Abstract
Chemotherapeutic treatment of cancers is a challenging endeavor, hindered by poor selectivity towards tumorous tissues over healthy ones. Preferentially delivering a given drug to tumor sites necessitates the use of targeting elements, of which there are a wide range in development. In this Review, we highlight recent examples of peptide-based targeting ligands that have been exploited to selectively deliver a chemotherapeutic payload to specific tumor-associated sites such as the vasculature, lymphatics, or cell surface. The advantages and limitations of such approaches will be discussed with a view to potential future development. Additionally, we will also examine how peptide-based ligands can be used diagnostically in the detection and characterization of cancers through their incorporation into imaging agents.
Keywords: Tumor targeting, cancer, peptides, ligands
1. RATIONALE BEHIND USING SMALL MOLECULE PEPTIDES FOR TARGETED THERAPIES
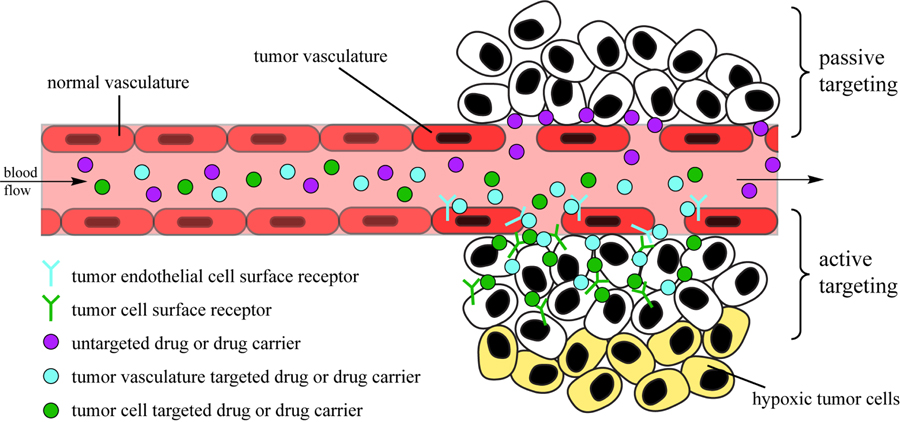
Cancer is a complex and deadly series of diseases [1–3]. In many cases, surgery and radiation therapy are deficient in their attempts to provide a positive, long-term prognosis, especially for instances where the primary tumor has metastasized and spread to multiple parts of the body [4]. Conventional anticancer drugs rely on a sufficiently long half-life in the blood stream to accumulate in cancerous tissues, exerting their desired biological effect through disruption of cell processes that are essential for cellular replication, such as DNA transcription [5, 6], cellular division [7] and metabolism [8]. Many anticancer drugs, however, possess no inherent means to distinguish healthy and diseased tissues, and it is only the unregulated, more rapid cell growth within tumors that results in a greater extent of action and thus gives the appearance of preferential targeting. Unfortunately, healthy cells that also grow rapidly, such as endothelial cells and leucocytes, are more strongly affected, leading to the notorious side-effects of chemotherapy, such as nausea, hair loss and a compromised immune system [9]. These unwanted cellular interactions, combined with the development of multi-drug resistance (MDR) [10] by cancer cells, necessitate the development of new therapies that specifically target tumors and cancerous tissues [11]. One strategy is the use of delivery vehicles, such as nanoparticles, that due to their size and other physicochemical characteristics are able to accumulate in tumors by traversing gaps in the endothelial cells lining the vascular wall [12, 13]. The utilization of this “leaky” vasculature surrounding tumors to deliver drugs is known as the enhanced permeability and retention (EPR) effect [14] and represents a passive targeting strategy (Fig. 1). These EPR-based therapies, though important, still possess several inherent limitations; for example, the same leaky vasculature responsible for the absorption of the therapeutics by solid tumors also leads to a non-uniform distribution of the drug [15]. Concerns about its effectiveness for the treatment of human metastatic cancers also exist [16, 17]. A major focus in recent years has been the development of “smart” drugs, which actively target tumors through the interaction of phenotype-specific ligands that are conjugated to the drug (or the drug-carrying delivery vector) [18, 19]. By binding with the corresponding receptors on the tumor and/or in its microenvironment, a greater build-up of drugs at the tumor site can be achieved than would otherwise be possible using a passive strategy (Fig. 1). Additionally, binding to certain receptors may help trigger internalization and potentially help avoid any drug resistance mechanisms that have arisen [20]. There is evidence to suggest that this internalization may play the major role in improving drug efficacy when compared with non-targeted counterparts [21, 22], rather than through accumulation alone, but at this stage it is unclear if this is a general trend or specific to certain tumors and/or targets.
Fig. (1).
Schematic illustration of targeting strategies in drug delivery. Passive targeting is based simply on extravasation of the drug or drug carrier through the leaky vasculature of the tumor. Active targeting is based on the binding of drug or carrier-conjugated ligand to its corresponding receptor on either the tumor endothelial cells (vasculature targeting) or tumor cell surface (tumor targeting).
Cells in the body possess a large number of protein-based receptors on their membrane surface that are responsible for communication between the intra- and extracellular environment and among different cells. These receptors bind signaling molecules (cytokines, hormones, and growth factors for instance) that then triggers some changes in the cell function. Cancer cells often express a great number and variety of receptors on their surface, particularly those that will facilitate important biological functions associated with tumor growth [23], migration [24], invasion and metastasis [25, 26]. In theory, this would make the tumor cell surface an attractive target for both therapy and imaging, but in practice it is difficult as many of the cells may not be accessible due to the heterogeneity of the tumor mass [15, 27]. Furthermore, cultured cells that are utilized for screening purposes may not possess the same characteristics as the corresponding in vivo tumor cells, i.e. they may lose tissue-specific characteristics or abnormally express molecules that in vivo cells would not. A more attractive proposition is the targeting of endothelial cells in the tumor vasculature rather than the tumor cells themselves [28–30]. This approach possesses several advantages, the most notable of which being the readily accessible nature of vasculature endothelial cells to intravenously introduced agents. Extensive studies have indicated that cancerous endothelial cells display a distinct pattern of surface receptors that is a product of their tissue type and microenvironment [31]. This differential expression of receptors therefore presents a viable strategy for the specific targeting of tumor endothelial cells over healthy endothelial cells. The challenge remains in discovering potential targets and optimizing suitable targeting ligands that can exploit them.
To target a specific receptor it is necessary to in some way mimic its natural ligand. One of the most effective means to do so is through the use of monoclonal antibodies (mAbs), which are possibly the closest entities to Paul Erlich’s “magic bullet” [32] that have been developed thus far. Naked mAbs, to which no chemotherapeutic is attached, can be used to target and block surface receptors involved in cell proliferation [33], e.g. trastuzumab (Herceptin®, Genentech/Roche) for the HER2 protein [34, 35] and Cetuximab (Erbitux®, Bristol-Myers Squib) for the EGFR protein [36, 37]. Furthermore, the use of mAbs has been shown to elicit an antitumor response from both the innate and adaptive immune system through their extra- and intracellular modes of action [38]. Utilizing the specificity of mAbs, the conjugation of a drug should in theory deliver the drug to only those cells which possess the target protein, though the effectiveness of this largely depends on the method and site(s) of conjugation and the tumor microenvironment [39]. As targeting elements, mAbs are not without issues of their own as they are expensive to produce, can be heterogeneous, often elicit an allergic response in the patient, and can have side-effects related to the targeted antigen. One targeting strategy of particularly great interest, and the focus of this review, is the use of small molecule peptides that, unlike mAbs, can be used in combination with drug delivery vehicles. Compared with mAbs, peptides have many important advantages: facile synthesis and design, lower risk of allergic response, their drug conjugates have better tumor penetrating properties, and an antigenic target is not required [40, 41]. Furthermore, they are naturally degraded by cellular catabolism. A major disadvantage to peptides, however, is their short half-life in plasma (on the order of minutes), suffering from both rapid degradation and renal clearance that limits their use as drugs outright and as targeting elements when conjugated to small molecular drugs. Fortunately, these otherwise adverse attributes are largely negated when the peptide is affixed to a larger entity such as a delivery vehicle, and chemical modification strategies exist that can prolong their circulation as smaller entities [42]. As a whole, peptides possess immense potential in the development of increasingly specific and advanced cancer therapies. We will venture to outline engineered systems that are harnessed by such therapies.
The rational design of synthetically engineered peptides for drug delivery applications is dependent upon finding amino acid sequences that can “home in” on the ligand of interest [43]. One of the most common methods employed to find homing peptides is the use of phage display libraries [44]. The phage display technique records how phages containing proteins on their surface interact with other ligands and biomarkers, thus shedding light on new protein- and peptide-based interactions [45]. This knowledge has proven invaluable in the discovery of ligands that selectively bind to specific tumor types and environments. A common strategy that employs this information is the development of antibodies with domains that preferentially bind to extracellular receptors of certain cancer phenotypes [46]. In addition, efforts in computational biology and the construction of phage display libraries have elucidated a host of information on the primary sequence and structure of these target ligands; as such, it has been possible to more easily synthesize small-molecule peptides that can preferentially bind to target tissues in the same manner as their larger antibody or protein counterparts [47]. These tumor-homing small-molecule peptides have served the field of drug delivery in various ways, the most common being the surface decoration of a cargo-carrying entity, such as dendrimers, liposomes, or microbubbles, with targeting peptides [48]. Alternatively, it has also been found that peptides conjugated directly to a therapeutic agent can function in a similarly selective manner (instances of which will be documented here). This review will seek to expound upon current approaches in the engineering of these peptides as the basis of cancer-targeting drug therapies.
2. HOMING PEPTIDES THAT TARGET THE TUMOR VASCULATURE
A critical process in tumor growth is the formation of new blood vessels, through angiogenesis, signaling the transition of tumors from a benign to malignant state [31]. Since angiogenesis is a process that strongly distinguishes cancerous cells from healthy ones [31], it is an attractive target for guided drug delivery strategies. Conveniently, cancer cells and tumor endothelial cells express their own distinct sets of receptors on their surfaces that vary significantly from normal vasculature and other healthy cells; this makes tumor vasculature a more realistic target for ligand-mediated delivery strategies [49]. Targeting tumor vasculature is also advantageous in that tumor endothelial cells are less drug resistant than more genetically unstable tumor cells; in addition, they are highly accessible to any intravenously delivered therapeutic [50]. An almost ubiquitous strategy to target the tumor vasculature is the use of integrin-binding peptides, with peptides based on the RGD motif a common sight in reports [51]. Given the wide breadth of literature available on this motif, we will instead focus on other strategies and direct the reader to a number of excellent reviews on the subject [52–56]. Table 1 contains a non-exhaustive summary of peptide ligands and their targets (where known) that have been used to target tumor vasculature.
Table 1.
Peptides that target tumor vasculature and lymphatics.
| Peptide Ligand | Cellular Target | Refs. |
|---|---|---|
| Targeting Tumor Vasculature | ||
| CDCRGDCFC (RGD-4C) | αv β3, αv β5 | [87, 88] |
| ACDCRGDCFCG | αv β3, αv β5 | [89] |
| CNGRCVSGCAGRC | Aminopeptidase N | [90] |
| TAASGVRSMH, LTLRWVGLMS | NG2 proteoglycan | [91] |
| CGSLVRC, CGLSDSC | Vasculature of various tumors | [28] |
| NRSLKRISNKRIRRK, LRIKRKRRKRKKTRK, NRSTHI | IC-12 rat tracheal tumor | [92] |
| SMSIARL, VSFLEYR | Mice prostate | [93] |
| CPGPEGAGC | Aminopeptidase P | [94] |
| ATWLPPR | VEGF | [95] |
| RRKRRR | VEGF | [96] |
| ASSSYPLIHWRPWAR | VEGF | [97] |
| CTTHWGFTLC | Gelatinase | [98] |
| Ciso DGRCG | αv β3, αv β5 | [99] |
| cyclo[(3R,6S)DKP-isoDGR], cyclo[(3S,6R)DKP-isoDGR] | αv β3, αv β5 | [100] |
| CTPSPFSHC | Unknown | [101] |
| CSRPRRSEC, CGKRK, CDTRL | Unknown | [102] |
| CKAAKNK, CKGAKAR, CRGRRST |
First two unknown Last one PDGF-β |
[103, 104] |
| CRGDK/RGPD/EC (iRGD) | αvβ3, αvβ5, neuropilin-1 | [79, 80] |
| CPRECESIC | Aminopeptidase A | [105] |
| CGNSNPKSC | Unknown | [106] |
| SVSVGMKPSPRP | Unknown | [107] |
| IFLLWQR | Annexin 1 | [108] |
| Targeting Tumor Lymphatics | ||
| CGNKRTRGC (LyP-1) | p32/gC1qR | [72, 109, 110] |
| CNRRTKAGC, CLSDGKRKC, CREAGRKAC, CAGRRSAYC | [110, 111] | |
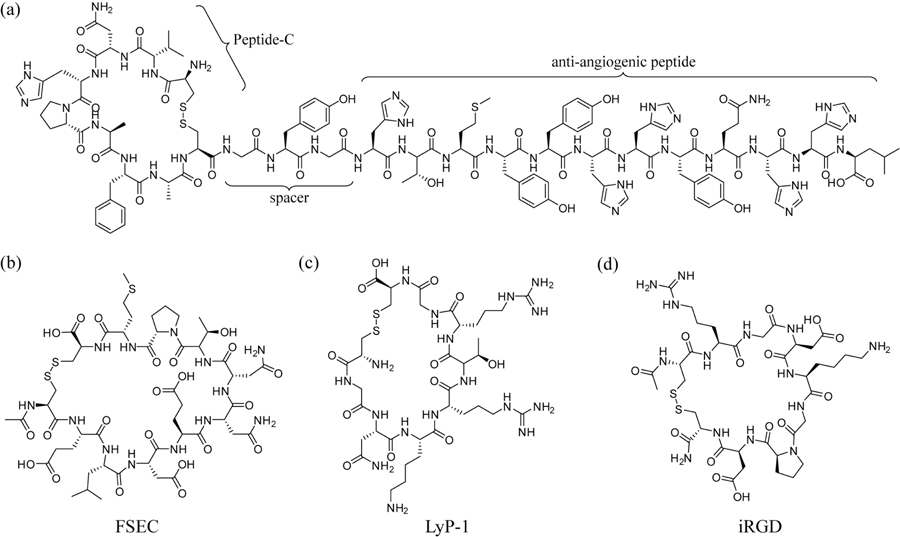
Given the importance of angiogenesis in tumor progression, it is desirable to develop peptide-based therapies that can inhibit this process and, therefore, prevent further tumor growth and metastasis [57]. One example of an angiogenesis-specific ligand was developed by Travassos et al. [58, 59]; using phage display, the group discovered a new melanoma-homing peptide termed peptide C, and conjugated it to a bioactive, anti-angiogenic peptide sequence (Fig. 2a). The resultant peptide was found to effectively slow tumor progression when systemically injected in mice; “proangiogenic” structures were reported to be reduced by 40 percent [59]. This newly created peptide sequence also binds the human sonic hedgehog protein, a protein prevalent in multiple tumor microenvironments, including melanoma [60]. The use of anti-angiogenic peptides, as it happens, is a valid strategy in the treatments of many types of high-risk tumors. One important example is given by Cohn et al., who show promising work in tumor-homing peptides called SPARC peptides (Secreted Protein, Acidic and Rich in Cysteine) that inhibit angiogenesis in neuroblastoma tumors [61]. Although its exact inhibitive mechanism is not fully understood, SPARC is able to interfere with a variety of growth factors, such as VEGF, PDGF, and bFGF, and reduce their ability to stimulate angiogenesis [62]. The same group then synthesized various peptides mimicking conserved SPARC domains, and found that one, named FSEC (Fig. 2b), not only potently inhibited angiogenesis but also inhibited neuroblastoma progression in a preclinical model [61].
Fig. (2).
Examples of tumor vasculature targeting peptides. (a) A Peptide-C based therapeutic that targets melanoma and inhibits angiogenesis through the conjugated anti-angiogenic peptide. (b) A shortened peptide mimic of a larger SPARC peptide that has tumor-homing properties. (c) LyP-1, a peptide that can be utilized to target a tumor’s lymphatic vasculature. (d) iRGD, a tumor penetrating peptide.
Targets for angiogenesis are not limited to endothelial cells, as proteins in the extracellular matrix such as collagen IV play an important role in the angiogenic process, and are therefore suitable candidates for tumor-targeting therapies [63, 64]. Essler et al. have discovered a tumor-homing peptide through phage library screening that specifically binds to collagen IV after it has been cleaved by matrix metalloproteinase 2 (MMP-2) [65]. This peptide, of sequence TLTYTWS, was found to block angiogenesis through the targeting of a molecular mechanism necessary for it to occur: the cleavage of the basement membrane by matrix metalloproteinases [66, 67]. This ability to selectively target different types of biomarkers, from overexpressed receptors to by-products of a molecular mechanism, demonstrates the versatility of using peptides as targeting entities.
Angiogenesis is not the only novel characteristic of the tumor microenvironment. Lymphatic vessels in tumors also possess specialized markers that differentiate them from their non-tumorigenic counterparts. Studies have shown that it is even possible to differentiate tumor types in terms of their lymphatic vasculature [68, 69]. In fact, the physical characteristics of a tumor’s lymphatics such as size, number, and expressed growth factors have been shown to be an important factor in tumor metastatic capability [70]. Consequently, metastasis may be combated by targeting not just tumor angiogenesis but also the lymphatic structure of tumors as well. This strategy was employed by Ruoslahti et al., who demonstrated the potential of a lytic peptide to reduce metastasis by targeting tumor lymphatics (Fig. 2c) [71]. The peptide in question, termed LyP-1 (CGNKRTRGC), was shown to preferentially accumulate in breast cancer xenografts following intravenous administration. In addition, LyP-1 was found to localize in hypoxic areas within the tumors, induce tumor cell apoptosis, destroy tumor lymphatics, and inhibit tumor growth [71].
Ruoslahti and coworkers later determined that LyP-1 binds to p32 (or gC1qR) [72], a cell surface protein that is overexpressed on tumor cells and stroma, and undergoes proteolysis to produce a C-terminal KRTR motif. It was this feature that was found to be responsible for the activity of LyP-1 [73], with a significant number of peptides possessing the consensus motif, R/KXXR/K, and similar cell internalization characteristics found by phage display. This tissue penetration motif was termed the C-end rule or CendR (“sender”) motif, and it is it’s interaction with neuropilin-1 (NRP-1) that triggers the activation of a transport pathway through both the vascular wall and tumorous tissues. It was postulated that this CendR tissue transport pathway may exist to facilitate the transfer of nutrients that are far from blood vessels or in some form of distress, i.e. hypoxic [74]. Overexpression of NRP-1 in tumors may be one way in which tumors exploit physiological pathways to promote their continued growth. The CendR pathway may also have been hijacked by viruses (e.g. Ebola [75], Crimean-Congo hemorrhagic fever [76]), microbial toxins (e.g. anthrax [77]) and venoms (melittin in bee venom [78], for example) to aid with cellular entry and spreading through tissues, as the CendR motif is a recurring theme in associated peptides/proteins.
Utilizing this knowledge of the CendR pathway, Ruoslahti’s group developed the tumor penetrating peptide, iRGD (internalizing-RGD, CRGDKGPDC) [79], in which the RGD motif targets overexpressed integrins in the tumor vasculature, with subsequent proteolytic cleavage revealing the CendR motif (-RGDK) that then enables tumor penetration. It was demonstrated that conjugation of the iRGD peptide to the clinically relevant therapeutic Abraxane (albumin-bound paclitaxel, Celgene) resulted in an eight-fold increase in the tumor concentration of the drug after intravenous injection when compared to Abraxane alone [79]. Remarkably, it was found that simply co-administering a drug or drug carrier with standalone iRGD resulted in increased tumor concentration of the therapeutic and enhanced their efficacy [80]. For instance, little difference between the effectiveness of Abraxane was found when co-administered with or conjugated to iRGD. The reasoning behind this is that the CendR pathway is a bulk transport pathway and allows greater systemic accessibility for other payloads to pass through at the same time. The obvious appeal of this observation is that it would remove the need for chemical conjugation, thereby greatly simplifying the preparation process. More recently, another tumor-penetrating peptide was developed in a de novo fashion, replacing the RGD targeting sequence of iRGD with the aminopeptidase N targeting NGR motif [28] to give iNGR (CRNGRGPDC) [81]. Similar to iRGD, iNGR can be coadministered with or conjugated to the therapeutic in order to enhance efficacy. The significance of iNGR, however, lies in its creation from known sequence elements that, in theory, points to the possibility of harnessing any targeting sequence provided that a ‘cryptic’ CendR motif can be incorporated and effectively unmasked upon proteolytic processing.
It should be noted that while the approaches described above have demonstrated great utility, it is important to understand the limitations that this tumor vasculature targeting strategy possesses. One such limitation is that there are many pathways involved in angiogenesis; targeting one in particular could force the tumor cells to adapt and use alternative pathways, resulting in resistance to drugs that seek to exploit the targeted pathway [29]. The heterogeneity of cancerous tissues, as well as their highly irregular vasculature, also raises difficulties for drug delivery strategies that operate by targeting angiogenesis or tumor lymphatics [82]. As the distance from the vasculature increases, there is a steep drop off in the availability of oxygen that can result in hypoxic regions (Fig. 1). In such environments, tumor cells can undergo drastic changes that allow them to avoid death and continue proliferating [83, 84]. Destruction of cancerous tissues nearer the vasculature can result in increased oxygenation that then promotes further tumor growth and invasion [85]. Furthermore, tumor cells that existed at the boundary between the oxygen-rich and poor regions can become drug resistant, as they may have been exposed to concentrations of the drug that were insufficient to induce apoptosis [86]. Tumor penetrating peptides, such as those developed by Ruoslahti, may provide a long-term answer to these challenges, though much work remains to be done.
3. HOMING PEPTIDES THAT TARGET SPECIFIC CANCERS
Just as the tumor microenvironment itself contains overexpressed receptors that become key identifiers, particular tumor types exhibit significant overexpression of specific extracellular receptors (e.g. growth factor receptors). As a result, monoclonal antibodies have been utilized as targeting moieties due to their high specificity for their target proteins, with a number of antibody-conjugated drugs (ADCs) already approved for clinical use [39], e.g. Brentuximab vedotin [112, 113] (Adcetris®, Seattle Genetics) and Adotrastuzumab emtansine [114] (Kadcyla® or T-DM1, Genentech/Roche). Tumor-homing peptides specific for certain cancers operate around the same concept, with the promise of overcoming some of the shortcomings of their monoclonal antibody counterparts: peptides have a comparatively facile synthesis, lower production cost, and increased efficacy in tumor targeting, penetration and internalization [41, 115]. It is the goal of many current research efforts to identify and develop peptides that can mimic the targeting function of antibodies in a more direct and cost-effective way. This section will seek to discuss the utilization of peptide-based therapies that target specific cancers on the basis of their affinity for certain cancer-specific extracellular biomarkers.
The engineering of peptides that can home in on specific cancers is predicated upon the knowledge of which biomarkers to target. Thus, ideal targets for tumor-homing therapies are those that are overexpressed or abundant in cancerous tissues, but not in healthy organs, providing the basis for differentiating cancer cells from their benign neighbors. As such, it is important that the appropriate markers be selected in order for the “smart” therapeutics to maintain the selectivity that distinguishes them from their unmodified counterparts. One of the most promising targets for the guided delivery of anticancer drugs is the luteinizing hormone-releasing hormone (LHRH) receptor; this G-protein coupled receptor has been found to be significantly overexpressed in a wide variety of cancer types, including prostate, ovarian, breast, pancreas, colorectal, kidney, and bladder cancers [116]. In addition to its significant up-regulation in cancer cells, the LHRH receptor is a particularly attractive candidate for guided drug delivery strategies because its expression levels in healthy organs is incredibly low. This provides a strong motivation to pursue LHRH agonist and antagonist-based therapies.
The overexpression of LHRH receptors is greatest in human prostate cancer, wherein it is reported that the hormone receptor is present in 86% of the cases studied [117]. The importance of LHRH in treating prostate cancer is also underscored by the morbidity and mortality for which the disease is responsible; as it stands, there is a great need in the treatment of prostate cancer for more versatile and effective treatments. In particular, cases wherein the cancer has proven to be castration-resistant have resulted in a remarkably poor prognosis, with an expected survival rate of less than nineteen months [118]. It has been shown that in such instances a lack of viable treatment options exists [119]. Given its prevalence in the majority of prostate cancer cases, LHRH receptor has become a promising avenue for the development of more effective treatments. In addition to its high up-regulation, the LHRH receptor has been demonstrated to maintain a steady expression level even after prolonged exposure to LHRH agonists [120]. While its specific role in the progression of cancers is still not clearly understood, its mere presence allows the use of LHRH receptor targeting moieties in cutting-edge therapies [120, 121].
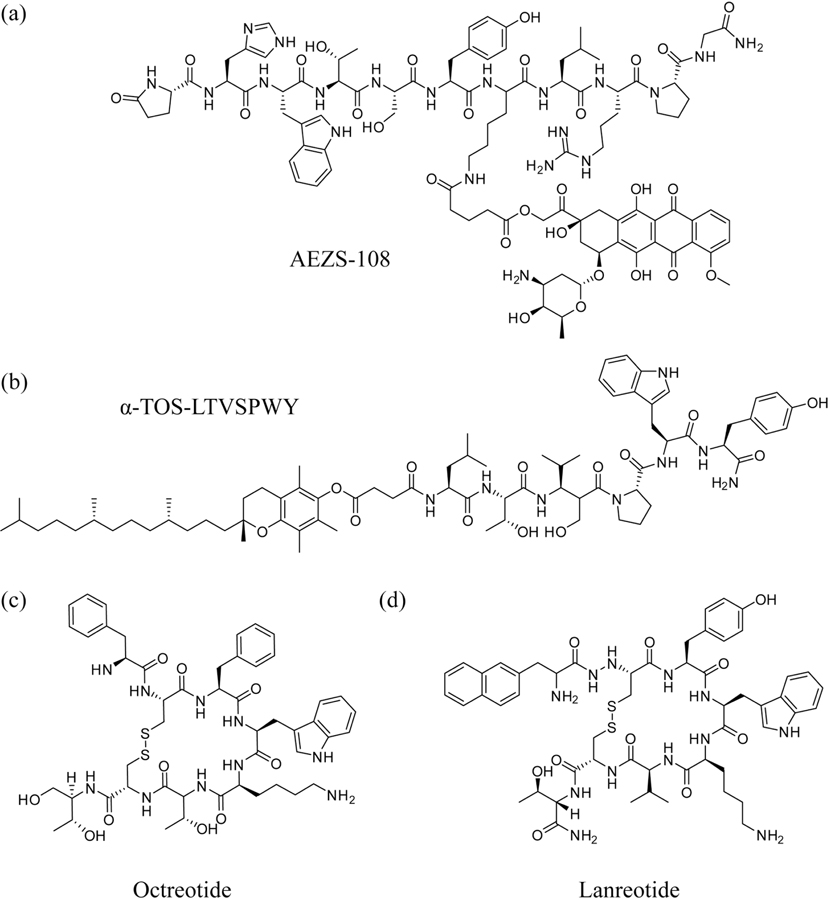
In the creation of LHRH receptor-targeted strategies, the most straight forward homing peptide that can be utilized is the corresponding antigen itself. LHRH is the relatively short polypeptide, pyroE-HWTSYGLRPG-NH2, which lends itself to facile synthesis and possesses the same advantages as other small-molecule compounds. Indeed, Minko et al. have shown that the use of LHRH as a targeting moiety in a drug delivery system (DDS) is sufficient to selectively target tumor cells and therefore increase the relative potency of the chemotherapeutic camptothecin (CPT) [122]. In their design, a LHRH receptor-targeted DDS was constructed by conjugating free CPT to a modified LHRH peptide via a polyethylene glycol (PEG) linker. Compared to the free drug, this new compound displayed increased efficacy by reducing tumor size in mice while decreasing harmful side effects to healthy organs [122]. Using modified LHRH peptides as targeting moieties has also shown great promise in clinical trials when conjugated to the chemotherapy drug doxorubicin (DOX); the compound AEZS-108 (also called AN-152) consists of a cytotoxic analog of LHRH that is conjugated to a molecule of DOX (Fig. 3a) and was shown to achieve disease stabilization in 90% of prostate cancer patients during Phase I trials [123]. It is currently being developed as a chemotherapy treatment option for a variety of cancer types [124, 125]. AEZS-108 exerts its cytotoxic effects by directing the DOX moiety to LHRH receptors, where the compound undergoes receptor-mediated endocytosis [126]. This contrasts with the passive diffusion across the plasma membrane that allows free DOX to internalize into cells [127]. The promise of AEZS-108 lies in its potential to provide a viable therapy to patients with castration-resistant prostate cancer (CRPC). Rick et al. have explored this potential in the treatment of DU-145 castration-resistant prostate tumors in nude mice, demonstrating that the peptide-drug conjugate had notably greater efficacy than unconjugated DOX or free LHRH analog alone in the inhibition of tumor growth [126]. The group’s results indicate that this formulation of doxorubicin allows for a delayed rate of intracellular uptake that changes DOX’s mechanism of action to additionally affect other extranuclear targets. These findings illustrate both the utility and importance of homing peptides in the development of therapies with poor prognoses.
Fig. (3).
Examples of tumor-homing peptides and peptide-drug conjugates. (a) AEZS-108, in which the chemotherapeutic doxorubicin is conjugated to a modified LHRH peptide for the treatment of castration-resistant prostate cancer. (b) Conjugation of the anti-apoptotic α-TOS to the HER2-targeting peptide, LTVSPWY. (c) Octreotide and (d) lanreotide, two somatostatin analogues developed as both anti-tumor agents and targeting moieties).
The LHRH receptor is but one of several important biomarkers that allow for targeted therapy. Another well-known cellular component that is overexpressed in several types of cancers is the human epidermal growth factor receptor 2 (HER2/neu or HER2). HER2 is a receptor tyrosine kinase of the EGFR family and a known oncogene that plays a role in several cancer types, including breast, gastric, ovarian, and prostate cancers [128]. In particular, HER2 has become an important biomarker in the diagnosis and treatment of human breast cancer, being overexpressed in approximately 30% of cases [129]. This plasma membrane-bound protein takes part in the transduction of multiple signaling pathways that, when uncontrolled, are characteristic of cancerous phenotypes [130]. Patients with HER2-positive cancers are associated with more aggressive disease and generally poorer prognoses; in light of this, it is desirable to develop HER2-specific therapies that can effectively treat these more threatening occurrences [129]. It has been demonstrated that the intracellular inhibition of HER2 in breast cancer cells where it is overexpressed promotes apoptosis [131]; thus, it can be reasoned that HER2 may prove to be a logical target for other guided therapies.
HER2 does not have any known corresponding binding ligand; however, cases have been documented that demonstrate the successful creation of artificial ligands which target the receptor [128, 132]. In line with these efforts, a monoclonal antibody known as Trastuzumab that interferes with the HER2 receptor has been developed as a first-line treatment against breast cancer [133]. Unfortunately, Trastuzumab-based therapies are susceptible to the same problems encountered by other therapeutic antibodies and antibody-targeted drugs, such as poor tumor and tissue penetration and inherent or acquired mechanisms of resistance [134]. Once again, peptide-based therapies offer significant advantages due to their small size (and consequently excellent tissue penetration), facile synthesis, and flexibility as targeting ligands. Murali et al. successfully created an anti-HER2/neu peptide (AHNP, -FCGDGFYACYMDV-) that acts as a mimetic for the full-chain antibody Trastuzumab. AHNP has been demonstrated to bind tightly to HER2 receptors and encourage apoptosis of tumor cells on an order comparable to its monoclonal antibody counterpart [135]. Treatment of HER2-positive cancers with AHNP also exhibited increased efficacy when used in conjunction with radiation or other chemotherapeutic agents such as doxorubicin [135]. The promise of this antibody-mimicking peptide has also allowed for its use as a targeting moiety for HER2-positive cancer cells, providing a versatile platform for the delivery of a variety of therapeutic cargo [136, 137]. Other artificial HER2-binding ligands have also emerged, including the peptide LTVSPWY, which was developed using phage display methods by Shadidi and Sioud [138]. They demonstrated the HER2-homing capability of the peptide by conjugation to an antisense nucleotide that was shown to be delivered preferentially to HER2-positive cancer cells [138]. This tumor-homing peptide also readily mediates delivery of pro-apoptotic agents which are otherwise non-selective in their cellular uptake. Neuzil et al. have conjugated LTVSPWY to the pro-apoptotic α-tocopheryl succinate (α-TOS) (Fig. 3b), which they found to effectively induce rapid apoptosis and slow growth in HER2-positive breast tumors [139]. This ability to enhance uptake of otherwise poorly or non-selective therapeutics in a rapid and biomarker concentration-dependent manner is at the heart of this shift in chemotherapy-based strategies that peptide-based therapies are leading.
Another biomarker of great interest to the field of peptide-based drug delivery is the hormone somatostatin, a cyclic polypeptide that is an important regulator of the endocrine system [140]. By binding with G-protein coupled somatostatin receptors, it inhibits the secretion of various secondary hormones depending on the location of the receptor, e.g. insulin release in the pancreas [141]. Somatostatin occurs naturally in two active forms consisting of 14 and 28 amino acids, respectively. Somatostatin is of key interest because the class of receptors through which it exerts its effects is overexpressed in a large number of tumor types, particularly neuroendocrine tumors [142]. In particular, somatostatin receptor 2 (SSTR2) was found to exist at high densities in 14 out of 16 common tumor cell lines, including breast, prostate, lung, pancreatic, ovarian, cervical cancers, leukemia and neuroblastoma [143]. Such ubiquity fosters a high level of interest in SSTRs for those attempting to develop novel peptide-based drug delivery systems.
Although somatostatin has a short half-life in the body [143], synthetic analogs of higher stability have been successfully created which can still effectively bind SSTRs with high affinity via a conserved sequence of amino acids [144, 145]. These somatostatin analogs have become useful tumor-localization agents, and analogs such as Lanreotide (Fig. 3c) and Octreotide (Fig. 3d) have also demonstrated antitumor effects in and of themselves [146]. These peptides have also been recognized for their potential as tumor-homing conjugates for chemotherapy agents; the use of Octreotide as a carrier of cytotoxins such as doxorubicin has been reported [147]. By simply pairing cytotoxic drugs with the analog Octreotide, significant tumor growth inhibition and general toxicity reduction in healthy organs can be achieved [148]. Inspired by these successes, Coy et al. developed a novel cytotoxic analog called JF-10-81 by conjugating camptothecin to the SST analog JF-07-69 via a degradable carbamate linker [149]. While selectively targeting SSTR2 and retaining its high binding affinity, this analog is rapidly internalized by tumor cells and inhibits proliferation and growth. This was demonstrated in neuroblastoma, pancreatic, leukemia, pancreatic carcinoid, and prostate tumors; this analog has also demonstrated the capacity to overcome multidrug resistance, inducing potent inhibitory effects in CPT-resistant BON pancreatic carcinoid tumors [149]. Other promising SST-analog-drug conjugates currently in development have displayed success in enhancing the efficacy of small-molecule drugs as well [150, 151].
Peptides may also play an important role in the targeting of many other overexpressed receptors, and even intracellular targets, in treating cancer [152, 153]. Table 2 lists many more examples of cancer-specific targeting peptides, but an in-depth summary of all biomarkers utilized in targeted cancer therapy is beyond the scope of this review. The emphasis here has been on some of the main successes in peptide-based targeting strategies that have shown potential to alter the core tenets of cancer treatment. The advantages that tumor-targeting peptides offer over traditional chemotherapy give them significant potential to change the face of a field that currently has a great need for both increased specificity and efficacy of treatment.
Table 2.
Peptides that target specific cancer types.
| Cancer Type | Peptide Ligand | Cellular Target | Refs. |
|---|---|---|---|
| Human lymphoma | KNGPWYAYTGRO, NWAVWXKR, YXXEDLRRR XXPVDHGL |
Surface idiotype of SUP-88 human B-cell lympoma | [104, 154] |
| LVRSTGQFV, LVSPSGSWT ALRPSGEWL, AIMASGQWL QILASGRWL, RRPSHAMAR DNNRPANSM, LQDRLRFAT PLSGDKSST |
Surface idiotype of human chronic lymphocytic leukaemia (CLL) | [155] | |
| IELLQAR | HL 60 human lymphoma & B-16 mouse melanoma | [156] | |
| SAKTAVSQRVWLPSHRGGEP, KSREHVNNSACPSKRITAAL, WLSEAGPVVTVRALRGTGSW |
A20 | [157] | |
| CAYHRLRRC | Molt-4 | [158] | |
| Myeloma | FDDARL, FSDARL, FSDMRL FVDVRL, FTDIRL, FNDYRL FSDTRL, PIHYIF, YIHYIF, RIHYIF |
Human multiple myeloma M-protein | [159] |
| Prostate cancer | CVFXXXYXXC CXFXXXYXYLMC CVXYCXXXXCYVC CVXYCXXXXCWXC |
Prostate-specific antigen (PSA) | [160] |
| DPRATPGS | LNCaP | [161] | |
| SHGFSRHSMTLI | Capan-2 (irradiated) | [162] | |
| FRPNRAQDYNTN | DU-145 | [163] | |
| IAGLATPGWSHWLAL | PC-3 | [164] | |
| CREAGRKAC (REA) | Prostate Tumors from TRAMP mice | [111] | |
| Breast cancer | VPWMEPAYQRFL | MDA-MB435 breast cancer | [165] |
| YQATPARFYTNT, CGWMGLELC | MDA-MB-231 | [166] | |
| LTVSPWY WNLPWYYSVSPT | SKBR3 | [138] | |
| CNGRCVSGCAGRC (NGR), CDCRGDCFC (RGD-4C) | MDA-MB-435, Amino-peptidase N & αvβ3, α5β1 | [28] | |
| Neuroblastoma | HLQIQPWYPQIS VPWMEPAYQRFL | WAC-2 human neuroblastoma | [165] |
| Head and neck cancer | TSPLNIHNGQKL | MDA Tu167 head and neck cancer lines | [167] |
| Laryngeal carcinoma | RLTGGKGVG | HEp-2 human laryngeal carcinoma | [168] |
| RLLDTNRPLLPY | NPC-TW 04 | [169] | |
| EDIKPKTSLAFR | Nasopharyngeal carcinoma (CNE-1) | [170] | |
| Hepatocarcinoma | TACHQHVRMVRP | BEL-7402 | [171] |
| KSLSRHDHIHHH | SMMC-7721 | [172] | |
| SFSIIHTPILPL | Mahlavu | [173] | |
| Melanoma | CTVALPGGYVRVC | GRP78 | [174] |
| TRTKLPRLHLQS | B16-F10 | [175] | |
| CLSDGKRKC (LSD) | C8161 Melanoma | [111] | |
| Gastric | GRRTRSRRLRRS | XGC9811-L4 | [176] |
| SMSIASPYIALE | GC9811-P | [177] | |
| SWKLPPS | AZ-P7a, α3β1 | [178] | |
| CGNSNPKSC | Human gastric adenocarcinoma, α3β1 | [179] | |
| Colon | CPIEDRPMC, | HT29 | [180] |
| HEWSYLAPYPWF, QIDRWFDAVQWL | WiDr | [166] | |
| VRPMPLQ | Human Colonic adenomas | [181] | |
| SPTKSNS | Resected human colon tumors | [182] | |
| VHLGYAT | SW480 | [183] | |
| VGLPEHTQ, ELRGDSLP, | |||
| Glioma | DSTKSGNM, DYDMTKNT, DLTKSTAP, ESRGDSYA, |
RG2 | [184] |
| MCPKHPLGC | U87-MG | [185] | |
| Cervical | CRLTGGKGVGC, CADPNSVRAMC, CAAHYRVGPWC | SiHa | [186] |
| Medullary Thyroid |
CHTFEPVGC | TT | [187] |
| SRESPHP | Medullary thyroid carcinoma (RET-C634R transgenic mice) |
[188] | |
| Rhabdomyosarcoma | CQQSNRGDRKRC, CMGNKRSAKRPC | αvβ3 | [138] |
| Leukemia | CPLDIDFYC | Kasumi-1 α4β1 | [189] |
| KDEPQRRSARLSAKPAPPKPEPK-PKKAPAKK | Human myeloid leukemia (HL-60) | [190] | |
| Lung cancer | VSQTMRQTAVPLLWFWTGSL, YAAWPASGAWTGTAPCSAGT, EHMALTYPFRPP |
H1299 (large cell) | [191–193] |
| RGDLATLRQLAQEDGVGVR | H2009 αvβ6 | [191] | |
| MTVCNASQRQAHAQATAVSL | A549 | [191] | |
| TDSILRSYDWTY | CL1-5 | [194] | |
| HVGGSSV | Lewis lung carcinoma (irradiated/SU11248) | [195] | |
| SVSVGMKPSPRP | Lung (CL1-5) | [107] | |
| Bladder | CSNRDARRC | HT-1376 | [196] |
| Pancreatic | KTLLPTP | Pancreatic ductal carcinomas arising from Kras/p52L/L mice, Plectin-1 |
[197] |
| CRGRRST, CRSRKG, CKAAKNK, CKGAKAR, FRVGVADV |
RIP1-Tag2 mice Pancreatic islets PDGFRβ | [103] | |
| Oral | SVSVGMKPSPRP, SNPFSKPYGLTV, WDSNTYTPRPLM |
Oral (SAS) | [107, 198] |
| Squamous carcinoma | CGKRK, CDTRL | K14-HPV16 mice basal cell | [102] |
4. TARGETING BRAIN TUMORS
Targeting tumors that form within the brain or central nervous system poses further challenges for effective therapies [199, 200]; an unfortunate happenstance given the most common form of primary brain tumor is the aggressive (WHO grade IV) diffuse glioma, glioblastoma multiforme (GBM) [201]. With a median survival time of 2–3 years (less for the more aggressive forms) [202], it is imperative that advances be made to enable more effective treatments that, if not curative, at least further extend survival time and allow a higher quality of life. The difficulty in treating gliomas arises from the unique environment of the brain, which has evolved to possess a protective barrier that prevents large proteins and bacteria from escaping the blood vessels and affecting the brain. The blood-brain barrier (BBB) results from the very tight junctions formed by the endothelial cells that prevent the diffusion of anything but small or gaseous molecules (water or carbon dioxide) and small (<400 Da) lipophilic molecules [203]. Additional protection against the latter is afforded by a range of efflux transporters expressed in the brain endothelium, the most abundant being the P-glycoprotein (P-gp), a protein that is commonly overexpressed in multidrug resistant cancers [204]. It should be noted that there has been some debate over whether the BBB is actually a hindrance in the treatment of brain tumors as, like other cancers, gliomas have irregular and leaky vasculature that disrupts the BBB in the tumor microenvironment, creating what is named the blood-brain-tumor barrier (BBTB) [199]. However, there is evidence to support that since it is a localized effect and gliomas readily metastasize to areas where the BBB remains intact, the BBB does in fact play a major role in the resistance of gliomas to conventional chemotherapies [205]. Regardless of the extent to which either barrier exists at the tumor site, it is clear that both present challenges to delivering a drug to brain tumors that must be addressed.
While the BBB exists to protect the brain by excluding small molecules, peptides, proteins and larger species, at that same time it is required that some of these entities can actually cross the barrier as they are essential for the brain to function properly. The brain needs fuel, for example, and so glucose must be able to be pass across the BBB, a task accomplished by glucose transporter proteins [206]. Accordingly, there is a wide range of active transporter proteins that line the brain endothelium and facilitate the import (and export) of vital metabolites and proteins. Exploiting these transport systems through selection of appropriate ligands should, therefore, allow the transport of a conjugated drug or drug carrier across the BBB via receptor-mediated endo or transcytosis.
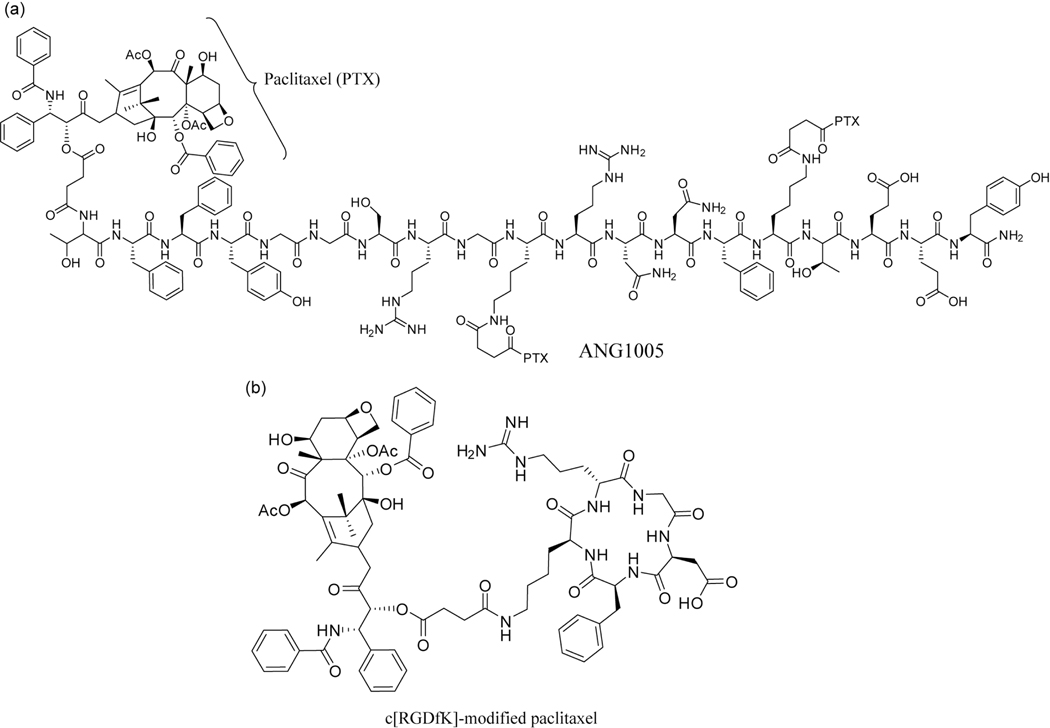
One family of receptors that have attracted great interest is the lipoprotein receptor-related proteins (LRPs), particularly those for low-density lipoproteins (LDLs) such as LRP-1. Béliveau and coworkers developed a family of peptides based on the Kunitz domain of aprotinin [207], a 6.5 kDA protease inhibitor that is a ligand for LRPs 1 and 2. These peptides, termed Angiopeps, demonstrated a greater transcytosis capacity than aprotinin, with Angiopep-2 (TFFYGGSRGKRNNFKTEEY) selected for further studies. One such study was the conjugation of three paclitaxel (Taxol, PTX) molecules to the N-terminal and lysine side-chain amines of Angiopep-2 (Fig. 4a) [208]. This peptide–drug conjugate, ANG1005, exhibited a 15% increase in life span in an intracerebral glioblastoma murine model [208] and is currently in Phase II clinical trials (ID NCT01967810), having successfully completed Phase I trials (as GRN1005) [209]. Xin et al. have also utilized Angiopep-2 to target their PEG-PCL nanoparticle drug delivery system to glioblastomas [210]. They demonstrated a potential dual targeting effect of the conjugated peptide that allows a PTX-loaded nanoparticle to cross the BBB and preferentially accumulate in the vicinity of glioma cells through recognition of overexpressed LPR-1 on their surface. This increased accumulation was confirmed through in vivo fluorescence imaging.
Fig. (4).
Examples of peptide-drug conjugates that are used to target brain tumors. (a) Beliveau’s PTX-conjugated Angiopep-2 therapeutic, ANG1005. (b) c[RGDfK]-modified paclitaxel that is loaded into transferrin-bearing micelles for targeting of gliomas.
Transferrin is an endogenous iron transport protein that is involved in maintaining iron concentrations within the brain [211]. Zhang et al. took advantage of the receptor for this protein using a dual targeting approach to deliver paclitaxel-loaded micelles to U87 MG glioblastomas in a mouse model [212]. Transferrin was grafted to a micelle loaded with a c[RGDfK] peptide-modified PTX (Fig. 4b). In circulation, the attached transferrin allows the micelles to cross the BBB where the micelle breaks down to release the modified drug. The cyclic RGD peptide ensures the drug exhibits a greater selectivity for gliomas over healthy tissues due to the overexpression of integrin proteins in the former [213]. In vivo studies showed a 26% increase in mean survival time when using the dual targeting strategy (43 days) over the free drug control and saline controls (34 and 35 days, respectively).
While the above approach to targeting the transferrin receptor relied on the use of the endogenous protein, Lee et al. used phage display to develop a small peptide, HAIYPRH (T7), which could effectively bind to the transferrin receptor [214]. Fusion of this peptide with the green fluorescent protein showed that this ligand could facilitate efficient internalization. Jiang and coworkers utilized this peptide to enable their doxorubicin-loaded PAMAM dendrimers to cross the BBB [215], demonstrating a significant improvement in tumor growth inhibition in vivo when compared to the non-targeted delivery vectors and saline.
Though not an example of cancer targeting, Son et al. exploited the nicotinic acetylcholine receptor (nAchR) on neuronal cells to deliver a polymer/DNA across the BBB and into neurons [216]. To accomplish this an nAchR-targeting RVG peptide (YTIWMPENPRPGTPCDIFTNSRGKfRASNG) was conjugated to the polyplex via reducible disulfide linkages in order to transport a gene sequence encoding luciferase into neuronal cells. Fluorescence imaging confirmed that transfection of the luciferase gene was successful, exhibiting fluorescence only when the RVG peptide was attached to the polymer/DNA complex. The potential of this approach to significantly improve targeted transfection for gene therapies is evident and will hopefully lead to and inspire other successful treatments in the future.
5. TARGETING PEPTIDES IN IMAGING AND THERANOSTICS
As we have seen, peptides possess the potential to play a powerful role in the treatment of cancer: they can act as delivery vectors, targeting moieties, and cell- and tumor-penetrating agents. One final aspect of peptide utility to be discussed herein is their role in the imaging and detection of cancer [217, 218]. This follows the logical progression that the effective treatment of disease is linked to, and informed by, the power to image the physiological area under treatment. Imaging methods such as PET, CT, and MRI have become the standard for approaching cancer cases, relying on the use of contrast agents to differentiate the various structures in the body. This section will seek to understand how peptides play a role in these tools to enhance current imaging capabilities.
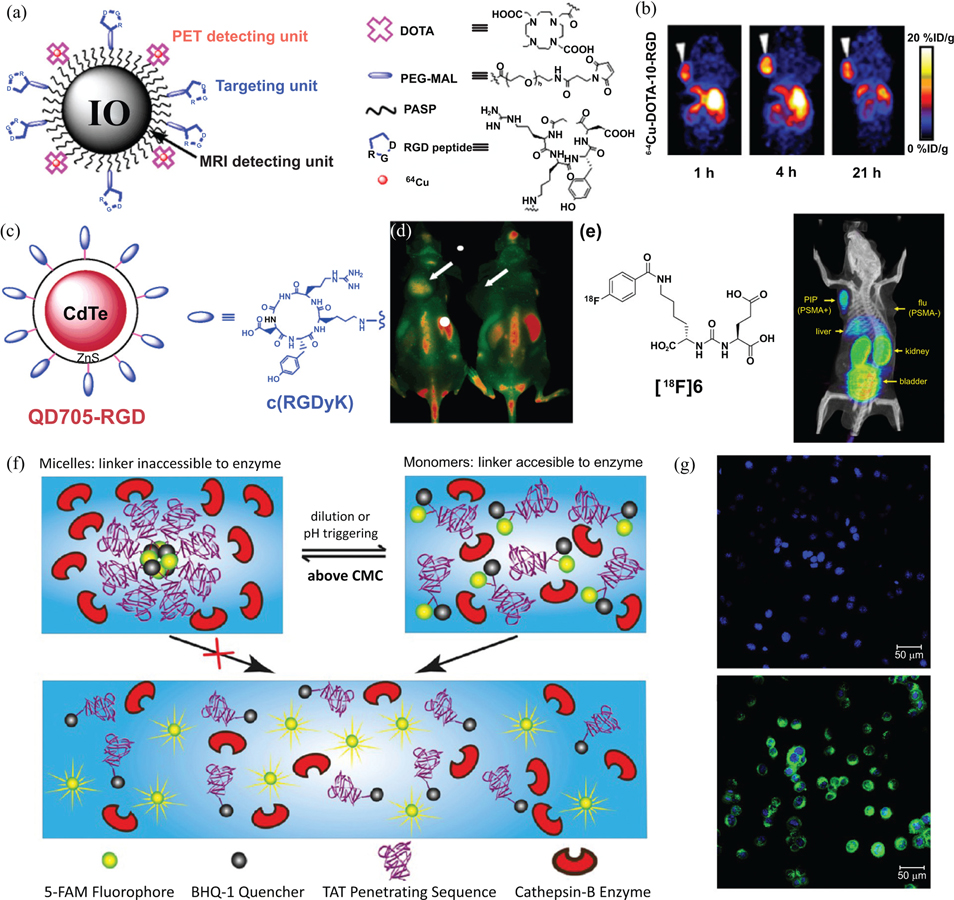
In recent years, it has been shown that contrast agents can be targeted specifically to tumors by employing similar strategies used to deliver therapeutics. This has included the functionalization of various nanoparticles as intracellular contrast agents via conjugation to a peptidic targeting or cell penetrating moiety [219]. One powerful example of this was demonstrated by Chen et al., who employed iron oxide (IO) nanoparticles as a molecular platform for simultaneous targeted PET and MRI imaging through the addition of functional peptide ligands (Fig. 5a) [220]. Polyaspartic acid-coated IO particles were conjugated with two functional groups: macrocyclic chelating agent 1,4,7,10-tetraazacyclododecane-N,N’,N”,N”’-tetraacetic acid (DOTA), and the Arginine-Glycine-Aspartate (RGD) peptide. This multifaceted nanoimaging agent contains three capabilities: the DOTA group chelates radioisotopes, such as [64] Cu for PET detection; the RGD peptide is a tumor-homing moiety that targets αvβ3 integrins overexpressed in tumor vasculature; the IO-nanoparticle core serves as a contrast agent for MRI imaging. These nanoparticles were found to localize specifically in αvβ3 integrin-positive tumors in vivo (Fig. 5b) [220]; theoretically, these multimodal probes may allow for early clinical tumor detection with high sensitivity. This innovative design is representative of the potential for tumor-homing and tumor-penetrating peptides to change the way that cancer is imaged.
Fig. (5).
Examples of nanoimaging agents that utilize peptides in their design. (a) A dual-modality imaging agent developed by Chen et al., possessing an iron oxide nanoparticle core for MRI imaging and conjugated 64Cu chelating DOTA groups for PET imaging. RGD peptide ligands target tumors displaying αvβ3 integrins. (b) Decay-corrected whole body coronal PET images of nude mouse bearing human U87MG tumor at 1, 4, and 21 h after injection of 3.7 MBq of the 64Cu-containing imaging agent. These images show that it can accumulate in the tumor xenograft (white arrows), with uptake by the liver also indicated. (c) A quantum dot (QD) based fluorescence imaging agent, QD705-RGD, developed by Chen et al. This agent consists of a CdTe/ZnS with a PEG-polymer coating. The surface was decorated with cyclic-RGD ligands to enable targeting of αvβ3 integrins. (d) In vivo NIR fluorescence image of U87MG tumor-bearing mice 6 h after treatment with 200 pmol of QD705-RGD (left) and QD705 (right), showing how the RGD ligand allows accumulation in the tumor relative to a control QD imaging agent. Prominent uptake was also seen in the liver, bone marrow and lymph nodes. (e) An example of Pomper’s urea-based PSMA-targeting ligand with an 18F radiolabel. Preferential uptake is seen in a PSMA+ (PIP) tumor, but not a PSMA− (flu) tumor via PET imaging. (f) Schematic illustration of Cui’s nanobeacon (NB) concept. In the assembled state, the cleavable linker (GFLG) is inaccessible to the Cathepsin B protease and remains intact. Upon breakdown of the nanostructure, triggered by either dilution or a change in pH, the linker becomes accessible and cleavage occurs. The separation of the 5-FAM fluorophore from the BHQ-1 quencher results in a measurable signal that can be used to probe the activity of the protease. (g) Fluorescence images of MCF-7 human breast cancer cells incubated with NBs after 0 h (top) and 1.5 h (bottom). The cell nuclei were stained with the blue dye Hoechst 33342. (Images in (a) and (b) were adapted from ref. [220], (c)-(d) were adapted from ref. [225], (e) from ref. [226], and (f)-(g) were adapted from ref. [242]).
In addition to their utility in the modification of radiological contrast agents, peptides can also function as targeting moieties in the development of molecular probes, such as quantum dots (QD). QDs are nanoscale crystals made of semiconductor material that, given a base solubility and biocompatibility, can be labeled with targeting ligands and utilized as fluorescent molecular probes [221, 222]. The potential use of quantum dots for imaging purposes was first demonstrated by Nie et al., who conjugated QDs to prostate-specific membrane antigen (PSMA) targeting monoclonal antibodies for the detection of prostate cancer [223]. Just as peptides possess advantages over antibodies as drug delivery vectors, the same principles apply to peptidic modification of nanoparticles in molecular probes; in addition, given their smaller size, a greater number of peptides may be conjugated to the surface of a QD than antibodies [224]. Chen et al. report the conjugation of ανβ3 integrin-targeting RGD peptides to the surface of QD705 (Fig. 5c), a quantum dot with a near-infrared emission maximum [225]. This new imaging agent was found to only bind to, and fluorescently visualize, integrin-positive cells, resulting in successful in vivo tumor vasculature imaging (Fig. 5d and e). It is once again evident that the ability of peptides to target imaging compounds selectively and effectively to cancerous sites represents a powerful new tool in cancer detection and consequent treatment.
Pomper and colleagues have developed peptidic urea-based inhibitors that can act as ligands for PSMA, imbuing a capacity for prostate cancer targeting [226]. Prostate-specific membrane antigen is a type II integral membrane glycoprotein that has close correlation with human prostate cancer. PSMA is highly expressed in primary prostate cancer and lymph node metastases [227–229], and recent evidence suggests that PSMA is also expressed in tumor-associated neovasculature [230], making PSMA a potential biomarker for prostate cancer imaging and therapeutics. PSMA has two natural ligands or substrates including N-acetyl-aspartylglutamate (NAAG, a neurotransmitter) and poly-γ-glutamated folate. However, these two peptidic ligands are readily degraded by PSMA due to its enzymatic activity [231, 232], preventing their use as targeting ligands for drug delivery and imaging. The synthesized ligands were labeled with 125I, 18F and 99mTc through different chemistries,226 and the complexes of these imaging agents with PSMA were studied by X-ray crystallography, revealing that all the ligands invariably bound with a glutarate moiety within the S1′ pocket of the enzyme [233]. The urea linkage that these ligands are based on prevents their PSMA-catalyzed degradation. In vivo studies showed that these urea-based reagents exhibited very specific binding to PSMA-overexpressed prostate tumors initiated by PC-3 PIP and LNCaP cell lines, but not to prostate tumors initiated by PC-3 (PSMA negative) and the breast cancer cell line MCF-7 (PSMA negative) [226, 234–236]. These results clearly indicate that these urea-based imaging contrasts have great potential in prostate cancer diagnosis.
Just as selectivity is important in the delivery of drugs exclusively to diseased tissues via recognition of certain biomarkers, it is also desirable to use molecular probes to visualize very specific molecular occurrences which play a part in the disease [237]. In recent years, activatable imaging probes that respond to specific biochemical events have been under development [238, 239]. Conventional “molecular beacons” consist of a fluorophore and a quencher connected to a functional peptide; they remain “quenched” until they are modified by a biochemical process, such as cleavage by a protease [240, 241]. A major hurdle for the development of reliable molecular beacons is their relatively short half-life under physiological conditions: for soluble beacon molecules, cleavable linkers designed to activate the fluorescent signal are often exposed to the environment, leading to non-specific proteolytic degradation (and, consequently, a positive signal with no correlation to the target physiological process). To address this problem, Cui et al. developed a new form of amphiphilic, self-assembling supramolecular nanobeacon (NB) that forms core-shell micelles under physiological conditions to protect the enzyme-cleavable segment (Fig. 5f) [242]. The NB molecule contains a fluorescent dye, 5-carboxyfluorescin (5-FAM), and a black hole quencher, BHQ-1, which form a hydrophobic core within the micelles. These molecules are conjugated to the hydrophilic, cell-penetrating TAT peptide to facilitate internalization of the nanobeacon. Once inside the cell and trafficked to the lysosome, the micelles dissociate into monomeric form, wherein the peptide linker, GFLG, that connects the 5-FAM dye to the amphiphile is cleaved specifically by the lysosomal enzyme Cathepsin B [243]. Cathepsin B is overexpressed in tumor cells and is important for tumor growth [244]. The 5-FAM entity thus dissociates from the BHQ-1 and generates a green fluorescent signal when irradiated. Experimental data indicated that the NB molecules not only effectively penetrated cells, but were also effectively activated intracellularly to produce a fluorescent signal (Fig. 5g) [242]. Although the field of peptide-based molecular imaging is still in its infancy, the facile synthesis and “tunability” of these supramolecular nanomaterials is incredibly promising for the future of imaging and diagnostic methods.
An important point to note is the arrangement of this review might suggest that targeted therapeutics and smart imaging and diagnostic methods are compartmentalized issues; the fact of the matter is the two ideas must be pursued in a concerted and holistic way. More simply put, the new concept of “theranostics” is interested in the development of technologies that address a spectrum of medical needs, from diagnosis to treatment [60, 245–252]. Current and future research areas focus on drug delivery systems which contain targeting, imaging, and therapeutic components. Such advances will undoubtedly cause a much needed paradigm shift in the field of cancer treatment.
FUTURE PROSPECTS
Incorporating onto a non-targeting object small molecule peptides capable of recognizing particular tumor types or tumor-relevant vasculature appears to be a logical choice in the construction of targeted drug delivery systems that promise both improved treatment efficacy and reduced side effects. In combination with recent progress in delivery technology that can facilitate release of the therapeutic cargo at only tumor sites, there is great hope that the synergy of the two strategies can realize Paul Erlich’s concept of the “magic bullet” for cancer therapy and diagnostics. However, there are still many hurdles ahead in translating this promising strategy into clinically useful therapies or tools. The major obstacles in this path include the individualized human anatomy and cancer pathophysiology, as well as the characteristic tumor heterogeneity, with the former preventing the development of consensus peptide sequences that would work for all patients diagnosed with the same tumor type, and the latter limiting effective dissemination of the delivered drugs throughout the tumor. The key to future successes in the field may lie in our ability to screen and identify targeting peptides at the individual patient level to develop customized medicines in a timely and cost-effective manner.
ACKNOWLEDGEMENTS
We thank the National Science Foundation (DMR 1255281) and Cohen Translational Fund for support of the project.
Biography

Honggang Cui
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Siegel R; Ma JM; Zou ZH; Jemal A Cancer statistics. CA-A Cancer J. Clin, 2014, 64, 9–29. [DOI] [PubMed] [Google Scholar]
- [2].Hanahan D; Weinberg RA Hallmarks of cancer: the next generation. Cell, 2011, 144, 646–674. [DOI] [PubMed] [Google Scholar]
- [3].Gerlinger M et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med, 2012, 366, 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chambers AF; Groom AC; MacDonald IC Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer, 2002, 2, 563–572. [DOI] [PubMed] [Google Scholar]
- [5].Minotti G; Menna P; Salvatorelli E; Cairo G; Gianni L Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev, 2004, 56, 185–229. [DOI] [PubMed] [Google Scholar]
- [6].Pommier Y Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer, 2006, 6, 789–802. [DOI] [PubMed] [Google Scholar]
- [7].Jordan MA; Wilson L Microtubules as a target for anticancer drugs. Nat. Rev. Cancer, 2004, 4, 253–265. [DOI] [PubMed] [Google Scholar]
- [8].Vander Heiden MG Targeting cancer metabolism: a therapeutic window opens. Nat. Rev. Drug Discov, 2011, 10, 671–684. [DOI] [PubMed] [Google Scholar]
- [9].Allen TM Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer, 2002, 2, 750–763. [DOI] [PubMed] [Google Scholar]
- [10].Liu Y; Yu J; Bitterman A; Giuliano A; Cabot M Antisense oligonucleotides targeting ceramide glycosylation overcome multidrug resistance in cancer cells. Eur. J. Cancer, 2002, 38, S142–S142.11858981 [Google Scholar]
- [11].Brannon-Peppas L; Blanchette JO Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev, 2004, 56, 1649–1659. [DOI] [PubMed] [Google Scholar]
- [12].Brigger I; Dubernet C and Couvreur P Nanoparticles in cancer therapy and diagnosis. Advan. Drug Deliv. Rev, 2002, 54, 631–651. [DOI] [PubMed] [Google Scholar]
- [13].Davis ME; Chen ZG; Shin DM Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov, 2008, 7, 771–782. [DOI] [PubMed] [Google Scholar]
- [14].Matsumura Y; Maeda HA New concept for macromolecular therapeutics in cancer-chemotherapy - mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res, 1986, 46, 6387–6392. [PubMed] [Google Scholar]
- [15].Jain RK; Stylianopoulos T Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol, 2010, 7, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prabhakar U; Maeda H; Jain RK; Sevick-Muraca EM; Zamboni W; Farokhzad OC; Barry ST; Gabizon A; Grodzinski P; Blakey DC Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res, 2013, 73, 2412–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fang J; Nakamura H; Maeda H The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Advan. Drug Deliv. Rev, 2011, 63, 136–151. [DOI] [PubMed] [Google Scholar]
- [18].Torchilin VP Passive and active drug targeting: drug delivery to tumors as an example. Handbook Exp. Pharmacol, 2010, 3–53. [DOI] [PubMed] [Google Scholar]
- [19].Byrne JD; Betancourt T; Brannon-Peppas L Active targeting schemes for nanoparticle systems in cancer therapeutics. Advan. Drug Deliv. Rev, 2008, 60, 1615–1626. [DOI] [PubMed] [Google Scholar]
- [20].Bareford LM; Swaan PW Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev, 2007, 59, 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kirpotin DB; Drummond DC; Shao Y; Shalaby MR; Hong K; Nielsen UB; Marks JD; Benz CC; Park JW Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res, 2006, 66, 6732–6740. [DOI] [PubMed] [Google Scholar]
- [22].Choi CHJ; Alabi CA; Webster P; Davis ME Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Nat. Acad. Sci. USA, 2010, 107, 1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Slamon DJ; Leyland-Jones B; Shak S; Fuchs H; Paton V; Bajamonde A; Fleming T; Eiermann W; Wolter J; Pegram M; Baselga J; Norton L Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med, 2001, 344, 783–792. [DOI] [PubMed] [Google Scholar]
- [24].Chaffer CL; Brennan JP; Slavin JL; Blick T; Thompson EW; Williams ED Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: Role of fibroblast growth factor receptor-2. Cancer Res, 2006, 66, 11271–11278. [DOI] [PubMed] [Google Scholar]
- [25].Feldmann G; Dhara S; Fendrich V; Bedja D; Beaty R; Mullendore M; Karikari C; Alvarez H; Iacobuzio-Donahue C; Jimeno A; Gabrielson KL; Matsui W; Maitra A Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: A new paradigm for combination therapy in solid cancers. Cancer Res, 2007, 67, 2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dunn SE; Ehrlich M; Sharp NJ; Reiss K; Solomon G; Hawkins R; Baserga R; Barrett JC A dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res, 1998, 58, 3353–3361. [PubMed] [Google Scholar]
- [27].Albanese A; Lam AK; Sykes EA; Rocheleau JV; Chan WCW Tumour-on-a-chip provides an optical window into nanoparticle tissue transport. Nat. Commun, 2013, 4, 2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Arap W; Pasqualini R; Ruoslahti E Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science, 1998, 279, 377–380. [DOI] [PubMed] [Google Scholar]
- [29].Ferrara N; Kerbel RS Angiogenesis as a therapeutic target. Nature, 2005, 438, 967–974. [DOI] [PubMed] [Google Scholar]
- [30].Carmeliet P; Jain RK Molecular mechanisms and clinical applications of angiogenesis. Nature, 2011, 473, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Carmeliet P; Jain RK Angiogenesis in cancer and other diseases. Nature, 407, 249–257, (2000). [DOI] [PubMed] [Google Scholar]
- [32].Strebhardt K; Ullrich A Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer, 2008, 8, 473–480. [DOI] [PubMed] [Google Scholar]
- [33].Scott AM; Wolchok JD; Old LJ Antibody therapy of cancer. Nat. Rev. Cancer, 2012, 12, 278–287. [DOI] [PubMed] [Google Scholar]
- [34].Hudziak RM; Lewis GD; Winget M; Fendly BM; Shepard HM; Ullrich A p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol. Cell. Biol, 1989, 9, 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Izumi Y; Xu L; di Tomaso E; Fukumura D; Jain RK Tumor biology - Herceptin acts as an anti-angiogenic cocktail. Nature, 2002, 416, 279–280. [DOI] [PubMed] [Google Scholar]
- [36].Aboud-Pirak E; Hurwitz E; Pirak ME; Bellot F; Schlessinger J; Sela M Efficacy of antibodies to epidermal growth factor receptor against KB carcinoma in vitro and in nude mice. J. Nat. Cancer Inst, 1988, 80, 1605–1611. [DOI] [PubMed] [Google Scholar]
- [37].Perrotte P; Matsumoto T; Inoue K; Kuniyasu H; Eve BY; Hicklin DJ; Radinsky R; Dinney CP Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin. Cancer Res, 1999, 5, 257–265. [PubMed] [Google Scholar]
- [38].Nuti M, Bellati F; Visconti V; Napoletano C; Domenici L; Caccetta J; Zizzari IG; Ruscito I; Rahimi H; Benedetti-Panici P; Rughetti A immune effects of trastuzumab. J. Cancer, 2011, 2, 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wu AM; Senter PD Arming antibodies: prospects and challenges for immunoconjugates. Nat. Biotechnol, 2005, 23, 1137–1146. [DOI] [PubMed] [Google Scholar]
- [40].Webber MJ; Berns EJ; Stupp SI Supramolecular nanofibers of peptide amphiphiles for medicine. Israel J. Chem, 2013, 53, 530–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stupp SI Self-assembly and biomaterials. Nano Lett, 2010, 10, 4783–4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pollaro L; Heinis C Strategies to prolong the plasma residence time of peptide drugs. Med. Chem. Comm, 2010, 1, 319–324. [Google Scholar]
- [43].Ruoslahti E Peptides as targeting elements and tissue penetration devices for nanoparticles. Advan. Mat, 2012, 24, 3747–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Teesalu T; Sugahara KN; Ruoslahti E Mapping of vascular zip codes by phage display. Methods Enzymol, 2012, 503, 35–56. [DOI] [PubMed] [Google Scholar]
- [45].Pasqualini R; Ruoslahti E Organ targeting in vivo using phage display peptide libraries. Nature, 1996, 380, 364–366. [DOI] [PubMed] [Google Scholar]
- [46].Winter G; Griffiths AD; Hawkins RE; Hoogenboom HR Making antibodies by phage display technology. Ann. Rev. Immunol, 1994, 12, 433–455. [DOI] [PubMed] [Google Scholar]
- [47].Ladner RC; Sato AK; Gorzelany J; de Souza M Phage display-derived peptides as a therapeutic alternatives to antibodies. Drug Discov. Today, 2004, 9, 525–529. [DOI] [PubMed] [Google Scholar]
- [48].Saad M; Garbuzenko OB; Ber E; Chandna P; Khandare JJ; Pozharov VP; Minko T Receptor targeted polymers, dendrimers, liposomes: Which nanocarrier is the most efficient for tumor-specific treatment and imaging? J. Control. Release, 2008, 130, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pasqualini R; Moeller BJ; Arap W Leveraging molecular heterogeneity of the vascular endothelium for targeted drug delivery and imaging. Semin. Thrombosis Hemostasis, 2010, 36, 343–351. [DOI] [PubMed] [Google Scholar]
- [50].Li ZJ; Cho CH Peptides as targeting probes against tumor vasculature for diagnosis and drug delivery. J. Transl. Med, 2012, 10, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ruoslahti E RGD and other recognition sequences for integrins. Ann. Rev. Cell Develop. Biol, 12, 697–715. [DOI] [PubMed] [Google Scholar]
- [52].Danhier F; Le Breton A; Preat V RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol. Pharm, 2012, 9, 2961–2973. [DOI] [PubMed] [Google Scholar]
- [53].Meyer A; Auemheimer J; Modlinger A; Kessler H Targeting RGD recognizing integrins: drug development, biomaterial research, tumor imaging and targeting. Curr. Pharm. Design, 2006, 12, 2723–2747. [DOI] [PubMed] [Google Scholar]
- [54].Chen K; Chen XY Integrin targeted delivery of chemotherapeutics. Theranostics, 2011, 1, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Temming K; Schiffelers RM; Molema G; Kok RJ RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resis. Updates, 2005, 8, 381–402. [DOI] [PubMed] [Google Scholar]
- [56].Liu S Radiolabeled cyclic RGD peptides as integrin alpha(v) beta(3)-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjugate Chem, 2009, 20, 2199–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Thorpe PE Vascular targeting agents as cancer therapeutics. Clin. Cancer Res, 2004, 10, 415–427. [DOI] [PubMed] [Google Scholar]
- [58].Matsuo AL; Tanaka AS; Juliano MA; Rodrigues EG; Travassos LR A novel melanoma-targeting peptide screened by phage display exhibits antitumor activity. J. Mol. Med, 2010, 88, 1255–1264. [DOI] [PubMed] [Google Scholar]
- [59].Matsuo AL; Juliano MA; Figueiredo CR; Batista WL; Tanaka AS; Travassos LR A new phage-display tumor-homing peptide fused to antiangiogenic peptide generates a novel bioactive molecule with antimelanoma activity. Mol. Cancer Res, 2011, 9, 1471–1478. [DOI] [PubMed] [Google Scholar]
- [60].Lammers T; Aime S; Hennink WE; Storm G; Kiessling F Theranostic nanomedicine. Acc. Chem. Res, 2011, 44, 1029–1038. [DOI] [PubMed] [Google Scholar]
- [61].Chlenski A; Guerrero LJ; Peddinti R; Spitz JA; Leonhardt PT; Yang Q; Tian Y; Salwen HR; Cohn SL Anti-angiogenic SPARC peptides inhibit progression of neuroblastoma tumors. Mol. Cancer, 2010, 9, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hasselaar P; Sage EH Sparc antagonizes the effect of basic fibroblast growth-factor on the migration of bovine aortic endothelial-cells. J. Cell. Biochem, 1992, 49, 272–283. [DOI] [PubMed] [Google Scholar]
- [63].Stetlerstevenson WG; Aznavoorian S; Liotta LA Tumor-cell interactions with the extracellular-matrix during invasion and metastasis. Ann. Rev. Cell Biol, 1993, 9, 541–573. [DOI] [PubMed] [Google Scholar]
- [64].Kessenbrock K; Plaks V; Werb Z Matrix metalloproteinases: regulators of the tumor microenvironment. Cell, 2010, 141, 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mueller J; Gaertner FC; Blechert B; Janssen KP; Essler M Targeting of tumor blood vessels: a phage-displayed tumor-homing peptide specifically binds to matrix metalloproteinase-2-processed collagen IV and blocks angiogenesis in vivo. Mol. Cancer Res, 2009, 7, 1078–1085. [DOI] [PubMed] [Google Scholar]
- [66].Rundhaug JE Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med, 2005, 9, 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fang JM; Shing Y; Wiederschain D; Yan L; Butterfield C; Jackson G; Harper J; Tamvakopoulos G; Moses MA Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc. Nat. Acad. Sci. U.S.A, 97, 3884–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Breiteneder-Geleff S; Soleiman A; Kowalski H; Horvat R; Amann G; Kriehuber E; Diem K; Weninger W; Tschachler E; Alitalo K; Kerjaschki D Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries - Podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol, 1999, 154, 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Banerji S; Ni J; Wang SX; Clasper S; Su J; Tammi R; Jones M; Jackson DG LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol, 1999, 144, 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Stacker SA; Caesar C; Baldwin ME; Thornton GE; Williams RA; Prevo R; Jackson DG; Nishikawa S; Kubo H; Achen MG VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med, 2001, 7, 186–191. [DOI] [PubMed] [Google Scholar]
- [71].Laakkonen P; Akerman ME; Biliran H; Yang M; Ferrer F; Karpanen T; Hoffman RM; Ruoslahti E Antitumor activity of a homing peptide that targets tumor lymphatics and tumor cells. Proc. Nat. Acad. Sci. U.S.A, 2004, 101, 9381–9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fogal V; Zhang L; Krajewski S; Ruoslahti E Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res, 2008, 68, 7210–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Teesalu T; Sugahara KN; Kotamraju VR; Ruoslahti E C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Nat. Acad. Sci. U.S.A, 2009, 106, 16157–16162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Teesalu T; Sugahara KN; Ruoslahti E Tumor-penetrating peptides. Front. Oncol, 2013, 3, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wool-Lewis RJ; Bates P Endoproteolytic processing of the ebola virus envelope glycoprotein: cleavage is not required for function. J. Virol, 1999, 73, 1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sanchez AJ; Vincent MJ; Erickson BR; Nichol ST Crimean-Congo hemorrhagic fever virus glycoprotein precursor is cleaved by furin-like and SKI-1 proteases to generate a novel 38-kilodalton glycoprotein. J. Virol, 2006, 80, 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Molloy SS; Bresnahan PA; Leppla SH; Klimpel KR; Thomas G Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J. Biol. Chem, 1992, 267, 16396–16402. [PubMed] [Google Scholar]
- [78].Tosteson MT; Tosteson DC The sting. melittin forms channels in lipid bilayers. Biophys. J, 1981, 36, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sugahara KN; Teesalu T; Karmali PP; Kotamraju VR; Agemy L; Girard OM; Hanahan D; Mattrey RF; Ruoslahti E Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell, 2009, 16, 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sugahara KN; Teesalu T; Karmali PP; Kotamraju VR; Agemy L; Greenwald DR; Ruoslahti E Co-administration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science, 2010, 328, 1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Alberici L; Roth L; Sugahara KN; Agemy L; Kotamraju VR; Teesalu T; Bordignon C; Traversari C; Rizzardi GP; Ruoslahti E De Novo design of a tumor-penetrating peptide. Cancer Res, 2013, 73, 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Goel S; Duda DG; Xu L; Munn LL; Boucher Y; Fukumura D; Jain RK Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev, 2011, 91, 1071–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Harris AL Hypoxia - A key regulatory factor in tumour growth. Nat. Rev. Cancer, 2002, 2, 38–47. [DOI] [PubMed] [Google Scholar]
- [84].Vaupel P; Mayer A Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev, 2007, 26, 225–239. [DOI] [PubMed] [Google Scholar]
- [85].Jang SH; Wientjes MG; Au JLS Enhancement of paclitaxel delivery to solid tumors by apoptosis-inducing pretreatment: Effect of treatment schedule. J. Pharm. Exp. Therap, 2001, 296, 1035–1042. [PubMed] [Google Scholar]
- [86].Tannock IF; Lee CM; Tunggal JK; Cowan DSM; Egorin MJ Limited penetration of anticancer drugs through tumor tissue: A potential cause of resistance of solid tumors to chemotherapy. Clin. Cancer Res, 2002, 8, 878–884. [PubMed] [Google Scholar]
- [87].Koivunen E; Wang BC; Ruoslahti E Phage libraries displaying cyclic-peptides with different ring sizes-ligand specificities of the Rgd-directed integrins. Bio-Technology, 1995, 13, 265–270. [DOI] [PubMed] [Google Scholar]
- [88].Brown KC Peptidic tumor targeting agents: the road from phage display peptide selections to clinical applications. Curr. Pharm. Design, 2010, 16, 1040–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Assa-Munt N; Jia X; Laakkonen P; Ruoslahti E Solution structures and integrin binding activities of an RGD peptide with two isomers. Biochem., 2001, 40, 2373–2378. [DOI] [PubMed] [Google Scholar]
- [90].Pasqualini R; Koivunen E; Kain R; Lahdenranta J; Sakamoto M; Stryhn A; Ashmun RA; Shapiro LH; Arap W; Ruoslahti E Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res, 2000, 60, 722–727. [PMC free article] [PubMed] [Google Scholar]
- [91].Burg MA; Pasqualini R; Arap W; Ruoslahti E; Stallcup WB NG2 proteoglycan-binding peptides target tumor neovasculature. Cancer Res,1999, 59, 2869–2874. [PubMed] [Google Scholar]
- [92].Kennel SJ; Mirzadeh S; Hurst GB; Foote LJ; Lankford TK; Glowienka KA; Chappell LL; Kelso JR; Davern SM; Safavy A; Brechbiel MW Labeling and distribution of linear peptides identified using in vivo phage display selection for tumors. Nucl. Med. Biol, 2000, 27, 815–825. [DOI] [PubMed] [Google Scholar]
- [93].Arap W; Haedicke W; Bernasconi M; Kain R; Rajotte D; Krajewski S; Ellerby HM; Bredesen DE; Pasqualini R; Ruoslahti E Targeting the prostate for destruction through a vascular address. Proc. Nat. Acad. Sci. U.S.A, 2002, 99, 1527–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Essler M; Ruoslahti E Molecular specialization of breast vasculature: A breast-homing phage-displayed peptide binds to aminopeptidase P in breast vasculature. Proc. Nat. Acad. Sci. U.S.A, 2002, 99, 2252–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Binetruy-Tournaire R; Demangel C; Malavaud B; Vassy R; Rouyre S; Kraemer M; Plouët J; Derbin C; Perret G; Mazié JC Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J, 2000, 19, 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bae DG; Gho YS; Yoon WH; Chae CB Arginine-rich anti-vascular endothelial growth factor peptides inhibit tumor growth and metastasis by blocking angiogenesis. J. Biol. Chem, 2000, 275, 13588–13596. [DOI] [PubMed] [Google Scholar]
- [97].Asai T; Nagatsuka M; Kuromi K; Yamakawa S; Kurohane K; Ogino K; Tanaka M; Taki T; Oku N Suppression of tumor growth by novel peptides homing to tumor-derived new blood vessels. FEBS Lett, 2002, 510, 206–210. [DOI] [PubMed] [Google Scholar]
- [98].Koivunen E; Arap W; Valtanen H; Rainisalo A; Medina OP; Heikkilä P; Kantor C; Gahmberg CG; Salo T; Konttinen YT; Sorsa T; Ruoslahti E; Pasqualini R Tumor targeting with a selective gelatinase inhibitor. Nat. Biotechnol, 1999, 17, 768–774. [DOI] [PubMed] [Google Scholar]
- [99].Curnis F; Sacchi A; Gasparri A; Longhi R; Bachi A; Doglioni C; Bordignon C; Traversari C; Rizzardi GP; Corti A Isoaspartate-glycine-arginine: A new tumor vasculature-targeting motif. Cancer Res, 2008, 68, 7073–7082. [DOI] [PubMed] [Google Scholar]
- [100].Mingozzi M; Dal Corso A; Marchini M; Guzzetti I; Civera M; Piarulli U; Arosio D; Belvisi L; Potenza D; Pignataro L; Gennari C Cyclic isoDGR peptidomimetics as low-nanomolar v3 integrin ligands. Chemistry, 2013, 19, 3563–3567. [DOI] [PubMed] [Google Scholar]
- [101].Li ZJ; Wu WK; Ng SS; Yu L; Li HT; Wong CC; Wu YC; Zhang L; Ren SX; Sun XG; Chan KM; Cho CH A novel peptide specifically targeting the vasculature of orthotopic colorectal cancer for imaging detection and drug delivery. J. Control. Rel, 2010, 148, 292–302. [DOI] [PubMed] [Google Scholar]
- [102].Hoffman JA; Giraudo E; Singh M; Zhang L; Inoue M; Porkka K; Hanahan D; Ruoslahti E Progressive vascular changes in a transgenic mouse model of squamous cell carcinoma. Cancer Cell, 2003, 4, 383–391. [DOI] [PubMed] [Google Scholar]
- [103].Joyce JA; Laakkonen P; Bernasconi M; Bergers G; Ruoslahti E; Hanahan D Stage-specific vascular markers revealed by phage display in a mouse model of pancreatic islet tumorigenesis. Cancer Cell, 2003, 4, 393–403. [DOI] [PubMed] [Google Scholar]
- [104].Renschler MF; Bhatt RR; Dower WJ; Levy R Synthetic peptide ligands of the antigen-binding receptor induce programmed cell-death in a human B-cell lymphoma. Proc. Nat. Acad. Sci. U.S.A, 1994, 91, 3623–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Marchio S; Lahdenranta J; Schlingemann RO; Valdembri D; Wesseling P; Arap MA; Hajitou A; Ozawa MG; Trepel M; Giordano RJ; Nanus DM; Dijkman HB; Oosterwijk E; Sidman RL; Cooper MD; Bussolino F; Pasqualini R; Arap W Aminopeptidase A is a functional target in angiogenic blood vessels. Cancer Cell, 2004, 5, 151–162. [DOI] [PubMed] [Google Scholar]
- [106].Hui XL; Han Y; Liang S; Liu Z; Liu J; Hong L; Zhao L; He L; Cao S; Chen B; Yan K; Jin B; Chai N; Wang J; Wu K; Fan D Specific targeting of the vasculature of gastric cancer by a new tumor-homing peptide CGNSNPKSC. J. Control. Release, 2008, 31, 86–93. [DOI] [PubMed] [Google Scholar]
- [107].Lee TY; Lin CT; Kuo SY; Chang DK & Wu HC Peptide-mediated targeting to tumor blood vessels of lung cancer for drug delivery. Cancer Res, 2007, 67, 10958–10965. [DOI] [PubMed] [Google Scholar]
- [108].Hatakeyama S; Sugihara K; Shibata TK; Nakayama J; Akama TO; Tamura N; Wong SM; Bobkov AA; Takano Y; Ohyama C; Fukuda M; Fukuda MN Targeted drug delivery to tumor vasculature by a carbohydrate mimetic peptide. Proc. Nat. Acad. Sci. U.S.A, 2011, 108, 19587–19592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Laakkonen P; Porkka K; Hoffman JA; Ruoslahti EA tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat. Med, 2002, 8, 751–755. [DOI] [PubMed] [Google Scholar]
- [110].Laakkonen P; Zhang LL; Ruoslahti E Peptide targeting of tumor lymph vessels. Lymp. Continuum Rev, 2008, 1131, 37–43. [DOI] [PubMed] [Google Scholar]
- [111].Zhang LL; Giraudo E; Hoffman JA; Hanahan D; Ruoslahti E Lymphatic zip codes in premalignant lesions and tumors. Cancer Res,2006, 66, 5696–5706. [DOI] [PubMed] [Google Scholar]
- [112].Oflazoglu E; Kissler KM; Sievers EL; Grewal IS; Gerber H-P Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br. J. Haematol, 2008, 142, 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Katz J; Janik JE; Younes A Brentuximab vedotin (SGN-35). Clin. Cancer Res, 2011, 17, 6428–6436. [DOI] [PubMed] [Google Scholar]
- [114].Lewis Phillips GD, Li G; Dugger DL; Crocker LM; Parsons KL; Mai E; Blättler WA; Lambert JM; Chari RV; Lutz RJ; Wong WL; Jacobson FS; Koeppen H; Schwall RH; Kenkare-Mitra SR; Spencer SD; Sliwkowski MX Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res, 2008, 68, 9280–9290. [DOI] [PubMed] [Google Scholar]
- [115].Firer MA; Gellerman G Targeted drug delivery for cancer therapy: the other side of antibodies. J. Hematol. Oncol, 2012, 5, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Harrison GS; Wierman ME; Nett TM; Glode LM Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocr. Relat. Cancer, 2004, 11, 725–748. [DOI] [PubMed] [Google Scholar]
- [117].Halmos G; Arencibia JM; Schally AV; Davis R; Bostwick DG High incidence of receptors for luteinizing hormone-releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. J. Urol, 2000, 163, 623–629. [PubMed] [Google Scholar]
- [118].Heidenreich A; Pfister D; Merseburger A; Bartsch G; Gwg-Crpc. Castration-resistant prostate cancer: where we stand in 2013 and what urologists should know. Eur. Urol, 2013, 64, 260–265. [DOI] [PubMed] [Google Scholar]
- [119].Garcia JA; Rini BI Castration-resistant prostate cancer: Many treatments, many options, many challenges ahead. Cancer, 2012, 118, 2583–2593. [DOI] [PubMed] [Google Scholar]
- [120].Liu SV; Liu SS; Pinski J Luteinizing hormone-releasing hormone receptor targeted agents for prostate cancer. Exp. Opin. Invest. Drugs, 20, 769–778. [DOI] [PubMed] [Google Scholar]
- [121].Schally AV; Engel JB; Emons G; Block NL; Pinski J Use of analogs of peptide hormones conjugated to cytotoxic radicals for chemotherapy targeted to receptors on tumors. Curr. Drug Deliv, 2011, 8, 11–25. [DOI] [PubMed] [Google Scholar]
- [122].Dharap SS; Wang Y; Chandna P; Khandare JJ; Qiu B; Gunaseelan S; Sinko PJ; Stein S; Farmanfarmaian A; Minko T Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc. Nat. Acad. Sci U.S.A, 2005, 102, 12962–12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Liu SV; Tsao-Wei DD; Xiong S; Groshen S; Dorff TB; Quinn DI; Tai YC; Engel J; Hawes D; Schally AV; Pinski JK Phase I, dose-escalation study of the targeted cytotoxic LHRH analog AEZS-108 in patients with castration- and taxane-resistant prostate cancer. Clin. Cancer Res, 2014, 20, 6277–6283. [DOI] [PubMed] [Google Scholar]
- [124].Letsch M; Schally AV; Szepeshazi K; Halmos G; Nagy A Preclinical evaluation of targeted cytotoxic luteinizing hormone-releasing hormone analogue AN-152 in androgen-sensitive and insensitive prostate cancers. Clin. Cancer Res, 2003, 9, 4505–4513. [PubMed] [Google Scholar]
- [125].Engel J; Emons G; Pinski J; Schally AV AEZS-108 : a targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors. Exp. Opin. Invest. Drugs, 2012, 21, 891–899. [DOI] [PubMed] [Google Scholar]
- [126].Popovics P; Schally AV; Szalontay L; Block NL; Rick FG Targeted cytotoxic analog of luteinizing hormone-releasing hormone (LHRH), AEZS-108 (AN-152), inhibits the growth of DU-145 human castration-resistant prostate cancer in vivo and in vitro through elevating p21 and ROS levels. Oncotarget., 2014, 5, 4567–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Speelmans G; Staffhorst RWHM; Dekruijff B; Dewolf FA Transport studies of doxorubicin in model membranes indicate a difference in passive diffusion across and binding at the outer and inner leaflets of the plasma-membrane. Biochem, 1994, 33, 13761–13768. [DOI] [PubMed] [Google Scholar]
- [128].Tai WY; Mahato R; Cheng K The role of HER2 in cancer therapy and targeted drug delivery. J. Control. Release, 2010, 146, 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Slamon DJ; Clark GM; Wong SG; Levin WJ; Ullrich A; McGuire WL Human-breast cancer-correlation of relapse and survival with amplification of the Her-2 neu oncogenE. Science, 1987, 235, 177–182. [DOI] [PubMed] [Google Scholar]
- [130].Alimandi M; Romano A; Curia MC; Muraro R; Fedi P; Aaronson SA; Di Fiore PP; Kraus MH Cooperative signaling of Erbb3 and Erbb2 in neoplastic transformation and human mammary carcinomas. Oncogene, 1995, 10, 1813–1821. [PubMed] [Google Scholar]
- [131].Yang G; Cai KQ; Thompson-Lanza JA; Bast RC; Liu JS Inhibition of breast and ovarian tumor growth through multiple signaling pathways by using retrovirus-mediated small interfering RNA against Her-2/neu gene expression. J. Biol. Chem, 2004, 279, 4339–4345. [DOI] [PubMed] [Google Scholar]
- [132].Rubin I; Yarden Y The basic biology of HER2. Ann. Oncol, 2001, 12, S3–8. [DOI] [PubMed] [Google Scholar]
- [133].Vogel CL et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol, 2002, 20, 719–726. [DOI] [PubMed] [Google Scholar]
- [134].Chames P; Van Regenmortel M; Weiss E; Baty D Therapeutic antibodies: successes, limitations and hopes for the future. Br. J. Pharm, 2009, 157, 220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Park BW; Zhang HT; Wu C; Berezov A; Zhang X; Dua R; Wang Q; Kao G; O’Rourke DM; Greene MI; Murali R Rationally designed anti-HER2/neu peptide mimetic disables P185HER2/neu tyrosine kinases in vitro and in vivo. Nat. Biotechnol, 2000, 18, 194–198. [DOI] [PubMed] [Google Scholar]
- [136].Guillemard V; Nedev HN; Berezov A; Murali R; Saragovi HU HER2-Mediated internalization of a targeted prodrug cytotoxic conjugate is dependent on the valency of the targeting ligand. DNA Cell Biol, 2005, 24, 350–358. [DOI] [PubMed] [Google Scholar]
- [137].Fantin VR; Berardi MJ; Babbe H; Michelman MV; Manning CM; Leder P A bifunctional targeted peptide that blocks HER-2 tyrosine kinase and disables mitochondrial function in HER-2-positive carcinoma cells. Cancer Res, 2005, 65, 6891–6900. [DOI] [PubMed] [Google Scholar]
- [138].Witt H, Hajdin K, Iljin K, Greiner O, Niggli FK, Schäfer BW, Bernasconi M Identification of a rhabdomyosarcoma targeting peptide by phage display with sequence similarities to the tumour lymphatic-homing peptide LyP-1. Int. J. Cancer, 2009, 124(9), 2026–2032. [DOI] [PubMed] [Google Scholar]
- [139].Wang XF; Birringer M; Dong LF; Veprek P; Low P; Swettenham E; Stantic M; Yuan LH; Zobalova R; Wu K; Ledvina M; Ralph SJ; Neuzil J A peptide conjugate of vitamin E succinate targets breast cancer cells with high ErbB2 expression. Cancer Res, 2007, 67, 3337–3344. [DOI] [PubMed] [Google Scholar]
- [140].Reichlin S Somatostatin 1. N. Engl. J. Med, 1983, 309, 1495–1501. [DOI] [PubMed] [Google Scholar]
- [141].Patel YC Somatostatin and its receptor family. Front. Neuroendocrinol, 1999, 20, 157–198. [DOI] [PubMed] [Google Scholar]
- [142].Volante M; Rosas R; Allìa E; Granata R; Baragli A; Muccioli G; Papotti M Somatostatin, cortistatin and their receptors in tumours. Mol. Cell. Endocr, 2008, 286, 219–229. [DOI] [PubMed] [Google Scholar]
- [143].Sun LC; Coy DH Somatostatin receptor-targeted anti-cancer therapy. Curr. Drug Deliv, 2011, 8, 2–10. [DOI] [PubMed] [Google Scholar]
- [144].Shimon I; Yan X; Taylor JE; Weiss MH; Culler MD; Melmed S Somatostatin receptor (SSTR) subtype-selective analogues differentially suppress in vitro growth hormone and prolactin in human pituitary adenomas - Novel potential therapy for functional pituitary tumors. J. Clin. Invest, 1997, 100, 2386–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Weckbecker G; Raulf F; Stolz B; Bruns C Somatostatin analogs for diagnosis and treatment of cancer. Pharmacol. Therap, 1993, 60, 245–264. [DOI] [PubMed] [Google Scholar]
- [146].Sideris L; Dube P; Rinke A Antitumor effects of somatostatin analogs in neuroendocrine tumors. Oncologist, 2012, 17, 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Schally AV; Szepeshazi K; Nagy A; Comaru-Schally AM; Halmos G New approaches to therapy of cancers of the stomach, colon and pancreas based on peptide analogs. Cell. Mol. Life Sci, 2004, 61, 1042–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Shen H; Hu D; Du J; Wang X; Liu Y; Wang Y; Wei JM; Ma D; Wang P; Li L Paclitaxel-octreotide conjugates in tumor growth inhibition of A549 human non-small cell lung cancer xenografted into nude mice. Eur. J. Pharmacol, 2008, 601, 23–29. [DOI] [PubMed] [Google Scholar]
- [149].Li-Chun Sun L, Vienna Mackey, Jing Luo, Fuselier Joseph A. & Coy DH Targeted chemotherapy using a cytotoxic somatostatin conjugate to inhibit tumor growth and metastasis in nude mice. Clin. Med.: Oncol, 2008, 2, 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kwekkeboom DJ; de Herder WW; Kam BL; van Eijck CH; van Essen M; Kooij PP; Feelders RA; van Aken MO; Krenning EP Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J. Clin. Oncol, 2008, 26, 2124–2130. [DOI] [PubMed] [Google Scholar]
- [151].Kwekkeboom DJ; Teunissen JJ; Bakker WH; Kooij PP; de Herder WW; Feelders RA; van Eijck CH; Esser JP; Kam BL; Krenning EP Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J. Clin. Oncol, 2005, 23, 2754–2762. [DOI] [PubMed] [Google Scholar]
- [152].Eldar-Finkelman H; Eisenstein M Peptide inhibitors targeting protein kinases. Curr. Pharm. Design, 2009, 15, 2463–2470. [DOI] [PubMed] [Google Scholar]
- [153].Nicolas P Multifunctional host defense peptides: intracellular-targeting antimicrobial peptides. FEBS J, 2009, 276, 6483–6496. [DOI] [PubMed] [Google Scholar]
- [154].Aina OH; Sroka TC; Chen ML; Lam KS Therapeutic cancer targeting peptides. Biopolymers, 2002, 66, 184–199. [DOI] [PubMed] [Google Scholar]
- [155].Buhl L; Szecsi PB; Gisselo GG; Schafer-Nielsen C Surface immunoglobulin on B lymphocytes as a potential target for specific peptide ligands in chronic lymphocytic leukaemia. Br. J. Haematol, 2002, 116, 549–554. [DOI] [PubMed] [Google Scholar]
- [156].Fukuda MN; Ohyama C; Lowitz K; Matsuo O; Pasqualini R; Ruoslahti E; Fukuda M A peptide mimic of E-selectin ligand inhibits sialyl Lewis X-dependent lung colonization of tumor cells. Cancer Res, 2000, 60, 450–456. [PubMed] [Google Scholar]
- [157].McGuire MJ; Samli KN; Chang YC; Brown KC Novel ligands for cancer diagnosis: Selection of peptide ligands for identification and isolation of B-cell lymphomas. Exp. Hematol, 2006, 34, 443–452. [DOI] [PubMed] [Google Scholar]
- [158].Nishimura S; Takahashi S; Kamikatahira H; Kuroki Y; Jaalouk DE; O’Brien S; Koivunen E; Arap W; Pasqualini R; Nakayama H; Kuniyasu A Combinatorial targeting of the macropinocytotic pathway in leukemia and lymphoma cells. J. Biol. Chem, 2008, 283, 11752–11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Szecsi PB; Riise E; Roslund LB; Engberg J; Turesson I; Buhl L; Schafer-Nielsen C Identification of patient-specific peptides for detection of M-proteins and myeloma cells. Br. J. Haematol, 1999, 107, 357–364. [DOI] [PubMed] [Google Scholar]
- [160].Wu P; Leinonen J; Koivunen E; Lankinen H; Stenman UH Identification of novel prostate-specific antigen-binding peptides modulating its enzyme activity. Eur. J. Biochem, 2000, 267, 6212–6220. [DOI] [PubMed] [Google Scholar]
- [161].Romanov VI; Durand DB; Petrenko VA Phage display selection of peptides that affect prostate carcinoma cells attachment and invasion. Prostate, 2001, 47, 239–251. [DOI] [PubMed] [Google Scholar]
- [162].Huang CH; Liu XY; Rehemtulla A; Lawrence TS Identification of peptides that bind to irradiated pancreatic tumor cells. Int. J. Radiat. Oncol. Biol. Physics, 2005, 62, 1497–1503. [DOI] [PubMed] [Google Scholar]
- [163].Zitzmann S; Mier W; Schad A; Kinscherf R; Askoxylakis V; Krämer S; Altmann A; Eisenhut M; Haberkorn U New prostate carcinoma binding peptide (DUP-1) for tumor imaging and therapy. Clin. Cancer Res, 2005, 11, 139–146. [PubMed] [Google Scholar]
- [164].Newton JR; Kelly KA; Mahmood U; Weissleder R; Deutscher SL In vivo selection of phage for the optical Imaging of PC-3 human prostate carcinoma in mice. Neoplasia, 2006, 8, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Zhang JB; Spring H; Schwab M Neuroblastoma tumor cell-binding peptides identified through random peptide phage display. Cancer Lett, 2001, 171, 153–164. [DOI] [PubMed] [Google Scholar]
- [166].Rasmussen UB; Schreiber V; Schultz H; Mischler F; Schughart K Tumor cell-targeting by phage-displayed peptides. Cancer Gene Ther, 2002, 9, 606–612. [DOI] [PubMed] [Google Scholar]
- [167].Hong FD; Clayman GL Isolation of a peptide for targeted drug delivery into human head and neck solid tumors. Cancer Res, 2000, 60, 6551–6556. [PubMed] [Google Scholar]
- [168].Ivanenkov VV; Felici F; Menon AG Targeted delivery of multivalent phage display vectors into mammalian cells. Biochimica Et Biophysica Acta-Mol. Cell Res, 1999, 1448, 463–472. [DOI] [PubMed] [Google Scholar]
- [169].Lee TY; Wu HC; Tseng YL; Lin CT A novel peptide specifically binding to nasopharyngeal carcinoma for targeted drug delivery. Cancer Res, 2004, 64, 8002–8008. [DOI] [PubMed] [Google Scholar]
- [170].Sun L; Chu TW; Wang Y & Wang XG Radiolabeling and biodistribution of a nasopharyngeal carcinoma-targeting peptide identified by in vivo phage display. Acta. Biochimica. Et Biophysica Sinica, 2007, 39, 624–632. [DOI] [PubMed] [Google Scholar]
- [171].Du B; Qian M; Zhou Z; Wang P; Wang L; Zhang X; Wu M; Zhang P; Mei B In vitro panning of a targeting peptide to hepatocarcinoma from a phage display peptide library. Biochem. Biophys. Res. Commun, 2006, 342, 956–962. [DOI] [PubMed] [Google Scholar]
- [172].Jiang YQ; Wang HR; Li HP; Hao HJ; Zheng YL; Gu J Targeting of hepatoma cell and suppression of tumor growth by a novel 12mer peptide fused to superantigen TSST-1. Mol. Med, 2006, 12, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Lo A; Lin CT; Wu HC Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. Mol. Cancer Therap, 2008, 7, 579–589. [DOI] [PubMed] [Google Scholar]
- [174].Kim Y; Lillo AM; Steiniger SC; Liu Y; Ballatore C; Anichini A; Mortarini R; Kaufmann GF; Zhou B; Felding-Habermann B; Janda KD Targeting heat shock proteins on cancer cells: Selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand. Biochemistry, 2006, 45, 9434–9444. [DOI] [PubMed] [Google Scholar]
- [175].Eriksson F; Culp WD; Massey R; Egevad L; Garland D; Persson MA; Pisa P Tumor specific phage particles promote tumor regression in a mouse melanoma model. Cancer Immunol. Immunother, 2007, 56, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Hu SJ; Guo X; Xie H; Du Y; Pan Y; Shi Y; Wang J; Hong L; Han S; Zhang D; Huang D; Zhang K; Bai F; Jiang H; Zhai H; Nie Y; Wu K; Fan D Phage display selection of peptides that inhibit metastasis ability of gastric cancer cells with high liver-metastatic potential. Biochem. Biophys. Res. Commun, 2006, 341, 964–972. [DOI] [PubMed] [Google Scholar]
- [177].Bai FH; Liang J; Wang J; Shi Y; Zhang K; Liang S; Hong L; Zhai H; Lu Y; Han Y; Yin F; Wu K; Fan D Inhibitory effec ts of a specific phage-displayed peptide on high peritoneal metastasis of gastric cancer. J. Mol. Med, 2007, 85, 169–180. [DOI] [PubMed] [Google Scholar]
- [178].Akita N; Maruta F; Seymour LW; Kerr DJ; Parker AL; Asai T; Oku N; Nakayama J; Miyagawa S Identification of oligopeptides binding to peritoneal tumors of gastric cancer. Cancer Sci, 2006, 97, 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Zhi M; Wu KC; Dong L; Hao ZM; Deng TZ; Hong L; Liang SH; Zhao PT; Qiao TD; Wang Y; Xu X; Fan DM Characterization of a specific phage-displayed peptide binding to vasculature of human gastric cancer. Cancer Biol. Ther, 2004, 3, 1232–1235. [DOI] [PubMed] [Google Scholar]
- [180].Kelly KA; Jones DA Isolation of a colon tumor specific binding peptide using phage display selection. Neoplasia, 2003, 5, 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Hsiung PL; Hardy J; Friedland S; Soetikno R; Du CB; Wu AP; Sahbaie P; Crawford JM; Lowe AW; Contag CH; Wang TD Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat. Med, 2008, 14, 585–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Kubo N; Akita N; Shimizu A; Kitahara H; Parker AL; Miyagawa S Identification of oligopeptide binding to colon cancer cells separated from patients using laser capture microdissection. J. Drug Target, 2008, 16, 396–404. [DOI] [PubMed] [Google Scholar]
- [183].Zhang YD; Chen J; Zhang Y; Hu Z; Hu D; Pan Y; Ou S; Liu G; Yin X; Zhao J; Ren L; Wang J Panning and identification of a colon tumor binding peptide from a phage display peptide library. J. Biomol. Screening, 2007, 12, 429–435. [DOI] [PubMed] [Google Scholar]
- [184].Samoylova TI; Petrenko VA; Morrison NE; Globa LP; Baker HJ; Cox NR Phage probes for malignant glial cells. Mol. Cancer Ther, 2003, 2, 1129–1137. [PubMed] [Google Scholar]
- [185].Spear MA; Breakefield XO; Beltzer J; Schuback D; Weissleder R; Pardo FS; Ladner R Isolation, characterization, and recovery of small peptide phage display epitopes selected against viable malignant glioma cells. Cancer Gene Ther, 2001, 8, 506–511. [DOI] [PubMed] [Google Scholar]
- [186].Robinson P; Stuber D; Deryckère F; Tedbury P; Lagrange M; Orfanoudakis G Identification using phage display of peptides promoting targeting and internalization into HPV-transformed cell lines. J. Mol. Recog, 2005, 18, 175–182. [DOI] [PubMed] [Google Scholar]
- [187].Bockmann M; Drosten M; Putzer BM Discovery of targeting peptides for selective therapy of medullary thyroid carcinoma. J. Gene Med, 2005, 7, 179–188. [DOI] [PubMed] [Google Scholar]
- [188].Bockmann M; Hilken G; Schmidt A; Cranston AN; Tannapfel A; Drosten M; Frilling A; Ponder BA; Pützer BM Novel SRESPHP peptide mediates specific binding to primary medullary thyroid carcinoma after systemic injection. Human Gene Ther, 2005, 16, 1267–1275. [DOI] [PubMed] [Google Scholar]
- [189].Jager S; Jahnke A; Wilmes T; Adebahr S; Vögtle FN; Delima-Hahn E; Pfeifer D; Berg T; Lübbert M; Trepel M Leukemia targeting ligands isolated from phage display peptide libraries. Leukemia, 2007, 21, 411–420. [DOI] [PubMed] [Google Scholar]
- [190].Porkka K; Laakkonen P; Hoffman JA; Bernasconi M; Ruoslahti E A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc. Nat. Acad. Sci. U.S.A, 2002, 99, 7444–7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Oyama T; Sykes KF; Samli KN; Minna JD; Johnston SA; Brown KC Isolation of lung tumor specific peptides from a random peptide library: generation of diagnostic and cell-targeting reagents. Cancer Lett, 2003, 202, 219–230. [DOI] [PubMed] [Google Scholar]
- [192].Oyama T; Rombel IT; Samli KN; Zhou X; Brown KC Isolation of multiple cell-binding ligands from different phage displayed-peptide libraries. Biosensors Bioelect, 2006, 21, 1867–1875. [DOI] [PubMed] [Google Scholar]
- [193].Zang LQ; Shi L; Guo J; Pan Q; Wu W; Pan X; Wang J Screening and Identification of a peptide specifically targeted to NCI-H1299 from a phage display peptide library. Cancer Lett, 2009, 281, 64–70. [DOI] [PubMed] [Google Scholar]
- [194].Chang DK; Lin CT; Wu CH; Wu HC A novel peptide enhances therapeutic efficacy of liposomal anti-cancer drugs in mice models of human lung cancer. PLoS One, 2009, 4, e4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Han Z; Fu A; Wang H; Diaz R; Geng L; Onishko H; Hallahan DE Noninvasive assessment of cancer response to therapy. Nat. Med, 2008, 14, 343–349. [DOI] [PubMed] [Google Scholar]
- [196].Lee SM; Lee EJ; Hong HY; Kwon MK; Kwon TH, Choi JY; Park RW; Kwon TG; Yoo ES; Yoon GS; Kim IS; Ruoslahti E; Lee BH Targeting bladder tumor cells in vivo and in the urine with a peptide identified by phage display. Mol. Cancer Res, 2007, 5, 11–19. [DOI] [PubMed] [Google Scholar]
- [197].Kelly KA; Bardeesy N; Anbazhagan R; Gurumurthy S; Berger J; Alencar H; Depinho RA; Mahmood U; Weissleder R Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med, 2008, 5, 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Chang DK; Chiu CY; Kuo SY; Lin WC; Lo A; Wang YP; Li PC; Wu HC Antiangiogenic targeting liposomes increase therapeutic efficacy for solid tumors. J. Biol. Chem, 2009, 284, 12905–12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].van Tellingen O; Yetkin-Arik B; de Gooijer MC.; Wesseling P; Wurdinger T; de Vries HE Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Update, 2015, 19, 1–12. [DOI] [PubMed] [Google Scholar]
- [200].Deeken JF; Loscher W The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin. Cancer Res, 2007, 13, 1663–1674. [DOI] [PubMed] [Google Scholar]
- [201].Siegel R; Ma J; Zou Z; Jemal A Cancer statistics, 2014. Cancer J. Clin, 2014, 64, 9–29. [DOI] [PubMed] [Google Scholar]
- [202].Ohgaki H; Dessen P; Jourde B; Horstmann S; Nishikawa T; Di Patre PL; Burkhard C; Schüler D; Probst-Hensch NM; Maiorka PC; Baeza N; Pisani P;Yonekawa Y; Yasargil MG; Lütolf UM; Kleihues P Genetic pathways to glioblastoma: A population-based study. Cancer Res, 2004, 64, 6892–6899. [DOI] [PubMed] [Google Scholar]
- [203].Begley DJ; Brightman MW Structural and functional aspects of the blood-brain barrier. Progress in drug research. Fortschritte der Arzneimittelforschung. Progres des Recherches Pharmaceutiques, 2003, 61, 39–78. [DOI] [PubMed] [Google Scholar]
- [204].Schinkel AH; Wagenaar E; Mol CA; van Deemter L P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Invest, 1996, 97, 2517–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Gilbertson RJ; Rich JN Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat. Rev. Cancer, 2007, 7, 733–736. [DOI] [PubMed] [Google Scholar]
- [206].Vannucci SJ; Maher F; Simpson IA Glucose transporter proteins in brain: Delivery of glucose to neurons and glia. Glia, 1997, 21, 2–21. [DOI] [PubMed] [Google Scholar]
- [207].Demeule M; Régina A; Ché C; Poirier J; Nguyen T; Gabathuler R; Castaigne JP; Béliveau R Identification and design of peptides as a new drug delivery system for the brain. J. Pharmaco. Exp. Therap, 2008, 324, 1064–1072. [DOI] [PubMed] [Google Scholar]
- [208].Régina A; Demeule M; Ché C; Lavallée I; Poirier J; Gabathuler R; Béliveau R; Castaigne JP Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br. J. Pharmacol, 2008, 155, 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Drappatz J; Brenner A; Wong ET; Eichler A; Schiff D; Groves MD; Mikkelsen T; Rosenfeld S; Sarantopoulos J; Meyers CA; Fielding RM; Elian K; Wang X; Lawrence B; Shing M; Kelsey S; Castaigne JP; Wen PY Phase I study of GRN1005 in recurrent malignant glioma. Clin. Cancer Res, 2013, 19, 1567–1576. [DOI] [PubMed] [Google Scholar]
- [210].Xin H; Jiang X; Gu J; Sha X; Chen L; Law K; Chen Y; Wang X; Jiang Y; Fang X Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials, 2011, 32, 4293–4305. [DOI] [PubMed] [Google Scholar]
- [211].Moos T; Morgan EH Transferrin and transferrin receptor function in brain barrier systems. Cell. Mol. Neurobiol, 2000, 20, 77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Zhang P; Hu L; Yin Q; Feng L; Li Y Transferrin-modified c[RGDfK]-paclitaxel loaded hybrid micelle for sequential blood-brain barrier penetration and glioma targeting therapy. Mol. Pharm, 2012, 9, 1590–1598. [DOI] [PubMed] [Google Scholar]
- [213].MacDonald TJ; Taga T; Shimada H; Tabrizi P; Zlokovic BV; Cheresh DA; Laug WE Preferential susceptibility of brain tumors to the antiangiogenic effects of an [alpha]v Integrin antagonist. Neurosurgery, 2001, 48, 151–157. [DOI] [PubMed] [Google Scholar]
- [214].Lee JH; Engler JA; Collawn JF; Moore BA Receptor mediated uptake of peptides that bind the human transferrin receptor. Eur. J. Biochem, 2001, 268, 2004–2012. [DOI] [PubMed] [Google Scholar]
- [215].Han L; Huang R; Liu S; Huang S; Jiang C Peptide-conjugated PAMAM for targeted doxorubicin delivery to transferrin receptor overexpressed tumors. Mol. Pharm, 2010, 7, 2156–2165. [DOI] [PubMed] [Google Scholar]
- [216].Son S; Hwang do W; Singha K; Jeong JH; Park TG; Lee DS; Kim WJ RVG peptide tethered bioreducible polyethylenimine for gene delivery to brain. J. Control. Release, 2011, 155, 18–25. [DOI] [PubMed] [Google Scholar]
- [217].Maecke HR; Hofmann M; Haberkorn U Ga-68-labeled peptides in tumor imaging. J. Nuclear Med, 2005, 46, 172s–178s. [PubMed] [Google Scholar]
- [218].Okarvi SM Recent progress in fluorine-18 labelled peptide radiopharmaceuticals. Eur. J. Nuclear Med, 2001, 28, 929–938. [DOI] [PubMed] [Google Scholar]
- [219].Nitin N; LaConte LEW; Zurkiya O; Hu X; Bao G Functionalization and peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. J. Biol. Inorganic Chem, 2004, 9, 706–712. [DOI] [PubMed] [Google Scholar]
- [220].Lee HY; Li Z; Chen K; Hsu AR; Xu C; Xie J; Sun S; Chen X PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD) - Conjugated radiolabeled iron oxide nanoparticles. J. Nucl. Med, 2008, 49, 1371–1379. [DOI] [PubMed] [Google Scholar]
- [221].Chan WCW; Nie SM Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science, 1998, 281, 2016–2018. [DOI] [PubMed] [Google Scholar]
- [222].Bruchez M; Moronne M; Gin P; Weiss S; Alivisatos AP Semiconductor nanocrystals as fluorescent biological labels. Science, 1998, 281, 2013–2016. [DOI] [PubMed] [Google Scholar]
- [223].Gao XH; Cui YY; Levenson RM; Chung LWK; Nie SM In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol, 2004, 22, 969–976. [DOI] [PubMed] [Google Scholar]
- [224].Mammen M; Choi SK; Whitesides GM Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angewandte Chemie-Int. Edition, 1998, 37, 2755–2794. [DOI] [PubMed] [Google Scholar]
- [225].Cai WB; Shin DW; Chen K;Gheysens O; Cao Q; Wang SX; Gambhir SS; Chen X Peptide-labeed near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett, 2006, 6, 669–676. [DOI] [PubMed] [Google Scholar]
- [226].Chen Y; Foss CA; Byun Y; Nimmagadda S; Pullambhatla M; Fox JJ; Castanares M; Lupold SE; Babich JW; Mease RC; Pomper MG Radiohalogenated prostate-specific membrane antigen (PSMA)-based ureas as imaging agents for prostate cancer. J. Med. Chem, 2008, 51, 7933–7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Silver DA; Pellicer I; Fair WR; Heston WDW, Cordon CC Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res, 1997, 3, 81–85. [PubMed] [Google Scholar]
- [228].Sweat SD; Pacelli A; Murphy GP; Bostwick DG Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology, 1998, 52, 637–640. [DOI] [PubMed] [Google Scholar]
- [229].Ross JS; Sheehan CE; Fisher HA; Kaufman RP Jr, Kaur P; Gray K; Webb I; Gray GS; Mosher R; Kallakury BV Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin. Cancer Res, 2003, 9, 6357–6362. [PubMed] [Google Scholar]
- [230].Chang SS; Reuter VE; Heston WD; Bander NH; Grauer LS; Gaudin PB Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res, 1999, 59, 3192–3198. [PubMed] [Google Scholar]
- [231].Carter RE; Feldman AR; Coyle JT Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc. Nat. Acad. Sci. U.S.A, 1996, 93, 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Pinto JT; Suffoletto BP; Berzin TM; Qiao CH; Lin S; Tong WP; May F; Mukherjee B; Heston WD Prostate-specific membrane antigen: A novel folate hydrolase in human prostatic carcinoma cells. Clin. Cancer Res, 1996, 2, 1445–1451. [PubMed] [Google Scholar]
- [233].Barinka C; Byun Y; Dusich CL; Banerjee SR; Chen Y; Castanares M; Kozikowski AP; Mease RC; Pomper MG; Lubkowski J Interactions between human glutamate carboxypeptidase II and urea-based inhibitors: structural characterization. J. Med. Chem, 2008, 51, 7737–7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Mease RC; Dusich CL; Foss CA; Ravert HT; Dannals RF; Seidel J; Prideaux A; Fox JJ; Sgouros G; Kozikowski AP; Pomper MG N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine, [18F]DCFBC: a new imaging probe for prostate cancer. Clin. Cancer Res, 2008, 14, 3036–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Foss CA; Mease RC; Fan H; Wang Y; Ravert HT; Dannals RF; Olszewski RT; Heston WD; Kozikowski AP; Pomper MG Radiolabeled small-molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer. Clin. Cancer Res, 2005, 11, 4022–4028. [DOI] [PubMed] [Google Scholar]
- [236].Hillier SM; Maresca KP; Femia FJ; Marquis JC; Foss CA; Nguyen N; Zimmerman CN; Barrett JA; Eckelman WC; Pomper MG; Joyal JL; Babich JW Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res, 2009, 69, 6932–6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Hoffman JM; Gambhir SS Molecular imaging: The vision and opportunity for radiology in the future. Radiology, 2007, 244, 39–47. [DOI] [PubMed] [Google Scholar]
- [238].Elias DR; Thorek DLJ; Chen AK; Czupryna J; Tsourkas A In vivo imaging of cancer biomarkers using activatable molecular probes. Cancer Biomarkers, 2008, 4, 287–305. [DOI] [PubMed] [Google Scholar]
- [239].Zhang P; Cheetham AG; Lock LL; Li Y; Cui H Activatable nanoprobes for biomolecular detection. Curr. Opin. Biotechnol, 2015, 34C, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Lee S; Xie J; Chen XY Activatable molecular probes for cancer imaging. Curr. Topics Med. Chem, 2010, 10, 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Pham W; Choi YD; Weissleder R; Tung CH Developing a peptide-based near-infrared molecular probe for protease sensing. Bioconjugate Chem, 2004, 15, 1403–1407. [DOI] [PubMed] [Google Scholar]
- [242].Lock LL; Cheetham AG; Zhang PC; Cui HG Design and construction of supramolecular nanobeacons for enzyme detection. ACS Nano, 2013, 7, 4924–4932. [DOI] [PubMed] [Google Scholar]
- [243].Duncan R; Cable HC; Lloyd JB; Rejmanova P; Kopecek J Polymers containing enzymatically degradable bonds. 7. Design of oligopeptide side-chains in poly N-(2-hydroypropyl) methacrylamide co-polymers to promote efficient degradation by lysosomal-enzymes. Makromolekulare Chemie-Macromolecular Chem. Phys, 1983, 184, 1997–2008. [Google Scholar]
- [244].Jedeszko C; Sloane BF Cysteine cathepsins in human cancer. Biological Chem, 2004, 385, 1017–1027. [DOI] [PubMed] [Google Scholar]
- [245].Janib SM; Moses AS; MacKay JA Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev, 2010, 62, 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Xie J; Lee S; Chen XY Nanoparticle-based theranostic agents. Adv. Drug Deliv. Revi, 2010, 62, 1064–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Xia YN; Li W; Cobley CM; Chen J; Xia X; Zhang Q; Yang M; Cho EC; Brown PK Gold nanocages: from synthesis to theranostic applications. Acc. Chem. Res, 2011, 44, 914–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Bardhan R; Lal S; Joshi A; Halas NJ Theranostic nanoshells: from probe design to imaging and treatment of cancer. Acc. Chem. Res, 2011, 44, 936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Al-Jamal WT; Kostarelos K Liposomes: From a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res, 2011, 44, 1094–1104. [DOI] [PubMed] [Google Scholar]
- [250].Ho D; Sun XL; Sun SH Monodisperse magnetic nanoparticles for theranostic applications. Acc. Chem. Res, 2011, 44, 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Yang K; Feng LZ; Shi XZ; Liu Z Nano-graphene in biomedicine: theranostic applications. Chem. Soc. Rev, 2013, 42, 530–547. [DOI] [PubMed] [Google Scholar]
- [252].Lee JE; Lee N; Kim T; Kim J; Hyeon T Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc. Chem. Res, 2011, 44, 893–902. [DOI] [PubMed] [Google Scholar]