Abstract
Germinal matrix hemorrhage-intraventricular hemorrhage (GMH-IVH) remains an important cause of brain injury in preterm infants, and is associated with high rates of mortality and adverse neurodevelopmental outcomes, despite the recent advances in perinatal care. Close neuroimaging is recommended for both the detection of GMH-IVH and for the follow-up of serious complications, such as post-hemorrhagic ventricular dilatation (PHVD). Although the question when best to treat PHVD remains a matter of debate, recent literature on this topic shows that later timing of interventions predicted higher rates of neurodevelopmental impairment, emphasizing the importance of a well-structured neuroimaging protocol and timely interventions. In this guideline, pathophysiologic mechanisms, preventive measures, and clinical presentations of GMH-IVH and PHVD will be presented, and a neuroimaging protocol as well as an optimal treatment approach will be proposed in light of the recent literature.
Keywords: Germinal matrix hemorrhage, intraventricular hemorrhage, complications
Introduction
Germinal matrix hemorrhage-intraventricular hemorrhage (GMH-IVH) remains an important cause of brain injury in preterm infants, and is associated with high rates of mortality and adverse neurodevelopmental outcomes, despite the recent advances in perinatal care. The incidence of GMH-IVH is inversely proportional to the degree of maturity, and the current incidence is reported to be around 20-25% in the very preterm population, while it affects around 45% of extremely preterm infants born under 26 weeks’ gestation.1-3 With the recent advances in neonatology, severe grades of GMH-IVH affect 5-10% of preterm infants who were born at <32 weeks of gestation.4-6
Pathophysiology
The germinal matrix (GM) is a gelatinous layer in the subependymal region of the preterm brain, with a dense vascular network, located between the caudate nucleus and thalamus at the level of the foramen of Monro. It is most active between 8 and 28 weeks of gestation and is the origin of neuronal precursors and glial cells.7,8 This transient anatomic region starts to regress beyond 28 weeks, and its full involution takes place at around 34 to 36 weeks.7-10 Arterial blood supply of the GM originates from the Heubner’s artery, a branch of the anterior cerebral artery and the terminal branches of the lateral striate artery, while the venous drainage merges with subependymal veins and medullary veins of the white matter, which then drain into the venous system through the terminal vein.6,11-13
The immature vascular bed of the GM region is the site of origin of GMH-IVH in very preterm infants, as the vasculature of the GM in the developing preterm brain is more fragile compared to the other cerebral veins.1,6,11,14 Endothelial tight junctions, astrocyte endfeet coverage, basal lamina, and pericytes are important building blocks of the stability of GM vasculature; however, GM vessels are poor in these elements, which contributes to the regional instability.7,15 It has been hypothesized that the paucity of endothelial tight junction proteins, and glial fibrillary acidic protein, both of which form the astrocyte endfeet coverage as the gestational week progresses, may predispose very preterm infants to GM hemorrhage.7,16 It has been shown that fibronectin in the extracellular matrix of GM vasculature is scarce, which may also play a role in the etiopathogenesis of GMH-IVH.17 The anatomic variations of the subependymal vein and narrow angulations of its terminal branch have also been shown to contribute to the development of GMH-IVH.6,18 All of these distinguishing characteristics of the GM create an increased risk of venous pressure and congestion, leading to GMH-IVH.11
Sudden fluctuations in cerebral blood flow may cause GMH-IVH in the preterm brain. The autoregulation of cerebral blood flow in preterm infants is more immature compared to the term infants, and this causes “pressure-passive” circulation, where cerebral blood flow cannot be maintained during fluctuations in systemic blood flow, resulting in damage to the fragile vasculature. The other common neonatal factors that can cause fluctuations in cerebral blood flow include pain, hypercarbia, acidosis, hypoglycemia, asphyxia, seizures, and rapid volume expansion.11,14,18-21
GMH-IVH leads to the development of two-stage brain damage. The primary brain injury occurs due to hemorrhagic insult, and secondary brain injury is due to subsequent neuroinflammation and neurotoxicity resulting in white matter damage. Previous studies have demonstrated that microglia and astrocyte activation, degraded blood components (e.g., extracellular hemoglobin, hemosiderin, methemoglobin, and iron) and the resulting toxic substances (e.g., bilirubin and carbon monoxide), coagulation products (e.g., thrombin, plasmin, fibrinogen, and platelets), infiltration of systemic inflammatory cells, necrotic process in neurons and glial cells, and the arrested maturation of the pre-oligodendrocytes caused by hemorrhage, result in brain injury in preterm infants affected by GMH-IVH.22-29
Clinical Findings and Classification Systems
While GMH-IVH commonly occurs in the first 3 days of life, the vast majority of the cases occur within the first 24 hours.6 The hemorrhage may progress over the course of the next 3 to 5 days after the onset of hemorrhage, in around 20-40% of the cases.1,11 Late-onset GMH-IVH is usually associated with sepsis and postnatal events causing hemodynamic fluctuations.30,31 Although rare, bleeding diatheses should also be considered in atypical cases.32 GMH-IVH can cause 3 distinct clinical presentations.11
Silent Presentation: The most common presentation without apparent clinical signs, that can only be detected by routine cranial ultrasound (cUS) scans.
Acute Presentation: Presents with feeding intolerance, respiratory problems, deterioration in the level of consciousness, and abnormal neurological examination findings that evolve gradually after the onset of GMH-IVH. While this presentation was previously the most common type, it is less commonly seen today, owing to routine cUS screening.
Catastrophic Presentation: The least common presentation, with a rapid deterioration of clinical condition within hours. Lethargy, hypotonia, abnormal posture, apnea, seizures, metabolic acidosis, and a sudden drop in the hemoglobin levels are among the common manifestations.4,11
While the Papile classification33 is still commonly used worldwide, the classification system developed by Joseph Volpe1 using cUS is recommended in these guidelines, with an aim to develop a common language. The details of this classification system are given in Table 1.
Table 1.
| Grade | Appearance in the parasagittal cUS section* |
| Grade I | Germinal matrix hemorrhage (GMH) This hemorrhage can extend into the ventricle; however, it occupies less than 10% of the ventricular space |
| Grade II | Hemorrhage occupying 10-50% of the ventricular space |
| Grade III | Hemorrhage occupying >50% of the ventricular space (usually accompanied by acute ventricular dilatation) |
| Periventricular hemorrhagic infarction | Parenchymal hemorrhage on the ipsilateral side |
*The amount of intraventricular hemorrhage is assessed on the parasagittal cUS views.
cUS, cranial ultrasonography.
Prevention
As prematurity is the single most important risk factor for GMH-IVH, the most effective method for the prevention of GMH-IVH is to prevent preterm birth.2,5,34-38 However, certain approaches that can be applied in the antenatal period, the delivery room, and the neonatal intensive care unit are also effective in preventing GMH-IVH.
Antenatal Practices
Antenatal Steroids: The role of antenatal steroids in the prevention of GMH-IVH in pregnant women at risk of preterm delivery has been shown in multiple studies.5,6,36,39-41 A substantial decrease in the risk of GMH-IVH has also been shown by antenatal steroid administration in a recent systematic review.42
Transport of the Newborn: Multiple studies show that the incidence of GMH-IVH is increased in preterm infants delivered in non- tertiary neonatal intensive care units (NICU) and transferred to a tertiary care center after delivery.36,41,43-46
Treatment of Chorioamnionitis: The association between clinical chorioamnionitis and GMH-IVH in premature infants has been shown in several studies.43,47-51 Histological chorioamnionitis, however, has not been shown to cause increase in GMH-IVH incidence, as seen in a recent large case series.52 The antenatal detection and treatment of clinical chorioamnionitis is an effective method for the prevention of GMH-IVH.48,51,53
Administration of Tocolytic Agents: Rescue tocolysis delays labor by up to 48 hours, and allows antenatal steroid administration and transfer of the pregnant woman to a tertiary center.40 However, maintenance tocolytics administered after this period have not been demonstrated to have an effect on the prevention of neonatal morbidities.54,55 The results of the EPIPAGE-II trial show a decrease in high-grade GMH-IVH after tocolytic administration to women with threatened preterm labor.56
Summary of Recommendations
The most effective method in the prevention of GMH-IVH is to prevent preterm birth (A1).
In cases where preterm delivery is unavoidable, antenatal steroid administration is one of the most effective methods in reducing GMH-IVH (A1).
In preterm infants <32 weeks who are anticipated to undergo inter-hospital transport, the aim should be intra-uterine transfer to the tertiary NICUs (B1).
Clinical chorioamnionitis should be diagnosed and treated to prevent GMH-IVH (B1).
Rescue tocolysis as indicated allows delaying preterm labor and completing antenatal steroid course, leading to reduction in the incidence of GMH-IVH (B2).
Delivery Room Practices
Method of Delivery: There is insufficient evidence to show that cesarean section provides reduction in the incidence of GMH-IVH in preterm infants.57 While several retrospective studies have shown that it provides reduction in the risk of GMH-IVH in preterm infants <32 weeks, it is clear that further studies are warranted.5,58
Delayed Cord Clamping (DCC): DCC for at least 30-60 seconds is a common practice in preterm infants who are not depressed at birth and do not require resuscitation.59 In a meta-analysis, DCC has been demonstrated to be effective in reducing GMH-IVH.60 However, in a recent prospective randomized controlled trial on 1566 preterm infants, DCC was not associated with risk reduction for high-grade GMH-IVH.61
Umbilical Cord Milking (UCM): A recent meta-analysis showed that UCM may be effective in reducing GMH-IVH in preterm infants who are depressed at birth and require delivery room resuscitation.62 In this meta-analysis of 7 randomized trials in 501 infants, UCM was shown to reduce GMH-IVH (RR: 0.62, 95% CI: 0.41-0.93).62 However, a prospective randomized controlled trial comparing the efficacy of UCM and DCC in preterm infants <32 weeks was prematurely terminated due to the increased incidence of severe GMH-IVH in infants <27 weeks undergoing UCM.63 Until the risks and benefits of this method are elucidated by further studies, UCM is not recommended as a routine practice, especially in preterm infants <29 weeks.64
Resuscitation: The preterm brain should be protected from cerebral blood flow that is too low or too high, as well as from sudden blood flow fluctuations, and the aim should be to support the transition period of preterm infants in the delivery room as much as possible. Hypotension and hypertension should be avoided in the delivery room, and optimum tissue perfusion should be the aim for the preterm infant.65 In preterm infants requiring resuscitation, the necessary procedures should be performed “gently,” and multiple intubations and painful stimuli should be avoided during resuscitation.66
Other Practices to Reduce the Risk of GMH-IVH: GMH-IVH can also be prevented in very preterm infants by avoiding hypothermia, hypoxia/hyperoxia, hypocarbia/hypercarbia, as well as multiple intubations in the delivery room. GMH-IVH has also been associated with fluid overload, inotrope use, and sodium bicarbonate infusion. Therefore, caution should be exercised to avoid these practices.36,53,67-71
Summary of Recommendations
There is insufficient evidence to show that cesarean section reduces the incidence of GMH-IVH. All risk factors for the mother and the baby should be considered, and prematurity alone should not be regarded as an indication for cesarean section (C2).
Delayed cord clamping for 30-60 seconds in very low-birthweight preterm infants who are not depressed and do not require delivery room resuscitation should be the standard practice (A1).
There is insufficient data to recommend UCM in preterm infants. Until this is elucidated by further studies, this practice should be avoided as it may increase the incidence of GMH-IVH (A1).
Supporting the transition period as much as possible in the delivery room should be preferred over resuscitation. In preterm infants requiring resuscitation, “gentle” resuscitation should be performed, and multiple intubations and painful stimuli should be avoided (B1).
Hypothermia, hypoxia/hyperoxia, hypocarbia/hypercarbia, and hypotension/hypertension should be avoided in the delivery room (B1).
Fluid loading and sodium bicarbonate infusion without clear indications should be avoided in the delivery room (C1).
For the infants with a gestational age of <32 weeks, an SpO2 of ≥80% and heart rate of >100/minute should be targeted within the first 5 minutes of postnatal life (C2).
NICU Practices
Prevention of Sudden Fluctuations of Cerebral Blood Flow: The avoidance of hypothermia, hypoxia/hyperoxia, and hypocarbia/hypercarbia initiated at the delivery room should be continued in the critical timeframe of the first 72 hours of postnatal life. Instead of treating hypotension based solely on numbers, it is recommended to closely monitor the parameters of adequate perfusion (e.g., capillary refill time, heart rate, diuresis, metabolic acidosis, and lactate levels).36,53,67-69 Near-infrared spectroscopy (NIRS) is frequently used in NICUs to monitor and optimize the cerebral blood flow; however, current evidence is insufficient to recommend its routine use.53,72-74
Prevention of Sodium and Glucose Imbalances: Cerebral blood flow is increased following hypoglycemia. It has also been shown that hyperglycemia and hyponatremia can increase the incidence of hemorrhage by elevating serum osmolarity. Attention should be paid to glucose and sodium metabolism, which should be kept within the normal range.75-78
Avoiding Off-label Use of Packed Red Blood Cell Transfusions: While the causality is not fully elucidated, it has been shown that avoiding off-label transfusions within the first week of life reduces the risk of GMH-IVH in very low-birthweight preterm infants.79
Correction of Coagulation Parameters: Routine use of fresh frozen plasma is a common practice to correct the impaired coagulation parameters in preterm infants.6 However, the literature is controversial, and there is insufficient evidence to support the routine correction of the coagulation parameters. Coagulation disorders should be treated as indicated.80-82
Management of Respiratory Disorders: Preferring non-invasive respiratory support modalities whenever possible in preterm infants, synchronizing the ventilator with the infant, and less invasive surfactant administration (LISA) in extremely preterm infants below 27 weeks reduce the incidence of GMH-IVH.83,84 The LISA method was also touched on in the 2019 European Consensus Guidelines on Respiratory Distress Syndrome, and if the practitioner is experienced, this technique of surfactant administration is recommended in preterm infants undergoing CPAP.65
Neutral Head Positioning: In the first 3 days of postnatal life when the head turns to one side, jugular venous return on ipsilateral side may diminish, which may put the infant at risk of GMH-IVH.53,85 However, there is not sufficient evidence in the literature yet to show the efficacy of the neutral head positioning method.86 On the other hand, it is worth noting that this practice is a part of bundle care in centers experienced in neonatal neurology with low GMH-IVH incidence in Europe and the United States. In a Dutch multicenter trial on this subject, it has been shown that the incidence of GMH-IVH is reduced by midline neutral head positioning, a slight upward tilting the head of the incubator, prevention of rapid administration of intravenous medications, gentle collection of blood samples for laboratory tests, and avoidance of rapid lifting of legs during care such as diaper change, as part of bundle care in the first 72 hours of postnatal life in preterm infants <30 weeks.87
Summary of Recommendations
The practice of preventing hypothermia, hypoxia/hyperoxia, and hypocarbia/hypercarbia should be continued during NICU stay. Significant fluctuations of blood glucose and serum sodium levels should be prevented (B1).
Hypotension and hypertension should be avoided, and attention should be paid to the clinical parameters of optimum tissue perfusion (B1). While the NIRS method is commonly used for this purpose, there is insufficient level of evidence for now to recommend its routine use (C2).
During the NICU admissions of preterm infants, off-label packed red blood cell transfusions should be avoided (C1). Coagulation disorders should be treated as indicated (C2).
When possible, non-invasive methods of ventilation should be preferred over invasive mechanical ventilation for the management of respiratory disorders (A1). Less invasive surfactant administration techniques can be preferred at the discretion of the physician (B2).
Minimum handling should be exercised within the first 72 hours of life for preterm infants <32 weeks. Bundle care methods including midline neutral head positioning, slightly tilting the head of the incubator upwards, preventing rapid administration of intravenous medications, gentle collection of blood samples and avoiding rapid lifting of legs during care and diaper changes are becoming increasingly common practices. However, for now, there is insufficient evidence to confirm the efficacy of these methods (C2).
Treatment
Currently, there is no treatment modality shown to be effective in reversing GMH-IVH once it has started; therefore, preventive strategies are of utmost importance. Treatment is largely based on supportive therapy, early detection of related complications, and identification of patients who are at risk of neurodevelopmental impairments, to support the neuroplastic capacity of the developing brain with early interventions.1,6,38,53
The principle of avoiding hypothermia, hypoxia/hyperoxia, hypocarbia/hypercarbia, hypotension/hypertension, and fluid-electrolyte and blood gas imbalances should be continued as supportive measures after hemorrhage is detected.36,53,67-69 GMH-IVH is a catabolic process, and secondary complications such as hypoglycemia should be prevented by maintaining optimum nutrition. Especially, pain should be diagnosed and treated using objective scoring systems. It is also recommended to treat the clinical/electrophysiological seizures which may occur after a hemorrhage.1,6
Summary of Recommendations
Supportive therapy should be given, and hypothermia, hypoxia/hyperoxia, hypocarbia/hypercarbia, significant hypotension/hypertension, and fluid-electrolyte and blood gas imbalances should be avoided. Pain should be detected with objective scoring systems and treated accordingly (C1).
Nutritional support should be optimized, and secondary nutritional complications such as hypoglycemia should be prevented (C1).
Clinical/electrophysiological seizures should be treated with anti-epileptic agents using doses adapted for preterm infants, to prevent further brain injury (C2).
Neuroimaging Protocol
Neuroimaging is recommended for both the detection of GMH-IVH and for the follow-up of complications.88 Computerized tomography should be avoided for this purpose, as it contains ionizing radiation. Cranial ultrasound (cUS) is an indispensable tool, and is the recommended neuroimaging modality for the diagnosis and follow-up of complications of GMH-IVH. It does not contain ionizing radiation, can be performed at the bedside, does not require sedation, is reproducible, and is highly sensitive in detecting intraventricular hemorrhage and its complications.36,87,89
Timing of cUS Screening: There is no study comparing the efficacy of different timings of imaging in preterm infants. The recommendations published by the American Academy of Neurology in 2002 on this subject are insufficient for the detection and monitoring of the complications of hemorrhage in extremely premature infants today.6,90
Today, we see that a number of centers specialized in neonatal neurology practices in the US, Canada, and Europe use variable protocols. Since some of these protocols require very frequent follow-up exams, their applicability might not be realistic for some centers. Therefore, we suggest that adhering to the protocol (explained in detail below) as much as possible is of paramount importance (Table 2). While it is an increasingly common global practice that cUS monitorization is performed by neonatologists, it can be performed legally by radiologists or pediatric radiologists in Turkey.
Table 2.
Cranial Ultrasonography Scan Protocol in Preterm Infants (D2)
| Gestational Age in Weeks | Within the First 24 hours | At the End of 3 days | At the End of the First Week | At the End of the Second Week | At the End of the Fourth Week | Subsequent Screenings |
|---|---|---|---|---|---|---|
| <280/7 | + | + | + | + | + | At 2-week intervals until postmenstrual age 34 and at discharge |
| 280/7-316/7 | - | + | + | + | + | At discharge |
*If GMH-IVH is detected at any time, until the hemorrhage and the PHVD are stabilized, cUS scans should be continued at least once a week for low-grade hemorrhages (Grade I and Grade II) and at least twice a week for high-grade hemorrhages (Grade III and PVHI).
**cUS should be repeated in infants with a new-onset hemodynamic impairment, sepsis, meningitis, severe respiratory disorders, and heart failure.
***cUS scan should be performed in case of any risk factor or conditions which may cause hemodynamic impairment such as sepsis, meningitis, severe respiratory disorders, or congestive heart failure in the preterm group above >320/7 weeks.
Preterm Infants <28 0/7 Weeks
First Screening: In this group, cUS should be performed within the first 24 hours after delivery if possible, following cardio-respiratory stabilization, completion of the necessary invasive interventions, initiation of total parenteral nutrition, and all necessary medical therapies. Antenatal hemorrhages and hemorrhages occurring during resuscitation in the delivery room can be detected using this screening. An important advantage of this screening is the fact that it provides prognostic information to the physician during family meetings.
Second Screening: Since the majority of GMH-IVH occurs within the first 72 hours in preterm infants, we recommend a second screening upon the completion of the first 72 hours.
Third Screening: To appreciate the full extent of hemorrhage, the cUS should be repeated at the end of the first week.
Subsequent Screenings: We recommend repeating the cUS screening when the postnatal 2nd week is completed, and then at 2-week intervals until postmenstrual age 34 weeks is reached. In the absence of an event that may cause cardio-respiratory deterioration (**explained below), the procedure should be repeated before the discharge, as part of the discharge preparation.
*cUS Screening When GMH-IVH is Detected: If GMH-IVH is detected at any time, cUS scans should be continued at least once a week for low-grade hemorrhages (Grade I and Grade II), and at least twice a week for high-grade hemorrhages (Grade III and PVHI) until the hemorrhage and the post-hemorrhagic ventricular dilatation (PHVD) are stabilized.
**cUS Screening After Concurrent Disorders: Reinitiating cUS screening is essential following sepsis, meningitis, cardio-respiratory events impacting the hemodynamic status, and systemic events deteriorating the clinical condition in a previously stable preterm infant.
Preterm Infants Between 28 0/7 and 31 6/7 weeks
First Screening: We recommend the first cUS screening to be performed at the end of the first 72 hours.
Second Screening: The screening should be repeated at the end of postnatal week 1.
Subsequent Screenings: We recommend repeating the cUS screening at the end of postnatal weeks 2 and 4. Afterwards, in the absence of severe intervening disease (**as explained above), the procedure should be repeated before the discharge of the preterm infant, as part of the discharge preparation.
Preterm Infants Above 32 0/7 weeks
Since the risk of GMH-IVH is low in this infant group and there are insufficient data in the literature to recommend a routine cUS scan, cUS screening should be performed if there is an antenatal event requiring an emergency cesarean with risk factors for hemorrhage being present, the need for resuscitation in the delivery room, sudden deterioration in the clinical condition, a stormy postnatal period, occurrence of cardio-respiratory events impairing hemodynamics and requiring fluid load or inotrope administration, and sepsis. If hemorrhage is detected, the screening should continue until the hemorrhage is stabilized, at least once a week for low-grade hemorrhages (Grade I and Grade II) and at least twice a week for high-grade hemorrhages (Grade III and PVHI).
Doppler Measurements in Addition to cUS Screening: Measuring flow velocity through the anterior or middle cerebral artery using Doppler ultrasonography, especially resistive index ([maximum systolic velocity−end-diastolic velocity]/maximum systolic velocity) measurement can provide invaluable information on brain hemodynamics, especially in cases with PHVD.91,92 The increase in resistive index above +2 standard deviation (SD) indicates diastolic flow loss due to ventricular dilatation, and therefore, serious impairment of brain perfusion.91,92 However, for now, there is insufficient evidence in the literature to recommend its routine use.
Assessment of the Cerebellum Through the Mastoid Window: The incidence of cerebellar hemorrhage accompanying GMH-IVH is between 2.2% and 19%, and the incidence of isolated cerebellar hemorrhage is around 2.5% in preterm infants <32 weeks.93,94 cUS scans through the anterior fontanelle are insufficient to detect cerebellar hemorrhages, which are at the furthest region to ultrasonography probe. Therefore, imaging through the mastoid window as a part of routine screening is becoming a common practice today. The cerebellum should especially be assessed in cases with GMH-IVH.
Role of Brain Magnetic Resonance Imaging in GMH-IVH: Brain MR imaging has become a common practice for preterm infants with high-grade GMH-IVH who reach postmenstrual 38-42 weeks, due to its superiority in predicting long-term prognosis. However, for now, there is insufficient evidence in the literature to recommend routine brain MR imaging to assess preterm infants with GMH-IVH when they reach term.95,96
Post-hemorrhagic Ventricular Dilatation
PHVD is the most common complication of GMH-IVH and is closely associated with mortality and neurodevelopmental impairments.38,97,98 Although it frequently accompanies Grade III GMH-IVH, it should be kept in mind that it may also rarely accompany low-grade hemorrhages.4 While PHVD occurs within 1-2 weeks following the hemorrhage, increased head circumference (generally accepted upper limit being 2 cm/week), bulging anterior fontanelle, split cranial sutures, sunset sign in the eyes, bradycardia, apnea, development of respiratory symptoms, and feeding difficulties occur later in the course, usually at around 3 to 4 weeks after the hemorrhage.97 There is compelling evidence showing that within this time period, cellular damage occurs due to compression of periventricular white matter tissues, and volume loss occurs in the basal ganglia and cerebellum due to inflammatory processes.98,99 Therefore, waiting for the clinical signs to occur in order to intervene in infants with PVHD will result in further neurological injury. However, the timing of the interventions is one of the most controversial issues in neonatal neurology.1,6
Measurement Technique and Reference Charts
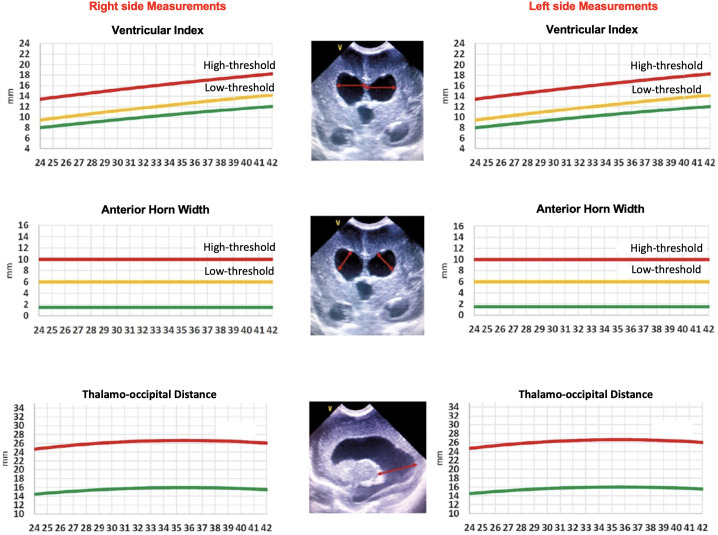
Certain sonographic measurement points were defined for the objective assessment of PHVD.100-104 These measurement points are described in Figure 1. After the measurements are performed, ventricular indices should be recorded on the charts separately for the right and the left lateral ventricles (Figure 2).100 Although the curves described by Levene are commonly used, they do not include the preterm infants at <27 weeks’ gestation.102-104 Therefore, the curves defined by Boyle M103 or Brouwer AJ104 can be used for infants below 27 weeks’ gestation. The charts below can be used for this purpose (Figure 2).
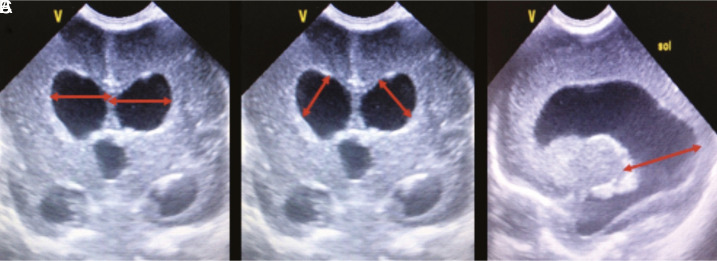
Figure 1.a-c.
Cranial ultrasound scans in a preterm infant with post-hemorrhagic ventricular dilatation. Figures represent the measurement points. (A) Ventricular index is the horizontal distance between the outermost part of the lateral ventricle and the interhemispheric fissure on the coronal scan at the level of the foramen of Monro. (B) Anterior horn width is the longest diagonal distance between the frontal horns of the lateral ventricles on the coronal scan at the level of the foramen of Monro. (C) Thalamo-occipital distance is the measurement between the most posterior portion of the thalamus and the occipital horn of the lateral ventricle in the parasagittal view.
Figure 2.
Curves indicating the ventricular index, anterior horn width, and thalamo-occipital distance. These measurements should be obtained in the right and left hemispheres separately, and these adapted charts should then be used to plot the measurements. These charts are adapted from El-Dib et al.111
Prevention of PVHD
There is no approach in the literature shown to prevent PHVD development in preterm infants with high-grade hemorrhage. There are meta-analyses showing that lumbar puncture (LP) interventions performed to prevent the development of PHVD are not effective on mortality and neurodevelopmental outcomes.105,106
Management of PVHD
In infants with PVHD, both mortality and long-term neurodevelopmental impairments can be reduced in properly managed cases. For this purpose, CSF drainage with ventricular taps through the anterior fontanelle is discouraged, as it causes cystic encephalomalacia in the white matter needle tracts.105 The intraventricular administration of fibrinolytic agents has become popular; however, studies have shown that it is ineffective, and later studies have shown that it leads to secondary hemorrhages.107,108 While the 2- and 10-year cognitive scores in these studies were favorable, this method is not routinely recommended as it is highly invasive, requires specific experience and equipment, and increases the risk of secondary hemorrhages.109,110
Watching for Clinical Signs of PHVD: The clinical parameters should be closely followed in preterm infants who develop PHVD. Although the signs of increased intracranial pressure occur after several weeks, these infants should be closely followed for an increase in the head circumference, bulging anterior fontanelle, and split cranial sutures, with daily assessments. Special attention should be paid to the presence of the sunset sign in the eyes, respiratory irregularities, resistant bradycardia, and feeding intolerance.1,6
Close cUS Monitorization: Close cUS monitorization is recommended in infants with PVHD. While there is no consensus in the literature on the frequency of cUS scans, it should be performed at least twice weekly in high-grade hemorrhages.1 These scans should be continued until PVHD is stabilized, and these infants should continue to be followed with cUS scans until they reach postmenstrual 34 weeks.111
Medications for the Treatment of PHVD: The efficacy and safety of medications such as furosemide and acetazolamide have not been demonstrated, and they have been shown to be ineffective. Furthermore, lower motor neurodevelopmental scores have been shown in patients undergoing furosemide therapy.112,113 The use of these 2 agents is not recommended to prevent progression of PHVD.114 Intraventricular fibrinolytic therapy cannot routinely be recommended after the onset of PHVD, as it can cause an increase in secondary hemorrhages, requires special experience and equipment, and is highly invasive. Moreover, when the neurodevelopmental outcomes of the DRIFT trial108,109,114 and the ELVIS trial97,99,115 are compared, larger ventricular measurements and a higher rate of neurodevelopmental impairments can be seen in the DRIFT trial.116
Serial Lumbar Punctures
It has been shown that after the development of PVHD, the removal of hemorrhagic cerebrospinal fluid (CSF) by serial LPs enables the removal of the cellular components of the blood and inflammatory mediators in the CSF, reduces the compression on the periventricular white matter, opens the blocked interventricular foramina, and facilitates resorption of CSF by reducing the obliteration of arachnoid villi. Serial LPs can be performed in 3 ways:
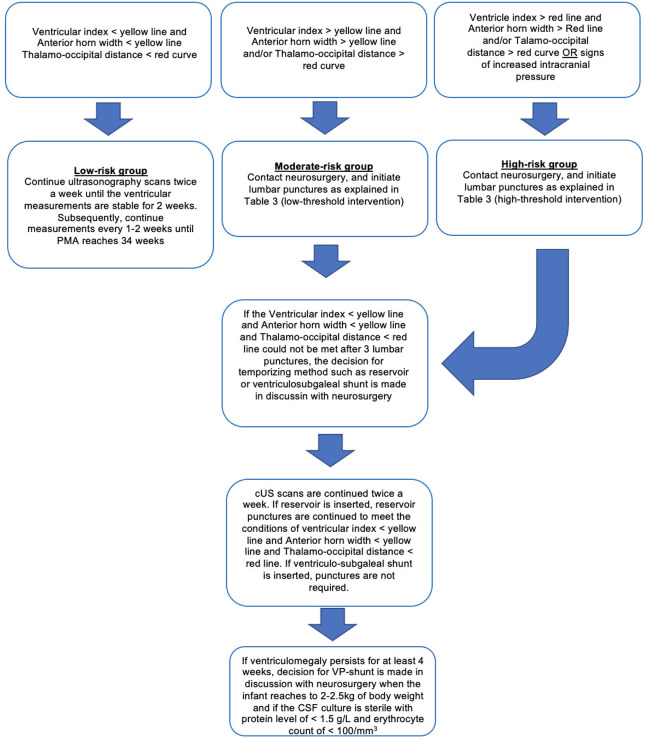
Low-threshold Intervention: Interventions are initiated when the bilateral ventricle indices exceed the 97th percentile (+2 SD curve) and move toward the 97th percentile + 4 mm line (+3 SD curve), and the anterior horn width is >6 mm and/or the thalamo-occipital distance exceeds 25 mm.
High-threshold Intervention: Interventions are initiated when the bilateral ventricle indices are > 97th percentile + 4 mm line (+3 SD curve) and the anterior horn width is >10 mm.
Waiting for Signs of Increased Intracranial Pressure: Interventions are initiated when there is a rapid increase in head circumference, bulging anterior fontanelle, split cranial sutures, sunset sign in the eyes, bradycardia, apnea, respiratory irregularities, and feeding problems, which are thought to be of neurological origin.
There is no consensus in the literature as to which of these 3 approaches is superior. However, lower rates of progression to a ventriculoperitoneal (VP) shunt,117,118 less injury in the brain parenchyma in the brain MRI,96 smaller ventricle-to-brain parenchyma ratio,99 lower ventricular volumes99 and similar neurodevelopmental scores, even in infants who go on to require a VP-shunt,117 have been demonstrated with low-threshold intervention. The results of the prospective, multicenter, randomized controlled ELVIS trial, which is the first trial comparing the low- and high-threshold interventions, can be summarized as follows:
There is no statistically significant difference between the low- and high-threshold intervention groups with respect to VP-shunt requirement.97
There is no statistically significant difference between the low- and high-threshold intervention groups with respect to the composite outcome of VP-shunt requirement/mortality.97
Significantly more LP procedures and reservoir insertions were performed in the low-threshold group compared to the high-threshold group.97
Significantly less brain injury (using Kidokoro scoring) was observed in the gray matter and white matter tissues of the low-threshold intervention group.99
The ventricle-to-brain parenchyma ratio and the frontal-occipital horn ratio, were lower in the low-threshold group, in other words, the ventricles remained smaller.99
Ventricular volumes (using the gold standard method, automated volume analysis) was significantly lower in the low-threshold intervention group.99
In the low-threshold group, cognitive and motor scores at 2 years in infants with and without a VP-shunt were similar, while in the high-threshold group infants who required a VP-shunt had lower cognitive and motor scores compared with those who did not require a VP-shunt.116
The composite adverse outcome of death/cerebral palsy/cognitive or motor score <-2 SD was seen in 35% of the infants in the low-threshold group, while the composite outcome was present at 51% in the high-threshold group. When the outcomes were adjusted for gestational age and severity of the hemorrhage, being in the low-threshold group significantly reduced the composite adverse outcome (OR: 0.24, 95% CI: 0.07-0.87, P = .03).116
In light of these data, adopting a low-threshold intervention (= early intervention) approach to initiate serial lumbar punctures causes patients to be exposed to an increased number of interventions, but reduces the risk of death/cerebral palsy/severe neurodevelopmental retardation by causing less damage in the brain parenchyma and resulting in smaller ventricular volumes. These favorable neurological outcomes seem to justify the increased number of invasive interventions. In a new international guideline prepared in light of the ELVIS trials, these favorable effects of low-threshold approach, in other words, early intervention, were emphasized.111 Until there are further studies on this topic, we recommend using the low-threshold approach in discussion with the parents, while also taking into account the clinical condition of the infant, risk of long-term neurodevelopmental impairment, and life expectancy of the infant. Also, due to above-listed reasons, the approach of delaying the interventions until the clinical signs of increased intracranial pressure should be abandoned (Table 3).
Table 3.
Summary of Interventional Approaches (B1)
| Low-threshold Interventional Approach | High-threshold Interventional Approach |
|---|---|
| Scanning: After the onset of PHVD, cUS scanning at least twice a week should be initiated. | Scanning: After the onset of PHVD, cUS scanning at least twice a week should be initiated. |
| Family Meeting: When the bilateral ventricular indices exceed the 97th percentile and anterior horn width exceeds 6mm and/or thalamo-occipital distance >25mm, a family meeting should be held and treatment plan should be clarified. | Family Meeting: When the bilateral ventricular indices are 97th percentile + 4 mm line and anterior horn width >10mm and/or thalamo-occipital distance >25mm, a family meeting should be held and treatment plan should be clarified. |
| The First Lumbar Puncture: If progression in the ventricular measurements (progression from the yellow curve to the red curve in Figure 2) is observed, the first LP is performed (10 mL/kg). | The First Lumbar Puncture: If this approach is adapted in the family meeting, the first LP is performed on the same day. LP volume is 10 mL/kg. |
| Subsequent Lumbar Punctures : If progression in the ventricular measurements continue 24-48 hours after the 1st LP, the 2nd LP is performed, and if the progression continues after 24-48 hours, the 3rd LP is performed. | Subsequent Lumbar Punctures: If there is a progression in the ventricular measurements 24-48 hours after the 1st LP, the 2nd LP is performed, if the progression continues after 24-48 hours, the 3rd LP is performed. |
| Target: Bilateral ventricular indices being <97th percentile and the anterior horn width <6mm and thalamo-occipital distance <25 mm. | Target: Bilateral ventricular indices being <97th percentile and the anterior horn width below 6mm and thalamo-occipital distance below 25 mm. |
| Maximum Number of Lumbar Punctures: 3 times. | Maximum Number of Lumbar Punctures: 3 times. |
| Subsequent Interventions: If the ventricles continue to enlarge after a total of 3 LPs, reservoir or ventriculo-subgaleal shunt or external ventricular drainage is performed, in discussion with the neurosurgery team. | Subsequent Interventions: If the ventricles continue to enlarge after a total of 3 LPs, reservoir or ventriculo-subgaleal shunt or external ventricular drainage is performed, in discussion with the neurosurgery team. |
A ventricular reservoir, a ventriculo-subgaleal shunt, or an external ventricular drainage system can be used as a temporizing method following LPs, with similar success rates.119 However, ventriculo-subgaleal shunts have been shown to reduce the need for daily taps.113 Based on the experience of the neurosurgeon, one of these methods can be preferred. If CSF drainage is still required 4 weeks after the temporizing methods, a VP-shunt is inserted, provided that the patient has reached a weight of 2-2.5 kg and the CSF culture is sterile, with a protein level of <1.5 g/dL and erythrocyte count of <100/mm3 (Figure 3).1,6
Figure 3.
Flowchart of the intervention options for infants with post-hemorrhagic ventricular dilatation.
Endoscopic third Ventriculostomy with Choroid Plexus Cauterization: While the method of early endoscopic third ventriculostomy with choroid plexus cauterization to reduce the rate of VP-shunt is becoming increasingly popular, there is insufficient evidence to recommend its routine use.1,6
Mesenchymal Stem Cell Treatment: Intraventricular or intravenous administration of mesenchymal stem cells has been shown to be promising in rat models, and a Phase-I human study has demonstrated that it is safe and tolerable.120-123 Further studies are warranted to recommend its routine use.
To achieve optimal outcomes and the highest quality of life for preterm infants with PHVD, it should be kept in mind that PHVD is a complex process requiring a multidisciplinary approach. A joint approach plan should be adopted with the neurosurgery team early in the course of patients who develop PHVD, and family participation in the decision process should be ensured.6
Mortality and Neurodevelopmental Outcomes
When informing the parents about the consequences of GMH-IVH and long-term neurological outcomes, one should be transparent, the clinical picture should be explained clearly, and possible treatment options should be explained to the families in plain language in light of the current literature. In family meetings, in addition to the description of GMH-IVH and related complications, it should also be explained in detail that prematurity per se is a negative risk factor for long-term adverse outcomes and that the comorbidities of prematurity will impact the long-term prognosis. It would also be more appropriate to share the mortality and morbidity data of the treatment center whenever possible. It should be emphasized that close follow-up after discharge is of paramount importance and initiating early rehabilitation programs following discharge for at-risk infants would provide the maximum benefit by preserving the neuroplasticity.1
Below, we present the current data on mortality and neurodevelopmental outcomes to guide the family meetings. In order to ensure that these data are easily remembered by physicians and can be easily absorbed by the parents, fractions have been avoided and numbers have been rounded.
Long-term Neurodevelopmental Outcomes
Neurodevelopmental impairments are closely associated with the gestation age, concurrent morbidities, severity of GMH-IVH, and requirement of a VP-shunt.
The association of low-grade hemorrhage (Grade I and Grade II) with neurodevelopmental outcomes is controversial. However, the general opinion is that they may cause neurodevelopmental retardation, although rare. In recent studies, the rate of cerebral palsy has been reported to be approximately 10% for Grade I and Grade II hemorrhages combined.124-126
The incidence of total cognitive and neuro-sensory impairments (cognitive/motor/hearing/visual problems) is around 20% for Grade I and Grade II hemorrhages.124-126
The incidence of cerebral palsy is around 30% for Grade III hemorrhages.125,126 The risk increases when Grade III hemorrhage is accompanied by PHVD. As described above, the risk of mortality/cerebral palsy/severe neurodevelopmental impairment is reduced, from half of the infants to a third of the infants with timely interventions.116
When PVHI (Papile Grade-IV) occurs, the risk of hemiparesis (=unilateral spastic cerebral palsy) increases substantially. The incidence is reported to be around 40% in the current literature.115
The risk of occurrence of neurodevelopmental impairments increases when GMH-IVH is bilateral, involves more than 1 lobe or causes midline shift. However, it should be kept in mind that with a good rehabilitation program, a majority of infants who go on to develop cerebral palsy after PVHI (Papile Grade-IV) can walk without support and around two-thirds of them will have normal cognitive outcomes.115
Mortality
Mortality is closely related with gestation age, accompanying comorbidities, and severity of GMH-IVH.4
In a recent large series, the mortality rate was reported to be 4% for Grade I and 10% for Grade II GMH-IVH.4 Mortality in low-grade GMH-IVH is usually a result of other underlying conditions.
The mortality rate is reported to be around 20% for Grade III hemorrhage, which may increase with concurrent PHVD.4,97
Mortality rates in PVHI (Papile Grade-IV) have decreased dramatically in the last 2 decades. The incidence is reported to be around 40% in the recent literature.115 The risk of mortality increases when the hemorrhage is bilateral, involves more than 1 lobe, or causes midline shift.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Peer Review: Externally peer-reviewed.
Author Contribution: Concept - M.N.Ç., M.A.A., E.Ö.; Design - M.N.Ç., M.A.A., E.Ö.; Supervision - E.Ö; Resource - M.N.Ç., M.A.A., E.Ö.; Materials - M.N.Ç., M.A.A., E.Ö.; Data Collection and/or Processing - M.N.Ç., M.A.A., E.Ö.; Analysis and/or Interpretation - M.N.Ç., M.A.A., E.Ö.; Literature Search - M.N.Ç., M.A.A.; Writing - M.N.Ç., M.A.A.; Critical Reviews - E.Ö.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. Inder TE Perlman JM, Volpe JJ. Preterm intraventricular hemorrhage/posthemorrhagic hydrocephalus. In: Volpe JJ.ed. Volpe’s Neurology of the Newborn. 6th ed. Philadelphia: Elsevier; 2018:637–698.. [Google Scholar]
- 2. Stoll BJ, Hansen NI, Bell EF, et al. Trends care pract morb mortal extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039–1051.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Su BH, Hsieh WS, Hsu CH, et al. Neonatal outcomes of extremely preterm infants from Taiwan: comparison with Canada, Japan, and the USA. Pediatr Neonatol. 2015;56(1):46–52.. 10.1016/j.pedneo.2014.05.002) [DOI] [PubMed] [Google Scholar]
- 4. Christian EA, Jin DL, Attenello F, et al. Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States, 2000-2010. J Neurosurg Pediatr. 2016;17(3):260–269.. 10.3171/2015.7.PEDS15140) [DOI] [PubMed] [Google Scholar]
- 5. Poryo M, Boeckh JC, Gortner L, et al. Ante-, peri- and postnatal factors associated with intraventricular hemorrhage in very premature infants. Early Hum Dev. 2018;116:1–8.. 10.1016/j.earlhumdev.2017.08.010) [DOI] [PubMed] [Google Scholar]
- 6. Leijser LM, de Vries LS. Preterm brain injury: germinal matrix-intraventricular hemorrhage and post-hemorrhagic ventricular dilatation. Handb Clin Neurol. 2019;162:173–199.. 10.1016/B978-0-444-64029-1.00008-4) [DOI] [PubMed] [Google Scholar]
- 7. Raets MM, Dudink J, Govaert P. Neonatal disorders of germinal matrix. J Matern Fetal Neonatal Med. 2015;28(suppl 1):2286–2290.. 10.3109/14767058.2013.796169) [DOI] [PubMed] [Google Scholar]
- 8. Kinoshita Y, Okudera T, Tsuru E, Yokota A. Volumetric analysis of the germinal matrix and lateral ventricles performed using MR images of postmortem fetuses. Am J Neuroradiol. 2001;22(2):382–388.. [PMC free article] [PubMed] [Google Scholar]
- 9. Scott JA, Habas PA, Kim K, et al. Growth trajectories of the human fetal brain tissues estimated from 3D reconstructed in utero MRI. Int J Dev Neurosci. 2011;29(5):529–536.. 10.1016/j.ijdevneu.2011.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corbin JG, Gaiano N, Juliano SL, et al. Regulation of neural progenitor cell development in the nervous system. J Neurochem. 2008;106(6):2272–2287.. 10.1111/j.1471-4159.2008.05522.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Vries LS, Leijser LM. Germinal matrix hemorrhage and intraventricular hemorrhage (GMH-İVH) in the newborn: pathogenesis, clinical presentation, and diagnosis. In: Martin R, Nordli DR, Kim S.eds. UpToDate. (https://www.uptodate.com/contents/germinal-matrix-hemorrhage-and-intraventricular-hemorrhage-GMH-%26%23304;VH-in-the-newborn-pathogenesis-clinical-presentation-and-diagnosis?search=germinal%2520matrix%2520hemorrhage%26source=search_result%26selectedTitle=2%7e10%26usage_type=default%26display_rank=2%23H22_type=default%26display_rank=2%23H22) [Google Scholar]
- 12. Ballabh P, Braun A, Nedergaard M. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res. 2004;56(1):117–124.. 10.1203/01.PDR.0000130472.30874.FF) [DOI] [PubMed] [Google Scholar]
- 13. Govaert P., Roehr C.C., Gressens P. Cranial ultrasound by neonatologists. Pediatr Res. 2020;87(suppl 1):1–2.. 10.1038/s41390-020-0779-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pape KE, Wigglesworth JS. Haemorrhage, ischaemia and perinatal brain. In: Pape K, Wigglesworth J.eds. Clinics in Developmental Medicine No. 69/70. London: SIMP/Heinemann; 1979:133–148.. [Google Scholar]
- 15. Braun A, Xu H, Hu F, et al. Paucity of pericytes in germinal matrix vasculature of premature infants. J Neurosci. 2007;27(44):12012–12024.. 10.1523/JNEUROSCI.3281-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Khoury N, Braun A, Hu F, et al. Astrocyte end-feet in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res. 2006;59(5):673–679.. 10.1203/01.pdr.0000214975.85311.9c) [DOI] [PubMed] [Google Scholar]
- 17. Xu H, Hu F, Sado Y, et al. Maturational changes in laminin, fibronectin, collagen IV, and perlecan in germinal matrix, cortex, and white matter and effect of betamethasone. J Neurosci Res. 2008;86(7):1482–1500.. 10.1002/jnr.21618) [DOI] [PubMed] [Google Scholar]
- 18. Ghazi-Birry HS, Brown WR, Moody DM. et al. Human germinal matrix: venous origin of hemorrhage and vascular characteristics. Am J Neuroradiol. 1997;18(2):219–229.. [PMC free article] [PubMed] [Google Scholar]
- 19. O’Leary H, Gregas MC, Limperopoulos C, et al. Elevated cerebral pressure passivity is associated with prematurity-related intracranial hemorrhage. Pediatrics. 2009;124(1):302–309.. 10.1542/peds.2008-2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soul JS, Hammer PE, Tsuji M, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61(4):467–473.. 10.1203/pdr.0b013e31803237f6) [DOI] [PubMed] [Google Scholar]
- 21. Noori S, Seri I. Hemodynamic antecedents of peri/intraventricular hemorrhage in very preterm neonates. Semin Fetal Neonatal Med. 2015;20(4):232–237.. 10.1016/j.siny.2015.02.004) [DOI] [PubMed] [Google Scholar]
- 22. Romantsik O, Bruschettini M, Ley D. Intraventricular hemorrhage and white matter injury in preclinical and clinical studies. NeoReviews. 2019;20(11):e636–e652.. 10.1542/neo.20-11-e636) [DOI] [PubMed] [Google Scholar]
- 23. Karimy JK, Zhang J, Kurland DB, et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 2017;23(8):997–1003.. 10.1038/nm.4361) [DOI] [PubMed] [Google Scholar]
- 24. Gram M, Sveinsdóttir S, Ruscher K, et al. Hemoglobin induces inflammation after preterm intraventricular hemorrhage by methemoglobin formation. J Neuroinflammation. 2013;10:100. 10.1186/1742-2094-10-100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen Q, Feng Z, Tan Q, et al. Post-hemorrhagic hydrocephalus: recent advances and new therapeutic insights. J Neurol Sci. 2017;375:220–230.. 10.1016/j.jns.2017.01.072) [DOI] [PubMed] [Google Scholar]
- 26. Gram M, Sveinsdóttir S, Cinthio M, et al. Extracellular hemoglobin: mediator of inflammation and cell death in the choroid plexus following preterm intraventricular hemorrhage. J Neuroinflammation. 2014;11:200. 10.1186/s12974-014-0200-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Z, Gao C, Hua Y. et al. Role of iron in brain injury after intraventricular hemorrhage. Stroke. 2011;42(2):465–470.. 10.1161/STROKEAHA.110.602755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F153–F161.. 10.1136/adc.2006.108837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agyemang AA, Sveinsdóttir K, Vallius S. et al. Cerebellar exposure to cell-free hemoglobin following preterm intraventricular hemorrhage: causal in cerebellar damage? Transl Stroke Res. 2017;8(5):461–473.. 10.1007/s12975-017-0539-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Evans N, Kluckow M. Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;75(3):F183–F186.. 10.1136/fn.75.3.f183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al-Abdi SY, Al-Aamri MA. A systematic review and meta-analysis of the timing of early intraventricular hemorrhage in preterm neonates: clinical and research implications. J Clin Neonatol. 2014;3(2):76–88.. 10.4103/2249-4847.134674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harteman JC, Groenendaal F, van Haastert IC, et al. Atypical timing and presentation of periventricular haemorrhagic infarction in preterm infants: the role of thrombophilia. Dev Med Child Neurol. 2012;54(2):140–147.. 10.1111/j.1469-8749.2011.04135.x) [DOI] [PubMed] [Google Scholar]
- 33. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534.. 10.1016/s0022-3476(78)80282-0) [DOI] [PubMed] [Google Scholar]
- 34. Handley SC, Passarella M, Lee HC, Lorch SA. Incidence trends and risk factor variation in severe intraventricular hemorrhage across a population based cohort. J Pediatr. 2018;200:24–29.e3.. 10.1016/j.jpeds.2018.04.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456.. 10.1542/peds.2009-2959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Szpecht D, Szymankiewicz M, Nowak I, Gadzinowski J. Intraventricular hemorrhage in neonates born before 32 weeks of gestation-retrospective analysis of risk factors. Childs Nerv Syst. 2016;32(8):1399–1404.. 10.1007/s00381-016-3127-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Howes A, Hilditch C, Keir A. What clinical practice strategies have been shown to decrease incidence rates of intraventricular haemorrhage in preterm infants? J Paediatr Child Health. 2019;55(10):1269–1278.. 10.1111/jpc.14613) [DOI] [PubMed] [Google Scholar]
- 38. Özek E, Ozek OMM. Germinal matrix-intraventricular hemorrhage and posthemorrhagic ventricular dilatation in the preterm infant. In: Pediatric Hydrocephalus. Berlin: Springer; 2018:1–28.. [Google Scholar]
- 39. Ment LR, Oh W, Ehrenkranz RA. et al. Antenatal steroids, delivery mode, and intraventricular hemorrhage in preterm infants. Am J Obstet Gynecol. 1995;172(3):795–800.. 10.1016/0002-9378(95)90001-2) [DOI] [PubMed] [Google Scholar]
- 40. Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. 10.1002/14651858.CD004454.pub3) [DOI] [PubMed] [Google Scholar]
- 41. Palmer KG, Kronsberg SS, Barton BA. et al. Effect of inborn versus outborn delivery on clinical outcomes in ventilated preterm neonates: secondary results from the NEOPAIN trial. J Perinatol. 2005;25(4):270–275.. 10.1038/sj.jp.7211239) [DOI] [PubMed] [Google Scholar]
- 42. Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3(3):CD004454. 10.1002/14651858.CD004454.pub3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thorp JA, Jones PG, Clark RH, Knox E, Peabody JL. Perinatal factors associated with severe intracranial hemorrhage. Am J Obstet Gynecol. 2001;185(4):859–862.. 10.1067/mob.2001.117355) [DOI] [PubMed] [Google Scholar]
- 44. Amer R, Moddemann D, Seshia M, et al. Neurodevelopmental outcomes of infants born at <29 weeks of gestation admitted to Canadian neonatal intensive care units based on location of birth. J Pediatr. 2018;196:31 .e1–37.e1.. 10.1016/j.jpeds.2017.11.038) [DOI] [PubMed] [Google Scholar]
- 45. Helenius K, Longford N, Lehtonen L, et al. Association of early postnatal transfer and birth outside a tertiary hospital with mortality and severe brain injury in extremely preterm infants: observational cohort study with propensity score matching. BMJ. 2019;367:l5678. 10.1136/bmj.l5678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mohamed MA, Aly H. Transport of premature infants is associated with increased risk for intraventricular haemorrhage. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F403–F407.. 10.1136/adc.2010.183236) [DOI] [PubMed] [Google Scholar]
- 47. Huang J, Meng J, Choonara I, et al. Antenatal infection and intraventricular hemorrhage in preterm infants: a meta-analysis. Medicine. 2019;98(31):e16665. 10.1097/MD.0000000000016665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol. 2009;200(4):372.e1-372.e6. 10.1016/j.ajog.2008.11.034) [DOI] [PubMed] [Google Scholar]
- 49. Oh KJ, Park JY, Lee J. et al. The combined exposure to intra-amniotic inflammation and neonatal respiratory distress syndrome increases the risk of intraventricular hemorrhage in preterm neonates. J Perinat Med. 2018;46(1):9–20.. 10.1515/jpm-2016-0348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chatzakis C, Papatheodorou S, Sarafidis K. et al. The effect of prophylactic antibiotics for preterm prelabor rupture of membranes on perinatal outcomes: a network meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2020;55(1):20–31.. 10.1002/uog.21884) [DOI] [PubMed] [Google Scholar]
- 51. Villamor-Martinez E, Fumagalli M, Mohammed Rahim O, et al. Chorioamnionitis is a risk factor for intraventricular hemorrhage in preterm infants: a systematic review and meta-analysis. Front Physiol. 2018;9:1253. 10.3389/fphys.2018.01253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bierstone D, Wagenaar N, Gano DL, et al. Association of histologic chorioamnionitis with perinatal brain injury and early childhood neurodevelopmental outcomes among preterm neonates. JAMA Pediatr. 2018;172(6):534–541.. 10.1001/jamapediatrics.2018.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lim J, Hagen E. Reducing germinal matrix-intraventricular hemorrhage: perinatal and delivery room factors. NeoReviews. 2019;20(8):e452–e463.. 10.1542/neo.20-8-e452) [DOI] [PubMed] [Google Scholar]
- 54. van Vliet E, Dijkema GH, Schuit E, et al. Nifedipine maintenance tocolysis and perinatal outcome: an individual participant data meta-analysis. BJOG. 2016;123(11):1753–1760.. 10.1111/1471-0528.14249) [DOI] [PubMed] [Google Scholar]
- 55. Aggarwal A, Bagga R, Girish B, Kalra J, Kumar P. Effect of maintenance tocolysis with nifedipine in established preterm labour on pregnancy prolongation and neonatal outcome. J Obstet Gynaecol. 2018;38(2):177–184.. 10.1080/01443615.2017.1331340) [DOI] [PubMed] [Google Scholar]
- 56. Pinto Cardoso G, Houivet E, Marchand-Martin L, et al. Association of intraventricular hemorrhage and death with tocolytic exposure in preterm infants. JAMA Netw Open. 2018;1(5):e182355. 10.1001/jamanetworkopen.2018.2355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Haque KN, Hayes AM, Ahmed Z, Wilde R, Fong CY. Caesarean or vaginal delivery for preterm very-low-birth weight (< or =1,250 g) infant: experience from a district general hospital in UK. Arch Gynecol Obstet. 2008;277(3):207–212.. 10.1007/s00404-007-0438-x) [DOI] [PubMed] [Google Scholar]
- 58. Humberg A, Härtel C, Paul P, et al. Delivery mode and intraventricular hemorrhage risk in very-low-birth-weight infants: observational data of the German Neonatal Network. Eur J Obstet Gynecol Reprod Biol. 2017;212:144–149.. 10.1016/j.ejogrb.2017.03.032) [DOI] [PubMed] [Google Scholar]
- 59. Manley BJ, Owen LS, Hooper SB, et al. Towards evidence-based resuscitation of the newborn infant. Lancet. 2017;389(10079):1639–1648.. 10.1016/S0140-6736(17)30547-0) [DOI] [PubMed] [Google Scholar]
- 60. Rabe H, Gyte GM, Díaz-Rossello JL, Duley L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2019;9(9):CD003248. 10.1002/14651858.CD003248.pub4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tarnow-Mordi W, Morris J, Kirby A, et al. Delayed versus immediate cord clamping in preterm infants. N Engl J Med. 2017;377(25):2445–2455.. 10.1056/NEJMoa1711281) [DOI] [PubMed] [Google Scholar]
- 62. Al-Wassia H, Shah PS. Efficacy and safety of umbilical cord milking at birth: a systematic review and meta-analysis. JAMA Pediatr. 2015;169(1):18–25.. 10.1001/jamapediatrics.2014.1906) [DOI] [PubMed] [Google Scholar]
- 63. Katheria A, Reister F, Essers J, et al. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA. 2019;322(19):1877–1886.. 10.1001/jama.2019.16004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Perlman JM, Wyllie J, Kattwinkel J, et al. Part 7: Neonatal resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with treatment recommendations. Circulation. 2015;132(16 Suppl 1):S204–S241.. [DOI] [PubMed] [Google Scholar]
- 65. Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatology. 2019;115(4):432–450.. 10.1159/000499361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oygür N, Önal E, Zenciroğlu A. Türk Neonatoloji Derneği doğum salonu Yaklaşımları. Internet. 2016. http://www.neonatology.org.tr/wp-content/uploads/2016/12/dogum_odasi_yonetimi.pdf. [Google Scholar]
- 67. Miller SS, Lee HC, Gould JB. Hypothermia in very low birth weight infants: distribution, risk factors and outcomes. J Perinatol. 2011;31(suppl 1):S49–S56.. 10.1038/jp.2010.177) [DOI] [PubMed] [Google Scholar]
- 68. Oei JL, Finer NN, Saugstad OD, et al. Outcomes of oxygen saturation targeting during delivery room stabilisation of preterm infants. Arch Dis Child Fetal Neonatal Ed. 2018;103(5):F446–F454.. 10.1136/archdischild-2016-312366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vesoulis ZA, Mathur AM. Cerebral autoregulation, brain injury, and the transitioning premature infant. Front Pediatr. 2017;5:64. 10.3389/fped.2017.00064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kaiser JR, Gauss CH, Pont MM, Williams DK. Hypercapnia during the first 3 days of life is associated with severe intra ventricular hemorrhage in very low birth weight infants. J Perinatol. 2006;26(5):279–285.. 10.1038/sj.jp.7211492) [DOI] [PubMed] [Google Scholar]
- 71. Sauer CW, Kong JY, Vaucher YE, et al. Intubation attempts increase the risk for severe intraventricular hemorrhage in preterm infants - a retrospective cohort study. J Pediatr. 2016;177:108–113.. 10.1016/j.jpeds.2016.06.051) [DOI] [PubMed] [Google Scholar]
- 72. Katheria AC, Harbert MJ, Nagaraj SB, et al. The Neu-Prem trial: neuromonitoring of brains of infants born preterm during resuscitation - a prospective observational cohort study. J Pediatr. 2018;198:209 .e3–213.e3.. 10.1016/j.jpeds.2018.02.065) [DOI] [PubMed] [Google Scholar]
- 73. Plomgaard AM, Alderliesten T, van Bel F, et al. No neurodevelopmental benefit of cerebral oximetry in the first randomised trial (SafeBoosC II) in preterm infants during the first days of life. Acta Paediatr. 2019;108(2):275–281.. 10.1111/apa.14463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hansen ML, Pellicer A, Gluud C, et al. Cerebral near-infrared spectroscopy monitoring versus treatment as usual for extremely preterm infants: a protocol for the SafeBoosC randomised clinical phase III trial. Trials. 2019;20(1):811. 10.1186/s13063-019-3955-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Barnette AR, Myers BJ, Berg CS, Inder TE. Sodium intake and intraventricular hemorrhage in the preterm infant. Ann Neurol. 2010;67(6):817–823.. 10.1002/ana.21986) [DOI] [PubMed] [Google Scholar]
- 76. Dalton J, Dechert RE, Sarkar S. Assessment of association between rapid fluctuations in serum sodium and intraventricular hemorrhage in hypernatremic preterm infants. Am J Perinatol. 2015;32(8):795–802.. 10.1055/s-0034-1396691) [DOI] [PubMed] [Google Scholar]
- 77. Bermick J, Dechert RE, Sarkar S. Does hyperglycemia in hypernatremic preterm infants increase the risk of intraventricular hemorrhage? J Perinatol. 2016;36(9):729–732.. 10.1038/jp.2016.86) [DOI] [PubMed] [Google Scholar]
- 78. Auerbach A, Eventov-Friedman S, Arad I, et al. Long duration of hyperglycemia in the first 96 hours of life is associated with severe intraventricular hemorrhage in preterm infants. J Pediatr. 2013;163(2):388–393.. 10.1016/j.jpeds.2013.01.051) [DOI] [PubMed] [Google Scholar]
- 79. Christensen RD, Baer VL, Lambert DK. et al. Association, among very-low-birthweight neonates, between red blood cell transfusions in the week after birth and severe intraventricular hemorrhage. Transfusion. 2014;54(1):104–108.. 10.1111/trf.12234) [DOI] [PubMed] [Google Scholar]
- 80. Tran TT, Veldman A, Malhotra A. Does risk-based coagulation screening predict intraventricular haemorrhage in extreme premature infants? Blood Coagul Fibrinolysis. 2012;23(6):532–536.. 10.1097/MBC.0b013e3283551145) [DOI] [PubMed] [Google Scholar]
- 81. Dani C, Poggi C, Ceciarini F. et al. Coagulopathy screening and early plasma treatment for the prevention of intraventricular hemorrhage in preterm infants. Transfusion. 2009;49(12):2637–2644.. 10.1111/j.1537-2995.2009.02328.x) [DOI] [PubMed] [Google Scholar]
- 82. Duppré P, Sauer H, Giannopoulou EZ, et al. Cellular and humoral coagulation profiles and occurrence of İVH in VLBW and ELWB infants. Early Hum Dev. 2015;91(12):695–700.. 10.1016/j.earlhumdev.2015.09.008) [DOI] [PubMed] [Google Scholar]
- 83. Kribs A, Roll C, Göpel W, et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 2015;169(8):723–730.. 10.1001/jamapediatrics.2015.0504) [DOI] [PubMed] [Google Scholar]
- 84. Legge NA, Shein D, Callander I. Methods of surfactant administration and early ventilation in neonatal intensive care units in New South Wales and the Australian Capital Territory. J Neonatal Perinatal Med. 2019;12(3):255–263.. 10.3233/NPM-180074) [DOI] [PubMed] [Google Scholar]
- 85. Kochan M, Leonardi B, Firestine A, et al. Elevated midline head positioning of extremely low birth weight infants: effects on cardiopulmonary function and the incidence of periventricular-intraventricular hemorrhage. J Perinatol. 2019;39(1):54–62.. 10.1038/s41372-018-0261-1) [DOI] [PubMed] [Google Scholar]
- 86. Romantsik O, Calevo MG, Bruschettini M. Head midline position for preventing the occurrence or extension of germinal matrix-intraventricular hemorrhage in preterm infants. Cochrane Database Syst Rev. 2017;7(7):CD012362. 10.1002/14651858.CD012362.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. de Bijl-Marcus K, Brouwer AJ, De Vries LS, Groenendaal F, Wezel-Meijler GV. Neonatal care bundles are associated with a reduction in the incidence of intraventricular haemorrhage in preterm infants: a multicentre cohort study. Arch Dis Child Fetal Neonatal Ed. 2020;105(4):419–424.. 10.1136/archdischild-2018-316692) [DOI] [PubMed] [Google Scholar]
- 88. Lowe LH, Bailey Z. State-of-the-art cranial sonography: Part 1, modern techniques and image interpretation. Am J Roentgenol. 2011;196(5):1028–1033.. 10.2214/AJR.10.6160) [DOI] [PubMed] [Google Scholar]
- 89. Guyatt G, Oxman AD, Akl EA. et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394.. 10.1016/j.jclinepi.2010.04.026) [DOI] [PubMed] [Google Scholar]
- 90. Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58(12):1726–1738.. 10.1212/wnl.58.12.1726) [DOI] [PubMed] [Google Scholar]
- 91. Forster DE, Koumoundouros E, Saxton V, Fedai G, Holberton J. Cerebral blood flow velocities and cerebrovascular resistance in normal-term neonates in the first 72 hours. J Paediatr Child Health. 2018;54(1):61–68.. 10.1111/jpc.13663) [DOI] [PubMed] [Google Scholar]
- 92. Couture A, Veyrac C, Baud C, Saguintaah M, Ferran JL. Advanced cranial ultrasound: transfontanellar Doppler imaging in neonates. Eur Radiol. 2001;11(12):2399–2410.. 10.1007/s00330-001-1150-z) [DOI] [PubMed] [Google Scholar]
- 93. Boswinkel V, Steggerda SJ, Fumagalli M, et al. The CHOPIn study: a multicenter study on cerebellar hemorrhage and outcome in preterm infants. Cerebellum. 2019;18(6):989–998.. 10.1007/s12311-019-01053-1) [DOI] [PubMed] [Google Scholar]
- 94. Hortensius LM, Dijkshoorn ABC, Ecury-Goossen GM. et al. Neurodevelopmental consequences of preterm isolated cerebellar hemorrhage: a systematic review. Pediatrics. 2018;142(5):e20180609. 10.1542/peds.2018-0609) [DOI] [PubMed] [Google Scholar]
- 95. Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am J Neuroradiol. 2013;34(11):2208–2214.. 10.3174/ajnr.A3521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Counsell SJ, Arichi T, Arulkumaran S, Rutherford MA. Fetal and neonatal neuroimaging. Handb Clin Neurol. 2019;162:67–103.. 10.1016/B978-0-444-64029-1.00004-7) [DOI] [PubMed] [Google Scholar]
- 97. de Vries LS, Groenendaal F, Liem KD, et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2019;104(1):F70–F75.. 10.1136/archdischild-2017-314206) [DOI] [PubMed] [Google Scholar]
- 98. Brouwer MJ, de Vries LS, Kersbergen KJ, et al. Effects of posthemorrhagic ventricular dilatation in the preterm ınfant on brain volumes and white matter diffusion variables at term-equivalent age. J Pediatr. 2016;168:41–49.e1.. 10.1016/j.jpeds.2015.09.083) [DOI] [PubMed] [Google Scholar]
- 99. Cizmeci MN, Khalili N, Claessens NHP, et al. Assessment of brain injury and brain volumes after posthemorrhagic ventricular dilatation: a nested substudy of the randomized controlled ELVIS trial. J Pediatr. 2019;208:191 .e2–197.e2.. 10.1016/j.jpeds.2018.12.062) [DOI] [PubMed] [Google Scholar]
- 100. Levene MI. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child. 1981;56(12):900–904.. 10.1136/adc.56.12.900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Davies MW, Swaminathan M, Chuang SL, Betheras FR. Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2000;82(3):F218–F223.. 10.1136/fn.82.3.f218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Brouwer AJ, Brouwer MJ, Groenendaal F. et al. European perspective on the diagnosis and treatment of posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed. 2012;97(1):F50–F55.. 10.1136/adc.2010.207837) [DOI] [PubMed] [Google Scholar]
- 103. Boyle M, Shim R, Gnanasekaran R, et al. Inclusion of extremes of prematurity in ventricular index centile charts. J Perinatol. 2015;35(6):439–443.. 10.1038/jp.2014.219) [DOI] [PubMed] [Google Scholar]
- 104. Brouwer MJ, de Vries LS, Groenendaal F, et al. New reference values for the neonatal cerebral ventricles. Radiology. 2012;262(1):224–233.. 10.1148/radiol.11110334) [DOI] [PubMed] [Google Scholar]
- 105. Whitelaw A, Lee-Kelland R. Repeated lumbar or ventricular punctures in newborns with intraventricular haemorrhage. Cochrane Database Syst Rev. 2017;4(4):CD000216. 10.1002/14651858.CD000216.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Whitelaw A. Repeated lumbar or ventricular punctures in newborns with intraventricular hemorrhage. Cochrane Database Syst Rev. 2001;1(1):CD000216. 10.1002/14651858.CD000216) [DOI] [PubMed] [Google Scholar]
- 107. Luciano R, Velardi F, Romagnoli C. et al. Failure of fibrinolytic endoventricular treatment to prevent neonatal post-haemorrhagic hydrocephalus. A case-control trial. Childs Nerv Syst. 1997;13(2):73–76.. 10.1007/s003810050045) [DOI] [PubMed] [Google Scholar]
- 108. Whitelaw A, Evans D, Carter M, et al. Randomized clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid. Pediatrics. 2007;119(5):e1071-e1078. 10.1542/peds.2006-2841) [DOI] [PubMed] [Google Scholar]
- 109. Whitelaw A, Jary S, Kmita G, et al. Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics. 2010;125(4):e852–e858.. 10.1542/peds.2009-1960) [DOI] [PubMed] [Google Scholar]
- 110. Luyt K, Jary S, Lea C, et al. Ten-year follow-up of a randomised trial of drainage, irrigation and fibrinolytic therapy (DRIFT) in infants with post-haemorrhagic ventricular dilatation. Health Technol Assess. 2019;23(4):1–116.. 10.3310/hta23040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. El-Dib M, Limbrick DD Jr, Inder T, Inder T, Whitelaw A, Kulkarni AV, Warf B, Volpe JJ, de Vries LS. Management of Post-hemorrhagic Ventricular Dilatation in the Infant Born Preterm . J Pediatr. 2020 Jul 30:S0022-3476(20)30978-1. 10.1016/j.jpeds.2020.07.079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shooman D, Portess H, Sparrow O. A review of the current treatment methods for posthaemorrhagic hydrocephalus of infants. Cerebrospinal Fluid Res. 2009;6:1. 10.1186/1743-8454-6-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mazzola CA, Choudhri AF, Auguste KI, et al. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: Management of posthemorrhagic hydrocephalus in premature infants. J Neurosurg Pediatr. 2014;14(suppl 1):8–23.. 10.3171/2014.7.PEDS14322) [DOI] [PubMed] [Google Scholar]
- 114. Whitelaw A, Kennedy CR, Brion LP. Diuretic therapy for newborn infants with posthemorrhagic ventricular dilatation. Cochrane Database Syst Rev. 2001;2(2):CD002270. 10.1002/14651858.CD002270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cizmeci MN, de Vries LS, Ly LG, et al. Periventricular hemorrhagic infarction in very preterm infants: characteristic sonographic findings and association with neurodevelopmental outcome at age 2 years. J Pediatr. 2020;217:79 .e1–85.e1.. 10.1016/j.jpeds.2019.09.081) [DOI] [PubMed] [Google Scholar]
- 116. Cizmeci MN, Groenendaal F, Liem KD, van Haastert IC, Benavente-Fernández I, van Straaten HLM, Steggerda S, Smit BJ, Whitelaw A, Woerdeman P, Heep A, de Vries LS; ELVIS study group. Randomized Controlled Early versus Late Ventricular Intervention Study in Posthemorrhagic Ventricular Dilatation: Outcome at 2 Years. J Pediatr. 2020 Aug 12:S0022-3476(20)30996-3. 10.1016/j.jpeds.2020.08.014) [DOI] [PubMed] [Google Scholar]
- 117. Leijser LM, Miller SP, van Wezel-Meijler G, et al. Posthemorrhagic ventricular dilatation in preterm infants: when best to intervene? Neurology. 2018;90(8):e698–e706.. 10.1212/WNL.0000000000004984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. de Vries LS, Liem KD, van Dijk K, et al. Early versus late treatment of posthaemorrhagic ventricular dilatation: results of a retrospective study from five neonatal intensive care units in the Netherlands. Acta Paediatr. 2002;91(2):212–217.. 10.1080/080352502317285234) [DOI] [PubMed] [Google Scholar]
- 119. Wellons 3rd JC, Shannon CN, Holubkov R, et al. Shunting outcomes in posthemorrhagic hydrocephalus: results of a Hydrocephalus Clinical Research Network prospective cohort study. J Neurosurg Pediatr. 2017;20(1):19–29.. 10.3171/2017.1.PEDS16496) [DOI] [PubMed] [Google Scholar]
- 120. Ahn SY, Chang YS, Sung DK, et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke. 2013;44(2):497–504.. 10.1161/STROKEAHA.112.679092) [DOI] [PubMed] [Google Scholar]
- 121. Ahn SY, Chang YS, Park WS. Mesenchymal stem cells transplantation for neuroprotection in preterm infants with severe intraventricular hemorrhage. Korean J Pediatr. 2014;57(6):251–256.. 10.3345/kjp.2014.57.6.251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ahn SY, Chang YS, Sung DK, et al. Optimal route for mesenchymal stem cells transplantation after severe intraventricular hemorrhage in newborn rats. PLoS ONE. 2015;10(7):e0132919. 10.1371/journal.pone.0132919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ahn SY, Chang YS, Sung SI, Park WS. Mesenchymal stem cells for severe intraventricular hemorrhage in preterm infants: phase I dose-escalation clinical trial. Stem Cells Transl Med. 2018;7(12):847–856.. 10.1002/sctm.17-0219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Beaino G, Khoshnood B, Kaminski M, et al. Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Dev Med Child Neurol. 2010;52(6):e119–e125.. 10.1111/j.1469-8749.2010.03612.x) [DOI] [PubMed] [Google Scholar]
- 125. Payne AH, Hintz SR, Hibbs AM, et al. Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA Pediatr. 2013;167(5):451–459.. 10.1001/jamapediatrics.2013.866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bolisetty S, Dhawan A, Abdel-Latif M, et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. 2014;133(1):55–62.. 10.1542/peds.2013-0372) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a