Abstract
Pseudoviruses are viral particles coated with a heterologous envelope protein, which mediates the entry of pseudoviruses as efficiently as that of the live viruses possessing high pathogenicity and infectivity. Due to the deletion of the envelope protein gene and the absence of pathogenic genes, pseudoviruses have no autonomous replication ability and can infect host cells for only a single cycle. In addition, pseudoviruses have the desired characteristics of high safety, strong operability, and can be easily used to perform rapid throughput detection. Therefore, pseudoviruses are widely employed in the mechanistic investigation of viral infection, the screening and evaluation of monoclonal antibodies and antiviral drugs, and the detection of neutralizing antibody titers in serum after vaccination. In this review, we will discuss the construction of pseudoviruses based on different packaging systems, their current applications especially in the research of SARS-CoV-2, limitations, and further directions.
Keywords: Pseudoviruses, Envelope protein, HIV, VSV, MLV, SARS-CoV-2
1. Introduction
As a commonly used viral tool, pseudoviruses facilitate the study of high-risk and highly pathogenic enveloped viruses that require biosafety level (BSL)‐3 or higher laboratories in a biosafety level (BSL)‐2 environment. Pseudoviruses are usually based on the genome of virus with low biological risk (e.g., murine leukemia virus, MLV and vesicular stomatitis virus, VSV) or modified virus (human immunodeficiency virus, HIV), in which the envelope protein genes required for infecting host cells are replaced by reporter genes that can be used for in vitro detection, such as GFP and luciferase genes. At the same time, plasmids or stably expressed cell lines are used to express the envelope protein of the high-risk virus to be studied. The core genome and envelope proteins derived from two different viruses are assembled in vitro to form a complete pseudovirus particle, which could be secreted into the cell culture supernatant. At this time, the supernatant is collected and can be used to infect the target cells. Thus, the pseudoviruses can simulate the process of live virus infection by using the envelope protein of highly infectious virus (Li et al., 2018).
Due to the characteristics of strong operability, low biological risk, convenient detection, and high sensitivity, pseudoviruses have been widely used in the research of highly pathogenic viruses, such as SARS (Kobinger et al., 2007), MERS (Fan et al., 2018), Ebola (Liu et al., 2017), Influenza (Lu and Jiang, 2013), Chikungunya (Wu et al., 2017), Hantan and Seoul Viruses (Ning et al., 2021), and especially in those newly discovered, high-infectious viruses. For example, during the outbreak of SARS-CoV-2, the research of live virus must be carried out in the biosafety level (BSL) 3 facilities, and mutant live viruses are very difficult to obtain. The pseudoviruses system has greatly promoted the relevant research of the SARS-CoV-2 and plays a significant role in the study of the mechanism of virus binding and recognition with cell receptors, in the screening of specific small molecule drugs, and in the evaluation of monoclonal antibodies and vaccine titers (Salazar-García et al., 2021). In addition, the neutralizing titers of antibodies and sera measured by pseudoviruses were highly correlated with those measured by live viruses (Wright et al., 2008, Zhou et al., 2016). Therefore, this paper summarizes the latest classification and application of pseudoviruses, particularly focusing on the application in SARS-CoV-2 in the past year, and expounds the advantages, disadvantages, and future development of pseudoviruses.
2. Classification of pseudoviruses
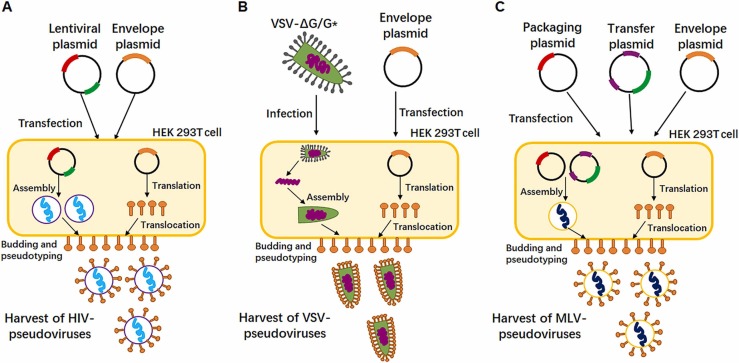
The surface of pseudoviruses can carry envelope proteins from different viruses according to diverse research needs. However, according to the different origin of its core genome, pseudoviruses can be roughly divided into three types, including pseudoviruses with HIV-1 genome as the core, pseudoviruses with VSV genome as the core, and pseudoviruses with MLV genome as the core. Fig. 1A-1C show the basic strategies to generate the SARS-CoV-2 pseudoviruses based on different systems. The packaging methods of pseudoviruses with the three types of viral core genomes are similar, but each has its advantages and disadvantages that are described below.
Fig. 1.
The schematic diagram of acquiring different pseudotyped-viruses based on different packaging systems. (A) HEK 293 T cells were transfected with a plasmid encoding lentiviral backbone and a plasmid expressing envelope protein. The transfected cells produced recombined pseudoviruses and these viral particles could be secreted to extracellular environment before harvesting. (B) HEK 293 T cells were firstly transfected with an envelope protein expression plasmid, twenty-four hours post-transfection, the cells were infected with VSV* ∆G encoding firefly luciferase or GFP. Pseudotyped particles were harvested 20 h post-inoculation. (C) HEK 293 T cells were co-transfected with an envelope protein encoding-plasmid, an MLV Gag-Pol packaging plasmid and the MLV transfer vector encoding a luciferase reporter. The transfected cells produced pseudotyped MLV particles like the HIV systems. Red bar in plasmid represents packaging elements such as gag and pol; green bar in plasmid represents reporter genes, such as GFP and Luciferase; orange bar in plasmid represents envelope protein gene; purple bar in plasmid represents packaging signals, 3’LTR and 5’LTR.
The HIV pseudoviruses system is the most widely used method. The HIV genome contains three structural genes (gag, pol, and env), three regulatory genes (tat, rev, and nef) and four helper genes (vif, vpr, vpu, and vpx) (Wain-Hobson et al., 1985), In order to reduce the chance of homologous recombination in vitro to form complete HIV, the different parts of HIV genome are cloned into different DNA expression vectors, and some dispensable elements of HIV genome, such as nef, are deleted. Meanwhile, the envelope protein gene env will be mutated by frame shift mutation or deletion in order to load the envelope proteins of other viruses. Hence, another plasmid heterologously expressing the envelope protein is required to form pseudoviruses based on HIV. Depending on the number of plasmids used in the system, the HIV pseudoviruses system can be classified into two-plasmid, three-plasmid, and four-plasmid systems. The preferred one is the two-plasmid system, which includes an expression plasmid and a packaging plasmid. The commonly used packaging plasmid is pSG3Δenv and pNL4–3(Bosch and Pawlita, 1990). The three-plasmid system divides the HIV skeleton into packaging plasmid and transfer plasmid based on the two-plasmid system. The packaging plasmid expresses Gag and Pol. The transfer plasmid contains HIV reverse transcriptase gene, cis regulatory elements required for integration, and reporter genes driven by CMV promoter (Dull et al., 1998). The commonly used packaging plasmid and transfer plasmid are psPAX2 and pLenti-GFP (Kretschmer et al., 2020, Ou et al., 2020). Noteworthily, the third generations of packing system not only evaluated the transactors but also improved cis elements. For better safety, the transferred plasmids need to be replication incompetent and render "self-inactivating" after integration by shortened 3'LTR. Although the system has been designed with many safety measures, the system still has potential threat to initiate tumor genes because the transgene sequence will insert into host genome by 2 long terminal repeat (LTR) via viral transduction (Mautino, 2002). The four-plasmid system further separates rev into an independent expression plasmid based on the three plasmid system to reduce the chance of HIV recombination in vitro and further improve the safety (Dull et al., 1998). Through transfecting the combined plasmids into 293 T cells, the HIV genome elements transcribed from the packaging plasmid and transfer plasmid are assembled into incomplete and safe HIV pseudoviruses particles, and the viral particles will be secreted into the extracellular area by exocytosis. During the secretion process, the pseudovirus particles will take the opportunity to integrate the heterologously expressed envelope proteins into the viral membrane, which is derived from cell membrane. Finally, collecting the cell supernatant and obtaining the pseudovirus through centrifugation, purification, and concentration will yield pseudovirus particles for subsequent research.
Pseudoviruses with VSV genome as the core are also widely used. VSV is an enveloped RNA virus which mainly infects animals. VSV genome is a non-segmented, negative strand, single strand RNA (ssRNA), with a size of about 11 kb. Five non-overlapping genes are arranged from the 3′ end to the 5′ end, encoding five different main proteins, including nuclear (N) protein, phosphate (P) protein, matrix (M) protein, sugar (G) protein and RNA polymerase (L) protein. VSV is also regarded as an ideal virus tool, because it has simple genome structure and can easily infect a wide variety of animal cells (Rodrı´guez, 2002, Ruedas and Connor, 2017). Studies in 1974 showed that when VSV and another virus co-infected host cells, the co-infection will produce a recombinant pseudovirus particle with the core genome of VSV and the envelope protein of another virus (Huang et al., 1974). Follow-up studies found that when VSV loses its surface glycoprotein G or G protein gene is replaced by other reporter genes, VSV can also complete the process of budding from cells if G protein or other virus-derived surface glycoproteins are expressed by plasmids (Whitt, 2010, Tani et al., 2011). Therefore, we can utilize G protein deficient VSV to load a variety of surface glycoproteins from others virus to assemble recombinant VSV pseudoviruses. In the preparation of recombinant VSV, we should firstly construct a ΔG transfer plasmid which can transcribe the negative strand RNA of VSV genome, and its transcript will be served as an RNA template for RNA-dependent RNA polymerase (RdRp) to synthesize viral RNA. The vector is co-transfected with four auxiliary vectors, which expressing VSV N, P, G and L proteins respectively, into packaging cells expressing phage T7 RNA polymerase inoculated with cowpox virus. The T7 RNA polymerase acts on the T7 promoter of the transfer plasmid to drive the transcription of the negative RNA VSV-ΔG genome. Recombinant VSV is assembled in and released from packaging cells. The cell supernatant is collected to obtain G-deficient VSV virus. Subsequently, we use it to re-infect with packaging cells expressing G glycoprotein to obtain high-yield VSV-ΔG/G* stock and we can use VSV-∆G virion coated with G protein to infect cells expressing other viral glycoproteins to generate the target pseudo virus. But the G protein of initial viral particles can be recycled on the newly produced particles which may cause super high background noise. So using an antibody against the G protein is critical for this type of pseudo virus (Almahboub et al., 2020).
Like HIV, MLV is also a retrovirus, which mainly infects mice and causes leukemia. MLV is an encapsulated positive strand RNA virus with a genome about 8000 nucleotides, including three structural genes (gag, pol and env), encoding viral capsid proteins [matrix (MA), capsid (CA) and nucleocapsid (NC)], protease [reverse transcriptase (RT), integrase (IN) and protease (PR)] and envelope protein (Fan, 1997). The early work of Witte’ team showed that when vesicular stomatitis virus (VSV) infected cells that had integrated MLV genomes, the harvested VSV pseudoviruses, although containing VSV genome, were resistant to VSV antiserum, which suggested that MLV also had the potential to package pseudoviruses (Witte and Baltimore, 1977). In subsequent studies, it was found that the G protein of VSV can also be independently integrated into MLV virus particles transcribed by plasmid when other VSV related genes did not exist (Emi et al., 1991). Since then, the genome of MLV had been split into 2 parts: one encoding gag‐pol and the other containing the reporter gene. The 2 gene sets were further cloned into plasmids to generate highly efficient MLV packaging systems (Soneoka et al., 1995). The packaging process of MLV pseudoviruses is similar to that of HIV. MLV structural genes gag and pol and a gene encoding heterologous virus envelope protein are transfected into cells by plasmid transfection. After intracellular recombination, the pseudoviruses particles carrying heterologous virus envelope protein will be secreted into the cell culture medium (Millet and Whittaker, 2014, Millet and Whittaker, 2016).
In general, each of the three methods discussed above have its own advantages and disadvantages. First, in terms of operational complexity, HIV and MLV packaging methods are simpler and less time-consuming than VSV packaging method. Second, in terms of virus yield, VSV and MLV packaging systems can achieve higher pseudovirus yields compared to HIV system (Li et al., 2018). For experiments with lower titer requirements, subsequent concentration and purification procedures will be omitted. Another advantage of VSV pseudovirus over their HIV and MLV counterparts is that the rapid intracellular replication of the VSV genome enables robust reporter gene expression to be detected within a few hours after infection (Schmidt et al., 2020). Finally, from a safety point of view, VSV and MLV systems are safer than HIV system, and live VSV and MLV themselves are less toxic than HIV.
3. The Role of pseudoviruses in the development of vaccine and antibody for SARS-CoV-2
At the end of 2019, a new type of acute pneumonia of unknown cause occurred in Wuhan, Hubei Province, China (Li et al., 2020b), Shortly after the outbreak, the unknown pathogen of the new pneumonia was isolated and identified as a bat originated New Coronavirus, belonging to the β Coronavirus genus which also includes the Severe Acute Respiratory Syndrome Coronavirus (SARS) and Middle East Respiratory Syndrome Coronavirus (MERS). Due to its high homology with SARS, the new coronavirus was named SARS-CoV-2 (Wu et al., 2020, Zhou et al., 2020). Due to the lack of effective therapeutic drugs for this new virus and the lax control measures in the early stage of outbreak, the novel coronavirus pneumonia caused by SARS-CoV-2, subsequently named as coronavirus disease 2019 (COVID-19), quickly became pandemic all over the world. As of December 2021, SARS-CoV-2 has caused about 260 million infections and 5 million deaths worldwide, and this figure is still rising (Organization, 2021). Therefore, there is an urgent need for COVID-19 specific therapeutic drugs and preventive vaccines all over the world. However, due to SARS-CoV-2’s high pathogenicity and infectivity, all test procedures using live viruses to evaluate drug efficacy must be carried out in biosafety level (BSL) 3 facilities, which undoubtedly hinders the development of related products. There is also an urgent need for standardized in vitro efficacy methods to evaluate preclinical and clinical antiviral products. In addition, the detection of neutralizing antibody against SARS-CoV-2 will help to understand the protective immune response status and the immune response of human body in COVID-19 patients and asymptomatic cases after vaccination. Because of the above reasons, the pseudoviruses system of SARS-CoV-2 plays an extremely important role during the outbreak of COVID-19, which greatly promotes the development of SARS-CoV-2 drug treatment, antibody screening, vaccine research, and receptor recognition, among others.
3.1. The pseudoviruses of SARS-CoV-2
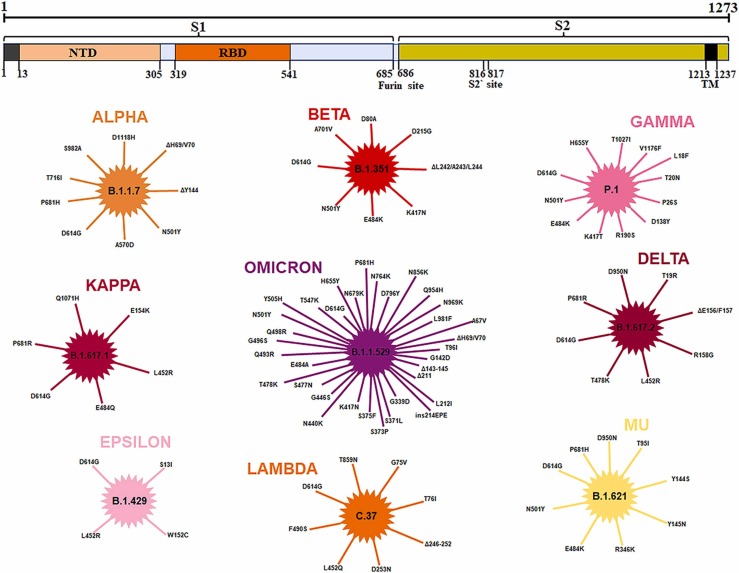
As mentioned above, pseudoviruses are composed of the core of one enveloped virus and the envelope glycoprotein of another enveloped virus. The envelope glycoprotein of the SARS-CoV-2 is the Spike protein on its surface. The Spike is responsible for recognizing the cell surface receptor ACE2 and mediating viruses’ entry, in which the activation of Spike is a prerequisite for the entry of SARS-CoV-2. This S protein activation is enabled through two proteolytic cleavage steps following the engagement of ACE2. The first of these is located at the S1-S2 boundary and is cleaved by furin in virus-producing cells, and the second is located at the S2' site in the S2 subunit ( Fig. 2), which is mainly cleaved by two major proteases, TMPRSS2 and cathepsin L. Since TMPRSS2 is present on the cell surface, TMPRSS2-mediated activation of the S protein occurs at the plasma membrane, whereas cathepsin-mediated activation occurs in the endolysosome (Hoffmann et al., 2020a, Hoffmann et al., 2020b, Walls et al., 2020, Bayati et al., 2021, Johnson et al., 2021). Therefore, S protein is the preferred target of many SARS-CoV-2 antiviral drugs and small molecule inhibitors. In addition, because the spike protein is the major factor on SARS-CoV-2 virion function as both receptor recognition and membrane fusion, and it is poor on antigenicity because of the sugar shelter (Walls et al., 2016), so S protein is also the preferred antigen of vaccine. For these reasons, pseudoviruses integrating SARS-CoV-2 S protein play an extremely important role and the research on these pseudoviruses is numerous.
Fig. 2.
An overview of mutations in the spike protein of 9 important SARS-CoV-2 variants. (Data from GISAID).
The three methods of packaging pseudoviruses mentioned above have all been used in the research of COVID-19. Among them, Crawford et al. showed in detail the process of packaging the SARS-CoV-2 pseudoviruses with HIV (Crawford et al., 2020), while Nie et al. and Almahboub et al. chose to package the SARS-CoV-2 pseudoviruses with VSV (Almahboub et al., 2020, Nie et al., 2020a), The SARS-CoV-2 MLV pseudovirus was packaged with three plasmids. The first plasmid is the packaging plasmid SV-Psi-Env-MLV. The second plasmid is L-LUC-SN, which encodes luciferase reporter and contains regulatory elements required for virus transfer. The third plasmid is the expression plasmid encoding proteins of SARS-CoV-2. When these three plasmids are co-transfected into cells, high titer pseudoviruses can be obtained (Zheng et al., 2021).
It is worth noting that the S protein of SARS-CoV-2 contains an endoplasmic reticulum retention signal (ERRS) with conserved KxHxx motif at the C terminal, which aggregates the mature S protein of SARS-CoV-2 to near the ER Golgi intermediate compartment (ERGIC) (Lontok et al., 2004, Stertz et al., 2007). In this compartment, S protein interacts with another structural protein M to drive S protein to participate in virus particle assembly and infiltrate into virus envelope (McBride et al., 2007, Ujike et al., 2016). In addition, some mature S proteins of SARS-CoV-2 reach the plasma membrane through secretory pathway, where they can mediate the fusion of infected cells and uninfected cells to fuse and form multinucleated giant cells (syncytia) (Malik, 2020). What's more, the pseudovirus is packaged with S proteins located on the plasma membrane of the cell. Therefore, several studies have shown that the S protein of SARS-CoV-2 without ERRS sequence not only can increase the infectivity of single cycle VSV pseudoviruses and VSV pseudoviruses with replication ability (Dieterle et al., 2020, Schmidt et al., 2020), but also enhances the infectivity of HIV pseudoviruses or other retroviral pseudoviruses (Schmidt et al., 2020, Yu et al., 2021). This deletion will enhance the localization of the S glycoprotein of SARS-CoV-2 on the cell membrane surface and promote the integration of more S protein into pseudoviruses particles. Previously, our group have used the pNL4–3. Luc.R-E plasmid and pVAX-S-ΔC19 with 19 amino acids deleted at the C-terminal to successfully package HIV SARS-CoV-2 pseudoviruses and applied it to antibody screening targeting the RBD region of S protein of SARS-CoV-2 (He et al., 2021).
3.2. Application of SARS-CoV-2 pseudotyped-viruses
At present, the pseudoviruses of SARS-CoV-2 has played an important role in the study of virus invasion mechanism, screening of antiviral drugs, evaluation of vaccine titer, determination of neutralizing antibody ability and so on. Hoffmann et al. used SARS-CoV-2 S pseudotyped VSV particles to demonstrate that angiotensin converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) are indispensable for the entry of SARS-CoV-2 (Hoffmann et al., 2020b),Mandel Clausen et al. also used the VSV pseudovirus system and found heparan sulfate is important to enhance the binding of Spike and ACE2 (Clausen et al., 2020). In addition, Congwen Wei et al. utilized HIV SARS-CoV-2 pseudoviruses to demonstrate that the high-density lipoprotein scavenger receptor B type 1 facilitates ACE2-dependent entry of SARS-CoV-2 (Wei et al., 2020). Using a luciferase-expressing pseudoviruses encoding SARS-CoV-2 S (G614) protein, He et al. identified 9 antiviral drug candidates with relatively high activity (EC50 <10 μM), low cytotoxicity (CC50 >20 μM), and high specificity (SI > 10, VSV-G EC50 >20 μM) from a library of 188 natural compounds (Chang-Long He1 et al., 2021). Yang et al. conducted a high throughput-screening assay for SARS-CoV-2 virus entry inhibitors using spike protein-pseudotyped HIV virions. 15 effective drugs were identified as specific entry inhibitors of SARS-CoV-2 pseudoviruses from an approved drug library including 1800 small molecular drugs (Yang et al., 2021). In terms of vaccine potency evaluation, in both preclinical and clinical stages, the determination of neutralizing antibody in serum after vaccination by pseudoviruses neutralization test is an essential experiment. A number of vaccines, including S RBD subunit protein vaccine (Yang et al., 2020a)developed by Sichuan University, full-length S glycoprotein vaccine (Tian et al., 2021)developed by Novavax, mRNA vaccine of Pfizer and Moderna (Corbett et al., 2020, Mulligan et al., 2020), adenovirus vector vaccine developed by Johnson and Oxford University (Mercado et al., 2020, van Doremalen et al., 2020) and inactivated vaccine developed by Sinovac Biotech and Beijing Institute of biological products (Qiang Gao1* et al., 2020, Wang et al., 2020), relied on pseudoviruses system to evaluate the neutralization ability of antibody induced after vaccination. Ju et al. further reported the isolation and characterization of 206 RBD-specific monoclonal antibodies derived from single B cells of eight SARS-CoV-2 infected individuals and used HIV pseudoviruses system to test the neutralization ability of these antibodies, in which the IC50 of multiple antibodies was less than 1 ug/mL (Ju et al., 2020). More examples of the applications of SARS-CoV-2 pseudoviruses are listed in Table 1.
Table 1.
The application of SARS-CoV-2 pseudovirus.
| Application | Packaging system | Reporter gene | Reference |
|---|---|---|---|
| Neutralizing Antibody Assay | VSV, HIV | Luciferase | (Cao et al., 2020, Lei et al., 2020) |
| Vaccine | HIV, VSV, MLV | Luciferase, GFP | (Erasmus and Fuller, 2020, Ma et al., 2020, Zhang et al., 2020, Sanchez-Felipe et al., 2021, Vogel et al., 2021) |
| Screen of Entry Inhibitors | HIV, MLV | Luciferase | (Zhu et al., 2020, Joseph et al., 2021, Xu et al., 2021) |
| Mechanisms of Virus Entry | VSV, MLV | Luciferase | (Daly et al., 2020, Yang et al., 2020b, Ou et al., 2021) |
| Variants of SARS-CoV-2 | HIV, VSV | Luciferase | (Hoffmann et al., 2021, Hu et al., 2021, Wang et al., 2021c) |
Another advantage of pseudoviruses is that when important mutations occur in the glycoprotein on the virus surface, which could improve the transmission ability and promote immune escape, and it is difficult to isolate the mutant live virus strain, scientists can quickly use the gene editing technology to perform site-directed mutagenesis on wild-type envelope proteins to obtain a single or multiple-point mutations in pseudoviruses to study its function (Li et al., 2020a). For example, during the COVID-19 pandemic, a variety of SARS-COV-2 mutant strains appeared all over the world, and the emergence of each new mutant strain has triggered a new wave of the epidemic, which has attracted widespread attention. First, in early March 2020, the first major mutant D614G of SARS-CoV-2 was detected, and amino acid at position 614 of spike changed from D to G. Subsequent studies showed that D614G could significantly improve the transmission ability of the virus (Hou et al., 2020, Korber et al., 2020). In August 2020, a new mutant strain B.1.1.7 was identified in the UK, and eight important mutations occurred in its S glycoprotein (Davies et al., 2021, Galloway et al., 2021). At the end of December 2020, a mutant B.1.351 from South Africa was reported, which contained 10 mutations on the S protein (Tegally et al., 2020). Variant P.1, mainly popular in Brazil, was detected from the end of 2020 to the beginning of 2021. In addition to D614G, P.1 also contains 11 important point mutations (Wang et al., 2021a). In early 2021, a new mutant B.1.617 appeared in India, and it produced two highly infectious progenies B.1.617.1 (Kappa) and B.1.617.2 (delta) (Ferreira et al., 2021). Recently, a brand new variant, B.1.1.529 variant (Omicron) containing almost all of the key mutation sites of these five mutants has been detected in Africa, which the World Health Organization (WHO) has classified as Variant of Concern (VOC) on November 26th 2021 (Roessler et al., 2021). Other mutants with their mutation sites are shown in Fig. 2. Based on this, scientists quickly constructed a series of pseudoviruses containing S protein of SARS-COV-2 with single point mutation or multi-point mutations and determined the effects of these mutants relative to the wild type.
By constructing pseudoviruses containing the S protein representing 10 globally circulating strains of SARS-CoV-2, Garcia-Beltran et al. evaluated the neutralization potency of sera from 99 individuals that received one or two doses of either BNT162b2 or mRNA-1273 vaccines. The results showed that 5 of the 10 pseudoviruses, harboring receptor binding domain mutations, including K417N/T, E484K, and N501Y, were highly resistant to neutralization (Garcia-Beltran et al., 2021). In addition, Kuzmina et al. monitored neutralization potency of convalescent or Pfizer-BTN162b2 post-vaccination sera against pseudoviruses displaying spike proteins derived from WT SARS-CoV-2, or its Alpha and Beta variants. Compared to convalescent sera, vaccination stimulated high titers of neutralizing antibodies, which exhibit efficient neutralization potential against pseudoviruses carrying WT SARS-CoV-2. However, while wild-type and N501Y pseudoviruses were similarly neutralized, those displaying N501Y/K417N/E484K spike mutations moderately resisted neutralization (Kuzmina et al., 2021). The situation of the SARS-CoV-2 Delta variant that was initially prevalent in India seems to be more worrying, because research indicated that Delta variant is sixfold less sensitive to serum neutralizing antibodies from recovered individuals, and eightfold less sensitive to vaccine-elicited antibodies, compared with wild-type bearing D614G (Mlcochova et al., 2021). What's more, sera from Beta and Gamma variant show markedly reduced neutralizing abilities against Delta variant (Liu et al., 2021). It is reported that Alpha variant is resistant to most monoclonal antibodies targeting the NTD domain of S protein, while it is relatively sensitive to monoclonal antibodies targeting the RBD structure of S protein. In addition, it is not highly resistant to convalescent plasma or vaccinated sera. The discovery of Beta variant is more worrying, because this variant is neither neutralized by most NTD mAbs, nor by multiple individual mAbs of the RBD, mainly due to the E484K mutation. In addition, the neutralizing ability of convalescent plasma and vaccinated serum against Beta variant decreased by 9.4 times and 10.3–12.4 times, respectively (Wang et al., 2021b). A latest research showed that in the serum of convalescence patients infected with the original SARS-CoV-2 strain, the mean neutralizing ED50 of their serum for Omicron decreased to 66, about 8.4 times that of the D614G reference strain (ED50 =556), while the neutralizing activity of other variant pseudoviruses decreased only about 1.2–4.5 times (Zhang et al., 2021a).
4. Limitations of pseudoviruses
Despite the advantages of pseudoviruses listed above, the system also has many limitations. Firstly, pseudoviruses can only be used in the study of viruses with specific envelope protein, such as influenza virus (Ao et al., 2008, Wang et al., 2008, Guo et al., 2009, Ferrara et al., 2012), coronavirus (Han et al., 2004, Simmons et al., 2004, Zhao et al., 2013, Wang et al., 2014), retrovirus (Su et al., 2013, Wang et al., 2013, Zhao et al., 2016), herpesvirus (Rogalin and Heldwein, 2016), flavivirus (Tani et al., 2010, Kretschmer et al., 2020), Ebola virus (Takada et al., 1997, Quinn et al., 2009) and so on. However, pseudoviruses system is difficult to function for viruses without envelope protein, such as rotavirus and poliovirus. Secondly, compared with the live virus, the characteristics simulated by pseudoviruses are also very limited. To a large extent, pseudoviruses can only simulate the role of the envelope protein of the live virus in mediating the virus into the cell in vitro, but the process of proliferation and release after entering the cell cannot be simulated. In addition, the shape of virus particles may affect the applicability of the constructed pseudoviruses. For example, SARS-CoV-2 is a spherical virus, and the S protein covers two different states of before and after fusion (Yao et al., 2020). However, among our commonly used pseudoviruses system, HIV-1 and MLV are spherical viruses, while VSV is a bullet virus, and the distribution of S protein on the surface of pseudoviruses is not clear. Therefore, the distribution, conformation and density pattern of heterologous viral glycoproteins on pseudoviruses may not reflect their "natural" state on the surface of live viruses (Li et al., 2018). In addition, it is inconvenient to calculate the titer of pseudoviruses quantitatively. When researchers use HIV to package pseudoviruses, they will choose to measure the level of HIV-1 p24 antigen by ELISA to quantify the titer of pseudoviruses (Deng et al., 2021, Motozono et al., 2021). Alternatively, the copies of pseudoviruses were expressed as numbers of viral RNA genomes per mL of viral stock solution determined by using reverse transcription fluorescence quantitative PCR. Other titer quantification methods include the 50% tissue culture infectious dose (TCID50) calculated by the Reed–Muench method (Nie et al., 2020b, Garcia-Beltran et al., 2021) and using reverse transcription fluorescence quantitative PCR to determine the copy number of the sample and comparing it with the standard (e.g., The P protein gene of VSV virus was cloned into the vector pCDNA3.1(+) as a plasmid standard) (Li et al., 2020a). However, among these methods, P24 Elisa assay or copy numbers seem to ignore a same problem, which is that what they measured is the amount of pseudoviruses core genome. Whereas the amount of envelope protein on pseudoviruses is not directly proportional to the copy number of core genome. Sometimes the outer membrane of pseudoviruses may not be successfully loaded with envelope protein. Nonetheless, the titers can be measured by P24 or qPCR, so the titer calculated by these methods loses its authenticity, especially when comparing the effects of S protein of different mutants on virus infection ability. Although the TCID50 method takes the factor of envelope proteins into consideration, there is still another problem: even when the infectious units are identical, the amount of membrane proteins may be inconsistent among different mutants possessing different infection abilities. For example, studies have shown that L452R mutation on S protein will improve the infection ability of pseudoviruses compared with WT (Deng et al., 2021, Motozono et al., 2021). Therefore, results from assays using pseudotyped viruses should be compared and validated against the live virus‐based assay, which remains the gold standard (Tamin et al., 2009).
5. Prospect
In order to overcome the shortcomings of pseudoviruses, many research groups have developed an infectious cDNA clone technology of SARS-CoV-2 (Xie et al., 2020, Ju et al., 2021, Rihn et al., 2021, Zhang et al., 2021b). The infectious clone of SARS-COV-2 contains almost all its own genes, and the reporter gene is inserted for easy detection. With regard to both pathogenicity and replication capacity, the infectious clone is similar to the situation of wild-type live virus infection. Meanwhile, it can also be operated in a biosafety level-2 environment, and the entire SARS-CoV-2 genome can be edited in a site-specific manner in vitro. Recently, a research group used infectious clone of SARS-CoV-2 to find that many—but not all—of the antibody products with Emergency Use Authorization should retain substantial efficacy against the prevailing variant strains of SARS-CoV-2 (Chen et al., 2021). However, this method is technically difficult and relatively expensive, and experiments may not be able to obtain successful infectious clones. Therefore, this method still has some limitations in its use. In summary, pseudoviruses system is an effective tool for screening and evaluation of monoclonal antibodies or antiviral drugs, detection of serum neutralizing antibodies and evaluation of vaccine titer. It will still be widely used in the future. This review summarizes three main methods of packaging pseudoviruses, and focuses on the application of pseudovirus in COVID-19 research, which has proper guiding significance for the future development and application of pseudoviruses.
CRediT authorship contribution statement
Qi Xiang: Writing-original draft, Writing-review & editing. Linhao Li: Writing-original draft. Jie Wu: Writing-original draft. Miao Tian: Writing-review & editing. Yang Fu: Conceptualization, Writing-original draft, Writing-review & editing.
Acknowledgment
This work was supported by Shenzhen Science and Technology Program (JCYJ20210324115611032) and Shenzhen Science and Technology Program (KQTD20200909113758004).
Conflicts of interest
The authors declare no conflict of interest.
References
- Almahboub S.A., Algaissi A., Alfaleh M.A., ElAssouli M.Z., Hashem A.M. Evaluation of neutralizing antibodies against highly pathogenic coronaviruses: a detailed protocol for a rapid evaluation of neutralizing antibodies using vesicular stomatitis virus pseudovirus-based assay. Front. Microbiol. 2020;11:2020. doi: 10.3389/fmicb.2020.02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao Z., Patel A., Tran K., He X., Fowke K., Coombs K., Kobasa D., Kobinger G., Yao X. Characterization of a trypsin-dependent avian influenza H5N1-pseudotyped HIV vector system for high throughput screening of inhibitory molecules. Antivir. Res. 2008;79(1):12–18. doi: 10.1016/j.antiviral.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayati A., Kumar R., Francis V., McPherson P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch V., Pawlita M. Mutational analysis of the human immunodeficiency virus type 1 env gene product proteolytic cleavage site. J. Virol. 1990;64(5):2337–2344. doi: 10.1128/JVI.64.5.2337-2344.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Su B., Guo X., Sun W., Deng Y., Bao L., Zhu Q., Zhang X., Zheng Y., Geng C., Chai X., He R., Li X., Lv Q., Zhu H., Deng W., Xu Y., Wang Y., Qiao L., Tan Y., Song L., Wang G., Du X., Gao N., Liu J., Xiao J., Su X.D., Du Z., Feng Y., Qin C., Qin C., Jin R., Xie X.S. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020;182(1):73–84 e16. doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Long He1 L.-Y.H., Wang 1 Kai, Gu2 Chen-Jian, Jie Hu1 G.-J.Z., Xu2 Wei, Xie 2,3 You-Hua, Tang1 Ni, Huang 1 Ai-Long. Identification of bis-benzylisoquinoline alkaloids as SARSCoV-2 entry inhibitors from a library of natural products. Signal Transduct. Target. Ther. 2021 doi: 10.1038/s41392-021-00531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.E., Winkler E.S., Case J.B., Aziati I.D., Bricker T.L., Joshi A., Darling T.L., Ying B., Errico J.M., Shrihari S., VanBlargan L.A., Xie X., Gilchuk P., Zost S.J., Droit L., Liu Z., Stumpf S., Wang D., Handley S.A., Stine W.B., Shi P.-Y., Davis-Gardner M.E., Suthar M.S., Knight M.G., Andino R., Chiu C.Y., Ellebedy A.H., Fremont D.H., Whelan S.P.J., Crowe J.E., Purcell L., Corti D., Boon A.C.M., Diamond M.S. In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains. Nature. 2021;596(7870):103–108. doi: 10.1038/s41586-021-03720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., Narayanan A., Majowicz S.A., Kwong E.M., McVicar R.N., Thacker B.E., Glass C.A., Yang Z., Torres J.L., Golden G.J., Bartels P.L., Porell R.N., Garretson A.F., Laubach L., Feldman J., Yin X., Pu Y., Hauser B.M., Caradonna T.M., Kellman B.P., Martino C., Gordts P., Chanda S.K., Schmidt A.G., Godula K., Leibel S.L., Jose J., Corbett K.D., Ward A.B., Carlin A.F., Esko J.D. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183(4):1043–1057 e15. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P., Flach B., O’Connell S., Bock K.W., Minai M., Nagata B.M., Andersen H., Martinez D.R., Noe A.T., Douek N., Donaldson M.M., Nji N.N., Alvarado G.S., Edwards D.K., Flebbe D.R., Lamb E., Doria-Rose N.A., Lin B.C., Louder M.K., O’Dell S., Schmidt S.D., Phung E., Chang L.A., Yap C., Todd J.M., Pessaint L., Van Ry A., Browne S., Greenhouse J., Putman-Taylor T., Strasbaugh A., Campbell T.A., Cook A., Dodson A., Steingrebe K., Shi W., Zhang Y., Abiona O.M., Wang L., Pegu A., Yang E.S., Leung K., Zhou T., Teng I.T., Widge A., Gordon I., Novik L., Gillespie R.A., Loomis R.J., Moliva J.I., Stewart-Jones G., Himansu S., Kong W.P., Nason M.C., Morabito K.M., Ruckwardt T.J., Ledgerwood J.E., Gaudinski M.R., Kwong P.D., Mascola J.R., Carfi A., Lewis M.G., Baric R.S., McDermott A., Moore I.N., Sullivan N.J., Roederer M., Seder R.A., Graham B.S. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford K.H.D., Eguia R., Dingens A.S., Loes A.N., Malone K.D., Wolf C.R., Chu H.Y., Tortorici M.A., Veesler D., Murphy M., Pettie D., King N.P., Balazs A.B., Bloom J.D. Protocol and reagents for pseudotyping lentiviral particles with SARS-CoV-2 spike protein for neutralization assays. Viruses. 2020;12(5) doi: 10.3390/v12050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J.L., Simonetti B., Klein K., Chen K.-E., Williamson M.K., Antón-Plágaro C., Shoemark D.K., Simón-Gracia L., Bauer M., Hollandi R., Greber U.F., Horvath P., Sessions R.B., Helenius A., Hiscox J.A., Teesalu T., Matthews D.A., Davidson A.D., Collins B.M., Cullen P.J., Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370(6518):861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., Wenseleers T., Gimma A., Waites W., Wong K.L.M., van Zandvoort K., Silverman J.D., Diaz-Ordaz K., Keogh R., Eggo R.M., Funk S., Jit M., Atkins K.E., Edmunds W.J. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(6538) doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Garcia-Knight M.A., Khalid M.M., Servellita V., Wang C., Morris M.K., Sotomayor-González A., Glasner D.R., Reyes K.R., Gliwa A.S., Reddy N.P., Sanchez San Martin C., Federman S., Cheng J., Balcerek J., Taylor J., Streithorst J.A., Miller S., Sreekumar B., Chen P.-Y., Schulze-Gahmen U., Taha T.Y., Hayashi J.M., Simoneau C.R., Kumar G.R., McMahon S., Lidsky P.V., Xiao Y., Hemarajata P., Green N.M., Espinosa A., Kath C., Haw M., Bell J., Hacker J.K., Hanson C., Wadford D.A., Anaya C., Ferguson D., Frankino P.A., Shivram H., Lareau L.F., Wyman S.K., Ott M., Andino R., Chiu C.Y. Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell. 2021;184(13) doi: 10.1016/j.cell.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterle M.E., Haslwanter D., Bortz R.H., 3rd, Wirchnianski A.S., Lasso G., Vergnolle O., Abbasi S.A., Fels J.M., Laudermilch E., Florez C., Mengotto A., Kimmel D., Malonis R.J., Georgiev G., Quiroz J., Barnhill J., Pirofski L.A., Daily J.P., Dye J.M., Lai J.R., Herbert A.S., Chandran K., Jangra R.K. A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition. Cell Host Microbe. 2020;28(3):486–496 e6. doi: 10.1016/j.chom.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T., Zufferey R., Kelly M., Mandel R.J., Nguyen M., Trono D., Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72(11):8463–8471. doi: 10.1128/JVI.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emi N., Friedmann T., Yee J.K. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J. Virol. 1991;65(3):1202–1207. doi: 10.1128/JVI.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erasmus J.H., Fuller D.H. An alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci. Transl. Med. 2020 doi: 10.1126/scitranslmed.abc9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C., Wu X., Liu Q., Li Q., Liu S., Lu J., Yang Y., Cao Y., Huang W., Liang C., Ying T., Jiang S., Wang Y. A human DPP4-knockin mouse’s susceptibility to infection by authentic and pseudotyped MERS-CoV. Viruses. 2018;10(9) doi: 10.3390/v10090448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H. Leukemogenesis by Moloney murine leukemia virus: a multistep process. Trends Microbiol. 1997;5(2):74–82. doi: 10.1016/S0966-842X(96)10076-7. [DOI] [PubMed] [Google Scholar]
- Ferrara F., Molesti E., Böttcher-Friebertshäuser E., Cattoli G., Corti D., Scott S.D., Temperton N.J. The human Transmembrane Protease Serine 2 is necessary for the production of Group 2 influenza A virus pseudotypes. J. Mol. Genet. Med. 2012:309–314. PMC3614188 (nih.gov) [PMC free article] [PubMed] [Google Scholar]
- Ferreira I.A.T.M., Kemp S.A., Datir R., Saito A., Meng B., Rakshit P., Takaori-Kondo A., Kosugi Y., Uriu K., Kimura I., Shirakawa K., Abdullahi A., Agarwal A., Ozono S., Tokunaga K., Sato K., Gupta R.K. SARS-CoV-2 B.1.617 mutations L452R and E484Q are not synergistic for antibody evasion. J. Infect. Dis. 2021;224(6):989–994. doi: 10.1093/infdis/jiab368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway S.E., Paul P., MacCannell D.R., Johansson M.A., Brooks J.T., MacNeil A., Slayton R.B., Tong S., Silk B.J., Armstrong G.L., Biggerstaff M., Dugan V.G. Emergence of SARS-CoV-2 B.1.1.7 Lineage - United States, December 29, 2020-January 12, 2021. Morb. Mortal. Wkly Rep. 2021;70(3):95–99. doi: 10.15585/mmwr.mm7003e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Beltran W.F., Lam E.C., St, Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., Feldman J., Pavlovic M.N., Gregory D.J., Poznansky M.C., Sigal A., Schmidt A.G., Iafrate A.J., Naranbhai V., Balazs A.B. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021 doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Rumschlag-Booms E., Wang J., Xiao H., Yu J., Wang J., Guo L., Gao G.F., Cao Y., Caffrey M., Rong L. Analysis of hemagglutinin-mediated entry tropism of H5N1 avian influenza. Virol. J. 2009;6:39. doi: 10.1186/1743-422X-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D.P., Kim H.G., Kim Y.B., Poon L.L.M., Cho M.W. Development of a safe neutralization assay for SARS-CoV and characterization of S-glycoprotein. Virology. 2004;326(1):140–149. doi: 10.1016/j.virol.2004.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Qu J., Wei L., Liao S., Zheng N., Liu Y., Wang X., Jing Y., Shen C.K.-F., Ji C., Luo G., Zhang Y., Xiang Q., Fu Y., Li S., Fan Y., Fang S., Wang P., Li L. Generation and effect testing of a SARS-CoV-2 RBD-targeted polyclonal therapeutic antibody based on a 2-D airway organoid screening system. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.689065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Pohlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell. 2020;78(4):779–784 e5. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280 e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Arora P., Gross R., Seidel A., Hornich B.F., Hahn A.S., Kruger N., Graichen L., Hofmann-Winkler H., Kempf A., Winkler M.S., Schulz S., Jack H.M., Jahrsdorfer B., Schrezenmeier H., Muller M., Kleger A., Munch J., Pohlmann S. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021 doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.J., Chiba S., Halfmann P., Ehre C., Kuroda M., Dinnon K.H., Leist S.R., Schäfer A., Nakajima N., Takahashi K., Lee R.E., Mascenik T.M., Graham R., Edwards C.E., Tse L.V., Okuda K., Markmann A.J., Bartelt L., de Silva A., Margolis D.M., Boucher R.C., Randell S.H., Suzuki T., Gralinski L.E., Kawaoka Y., Baric R.S. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370(6523):1464–1468. doi: 10.1126/science.abe8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Peng P., Wang K., Fang L., Luo F.-Y., Jin A.-S., Liu B.-Z., Tang N., Huang A.-L. Emerging SARS-CoV-2 variants reduce neutralization sensitivity to convalescent sera and monoclonal antibodies. Cell. Mol. Immunol. 2021;18(4):1061–1063. doi: 10.1038/s41423-021-00648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A.S., Palma E.L., Hewlett N., Roizman B. Pseudotype formation between enveloped RNA and DNA viruses. Nature. 1974;252(5485):743–745. doi: 10.1038/252743a0. [DOI] [PubMed] [Google Scholar]
- Johnson B.A., Xie X., Bailey A.L., Kalveram B., Lokugamage K.G., Muruato A., Zou J., Zhang X., Juelich T., Smith J.K., Zhang L., Bopp N., Schindewolf C., Vu M., Vanderheiden A., Winkler E.S., Swetnam D., Plante J.A., Aguilar P., Plante K.S., Popov V., Lee B., Weaver S.C., Suthar M.S., Routh A.L., Ren P., Ku Z., An Z., Debbink K., Diamond M.S., Shi P.Y., Freiberg A.N., Menachery V.D. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature. 2021 doi: 10.1038/s41586-021-03237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Karthika T., Das V.R.A., Raj V.S. The use of pseudotyped coronaviruses for the screening of entry inhibitors: green tea extract inhibits the entry of SARS-CoV-1, MERS-CoV, and SARS-CoV-2 by blocking receptor-spike interaction. Curr. Pharm. Biotechnol. 2021 doi: 10.2174/1389201022666210810111716. [DOI] [PubMed] [Google Scholar]
- Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., Tang X., Yu J., Lan J., Yuan J., Wang H., Zhao J., Zhang S., Wang Y., Shi X., Liu L., Zhao J., Wang X., Zhang Z., Zhang L. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020 doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- Ju X., Zhu Y., Wang Y., Li J., Zhang J., Gong M., Ren W., Li S., Zhong J., Zhang L., Zhang Q.C., Zhang R., Ding Q. A novel cell culture system modeling the SARS-CoV-2 life cycle. PLoS Pathog. 2021;17(3) doi: 10.1371/journal.ppat.1009439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobinger G.P., Limberis M.P., Somanathan S., Schumer G., Bell P., Wilson J.M. Human immunodeficiency viral vector pseudotyped with the spike envelope of severe acute respiratory syndrome coronavirus transduces human airway epithelial cells and dendritic cells. Hum. Gene Ther. 2007;18(5):413–422. doi: 10.1089/hum.2006.194. [DOI] [PubMed] [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M., de Silva T.I., McDanal C., Perez L.G., Tang H., Moon-Walker A., Whelan S.P., LaBranche C.C., Saphire E.O., Montefiori D.C. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4) doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer M., Kadlubowska P., Hoffmann D., Schwalbe B., Auerswald H., Schreiber M., Zikavirus M.E. Envelope pseudotyped human immunodeficiency virus type-1 as a novel tool for glioblastoma-directed virotherapy. Cancers. 2020;12(4) doi: 10.3390/cancers12041000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmina A., Khalaila Y., Voloshin O., Keren-Naus A., Boehm-Cohen L., Raviv Y., Shemer-Avni Y., Rosenberg E., Taube R. SARS-CoV-2 spike variants exhibit differential infectivity and neutralization resistance to convalescent or post-vaccination sera. Cell Host Microbe. 2021;29(4) doi: 10.1016/j.chom.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C., Qian K., Li T., Zhang S., Fu W., Ding M., Hu S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat. Commun. 2020;11(1):2070. doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Liu Q., Huang W., Li X., Wang Y. Current status on the development of pseudoviruses for enveloped viruses. Rev. Med. Virol. 2018;28(1) doi: 10.1002/rmv.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wu J., Nie J., Zhang L., Hao H., Liu S., Zhao C., Zhang Q., Liu H., Nie L., Qin H., Wang M., Lu Q., Li X., Sun Q., Liu J., Zhang L., Li X., Huang W., Wang Y. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell. 2020 doi: 10.1016/j.cell.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Ginn H.M., Dejnirattisai W., Supasa P., Wang B., Tuekprakhon A., Nutalai R., Zhou D., Mentzer A.J., Zhao Y., Duyvesteyn H.M.E., López-Camacho C., Slon-Campos J., Walter T.S., Skelly D., Johnson S.A., Ritter T.G., Mason C., Costa Clemens S.A., Gomes Naveca F., Nascimento V., Nascimento F., Fernandes da Costa C., Resende P.C., Pauvolid-Correa A., Siqueira M.M., Dold C., Temperton N., Dong T., Pollard A.J., Knight J.C., Crook D., Lambe T., Clutterbuck E., Bibi S., Flaxman A., Bittaye M., Belij-Rammerstorfer S., Gilbert S.C., Malik T., Carroll M.W., Klenerman P., Barnes E., Dunachie S.J., Baillie V., Serafin N., Ditse Z., Da Silva K., Paterson N.G., Williams M.A., Hall D.R., Madhi S., Nunes M.C., Goulder P., Fry E.E., Mongkolsapaya J., Ren J., Stuart D.I., Screaton G.R. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184(16) doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Fan C., Li Q., Zhou S., Huang W., Wang L., Sun C., Wang M., Wu X., Ma J., Li B., Xie L., Wang Y. Antibody-dependent-cellular-cytotoxicity-inducing antibodies significantly affect the post-exposure treatment of Ebola virus infection. Sci. Rep. 2017;7:45552. doi: 10.1038/srep45552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lontok E., Corse E., Machamer C.E. Intracellular targeting signals contribute to localization of coronavirus spike proteins near the virus assembly site. J. Virol. 2004;78(11):5913–5922. doi: 10.1128/JVI.78.11.5913-5922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Jiang T. Pseudovirus-based neuraminidase inhibition assays reveal potential H5N1 drug-resistant mutations. Protein Cell. 2013;4(5):356–363. doi: 10.1007/s13238-013-2125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Zou F., Yu F., Li R., Yuan Y., Zhang Y., Zhang X., Deng J., Chen T., Song Z., Qiao Y., Zhan Y., Liu J., Zhang J., Zhang X., Peng Z., Li Y., Lin Y., Liang L., Wang G., Chen Y., Chen Q., Pan T., He X., Zhang H. Nanoparticle vaccines based on the receptor binding domain (RBD) and heptad repeat (HR) of SARS-CoV-2 elicit robust protective immune responses. Immunity. 2020 doi: 10.1016/j.immuni.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik Y.A. Properties of coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020 [PubMed] [Google Scholar]
- Mautino M.R. Lentiviral vectors for gene therapy of HIV-1 infection. Curr. Gene Ther. 2002;2(1):23–43. doi: 10.2174/1566523023348165. [DOI] [PubMed] [Google Scholar]
- McBride C.E., Li J., Machamer C.E. The cytoplasmic tail of the severe acute respiratory syndrome coronavirus spike protein contains a novel endoplasmic reticulum retrieval signal that binds COPI and promotes interaction with membrane protein. J. Virol. 2007;81(5):2418–2428. doi: 10.1128/JVI.02146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H., He X., Martinez D.R., Rutten L., Bos R., van Manen D., Vellinga J., Custers J., Langedijk J.P., Kwaks T., Bakkers M.J.G., Zuijdgeest D., Huber S.K.R., Atyeo C., Fischinger S., Burke J.S., Feldman J., Hauser B.M., Caradonna T.M., Bondzie E.A., Dagotto G., Gebre M.S., Hoffman E., Jacob-Dolan C., Kirilova M., Li Z., Lin Z., Mahrokhian S.H., Maxfield L.F., Nampanya F., Nityanandam R., Nkolola J.P., Patel S., Ventura J.D., Verrington K., Wan H., Pessaint L., Ry A.V., Blade K., Strasbaugh A., Cabus M., Brown R., Cook A., Zouantchangadou S., Teow E., Andersen H., Lewis M.G., Cai Y., Chen B., Schmidt A.G., Reeves R.K., Baric R.S., Lauffenburger D.A., Alter G., Stoffels P., Mammen M., Hoof J.V., Schuitemaker H., Barouch D.H. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020 doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. USA. 2014;111(42):15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Murine leukemia virus (MLV)-based coronavirus spike-pseudotyped particle production and infection. Bio Protoc. 2016;6(23) doi: 10.21769/BioProtoc.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlcochova P., Kemp S.A., Dhar M.S., Papa G., Meng B., Ferreira I.A.T.M., Datir R., Collier D.A., Albecka A., Singh S., Pandey R., Brown J., Zhou J., Goonawardane N., Mishra S., Whittaker C., Mellan T., Marwal R., Datta M., Sengupta S., Ponnusamy K., Radhakrishnan V.S., Abdullahi A., Charles O., Chattopadhyay P., Devi P., Caputo D., Peacock T., Wattal C., Goel N., Satwik A., Vaishya R., Agarwal M., Mavousian A., Lee J.H., Bassi J., Silacci-Fegni C., Saliba C., Pinto D., Irie T., Yoshida I., Hamilton W.L., Sato K., Bhatt S., Flaxman S., James L.C., Corti D., Piccoli L., Barclay W.S., Rakshit P., Agrawal A., Gupta R.K. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599(7883):114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motozono C., Toyoda M., Zahradnik J., Saito A., Nasser H., Tan T.S., Ngare I., Kimura I., Uriu K., Kosugi Y., Yue Y., Shimizu R., Ito J., Torii S., Yonekawa A., Shimono N., Nagasaki Y., Minami R., Toya T., Sekiya N., Fukuhara T., Matsuura Y., Schreiber G., Ikeda T., Nakagawa S., Ueno T., Sato K. SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe. 2021;29(7) doi: 10.1016/j.chom.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Tureci O., Tompkins K.R., Walsh E.E., Frenck R., Falsey A.R., Dormitzer P.R., Gruber W.C., Sahin U., Jansen K.U. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020 doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- Nie J., Li Q., Wu J., Zhao C., Hao H., Liu H., Zhang L., Nie L., Qin H., Wang M., Lu Q., Li X., Sun Q., Liu J., Fan C., Huang W., Xu M., Wang Y. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 2020 doi: 10.1038/s41596-020-0394-5. [DOI] [PubMed] [Google Scholar]
- Nie J., Li Q., Wu J., Zhao C., Hao H., Liu H., Zhang L., Nie L., Qin H., Wang M., Lu Q., Li X., Sun Q., Liu J., Fan C., Huang W., Xu M., Wang Y. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020;9(1):680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning T., Wang L., Liu S., Ma J., Nie J., Huang W., Li X., Li Y., Wang Y. Monitoring neutralization property change of evolving Hantaan and Seoul viruses with a novel pseudovirus-based assay. Virol. Sin. 2021;36(1):104–112. doi: 10.1007/s12250-020-00237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH. WHO Coronavirus (COVID-19) Dashboard 2021. WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data.
- Ou T., Mou H., Zhang L., Ojha A., Choe H., Farzan M. Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2. PLoS Pathog. 2021;17(1) doi: 10.1371/journal.ppat.1009212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang Gao1 et al., 2020.Qiang Gao1* L.B., Mao3* Haiyan, Wang1* Lin, Xu4* Kangwei, Yang5* Minnan, Li1 Yajing, Zhu5 Ling, Wang5 Nan, Lv5 Zhe, Gao2 Hong, Ge1 Xiaoqin, Kan6 Biao, Hu1 Yaling, Liu2 Jiangning, Cai1 Fang, Jiang1 Deyu, Yin1 Yanhui, Qin7 Chengfeng, Li1 Jing, Gong1 Xuejie, Lou3 Xiuyu, Shi3 Wen, Wu1 Dongdong, Zhang1 Hengming, Zhu1 Lang, Deng2 Wei, Li1 Yurong, Lu6† Jinxing, Li4† Changgui, Wang5† Xiangxi, Yin1† Weidong, Zhang3† Yanjun, Qin2† Chuan. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020 doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn K., Brindley M.A., Weller M.L., Kaludov N., Kondratowicz A., Hunt C.L., Sinn P.L., McCray P.B., Stein C.S., Davidson B.L., Flick R., Mandell R., Staplin W., Maury W., Chiorini J.A. Rho GTPases modulate entry of Ebola virus and vesicular stomatitis virus pseudotyped vectors. J. Virol. 2009;83(19):10176–10186. doi: 10.1128/JVI.00422-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihn S.J., Merits A., Bakshi S., Turnbull M.L., Wickenhagen A., Alexander A.J.T., Baillie C., Brennan B., Brown F., Brunker K., Bryden S.R., Burness K.A., Carmichael S., Cole S.J., Cowton V.M., Davies P., Davis C., De Lorenzo G., Donald C.L., Dorward M., Dunlop J.I., Elliott M., Fares M., da Silva Filipe A., Freitas J.R., Furnon W., Gestuveo R.J., Geyer A., Giesel D., Goldfarb D.M., Goodman N., Gunson R., Hastie C.J., Herder V., Hughes J., Johnson C., Johnson N., Kohl A., Kerr K., Leech H., Lello L.S., Li K., Lieber G., Liu X., Lingala R., Loney C., Mair D., McElwee M.J., McFarlane S., Nichols J., Nomikou K., Orr A., Orton R.J., Palmarini M., Parr Y.A., Pinto R.M., Raggett S., Reid E., Robertson D.L., Royle J., Cameron-Ruiz N., Shepherd J.G., Smollett K., Stewart D.G., Stewart M., Sugrue E., Szemiel A.M., Taggart A., Thomson E.C., Tong L., Torrie L.S., Toth R., Varjak M., Wang S., Wilkinson S.G., Wyatt P.G., Zusinaite E., Alessi D.R., Patel A.H., Zaid A., Wilson S.J., Mahalingam S. A plasmid DNA-launched SARS-CoV-2 reverse genetics system and coronavirus toolkit for COVID-19 research. PLoS Biol. 2021;19(2) doi: 10.1371/journal.pbio.3001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrı´guez L.L. Emergence and re-emergence of vesicular stomatitis in the United States. Virus Res. 2002 doi: 10.1016/s0168-1702(02)00026-6. [DOI] [PubMed] [Google Scholar]
- Roessler A., Riepler L., Bante D., von Laer D., Kimpel J. SARS-CoV-2 B.1.1.529 variant (Omicron) evades neutralization by sera from vaccinated and convalescent individuals. medRxiv. 2021 doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalin H.B., Heldwein E.E. Characterization of vesicular stomatitis virus pseudotypes bearing essential entry glycoproteins gB, gD, gH, and gL of herpes simplex virus 1. J. Virol. 2016;90(22):10321–10328. doi: 10.1128/JVI.01714-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedas J.B., Connor J.H. Generating recombinant vesicular stomatitis viruses for use as vaccine platforms. Methods Mol. Biol. 2017;1581:203–222. doi: 10.1007/978-1-4939-6869-5_12. [DOI] [PubMed] [Google Scholar]
- Salazar-García M., Acosta-Contreras S., Rodríguez-Martínez G., Cruz-Rangel A., Flores-Alanis A., Patiño-López G., Luna-Pineda V.M. Pseudotyped vesicular stomatitis virus-severe acute respiratory syndrome-coronavirus-2 spike for the study of variants, vaccines, and therapeutics against coronavirus disease 2019. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.817200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Felipe L., Vercruysse T., Sharma S., Ma J., Lemmens V., Van Looveren D., Arkalagud Javarappa M.P., Boudewijns R., Malengier-Devlies B., Liesenborghs L., Kaptein S.J.F., De Keyzer C., Bervoets L., Debaveye S., Rasulova M., Seldeslachts L., Li L.-H., Jansen S., Yakass M.B., Verstrepen B.E., Böszörményi K.P., Kiemenyi-Kayere G., van Driel N., Quaye O., Zhang X., Ter Horst S., Mishra N., Deboutte W., Matthijnssens J., Coelmont L., Vandermeulen C., Heylen E., Vergote V., Schols D., Wang Z., Bogers W., Kuiken T., Verschoor E., Cawthorne C., Van Laere K., Opdenakker G., Vande Velde G., Weynand B., Teuwen D.E., Matthys P., Neyts J., Jan Thibaut H., Dallmeier K. A single-dose live-attenuated YF17D-vectored SARS-CoV-2 vaccine candidate. Nature. 2021;590(7845):320–325. doi: 10.1038/s41586-020-3035-9. [DOI] [PubMed] [Google Scholar]
- Schmidt F., Weisblum Y., Muecksch F., Hoffmann H.H., Michailidis E., Lorenzi J.C.C., Mendoza P., Rutkowska M., Bednarski E., Gaebler C., Agudelo M., Cho A., Wang Z., Gazumyan A., Cipolla M., Caskey M., Robbiani D.F., Nussenzweig M.C., Rice C.M., Hatziioannou T., Bieniasz P.D. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 2020;217(11) doi: 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J., Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. USA. 2004;101(12):4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneoka Y., Cannon P.M., Ramsdale E.E., Griffiths J.C., Romano G., Kingsman S.M., Kingsman A.J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23(4):628–633. doi: 10.1111/j.1745-7270.2007.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stertz S., Reichelt M., Spiegel M., Kuri T., Martínez-Sobrido L., García-Sastre A., Weber F., Kochs G. The intracellular sites of early replication and budding of SARS-coronavirus. Virology. 2007;361(2):304–315. doi: 10.1016/j.virol.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Zhang B., Su L. CD4 detected from Lactobacillus helps understand the interaction between Lactobacillus and HIV. Microbiol. Res. 2013;168(5):273–277. doi: 10.1016/j.micres.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Takada A., Robison C., Goto H., Sanchez A., Murti K.G., Whitt M.A., Kawaoka Y. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. USA. 1997;94(26):14764–14769. doi: 10.1073/pnas.94.26.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamin A., Harcourt B.H., Lo M.K., Roth J.A., Wolf M.C., Lee B., Weingartl H., Audonnet J.-C., Bellini W.J., Rota P.A. Development of a neutralization assay for Nipah virus using pseudotype particles. J. Virol. Methods. 2009;160(1–2):1–6. doi: 10.1016/j.jviromet.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H., Morikawa S., Matsuura Y. Development and applications of VSV vectors based on cell tropism. Front. Microbiol. 2011;2:272. doi: 10.3389/fmicb.2011.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H., Shiokawa M., Kaname Y., Kambara H., Mori Y., Abe T., Moriishi K., Matsuura Y. Involvement of ceramide in the propagation of Japanese encephalitis virus. J. Virol. 2010;84(6):2798–2807. doi: 10.1128/JVI.02499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., Doolabh D., Pillay S., San E.J., Msomi N., Mlisana K., von Gottberg A., Walaza S., Allam M., Ismail A., Mohale T., Glass A.J., Engelbrecht S., Van Zyl G., Preiser W., Petruccione F., Sigal A., Hardie D., Marais G., Hsiao M., Korsman S., Davies M.-A., Tyers L., Mudau I., York D., Maslo C., Goedhals D., Abrahams S., Laguda-Akingba O., Alisoltani-Dehkordi A., Godzik A., Wibmer C.K., Sewell B.T., Lourenço J., Alcantara L.C.J., Pond S.L.K., Weaver S., Martin D., Lessells R.J., Bhiman J.N., Williamson C., de Oliveira T. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. MedRxiv. 2020 doi: 10.1101/2020.12.21.20248640. [DOI] [Google Scholar]
- Tian J.H., Patel N., Haupt R., Zhou H., Weston S., Hammond H., Logue J., Portnoff A.D., Norton J., Guebre-Xabier M., Zhou B., Jacobson K., Maciejewski S., Khatoon R., Wisniewska M., Moffitt W., Kluepfel-Stahl S., Ekechukwu B., Papin J., Boddapati S., Jason Wong C., Piedra P.A., Frieman M.B., Massare M.J., Fries L., Bengtsson K.L., Stertman L., Ellingsworth L., Glenn G., Smith G. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021;12(1):372. doi: 10.1038/s41467-020-20653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujike M., Huang C., Shirato K., Makino S., Taguchi F. The contribution of the cytoplasmic retrieval signal of severe acute respiratory syndrome coronavirus to intracellular accumulation of S proteins and incorporation of S protein into virus-like particles. J. Gen. Virol. 2016;97(8):1853–1864. doi: 10.1099/jgv.0.000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., Avanzato V.A., Bushmaker T., Flaxman A., Ulaszewska M., Feldmann F., Allen E.R., Sharpe H., Schulz J., Holbrook M., Okumura A., Meade-White K., Perez-Perez L., Edwards N.J., Wright D., Bissett C., Gilbride C., Williamson B.N., Rosenke R., Long D., Ishwarbhai A., Kailath R., Rose L., Morris S., Powers C., Lovaglio J., Hanley P.W., Scott D., Saturday G., de Wit E., Gilbert S.C., Munster V.J. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020 doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A.B., Kanevsky I., Che Y., Swanson K.A., Muik A., Vormehr M., Kranz L.M., Walzer K.C., Hein S., Güler A., Loschko J., Maddur M.S., Ota-Setlik A., Tompkins K., Cole J., Lui B.G., Ziegenhals T., Plaschke A., Eisel D., Dany S.C., Fesser S., Erbar S., Bates F., Schneider D., Jesionek B., Sänger B., Wallisch A.-K., Feuchter Y., Junginger H., Krumm S.A., Heinen A.P., Adams-Quack P., Schlereth J., Schille S., Kröner C., de la Caridad Güimil Garcia R., Hiller T., Fischer L., Sellers R.S., Choudhary S., Gonzalez O., Vascotto F., Gutman M.R., Fontenot J.A., Hall-Ursone S., Brasky K., Griffor M.C., Han S., Su A.A.H., Lees J.A., Nedoma N.L., Mashalidis E.H., Sahasrabudhe P.V., Tan C.Y., Pavliakova D., Singh G., Fontes-Garfias C., Pride M., Scully I.L., Ciolino T., Obregon J., Gazi M., Carrion R., Alfson K.J., Kalina W.V., Kaushal D., Shi P.-Y., Klamp T., Rosenbaum C., Kuhn A.N., Türeci Ö., Dormitzer P.R., Jansen K.U., Sahin U. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592(7853):283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985;40(1) doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292 e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Tortorici M.A., Frenz B., Snijder J., Li W., Rey F.A., DiMaio F., Bosch B.-J., Veesler D. Glycan shield and epitope masking of a coronavirus spike protein observed by cryo-electron microscopy. Nat. Struct. Mol. Biol. 2016;23(10):899–905. doi: 10.1038/nsmb.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., Xu W., Zhao Y., Li N., Zhang J., Liang H., Bao L., Xu Y., Ding L., Zhou W., Gao H., Liu J., Niu P., Zhao L., Zhen W., Fu H., Yu S., Zhang Z., Xu G., Li C., Lou Z., Xu M., Qin C., Wu G., Gao G.F., Tan W., Yang X. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182(3):713–721 e9. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Casner R.G., Nair M.S., Wang M., Yu J., Cerutti G., Liu L., Kwong P.D., Huang Y., Shapiro L., Ho D.D. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29(5) doi: 10.1016/j.chom.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., Graham B.S., Mascola J.R., Chang J.Y., Yin M.T., Sobieszczyk M., Kyratsous C.A., Shapiro L., Sheng Z., Huang Y., Ho D.D. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021 doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- Wang Q., Qi J., Yuan Y., Xuan Y., Han P., Wan Y., Ji W., Li Y., Wu Y., Wang J., Iwamoto A., Woo P.C.Y., Yuen K.-Y., Yan J., Lu G., Gao G.F. Bat origins of MERS-CoV supported by bat coronavirus HKU4 usage of human receptor CD26. Cell Host Microbe. 2014;16(3):328–337. doi: 10.1016/j.chom.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Nie J., Prochnow C., Truong C., Jia Z., Wang S., Chen X.S., Wang Y. A systematic study of the N-glycosylation sites of HIV-1 envelope protein on infectivity and antibody-mediated neutralization. Retrovirology. 2013;10:14. doi: 10.1186/1742-4690-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Butler E.N., Veguilla V., Vassell R., Thomas J.T., Moos M., Ye Z., Hancock K., Weiss C.D. Establishment of retroviral pseudotypes with influenza hemagglutinins from H1, H3, and H5 subtypes for sensitive and specific detection of neutralizing antibodies. J. Virol. Methods. 2008;153(2):111–119. doi: 10.1016/j.jviromet.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., Schaefer-Babajew D., Cipolla M., Gaebler C., Lieberman J.A., Oliveira T.Y., Yang Z., Abernathy M.E., Huey-Tubman K.E., Hurley A., Turroja M., West K.A., Gordon K., Millard K.G., Ramos V., Silva J.D., Xu J., Colbert R.A., Patel R., Dizon J., Unson-O'Brien C., Shimeliovich I., Gazumyan A., Caskey M., Bjorkman P.J., Casellas R., Hatziioannou T., Bieniasz P.D., Nussenzweig M.C. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021 doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Wan L., Yan Q., Wang X., Zhang J., Yang X., Zhang Y., Fan C., Li D., Deng Y., Sun J., Gong J., Yang X., Wang Y., Wang X., Li J., Yang H., Li H., Zhang Z., Wang R., Du P., Zong Y., Yin F., Zhang W., Wang N., Peng Y., Lin H., Feng J., Qin C., Chen W., Gao Q., Zhang R., Cao Y., Zhong H. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat. Metab. 2020 doi: 10.1038/s42255-020-00324-0. [DOI] [PubMed] [Google Scholar]
- Whitt M.A. Generation of VSV pseudotypes using recombinant ΔG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J. Virol. Methods. 2010;169(2):365–374. doi: 10.1016/j.jviromet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O.N., Baltimore D. Mechanism of formation of pseudotypes between vesicular stomatitis virus and murine leukemia virus. Cell. 1977;11(3):505–511. doi: 10.1016/0092-8674(77)90068-x. [DOI] [PubMed] [Google Scholar]
- Wright E., Temperton N.J., Marston D.A., McElhinney L.M., Fooks A.R., Weiss R.A. Investigating antibody neutralization of lyssaviruses using lentiviral pseudotypes: a cross-species comparison. J. Gen. Virol. 2008;89(Pt 9):2204–2213. doi: 10.1099/vir.0.2008/000349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Zhao C., Liu Q., Huang W., Wang Y. Development and application of a bioluminescent imaging mouse model for Chikungunya virus based on pseudovirus system. Vaccine. 2017;35(47):6387–6394. doi: 10.1016/j.vaccine.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Xie X., Muruato A., Lokugamage K.G., Narayanan K., Zhang X., Zou J., Liu J., Schindewolf C., Bopp N.E., Aguilar P.V., Plante K.S., Weaver S.C., Makino S., LeDuc J.W., Menachery V.D., Shi P.Y. An Infectious cDNA Clone of SARS-CoV-2. Cell Host Microbe. 2020;27(5):841–848 e3. doi: 10.1016/j.chom.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Pradhan M., Gorshkov K., Petersen J.D., Shen M., Guo H., Zhu W., Klumpp-Thomas C., Michael S., Itkin M., Itkin Z., Straus M.R., Zimmerberg J., Zheng W., Whittaker G.R., Chen C.Z. A high throughput screening assay for inhibitors of SARS-CoV-2 pseudotyped particle entry. bioRxiv: Prepr. Serv. Biol. 2021 doi: 10.1101/2021.10.04.463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Wang W., Chen Z., Lu S., Yang F., Bi Z., Bao L., Mo F., Li X., Huang Y., Hong W., Yang Y., Zhao Y., Ye F., Lin S., Deng W., Chen H., Lei H., Zhang Z., Luo M., Gao H., Zheng Y., Gong Y., Jiang X., Xu Y., Lv Q., Li D., Wang M., Li F., Wang S., Wang G., Yu P., Qu Y., Yang L., Deng H., Tong A., Li J., Wang Z., Yang J., Shen G., Zhao Z., Li Y., Luo J., Liu H., Yu W., Yang M., Xu J., Wang J., Li H., Wang H., Kuang D., Lin P., Hu Z., Guo W., Cheng W., He Y., Song X., Chen C., Xue Z., Yao S., Chen L., Ma X., Chen S., Gou M., Huang W., Wang Y., Fan C., Tian Z., Shi M., Wang F.S., Dai L., Wu M., Li G., Wang G., Peng Y., Qian Z., Huang C., Lau J.Y., Yang Z., Wei Y., Cen X., Peng X., Qin C., Zhang K., Lu G., Wei X. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020 doi: 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]
- Yang L., Pei R.-J., Li H., Ma X.-N., Zhou Y., Zhu F.-H., He P.-L., Tang W., Zhang Y.-C., Xiong J., Xiao S.-Q., Tong X.-K., Zhang B., Zuo J.-P. Identification of SARS-CoV-2 entry inhibitors among already approved drugs. Acta Pharmacol. Sin. 2021;42(8):1347–1353. doi: 10.1038/s41401-020-00556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Hughes T.A., Kelkar A., Yu X., Cheng K., Park S., Huang W.-C., Lovell J.F., Neelamegham S. Inhibition of SARS-CoV-2 viral entry upon blocking N- and O-glycan elaboration. eLife. 2020;9 doi: 10.7554/eLife.61552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Song Y., Chen Y., Wu N., Xu J., Sun C., Zhang J., Weng T., Zhang Z., Wu Z., Cheng L., Shi D., Lu X., Lei J., Crispin M., Shi Y., Li L., Li S. Molecular architecture of the SARS-CoV-2 virus. Cell. 2020;183(3):730–738 e13. doi: 10.1016/j.cell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Li Z., He X., Gebre M.S., Bondzie E.A., Wan H., Jacob-Dolan C., Martinez D.R., Nkolola J.P., Baric R.S., Barouch D.H. Deletion of the SARS-CoV-2 spike cytoplasmic tail increases infectivity in pseudovirus neutralization assays. J. Virol. 2021 doi: 10.1128/JVI.00044-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Li Q., Liang Z., Li T., Liu S., Cui Q., Nie J., Wu Q., Qu X., Huang W., Wang Y. The significant immune escape of pseudotyped SARS-CoV-2 Variant Omicron. Emerg. Microbes Infect. 2021 doi: 10.1080/22221751.2021.2017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N.-N., Li X.-F., Deng Y.-Q., Zhao H., Huang Y.-J., Yang G., Huang W.-J., Gao P., Zhou C., Zhang R.-R., Guo Y., Sun S.-H., Fan H., Zu S.-L., Chen Q., He Q., Cao T.-S., Huang X.-Y., Qiu H.-Y., Nie J.-H., Jiang Y., Yan H.-Y., Ye Q., Zhong X., Xue X.-L., Zha Z.-Y., Zhou D., Yang X., Wang Y.-C., Ying B., Qin C.-F. A thermostable mRNA vaccine against COVID-19. Cell. 2020 doi: 10.1080/22221751.2021.2017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Liu Y., Liu J., Bailey A.L., Plante K.S., Plante J.A., Zou J., Xia H., Bopp N.E., Aguilar P.V., Ren P., Menachery V.D., Diamond M.S., Weaver S.C., Xie X., Shi P.-Y. A trans-complementation system for SARS-CoV-2 recapitulates authentic viral replication without virulence. Cell. 2021 doi: 10.1016/j.cell.2021.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Du L., Ma C., Li Y., Li L., Poon V.K., Wang L., Yu F., Zheng B.-J., Jiang S., Zhou Y. A safe and convenient pseudovirus-based inhibition assay to detect neutralizing antibodies and screen for viral entry inhibitors against the novel human coronavirus MERS-CoV. Virol. J. 2013;10:266. doi: 10.1186/1743-422X-10-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Nie J., Jiao Y., Li L., Zhang T., Liu Q., Huang W., Wu H., Wang Y. Effect of the maturation of neutralizing antibodies on human immunodeficiency virus (HIV) envelope evolution in HIV-infected subjects. Infect. Genet. Evol. J. Mol. Epidemiol. Evolut. Genet. Infect. Dis. 2016;38:82–89. doi: 10.1016/j.meegid.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Larragoite E.T., Williams E.S.C.P., Lama J., Cisneros I., Delgado J.C., Slev P., Rychert J., Innis E.A., Coiras M., Rondina M.T., Spivak A.M., Planelles V. Neutralization assay with SARS-CoV-1 and SARS-CoV-2 spike pseudotyped murine leukemia virions. Virol. J. 2021;18(1):1. doi: 10.1186/s12985-020-01472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Liu Q., Wu X., Chen P., Wu X., Guo Y., Liu S., Liang Z., Fan C., Wang Y. A safe and sensitive enterovirus A71 infection model based on human SCARB2 knock-in mice. Vaccine. 2016;34(24):2729–2736. doi: 10.1016/j.vaccine.2016.04.029. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Yu D., Yan H., Chong H., He Y. Design of potent membrane fusion inhibitors against SARS-CoV-2, an emerging coronavirus with high fusogenic activity. J. Virol. 2020;94(14) doi: 10.1128/JVI.00635-20. [DOI] [PMC free article] [PubMed] [Google Scholar]