Abstract
Context
The growing number of systematic reviews/meta-analyses (SR/MAs) on vitamin D (± calcium) for fracture prevention has led to contradictory guidelines.
Objective
This umbrella review aims to assess the quality and explore the reasons for the discrepancy of SR/MAs of trials on vitamin D supplementation for fracture risk reduction in adults.
Methods
We searched 4 databases (2010-2020), Epistemonikos, and references of included SRs/MAs, and we contacted experts in the field. We used A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR-2) for quality assessment. We compared results and investigated reasons for discordance using matrices and subgroup analyses (PROSPERO registration: CRD42019129540). We included 13 SR/MAs on vitamin D and calcium (Ca/D) and 19 SR/MAs on vitamin D alone, compared to placebo/control.
Results
Only 2 from 10 SRs/MAs on Ca/D were of moderate quality. Ca/D reduced the risk of hip fractures in 8 of 12 SRs/MAs (relative risk [RR] 0.61-0.84), and any fractures in 7 of 11 SR/MAs (RR 0.74-0.95). No fracture risk reduction was noted in SRs/MAs exclusively evaluating community-dwelling individuals or in those on vitamin D alone compared to placebo/control. Discordance in results between SRs/MAs stems from inclusion of different trials, related to search periods and eligibility criteria, and varying methodology (using intention to treat, per-protocol, or complete case analysis from individual trials).
Conclusion
Ca/D reduces the risk of hip and any fractures, possibly driven by findings from institutionalized individuals. Individual participant data meta-analyses of patients on Ca/D with sufficient follow-up periods, and subgroup analyses, would unravel determinants for a beneficial response to supplementation.
Keywords: vitamin D, fractures, adults, umbrella review
Vitamin D plays a critical role in musculoskeletal health through its effects on mineral homeostasis and bone metabolism (1, 2). Vitamin D deficiency is common among older individuals because of reduced cholecalciferol synthesis in the skin, reduced intestinal calcium (Ca) absorption, and changes in lifestyle favoring lower exposure to ultraviolet radiation (3). This deficiency may contribute to the observed steep rise in the risk of fractures with older age, particularly at the hip (4). This is of particular importance given that the estimated 1-year mortality following hip fracture is around 30% (5).
The benefit of vitamin D supplementation on fracture prevention has been extensively assessed, with an exponential rise in the number of systematic reviews/meta-analyses (SRs/MAs) reporting discordant conclusions (6). A recent review in 2017 identified more than 40 international vitamin D guidelines with highly variable recommendations (7). There is still conflicting evidence with regards to the extent of vitamin D’s benefit in fracture prevention, the target population likely to benefit the most, the desirable serum 25-hydroxyvitamin D (25[OH]D) concentration, the optimal vitamin D dose, and the need for coadministration of Ca (7-9).
We therefore conducted an umbrella review of SRs/MAs of vitamin D supplementation randomized clinical trials (RCTs) evaluating fracture risk reduction in adults, to assess the quality of each SR/MA, explore similarities and differences between them, investigate the reasons for any discrepancy, and formulate a reliable conclusion on the topic. Such an approach would bring clarity to much confusion, paving the path for the formulation of informed population-tailored conclusions regarding the benefit of vitamin D supplementation in preventing fractures.
Methods and Literature Search
We followed the guidance provided by Pollock et al (10) to develop the protocol for this umbrella review. We registered the protocol on PROSPERO (CRD42019129540) (11). We included SRs/MAs of RCTs of adults aged 18 years and older evaluating the risk of hip and any fracture, with vitamin D supplementation, alone or in combination with Ca, compared with placebo/control.
We searched MEDLINE, PubMed, Embase, Cochrane, and Epistemonikos databases, without language restriction, from January 1, 2010 until October 23, 2020, with the help of a medical librarian (A.F.), to cover the reviews published following vitamin D guidelines issued by 2 major societies, the Institute of Medicine in 2009 (9) and the Endocrine Society in 2011 (8).We used Medical Subject Headings terms and keywords relevant to vitamin D, fractures, meta-analysis, systematic review, and randomized controlled trials. We reviewed the citations of included SRs/MAs and of narrative reviews on the topic, and contacted experts in the field (Supplementary data 1) (12).
Pairs of reviewers (C.G. and F.K., D.B. and S.A., Y.J. and M.A., A.B. and S.A.) completed title/abstract and full-text screening, and data abstraction in duplicate and independently, after a calibration exercise. Disagreement was resolved through discussion with a content expert (G.E.H.F. or M.C.).
We collected information on study population, intervention(s), comparator(s), and outcome(s), in addition to the methodology of each SR/MA. We constructed, for each of hip and any fractures, figures displaying the effect size estimates derived from each SR/MA, 95% CIs, and degree of heterogeneity using I2. We constructed matrices to compare and contrast the SR/MAs’ respective results, including RCTs and respective data, and quality ratings. We calculated the “corrected covered area” (CCA) for each of hip and any fracture, as suggested by Pieper et al (13), using the following formula: CCA = (N – r)/[rc – r], where N is the total number of included publications (including double counting) as displayed in the matrix for all the SR/MA, r is the number of RCTs included at least once in the matrix, and c is the number of SR/MAs. We also collected data on subgroup analyses by baseline serum 25(OH)D concentration (prespecified), and on other subgroups as available in each SR/MA.
We assessed the quality of SR/MAs independently in triplicate using A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR-2) (14).
Results
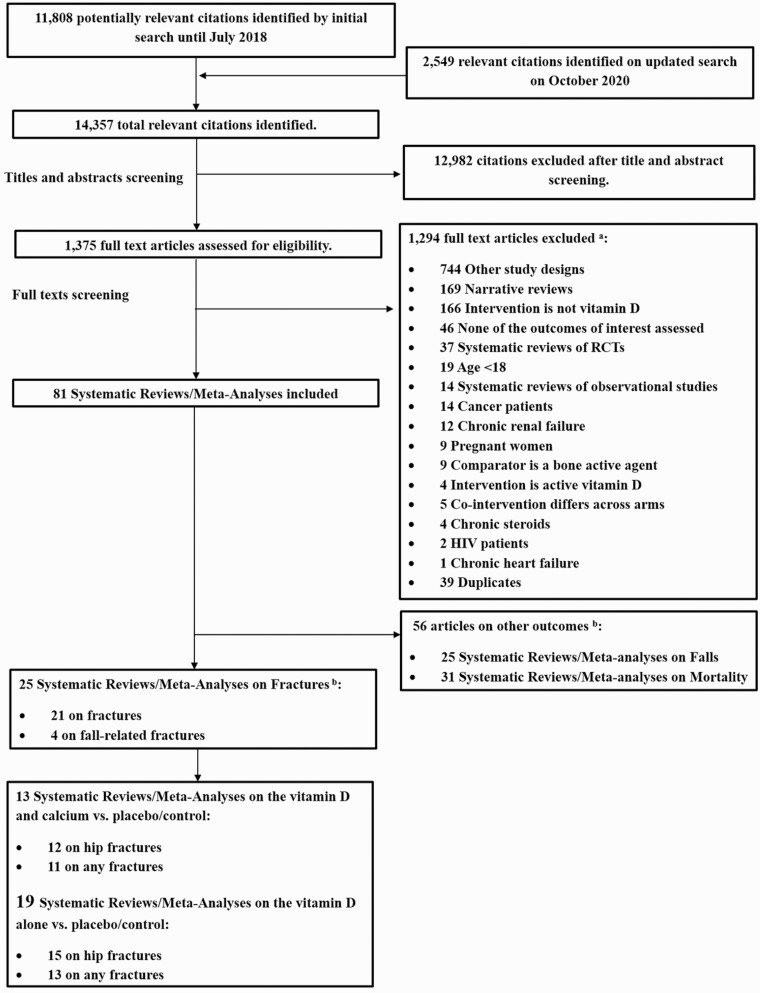
The search strategy retrieved 14 357 citations. We reviewed 1375 full-text papers. We identified 81 SRs/MAs of RCTs on vitamin D supplementation, 25 of which assessed fractures (n = 21) (15-35) or fall-related fractures (n = 4) (36-39), all in the English language. We included SRs/MAs on vitamin D with concomitant Ca supplementation (Ca/D) (13 SR/MAs) (15-17, 19, 22-24, 28-31, 37, 39), or vitamin D alone (19 SR/MAs) (15, 16, 19-21, 23-28, 30-32, 34-37, 39), compared to placebo/control; 9 SR/MAs included other comparisons (15, 17, 18, 20, 21, 33, 37-39) that were beyond the focus of this manuscript (Fig. 1).
Figure 1.
Flow diagram of the included studies. aList of excluded articles is available on request. bThese will be included in separate manuscripts.
Results on Calcium and Vitamin D Supplementation vs Placebo/Control
We identified 12 SRs/MAs assessing hip fracture risk and 11 assessing any fracture risk, with Ca/D supplementation compared with placebo/control, including one individual participant data meta-analysis (IPD-MA) (23). The mean age of participants ranged between 62 and 85 years. The vitamin D dose was 400 to 800 IU/day, and 6 SR/MAs included 1 trial providing a high dose of 300 000 IU once (15, 16, 19, 24, 31, 37). The Ca dose was 500 to 1200 mg/day. The included trials extended from 1 to 7 years. Five SRs/MAs reported on baseline 25(OH)D concentration (20.9-83.8 nmol/L) (15, 19, 24, 31, 37), and the achieved concentration (15-112.3 nmol/L) (19) (Table 1).
Table 1.
Characteristics of populations and interventions in the included systematic reviews/meta-analyses on vitamin D and concomitant calcium supplementation compared to placebo/control
| Characteristics of populations and interventions | No. (%) or range |
|---|---|
| Mean age, y | 62-85.2 |
| Sex | |
| • Men and women | 9 (69) |
| • Women only | 2 (15) |
| • Not reported | 2 (15) |
| Residency status | |
| • Community-dwelling only | 3 (23) |
| • Institutionalized only | 0 (0) |
| • Both | 8 (62) |
| • Not reported | 2 (15) |
| Mean serum 25(OH)D concentration at baseline, nmol/L | 11.9-84 |
| • Reported | 5 (38) |
| • Not reported | 8 (62) |
| Mean serum 25(OH)D concentration at follow-up, nmol/L | 15.0-112 |
| • Reporteda | 1 (8) |
| • Not reported | 12 (92) |
| Vitamin D dose frequency | |
| • Daily, IU/d | 400-1600 |
| • Once, IU | 300 000 |
| Calcium dose, mg/d | 500-1200 |
| • Daily | |
| Vitamin D route of administration | |
| • Oral supplementation only | 1 (8) |
| • Oral and parenteral supplementation | 4 (31) |
| • Administration mode not mentioned | 8 (62) |
| Individual trial duration | |
| • SR/MA including ≥ 1 trial < 12 mo | 0 (0) |
| • SR/MA including all trials ≥ 12 mo | 9 (69) |
| • Not reported | 4 (31) |
| Fracture siteb | |
| • Hip | 12 |
| • Total/Any/Other | 11 |
| • Vertebral | 6 |
| • Nonvertebral | 5 |
| SR/MAs with trials on individuals at high risk of fracturec | 9 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; IU, international units; SR/MA, systematic review and meta-analysis.
a Yao et al (30) reported change rather than follow up 25-hydroxyvitamin D concentration.
b Some MAs may include more than one fracture site.
c Previous fracture or osteopenia/osteoporosis.
Hip fractures
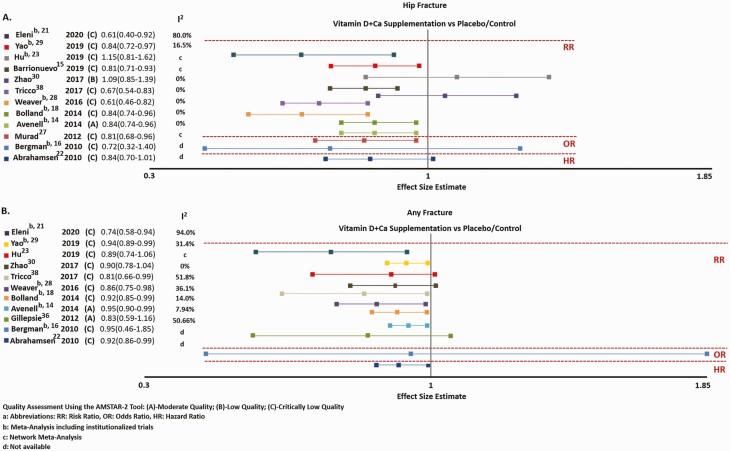
Most SRs/MAs had the same direction, but were discrepant both in magnitude of effect and statistical significance. Eight of 12 SRs/MAs reported a significant reduction in hip fracture risk when combining institutionalized and community-dwelling individuals (15, 16, 19, 22, 28-30, 39) (Fig. 2A). Only one Cochrane SR/MA, by Avenell et al (15), was of moderate quality, and was the only one evaluating the quality of evidence, reported as high, with a 16% relative risk reduction (RRR) in hip fracture, with Ca/D supplementation compared with placebo/control. The other 7 SR/MAs, of critically low quality, showed a 16% to 39% RRR in hip fracture (16, 19, 22, 28-30, 39). Two other SR/MAs (17, 23), including 1 IPD-MA (23), and the 2 SRs/MAs that exclusively evaluated RCTs in community-dwelling individuals (24, 31), failed to show any significant fracture reduction. They were of low (31) to critically low quality (17, 23, 24) (Supplementary data 2) (12).
Figure 2.
Effect size estimates and 95% CI for A, hip, and B, any fracture risk with vitamin D and calcium supplementation vs placebo/control. Quality assessment using A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR-2) tool described between parenthesis: A, moderate quality; B, low quality; and C, critically low quality. aHR, hazard ratio; OR, odds ratio; RR, risk ratio. bMeta-analyses that included institutionalized trials. cNetwork meta-analyses. dNot available.
Nine SRs/MAs on hip fracture were included in the matrix because they provided the needed information (Table 2). The population consisted of older women and/or men, with (40-44) or without a history of fracture (45-49). The Women’s Health Initiative (WHI) trial (N = 36 282, Ca/D dose per day 1000 mg/400 IU) (48) and the RECORD trial (N = 5292, Ca/D dose per day 1000 mg/800 IU) (41) contributed with the largest weights for the findings in the community. The 2 trials by Chapuy et al (45, 46) were the only trials on institutionalized individuals included in all SRs/MAs (N = 3847, Ca/D dose per day 1200 mg/800 IU) (see Table 2).
Table 2.
Comparison of individual trials included in traditional systematic reviews/meta-analysesa and reasons for exclusionb of trials by some systematic reviews/meta-analyses investigating hip fracture risk with calcium and vitamin D supplementation
| Bergman et al (17) 2010 |
Avenell et al (15) 2014 |
Bolland et al (19) 2014 |
Weaver et al (29) 2016 |
Zhao et al (31) 2017 |
Barrionuevo et al (16) 2019 |
Hu et al (24) 2019 |
Yao et al (30) 2019 |
Eleni and Panagiotis (22) 2020 |
|
|---|---|---|---|---|---|---|---|---|---|
| Pooled RR | 0.72 (0.32-1.40) | 0.84 (0.74-0.96) | 0.84 (0.74-0.96) | 0.61 (0.46-0.82) | 0.81 (0.71-0.93) | 0.84 (0.72-0.97) | 0.61 (0.40-0.92) | ||
| Institutionalized RR | 0.75 (0.62-0.92)c | 0.69 (0.53-0.90)d | |||||||
| Community RR | 0.91 (0.77-1.09) | 1.09 (0.85-1.39) | 1.15 (0.81-1.62)e | 0.92 (0.77-1.10) | |||||
| AMSTAR Quality Assessment | Critically low | Moderate | Critically low | Critically low | Low | Critically low | Critically low | Critically low | Critically low |
| Included trials | |||||||||
| Chapuy et al (45) 1992 |
NA 80/1387 110/1403 NA |
38.24% 137/1634 178/1636 0.77 (0.62-0.95) |
38% 137/1634 178/1636 NA |
NA 21/877 37/888 0.57 (0.34-0.97) |
Excluded institutionalized trials | 42.77% NA NA 0.77 (0.62-0.95) |
Excluded institutionalized trials | 25.5% 80/1634 110/1636 0.72 (0.53-0.96) |
15.2% 21/1387 37/1403 0.57 (0.34-0.98) |
| Chapuy et al (46) 2002 |
NA 27/393 21/190 NA |
6.02% 27/389 21/194 0.64 (0.37-1.10) |
6% 27/389 21/194 NA |
NA 27/393 21/190 0.62 (0.36-1.07) |
Excluded institutionalized trials | 6.50% NA NA 0.64 (0.37-1.10) |
Excluded institutionalized trials | 5.5% 27/393 21/190 0.58 (0.31-1.08) |
15.0% 27/393 21/190 0.62 (0.36-1.07) |
| Dawson-Hughes et al (47) 1997 |
Excluded trials including men | 0.31% 0/187 1/202 0.36 (0.01-8.78) |
0.2% 0/187 1/202 NA |
NA 0/170 1/148 0.29 (0.01-7.08) |
0.6% 0/187 1/202 0.36 (0.01-8.78) |
Excluded trials including men | NA 0/187 1/202 NA |
Excluded trials with < 500 participants | 1.5% 0/187 1/202 0.36 (0.01-8.78) |
| Larsen et al (43) 2004 |
NA | “No treatment group received vitamin D & calcium alone”f | NA | NA | “no treatment group received vitamin D and calcium alone” | NA | NA | “Not randomized (cluster randomized factorial design)” | 18.6% 87/4957 114/2116 0.33 (0.25-0.43) |
| Avenell et al (40) 2004 |
Excluded trials including men | 0.21% 1/35 1/35 1.0 (0.07-15.36) |
0.2% 1/35 1/35 NA |
“Doesn’t qualify as an RCT of Calcium and vitamin D” | 0.8% 1/35 1/35 1.0 (0.07-15.36) |
Excluded trials including men | NA 1/35 1/35 NA |
Excluded trials with < 500 participants | “It assessed the open trial design and not explicitly the combination of Ca & vitamin D” |
| Harwood et al (NoNOF) (42) 2004 |
Excluded trials on post–hip fracture | 0.29% 1/75 1/37 0.49 (0.03-7.67) |
0.2% 1/75 1/37 NA |
No data on hip fracture per SR/MA definition | 0.8% 1/75 1/37 0.49 (0.03-7.67) |
0.26% NA NA 0.49 (0.03-7.67) |
NA 1/75 1/37 NA |
Excluded trials with < 500 participants | NA |
| Grant et al (RECORD) (41) 2005 |
Included in sensitivity analysis | 8.73% 46/1306 41/1332 1.14 (0.76-1.73) |
10% 46/1306 41/1332 NA |
No data on hip fracture per SR/MA definition | 35.2% 46/1306 41/1332 1.14 (0.76-1.73) |
Excluded trials including men | NA 46/1306 41/1332 NA |
12% 46/1306 41/1332 1.15 (0.75-1.76) |
NA |
| Porthouse et al (44) 2005 |
NA 8/1321 17/1993 NA |
2.91% 8/1321 17/1993 0.71 (0.31-1.64) |
2% 8/1321 17/1993 NA |
NA 5/607 2/602 2.48 (0.48-12.78)g |
8.6% 8/1321 17/1993 0.71 (0.31-1.64) |
2.74% NA NA 0.71 (0.31-1.64) |
NA 8/1321 17/1993 NA |
3.4% 8/1321 17/1993 0.72 (0.32-1.61) |
11.0% 8/1321 17/1993 0.71 (0.31-1.64) |
| Jackson et al (WHI) (48) 2006 |
Excluded interventions incorporating hormone therapy | 42.86% 175/18176 199/18106 0.88 (0.72-1.07) |
42% 175/18176 199/18106 NA |
Excluded because used WHI post hoc analysis by Prentice et al (50) instead | 52% 70/4015 61/3957 1.13 (0.8-1.59) |
47.06% NA NA 0.88 (0.72-1.07) |
NA 70/4015 61/3957 NA |
52.7% 175/18176 199/18106 0.87 (0.71-1.07) |
19.4% 175/18176 199/18106 0.88 (0.72-1.07) |
| Salovaara et al (OSTPRE-FPS) (49) 2010 |
Beyond search period | 0.43% 4/1586 2/1609 2.03 (0.37-11.06) |
0.6% 4/1718 2/1714 NA |
NA 4/1586 2/1609 2.03 (0.37-11.06) |
2.1% 4/1718 2/1714 2.0 (0.37-10.88) |
0.67% NA NA 2.03 (0.37-11.06) |
NA 4/1718 2/1714 NA |
0.9% 4/1586 2/1609 1.98 (0.40-9.81) |
4.5% 4/1586 2/1609 2.03 (0.37-11.06) |
| Prentice et al (50) 2013 |
Beyond search period | NA | NA | NA 19/7530 35/7406 0.53 (0.31-0.93) |
NA | Post hoc analysis of WHI trial | NA | NA | 14.8% 19/7530 35/7801 0.56 (0.32-0.98) |
The trials by Chapuy et al (45, 46) are the only ones conducted in institutionalized populations. All the other trials are conducted in community-dwelling populations. Individual rows within cells are filled with the following: Percentage is weight of each trial taken from the forest plot of the corresponding SR/MA, number of fractures/total number of participants in the treatment group, number of fractures/total number of participants in control group, and effect size estimate of the trial taken from the forest plot of the corresponding SR/MA.
Abbreviations: Ca, calcium; D, vitamin D; MA, meta-analysis; NA, not available; RCT, randomized clinical trial; RR, relative risk; SR, systematic review; WHI, Women’s Health Initiative.
a MAs not included in the matrix: DIPART, 2010: individual participant data without details about individual RCTs (23); Murad et al, 2012: Network MA without details about Ca/D vs placebo (28); Tricco et al, 2017: Network MA without details about individual RCTs (39).
b Reasons for exclusion of some trials by SRs/MAs either as exactly reported or derived from inclusion/exclusion criteria.
c “The test for subgroup differences was not significant (P = .15).”
d “P for heterogeneity: .07”; P value for interaction term was not provided.
e This RR is for high D (≥ 800 IU/day) + high Ca (≥ 800 mg/day) vs placebo. RR for low D (< 800 IU/day) + high Ca (≥ 800 mg/day) is 1.13 (0.78-1.63). RR for low D (< 800 IU/day) + low Ca (< 800 mg/day) is 0.36 (0.01-8.81).
f Avenell excluded Larsen because participants in each of the 3 treatment clusters received 1 or more cointerventions designed to reduce falls (medication review, environmental hazard, and health assessment, and osteoporosis/fall prevention leaflets) but the control group received no intervention. No treatment group received D and Ca alone.
g CI for “equally allocated” subgroup.
There was a substantial overlap in the RCTs conducted in community-dwelling individuals, included in the SRs/MAs by Avenell and colleagues and Bolland et al (15, 19), Zhao et al and Hu et al (24, 31), Yao et al and Barrionuevo and colleagues (16, 30), thus explaining their very similar effect size estimates. Conversely, the overlap varied across other SRs/MAs. One study (Porthouse et al 2005) (44) was included in all SRs/MAs, whereas another one (Larsen et al 2004) (43) was included only in the most recent one (22). This is explained by differences in either the search period or the eligibility criteria. Some SRs/MAs included RCTs with a relatively large sample size (> 500 [30] or > 1000 participants [23]), or exclusively from the community (23, 24, 31), or exclusively women (16, 17), or excluded specific trials or trial arms if the intervention was a single high vitamin D dose (22, 27), Ca only, vitamin D only (15, 19, 24, 30, 31), or hormone replacement therapy (17) (Supplementary data 3) (12). One SR/MA included duplicate data from the WHI trial (22) (primary [48] and post hoc analyses [50]). The CCA for SRs/MAs on hip fracture was 57%.
For the same RCTs, the derived effect size estimates varied across SRs/MAs. For instance, for Chapuy 1992 (45), the sample size in the intervention and the control varied, 877 to 1634, and 888 to 1636, respectively. Similarly, the number of hip fractures considered in the analysis ranged between 21 and 137 in the intervention arm, and 37 to 178 in the control arm. Therefore, the effect size was also variable at 0.57 to 0.77 (see Table 2). Such findings are related to the data abstraction methods, targeting intention to treat (17, 22), per-protocol analyses (29), complete case analyses (15, 16, 19, 30), or excluding deaths (15, 16, 22, 29, 30). Furthermore, the methods used for pooling data were also heterogeneous; while the majority used a traditional meta-analysis, few conducted a network (16, 24, 27, 28, 39) or an IPD-MA (23) (see Supplementary data 3) (12).
Subgroup Analyses
Residency status as a predictor of response was scrutinized in 2 SRs/MAs. Although the CI of the effect size estimate in the subgroup of institutionalized individuals in the Cochrane SR/MA (15) did not cross 1.0, subgroup effect by residency was not significant, with a P interaction of .15 (see Table 2). Similarly, another SR/MA reported no significant heterogeneity across residence subgroups (15, 30), without providing an interaction P value (see Table 2). The Cochrane SR/MA reported an overall 16% RRR in hip fractures (15). The absolute risk reduction therefore varied depending on the baseline risk. It was 1 of 1000 (0/1000-2/1000) for a lower-risk population derived from the community (baseline hip fracture risk of 8/1000), while it was 9/1000 (2/1000-14/1000) for a higher-risk population derived from institutional residence (baseline risk of 54/1000) (15). Age 80 years and older and a baseline serum 25(OH)D concentration of 50 nmol/L or less approached significance, as modifiers. Conversely, subgroup analyses based on sex (31), osteoporotic fracture history (15, 30), and vitamin D dose (23, 31) did not (Table 3).
Table 3.
Subgroup analyses of vitamin D with calcium vs placebo on risk of hip and any fracture
| Hip fracture | Any fracture | |
|---|---|---|
| By age, y | Yao et al, 2019 (30): < 80: 0.92 (0.77-1.10) ≥ 80: 0.69 (0.53-0.90) P (interaction): NA P (heterogeneity): .07 |
Yao et al, 2019 (30): < 80: 0.96 (0.90-1.02) ≥ 80: 0.76 (0.62-0.92) P (interaction): NA P (heterogeneity): .02 |
| By sex | Zhao et al, 2017 (31): Women only: 1.07 (0.79-1.46) Both: 1.12 (0.75-1.68) P (interaction): .86 |
Zhao et al, 2017 (31): Women only: 0.88 (0.71-1.08) Both: 0.83 (0.48-1.42) P (interaction): .84 |
| By history of osteoporotic fracture | Zhao et al, 2017 (31): Yes: 1.12 (0.75-1.68) Othera: 1.07 (0.79-1.46) P (interaction): .86 |
Zhao et al, 2017 (31): Yes: 0.92 (0.76-1.11) Othera: 0.88 (0.71-1.09) P (interaction): .78 |
| Avenell et al, 2014 (15): Yes: 1.02 (0.71, 1.47) No: 0.82 (0.71, 0.94) P (interaction): .26 |
Avenell et al, 2014 (15): Yes: 0.93 (0.79-1.10) No: 0.95 (0.90-1.00) P (interaction): .84 |
|
| By baseline serum 25(OH)D concentration, nmol/L | Zhao et al, 2017 (31): ≥50: 0.36 (0.01-8.78) < 50: 1.14 (0.88-1.48) P (interaction): .48 |
Zhao et al, 2017 (31): ≥50: 1.87 (0.32-11.02) < 50: 0.90 (0.76-1.05) P (interaction): .42 |
| Bolland et al, 2014 (19): ≥ 50: 0.36 (0.02-8.79) < 50: 0.84 (0.74-0.97) P (interaction): .82 |
Bolland et al, 2014 (19): ≥ 50: 1.50 (1.02-2.19) < 50: 0.75 (0.43-1.31) P (interaction): .24 |
|
| By D dose | Zhao et al (with Ca), 2017 (31): ≥ 1 g and ≥ 800 IU: 1.06 (0.74-1.51) Another dose: 1.12 (0.80-1.57) P (interaction): .83 |
Zhao et al (with Ca), 2017 (31): ≥ 1g and ≥ 800 IU: 0.90 (0.78-1.04) Another dose: 1.15 (0.28-4.74) P (interaction): .74 |
| DIPART, 2010 (23): 10 μg: 0.74 (0.60-0.91) 20 μg: 1.30 (0.88-1.91) P (interaction): NA |
DIPART, 2010 (23): 10 μg: 0.91 (0.85-0.99) 20 μg: 0.95 (0.80, 1.14) P (interaction): NA |
|
| Other subgroup analyses | Yao et al, 2019 (30): region, open-label, Ca dose (1000 mg-1200 mg), treatment difference in 25(OH)D (< 50 nmol/L-≥ 50 nmol/L) Bolland et al, 2014 (19): achieved 25(OH)D (nmol/L), duration of trial Zhao et al, 2017 (31): Ca dose (< 1 g-≥ 1 g), dietary Ca intake (< 900 mg-≥ 900 mg), dose frequency Weaver et al, 2016 (29): use of personal supplements, adherence to assigned study pills in WHI |
Units listed as reported; to convert from nmol/L to ng/mL, divide by 2.496.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; C, community-dwelling individuals; Ca, calcium; D, vitamin D; I, institutionalized; NA, not available; WHI, Women’s Health Initiative.
a Other includes no fracture, partial fracture, or missing fracture data.
Any Fracture
Eleven SRs/MAs assessed the benefit of Ca/D on any fractures (15, 17, 19, 22-24, 29-31, 37, 39). They had the same direction but were discrepant both in magnitude of effect and statistical significance. Seven SRs/MAs reported a significant reduction in any fracture risk in analyses combining institutionalized and community-dwelling individuals (15, 19, 22, 23, 29, 30, 39) (Fig. 2B). Only 2 SRs/MAs, by the Cochrane Group, were of moderate quality (15, 37). The first on fall-related fracture included exclusively RCTs from the community and showed no effect (37). The second combined institutionalized and community-dwelling individuals and was the only one evaluating the quality of the evidence. It reported a high quality of evidence for a 5% RRR in any fractures (15). The remaining 6 SRs/MAs, of critically low quality, reported an RRR ranging between 6% and 29% (19, 22, 23, 29, 30, 39), one of which was a network MA (39) and one an IPD-MA (23). Three other SRs/MAs on community-dwelling individuals were of critically low quality and did not show evidence of fracture reduction (17, 24, 31).
Ten SRs/MAs were included in the matrix (Table 4). The population consisted mostly of older women and/or men with (40-44, 49, 51) or without (45-48, 52) a history of fracture. The RCTs with the largest weights were the community-based WHI and RECORD trials (41, 48). The 2 trials by Chapuy et al (45, 46) were the only ones on institutionalized individuals included in SRs/MAs. The overlap of included RCTs from the community was variable. As reported for the hip fracture, the variability in the inclusion of RCTs and the discrepancy in their individual effect size estimates across SR/MAs are explained by the difference in the search period, eligibility criteria, and analysis methods. The CCA for any fracture was 39%.
Table 4.
Comparison of individual trials included in traditional systematic reviews/meta-analysesa and reasons for exclusionb of trials by some systematic reviews/meta-analyses investigating any fracture risk with calcium and vitamin D supplementation
| Bergman et al (17) 2010 |
Gillespie et al (37) 2012 |
Avenell et al (15) 2014 |
Bolland et al (19) 2014 |
Weaver et al (29) 2016 |
Zhao et al (31) 2017 |
Hu et al (24) 2019 |
Yao et al (30) 2019 |
Eleni and Panagiotis (22) 2020 |
|
|---|---|---|---|---|---|---|---|---|---|
| Pooled RR | 0.95 (0.46-1.85) | 0.95 (0.9-0.99) | 0.92 (0.85-0.99) | 0.86 (0.75-0.98) | 0.94 (0.89-0.99) | 0.74 (0.58-0.94) | |||
| Institutionalized RR | 0.85 (0.74-0.98)c | 0.67 (0.52-0.88)d | 0.76 (0.62-0.92)e | ||||||
| Community RR | 0.83 (0.59 and 1.16) | 0.96 (0.91-1.01) | 0.95 (0.85-1.06) | 0.90 (0.78-1.04) | 0.89 (0.74-1.06)f | 0.96 (0.90-1.02) | |||
| AMSTAR Quality assessment | Critically low | Moderate | Moderate | Critically low | Critically low | Low | Critically low | Critically low | Critically low |
| Total fx definition | Any fx excluding hip and NV fx | Fall-related fx at any site | Any fx excluding hip, V, and NV fx | Any fx excluding hip fx | Any fx excluding hip fx | Any fx excluding hip, V, & NV fx | Any fx excluding hip and V fx | Any fx including hip fxg | Any fx excluding hip and wrist fx |
| Trials | |||||||||
| Chapuy (45) 1992 |
NA 80/1387 115/1403 NA |
Excluded institutionalized trials | 10.67% 255/1634 308/1636 0.83 (0.71-0.96) |
19% 255/1634 308/1636 NA |
NA 66/877 97/888 0.69 (0.51-0.93) |
Excluded institutionalized trials | Excluded institutionalized trials | 7.0 160/1634 215/1636 0.72 (0.58-0.89) |
11.1% 80/1387 110/1403 0.74 (0.56-0.97) |
| Chapuy (46) 2002 |
NA 70/393 30/190 NA |
Excluded institutionalized trials | 1.57% 69/389 34/194 1.01 (0.7-1.47) |
4% 69/389 34/194 NA |
No data on any fracture as per SR/MA definition | Excluded institutionalized trials | Excluded institutionalized trials | 1.6 70/393 35/190 0.96 (0.61-1.51) |
7.9% 27/393 21/190 0.62 (0.36-1.07) |
| Inkovaara (83) 1983 |
NA | Excluded trials not reporting fall outcome | “Unclear whether the data represent fractures or participants with fractures” | NA | NA | 0.2% 0/46 3/42 0.13 (0.01-2.46) |
NA | Excluded trials with total fx events < 10 | NA |
| Dawson-Hughes (47) 1997 |
Excluded trials including men | Excluded trials not reporting fall outcome | 0.87% 11/187 26/202 0.46 (0.23-0.9) |
1% 11/187 26/202 NA |
NA 11/170 26/148 0.37 (0.19-0.72) |
No data on any fracture as per SR/MA definition | No data on any fracture as per SR/MA definition | Excluded trials with < 500 participants | 6.5% 11/187 26/202 0.46 (0.23-0.90) |
| Larsen et al (43) 2004 |
NA | “The outcome is only ‘severe’ falls leading to acute hospital admission” | “no treatment group received vitamin D & calcium alone” | “It’s a cluster randomized controlled trial” | NA | “No treatment group received vitamin D and calcium alone” | NA | “Not randomized (cluster randomized factorial design)” | 12.3% 217/4957 270/2116 0.34 (0.29-0.41) |
| Avenell et al (40) 2004 |
Excluded trials including men | Excluded trials not reporting on fall outcome | 0.14% 2/35 4/35 0.5 (0.10-2.56) |
0.3% 3/35 5/35 NA |
“Doesn’t qualify as an RCT of Calcium and vitamin D” | 0.8% 2/35 4/35 0.5(0.10,2.56) |
NA 2/35 4/35 NA |
Excluded trials with < 500 participants | “It assessed the open trial design and not explicitly the combination of Ca & vitamin D” |
| Harwood et al (NoNOF) (42) 2004 |
Excluded trials on post–hip fracture | 1.5% NA NA 0.5 (0.16-1.56) |
0.23% 6/75 5/37 0.59 (0.19-1.81) |
0.5% 6/75 5/37 NA |
NA 3/29 5/35 0.72 (0.19-2.78) |
1.6% 6/75 5/37 0.59 (0.19-1.81) |
NA 6/75 5/37 NA |
Excluded trials with < 500 participants | 2.5% 3/39 5/37 0.57(0.15,2.22) |
| Grant et al (RECORD) (41) 2005 |
Excluded trials including men | 19.96% N = 5292h 1.02 (0.87-1.19) |
6.14% 165/1306 179/1332 0.94 (0.77-1.15) |
14% 179/1306 192/1332 NA |
NA 179/1306 192/1332 0.95 (0.79-1.15) |
52.6% 165/1306 179/1332 0.94 (0.77-1.15) |
NA 165/1306 179/1332 NA |
6.6% 179/1306 192/1332 0.94 (0.76-1.17) |
12.6% 387/2649 377/2643 1.02 (0.90-1.17) |
| Porthouse et al (44) 2005 |
NA 29/1321 38/1993 NA |
10.14% N = 3314h 1.01 (0.71-1.44) |
2.52% 58/1321 91/1993 0.96 (0.7-1.33) |
5% 58/1321 91/1993 NA |
NA 24/607 22/602 1.08 (0.61-1.91)i |
19.8% 58/1321 91/1993 0.96 (0.7-1.33) |
NA 58/1321 91/1993 NA |
2.8% 58/1321 91/1993 0.96 (0.69-1.34) |
10.6% 58/1321 91/1993 0.96 (0.70-1.33) |
| Jackson et al (WHI) (48) 2006 |
Excluded interventions incorporating hormone therapy | Excluded trials not reporting fall outcome | 74.96% 2102/18176 2158/18106 0.97 (0.92-1.03) |
49% 1921/18176 1961/18106 NA |
Used post hoc analysis by Prentice et al (50) instead | No data on any fracture per SR/MA definition | NA | 78.3% 2102/18176 2158/18106 0.97 (0.91-1.03) |
13.0% 2102/18176 2158/18106 0.97 (0.92-1.03) |
| Bischoff-Ferrari et al (84) 2006 |
NA | 3.75% N = 3890h 0.46 (0.23-0.91) |
NA | NA | NA | NA | NA | NA | NA |
| Bolton-Smith et al (85) 2007 |
NA | Excluded trials not reporting fall outcome | 0.07% 2/62 2/61 0.98 (0.14-6.76) |
0.2% 2/62 2/61 NA |
NA | No data on any fracture per SR/MA definition | NA | Excluded trials with < 500 participants | Excluded because “confounded by the use of vitamin K1 in the therapeutic regimen” |
| Salovaara et al (49) 2010 |
Beyond search period | 11.63% N = 3195h 0.89 (0.65-1.21) |
2.82% 71/1586 82/1609 0.88 (0.64-1.2) |
6% 78/1718 94/1714 NA |
NA 78/1586 94/1609 0.84 (0.63-1.13) |
23.9% 78/1718 94/1714 0.83 (0.62-1.11) |
NA 78/1718 94/1714 NA |
3.7% 86/1586 103/1609 0.84 (0.63-1.13) |
11.0% 78/1586 94/1609 0.84 (0.63-1.13) |
| Prentice et al (50) 2013 |
Beyond search period | Beyond search period | NA | NA | NA 405/7530 458/7801 0.92 (0.80-1.04) |
NA | NA | NA | 12.6% 405/7530 458/7801 0.92 (0.80-1.04) |
| Liu et al (52) 2015 |
Beyond search period | Beyond search period | Beyond search period | Beyond search period | NA | 0.4% 1/50 2/48 0.48 (0.04-5.12) |
NA 1/50 2/48 NA |
Excluded trials with < 500 participants | NA |
| Xue et al (51) 2017 |
Beyond search period | Beyond search period | Beyond search period | Beyond search period | Beyond search period | 0.7% 3/139 2/173 1.87 (0.32-11.02) |
NA 3/139 2/173 NA |
Excluded trials with < 500 participants | NA |
The trials by Chapuy et al (45, 46) are the only ones conducted in institutionalized populations. All the other trials are conducted in community-dwelling populations. Individual cells are filled with the following: Percentage is weight of each trial, number of fractures/treatment group, number of fractures/control group, and effect size estimate.
Abbreviations: AMSTAR-2, A MeaSurement Tool to Assess systematic Reviews 2; Ca, calcium; D, vitamin D; fx, fracture; MA, meta-analysis; NA, not available; NV, nonvertebral; RCT, randomized clinical trial; RR, relative risk; SR, systematic review; V, vertebral; WHI, Women’s Health Initiative.
a MAs not included in matrix: DIPART, 2010: individual participant data without details about individual RCTs (23); Tricco et al, 2017: Network MA without details about individual RCTs (39).
b Reasons for exclusion of some trials by systematic reviews/meta-analyses either as exactly reported or derived from inclusion/exclusion criteria.
c “The test for subgroup differences was not significant (P = .13).”
d Not a subgroup analysis; community and institutionalized were each analyzed separately.
e “P for heterogeneity: 0.02.”
f This RR is for high D (≥ 800 IU/day) + high Ca (≥ 800 mg/day) vs placebo. RR for low D (< 800 IU/day) + high Ca (≥ 800 mg/day) is 0.48 (0.04-5.16). RR for high D (≥ 800 IU/day) + low Ca (< 800 mg/day) is 1.87 (0.31-11.13).
g Any fracture is defined as an fx occurring at any site, but if an RCT reported only hip fx, these were also counted as any fx.
h Only total number available (number of events per treatment/control is not available).
i CI for equally allocated subgroup.
Subgroup Analyses
Two SRs/MAs conducted subgroup analysis by residency (15, 30). Although there was a trend for a higher effect in institutionalized individuals, none of the SRs/MAs found a subgroup effect (15, 30). The Cochrane SR/MA reported a 5% RRR in any fracture and an absolute risk reduction varying from 1 of 1000 for a lower baseline risk population (of 26/1000), to 4 of 1000 for a higher baseline risk population (of 74/1000) (15). Weaver et al (29) conducted 2 primary analyses according to residency and the effect size CIs were overlapping. There was a subgroup effect by age in one SR/MA; P heterogeneity of .02, P of interaction not reported (30). Other subgroup analyses did not show any significant effect (see Table 4).
Results of Systematic Review/Meta-Analysis on Vitamin D Alone vs Placebo
Out of the 19 SRs/MAs on vitamin D alone vs placebo/control, only 3 were of moderate quality, published by the Cochrane Group (15, 36, 37), 15 evaluated the effect on hip fractures (15, 16, 19, 20, 23-28, 30-32, 35, 39), 13 on any fractures (15, 19, 21, 23, 24, 27, 30-32, 34, 36, 37, 39), and 2 on fall-related fracture (36, 37). They were all concordant in direction and statistical significance. They showed a trend for an increased risk of hip (RR 1.10-1.30) and any fracture (RR 1.01-1.09), but none reached statistical significance.
Discussion
In this umbrella review, we synthesize, compare, and contrast methodological quality and findings, and investigate reasons for discordance of SRs/MAs of RCTs evaluating the efficacy of vitamin D supplementation, with or without Ca supplementation, on fracture risk. Importantly, the majority of the identified SRs/MAs were rated of low to critically low quality, secondary to the lack of or insufficient data in one or more of the critical AMSTAR-2 domains, specifically limitations in the search strategy, the lack of a list for excluded trials, the absence of an appropriate bias assessment or accounting for it in the interpretation of results, and the lack of investigation of publication bias. Several of these items were added to the AMSTAR-2 tool, but were not available in AMSTAR-1, which was available when many of the included SRs/MAs were conducted and/or published (14).
Most of the included SRs/MAs showed a protective effect of Ca/D compared with placebo/control. The discordance in results stemmed from differences in the magnitude of the effect size and statistical significance, both for hip and any fracture. SRs/MAs that exclusively considered RCTs in community-dwelling individuals failed to show any significant reductions in fractures. We identified only one SR/MA of moderate quality, pooling data from institutionalized and community-dwelling individuals, and that evaluated the quality of evidence (15). It reported high-quality evidence of a small RRR in hip fracture by 16% and any fracture by 5% with vitamin D dose of 400 to 800 IU/day, coadministered with Ca, compared with placebo/control. Fracture risk reduction in institutionalized individuals was derived from 2 RCTs, included in all SRs/MAs except those exclusively targeting the community (31, 38). These 2 RCTs enrolled older women (mean age 85 years) living in nursing homes, with mean serum 25(OH)D concentration 22.5 to 40 nmol/L, randomly assigned to daily Ca/D 1200 mg/800 IU or placebo, for 18 to 24 months (45, 46). Each of these RCTs demonstrated a beneficial effect of Ca/D in reducing the risk of hip and nonvertebral fractures (45, 46).
Subgroup analyses by residency status were performed in 2 SRs/MAs. The first revealed an RR of 0.75 (0.62-0.92) (15), and the second of 0.69 (0.53-0.90) (30), in institutionalized individuals, but the interaction term was not significant for the former, thus ruling out an effect of residency on risk reduction, and was not provided for the latter. The lack of evidence for a subgroup effect by residency may be due to the imprecise effect size estimates, with large CIs, despite a large number of trials and sample sizes (2000-3500 [41, 44, 53], and > 36 000 participants in the WHI trial [48]), explained by the very low baseline fracture risk (< 1%) in this population (47-50). Indeed, the neutral results of subgroup analyses could imply a lack of effect, or it may also be affected by the evaluation of subgroup effect within vs between trials, and prespecifying subgroup analysis before the conduct of the SR/MA, among others (54).
Vitamin D alone does not appear to protect against fractures when compared with placebo, and this is consistent with the findings of a previous umbrella review (55). Concomitant Ca supplementation is needed (56), as the combination has a synergistic effect on intestinal Ca absorption (57), on reversal of secondary hyperparathyroidism, especially if 25(OH)D is less than 25 nmol/L (58), and on reducing body sway and fall risk (59).
The discordance in results between SRs/MAs is multifactorial (60), including the clinical question investigated, search period, eligibility criteria, intervention, cointervention, and outcome. For hip fracture outcome, few SRs/MAs aimed to assess interventions that reduce fracture risk in individuals with osteoporosis or at risk for osteoporosis or fragility fractures (16, 25, 28), while for others the population consisted of postmenopausal women and older men. Despite this variability in the population of interest, there was a significant overlap (CCA > 15% both for hip and any fracture) of the included RCTs, many of which had patients with previous fractures (15-17, 23, 24, 26, 29-31, 37). Fracture definition varied across SRs/MAs. Some SRs/MAs included proximal femoral fractures as hip fractures (15, 19, 24, 31), whereas others did not (29). “Any fracture” included fall-related fractures (36, 37), or nonvertebral fractures (15, 20), or fractures defined at specific sites (hip, spine, or wrist) (21), or fractures at any site (30), or without site specification (31). None of the SRs/MAs targeted fragility fractures per se, and few excluded nonosteoporotic fractures (skull, carpal, metacarpal, tarsal, or metatarsal bones) (61-63). There was heterogeneity in the measure of association used. While the majority of studies used RR, the preferred tool, a few studies used hazard ratio or odds ratio; the latter tends to inflate the association (64). Similarly, there was variability in the methods used for pooling of effect sizes and in the data derived from each trial (6, 60). Although all SRs/MAs used a complete case analysis, which is the appropriate analysis method to deal with missing data at the trial level in the primary analysis (65), some used intention to treat or per-protocol analyses for one or more RCTs.
There are 2 previous umbrella reviews on vitamin D supplementation. Theodoratou et al (55) included observational and interventional SR/MAs until 2013, addressing 137 health outcomes. Stubbs and colleagues (66) assessed interventions preventing falls in the community, among which was the efficacy of vitamin D supplementation on fall-related fractures. The first concluded there was no evidence, and the second commented on conflicting evidence for a fracture risk reduction. Neither delved into a critical appraisal of the quality of SRs/MAs, or importantly into sources for any discrepancy in results from various SRs/MAs, and their heterogeneity led to conflicting conclusions.
Pollock et al (67) recognized several methodologic challenges in the overview of reviews. When a meta-analysis of SR/MA is conducted, overlap between reviews is a major consideration, as it is associated with the risk of “double counting” studies included in more than one SR/MA (67). Therefore, some experts select only one SR/MA when several are eligible, such as the most recent one, or the one conducted by the Cochrane Group (67). We have quantified the overlap between the SRs/MAs included in our umbrella review. However, we did not pool their results, and therefore, we do not expect an amplification of the related bias in the interpretation of the results. We have used the AMSTAR-2 tool to evaluate the quality of the included SRs/MAs. Given that this tool implies some subjectivity, the assessment was conducted by 3 reviewers (including content experts M.C. and G.E.H.F.), and the rationale for this decision is detailed in Supplementary data 3 (12), as previously suggested (67). We did not use the GRADE approach to summarize our findings because to date there is no clear guidance on how to apply the GRADE principles in an umbrella review (67, 68). We did not assess for publication bias because this remains one of the challenges of umbrella reviews without a validated tool to assess it (67).
The limitations of our findings are in large part due to the limitation of the evidence available. Baseline vitamin D status was reported in only 14 SRs/MAs (15, 19, 21, 24-26, 30-32, 35-37), and fewer reported follow-up status (19, 21, 30, 32, 35). The large variation in 25(OH)D assays was not addressed. Several SRs/MAs on vitamin D alone compared with placebo/control included trials extending over 1 to 10 months (15, 17, 19, 21, 30-32, 35), an insufficient period to demonstrate any significant effect on fractures. For SRs/MAs on Ca/D, a narrow range of vitamin D dose was used in the included trials, and therefore a dose effect cannot be investigated. Some discrepancies in SRs/MAs might be related to human errors; however, we did not investigate this possibility because verifying trial data was beyond our aim.
To our knowledge this is the only umbrella review on a controversial topic that is exclusively focusing on SRs/MAs analyses of RCTs, and solely on fractures. Our umbrella review fulfills the Joanna Briggs Institute guidance on conducting umbrella reviews (69) (Supplementary data 4) (12). Furthermore, the quality assessment of SRs/MAs, the matrices to dissect their similarities and differences, and important subgroup analyses allow a better understanding of the evidence available and contradicting results, and provides a path forward.
There is a wide variability in the development methods of the current vitamin D guidelines (70) and in their recommended vitamin D doses. Despite the lack of solid evidence of a protective effect of vitamin D supplementation in younger adults (age 50-65 years) and noninstitutionalized individuals, the majority of the guidelines recommend vitamin D supplementation for adults (8, 9, 71-77). Only a few guidelines target older adults, those older than 60 to 70 years (78-80), or older institutionalized individuals (81, 82), or recommend a higher dose in older individuals (9, 75-77), populations in which the evidence is most convincing, namely institutionalized individuals, or most suggestive (age as a high risk).
Therefore, there is a need to improve the methodologic rigor of vitamin D guidelines in order to issue recommendations that are evidence based (70).
Conclusions
Ca/D reduces the risk of hip and any fracture in analyses combining institutionalized and community-dwelling individuals. High-risk individuals, such as those older, institutionalized, or with low vitamin D status, may benefit most, although this could not be unequivocally demonstrated in the few SRs/MAs that evaluated such predictors, most likely because of low power. An IPD-MA restricted to individuals with sufficient follow-up, rather than pooled data, will be more powerful to evaluate treatment efficacy and predictors of fracture reduction. These would include age, residency status, baseline vitamin D level, and regimen used (daily, weekly, or monthly vitamin D).
Acknowledgments
We would like to thank the investigators who replied to our emails and provided information whenever available: Drs B. Abrahamsen, A. Avenell, H. Bischoff Ferrari, L. Kahwati, L. Renjmark, and C. Weaver. We would like to thank Miss Aida Farha, senior librarian at the American University of Beirut, who has provided advice of the search strategy, and Mrs Nariman Chamoun, who built the search strategy.
Glossary
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- AMSTAR-2
A MeaSurement Tool to Assess systematic Reviews 2
- Ca/D
vitamin D and calcium supplementation
- CCA
corrected covered area
- IPD
individual participant data
- MA
meta-analysis
- RCT
randomized clinical trial
- RR
risk ratio
- RRR
relative risk reduction
- SR
systematic review
- WHI
Women’s Health Initiative
Financial Support
This work was supported in part by internal institutional funds from the Medical Practice Plan at the American University of Beirut Medical Center. Research reported in this publication was supported in part by the Fogarty International Center and Office of Dietary Supplements of the National Institutes of Health (NIH; award No. D43 TW009118 to principal investigator Ghada El-Hajj Fuleihan). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design and conducting of this project, the interpretation of the results, the manuscript write-up, or submission for publication.
Disclosures
Drs M. Chakhtoura, D. Bacha, C. Gharios, Y. Jabbour, F. Kahale, A. Bassatne, S. Antoun, E. Akl, and G. El Hajj Fuleihan, Ms S. Ajjour, and M. Assaad have nothing to disclose. Dr P. Ebeling reports grants and other compensation from Amgen, grants from Eli Lilly, grants from Alexion, and other compensation from Sanofi, outside the submitted work. Dr R. Bouillon reports receiving small lecture fees from FAES (Spain), Procter & Gamble (Belgium), Abiogen (Italy), and support for attending meetings and/or travel from FAES (Spain) and Abiogen (Italy). Dr P. Lips reports receiving travel costs for a vitamin D workshop from Abiogen.
Additional Information
Disclosures: Drs M. Chakhtoura, D. Bacha, C. Gharios, Y. Jabbour, F. Kahale, A. Bassatne, S. Antoun, E. Akl and G. El Hajj Fuleihan, and Ms S. Ajjour and M. Assaad have no conflict of interest. Dr P. Ebeling reports grants and other from Amgen, grants from Eli-Lilly, grants from Alexion, other from Sanofi, outside the submitted work. Dr R. Bouillon reports receiving small lecture fees from FAES (Spain), Proctor & Gamble (Belgium), Abiogen (Italy), and support for attending meetings and/or travel from FAES (Spain), Abiogen (Italy). Dr P. Lips reports receiving travel cost for vitamin D workshop from Abiogen.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christakos S, Li S, De La Cruz J, Bikle DD. New developments in our understanding of vitamin metabolism, action and treatment. Metabolism. 2019;98:112-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cesari M, Incalzi RA, Zamboni V, Pahor M. Vitamin D hormone: a multitude of actions potentially influencing the physical function decline in older persons. Geriatr Gerontol Int. 2011;11(2):133-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl DA, Cooper C; IOF Working Group on Epidemiology and Quality of Life . A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23(9):2239-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lund CA, Møller AM, Wetterslev J, Lundstrøm LH. Organizational factors and long-term mortality after hip fracture surgery. A cohort study of 6143 consecutive patients undergoing hip fracture surgery. PLoS One. 2014;9(6):e99308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bolland MJ, Grey A. A case study of discordant overlapping meta-analyses: vitamin D supplements and fracture. PLoS One. 2014;9(12):e115934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13(8):466-479. [DOI] [PubMed] [Google Scholar]
- 8. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. ; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. [DOI] [PubMed] [Google Scholar]
- 9. Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. The National Academies Collection: Reports funded by National Institutes of Health. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press, National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 10. Pollock M, Fernandes RM, Becker LA, Featherstone R, Hartling L. What guidance is available for researchers conducting overviews of reviews of healthcare interventions? A scoping review and qualitative metasummary. Syst Rev. 2016;5(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chakhtoura M, Ajjour S, Assad M, et al. The impact of vitamin D supplementation on fractures, falls and mortality: an umbrella review of systematic reviews and meta-analyses of randomized controlled trials. April 2019. Accessed May 2021. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019129540
- 12. Chakhtoura M.Supplementary data for “ Vitamin D supplementation and fractures in adults: a systematic umbrella review of meta-analyses of randomized controlled trials.” Appendices. Deposited September 9, 2021. doi: 10.6084/m9.figshare.16595090.v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pieper D, Antoine SL, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67(4):368-375. [DOI] [PubMed] [Google Scholar]
- 14. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;2014(4):CD000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barrionuevo P, Kapoor E, Asi N, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab. 2019;104(5):1623-1630. [DOI] [PubMed] [Google Scholar]
- 17. Bergman GJ, Fan T, McFetridge JT, Sen SS. Efficacy of vitamin D3 supplementation in preventing fractures in elderly women: a meta-analysis. Curr Med Res Opin. 2010;26(5):1193-1201. [DOI] [PubMed] [Google Scholar]
- 18. Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40-49. [DOI] [PubMed] [Google Scholar]
- 19. Bolland MJ, Grey A, Gamble GD, Reid IR. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(7):573-580. [DOI] [PubMed] [Google Scholar]
- 20. Bolland MJ, Leung W, Tai V, et al. Calcium intake and risk of fracture: systematic review. BMJ. 2015;351:h4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(12):827-838. [DOI] [PubMed] [Google Scholar]
- 22. Eleni A, Panagiotis P. A systematic review and meta-analysis of vitamin D and calcium in preventing osteoporotic fractures. Clin Rheumatol. 2020;39(12):3571-3579. [DOI] [PubMed] [Google Scholar]
- 23. DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu ZC, Tang Q, Sang CM, et al. Comparison of fracture risk using different supplemental doses of vitamin D, calcium or their combination: a network meta-analysis of randomised controlled trials. BMJ Open. 2019;9(10):e024595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US preventive services task force. JAMA. 2018;319(15):1600-1612. [DOI] [PubMed] [Google Scholar]
- 26. Lai JK, Lucas RM, Clements MS, Roddam AW, Banks E. Hip fracture risk in relation to vitamin D supplementation and serum 25-hydroxyvitamin D levels: a systematic review and meta-analysis of randomised controlled trials and observational studies. BMC Public Health. 2010;10:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li S, Xi C, Li L, et al. Comparisons of different vitamin D supplementation for prevention of osteoporotic fractures: a Bayesian network meta-analysis and meta-regression of randomised controlled trials. Int J Food Sci Nutr. 2021;72(4):518-528. [DOI] [PubMed] [Google Scholar]
- 28. Murad MH, Drake MT, Mullan RJ, et al. Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2012;97(6):1871-1880. [DOI] [PubMed] [Google Scholar]
- 29. Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27(1):367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yao P, Bennett D, Mafham M, et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(12):e1917789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao JG, Zeng XT, Wang J, Liu L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318(24):2466-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6(11):847-858. [DOI] [PubMed] [Google Scholar]
- 33. LeBlanc ES, Zakher B, Daeges M, Pappas M, Chou R. Screening for vitamin D deficiency: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(2):109-122. [DOI] [PubMed] [Google Scholar]
- 34. Thanapluetiwong S, Chewcharat A, Takkavatakarn K, Praditpornsilpa K, Eiam-Ong S, Susantitaphong P. Vitamin D supplement on prevention of fall and fracture: a meta-analysis of randomized controlled trials. Medicine. 2020;99(34):e21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng YT, Cui QQ, Hong YM, Yao WG. A meta-analysis of high dose, intermittent vitamin D supplementation among older adults. PLoS One. 2015;10(1):e0115850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;2012(9):CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Michael YL, Lin JS, Whitlock EP, et al. Interventions to Prevent Falls in Older Adults: An Updated Systematic Review. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews; 2010. [Google Scholar]
- 39. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318(17):1687-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Avenell A, Grant AM, McGee M, McPherson G, Campbell MK, McGee MA; RECORD Trial Management Group . The effects of an open design on trial participant recruitment, compliance and retention—a randomized controlled trial comparison with a blinded, placebo-controlled design. Clin Trials. 2004;1(6):490-498. [DOI] [PubMed] [Google Scholar]
- 41. Grant AM, Avenell A, Campbell MK, et al. ; RECORD Trial Group . Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621-1628. [DOI] [PubMed] [Google Scholar]
- 42. Harwood RH, Sahota O, Gaynor K, Masud T, Hosking DJ; Nottingham Neck of Femur (NONOF) Study . A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: the Nottingham Neck of Femur (NONOF) Study. Age Ageing. 2004;33(1):45-51. [DOI] [PubMed] [Google Scholar]
- 43. Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. J Bone Miner Res. 2004;19(3):370-378. [DOI] [PubMed] [Google Scholar]
- 44. Porthouse J, Cockayne S, King C, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330(7498):1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327(23):1637-1642. [DOI] [PubMed] [Google Scholar]
- 46. Chapuy MC, Pamphile R, Paris E, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13(3):257-264. [DOI] [PubMed] [Google Scholar]
- 47. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670-676. [DOI] [PubMed] [Google Scholar]
- 48. Jackson RD, LaCroix AZ, Gass M, et al. ; Women’s Health Initiative Investigators . Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683. [DOI] [PubMed] [Google Scholar]
- 49. Salovaara K, Tuppurainen M, Kärkkäinen M, et al. Effect of vitamin D(3) and calcium on fracture risk in 65- to 71-year-old women: a population-based 3-year randomized, controlled trial—the OSTPRE-FPS. J Bone Miner Res. 2010;25(7):1487-1495. [DOI] [PubMed] [Google Scholar]
- 50. Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24(2):567-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xue Y, Hu Y, Wang O, et al. Effects of enhanced exercise and combined vitamin D and calcium supplementation on muscle strength and fracture risk in postmenopausal Chinese women. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017;39(3):345-351. [DOI] [PubMed] [Google Scholar]
- 52. Liu BX, Chen SP, Li YD, et al. The effect of the modified eighth section of Eight-section Brocade on osteoporosis in postmenopausal women: a prospective randomized trial. Medicine (Baltimore). 2015;94(25):e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kärkkäinen MK, Tuppurainen M, Salovaara K, et al. Does daily vitamin D 800 IU and calcium 1000 mg supplementation decrease the risk of falling in ambulatory women aged 65-71 years? A 3-year randomized population-based trial (OSTPRE-FPS). Maturitas. 2010;65(4):359-365. [DOI] [PubMed] [Google Scholar]
- 54. Schandelmaier S, Briel M, Varadhan R, et al. Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ. 2020;192(32):E901-E906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92(4):1415-1423. [DOI] [PubMed] [Google Scholar]
- 57. Heaney RP. Vitamin D and calcium interactions: functional outcomes. Am J Clin Nutr. 2008;88(2):541S-544S. [DOI] [PubMed] [Google Scholar]
- 58. Lips P. Interaction between vitamin D and calcium. Scand J Clin Lab Invest Suppl. 2012;243:60-64. [DOI] [PubMed] [Google Scholar]
- 59. Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15(6):1113-1118. [DOI] [PubMed] [Google Scholar]
- 60. Jadad AR, Cook DJ, Browman GP. A guide to interpreting discordant systematic reviews. CMAJ. 1997;156(10):1411-1416. [PMC free article] [PubMed] [Google Scholar]
- 61. FitzGerald G, Boonen S, Compston JE, et al. ; GLOW Investigators . Differing risk profiles for individual fracture sites: evidence from the Global Longitudinal Study of Osteoporosis in Women (GLOW). J Bone Miner Res. 2012;27(9):1907-1915. [DOI] [PubMed] [Google Scholar]
- 62. Morin SN, Lix LM, Leslie WD. The importance of previous fracture site on osteoporosis diagnosis and incident fractures in women. J Bone Miner Res. 2014;29(7):1675-1680. [DOI] [PubMed] [Google Scholar]
- 63. Delmas PD, Marin F, Marcus R, Misurski DA, Mitlak BH. Beyond hip: importance of other nonspinal fractures. Am J Med. 2007;120(5):381-387. [DOI] [PubMed] [Google Scholar]
- 64. Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, Groenwold RH. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. CMAJ. 2012;184(8):895-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Akl EA, Kahale LA, Agoritsas T, et al. Handling trial participants with missing outcome data when conducting a meta-analysis: a systematic survey of proposed approaches. Syst Rev. 2015;4:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stubbs B, Brefka S, Denkinger MD. What works to prevent falls in community-dwelling older adults? Umbrella review of meta-analyses of randomized controlled trials. Phys Ther. 2015;95(8):1095-1110. [DOI] [PubMed] [Google Scholar]
- 67. Pollock A, Campbell P, Brunton G, Hunt H, Estcourt L. Selecting and implementing overview methods: implications from five exemplar overviews. Syst Rev. 2017;6(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 2—risk of bias assessment; synthesis, presentation and summary of the findings; and assessment of the certainty of the evidence. Syst Rev. 2018;7(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aromataris EMZE. JBI Manual for Evidence Synthesis. JBI 2020. Accessed September 2021. 10.46658/JBIMES-20-01 [DOI]
- 70. Dai Z, McKenzie JE, McDonald S, et al. Assessment of the methods used to develop vitamin D and calcium recommendations—a systematic review of bone health guidelines. Nutrients. 2021;13(7):2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hanley DA, Cranney A, Jones G, et al. ; Guidelines Committee of the Scientific Advisory Council of Osteoporosis Canada . Vitamin D in adult health and disease: a review and guideline statement from Osteoporosis Canada. CMAJ. 2010;182(12):E610-E618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. German Nutrition Society. New reference values for vitamin D. Ann Nutr Metab. 2012;60(4):241-246. [DOI] [PubMed] [Google Scholar]
- 73. Płudowski P, Karczmarewicz E, Bayer M, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol. 2013;64(4):319-327. [DOI] [PubMed] [Google Scholar]
- 74. EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on the tolerable upper intake level of vitamin D. European Food Safety Authority; 2012. Accessed September 2021. http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2012.2813/epdf
- 75. Haq A, Wimalawansa SJ, Pludowski P, Anouti FA. Clinical practice guidelines for vitamin D in the United Arab Emirates. J Steroid Biochem Mol Biol. 2018;175:4-11. [DOI] [PubMed] [Google Scholar]
- 76. Lötscher KQ, l’Allemand D, Bischoff-Ferrari HA, Burckhardt P. Vitamin-D deficiency: evidence, safety, and recommendations for the Swiss population. 2012. Accessed September 2021. https://www.zora.uzh.ch/id/eprint/73029/
- 77. Weggemans RM, Kromhout D, van Weel C. New dietary reference values for vitamin D in the Netherlands. Eur J Clin Nutr. 2013;67(6):685. [DOI] [PubMed] [Google Scholar]
- 78. Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21(7):1151-1154. [DOI] [PubMed] [Google Scholar]
- 79. National Institute for Health And Clinical Excellence. 2008. Accessed August 2021. https://www.nice.org.uk/guidance/ph56/documents/implementing-vitamin-d-guidance-final-scope-2
- 80. National Osteoporosis Society. Vitamin D and Bone Health: A Practical Clinical Guideline for Patient Management. 2013. Accessed September 2021. http://www.aub.edu.lb/fm/cmop/downloads/Vitamin-D-Bone-Health.pdf
- 81. Lips P, Cashman KD, Lamberg-Allardt C, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):P23-P54. [DOI] [PubMed] [Google Scholar]
- 82. American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society consensus statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62(1):147-152. [DOI] [PubMed] [Google Scholar]
- 83. Inkovaara J, Gothoni G, Halttula R, Heikinheimo R, Tokola O. Calcium, vitamin D and anabolic steroid in treatment of aged bones: double-blind placebo-controlled long-term clinical trial. Age Ageing. 1983;12(2):124-130. [DOI] [PubMed] [Google Scholar]
- 84. Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med. 2006;166(4):424-430. [DOI] [PubMed] [Google Scholar]
- 85. Bolton-Smith C, McMurdo ME, Paterson CR, et al. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res. 2007;22(4): 509-519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”