Abstract
Introduction
COVID-19 first struck New York City in the spring of 2020, resulting in an unprecedented strain on our healthcare system and triggering multiple changes in public health policy governing hospital operations as well as therapeutic approaches to COVID-19. We examined inpatient mortality at our centre throughout the course of the pandemic.
Methods
This is a retrospective chart review of clinical characteristics, treatments and outcome data of all patients admitted with COVID-19 from 1 March 2020 to 28 February 2021. Patients were grouped into 3-month quartiles. Hospital strain was assessed as per cent of occupied beds based on a normal bed capacity of 1491.
Results
Inpatient mortality decreased from 25.0% in spring to 10.8% over the course of the year. During this time, use of remdesivir, steroids and anticoagulants increased; use of hydroxychloroquine and other antibiotics decreased. Daily bed occupancy ranged from 62% to 118%. In a multivariate model with all year’s data controlling for demographics, comorbidities and acuity of illness, percentage of bed occupancy was associated with increased 30-day in-hospital mortality of patients with COVID-19 (0.7% mortality increase for each 1% increase in bed occupancy; HR 1.007, CI 1.001 to 1.013, p=0.004)
Conclusion
Inpatient mortality from COVID-19 was associated with bed occupancy. Early reduction in epicentre hospital bed occupancy to accommodate acutely ill and resource-intensive patients should be a critical component in the strategic planning for future pandemics.
Keywords: COVID-19, public health, epidemiology
Strengths and limitations of this study.
This is a large cohort study with 7390 patients with COVID-19.
This is a longitudinal analysis over 1 year of management and hospital policy changes.
The study analyses mortality changes after adjustment for different therapies and clinical parameters.
The study identified the association between level of hospital system stress and mortality, with important public health ramifications.
Data on most recent variants were not included.
Introduction
COVID-19 was declared a global pandemic by the WHO on 11 March 2020.1 In the USA, after a cluster of cases reported from Washington state,2 New York State quickly became the initial epicentre of this pandemic, with over 1.27 million of cases to date and over 50 000 fatalities, with the highest concentration in the Bronx and Queens boroughs of New York City.3 Montefiore Einstein, with its three principal teaching hospitals and combined adult bed capacity of 1491, is the primary healthcare provider for the large, nearly 1.5 million diverse population of the Bronx4 and experienced a ‘first wave’ of COVID-19 admissions in the spring of 2020,3 followed by a significant reduction of cases until a second surge in hospitalisations was noted in the winter of 2020. Throughout the course of the year, multiple public health measures, including those adapting hospital operation to a disaster-level pandemic, such as cancellation of all elective procedures and waiver of state-specific licensing for healthcare providers, were put in place. In addition, the understanding of COVID-19 pathophysiology improved,5 6 new treatments were developed,7–10 parts of the general population11 12 as well as hospital personnel developed antibodies after COVID-19 illness,13 and our hospital system adapted to and then recovered from crisis mode.14 Here, we report the outcomes of patients hospitalised with COVID-19 through 1 year since the first case, focusing on the differences observed between the spring and the winter surges.
Methods
Study population
We retrospectively reviewed all adult patients admitted to Montefiore Medical Center with a real-time reverse transcription-PCR assay positive for COVID-19 between 1 March 2020 and 28 February 2021. We divided this timeframe into four 3-month seasons based on the northern hemisphere calendar: spring (from 1 March 2020 to 31 May 2020), summer (from 1 June 2020 to 30 August 2020), fall (from 1 September 2020 to 30 November 2020) and winter (1 December 2020 to 28 February 2021).
Data collection
Medical data including demographic, clinical and laboratory variables were extracted from the electronic medical record system. The primary outcome was 30-day in-hospital mortality.
Statistical analysis
Continuous variables are displayed as mean±SD or median (25%–75% IQR) and compared with the Student’s t-test or Wilcoxon rank-sum, as appropriate. Categorical data are presented as per cent and compared by χ2 test. We estimated the cumulative incidence of the primary endpoint in-hospital mortality for each season, treating hospital discharge as a competing event.15 To avoid any bias due to differential follow-up length, we censored the follow-up time at 30 days after admission.
A multivariable competing risk proportional hazard model was used to estimate the subdistribution HR16 17 for time to in-hospital death. The covariates in the multivariable analyses included factors present in >90% of our data set, known to be associated with in-hospital COVID-19 mortality based on prior literature,6 18 19 or with a univariate association with in-hospital mortality (p<0.05) and a clinical (relative difference >5%) difference between survivors and non-survivors (online supplemental table 1). These variables included age, sex, body mass index (BMI), vital signs at presentation (temperature, systolic and diastolic blood pressure, heart rate, respiratory rate, pulse oxygen saturation), platelet count, white cell count, potassium, bicarbonate, creatinine, glucose, alanine transaminase, aspartate transaminase, history of hypertension, dyslipidaemia, chronic kidney disease, heart failure, coronary artery disease, asthma/chronic obstructive pulmonary disease, diabetes mellitus and statin use. Additionally, lactic acid level and per cent of hospital bed saturation were forced into the model as markers of illness severity and level of hospital stress, respectively.
bmjopen-2021-058171supp001.pdf (361.5KB, pdf)
Then we focused on examining the difference in in-hospital death between patients admitted in the spring and in the winter, as they represented the two largest and most temporal distant waves of the COVID-19 pandemic occurring before and after public health policies, specific therapeutic approaches and hospital management changes had been implemented. The selection method for covariates is presented in online supplemental table 2.
The proportionality assumption was examined20 and no violation was identified. A two-sided p<0.05 was considered statistically significant.
Propensity score analysis
To fully control the potential differences in patient population and hospital stress between spring and winter patients with COVID-19, we also used propensity score (PS) matching to compare the 30-day in-hospital mortality between spring and winter admissions. The same covariates used for the multivariable competing risk regression were used for PS matching. PS matching was carried out through a 1:1 greedy matching algorithm, with a calliper width of 0.1 SD. We then stratified on matched pair in the competing risk regression model.21 22 Because one-to-one matching led to a reduction in sample size, we used this analysis as a sensitivity analysis.
All statistical analyses was performed with SPSS V.25 and the R packages cmprsk and crrSC (V.3.5; R Foundation for Statistical Computing).
Patient and public involvement
Given the retrospective nature of our analysis, it was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting or dissemination plans of our research.
Results
There were 7390 COVID-19-positive adult patients admitted between 1 March 2020 and 28 February 2021 (figure 1). Of these, 4495 patients were admitted during the spring, 264 during the summer, 377 during the fall and 2254 during the winter.
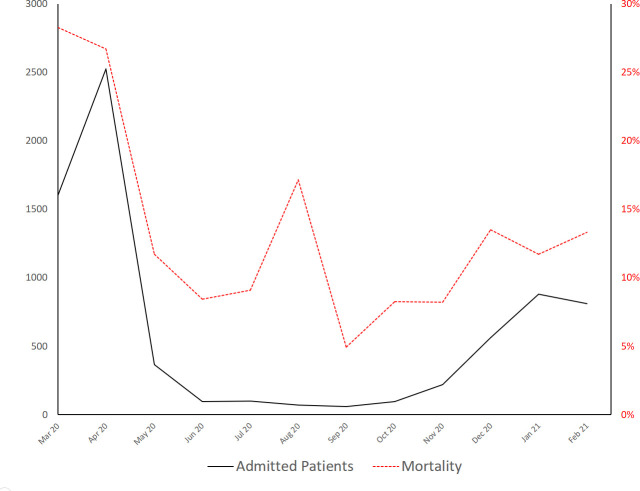
Figure 1.
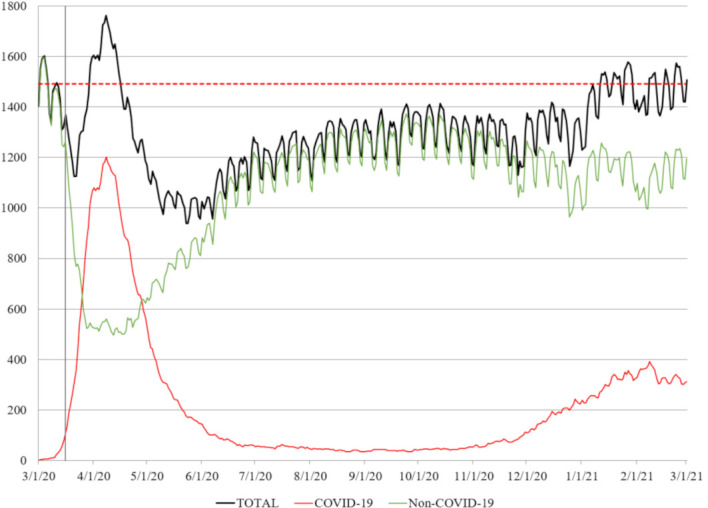
Simultaneously admitted patients. This graph includes hospitalised and admitted patients in the emergency department waiting for a bed. A precipitous decline of non-COVID-19 admissions began on 16 March 2020 (vertical grey line) coinciding with gubernatorial healthcare-associated directives in New York State. The dotted red line indicates the nominal bed capacity of our institution (1491 beds).
On 8 April 2020, the peak of the spring season, the total number of simultaneously adult patients admitted to our hospital (including those admitted to emergency adult wards at our children’s hospital23) was 1762 (118% of nominal bed capacity); 1201 of them (68.2%) were patients with COVID-19. On 8 February 2021, the peak of winter season, 1512 patients (101% of nominal bed capacity) were admitted to our hospital and 393 of them (26.0%) were patients with COVID-19 (figure 1). Following cancellation of elective procedures, bed occupancy decreased to 70% by the end of the spring season and remained at 90% until the beginning of the winter season, when the second wave occurred in December 2020. Unadjusted mortality of patients admitted at the beginning of spring, end of spring, beginning of winter and end of winter was 28%, 8%, 14% and 13%, respectively (figure 2).
Figure 2.
Cumulative monthly admissions (black line, left axis) and mortality (dotted red line, right axis) over the year.
Patient population
Demographics, medical history and vital signs at arrivals are presented in table 1. Initial laboratory blood tests are presented in online supplemental table 3. Overall, the median age was 66 (55–77) years, 3835 (51.9%) patients were male and 5519 (74.2%) were of black race and/or Hispanic ethnicity. The median age ranged from 63 years in fall to 67 years in winter. Sex distribution was similar throughout the year. Summer and fall patients had the lowest and the highest BMI: 26.7 kg/m2 and 28.6 kg/m2, respectively.
Table 1.
Demographics, medical history and vital signs of admitted patients
| Spring (n=4495) | Summer (n=264) | Fall (n=377) | Winter (n=2254) | |
| 30-day hospital outcome, n (%) | ||||
| Still admitted | 194 (4.3) | 6 (2.3) | 15 (4.0) | 103 (4.6) |
| Discharged alive | 3177 (70.7) | 229 (86.7) | 336 (89.1) | 1893 (84.0) |
| Died in the hospital | 1124 (25.0) | 29 (11.0) | 26 (6.9) | 258 (11.4) |
| Demographics | ||||
| Age (IQR), years | 66 (55–77) | 66 (50–76) | 63 (50–73) | 67 (56–77) |
| Male sex, n (%) | 2377 (52.9) | 138 (52.3) | 198 (52.5) | 1122 (49.8) |
| Black race and/or Hispanic ethnicity, n (%) | 3345 (74.4) | 219 (83.0) | 286 (75.9) | 1635 (74.2) |
| Body mass index (IQR), kg/m2 | 28.4 (24.6–33) | 27.6 (22.5–32.7) | 28.6 (25–34.1) | 28.2 (24.4–33.1) |
| Hospital bed saturation, % (IQR) | 97.4 (86.5–107.6) | 81.7 (76.3–85.8) | 87.6 (83.2–90.2) | 95.3 (91.9–101.8) |
| Medical history, n (%) | ||||
| Hypertension | 3370 (75) | 197 (74.6) | 254 (67.4) | 1713 (76) |
| Sleep apnoea | 521 (11.6) | 28 (10.6) | 47 (12.5) | 270 (12) |
| Hyperlipidaemia | 2609 (58) | 153 (58) | 199 (52.8) | 1380 (61.2) |
| Atrial fibrillation | 449 (10) | 30 (11.4) | 35 (9.3) | 267 (11.8) |
| Chronic kidney disease | 1406 (31.3) | 70 (26.5) | 85 (22.5) | 620 (27.5) |
| Heart failure | 980 (21.8) | 72 (27.3) | 66 (17.5) | 519 (23) |
| Coronary artery disease | 1316 (29.3) | 95 (36) | 108 (28.6) | 721 (32) |
| Asthma/COPD | 1371 (30.5) | 84 (31.8) | 98 (26) | 753 (33.4) |
| Diabetes mellitus | 2522 (56.1) | 148 (56.1) | 187 (49.6) | 1244 (55.2) |
| Vitals at presentation | ||||
| Temperature (IQR), °C | 37.2 (36.8–37.8) | 36.9 (36.6–37.2) | 37.1 (36.7–37.7) | 37.1 (36.7–37.7) |
| SBP (IQR), mm Hg | 131 (114–148) | 132 (117–149) | 131 (117–147) | 132 (117–148) |
| DBP (IQR), mm Hg | 75 (65–84) | 77 (67–87) | 74 (68–84) | 75 (67–84) |
| HR (IQR), beats per minute | 98 (85–112) | 92.5 (76.3–105) | 94 (80–107) | 95 (82–107) |
| Oxygen saturation (IQR), % | 95 (91–98) | 98 (96–99) | 96 (94–98) | 96 (92–98) |
| Respiratory rate (IQR), breaths per minute | 20 (18–22) | 18 (17–20) | 18 (18–20) | 19 (18–22) |
COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
Pharmacotherapy
Changes in pharmacological approach are presented in online supplemental table 4 and figure 3.
Figure 3.
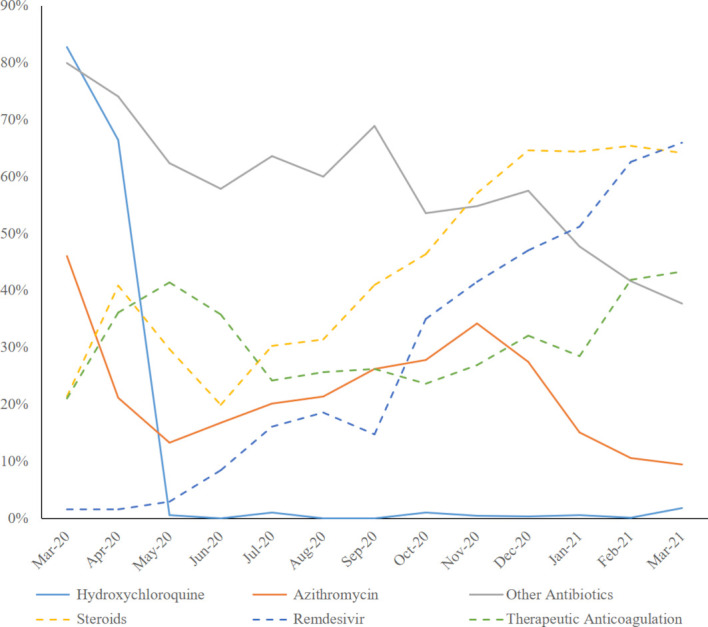
Change in therapies: per cent of patients receiving specific therapies over the year.
Spring patients were more likely to receive hydroxychloroquine, azithromycin and other antibiotics. The use of remdesivir substantially increased throughout the year (from less than 2% during spring to almost 70% by the end of the winter). Steroids prescription (from 33% during spring to almost 70% in February 2021), therapeutic anticoagulation therapy, as well as use of statins, ACE inhibitors (ACE-I) or angiotensin receptor blockers (ARBs) also increased.
Death, intubation and length of stay
Over the course of a year, 1437 (19.4%) died while hospitalised. Patients who died were older, had more comorbidities and were more acutely ill, consistent within prior reports on risk factors for death in COVID-195 6 (online supplemental table 1). The average unadjusted monthly mortality is presented in figure 2. The 30-day in-hospital mortality (figure 4A) was 25.0% for spring patients, 11.0% for summer patients, 6.9% for fall patients and 11.4% for winter patients (p<0.001). On average, spring patients died 6.4 (3.2–12.9) days after arrival to the emergency department, summer patients 7.2 (3.0–15.7) days after arrival, fall patients 13.4 (8.7–21.6) days after arrival and winter patients 13.3 (6.8–20.7) days after arrival (p<0.001). The frequency of invasive ventilatory support was higher during the spring, with 892 patients (19.4%) intubated, vs 27 (10.2%) in summer, 36 (9.5%) in fall and 268 (11.9%) in winter (p<0.001). The median time from arrival to intubation was 0.7 (0.1–4.1) days for spring patients, 0.6 (0.1–8.1) days for summer patients, 2.2 (0.1–7.3) days for fall patients and 2.8 (0.3–7.0) days for winter patients (p<0.001). The median length of stay was 6.1 (3.5–11.1) days during spring, 5.1 (2.7–10.1) days during summer, 5.0 (3.0–10.1) days during fall and 6.3 (3.8–12.0) days during winter (p<0.001).
Figure 4.
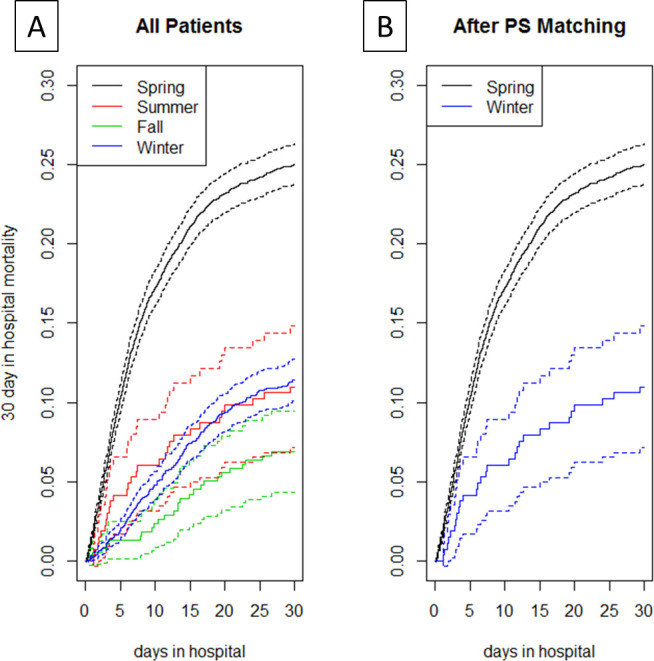
Cumulative incidence: 30-day in-hospital mortality (A) by season and (B) spring vs winter after propensity score matching. PS, propensity score.
Bed saturation and mortality
We defined bed saturation as the percentage of bed occupancy calculated from the ratio between the number of admitted patients over the nominal bed capacity of our institution (1491).
In the multivariable competing risk proportional hazard model of the entire cohort, per cent of bed occupancy was associated with increased 30-day in-hospital mortality (HR 1.007, CI 1.001 to 1.013, p=0.004); that is, mortality increases by 0.7 % for each 1% increase in bed occupancy. Consistent results were observed per level increase in bed occupancy quartile (HR 1.086, CI 1.026 to 1.148, p value for linear trend=0.004). The results of the competing risk regression analysis are presented in table 2.
Table 2.
Association with in‐hospital mortality (regression models with competing risks)
| Variable | Multivariable | |
| HR (95% CI) | P value | |
| Age, years | 1.046 (1.04 to 1.051) | <0.001 |
| Male sex, yes/no | 1.352 (1.187 to 1.54) | <0.001 |
| Body mass index, kg/m2 | 1.022 (1.012 to 1.032) | <0.001 |
| Temperature, °C | 1.129 (1.063 to 1.200) | <0.001 |
| SBP, mm Hg | 0.994 (0.991 to 0.997) | <0.001 |
| DBP, mm Hg | 0.996 (0.991 to 1.001) | 0.14 |
| HR, beats per minute | 1.003 (0.999 to 1.006) | 0.11 |
| Oxygen saturation, % | 0.967 (0.961 to 0.972) | <0.001 |
| Respiratory rate, breaths per minute | 1.027 (1.019 to 1.035) | <0.001 |
| White cell count, ×109/L | 1.008 (1.001 to 1.016) | 0.02 |
| Glucose, mg/dL | 1.001 (1 to 1.001) | 0.001 |
| Aspartate aminotransferase, U/L | 1 (1 to 1.001) | 0.21 |
| Alanine aminotransferase, U/L | 1 (0.999 to 1) | 0.25 |
| Lactic acid, mmol/L | 1.071 (1.036 to 1.107) | <0.001 |
| Platelet count, k/µL | 0.999 (0.998 to 0.999) | <0.001 |
| Potassium, mEq/L | 1.096 (1.028 to 1.168) | 0.0052 |
| Bicarbonates, mEq/L | 0.957 (0.944 to 0.971) | <0.001 |
| Creatinine, mg/dL | 1.023 (0.998 to 1.049) | 0.069 |
| HTN, yes/no | 1.008 (0.851 to 1.194) | 0.93 |
| HLD, yes/no | 1.196 (1.02 to 1.401) | 0.027 |
| CKD, yes/no | 1.263 (1.09 to 1.462) | 0.002 |
| HF, yes/no | 1.33 (1.146 to 1.543) | <0.001 |
| COPD/asthma, yes/no | 0.948 (0.827 to 1.088) | 0.45 |
| DM, yes/no | 0.946 (0.819 to 1.093) | 0.45 |
| CAD, yes/no | 1.101 (0.955 to 1.271) | 0.19 |
| Statin use, % | 0.577 (0.501 to 0.664) | <0.001 |
| Bed occupancy, % | 1.007 (1.001 to 1.013) | 0.004 |
CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; DM, diabetes mellitus; HF, heart failure; HLD, hyperlipidaemia; HR, heart rate; HTN, hypertension; SBP, systolic blood pressure.
Spring versus winter mortality comparison and propensity matched analysis
In the multivariable competing risk proportional hazard model comparing spring and winter season, the 30-day in-hospital mortality was lower in winter (HR 0.520, CI 0.448 to 0.604, p<0.001) when compared with spring. After PS calliper matching, there were 1722 matched pairs. Spring and winter patients had similar distribution of PS (online supplemental figure 1) and the standardised average difference among covariates was greatly reduced. PS analysis showed a significant reduction in in-hospital mortality during winter (HR 0.580, CI 0.507 to 0.663, p<0.001), confirming what we observed in the multivariable adjusted analysis (figure 4B).
Discussion
We examined inpatient mortality from COVID-19 over the course of a 1-year pandemic at our hospital system in New York City. Our principal findings are as follows. First, we observed a substantial reduction in in-hospital mortality, coinciding with multiple pandemic-related public health measures focusing on hospital resource management—and preceding comprehensive changes in pharmacotherapy—towards the end of the first surge. Second, we describe for the first time hospital bed occupancy as an independent risk factor for inpatient mortality from COVID-19.
Public health measures in response to COVID-19
After declaring a state of disaster emergency (7 March 2020), New York State introduced different measures to limit the spread of the disease, including public school closures (16 March 2020), limitation in indoor dining (17 March 2020), stay-home order for non-essential workers (22 March 2020), mandatory face coverings in public (15 April 2020) and night subway closure (30 April 2020).24 Despite these measures to limit the diffusion of the disease and a generalised reduction of movements around New York City (as evidenced by a more than 90% reduction of subway ridership compared with 2019),25 more than 30% of Bronx residents were found to have positive antibodies (and thus possibly temporary immunity) against SARS-CoV-2 in August 2020.26
Specifically relevant to hospital operations, executive order number 202.5 (16 March 2020)27 allowed healthcare providers not licensed or registered in New York State to temporarily work in the state, and executive order number 202.10 (22 March 2020)27 suspended elective operations. These executive orders were associated with a dramatic drop in non-COVID-19 admissions at our institution beginning 16 March 2020 (figure 1). On 26 March 2020 New York State Governor Cuomo additionally mandated all hospitals to increase their bed capacity by 50% to accommodate the surge of patients with COVID-19.27 Despite this order, the actual bed occupancy at our institution (while accommodating all patients with COVID-19 presenting to our hospitals) remained below the usual operating capacity until December 2020.
Notably, COVID-19 mortality remained stable throughout the summer and fall of 2020, with low case counts and increased utilisation of steroids, anticoagulation and remdesivir. Although randomised controlled trials have shown morbidity benefits with the use of remdesivir7 and mortality reduction with steroids,8 the magnitude of these effects cannot explain the more than 50% reduction in mortality we observed. Furthermore, pharmacotherapy, with the exception of hydroxychloroquine elimination, did not materially change within the spring season, by the end of which mortality was already decreased. Steroid, remdesivir and therapeutic anticoagulation were used in 10%–20% of patients by May 2020, but they reached 30%–70% only in the winter season. Despite this, unadjusted mortality began to increase again in December 2020 during the second wave. Of note, bed occupancy also increased at that time and proved to be an independent risk factor for COVID-19 mortality in our cohort of nearly 8000 patients.
Change in therapeutic approach
The initial widespread (more than two-thirds of first spring patients) use of hydroxychloroquine, an agent eventually proven to be ineffective28 to treat COVID-19, probably represents the most obvious pandemic-associated deviation from the usual multiphase clinical trial standards of therapeutic paradigm development. Only 8 of 2254 patients received hydroxychloroquine during the winter wave. Similarly, we observed a reduction in the use of azithromycin and other antibiotics, the latter possibly reflecting a more careful assessment of the need to treat superimposed bacterial infections during the second wave. Steroid therapy8 29 and therapeutic anticoagulation9 were implemented in the majority of patients during the winter after the knowledge on the likely disease modulating inflammatory proprieties and prothrombotic effect of COVID-19 had been recognised30 and, in the case of steroids, a therapeutic effect had been proven.8 Remdesivir, an inhibitor of the viral RNA-dependent RNA polymerase that showed shortening of recovery time in hospitalised patients with COVID-19,7 received emergency approval from the Food and Drug Administration on 22 October 202031 and was administered to almost half of the admitted patients during the winter. If initial concerns of possible interactions between ACE-I or ARBs and SARS-CoV-232 led to a possible underutilisation or discontinuation of these drugs during the spring, we observed a significant increase in their use during the following months, after no increased risks were reported.33 34
Similarly, after several reports showed a possible protective effect associated with the use of statins,35 36 their utilisation markedly increased during the winter.
Lastly, after the spring wave provided anecdotal evidence for early proning in COVID-19 pneumonia, an approach strongly favouring non-invasive ventilation and avoiding intubation was developed to address respiratory distress in COVID-19; more data about such an approach have since accumulated.10 37 The cumulative effect of these therapeutic changes, in combination with a better preparedness to respond to a pandemic, can be estimate from the different mortality between the first surge (spring) and the second surge (winter). After matching the two groups in demographic and clinical variables, as well as in elements indicative of hospital distress (bed occupancy), a significant reduction in mortality was observed during the winter trimester.
Change in hospital stress load
At the peak of the pandemic, the hospital saturation reached the 118% of the nominal bed capacity and patients with COVID-19 accounted for 68.2% of all admitted patients. This increase in acutely ill patients created significant excess demand on the rest of the hospital infrastructure best characterised by the surge in the need for intensive care unit (ICU) beds and transformation of other hospital areas to ICUs.14 23 Despite increased patient load, the number of standard ICU beds, as well as laboratories, diagnostic equipment and available personnel, remained the same as before the pandemic. This unmatched patient overload resulted in a 0.7% mortality increase for each 1% increment in hospital bed saturation. In light of these results, strategies to minimise the bed occupancy for patients without COVID-19 or non-life-saving admission should be adopted to diverge resources to improve the outcome of admitted patients with COVID-19.
Limitations
Our study has the shortcomings of a retrospective investigation, but there are some very specific aspects limiting the interpretation of our results. First, it is difficult to assess the true effects of pharmacotherapy given the dynamic changes in indications, doses and usage that happened over the course of the year. Regardless, we believe the propensity matched comparison between the spring and the winter waves provides compelling evidence for the validity of our principal observation of inpatient COVID-19 mortality reduction disproportionate to advances in pharmacotherapy. We chose total bed occupancy as a metric for hospital stress assuming that other resources per bed remained static. Notably, the ratio of patients with COVID-19 to those without COVID-19, ICU bed saturation and staff shortages are unaccounted for in this model. Regrettably, an indepth analysis of these metrics is beyond our ability in this retrospective pandemic analysis with disaster elements. Additionally, a significant number of patients received ICU-level-of-care interventions (mechanical ventilatory support, dialysis, vasopressor titration) on regular floors; therefore, the concept of ICU bed saturation might have been not truly representative of the burden.
However, we feel our data are sufficiently strong to support the notion that bed capacity expansion alone is not the answer. Rather, a smaller number of beds with higher staffing accomplished by drastic reductions in all non-emergent procedures and activities is likely a better approach. Although offering fewer beds in a pandemic situation appears initially quite counterintuitive, in practice we observed that mortality began to decrease once beds and resources were allocated specifically to patients with COVID-19 by executive orders 202.5 and 202.10, and most importantly that bed occupancy never exceeded 100% once hospital operations focused on the COVID-19 pandemic only. It is conceivable that an uptrend in mortality observed late in the pandemic with established treatment paradigms could be due to new viral strains or a sicker patient population. Although we are unable to provide detailed strain analysis for our study population, a meaningful number of new (and possibly more virulent) strains were not yet observed in the Bronx, where our study was conducted.38 The small sample size of patients in summer and fall does not allow meaningful propensity matched comparisons, and when comparing summer, fall and winter populations there do not appear to be clinically meaningful differences. Lastly, single-patient data on vaccination status were not available. At the conclusion of the study, only 13.8% of the population of New York State have received at least one dose and 7.4% have received two doses.39 Given the heterogeneous distribution of vaccination within the state (and the city of New York), it is impossible to meaningfully account for these parameters.
Conclusions
Inpatient mortality from COVID-19 decreased to a degree disproportionate to advances in disease-specific therapeutics. Increased bed occupancy was associated with higher in-hospital mortality. Implementation of non-pharmacological approaches and other seasonal variations might also had a role in mortality reduction. Early reduction in epicentre hospital bed occupancy to accommodate acutely ill and resource-intensive patients should be a critical component in the strategic planning for future pandemics.
Supplementary Material
Footnotes
Twitter: @FraCastagna_MD
Contributors: Design of the project: FC, XX and UPJ. Underlying data verification: FC, XX and UPJ. Acquisition, analysis and interpretation of data: FC, XX, OS, RK, YAP, SRP, MJG, ADR, DBS and UPJ. Statistical analysis: FC and XX. Obtained funding: UPJ. Manuscript writing: FC, XX and UPJ. Critical revision of the manuscript for important intellectual content: FC, XX, OS, RK, YAP, SRP, MJG, ADR, DBS and UPJ. Supervision: UPJ. Guarantor: UPJ.
Funding: FC is supported by a grant from the National Institutes of Health (T32HL144456) and the National Center for Advancing Translational Science (NCATS) Clinical and Translational Science Award at Einstein-Montefiore (UL1TR001073). OS is supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute (K23HL145140) and the National Center for Advancing Translational Science (NCATS) Clinical and Translational Science Award at Einstein-Montefiore (UL1TR001073). UPJ is supported by the McAdam Family Foundation.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Request for de-identified data and dictionaries will be evaluated on a case-by-case basis after the submission of a research proposal to the corresponding author and the signature of a data access agreement. Data will be available after manuscript publication.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The Office of Human Research Affairs at Albert Einstein College of Medicine approved this study (#2020-11308). Patient consent and HIPAA forms were waived by our IRB due to the retrospective nature of our research.
References
- 1.World Health Organization . WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020. Geneva, Switzerland, 2020. [Google Scholar]
- 2.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region — case series. N Engl J Med 2020;382:2012–22. 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John Hopkins University . COVID-19 Dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU), 2020. Available: https://coronavirus.jhu.edu/map.html [Accessed 20 Jan 2021].
- 4.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 7.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 2020;383:1813–26. 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 2020;18:1023–6. 10.1111/jth.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shelhamer MC, Wesson PD, Solari IL, et al. Prone positioning in moderate to severe acute respiratory distress syndrome due to COVID-19: a cohort study and analysis of physiology. J Intensive Care Med 2021;36:241–52. 10.1177/0885066620980399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg ES, Tesoriero JM, Rosenthal EM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol 2020;48:23–9. 10.1016/j.annepidem.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020;183:e14:158–68. 10.1016/j.cell.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fill Malfertheiner S, Brandstetter S, Roth S, et al. Immune response to SARS-CoV-2 in health care workers following a COVID-19 outbreak: a prospective longitudinal study. J Clin Virol 2020;130:104575. 10.1016/j.jcv.2020.104575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez Villela M, Boucher T, Terre J, et al. Surge-in-Place: conversion of a cardiac catheterization laboratory into a COVID-19 intensive care unit and back again. J Invasive Cardiol 2021;33:E71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389–430. 10.1002/sim.2712 [DOI] [PubMed] [Google Scholar]
- 16.Gray RJ. A class of $K$-sample tests for comparing the cumulative incidence of a competing risk. Ann Stats 1988;16:1141–54. 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the Subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 18.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med 2020;173:773–81. 10.7326/M20-3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–41. 10.1093/biomet/69.1.239 [DOI] [Google Scholar]
- 21.Austin PC, Fine JP. Propensity-score matching with competing risks in survival analysis. Stat Med 2019;38:751–77. 10.1002/sim.8008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou B, Latouche A, Rocha V, et al. Competing risks regression for stratified data. Biometrics 2011;67:661–70. 10.1111/j.1541-0420.2010.01493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philips K, Uong A, Buckenmyer T, et al. Rapid implementation of an adult coronavirus disease 2019 unit in a children's Hospital. J Pediatr 2020;222:22–7. 10.1016/j.jpeds.2020.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.New York City Office of the Mayor . News. Available: https://www1.nyc.gov/office-of-the-mayor/news.page [Accessed 17 Jan 2021].
- 25.Metropolitan Transportation Authority . Fare data. Available: http://web.mta.info/developers/fare.html [Accessed 17 Jan 2021].
- 26.New York City Department of Health and Mental Hygiene . COVID-19: data, 2021. Available: https://www1.nyc.gov/site/doh/covid/covid-19-data.page [Accessed 17 Jan 2021].
- 27.State of New York . Executive orders. Available: https://www.governor.ny.gov/keywords/executive-order [Accessed 17 Jan 2021].
- 28.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med 2020;383:2041–52. 10.1056/NEJMoa2019014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020;324:1330–41. 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 2020;20:1135–40. 10.1016/S1473-3099(20)30434-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.United States Federal Drug Administration . FDA’s approval of Veklury (remdesivir) for the treatment of COVID-19—The Science of Safety and Effectiveness. Available: https://www.fda.gov/drugs/drug-safety-and-availability/fdas-approval-veklury-remdesivir-treatment-covid-19-science-safety-and-effectiveness [Accessed 17 Jan 2021].
- 32.Esler M, Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens 2020;38:781–2. 10.1097/HJH.0000000000002450 [DOI] [PubMed] [Google Scholar]
- 33.Mancia G, Rea F, Ludergnani M, et al. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020;382:2431–40. 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020;126:1671–81. 10.1161/CIRCRESAHA.120.317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan WYT, Young BE, Lye DC, et al. Statin use is associated with lower disease severity in COVID-19 infection. Sci Rep 2020;10:17458. 10.1038/s41598-020-74492-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saeed O, Castagna F, Agalliu I, et al. Statin use and in-hospital mortality in patients with diabetes mellitus and COVID-19. J Am Heart Assoc 2020;9:e018475. 10.1161/JAHA.120.018475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul V, Patel S, Royse M, et al. Proning in Non-Intubated (PINI) in Times of COVID-19: case series and a review. J Intensive Care Med 2020;35:818–24. 10.1177/0885066620934801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.New York City Department of Health and Mental Hygiene . Health department releases detailed report on COVID-19 variants, 2021. Available: https://www1.nyc.gov/site/doh/about/press/pr2021/health-department-releases-detailed-report-on-covid-19-variants.page [Accessed 12 Apr 2021].
- 39.New York City Department of Health and Mental Hygiene . COVID-19: Data - Vaccines: New York City Department of Health and Mental Hygiene, 2021. Available: https://www1.nyc.gov/site/doh/covid/covid-19-data-vaccines.page#dosestrend [Accessed 10 Dec 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058171supp001.pdf (361.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Request for de-identified data and dictionaries will be evaluated on a case-by-case basis after the submission of a research proposal to the corresponding author and the signature of a data access agreement. Data will be available after manuscript publication.