Abstract
Objective
Electronic health records (EHR) hold promise for conducting large-scale analyses linking individual characteristics to health outcomes. However, these data often contain a large number of missing values at both the patient and visit level due to variation in data collection across facilities, providers, and clinical need. This study proposes a stepwise framework for imputing missing values within a visit-level EHR dataset that combines informative missingness and conditional imputation in a scalable manner that may be parallelized for efficiency.
Results
For this study we use a subset of data from AMPATH representing information from 530,812 clinic visits from 16,316 Human Immunodeficiency Virus (HIV) positive women across Western Kenya who have given birth. We apply this process to a set of 84 clinical, social and economic variables and are able to impute values for 84.6% of variables with missing data with an average reduction in missing data of approximately 35.6%. We validate the use of this imputed dataset by predicting National Hospital Insurance Fund (NHIF) enrollment with 94.8% accuracy.
Keywords: Electronic medical records, HIV, Imputation, Big data
Introduction
Electronic Health Records (EHRs) are systematized sources of patient data that medical providers collect and store using digital tools. They have the potential to improve patient care by providing access to rich, longitudinal, patient-level data that may be used to advance precision medicine and lead to more personalized care [1–5]. In addition to facilitating more customized care, EMRs can be used to build machine learning models and generate new insights regarding patient behavior, biology and health outcomes [6–8].
One of the primary challenges posed by EHRs is that they often contain large amounts of missing data [2, 4, 9]. Practitioners entering data for a clinical-encounter may only elect to enter fields relevant to the patient’s clinical needs at that time, and data may be aggregated across sites that have varying standards for record-keeping [10]. By proposing a scalable, stepwise system for imputing considerable volumes of missing values we hope to make EMR data more accessible researchers interested in leveraging the big data aspects of these records, and promote collaboration between medical researchers and data scientists.
Handling missing data in electronic health records
Approaches toward managing missing EHR data vary. In summarizing the use of EHR data to develop risk prediction models, Goldstein et al. [9] found that only 58 of the 90 studies evaluated addressed missing data prior to analysis. The simplest approaches toward managing missing values involve selecting subsets of the data that contain complete information [11, 12], and using stratified mean imputation used to fill-in missing values [13]. Others have designed functions to interpolate longitudinal variables with limited individual-level variability that are typically not dependent on other covariates [14]. This approach is applicable only to continuous measures. Few studies using EHR utilize ‘informative observations’ where the presence of a variable is meaningful for associated, possibly missing values [9].
Simpler approaches toward EHR imputation must consider whether missing values are missing completely at random (MCAR), missing at random (MAR), or missing not at random (MNAR) [14]. Conditional imputation methods may be used to account for these dependencies, most effectively if missing data are MAR [10, 12, 15]. While they may improve completeness and predictive precision, these methods may be computationally intensive when applied to large-scale EHR data with significant amounts of missing values.
Research objectives
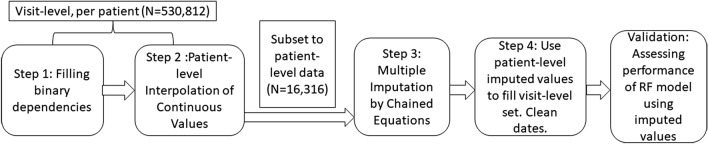
Our goal in this study is to design and utilize a scalable, multi-step approach toward imputing missing values in EHR data (Fig. 1). Our approach recognizes that EHR data is both patient- and time-variant, but that collection methods do not produce data that closely resembles repeated measures from a random population [16]. To assess results, we measure differences in response coverage for each variable, and feed our results into a decision tree classifier designed to predict NHIF enrollment. The ability to accurately predict National Hospital Insurance Fund (NHIF) enrollment serves as validation for the utility of using predicted values in research.
Fig. 1.
Visual representation of workflow, noting unit of analysis shifts
Data used
For this study we use data from AMPATH—a robust EHR system containing health records for over 150,000 HIV + individuals across Western Kenya. Past research has focused on cohort studies of AMPATH data, selecting measures and observations that minimize the missingness of these data [17]. We specifically focus on a subset of these data that contain longitudinal information from 530,812 clinic visits for 16,316 HIV positive women who have given birth. Clinic visits span from December 19th, 2001 to September 2nd, 2013. The average number of clinic visits per patient is 32 with a standard deviation of 23. The maximum number of visits across this dataset is 162.
This analysis focuses on a set of 84 variables of interest to researchers studying HIV diagnosis and maternal health outcomes among HIV positive Kenyan women [17, 18]. Approximately 93% of these variables contain missing values across visits. Among these, 42% are missing more than half of possible recorded values. Variables included access factors such as antiretroviral (ARV) medication regimen, clinical HIV wellness information such as CD4 count and viral load, diagnosis of respiratory illness, delivery information, social and economic background information about the mother, and NHIF enrollment.
Main text
Methods
Step 1: filling binary variable dependencies in visit-level data
Missing values in the dataset may be non-applicable, or they may represent “no” values depending on the interface available or the decisions of the individual entering data. Using domain expertise, we identify dependencies between variables and develop a function that uses these dependencies to fill missing “no” values within binary variables. This step is performed using visit level data for each patient. Not only does this cleaning step improve data coverage, it provides variance that may be helpful when filling in other values. Figure 1 summarizes this and all subsequent analytic steps.
Step 2: patient-level interpolation of continuous values
For continuous, individual-level variables that are missing instances of reporting throughout the dataset, we rely on linear interpolation. Examples of variables imputed by this step include body mass index (BMI), systolic and diastolic blood pressure, arterial oxygen saturation (Sa02). We elect to build linear models using observed data and predict values for gaps based on this relationship. For this step, we utilize visit level data for each patient. Because this step relies on individuals’ data, it can be completed in parallel and is highly scalable for larger datasets.
Step 3: multiple imputation by chained equations for patient-level data
Using the dataset that has been partially filled based on the previous two steps, we use multiple imputation by chained equations (MICE) to fill the remaining values. First, due the challenge of accounting for temporal variation in this dataset, we convert the data from visit-level to patient level prior to MICE imputation by randomly selecting one observation to represent an individual. MICE is a conditional imputation approach that has been shown to be effective for imputing EHR data with low error [10, 19]. This imputation process leverages five distinct steps. First, it creates multiple copies of the dataset and replaces missing values with randomly selected, temporary ‘placeholder’ variables. Separate regression models are used to impute missing values across data copies for each variable. These predictions are then pooled, creating a set number of candidate, imputed datasets from which we randomly select a value. Given that the majority of responses are categorical and variation is minimal, we do not anticipate random selection to change the structure of the data. To complete this step, we use the package mice in R [20], and specify the use of classification and regression trees with five data copies to fill values.
Step 4: filling visit-level dataset and cleaning date variables
To create values that represent individuals and reduce the possible impact of outliers, we randomly select one row per patient. Finally, because we note that MICE incorrectly fills in date values for two variables—delivery date and CD4 count date—we elect to complete our data generation process by carrying the last available date forward for each patient.
Validation using random forest modeling
We validate imputed values by building a random forest model designed to predict enrollment in the NHIF. This measure is both patient- and time-variant, and it is present for 107,566 visits within the dataset. We randomly select 80% of the dataset to use as a training set, and 20% to use as a test or validation set. The random forest model includes fourteen measures expected to correlate with NHIF enrollment, all of which were included in the imputation process. We then measure the performance of this classifier by comparing the accuracy and F1-scores of the predicted and observed test set enrollment values (Table 1).
Table 1.
Variables imputed using value dependencies
| Dependency 1 | Dependency 2 | Variable of interest | Original variation | Imputed variation | ||
|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||
| On ARV | NA | Change in ARV regimen | 0 | 1375 | 386,984 | 1375 |
| On ARV | Change in ARV regimen | ARV stop: Completed T-pMTCT | 0 | 2588 | 384,488 | 2588 |
| On ARV | Change in ARV regimen | ARV stop/change due to regiment failure | 0 | 352 | 386,937 | 352 |
| On ARV | Change in ARV regimen | ARV stop/change due to toxicity | 0 | 1696 | 385,509 | 1696 |
| On ARV | Change in ARV regimen | ARV stop/change due to weight change | 0 | 10 | 386,974 | 10 |
| On ARV | Change in ARV regimen | ARV stop/change due to other reason | 0 | 2002 | 385,266 | 2002 |
| On ARV | Change in ARV regimen | ARV stop/change due to new TB | 0 | 43 | 386,942 | 43 |
| On ARV | Change in ARV regimen | ARV stop/change due to non-adherence | 0 | 358 | 386,637 | 358 |
| On ARV | Change in ARV regimen | ARV stop/change due to out of stock | 0 | 63 | 386,927 | 63 |
Results
Filling binary variable dependencies in visit-level data
The majority of dependency-filled variables relate to ARV medication regimens. We assume that if individuals report: (a) being on ARV but; (b) have no reported change in ARV regimen, then we mark all subsequent change behaviors as values as “no” (Table 1). This approach led to an average 72.4% increase in the number of visit-level values filled.
Step 2: patient-level interpolation of continuous values
Using the parameters identified, we interpolated values for 33 variables in our dataset. These variables represent variables that are numeric and have at least ten unique values across the total dataset. The majority of these values are continuous values with variance that depends only on the individual and not on the cohort or clinic. Because we have a limited number of observations for many individuals, this limits our degrees of freedom and inhibits the inclusion of control variables and smoothing parameters.
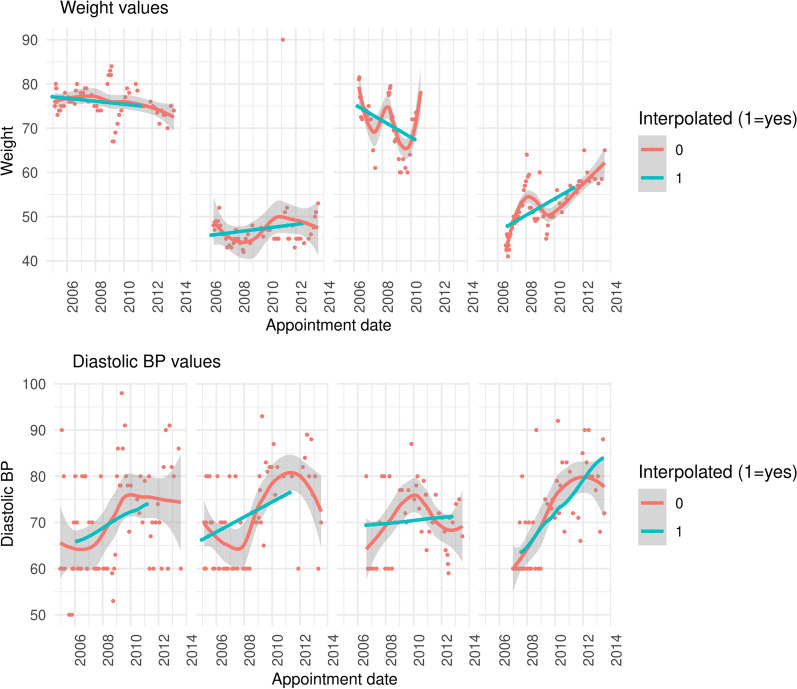
Variables addressed using this technique include continuous measures such as: body weight, CD4 count, and diastolic/systolic blood pressure. Using these four variables as an illustration, we find that within the visit-level dataset, approximately 10.9% and 11.0% of entries are missing for systolic and diastolic blood pressure, respectively, 10.1% are missing for body weight, and 84.9% are missing for CD4 count. Interpolating values per patient drops the number of missing visit-level values to zero for all variables. Figure 2 illustrates that filling visit-level data gaps with time-based linear interpolation produces results that follow patterns similar to those observed for patients.
Fig. 2.
Patient weight and Diastolic BP over time for several patients among a random subset of 100 patients. Red indicates observed weight, and blue indicates imputed weight
Steps 3 and 4: MICE for patient level data, filling and cleaning visit-level dataset
We next reduce our dataset from visit level to patient-level, apply MICE imputation, and use generated values to fill gaps in the visit-level dataset. Comparing the original dataset to the final, imputed dataset, we note that 78 focal variables required imputation. At least some missing values were filled for 66 (84.6%) of variables. Only 12 variables saw no reduction in missing values. Figure 3 displays the change in percent missing per variable pre- and post-imputation. We note that 20 variables saw a percent reduction in missing values of 80% or more. In terms of processing time, we completed all cleaning and imputation steps within 6 hours by parallelizing each component.
Fig. 3.
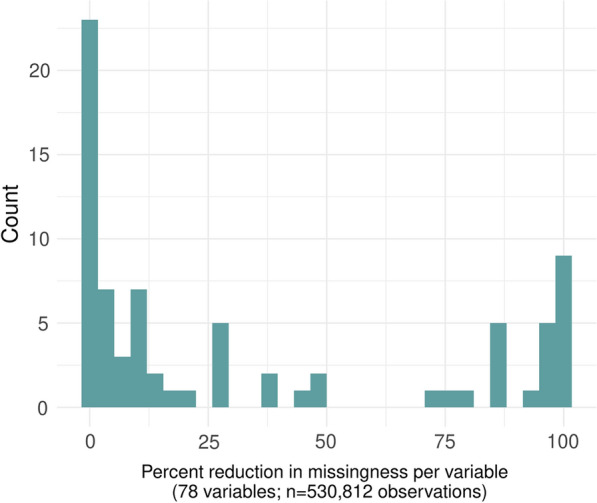
Percent reduction in missing values pre- and post-imputation among focal variables
Validation
We validate our data by predicting visit-level NHIF enrollment using a random forest algorithm. Within our data, an estimated 7% percent of women are enrolled, which is in line with national estimates of approximately 11% [21]. Our model uses the following measures as predictors: pregnancy outcomes and location/delivery help; the state of illness as defined by WHO weight loss state, respiratory infection, viral load, and ARV medication regimen; socioeconomic status as measured by educational attainment and, and select background characteristics including age, age at first pregnancy, and number of children under 18 months of age. We find that despite class imbalance, our classifier performs well and achieves an accuracy of 94.8% and an F1 score of 0.587 (Appendix: Table 2).
Table 2.
NIHF prediction classifier performance
| Yes | No | |
|---|---|---|
| Yes | 791 | 780 |
| No | 333 | 19,608 |
| Accuracy | 0.948 | |
| Precision | 0.504 | |
| Recall | 0.704 | |
| F1 | 0.587 | |
Furthermore, indications of component variable significance (Appendix: Table 3), illustrate that indicators of illness progression such as BMI and later WHO weight loss stages are significant predictors of NHIF enrollment. Education is also a key predictor; a bivariate analysis indicates that those with recorded insurance have an average of 9 years of education versus 7.5 years of education among those who do not (t = 112.54, p < 0.001). These factors correspond with existing research addressing determinants of healthcare access among rural Kenyan women [18, 22].
Table 3.
Predictive importance of key variables
| Variable | Generalized cross-validation (GCV) estimate of error |
|---|---|
| Age at fist pregnancy | 100 |
| Body Mass Index (BMI) | 69.9913 |
| Viral load | 53.1357 |
| Age at first pregnancy | 29.11793 |
| Years of school | 28.06922 |
| Children under 18 months: 1 | 6.261627 |
| Travel time to clinic: 30–60 min | 5.991079 |
| WHO weight loss stage: 3 | 5.981254 |
| Place of delivery 2 | 5.827017 |
| Pregnancy outcome: unknown/not documented | 5.822529 |
| WHO weight loss stage2 | 5.179188 |
| Visits urban clinic | 5.064835 |
| On ARV | 5.025331 |
| Travel time to clinic: 1–2 h | 4.986507 |
| Delivery assistance | 4.309087 |
| Place of delivery 4 | 4.232822 |
| Delivery assistance 4 | 3.88988 |
| Travel time to clinic: > 2 h | 3.462557 |
| WHO weight loss stage: 4 | 2.662623 |
| Delivery assistance 5 | 1.985101 |
Discussion
The scale of EHR data offer numerous advantages for health researchers. The primary challenge of using these data, however, is that they typically contain a number of missing values [2, 4, 9]. By leveraging this step-wise cleaning and imputation process, we are able to fill values for 84.6% of selected variables with initial non-zero missingness on a dataset that contains information on 530,812 visits for 16,316 patients. A predictive model utilizing these data is highly accurate and validates what is known about health insurance enrollment among rural Kenyan women [17, 18, 21].
One of the goals underlying this work is to encourage researchers across disciplines to leverage the ‘big data’ aspects of EHR [4, 5]. Rather than reduce their dataset to a handful of predictors or a single cohort of observations, researchers may use the full dataset and engage in more data-driven, predictive model building and gain new insights into the association between healthcare provision, patient characteristics and behavior, and health outcomes.
Limitations
Two key limitations of this approach is that our MICE imputation model is time-invariant, and that it does not account for spatial autocorrelation among nearby clinics. In the future, we may adapt approaches for imputing panel data to suit this task. There are also variables for which we have too few observations to impute. Future work may explore methods of predicting these values with little to no ground truth data.
Acknowledgements
We are grateful to the Academic Model Providing Access to Healthcare (AMPATH)—Kenya for providing the data used in this study. We would also like to thank Dr. Sara Lodi of Boston University’s School of Public Health for reviewing drafts of this manuscript and providing valuable insight and direction.
Abbreviations
- EHR
Electronic health records
- AMPATH
Academic Model Providing Access to Healthcare
- MICE
Multiple imputation by chained equations
- NHIF
National Hospital Insurance Fund
Appendix
Authors’ contributions
NC and LPOW contributed to the conception of the paper, data analysis and interpretation, drafting of the paper and subsequent revisions. Both authors read and approved the final manuscript.
Funding
This work is supported by: (CFAR) NIH/NIAID fund P30AI042853. None of the funders were involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The de-identified datasets used and/or analyzed in this current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All the data analyzed in this study was from the AMPATH Medical Records System (AMRS) and was de-identified. De-identification was done by data managers and programmers at AMPTH who extracted the data from the AMRS and removed identifiers including: names; Kenya national identification numbers; dates of birth; mobile phone numbers; and residential information. Thus, this study was not considered human subjects research. Nevertheless, the Institutional Research and Ethics Committee (IREC) at Moi University/Moi Teaching and Referral hospital in Eldoret, Kenya approved the study. Anonymized data were stored on a secure, password protected server and transferred between collaborators via encrypted email.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abul-Husn NS, Kenny EE. Personalized medicine and the power of electronic health records. Cell. 2019;177:58–69. doi: 10.1016/j.cell.2019.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter JT. Electronic medical records and quality improvement. Neurosurg Clin N Am. 2015;26(245–51):ix. doi: 10.1016/j.nec.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Evans RS. Electronic health records: then, now, and in the future. Yearb Med Inform. 2016 doi: 10.15265/IYS-2016-s006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemingway H, Asselbergs FW, Danesh J, Dobson R, Maniadakis N, Maggioni A, et al. Big data from electronic health records for early and late translational cardiovascular research: challenges and potential. Eur Heart J. 2018;39:1481–1495. doi: 10.1093/eurheartj/ehx487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ristevski B, Chen M. Big data analytics in medicine and healthcare. J Integr Bioinforma. 2018 doi: 10.1515/jib-2017-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golas SB, Shibahara T, Agboola S, Otaki H, Sato J, Nakae T, et al. A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: a retrospective analysis of electronic medical records data. BMC Med Inform Decis Mak. 2018;18:44. doi: 10.1186/s12911-018-0620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin H, Long E, Ding X, Diao H, Chen Z, Liu R, et al. Prediction of myopia development among Chinese school-aged children using refraction data from electronic medical records: a retrospective, multicentre machine learning study. PLoS Med. 2018;15:e1002674. doi: 10.1371/journal.pmed.1002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz JT, Gao M, Geng EA, Mody KS, Mikhail CM, Cho SK. Applications of machine learning using electronic medical records in spine surgery. Neurospine. 2019;16:643–653. doi: 10.14245/ns.1938386.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein BA, Navar AM, Pencina MJ, Ioannidis JPA. Opportunities and challenges in developing risk prediction models with electronic health records data: a systematic review. J Am Med Inform Assoc. 2017;24:198–208. doi: 10.1093/jamia/ocw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaulieu-Jones BK, Lavage DR, Snyder JW, Moore JH, Pendergrass SA, Bauer CR. Characterizing and managing missing structured data in electronic health records: data analysis. JMIR Med Inform. 2018;6:e11. doi: 10.2196/medinform.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloomfield GS, Hogan JW, Keter A, Holland TL, Sang E, Kimaiyo S, et al. Blood pressure level impacts risk of death among HIV seropositive adults in Kenya: a retrospective analysis of electronic health records. BMC Infect Dis. 2014;14:284. doi: 10.1186/1471-2334-14-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martín-Merino E, Calderón-Larrañaga A, Hawley S, Poblador-Plou B, Llorente-García A, Petersen I, et al. The impact of different strategies to handle missing data on both precision and bias in a drug safety study: a multidatabase multinational population-based cohort study. Clin Epidemiol. 2018;10:643–654. doi: 10.2147/CLEP.S154914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalton A, Bottle A, Soljak M, Okoro C, Majeed A, Millett C. The comparison of cardiovascular risk scores using two methods of substituting missing risk factor data in patient medical records. J Innov Health Inform. 2011;19:225–232. doi: 10.14236/jhi.v19i4.817. [DOI] [PubMed] [Google Scholar]
- 14.Kenward MG, Molenberghs G. Missing data in clinical studies. Hoboken: Wiley; 2007. [Google Scholar]
- 15.Garies S, Cummings M, Quan H, McBrien K, Drummond N, Manca D, et al. Methods to improve the quality of smoking records in a primary care EMR database: exploring multiple imputation and pattern-matching algorithms. BMC Med Inform Decis Mak. 2020;20:56. doi: 10.1186/s12911-020-1068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honaker J, King G. What to do about missing values in time-series cross-section data. Am J Polit Sci. 2010;54:561–581. doi: 10.1111/j.1540-5907.2010.00447.x. [DOI] [Google Scholar]
- 17.Were LPO, Hogan JW, Galárraga O, Wamai R. Predictors of health insurance enrollment among HIV positive pregnant women in Kenya: potential for adverse selection and implications for HIV treatment and prevention. Int J Environ Res Public Health. 2020;17:2892. doi: 10.3390/ijerph17082892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Were LPO, Were E, Wamai R, Hogan J, Galarraga O. Effects of social health insurance on access and utilization of obstetric health services: results from HIV+ pregnant women in Kenya. BMC Public Health. 2020;20:87. doi: 10.1186/s12889-020-8186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Buuren S. Package “mice”. 2020. https://cran.r-project.org/package=mice. Accessed 26 Aug 2020.
- 21.Barasa E, Rogo K, Mwaura N, Chuma J. Kenya National Hospital Insurance Fund Reforms: implications and lessons for universal health coverage. Health Syst Reform. 2018;4:346–361. doi: 10.1080/23288604.2018.1513267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maina JM, Kithuka P, Tororei S. Perceptions and uptake of health insurance for maternal care in rural Kenya: a cross sectional study. Pan Afr Med J. 2016;23:125. doi: 10.11604/pamj.2016.23.125.8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The de-identified datasets used and/or analyzed in this current study are available from the corresponding author on reasonable request.