Abstract
In 2021, mRNA vaccines against COVID-19 were approved by the Food and Drug Administration. mRNA vaccines are important for preventing severe COVID-19 and returning to normal life. The development of RNA-delivery technology, including mRNA vaccines, has been investigated worldwide for ~30 years. Lipid nanoparticles (LNPs) are a breakthrough technology that stably delivers RNA to target organs, and RNA-loaded LNP-based nanomedicines have been studied for the development of vaccines and nanomedicines for RNA-, gene-, and cell-based therapies. Recently, microfluidic devices and technologies have attracted attention for the production of LNPs, particularly RNA-loaded LNPs. Microfluidics provides many advantages for RNA-loaded LNP production, including precise LNP size controllability, high reproducibility, high-throughput optimization of LNP formulation, and continuous LNP-production processes. In this review, we summarize microfluidic-based RNA-loaded LNP production and its applications in RNA-based therapy and genome editing.
Keywords: Lipid nanoparticles, RNA delivery, Microfluidic device, mRNA vaccine
Graphical abstract
1. Introduction
In recent years, RNA-delivery technologies have been investigated for use in vaccines and nucleic acid therapies [[1], [2], [3], [4]]. In 2020, mRNA vaccines for COVID-19 were authorized by the Food and Drug Administration (FDA) under an emergency use authorization. The mRNA vaccines BNT16b2 and mRNA-1273 produced by Pfizer–BioNTech and Moderna can prevent severe cases of COVID-19 worldwide [[5], [6], [7], [8], [9]]. The mRNA vaccine was officially approved by the FDA in 2021, and other mRNA vaccines against malaria, human immunodeficiency virus, influenza, and cancer are also being developed.
RNA-delivery technology using lipid nanoparticles (LNPs) plays an essential role in the practical application of mRNA vaccines and RNA-based therapies. The encapsulation of RNA into the LNP prevents RNA cleavage by RNases in the blood. Additionally, LNP-based drug-delivery technologies enable the delivery of RNA to target organs. LNP characteristics, including lipid composition, the molar ratio of nitrogen/phosphate (N/P), LNP size, LNP size distribution, Z-potential, and RNA-encapsulation efficiency, affect biodistribution and therapeutic effects, suggesting that these conditions should be optimized to maximize the therapeutic effect. Therefore, the development of RNA-loaded LNP-production technologies with high reproducibility is strongly desired.
Microfluidic devices provide many advantages, such as high-throughput screening, reduced use of expensive samples, precise control of reaction times, wearable sensing, and on-site analysis in the biochemical, biomedical, diagnostics, drug-design, and pharmaceutical fields [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. Microfluidic devices and technologies promise precise size controllability with high reproducibility for the production of microdroplets, emulsions, microcapsules, microparticles, and NPs [[21], [22], [23], [24], [25], [26]]. Additionally, microfluidic devices have been employed for liposome and LNP production [[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51]]. Microfluidic device features, including continuous flow, precise control of reaction time, high-temperature controllability, and shorter diffusion distance in a microchannel, are advantageous for the production of microparticles and NPs. Other aspects of a microfluidic device suitable for LNP production include rapid optimization of LNP-production conditions and ease of scaling up. These microfluidics features provide outstanding contributions to LNP production and LNP-based RNA-delivery technology and enable transition from laboratory-scale use to practical applications. Recently, various microfluidic devices have been developed to produce LNPs and applied to RNA, DNA, ribonucleoprotein (RNP), drugs, and other NP-delivery platforms, as well as the development of Onpattro, the first approved RNA-interference therapeutic drug, employing a microfluidic device [52]. Furthermore, several microfluidic devices, including NanoAssemblr and iLiNP, are commercially available and used to study RNA delivery. In the future, microfluidic devices and technologies will be the gold standard for LNP production.
This review focuses on RNA-delivery technologies using LNPs produced by microfluidic devices. We briefly introduce the characteristics of microfluidic devices used for LNP production and the mechanism of LNP formation using microfluidic devices. Additionally, we provide an overview of LNPs used for small-interfering (si)RNA, mRNA, and RNP delivery and produced by microfluidic devices.
2. Microfluidic devices for RNA-loaded LNP production
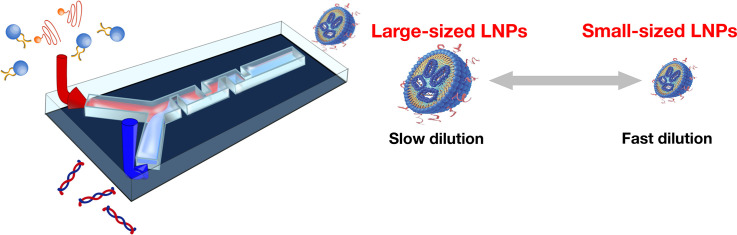
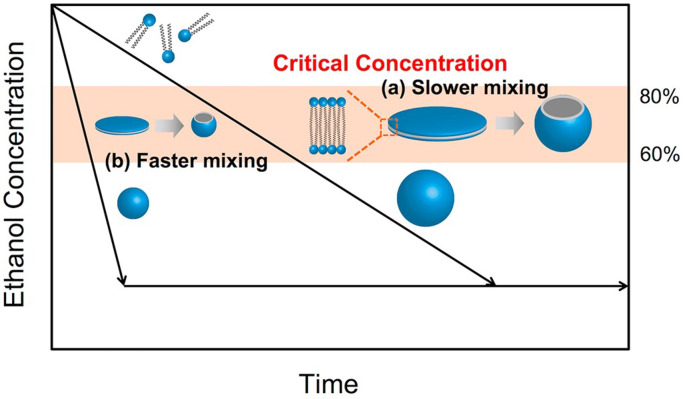
Generally, the ethanol-dilution method is used for LNP production using a microfluidic device. The lipid solution is dissolved in ethanol, and RNA is dissolved in appropriate buffer solutions, such as acetate, citrate, or malic acid buffer. To encapsulate RNAs into LNPs, a cationic lipid or pH-sensitive cationic lipid is employed for the lipid components. The lipid solution and RNA/buffer solutions are then introduced into the microfluidic device, where positively-charged lipids and negatively-charged RNAs form complexes via electrostatic interactions. The RNA–cationic lipid complexes are then assembled with other lipids to form LNPs. Lipids dissolved in ethanol are self-assembled by diluting ethanol with a buffer solution. Therefore, the performance of microfluidic devices in ethanol-diluted solutions is a significant factor in controlling LNP size and size distribution. A conceptual illustration of LNP-formation behavior and fluid dynamics is shown in Fig. 1 . When ethanol is rapidly diluted with a buffer solution to a critical ethanol concentration, small-sized LNPs form, whereas large-sized LNPs form under slow ethanol-dilution conditions as shown in Fig. 2 [30]. In the previous work, we hypothesized the ethanol concentration of 60–80% is critical for producing the small-sized LNPs. In the batch-wise (vortex mixing) method, the nonuniform dilution of ethanol is a major reason for LNP variation.
Fig. 1.
Conceptual illustration of the relationship between LNP-formation behavior and fluid dynamics.
Fig. 2.
Schematic illustration of LNP formation process in a microfluidic device at (a) slower and (b) faster mixing. Reprinted from [30] with the permission of the Public Library of Science.
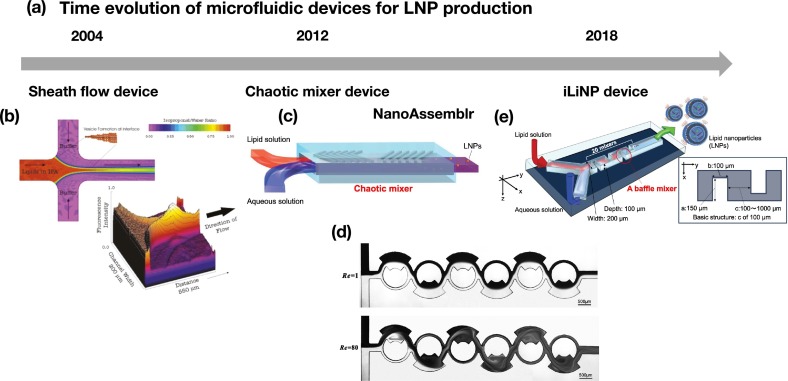
Microfluidic devices for LNP production can be classified into several types (Fig. 3 ). First, typical T- or Y-shaped microfluidic devices are the simplest structures for LNP production. The lipid and buffer solutions are fed into the microfluidic device, and LNPs form at the liquid–liquid interface by diffusion-based ethanol dilution. These types of microfluidic devices tend to produce large-sized LNPs owing to the slow ethanol dilution. Additionally, an ethanol-concentration gradient forms at the liquid–liquid interface, which induces LNP size variation. A previous study employed a sheath-flow (three inlet)-type microfluidic device for LNP production to improve the ethanol-dilution rate [Fig. 3(b)] [[53], [54], [55]]. Jahn et al. used the sheath-flow-type microfluidic device and with a depth of 40 μm, a maximum width of 200 μm, and a minimum width of 147 μm [53]. Hood et al. reported several types of heath-flow-type microfluidic devices with different aspect ratio. The microchannel dimensions were 50 μm wide and either 25 or 250 μm high in the mixing region [55]. Compared with T- or Y-shaped microfluidic devices, ethanol is diluted rapidly in this system, because the sheath flow increases the ethanol-buffer solution interface.
Fig. 3.
(a) Time revolution of the microfluidic device for LNP production. (b) A sheath-flow (3 inlets)-type microfluidic device. Reprinted from [54] with the permission of the American Chemical Society. (c) A chaotic mixer device. Reprinted from [28] with the permission of the American Chemical Society. (d) A planar asymmetric split-and-recombine micromixer. Reprinted from [58] with the permission of the American Chemical Society. (e) The iLiNP device. Reprinted from [28] with the permission of the American Chemical Society.
A chaotic mixer device is one of the most commonly used microfluidic devices for LNP production and employed for NanoAssemblr [Fig. 3(b)] [31,36,39,56]. Stroock et al. [57] described the concept of a chaotic mixer device in 2002, with this device subsequently widely used in the microfluidics field. The herringbone structures affect the mixing performance. Chen et al. used the chaotic mixer device with a 200 μm wide and 79 μm high mixing channel with 31 μm high herringbone structures for siRNA-loaded LNPs [39]. Generally, chaotic mixer structures are suitable for mixing solutions at low flow rates, which increased LNP size controllability relative to that of T- or Y-shaped microfluidic devices and the sheath-flow device. From the viewpoint of fluid dynamics, the mixing performance of the chaotic mixer device is reduced under high-flow-rate conditions; however, the solutions pass through chaotic mixer structures in a short time, resulting in rapid mixing of the solution, even under high-flow-rate conditions [30]. Recently, the NanoAssemblr Ignite system was released from Precision NanoSystems (Vancouver, BC, Canada). The Ignite system employs planar asymmetric split-and-recombine micromixers reported by Xia et al. in 2012. [Fig. 3(d)] [58], with the solutions mixed using the Dean vortex. This type of microchannel structure is suitable for high-flow-rate conditions, because the Dean vortex is more easily generated under these conditions relative to low-flow-rate conditions.
We previously developed a microfluidic device with baffle structures (iLiNP) suitable for LNP production [29]. Standard dimensions of the baffle structure were width (a) of 150 μm, depth (b) of 100 μm, and the interval (c) of 100 μm. The width and height of the microchannel were 200 and 100 μm [Fig. 3(e)]. The iLiNP device has a simple structure and microchannel fluid dynamics that differ from those in the chaotic mixer. The chaotic mixer device induces homogeneous mixing in the microchannel by chaotic convection, whereas the iLiNP device maintains a layered flow of ethanol and buffer solution in the microchannel. However, ethanol is diluted rapidly at the liquid–liquid interface by secondary flow at the baffle structures. Because LNPs are formed at the liquid–liquid interface by diluting ethanol, homogeneous mixing is unnecessary for LNP production. Contrary to the chaotic mixer device, the iLiNP device shows preferable ethanol dilution performance at the high flow rate condition [29]. Previous studies have reported several types of iLiNP devices capable of improving LNP size controllability and integrating LNP-production processes [28,32,33]. We found that the post-treatment process of LNP production affected the final production size of LNPs. Other types of microfluidic devices for LNP production have been reported, including capillary-based devices [59]; those involving electrohydrodynamics [46] and ultrasound [47]; 3D-printing micromixers [41]; and other types of microchannel structures [43,44].
3. Application of microfluidic devices for RNA delivery
3.1. Microfluidic preparation of RNA-loaded LNPs
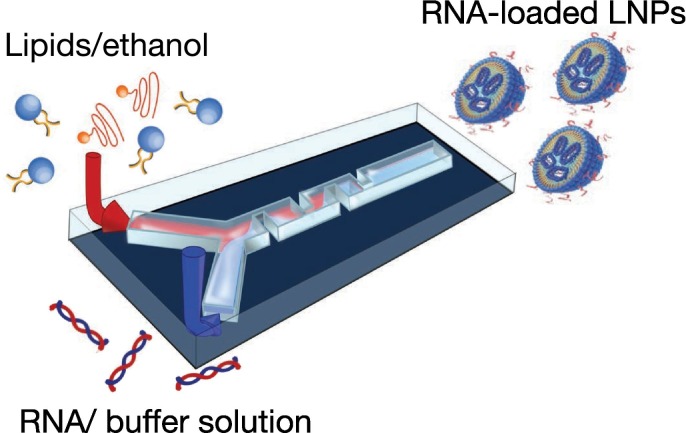
Fig. 4 shows a schematic illustration of the preparation method for RNA-loaded LNPs using a microfluidic device. A microfluidic device for RNA-loaded LNPs was designed with two inlets for a lipid/ethanol solution and an RNA/buffer solution. The lipid solution contains a cationic lipid or a pH-sensitive lipid as the main component, and other lipids, such as phospholipids, cholesterol, and polyethylene glycol (PEG)ylated lipids, are mixed into the solutions. The pKa of typical pH-sensitive lipids is lower than physiological pH in order to prevent electrostatic interactions between the LNP and serum proteins in blood. When pH-sensitive cationic lipid-based LNPs are taken up by cells via endocytosis, the LNPs show a positive charge at the late endosome, which enables fusion of positively-charged LNPs with the endosomal membrane and subsequent RNA release into the cytosol. The lipid solution is diluted with RNA/buffer solution to allow the formation of RNA-loaded LNPs, and acidic buffer solutions are used to encapsulate RNAs into pH-sensitive cationic lipid-based LNPs. RNA-loaded LNPs are collected at the outlet of the microfluidic device, after which the collected LNP suspension is dialyzed against appropriate buffer solutions to remove ethanol.
Fig. 4.
Schematic illustration of the RNA-loaded LNP-preparation method using a microfluidic device. Introduction of the lipid/ethanol and RNA/buffer solutions into a microfluidic device.
For RNA delivery, LNP characteristics, including size, size distribution, the polydispersity index (PDI), Z-potential, and RNA-encapsulation efficiency, are controlled by lipid composition and concentration, preparation methods, and the N/P ratio (the ratio between the positively-charged amine in lipid to the negatively-charged phosphate in RNA) [60,61]. Recently, the influences of these critical formulation parameters on the LNP size and mRNA vaccine immunogenicity have been reported using microfluidic devices. A variety of nucleic acids, such as plasmid DNA, oligonucleotides, siRNA, mRNA, and RNPs, can be encapsulated into LNPs using microfluidic devices. In this review, we provide an overview of siRNA, mRNA, and RNP delivery by LNPs prepared using microfluidic devices.
3.2. siRNA-loaded LNPs prepared by microfluidic devices
siRNA is a double-stranded RNA that marks mRNA for degradation through binding to a complementary sequence in the mRNA. This process is applied by Onpattro, the first approved siRNA drug. Table 1 summarizes the characteristics of siRNA-loaded LNPs prepared using microfluidic devices [29,[37], [38], [39],[62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78]]. Several types of microfluidic devices have been used for the production of siRNA-loaded LNPs.
Table 1.
siRNA-loaded LNPs prepared by microfluidic devices.
| Lipid | Device | Size [nm] | PDI [−] | Z potential [mV] | RNA EE [%] | In vitro target | In vivo target | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cationic lipid/DSPC/cholesterol/mPEG2000-DMG = 2.0: 0.28: 0.52: 0.13 mg/mL | CM | 60–90 | – | – | 80 | Firefly luciferase in dual-glow HeLa cells | VII factor in mouse liver | [39] |
| DLinKC2-DMA/DSPC/cholesterol/PEG-c-DMA = 40: 11.5: 47.5-x: 1 + x mol% | CM | 28–54 | > 0.1 | – | > 95 | – | VII factor in mouse liver | [37] |
| YSK05/Chol/DMG-PEG = 50: 50: 1 | iLiNP | 40 | – | – | > 90 | – | VII factor in mouse liver | [29] |
| CLinDMA/Cholesterol/PEG-DMG = 50: 44: 6 mol% | T-junction | 140± | – | – | 82% | – | mRNA in liver and spleen | [62] |
| KC2/DSPC/Cholesterol/PEG = 50: 10: 38.5: 1.5 mol% | T-junction | 20–60 | – | – | – | – | – | [63] |
| DLin-KC2-DMA/DSPC/Cholesterol/PEG = 20: 31.5: 47.5: 1 mol/mol | CM | – | – | – | – | – | – | [38] |
| DMAP-BLP/DSPC/Cholesterol/PEG = 50: 10: 39.5–39.75: 0.25–5 mol/mol | CM | 27–117 | – | – | – | – | VII factor in mouse | [64] |
| Dlin-MC3-DMA/DSPC/Cholesterol = 51: 10: 39 mol% DMG-PEG (a)1.4–1.5% (b) 4–5% (c) 6–8% | T-junction | (a) 58 (b) 47 (c) 46 |
– | – | (a) 96 (b) 81 (c) 82 |
(Cytokin in human whole blood) | (Cytokin in human whole blood) VII factor in mouse |
[65] |
| pPB-PEG-DSPE/DlinMC3 PEG-DMG/DSPC/Cholesterol = 1: 40: 1: 10: 48 mol% | T-junction | 110–130 | – | – | – | gp46 in NIH3T3 cells | gp46 mRNA in mouse liver | [66] |
| DMAP-BLP/DSPC/Cholesterol/PEG-DSG = 40: 17.5: 40: 2.5 mol% | CM | 84.5 ± 32.5 | – | – | – | LNCaP cells | AR and PSMA mRNA | [67] |
| DOPE/DSPE-PEG/Cholesterol = 13:1:13 | Original | 120.2 ± 1.4 | 0.18 ± 0.04 | −8.8 ± 1.6 | 98 ± 1 | EGFR mRNA in PC-3 cells | EGFR mRNA in mouse | [68] |
| C12–200/DSPC/Cholesterol/PEG2000-PE = 50: 10: 38.5: 1.5 mol% | CM (Original) | siRNA: 82.2 ± 21.4 mRNA: 82.4 ± 24.6 |
siRNA: 0.066 ± 0.018 mRNA: 0.089 ± 0.040 |
– | siRNA: 95.4 ± 0.5 mRNA: 81.1 ± 7.3 |
Luciferase in HeLa cells | VII factor in mouse | [69] |
| YSK05/DOPE/Cholesterol/PEG-SP94/PEG = 5: 2: 3: 1: 0.3 | iLiNP | 60.47 ± 6.9 | 0.101 ± 0.011 | −17.4 ± 5 | 94.5 ± 6.5 | Midkine gene in HepG2 cells | HCC in mouse | [70] |
| DODAP/Cholesterol/HSPC/PEG-DSPE = 50: 10: 39: 1 | CM | – | – | – | > 90 | – | – | [71] |
| DODMA/DOTMA/egg PC/Chol/mPEG-Chol = 40: 5: 18: 35: 2 | CM (Original) | 132.6 ± 1.6 | 0.129 | 7.3 ± 0.7 | – | Tf receptor in HepG-2 | Survivin mRNA in mouse | [72] |
| Dlin-MC3-DMA/Chol/DSPC/PEG-DMG = 50: 38: 10.5: 1.5 | NanoAssembler | siRNA: 73 ± 6.04 mRNA: 66 ± 3.94 |
siRNA: 0.1 ± 0.06 mRNA: 0.13 ± 0.07 |
siRNA: −0.55 ± 0.1 mRNA: 0.62 ± 0.47 |
siRNA: 95 ± 0.01 mRNA: 95 ± 0.02 |
CD44 in CLL cells | – | [73] |
| MC3/DSPC/Chol/DMG-PEG/DSPE-PEG = 50: 10: 38: 1.5: 0.5 | NanoAssembler | 129 ± 5 | 0.12 ± 0.02 | −10 ± 0.5 | 95 ± 9 | CD4 T cells | CD45 in CD4 T cells of mouse | [74] |
| Dendrimer/DSPC/Cholesterol/PEG-GnCm = 50: 10: 38: 2 | CM | 50–100 | – | – | > 90 | Luciferase in HeLa cells | VII factor in mouse | [75] |
| Dlin-MC3-DMA/DSPC/Chol/DMG-PEG = 50: 10: 38.5: 1.5 | NanoAssembler | 36.93 | 0.049 | – | 91.87 ± 0.4976 | SOST-mRNA in MEF | SOST gene in mouse | [76] |
| DMAP-BLP/DSPC/Chol/DMG-PEG or PEG DSG = 50: 10: (39.75-x): (0.25 + x) mol% | NanoAssembler | 30–115 | – | – | – | – | VII factor in mouse liver | [77] |
| EPC(eggPC)/DOTAP/DOPE = 2: 1: 1 | Original | 350–1400 | 0.1–0.4 | < 40 | – | Luciferase in HeLa cells | – | [78] |
Abrams et al. [62] investigated the biodistribution of an siRNA-loaded LNP (LNP201) the its associated induction of inflammation. LNP preparation was performed using a T-type microfluidic device and a high-performance liquid chromatography pump with a solution-flow rate of 40 mL/min, which resulted in 140-nm-sized LNPs. Following intravenous administration of 9 mg/kg LNP201 to mice and targeting to the liver and spleen, the authors observed maximum reductions of mRNA in the liver and spleen of 89% and 33%, respectively, with subsequent confirmation of organ-specific differences in gene-silencing efficiency. Additionally, the authors demonstrated the effect of dexamethasone on inhibiting the inflammatory responses caused by CLinDMA.
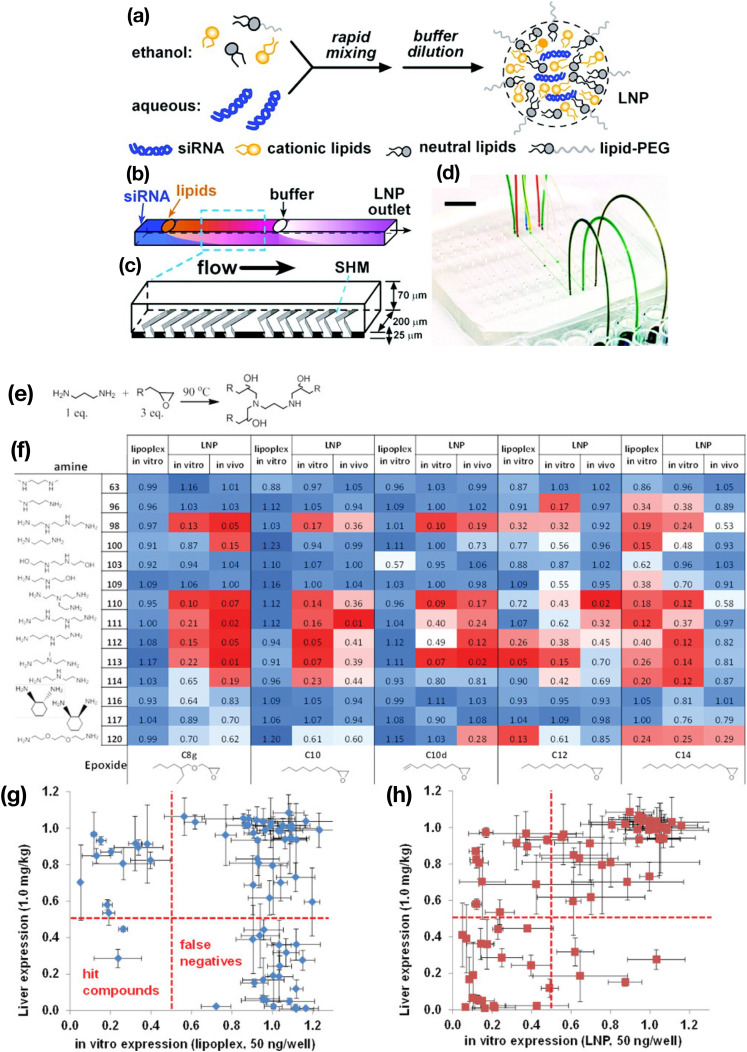
Chen et al. [39] reported the rapid screening of lipid-like materials (lipidoids) for siRNA delivery using a chaotic mixer device (Fig. 5 ) capable of producing 60–90-nm-sized LNPs depending on the total flow rate. A total of 70 compounds were synthesized for the lipidoid library, followed by evaluation of the gene-silencing activities of the siRNA-loaded LNPs and lipoplexes prepared by the lipidoids in vitro and in vivo. Interestingly, the number of hit compounds was higher for LNPs than for the lipoplexes, suggesting that microfluidic technologies improved the screening of carrier materials for RNA delivery.
Fig. 5.
(a) Concept of siRNA-loaded LNP production. (b, c) Schematic illustration of microchannel design and chaotic micromixers. (d) Photograph of the microfluidic device. (e) The lipidoid-synthesis reaction described in this study. (f) Heatmap of lipidoid screening by in vitro and in vivo experiments. (g, h) Comparison of the correlation between in vitro and in vivo gene expression associated with (g) lipoplex and (h) LNPs, respectively. Reprinted from [39] with the permission of the American Chemical Society.
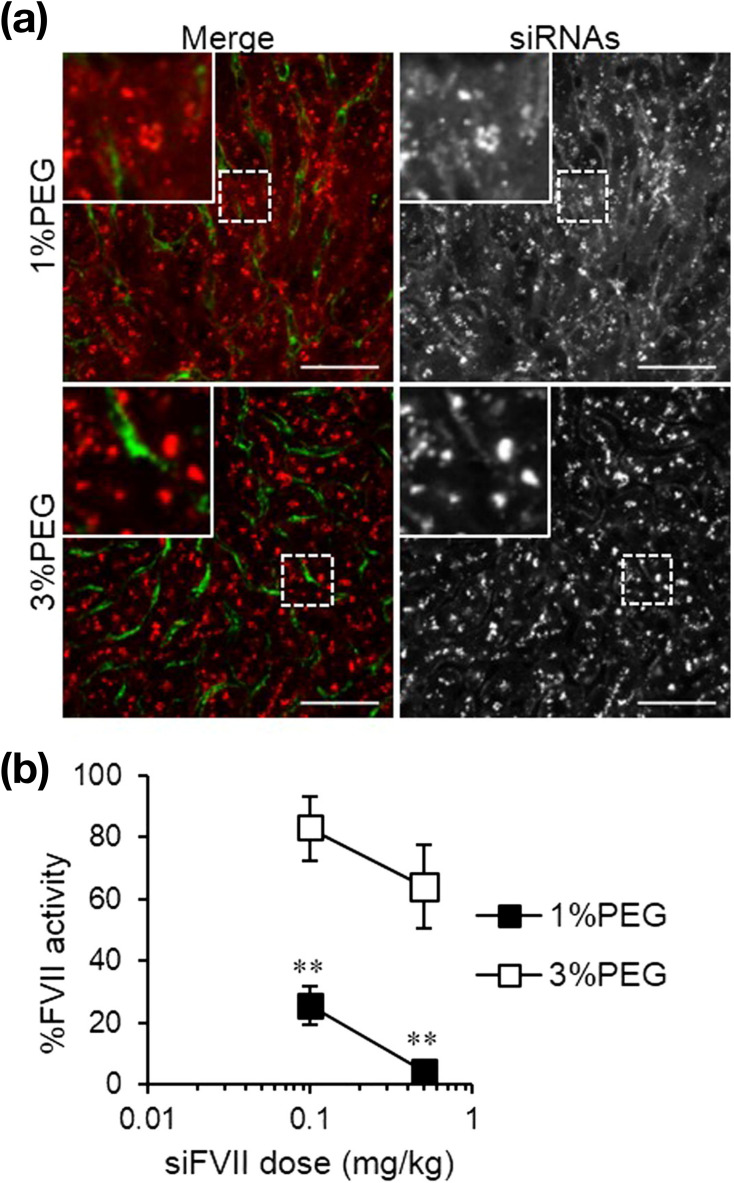
PEGylation of LNPs is indispensable for ensuring circulation time in blood. Additionally, PEGylated lipids play an important role in controlling LNP size. Belliveau et al. [37] demonstrated the effect of PEGylated molecule concentration on LNP size using a chaotic mixer device, D-LinKC2-DMA as a pH-sensitive cationic lipid, and PEG-C-DMA as a PEGylated lipid. Increase in the concentration of PEGylated lipids to a range of 1 mol% to 5 mol% reduced LNP size from 54 nm to 28 nm, and smaller-sized LNPs forming at high concentrations of PEGylated lipids. Their results showed that the combination of the microfluidic device and the molar ratio of PEGylated lipid allowed control of the size of siRNA-loaded LNPs (20–100 nm). Moreover, D-LinKC2-DMA-based LNPs showed 50% factor VII (FVII) silencing at a dose of 0.01 mg/kg in mice. Other studies demonstrated siRNA-loaded LNP-mediated gene silencing in hepatocytes using various sizes of siRNA-loaded LNPs, sizes of which were controlled according to PEG– dimyristolglycerol (DMG) concentration [64,77]. The authors reported that lipids including PEG-DMG resulted in a faster dissociation rate of 45-nm-sized LNPs relative to that observed for 80-nm-sized LNPs, with the dissociation rate affecting gene-knockdown activity. Additionally, Kumar et al. [63] studied the effect of PEGylated lipids on LNP physicochemical properties using a T-type microfluidic device and DLin-MC3-DMA as a pH-sensitive cationic lipid. They first mixed a DLin-MC3-DMA-based lipid/ethanol solution with an aqueous siRNA solution using the microfluidic device. Following LNP formation, the prepared siRNA–LNPs were mixed with PEG–DMG solution in ethanol using the microfluidic device, with PEG–DMG adjusted to control PEG density on the LNP surface. After separation of the siRNA-loaded LNPs free PEG–DMG by size-exclusion chromatography, they found that the charge-shielding effect and reduction in hemolytic activity depended on PEG density. Moreover, LNPs with high PEG–DMG density reduced LNP-induced immunostimulation but inhibited FVII silencing, suggesting that the PEG–lipid molar ratio is an essential parameter not only for LNP size but also LNP efficacy. Sato et al. [56] also investigated the physicochemical properties of different-sized LNPs composed of their synthesized pH-sensitive cationic lipid (YSK-05). Using small-sized (30–35 nm) and large-sized (50–70 nm) LNPs, they observed that the small-sized LNPs were well distributed in hepatocytes relative to the large-sized LNPs (Fig. 6 ), although the large-sized LNPs showed higher FVII-silencing activity. As mentioned earlier, lipid composition other than PEGylated lipid has a crucial role in the siRNA-loaded LNP formulation. Kulkarni et al. reported the importance of helper lipids, DSPC and cholesterol, on the LNP size and siRNA encapsulation [79]. The helper lipid composition lower than 40% formed large-sized LNPs and the siRNA encapsulation efficiency was reduced to 40%. The low siRNA encapsulation efficiency might induce the lower gene silencing performance.
Fig. 6.
(a) Intrahepatic distribution of siRNA. Blood vessels and siRNA are colored green and red, respectively. Scale bar represents 50 μm. (b) FVII-silencing activity of the 1% and 3% PEG-LNPs. Reprinted from [56] with the permission of Elsevier. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Surface-modified or ligand-conjugated LNPs allow specific delivery and uptake for cell/organ targeting, with these modified LNPs also having been produced using microfluidic devices. Lee et al. [67] developed Glu-urea-Lys ligand-conjugated siRNA-loaded LNPs using a chaotic mixer device to target prostate-specific membrane antigen (PSMA), subsequently confirming a significant decrease in PSA mRNA levels in PSMA-targeted LNP-treated tumor tissues according to quantitative real-time polymerase chain reaction. Additionally, Li et al. [72] reported the single-step production of transferrin-conjugated LNPs (Tf-LNPs) for siRNA delivery. They first formed siRNA-loaded LNPs in the microchannel by mixing an siRNA solution with a lipid solution, followed by introduction of Tf–PEG-cholesterol from the branched microchannel downstream of the microfluidic device. They found that the size of the Tf-LNPs was larger than that of LNPs without Tf conjugation, and that compared with the bulk-mixing method, the microfluidics method produced small-sized LNPs with a narrow size distribution. Furthermore, the Tf-LNPs produced by the microfluidic device inhibited the expression of survivin mRNA [72]. Moreover, peptide- and antibody-conjugated LNPs were also produced using a microfluidic device for targeting hepatic fibrosis [66] and T lymphocytes [74].
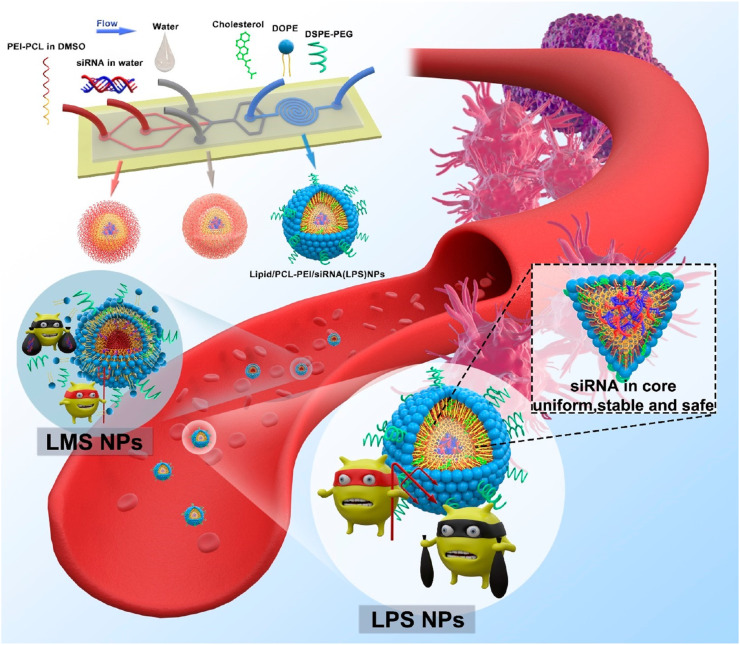
Microfluidic devices have been applied for other applications, including the production of functional NPs [28,38,68,70,73,76], investigating the mechanisms of siRNA-loaded LNP formation [63] and their associated characteristics [75], and developing new approaches for LNP production [69,71,80]. Wei et al. [68] produced hybrid NPs using siRNA, polycaprolactone–polyethylenimine (PCL–PEI), cholesterol, DOPE, and DSPE–PEG. In the first step, siRNA and PCL–PEI complexes were prepared by microfluidic mixing, and the complex was coated with lipids in the microchannel (Fig. 7 ), resulting in NPs of ~120 nm with a PDI of 0.18, the properties of which were well controlled by microfluidic production as compared with bulk mixing. Their results showed that siRNA-loaded hybrid NPs targeting epidermal growth factor receptor (EGFR) mRNA resulted in reduced mRNA levels and a 57% decreases in tumor-growth rate. Additionally, we previously reported one-step production of siRNA-loaded exosome-like NPs using the iLiNP device [28]. Because encapsulation of negatively-charged siRNA using the non-cationic lipid system is a major challenge due to the lack of a driving force for RNA encapsulation (i.e., electrostatic interactions), the iLiNP-based approach enabled production of size-controlled siRNA-loaded exosome-like NPs, which demonstrated luciferase knockdown in HeLa cells that was proportional to NP size.
Fig. 7.
Schematic illustration of the preparation steps for lipid/PCL-PEI/siRNA (LPS) nanoparticles using a microfluidic device. Reprinted from [68] with the permission of the American Chemical Society.
3.3. Microfluidics for mRNA-delivery applications using LNPs
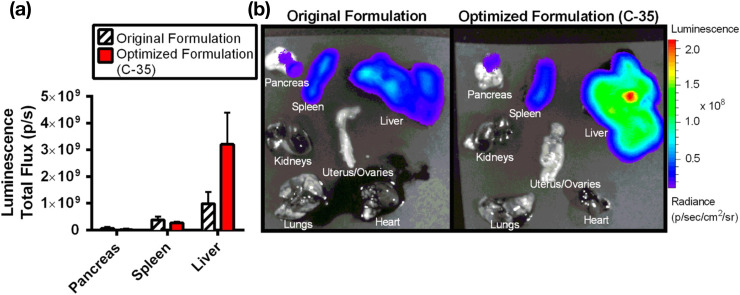
mRNA delivery using LNPs is a popular research topic worldwide, and mRNA-loaded LNPs are prepared similar to siRNA-loaded LNPs. Table 2 summarizes the mRNA-loaded LNPs produced by microfluidic devices [3,35,[81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99]]. Kauffman et al. [81] reported an optimization strategy for lipid formulation using a microfluidic device. They optimized the types of lipids, molar ratio of the lipids, and N/P ratio for mRNA delivery to the liver in vivo, which resulted in a 3-fold improvement in the delivery and expression of luciferase mRNA (1653 nucleotides) relative to the original formulation (Fig. 8 ). For a shorter mRNA (583 nucleotides) encoding erythropoietin (EPO), the optimized LNP formulation showed 7-fold higher EPO expression in the liver relative to that of the original LNP formulation. Interestingly, the FVII-silencing activities of the original and optimized formulations for siRNA-loaded LNPs were the same. The results demonstrated the combined use of microfluidics and design-of-experiment (DoE) approaches for optimizing the LNP formulation. Additionally, Sato et al. [35] reported a two-step DoE approach for preparing liver-targeted mRNA-loaded LNPs using original pH-sensitive cationic lipids (CL4H6 and CL15H6) and the iLiNP device. They optimized several formulation parameters, including the mRNA/lipid ratio, cationic lipid concentration, phospholipid concentration, and molar ratios of lipids, to design mRNA-loaded LNP libraries based on previously reported siRNA-loaded LNPs and DoEs. The results identified LNP size and the PEG–DMG/phospholipid ratio as critical factors, which was similar to other lipid formulations, and that the optimized mRNA-loaded LNPs promoted liver-specific gene expression. Moreover, mRNA-loaded CL4H6-based LNPs showed higher protein expression in hepatocytes than the mRNA-loaded MC3-LNPs [35].
Table 2.
mRNA-loaded LNPs produced by microfluidic devices.
| Lipid | Lipid: mRNA | Device | Size [nm] | PDI [−] | Z potential [mV] | RNA EE [%] | pKa | In vitro | In vivo | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| CL4H6/ESM/chol/PEG-DMG (60/5/35/1.5 mol%) | mRNA/lipid = 18.3 (g/mol) | iLiNP | 66.9 | 0.129 | 6.2 | 95.1 | – | – | ICR mouse(4 weeks year), BALB/c mouse (4–5 weeks year), C.KOR/Stm Slc-Apoe (4 − 5 weeks year) | [35] |
| C12–200/DOPE/Chol/C14-PEG2k (35/16/46.5/2.5 mol%) | C12–200/mRNA = 10/1 (w/w) | CM | 102 | 0.158 | −5 | 43 | 6.96 | – | C57BL/6 mouse (18–22 g) | [81] |
| X/DSPC/Chol/DMG-PEG2k (50/10/38.5/1.5 mol%) X: selected from library | – | CM | 86.2 ± 1.7 | 0.04 ± 0.06 | – | 97.5 ± 0.2 | 6.56 ± 0.13 | – | CD-1 mouse (18–22 g), Sprague-Dawley rat (225–250 g), Naive cynomolgus monkeys (2–4 years old, 2–6 kg) | [82] |
| MC3/DSPC/Chol/DMG-PEG2k (50/10/38.5/1.5 mol%) | – | CM | 80–100 | – | – | ≧ 90 | – | HeLa | BALB/c mouse (5–8 weeks old), Ferret (13–15 weeks old), Naive cynomolgus monkeys (2–4 years old, 2–6 kg) | [3] |
| X/DSPC/Y/DMG-PEG2k (50/10/38.5/1.5 mol%) X,Y: selected from library | N/P = 5.67 | CM | – | – | – | – | – | HeLa | – | [83] |
| Selected from library | Total lipid/mRNA = 40/1 (w/w) | CM | – | – | – | – | – | – | C57BL/6 mouse (18–20 g) | [84] |
| OF-Deg-Lin/DOPE/Chol/C14-PEG2k (35/16/46.5/2.5 mol%) | – | CM | 75 ± 10 | 0.197 | – | – | 5.7 | – | C57BL/6 mouse (18–22 g) | [85] |
| APE/DOPE/Chol/C14-PEG2k (50/25/23.5/1.5 mol%)APE:selected from several types | N/P = 8 | CM | 65–100 | 0.136–0.151 | −2.1–10 | 93–98 | – | HeLa | C57BL/6 mouse (18–22 g) | [86] |
| C12–200/DOPE/Chol/C14-PEG2k (35/16/46.5/2.5 mol%) | – | CM | 80± | – | – | 55–65 | – | HeLa | C57BL/6 mouse (16–20 g) | [87] |
| C14–4/DOPE/Chol/C14-PEG (35/16/46.5/2.5 mol%) | – | CM | 65.19–70.17 | 0.176–0.189 | – | 86.3–92.53 | 6.143–6.505 | Jurkat cell, Nalm-6 cell | – | [88] |
| X/DSPC/Chol/DMG-PEG2k (50/10/38.5/1.5 mol%) X: selected from library | – | CM | 75–95 | – | – | 69–100 | 6.6–6.9 | – | BALB/c mouse (5–8 weeks old), CD-1 mouse, Sprague-Dawley rat, Naive cynomolgus monkeys (2–5 years old, 2–3 kg) | [89] |
| X/DSPC/Chol/DMG-PEG2k (50/10/38.5/1.5 mol%) X: N/A | – | CM | 78 ± 3.5 | 0.15 ± 0.03 | – | 95.8 ± 0.5 | – | Human fibroblasts | CD1 mouse | [90] |
| X/DSPC/Chol/DMG-PEG2k (50/10/38.5/1.5 mol%) X: N/A | N/P = 5.67 | CM | 80–100 | – | – | ≧ 90 | – | HeLa | mouse, rabbit, non human primates | [91] |
| DMAP-BLP/DSPC/Chol/DMG-PEG2k (50/10/38.5/1.5 mol%) | – | CM | 80–100 | – | – | ≧ 90 | – | K562 cell, Vero cell | AG129 mouse, C57BL/6 mouse, BALB/c mouse (8 weeks old) | [92] |
| ATX/DSPC/Chol/DMG-PEG2k (50/7/40/3 mol%) | – | CM | N/A | – | – | N/A | – | – | CD-1 mouse, C57Bl6 mouse (6–8 weeks old), BALB/c mouse (7 weeks old) | [93] |
| X/DOPE/Chol/C14-PEG2k (35/16/46.5/2.5 mol%) X: selected from library | – | CM | 82.5–135.2 | – | – | 74.0–98.0 | 5.05–7.14 | Human dendritic cells | BALB/c mouse, C57BL/6 mouse | [94] |
| TT3/DOPE/Chol/DMG-PEG2k (20/30/40/0.75 mol%) | – | CM | 99–178 | < 0.2 | ≧ 0 | 15–82 | – | Hep3B cell | C57BL/6 mouse (6–8 weeks old) | [95] |
| Ionizable lipid/DSPC/Chol/DMG-PEG2k or pSar (40/10/50-x/x mol%) | N/P = 4 | CM | PEG: 90 pSar: 150 |
< 0.25 | – | – | – | HepG2 | BALB/C mouse | [96] |
| TT3/DOPE/Gd-DTPA-BSA/Chol/DMG-PEG2k (20/12/18/40/0.75 mol%) | – | CM | Gd18: 110± | – | 6± | 91 | – | Hep3B | C57BL/6 mouse (6–8 weeks old) | [97] |
| MC3/DSPC/Chol/DMPE-PEG2k (50/10/38.5/1.5 mol%) | N/P = 3 | CM | 81–87 | 0.020–0.13 | – | 95–98 | – | – | – | [98] |
| Ionizable lipid/DSPC/Chol/DMG-PEG2k (40/10.5/47.5/2 mol%) | Ionizable lipid/mRNA = 23/1 (w/w) | CM | Lipid14: 50± | 0.2 ± | – | – | – | VeroE6 cell | BALB/c mouse (6–8 weeks old) | [99] |
Fig. 8.
(a) Comparison of luciferase-expression efficiency between the original and optimized LNP formulations (C-35). (b) Biodistribution images of luciferase expression for the original and optimized mRNA-loaded LNPs. Reprinted from [81] with the permission of Elsevier.
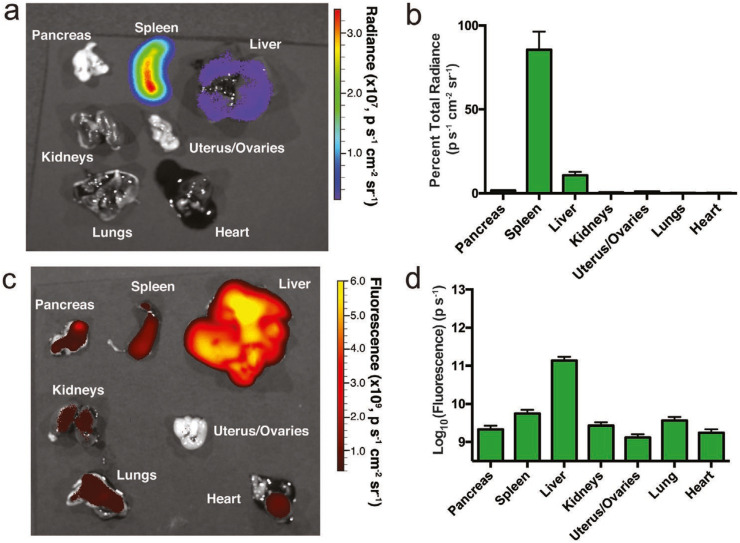
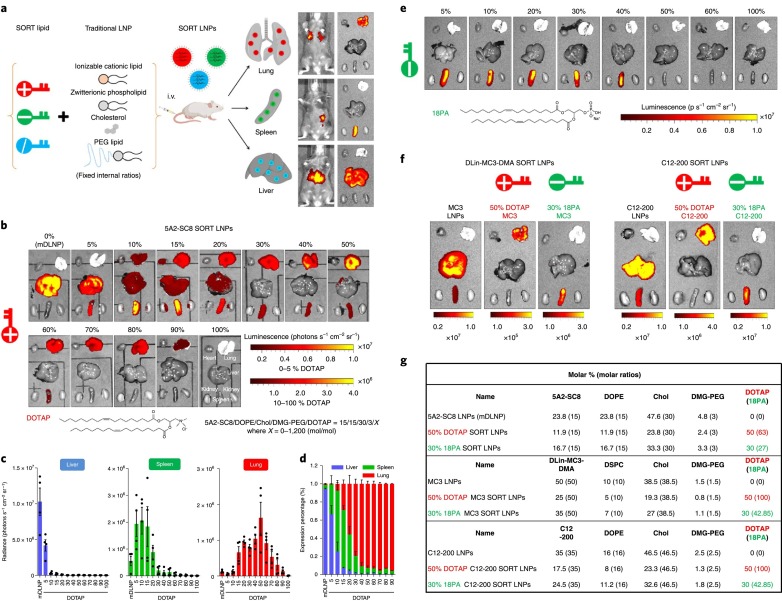
Specific organ targeting and protein expression are essential advantages of LNP-based RNA-delivery technology; therefore, the development of mRNA-delivery technology for targeted organs is strongly desired for protein-replacement therapy. B lymphocytes play an important role in antibody production, and dysregulation of B-cells induces autoimmune disorders. B-cells are attractive targets for gene delivery in order to modulate their functions; however, in vivo mRNA-delivery systems targeting B-cells to allow sufficient protein expression have not been demonstrated. Fenton et al. [85] synthesized a novel lipid (OF-Deg-Lin) for spleen-selective mRNA delivery and confirmed that OF-Deg-Lin-based LNPs could specifically produce proteins in B-cells. OF-Deg-Lin is an ionizable lipid that contains degradable linkers. The authors prepared Cy5-labeled mRNA-loaded LNPs and evaluated their biodistribution and luciferase expression, revealing that OF-Deg-Lin-based LNPs were mainly delivered to the liver; however, luciferase-expression efficiency was lower than that of the spleen, which demonstrated >85% of detected luciferase expression (Fig. 9 ). They hypothesized that the organ-dependent degradation of OF-Deg-Lin ester linkers affected spleen-specific luciferase expression and tested this hypothesis by preparing non-degradable OF-02-based mRNA-loaded LNPs. They found that OF-02-based mRNA-loaded LNPs were primarily distributed in the liver along with observed luciferase expression. Their findings suggested the importance of designing lipid molecules for selective targeting and protein expression [85]. Additionally, Cheng et al. [84] investigated selective organ-targeting (SORT) NPs for mRNA and CRISPR-Cas9 delivery, demonstrating SORT of lung, spleen, and liver by LNPs as a result of controlling internal and/or external charge. The LNPs were formulated with ionizable cationic lipids (5A2-Sc8, DLin-MC3-DMA, or C12–200), phospholipids, cholesterol, and PEGylated lipids, with the SORT LNPs harboring supplemental components relative to basic LNP formulations (Fig. 10 ). These included positively-charged lipids, such as DOTAP, DDAB, and EPC, that play an important role in lung targeting, whereas negatively-charged lipids, including 14PA, 18PA, and 18BMP, promote specific delivery to the spleen and show a high level of luciferase expression. Additionally, liver-specific SORT LNPs can be produced by adding other ionizable lipids. The authors confirmed that the primary ionizable lipid did not affect SORT according to supplemental lipid component [84].
Fig. 9.
(a) Biodistribution of luciferase expression and (b) spleen-selective luciferase expression by FLuc mRNA-loaded OF-Deg-Lin LNPs. (c) Biodistribution of non-translating Cy5 mRNA delivered by OF-Deg-Lin LNPs. (d) Delivery of non-translating Cy5 mRNA-loaded LNPs to the liver was ~100-fold higher as compared with other organs. Reprinted from [85] with the permission of Wiley-VCH.
Fig. 10.
(a) LNPs used for SORT. (b) Effect of cationic lipid (DOTAP) molar ratio on lung-targeted delivery using 5A2-SC8 SORT LNPs. (c, d) Importance of a cationic lipid (DOTAP) on lung-targeted delivery and luciferase expression. (e) Induction of mRNA delivery of an anion SORT molecule (18PA) to the spleen. (f) Luciferase expression in major organs administrated by D-Lin-MC3-DMA SORT LNPs and C12–200 SORT LNPs. (g) Detailed lipid formulations of SORT molecules. Reprinted from [81] with the permission of Springer Nature Publishing Group.
To improve mRNA delivery and protein expression, the development of new ionizable lipids is a major research topic in the field of drug delivery; however, the development and role of helper lipids, such as phospholipids and cholesterol, have not been well investigated. Patel et al. [83] studied the structural effects of cholesterol derivatives on mRNA delivery in combination with different LNP compositions. The authors used DLin-MC3-DMA, lipid 9, and DODMA as ionizable lipids along with a fixed LNP formulation at molar ratios of 50/38.5/10/1.5 mol% [ionizable lipid/cholesterol-derivative/distearoylphosphatidylcholine (DSPC)/PEG–DMG].
Antibody-dependent enhancement (ADE) is a significant problem in the development of vaccines against infections, because ADE exacerbates symptoms caused by the production of non-neutralizing antibodies. For example, ADE has been reported for dengue vaccines [100]. Richner et al. [92] demonstrated the induction of neutralizing antibodies and reduction of non-neutralizing antibodies by mRNA-loaded LNPs for ZIKV infection. The authors used DAMP–BLP/DSPC/cholesterol/PEG–DMG at a molar ratio of 50/10/38.5/1.5 and evaluated the effects of dose and boosting on survival rate, antibody titer, and weight change. They found that two doses of mRNA-loaded LNPs induced high neutralizing-antibody titers that protected against ZIKV infection and conferred sterilizing immunity [100]. mRNA delivery for human T cells is essential for producing chimeric antigen receptor (CAR) T cells to develop non-viral cell-based therapies. Conventional CAR T cell engineering uses viral vectors or electroporation, which can induce adverse effects and cytotoxicity. Billingsley et al. [88] described mRNA-loaded LNP-mediated mRNA delivery for CAR T cell engineering. They synthesized 24 ionizable lipid libraries for mRNA delivery to Jurkat cells, finding that C14–4 showed the best mRNA-delivery and luciferase-expression performance. Furthermore, T cells treated with C14–4-based LNPs killed tumor cells to a degree similar to those treated by electroporation or lentivirus and with low cytotoxicity [88].
Other applications of mRNA-loaded LNPs have been studied using microfluidics. Sebastiani et al. [98] investigated the structural and compositional effects of apolipoprotein (ApoE) on mRNA-loaded LNPs by evaluating mRNA-loaded LNPs using small-angle neutron scattering and small-angle X-ray scattering, with their findings revealing that ApoE binding to the LNP induced a redistribution of the lipid. Additionally, Luo et al. [97] demonstrated co-delivery of mRNA and magnetic resonance imaging contrast agents [gadolinium (Gd)-based contrast agents] for theranostics. The particle size of TT3-Gd lipid-like NPs was almost 100 nm and resulted in encapsulation efficiencies of mRNA and the Gd contrast agent of 91% and 74%, respectively. Moreover, the TT3-Gd lipid-like NPs showed good stability over the course of 1 month at 4 °C, and the authors confirmed the ability of the optimized lipid-like NPs to co-deliver mRNA and Gd in vitro and in vivo [97].
3.4. RNA delivery for genome editing
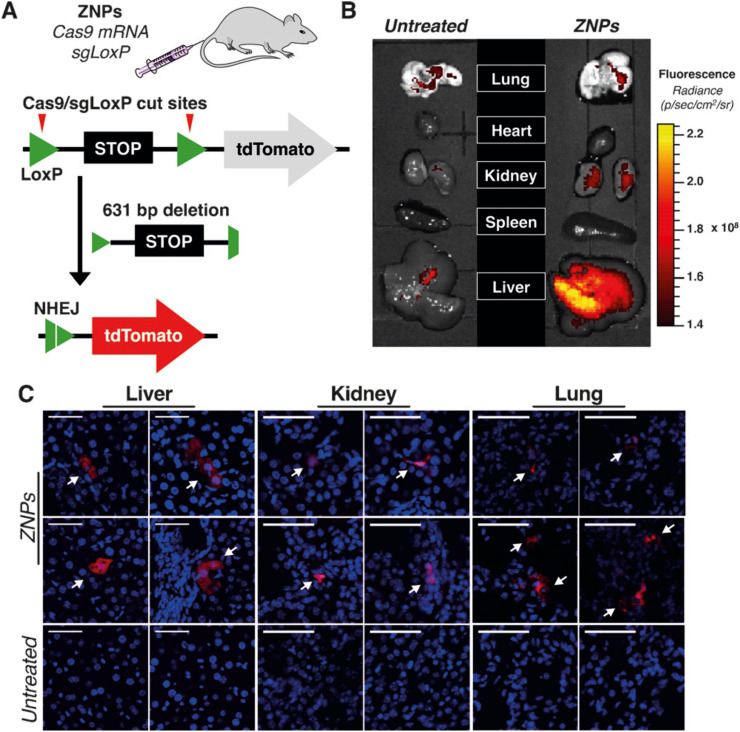
For genome editing-based therapy, the CRISPR/Cas9 RNP is a major target of LNP-based delivery systems. However, LNP-based RNA delivery has primarily focused on siRNA and mRNA. Therefore, there are few studies on the application of RNPs delivery using LNPs prepared by microfluidic devices as compared with siRNA and mRNA delivery. Table 3 summarizes the RNP-loaded LNPs prepared using microfluidic devices [34,84,[101], [102], [103], [104], [105], [106], [107], [108], [109], [110]]. Miller et al. [101] demonstrated the first non-viral co-delivery of Cas9 mRNA and single-guide (sg)RNA using LNPs in vitro and in vivo. They designed a new lipid-like material (ZA3-Ep10; a zwitterionic amino lipid) suitable for the delivery of long nucleic acids and prepared LNPs using NanoAssemblr. Treatment of HeLa-Luc-Cas9 cells with sgRNA-loaded LNPs and siRNA (siLuc) resulted in similar luciferase-silencing activity by the sgRNA-loaded LNPs relative to that observed by the siLuc-loaded LNPs. Additionally, co-delivery of Cas9 mRNA and sgRNA using LNPs was confirmed in vitro and in vivo. Fig. 11 shows CRISPR/Cas9 editing by co-delivery of Cas9 mRNA and sgRNA using LNPs in vivo. The authors showed that Cas9 mRNA and sgRNA-loaded LNPs were able to delete the target area in the genome and induce the expression of tdTomato [101]. Their findings demonstrated the ability to deliver multiple long RNAs using LNPs.
Table 3.
RNP-loaded LNPs prepared by microfluidic devices.
| Lipid | Device | Size [nm] | PDI [−] | Z potential [mV] | Cargo | RNA EE [%] | In vitro target | In vivo target | Ref |
|---|---|---|---|---|---|---|---|---|---|
| ZA3-Ep10 (zwitterionic amino lipid)/cholesterol/PEG-lipid = 100/77/1 mol/mol | NanoAssemblr | – | – | – | Cas9-mRNA/sgRNA | – | HeLa-Luc A549-Luc |
LoxP in mouse | [101] |
| TT3 lipids/cholesterol/DOPE/DMG-PEG2K = 15/25/45/0.75 mol% | NanoAssemblr | – | – | – | Cas9-mRNA/sgRNA | – | HEK293-EGFP | HBV DNA, Pcsk9 in mouse | [102] |
| MPA-A(Ab) (biodegradable lipidlike compounds)/DOPE/cholesterol/DMG-PEG2K = 20/30/40/0.75 mol/mol | NanoAssemblr | 119〜184 nm | <0.3 | – | Cas9-mRNA/sgRNA | <94 | 293 T-EGFP | Xenograft model (293 T-EGFP) | [103] |
| LP01 lipid (biodegradable, ionizable lipid)/cholesterol/DSPC/DMG-PEG2K = 45/44/9/2 mol% | NanoAssemblr | 105 | 0.06 | – | Cas9-mRNA/sgRNA | 97 | Mouse primary hepatocytes | Ttr in mouse | [104] |
| Lipid8 (ionizable lipid)/cholesterol/DSPC//DMG-PEG/DSPE-PEG = 50/10.5/38/1.4/0.1 mol/mol | NanoAssemblr | 79.3 ± 1.7 | 0.085 | 7.6 ± 0.4 | Cas9-mRNA/sgRNA | 90< | HEK293-GFP | PLK1 in mouse | [105] |
| ssPalms (self-degradable ionizable lipidlike compounds)/DOPC/cholesterol = 52.5/7.5/40 with additional 3 mol% of DMG-PEG2K | NanoAssemblr | 58.8 | 0.11 | −6.0 | Cas9-mRNA/sgRNA | 94 | – | Ttr in mouse | [106] |
| 306-O12B (bioreducible lipidoid)/cholesterol/DOPC/DMG-PEG = 50/38.5/10/1.5 mol% | NanoAssemblr | 110 | – | – | Cas9-mRNA/sgRNA | – | – | LoxP, Angptl3 in mouse | [107] |
| C12–200 (lipidoid)/cholesterol/C14PEG2000/DOPE = 50/20/10/10/10 in a weight ratio | Chaotic Mixer | 120 | – | – | Cas9-mRNA | – | 293 T-GFP | Fahmut/mut mouse | [108] |
| cKK-E12 (ionizable lipid)/cholesterol/C14-PEG 2000/DOPE = 35/46.5/2.5/16 mol/mol | Chaotic Mixer | – | – | – | Cas9-mRNA/sgRNA | – | 293 T-GFP | Pcsk9 in mouse | [109] |
| Core: PLGA/DOTAPShell: DOTAP/cholesterol/DOPE/DSPE-PEG2K = 4.4/0.96/4.7/1.7 in a weight ratio | Original Device + Pipetting | – | – | – | Cas9-mRNA/sgRNA-cationic LNP complex | – | B16 J774A.1 B16-GFP |
– | [110] |
| (a) CL4H6/DOPE/chol = 40/20/40 with additional 2 mol% of DMG-PEG2K(b) CL4H6/DOPE/= 50/50 with additional 2 mol% of DMG-PEG2K | iLiNP Device (2 inlets, 3 inlets) | (a) 219.5 (b) 179.0 |
(a) 0.083 (b) 0.194 |
(a) −0.85 (b) 0.42 |
RNP-ssON complex | (a) 84.4 (b) 94.1 |
HeLa-GFP HEK293-GFP HBV-infected HepG2-hNTCP-30 |
– | [34] |
Fig. 11.
Non-viral CRISPR/Cas9 genome editing using zwitterionic amino lipid nanoparticles (ZNPs). (a) Schemes of Cas9 mRNA and sgRNA co-delivery and evaluation. (b) Comparison of tdTomato protein expression in organs between untreated mice and ZNP-administered mice. (c) Confocal fluorescence microscopy images of liver, kidney, and lung tissues. Reprinted from [101] with the permission of Wiley-VCH.
Rosenblum et al. [105] reported genome editing for cancer therapy using Cas9 mRNA and sgRNA-loaded LNPs (sgPLK1-cLNPs). The optimized lipid system enabled up to 70% gene editing of polo-like kinase I (PLK1), inhibited tumor growth by 50%, and improved the survival rate of mice by 30%. Additionally, they developed EGFR-targeting sgPLK1-cLNPs, which improved overall survival by 80% [105]. Additionally, Qiu et al. [107] described co-delivery of Cas9 mRNA and gRNA for liver-specific in vivo genome editing by designing tail-branched biodegradable lipidoids to explore suitable LNP formulations for liver targeting. They found that 306-O12B LNP showed higher knockdown performance than LNPs with MC-3, which is the gold standard lipid for liver-targeted delivery.
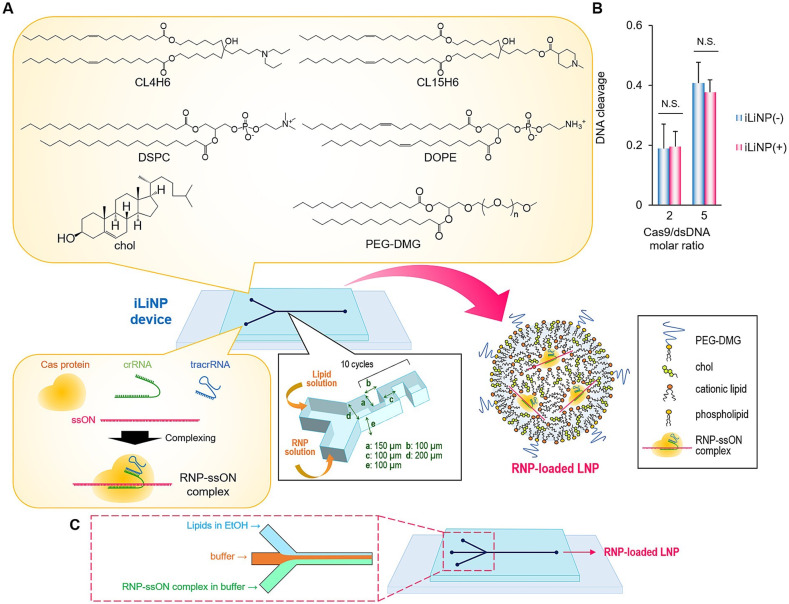
LNP-based CRISPR-Cas9 RNP delivery provides several advantages relative to Cas9 mRNA and sgRNA co-delivery using LNPs. RNP delivery does not require expression of Cas9 protein or complexation of CRISPR, Cas9, and sgRNA in the cell. Therefore, RNPs should enable higher genome-editing efficacy than co-delivery systems, such as Cas9 mRNA/pDNA and sgRNA. Additionally, an RNP-delivery system can minimize off-target effects, because Cas9 protein does not continue to be expressed in cells. However, critical problems include RNP denaturation during the production process and issues with the encapsulation efficiency of large-sized RNPs into LNPs. Specifically, the principle method for microfluidic-based LNP production involves ethanol dilution; however, RNPs denatures upon contact with ethanol. Therefore, rapid dilution of ethanol is important to avoid RNP denaturation. Suzuki et al. [34] reported the delivery of an RNP/single-stranded oligonucleotide (ssON) complex using an iLiNP device. Fig. 12 shows a schematic illustration of the RNP-loaded LNP preparation using a three-inlet iLiNP device. Inclusion of ssON added an additional negative charge following complexation with RNP, thereby promoting LNP encapsulation via strong electrostatic interactions with cationic lipids. The three-inlet iLiNP device reduced RNP denaturation by introducing a buffer solution between the lipid flow stream and the RNP–ssON complex flow stream. Their results showed that the optimized RNP-loaded LNPs successfully convert the EGFP gene sequence into one encoding blue fluorescent protein. Moreover, they demonstrated 95% EGFP knockout using 5 nM of spCas9 RNP in HEK-GFP cells [34]. The optimized RNP-loaded LNPs were subsequently applied for hepatitis B virus (HBV) inhibition, showing effective suppression of both HBV DNA and covalently-closed circular DNA in HBV-infected human liver cells as compared with effects on adeno-associated virus type 2. Based on the advantages of RNP delivery using LNPs, the applications of microfluidic technologies for RNP-loaded LNP production should be expanded in the future.
Fig. 12.
(a) Schematic illustration of RNP-loaded LNPs using the iLiNP device. (b) DNA-cleavage activity of Cas9 before and after introduction into the iLiNP device. (c) Schematic illustration of the three-inlet iLiNP device for RNP-loaded LNP preparation. Reprinted from [34] with the permission of Elsevier.
4. Conclusion and outlook
In this review, we focused on microfluidic approaches for LNP-based RNA-delivery systems and their applications. Microfluidic devices and technologies allow the production of a variety of RNA-encapsulated LNPs, including those for siRNA, mRNA, and RNPs. Compared with conventional RNA-loaded LNP-production methods, microfluidic approaches can produce homogeneous-sized LNPs with high reproducibility. Additionally, optimized LNP-production conditions at a laboratory scale did not change when applied for mass production. These findings suggest that microfluidic devices have accelerated the development of LNP-based nanomedicines.
The LNP-production performance of microfluidics should be improved for vaccine production. Numbering-up and piling-up techniques can be expanded to increase LNP-production rates using microfluidic devices. A PDMS-based microfluidic device is usually used in laboratory studies, because it can be fabricated by rapid prototyping and under reasonable production costs. The pressure resistance and rigidity of the microfluidic device are required for LNP mass production. For this reason, microfluidic devices made from glass or metal are expected to be developed for LNP mass production. For the development of glass or metal-based microfluidic devices, a microchannel design is essential to allow fabrication of micrometer-scale structures via microfabrication processes, such as chemical etching and micromachining. We consider that the simple structure of the iLiNP device is suitable for fabricating glass or metal-based microfluidic devices.
In the future, RNA delivery using microfluidic devices will be indispensable for personalized nanomedicine. In particular, a combination of digital twin and personalized nanomedicine can lead to the next generation of RNA, cell, and gene therapies. We believe that microfluidic technologies are a critical and core technology for next-generation therapies and can potentially promote a global paradigm shift.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
This work was supported by JST CREST (grant No. JPMJCR17H1, Japan), JST PRESTO (grant No. JPMJPR19K8, Japan), JST SCORE (grant No. JPMJST2077, Japan) and the Special Education and Research Expenses from the Ministry of Education, Culture, Sports, Science and Technology, AMED Grant Number JP21zf0127004, JSPS KAKENHI; grant No. JP19KK0140, and the Iketani Science and Technology Foundation.
References
- 1.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021:1–17. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines – a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahl K., Senn J.J., Yuzhakov O., Bulychev A., Brito L.A., Hassett K.J., Laska M.E., Smith M., Almarsson O., Thompson J., Ribeiro A.M., Watson M., Zaks T., Ciaramella G. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol. Ther. 2017;25:1316–1327. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 5.Schoenmaker L., Witzigmann D., Kulkarni J.A., Verbeke R., Kersten G., Jiskoot W., Crommelin D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int. J. Pharm. 2021;601 doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., Myers R., Campbell C.N.J., Amirthalingam G., Edmunds M., Zambon M., Brown K.E., Hopkins S., Chand M., Ramsay M. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukherjee R. Global efforts on vaccines for COVID-19: since, sooner or later, we all will catch the coronavirus. J. Biosci. 2020;45 doi: 10.1007/s12038-020-00040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung Y.H., Beiss V., Fiering S.N., Steinmetz N.F. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14:12522–12537. doi: 10.1021/acsnano.0c07197. [DOI] [PubMed] [Google Scholar]
- 9.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 10.Maeki M., Ito S., Takeda R., Ueno G., Ishida A., Tani H., Yamamoto M., Tokeshi M. Room-temperature crystallography using a microfluidic protein crystal array device and its application to protein-ligand complex structure analysis. Chem. Sci. 2020;11:9072–9087. doi: 10.1039/d0sc02117b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishiyama K., Fukuyama M., Maeki M., Ishida A., Tani H., Hibara A., Tokeshi M. One-step non-competitive fluorescence polarization immunoassay based on a Fab fragment for C-reactive protein quantification. Sensors Actuators B Chem. 2021;326 [Google Scholar]
- 12.Komatsu T., Maeki M., Ishida A., Tani H., Tokeshi M. Paper-based device for the facile colorimetric determination of lithium ions in human whole blood. ACS Sens. 2020;5:1287–1294. doi: 10.1021/acssensors.9b02218. [DOI] [PubMed] [Google Scholar]
- 13.Heikenfeld J., Jajack A., Rogers J., Gutruf P., Tian L., Pan T., Li R., Khine M., Kim J., Wang J., Kim J. Wearable sensors: modalities, challenges, and prospects. Lab Chip. 2018;18:217–248. doi: 10.1039/c7lc00914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J., Gutruf P., Chiarelli A.M., Heo S.Y., Cho K., Xie Z., Banks A., Han S., Jang K.I., Lee J.W., Lee K.T., Feng X., Huang Y., Fabiani M., Gratton G., Paik U., Rogers J.A. Miniaturized battery-free wireless systems for wearable pulse oximetry. Adv. Funct. Mater. 2017;27 doi: 10.1002/adfm.201604373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komatsu T., Sato Y., Maeki M., Ishida A., Tani H., Tokeshi M. Rapid, sensitive universal paper-based device enhances competitive immunoassays of small molecules. Anal. Chim. Acta. 2021;1144:85–95. doi: 10.1016/j.aca.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y., Noviana E., Nguyen M.P., Geiss B.J., Dandy D.S., Henry C.S. Paper-based microfluidic devices: emerging themes and applications. Anal. Chem. 2017;89:71–91. doi: 10.1021/acs.analchem.6b04581. [DOI] [PubMed] [Google Scholar]
- 17.Maeki M., Yamazaki S., Takeda R., Ishida A., Tani H., Tokeshi M. Real-time measurement of protein crystal growth rates within the microfluidic device to understand the microspace effect. ACS Omega. 2020;5:17199–17206. doi: 10.1021/acsomega.0c01285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Y., Lin B., Tian T., Xu X., Wang W., Ruan Q., Guo J., Zhu Z., Yang C. Recent progress in microfluidics-based biosensing. Anal. Chem. 2019;91:388–404. doi: 10.1021/acs.analchem.8b05007. [DOI] [PubMed] [Google Scholar]
- 19.Shi H.H., Xiao Y., Ferguson S., Huang X., Wang N., Hao H.X. Progress of crystallization in microfluidic devices. Lab Chip. 2017;17:2167–2185. doi: 10.1039/c6lc01225f. [DOI] [PubMed] [Google Scholar]
- 20.Choi A., Seo K.D., Kim D.W., Kim B.C., Kim D.S. Recent advances in engineering microparticles and their nascent utilization in biomedical delivery and diagnostic applications. Lab Chip. 2017;17:591–613. doi: 10.1039/c6lc01023g. [DOI] [PubMed] [Google Scholar]
- 21.Kopp M.R.G., Linsenmeier M., Hettich B., Prantl S., Stavrakis S., Leroux J.C., Arosio P. Microfluidic shrinking droplet concentrator for analyte detection and phase separation of protein solutions. Anal. Chem. 2020;92:5803–5812. doi: 10.1021/acs.analchem.9b05329. [DOI] [PubMed] [Google Scholar]
- 22.Chung M.T., Kurabayashi K., Cai D. Single-cell RT-LAMP mRNA detection by integrated droplet sorting and merging. Lab Chip. 2019;19:2425–2434. doi: 10.1039/c9lc00161a. [DOI] [PubMed] [Google Scholar]
- 23.Utharala R., Tseng Q., Furlong E.E.M., Merten C.A., Versatile A. Low-cost, multiway microfluidic sorter for droplets, cells, and embryos. Anal. Chem. 2018;90:5982–5988. doi: 10.1021/acs.analchem.7b04689. [DOI] [PubMed] [Google Scholar]
- 24.Shang L., Cheng Y., Zhao Y. Emerging droplet microfluidics. Chem. Rev. 2017;117:7964–8040. doi: 10.1021/acs.chemrev.6b00848. [DOI] [PubMed] [Google Scholar]
- 25.Liu D., Zhang H., Cito S., Fan J., Makila E., Salonen J., Hirvonen J., Sikanen T.M., Weitz D.A., Santos H.A. Core/Shell nanocomposites produced by superfast sequential microfluidic nanoprecipitation. Nano Lett. 2017;17:606–614. doi: 10.1021/acs.nanolett.6b03251. [DOI] [PubMed] [Google Scholar]
- 26.Yang X.L., Ju X.J., Mu X.T., Wang W., Xie R., Liu Z., Chu L.Y. Core-shell chitosan microcapsules for programmed sequential drug release. ACS Appl. Mater. Interfaces. 2016;8:10524–10534. doi: 10.1021/acsami.6b01277. [DOI] [PubMed] [Google Scholar]
- 27.Maeki M., Kimura N., Sato Y., Harashima H., Tokeshi M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018;128:84–100. doi: 10.1016/j.addr.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Kimura N., Maeki M., Ishida A., Tani H., Tokeshi M. One-step production using a microfluidic device of highly biocompatible size-controlled noncationic exosome-like nanoparticles for RNA delivery. ACS Appl. Bio Mater. 2021;4:1783–1793. doi: 10.1021/acsabm.0c01519. [DOI] [PubMed] [Google Scholar]
- 29.Kimura N., Maeki M., Sato Y., Note Y., Ishida A., Tani H., Harashima H., Tokeshi M. Development of the iLiNP device: fine tuning the lipid nanoparticle size within 10 nm for drug delivery. ACS Omega. 2018;3:5044–5051. doi: 10.1021/acsomega.8b00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeki M., Fujishima Y., Sato Y., Yasui T., Kaji N., Ishida A., Tani H., Baba Y., Harashima H., Tokeshi M. Understanding the formation mechanism of lipid nanoparticles in microfluidic devices with chaotic micromixers. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeki M., Saito T., Sato Y., Yasui T., Kaji N., Ishida A., Tani H., Baba Y., Harashima H., Tokeshi M. A strategy for synthesis of lipid nanoparticles using microfluidic devices with a mixer structure. RSC Adv. 2015;5:46181–46185. [Google Scholar]
- 32.Kimura N., Maeki M., Sato Y., Ishida A., Tani H., Harashima H., Tokeshi M. Development of a microfluidic-based post-treatment process for size-controlled lipid nanoparticles and application to siRNA delivery. ACS Appl. Mater. Interfaces. 2020;12:34011–34020. doi: 10.1021/acsami.0c05489. [DOI] [PubMed] [Google Scholar]
- 33.Kimura N., Maeki M., Sasaki K., Sato Y., Ishida A., Tani H., Harashima H., Tokeshi M. Three-dimensional, symmetrically assembled microfluidic device for lipid nanoparticle production. RSC Adv. 2021;11:1430–1439. doi: 10.1039/d0ra08826a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki Y., Onuma H., Sato R., Sato Y., Hashiba A., Maeki M., Tokeshi M., Kayesh M.E.H., Kohara M., Tsukiyama-Kohara K., Harashima H. Lipid nanoparticles loaded with ribonucleoprotein-oligonucleotide complexes synthesized using a microfluidic device exhibit robust genome editing and hepatitis B virus inhibition. J. Control. Release. 2021;330:61–71. doi: 10.1016/j.jconrel.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Hashiba A., Toyooka M., Sato Y., Maeki M., Tokeshi M., Harashima H. The use of design of experiments with multiple responses to determine optimal formulations for in vivo hepatic mRNA delivery. J. Control. Release. 2020;327:467–476. doi: 10.1016/j.jconrel.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Zhigaltsev I.V., Belliveau N., Hafez I., Leung A.K.K., Huft J., Hansen C., Cullis P.R. Bottom-up design and synthesis of limit size lipid nanoparticle systems with aqueous and triglyceride cores using millisecond microfluidic mixing. Langmuir. 2012;28:3633–3640. doi: 10.1021/la204833h. [DOI] [PubMed] [Google Scholar]
- 37.Belliveau N.M., Huft J., Lin P.J., Chen S., Leung A.K., Leaver T.J., Wild A.W., Lee J.B., Taylor R.J., Tam Y.K., Hansen C.L., Cullis P.R. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Mol. Ther. Nucleic Acids. 2012;1 doi: 10.1038/mtna.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung A.K., Tam Y.Y., Chen S., Hafez I.M., Cullis P.R. Microfluidic mixing: a general method for encapsulating macromolecules in lipid nanoparticle systems. J. Phys. Chem. B. 2015;119:8698–8706. doi: 10.1021/acs.jpcb.5b02891. [DOI] [PubMed] [Google Scholar]
- 39.Chen D., Love K.T., Chen Y., Eltoukhy A.A., Kastrup C., Sahay G., Jeon A., Dong Y., Whitehead K.A., Anderson D.G. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J. Am. Chem. Soc. 2012;134:6948–6951. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- 40.Tahir N., Madni A., Li W., Correia A., Khan M.M., Rahim M.A., Santos H.A. Microfluidic fabrication and characterization of Sorafenib-loaded lipid-polymer hybrid nanoparticles for controlled drug delivery. Int. J. Pharm. 2020;581 doi: 10.1016/j.ijpharm.2020.119275. [DOI] [PubMed] [Google Scholar]
- 41.Li Y., Botker J., Rantanen J., Yang M., Bohr A. In silico design and 3D printing of microfluidic chips for the preparation of size-controllable siRNA nanocomplexes. Int. J. Pharm. 2020;583 doi: 10.1016/j.ijpharm.2020.119388. [DOI] [PubMed] [Google Scholar]
- 42.Aghaei H., Solaimany Nazar A.R., Varshosaz J. Double flow focusing microfluidic-assisted based preparation of methotrexate–loaded liposomal nanoparticles: encapsulation efficacy, drug release and stability. Colloids Surf. A Physicochem. Eng. Asp. 2021;614 [Google Scholar]
- 43.Lopez R.R., G.F.d.R. P, Sanchez L.M., Tsering T., Alazzam A., Bergeron K.F., Mounier C., Burnier J.V., Stiharu I., Nerguizian V. The effect of different organic solvents in liposome properties produced in a periodic disturbance mixer: Transcutol(R), a potential organic solvent replacement. Colloids Surf. B: Biointerfaces. 2021;198 doi: 10.1016/j.colsurfb.2020.111447. [DOI] [PubMed] [Google Scholar]
- 44.Lopez R.R., Ocampo I., Font de Rubinat P.G., Sanchez L.M., Alazzam A., Tsering T., Bergeron K.F., Camacho-Leon S., Burnier J.V., Mounier C., Stiharu I., Nerguizian V. Parametric study of the factors influencing liposome physicochemical characteristics in a periodic disturbance mixer. Langmuir. 2021;37:8544–8556. doi: 10.1021/acs.langmuir.1c01005. [DOI] [PubMed] [Google Scholar]
- 45.Arduino I., Liu Z., Rahikkala A., Figueiredo P., Correia A., Cutrignelli A., Denora N., Santos H.A. Preparation of cetyl palmitate-based PEGylated solid lipid nanoparticles by microfluidic technique. Acta Biomater. 2021;121:566–578. doi: 10.1016/j.actbio.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 46.Modarres P., Tabrizian M. Electrohydrodynamic-driven micromixing for the synthesis of highly monodisperse nanoscale liposomes. ACS Appl. Nano Mater. 2020;3:4000–4013. [Google Scholar]
- 47.Bolze H., Riewe J., Bunjes H., Dietzel A., Burg T.P. Continuous production of lipid nanoparticles by ultrasound-assisted microfluidic antisolvent precipitation. Chem. Eng. Technol. 2021;44:1641–1650. [Google Scholar]
- 48.Firmino P., Vianna S.S.V., da Costa O., Malfatti-Gasperini A.A., Gobbi A.L., Lima R.S., de la Torre L.G. 3D micromixer for nanoliposome synthesis: a promising advance in high mass productivity. Lab Chip. 2021;21:2971–2985. doi: 10.1039/d1lc00232e. [DOI] [PubMed] [Google Scholar]
- 49.Webb C., Forbes N., Roces C.B., Anderluzzi G., Lou G., Abraham S., Ingalls L., Marshall K., Leaver T.J., Watts J.A., Aylott J.W., Perrie Y. Using microfluidics for scalable manufacturing of nanomedicines from bench to GMP: a case study using protein-loaded liposomes. Int. J. Pharm. 2020;582 doi: 10.1016/j.ijpharm.2020.119266. [DOI] [PubMed] [Google Scholar]
- 50.Khadke S., Roces C.B., Cameron A., Devitt A., Perrie Y. Formulation and manufacturing of lymphatic targeting liposomes using microfluidics. J. Control. Release. 2019;307:211–220. doi: 10.1016/j.jconrel.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Joshi S., Hussain M.T., Roces C.B., Anderluzzi G., Kastner E., Salmaso S., Kirby D.J., Perrie Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016;514:160–168. doi: 10.1016/j.ijpharm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 52.Akinc A., Maier M.A., Manoharan M., Fitzgerald K., Jayaraman M., Barros S., Ansell S., Du X., Hope M.J., Madden T.D., Mui B.L., Semple S.C., Tam Y.K., Ciufolini M., Witzigmann D., Kulkarni J.A., van der Meel R., Cullis P.R. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019;14:1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 53.Jahn A., Vreeland W.N., Gaitan M., Locascio L.E. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J. Am. Chem. Soc. 2004;126:2674. doi: 10.1021/ja0318030. [DOI] [PubMed] [Google Scholar]
- 54.Jahn A., Stavis S.M., Hong J.S., Vreeland W.N., DeVoe D.L., Gaitan M. Microfluidic mixing and the formation of nanoscale lipid vesicles. ACS Nano. 2010;4:2077–2087. doi: 10.1021/nn901676x. [DOI] [PubMed] [Google Scholar]
- 55.Hood R.R., DeVoe D.L. High-throughput continuous flow production of nanoscale liposomes by microfluidic vertical flow focusing. Small. 2015;11:5790–5799. doi: 10.1002/smll.201501345. [DOI] [PubMed] [Google Scholar]
- 56.Sato Y., Note Y., Maeki M., Kaji N., Baba Y., Tokeshi M., Harashima H. Elucidation of the physicochemical properties and potency of siRNA-loaded small-sized lipid nanoparticles for siRNA delivery. J. Control. Release. 2016;229:48–57. doi: 10.1016/j.jconrel.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 57.Stroock A.D., Dertinger S.K., Ajdari A., Mezic I., Stone H.A., Whitesides G.M. Chaotic mixer for microchannels. Science. 2002;295:647–651. doi: 10.1126/science.1066238. [DOI] [PubMed] [Google Scholar]
- 58.Xia G., Li J., Tian X., Zhou M. Analysis of flow and mixing characteristics of planar asymmetric split-and-recombine (P-SAR) micromixers with Fan-shaped cavities. Ind. Eng. Chem. Res. 2012;51:7816–7827. [Google Scholar]
- 59.Hood R.R., DeVoe D.L., Atencia J., Vreeland W.N., Omiatek D.M. A facile route to the synthesis of monodisperse nanoscale liposomes using 3D microfluidic hydrodynamic focusing in a concentric capillary array. Lab Chip. 2014;14:2403–2409. doi: 10.1039/c4lc00334a. [DOI] [PubMed] [Google Scholar]
- 60.Roces C.B., Lou G., Jain N., Abraham S., Thomas A., Halbert G.W., Perrie Y. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hassett K.J., Higgins J., Woods A., Levy B., Xia Y., Hsiao C.J., Acosta E., Almarsson O., Moore M.J., Brito L.A. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J. Control. Release. 2021;335:237–246. doi: 10.1016/j.jconrel.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 62.Abrams M.T., Koser M.L., Seitzer J., Williams S.C., DiPietro M.A., Wang W., Shaw A.W., Mao X., Jadhav V., Davide J.P., Burke P.A., Sachs A.B., Stirdivant S.M., Sepp-Lorenzino L. Evaluation of efficacy, biodistribution, and inflammation for a potent siRNA nanoparticle: effect of dexamethasone co-treatment. Mol. Ther. 2010;18:171–180. doi: 10.1038/mt.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kulkarni J.A., Darjuan M.M., Mercer J.E., Chen S., van der Meel R., Thewalt J.L., Tam Y.Y.C., Cullis P.R. On the formation and morphology of lipid nanoparticles containing Ionizable cationic lipids and siRNA. ACS Nano. 2018;12:4787–4795. doi: 10.1021/acsnano.8b01516. [DOI] [PubMed] [Google Scholar]
- 64.Chen S., Tam Y.Y.C., Lin P.J.C., Sung M.M.H., Tam Y.K., Cullis P.R. Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA. J. Control. Release. 2016;235:236–244. doi: 10.1016/j.jconrel.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 65.Kumar V., Qin J., Jiang Y., Duncan R.G., Brigham B., Fishman S., Nair J.K., Akinc A., Barros S.A., Kasperkovitz P.V. Shielding of lipid nanoparticles for siRNA delivery: impact on physicochemical properties, cytokine induction, and efficacy. Mol Ther Nucleic Acids. 2014;3 doi: 10.1038/mtna.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jia Z., Gong Y., Pi Y., Liu X., Gao L., Kang L., Wang J., Yang F., Tang J., Lu W., Li Q., Zhang W., Yan Z., Yu L. pPB peptide-mediated siRNA-loaded stable nucleic acid lipid nanoparticles on targeting therapy of hepatic fibrosis. Mol. Pharm. 2018;15:53–62. doi: 10.1021/acs.molpharmaceut.7b00709. [DOI] [PubMed] [Google Scholar]
- 67.Lee J.B., Zhang K., Tam Y.Y.C., Quick J., Tam Y.K., Lin P.J., Chen S., Liu Y., Nair J.K., Zlatev I., Rajeev K.G., Manoharan M., Rennie P.S., Cullis P.R. A Glu-urea-Lys ligand-conjugated lipid nanoparticle/siRNA system inhibits androgen receptor expression in vivo. Mol. Ther. Nucleic Acids. 2016;5 doi: 10.1038/mtna.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei W., Sun J., Guo X.Y., Chen X., Wang R., Qiu C., Zhang H.T., Pang W.H., Wang J.C., Zhang Q. Microfluidic-based holonomic constraints of siRNA in the kernel of lipid/polymer hybrid nanoassemblies for improving stable and safe in vivo delivery. ACS Appl. Mater. Interfaces. 2020;12:14839–14854. doi: 10.1021/acsami.9b22781. [DOI] [PubMed] [Google Scholar]
- 69.Shepherd S.J., Warzecha C.C., Yadavali S., El-Mayta R., Alameh M.G., Wang L., Weissman D., Wilson J.M., Issadore D., Mitchell M.J. Scalable mRNA and siRNA lipid nanoparticle production using a parallelized microfluidic device. Nano Lett. 2021;21:5671–5680. doi: 10.1021/acs.nanolett.1c01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Younis M.A., Khalil I.A., Elewa Y.H.A., Kon Y., Harashima H. Ultra-small lipid nanoparticles encapsulating sorafenib and midkine-siRNA selectively-eradicate sorafenib-resistant hepatocellular carcinoma in vivo. J. Control. Release. 2021;331:335–349. doi: 10.1016/j.jconrel.2021.01.021. [DOI] [PubMed] [Google Scholar]
- 71.Terada T., Kulkarni J.A., Huynh A., Chen S., van der Meel R., Tam Y.Y.C., Cullis P.R. Characterization of lipid nanoparticles containing ionizable cationic lipids using design-of-experiments approach. Langmuir. 2021;37:1120–1128. doi: 10.1021/acs.langmuir.0c03039. [DOI] [PubMed] [Google Scholar]
- 72.Li Y., Lee R.J., Huang X., Li Y., Lv B., Wang T., Qi Y., Hao F., Lu J., Meng Q., Teng L., Zhou Y., Xie J., Teng L. Single-step microfluidic synthesis of transferrin-conjugated lipid nanoparticles for siRNA delivery. Nanomedicine. 2017;13:371–381. doi: 10.1016/j.nano.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 73.Kon E., Hazan-Halevy I., Rosenblum D., Cohen N., Chatterjee S., Veiga N., Raanani P., Bairey O., Benjamini O., Nagler A., Peer D. Resveratrol enhances mRNA and siRNA lipid nanoparticles primary CLL cell transfection. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramishetti S., Kedmi R., Goldsmith M., Leonard F., Sprague A.G., Godin B., Gozin M., Cullis P.R., Dykxhoorn D.M., Peer D. Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano. 2015;9:6706–6716. doi: 10.1021/acsnano.5b02796. [DOI] [PubMed] [Google Scholar]
- 75.Zhou K., Johnson L.T., Xiong H., Barrios S., Minnig J.T., Yan Y., Abram B., Yu X., Siegwart D.J. Hydrophobic domain structure of linear-dendritic poly(ethylene glycol) lipids affects RNA delivery of lipid nanoparticles. Mol. Pharm. 2020;17:1575–1585. doi: 10.1021/acs.molpharmaceut.9b01288. [DOI] [PubMed] [Google Scholar]
- 76.Basha G., Ordobad M., Scott W.R., Cottle A., Liu Y., Wang H., Cullis P.R. Lipid nanoparticle delivery of siRNA to osteocytes leads to effective silencing of SOST and inhibition of sclerostin in vivo. Mol. Ther. Nucleic Acids. 2016;5:2363. doi: 10.1038/mtna.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen S., Tam Y.Y., Lin P.J., Leung A.K., Tam Y.K., Cullis P.R. Development of lipid nanoparticle formulations of siRNA for hepatocyte gene silencing following subcutaneous administration. J. Control. Release. 2014;196:106–112. doi: 10.1016/j.jconrel.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 78.Eş I., Ok M.T., Puentes-Martinez X.E., de Toledo M.A.S., de Pinho Favaro M.T., Cavalcanti L.P., Cassago A., Portugal R.V., Azzoni A.R., de la Torre L.G. Evaluation of siRNA and cationic liposomes complexes as a model for in vitro siRNA delivery to cancer cells. Colloids Surf. A Physicochem. Eng. Asp. 2018;555:280–289. [Google Scholar]
- 79.Kulkarni J.A., Witzigmann D., Leung J., Tam Y.Y.C., Cullis P.R. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale. 2019;11:21733–21739. doi: 10.1039/c9nr09347h. [DOI] [PubMed] [Google Scholar]
- 80.Kulkarni J.A., Thomson S.B., Zaifman J., Leung J., Wagner P.K., Hill A., Tam Y.Y.C., Cullis P.R., Petkau T.L., Leavitt B.R. Spontaneous, solvent-free entrapment of siRNA within lipid nanoparticles. Nanoscale. 2020;12:23959–23966. doi: 10.1039/d0nr06816k. [DOI] [PubMed] [Google Scholar]
- 81.Kauffman K.J., Dorkin J.R., Yang J.H., Heartlein M.W., DeRosa F., Mir F.F., Fenton O.S., Anderson D.G. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15:7300–7306. doi: 10.1021/acs.nanolett.5b02497. [DOI] [PubMed] [Google Scholar]
- 82.Sabnis S., Kumarasinghe E.S., Salerno T., Mihai C., Ketova T., Senn J.J., Lynn A., Bulychev A., McFadyen I., Chan J., Almarsson O., Stanton M.G., Benenato K.E. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018;26:1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel S., Ashwanikumar N., Robinson E., Xia Y., Mihai C., Griffith J.P., 3rd, Hou S., Esposito A.A., Ketova T., Welsher K., Joyal J.L., Almarsson O., Sahay G. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020;11:983. doi: 10.1038/s41467-020-14527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020;15:313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fenton O.S., Kauffman K.J., Kaczmarek J.C., McClellan R.L., Jhunjhunwala S., Tibbitt M.W., Zeng M.D., Appel E.A., Dorkin J.R., Mir F.F., Yang J.H., Oberli M.A., Heartlein M.W., DeRosa F., Langer R., Anderson D.G. Synthesis and biological evaluation of Ionizable lipid materials for the in vivo delivery of messenger RNA to B lymphocytes. Adv. Mater. 2017;29:1606944. doi: 10.1002/adma.201606944. [DOI] [PubMed] [Google Scholar]
- 86.Kowalski P.S., Capasso Palmiero U., Huang Y., Rudra A., Langer R., Anderson D.G. Ionizable amino-polyesters synthesized via ring opening polymerization of tertiary amino-alcohols for tissue selective mRNA delivery. Adv. Mater. 2018;30:1801151. doi: 10.1002/adma.201801151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kauffman K.J., Mir F.F., Jhunjhunwala S., Kaczmarek J.C., Hurtado J.E., Yang J.H., Webber M.J., Kowalski P.S., Heartlein M.W., DeRosa F., Anderson D.G. Efficacy and immunogenicity of unmodified and pseudouridine-modified mRNA delivered systemically with lipid nanoparticles in vivo. Biomaterials. 2016;109:78–87. doi: 10.1016/j.biomaterials.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Billingsley M.M., Singh N., Ravikumar P., Zhang R., June C.H., Mitchell M.J. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20:1578–1589. doi: 10.1021/acs.nanolett.9b04246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hassett K.J., Benenato K.E., Jacquinet E., Lee A., Woods A., Yuzhakov O., Himansu S., Deterling J., Geilich B.M., Ketova T., Mihai C., Lynn A., McFadyen I., Moore M.J., Senn J.J., Stanton M.G., Almarsson O., Ciaramella G., Brito L.A. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.An D., Schneller J.L., Frassetto A., Liang S., Zhu X., Park J.S., Theisen M., Hong S.J., Zhou J., Rajendran R., Levy B., Howell R., Besin G., Presnyak V., Sabnis S., Murphy-Benenato K.E., Kumarasinghe E.S., Salerno T., Mihai C., Lukacs C.M., Chandler R.J., Guey L.T., Venditti C.P., Martini P.G.V. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2017;21:3548–3558. doi: 10.1016/j.celrep.2017.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang L., Berraondo P., Jerico D., Guey L.T., Sampedro A., Frassetto A., Benenato K.E., Burke K., Santamaria E., Alegre M., Pejenaute A., Kalariya M., Butcher W., Park J.S., Zhu X., Sabnis S., Kumarasinghe E.S., Salerno T., Kenney M., Lukacs C.M., Avila M.A., Martini P.G.V., Fontanellas A. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat. Med. 2018;24:1899–1909. doi: 10.1038/s41591-018-0199-z. [DOI] [PubMed] [Google Scholar]
- 92.Richner J.M., Himansu S., Dowd K.A., Butler S.L., Salazar V., Fox J.M., Julander J.G., Tang W.W., Shresta S., Pierson T.C., Ciaramella G., Diamond M.S. Modified mRNA vaccines protect against Zika virus infection. Cell. 2017;168:1114–1125 e1110. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramaswamy S., Tonnu N., Tachikawa K., Limphong P., Vega J.B., Karmali P.P., Chivukula P., Verma I.M. Systemic delivery of factor IX messenger RNA for protein replacement therapy. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E1941–E1950. doi: 10.1073/pnas.1619653114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Riley R.S., Kashyap M.V., Billingsley M.M., White B., Alameh M.G., Bose S.K., Zoltick P.W., Li H., Zhang R., Cheng A.W., Weissman D., Peranteau W.H., Mitchell M.J. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci. Adv. 2021;7:eaba1028. doi: 10.1126/sciadv.aba1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li B., Luo X., Deng B., Wang J., McComb D.W., Shi Y., Gaensler K.M., Tan X., Dunn A.L., Kerlin B.A., Dong Y. An orthogonal array optimization of lipid-like nanoparticles for mRNA delivery in vivo. Nano Lett. 2015;15:8099–8107. doi: 10.1021/acs.nanolett.5b03528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nogueira S.S., Schlegel A., Maxeiner K., Weber B., Barz M., Schroer M.A., Blanchet C.E., Svergun D.I., Ramishetti S., Peer D., Langguth P., Sahin U., Haas H. Polysarcosine-functionalized lipid nanoparticles for therapeutic mRNA delivery. ACS Appl. Nano Mater. 2020;3:10634–10645. [Google Scholar]
- 97.Luo X., Li B., Zhang X., Zhao W., Bratasz A., Deng B., McComb D.W., Dong Y. Dual-functional lipid-like nanoparticles for delivery of mRNA and MRI contrast agents. Nanoscale. 2017;9:1575–1579. doi: 10.1039/c6nr08496f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sebastiani F., Yanez Arteta M., Lerche M., Porcar L., Lang C., Bragg R.A., Elmore C.S., Krishnamurthy V.R., Russell R.A., Darwish T., Pichler H., Waldie S., Moulin M., Haertlein M., Forsyth V.T., Lindfors L., Cardenas M. Apolipoprotein E binding drives structural and compositional rearrangement of mRNA-containing lipid nanoparticles. ACS Nano. 2021;15:6709–6722. doi: 10.1021/acsnano.0c10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elia U., Ramishetti S., Rosenfeld R., Dammes N., Bar-Haim E., Naidu G.S., Makdasi E., Yahalom-Ronen Y., Tamir H., Paran N., Cohen O., Peer D. Design of SARS-CoV-2 hFc-conjugated receptor-binding domain mRNA vaccine delivered via lipid nanoparticles. ACS Nano. 2021;15:9627–9637. doi: 10.1021/acsnano.0c10180. [DOI] [PubMed] [Google Scholar]
- 100.Sridhar S., Luedtke A., Langevin E., Zhu M., Bonaparte M., Machabert T., Savarino S., Zambrano B., Moureau A., Khromava A., Moodie Z., Westling T., Mascarenas C., Frago C., Cortes M., Chansinghakul D., Noriega F., Bouckenooghe A., Chen J., Ng S.P., Gilbert P.B., Gurunathan S., DiazGranados C.A. Effect of dengue Serostatus on dengue vaccine safety and efficacy. N. Engl. J. Med. 2018;379:327–340. doi: 10.1056/NEJMoa1800820. [DOI] [PubMed] [Google Scholar]
- 101.Miller J.B., Zhang S., Kos P., Xiong H., Zhou K., Perelman S.S., Zhu H., Siegwart D.J. Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew. Chem. Int. Ed. Eng. 2017;56:1059–1063. doi: 10.1002/anie.201610209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang C., Mei M., Li B., Zhu X., Zu W., Tian Y., Wang Q., Guo Y., Dong Y., Tan X. A non-viral CRISPR/Cas9 delivery system for therapeutically targeting HBV DNA and pcsk9 in vivo. Cell Res. 2017;27:440–443. doi: 10.1038/cr.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang X., Li B., Luo X., Zhao W., Jiang J., Zhang C., Gao M., Chen X., Dong Y. Biodegradable amino-ester nanomaterials for Cas9 mRNA delivery in vitro and in vivo. ACS Appl. Mater. Interfaces. 2017;9:25481–25487. doi: 10.1021/acsami.7b08163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Finn J.D., Smith A.R., Patel M.C., Shaw L., Youniss M.R., van Heteren J., Dirstine T., Ciullo C., Lescarbeau R., Seitzer J., Shah R.R., Shah A., Ling D., Growe J., Pink M., Rohde E., Wood K.M., Salomon W.E., Harrington W.F., Dombrowski C., Strapps W.R., Chang Y., Morrissey D.V. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 2018;22:2227–2235. doi: 10.1016/j.celrep.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 105.Rosenblum D., Gutkin A., Kedmi R., Ramishetti S., Veiga N., Jacobi A.M., Schubert M.S., Friedmann-Morvinski D., Cohen Z.R., Behlke M.A., Lieberman J., D. Peer, CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020;6:eabc9450. doi: 10.1126/sciadv.abc9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tanaka H., Takahashi T., Konishi M., Takata N., Gomi M., Shirane D., Miyama R., Hagiwara S., Yamasaki Y., Sakurai Y., Ueda K., Higashi K., Moribe K., Shinsho E., Nishida R., Fukuzawa K., Yonemochi E., Okuwaki K., Mochizuki Y., Nakai Y., Tange K., Yoshioka H., Tamagawa S., Akita H. Self-degradable lipid-like materials based on “hydrolysis accelerated by the intra-particle enrichment of reactant (HyPER)” for messenger RNA delivery. Adv. Funct. Mater. 2020;30:1910575. [Google Scholar]
- 107.Qiu M., Glass Z., Chen J., Haas M., Jin X., Zhao X., Rui X., Ye Z., Li Y., Zhang F., Xu Q. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2020401118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yin H., Song C.Q., Dorkin J.R., Zhu L.J., Li Y., Wu Q., Park A., Yang J., Suresh S., Bizhanova A., Gupta A., Bolukbasi M.F., Walsh S., Bogorad R.L., Gao G., Weng Z., Dong Y., Koteliansky V., Wolfe S.A., Langer R., Xue W., Anderson D.G. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat. Biotechnol. 2016;34:328–333. doi: 10.1038/nbt.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yin H., Song C.Q., Suresh S., Wu Q., Walsh S., Rhym L.H., Mintzer E., Bolukbasi M.F., Zhu L.J., Kauffman K., Mou H., Oberholzer A., Ding J., Kwan S.Y., Bogorad R.L., Zatsepin T., Koteliansky V., Wolfe S.A., Xue W., Langer R., Anderson D.G. Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nat. Biotechnol. 2017;35:1179–1187. doi: 10.1038/nbt.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li X., Feng Q., Han Z., Jiang X. Enhancing gene editing efficiency for cells by CRISPR/Cas9 system-loaded multilayered nanoparticles assembled via microfluidics. Chin. J. Chem. Eng. 2021;38:216–220. [Google Scholar]