth and Adolescence, 49, 1545–57. doi: 10.1007/s10964-020-01193-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel C., Somerville L.H. (2018). Asymmetric neural tracking of gain and loss magnitude during adolescence. Social Cognitive and Affective Neuroscience, 13(8), 785–96. doi: 10.1093/SCAN/NSY058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster-Hale J., Richardson H., Velez N., Asaba M., Young L., Saxe R. (2017). Mentalizing regions represent distributed, continuous, and abstract dimensions of others’ beliefs. NeuroImage, 161, 9–18. doi: 10.1016/j.neuroimage.2017.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin I.P., Hart S.S. (2003). Risk preferences in young children: early evidence of individual differences in reaction to potential gains and losses. Journal of Behavioral Decision Making, 16(5), 397–413. doi: 10.1002/bdm.453 [DOI] [Google Scholar]
- Ma N., Vandekerckhove M., Van Hoeck N., Van Overwalle F. (2012). Distinct recruitment of temporo-parietal junction and medial prefrontal cortex in behavior understanding and trait identification. Social Neuroscience, 7(6), 591–605. doi: 10.1080/17470919.2012.686925 [DOI] [PubMed] [Google Scholar]
- McElhaney K.B., Allen J.P., Stephenson J.C., Hare A.L. (2009). Attachment and autonomy during adolescence. In: Lerner, R.M., Steinberg, L., editors. Handbook of Adolescent Psychology: Individual Bases of Adolescent Development, Hoboken, NJ: John Wiley & Sons Inc, 358–403. doi: 10.1002/9780470479193.adlpsy001012 [DOI] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage, 61(4), 1277–86. doi: 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Lalonde F., Clasen L.S., Giedd J.N., Blakemore S.J. (2014). Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience, 9(1), 123–31. doi: 10.1093/scan/nss113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Borofsky L.A., Dapretto M., Fuligni A.J., Lieberman M.D. (2009). Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Development, 80(4), 1016–38. doi: 10.1111/j.1467-8624.2009.01314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Peake S.J. (2012). Self-development: integrating cognitive, socioemotional, and neuroimaging perspectives. Developmental Cognitive Neuroscience, 2(1), 55–69. doi: 10.1016/j.dcn.2011.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S.W. (2004). HLM 6: Hierarchical Linear and Nonlinear Modeling. Lincolnwood, IL: Scientific Software International. [Google Scholar]
- Raudenbush S.W., Bryk A.S. (2002). Hierarchical Linear Models: Applications and Data Analysis Methods. Vol. 1. Newbury Park, CA: Sage. [Google Scholar]
- Somerville L.H., Haddara N., Sasse S.F., Skwara A.C., Moran J.M., Figner B. (2019). Dissecting “peer presence” and “decisions” to deepen understanding of peer influence on adolescent risky choice. Child Development, 90(6), 2086–103. doi: 10.1111/cdev.13081 [DOI] [PubMed] [Google Scholar]
- Tohka J., Foerde K., Aron A.R., Tom S.M., Toga A.W., Poldrack R.A. (2008). Automatic independent component labeling for artifact removal in fMRI. NeuroImage, 39(3), 1227–45. doi: 10.1016/j.neuroimage.2007.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W., van Dijk E., Westenberg M., Rombouts S.A., Crone E.A. (2011). Changing brains, changing perspectives: the neurocognitive development of reciprocity. Psychological Science, 22(1), 60–70. doi: 10.1177/0956797610391102 [DOI] [PubMed] [Google Scholar]
- van der Cruijsen R., Buisman R., Green K., Peters S., Crone E. (2019). Neural responses for evaluating self and mother traits in adolescence depend on mother-adolescent relationships. Social Cognitive and Affective Neuroscience, 14(5), 481–92. doi: 10.1093/scan/nsz023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde A.C., Huigenza, H.M., Somerville, L.H., et al. (2015). Neural correlates of expected risks and returns in risky choice across development. The Journal of Neuroscience, 35(4), 1549–60. doi: 10.1523/JNEUROSCI.1924-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoorn J., Shablack H., Lindquist K.A., Telzer E.H. (2019). Incorporating the social context into neurocognitive models of adolescent decision-making: a neuroimaging meta-analysis. Neuroscience and Biobehavioral Reviews, 101, 129–42. doi: 10.1016/j.neubiorev.2018.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Overwalle F. (2009). Social cognition and the brain: a meta-analysis. Human Brain Mapping, 30(3), 829–58. doi: 10.1002/hbm.20547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waytz A., Mitchell J.P. (2011). Two mechanisms for simulating other minds: dissociations between mirroring and self-projection. Current Directions in Psychological Science, 20(3), 197–200. doi: 10.1177/0963721411409007 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


