Abstract
Many medicinal plants such as a Panax ginseng and Morus alba (mulberry tree) have been widely used as depigmenting agents in Asia. To maximize their synergistic effects on melanogenesis, new herbal decoctions were created by mixing Ginseng Radix Alba (GR) and Mori Radicis Cortex (MC) at a ratio of 3:2 which called GMC decoction. A decoction of GR and Mori Ramulus (MR), which called GMR, was also formulated in order to compare the anti-melanogenic capacity. Combined decoctions, GMC and GMR, significantly decreased mushroom tyrosinase activity in vitro; however, single extracts, including MC and MR, showed weaker inhibitory activity. Melanin content assay and Fontana–Masson staining confirmed that two decoctions showed stronger inhibitory effects on the forskolin-induced melanin level in B16 cells, without cytotoxicity. Our findings suggest that ginseng in combination with mulberry tree enhances the anti-melanogenic effect in vitro.
Keywords: Herbal decoction, Ginseng radix alba extract, Mori radicis cortex extract, Mori ramulus extract, Tyrosinase, Melanin
Herbal decoction, Ginseng Radix Alba extract, Mori Radicis Cortex extract, Mori Ramulus extract, Tyrosinase, Melanin.
1. Introduction
Melanin is an insoluble pigment that determines the color of the skin, hair, iris, and other tissues (Dubey and Roulin, 2014). Most natural melanin is a mixture of eumelanin, which has a brown-to-black color, and pheomelanin, which has a yellow-to-reddish brown color (Wakamatsu et al., 2021), in varying quantities and ratios. This overall process is known as “mixed melanogenesis.” A previous study revealed the “Raper–Mason pathway” in which L-tyrosine and L-3,4-dihydroxyphenylalanine (L-DOPA) are the initial substrates for the biosynthesis of melanin in mammals (D'Mello et al., 2016). Hydroxylated L-tyrosine is converted to L-DOPA by tyrosinase, after which L-DOPA is oxidized into dopaquinone (orthoquinone of L-DOPA), which is the main precursor in the synthesis of both eumelanin and pheomelanin (Ito and Wakamatsu, 2011; Pillaiyar et al., 2018). This is the rate-limiting step of melanogenesis (Ito and Wakamatsu, 2011). Furthermore, tyrosinase activity is used for determining the ratio of eumelanin to pheomelanin in vitro (Hunt et al., 1995) and in vivo (Burchill et al., 1986). Therefore, it is proposed that the regulation of tyrosinase would be an effective strategy to control the production of melanin pigments affected by the levels of L-tyrosine and L-DOPA (D'Mello et al., 2016). Most of the pathway of melanogenesis is closely related to tyrosinase upregulation, which is switched on by various extrinsic and intrinsic factors (Qian et al., 2020). These include cyclic AMP, which acts through the upregulation of protein kinase A (PKA)/CREB, and the essential transcription factor MITF, which is involved in tyrosinase expression and activity (D'Mello et al., 2016). Therefore, various efforts have been made to discover natural substances modulating the cAMP pathway so as to develop a novel skin-whitening agent.
Medicinal herbs modulate melanin synthesis by affecting melanogenesis-related genes or signaling pathways in complex ways (Li et al., 2019). In particular, Ginseng Radix Alba (GR) and Morus alba (mulberry tree) have shown good potential for treating skin pigmentation-related symptoms. Several studies have reported that various types of ginseng products have been able to reduce melanin synthesis in vitro as well as in vivo (Jiang et al., 2017; Kim, 2015). The wood of M. alba reportedly possesses powerful anti-melanogenic potential (Chaita et al., 2017). It has also been documented that diverse extracts of M. alba can inhibit tyrosinase activity and melanin synthesis (Chang et al., 2011; Lee et al., 2003). However, comprehensive studies on herbal decoctions of GR and detailed parts of M. alba wood have not yet been performed. Moreover, the synergistic effects of the components of combination decoctions require further investigation to maximize their pharmacological effects and minimize unnecessary side effects. Despite many pharmacological properties of Mori Radicis Cortex (MC) and Mori Ramulus (MR), their synergistic efficacies against melanogenesis in combination with ginseng remain to be elucidated.
In the present study, we formulated novel herbal decoctions, GR and MC (GMC) and GR and MR (GMR), and investigated their inhibitory effects on melanogenesis in vitro. In addition, benefits of using the herbal cocktails was proven by comparing the anti-melanogenic activity of GMC and GMR with that of single extracts on B16 melanoma cells.
Chemical compounds
L-tyrosine (PubChem CID: 6057), L-dopa (PubChem CID: 6047), kojic acid (PubChem CID: 3840), forskolin (PubChem CID: 47936), ginsenoside Rg1 (PubChem CID: 441923), oxyresveratrol (PubChem CID: 5281717), mulberroside A (PubChem CID: 6443484), morusin (PubChem CID: 5281671).
2. Materials and methods
2.1. Plant source
All dried herbs, including GR and MC (root bark of mulberry tree), were purchased at Yeoncheon Hyundai Herbal Market (Yeoncheon, Republic of Korea). MR (young twigs of mulberry tree) were purchased from Bomyeong Herbal Market (Seoul, Republic of Korea) (Kim et al., 2021).
2.2. Reagents and chemicals
L-tyrosine, L-DOPA, dimethyl sulfoxide (DMSO), mushroom tyrosinase, forskolin, all standard compounds (ginsenoside Rg1, mulberroside A, morusin, oxyresveratrol) and trifluoroacetic acid for HPLC analysis were purchased from Sigma-Aldrich (St. Louis, MO, United States). Kojic acid was obtained from ChemFaces (Wuhan, China). All solutions for cell culture such as Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), 0.25% trypsin-ethylenediaminetetraacetic acid, and penicillin–streptomycin (P/S) was purchased from Hyclone Laboratories Inc. (Chicago, IL, United States). Acetonitrile for HPLC analysis was obtained from Merck KGaA (Darmstadt, Germany).
2.3. Preparation of standard and sample solutions
To prepare herbal extracts of MC, MR, GMC, and GMR, 50 g of each plant was ground into a powder and then extracted in 500 mL of 70% ethanol in a 37 °C shaking incubator at 100 rpm for 24 h. The novel herbal decoctions, GMC and GMR, were prepared by mixing the powder of two agents (GR and MC or GR and MR) at a dry weight ratio of 3:2. Each extract was filtered by Whatman filter (Piscataway, NJ, United States) and concentrated using a rotary vacuum evaporator (Buchi, Tokyo, Japan). The freeze-dried powder of each sample was collected, with MC, MR, GMC, and GMR weighing 7.1 g, 2.2 g, 2.8 g, and 2.7 g and giving yields of 14.21%, 4.46%, 5.60%, and 5.40%, respectively. For biological activity assays, each herb powder was dissolved by an ultrasonicator from JAC Ultrasonic (Yongin, Republic of Korea). In order to make a final concentration to 100 mg/mL, 50% dimethyl sulfoxide (v/v) were added as a solvent. All solutions were filtered by a 0.22 μm membrane, and then stored at −20 °C until use. For HPLC analysis, a total of 20 mg of MC or MR, and 50 mg of GMC or GMR was accurately weighed according to the weight combination ratio. A standard solution of each compound was prepared at final concentration of 1.0 mg/mL. Each samples and standard solutions were dissolved in 1 mL of methanol for 30 min in JAC Ultrasonic ultrasonicator. After filtering via a 0.22 μm membrane, a 10 μL aliquot of the filtrate was analyzed by HPLC utilizing the Chromeleon 7 software from Thermo Scientific™ (Waltham, MA, United States).
2.4. HPLC conditions
The HPLC-diode-array detector (DAD) was consisted of the Thermo Scientific UltiMate 3000 system (binary pump, auto-sampler, DAD detector, and column oven). HPLC analysis was performed on an X bridge C18 column (250 mm × 4.6 mm, 5 μm) coupled with a guard cartridge C18 (4.0 × 3.0 mm). The mobile phase consisted of 0.1% trifluoroacetic acid in water (eluent A) and acetonitrile (eluent B), which were applied in the gradient elution as follows: 0–3 min, 10% B; 3–20 min, 10%–45% B; 20–55 min, 45%–80% B; and 55–60 min, 80% B. The chromatogram was monitored at 210 nm and 280 nm, the flow rate was 1.0 mL/min, and the injection volume was 10 μL (Table 1). The established HPLC–DAD method was suitable for analysis of each extract and standard constituents (Kim et al., 2021).
Table 1.
HPLC conditions for analysis.
| HPLC Conditions | |||
|---|---|---|---|
| Detector | 210 nm, 280 nm | ||
| Column | X bridge C18 column (250 mm × 4.6 mm, 5 μm) | ||
| Column Temperature | 30 °C | ||
| Injection Volume | 10 μL | ||
| Flow rate | 1.0 mL/min | ||
| Mobile phase |
Time (min) |
A |
B |
| A: 0.1% TFA in Water B: ACN |
0.0 | 90 | 10 |
| 3.0 | 90 | 10 | |
| 20.0 | 55 | 45 | |
| 55.0 | 20 | 80 | |
| 60.0 | 20 | 80 | |
2.5. In vitro tyrosinase activity (diphenolase activity)
The mushroom tyrosinase activity was measured by the oxidation of o-diphenol (L-DOPA) to o-quinone and dopachrome. Briefly (Lee et al., 2017), 80 μL of 0.1 M potassium phosphate buffer (pH 6.8) was prepared in a 96-well plate. Next, 10 μL of test samples or solvents were added before being mixed with 10 μL of mushroom tyrosinase (200 units/mL in buffer). After adding 100 μL of 1 mM L-DOPA solution to each well as a substrate, the reaction was performed at 37 °C. The relative tyrosinase activity was expressed as % activity using the following formula: [(A − B)/A] × 100. Here, A represents the absorbance in the vehicle-treated group and B represents the absorbance in the test sample-treated group.
2.6. In vitro tyrosinase activity assay (monophenolase activity)
The monophenolase activity was determined by measuring the hydroxylation of monophenol (L-tyrosine) to dopachrome (a precursor of melanin). Briefly, 80 μL of 0.1 M potassium phosphate buffer (pH 6.8) was prepared in a 96-well plate. Next, 10 μL of test samples or solvents, and 10 μL of mushroom tyrosinase (1000 units/mL in buffer) were mixed. After adding 100 μL of 1 mM L-tyrosine solution to each well as a substrate, the reaction was performed at 37 °C. The formation of dopachrome was measured by evaluating absorbance at 475 nm using a microplate reader (Molecular Devices, San Jose, CA, USA). The relative tyrosinase activity was expressed as % activity using the following formula: [(A − B)/A] × 100. Here, A represents the absorbance in the vehicle-treated group and B represents the absorbance in the test sample-treated group.
2.7. Cell culture
Murine B16F10 cells were obtained from the Korean Cell Line Bank (KCLB, Seoul, Republic of Korea). As described in previous study (Kim et al., 2017), cells were cultured in DMEM supplemented with 10% FBS and 1% P/S at 37 °C in a 5% CO2 incubator.
2.8. CCK-8 assay
Cell viability was analyzed using the Cell Counting Kit-8, as per manufacturer's instructions from Dojindo Molecular Technologies Inc. (Rockville, MD, United States). Briefly (Kim et al., 2020), cells were seeded into 96-well plates at a density of 1 × 104 cells/100 μL. In order to make stabilization, cells were incubated overnight at 37 °C. Each samples were treated to the wells at indicated concentrations for the indicated time. After adding 10 μL of CCK solutions into wells, cells were further incubated for 2 h at 37 °C. The amount of formazan dye was changed by cellular dehydrogenases, which is soluble in the cell culture medium, represented the number of living cells. Color density was measured at 450 nm using a microplate reader (Molecular Devices i3).
2.9. Melanin content assay
Melanin content assay was performed to elucidate the effect of test samples on cellular melanin levels (Kim et al., 2018). Briefly, B16F10 cells (1.5 × 104 cells/2 mL) were seeded into each well of a 6-well plate. After 24 h, B16F10 cells were pretreated with samples at the indicated concentrations for 1 h before exposure to forskolin (20 μM) for 6 days. Then, extracellular melanin levels were calculated by measuring the absorbance at 405 nm using a microplate reader (Molecular Devices i3).
2.10. Fontana–Masson staining
As reported in previous study (Kim et al., 2018), cells were fixed in 10% formaldehyde for 10 min at room temperature, and intracellular melanin was then stained using a staining kit from American Master Tech Scientific, Inc. (Lodi, CA, United States). Stained cells were observed under an Olympus inverted microscope (Tokyo, Japan). Images were captured at 100× magnification.
2.11. Statistical analysis
As described in previous study (Kim et al., 2021), data are represented as mean ± SEM. The significance of differences in the mean values between the treatment and control groups was analyzed by one-way analysis of variance (ANOVA) with Tukey's post hoc test for multiple comparisons. Analyses were conducted by GraphPad Prism software® Version 5.02 (La Jolla, CA, United States). ∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05 indicated statistical significance.
3. Results
3.1. Optimizing the mixing ratio for new herbal decoctions
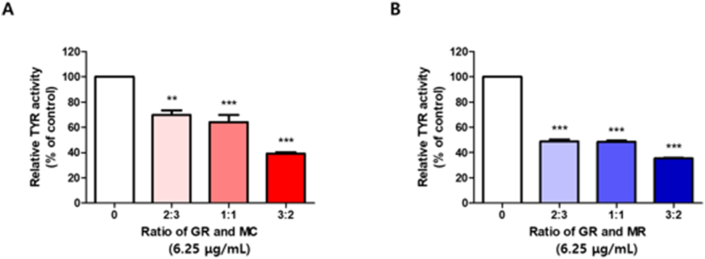
To determine the optimal extraction conditions for herbal decoctions, GR and each herbal extract with MC or MR were mixed as per various combination ratios (2:3, 1:1, and 3:2). First, GMC extracts were formulated by mixing GR and MC together, and they were then subjected to mushroom tyrosinase assay at single dose of 6.25 μg/mL. As shown in Figure 1A, tyrosinase activity was reduced up to 69.83% by the treatment with GMC at the ratio of 2:3. Similarly, GMC at the ratio of 1:1 exhibited anti-tyrosinase activity (reduced by 63.93%). Notably, GMC at the ratio of 3:2 showed the strongest inhibitory effects on tyrosinase activity (reduced by up to 39.3%). Similar results were obtained for the optimization ratio of GR and MR (Figure 1B). GMR at the ratio of 2:3 and 1:1 significantly inhibited tyrosinase activity by up to 48.87% and 48.40 %, respectively. However, treatment with GMR at the ratio of 3:2 reduced diphenolase activity by up to 35.37%. Therefore, all subsequent experiments were conducted by GMC or GMR herbal decoctions at the ratio of 3:2.
Figure 1.
Optimization of extraction condition for new herbal decoctions. (A) GMC (GR and MC) and (B) GMR (GR and MR) were extracted according to the indicated mixing ratios. The mushroom tyrosinase activity was determined using 1 mM L-dopa as the substrate in the absence and presence of each extract. Bar graph (mean ± SEM) data from three experiments were determined using one-way ANOVA with Tukey's post hoc test, ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05, compared with the vehicle group.
3.2. Effects of herbal decoctions on diphenolase activity tyrosinase activity in vitro
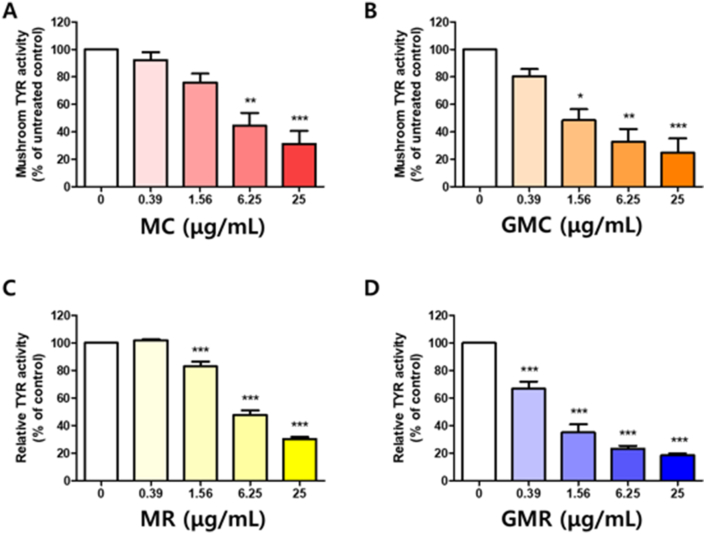
Tyrosinase plays a crucial role in synthesizing melanin by catalyzing two initial substrates L-tyrosine and L-DOPA to melanin in the presence of oxygen. To obtain more detail on the mechanisms of action of each extract, we compared tyrosinase activity by using two substrates. Results showed significant inhibitory capacity of single extracts in catalyzing the oxidation of L-DOPA (Figure 2), as well as in the oxidation of L-tyrosine (Figure 3). Treatment with MC at 6.25 μg/mL and 25 μg/mL reduced the tyrosinase activity to 75.97 and 44.6% (Figure 2A). In addition, GMC dose-dependently decreased the tyrosinase activity up to 43.0% and 7.8%, respectively (Figure 2B). Similarly, MR at 6.25 μg/mL and 25 μg/mL altered the levels of the oxidation of L-DOPA to 47.68% and 30.20%, respectively (Figure 2C). Treatment with GMR decreased diphenolase activity in a dose-dependent manner by up to 23.10% and 18.40%, respectively (Figure 2D). These results indicated that both herbal decoctions had a more potent inhibitory effect on tyrosinase activity than single extract MR or MC.
Figure 2.
Effect of GMC or GMR on mushroom tyrosinase activity in vitro. The tyrosinase activity was tested using 1 mM L-dopa as the substrate in the absence and presence of (A) MC (B) GMC (C) MR (D) GMR. Bar graph (mean ± SEM) data from three experiments were determined using one-way ANOVA with Tukey's post hoc test, ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05, compared with the vehicle group.
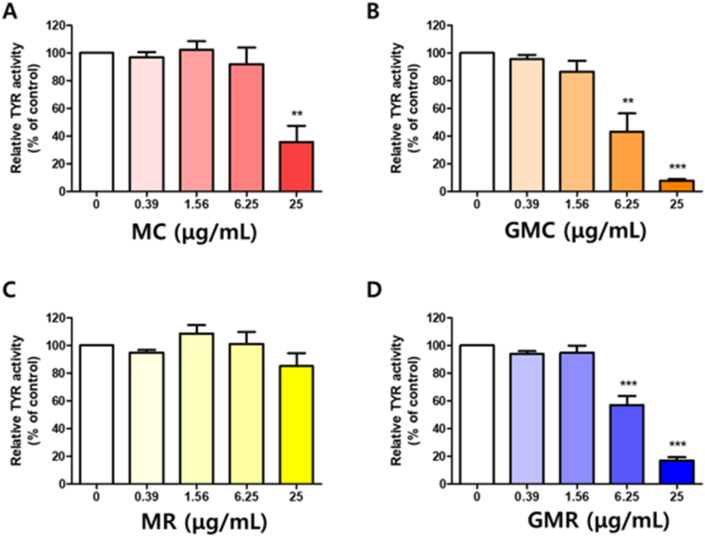
Figure 3.
Effect of GMR or GMC on mushroom tyrosinase activity in vitro. The tyrosinase activity was tested using 1 mM L-tyrosine as the substrate in the absence and presence of (A) MC (B) GMC (C) MR (D) GMR. Bar graph (mean ± SEM) data from three experiments were determined using one-way ANOVA with Tukey's post hoc test, ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05, compared with the vehicle group.
3.3. Effects of herbal decoctions on monophenolase activity of tyrosinase activity in vitro
Tyrosinase monophenolase activity assay were performed using L-tyrosine as the substrate in order to elucidate whether herbal decoctions are also involved in the hydroxylation of L-tyrosine (monophenol) to dopachrome. MC reduced the monophenolase activity of tyrosinase to only 35.63% at 25 μg/mL (Figure 3A). In contrast, GMC significantly decreased the monophenolase activity of tyrosinase at 6.25 μg/mL and 25 μg/mL concentrations to 43.0% and 7.8%, respectively (Figure 3B). Treatment with MR did not affect the monophenolase activity of tyrosinase at all tested concentrations (Figure 3C). However, GMR considerably decreased the hydroxylation of the substrate L-tyrosine to 57.10% and 16.83% at 6.25 μg/mL and 25 μg/mL, respectively (Figure 3D). These results consistently confirmed that both GMC and GMR also closely involved in the inhibition of initial stage for melanin production by tyrosinase.
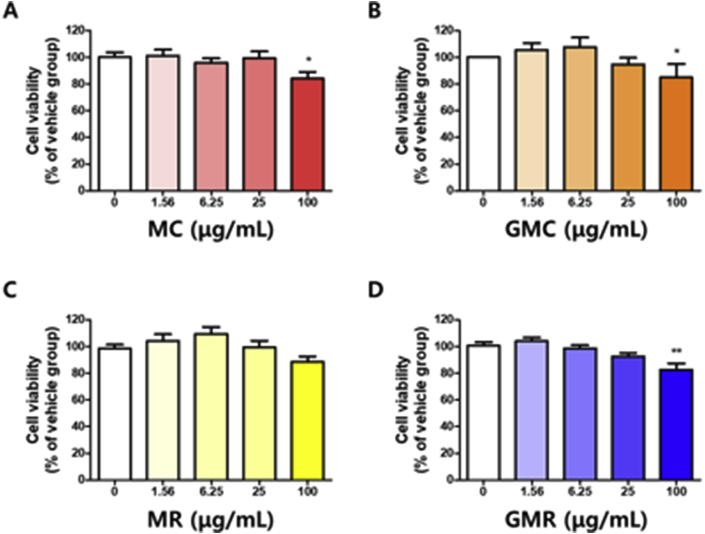
3.4. Effects of herbal decoctions on cell viability in B16 cells
B16 melanoma cells are a well-established cell model system derived from C57BL/6J mice for evaluating melanin formation. Therefore, B16 cells are generally used to measure the cytotoxicity of various drugs for skin-whitening. To the best of our knowledge, there are no previous reports on the herbal decoction of ginseng in combination with extracts from mulberry tree parts. Therefore, ginseng and mulberry tree extracts, alone and in combination, were tested for cytotoxicity after exposure to B16F10 cells at various concentrations for 72 h. The results regarding cell viability showed slight toxic effects by MC or GMC at 100 μg/mL in B16 cells (Figure 4A and B). However, the proliferation was not affected by treatment with MR or GMR at 100 μg/mL (Figure 4C and D). Thus, subsequent experiments were performed at doses lower than 100 μg/mL.
Figure 4.
Determination of the cytotoxicity in B16 cells. Cells were treated with (A) MC (B) GMC (C) MR (D) GMR for 3 days. And then, the cell viability was assessed using a CCK-8 assay. Absorbance was measured using a microplate reader (470 nm). Bar graph (mean ± SEM) data from three experiments were determined using one-way ANOVA with Tukey's post hoc test, ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05, compared with the vehicle group.
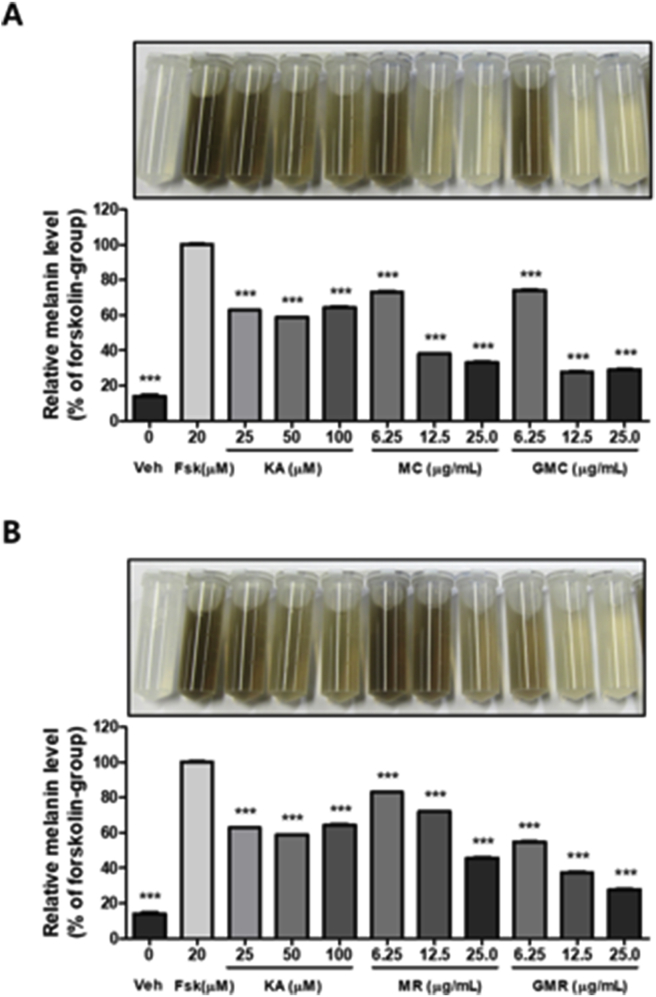
3.5. Effects of herbal decoctions on forskolin-induced melanogenesis in B16 cells
Previous studies indicated that there are various substances that regulate melanogenesis and tyrosinase activity genetically, biochemically, and pharmacologically (D'Mello et al., 2016). In particular, forskolin is an extrinsic factor known to activate the adenylyl cyclase and cAMP-dependent pathway, which is the major pathway for melanogenic signaling. Therefore, to confirm the effects of GMC and GMR on melanin synthesis and secretion, in vitro assays were performed to measure the amount of melanin in B16 cells using forskolin. Kojic acid was used as a positive control to compare the depigmenting efficacy of GMC and GMR. Treatment with GMC and GMR synergistically reduced the forskolin-induced melanin level in a dose-dependent manner (Figure 5A). GMC reduced melanin secretion up to 27.73% and 29.15% at 1.56 and 6.25 μg/mL, respectively. MC also inhibited melanin secretion up to 38.12% and 33.28% at the same dose, respectively. Similarly, melanin level was substantially decreased by GMR up to 37.38% and 27.83%, respectively (Figure 5B). Additionally, MR at the same doses showed weaker inhibitory effect than GMR (72.03% and 45.79% at 1.56 and 6.25 μg/mL, respectively). Collectively, these results indicate the superior effects of herbal formulas rather than treatments with a single extract.
Figure 5.
Effects of herbal decoctions on melanin synthesis in B16 cells. Cells were pretreated with (A) MC and GMC or (B) MR or GMR at the indicated concentrations (0, 6.25, 12.5, and 25 μg/mL) for 1 h before exposure to forskolin (20 μM) for 6 days. Kojic acid was used as the positive control. The secreted melanin levels were analyzed by measuring absorbance using a microplate reader (420 nm). Bar graph (mean ± SEM) data from three experiments were determined using one-way ANOVA with Tukey's post hoc test, ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05, compared with the vehicle group.
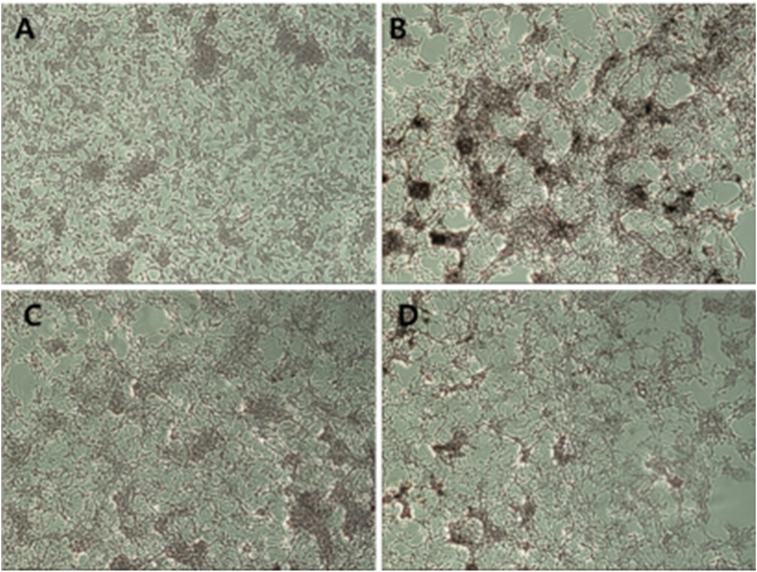
3.6. Effects of herbal decoctions on forskolin-induced intracellular melanin in B16 cells
Fontana–Masson staining is a well-established method to visualize intracellular melanin in melanocytes or skin tissues. Herein we assessed the anti-melanogenic effects of decoctions by analyzing pigmented cells. In comparison with the vehicle-treated group (Figure 6A), forskolin significantly increased the number of dark spots and pigmented cells areas (Figure 6B). Meanwhile, considerable changes, including fewer dark spots and smaller pigmented areas, were observed in the GMC-treated group (Figure 6B). Similar to the effects in the vehicle group, the number of pigmented cells was significantly attenuated in the GMR-treated group (Figure 6D). Thus, our results revealed that GMC and GMR at 25 μg/mL attenuated intracellular melanin synthesis stimulated by forskolin in B16F10 cells, appearing as powerful melanogenesis inhibitors in cell models.
Figure 6.
Representative images of Fontana–Masson stained B16F10 cells. Cells were pretreated with (A) vehicle, (B) forskolin 20 μM, (C) forskolin 20 μM and GMC 25 μg/mL, and (D) forskolin 20 μM and GMR 25 μg/mL for 4 days. Images were captured at ×100 magnification under a microscope.
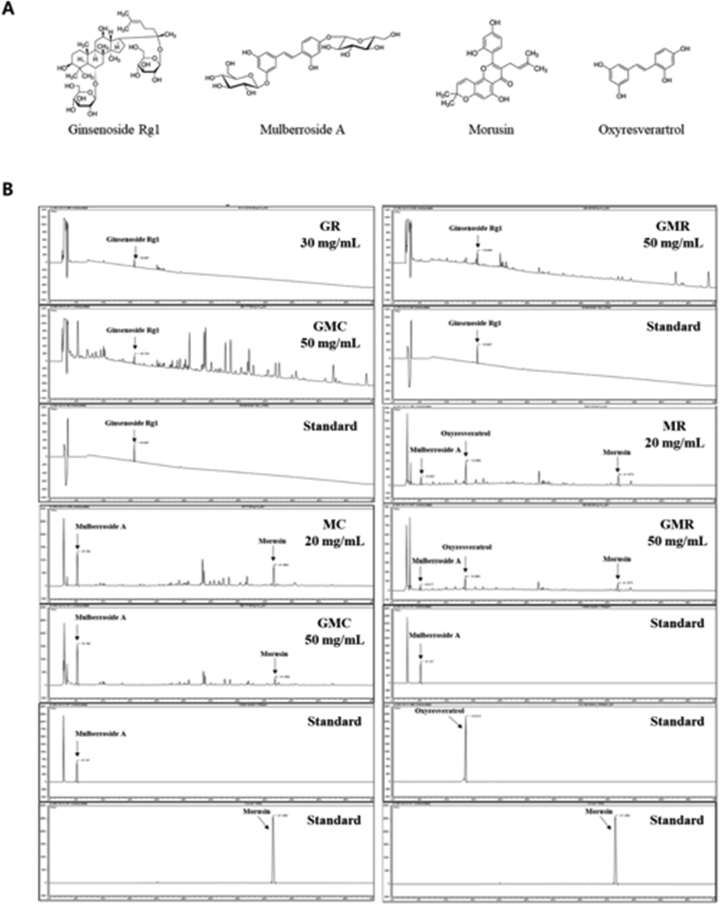
3.7. Content of major compounds in GMC and GMR
The established HPLC system analyzed major compounds of GMC and GMR. A calibration curve of the major compounds was prepared to detect the amount of each component including ginsenoside Rg1, mulberroside A, morusin and oxyresvertrol (Figure 7). The calibration curve linearity of the four major compounds was good at the tested concentration range (Table 2). Each compound area mean value in GMC and GMR was calculated using the calibration curve equation, which was prepared at the tested concentration range. The result showed that GMC contains 1.64% ginsenoside Rg1, 12.54% mulberroside A, 0.16% morusin (Table 3). In case of GMR, the contents were ginsenoside 2.48% Rg1, 0.36% mulberroside A, 0.05% morusin and 0.24% oxyresvertrol (Table 4).
Figure 7.
The structure of compounds and HPLC chromatogram at 210 and 280 nm. (A) Chemical structures of the main components of GMC and GMR including ginsenoside Rg1, mulberroside A, morusin, and oxyresveratrol. (b) HPLC–DAD chromatograms of each extract.
Table 2.
Calibration curves of marker compounds.
| Compounds | Range (ug/mL,ppm) | Regression equation | r2 | LOD (μg/ml) | LOQ (μg/ml) |
|---|---|---|---|---|---|
| 1 | 10.0–200.0 | y = 1.0380x + 1.0470 | 0.9995 | 0.0035 | 0.0106 |
| 2 | 50.0–1000.0 | y = 0.0407x + 0.2627 | 0.9990 | 0.0892 | 0.2701 |
| 3 | 50.0–2500.0 | y = 0.0860x – 1.0593 | 0.9991 | 0.0838 | 0.2539 |
| 4 | 10.0–200.0 | y = 0.3119x + 0.2779 | 0.9985 | 0.0097 | 0.0295 |
Morusin (1); Ginsenoside Rg1 (2); Mulberroside A (3); Oxyresveratrol (4).
LOD = 3.3 × σ/S. LOQ = 10 × σ/S. σ is the standard deviation of the intercept from the regression equation and S is the slope of the calibration curve.
Table 3.
Levels of ginsenoside Rg1, mulberroside A, and morusin in GMC.
| Contents (%) | ||||
|---|---|---|---|---|
| MC (20 mg/ml) | GR (30 mg/ml) | GMC (50 mg/ml) | Fold | |
| Ginsenoside Rg1 | – | 1.33 | 1.64 | 1.23 |
| Morusin | 0.64 | – | 0.16 | 0.25 |
| Mulberroside A | 8.23 | – | 12.54 | 1.52 |
Table 4.
Levels of ginsenoside Rg1, mulberroside A, morusin, and oxyresveratrol in GMR.
| Contents (%) | ||||
|---|---|---|---|---|
| MR (20 mg/ml) | GR (30 mg/ml) | GMR (50 mg/ml) | Fold | |
| Ginsenoside Rg1 | – | 1.33 | 2.48 | 1.86 |
| Mulberroside A | 0.82 | – | 0.36 | 0.44 |
| Morusin | 0.11 | – | 0.05 | 0.45 |
| Oxyresveratrol | 0.69 | 0.24 | 0.35 | |
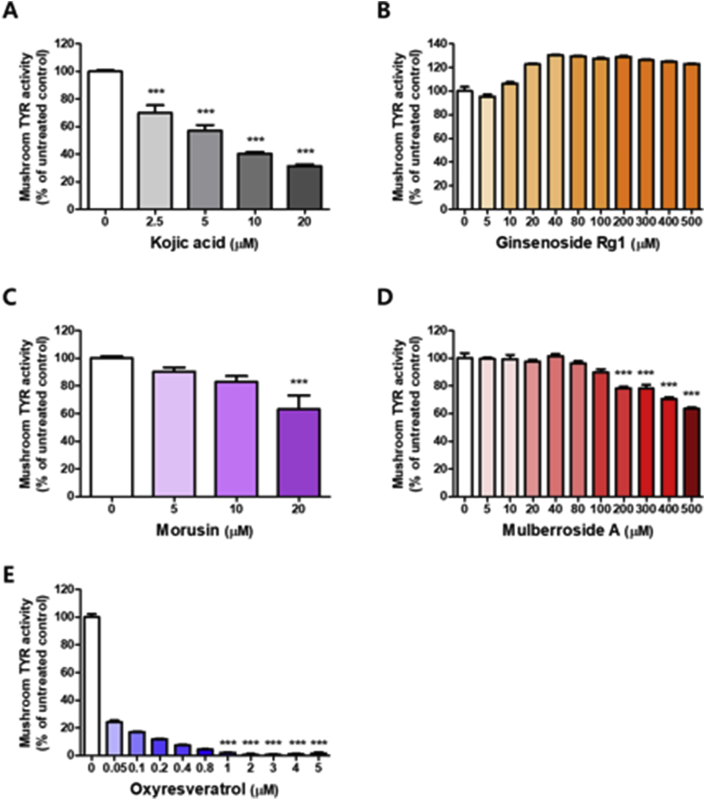
3.8. Effects of GMC and GMR compounds
HPLC analysis confirmed that the main components of GMC were ginsenoside Rg1, mulberroside A, and morusin (Figure 7 and Table 3). In order to elucidate the activity of these compounds, mushroom tyrosinase assay were conducted by comparing kojic acid as a positive control. Kojic acid significantly reduced tyrosinase activity in a dose-dependent manner (Figure 8A), however, ginsenoside Rg1 did not inhibit tyrosinase activity up to 500 μM (Figure 8B). Treatment of morusin exhibited moderate anti-tyrosinase activity at 20 μM, with a reduction to 63.16% (Figure 8C). Mulberroside A did not inhibit tyrosinase activity up to 100 μM, but its treatment at high concentrations (200–500 μM) reduced tyrosinase activity in a dose-dependent manner (Figure 8D). Our results suggested that mulberroside A and morusin are major components for inhibiting anti-tyrosinase activity of GMC. In the case of GMR, oxyresveratrol was additionally identified as the major constituent and showed very powerful anti-tyrosinase activity at the tested doses (Figure 8E and Table 4). These results support that the anti-melanogenic activity of GMR was derived from oxyresveratrol as well as morusin.
Figure 8.
Anti-tyrosinase activity of the main components in GMC and GMR. The mushroom tyrosinase activity of tyrosinase was determined using 1 mM L-dopa as the substrate in the absence and presence of each compound: (A) kojic acid, (B) ginsenoside Rg1 (C) morusin (D) mulberroside A (E) oxyresveratrol. Bar graph (mean ± SEM) data were determined from three experiments using one-way ANOVA with Tukey's post hoc test, ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05, compared with the vehicle group.
4. Discussion
GR has been extensively used as a traditional folk medicine for centuries and a nourishing tonic for health. Many prestigious Asian skin care products reinforce their product's formulation by supplementing GR as an active ingredient (Hu et al., 2020). Moreover, its skin-related beneficial effects also have been elucidated by previous studies (Meng et al., 2021; Song et al., 2011). In the present work, MC and MR were selected due to the their powerful anti-tyrosinase activity observed by us and reported in previous study (Lee et al., 2003). However, their synergistic efficacies against melanogenesis have not been elucidated in combination with ginseng. In the present study, our aim was to compare the anti-melanogenic activity of the novel herbal decoctions, GMC and GMR, with that of single extracts. To optimize the extraction condition of new herbal decoctions, the mixing ratios of ginseng and MC were changed to 2:3, 1:1, and 3:2. As shown in Figure 1A, GMC at the ratio of 3:2 showed the highest inhibitory effect on tyrosinase activity. GMR was also formulated using the same combination ratio with GMC. The anti-tyrosinase activity of GMC was the highest at the ratio of 3:2 (Figure 1B). Therefore, subsequent experiments were performed using formulated herbal decoctions at the ratio of 3:2.
It is known that tyrosinase catalyzes the hydroxylation of L-tyrosine (monooxidase activity) and the oxidation of L-DOPA (diphenolase activity) (Chou et al., 2013). A previous report suggested that the ethanolic extract of mulberry twig (MR) can act as a potent tyrosinase inhibitor for the oxidation of L-DOPA and is superior to the ethanolic extract of mulberry root bark (MC) in this regard (Chang et al., 2011). To obtain more detail on the mechanisms of action of each extract, we compared tyrosinase activity by using L-DOPA. Similar to a previous report, our results also showed significant inhibitory capacity of single extracts in catalyzing the oxidation of L-DOPA (Figure 2), as well as in the oxidation of L-tyrosine (Figure 3). Furthermore, both GMC and GMR were more effective at inhibiting diphenolase activity than the monophenolase activity of mushroom tyrosinase at 6.25 μg/mL. These results suggest that the novel herbal decoctions are more effective than single extracts as tyrosinase inhibitors.
To the best of our knowledge, there are no previous reports on the herbal decoction of ginseng in combination with extracts from mulberry tree parts. At non-cytotoxic concentrations (Figure 4), GMC and GMR significantly attenuated melanin synthesis stimulated by forskolin in B16 cells (Figure 5). Fontana–Masson staining also reconfirmed their superior anti-melanogenic efficacy in B16 cells (Figure 6). Furthermore, HPLC results confirmed that the main components of GMC were ginsenoside Rg1, mulberroside A, and morusin (Figure 7). Previous report confirmed that ethyl acetate extract from GR was reported to inhibit melanogenesis by attenuating oxidative stress (Jiang et al., 2017). Considering these results, we suggest that the anti-melanogenic effect of GR is derived from modulation of a cellular signaling pathway rather than tyrosinase activity directly. Previous studies also demonstrated that morusin and mulberroside A is the major tyrosinase inhibitory compound in mulberry (Inyai et al., 2015; Park et al., 2011), indicating the consistent results with anti-melanogenic capacity of GMC in the present study. In addition, oxyresveratrol was reported to suppress melanogenesis by reducing oxidative stress, tyrosinase expression and activity, and melanogenic signaling pathways (Chaita et al., 2017; Shin et al., 1998). These observations suggest that the anti-melanogenic activity of GMC was mainly due to mulberroside A, whereas that of GMR was derived from oxyresveratrol as well as morusin (Figure 8). Based on the observed results, we speculate that these compounds may contribute to the enhanced anti-melanogenic activity of herbal decoctions by acting synergistically.
5. Conclusions
In this study, we established novel herbal decoctions, GMC and GMR, by combining ginseng and mulberry twigs and root bark and then determined the optimal combination ratios for the decoctions. GMR and GMC exhibited stronger efficacy in terms of monophenolase and diphenolase activities than single extracts of GR, MC, and MR. Furthermore, the improved anti-melanogenic activities of GMC and GMR were demonstrated in the B16 cell model. We speculate that these compounds may contribute to the enhanced anti-melanogenic activity of herbal decoctions by acting synergistically. Our results suggest the potential of GMC and GMR as new anti-melanogenic agent for applications in cosmetics and functional foods for skin pigmentation.
Declarations
Author contribution statement
Ji Hye Kim; Tae In Kim:conceived and designed the experiments; performed the experiments; analyzed and interpreted the data; wrote the paper.
Jin-Yeul Ma: contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Korea Institute of Oriental Medicine, the Ministry of Science and ICT, Republic of Korea (grant number KSN2021230, KSN1812101).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Burchill S.A., Thody A.J., Ito S. Melanocyte-stimulating hormone, tyrosinase activity and the regulation of eumelanogenesis and phaeomelanogenesis in the hair follicular melanocytes of the mouse. J. Endocrinol. 1986;109:15–21. doi: 10.1677/joe.0.1090015. [DOI] [PubMed] [Google Scholar]
- Chaita E., Lambrinidis G., Cheimonidi C., Agalou A., Beis D., Trougakos I., Mikros E., Skaltsounis A.L., Aligiannis N. Anti-melanogenic properties of Greek plants. A novel depigmenting agent from Morus alba wood. Molecules. 2017;22 doi: 10.3390/molecules22040514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L.W., Juang L.J., Wang B.S., Wang M.Y., Tai H.M., Hung W.J., Chen Y.J., Huang M.H. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food Chem. Toxicol. 2011;49:785–790. doi: 10.1016/j.fct.2010.11.045. [DOI] [PubMed] [Google Scholar]
- Chou S.T., Chang W.L., Chang C.T., Hsu S.L., Lin Y.C., Shih Y. Cinnamomum cassia essential oil inhibits alpha-MSH-induced melanin production and oxidative stress in murine B16 melanoma cells. Int. J. Mol. Sci. 2013;14:19186–19201. doi: 10.3390/ijms140919186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello S.A., Finlay G.J., Baguley B.C., Askarian-Amiri M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey S., Roulin A. Evolutionary and biomedical consequences of internal melanins. Pigment Cell Melanoma Res. 2014;27:327–338. doi: 10.1111/pcmr.12231. [DOI] [PubMed] [Google Scholar]
- Hu S., Wolfe S., Laughter M.R., Sadeghpour M. The use of botanical extracts in East Asia for treatment of hyperpigmentation: an evidenced-based review. J. Drugs Dermatol. JDD. 2020;19:758–763. doi: 10.36849/JDD.2020.4776. [DOI] [PubMed] [Google Scholar]
- Hunt G., Kyne S., Wakamatsu K., Ito S., Thody A.J. Nle4DPhe7 alpha-melanocyte-stimulating hormone increases the eumelanin:phaeomelanin ratio in cultured human melanocytes. J. Invest. Dermatol. 1995;104:83–85. doi: 10.1111/1523-1747.ep12613565. [DOI] [PubMed] [Google Scholar]
- Inyai C., Komaikul J., Kitisripanya T., Tanaka H., Sritularak B., Putalun W. Development of a rapid immunochromatographic strip test for the detection of mulberroside A. Phytochem. Anal. 2015;26:423–427. doi: 10.1002/pca.2576. [DOI] [PubMed] [Google Scholar]
- Ito S., Wakamatsu K. Human hair melanins: what we have learned and have not learned from mouse coat color pigmentation. Pigment Cell Melanoma Res. 2011;24:63–74. doi: 10.1111/j.1755-148X.2010.00755.x. [DOI] [PubMed] [Google Scholar]
- Jiang R., Xu X.H., Wang K., Yang X.Z., Bi Y.F., Yan Y., Liu J.Z., Chen X.N., Wang Z.Z., Guo X.L., et al. Ethyl acetate extract from Panax ginseng C.A. Meyer and its main constituents inhibit alpha-melanocyte-stimulating hormone-induced melanogenesis by suppressing oxidative stress in B16 mouse melanoma cells. J. Ethnopharmacol. 2017;208:149–156. doi: 10.1016/j.jep.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Kim D.H., Cho K.M., Kim K.H., Kang N.J. Effect of 3,6-anhydro-l-galactose on alpha-melanocyte stimulating hormone-induced melanogenesis in human melanocytes and a skin-equivalent model. J. Cell. Biochem. 2018;119:7643–7656. doi: 10.1002/jcb.27112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Kim Y.S., Kim T.I., Li W., Mun J.G., Jeon H.D., Kee J.Y., Choi J.G., Chung H.S. Unripe black raspberry (rubus coreanus miquel) extract and its constitute, ellagic acid induces T cell activation and antitumor immunity by blocking PD-1/PD-L1 interaction. Foods. 2020;9 doi: 10.3390/foods9111590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Yun E.J., Yu S., Kim K.H., Kang N.J. Different levels of skin whitening activity among 3,6-Anhydro-l-galactose, agarooligosaccharides, and neoagarooligosaccharides. Mar. Drugs. 2017;15 doi: 10.3390/md15100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. Effect of ginseng and ginsenosides on melanogenesis and their mechanism of action. J. Ginseng Res. 2015;39:1–6. doi: 10.1016/j.jgr.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.I., Kwon E.B., Oh Y.C., Go Y., Choi J.G. Mori ramulus and its major component morusin inhibit herpes simplex virus type 1 replication and the virus-induced reactive oxygen species. Am. J. Chin. Med. 2021;49:163–179. doi: 10.1142/S0192415X21500099. [DOI] [PubMed] [Google Scholar]
- Lee B., Moon K.M., Lee B.S., Yang J.H., Park K.I., Cho W.K., Ma J.Y. Swertiajaponin inhibits skin pigmentation by dual mechanisms to suppress tyrosinase. Oncotarget. 2017;8:95530–95541. doi: 10.18632/oncotarget.20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.T., Lee K.S., Jeong J.H., Jo B.K., Heo M.Y., Kim H.P. Inhibitory effects of Ramulus mori extracts on melanogenesis. J. Cosmet. Sci. 2003;54:133–142. [PubMed] [Google Scholar]
- Li Y., Huang J., Lu J., Ding Y., Jiang L., Hu S., Chen J., Zeng Q. The role and mechanism of Asian medicinal plants in treating skin pigmentary disorders. J. Ethnopharmacol. 2019;245:112173. doi: 10.1016/j.jep.2019.112173. [DOI] [PubMed] [Google Scholar]
- Meng H., Liu X.K., Li J.R., Bao T.Y., Yi F. Bibliometric analysis of the effects of ginseng on skin. J. Cosmet. Dermatol. 2021 doi: 10.1111/jocd.14450. [DOI] [PubMed] [Google Scholar]
- Park K.T., Kim J.K., Hwang D., Yoo Y., Lim Y.H. Inhibitory effect of mulberroside A and its derivatives on melanogenesis induced by ultraviolet B irradiation. Food Chem. Toxicol. 2011;49:3038–3045. doi: 10.1016/j.fct.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Pillaiyar T., Namasivayam V., Manickam M., Jung S.H. Inhibitors of melanogenesis: an updated review. J. Med. Chem. 2018;61:7395–7418. doi: 10.1021/acs.jmedchem.7b00967. [DOI] [PubMed] [Google Scholar]
- Qian W., Liu W., Zhu D., Cao Y., Tang A., Gong G., Su H. Natural skin-whitening compounds for the treatment of melanogenesis (Review) Exp. Ther. Med. 2020;20:173–185. doi: 10.3892/etm.2020.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N.H., Ryu S.Y., Choi E.J., Kang S.H., Chang I.M., Min K.R., Kim Y. Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 1998;243:801–803. doi: 10.1006/bbrc.1998.8169. [DOI] [PubMed] [Google Scholar]
- Song M., Mun J.H., Ko H.C., Kim B.S., Kim M.B. Korean red ginseng powder in the treatment of melasma: an uncontrolled observational study. J. Ginseng Res. 2011;35:170–175. doi: 10.5142/jgr.2011.35.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu K., Zippin J.H., Ito S. Chemical and biochemical control of skin pigmentation with special emphasis on mixed melanogenesis. Pigment Cell Melanoma Res. 2021;34:730–747. doi: 10.1111/pcmr.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.