Abstract
Female mice homozygous for an engineered Gnrhr E90K mutation have reduced gonadotropin-releasing hormone signaling, leading to infertility. Their ovaries have numerous antral follicles but no corpora lutea, indicating a block to ovulation. These mutants have high levels of circulating estradiol and low progesterone, indicating a state of persistent estrus. This mouse model provided a unique opportunity to examine the lack of cyclic levels of ovarian hormones on uterine gland biology. Although uterine gland development appeared similar to controls during prepubertal development, it was compromised during adolescence in the mutants. By age 20 weeks, uterine gland development was comparable to controls, but pathologies, including cribriform glandular structures, were observed. Induction of ovulations by periodic human chorionic gonadotropin treatment did not rescue postpubertal uterine gland development. Interestingly, progesterone receptor knockout mice, which lack progesterone signaling, also have defects in postpubertal uterine gland development. However, progesterone treatment did not rescue postpubertal uterine gland development. These studies indicate that chronically elevated levels of estradiol with low progesterone and therefore an absence of cyclic ovarian hormone secretion disrupts postpubertal uterine gland development and homeostasis.
Keywords: GnRHR, estrogen, progesterone, endometrium, adenogenesis, hydrometria
Endometrial or uterine glands produce and transport secretions during gestation that are essential for embryo survival and development (1). This is especially true during preimplantation development, when uterine glands are the primary source of nutrients for the embryo. The uterine secretome has been characterized and includes nutrients, cytokines, hormones, and a variety of other factors (2-4). Blocking uterine gland formation causes infertility due to failed implantation, highlighting the essential role for uterine glands in early conceptus development (5-7).
Uterine gland development proceeds in 2 waves. In most species studied, uterine glands are not present at birth when the uterus consists of a simple epithelium surrounded by undifferentiated mesenchyme (8, 9). Shortly after birth, the uterus differentiates into separate layers, an endometrial stromal layer surrounded by 2 myometrial smooth muscle layers (9). At birth, the endometrium consists of a simple, columnar epithelium, lining the lumen of the uterus (luminal epithelium) and the underlying mesenchyme. In the mouse, the first wave of uterine gland development occurs within 1 week after birth, when numerous foci within the luminal epithelium begin to invaginate and elongate through the stroma toward the myometrium (10, 11). During this prepubertal period, uterine gland development occurs in an ovary- and hormone-independent manner (12, 13). The second wave of uterine gland development occurs after puberty (adolescence) and is dependent on ovarian hormones (14). At this stage, uterine gland growth elaborates branched structures within the stroma (15, 16). In primates and a subset of other mammalian species, a third period of growth occurs with every menstrual cycle as the endometrium is regenerated (17-19).
The gonadotropin-releasing hormone receptor (GnRHR) is a 7-transmembrane G-protein coupled receptor primarily expressed by gonadotroph cells in the anterior pituitary (20). GnRHR signaling stimulates the release of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), in response to its ligand, GnRH, produced in the hypothalamus. The pulsatile release of LH and FSH is essential for ovarian follicle selection, maturation, ovulation and luteinization, and production of the ovarian steroid hormones, estradiol and progesterone. Steroid hormones form a negative feedback loop for GnRH release. This system is referred to as the hypothalamic-pituitary-gonadal axis, which is essential for cyclicity, fertility, and pregnancy. GnRHR signaling stimulation by GnRH agonists has been used to treat disorders in women, including endometriosis, uterine fibroids, and polycystic ovarian syndrome (PCOS) (21-23). However, GnRH agonist treatment can increase the risk of pathologies, including diabetes mellitus and cardiovascular disease (24). Inhibition of GnRH signaling by GnRH antagonists has been used during ovarian hyperstimulation for fertility treatments (25). GnRH antagonist treatment can prevent the development of diabetes in susceptible rodents (26). It has also been used to reduce testosterone levels in prostate cancer patients (27). Thus, modulating GnRH signaling regulates a variety of physiological processes and can lead to pathologies.
Previously, we engineered mice with a single amino acid substitution in the Gnrhr gene (Gnrhr E90K) to mimic a human homozygous mutation that causes hypogonadotropic hypogonadism in males (28). The E90K substitution prevents formation of a salt bridge between E90 and K121 in the first and second transmembrane domains (29). This folding defect is recognized and retained in the endoplasmic reticulum by the quality control system. Interestingly, if correctly routed to the plasma membrane, the E90K mutant responds to GnRH and functions appropriately (30). The net result of the E90K mutant is reduced plasma membrane expression of GnRHR and therefore reduced signaling. GnrhrE90K homozygous female mice are infertile because of a block in ovulation. Their ovaries have multiple, antral follicles but no corpora lutea (CL) (28). Thus, GnrhrE90K homozygous females do not cycle, suggesting that they are in a persistent state of estrus. These mice provide a unique model to determine the consequence of preventing cyclic ovarian hormone production on uterine gland development. In these mutant mice, postpubertal uterine gland development was impaired and disease-related phenotypes occurred, including hydrometria and glandular hyperplasia. These results suggest that cyclic steroid hormone production is essential for postpubertal uterine gland development and endometrial homeostasis.
Materials and Methods
Mice
The GnrhrE90K and Pgrtm2(cre)Lyd mice were maintained on a C57BL/6J × 129/SvEv mixed background. GnrhrE90K and Pgrtm2(cre)Lyd mice were genotyped as previously described (28, 31). Mice for experimental analysis were obtained by crossing GnrhrE90K/+ females with GnrhrE90K/E90K males. The mutants were GnrhrE90K/E90K and the controls were GnrhrE90K/+. For Pgrtm2(cre)Lyd (PgrCre) mice, PgrCre/+ females were bred with PgrCre/Cre males. The mutants were PgrCre/Cre (referred to as PRKO) and the controls were PgrCre/+ (referred to as controls) All experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center.
Estrous Cycle Assessment
Vaginal washes of sexually mature control and experimental females were examined by light microscopy to determine the stage of the estrous cycle (32). Because the GnrhrE90K/E90K mutant females exhibited ovarian and hormonal characteristics of persistent estrus, we used only control females at the estrous stage of the cycle.
Hormone Treatments
Human chorionic gonadotropin (hCG) (Sigma) or phosphate-buffered saline (PBS) was injected intraperitoneally every 4 days from age 5 to 8 weeks. Slow-release implants were prepared with or without 0.5 g/mL progesterone in peanut oil in silastic tubing (Fisher Scientific 11-189-15G) with a 1-cm release space. The ends of the implants were heat-sealed with polyethylene tubing inserted into the silastic tube (33-35).
Tissue Collection
Tissues were dissected in PBS immediately after euthanasia. Uterine horn length was measured from the uterotubal junction to the uterine bifurcation on freshly dissected tissues. The number of CL was counted in freshly dissected ovaries. Uteri and ovaries were fixed in 4% paraformaldehyde in PBS for 24 hours at 4 °C and rinsed in 70% ethanol for 24 hours or more before tissue processing. All tissues were dehydrated through a standard ethanol gradient, cleared in Histoclear (National Diagnostics), and embedded in Paraplast Plus Tissue Embedding Medium (StatLab).
Histological Analyses and Glandular Tissue Quantification
Five µm sections were cut from paraffin-embedded tissues. Hematoxylin-eosin (H&E) staining was performed using a standard protocol. Uterine glandular tissue abundance was performed by counting the number of endometrial gland cross-sections (5 slides, 6 sections per slide for each animal) of the uterus from mice of each genotype at various time points or following hormone treatment (n = 4-9) in a blind experiment.
Immunohistochemistry
Mouse monoclonal antiestrogen receptor antibody (Biocare Medical catalog No. ACA 054C, RRID:AB_2651037, http://antibodyregistry.org/AB_2651037, 1:100) and rabbit anti-progesterone receptor antibody (Cell Signaling catalog No. 8757, RRID:AB_2797144, http://antibodyregistry.org/AB_2797144, 1:400) were used for immunohistochemistry. Rabbit anti-FOXA2 antibody (Seven Hills BioReagents, catalog No. WRAB-1200, RRID:AB_451718, http://antibodyregistry.org/AB_451718, 1:2000) was used for immunofluorescence.
Microscopy
Gross images were acquired using a Leica MZ10F stereomicroscope and the extended depth of focus feature of the LAS v3.7 software (Leica Microsystems). Brightfield images of tissues sections were obtained using a Nikon 80i microscope equipped with a DS-Fi1 5-megapixel color camera (Nikon Instruments) and NIS Elements AR 3.1 software v4.13 (Nikon Instruments). Fluorescent images of tissue sections were acquired using an A1 Nikon confocal microscope.
Real-time Polymerase Chain Reaction
Total RNA for quantitative polymerase chain reaction qPCR was extracted using TRIzol reagent according to the manufacturer’s protocol (Life Technologies). Relative messenger RNA (mRNA) levels were measured using TaqMan gene expression assays with 6-carboxyfluorescein (FAM)-labeled probes. Primer probe sets were Cyp19a1 (Mm00484049(m1), Cyp11a1 (Mm00490735_m1), Esr1 (Mm00433149_m1), Pgr (Mm00435625_m1), Foxa2 (Mm00839704_mH), and Ihh (Mm00439613_m1). Gapdh mRNA expression levels were used for normalization. The PCR was run using an ABI Prism 7900HT thermocycler and SDS2.1 software (Applied Biosystems). Data were analyzed by the comparative ∆∆CT method.
Serum Hormone Levels
Serum levels of progesterone, estrogen, and testosterone were measured by enzyme-linked immunosorbent assay by the Ligand Assay and Analysis Core at the University of Virginia.
Statistical Analyses
For experiments with more than 2 experimental groups, quantitative data were subjected to one-way analysis of variance (ANOVA) using MedCalc v14.12.0 software (MedCalc Software). If the analysis of variance was positive (P < .05), a post hoc Student-Newman-Keuls test was performed for pairwise comparison of subgroups. For studies with 2 experimental groups, an independent-sample t test was performed. A P value of less than .05 was considered statistically significant for all tests.
Results
Ovulation Failure in GnrhrE90K Homozygotes Results in Persistent Estrus and Low Levels of Progesterone
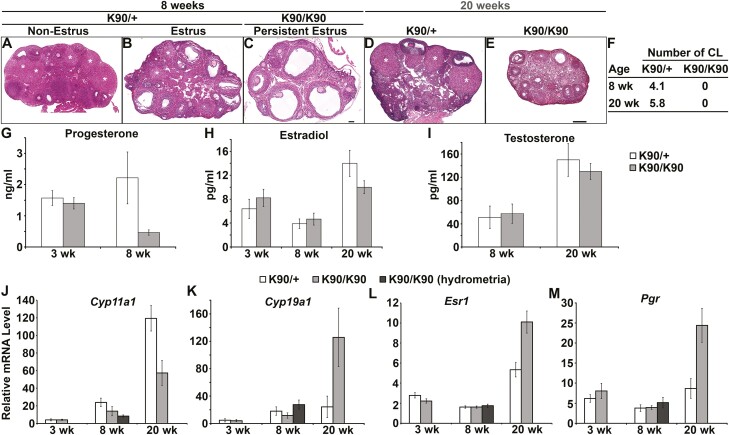
Previously, we reported that female mice homozygous for GnrhrE90K are infertile because of ovulation failure (28). In this study, our objective was to determine how ovulation failure affected cyclic hormone levels and uterine gland development. At age 8 weeks, female mice heterozygous for GnrhrE90K (controls) were fertile and cycled. Ovarian histology showed various stages of folliculogenesis with multiple CL (Fig. 1A). At estrus, GnrhrE90K heterozygotes exhibited multiple antral follicles and CL (Fig. 1B). In contrast, all GnrhrE90K homozygous females had multiple antral follicles with no CL (Fig. 1C). Similar results were found at age 20 weeks. GnrhrE90K heterozygotes at estrus exhibited a mixture of follicles at various stages and CL (Fig. 1D). However, no CL were observed on the ovaries of GnrhrE90K homozygotes (Fig. 1E). The average number of CL per ovary for mutants and controls is summarized in Fig. 1F.
Figure 1.
Ovulation failure in GnrhrE90K homozygotes results in persistent estrus and low levels of progesterone. A to E, Hematoxylin-eosin–stained ovarian sections. Corpora lutea (CL) (white asterisks) were abundant in heterozygous controls (A, B, and D), but absent in GnrhrE90K homozygotes (C and E). A to C, scale bar = 100μm; D and E, scale bar = 250 µm. F, Average number of CL per ovary (± SEM). G to I, Steroid hormone levels. J to M, Real-time polymerase chain reaction gene expression levels (± SEM) in the ovary. *P less than .05, *** P less than .005, t test. K90/+, GnrhrE90K heterozygotes; K90/K90, GnrhrE90K homozygotes.
We next determined the effect of the GnRHR E90K-induced ovulation block on blood levels of ovarian steroid hormones. Serum was collected at estrus for all sexually mature groups to normalize for the persistent estrus observed in GnrhrE90K homozygotes. At age 3 weeks, progesterone levels were comparable between mutants and controls (Fig. 1G, P = .29). As expected, owing to the lack of CL in the mutants, progesterone levels were reduced at age 8 weeks (Fig. 1G, P < .05). Estradiol levels were similar between mutants and controls at ages 3, 8, and 20 weeks (Fig. 1H, P = .21, P = .32, and P = .1, respectively). Testosterone levels were similar between mutants and controls at ages 8 and 20 weeks (Fig. 1I, P = .41 and P = .24, respectively). These data demonstrate that GnrhrE90K homozygotes have estradiol levels comparable to controls in estrus and low progesterone levels, suggesting that the mutants are in a persistent state of estrus.
Next, we tested the effect of GnRHR E90K on the expression of genes encoding steroidogenic enzymes (Cyp11a1 and Cyp19a1, encoding P450scc and aromatase, respectively) and steroid hormone receptors (Esr1 and Pgr, encoding estrogen receptor alpha and progesterone receptor, respectively) in the ovary (Fig. 1J-1M). P450scc catalyzes the first biosynthetic step in steroidogenesis, converting cholesterol to pregnenolone. Aromatase converts testosterone to estradiol.
At age 3 weeks, no differences in the levels of Cyp11a1 (P = .47), Cyp19a1 (P = .42), or Pgr (P = .18) were observed between mutants and controls; however, Esr1 was reduced in the mutants (P < .001) (Fig. 1J-1M). At ages 8 and 20 weeks, Cyp11a1 was downregulated in the mutants compared to controls (Fig. 1J, P < .005 for both time points). Cyp19a1 was upregulated at age 20 weeks (Fig. 1K, P = .01). At age 8 weeks, Esr1 (P = .48) and Pgr (P = .44) expression levels were comparable between the mutants and controls but were elevated in the mutants at age 20 weeks (Figs. 1L and 1M, P < .001 and P < .002, respectively).
Chronic Estrus Results in Compromised Uterine Gland Development During Adolescence
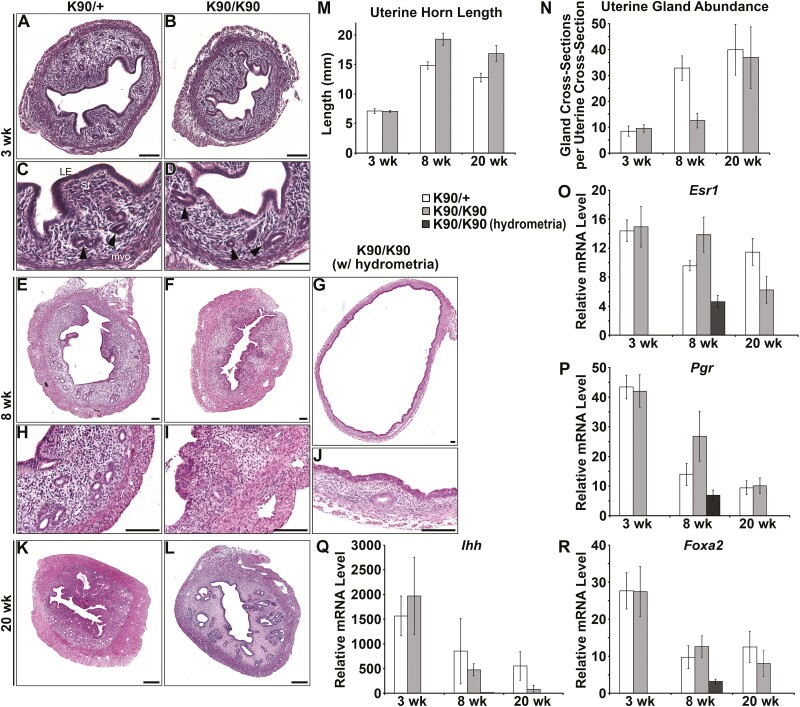
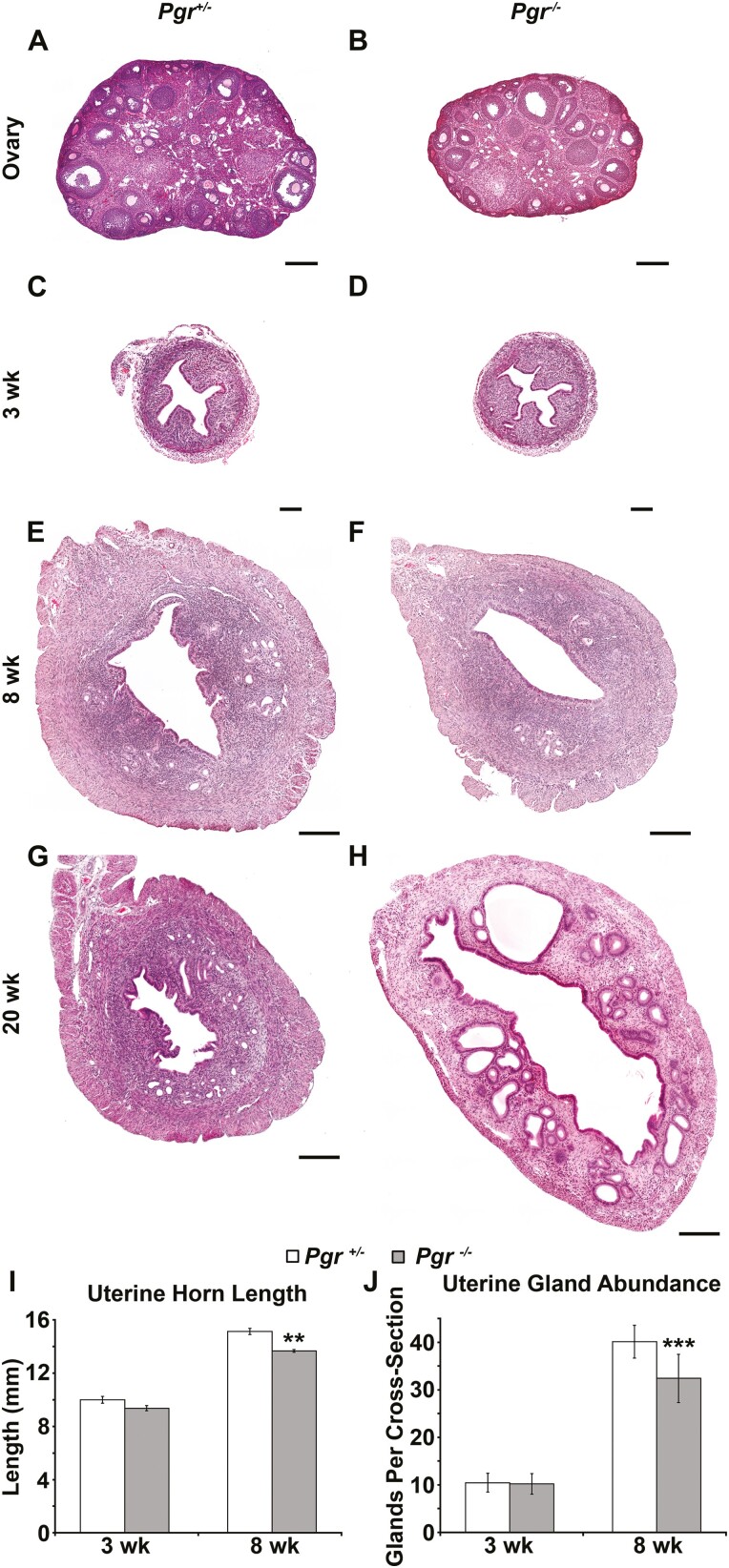
Uterine gland development proceeds in 2 waves. The first glands form as buds from the luminal epithelium shortly after birth (9, 11). This initial gland structure is elaborated during adolescence. Postnatal uterine gland development in GnrhrE90K homozygous females was similar to controls as determined by histology (Fig. 2A-2D), measurements of uterine horn length (Fig. 2M, P = .41), and glandular tissue abundance (Fig. 2N, P = .23). By age 8 weeks (post puberty), uterine histoarchitecture was affected in GnrhrE90K homozygotes (Fig. 2E-2J). About half the GnrhrE90K homozygotes exhibited uteri that appeared similar to controls except with statistically significantly decreased uterine glandular tissue abundance (Figs. 2E, 2F, 2H, 2I, and 2N, P < .001). However, the average area of the endometrium of GnrhrE90K homozygotes (n = 7) was not statistically significantly different from controls (n = 8). The other half of the mutants had hydrometria (fluid-filled uterine horns) accompanied by a reduction in myometrial and endometrial layer width and very few uterine glands (Figs. 2G and 2J). At age 8 weeks, uterine horn length was increased for GnrhrE90K homozygotes compared to controls (Fig. 2M, P < .001). These data indicate impaired uterine gland development during adolescence in GnrhrE90K homozygous females that coincides with a loss of steroid hormone cycling.
Figure 2.
Compromised uterine gland development during adolescence. (A to L), Hematoxylin-eosin–stained uterine sections. (A, C, E, H, and K) GnrhrE90K heterozygous controls. (B, D, F, G, I, J, and L) GnrhrE90K homozygous mutants. (A to D) Uterine histoarchitecture and gland development was comparable between mutants and controls at age 3 weeks. (E to J) Uterine gland development was perturbed in the mutants by age 8 weeks. (G and J) Some GnrhrE90K homozygotes exhibited hydrometria. (K and L) By age 20 weeks, uterine gland development was similar between mutants and controls. Scale bars = 100 μm. Black arrowheads, uterine gland tissue. M, GnrhrE90Khomozygotes displayed increased uterine horn length after age 3 weeks. N, The nonhydrometria mutants had decreased numbers of uterine glands at age 8 weeks. (O to R) Expression levels of Esr1, Pgr, Ihh, and Foxa2. *P less than .05, *** P less than .005, t test.
Interestingly, the uteri of GnrhrE90K homozygous females at age 20 weeks were similar to controls. No hydrometria was detected and uterine histoarchitecture was comparable to controls (Figs. 2K and 2L). Uterine horn length was still increased in the mutants compared to controls (Fig. 2M, P < .001), but uterine glandular tissue abundance was similar to controls (Fig. 2N, P = .27). However, pathologies were observed (discussed next). These data suggest the postpubertal wave of uterine gland development is delayed in the absence of cyclic steroid hormones.
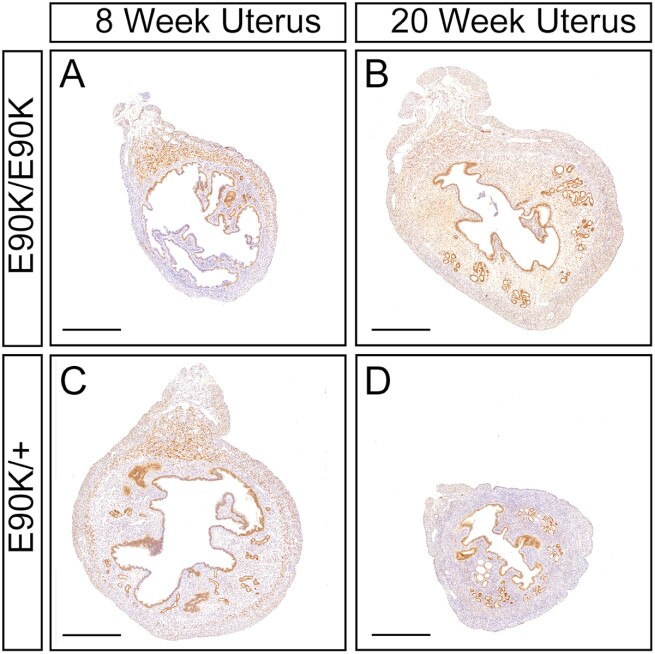
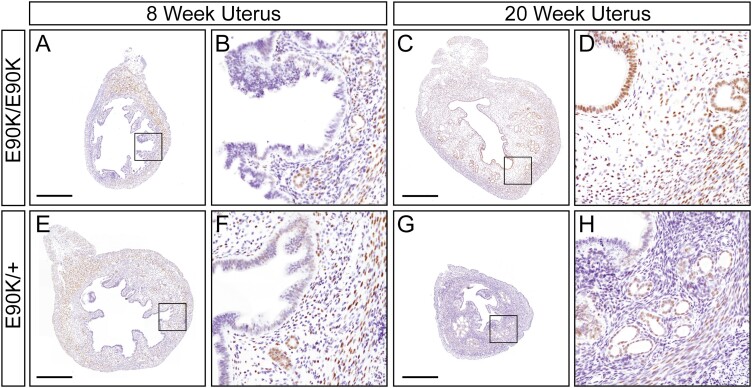
We next examined the expression of Esr1 and Pgr transcript levels in the uterus by real-time PCR (Figs. 2O and 2P). At age 3 weeks, uterine expression of Esr1 (P = .42) and Pgr (P = .41) was similar, consistent with histological assays. At age 8 weeks, Esr1 mRNA levels were elevated in nonhydrometria uteri of mutants (P = .04). However, Esr1 mRNA levels were decreased in hydrometria-mutant uteri relative to controls (P < .001). At age 8 weeks, Pgr mRNA levels were not significantly different between mutants and controls (P = .069). Pgr mRNA levels were reduced in hydrometria-mutant uteri relative to nonhydrometria-mutant uteri (P = .02). At age 20 weeks, Esr1 expression was decreased (P = 0.03), but Pgr was similar to controls (Fig. 2O, P = .21). Although Esr1 transcript levels in the uterus were elevated at age 8 weeks and decreased at age 20 weeks, estrogen receptor protein localization in uterine tissues was similar to controls (Fig. 3). In addition, progesterone receptor protein localization in uterine tissues was also similar to controls (Fig. 4).
Figure 3.
Immunohistochemical localization of estrogen receptor (ER) in the uterus of GnrhrE90K homozygotes and heterozygous controls mice at ages 8 and 20 weeks. A and B, GnrhrE90K homozygotes; C and D, GnrhrE90K heterozygous controls. ER, brown; hematoxylin, purple. Mesometrium, top. Scale bar = 500 μm.
Figure 4.
Immunohistochemical localization of progesterone receptor (PR) in the uterus of GnrhrE90K homozygotes and heterozygous controls mice at ages 8 and 20 weeks. A to D, GnrhrE90K homozygotes; E to H, GnrhrE90K heterozygous control mice. PR brown; hematoxylin, purple. Mesometrium, top. Scale bar = 500 μm.
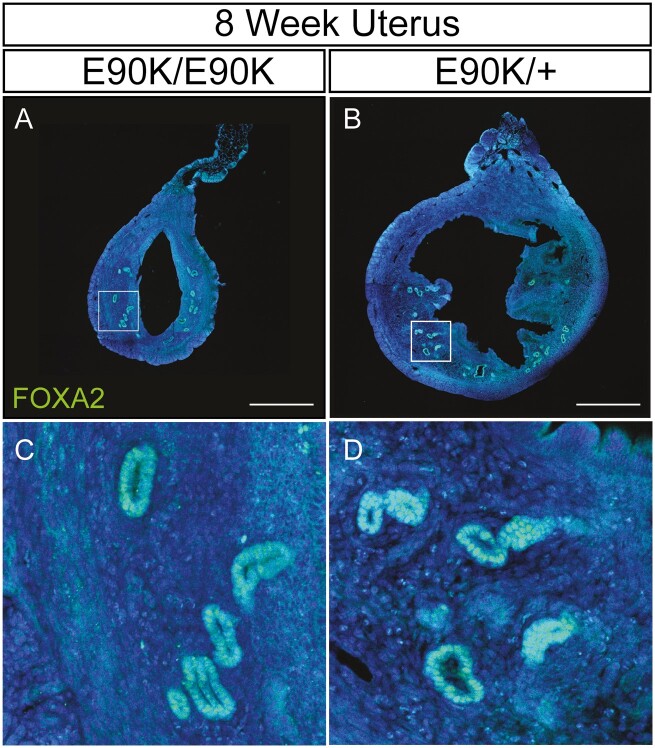
Ihh is a progesterone-responsive gene and mediator of progesterone action for epithelial to stromal interactions in the mouse uterus (36, 37). Expression of Ihh in mutants and controls was similar at all 3 time points (Fig. 2Q, P = .31, P = .29, and P = .07, respectively). Foxa2 is a molecular marker of the uterine glands (38). Foxa2 expression was similar between mutants and controls at ages 3, 8, and 20 weeks (P = .49, P = .24, and P = .29, respectively), but was lower in hydrometria-mutant uteri at age 8 weeks (Fig. 2R, P = .04). FOXA2 protein was localized to nuclei of uterine glandular epithelium of both nonhydrometria mutants and controls at age 8 weeks (Fig. 5).
Figure 5.
Immunofluorescent localization of FOXA2 in uterine gland of GrnhrE90K homozygotes and heterozygous control mice at age 8 weeks. A and C, GrnhrE90K homozygous mutant; B and D, GrnhrE90K heterozygous control. Mesometrium, top. FOXA2, green; DAPI, blue. Scale bar = 500 μm.
Uterine Pathologies in GnrhrE90K Homozygotes
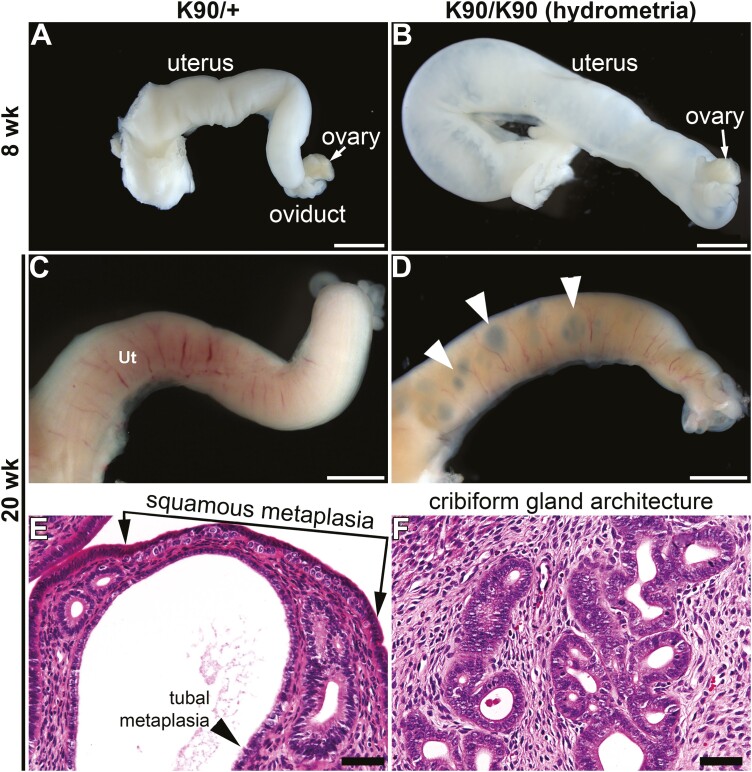
At age 8 weeks of age, the gross morphology of uteri from GnrhrE90K homozygotes appeared similar to controls except for those mutants exhibiting swelling due to hydrometria (Fig. 6A and 6B). By age 20 weeks, we noticed dark focal regions within the uterine wall of the mutants that were visible under the stereo dissecting microscope (Figs. 6C and 6D). Histological analysis showed enlarged cavities peripheral to the uterine lumen associated with the glandular epithelium (Figs. 2K and 2L). In addition, there was squamous metaplasia, tubal metaplasia, and cribriform gland architecture, indicating glandular hyperplasia (Figs. 6E and 6F). Thus, by age 20 weeks persistent estrous levels of estrogen and low progesterone led to uterine epithelial pathologies.
Figure 6.
Uterine pathologies in GnrhrE90K homozygotes. A to D, Dissecting stereomicroscopic images of uterine horns. A and C, GnrhrE90/+ controls; B and D, GnrhrE90K homozygous mutants. B, Hydrometria in GnrhrE90K homozygous mutant. Focal abnormalities appeared by age 20 weeks (white arrowheads in D). E and F, Hematoxylin-eosin–stained sections of uteri at age 20 weeks showing pathologies. A to D, scale bars = 2 mm; E and F, 50 μm.
Induced Ovulation Eliminates Hydrometria, but Does not Rescue Postpubertal Uterine Gland Defects in GnrhrE90K Homozygous Females
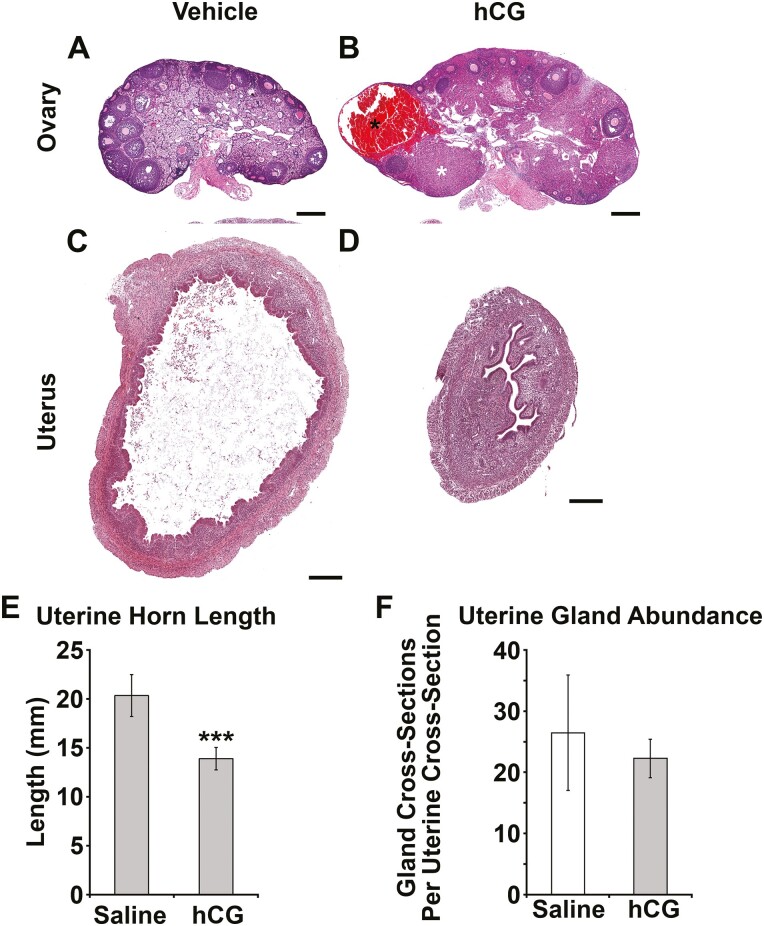
To test whether repeated inductions of ovulation could rescue postpubertal uterine gland development, GnrhrE90K homozygous females were treated with hCG or vehicle every 4 days from ages 5 to 8 weeks. Administration of hCG induced ovulation and formation of CL in the mutants (Fig. 7A and 7B and Fig. 8). Hydrometria was observed in approximately 50% of the mutants treated with vehicle. In contrast, hydrometria was not observed in the hCG-treated mutants as shown by histology (Figs. 7C and 7D), and reduced uterine horn length (Fig. 7E, P = .01). However, the repeated induced ovulations did not rescue the postpubertal uterine gland mutant phenotype (Fig. 7F, P = .12). The lack of hydrometria in the hCG-treated mutants suggests that this treatment regimen was sufficient to alter hormone levels, but not to rescue adolescent uterine gland development.
Figure 7.
Induced ovulation eliminates uterine edema, but does not rescue uterine gland development in GnrhrE90K mice. A and B, Administration of human chorionic gonadotropin (hCG) induced ovulation as evident by development of corpora hemorrhagica (black asterisk) and corpora lutea (white asterisk) in GnrhrE90K homozygotes. C and D, Administration of hCG restored uterine histoarchitecture in GnrhrE90K homozygotes. Scale bars = 250 μm. E, Uterine horn length was reduced in mice that received hCG. F, Uterine glandular tissue abundance was unaffected by hCG treatment. ***P less than .01, t test.
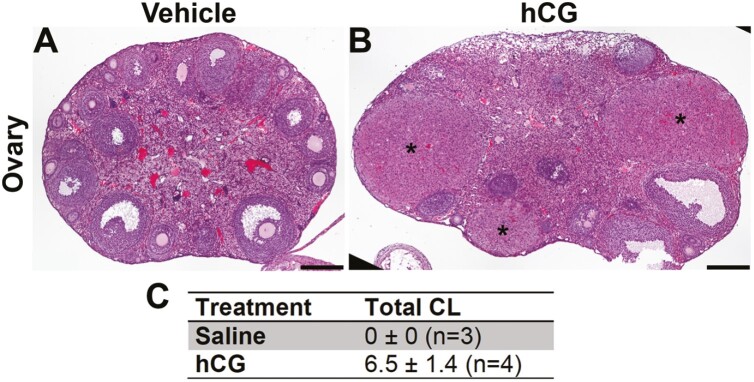
Figure 8.
A single injection of human chorionic gonadotropin (hCG) induces ovulation in GrnhrE90K homozygous mice. A and B, Hematoxylin-eosin–stained ovarian sections. Corpora lutea (CL) were absent from A, controls, but abundant in the ovaries of B, mice that received hCG (black asterisks). C, Average number of CL per female (both ovaries) (± SEM). Scale bars = 200 µm.
Progesterone Receptor Knockout Mice Also Have Reduced Postpubertal Uterine Gland Development
Gnrhr E90K homozygous females fail to ovulate or form CL. In addition to estrous levels of estradiol, they have low levels of circulating progesterone. Thus, we hypothesized that the delayed postpubertal gland development was due to insufficient progesterone signaling within the endometrium. To test this idea, we determined if postpubertal gland development was also impaired in Pgr knockout (PRKO) mice. As previously reported (39), the ovaries of PRKO females showed no evidence for ovulation (no CL were present in histological sections) (Fig. 9A and 9B). At age 3 weeks, uterine histology was similar between PRKO females and controls (Figs. 9C and 9D). There was no difference in uterine horn length (Fig. 9I, P = .13) or glandular tissue abundance between mutants and controls (Fig. 9J, P = .40). By age 8 weeks, uterine gland development was noticeably impaired (Fig. 9E and 9F), including reduced uterine horn length (Fig. 9I, P < .005) and less glandular tissue (Fig. 9J, P < .001). Similar to GnrhrE90K homozygotes, uterine gland development was comparable to controls by age 20 weeks. However, PRKO uterine glands were dilated (Fig. 9G and 9H). These data suggest that progesterone signaling is important for uterine gland development during the postpubertal period.
Figure 9.
Pgr Cre/Cre (PRKO) mice also display reduced uterine gland development at age 8 weeks. A and B, Hematoxylin-eosin (H&E)-stained ovarian sections. Similar to GnrhrE90K homozygotes, PRKO mice exhibit ovulation failure. C to H, H&E-stained uterine sections. The uteri of PRKO mice appeared morphologically similar at C and D, age 3 weeks, but uterine glandular tissue was less abundant at E and F, age 8 weeks. G and H, Similar to GnrhrE90K homozygotes, uterine glands were large and dilated at age 20 weeks. A and B, scale bars = 250 μm; C to H, scale bars = 200 µm. I, Uterine horn length was reduced for PRKO females at age 8 weeks. J, Uterine glandular tissue abundance was reduced for PRKO females at age 8 weeks. **P less than .005, ***P less than .001, t test.
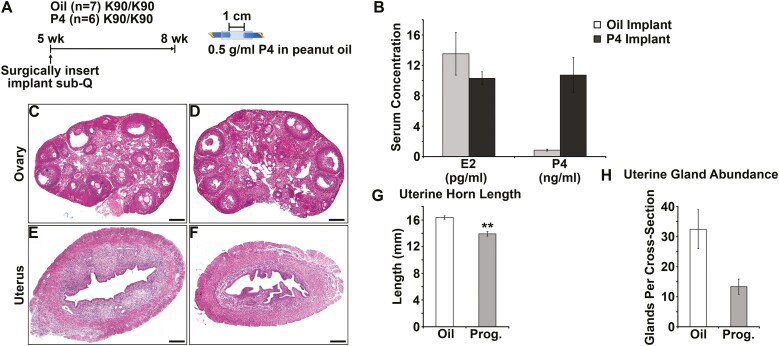
Progesterone Treatment Does not Rescue Postpubertal Uterine Gland Development in GnrhrE90K Homozygous Mice
Next, we tested the idea that low progesterone levels were responsible for delayed postpubertal uterine gland development in GnrhrE90K homozygous mice. GnrhrE90K homozygous females were given vehicle or progesterone implants at age 5 weeks and tissues were collected at age 8 weeks (Fig. 10A). We confirmed elevated progesterone blood levels in the progesterone-treated group by radioimmunoassay (Fig. 10B, P < .001). There was no effect of progesterone treatment on circulating levels of estradiol (see Fig. 10B, P = .16). Progesterone treatment did not affect ovarian histology as abundant antral follicles and no CL were observed in vehicle or progesterone-treated mutants (Fig. 10C and 10D). Surprisingly, progesterone treatment did not rescue postpubertal uterine gland development (Fig. 10E and 10F). Uterine horn length was reduced in the progesterone-treated group (Fig. 10G, P = .01), perhaps because hydrometria was not observed. Uterine glandular tissue abundance was reduced by progesterone treatment (Fig. 10H, P < .001). Thus, continual progesterone release relieved hydrometria, but negatively affected postpubertal uterine gland development.
Figure 10.
Progesterone supplement is unable to rescue uterine gland development in GnrhrE90K mice. A, Experimental design. Slow-release hormone implants were administered at age 5 weeks and tissues harvested at age 8 weeks. Only GnrhrE90Khomozygotes were used for this study. B, Administration of the progesterone implants increased serum progesterone levels, but did not affect estradiol levels. C and D, Administration of progesterone did not affect the persistent antral follicle phenotype. E and F, Overall uterine morphology was similar between groups; however, mice in the progesterone-treated group appear to have less glandular tissue. G, Uterine horn length was reduced in progesterone-treated mice. H, Uterine glandular tissue abundance was reduced in progesterone-treated mice. Scale bars = 200 μm. **P less than .05, ***P less than .005, t test.
Discussion
The GnrhrE90K mouse model offers a unique opportunity to observe the consequences of reduced plasma membrane expression of GnRHR on the hypothalamic-pituitary-gonadal axis. Gonadotropes synthesize and release LH and FSH in response to the GnRHR ligand GnRH. GnRHR plasma membrane levels vary throughout the estrous cycle, peaking at proestrus in close correlation with the preovulatory LH surge (40). At proestrus, GnRH release becomes less pulsatile and more sustained. GnrhrE90K homozygous females fail to produce an LH surge and therefore do not ovulate, resulting in an ovarian cycle that is arrested at the antral follicle stage. These observations support the idea that high levels of GnRHR plasma membrane expression are required to decode the switch from pulsatile to sustained GnRH. Thus, there is a close relationship between plasma membrane levels of GnRHR and gonadotropin release. Importantly, a threshold level of plasma membrane-associated GnRHR must be achieved to induce the LH surge.
The ovarian cycle of GnrhrE90K females is effectively arrested at the antral follicle stage because of ovulation failure. As a consequence, ovarian steroid hormones are not produced cyclically. We found continual production of estradiol at levels similar to those of an animal in estrus and very low progesterone. Low progesterone levels are a consequence of the inability to form CL. Thus, GnrhrE90K homozygous females exhibit a unique endocrine phenotype in which the estrous cycle is arrested. This mutant phenotype (arrested antral follicles, estrous levels of estradiol with low progesterone) is reminiscent of human PCOS, which is also characterized by ovulation failure in the presence of multiple antral follicles (polycystic ovaries) (41). However, one key characteristic of human PCOS that the GnrhrE90K females did not exhibit was elevated androgens. The GnrhrE90K females showed metaplastic changes in their uterine epithelium and glandular hypertrophy by age 20 weeks. Women with PCOS have an increased risk of developing endometrial cancer (42). Our data suggest that in humans PCOS may be detrimental to uterine gland growth and maintenance of pregnancy. Thus, even if ovulation is restored, the ability of the uterus to support peri-implantation growth and development might be compromised. This could account for the increased prevalence of low birth weight babies born to women with PCOS (43).
Uterine gland development occurs in 2 waves. The neonatal wave is considered to be ovarian hormone independent, whereas the postpubertal (adolescent) wave is hormone dependent. In GnrhrE90K homozygous females, the hormone-independent neonatal wave proceeded similarly to controls, but the second wave of development was impaired. These results support the idea that the neonatal wave is hormone independent and expand our understanding of the postpubertal wave by illustrating the importance of cyclic, rather than continual, hormone production for proper uterine gland growth and development.
Not only was uterine gland development impaired, but disease-related phenotypes manifested, including hydrometria and glandular hyperplasia. Interestingly, hydrometria appeared in about 50% of GnrhrE90K homozygotes at age 8 weeks, but in none of the E90K mice at age 20 weeks. Thus, the absence of hydrometria correlated with appearance of tissue pathologies. Perhaps persistent high levels of estradiol first stimulated hydrometria and then, with time, promoted metaplasia/hyperplasia. In one mouse model, estrogen treatment resulted in hydrometria (44). These observations illustrate the negative consequence of persistent estrous levels of estradiol in the absence of progesterone.
PRKO mice shared reproductive organ phenotypes with GnrhrE90K, including reduced uterine gland development and hydrometria, but to a lesser extent. The PRKO uterine phenotype may be the result of similar endocrine alterations, including a lack of progesterone signaling and elevated estradiol. These data support the idea that progesterone signaling in the uterus counteracts the effects of estradiol on hydrometria.
We tested whether progesterone treatment could rescue the uterine defects in the GnrhrE90K mutants by administering slow-release progesterone implants between ages 5 and 8 weeks. Progesterone treatment eliminated hydrometria, but did not rescue the second wave of uterine gland development. Thus, progesterone counteracted the effects of estradiol to suppress hydrometria, but was not sufficient to rescue postpubertal uterine gland development. This may be due to the continual release of progesterone by the implant. Progesterone levels rise and fall during the estrous cycle in an inverse relationship to estradiol. Perhaps cyclic—not continuous—hormone secretion is necessary for correct uterine gland growth during adolescence.
We attempted to rescue the uterine defects of the GnrhrE90K homozygotes by administering hCG every 4 days to restore cycling. As expected, this protocol induced ovulation and luteinization in the mutants. Hydrometria was eliminated, but the second wave of gland development was not rescued. These observations indicate that the hydrometria is fueled by chronic estradiol secretion from the persistent antral follicles. Furthermore, these data suggest that uterine gland growth is very sensitive to the endogenous (optimal) pattern of hormone secretion, which cannot be fully replicated by hCG injection. hCG injections and progesterone implants both prevented hydrometria in GnrhrE90K homozygous females. The hCG injections induced ovulation, eliminating the persistent antral follicles, and the progesterone treatment likely counteracted the effects of chronic estradiol. Thus, both experiments suggest that persistent antral follicles are the cause of the hydrometria in these mice. To our knowledge hydrometria has either not been reported or not studied in women with PCOS; however, fluid retention and general edema are common. Women with PCOS are typically treated with progesterone or cyclic birth control to simulate an estrous cycle. Our data suggest that these hormone treatments may be sufficient to overcome possible uterine edema, but not to rescue uterine gland numbers in women with PCOS.
Numerous mouse models have been generated that recapitulate various aspects of human PCOS (45). These mouse models include those induced by hormone treatments and also genetic models. PCOS is a heterogeneous disease and it is likely that no single mouse model can recapitulate the phenotypes found in women with PCOS. The same is true for the Gnrhr E90K mouse model, primarily because it does not have increased androgen levels. Perhaps combining multiple mouse PCOS models could reproduce more aspects of the human condition.
In conclusion, postpubertal uterine gland growth and homeostasis require proper regulation of cyclic ovarian hormone secretion. In our mouse model, anovulation had detrimental effects on postpubertal uterine gland growth, which potentially could impair peri-implantation conceptus development. Finally, the absence of cyclic steroid hormone production can lead to epithelial pathologies of the uterus, including neoplasia.
Acknowledgments
We are grateful to Franco DeMayo (National Institute of Environmental Health Sciences) for generously providing Pgrtm2(cre)Lyd mice and advice on immunohistochemistry. We dedicate these studies to Dr P. Michael Conn. We thank Dr Adriana Paulucci-Holthauzen for confocal microscopy training.
Glossary
Abbreviations
- CL
corpora lutea
- Control
Pgr Cre/+
- FSH
follicle-stimulating hormone;
- GnRH
gonadotropin-releasing hormone
- GnRHR
gonadotropin-releasing hormone receptor
- hCG
human chorionic gonadotropin
- LH
luteinizing hormone
- mRNA
messenger RNA
- PBS
phosphate-buffered saline
- PCOS
polycystic ovarian syndrome
- PR
progesterone hormone
- PRKO
Pgr Cre/Cre
- qPCR
quantitative polymerase chain reaction
Financial Support
This work was supported by the US National Institutes of Health (NIH) (grant No. HD030284) and the Ben F. Love Endowment (to R.R.B.), the American Heart Association grant No. 12BGIA11860006), and the University of Houston Division of Research Small Grants Program (grant No. 1HOU08 to M.D.S). B.K.K. was supported by NIH grant number T32 HD098068-01A1. Confocal microscopy was supported by an NIH shared instrument grant number OD024976. Veterinary resources were supported by NIH grant number CA16672.
Author Contributions
C.A.S. conceived the study, performed experiments, analyzed data, and wrote the paper. M.D.S., R.L., and Y.L. performed experiments and analyzed data. Y.W., R.M., and B.K. performed histology and acquired microscopic images. R.B. analyzed data and wrote the paper.
Disclosures
The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
- 1. Gray CA, Bartol FF, Tarleton BJ, et al. . Developmental biology of uterine glands. Biol Reprod. 2001;65(5):1311-1323. [DOI] [PubMed] [Google Scholar]
- 2. Bhusane K, Bhutada S, Chaudhari U, Savardekar L, Katkam R, Sachdeva G. Secrets of endometrial receptivity: some are hidden in uterine secretome. Am J Reprod Immunol. 2016;75(3):226-236. [DOI] [PubMed] [Google Scholar]
- 3. Bhutada S, Katkam RR, Nandedkar T, et al. . Uterine secretome and its modulation in rat (Rattus norvegicus). Reproduction. 2013;146(1):13-26. [DOI] [PubMed] [Google Scholar]
- 4. Salamonsen LA, Evans J, Nguyen HP, Edgell TA. The microenvironment of human implantation: determinant of reproductive success. Am J Reprod Immunol. 2016;75(3):218-225. [DOI] [PubMed] [Google Scholar]
- 5. Dunlap KA, Filant J, Hayashi K, et al. . Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod. 2011;85(2):386-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction. 2002;124(2):289-300. [PubMed] [Google Scholar]
- 7. Gray CA, Taylor KM, Ramsey WS, et al. . Endometrial glands are required for preimplantation conceptus elongation and survival. Biol Reprod. 2001;64(6):1608-1613. [DOI] [PubMed] [Google Scholar]
- 8. Cooke PS, Spencer TE, Bartol FF, Hayashi K. Uterine glands: development, function and experimental model systems. Mol Hum Reprod. 2013;19(9):547-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu J, Gray CA, Spencer TE. Gene expression profiling of neonatal mouse uterine development. Biol Reprod. 2004;70(6):1870-1876. [DOI] [PubMed] [Google Scholar]
- 10. Cooke PS, Ekman GC, Kaur J, et al. . Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice. Biol Reprod. 2012;86(3):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vue Z, Gonzalez G, Stewart CA, Mehra S, Behringer RR. Volumetric imaging of the developing prepubertal mouse uterine epithelium using light sheet microscopy. Mol Reprod Dev. 2018;85(5):397-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ogasawara Y, Okamoto S, Kitamura Y, Matsumoto K. Proliferative pattern of uterine cells from birth to adulthood in intact, neonatally castrated, and/or adrenalectomized mice, assayed by incorporation of [125I]iododeoxyuridine. Endocrinology. 1983;113(2):582-587. [DOI] [PubMed] [Google Scholar]
- 13. Branham WS, Sheehan DM. Ovarian and adrenal contributions to postnatal growth and differentiation of the rat uterus. Biol Reprod. 1995;53(4):863-872. [DOI] [PubMed] [Google Scholar]
- 14. Stewart CA, Fisher SJ, Wang Y, et al. . Uterine gland formation in mice is a continuous process, requiring the ovary after puberty, but not after parturition. Biol Reprod. 2011;85(5):954-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arora R, Fries A, Oelerich K, et al. . Insights from imaging the implanting embryo and the uterine environment in three dimensions. Development. 2016;143(24):4749-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yuan J, Deng W, Cha J, Sun X, Borg JP, Dey SK. Tridimensional visualization reveals direct communication between the embryo and glands critical for implantation. Nat Commun. 2018;9(1):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maybin JA, Critchley HO. Progesterone: a pivotal hormone at menstruation. Ann N Y Acad Sci. 2011;1221(1):88-97. [DOI] [PubMed] [Google Scholar]
- 18. Rasweiler JJ. Spontaneous decidual reactions and menstruation in the black mastiff bat, Molossus ater. Am J Anat. 1991;191(1):1-22. [DOI] [PubMed] [Google Scholar]
- 19. van der Horst C, Gillman J. The menstrual cycle in Elephantulus. S Afr J Med Sci. 1941;6:27-47. [Google Scholar]
- 20. Stamatiades GA, Kaiser UB. Gonadotropin regulation by pulsatile GnRH: signaling and gene expression. Mol Cell Endocrinol. 2018;463:131-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hackenberg R, Gesenhues T, Deichert U, Duda V, Schmidt-Rhode P, Schulz KD. The response of uterine fibroids to GnRH-agonist treatment can be predicted in most cases after one month. Eur J Obstet Gynecol Reprod Biol. 1992;45(2):125-129. [DOI] [PubMed] [Google Scholar]
- 22. Marschalek J, Ott J, Husslein H, et al. . The impact of GnRH agonists in patients with endometriosis on prolactin and sex hormone levels: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2015;195:156-159. [DOI] [PubMed] [Google Scholar]
- 23. Tsai HW, Wang PH, Lin LT, Chen SN, Tsui KH. Using gonadotropin-releasing hormone agonist before frozen embryo transfer may improve ongoing pregnancy rates in hyperandrogenic polycystic ovary syndrome women. Gynecol Endocrinol. 2017;33(9):686-689. [DOI] [PubMed] [Google Scholar]
- 24. Palomba S, Russo T, Orio F Jr, et al. . Lipid, glucose and homocysteine metabolism in women treated with a GnRH agonist with or without raloxifene. Hum Reprod. 2004;19(2): 415-421. [DOI] [PubMed] [Google Scholar]
- 25. Lee TH, Lin YH, Seow KM, Hwang JL, Tzeng CR, Yang YS. Effectiveness of cetrorelix for the prevention of premature luteinizing hormone surge during controlled ovarian stimulation using letrozole and gonadotropins: a randomized trial. Fertil Steril. 2008;90(1):113-120. [DOI] [PubMed] [Google Scholar]
- 26. Ansari MA, Dhar M, Spieker S, et al. . Modulation of diabetes with gonadotropin-releasing hormone antagonists in the nonobese mouse model of autoimmune diabetes. Endocrinology. 2004;145(1):337-342. [DOI] [PubMed] [Google Scholar]
- 27. Steinberg M. Degarelix: a gonadotropin-releasing hormone antagonist for the management of prostate cancer. Clin Ther. 2009;31(Pt 2):2312-2331. [DOI] [PubMed] [Google Scholar]
- 28. Stewart MD, Deng JM, Stewart CA, et al. . Mice harboring Gnrhr E90K, a mutation that causes protein misfolding and hypogonadotropic hypogonadism in humans, exhibit testis size reduction and ovulation failure. Mol Endocrinol. 2012;26(11):1847-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Janovick JA, Patny A, Mosley R, et al. . Molecular mechanism of action of pharmacoperone rescue of misrouted GPCR mutants: the GnRH receptor. Mol Endocrinol. 2009;23(2):157-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Janovick JA, Stewart MD, Jacob D, et al. . Restoration of testis function in hypogonadotropic hypogonadal mice harboring a misfolded GnRHR mutant by pharmacoperone drug therapy. Proc Natl Acad Sci U S A. 2013;110(52):21030-21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soyal SM, Mukherjee A, Lee KY, et al. . Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58-66. [DOI] [PubMed] [Google Scholar]
- 32. Gonzalez G. Determining the stage of the estrous cycle in female mice by vaginal smear. Cold Spring Harb Protoc. 2016;2016(8):732-734. doi: 10.1101/pdb.prot094474 [DOI] [PubMed] [Google Scholar]
- 33. Cohen PE, Milligan SR. Silastic implants for delivery of oestradiol to mice. J Reprod Fertil. 1993;99(1):219-223. [DOI] [PubMed] [Google Scholar]
- 34. Milligan SR, Cohen PE. Silastic implants for delivering physiological concentrations of progesterone to mice. Reprod Fertil Dev. 1994;6(2):235-239. [DOI] [PubMed] [Google Scholar]
- 35. Yellon SM, Burns AE, See JL, Lechuga TJ, Kirby MA. Progesterone withdrawal promotes remodeling processes in the nonpregnant mouse cervix. Biol Reprod. 2009;81(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee K, Jeong J, Kwak I, et al. . Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38(10):1204-1209. [DOI] [PubMed] [Google Scholar]
- 37. Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol. 2002;16(10):2338-2348. [DOI] [PubMed] [Google Scholar]
- 38. Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns. 2004;5(2):193-208. [DOI] [PubMed] [Google Scholar]
- 39. Lydon JP, DeMayo FJ, Funk CR, et al. . Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266-2278. [DOI] [PubMed] [Google Scholar]
- 40. Marian J, Cooper RL, Conn PM. Regulation of the rat pituitary gonadotropin-releasing hormone receptor. Mol Pharmacol. 1981;19(3):399-405. [PubMed] [Google Scholar]
- 41. Bellver J, Rodríguez-Tabernero L, Robles A, et al. . Group of Interest in Reproductive Endocrinology (GIER) of the Spanish Fertility Society (SEF) . Polycystic ovary syndrome throughout a woman’s life. J Assist Reprod Genet. 2018;35(1):25-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids. 2013;78(8):782-785. [DOI] [PubMed] [Google Scholar]
- 43. Sir-Petermann T, Hitchsfeld C, Maliqueo M, et al. . Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20(8):2122-2126. [DOI] [PubMed] [Google Scholar]
- 44. Wu B, Shi Y, Gong X, et al. . Evaluation of follicular synchronization caused by estrogen administration and its reproductive outcome. PLoS One. 2015;10(5):e0127595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Houten ELAF, Visser JA. Mouse models to study polycystic ovary syndrome: a possible link between metabolism and ovarian function? Reprod Biol. 2014;14(1):32-43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.