Abstract
Background:
Differential diagnosis of active tuberculosis (ATB) and latent tuberculosis infection (LTBI) has been a challenge for clinicians in high TB burden countries. The purpose of this study was to improve the accuracy of differential diagnosis of ATB and LTBI by using fluorescent immunospot (FluoroSpot) assay to detect specific Th1 cell immune responses. The novel mycobacterium tuberculosis (MTB) latency-associated antigens Rv1733c and synthetic long peptides derived from Rv1733c (Rv1733c SLP) were used based on virulence factors early secreting antigen target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10).
Methods:
Fifty-seven ATB cases, including 20 pathogen-confirmed ATB and 37 clinically diagnosed ATB, and 36 LTBI cases, were enrolled between January and December 2017. FluoroSpot assay was used to detect the interferon γ (IFN-γ) and interleukin 2 (IL-2) secreted by the specific T cells after being stimulated with MTB virulence factors ESAT-6 and CFP-10, MTB latency-associated antigens Rv1733c and Rv1733c SLP. The receiver operating characteristic (ROC) curve was used to define the best cutoff value of latency-associated antigens in the use of differentiating ATB and LTBI. The sensitivity, specificity, predictive value, and likelihood ratio of ESAT-6 and CFP-10-FluoroSpot combined with latency-associated antigen in the differential diagnosis of ATB and LTBI were also calculated.
Results:
Following the stimulation with Rv1733c and Rv1733c SLP, the frequency of single IL-2-secreting T cells stimulated by Rv1733c SLP had the largest area under the ROC curve, which was 0.766. With a cutoff value of 1 (spot-forming cells [SFCs]/2.5 × 105 peripheral blood mononuclear cells) for frequency, the sensitivity and specificity of distinguishing ATB from LTBI were 72.2% and 73.7%, respectively. ESAT-6 and CFP-10-FluoroSpot detected the frequency and proportion of single IFN-γ-secreting T cells; the sensitivity and specificity of distinguishing ATB from LTBI were 82.5% and 66.7%, respectively. Combined with the frequency of single IL-2-secreting T cells stimulated by Rv1733c SLP on the basis of ESAT-6 and CFP-10-FluoroSpot, the sensitivity and specificity increased to 84.2% and 83.3%, respectively.
Conclusion:
Rv1733c SLP, combined with ESAT-6 and CFP-10, might be used as a candidate antigen for T cell-based tuberculosis diagnostic tests to differentiate ATB from LTBI.
Keywords: Mycobacterium tuberculosis latency-associated antigens, Active tuberculosis, Latent tuberculosis infection, Differential diagnosis
Introduction
Tuberculosis (TB), caused by mycobacterium tuberculosis (MTB) infection, remains one of the leading causes of death from a single infectious agent and one of the most critical public health problems worldwide.[1] According to the 2020 Global Tuberculosis Report, there were 10 million new cases of TB in 2019. After India (26%) and Indonesia (8.5%), China has the highest number of TB patients accounting for 8.4%.[1]
The TB skin test (TST) and TB blood tests are the most common diagnostic methods for TB. Other methods include chest radiographs and diagnostic microbiology. Yet, all these methods have certain limitations. For example, smear antacid staining detection sensitivity is poor, and the bacterial culture procedure is often time-consuming. The Xpert MTB/Rifampicin (RIF) assay is a novel and revolutionizing TB control approach. The Xpert MTB/RIF results can be obtained within hours; nevertheless, this approach cannot be applied in 30% to 50% of cases due to the lack of qualified samples. Moreover, our previous study showed that <30% of active tuberculosis (ATB) diagnoses were confirmed by the pathogen.[2] Therefore, new and accurate methods are urgently needed to aid clinicians in diagnosing patients suspected of ATB without available etiological evidence.
In the past 20 years, interferon-γ release assays (IGRAs) that use early secreting antigen target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10) as specific antigens have been widely used in the diagnosis of TB infection, especially in the immunosuppressive population and global regions where Bacillus Calmette-Guerin (BCG) vaccines are included in the neonatal vaccination plan. The accuracy of IGRAs in the diagnosis of TB infection has been shown to be superior to that of the TST test.[3–5] However, IGRAs fail to distinguish ATB from latent tuberculosis infection (LTBI). Compared to ATB patients, persons with LTBI do not feel sick, do not have any symptoms, and are not infectious (cannot spread TB infection to others). In China, there are many large number of LTBI.[6] Therefore, when the IGRAs result is positive, differentiating ATB from LTBI is of extreme importance.
Rv1733c is a hypoxia-related latent antigen, which can induce the expression of different cytokine lineages in ATB and LTBI.[7,8] Previous studies suggested a preferential recognition of Rv1733c by T cells in subjects with LTBI.[9,10] Moreover, after stimulation with Rv1733c antigen, the level of interferon γ (IFN-γ) in the peripheral blood of LTBI increases.[11] Animal experiments showed that the synthetic long peptides derived from Rv1733c (Rv1733c SLP) caused a stronger immune response and produced higher levels of IFN-γ in mice compared to animals treated with Rv1733c.[12] Thus, it is worth assessing whether Rv1733c and Rv1733c SLP can be used as an alternative antigen for the differential diagnosis of ATB and LTBI.
As an improvement of the traditional enzyme-linked immunospot assay, the FluoroSpot assay can simultaneously detect multiple cytokines at the single-cell level without causing spotted color mixing.[13] In our previous studies, we confirmed that by using ESAT-6 and CFP-10 peptides as stimulators, the Fluorospot might be used to detect the secretion of IFN-γ and interleukin 2 (IL-2), which, in turn, is helpful for the differential diagnosis of ATB and LTBI.[14,15] In this study, we further improved the diagnostic accuracy by combining the MTB latency-associated antigen Rv1733c and Rv1733c SLP based on ESAT-6 and CFP-10-FluoroSpot to differentiate ATB from LTBI.
Methods
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Peking Union Medical College Hospital (PUMCH) (No: S-715). Informed written consent was obtained from all patients prior to their enrollment in this study.
Study design and subjects
The study enrolled pathogen-confirmed ATB and clinically diagnosed ATB cases admitted to the PUMCH and Beijing Chest Hospital between January and December 2017, whereas those with LTBI were used as a control group. The inclusion criteria for pathogen-confirmed ATB group were: (1) age 18 to 75 years; (2) subjects with ATB manifestations, such as fever, cough; (3) positive results of smear acid-fast stain or culture of MTB, MTB nucleic acid, or Xpert MTB/RIF; (4) no anti-TB treatment at enrollment. The inclusion criteria for the clinically diagnosed ATB group were: (1) age 18 to 75 years; (2) subjects with ATB manifestations, such as fever, cough; (3) laboratory results and radiologic features suggesting ATB; (4) anti-TB treatment is effective. The inclusion criteria for the LTBI group were: (1) age 18 to 75 years; (2) no clinical manifestations of ATB; (3) no history of TB, no manifestations of the previous TB in the chest radiogram; (4) positive results of T-cell spot test for tuberculosis infection (T-SPOT.TB). Subjects who were pregnant or lactating or were HIV-positive were excluded from the study.
Synthetic peptides
Jill Biochemical (Shanghai) Co., Ltd. (China) synthesized and purified MTB hypoxia-associated latency antigens Rv1733c and Rv1733c SLP. Rv1733c contains 19 peptides, and each peptide has 20 amino acids, with both ends overlapping on ten amino acids. Rv1733c SLP contains 19 peptides, and each peptide has 28 amino acids with 14 amino acids overlapping on both ends. Homogeneity and purity were confirmed by analytical high-pressure liquid chromatography and by mass spectrometry. The purity of all peptides was ≥80%.
IFN-γ/IL-2 FluoroSpot assay
Four milliliters of peripheral blood were collected from each patient. IFN-γ/IL-2 FluoroSpot (AID, Straßberg, Germany) assays were performed within 4 h according to the manufacturer instructions. Peripheral blood mononuclear cells (PBMCs) were firstly isolated by gradient centrifugation. The AIM-V medium (Gibco™ AIM V Medium liquid, Invitrogen, USA) was used to prepare a cell suspension with a concentration of 2.5 × 106 PBMCs/mL. A 96-well plate was then pre-coated with monoclonal antibodies against IFN-γ and IL-2. A 50 μL AIM-V cell culture medium was used as a negative control and 5 μg/mL phytohemagglutinin as a positive control; ESAT-6 peptides, CFP-10 peptides, Rv1733c peptides, and Rv1733c SLP peptides as antigens, respectively.
A 2.5 × 105 PBMCs and anti-CD28 (0.5 μg/mL, AID) were added to each well. The plates were then incubated for 16 to 20 h at 37°C in 5% CO2. The plates were incubated with IFN-γ-FITC and IL-2-biotin tagged with fluorescein and further incubated with fluorophore-labeled secondary antibodies and fluorescent enhancer. The frequencies of IFN-γ-, IL-2- and dual IFN-γ/IL-2-secreting T cells were counted by an automated fluorescence plate reader (AID-iSpot, Straßberg, Germany).
Data analysis
Statistical analysis was performed using SPSS 26.0 (IBM, Armonk, NY, USA). The Kolmogorov–Smirnov test was adopted to examine whether the variable data followed a normal distribution. The variables with normal distribution were denoted as the mean ± standard deviation, whereas the variables with non-normal distribution were denoted as the median and interquartile range. The enumeration data were presented as percentages and 95% confidence intervals (95% CIs). The number of T cells between groups was compared using an independent sample rank-sum test. To evaluate the diagnostic accuracy of Rv1733c SLP combined with ESAT-6 and CFP-10 to differentiate ATB from LTBI, the sample size was determined by the following formula. We assumed that the sensitivity of IFN-γ/IL-2 FluoroSpot to differentiate ATB from LTBI was 85%, the specificity was 90%, the type I error was 0.05, and the power was 0.90. According to these assumptions, the ATB group and LTBI group minimum sample sizes were 50 and 35, respectively.
The cutoff of ESAT-6 and CFP-10-FluoroSpot in the differential diagnosis of ATB and LTBI was defined according to our previous research results. The frequency of single IFN-γ-secreting T cells was 25 (SFCs/2.5 × 105 PBMCs), and the proportion of single IFN-γ-secreting T cells was 43.6%.[15] Receiver operating characteristic (ROC) was used to define the best cutoff value of Rv1733c(SLP)-FluoroSpot in the differential diagnosis of ATB and LTBI. Binary logistic regression model was used for fitting joint diagnostic parameters. The sensitivity, specificity, predictive value, likelihood ratio, and 95% CI of ATB and LTBI were calculated by medcalc software (MedCalc Software Ltd., Ostend, Belgium). A P < 0.05 (two-sided) was considered to be statistically significant.
Results
Demographic and clinical characteristics
Ninety-three subjects were included in the study, including 57 patients with ATB and 36 patients with LTBI. Among ATB patients, 20 cases were pathologically confirmed, and 37 cases were clinically diagnosed. The average age of the subjects was 46 ± 18 years, and males represented 65.6% of total cases. The basic information of the subjects is shown in [Table 1].
Table 1.
Demographic and characteristics of the study subjects.
| Pathogen-confirmed ATB | Clinically diagnosed ATB | LTBI | |
| Characteristics | (n = 20) | (n = 37) | (n = 36) |
| Age (years) | 46 ± 18 | 43 ± 19 | 48 ± 15 |
| Gender | |||
| Male | 12 (60.0) | 28 (75.7) | 21 (58.3) |
| Female | 8 (40.0) | 9 (24.3) | 15 (41.7) |
| Site | |||
| Lung TB | 18 (90) | 31 (83.8) | – |
| Lung TB and intestinal TB | 2 (10) | 0 | – |
| Lung TB and lymphatic TB | 0 | 2 (5.4) | |
| Lung TB and joint TB | 0 | 1 (2.7) | |
| Bone TB | 0 | 1 (2.7) | |
| Unknown | 0 | 2 (5.4) | |
| Method | |||
| Acid-fast stain positive | 1 (5) | – | – |
| Culture positive | 19 (95) | – | – |
Values are presented as n (%) or mean ± SD.ATB: Active tuberculosis; LTBI: Latent tuberculosis infection; SD: Standard deviation; TB: Tuberculosis; –: No data.
Comparison of frequencies of MTB IFN-γ/IL-2-secreting T cells in each group
Following stimulation by the MTB-specific ESAT-6 and CFP-10 peptides, the frequency (P = 0.003) and proportion (P < 0.001) of single IFN-γ-secreting T cells were significantly higher in the ATB group than those noted in the LTBI group. By contrast, the frequency (P = 0.004) and proportion (P < 0.001) of single IL-2-secreting T cells were significantly lower in the ATB group than that in the LTBI group [Table 2].
Table 2.
Comparisons of the frequency and proportion of IFN-γ-, IL-2-secreting T cells when stimulated by ESAT-6 and CFP-10 (iSFCs/250,000 PBMC).
| Parameters | Cytokines | ATB | LTBI | Statistics | P value∗ |
| Frequency | Single-IL-2 | 3 (1–6) | 7 (3–21) | 659.0 | 0.004 |
| Single-IFN-γ | 55 (11–138) | 14 (5–39) | 647.5 | 0.003 | |
| Total-IL-2 | 13 (7–46) | 26 (10–76) | 809.5 | 0.088 | |
| Total-IFN-γ | 79 (18–157) | 32 (13–91) | 809.0 | 0.087 | |
| Dual IFN-γ/IL-2 | 9 (4–40) | 19 (7–57) | 872.0 | 0.224 | |
| Proportion (%) | Single-IL-2 | 4.2 (1.6–10.3) | 14.4 (9.1–27.9) | 503.5 | <0.001 |
| Single-IFN-γ | 72.2 (50.9–85.3) | 34.0 (20.8–58.5) | 431.5 | <0.001 | |
| Dual IFN-γ/IL-2 | 20.8 (8.3–30.2) | 42.9 (30.0–56.4) | 466.0 | <0.001 |
Values are presented as median (range).
P values were calculated by Mann-Whitney U test.ATB: Active tuberculosis; CFP-10: Culture filtrate protein-10; ESAT-6: Early secreting antigen target-6; IFN-γ: Interferon γ; IL-2: Interleukin 2; IQR: Interquartile range; LTBI: Latent tuberculosis infection; PBMC: Peripheral blood mononuclear cell.
Following stimulation by the MTB latency-associated antigen Rv1733c (P = 0.008 and P = 0.010) and Rv1733c SLP (P < 0.001 and P < 0.001) peptides, the frequencies of single and total IL-2-secreting T cells were all higher in the LTBI group than those noted in the ATB group [Table 3].
Table 3.
Comparisons of frequencies of IFN-γ-, IL-2-secreting T cells when stimulated by Rv1733c, Rv1733c SLP (iSFCs/250,000 PBMC).
| Antigens | Cytokines | ATB | LTBI | Statistics | P value∗ |
| Rv1733c SLP | Single-IL-2 | 0 (0–1) | 1 (0–4) | 480.0 | <0.001 |
| Single-IFN-γ | 0 (0–1) | 0 (0–3) | 989.5 | 0.731 | |
| Total-IL-2 | 0 (0–1) | 2 (0–4) | 563.0 | <0.001 | |
| Total-IFN-γ | 0 (0–2) | 0 (0–3) | 940.5 | 0.430 | |
| Dual IFN-γ/IL-2 | 0 (0–0) | 0 (0–0) | 896.5 | 0.110 | |
| Rv1733c | Single-IL-2 | 0 (0–3) | 3 (0–6) | 687.0 | 0.004 |
| Single-IFN-γ | 0 (0–0) | 0 (0–0) | 981.5 | 0.611 | |
| Total-IL-2 | 0 (0–3) | 3 (0–7) | 722.5 | 0.010 | |
| Total-IFN-γ | 0 (0–0) | 0 (0–0) | 1011.5 | 0.873 | |
| Dual IFN-γ/IL-2 | 0 (0–0) | 0 (0–0) | 1018.5 | 0.912 |
Values are presented as median (range).
P values were calculated by Mann-Whitney U test.ATB: Active tuberculosis; IFN-γ: Interferon γ; IL-2: Interleukin 2; IQR: Interquartile range; LTBI: Latent tuberculosis infection; PBMC: Peripheral blood mononuclear cell; Rv1733c SLP: Synthetic long peptides derived from Rv1733c.
Diagnostic accuracy of the IFN-γ/IL-2 FluoroSpot assay of MTB latency-associated antigen for distinguishing ATB from LTBI
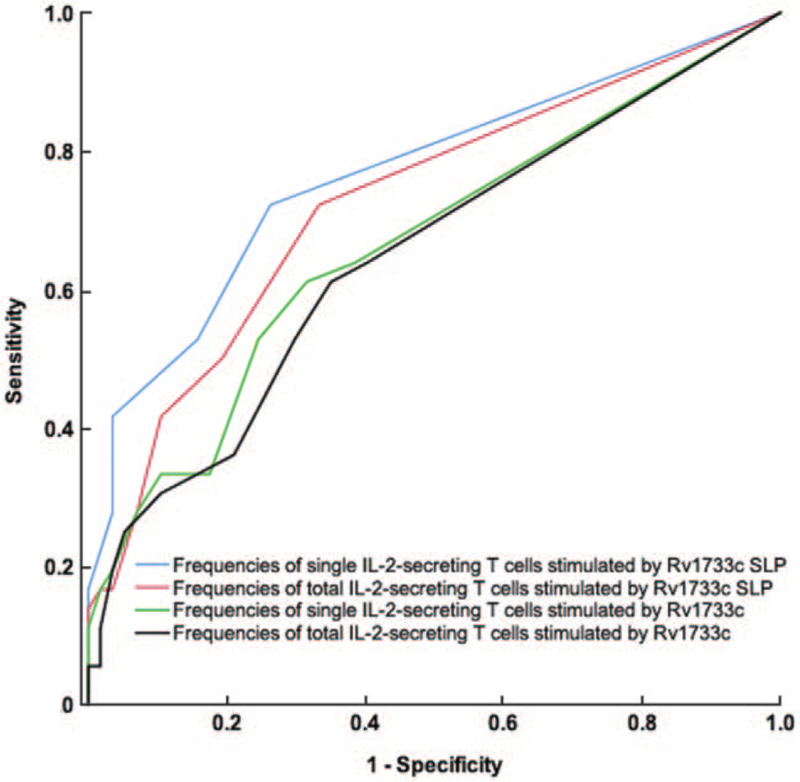
The ROC curves were drawn by the frequencies of single IL-2-, total IL-2-secreting T cell stimulated by Rv1733c and Rv1733c SLP peptides. The AUROC of 0.766 (95% CI, 0.662–0.870), drawn by the frequency of single IL-2-secreting T cells stimulated by Rv1733c SLP, was the largest. When the cutoff was 1 (SFCs/2.5 × 105 PBMCs), the sensitivity and specificity of the differential diagnosis of ATB and LTBI were 72.2% (95% CI 54.8–85.8%) and 73.7% (95% CI 60.3–84.5%), respectively [Figure 1].
Figure 1.
ROC curve of IFN-γ/IL-2 FluoroSpot assay of Rv1733c and Rv1733c SLP for differentiating ATB from LTBI. LTBI is the State variable. The blue line is drawn with the frequency of single IL-2-secreting T cells when stimulated by Rv1733c SLP, and the AUC is 0.766 (95% CI, 0.662–0.870). The red line is drawn with the frequency of total IL-2-secreting T cells when stimulated by Rv1733c SLP, and the AUC is 0.726 (95% CI, 0.617–0.834). The green line is drawn with the frequency of single IL-2-secreting T cells when stimulated by Rv1733c, and the AUC is 0.665 (95% CI, 0.549–0.782). The black line is drawn with the frequency of total IL-2-secreting T cells when stimulated by Rv1733c, and the AUC is 0.648 (95% CI, 0.530–0.765). ATB: Active tuberculosis; IFN-γ: Interferon γ; IL-2: Interleukin 2; LTBI: Latent tuberculosis infection; Rv1733c SLP: Synthetic long peptides derived from Rv1733c.
Diagnostic accuracy of ESAT-6 and CFP-10-FluoroSpot combined with Rv1733c SLP for distinguishing ATB from LTBI
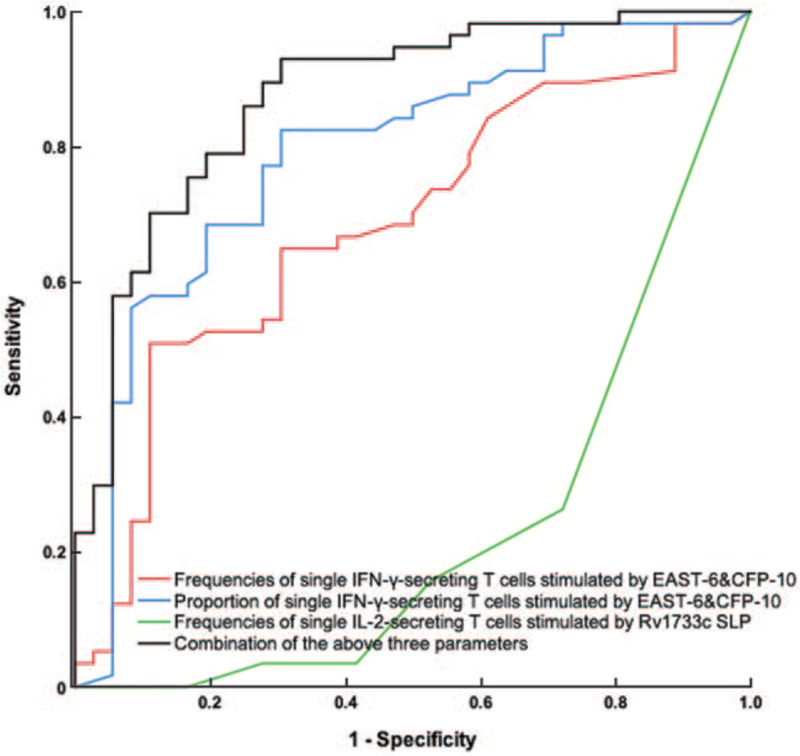
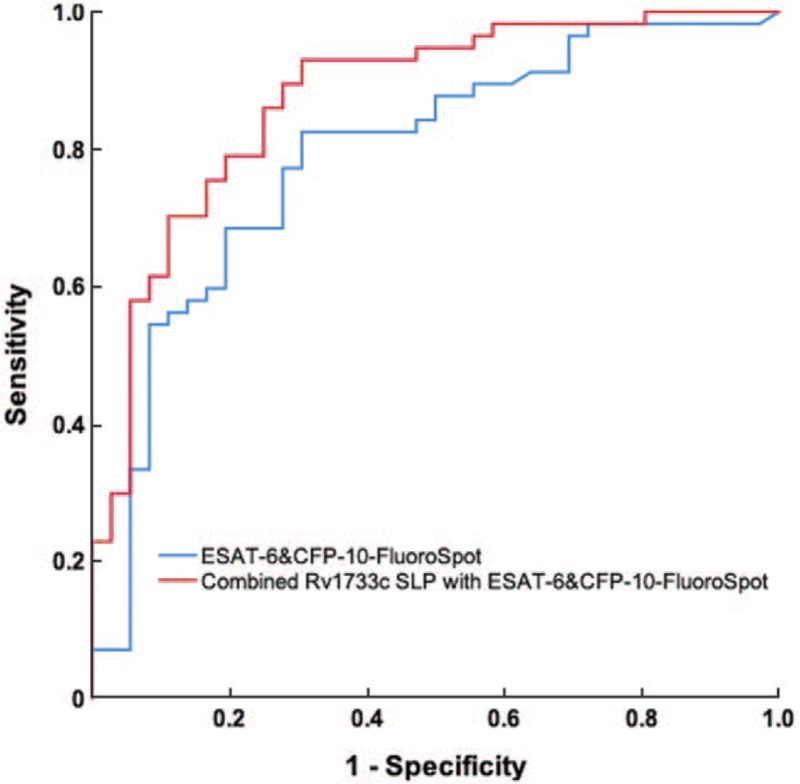
After stimulation by the MTB-specific antigen ESAT-6 and CFP-10 peptides, combined with the frequency and proportion of single IFN-γ-secreting T cells stimulated by ESAT-6 and CFP-10, the sensitivity and specificity of ESAT-6 and CFP-10-FluoroSpot for differential diagnosis of ATB and LTBI were 82.5% (95% CI 70.1–91.3%) and 66.7% (95% CI 49.0–81.4%), respectively. Based on the basis of ESAT-6 and CFP-10-FluoroSpot, the combination with Rv1733c SLP could increase sensitivity to 84.2% (95% CI 72.1–92.5%) and specificity to 83.3% (95% CI 67.2–93.4%) [Figures 2 and 3; Table 4].
Figure 2.
ROC curve of IFN-γ/IL-2 FluoroSpot assay of ESAT-6 and CFP-10 and Rv1733c SLP for differentiating ATB from LTBI. ATB is the State variable. The red line is drawn with the frequencies of single IFN-γ-secreting T cells stimulated by EAST-6 and CFP-10, and the AUC is 0.699 (95% CI, 0.592–0.805). The blue line is drawn with the proportion of single IFN-γ-secreting T cells stimulated by EAST-6 and CFP-10, and the AUC is 0.746 (95% CI, 0.638–0.853). The green line is drawn with the frequency of single IL-2-secreting T cells when stimulated by Rv1733c SLP, and the AUC is 0.234 (95% CI, 0.130–0.338). The black line is drawn with the combination of the above three parameters, and the AUC is 0.874 (95% CI, 0.799–0.948). ATB: Active tuberculosis; CFP-10: Culture filtrate protein-10; ESAT-6: Early secreting antigen target-6; IFN-γ: Interferon γ; IL-2: Interleukin 2; LTBI: Latent tuberculosis infection; ROC: Receiver operating characteristic; Rv1733c SLP: Synthetic long peptides derived from Rv1733c.
Figure 3.
The ROC curve for differentiating ATB from LTBI. ATB was the State variable. The blue line is drawn with a combination of the frequency and proportion of single IFN-γ-secreting T cells when stimulated by ESAT-6 and CFP-10, and the AUC is 0.790 (95% CI, 0.693–0.887). The blue line is drawn with a combination of the frequency of single IL-2-secreting T cells when stimulated by Rv1733c SLP with ESAT-6 and CFP-10-FluoroSpot, and the AUC is 0.874 (95% CI, 0.799–0.948). ATB: Active tuberculosis; CFP-10: Culture filtrate protein-10; ESAT-6: Early secreting antigen target-6; IFN-γ: Interferon γ; IL-2: Interleukin 2; LTBI: Latent tuberculosis infection; ROC: Receiver operating characteristic; Rv1733c SLP: Synthetic long peptides derived from Rv1733c.
Table 4.
Diagnostic value of FluoroSpot method to differentiate ATB and LTBI.
| Parameters | Sensitivity (%, 95% CI) | Specificity (%, 95% CI) | PLR (%, 95% CI) | NLR (%, 95% CI) | PPV (95% CI) | NPV (95% CI) |
| Frequencies of single IFN-γ-secreting T cells stimulated by EAST-6 and CFP-10 | 50.9 (37.3–64.4) | 88.9 (73.9–96.9) | 4.58 (1.76–11.94) | 0.55 (0.41–0.74) | 87.8 (71.8–96.6) | 53.3 (40.0–66.3) |
| Proportion of single IFN-γ-secreting T cells stimulated by EAST-6 and CFP-10 | 82.5 (70.1–91.3) | 62.2 (43.6–76.1) | 2.97 (1.73–5.09) | 0.24 (0.13–0.44) | 82.5 (70.1–91.3) | 64.2 (46.8–75.6) |
| Frequencies of single IL-2-secreting T cells stimulated by Rv1733c SLP | 72.2 (54.8–85.8) | 73.7 (60.3–84.5) | 2.65 (1.53–4.59) | 0.36 (0.23–0.59) | 80.8 (67.5–90.4) | 63.4 (46.9–77.9) |
| EAST-6 and CFP-10-FluoroSpot | 82.5 (70.1–91.3) | 66.7 (49.0–81.4) | 2.47 (1.53–3.99) | 0.26 (0.14–0.48) | 79.7 (67.2–89.0) | 70.6 (52.5–84.9) |
| Combined Rv1733c SLP with EAST-6 and CFP-10-FluoroSpot | 84.2 (72.1–92.5) | 83.3 (67.2–93.4) | 5.05 (2.41–10.58) | 0.19 (0.10–0.35) | 88.9 (77.4–95.8) | 76.9 (60.7–88.9) |
ATB: Active tuberculosis; CFP-10: Culture filtrate protein-10; ESAT-6: Early secreting antigen target-6; IFN-γ: Interferon γ; IL-2: Interleukin 2; IQR: Interquartile range; LTBI: Latent tuberculosis infection; NLR: Negative likelihood ratio; NPV: Negative predictive value; PLR: Positive likelihood ratio; PPV: Positive predictive value; Rv1733c SLP: Synthetic long peptides derived from Rv1733c.
Discussion
In the present study, we conducted a preliminary investigation of the diagnostic value of the MTB latency-associated antigen Rv1733c and Rv1733c SLP, combined with the EAST-6 and CFP-10-FluoroSpot, for differentiating ATB from LTBI.
In LTBI, MTB is usually present in macrophages as dormant bacteria. Inflammatory cells are recruited at the infected site, where they gather and form granuloma. The microenvironment of granuloma tissue with hypoxia, insufficient nutrition, and low pH inhibits the growth of MTB encapsulated in it.[16,17] Similarly, MTB can adapt to the host internal environment by changing its own characteristics and metabolic pathways, escaping the body immune response, and surviving in the body for a long time, thus making the body enter the latent infection state. However, when the host immunity or the signals maintaining granuloma structure are reduced, dormant MTB is activated, followed by proliferation and dissemination, eventually resulting in ATB.[18,19] Although bacterial and host factors that induce and maintain latent MTB infection are ill-defined, recent studies have revealed that during the dormancy of MTB, dormancy survival regulon (DosR) expression is upregulated. Through the interaction of dormancy-associated proteins with macrophages, the formatting and maintenance of the granuloma structure are necessary to allow MTB to transit into a stage of dormancy.[20] Interestingly, the immune response to the DosR regulon-encoded antigens have been associated with the containment during latent phases of MTB infection since the antigens encoded by several DosR regulons can be preferentially recognized by the T cells of LTBI subjects in order to induce the activation of T cells associated with the control of MTB infection. This process promotes various cytokine production, such as IFN-γ, TNF, and IL-2, thus preventing the progression of ATB.[21,22] Similarly, a preventive vaccine against DosR regulon-encoded antigens could alleviate the MTB infection in the lung.[23] Therefore, the immune response against these antigens may contribute to control latent MTB infection and preventing the reactivation of TB.[24,25] Previous studies have reported that while responsiveness to ESAT-6 is an optimal indicator of MTB infection, a strong response to the dormant antigen is largely restricted to latently infected individuals, offering the possibility of differential immunodiagnosis of TB and making it a potential vaccine candidate against TB.[26]
Rv1733c has been identified as the most common latency antigen in household contacts exposed to MTB in South Africa, Gambia, and Uganda.[9] Leyten et al[21] stimulated human PBMCs with 25 types of proteins expressed by DosR regulators, reporting that Rv1733c-stimulated LTBI subjects produced higher levels of IFN-γ compared to those of ATB patients. In the context of therapeutic vaccination, SLPs have been proven to induce better response and protection against tumors compared to short peptides.[27] Furthermore, Coppola et al[12] synthesized the SLP derived from Rv1733c (Rv1733c SLP) and observed that Rv1733c SLP caused a stronger immune response and could induce specific CD4+T cells to produce higher levels of IFN-γ in mice than Rv1733c.
The present study compared the difference of cytokines secreted by ATB and LTBI groups after stimulation by MTB latency-associated antigens Rv1733c and Rv1733c SLP peptides. The obtained results revealed that Rv1733c and Rv1733c SLP could induce more T cells to produce higher levels of IL-2 in LTBI than ATB. In addition, we found that Rv1733c SLP caused more extensive immune responses than Rv1733c, and the diagnostic accuracy of differentiating ATB from LTBI was better. SLP may prolong the epitope expression of antigen in vivo, increasing the clonal expansion and cytokine production by effector T cells.[28] The significant difference of IL-2 secretion between the ATB group and LTBI group stimulated by latency-associated Rv1733c SLP may be helpful for differentiation.
The results of the ESAT-6 and CFP-10-Fluorospot revealed that the frequency and proportion of single IFN-γ-secreting T cells in the ATB group were significantly higher than those in the LTBI group (P = 0.003 and P < 0.001), whereas the frequency and proportion of single IL-2-secreting T cells in the ATB group were significantly lower than those in the LTBI group (P = 0.004 and P < 0.001), which is consistent with our previous results.[15] The study demonstrated that the combination of the ESAT-6 and CFP-10-FluoroSpot with the Rv1733c SLP resulted in the specificity increased from 66.7% to 83.3%, the positive likelihood ratio increased from 2.47 to 5.05, and the positive predictive value increased from 79.7% to 88.9%. Rv1733c SLP combined with ESAT-6 and CFP-10-FluoroSpot could improve the accuracy of differential diagnosis between ATB and LTBI. Therefore, for patients who are IGRA positive and suspected ATB, the latency-associated antigen Rv1733c SLP based on ESAT-6 and CFP-10-FluoroSpot could be used to provide clues for the differential diagnosis of ATB and LTBI. It has been reported that Rv1733c and Rv1733c SLP can be applied to the development of vaccines and has broad application prospects.[12,29] Therefore, it is worth noting that if this vaccine becomes widely used in the future, the discrimination ability of Rv1733c SLP will be affected by this vaccine, which means that Rv1733c SLP detection may not be used for the differential diagnosis of ATB and LTBI.
The advantages of our study can be summed as follows: (1) based on the virulence factor ESAT-6 and CFP-10, MTB latency-associated antigens Rv1733c and Rv1733c SLP can be added as stimulators to improve the accuracy of differential diagnosis; (2) the IFN-γ/IL-2 FluoroSpot method was used to simultaneously detect the secretion of IFN-γ and IL-2 at the single-cell level, saving workforce and blood samples to the greatest extent.
The limitations of our study can be summarized as follows: (1) In this study, the differential diagnosis was based on the difference of specific Th1 cytokine secretion spectrum in different TB infection states. There is still overlapping in cytokine responses, which may inevitably reduce the accuracy of differential diagnosis. (2) This study adopted a case–control study design and probably overestimated the accuracy of differential diagnosis. This is only a preliminary study on the diagnostic value of latency-associated antigen, and our conclusions must be verified by further prospective cohort studies.
In conclusion, the latency-associated antigen Rv1733c SLP can be used as an alternative antigen for the TB diagnostic test based on the T-cell immune reaction. Based on the MTB-specific antigens ESAT-6 and CFP-10, the combination with Rv1733c SLP may be helpful for the differential diagnosis of ATB and LTBI. However, this conclusion needs to be further verified by prospective studies with a larger sample size.
Acknowledgements
The authors acknowledge all of the study staff and participants who contributed to this project.
Funding
This study was supported by grants from the National Science and Technology Major Project of China (No. 2017ZX10201302) and the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (Nos. 2016-I2M-1-013, 2019-I2M-2-005).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang L, Ma H, Wan S, Zhang Y, Gao M, Liu X. Mycobacterium tuberculosis latency-associated antigen Rv1733c SLP improves the accuracy of differential diagnosis of active tuberculosis and latent tuberculosis infection. Chin Med J 2022;135:63–69. doi: 10.1097/CM9.0000000000001858
Lifan Zhang and Huimin Ma contributed equally to this study.
References
- 1.WHO Global tuberculosis report 2020. http://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.Zhang L, Zhang Y, Shi X, Zhang Y, Deng G, Lalvani A, et al. Utility of T-cell interferon-( release assays for diagnosing tuberculous serositis: a prospective study in Beijing, China. PLoS One 2014; 9:e85030.doi: 10.1371/journal.pone.0085030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Sun Y, He C, Qiu X, Zhou D, Ye Z, et al. The immune characterization of interferon-( responses in tuberculosis patients. Microbiol Immunol 2018; 62:281–290. doi: 10.1111/1348-0421.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014; 27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 6.Gao L, Lu W, Bai L, Wang X, Xu J, Catanzaro A, et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis 2015; 15:310–319. doi: 10.1016/S1473-3099(14)71085-0. [DOI] [PubMed] [Google Scholar]
- 7.Meier NR, Jacobsen M, Ottenhoff THM, Ritz N. A systematic review on novel Mycobacterium tuberculosis antigens and their discriminatory potential for the diagnosis of latent and active tuberculosis. Front Immunol 2018; 9:2476.doi: 10.3389/fimmu.2018.02476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassa D, Ran L, Geberemeskel W, Tebeje M, Alemu A, Selase A, et al. Analysis of immune responses against a wide range of Mycobacterium tuberculosis antigens in patients with active pulmonary tuberculosis. Clin Vaccine Immunol 2012; 19:1907–1915. doi: 10.1128/CVI.00482-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black GF, Thiel BA, Ota MO, Parida SK, Adegbola R, Boom WH, et al. Immunogenicity of novel DosR regulon-encoded candidate antigens of Mycobacterium tuberculosis in three high-burden populations in Africa. Clin Vaccine Immunol 2009; 16:1203–1212. doi: 10.1128/CVI.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esmail H, Barry CE, 3rd, Wilkinson RJ. Understanding latent tuberculosis: the key to improved diagnostic and novel treatment strategies. Drug Discov Today 2012; 17:514–521. doi: 10.1016/j.drudis.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutherland JS, Lalor MK, Black GF, Ambrose LR, Loxton AG, Chegou NN, et al. Analysis of host responses to Mycobacterium tuberculosis antigens in a multisite study of subjects with different TB and HIV infection states in sub-Saharan Africa. PLoS One 2013; 8:e74080.doi: 10.1371/journal.pone.0074080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppola M, van den Eeden SJ, Wilson L, Franken KLMC, Ottenhoff THM, Geluk A. Synthetic long peptide derived from Mycobacterium tuberculosis latency antigen Rv1733c protects against tuberculosis. Clin Vaccine Immunol 2015; 22:1060–1069. doi: 10.1128/CVI.00271-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazagne A, Claret E, Wijdenes J, Yssel H, Bousquet F, Levy E, et al. A fluorospot assay to detect single T lymphocytes simultaneously producing multiple cytokines. J Immunol Methods 2003; 283:91–98. doi: 10.1016/j.jim.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Cheng X, Bian S, Song Y, Li Q, Gao M, et al. Utility of Th1-cell immune responses for distinguishing active tuberculosis from non-active tuberculosis: a case-control study. PLoS One 2017; 12:e0177850.doi: 10.1371/journal.pone.0177850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Wan S, Ye S, Cheng X, Zhang Y, Shi X, et al. Application of IFN-(/IL-2 FluoroSpot assay for distinguishing active tuberculosis from non-active tuberculosis: a cohort study. Clin Chim Acta 2019; 499:64–69. doi: 10.1016/j.cca.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Kaul A, Tsolaki AG, Kishore U, Bhakta S. Mycobacterium tuberculosis: immune evasion, latency and reactivation. Immunobiology 2012; 217:363–374. doi: 10.1016/j.imbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Saunders BM, Britton WJ. Life and death in the granuloma: Immunopathology of tuberculosis. Immunol Cell Biol 2007; 85:103–111. doi: 10.1038/sj.icb.7100027. [DOI] [PubMed] [Google Scholar]
- 18.Kondratieva T, Azhikina T, Nikonenko B, Kaprelyants A, Apt A. Latent tuberculosis infection: what we know about its genetic control? Tuberculosis (Edinb) 2014; 94:462–468. doi: 10.1016/j.tube.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Gengenbacher M, Kaufmann SHE. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol Rev 2012; 36:514–532. doi: 10.1111/j.1574-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serra-Vidal MM, Latorre I, Franken KLCM, Díaz J, de Souza-Galvão ML, Casas I, et al. Immunogenicity of 60 novel latency-related antigens of Mycobacterium tuberculosis. Front Microbiol 2014; 5:517.doi: 10.3389/fmicb.2014.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leyten EMS, Lin MY, Franken KLMC, Friggen AH, Prins C, van Meijgaarden KE, et al. Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosis. Microbes Infect 2006; 8:2052–2060. doi: 10.1016/j.micinf.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Roupie V, Romano M, Zhang L, Korf H, Lin MY, Franken KLMC, et al. Immunogenicity of eight dormancy regulon-encoded proteins of Mycobacterium tuberculosis in DNA-vaccinated and tuberculosis-infected mice. Infect Immun 2007; 75:941–949. doi: 10.1128/IAI.01137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol 2001; 1:20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- 24.Commandeur S, Lin MY, van Meijgaarden KE, Friggen AH, Franken KLMC, Drijfhout JW, et al. Double- and monofunctional CD4(+) and CD8(+) T-cell responses to Mycobacterium tuberculosis DosR antigens and peptides in long-term latently infected individuals. Eur J Immunol 2011; 41:2925–2936. doi: 10.1002/eji.201141602. [DOI] [PubMed] [Google Scholar]
- 25.Riaño F, Arroyo L, París S, Rojas M, Friggen AH, van Meijgaarden KE, et al. T cell responses to DosR and Rpf proteins in actively and latently infected individuals from Colombia. Tuberculosis (Edinb) 2012; 92:148–159. doi: 10.1016/j.tube.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Demissie A, Leyten EMS, Abebe M, Wassie L, Aseffa A, Abate G, et al. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin Vaccine Immunol 2006; 13:179–186. doi: 10.1128/CVI.13.2.179-186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quakkelaar ED, Melief CJM. Experience with synthetic vaccines for cancer and persistent virus infections in nonhuman primates and patients. Adv Immunol 2012; 114:77–106. doi: 10.1016/B978-0-12-396548-6.00004-4. [DOI] [PubMed] [Google Scholar]
- 28.Strauss G, Vignali DA, Schonrich G, Hammerling GJ. Negative and positive selection by HLA-DR3(DRw17) molecules in transgenic mice. Immunogenetics 1994; 40:104–108. [PubMed] [Google Scholar]
- 29.Ashayeri-Panah M, Eftekhar F, Kazemi B, Joseph J. Cloning, optimization of induction conditions and purification of Mycobacterium tuberculosis Rv1733c protein expressed in Escherichia coli. Iran J Microbiol 2017; 9:64–73. [PMC free article] [PubMed] [Google Scholar]