Abstract
Background:
It is not known which social determinants of health (SDOH) impact 30-day readmission after a heart failure (HF) hospitalization among older adults. We examined the association of 9 individual SDOH with 30-day readmission after a HF hospitalization.
Methods and Results:
Using The REasons for Geographic and Racial Differences in Stroke (REGARDS) Study, we included Medicare beneficiaries who were discharged alive after a HF-hospitalization between 2003 and 2014. We assessed 9 SDOH based on the HealthyPeople 2030 Framework: race, education, income, social isolation, social network, residential poverty, Health Professional Shortage Area (HPSA), rural residence, and state public health infrastructure. The primary outcome was 30-day all-cause readmission. For each SDOH, we calculated incidence per 1000-person years and multivariable-adjusted hazard ratios of readmission. Among 690 participants, the median age was 76 years at hospitalization (IQR 71–82), 44.3% were female, 35.5% were Black, 23.5% had low educational attainment, 63.0% had low income, 21.0% had zip code-level poverty, 43.5% resided in HPSAs, 39.3% lived in states with poor public health infrastructure, 13.1% were socially isolated, 13.3% had poor social networks, and 10.2% lived in rural areas. The 30-day readmission rate was 22.4%. In an unadjusted analysis, only HPSA was significantly associated with 30-day readmission; in a fully-adjusted analysis, none of the 9 SDOH were individually associated with 30-day readmission.
Conclusions:
In this modestly sized national cohort, although prevalent, none of the SDOH were associated with 30-day readmission after a HF-hospitalization. Policies or interventions that only target individual SDOH to reduce readmissions after HF hospitalizations may not be sufficient to prevent readmission among older adults.
Keywords: Heart failure, quality and outcomes, health equity
Background
Heart failure (HF) is the leading cause for hospitalization and readmission among Medicare beneficiaries.1 Readmissions are associated with patient morbidity and mortality and are costly for health care systems.2 Despite the implementation of the Hospital Readmissions Reduction Program, research, and interventions implemented at the patient- and hospital-level, risk-adjusted HF readmission rates remain high.3 Among Medicare beneficiaries, more than 20% of patients admitted for HF are readmitted within 30 days.4, 5 Thus, preventing readmissions among older adults hospitalized for HF patients remains an important priority for patients, clinicians, health systems, and policy makers.
Social determinants of health (SDOH) -- defined as the conditions in which people are born, grow, work, live, and age6 – are an important area of investigation with respect to improving post-discharge outcomes for HF patients.7 Studies have found that certain individual (e.g. income, education, and race/ethnicity) and neighborhood-level (e.g. poverty) SDOH are associated with hospitalization and readmission risk.8–10 As such, policy makers and medical societies have called for upstream interventions and policies which could address SDOH to mitigate downstream readmission risk, such as investing in housing or education programs.11, 12 Yet, most HF studies have only examined a select number of SDOH within the same individual or have not included individual and neighborhood-level SDOH.7 Owing to this, it is unclear which SDOH should be prioritized, especially among older adults who are particularly vulnerable to adverse outcomes.
To that end, based on the HealthyPeople 2030 Framework,13 we examined a comprehensive group of SDOH and aimed to determine which were independently associated with 30-day all-cause readmission among Medicare beneficiaries hospitalized for HF in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. A more nuanced understanding of which SDOH influence readmission risk among older adults hospitalized for HF is critical for optimal post-discharge management.
Methods
In order to abide by its obligations with NIH/NINDS and the IRB of the University of Alabama at Birmingham, REGARDS facilitates data sharing through formal data use agreements. Any investigator is welcome to access the REGARDS data and documentation through this process. Requests for data access may be sent to the REGARDS study at regardsadmin@uab.edu
Study Population
Details of the REGARDS study have been described previously.14 Briefly, REGARDS is a national, prospective, observational cohort of 30,239 community-dwelling Black and White men and women aged ≥45 years from the United States. REGARDS was initially designed to investigate racial and geographic disparities in stroke mortality. Participants were recruited from 2003–2007 with ongoing follow-up.14 Black adults and residents of the Stroke Belt, an area in the southeastern US with high burden of cardiovascular disease and mortality, were oversampled by design. Participants completed a telephone interview ascertaining medical history followed by an in-home examination assessing blood pressure levels, height and weight, obtaining ECGs, anthropomorphic measures, and blood and urine samples along with a medication inventory. At six-month intervals, participants are contacted by phone to ask about general health status and potential study endpoints such as HF hospitalizations. Medical records for HF hospitalizations were retrieved and adjudicated by 2 clinicians experts using a structured approach based on well-established epidemiologic criteria.15 Disagreements were resolved by committee consensus.
For this study, we included REGARDS participants aged ≥ 65 years at the time of first adjudicated HF-hospitalization between 2003 and 2014 who had continuous Medicare Part A for 6 months prior to their hospitalization and for the 90 days following their hospitalization. To be included, participants had to be at least 65 years of age at the time of the first adjudicated HF-hospitalization. Medicare linkage was necessary for this study because, it permitted the detection of all readmissions including those with cardiovascular and non-cardiovascular etiology. In this study, we excluded individuals discharged to hospice.
The study protocol was reviewed and approved by the University of Alabama at Birmingham Institutional Review Board and the Weill Cornell Medical College Institutional Review Board. All participants provided written informed consent.
Outcome: 30-Day All-cause Readmission
The outcome of interest for this study was all-cause 30-day readmission after discharge from a HF hospitalization. Hospital discharge dates were obtained from Medicare inpatient claims. We obtained readmission data from Medicare claims.
Main Exposure(s): SDOH
We used the HealthyPeople2030 conceptual framework to guide our analyses, categorizing SDOH into 5 domains: 1) social and community context; 2) education; 3) economic stability; 4) neighborhood and built environment; 5) health and health care (Supplemental Figure 1).13 Using this framework, we considered 9 SDOH: Black race; social isolation (defined as having 0–1 visits from family or friends in the past month); social network (defined as having someone to care for them if ill); low educational attainment (<high school education); low annual household income (<$35,000); living in a rural areas (living in an isolated or small rural area based off of Rural Urban Commuting Area Codes); living in a zip code with high poverty (>25% of residence below the federal poverty line); living in a complete or partial Health Professional Shortage Area (HPSA) county; and public health infrastructure (assessed with America’s Health Ranking,16 which ranked states from 1993–2002 based on their contribution to lifestyle, access to care, and disability; states ranked in the bottom 20th percentile for ≥8 years were considered to have poor infrastructure)..
All SDOH were assessed at during the REGARDS baseline interview and dichotomized as yes/no; some SDOH were self-reported, while others were based on geocoded addresses. Of note, we did not include sex or age as SDOH since they are biologically determined; instead, we accounted for them as covariates in our analyses.
Covariates
We selected covariates using a framework by Calvillo-King et al. (2013) which conceptualized the impact of social factors on hospital readmission and mortality in HF.7, 17 Using this framework, we included demographics, clinical comorbidities, health behaviors, self-reported health, hospitalization factors, and hospital characteristics, as we have done in prior studies.17 Data were collected from four sources: the REGARDS baseline assessment; medical charts from each HF adjudicated hospitalization; the American Hospital Association annual survey database;18 and Medicare’s Hospital Compare Website.19
Demographics (sex, region of residence), health behaviors (smoking status), and self-reported health were collected from the REGARDS baseline assessment. Physical and mental health were assessed using the Short Form-12’s Physical and Mental Component Summary (PCS and MCS) scores. MCS and PCS scores range from 0 to 100 with higher scores representing better health.20 Cognition was assessed with the Six-Item Screener (SIS), which is performed annually on REGARDS participants.21 Cognitive impairment was defined as a SIS score of <5. Of note, the SIS performed prior to and as close to the adjudicated HF hospitalization as possible, was used. Age at the time of HF hospitalization, the Charlson comorbidity index, echocardiogram parameters, discharge disposition (home vs. nursing home/rehabilitation) and length of stay were abstracted from chart review at the time of the index hospitalization. We defined heart failure with preserved ejection fraction (HFpEF) as those with left ventricular ejection fraction (LVEF) > 50% or a qualitative description of normal systolic function; and heart failure with reduced ejection fraction (HFrEF) as those with an LVEF < 50% or a qualitative description of abnormal systolic function. We grouped individuals with LVEF between 40 and 50% with individuals with HFrEF.22
Hospitalization characteristics included: echocardiogram parameters, discharge disposition, length of stay (LOS), intensive care unit (ICU) stay, and consultation with a cardiologist, all abstracted from medical records. Hospital characteristics including bed size, teaching status (ascertained from the American Hospital Association survey) and hospital quality (ascertained from the Medicare’s Hospital Compare website).17 For this study, we examined hospital rating, which is a summary measure comprised of several different quality metrics used to compare hospitals. Hospital ratings are scored within a range of 1–5, with 3 being average and higher scores reflecting higher quality care.
Statistical analysis
First, we compared the characteristics of participants by their 30-day readmission status using Chi square tests for categorical variables and t-tests or Wilcoxon rank sum tests for continuous variables. Next, for each SDOH, we calculated the incidence per 1000-person years of 30-day readmission using Poisson regression. Prior work has demonstrated that these individual SDOH are not collinear. To confirm this in our study, however, we assessed multicollinarity among the 9 SDOH using variance inflation factors and by investigating correlations. We then estimated hazard ratios (HR) and 95% confidence intervals (CI) in separate Cox models to determine the association between each individual SDOH and 30-day readmission (crude model). After examining the crude associations for each SDOH and risk of 30-day readmission, we adjusted for each of the other SDOH in Model 1. In a fully adjusted model (Model 2), we adjusted for additional covariates (demographics, clinical comorbidities, health behaviors, physical and mental functioning, hospitalization and hospital characteristics) to determine the independent effect of each SDOH on 30-day readmission; covariates included in Model 2 were based on a p<0.10 cutoff value from Table 1. We tested for interactions between all SDOH and age, sex, and HF-subtype using the Wald test. We used Shoenfeld residuals to test the proportionality assumption for Cox models as a whole as well as for the individual exposures of interest. To account for the competing risk of death, we performed a sensitivity analysis using the Fine and Gray approach. We used multiple imputation by chained equations to minimize bias attributed to missing data;23 the largest sources of missing data were income (n=92, 13.3%), rural residence (n=62, 9.0%), cognition (n=56, 8.1%), and social network (n=50, 7.2%). We used two-sided hypothesis testing with p-value <0.05 for all analyses. Analyses were conducted in SAS 9.4 and Stata 14.
Table 1.
Characteristics of REGARDS Participants Hospitalized for HF by Readmission Status.
| Characteristics | Total (N=690) | No 30-day Readmission (n=535) | 30-day Readmission (n=155) | p-value |
|---|---|---|---|---|
| SDOH | ||||
| Black Race | 245 (35.5%) | 185 (34.6%) | 60 (38.7%) | 0.34 |
| Low Educational Attainment | 162 (23.5%) | 124 (23.2%) | 38 (24.5%) | 0.73 |
| Low Income | 377 (63.0%) | 295 (63.3%) | 82 (62.1%) | 0.8 |
| Zip code level poverty | 143 (21.0%) | 109 (20.6%) | 34 (22.4%) | 0.64 |
| Health Professional Shortage Area | 300 (43.5%) | 219 (40.9%) | 81 (52.3%) | 0.01 |
| Poor Public Health Infrastructure | 271 (39.3%) | 213 (39.8%) | 58 (37.4%) | 0.59 |
| Social Isolation | 88 (13.1%) | 64 (12.3%) | 24 (15.8%) | 0.26 |
| Social Network | 85 (13.3%) | 67 (13.5%) | 18 (12.6%) | 0.78 |
| Rural residence | 64 (10.2%) | 47 (9.7%) | 17 (12.0%) | 0.43 |
| Demographics | ||||
| Age at hospitalization, median (IQR) | 76.0 (71.0, 82.0) | 76.0 (72.0, 82.0) | 75.0 (70.0, 81.0) | 0.10 |
| Female | 306 (44.3%) | 244 (45.6%) | 62 (40.0%) | 0.22 |
| Region of residence | 0.50 | |||
| Belt | 250 (36.2%) | 193 (36.1%) | 57 (36.8%) | |
| Buckle | 152 (22.0%) | 123 (23.0%) | 29 (18.7%) | |
| Non-belt | 288 (41.7%) | 219 (40.9%) | 69 (44.5%) | |
| Health Behaviors and Medical Conditions | ||||
| Current Smoking | 66 (9.6%) | 43 (8.0%) | 23 (14.8%) | 0.01 |
| Charlson Comorbidity Index, median (IQR) | 4.0 (3.0, 5.0) | 3.5 (2.5, 5.0) | 4.0 (3.0, 5.0) | 0.09 |
| Physical and Mental Functioning | ||||
| Impaired Cognition* | 113 (17.8%) | 82 (16.5%) | 31 (22.8%) | 0.09 |
| PCS- Physical Health†, median (IQR) | 41.7 (31.6, 49.9) | 41.8 (31.4, 50.4) | 41.3 (32.3, 49.2) | 0.63 |
| MCS-Mental Health†, median (IQR) | 56.7 (49.5, 59.9) | 56.7 (49.6, 59.9) | 56.0 (48.3, 60.2) | 0.85 |
| Hospital Characteristics and Transitions to Care | ||||
| ICU stay during hospitalization | 145 (21.0%) | 109 (20.4%) | 36 (23.2%) | 0.44 |
| MI during hospitalization | 111 (16.1%) | 80 (15.0%) | 31 (20.0%) | 0.13 |
| Revascularization during hospitalization | 79 (11.4%) | 57 (10.7%) | 22 (14.2%) | 0.22 |
| Consult with cardiologist | 211 (30.6%) | 171 (32.0%) | 40 (25.8%) | 0.14 |
| Discharged to nursing home | 85 (12.5%) | 59 (11.2%) | 26 (17.0%) | 0.05 |
| Length of stay, median (IQR) | 5.0 (3.0, 8.0) | 5.0 (3.0, 7.0) | 6.0 (4.0, 9.0) | 0.01 |
| Ejection Fraction < 50‡, | 280 (55.0%) | 218 (54.1%) | 62 (58.5%) | 0.42 |
| Hospital Characteristics | ||||
| Bed size||, median (IQR) | 348.5 (201.0, 564.0) | 363.5 (213.0, 580.0) | 328.0 (180.0, 509.0) | 0.08 |
| Teaching status# | 324 (47.1%) | 254 (47.6%) | 70 (45.5%) | 0.64 |
| Hospital Quality Rating**, mean (SD) | 2.9 (0.9) | 2.9 (0.9) | 2.8 (0.9) | 0.42 |
Data were collected from four sources: the REGARDS baseline assessment; medical charts from each HF-adjudicated hospitalization; the American Hospital Association annual survey database; and Medicare’s Hospital Compare website. Region of residence: Stroke Buckle (coastal North and South Carolina and Georgia), Stroke Belt (the remainder of North and South Carolina and Georgia, and Alabama, Mississippi, Louisiana, Arkansas, and Tennessee), and Non-Belt.
Cognitive impairment was defined as a Six-item Screener score of <5. Note, the SIS performed prior to and as close to the adjudicated HF hospitalization was used.
Physical and mental health were assessed with the Short-Form (SF-12) which comprises of the Physical Component Summary (PCS) and Mental Component Summary (MCS). MCS and PCS scores range from 0 to 100 with higher scores representing better health.
Heart failure was classified as preserved ejection fraction (HFpEF) for those with left ventricular ejection fraction (LVEF) > 50% or a qualitative description of normal systolic function; and heart failure with ejection fraction (HFrEF) for those with a LVEF < 50% or a qualitative description of abnormal systolic function. We grouped individuals with LVEF between 40 and 50% with individuals with HFrEF.
Small hospital size was defined as <200 beds.
Teaching status was defined as the academic status of the hospital.
Hospital quality was determined using the Medicare’s Hospital Compare website which lists publicly available information regarding the quality of care of over 4,000 Medicare-certified hospitals. Hospital ratings are scored within a range of 1–5, with 3 being average and higher scores reflecting higher quality care.
Largest sources of missing data: Income (n=92), zip code level poverty (n=9), social isolation (n=17), social network (n=50), rural residence (n=62), Charlson (n=30, cog impairment (n=56), pcs (n=47), mcs (n=47), discharged to nursing home (n=10), bed size (n=2), and hospital quality rating (n=38).
Results
A total of 690 participants were hospitalized for HF at 440 unique hospitals across the US (Supplemental Figure 2). HF hospitalization events occurred within a median of 3.7 years (interquartile range [IQR] 1.8–6.0) of participants’ baseline REGARDS interview. With respect to SDOH, 44.3% were female, 35.5% were Black, 23.5% had low educational attainment, 63.0% had low income, 21.0% had zip code-level poverty, 43.5% resided in HPSAs, 39.3% lived in states with poor public health infrastructure, 13.1% were socially isolated, 13.3% had poor social networks, and 10.2% lived in rural areas (Table 1). At admission, they had a median age of 76 years (IQR:71–82) and pre-HF hospitalization median PCS of 41.7 (IQR:31.6–49.9), MCS of 56.7 (IQR:49.5–59.9) and CCI of 4 (IQR:3.0–5.0). During the hospitalization, median LOS was 5 days (IRQ:3.0–8.0), 21% were admitted to the ICU, and 12.5% were discharged to a nursing home (Table 1).
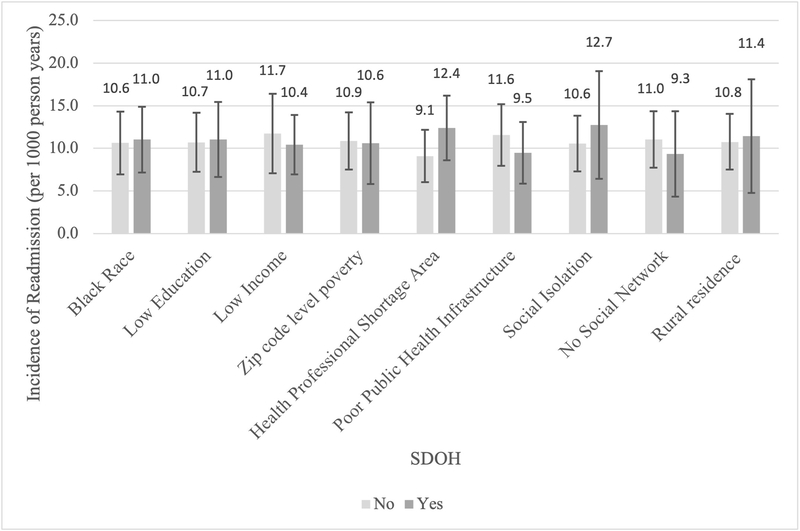
Of the 690 participants admitted for HF and discharged alive, 155 participants were readmitted within 30 days (22.5%) and 19 died (2.7%) during the study period. There was no evidence of multicollinearity among the 9 SDOH; variance inflation factors were low, ranging from 1.04–1.42 and the correlation coefficients were weak to moderate, with absolute values ranging from 0 to 0.34 (Supplemental Figure 3). Among the 9 SDOH, 30-day readmission status differed only by HPSA (52.3% with readmission vs. 40.9% without, p=0.01); incidence rates of 30-day readmission did not differ significantly by any of the 9 SDOH (Figure 1). Participants who were readmitted were more likely to have been discharged to a nursing home (17.0% vs. 11.2%, p <0.05) and have longer LOS (6.0 vs. 5.0 days, p<.01) during the HF hospitalization compared to those who were not readmitted. Those who were readmitted also had more comorbidities (4.0 vs. 3.5, p<0.09) and worse cognition (22.8% vs. 16.5%, p<0.09).
Figure 1 (Central Figure). Adjusted Incidence of 30-Day Readmission by Individual Social Determinants of Health (SDOH) among REGARDS Participants Hospitalized for Heart Failure (HF).
Incidence rate of 30-day readmission for each individual SDOH, adjusted for SDOH, age, Charlson comorbidity index, current smoking, impaired cognition, discharge from nursing home, length of stay,, and bed size of admitting hospital.
Bars indicate 95% Confidence Intervals
“No” indicates not having the specific SDOH of interest
In a crude model which examined the association between each SDOH and the risk of 30-day readmission, residing in a HPSA was associated with a 47% higher risk of 30-day readmission (HR 1.47, 95% CI:1.07–2.01, p<0.02) (Table 2). While Black race (HR: 1.17, 95% CI:0.84–1.61), social isolation (HR 1.30, CI:0.84–2.00), and rural residence (HR: 1.20, CI:0.72–1.99) were associated with greater readmission risk, these associations did not achieve statistical significance. In a fully adjusted model, none of the SDOH was independently associated with higher 30-day readmission risk (Table 2). Although we a priori intended to test for interactions of SDOH by age, gender, and HF sub-type, we dropped these analyses given inadequate sample size in these groups.
Table 2.
Crude and Fully Adjusted HR of 30-Day Readmission Among REGARDS Participants Admitted for Heart Failure in REGARDS
| SDOH | Crude | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Less education | 1.08 | (0.75, 1.56) | 0.68 | 1.07 | (0.72, 1.58) | 0.75 | 1.03 | (0.69, 1.55) | 0.88 |
| Health Professional Shortage Area (HPSA) | 1.47 | (1.07, 2.01) | 0.02 | 1.46 | (1.05, 2.02) | 0.02 | 1.35 | (0.96, 1.9) | 0.09 |
| Low Income | 0.97 | (0.68, 1.37) | 0.86 | 0.94 | (0.64, 1.38) | 0.75 | 0.90 | (0.61, 1.32) | 0.59 |
| Poor public health infrastructure | 0.91 | (0.66, 1.26) | 0.56 | 0.87 | (0.62, 1.22) | 0.42 | 0.83 | (0.56, 1.22) | 0.34 |
| Black race | 1.17 | (0.84, 1.61) | 0.35 | 1.13 | (0.78, 1.62) | 0.52 | 1.03 | (0.71, 1.49) | 0.89 |
| Rural residence | 1.20 | (0.72, 1.99) | 0.49 | 1.15 | (0.68, 1.94) | 0.61 | 1.06 | (0.61, 1.84) | 0.84 |
| Social Network | 0.97 | (0.59, 1.59) | 0.90 | 0.92 | (0.56, 1.52) | 0.75 | 0.85 | (0.51, 1.41) | 0.52 |
| Social isolation | 1.30 | (0.84, 2.00) | 0.24 | 1.26 | (0.81, 1.95) | 0.31 | 1.21 | (0.76, 1.91) | 0.42 |
| Zip code level poverty | 1.09 | (0.75, 1.60) | 0.65 | 0.99 | (0.65, 1.51) | 0.96 | 0.98 | (0.64, 1.5) | 0.92 |
Crude – Individual SDOH: Education, HPSA, low income, poor public health infrastructure, black race, rural residence, social network, social isolation, zip code level poverty
Model 1- adjusted for other SDOH
Model 2 - adjusted for other SDOH, age, Charlson comorbidity index Current smoking, Impaired Cognition, discharge from nursing home, length of stay, and bed size of admitting hospital
Finally, the competing risk analyses which accounted for death did not differ from the main analyses that did not (Supplemental Table 1).
Discussion
In this prospective cohort study of Medicare beneficiaries hospitalized for HF, we observed a high prevalence of each of the 9 SDOH, ranging from 10% (rural residence) to 63% (low income) among participants. While some of the SDOH we examined, such as living in a HPSA, Black race, and social isolation, were associated with higher risk of 30-day readmission in unadjusted models, none of the 9 SDOH were independently associated with 30-day readmission risk in fully adjusted models in this modestly sized sample. In the context of increased appreciation for the influence of SDOH on cardiovascular outcomes, our findings suggest that policies or interventions which target individual SDOH alone may not suffice in reducing 30-day readmissions among older adults hospitalized for HF. Rather, a more nuanced approach to screening for and addressing certain SDOH, alongside other factors, may be required to reduce readmissions in this patient population.24
Prior studies which have examined SDOH and readmission risk among Medicare beneficiaries hospitalized for HF have found Black race,25 Hispanic ethnicity,26 residing in a disadvantaged neighborhood,10, 27 and poor social support28 to be associated with higher all-cause 30-day readmission.29 Similarly, we also found that being Black, residing in a HPSA, and having an inadequate social support were each associated with greater risk of 30-day readmission. However, our study differs from many others in that after adjusting for other individual and neighborhood-level SDOH, alongside participants’ demographic, clinical, hospitalization and hospital characteristics, these associations were attenuated. This suggests that among older adults hospitalized for HF, SDOH may be important, but targeting individual SDOH alone may not be enough to reduce readmissions. Alternatively, and in line with a recent study which has found that multiple within-person SDOH are associated with increased mortality following a HF hospitalization,17 future larger studies ought to examine how a cumulative burden of SDOH effect 30-day readmission risk.
Our findings also underscore the need for HF readmission studies to include other factors, beyond traditional SDOH, that are likely to affect post-discharge outcomes among older adults with HF. Medicare beneficiaries hospitalized with HF have multiple comorbidities, are frail, and have functional, cognitive, and sensory deficits.30–32 As such, many 30-day readmissions may not due to HF itself or may be due to patients’ inability to comply with HF self-care, thus minimizing the influence of SDOH in the short-term.33 Additionally, other factors such as health literacy,34 support from unpaid and paid caregivers,35 medication burden and adherence,36 severity of the HF hospitalization (as measured by laboratory data such as BNP and/or devices received),37 and outpatient follow-up after discharge may also impact 30-day readmissions for this patient population, and warrant further investigation, as we were unable to study these in our cohort.
Strengths and Limitations
Strengths of our study include its national, biracial sample with rigorously collected data and adjudicated HF hospitalizations. In addition to studying demographic and health-related characteristics of participants, we were able to assess characteristics of the HF hospitalization and the hospital to which patients were admitted. We assessed a comprehensive number of individual and community-level SDOH from each of the HealthyPeople2030 domains. Limitations, however, exist. First, we were unable to assess some SDOH including neighborhood and physical environment (e.g. food and housing, transportation, and safety) and racial discrimination which may be associated with readmission risk. Second, our sample size was relatively modest; it is likely that the small number of readmission events, and thus low statistical power, may have limited our ability to detect associations between SDOH and 30-day readmissions in this cohort. The modest sample size also limits our ability to conduct additional analyses that could examine the cumulative burden of SDOH within individuals on readmission risk or analyses that could account for intersectionality among the various SDOH assessed here. Third, we only had access to data through 2014; there is a need for more contemporary studies to build on our findings. Finally, we used the baseline REGARDS interview to assess the majority of SDOH, which may have occurred several years before the adjudicated HF hospitalization.
Conclusion
Despite the high prevalence of SDOH in this national cohort of Medicare beneficiaries hospitalized for HF, we did not observe an increased risk of 30-day re-admission among individuals with SDOH with the exception of residence in a HPSA, which diminished after adjustment for clinical, hospital, and HF hospitalization characteristics. Our findings suggest that although SDOH are highly prevalent and important, other factors may also be influential in driving readmission risk among older adults with HF. Additionally, policies or interventions that only target individual SDOH to reduce readmissions after HF hospitalizations may not be sufficient to improve post-discharge outcomes among older Medicare beneficiaries.
Supplementary Material
What is new?
Social determinants of health (SDOH) may be important contributors to post-discharge outcomes among adults hospitalized for heart failure (HF). However, their impact on 30-day readmissions among older adults is not known. Informed by the Healthy People 2030 Framework, we examined the association between 9 individual SDOH and 30-day readmission risk among Medicare Beneficiaries hospitalized for HF in the REGARDS cohort. In this modestly sized sample, we found that while the prevalence of many of the SDOH were high, none of the 9 SDOH were independently associated with 30-day readmission risk in fully adjusted models.
What are the clinical implications?
These observational findings suggest that screening for SDOH among older adults hospitalized for HF is important, as the prevalence of many SDOH were high. However, policies or interventions that aim to reduce readmissions by only targeting individual SDOH may not be enough to reduce 30-day readmissions among Medicare beneficiaries.
Acknowledgements:
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
Sources of Funding:
This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis or interpretation of the data. Additional funding was provided by NHLBI grant R01HL080477 (Safford) and NIA grant R03AG056446 (Goyal). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Dr. Sterling is supported by the National Heart, Lung, and Blood Institute (K23HL150160). The views expressed here do not reflect those of the National Heart, Lung, and Blood Institute.
Conflicts and Disclosures:
Dr. Sterling, Ms. Bryan, Dr. Pinheiro, and Dr. Phillips have no conflicts to report. Dr. Goyal, Dr. Levitan, Dr. Safford, and Dr. Brown receive research support from Amgen. Dr. Levitan also received support from Amgen advisory boards and Novartis. Dr. Brown serves as site PI for a clinical trial funded by Omthera Pharmaceuticals.
Footnotes
Presentations: This study was presented (virtually) at the 2020 Society of General Internal Medicine (SGIM) Annual Meeting on May 8–11, 2020. Dr. Sterling was awarded ‘Best Scientific Abstract’ (2020 Milton W. Hamolsky Award) among junior faculty by the SGIM.
References
- 1.Chen J, Normand SL, Wang Y and Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. Jama. 2011;306:1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV and Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal MA, Fonarow GC, Ziaeian B. National Trends in Heart Failure Hospitalizations and Readmissions From 2010 to 2017. JAMA Cardiol. 2021; 10:e207472. doi: 10.1001/jamacardio.2020.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziaeian B and Fonarow GC. The Prevention of Hospital Readmissions in Heart Failure. Prog Cardiovasc Dis. 2016;58:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergethon KE, Ju C, DeVore AD, Hardy NC, Fonarow GC, Yancy CW, Heidenreich PA, Bhatt DL, Peterson ED and Hernandez AF. Trends in 30-Day Readmission Rates for Patients Hospitalized With Heart Failure: Findings From the Get With The Guidelines-Heart Failure Registry. Circulation Heart failure. 2016; 9: e002594. 10.1161/CIRCHEARTFAILURE.115.002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO | Health equity. WHO. 2017. Available at https://www.who.int/topics/health_equity/en/; accessed May 15, 2019. [Google Scholar]
- 7.Calvillo-King L, Arnold D, Eubank KJ, Lo M, Yunyongying P, Stieglitz H and Halm EA. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28:269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knighton AJ, Savitz LA, Benuzillo J, VanDerslice JA. It takes a village: Exploring the impact of social determinants on delivery system outcomes for heart failure patients. Healthc (Amst). 2018;6:112–116. [DOI] [PubMed] [Google Scholar]
- 9.Rathore SS, Masoudi FA, Wang Y, Curtis JP, Foody JM, Havranek EP, Krumholz HM. Socioeconomic status, treatment, and outcomes among elderly patients hospitalized with heart failure: findings from the National Heart Failure Project. Am Heart J. 2006;152:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C, Smith M. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014. Dec 2;161:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byhoff E, Kangovi S, Berkowitz SA, DeCamp M, Dzeng E, Earnest M, Gonzalez CM, Hartigan S, Karani R, Memari M, Roy B, Schwartz MD, Volerman A, DeSalvo K; Society of General Internal Medicine. A Society of General Internal Medicine Position Statement on the Internists’ Role in Social Determinants of Health. J Gen Intern Med. 2020;35:2721–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White-Williams C, Rossi LP, Bittner VA, Driscoll A, Durant RW, Granger BB, Graven LJ, Kitko L, Newlin K, Shirey M; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Epidemiology and Prevention. Addressing Social Determinants of Health in the Care of Patients With Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2020; 141:e841–e863. [DOI] [PubMed] [Google Scholar]
- 13.About Healthy People. 2018. Available at: https://www.healthypeople.gov/2020/About-Healthy-People. Accessed June 20, 2018.
- 14.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS and Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 15.Goyal P, Mefford MT, Chen L, Sterling MR, Durant RW, Safford MM, Levitan EB. Assembling and validating a heart failure-free cohort from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. BMC Med Res Methodol. 2020; 20: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American’s Health Rankings. 2018. Available at: https://www.americashealthrankings.org/. Accessed June 5, 2019.
- 17.Sterling MR, Ringel JB, Pinheiro LC, Safford MM, Levitan EB, Phillips E, Brown TM, Goyal P. Social Determinants of Health and 90-Day Mortality After Hospitalization for Heart Failure in the REGARDS Study. J Am Heart Assoc. 2020; 9 :e014836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AHA Annual Survey Database. Available at: https://www.aha.org/data-insights/aha-data-products. Accessed June 20, 2018.
- 19.Hospital Compare. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/HospitalCompare. Accessed June 20, 2018.
- 20.Ware J Jr., Kosinski Mand Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 21.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ and Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. [DOI] [PubMed] [Google Scholar]
- 22.Butler J, Anker SD and Packer M. Redefining Heart Failure With a Reduced Ejection Fraction. JAMA. 2019. Nov 12;322:1761–1762. [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10:585–598. [DOI] [PubMed] [Google Scholar]
- 24.Jain SH, Chandrashekar P Implementing a targeted approach to social determinants of health interventions. The American journal of managed care. 2020;26: 502–504. [DOI] [PubMed] [Google Scholar]
- 25.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305: 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez F, Joynt KE, López L, Saldaña F, Jha AK. Readmission rates for Hispanic Medicare beneficiaries with heart failure and acute myocardial infarction. Am Heart J. 2011; 162: 254–261.e3. [DOI] [PubMed] [Google Scholar]
- 27.Akwo EA, Kabagambe EK, Harrell FE Jr., Blot WJ, Bachmann JM, Wang TJ, Gupta DK and Lipworth L Neighborhood Deprivation Predicts Heart Failure Risk in a Low-Income Population of Blacks and Whites in the Southeastern United States. Circulation Cardiovascular quality and outcomes. 2018;11:e004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wray CM, Vali M, Byers A, Keyhani S. Examining the Association of Social Determinants of Health with Missed Clinic Visits in Patients with Heart Failure in the Veterans Health Administration. J Gen Intern Med. 2020; 35: 1591–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joynt Maddox KE, Reidhead M, Hu J, Kind AJH, Zaslavsky AM, Nagasako EM and Nerenz DR. Adjusting for social risk factors impacts performance and penalties in the hospital readmissions reduction program. Health services research. 2019;54:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorodeski EZ, Goyal P, Hummel SL, Krishnaswami A, Goodlin SJ, Hart LL, Forman DE, Wenger NK, Kirkpatrick JN and Alexander KP. Domain Management Approach to Heart Failure in the Geriatric Patient: Present and Future. Journal of the American College of Cardiology. 2018;71:1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterling MR, Jannat-Khah D, Bryan J, Banerjee S, McClure LA, Wadley VG, Unverzagt FW, Levitan EB, Goyal P, Peterson JC, Manly JJ, Levine DA and Safford MM. The Prevalence of Cognitive Impairment Among Adults with Incident Heart Failure: The REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Journal of cardiac failure.2019; 25: 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sterling MR, Lin FR, Jannat-Khah DP, Goman AM, Echeverria SE and Safford MM. Hearing Loss Among Older Adults With Heart Failure in the United States: Data From the National Health and Nutrition Examination Survey. JAMA otolaryngology-- head & neck surgery. 2018. 144: 273–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyers AG, Salanitro A, Wallston KA, Cawthon C, Vasilevskis EE, Goggins KM, Davis CM, Rothman RL, Castel LD, Donato KM, Schnelle JF, Bell SP, Schildcrout JS, Osborn CY, Harrell FE and Kripalani S. Determinants of health after hospital discharge: rationale and design of the Vanderbilt Inpatient Cohort Study (VICS). BMC Health Serv Res. 2014;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu JR, Moser DK, DeWalt DA, Rayens MK and Dracup K. Health Literacy Mediates the Relationship Between Age and Health Outcomes in Patients With Heart Failure. Circulation Heart failure. 2016;9:e002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterling MR, Kern LM, Safford MM, Jones CD, Feldman PH, Fonarow GC, Sheng S, Matsouaka RA, DeVore AD, Lytle B, Xu H, Allen LA, Deswal A, Yancy CW, Albert NM. Home Health Care Use and Post-Discharge Outcomes After Heart Failure Hospitalizations. JACC Heart Fail. 2020: S2213–1779(20)30385–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goyal P, Bryan J, Kneifati-Hayek J, Sterling MR, Banerjee S, Maurer MS, Lachs MS and Safford MM. Association Between Functional Impairment and Medication Burden in Adults with Heart Failure. J Am Geriatr Soc. 2019;67:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ and Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.