Summary
Intracerebral haemorrhage (ICH) is the second most common type of stroke and a major cause of mortality and disability worldwide. Despite advances in surgical interventions and acute ICH management, there is currently no effective therapy to improve functional outcomes in patients. Recently, there has been tremendous progress uncovering new pathophysiological mechanisms underlying ICH that may pave the way for the development of therapeutic interventions. Here, we highlight emerging targets, but also existing gaps in preclinical animal modelling that prevent their exploitation. We particularly focus on (1) ICH aetiology, (2) the haematoma, (3) inflammation, and (4) post-ICH pathology. It is important to recognize that beyond neurons and the brain, other cell types and organs are crucially involved in ICH pathophysiology and successful interventions likely will need to address the entire organism. This review will spur the development of successful therapeutic interventions for ICH and advanced animal models that better reflect its aetiology and pathophysiology.
Keywords: Animal models, Brain haemorrhage, Aetiology, Haematoma, Inflammation, Recovery
Introduction
Intracerebral haemorrhage (ICH) is caused by a loss of vascular integrity leading to bleeding within the brain parenchyma. ICH accounts for ∼28% of all strokes and has the highest mortality rates among all stroke types.1 ICH also contributes the largest proportion of disability-adjusted life-years of all neurologic disorders.2 Despite advances in medical care, the mortality and morbidity rates remain high and effective treatment options are lacking. This review gives an update on emerging targets and treatments for spontaneous (nontraumatic) ICH focusing on the past three years and attempts to uncover novel research avenues including filling gaps in animal modelling.
Aetiology of ICH
Hypertension is the most common risk factor of spontaneous ICH, exhibited by up to 70% of ICH patients and antihypertensive treatment significantly reduces ICH risk. Animal models may help to reveal underlying cellular and molecular mechanisms of vascular instability prior to first ICH occurrence. Mouse models of hypertension-related spontaneous ICH are available (8% high-salt diet + the nitric oxide synthase inhibitor L-NAME in drinking water on a double transgenic background of renin and angiotensinogen overexpression3; angiotensin II pump infusion + L-NAME in drinking water + injection of angiotensin II or norepinephrine in C57BL/6 mice).4 These models better reflect the more complex rupture of a blood vessel than what is modeled by cerebral collagenase injection.5 Major drawbacks are the relatively small ICH volume and thus comparatively short haemorrhage resolution time, as well as the unpredictable time of ICH onset.
Smooth muscle cell degeneration at cerebral arterioles is common in cerebral small vessel disease that frequently underlies ICH. It is intuitive to hypothesize that weakened arterioles eventually burst when exposed to high perfusion pressure, most commonly in deep brain regions where penetrating arterioles are in close proximity to the circle of Willis. However, smooth muscle cell degeneration alone may not be sufficient to induce blood vessel rupture. In mice with a mutation in the α1 chain of collagen type IV (COL4A1), a model of spontaneous ICH, hypermuscularization in the transitional segment between arterioles and capillaries led to an increase in intravascular pressure in the upstream arteriole that bursts at sites of smooth muscle cell loss.6 Similarly, combining angiotensin II-induced hypertension with low serum uric acid levels worsened the disruption of the smooth muscle cell-elastin contractile unit in cerebral vessels and ICH progression in mice.7 This highlights the importance of using spontaneous models of ICH for investigating aetiology.
Cerebral amyloid angiopathy (CAA) is characterised by amyloid β deposits in leptomeningeal and cerebral blood vessel walls decreasing vessel diameter and leading to microaneurysms which can cause ICH. Patients with CAA-related ICH also have a greater risk for ICH recurrence.8 CAA is mainly (80%) sporadic, but hereditary forms exist. Only the hereditary forms can be sufficiently modelled in animals (Swedish K670N/M671L and Dutch/Iowa E693Q/D694N mutations in the amyloid precursor protein9), which to some extent can also mimic sporadic CAA. Whereas these transgenic mice display cerebral microbleeds, preclinical models of CAA leading to large ICH are lacking. Promising therapeutic approaches have been developed around amyloid β clearance.10
Oral anticoagulant use increases the risk of ICH 7- to 10-fold. However, that risk is reduced in patients treated with direct oral anticoagulants (DOACs) compared to warfarin (Supplementary Table).11 Preclinical studies using collagenase- or laser-induced ICH in rodents have replicated the benefit of DOACs12 and the effectiveness of reversing anticoagulation was examined in animal models.13 In mice subjected to cerebral microbleeds, warfarin promoted deadly ICH, whereas DOACs increased microbleed burden without triggering long-term cognitive impairment.14 Whether anticoagulant treatment provokes ICH by aggravating existing microbleeds in humans remains unknown.
Vascular malformations including cerebral cavernous (CCMs) and arteriovenous malformations (AVMs) are major risk factors for ICH. CCMs are clusters of abnormal venous capillaries. There is no structural support of blood vessels from smooth muscle cells making CCMs vulnerable to rupture. Although most CCMs develop sporadically, several mutations causing CCMs have been identified (KRIT1/CCM1, malcavernin/CCM2, PDCD10/CCM3) and respective mouse models are available.15,16 AVMs are congenital entanglements of arterial vessels directly connected to the venous system without an intermediate capillary bed. Excessive vascular endothelial growth factor (VEGF) signalling is believed to promote AVMs, and Ras plays an important role for physiological VEGF signalling. Mutations in genes involved in the RAS/MAPK pathway such as KRAS have been observed in AVM endothelial cells in human patients.17 A mouse model of controllable Ras overactivation in endothelial cells has been created, in which cerebral AVMs and spontaneous ICH are observed.18
Hypercholesterolaemia is associated with an increased risk for cardiovascular diseases, but decreases the risk for ICH in both sexes.19 However, hypercholesterinaemia can aggravate neuroinflammatory reactions after ICH similar to hypertension. An increased recruitment of neutrophils and monocytes is observed in dyslipidaemic mice, leading to poor functional outcome and exacerbated perihaematomal oedema.20 Because of the high prevalence of hypercholesterinaemia in humans, further investigations in dyslipidaemic ICH models are warranted.
Haematoma
Haematoma expansion occurs in 20–40% of patients over the first day after ICH. Haematoma volume is a major determinant of outcome, and clinical trials have focused on limiting haematoma expansion. Phase III clinical trials (INTERACT2, NCT00716079; ATACH-II, NCT01176565) have investigated pharmacological blood pressure lowering, including intensive approaches, after ICH (Supplementary Table). Current recommendations strongly suggest that elevated blood pressure should be treated as early as possible in patients with acute ICH.21 A recent phase II trial (ICH-ADAPT II, NCT02281838, Supplementary Table) aims to identify the benefit of aggressive vs. conservative blood pressure lowering. It should be noted that studies focused on reducing blood pressure to limit secondary haematoma expansion after ICH have focused on reducing systolic or mean arterial blood pressure. It may be that fluctuations in pressure and blood flow play a role in inducing continued bleeding. Other trials to limit haematoma expansion include using haemostatic agents (tranexamic acid (TRANSACT, NCT03044184; STOP-MSU, NCT03385928) and recombinant Factor VIIa (FAST, NCT00127283; FASTEST, NCT03496883, Supplementary Table). Preclinical studies on haematoma expansion are hampered by available models. Yet, intracerebral injection of collagenase or autologous blood does produce a haematoma that initially expands and that can be exacerbated by hypertension and hyperglycaemia.5,22 However, there are concerns that the underlying mechanisms (e.g., gradual degradation of the endothelial basement membrane) do not reflect those occurring in ICH patients. A model using liquid polymer gel that coagulates on contact with tissue has recently been devised to create a mass that causes secondary bleeding, the extent of which is blood pressure-dependent.23 However, this model is lacking haemolysis-induced toxicity. Nevertheless, it may be useful for comparing different methods for limiting haematoma expansion.
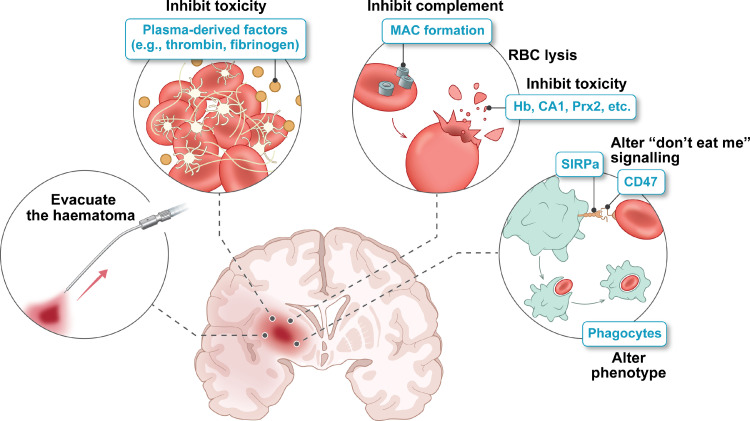
The impact of physical haematoma evacuation (Figure 1) has been examined in many clinical trials with, as yet, no evidence of improved neurological outcome (STICH II, ISRCTN22153967; MISTIE-III, NCT01827046, Supplementary Table).24,25 An alternate approach may be to accelerate endogenous haematoma resolution. Currently, in rodents, multiple agents such as peroxisome proliferator-activated receptor-γ and retinoid X receptor agonists have been shown to alter microglia/macrophage phenotype, enhance phagocytosis, speed haematoma clearance, and improve neurological outcome.26,27 Endogenous IL-4/Stat6 signalling is important in regulating haematoma resolution. Intranasal delivery of IL-4 nanoparticles also speeds resolution and improves neurological outcomes.28 An alternate approach is to block ‘don't-eat-me’ signals expressed on erythrocytes that normally suppress phagocytosis (e.g., using a CD47 antibody29). Whereas rodent ICH models have provided insight into mechanisms regulating endogenous haematoma clearance, studies on gyrencephalic species with larger brain sizes (e.g., pig, sheep) are needed to examine the effects of haematoma size and species physiological differences on endogenous clearance. Translation to the clinic may require a combination with physical evacuation to debulk the haematoma with accelerated endogenous clearance mechanisms to remove residual haematoma.
Figure 1.
The haematoma as a therapeutic target in ICH. The haematoma can cause brain injury via mass effect and the release of potentially toxic, plasma- or erythrocyte-derived factors. Surgical haematoma evacuation and accelerating endogenous haematoma clearance via phagocytosis are potential approaches, as is delaying erythrocyte lysis and inhibiting deleterious effects of clot-derived factors. RBC, red blood cell; MAC, membrane attack complex; Hb, haemoglobin; CA1, carbonic anhydrase 1; Prx2, peroxiredoxin-2; SIRPα, signal regulatory protein alpha.
White matter (WM) injury with demyelination and axonal degeneration commonly occurs in human ICH. Despite this, WM injury has received less attention in preclinical ICH models in part because rodents have limited WM. Evidence indicates that ICH induces WM injury via multiple mechanisms including mechanical injury, oxidative stress (in part haemoglobin-/iron-mediated), neuroinflammation, excitotoxicity, and blood-brain barrier (BBB) disruption.30 Changes in the axonal cytoskeleton after ICH not only impact the physical structure of the axon but also mitochondrial transport and function leading to degeneration.31 Recent studies have suggested that the inhibition of histone deacetylases (HDACs) with scriptaid or conditional knockout of HDAC2 in microglia can reduce ICH-induced neuroinflammation and WM injury in mice.32 Other studies have targeted neuroinflammation with the antibiotic and inhibitor of microglial activation minocycline in piglets,33 and the sphingosine-1-phosphate receptor modulator FTY720/fingolimod in mice.34 Both approaches reduced WM injury. It should be noted that fingolimod is currently undergoing clinical testing for ICH (FITCH, NCT04088630, Supplementary Table). A pilot study on minocycline (MACH, NCT01805895, Supplementary Table) demonstrated that 400 mg of minocycline were safe and resulted in neuroprotective serum concentrations.35 Further clinical trials are needed to demonstrate the efficacy of minocycline in treatment of ICH.
Cell death is a hallmark of ICH. Whereas the haematoma causes immediate damage to cells, clot-derived breakdown products induce cell death hours to days after the initial bleed. Importantly, different cell death subroutines occur after ICH, including autophagy, necroptosis, and ferroptosis.36,37 Ferroptosis is an iron-dependent, non-apoptotic form of regulated cell death that is driven by lipid peroxidation and critically depends on glutathione peroxidase 4 (GPX4). Lipid peroxidation has been demonstrated in animal models of ICH.38,39 Knockout of 5-lipoxygenase improved functional recovery and N-acetylcysteine inhibited its toxic arachidonic products.38 Furthermore, the lipid peroxidation inhibitors ferrostatin-1 and liproxstatin-1 reduced neurological deficits, memory impairment, brain atrophy, lesion volume, and neuronal cell death in collagenase and autologous blood infusion models in mice.40,41 Increasing the expression of selenoproteins including GPX4 by selenium supplementation or a brain-penetrant selenopeptide abrogated ferroptosis and improved functional outcome in collagenase-induced ICH in mice.42 In ICH patients, ferroptotic gene expression is increased,41 but studies demonstrating functional benefit of anti-ferroptotic drugs for patients are still required. It should be noted that a recent analysis of the iDEF trial (NCT02175225, Supplementary Table) indicated a benefit of deferoxamine in patients with moderate (10–30 ml) haematomas.43
Besides neurons, brain endothelial cells also undergo cell death after ICH.44 However, the underlying pathways are not yet fully elucidated. Endothelial dysfunction and BBB disruption have been demonstrated in collagenase and autologous blood infusion models of ICH in mice45 as well as in sheep in regions distant from the haematoma.46 The impairment of the BBB in ICH leads to brain oedema formation, the infiltration of immune cells, and the leakage of neurotoxic, pro-inflammatory, and vasoactive molecules.47
ICH-induced cytotoxic and vasogenic brain oedema are intimately connected to parenchymal cell injury and vascular injury/BBB disruption. Currently, oedema treatments are generally limited to hyperosmotic solutions (mannitol/hypertonic saline) or hyperventilation. Both types of oedema are associated with a brain build-up of ions (e.g., Na+ and Cl−) and there has been considerable interest in the use of glibenclamide, an inhibitor of the sulfonylurea receptor 1-regulated ion channels, for reducing perihaematomal oedema including a recently completed trial in ICH (GATE-ICH, NCT03741530, Supplementary Table). It should be noted that there is some disagreement in the preclinical literature about the effectiveness of glibenclamide in ICH.48,49
Inflammation in ICH
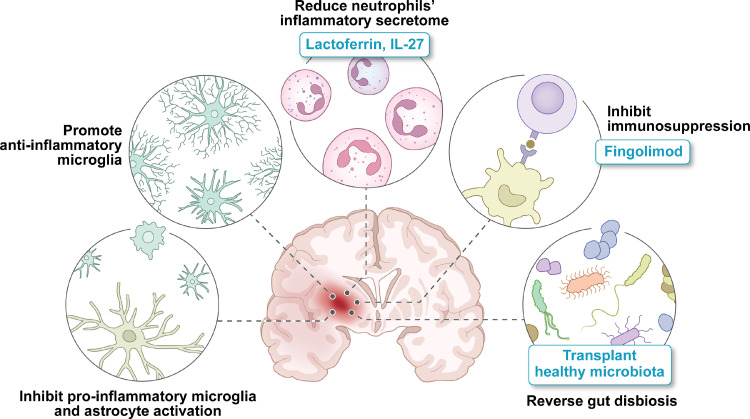
Inflammatory events can be triggered by intraparenchymal blood (Figure 2), and partly resemble that in ischaemic stroke. They are, however, incompletely investigated and their temporal profile is not well understood. Local microglia and astrocytes are first responders and their proinflammatory activation promotes circulating immune cell influx, predominantly of macrophages. There is an increased release of inflammatory cytokines (e.g., interleukin-1β, tumor necrosis factor), free radicals, and chemokines. This attracts and activates lymphocytes. Importantly, these processes also contribute to perihaematomal oedema formation and potentially ICH growth by compromising BBB integrity.50 Most ICH models in immunocompetent animals allow investigating post-ICH inflammation impact and kinetics, but more specialised models exhibiting relevant comorbidities may provide better insights into inflammation post ICH.
Figure 2.
Inflammation as a therapeutic target in ICH. The haematoma induces local inflammatory events, mediated by microglia and astrocytes, as well as acute systemic inflammation, resulting in the recruitment of immune cells to the brain. The injured CNS then triggers systemic immunosuppression and changes in the gut-brain axis.
Proinflammatory microglia/macrophages (often referred to as the M1 subtype) are considered promising therapeutic targets in early ICH as they drive neuroinflammation that is mainly associated with inferior outcome. Beneficial effects were observed with minocycline, a central nervous system (CNS)-penetrant tetracycline with inhibitory activity on pro-inflammatory microglia/macrophages, in rodent ICH models, but early-stage clinical studies were inconclusive.51 At later stages, anti-inflammatory (M2) microglia/macrophage subtypes exert beneficial effects for instance by contributing to haematoma removal as well as perihaematomal oedema resorption. M2 microglia/macrophages also promote local white matter integrity, and even exert neuroprotective effects. Thus, an alternative approach with a potentially wider therapeutic time window would be timely, sub-acute promotion of anti-inflammatory microglia/macrophages.52 However, it is important to stress that neuroinflammation after ICH is a complex and continuous rather than dichotomous process that can have both detrimental and beneficial effects depending on the respective timepoint and pathobiological process affected. This should be taken into thorough consideration when developing precise experimental intervention strategies targeting neuroinflammation.
Inflammation and cytotoxicity driven by neutrophils are generally thought to exacerbate ICH injury, but neutrophils also exert beneficial and protective functions. For instance, the secretion of iron-scavenging lactoferrin can contribute to perihaematomal oedema reduction and increased haematoma clearance. Interleukin 27 reduces the amount of inflammatory/cytotoxic products in the neutrophil secretome and this increases the production of iron scavengers, thus shifting the balance in neutrophil action towards beneficial and protective effects.53 To note, this is a systemic process as the shift in the neutrophil secretome is induced in the bone marrow. Fusing human lactoferrin with the Fc domain of human IgG resulted in a molecule with a long plasma half-life and superior therapeutic outcome.54
To counterbalance inflammation, the injured CNS induces systemic immunosuppression. The overactivation of the sympathetic and parasympathetic nervous system in ICH results in spleen shrinkage and rapid lymphopenia. The degree of spleen shrinkage in ICH patients correlates with haematoma size, highlighting the link between ICH severity and immunosuppression, which in turn, predicts the likelihood of infections and impacts long-term outcome.55 Whereas the numbers of circulating (CD4+) T- and natural killer (NK) cells are decreased,55 there is a prominent invasion into the brain.56,57 Immunosuppression after ICH has been investigated in rodent ICH models using cerebral injection of autologous blood58 or collagenase,59 revealing programmed death ligand 1 (PD-L1) and metoprolol as potential therapeutic options. However, haematoma location and size vary considerably in patients and may influence how the haematoma regulates immune responses after ICH. Hence, there is a need for future models resembling the clinical situation more closely. Of note, the immunosuppressive effects of fingolimod (FTY720), currently in clinical trial for ICH treatment (FITCH, NCT04088630, Supplementary Table), rely mainly on inhibiting helper (CD4+) and effector (CD8+) T cells as well as CD19+ B cells.60 Hence, post-ICH immunosuppression should be considered when designing immunomodulatory interventions for ICH.
Modulating immune cells in the CNS also affects the microbiome via the gut-brain-axis. The microbiome is well-studied in several CNS pathologies, including ischaemic stroke and plays a role in neuroinflammation, neuroplasticity and autoimmunity. However, little is known about the cross-talk between the gut and the brain in ICH. In collagenase-induced ICH in mice, reduced gastrointestinal motility and microbiota dysbiosis have been demonstrated, and adversely affected outcome. Recolonising ICH mice with healthy microbiota has shown promising results.61 It would be important to further investigate microbiome changes in ICH patients as well as microbiome transplantations. A human stool bank for healthy microbiota already exists (openbiome.org) and would be a powerful tool to leverage.
ICH sequelae and improving recovery
Cardiac complications are common after ICH and higher heart rate variability in the acute phase is associated with poorer 3-month outcomes.62 Preclinical studies using autologous blood or collagenase-induced ICH in mice have replicated cardiac complications59,63 and metoprolol reduced cardiac damage by abrogating sympathetic overactivation in addition to its immunosuppressive effects.59 Furthermore, splenectomy reduced cardiac dysfunction along with improving neurological outcome after autologous blood-induced ICH in mice.64 Due to their prevalence, cardiac complications should be assessed when testing novel therapeutic interventions preclinically.
Enhancing post-ICH recovery is an important aspect in therapeutic research. There is a plethora of well-established tests of motor, sensory, and cognitive functions for rodent ICH models.65 However, evidence for cognitive deficits is limited and spontaneous recovery of motor function and compensatory mechanisms in rodents may overestimate the impact of therapeutic interventions after brain injury; some tests may not be able to discriminate between these effects. Sensitive tests such as automated gait assessment or kinematic measures can provide valuable insights into functional recovery.66 Interestingly, it seems that rehabilitation strategies predominantly focusing on gross motor function may not be optimal in rodents,67 an important parallel to human patients.68 However, more complex neurorehabilitation strategies are under-investigated for ICH. A combined application of enriched environment and task-specific motor training showed improved outcome in a rat model of striatal ICH more than a decade ago,69 but more detailed research on optimal neurorehabilitation strategies is currently missing. It is known though that very early and intense rehabilitation can even impair functional recovery. This was shown in the phase III A Very Early Rehabilitation Trial after stroke (AVERT) which enrolled both patients with ischaemic and haemorrhagic stroke.70 Thus, timing and intensity of rehabilitation strategies requires further research. Interestingly, ICH location also impacts functional recovery. For instance, long-lasting deficits were observed after ICH in the internal capsule as compared to striatal lesions, despite a smaller lesion volume.71 ICH models targeting the internal capsule may therefore be well suited to investigate advanced rehabilitation or even restorative strategies. Detailed imaging protocols can be applied in ICH models46 and may reveal valuable morphological information related to functional recovery.
Depressive symptoms following ICH are common. Post-ICH depression may occur in up to 20% of patients and is associated with poorer long-term outcomes. Whereas treating depression is a priority, there is also an increased risk of secondary events associated with selective serotonin reuptake inhibitors.72 Furthermore, there has been interest in taking advantage of pleiotropic regenerative effects that drugs such as fluoxetine may offer. Three recent randomized clinical trials (FOCUS, ISRCTN83290762; AFFINITY, ACTRN12611000774921; EFFECTS, NCT02683213, Supplementary Table) assess fluoxetine for stroke recovery and all included ICH patients. They demonstrated that, whereas post-stroke depression was decreased, the risk of bone fractures and hyponatremia was increased and functional outcome was not improved. It is clear that post-ICH depression represents an important unmet need and novel treatments may require improved knowledge of the underlying mechanisms. This effort is hampered by the sparsity of preclinical research. Some studies suggest rodents with ICH exhibit deficits in the elevated plus maze, sucrose preference, tail suspension, open field, and forced swim tests,73,74 but others have failed75 resulting in uncertainty whether animal models accurately reflect cognition and depression.66 Recent studies have investigated genetic links between ICH and depression76 and the results support the monoaminergic hypothesis, as well as the idea that pro-inflammatory cytokines may stimulate the hypothalamic pituitary adrenal axis.77
Emerging therapeutic approaches
Small RNAs (size <200 nucleotides) are readily accessible in body fluids. The most abundantly investigated in ICH diagnosis and therapy are micro-RNAs (miRNAs, 20–25 nucleotides). Their expression profile not only distinguishes ICH patients from healthy controls but also ischaemic stroke and subarachnoid haemorrhage patients, which makes them valuable diagnostic (e.g., miR-124-3p) and prognostic markers (e.g., miR-130a).78 Dysregulated miRNAs and their putative mRNA targets are commonly associated with pathways regulating neuroinflammation, cell death, vascular smooth muscle and focal adhesion.79 Normalising imbalanced miRNA levels in ICH using miRNA mimics or antagomirs leads to improved outcome after ICH in animals and in vitro models, including in models of the BBB. In addition, miRNA mimics and antagomirs in ICH therapeutics commonly have anti-inflammatory functions, reduce perihaematomal oedema and haematoma size (miR130a,78 miR-223,78 miR-194-5p,80 miR-152,81 miR-7-5p79), regulate BBB permeability (miR-130a,78 miR-27a,82 miR-126-3p83), promote neuronal survival (miR-27a,82 miR-15281), foster stem cell proliferation and migration, and improve endothelial function (miR-19584). This suggests that miRNA-based therapies can be successful in improving ICH outcome in the clinical context when administered after ICH if appropriate delivery can be achieved.
Whereas the role and therapeutic potential of miRNAs is actively investigated, the potential of other small RNAs is relatively unexplored. These small RNAs include PIWI-interacting RNAs (piRNAs), transfer RNA derived small RNAs (tsRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), and small Cajal body RNAs (scaRNAs). Seven tsRNAs have been demonstrated to be significantly changed in a rat model of collagenase-induced ICH and were involved in pathways participating in the oxidative stress response, endocytosis, and the regulation of G protein-coupled receptor signalling.85 Emerging clinical data highlights the differential expression of ribosomal and tRNA-derived fragments86 as well as snoRNAs79 in ICH compared with ischaemic stroke patients and healthy controls, respectively. The role of these dysregulated small RNAs in ICH is a field for future extensive studies and carries potential to open new avenues in understanding ICH pathobiology and developing ICH treatments.
Exosomes, also known as extracellular vesicles, are important mediators of regenerative mechanisms and exert therapeutic impact similar to that of regenerative cell populations. They are increasingly described in neurodegenerative disease, but knowledge in ICH is limited. Importantly, there is preliminary evidence for exosome-mediated anti-inflammatory mechanisms in mice87 and humans.88 Exosomes are frequently derived from mesenchymal stem cells (MSCs) as these cells are known to exert beneficial effects after ICH which are believed to be at least partly mediated by exosomes. Moreover, obtaining exosomes from MSCs is a well-established procedure. Specifically, exosomes obtained from bone marrow MSCs enriched with miR-146a-5p inhibited neuronal cell death and microglial M1 polarization compared to exosomes without enrichment.89 Exosomes from miR-19b-3p-mimic transfected adipose-derived stem cells were demonstrated to abrogate post-ICH ferroptosis in comparison to negative control mimic.90 Systemic delivery of miR-133b containing exosomes compared to miR-control reduced neurodegeneration by inhibiting RhoA and activating ERK1/2/CREB pathway even when administered 72 h after ICH,91 suggesting a clinical potential for this miRNA or exosomes as a delivery agent. A thorough characterisation of exosomes, including their cellular origin and content in the context of ICH will be important, and future research will also have to investigate therapeutic effects of exosomes derived from other (stem) cell populations.
Nanoparticle-based treatments, like many ICH-targeting therapies, have focused on mitigating pathogenic processes associated with the breakdown of blood in the brain. The most widely used approach has been to use nanoparticles to provide anti-oxidant benefits. Several nanoparticles have inherent anti-oxidant properties. For example, cerium oxide nanoparticles improved outcome in rodent models of ICH, with and without conjugation to polyethylene glycol (PEG), by reducing inflammatory activity and perihaematomal oedema formation.92,93 Several nanoparticle formulations are also amenable to functionalisation and/or modification to deliver therapeutic payloads. PEGylated hydrophilic carbon cluster nanoparticles have been bound to the iron chelator deferoxamine to further target ICH.94 Polymer-based nanoparticles remain popular as they are straightforward to manufacture, have multiple surface modification strategies, and are relatively stable. They have been modified to deliver oxidative therapies such as resveratrol93 and edaravone. The latter has potential for more rapid translation as edaravone has been used to treat ICH. Edavarone-containing nanoparticles further reduced perihaematomal oedema in patients with haematoma removal when compared to edaravone alone.95
Limitations of animal models
As outlined above and summarized in Table 1, animal models have been used successfully to investigate some of the aetiological and pathophysiological mechanisms of ICH, but all have limitations. There are indeed limitations that are common across animal models, such as an extraordinary spontaneous recovery of sensorimotor function in the rodent, as well as limited evidence for cognitive deficits. While translational validity is an important consideration, no model perfectly recapitulates the complex aetiology of spontaneous ICH in humans. Models should always be chosen with this in mind according to the specific research question. Furthermore, the inclusion of multiple comorbidities within models (including hypertension, dyslipidaemia, diabetes, arteritis) is recommended in order to better reflect the clinical scenario, and when testing new therapeutic approaches, the use of at least two models reduces considerations surrounding limitations and increases security that mechanisms of interest are widely applicable.96 Moreover, age and sex should be more often considered in animal studies as they impact the pathophysiology and clinical outcomes.97,98 In addition to behavioural/functional outcome assessments, non-invasive imaging methods such as magnetic resonance imaging may give further and longitudinal insights into the underlying mechanisms such as white matter injury, axonal degeneration, and haematoma expansion as well as when assessing potential treatment candidates. Thorough outcome analysis requires sufficient time to allow the lesion to be finally organized and for potential improvements to plateau. Post-injury/-treatment surveillance time should be at least 3 weeks according to the Stroke Therapy Academic Industry Roundtable (STAIR) guidelines for preclinical research in ischaemic stroke using behavioural and structural or histological endpoints.99 Similar post-injury surveillance times are recommended for ICH.
Table 1.
ICH animal models to address aetiological and pathophysiological mechanisms and their limitations. AVM, arteriovenous malformations; BBB, blood-brain barrier; CAA, cerebral amyloid angiopathy; CCM, cerebral cavernous malformation; COL4A1, α1 chain of collagen type IV; ICH, intracerebral haemorrhage; L-NAME, Nω-nitro-L-arginine methyl ester.
| (A) Rodent models | |
|---|---|
| Model | Aetiological or pathophysiological mechanisms addressed, translational value |
| Autologous blood injection | |
| Collagenase injection | |
| Injection of blood components | |
| Laser-induced rupture of vessels | |
| Cyclodextrin nanoparticle injection | |
| Liquid polymer gel | |
| High salt diet + L-NAME in drinking water in mice overexpressing human renin and angiotensinogen |
|
| Chronic angiotensin II infusion + L-NAME in drinking water + acute angiotensin II injection in mice | |
| Chronic Angiotensin II infusion + L-NAME in drinking water + low serum uric acid levels in mice | |
| COL4A1 mutation in mice | |
| CAA-related transgenic mice | |
| CCM/AVM-related transgenic mouse models | |
| (B) Non-rodent models | |
| Autologous blood injection in rabbits | |
| Autologous blood injection in cats |
|
| Autologous blood injection in dogs | |
| Collagenase injection in dogs |
|
| Vessel puncture in dogs | |
| Autologous blood injection in pigs | |
| Autologous blood injection in sheep | |
| Naturally occurring CAA in dogs |
|
| CAA-related transgenic squirrel monkeys | |
Conclusion
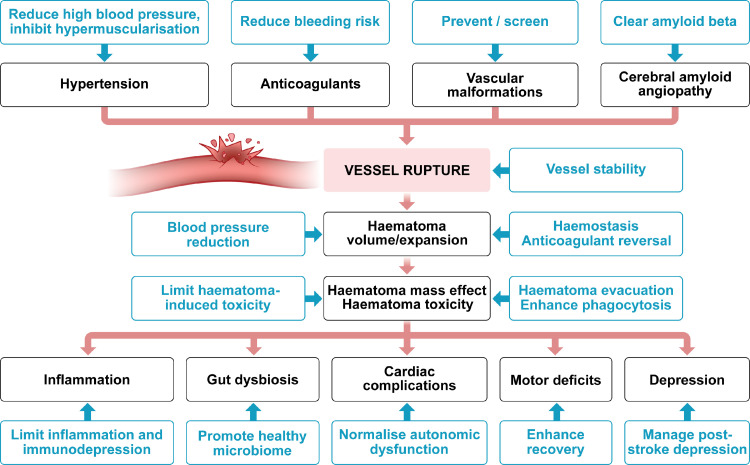
Over the past two decades, it has become clear that ICH was previously neglected as a stroke type being distinct in its pathophysiology and treatment needs. We here summarized current therapeutical approaches and unaddressed targets that will help researchers in clinical translation (Figure 3). Importantly, there is increasing knowledge about the involvement of other cell types and organs that need more thorough investigation and to be considered for successful interventions. This also means that animal modelling needs to reflect this situation more closely to target the aetiology and pathophysiology of ICH better.
Figure 3.
Therapeutic targets and approaches in ICH.
Outstanding questions
Whereas much has been learned about the pathological mechanisms involved in ICH-induced brain injury, translating that information into novel therapies remains a challenge. Some of the mechanisms elucidated may have beneficial as well as detrimental effects (e.g., inflammation), the impact of which may vary with haematoma size and time after ictus. In addition, the importance of different injury mechanisms may differ between human and animal models. The use of large gyrencephalic species in addition to rodents for ICH modelling may address some concerns, but even they do not perfectly replicate human ICH size and time course. Thus, it is imperative to select the ICH model best suited to the respective research question or therapeutic target for best translational value.
Search strategy and selection criteria
Data for this review were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles using the search terms “intracerebral hemorrhage”, “hypertension”, “smooth muscle cells”, “hypercholesterolaemia”, “anticoagulants”, “haematoma expansion”, “haematoma evacuation”, “haematoma resolution”, “oedema”, “white matter injury”, “demyelination”, “axonal degeneration”, “ferroptosis”, “lipid peroxidation”, “brain endothelial cells”, “inflammation”, “small RNA”, “miRNA”, “exosomes”, “nanoparticles”, “cardiac complication”, “brain-heart axis”, “immunosuppression”, “gut-brain axis”, “recovery”, and “depression”. Only articles published in English were included with a particular focus on the past 3 years.
Contributions
All authors contributed to the writing of the article and approved the final version of the manuscript.
Declaration of interests
The authors declare that they have no conflicts of interests.
Acknowledgements
We would like to thank Dr. Fan Xia the support in designing Figure 1 and Dr. Larisa Bulavina, a neuroscientist and professional medical illustrator (www.larisabulavina.com), for her invaluable help in graphical artwork.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103880.
Contributor Information
Marietta Zille, Email: marietta.zille@univie.ac.at.
Johannes Boltze, Email: johannes.boltze@warwick.ac.uk.
Appendix. Supplementary materials
References
- 1.GBD Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin J.H., Vora N. The global burden of neurologic diseases. Neurology. 2014;83(4):349–351. doi: 10.1212/WNL.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iida S., Baumbach G.L., Lavoie J.L., et al. Spontaneous stroke in a genetic model of hypertension in mice. Stroke. 2005;36(6):1253–1258. doi: 10.1161/01.str.0000167694.58419.a2. [DOI] [PubMed] [Google Scholar]
- 4.Wakisaka Y., Chu Y., Miller J.D., et al. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab. 2010;30(1):56–69. doi: 10.1038/jcbfm.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia P.M., Chamberlain R., Luo X., et al. Elevated blood pressure causes larger hematoma in a rat model of intracerebral hemorrhage. Transl Stroke Res. 2012;3(4):428–434. doi: 10.1007/s12975-012-0199-0. [DOI] [PubMed] [Google Scholar]
- 6.Ratelade J., Klug N.R., Lombardi D., et al. Reducing hypermuscularization of the transitional segment between arterioles and capillaries protects against spontaneous intracerebral hemorrhage. Circulation. 2020;141(25):2078–2094. doi: 10.1161/CIRCULATIONAHA.119.040963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao N., Liu T.L., Li H., et al. Low serum uric acid levels promote hypertensive intracerebral hemorrhage by disrupting the smooth muscle cell-elastin contractile unit and upregulating the Erk1/2-MMP axis. Transl Stroke Res. 2020;11(5):1077–1094. doi: 10.1007/s12975-020-00791-3. [DOI] [PubMed] [Google Scholar]
- 8.Pinho J., Araujo J.M., Costa A.S., et al. Intracerebral hemorrhage recurrence in patients with and without cerebral amyloid angiopathy. Cerebrovasc Dis Extra. 2021;11(1):15–21. doi: 10.1159/000513503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alharbi B.M., Tso M.K., Macdonald R.L. Animal models of spontaneous intracerebral hemorrhage. Neurol Res. 2016;38(5):448–455. doi: 10.1080/01616412.2016.1144671. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y., Ando Y., Misumi Y., et al. Current management and therapeutic strategies for cerebral amyloid angiopathy. Int J Mol Sci. 2021;22(8) doi: 10.3390/ijms22083869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai Y., Deng H., Shantsila A., et al. Rivaroxaban versus dabigatran or warfarin in real-world studies of stroke prevention in atrial fibrillation: systematic review and meta-analysis. Stroke. 2017;48(4):970–976. doi: 10.1161/STROKEAHA.116.016275. [DOI] [PubMed] [Google Scholar]
- 12.Foerch C., Lo E.H., van Leyen K., et al. Intracerebral hemorrhage formation under direct oral anticoagulants. Stroke. 2019;50(4):1034–1042. doi: 10.1161/STROKEAHA.118.023722. [DOI] [PubMed] [Google Scholar]
- 13.Na S.Y., Mracsko E., van Ryn J., et al. Idarucizumab improves outcome in murine brain hemorrhage related to dabigatran. Ann Neurol. 2015;78(1):137–141. doi: 10.1002/ana.24421. [DOI] [PubMed] [Google Scholar]
- 14.Petrault M., Ouk T., Petrault O., et al. Safety of oral anticoagulants on experimental brain microbleeding and cognition. Neuropharmacology. 2019;155:162–172. doi: 10.1016/j.neuropharm.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso C., Arnould M., De Luca C., et al. Novel chronic mouse model of cerebral cavernous malformations. Stroke. 2020;51(4):1272–1278. doi: 10.1161/STROKEAHA.119.027207. [DOI] [PubMed] [Google Scholar]
- 16.McDonald D.A., Shenkar R., Shi C., et al. A novel mouse model of cerebral cavernous malformations based on the two-hit mutation hypothesis recapitulates the human disease. Hum Mol Genet. 2011;20(2):211–222. doi: 10.1093/hmg/ddq433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikolaev S.I., Vetiska S., Bonilla X., et al. Somatic activating KRAS mutations in arteriovenous malformations of the brain. New Engl J Med. 2018;378(3):250–261. doi: 10.1056/NEJMoa1709449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q.F., Decker-Rockefeller B., Bajaj A., et al. Activation of ras in the vascular endothelium induces brain vascular malformations and hemorrhagic stroke. Cell Rep. 2018;24(11):2869–2882. doi: 10.1016/j.celrep.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Rist P.M., Buring J.E., Ridker P.M., et al. Lipid levels and the risk of hemorrhagic stroke among women. Neurology. 2019;92(19):e2286. doi: 10.1212/WNL.0000000000007454. -e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Cheng X., Wang X., et al. Dyslipidemic diet induces mobilization of peripheral neutrophils and monocytes that exacerbate hemorrhagic brain injury and neuroinflammation. Front Cell Neurosci. 2020;14:154. doi: 10.3389/fncel.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandset E.C., Anderson C.S., Bath P.M., et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J. 2021;6(2):XLVIII–LXXXIX. doi: 10.1177/23969873211012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu H., Gao Z., Huang C., et al. Relationship between hematoma expansion induced by hypertension and hyperglycemia and blood-brain barrier disruption in mice and its possible mechanism: role of aquaporin-4 and connexin43. Neurosci Bull. 2020;36(11):1369–1380. doi: 10.1007/s12264-020-00540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlunk F., Böhm M., Boulouis G., et al. Secondary bleeding during acute experimental intracerebral hemorrhage. Stroke. 2019;50(5):1210–1215. doi: 10.1161/STROKEAHA.118.021732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanley D.F., Thompson R.E., Rosenblum M., et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393(10175):1021–1032. doi: 10.1016/S0140-6736(19)30195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendelow A.D., Gregson B.A., Rowan E.N., et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382(9890):397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang C.F., Massey J., Osherov A., et al. Bexarotene enhances macrophage erythrophagocytosis and hematoma clearance in experimental intracerebral hemorrhage. Stroke. 2020;51(2):612–618. doi: 10.1161/STROKEAHA.119.027037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang G., Li T., Duan S.N., et al. PPAR-γ promotes hematoma clearance through haptoglobin-hemoglobin-CD163 in a rat model of intracerebral hemorrhage. Behav Neurol. 2018;2018 doi: 10.1155/2018/7646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J., Chen Z., Yu F., et al. IL-4/STAT6 signaling facilitates innate hematoma resolution and neurological recovery after hemorrhagic stroke in mice. Proc Natl Acad Sci USA. 2020;117(51):32679–32690. doi: 10.1073/pnas.2018497117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jing C., Bian L., Wang M., et al. Enhancement of hematoma clearance with CD47 blocking antibody in experimental intracerebral hemorrhage. Stroke. 2019;50(6):1539–1547. doi: 10.1161/STROKEAHA.118.024578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu X., Zhou G., Zhuang J., et al. White matter injury after intracerebral hemorrhage. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.562090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y., Chen X., Feng Z., et al. MEC17-induced alpha-tubulin acetylation restores mitochondrial transport function and alleviates axonal injury after intracerebral hemorrhage in mice. J Neurochem. 2022;160(1):51–63. doi: 10.1111/jnc.15493. [DOI] [PubMed] [Google Scholar]
- 32.Yang H., Ni W., Wei P., et al. HDAC inhibition reduces white matter injury after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2021;41(5):958–974. doi: 10.1177/0271678X20942613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H., Gao X.J., Li Y.J., et al. Minocycline reduces intracerebral hemorrhage-induced white matter injury in piglets. CNS Neurosci Ther. 2019;25(10):1195–1206. doi: 10.1111/cns.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Z., Dong S., Zheng Q., et al. FTY720 attenuates iron deposition and glial responses in improving delayed lesion and long-term outcomes of collagenase-induced intracerebral hemorrhage. Brain Res. 2019;1718:91–102. doi: 10.1016/j.brainres.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 35.Fouda A.Y., Newsome A.S., Spellicy S., et al. Minocycline in acute cerebral hemorrhage: an early phase randomized trial. Stroke. 2017;48(10):2885–2887. doi: 10.1161/STROKEAHA.117.018658. [DOI] [PubMed] [Google Scholar]
- 36.Zille M., Karuppagounder S.S., Chen Y., et al. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke. 2017;48(4):1033–1043. doi: 10.1161/STROKEAHA.116.015609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q., Weiland A., Chen X., et al. Ultrastructural characteristics of neuronal death and white matter injury in mouse brain tissues after intracerebral hemorrhage: coexistence of ferroptosis, autophagy, and necrosis. Front Neurol. 2018;9:581. doi: 10.3389/fneur.2018.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karuppagounder S.S., Alin L., Chen Y., et al. N-acetylcysteine targets 5 lipoxygenase-derived, toxic lipids and can synergize with prostaglandin E2 to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Ann Neurol. 2018;84(6):854–872. doi: 10.1002/ana.25356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou S.Y., Cui G.Z., Yan X.L., et al. Mechanism of ferroptosis and its relationships with other types of programmed cell death: insights for potential interventions after intracerebral hemorrhage. Front Neurosci. 2020;14 doi: 10.3389/fnins.2020.589042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q., Han X., Lan X., et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2(7):e90777. doi: 10.1172/jci.insight.90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen B., Chen Z., Liu M., et al. Inhibition of neuronal ferroptosis in the acute phase of intracerebral hemorrhage shows long-term cerebroprotective effects. Brain Res Bull. 2019;153:122–132. doi: 10.1016/j.brainresbull.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Alim I., Caulfield J.T., Chen Y., et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 2019;177(5):1262–1279. doi: 10.1016/j.cell.2019.03.032. e25. [DOI] [PubMed] [Google Scholar]
- 43.Selim M., Foster L.D., Moy C.S., et al. Deferoxamine mesylate in patients with intracerebral haemorrhage (i-DEF): a multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2019;18(5):428–438. doi: 10.1016/S1474-4422(19)30069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zille M., Ikhsan M., Jiang Y., et al. The impact of endothelial cell death in the brain and its role after stroke: a systematic review. Cell Stress. 2019;3(11):330–347. doi: 10.15698/cst2019.11.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia P., He J., Li Z., et al. Profiling of blood-brain barrier disruption in mouse intracerebral hemorrhage models: collagenase injection vs. autologous arterial whole blood infusion. Front Cell Neurosci. 2021;15 doi: 10.3389/fncel.2021.699736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boltze J., Ferrara F., Hainsworth A.H., et al. Lesional and perilesional tissue characterization by automated image processing in a novel gyrencephalic animal model of peracute intracerebral hemorrhage. J Cereb Blood Flow Metab. 2019;39(12):2521–2535. doi: 10.1177/0271678X18802119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keep R.F., Andjelkovic A.V., Xiang J., et al. Brain endothelial cell junctions after cerebral hemorrhage: changes, mechanisms and therapeutic targets. J Cereb Blood Flow Metab. 2018;38(8):1255–1275. doi: 10.1177/0271678X18774666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kung T.F.C., Wilkinson C.M., Dirks C.A., et al. Glibenclamide does not improve outcome following severe collagenase-induced intracerebral hemorrhage in rats. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu F., Shen G., Su Z., et al. Glibenclamide ameliorates the disrupted blood-brain barrier in experimental intracerebral hemorrhage by inhibiting the activation of NLRP3 inflammasome. Brain Behav. 2019;9(4):e01254. doi: 10.1002/brb3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue M., Yong V.W. Neuroinflammation in intracerebral haemorrhage: immunotherapies with potential for translation. Lancet Neurol. 2020;19(12):1023–1032. doi: 10.1016/S1474-4422(20)30364-1. [DOI] [PubMed] [Google Scholar]
- 51.Bai Q., Xue M., Yong V.W. Microglia and macrophage phenotypes in intracerebral haemorrhage injury: therapeutic opportunities. Brain J Neurol. 2020;143(5):1297–1314. doi: 10.1093/brain/awz393. [DOI] [PubMed] [Google Scholar]
- 52.Tschoe C., Bushnell C.D., Duncan P.W., et al. Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J Stroke. 2020;22(1):29–46. doi: 10.5853/jos.2019.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao X., Ting S.M., Liu C.H., et al. Neutrophil polarization by IL-27 as a therapeutic target for intracerebral hemorrhage. Nat Commun. 2017;8(1):602. doi: 10.1038/s41467-017-00770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao X., Kruzel M., Ting S.M., et al. Optimized lactoferrin as a highly promising treatment for intracerebral hemorrhage: pre-clinical experience. J Cereb Blood Flow Metab. 2021;41(1):53–66. doi: 10.1177/0271678X20925667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J., Shi K., Li Z., et al. Organ- and cell-specific immune responses are associated with the outcomes of intracerebral hemorrhage. FASEB J. 2018;32(1):220–229. doi: 10.1096/fj.201700324R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goods B.A., Askenase M.H., Markarian E., et al. Leukocyte dynamics after intracerebral hemorrhage in a living patient reveal rapid adaptations to tissue milieu. JCI Insight. 2021;6(6):e145857. doi: 10.1172/jci.insight.145857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mei S., Shao Y., Fang Y., et al. The changes of leukocytes in brain and blood after intracerebral hemorrhage. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.617163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han R., Luo J., Shi Y., et al. PD-L1 (programmed death ligand 1) protects against experimental intracerebral hemorrhage-induced brain injury. Stroke. 2017;48(8):2255–2262. doi: 10.1161/STROKEAHA.117.016705. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L., Wuri J., An L., et al. Metoprolol attenuates intracerebral hemorrhage-induced cardiac damage by suppression of sympathetic overactivity in mice. Auton Neurosci Basic Clin. 2021;234 doi: 10.1016/j.autneu.2021.102832. [DOI] [PubMed] [Google Scholar]
- 60.Fu Y., Hao J., Zhang N., et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 2014;71(9):1092–1101. doi: 10.1001/jamaneurol.2014.1065. [DOI] [PubMed] [Google Scholar]
- 61.Yu X., Zhou G., Shao B., et al. Gut microbiota dysbiosis induced by intracerebral hemorrhage aggravates neuroinflammation in mice. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.647304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rass V., Lindner A., Ianosi B.A., et al. Early alterations in heart rate are associated with poor outcome in patients with intracerebral hemorrhage. J Crit Care. 2021;61:199–206. doi: 10.1016/j.jcrc.2020.10.027. [DOI] [PubMed] [Google Scholar]
- 63.Li W., Li L., Chopp M., et al. Intracerebral hemorrhage induces cardiac dysfunction in mice without primary cardiac disease. Front Neurol. 2018;9:965. doi: 10.3389/fneur.2018.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W., Li L., Li W., et al. Spleen associated immune-response mediates brain-heart interaction after intracerebral hemorrhage. Exp Neurol. 2020;327 doi: 10.1016/j.expneurol.2020.113209. [DOI] [PubMed] [Google Scholar]
- 65.Shi X., Bai H., Wang J., et al. Behavioral assessment of sensory, motor, emotion, and cognition in rodent models of intracerebral hemorrhage. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.667511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balkaya M.G., Trueman R.C., Boltze J., et al. Behavioral outcome measures to improve experimental stroke research. Behav Brain Res. 2018;352:161–171. doi: 10.1016/j.bbr.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 67.Tamakoshi K., Ishida K., Hayao K., et al. Behavioral effect of short- and long-term exercise on motor functional recovery after intracerebral hemorrhage in rats. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2018;27(12):3630–3635. doi: 10.1016/j.jstrokecerebrovasdis.2018.08.038. [DOI] [PubMed] [Google Scholar]
- 68.Okuda Y., Nakata T. Effect of intensive rehabilitation on improvement of activity of daily living after intracerebral hemorrhage: a retrospective observational study. Int J Rehabil Res. 2020;43(1):37–40. doi: 10.1097/MRR.0000000000000381. Int Zeitschrift fur Rehabilitationsforschung Revue internationale de recherches de readaptation. [DOI] [PubMed] [Google Scholar]
- 69.MacLellan C.L., Plummer N., Silasi G., et al. Rehabilitation promotes recovery after whole blood-induced intracerebral hemorrhage in rats. Neurorehabil Neural Repair. 2011;25(5):477–483. doi: 10.1177/1545968310395602. [DOI] [PubMed] [Google Scholar]
- 70.Langhorne P., Wu O., Rodgers H., et al. A Very Early Rehabilitation Trial after stroke (AVERT): a phase III, multicentre, randomised controlled trial. Health Technol Assess. 2017;21(54):1–120. doi: 10.3310/hta21540. (Rockv) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y., Lu G., Su X.W., et al. Characterization of axon damage, neurological deficits, and histopathology in two experimental models of intracerebral hemorrhage. Front Neurosci. 2018;12:928. doi: 10.3389/fnins.2018.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kubiszewski P., Sugita L., Kourkoulis C., et al. Association of selective serotonin reuptake inhibitor use after intracerebral hemorrhage with hemorrhage recurrence and depression severity. JAMA Neurol. 2020;78(1):1–8. doi: 10.1001/jamaneurol.2020.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu W., Gao Y., Wan J., et al. Changes in motor function, cognition, and emotion-related behavior after right hemispheric intracerebral hemorrhage in various brain regions of mouse. Brain Behav Immun. 2018;69:568–581. doi: 10.1016/j.bbi.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh N., Bansal Y., Bhandari R., et al. Naringin reverses neurobehavioral and biochemical alterations in intracerebroventricular collagenase-induced intracerebral hemorrhage in rats. Pharmacology. 2017;100(3-4):172–187. doi: 10.1159/000453580. [DOI] [PubMed] [Google Scholar]
- 75.MacLellan C.L., Langdon K.D., Churchill K.P., et al. Assessing cognitive function after intracerebral hemorrhage in rats. Behav Brain Res. 2009;198(2):321–328. doi: 10.1016/j.bbr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Zou C., Huang X., Lan X., et al. Potential genes and mechanisms linking intracerebral hemorrhage and depression: a bioinformatics-based study. Int J Gen Med. 2021;14:1213–1226. doi: 10.2147/IJGM.S302916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Y., Wang L., Hu K., et al. Mechanisms and therapeutic targets of depression after intracerebral hemorrhage. Front Psychiatry. 2018;9:682. doi: 10.3389/fpsyt.2018.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martinez B., Peplow P.V. Blood microRNAs as potential diagnostic markers for hemorrhagic stroke. Neural Regen Res. 2017;12(1):13–18. doi: 10.4103/1673-5374.198965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng X., Ander B.P., Jickling G.C., et al. MicroRNA and their target mRNAs change expression in whole blood of patients after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2020;40(4):775–786. doi: 10.1177/0271678X19839501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wan S.Y., Li G.S., Tu C., et al. MicroNAR-194-5p hinders the activation of NLRP3 inflammasomes and alleviates neuroinflammation during intracerebral hemorrhage by blocking the interaction between TRAF6 and NLRP3. Brain Res. 2021;1752 doi: 10.1016/j.brainres.2020.147228. [DOI] [PubMed] [Google Scholar]
- 81.Hu L., Zhang H., Wang B., et al. MicroRNA-152 attenuates neuroinflammation in intracerebral hemorrhage by inhibiting thioredoxin interacting protein (TXNIP)-mediated NLRP3 inflammasome activation. Int Immunopharmacol. 2020;80 doi: 10.1016/j.intimp.2019.106141. [DOI] [PubMed] [Google Scholar]
- 82.Xi T., Jin F., Zhu Y., et al. miR-27a-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. J Biol Chem. 2018;293(52):20041–20050. doi: 10.1074/jbc.RA118.001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fu X., Niu T., Li X. MicroRNA-126-3p attenuates intracerebral hemorrhage-induced blood-brain barrier disruption by regulating VCAM-1 expression. Front Neurosci. 2019;13:866. doi: 10.3389/fnins.2019.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng H.Y., Wang Y.S., Hsu P.Y., et al. miR-195 Has a Potential to Treat Ischemic and Hemorrhagic Stroke through Neurovascular Protection and Neurogenesis. Mol Ther Methods Clin Dev. 2019;13:121–132. doi: 10.1016/j.omtm.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li P.F., Guo S.C., Liu T., et al. Integrative analysis of transcriptomes highlights potential functions of transfer-RNA-derived small RNAs in experimental intracerebral hemorrhage. Aging (Albany NY) 2020;12(22):22794–22813. doi: 10.18632/aging.103938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nguyen T.T.M., van der Bent M.L., Wermer M.J.H., et al. Circulating tRNA fragments as a novel biomarker class to distinguish acute stroke subtypes. Int J Mol Sci. 2020;22(1):135. doi: 10.3390/ijms22010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li M., Li X., Wang D., et al. Inhibition of exosome release augments neuroinflammation following intracerebral hemorrhage. FASEB J. 2021;35(6):e21617. doi: 10.1096/fj.202002766R. [DOI] [PubMed] [Google Scholar]
- 88.Walsh K.B., Campos B., Hart K., et al. M2 monocyte microparticles are increased in intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2017;26(10):2369–2375. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.027. the official journal of National Stroke Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duan S., Wang F., Cao J., et al. Exosomes derived from MicroRNA-146a-5p-enriched bone marrow mesenchymal stem cells alleviate intracerebral hemorrhage by inhibiting neuronal apoptosis and microglial M1 polarization. Drug Des Dev Ther. 2020;14:3143–3158. doi: 10.2147/DDDT.S255828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yi X., Tang X. Exosomes from miR-19b-3p-modified ADSCs inhibit ferroptosis in intracerebral hemorrhage mice. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.661317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shen H., Yao X., Li H., et al. Role of exosomes derived from miR-133b modified MSCs in an experimental rat model of intracerebral hemorrhage. J Mol Neurosci. 2018;64(3):421–430. doi: 10.1007/s12031-018-1041-2. [DOI] [PubMed] [Google Scholar]
- 92.Kang D.W., Kim C.K., Jeong H.G., et al. Biocompatible custom ceria nanoparticles against reactive oxygen species resolve acute inflammatory reaction after intracerebral hemorrhage. Nano Res. 2017;10(8):2743–2760. [Google Scholar]
- 93.Mo Y., Duan L., Yang Y., et al. Nanoparticles improved resveratrol brain delivery and its therapeutic efficacy against intracerebral hemorrhage. Nanoscale. 2021;13(6):3827–3840. doi: 10.1039/d0nr06249a. [DOI] [PubMed] [Google Scholar]
- 94.Dharmalingam P., Talakatta G., Mitra J., et al. Pervasive genomic damage in experimental intracerebral hemorrhage: therapeutic potential of a mechanistic-based carbon nanoparticle. ACS Nano. 2020;14(3):2827–2846. doi: 10.1021/acsnano.9b05821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dang L., Dong X., Yang J. Influence of nanoparticle-loaded edaravone on postoperative effects in patients with cerebral hemorrhage. J Nanosci Nanotechnol. 2021;21(2):1202–1211. doi: 10.1166/jnn.2021.18668. [DOI] [PubMed] [Google Scholar]
- 96.Hemorrhagic Stroke Academia Industry Roundtable Participants Basic and translational research in intracerebral hemorrhage: limitations, priorities, and recommendations. Stroke. 2018;49(5):1308–1314. doi: 10.1161/STROKEAHA.117.019539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gokhale S., Caplan L.R., James M.L. Sex differences in incidence, pathophysiology, and outcome of primary intracerebral hemorrhage. Stroke. 2015;46(3):886–892. doi: 10.1161/STROKEAHA.114.007682. [DOI] [PubMed] [Google Scholar]
- 98.Hsieh J.T., Ang B.T., Ng Y.P., et al. Comparison of gender differences in intracerebral hemorrhage in a multi-ethnic Asian population. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0152945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fisher M., Feuerstein G., Howells D.W., et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang Y., Chen X., Feng Z., et al. MEC17-induced alpha-tubulin acetylation restores mitochondrial transport function and alleviates axonal injury after intracerebral hemorrhage in mice. J Neurochem. 2021;160(1):51–63. doi: 10.1111/jnc.15493. [DOI] [PubMed] [Google Scholar]
- 101.Zhu W., Gao Y., Chang C.F., et al. Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippocampus by injections of autologous blood or collagenase. PLoS One. 2014;9(5):e97423. doi: 10.1371/journal.pone.0097423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng J., Shi L., Liang F., et al. Sirt3 ameliorates oxidative stress and mitochondrial dysfunction after intracerebral hemorrhage in diabetic rats. Front Neurosci. 2018;12:414. doi: 10.3389/fnins.2018.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liesz A., Middelhoff M., Zhou W., et al. Comparison of humoral neuroinflammation and adhesion molecule expression in two models of experimental intracerebral hemorrhage. Exp Transl Stroke Med. 2011;3(1):11. doi: 10.1186/2040-7378-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bahader G.A., Nash K.M., Almarghalani D.A., et al. Type-I diabetes aggravates post-hemorrhagic stroke cognitive impairment by augmenting oxidative stress and neuroinflammation in mice. Neurochem Int. 2021;149 doi: 10.1016/j.neuint.2021.105151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hijioka M., Anan J., Matsushita H., et al. Axonal dysfunction in internal capsule is closely associated with early motor deficits after intracerebral hemorrhage in mice. Neurosci Res. 2016;106:38–46. doi: 10.1016/j.neures.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 106.Lakovic K., Ai J., D'Abbondanza J., et al. Bilirubin and its oxidation products damage brain white matter. J Cereb Blood Flow Metab. 2014;34(11):1837–1847. doi: 10.1038/jcbfm.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lauer A., Cianchetti F.A., Van Cott E.M., et al. Anticoagulation with the oral direct thrombin inhibitor dabigatran does not enlarge hematoma volume in experimental intracerebral hemorrhage. Circulation. 2011;124(15):1654–1662. doi: 10.1161/CIRCULATIONAHA.111.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meissner A., Minnerup J., Soria G., et al. Structural and functional brain alterations in a murine model of Angiotensin II-induced hypertension. J Neurochem. 2017;140(3):509–521. doi: 10.1111/jnc.13905. [DOI] [PubMed] [Google Scholar]
- 109.Ratelade J., Mezouar N., Domenga-Denier V., et al. Severity of arterial defects in the retina correlates with the burden of intracerebral haemorrhage in COL4A1-related stroke. J Pathol. 2018;244(4):408–420. doi: 10.1002/path.5023. [DOI] [PubMed] [Google Scholar]
- 110.Chen M., Yan Q., Sun J., et al. Investigating the relationship between cerebrospinal fluid and magnetic induction phase shift in rabbit intracerebral hematoma expansion monitoring by MRI. Sci Rep. 2017;7(1):11186. doi: 10.1038/s41598-017-11107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu G., Wu J., Jiao Y., et al. Rosiglitazone infusion therapy following minimally invasive surgery for intracerebral hemorrhage evacuation decreases matrix metalloproteinase-9 and blood-brain barrier disruption in rabbits. BMC Neurol. 2015;15:37. doi: 10.1186/s12883-015-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin X., Tang Y., Sun B., et al. Cerebral glucose metabolism: Influence on perihematomal edema formation after intracerebral hemorrhage in cat models. Acta Radiol. 2010;51(5):549–554. doi: 10.3109/02841851003660065. [DOI] [PubMed] [Google Scholar]
- 113.Wu G., Wang F., Wang L., et al. Minimally invasive surgery for evacuating the intracerebral hematoma in early stages decreased secondary damages to the internal capsule in dog model of ICH observed by diffusion tensor imaging. J Stroke Cerebrovasc Dis. 2017;26(4):701–710. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.013. the official journal of National Stroke Association. [DOI] [PubMed] [Google Scholar]
- 114.Wu G., Zhong W. Effect of minimally invasive surgery for cerebral hematoma evacuation in different stages on motor evoked potential and thrombin in dog model of intracranial hemorrhage. Neurol Res. 2010;32(2):127–133. doi: 10.1179/016164109X12478302362617. [DOI] [PubMed] [Google Scholar]
- 115.An D., Park J., Shin J.I., et al. Temporal Evolution of MRI characteristics in dogs with collagenase-induced intracerebral hemorrhage. Comp Med. 2015;65(6):517–525. [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou X., Chen L., Feng C., et al. Establishing an animal model of intracerebral hemorrhage under the guidance of ultrasound. Ultrasound Med Biol. 2013;39(11):2116–2122. doi: 10.1016/j.ultrasmedbio.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 117.Cao S., Zheng M., Hua Y., et al. Hematoma changes during clot resolution after experimental intracerebral hemorrhage. Stroke. 2016;47(6):1626–1631. doi: 10.1161/STROKEAHA.116.013146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu S., Hua Y., Keep R.F., et al. Deferoxamine therapy reduces brain hemin accumulation after intracerebral hemorrhage in piglets. Exp Neurol. 2019;318:244–250. doi: 10.1016/j.expneurol.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang M., Xia F., Wan S., et al. Role of complement component 3 in early erythrolysis in the hematoma after experimental intracerebral hemorrhage. Stroke. 2021;52(8):2649–2660. doi: 10.1161/STROKEAHA.121.034372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gu Y., Hua Y., Keep R.F., et al. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40(6):2241–2243. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu R., Cao S., Hua Y., et al. CD163 expression in neurons after experimental intracerebral hemorrhage. Stroke. 2017;48(5):1369–1375. doi: 10.1161/STROKEAHA.117.016850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xie Q., Gu Y., Hua Y., et al. Deferoxamine attenuates white matter injury in a piglet intracerebral hemorrhage model. Stroke. 2014;45(1):290–292. doi: 10.1161/STROKEAHA.113.003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Uchida K., Miyauchi Y., Nakayama H., et al. Amyloid angiopathy with cerebral hemorrhage and senile plaque in aged dogs. Nihon Juigaku Zasshi Jpn J Vet Sci. 1990;52(3):605–611. doi: 10.1292/jvms1939.52.605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.