Abstract
To obtain a rapid genotyping method of Candida albicans, three polymorphic microsatellite markers were investigated by multiplex PCR. The three loci, called CDC3, EF3, and HIS3, were chosen because they are on different chromosomes so as to improve the chances of finding polymorphisms. One set of primers was designed for each locus, and one primer of each set was dye-labeled to read PCR signals by using an automatic sequencer. Amplifications were performed directly from the colonies harvested on the agar plate without a sophisticated DNA extraction step. At total of 27 reference strains and 73 clinical independent isolates were tested. The numbers of allelic associations were 10, 22, and 25 for the loci CDC3, EF3, and HIS3, respectively. The combined discriminatory power of the three microsatellites markers was 0.97. The markers were stable after 25 subcultures, and the amplifications were specific for C. albicans. An initial study of 17 clinical isolate pairs, including blood culture and peripheral sites, showed a similar genotype for 15 of them, confirming that candidemia usually originates from the colonizing isolate. Therefore, microsatellite marker analysis with multiplex PCR and automated procedures has a high throughput and should be suitable for large epidemiologic studies of C. albicans.
Among the yeasts that have emerged as major fungal pathogens in recent years (3), the commensal Candida albicans is the most prevalent and acts as an opportunistic agent in immunocompromised patients. The ability to discriminate strains of the organism has been developed for a better understanding of the epidemiology of this yeast. Thus, the route of acquisition (21), nosocomial transmission (16), or the emergence of antifungal-resistant strains (6, 25) can be identified by using DNA-based methods already used for typing C. albicans. Strain-typing techniques such as restriction length polymorphic DNA (RFLP) with hybridization with a C. albicans-specific probe and the random amplified polymorphic DNA (RAPD) have been recently reviewed (23). The RFLP technique is very informative but is time-consuming since Southern blots are needed. The PCR-based RAPD technique is rapid but poorly reproducible, especially between laboratories. Other authors have developed allele-specific oligonucleotide probes in Southern hybridizations with PCR-amplified DNA regions (6). Another PCR-based method is the analysis of microsatellites, defined as tandemly repetitive stretches of two to five nucleotides. Since most microsatellites show a substantial level of polymorphism between individuals, microsatellites are extensively used for physical mapping in humans (28). Moreover, since microsatellites test the presence of different alleles at a given locus, distinguishing heterozygotes in diploid organisms such as C. albicans is possible in contrast to the RFLP and RAPD methods. Several studies have already reported the application of this technique for the genotyping of C. albicans (4, 7, 10, 13, 22).
One microsatellite marker in the EF3 promoter sequence of C. albicans was previously reported (4). Reliability was achieved by automated procedures by use of fluorescent probes analyzed with an automatic sequencer. The discriminatory power of this single microsatellite marker was 0.86. To obtain greater resolution, we searched for new microsatellite markers located on different chromosomes. We secondarily optimized our PCR conditions to perform the analysis in a multiplex reaction to increase the throughput of the typing system. We subsequently evaluated the performance of this typing system on collection and clinical strains.
MATERIALS AND METHODS
Primers and amplification.
A search for repeated sequences containing at least five contiguous identical motifs of one to five nucleotides was performed on the sequences of C. albicans available in GenBank. Along with the microsatellite marker already described in the upstream sequence of the elongation factor 3 gene (EF3), located on chromosome 5 (20), two other markers were selected: one downstream of the cell division cycle protein gene (CDC3), located on chromosome 1 (8), and one downstream of the coding sequence for the imidazole glycerol phosphate deshydratase gene (HIS3), located on chromosome 2 (15). The PCRs were subsequently referred to as EF3, CDC3, and HIS3, respectively. Primers were designed to amplify these microsatellite markers (Table 1), and one primer of each set was 5′ labeled with different dyes. The antisense primer of EF3 was labeled with 6-carboxyfluorescein, while the sense primer of CDC3 was labeled with 4,7,2′,4′,5′,7′-hexachloro-6-carboxyfluorescein, and the sense primer of HIS3 was labeled with 4,7,2′,7′-tetrachloro-6-carboxyfluorescein. This type of labeling allows for multiplex PCRs and sizing of the PCR products with an automatic sequencer.
TABLE 1.
Features of the three sets of primers retained in the upstream sequence of the elongation factor 3 gene (EF3), downstream of the cell division cycle protein gene (CDC3), and downstream of the coding sequence for the imidazole glycerol phosphate deshydratase gene (HIS3)
| Locus (GenBank accession no.), chromosome | Microsatellite sequence | Primer sequences (forward and reverse) |
|---|---|---|
| CDC3 (Z25869), chromosome 1 | (AGTA)8 | 5′-CAGATGATTTTTTGTATGAGAAGAA-3′ |
| 5′-CAGTCACAAGATTAAAATGTTCAAG-3′ | ||
| EF3 (Z11484), chromosome 5 | (TTTC)5(TTC)5 | 5′-TTTCCTCTTCCTTTCATATAGAA-3′ |
| 5′-GGATTCACTAGCAGCAGACA-3′ | ||
| HIS3 (AF006605), chromosome 2 | (ATTT)13 | 5′-TGGCAAAAATGATATTCCAA-3′ |
| 5′-TACACTATGCCCCAAACACA-3′ |
Amplifications were directly performed on C. albicans colonies from Sabouraud plates. The colonies were harvested with a single use plastic tip, and cells were then suspended in 20 μl of the reaction mixture, including 1× PCR buffer, 3.25 mM MgCl2, a 0.2 mM concentration of each deoxynucleoside triphosphate, 2 pmol of each of the six primers, and 1.25 U of AmpliTaq Gold (all from Applied Biosystems, les Ulis, France). The samples were initially incubated for 10 min at 95°C to activate the AmpliTaq Gold and to denature the DNA. The temperature cycling (30 cycles at 95°C for 15 s, 52°C for 1 min, and 72°C each) was performed in a 24-well thermal cycler (Applied Biosystems/Perkin-Elmer Cetus 2400). The final cycle was followed by an additional 7 min at 72°C to complete partial polymerization. PCR products were diluted 1/5 in water, and 1 μl of each was run on a 36-cm acrylamide urea gel (Sequagel; National Diagnostics) for 2 h under 3,000 V. An internal standard labeled with 6-carboxy-X-rhodamine dye (GenScan-500 Rox; Applied Biosystems) was loaded into each well, along with the PCR products. Signals were read by using a 377 automatic sequencer (Applied Biosystems), and the data were stored and analyzed with the 372 Genescan software (Applied Biosystems). To ensure the reproducibility of the results, reference strain H12 was systematically run as a control in each gel.
C. albicans strains and isolates.
To evaluate the discriminatory power of the three microsatellite markers, 100 independent C. albicans strains were genotyped, including 27 reference strains and 73 clinical isolates (Table 2). These isolates were collected from different patients in different wards in two different hospitals and from different anatomical sites. To compare C. albicans isolates responsible for invasive infections and the corresponding isolates from peripheral anatomical sites in a given patient, 18 pairs of isolates were genotyped: 9 blood culture-peripheral sites and 9 central catheter-peripheral sites.
TABLE 2.
Origin and genotype of the 100 isolates tested, including 27 reference strains and 73 independent clinical isolates with the number of isolates (n) with the same profile for calculation of the discriminatory power
| Isolate origin | Length (bp) determined by PCR analysis of:
|
n | ||||||
|---|---|---|---|---|---|---|---|---|
| CDC3 marker
|
EF3 marker
|
HIS3 marker
|
||||||
| Allele 1a | Allele 2 | Allele 1 | Allele 2 | Allele 1 | Allele 2 | |||
| Reference strain Ca4918 | 113 | 113 | 130 | 136 | 150 | 162 | 1 | |
| Anal swab | 113 | 117 | 130 | 130 | 150 | 166 | 1 | |
| Abdominal drainage | 113 | 117 | 130 | 136 | 150 | 162 | 1 | |
| Abdominal drainage | 113 | 117 | 130 | 136 | 150 | 162 | 3 | |
| Expectoration | 113 | 117 | 130 | 136 | 150 | 162 | ||
| Reference strain IP887/65 | 113 | 117 | 130 | 136 | 150 | 162 | ||
| Stools | 113 | 117 | 130 | 136 | 150 | 166 | 1 | |
| Oral mucosa | 113 | 117 | 130 | 136 | 162 | 162 | 1 | |
| Vascular catheter | 117 | 117 | 126 | 126 | 162 | 162 | 1 | |
| Expectoration | 117 | 117 | 130 | 136 | 150 | 162 | 1 | |
| Urine | 117 | 117 | 130 | 136 | 154 | 194 | 1 | |
| Reference strain IP1548/84 | 117 | 117 | 130 | 136 | 178 | 178 | 2 | |
| Reference strain IP1407/82 | 117 | 117 | 130 | 136 | 178 | 178 | ||
| Anal swab | 117 | 117 | 131 | 131 | 162 | 162 | 1 | |
| Urine | 117 | 117 | 131 | 131 | 162 | 182 | 1 | |
| Tracheal aspirate | 117 | 117 | 133 | 140 | 154 | 154 | 1 | |
| Reference strain 38696 | 117 | 117 | 133 | 144 | 154 | 154 | 1 | |
| Tracheal aspirate | 117 | 117 | 133 | 144 | 154 | 186 | 2 | |
| Stools | 117 | 117 | 133 | 144 | 154 | 186 | ||
| Blood culture | 117 | 117 | 136 | 136 | 150 | 154 | 1 | |
| Anal swab | 117 | 117 | 137 | 139 | 182 | 182 | 1 | |
| Stools | 117 | 117 | 146 | 146 | 154 | 154 | 1 | |
| Abdominal drainage | 117 | 121 | 133 | 136 | 154 | 154 | 1 | |
| Reference strain IP1876/89 | 117 | 121 | 135 | 135 | 162 | 162 | 2 | |
| Reference strain IP1877/89 | 117 | 121 | 135 | 135 | 162 | 162 | ||
| Anal swab | 117 | 121 | 136 | 136 | 150 | 150 | 1 | |
| Urine | 117 | 125 | 126 | 126 | 162 | 162 | 1 | |
| Stools | 117 | 125 | 126 | 126 | 162 | 238 | 1 | |
| Anal swab | 117 | 125 | 126 | 131 | 162 | 170 | 1 | |
| Anal swab | 117 | 125 | 126 | 135 | 150 | 150 | 1 | |
| Tracheal aspirate | 117 | 125 | 126 | 135 | 158 | 158 | 1 | |
| Vascular catheter | 117 | 125 | 126 | 135 | 162 | 162 | 17 | |
| Blood culture | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Anal swab | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Abdominal drainage | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Tracheal aspirate | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Stools | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Stools | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Surgical wound | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Tracheal aspirate | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Reference strain SC5314 | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Reference strain 3153A | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Reference strain 441B | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Reference strain A81Pu | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Reference strain IP884/65 | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Reference strain IP993/69 | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Reference strain IP996/69 | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Reference strain IP2146/93 | 117 | 125 | 126 | 135 | 162 | 162 | ||
| Vascular catheter | 117 | 125 | 126 | 135 | 162 | 170 | 1 | |
| Stools | 117 | 125 | 126 | 135 | 162 | 182 | 1 | |
| Stools | 117 | 125 | 126 | 135 | 162 | 194 | 2 |
| Oral mucosa | 117 | 125 | 126 | 135 | 162 | 194 | |
| Stools | 117 | 125 | 126 | 135 | 162 | 198 | 1 |
| Stools | 117 | 125 | 126 | 135 | 162 | 206 | 1 |
| Vascular catheter | 117 | 125 | 126 | 135 | 162 | 210 | 1 |
| Vascular catheter | 117 | 125 | 126 | 135 | 162 | 214 | 1 |
| Stools | 117 | 125 | 126 | 135 | 162 | 218 | 1 |
| Stools | 117 | 125 | 126 | 135 | 170 | 218 | 1 |
| Vascular catheter | 117 | 125 | 126 | 135 | 210 | 210 | 2 |
| Reference strain 1332/82 | 117 | 125 | 126 | 135 | 210 | 210 | |
| Tracheal aspirate | 117 | 125 | 130 | 144 | 154 | 154 | 1 |
| Stools | 117 | 125 | 131 | 131 | 162 | 162 | 4 |
| Anal swab | 117 | 125 | 131 | 131 | 162 | 162 | |
| Reference strain IP1880/89 | 117 | 125 | 131 | 131 | 162 | 162 | |
| Reference strain IP1878/89 | 117 | 125 | 131 | 131 | 162 | 162 | |
| Vascular catheter | 117 | 125 | 131 | 131 | 162 | 186 | 1 |
| Surgical wound | 117 | 125 | 131 | 131 | 162 | 190 | 2 |
| Stools | 117 | 125 | 131 | 131 | 162 | 190 | |
| Vascular catheter | 117 | 125 | 131 | 131 | 166 | 166 | 2 |
| Skin | 117 | 125 | 131 | 131 | 166 | 166 | |
| Vascular catheter | 117 | 125 | 131 | 131 | 166 | 186 | 2 |
| Blood culture | 117 | 125 | 131 | 131 | 166 | 186 | |
| Bile aspirate | 117 | 125 | 133 | 144 | 154 | 182 | 1 |
| Anal swab | 117 | 125 | 136 | 139 | 154 | 154 | 2 |
| Blood culture | 117 | 125 | 136 | 139 | 154 | 154 | |
| Stools | 117 | 129 | 130 | 145 | 170 | 170 | 1 |
| Urine | 117 | 129 | 136 | 146 | 154 | 154 | 2 |
| Blood culture | 117 | 129 | 136 | 146 | 154 | 154 | |
| Urine | 117 | 129 | 136 | 146 | 154 | 170 | 1 |
| Reference strain IP1213/80 | 121 | 121 | 130 | 130 | 154 | 154 | 1 |
| Vaginal swab | 121 | 121 | 130 | 131 | 154 | 154 | 1 |
| Anal swab | 121 | 121 | 130 | 144 | 154 | 166 | 2 |
| Stools | 121 | 121 | 130 | 144 | 154 | 166 | |
| Stools | 121 | 121 | 130 | 145 | 154 | 166 | 1 |
| Reference strain IP1180/79 | 121 | 121 | 131 | 131 | 178 | 178 | 1 |
| Reference strain IP1663/86 | 121 | 121 | 131 | 135 | 170 | 170 | 1 |
| Reference strain H12 | 121 | 125 | 130 | 144 | 154 | 154 | 1 |
| Reference strain B311 | 121 | 125 | 130 | 144 | 154 | 166 | 1 |
| Tracheal aspirate | 125 | 125 | 130 | 130 | 154 | 166 | 1 |
| Reference strain B792 | 125 | 125 | 131 | 139 | 166 | 178 | 3 |
| Reference strain IP886/65 | 125 | 125 | 131 | 139 | 166 | 178 | |
| Reference strain 28367 | 125 | 125 | 131 | 139 | 166 | 178 | |
| Blood culture | 125 | 125 | 131 | 139 | 186 | 186 | 1 |
| Stools | 125 | 125 | 131 | 139 | 194 | 194 | 1 |
| Abdominal drainage | 125 | 125 | 136 | 136 | 150 | 150 | 1 |
| Reference strain 10231 | 125 | 125 | 139 | 139 | 190 | 190 | 1 |
| Oral mucosa | 125 | 129 | 131 | 139 | 186 | 194 | 1 |
| Stools | 129 | 129 | 136 | 141 | 150 | 162 | 2 |
| Abdominal drainage | 129 | 129 | 136 | 141 | 150 | 162 | |
| Vascular catheter | 129 | 129 | 136 | 141 | 150 | 166 | 1 |
The terms allele 1 and allele 2 indicate two different alleles for a given locus.
RESULTS
For each marker and for a given isolate, one or two bands were observed. Since C. albicans is diploid and since each marker tested a single locus, each band observed was assigned to an allele. For a given isolate, identical results were obtained upon two different amplifications of the same DNA preparation and upon two different preparations of DNA of the same colony. Four reference strains (B792, Ca 4918, H12, and ATCC 38696) were subcultured 25 times in yeast potato dextrose, corresponding roughly to more than 300 generations, and the alleles were unchanged. The amplifications were specific for C. albicans, since no bands were observed upon amplification of clinical isolates of C. tropicalis, C. glabrata, and C. dubliniensis (F. Mühlschlegel) and a C. stellatoidea reference strain (IP2814).
As already done for the EF3 locus (4), we determined that the differences in length observed in the CDC3 and the HIS3 systems were due to the different number of repeats of the microsatellites. We performed direct sequencing of four alleles obtained from four homozygous reference strains: strains 28367 and 38696 for the CDC3 microsatellite and strains IP1548/84 and 10231 for the HIS3 microsatellite. The sequencing showed that the differences in length observed were due to the different number of microsatellites. However, we did not express our results as a number of repeats at a given locus because we cannot completely exclude that differences in base composition outside the microsatellite sequence could occur for some isolates (17).
Each isolate was therefore characterized by a profile of six alleles. Among the 100 independent C. albicans tested, i.e., 200 chromosomes since C. albicans is diploid, the number of alleles detected was 5, 12, and 18 in the CDC3, EF3, and HIS3 systems, respectively (Table 2). These alleles were differently associated and 10, 22, and 25 allele associations were observed in the CDC3, EF3, and HIS3 systems, respectively (Table 2). The numerical index of discriminatory power (DP), based on the probability that two unrelated isolates sampled from the test population will be placed into different typing groups, was calculated for each microsatellite marker from the formula (12):
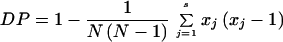 |
where s is the number of profiles, xj is the number of the population falling into the jth type, and N is the size of the population (N = 100). The DP of EF3 was 0.86, identical to the DP previously observed with different isolates (4), the DP of HIS3 was 0.91, and the DP of CDC3 was 0.77. When the three markers are combined, the DP was 0.97. An index greater than 0.90 is desirable if the typing results are to be interpreted with confidence (12).
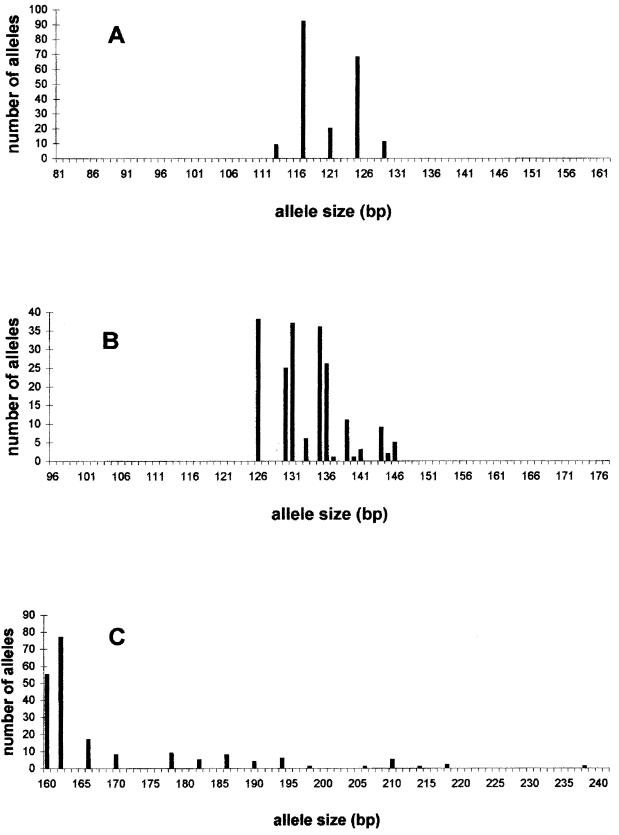
Heterogeneity was observed among the frequency of the alleles. At a given locus, most of the possible number of repeats were present demonstrating a continuum for increasing or decreasing the numbers of repeats (Fig. 1). However, the distribution of the alleles was not normal, and some alleles were overrepresented. Among the profile associations, a group of 17 isolates could not be distinguished by the three microsatellite markers (Table 2). Besides, most of the isolates tested were heterozygous for at least one locus since only three isolates were homozygous at the three loci. No definitive conclusion on the ploidy of these isolates could be drawn. However, they were considered homozygous in Table 2 and Fig. 1.
FIG. 1.
Allele size distribution at the microsatellite loci CDC3 (A), EF3 (B), and HIS3 (C) upon analysis of 200 alleles of 73 C. albicans isolates and 27 reference strains.
To explore the origin of blood-borne infections in 17 patients, eight positive blood cultures and nine cultures from central catheters, as well as the corresponding infective and/or colonizing isolates obtained from peripheral anatomical sites at the same time, were genotyped. We observed an identity of the genotypes in 15 of these patients (Table 3). For patients 7 and 17, the genotype of the C. albicans from the central catheters was different from the genotype observed in urine or on the skin (Table 3). This finding suggested an exogenous source for the contamination of the catheter. It is also noteworthy that one new allelic association was found for patient 7. This demonstrated that the allelic associations reported above with the 100 independent isolates (Table 2) do not represent all of the possibilities and that the typing system is not saturated.
TABLE 3.
Comparison of 17 pairs of isolates from blood or central catheter and the corresponding isolates from peripheral anatomical sites collected at the same timea
| Patient | Isolate origin | Length (bp) determined by PCR analysis
|
|||||
|---|---|---|---|---|---|---|---|
| CDC3 marker
|
EF3 marker
|
HIS3 marker
|
|||||
| Allele 1b | Allele 2 | Allele 1 | Allele 2 | Allele 1 | Allele 2 | ||
| Patient 1 | Blood culture | 125 | 125 | 136 | 136 | 150 | 150 |
| Surgical wound | 125 | 125 | 136 | 136 | 150 | 150 | |
| Patient 2 | Central catheter | 117 | 117 | 126 | 126 | 162 | 162 |
| Oral mucosa | 117 | 117 | 126 | 126 | 162 | 162 | |
| Patient 3 | Blood culture | 117 | 117 | 131 | 131 | 162 | 182 |
| Urine | 117 | 117 | 131 | 131 | 162 | 182 | |
| Patient 4 | Blood culture | 125 | 129 | 131 | 139 | 186 | 194 |
| Oral mucosa | 125 | 129 | 131 | 139 | 186 | 194 | |
| Patient 5 | Blood culture | 117 | 125 | 131 | 131 | 166 | 186 |
| Stools | 117 | 125 | 131 | 131 | 166 | 186 | |
| Patient 6 | Central catheter | 117 | 125 | 126 | 135 | 158 | 158 |
| Tracheal aspirate | 117 | 125 | 126 | 135 | 158 | 158 | |
| Patient 7 | Central catheter | 117 | 129 | 136 | 146 | 154 | 170 |
| Urine | 117 | 125 | 131 | 131 | 190 | 190 | |
| Patient 8 | Central catheter | 113 | 117 | 130 | 136 | 162 | 162 |
| Oral mucosa | 113 | 117 | 130 | 136 | 162 | 162 | |
| Patient 9 | Blood culture | 117 | 125 | 126 | 135 | 162 | 210 |
| Urine | 117 | 125 | 126 | 135 | 162 | 210 | |
| Patient 10 | Central catheter | 117 | 125 | 126 | 135 | 210 | 210 |
| Rectal swab | 117 | 125 | 126 | 135 | 210 | 210 | |
| Patient 11 | Central catheter | 129 | 129 | 136 | 141 | 150 | 166 |
| Tracheal aspirate | 129 | 129 | 136 | 141 | 150 | 166 | |
| Patient 12 | Central catheter | 117 | 125 | 126 | 135 | 162 | 194 |
| Oral mucosa | 117 | 125 | 126 | 135 | 162 | 194 | |
| Patient 13 | Blood culture | 125 | 125 | 131 | 139 | 186 | 186 |
| Stools | 125 | 125 | 131 | 139 | 186 | 186 | |
| Patient 14 | Blood culture | 117 | 125 | 126 | 135 | 162 | 214 |
| Urine | 117 | 125 | 126 | 135 | 162 | 214 | |
| Patient 15 | Blood culture | 121 | 121 | 130 | 144 | 154 | 166 |
| Stools | 121 | 121 | 130 | 144 | 154 | 166 | |
| Patient 16 | Central catheter | 117 | 125 | 131 | 131 | 162 | 186 |
| Tracheal aspirate | 117 | 125 | 131 | 131 | 162 | 186 | |
| Patient 17 | Central catheter | 117 | 125 | 126 | 135 | 162 | 162 |
| Skin | 117 | 129 | 136 | 146 | 154 | 154 | |
All but patients 7 and 17 had identical genotypes. The genotype of the urine isolate of patient 7 is a new genotype compared with those in Table 2.
The terms allele 1 and allele 2 indicate two different alleles for a given locus.
DISCUSSION
To be utilized as a typing system, a method must fulfill several biological and technical criteria, such as high polymorphism, reproducibility, and feasibility (23). The microsatellite markers fulfill these criteria. First, the three microsatellite markers in the present study have a high discriminatory power of 0.97. The loci tested are not clustered but are located on different chromosomes. Second, these markers are stable over many generations and do not change, similar to Aspergillus fumigatus (1) and Saccharomyces cerevisiae (11). The data are reliable if an automatic sequencer is used to measure the length of the alleles. The amplification of short DNA sequences at a high annealing temperature, as used for the analysis of microsatellites, in contrast to RAPD, increases reproducibility upon sequential tests and between laboratories. Indeed, similar results have been obtained by two different teams (4, 7), and the lengths of the alleles are numeric data that are easy to compare. However, some PCR artifacts can occur due to the addition of extra nucleotides by some Taq polymerases (5). This problem can be controlled by the systematic use of a reference strain in each experiment. Since the expected length is known, it is possible to detect an artifact and to correct the sizing of the alleles. Third, the throughput of this technique is high. There is no need for sophisticated DNA extraction procedures since heating of the colonies releases enough DNA for amplification. Moreover, multiplex PCR tests are possible when different fluorogenic dyes are used, as done in the present work to save time in preparing electrophoresis gels.
The analysis of microsatellite markers is therefore suitable for addressing medical questions such as the origin of the infective strains. For instance, the study of 17 pairs of isolates from blood or central catheter and peripheral anatomical sites showed that, in 15 cases, the genotype was identical, confirming that the patient was well infected with his or her own colonizing strain (13, 16, 26). For the other two patients, a nosocomial transmission from the medical staff or from unidentified fomites can be hypothesized. This finding can lead to an investigation of the source of the infection.
Among all possible allele associations, some account for more isolates than others. For the EF3 marker, the associations 126-135, 130-136, and 131-131 represented 31, 21, and 13% of the genotypes, respectively. These figures are close to those previously observed on 60 independent isolates by our laboratory and on 96 isolates by a different team: 25, 15, and 15% (4) and 28, 17, and 16% (7), respectively. The fact that some clusters are more prominent has already been observed with other typing systems (16, 29). Adding other microsatellite markers led to a smaller size of groups with the same genotype. Thus, the 131-131 EF3 genotype was resolved in five different genotypes, and the 130-136 EF3 genotype in four different genotypes. Interestingly, the 125-136 EF3 genotype was reduced, but the number of undistinguishable C. albicans remained at 17%. To know whether this genotype represents a population with some selective advantage warrants additional studies, especially in animals and in healthy individuals since all of the strains tested were from human patients. In the same way, specific studies focused on the minor genotypes should be designed to know whether some genotypes are more pathogenic than others, which does not appear to be the case (7, 14).
Presently, the microsatellite markers must be used cautiously for phylogenetic studies (24). If the main mechanism leading to the polymorphism observed is thought to be replication slippage, other mechanisms are hypothetized such as gene conversion (9). The mutation rate of microsatellites is also variable. It depends on the length of the individual microsatelitte (27). The average repeat number at a locus is directly proportional to the degree of length polymorphism, indicating that long loci mutate more often than short loci. This observation was confirmed with C. albicans since the more polymorphic microsatellitte is the one with the higher number of repeats (see Tables 1 and 2). Moreover, the mutation rate of microsatellites is probably not equal for each species. For instance, the mean rate in Drosophila melanogaster is lower than the mean rate in humans (9). Therefore, it is currently impossible to determine the origin of a given allele from another specific one and comparisons with other genetic markers are needed. Convergence may explain some groupings within the microsatellite markers, whereas other genetic markers can give divergent results as reported for Escherichia coli (18). Discrepancies have already been reported for RAPD and microsatellite typing for C. albicans in human immunodeficiency virus-infected patients (19). However, another recent study of A. fumigatus isolates comparing RFLP, followed by hybridization with a specific probe and microsatellite markers, allowed similar typing (2).
Whatever the cause of the different number of repeats of the microsatellites, these markers are stable, easy to assay, adaptable to a large series, and discriminatory enough to be used as a typing system to investigate clinical issues, such as the nosocomial transmission of C. albicans. This typing system should be also developed for typing other medically important yeasts such as C. glabrata and C. parapsilosis to know whether their epidemiology differs from that of C. albicans.
ACKNOWLEDGMENT
We thank Richard Calderone from Georgetown University, Washington, D.C., for critical reading of the manuscript.
REFERENCES
- 1.Bart-Delabesse E, Humbert J F, Delabesse E, Bretagne S. Microsatellite markers for typing Aspergillus fumigatus isolates. J Clin Microbiol. 1998;36:2413–2418. doi: 10.1128/jcm.36.9.2413-2418.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bart-Delabesse E, Sarfati J, Debeaupuis J P, van Leeuwen W, van Belkum A, Bretagne S, Latgé J P. Comparison of restriction fragment length polymorphism, microsatellite length polymorphism, and random amplification of polymorphic DNA analyses for fingerprinting Aspergillus fumigatus isolates. J Clin Microbiol. 2001;39:2683–2686. doi: 10.1128/JCM.39.7.2683-2686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck-Sagué C M, Jarvis W R National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 4.Bretagne S, Costa J M, Besmond C, Carsique R, Calderone R. Microsatellite polymorphism in the promoter sequence of the elongation factor 3 gene of Candida albicans as the basis for a typing system. J Clin Microbiol. 1997;35:1777–1780. doi: 10.1128/jcm.35.7.1777-1780.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownstein M J, Carpten J D, Smith J R. Modulation of non-templated nucleotide addition by Taq DNA polymerase: primer modifications that facilitate genotyping. BioTechniques. 1996;20:1004–1010. doi: 10.2144/96206st01. [DOI] [PubMed] [Google Scholar]
- 6.Cowen L E, Sirjusingh C, Summerbell R C, Walmsley S, Richardson S, Kohn L M, Anderson J B. Multilocus genotypes and DNA fingerprints do not predict variation in azole resistance among clinical isolates of Candida albicans. Antimicrob Agents Chemother. 1999;43:2930–2938. doi: 10.1128/aac.43.12.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalle F, Franco N, Lopez J, Vagner O, Caillot D, Chavanet P, Cuisenier B, Aho S, Lizard S, Bonnin A. Comparative genotyping of Candida albicans bloodstream and nonbloodstream isolates at a polymorphic microsatellite locus. J Clin Microbiol. 2000;38:4554–4559. doi: 10.1128/jcm.38.12.4554-4559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiDomenico B J, Brown N H, Lupisella J, Greene J R, Yanko M, Koltin Y. Homologs of the yeast neck filament associated genes: isolation and sequence analysis of Candida albicans CDC3 and CDC10. Mol Gen Genet. 1994;242:689–698. doi: 10.1007/BF00283424. [DOI] [PubMed] [Google Scholar]
- 9.Ellegren H. Microsatellite mutations in the germline: implications for evolutionary inference. Trends Genet. 2000;16:551–558. doi: 10.1016/s0168-9525(00)02139-9. [DOI] [PubMed] [Google Scholar]
- 10.Field D, Eggert L, Metzgar D, Rose R, Wills C. Use of polymorphic short and clustered coding-region microsatellites to distinguish strains of Candida albicans. FEMS Immunol Med Microbiol. 1996;15:73–79. doi: 10.1111/j.1574-695X.1996.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 11.Hennequin C, Thierry A, Richard G F, Lecointre G, Nguyen H V, Gaillardin C, Dujon B. Microsatellite typing as a new tool for identification of Saccharomyces cerevisiae strains. J Clin Microbiol. 2001;39:551–559. doi: 10.1128/JCM.39.2.551-559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter P R, Gaston M A. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol. 1988;26:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lunel F V, Licciardello L, Stefani S, Verbrugh H A, Melchers W J, Meis J F, Scherer S, van Belkum A. Lack of consistent short sequence repeat polymorphisms in genetically homologous colonizing and invasive Candida albicans strains. J Bacteriol. 1998;180:3771–3778. doi: 10.1128/jb.180.15.3771-3778.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luu L N, Cowen L E, Sirjusingh C, Kohn L M, Anderson J B. Multilocus genotyping indicates that the ability to invade the bloodstream is widespread among Candida albicans isolates. J Clin Microbiol. 2001;39:1657–1660. doi: 10.1128/JCM.39.4.1657-1660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magee B B, Koltin Y, Gorman J A, Magee P T. Assignment of cloned genes to the seven electrophoretically separated Candida albicans chromosomes. Mol Cell Biol. 1988;8:4721–4726. doi: 10.1128/mcb.8.11.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marco F, Lockhart S R, Pfaller M A, Pujol C, Rangel-Frausto M S, Wiblin T, Blumberg H M, Edwards J E, Jarvis W, Saiman L, Patterson J E, Rinaldi M G, Wenzel R P, Soll D R. Elucidating the origins of nosocomial infections with Candida albicans by DNA fingerprinting with the complex probe Ca3. J Clin Microbiol. 1999;37:2817–2828. doi: 10.1128/jcm.37.9.2817-2828.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metzgar D, Field D, Haubrich R, Wills C. Sequence analysis of a compound coding-region microsatellite in Candida albicans resolves homoplasies and provides a high-resolution tool for genotyping. FEMS Immunol Med Microbiol. 1998;20:103–109. doi: 10.1111/j.1574-695X.1998.tb01116.x. [DOI] [PubMed] [Google Scholar]
- 18.Metzgar D, Thomas E, Davis C, Field D, Wills C. The microsatellites of Escherichia coli: rapidly evolving repetitive DNAs in a non-pathogenic prokaryote. Mol Microbiol. 2001;39:183–190. doi: 10.1046/j.1365-2958.2001.02245.x. [DOI] [PubMed] [Google Scholar]
- 19.Metzgar D, van Belkum A, Field D, Haubrich R, Wills C. Random amplification of polymorphic DNA and microsatellite genotyping of pre- and posttreatment isolates of Candida spp. from human immunodeficiency virus-infected patients on different fluconazole regimens. J Clin Microbiol. 1998;36:2308–2313. doi: 10.1128/jcm.36.8.2308-2313.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers K K, Fonzi W A, Sypherd P S. Isolation and sequence analysis of the gene for translation elongation factor 3 from Candida albicans. Nucleic Acids Res. 1992;20:1705–1710. doi: 10.1093/nar/20.7.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redding S W, Zellars R C, Kirkpatrick W R, McAtee R K, Caceres M A, Fothergill A W, Lopez-Ribot J L, Bailey C W, Rinaldi M G, Patterson T F. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J Clin Microbiol. 1999;37:3896–3900. doi: 10.1128/jcm.37.12.3896-3900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richard G F, Hennequin C, Thierry A, Dujon B. Trinucleotide repeats and other microsatellites in yeasts. Res Microbiol. 1999;150:589–602. doi: 10.1016/s0923-2508(99)00131-x. [DOI] [PubMed] [Google Scholar]
- 23.Soll D R. The ins and outs of DNA fingerprinting the infectious fungi. Clin Microbiol Rev. 2000;13:332–370. doi: 10.1128/cmr.13.2.332-370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor J W, Geiser D M, Burt A, Koufopanou V. The evolutionary biology and population genetics underlying fungal strain typing. Clin Microbiol Rev. 1999;12:126–146. doi: 10.1128/cmr.12.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Belkum A, Melchers W, de Pauw B E, Scherer S, Quint W, Meis J F. Genotypic characterization of sequential Candida albicans isolates from fluconazole-treated neutropenic patients. J Infect Dis. 1994;169:1062–1070. doi: 10.1093/infdis/169.5.1062. [DOI] [PubMed] [Google Scholar]
- 26.Voss A, Hollis R J, Pfaller M A, Wenzel R P, Doebbeling B N. Investigation of the sequence of colonization and candidemia in nonneutropenic patients. J Clin Microbiol. 1994;32:975–980. doi: 10.1128/jcm.32.4.975-980.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber J L, Wong C. Mutation of human short tandem repeats. Hum Mol Genet. 1993;2:1123–1128. doi: 10.1093/hmg/2.8.1123. [DOI] [PubMed] [Google Scholar]
- 28.Weissenbach J, Gyapay G, Dib C, Virginal A, Morissette J, Millasseau P, Vaysseix G, Lathrop M. A second-generation linkage map of the human genome. Nature. 1992;359:794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Mitchell T G, Vilgalys R. PCR-restriction fragment length polymorphism (RFLP) analyses reveal both extensive clonality and local genetic differences in Candida albicans. Mol Ecol. 1999;8:59–73. doi: 10.1046/j.1365-294x.1999.00523.x. [DOI] [PubMed] [Google Scholar]