Summary
The central nervous system (CNS) has historically been viewed as an immunologically privileged site, but recent studies have uncovered a vast landscape of immune cells that reside primarily along its borders. While microglia are largely responsible for surveying the parenchyma, CNS barrier sites are inhabited by a plethora of different innate and adaptive immune cells that participate in everything from the defense against microbes to the maintenance of neural function. Static and dynamic imaging studies have revolutionized the field of neuroimmunology by providing detailed maps of CNS immune cells as well as information about how these cells move, organize, and interact during steady state and inflammatory conditions. These studies have also redefined our understanding of neural immune interactions at a cellular level and reshaped our conceptual view of immune privilege in this specialized compartment. This review will focus on insights gained using imaging techniques in the field of neuroimmunology, with an emphasis on anatomy and CNS immune dynamics during homeostasis, infectious diseases, injuries, and aging.
Keywords: two-photon microscopy, intravital, meninges, brain, infection, virus, neuroimmunology, microglia, monocytes, neutrophils, vasculature, traumatic brain injury, stroke, Alzheimer’s, glymphatics, lymphatics, dura, sinuses
1. Introduction
The central nervous system (CNS) has traditionally been viewed as an immune privileged site with tolerance to normally rejected foreign antigens and the lack of an immune response to CNS antigens1,2. This theory was first described by Peter Medawar with experiments demonstrating delayed graft rejection of skin allographs in the brain parenchyma3. Immune privilege in the CNS was attributed to either poor immunosurveillance or modulation of the immune response. Poor immunosurveillance was thought to be due to isolation of CNS antigens from the peripheral immune system by a complex set of barriers, a lack of draining lymphatics, and poor antigen presenting capabilities, among other factors1,2,4–6. However, further investigations have shown that the CNS is an immunologically specialized tissue with tight regulation of both innate and adaptive immune cells. This regulation is in place to limit damage to post-mitotic neural cells while allowing for immune cells to protect against infections and to participate in CNS remodeling after injury2,6,7.
A contemporary understanding of neuroimmunology requires a detailed knowledge of the anatomical sub-compartments within the CNS and the immune response that each can support (Figure 1). In fact, the nuances of CNS anatomy often dictate immune composition at steady state and the type of immune cells recruited under different inflammatory conditions5–7. A careful balance must be maintained between protecting the parenchyma from infections and the injurious effects of inflammation while permitting immunosurveillance and establishment of robust immune responses at barrier sites, such as the cerebral spinal fluid (CSF) filled spaces of the meninges and the choroid plexus (CP) within the ventricles7,8. Unlike the parenchyma, the meninges contain a diverse repertoire of immune cells as well as lymphatic drainage to the cervical lymph nodes7,9–12. The differing immune response at these sites is highlighted by early studies demonstrating rapid rejection of foreign tissue grafts injected along the fluid filled ventricles as compared to delayed graft rejection within the brain parenchyma3. Since a detailed knowledge of CNS anatomy is key to our understanding of neuroimmunology6, techniques that allow direct visualization of immune cells in their native environment have been revolutionary to the field. Studies using high resolution static imaging and intravital microscopy have shown that neuroimmune interactions are crucial for normal development and brain functioning, help protect against infection, and promote recovery from injury13–15. Dysregulation of CNS immune response contributes to the development of autoimmunity (e.g., multiple sclerosis) and has also been associated with aging and neurodegeneration16.
Figure 1. The immune defense of different CNS barriers.
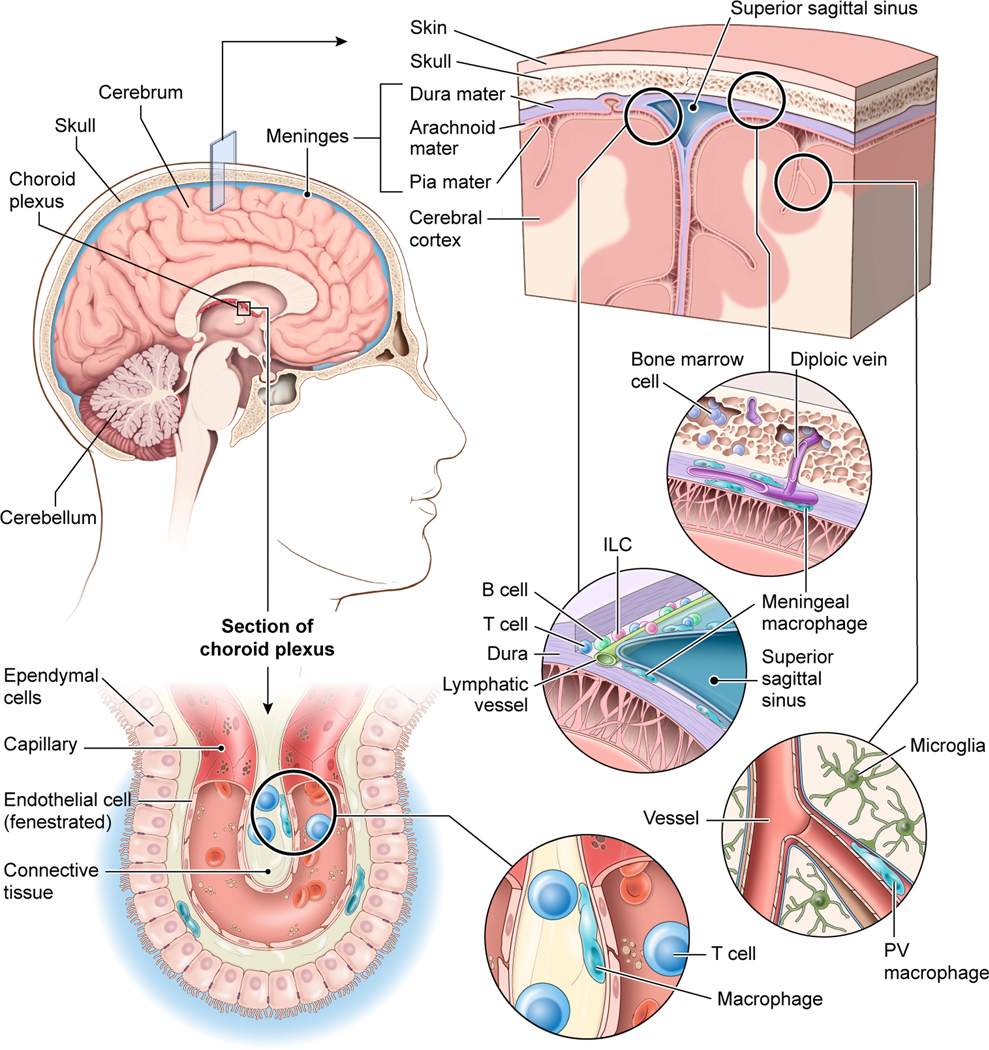
The brain is confined by the skull bone which contains pockets of bone marrow connected to the dura mater via diploic veins. The dura mater is vascularized by vessels that resemble those found in the periphery (i.e., they do not have tight junctions). These ‘open’ vessels are lined by meningeal macrophages. The dura mater also has large venous drains referred to as sinuses. These fenestrated sinuses are lined by lymphatic vessels as well as a diverse collection of immune cells, including macrophages, T cells, B cells, and innate lymphoid cells (ILCs), among others. Beneath the dura mater are the other two meningeal layers – the arachnoid mater and pia mater – which together are referred to as the leptomeninges. Blood vessels within the leptomeninges and brain parenchyma are sealed by tight junctions, which represents an important CNS barrier. Perivascular (PV) macrophages reside in the spaces between blood vessels that enter the brain and astrocytic foot processes comprising the blood brain barrier. The brain parenchyma itself is surveilled by microglia. The choroid plexus is another CNS barrier responsible for producing cerebral spinal fluid. It contains fenestrated vessels that reside behind ependymal cells connected by tight junctions. The choroid plexus is protected by macrophages and T cells as well as other immune cells.
Techniques used for direct visualization of neuroimmune interactions in vivo include static imagery such as confocal microscopy as well as intravital imaging approaches. Comprehensive review of these techniques, which is outside the scope of the current review, is available elsewhere17–21. In addition to the development of advanced microscopy techniques, the generation of reporter mice to visualize different immune and neural cells in vivo has been crucial for monitoring neuroimmune interactions at the cellular level11,22. T cell activation and calcium flux can even be visualized in real time using calcium reporter mice22,23. Direct visualization of immune cells in vivo allows for studying the interaction of immune cells within their native environment, dynamics of immune cells in the context of vasculature and blood flow, and interactions with other resident or recruited cells. These techniques also avoid biases introduced by cellular isolation and selection methods. Instead, intravital imaging approaches such as two-photon laser scanning microscopy (TPLSM) enable spatial and temporal observations of immune cell behavior in real time under homeostatic conditions as well as during inflammatory states. Improved surgical techniques such as imaging through a thinned skull instead of a glass window permits visualization of the intact meninges and neocortex while minimizing confounders such as tissue injury and subsequent immune cell activation24–26. While TPLSM is one option for imaging the meninges, another complementary ex vivo approach involves imaging meningeal whole mounts in 3D by confocal microscopy (Figure 2)27. This technique does not provide insights into immune cell dynamics but provides a spatial map of the meninges and sinuses that cannot be offered by imaging sagittal or coronal brain sections which typically damage the meninges. Given the dynamic nature of immune responses, it is important to use a combination of static and dynamic imaging techniques to construct a complete picture of CNS immunology.
Figure 2. Anatomy of the meninges during steady state.
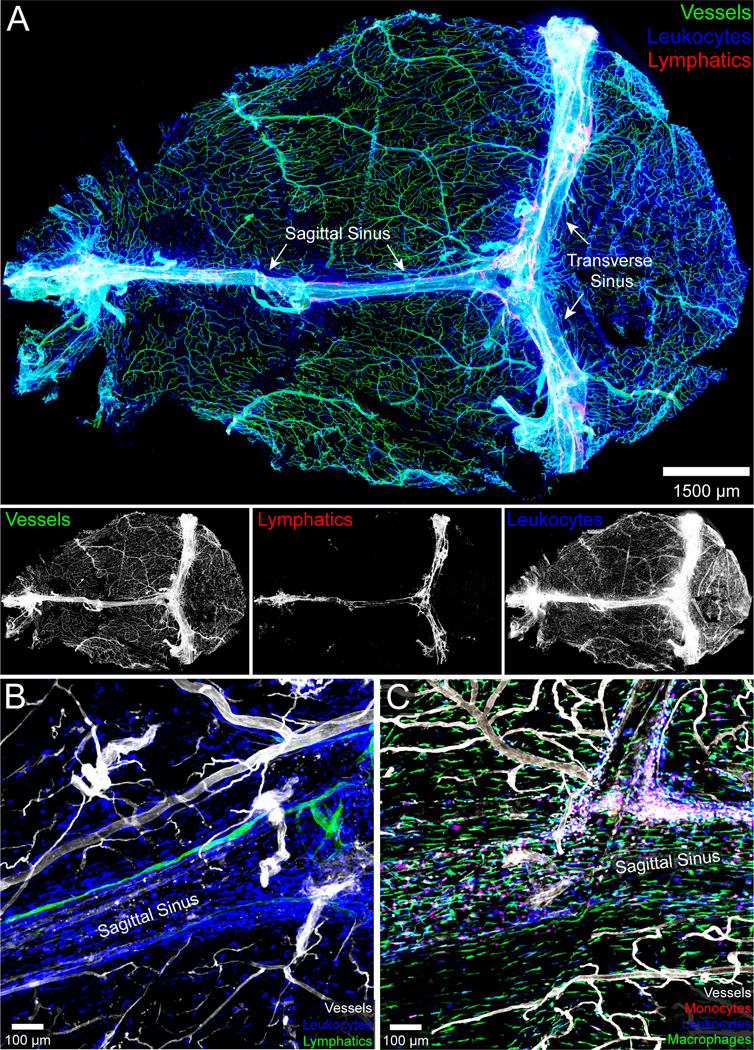
A.) The naïve meninges beneath the skull bone of an 8-week-old C57BL/6J mouse were harvested and imaged in 3D using confocal microscopy. The whole mount consisting primarily of dura and arachnoid mater shows the distribution of blood vessels labeled intravenously with fluorescent tomato lectin (green), CD45+ leukocytes (blue), and Lyve1+ lymphatic vessels (red). The individual grayscale images for each channel are shown beneath the 3-color overlay. The sagittal and transverse sinuses are also labeled. Note that the meningeal lymphatics are juxtaposed to the dural venous sinuses. B.) A three color zoomed confocal image from a meningeal whole mount shows a Lyve1+ lymphatic vessel adjacent to the superior sagittal sinus. CD45+ leukocytes are shown in blue and tomato lectin+ blood vessels in white. C.) A confocal image captured from a meningeal whole mount shows the distribution of CCR2rfp/+ monocytes (red) and CX3CR1gfp/+ meningeal macrophages (green) along the superior sagittal sinus. Blood vessels are shown in white.
2. The immunology of CNS barriers and anatomy
The brain and spinal cord are protected by a unique series of barriers that not only provide structural protection from mechanical injury but also restrict access to the parenchyma5,28. To maintain proper function and homeostasis, it is imperative that the CNS limit and tightly regulate the entry of cells and soluble mediators into the parenchyma. The CNS borders, however, are more permissive and tolerant to immune reactions and each compartment must therefore be considered separately to understand how responses are tailored to the anatomy. This section will cover CNS barriers including the skull, meninges, blood-brain barrier (BBB), blood-CSF barrier, CSF-interstitial fluid barrier, and the parenchyma as well as how the associated anatomy tailors specialized immune responses (Figure 1).
2.1. Skull
The first layer of protection for the brain and spinal cord is the skull and vertebral bodies. The skull is created by separate bones that are joined via cranial sutures to create the protective cranial cavity. The vertebrae join to create the vertebral column and protect the spinal cord. However, recent studies have shown that the skull and vertebral bodies are not simply structural barriers that protect against mechanical injuries; they contain specialized pockets of bone marrow that are connected to dural sinuses and meningeal veins (Figure 1). These bone marrow niches maintain populations of immune cells in the meninges during homeostasis and allow for rapid recruitment in response to injury or autoimmunity29–32. Microscopy enables visualization of immune traffic through specialized ossified vascular channels29. These specialized bone marrow niches can imprint developing cells with distinct properties and play a role in tolerance to CNS antigens30,32,33. Furthermore, the skull may also play a role in CSF outflow and immunosurveillance. Lymphatic vessels have been found outside foramina in the skull, and elegant studies using high resolution noninvasive imaging with lymphatic vessel reporter mice and near-infrared tracers demonstrated CNS outflow primarily through these perineural routes34. The role of the calvaria bone marrow in the immune response will be further discussed in Section 3.4.
2.2. Meninges
The meninges are a three-layered membranous structure that surrounds the brain and spinal cord and provides further protection to the CNS parenchyma (Figure 1). The meninges cover both the dorsal and basal surfaces of the brain. The dorsal meninges are more accessible to in vivo imaging via TPLSM and can be easily dissected as whole mounts for ex vivo static imaging, although the basal meninges can also be imaged ex vivo12,26,27. The meninges consist of three layers. The outermost layer adjacent to the skull is referred to as the dura mater, the middle layer is the arachnoid mater, and the innermost layer adjacent to the brain and spinal cord parenchyma is the pia mater. The meninges serve as another physical barrier protecting the CNS parenchyma and are also capable of supporting immune responses7,11,35. The meninges contain a wide variety of immune cells under steady state that include innate cells such as macrophages, dendritic cells (DCs), mast cells, natural killer (NK) cells, innate lymphoid cells (ILCs), neutrophils, and monocytes as well as T and B cells of the adaptive immune system36–41 (Figure 1,2). Under conditions of infection, inflammation, or injury, immune cells can be recruited to the meninges via extravasation from the blood stream or by recruitment from the skull or vertebral bone marrow niches30,37,38,42. Parenchymal immune responses often originate within the meninges, and immune cells can be recruited from the meninges to the parenchyma. This section will provide a basic review of the anatomy and protective role of the meninges.
The outermost layer of the meninges is the dura mater, which is a thick collagenous membrane formed by the periosteal layer adjacent to the skull and the meningeal layer adjacent to the arachnoid mater6,7. The periosteal and meningeal layer are fused in most areas, but separate to form the dural sinus cavity, which contains venous blood in route back to the heart11. The dura mater is the layer of the meninges that is most supportive to inflammation and similar to a peripheral tissue. Unlike the other layers, the dura is highly vascularized (Figure 1,2), and the blood vessels within are fenestrated and lack tight junctions, allowing materials and cells from peripheral circulation to enter6. Additionally, the dura mater is innervated and contains sensory, sympathetic, and parasympathetic fibers7. Sensory fibers can respond to changes in the environment and even interact with immune cells or affect the permeability of dural vasculature7. The dural venous sinuses are also regional hubs for immune activation and surveillance; there is a high density and diversity of immune cells along the dural sinuses to survey these large open drains. In addition, the recently ‘rediscovered’ meningeal lymphatic vessels lie parallel to the venous sinuses and are likely in this position to sample their contents (Figure 1,2)6,9,10,37,43–45. Imaging studies have also shown that there is drainage of CSF along the dural sinuses, and antigen presenting cells (APCs) can present CSF-antigens to circulating T cells37. Therefore, the dura mater appears capable of immunologically surveying both the sinuses and CSF, draining material to the cervical lymph nodes, especially the deep cervical lymph nodes which can participate in initiating immune responses against CNS antigens46. The role of the venous sinuses and dural lymphatics will be further discussed in later sections.
The arachnoid and pia mater together are referred to as the leptomeninges, and these layers enclose the subarachnoid space (SAS) that contains CSF11. CSF within the SAS provides buoyancy and protection from mechanical damage. Unlike the dura mater, the leptomeninges are a more tightly regulated compartment and are less accessible to the periphery. The arachnoid mater contains a layer of cells connected by tight junctions, which forms a barrier between the CSF and relatively permissive and open environment of the dura mater. The tight junctions of the arachnoid mater form a component of the blood-CSF barrier6. Projections of arachnoid mater into the dural sinuses, termed arachnoid granulations, participate reabsorption of CSF into systemic circulation, although this is not the only route of CSF reabsorption47,48. The pia mater is adherent to the CNS parenchyma and semipermeable to CSF, which facilitates mixing of CSF and brain interstitial fluid. This process is important for waste and fluid management in the parenchyma as well as immune cell exposure to CNS antigens47.
2.3. Blood brain barrier
Proper CNS function requires a precisely regulated microenvironment, and the BBB selectively restricts passage of cells and soluble mediators from the blood into the parenchyma. The specialized anatomy and function of CNS vasculature in the context of immunology has been reviewed previously and will only be discussed briefly in this section6,49. The BBB consists of various structures and cell types, including vascular endothelial cells, pericytes, the glia limitans perivascularis (astrocytes), and microglia (Figure 1). Immunofluorescent imaging, intravital imaging, and electron microscopy have further defined the structure and function of the BBB. Maintenance of the BBB is essential for compartmentalizing CNS immune responses and protecting the parenchyma. However, BBB breakdown does occur during many immunopathological conditions, and rapid restoration is needed for return to homeostasis50,51.
Specialized endothelial cells comprising CNS vasculature are the main unit of the BBB. These cells are distinct from endothelial cells of the peripheral blood vessels, including those found in the dura mater. For example, they lack fenestrations, contain tight junctions, express specific transporters, and have low levels of transcytosis6. The endothelial cells are also surrounded by endothelial basement membrane as well as pericytes. Beyond this initial barrier is the glia limitans that forms the perivascular space with basement membrane and is composed of astrocytic endfeet6. This vascular ensheathment by astrocytic foot processes is referred to as the glia perivascularis52, which is different from the glia limitans superficialis – a layer of surface associated astrocytes that reside beneath the pia mater and protect the CNS parenchyma. Penetrating subpial vessels and parenchymal blood vessels are lined by perivascular macrophages that are dynamic and uniquely positioned to sample the perivascular environment and rapidly respond to blood-borne challenges (Figure 1)53. Furthermore, to reach the brain parenchyma from the blood, immune cells must traverse both the vascular endothelium and the glia limitans perivascularis52. This feat is made even more challenging by the low expression of adhesion molecules on steady state CNS vasculature, further limiting entry of peripheral immune cells to the CNS parenchyma54. The role of the BBB and its breakdown under immunopathological conditions will be further discussed in the sections that follow.
2.4. The choroid plexus and blood-CSF barrier
The choroid plexus (CP) is a specialized structure found within the ventricles and is the main producer of CSF (Figure 1). Recent studies have demonstrated that the CP can also host diverse immune responses55–57. Like the meninges, the CP contains fenestrated vasculature and is relatively open to the periphery. It therefore serves an important interface between the brain and circulation; however, it is important to remember that this interface is not completely open. Much the same as the tight junction expressing arachnoid mater protects the CNS from open dural vasculature, an epithelial layer with tight junctions protects the CSF from open blood vessels in the choroid plexus58. This is referred to as the blood-CSF barrier – a structure that regulates entry of cells and soluble factors into the CSF59. During homeostasis, the CP contains resident immune cells including DCs, macrophages, innate lymphoid cells, and CD4+ T cells, among others (Figure 1)55. Interestingly, many T cells found in the CP are effector memory cells with T cell receptors that recognize CNS antigens60. Confocal microscopy studies have revealed that the CP can support activation of the adaptive immune system. In fact, CD4+ T cells in the CP of naïve mice are often found adjacent to MHC II-expressing cells8,60. Furthermore, a robust immune response can be mounted under pathological conditions, during which T cells undergo antigen specific activation and proliferation within the CP8. In the experimental autoimmune encephalomyelitis (EAE) model of MS, one route of T helper 17 (Th17) cell entry into the CNS parenchyma is through epithelial cells of the choroid plexus61,62. Thus, the CP provides at least one gateway for immune cells to access the CNS, assuming they are able to cross the surrounding epithelial barrier.
2.5. The CSF interface with parenchymal interstitial fluid (ISF)
In addition to forming the BBB, the glia limitans and associated basement membrane form a component of the barrier that separates CSF from parenchymal ISF. Despite this barrier, the CSF is known to clear at least some waste products from the CNS parenchyma and ISF, which suggests that there is some mixing between the CSF and ISF47. The amount of ISF efflux into CSF varies per brain region tested but can be as low as 10%63. As lymphatic vessels do not directly contact or drain the CNS parenchyma, other pathways must exist to clear and return ISF to vascular circulation. Recent studies using injected fluorescent tracers and confocal / intravital microscopy have further enhanced our understanding of the CSF-ISF interface47,48,64–66. This system will be discussed in more depth in Section 3.1.
2.6. The CNS parenchyma and innate immune sentinels
The CNS parenchyma is largely devoid of traditional immune cells during steady-state conditions but is protected by resident microglia (Figure 1). Microglia are innate immune sentinels that are derived from the primitive yolk sac during embryonic development and are crucial for parenchymal surveillance67,68. Microglia are classified as antigen presenting cells (APCs) capable of presenting antigens on MHC class II molecules as well as cross-presenting peptides on MHC class I under certain conditions69,70. Two-photon imaging studies relying on different reporter mice (e.g., CX3CR1gfp/+)71 have revealed a range of different dynamic states that microglia utilize under steady-state and inflammatory conditions. At rest, microglia have a small cell body with multiple, highly dynamic branches that continuously scan the parenchyma72,73. This homeostatic program gives microglia the ability to continually scan the entire parenchyma and rapidly respond when needed to challenges such as infections and injuries51,70,73–75. Monocytes by contrast are infrequently found in healthy parenchyma53, but can be quickly recruited during states of inflammation and differentiate into macrophages or dendritic cells (DCs).
3. CNS immune surveillance and maintenance of homeostasis
The CNS is an immunologically specialized tissue that tightly regulates innate and adaptive immune cells. Despite this tight regulation, neuroimmune interactions are crucial for maintaining neural homeostasis, protecting the tissue from pathogens, limiting the spread of neurodegenerative processes, and remodeling after injury. While CNS immune surveillance aids in the maintenance of tolerance to local self-proteins, the system must be nimble enough to mount a rapid productive response to either an injury or infection. This section will highlight recent insights into our understanding of CNS immune privilege and the maintenance of tolerance / homeostasis that are based in part on imaging studies.
3.1. Mechanisms of CNS fluid and waste clearance
Maintenance of CNS homeostasis requires a mechanism of fluid transport that allows interstitial solutes, waste, and CNS derived antigens to be removed. Lymphatic vessels traditionally play this role in other tissues; however, the CNS parenchyma is devoid of traditional lymphatic vessels. One proposed mechanism for waste and fluid clearance is an exchange between the ISF and CSF. Early studies involving direct injection of horseradish peroxidase or Evans blue labeled albumin into different brain regions revealed some degree of ISF drainage into the bulk CSF that varied per brain region63. Drainage was demonstrated to occur along cerebral arteries and enter CSF within the subarachnoid space. More recently, a series of imaging studies offered another view of the CSF-ISF interface referred to as the glymphatic system47. In these studies, small fluorescent tracers injected into the cisterna magna were observed by two-photon and confocal microscopy to move from the CSF into the brain parenchyma47. The influx occurred along cerebral arteries, and the authors proposed that fluid movement was propelled by arterial pulsations76. A central tenet of this model is the ability of CSF to move into the interstitial space and mix with ISF via a process facilitated by aquaporin 4 (AQP4) water channels present on the astrocytic endfeet47. However, the involvement of AQP4 in CSF-ISF exchange is controversial, with some studies showing that this water channel does play a role47,77 and others demonstrating that it does not78,79. The advective flow of CSF from periarterial to perivenous as proposed by the glymphatic theory has also been questioned by studies demonstrating that solute movement in the parenchyma is non-directional and occurs by diffusion78. While it is unclear which model will prevail80, CSF-ISF exchange is an important area of investigation that has implications for our understanding of parenchymal antigen drainage, immune surveillance, and CNS disorders. Defects in CNS waste management have been implicated in models of Alzheimer’s disease, subarachnoid hemorrhage, traumatic brain injury (TBI), cognitive decline, and aging, among others 47,66,81–84. It has also been shown that this system is enhanced during sleep65.
3.2. Meningeal lymphatic vessels
Although the CNS is devoid of a traditional lymphatic system, recent work has revealed functional lymphatic vessels within the dorsal and basal dura mater of both mice and humans9,10,12,85. These vessels reside primarily along venous sinuses that drain blood from the brain (Figure 1, 2A,B). In the spinal cord, lymphatic vessels were found between individual vertebra where they are thought to drain the epidural space and neighboring dura mater86. It should be noted that both the brain and spinal cord lymphatics reside primarily outside the arachnoid barrier in an environment resembling the periphery, and it is likely that the main function of these vessels is to sample the contents of the fenestrated venous drains they are adjacent to in the dura mater. However, studies have also suggested that these lymphatic vessels can sample the ISF that mixes with CSF. For example, dyes injected into the CSF or brain parenchyma were found within dural lymphatic vessels and eventually shuttled to the deep cervical lymph nodes9,10,12. Interestingly, there are morphological and functional differences in the lymphatic vessels present in the dorsal9,10 and basal12 meninges. Basal meningeal lymphatic vessels have features of classical lymphatics found in peripheral organs. These vessels have lymphatic valves, larger diameters, and more frequent capillary branches than dorsal vessels. They are also closer to the subarachnoid space and are more efficient at sampling the CSF than the dorsal vessels12. While dural lymphatic vessels play a role in clearing macromolecules,9,10 they appear dispensable for intracerebral fluid management, as no change in intracranial pressure or brain water content was observed in mice lacking them10. Under steady state meningeal lymphatics are thought to participate in sampling macromolecules, efflux of immune cells from the CNS, and possibly the maintenance CNS tolerance. Meningeal lymphatic vessels have also been implicated in many different pathophysiological states such as aging, Alzheimer’s disease, infections, TBI, and autoimmunity, which will be discussed further in later sections2,9,10,12,87–90.
3.3. Immunology of dural venous sinuses
The dural venous sinuses are slow flowing blood-filled cavities with fenestrations located in between the periosteal and meningeal layer of the dura mater. Juxtaposed to the dural sinuses are meningeal lymphatic vessels that assist in the egress of immune cells and macromolecules to the deep cervical lymph nodes as discussed previously9,10,12. The greatest concentration of immune cells within the dura mater is located along the sinuses, where there is a high concentration of CD45+ hematopoietic cells including T cells, B cells, macrophages, DCs, and innate lymphoid cells36,37,91 (Figure 1, 2). The microenvironment within the dural sinuses is conducive to immune surveillance with availability of CNS antigens derived from the CSF to antigen presenting cells and T / B cells.
One of the most abundant cell populations in the meninges is meningeal macrophages38,92,93 (Figure 1, 2C). Meningeal macrophages are primarily derived from early embryonic precursors and are long-lived cells. Similar to barrier macrophages located within the choroid plexus, meningeal macrophages also receive input from peripheral monocytes during inflammation, which likely occurs due to fenestrated blood vessels within the meninges38,92,94. At steady state, meningeal macrophages are situated throughout the meninges juxtaposed to blood vessels and at a high concentration along the dural venous sinuses38,53. These macrophages express common macrophage markers but can also serve as antigen presenting cells by expressing costimulatory molecules (e.g., CD80) and antigen-presenting machinery (e.g., MHC I, MHC II). Under homeostatic conditions, meningeal macrophages imaged through a thinned skull window by TPLSM are dynamic and actively survey their surroundings similar to microglia38,72. Morphologically, however, meningeal macrophages look very different from microglia. They have an elongated cell body and few processes (Figure 2C). Furthermore, steady state meningeal macrophages are skewed towards an anti-inflammatory state that is likely conducive to the maintenance of CNS homeostasis and neurological function, including learning and memory95.
The anatomy of the dural sinuses with close proximity of venous blood flow, lymphatic vasculature, and immune cells also provides an environment conducive to activation of the adaptive immune system (Figure 1, 2). Meningeal T cells likely circulate from the blood into the meninges and then exit through the lymphatic vasculature into the draining cervical lymph nodes37,96. T cells are enriched along the dural sinuses, in part due to expression of adhesion molecules that facilitate T cell adhesion and extravasation37. Steady state T cell trafficking in the meninges was recently monitored by intravital imaging, which revealed a preference of T cells to survey the dural venous sinuses relative to other meningeal vasculature37. A lack of vascular tight junctions in the dura mater also permits fluid exchange between the dural venous sinuses and juxtaposed lymphatic vessels. Within this niche, T cells can survey antigen presenting cells displaying local antigens derived from the CSF / blood and also traffic to the draining cervical lymph nodes via CC-chemokine receptor 7 (CCR7) mediated migration37,88.
The meninges also harbor a population of meningeal B cells, including B cell progenitors as well as terminally differentiated plasma cells (Figure 1)32,33,91,97. Meningeal B cells are enriched along the dural sinuses and have also been found within the human meninges, especially along the superior sagittal sinus. Similar to meningeal macrophages, under homeostatic conditions, some meningeal B cells are long term tissue residents, and studies have shown that these B cells are derived primarily from bone marrow pockets within the skull (discussed further in section 3.4). However, with age, there is an increase in meningeal B cells that infiltrate from the peripheral blood. While studies have shown that B cells with self-reactivity to CNS antigens can undergo negative selection in the meninges32,33, it is also clear that the dura mater harbors B cells to protect its vulnerable ‘open’ vascular system91. Terminally differentiated plasma cells reside along the dural sinuses of mice and humans32,91. Interestingly, most of these plasma cells are IgA-producers91, which are crucial for protection of other peripheral barrier surfaces and mucosal tissues such as the gut98. A recent imaging study demonstrated that IgA plasma cells residing along the dural venous sinuses increased with age and were originally educated in the gut by the microbiome91. These cells were shown to protect the underlying brain parenchyma from blood borne pathogens. Intravenous inoculation of mice with the fungus, Candida albicans, resulted in IgA dependent entrapment in the dural venous sinuses. Specific depletion of meningeal IgA cells eliminated this entrapment, allowing fungi to enter the brain parenchyma and cause a fatal meningoencephalitis91. Together these data demonstrate that the meninges participate in tolerizing skull bone marrow-derived B cells against CNS antigens but also harbor a population of differentiated plasma cells to help protect vulnerable vasculature from blood borne pathogens.
3.4. CNS bone marrow niches
Both the skull and vertebral bodies contain pockets of bone marrow, and hematopoiesis occurs within these specialized niches. CNS associated bone marrow is involved in the maintenance of certain immune cell populations within the meninges and allows for rapid recruitment of immune cells under pathological conditions29,30,32. A role for skull bone marrow in CNS immunity was first discovered in studies focused on ischemic stroke and chemically induced cerebral inflammation in mice29. It was demonstrated during states of CNS inflammation that a higher percentage of meningeal neutrophils were derived from the skull bone marrow relative to a distal bone marrow site (the tibia)29. Confocal imaging further revealed ossified vascular channels that create a direct connection between skull bone marrow niches and the dura mater. It is postulated that calvaria bone marrow provides a reservoir of cells available for rapid recruitment to the dura mater that is independent of systemic circulation29,30,32. Vascular channels were also found in humans using high resolution X-ray computed tomography of ex vivo occipital skull bone samples29 and have been demonstrated within vertebral bone connecting bone marrow niches to spinal cord dura mater30.
Bone marrow within the skull and vertebral bodies are also involved in maintenance of the meningeal immune cell repertoire during homeostasis. Recent studies have shown, for example, that the skull bone marrow helps maintain both innate (myeloid cells) and adaptive (B cells) immune cells in the meninges30,32,33. Under steady state, a substantial number of meningeal monocytes, neutrophils, and B cells were found to originate from the bone marrow of the skull or vertebral bodies rather than the blood. However, this is not universal for all immune cells within the meninges. The majority of T cells are derived from the blood, and meningeal IgA plasma cells are educated in the gut37,91.
It is clear from recent studies that skull and vertebral bone marrow imprint meningeal immune cells with distinct properties and participate in their education30,32,33,97. Immature B cells from the calvaria bone marrow migrate to the meninges through specialized channels and likely complete their differentiation within the meninges with support from dural fibroblasts. Developing B cells are exposed to CNS self-antigens in the meninges, and high affinity autoreactive B cells can undergo local negative selection, resulting in elimination from the repertoire32,33. Myeloid cells recruited from the calvaria bone marrow may also have distinct properties30. In mice with experimental autoimmune encephalomyelitis, myeloid cells recruited to the spinal cord from the blood exhibited a more proinflammatory phenotype as compared to cells recruited from the vertebral bone marrow30. It remains to be determined what additional specializations and functions the bone marrow niches within the calvaria and vertebrae contribute to meningeal immune cells.
4. Immune response to injury, infection, and neurodegeneration
Neuroimmune interactions are crucial for recovery from injuries as well as infections, and dysregulation plays a role in neurodegeneration and aging. This section will review the immune response under pathological conditions, with special attention to insights gained from static and intravital imaging techniques. Understanding the role of the neural immune interface during CNS disorders is important for the development and appropriate delivery of new therapeutic interventions.
4.1. Traumatic brain injury
Traumatic brain injury (TBI) is the most common brain injury in humans and causes significant morbidity, mortality, and socioeconomic costs. TBI encompasses a wide range of injuries ranging from mild focal injuries and concussion to diffuse injuries with vascular edema, hemorrhage, and axonal damage99. The pathogenesis of TBI includes the initial phase of primary mechanical injury followed by secondary damage that is caused by many factors, including pathogenic signaling cascades and unresolved inflammation. Activation of the immune response occurs immediately after TBI, and inflammation can have both a protective role in healing as well as pathological role in perpetuating damage14,100,101. Intravital imaging has been key to unraveling the complexities of the immune response after TBI. Because the TBI immune response varies based on time and anatomical location, crucial information is lost when static imaging is performed at a single time point or when CNS tissue is harvested and homogenized for ex vivo cellular analyses. Visualization of the immune response at a cellular and subcellular level has led to new insights into the role of the TBI immune response, which will be further discussed in this section.
Breakdown and damage to CNS barriers including the meninges, BBB, and blood-CSF barrier is common after TBI. Repair of vasculature and rapid restoration of barriers is essential for recovery, and this repair is further impaired by repeated injury50,74,102. Resident microglial have a small cell body and long processes during steady state, but rapidly respond to injury by changing their morphology. Intravital imaging of a mouse model of mild TBI (mTBI) that involves focal meningeal compression uncovered an important role for microglia in the immediate neuroprotective response to injury. Two-photon imaging through a thinned skull window revealed that microglia transition within an hour of mTBI from a naïve, ramified state into cells with “honeycomb” and “jellyfish” like morphologies that help seal the glial limitans superficialis and clear dead cells, respectively74 (Figure 3A,B). Interestingly, this microglia response failed when a second mTBI was encountered within a day of the first injury, leading to enhanced leakiness of the glia limitans superficialis and increased cell death in the neocortex102. Focal laser models of brain damage have demonstrated that microglial rapidly extend processes (on a time scale of minutes) and attempt to wall off regions of cerebrovascular or parenchymal damage by responding to extracellular purines like adenosine triphosphate (ATP)72–74,103. Purines can be released by dying cells or actively pumped into the extracellular space as an immunological alarmin by pannexin or connexin hemichannels104,105. Distressed astrocytes along the glia limitans superficialis and perivascularis are known to release ATP via connexin hemichannels73,74. Microglia detect and project processes toward sources of purines using purinergic receptors67,105. Inhibition of purinergic receptors (P2RY12, P2RX4, and P2RY6) or astrocytic connexin hemichannels all impede aspects of the microglial response to mTBI or focal brain injury, leading to a failure in damage containment and elevated parenchymal cell death. A rapid purinergic receptor dependent microglia response is required to limit the extent the damage after brain injuries involving barrier breaches.
Figure 3. Microglia responses to mTBI and cerebrovascular injury.
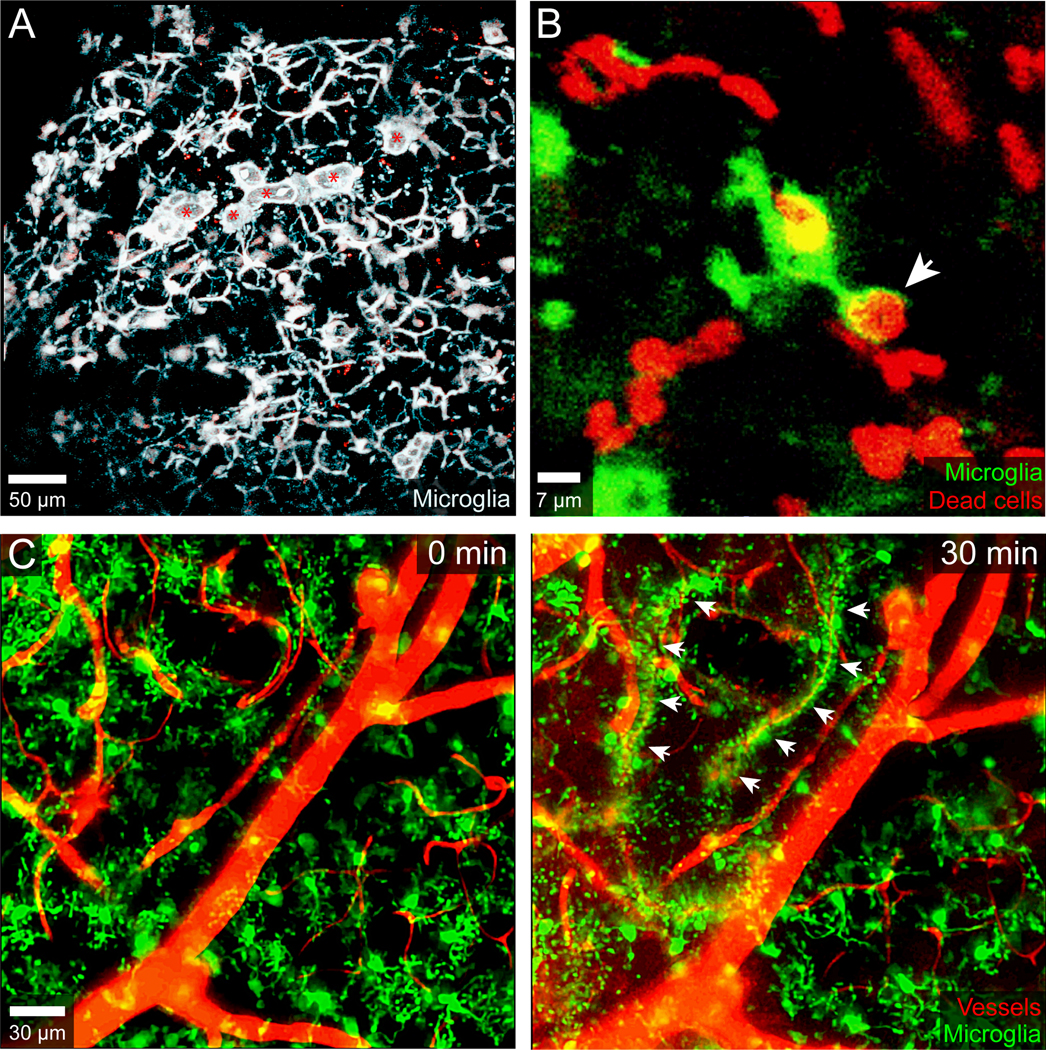
A.) Intravital two-photon microscopy was used to image the mTBI response in a CX3CR1gfp/+ CCR2rfp/+ mouse immediately following a focal meningeal compression injury. A 3D time lapse was captured through a 20μm thinned skull window for the first hour after injury. This maximal projection image at 30 min post-injury shows the microglial response along the glia limitans superficialis. Most of the microglia (white) in the image are forming honeycomb-like structures designed to seal the gaps between individual surface associated astrocytes (not labeled). The amoeboid microglia denoted with red asterisks and resembling ‘jellyfish’ are responsible for clearing debris. B.) A CX3CR1gfp/+ microglia (green) captured in another two-photon time lapse after mTBI is shown acquiring a dead cell (red) labeled transcranially with propidium iodide. The microglial process acquiring the dead cell is denoted with a white arrowhead. C.) Two-imaging was used to capture a time lapse through the thinned skull window of CX3CR1gfp/+ mouse immediately after inducing a cerebrovascular injury in the neocortex with transcranial ultrasound + intravenous microbubbles. Two time points are shown in the time lapse: 0- and 30-minutes post-injury. The microglia are naïve at time point zero but then extend processes and envelop individual damaged blood vessels forming rosettes by 30 min (denoted with white arrowheads). Blood vessels (red) were visualized by injecting Evans Blue intravenously.
The meninges also play a crucial role in the pathogenesis of TBI. Meningeal vascular damage has been demonstrated after mTBI in both humans and mice50,74. In humans, 50% of patients with mTBI injected intravenously with gadolinium showed evidence of meningeal enhancement on post-contrast MRI fluid-attenuated inversion recovery (FLAIR) images, which is indicative of meningeal vascular damage50,74. Most patients had resolution of enhancement within ~3 weeks of injury; however, enhancement persisted in 17% of patients for more than 3 months, suggesting a failure to repair meningeal vasculature. In mice, whole mount meningeal imaging revealed damage to meningeal vessels after mTBI and peripheral monocyte-mediated repair within 1 week. Immune-mediated repair of meningeal vasculature explains why many mTBI patients resolve their MRI findings within a few weeks. However, unresolved meningeal damage would almost certainly cause harm to the underlying brain parenchyma. In murine studies, mechanical damage to the meninges was shown to promote secondary injury via release of reactive oxygen species (ROS). High concentrations of meningeal ROS released after primary and secondary mTBI break down the glial limitans superficialis and cause cell death in the brain parenchyma50,74,102. Importantly, treatment with the free radical scavenger, glutathione, was shown to markedly reduce brain damage following both primary and secondary mTBI74,102.
In addition to vascular damage, meningeal lymphatics also play a role in TBI pathogenesis. In a closed-skull model of mTBI, an impairment in meningeal lymphatic drainage was observed, with compensatory lymphangiogenesis evidenced by imaging studies showing an increased area of lymphatic vessels and associated morphological changes90. Furthermore, lymphatic vessel dysfunction or ablation led to exacerbation of inflammation and worsened cognitive outcomes in mice after TBI90. These data suggest that the dysfunctions in meningeal lymphatics, such as those that occur with age, may exacerbate TBI pathogenesis.
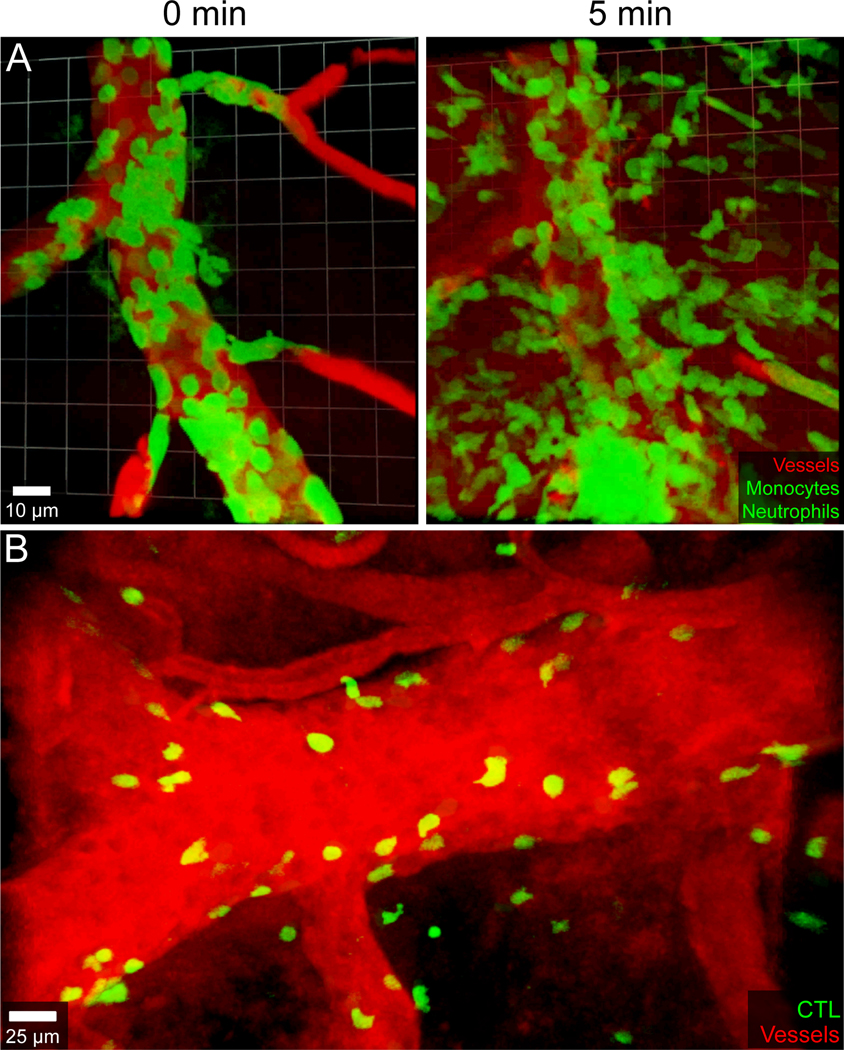
Another major component of a TBI induced immune response is recruitment of peripheral myelomonocytic cells (i.e., monocytes and neutrophils). Two-photon imaging studies in a murine mTBI model revealed that LysMgfp/+ neutrophils enter the damaged meninges with 1 to 3 hours of injury and interact with dead cells74. These cells remained exclusively in the meninges and were recruited in a P2RX7 dependent manner. Additional intravital imaging studies using Cx3cr1GFP/+ Ccr2RFP/+ dual reporter mice demonstrated that peripheral blood monocytes entered the damaged meninges within 24 hours and played an important role in meningeal repair50. Investigation of the myeloid cell response using Cx3cr1GFP/+ Ccr2RFP/+ dual reporter mice facilitated identification of macrophages (CX3CR1hi CCR2low-neg) and monocytes (CX3CR1low-neg CCR2hi) in the injured meninges by intravital microscopy. Initial invasion of the lesion core by monocytes at day 1 was followed by conversion into macrophages by day 4. At day 4 macrophage subsets with different functions localized to distinct anatomical niches within the meningeal mTBI lesion. Inflammatory macrophages derived from classical monocytes localized to the lesion core, whereas wound healing (CD206+) macrophages derived from nonclassical monocytes were found along the lesion perimeter at sites of new blood vessel formation50. Specific depletion strategies in conjunction with intravital and meningeal whole mount imaging studies revealed that these two different macrophage subsets had specific, non-overlapping roles in repair and recovery after injury. Perimeter wound healing macrophages promoted angiogenesis via matrix remodeling while inflammatory macrophages promoted clearance of dead cells in the lesion core50. Interestingly, repetitive mTBI was found to disrupt this reparative process entirely if the initial injury did not have sufficient time to recover. A second mTBI encountered within 1 day of the first impeded angiogenesis, elevated cell death / barrier leakage, and promoted a massive recruitment of inflammatory myelomonocytic cells50,102. Whether the calvaria bone marrow29,30 plays a role in the rapid recruitment of infiltrative cells after mTBI is unknown, but this seems likely given the direct vascular channels from the skull bone marrow to the meninges.
Because the innate immune system plays such a crucial role in CNS repair after injury, it is important to keep in mind that these normal repair mechanisms can be easily disrupted in the setting of systemic inflammation or infection106, which might explain why some TBI patients fail to recover properly50. Systemic infections are known to contribute to poor outcomes in TBI patients107. It was discovered in a recent imaging study that the mechanism by which infections disrupt reparative immune programming after TBI is shared by a broad range of pathogens106. Systemically introduced microorganisms or microbial products representing viruses, bacteria, and fungi all impeded meningeal vascular repair in mice that experienced a mTBI. This impairment was due to induction of type I interferon (IFN-I) signaling in myeloid cells, which led to alterations in angiogenetic programming and disrupted the anatomical distribution / function of reparative macrophages in the meninges106. Importantly, meningeal vascular repair was restored in systemically infected mTBI mice by inhibiting or genetically deleting IFN-I signaling. These findings demonstrate a common mechanism by which many pathogens disrupt immune-mediated CNS repair and explain why systemically infected TBI patients experience poor outcomes.
Currently, there are no approved treatments that improve outcomes after TBI, and a deeper understanding of the immune response and repair process in patients with these injuries is essential. Intravital imaging in rodents has shown that treatment with the ROS scavenger, glutathione, is an excellent therapeutic, especially when applied transcranially to the mTBI lesion74,100,102. It preserves integrity of the glia limitans superficialis, which in turn reduces immune cell activation / recruitment and cell death in the underlying brain parenchyma. The efficacy of many other therapeutic options could be evaluated in a similar manner using intravital microscopy. Imaging studies can also be designed to determine how commonly used clinical drugs might impede recovery from brain injury. For example, the P2RY12 inhibitor, clopidogrel, which is routinely used in clinical practice, was shown to impede the barrier preserving microglial response to cerebrovascular injury103. Learning more about the mechanisms that guide reparative immune activities after TBI should facilitate decisions about which drugs might aid versus deter the recovery process. Intravital imaging studies can offer advanced insights into this decision-making process by showing how drugs function locally and temporally.
4.2. Cerebrovascular disease and stroke
Cerebrovascular disease and stroke are a significant cause of morbidity and mortality worldwide108. A commonly used animal model for stroke is middle cerebral artery occlusion (MCAO) followed by reperfusion, although other models for cerebrovascular injury are also studied51,106,109. The role of the immune response after ischemic stroke is controversial and like TBI is thought to have both beneficial and detrimental components110,111. Intravital imaging studies have helped unravel the precise temporal, spatial, and functional aspects of immune responses elicited by cerebrovascular injuries (CVI)51,112–114.
Resident microglia are among the first immune responders to ischemic injury. They become activated even prior to neuronal cell death and demonstrate sequential morphological changes115. Studies have shown both neuroprotective and neurotoxic roles for microglia that depend in part on the CVI model, timing, and precise location within the injured CNS111. In response to cerebrovascular injury, microglia are activated and change morphology, assist with removal of cell debris, and promote repair / angiogenesis, and depletion of microglia was shown to worsen inflammation and brain injury51,68,116,117. However, microglia can also release cytotoxic factors like free radicals that can exacerbate damage. Thus, it is very important to evaluate what microglia are doing spatially and over time when CNS blood vessels become injured. Therapeutically, the goal should be to enhance reparative functions (e.g. angiogenesis) while deterring those that promote pathology (e.g. free radical release).
To study the innate immune response to cerebrovascular injury, a recent study developed a new injury paradigm in mice involving transcranial application of low-intensity pulse ultrasound to a thinned skull after intravenous microbubble injection51. This method promotes rapid intraparenchymal hemorrhage and glial limitans breakdown beneath the thinned skull window that can be imaged by intravital two-photon microscopy. Within 20 minutes of cerebrovascular injury, intravital imaging revealed that microglia transformed from their resting morphology and extended processes, forming tubular “rosette” structures that enveloped and sealed leaking blood vessels51 (Figure 3C). The formation of these structures was dependent on ATP release via astrocytic connexin hemichannels, which was sensed by the purinergic receptor, P2RY12, on microglia. This mechanism provides a way for BBB astrocytes to quickly notify neighboring microglia that a blood vessel has been damaged, and the microglia in turn attempt to wall off the vessel and contain the extent of vascular leak. In fact, depletion of microglia or inhibition of the ATP detection system resulted in extensive vascular leakage and parenchymal damage in this model of cerebrovascular injury. These data demonstrate that, similar to the response observed following TBI, microglia play a crucial role in restoring barrier function after cerebrovascular injury, and rapid restoration of the BBB is crucial for optimal recovery and repair.
Cerebrovascular injury also induces a peripheral immune response that involves massive recruitment of myelomonocytic cells from the blood. Intravital imaging studies revealed that these cells begin entering the damaged brain within hours of injury and cause extensive edema51. Brain swelling or edema is a potentially life-threatening condition observed in some TBI, intracerebral hemorrhage (ICH), and ischemic stroke patients. Imaging studies in rodents demonstrated that myelomonocytic cell extravasation is at least one mechanism by which fatal edema is generated in the brain after cerebrovascular injury. In fact, myelomonocytic cell extravasation can be blocked therapeutically by intravenously injecting antibodies against the adhesion molecules Lymphocyte Function-associated Antigen-1 (LFA-1) and Very Late Antige-4 (VLA-4). Administration of these antibodies within 6 hours of cerebrovascular injury prevented edema and promoted survival51. While it is clear from these studies that myelomonocytic cells can promote brain pathology, it is important to emphasize that they are also required for proper vascular repair. After initially forming an acute barrier to seal damaged blood vessels, microglia eventually become pro-angiogenic VEGF-A expressing cells that have been termed repair associated microglia (RAM)51. RAM are essential for the formation of new blood vessels after a cerebrovascular injury, and they acquire their pro-angiogenic programming from infiltrating CCR2+ monocytes. Interference with the recruitment of these cells into the brain prevents RAM generation and blocks cerebrovascular repair. Collectively, these data demonstrate that the innate myeloid response to cerebrovascular injury is complex, possessing both reparative and pathogenic components that must be considered when designing therapeutic interventions.
The meninges are also involved in the immune response after stroke, and immune cell infiltration of the meninges often precedes that observed in the parenchyma. Neutrophils are among the earliest immune cells to infiltrate the brain parenchyma after ischemic stroke118. In the MCAO model, imaging studies have demonstrated that infiltrating neutrophils are derived in part from the calvaria bone marrow and migrate through vascular channels that connect skull bone marrow pockets to the dura mater. Extravasated neutrophils can be found within the dura mater after stroke and likely enter the CNS parenchyma from the meninges29. Other studies have found that mast cells within the dura mater contribute to stroke pathogenesis by promoting barrier breakdown and furthering ischemic damage119. While these data provide some insights into the how meninges participate in stroke immunology, additional studies are required to generate a more comprehensive picture of how the meninges and parenchyma interact with one other. It is also worth considering that meningeal and parenchymal vasculature are not the same structurally and rely on different immune-mediated repair mechanisms following injury6,50,51.
4.3. Aging and neurodegeneration
Changes to the immune repertoire within the brain and at barrier sites during normal aging as well as under conditions of neurodegeneration remains incompletely understood. It is well established that immune dysregulation occurs during aging in the peripheral immune system, with elevated oxidative stress and inflammation as well as impaired adaptive immune system functions due to immune senescence. Unraveling neuroimmune interactions during aging and neurodegeneration is crucial for developing new therapeutic strategies. Recent work has highlighted changes that occur with normal aging along CNS parenchymal barriers (e.g., the meninges) and in waste clearance may contribute to the development of neurodegenerative diseases like Alzheimer’s and even affect therapeutic interventions83,87,120.
During normal aging, there are significant changes to the CNS immune repertoire that have been identified using high dimensional single cell mass and fluorescent cytometry40. A subset of microglia has been found in the aged brain that expresses higher levels of CD11c and CD14, which is suggestive of inflammatory programming40,121. Within the brain parenchyma, intravital microscopy has revealed that aged microglia have a distinct morphology consisting of smaller and less branched dendritic arbors. Their processes also move more slowly than those of young microglia, which may compromise steady state parenchymal surveillance. Notably, aged microglia have an impaired and delayed response to injury122. This finding is relevant given the speed required for microglia to quickly seal damaged CNS barriers following TBI and cerebrovascular injury51,74. Any delay in microglial process extension could render the CNS parenchyma more susceptible to damage after injury. In addition to microglia, changes in the adaptive immune system have also been observed in the parenchyma of aged mice. Although the CNS parenchyma is usually devoid of peripheral immune cells such as T cells, these cells are found in the aged brain. For example, in monkeys an age-related increase in perivascular T cells was observed within the white matter parenchyma123. Studies have also demonstrated the presence of clonally expanded interferon-gamma(IFNγ)-producing CD8+ T cells in the subventricular zone of aged mice. The subventricular zone is a brain region that contains neural stem cells, and it is thought that T cell derived IFNγ may interfere with neurogenesis, further contributing to age related cognitive decline39.
CNS barriers such as the choroid plexus and meninges also show notable changes in immune composition with age. Recent studies have demonstrated that meningeal T and B cells increase in number and undergo functional changes30,37,40. For example, an increased frequency of CD4+ and CD8+ T cells was observed in the CNS of aged mice37,40. While these T cells localized primarily along the dural venous sinuses (similar to the distribution in young adult mice), an increase in parenchymal and non-sinus T cells was found with age37. These aged-related changes in T cell distribution could be linked to elevated expression of the endothelium adhesion molecules like Vascular Cell Adhesion Molecule-1 (VCAM1) and Intercellular Adhesion Molecule 1 (ICAM1) on non-sinus vasculature as well as increased extracellular matrix components that foster T cell adhesion37.
In addition to the change in meningeal T cell distribution and frequency, aged mice demonstrate increased regulatory T cells (Treg) and a smaller percentage of CCR7 expressing T cells. Decreased CCR7 expression may impair migration to draining lymph nodes and promote T cell accumulation within the meninges124. Because cytokines produced by meningeal T cells are known to influence neuronal function, learning, and memory, it is conceivable that changes in the meningeal immune landscape may play a role in age related behavioral alterations13,125–129. Modulation of neuronal function by cytokines is not limited to T cells but can be mediated by other immune cell populations. Innate lymphoid cells (ILCs), for example, are the innate counterpart to T cells but lack an antigen specific T cell receptor. These cells change with age, and studies have shown that group 2 ILCs (ILC2s), which produce similar cytokines to TH2 CD4+ T cells, are increased in the choroid plexus and meninges of aged mice but decreased in a murine model of Alzheimer’s disease129,130. Infusion of activated ILC2s or IL-5 treatment improved cognitive function in aged animals, suggesting that the ILC2s at CNS barrier sites play a role in neural homeostasis and that their function declines over time129,130.
B cells and plasma cells are also increased in the meninges of aged mice, and these cells localize primarily along the dural venous sinuses32,91. In younger mice, dural plasma cells are mostly IgA-producers, but an increased prevalence of IgM plasma cells was observed in aged mice32,91. The significance of this shift from IgA to IgM plasma cells is unclear but is likely to have an impact on the meningeal defense against vascular pathogens. It was also recently shown that the meninges of young adult mice harbor immature B cell populations and that this allows for maintenance of immune tolerance to CNS antigens as developing B cells are educated locally30,32,33. However, in aged mice, there is an accumulation of antigen experienced B cells that infiltrate the meninges from the periphery. These cells can undergo terminal differentiation into plasma cells, potentially differentiating into CNS reactive plasma cells that may contribute to autoimmunity and inflammation.
Clearance of metabolites and waste from the CNS also becomes impaired during the normal process of aging34,81,87. Studies have suggested that this age-related impairment in clearance contributes to Alzheimer’s disease pathology and age-related cognitive decline. Using intravital imaging as well as ex vivo fluorescent microscopy, a recent study demonstrated that parenchymal penetration of CSF tracers injected into the cisterna magna was significantly reduced in aged mice relative to young-adult mice81. Furthermore, clearance of interstitial solutes was also impaired81. Alterations in CNS fluid movement with age could be linked to reductions in arterial pulsatility, decreased astrocytic AQP4 expression, changes in the function of meningeal lymphatics, and / or deterioration of the CNS venous drainage system. Interestingly, treatment with vascular endothelial growth factor C, which is known to enhance meningeal lymphatic function, improved cognitive performance in aged mice87.
4.3. Infectious disease
The CNS has a unique set of barriers to protect itself from pathogen invasion because loss of postmitotic neuronal cells (resulting from infection) can have devastating consequences. The immune response to a CNS infection must be carefully orchestrated, as antimicrobial inflammation is known to cause tissue pathology in the periphery that is not well tolerated in the CNS. Static and intravital imaging approaches have provided novel insights into the protection of the CNS from infections and the unique immune response that occurs within the different CNS compartments. The immune defense systems found in CNS barriers including the BBB, meninges and choroid plexus help protect the parenchyma from infection. However, infections can still occur via the meninges, breaches in the BBB, retrograde transport via peripheral nerves, or infiltrating immune cells15,131. The meninges host a diverse repertoire of immune cells that are likely in place to protect the underlying brain parenchyma from a vulnerable vascular system. The dura mater contains fenestrated vasculature without tight junctions as well as large venous sinuses with slow moving blood. It is therefore no surprise that the lymphatic drainage system is juxtaposed to the dural venous sinuses and that the entire dural vascular system is heavily defended by a full armament of immune cells6,7,9,36,132. Any blood borne pathogen has the potential to enter the dura mater and from there the underlying CNS parenchyma. A similar vulnerability exists in the choroid plexus and circumventricular organs due to their ‘open’ vasculature6.
Intravital microscopy has provided important insights into the interactions between CNS vasculature and innate / adaptive immune cells in different models of infectious disease, which has unveiled new modes of pathogenesis. Lymphocytic choriomeningitis virus (LCMV) is a noncytopathic arenavirus and a natural pathogen in humans and mice. The virus can invade the meninges, infecting stromal cells, meningeal macrophages, and infiltrating leukocytes, leading to immune-mediated meningitis38,42,133–135. Intracerebral inoculation of mice with LCMV causes fatal choriomeningitis with convulsive seizures at day 6 post-infection135. The cause of death in this model is brainstem herniation resulting from immune-mediated cerebral edema136. The study of LCMV meningitis has advanced our understanding of basic meningeal immunity and revealed the mechanisms underlying the pathogenesis of this fatal disease. LCMV infection promotes a robust meningeal immune response, including infiltration by CD8+ cytotoxic T lymphocytes (CTLs) and myelomonocytic cells, both of which are important for disease pathogenesis42,137. Light microscopy has helped to define the role of these two different immune subsets in disease pathogenesis. To better understand this disease, one early study visualized virus-specific CTL in the meninges of LCMV infected mice at day 6 post-infection using in situ tetramer staining as well as fluorescently tagged CTL138. This study was the first to demonstrate that virus-specific CTL form immunological synapses with LCMV infected meningeal targets in vivo and could also engage multiple targets simultaneously, thereby increasing their efficiency. It was discovered in a separate study that LCMV-specific CTL could divide in situ within the meningeal space139. Together these data supported the long-held notion that MHC I restricted virus-specific CD8+ T cells were essential for disease pathogenesis137,140. However, the exact role of CD8+ T cells became clearer after they were imaged in relation to meningeal pathology by intravital two-photon microscopy through a thinned skull42. Intravital imaging of symptomatic LCMV-infected mice at the peak of disease revealed evidence of profound meningeal vascular damage that could not be explained by direct CTL engagement of the vessel wall. Instead, meningeal vascular breakdown and leakage was caused by synchronous extravasation of myelomonocytic cells (Figure 4A), and CTL were found to participate in the recruitment of these cells through the release of chemokines42. CTL are typically thought to cause tissue pathology by killing infected target cells, but these data demonstrated that CTL could also cause vascular damage and fatal brain swelling by recruiting vessel-damaging myelomonocytic cells.
Figure 4. Immune mediated damage to CNS vasculature during viral meningitis and cerebral malaria.
A.) An intravital two-photon time lapse captured through the thinned skull window of a LysMgfp/+ mouse six days following intracerebral inoculation of LCMV Armstrong shows myelomonocytic cells (green) synchronously extravasating from a meningeal blood vessel. Quantum dots (red) were injected intravenously to visualize blood vessels. Note the massive extravasation of myelomonocytic cells at 5 min, which coincides with leakage of quantum dots into the extravascular space. B.) Parasite-specific cytotoxic lymphocytes (CTL, green) tagged with a fluorescent protein were visualized at the peak of disease (day 6) in a C57BL/6J mouse infected intravenously with 106 red blood cells parasitized by Plasmodium berghei ANKA. CTL were observed attacking meningeal and cerebral vasculature, arresting along both the luminal and abluminal surfaces. These CTL engagements are responsible for fatal vascular breakdown during cerebral malaria in both mice and humans. Blood vessels were visualized with Evans Blue (red).
Cerebral malaria is another disease associated with cerebrovascular damage, which is linked to the activity of CD8+ T cells. Like LCMV meningitis, mice and humans die from brainstem herniation resulting from brain swelling141,142. Cerebral malaria primarily affects children and is caused by the parasite plasmodium falciparum in humans and plasmodium berghei ANKA in mice. Parasite-specific CD8+ T cells are essential for BBB breakdown, edema, and fatal brainstem herniation in mice141,143, and it was theorized based on the mechanism of vascular pathogenesis observed during LCMV meningitis42 that myelomonocytic cells would play a role in cerebral malaria. However, two intravital imaging studies uncovered a different mode of pathogenesis that involved direct engagement of meningeal and cerebral vasculature by parasite specific CD8+ T cells141,144. CD8+ T cells were observed slowly crawling or arresting along the luminal and abluminal surfaces of CNS blood vessels at the peak of disease, and these interactions were directly associated with vascular breakdown (Figure 4B). Myelomonocytic cells were found to have no role in the disease pathogenesis. In addition, it was possible to treat symptomatic mice and prevent fatal disease by intravenously administering anti-LFA-1 and VLA-4 antibodies, which were shown by intravital microscopy to displace parasite-specific CD8+ T cells from CNS blood vessels141. These imaging data in rodents demonstrated that CD8+ T cells can play a major role in a CNS disease by directly targeting CNS vasculature. Importantly, this finding was confirmed in a separate static imaging study that examined brain tissue from children that died of cerebral malaria145. In this study, CD8+ T cells were observed engaging and releasing lytic effector molecules onto cerebrovasculature – identical to that observed in the rodent model of the disease141,145. This study ended a longstanding debate in the cerebral malaria field by showing that CD8+ T cells were similarly responsible for disease both in mice and humans146.
The study of meningeal infections by light microscopy has also shed light on how pathogens can imprint this compartment with long term alterations in immune repertoire and function38. Meningeal macrophages residing in the dura mater are originally derived from yolk sac erythromyeloid precursors but similar to the choroid plexus macrophages are replaced over time by peripheral blood monocytes92–94. During LCMV meningitis, meningeal macrophages are infected by the virus and upregulate genes associated with antigen presentation, antiviral activity, and phagocytosis7,38. Infected meningeal macrophages are also engaged and killed by virus-specific CTL, which leads to their depletion. Wild type LCMV injected intracerebrally causes fatal meningitis in all mice by day 6 post-infection135. To study the long-term effects on the meningeal immune landscape, a non-lethal model of meningitis was developed that involved intracerebral inoculation with an attenuated recombinant of the virus147. Infection with attenuated LCMV resulted in non-lethal meningitis. Study of dural meningeal macrophages in this model revealed that their early depletion after infection was followed by restoration of the compartment by day 30. This recovery was not due to proliferation of local myeloid precursors but instead due to engraftment by peripheral inflammatory monocytes that differentiated into meningeal macrophages. Although the number, distribution, and dynamics of the engrafted meningeal macrophages visualized by different imaging techniques were indistinguishable from the non-engrafted tissue residents, key differences in gene expression were observed using microarrays38. For example, reduced expression of the microbial sensor CD209b/SIGNR-1 and the nicotinic cholinergic receptor (Chnrb4) was found on engrafting monocyte-derived meningeal macrophages following resolution of LCMV meningitis. These changes impeded the ability of engrafted meningeal macrophages to respond to a new microbial challenge or dampen excessive inflammation, respectively38. This study highlighted the lasting imprint an infection can have on the CNS immune landscape and offered a new possibility for how CNS diseases and susceptibility to secondary infections might develop long after a pathogen is cleared.
Infection of the CNS parenchyma can also occur via invasion of peripheral nerves, which bypasses many protective barriers such as the BBB and meninges15,131,132. Vesicular stomatitis virus (VSV) is a neurotropic rhabdovirus that upon intranasal inoculation infects olfactory sensory neurons in the nasal airway and travels through the cribriform plate via the axons of these nasal neurons to infect the olfactory bulb (OB)148. This is a common route by which many nasal pathogens enter the brain131,132. The innate and adaptive immune defense mounted within the OB parenchyma is crucial for protecting the CNS from viral spread and development of fatal encephalitis70,149. Steady state microglia have small cell bodies with highly dynamic processes that allow them to continuously scan the CNS parenchyma and rapidly respond to infections and injuries53,67,72,73,150. Following an intranasal VSV infection, OB microglia locally proliferated and showed immediate signs of activation that included upregulation of antigen presenting and costimulatory molecules70,75. Although microglia are not infected by VSV in adult mice, a recent imaging study demonstrated that microglia are capable of cross-presenting antigens acquired from adjacent VSV-infected neurons38,70. Intravital imaging of the infected OB through a thinned skull window showed that VSV-specific CD8+ T cells recognized their cognate antigen, fluxed calcium, and arrested upon engagement of OB microglia even though neurons (not microglia) were infected by the virus70. This study also demonstrated that microglia could acquire antigens from adjacent neurons and that neuronal antigen presentation via MHC I was not required for CD8+ T cells to perform their antiviral functions after VSV infection. Instead, interactions between CD8+ T cells and cross-presenting OB microglia were found to be important for protecting mice from CNS viral spread and fatal encephalitis70,149. These studies uncovered an important new mechanism by which the innate (microglia) and adaptive (CD8+ T cells) immune system interact to protect the brain from a nasal pathogen that attempts to enter via peripheral nerves. CTL engagement of cross-presenting microglia also helps protect and preserve infected OB neurons by allowing them to be cleared by well described noncytolytic cytokine-mediated effector mechanisms instead of direct CTL killing151.
5. Concluding Remarks
Imaging techniques have illuminated an exciting new world of immunological specializations within the CNS, especially at its borders. While it is important to remember that the CNS is still an immunoprivileged site that does not recognize foreign grafts or tumors unless the meningeal lymphatics are artificially enhanced3,152, there is impressive immunological diversity at specialized CNS barrier sites such as the meninges, dural venous sinuses, choroid plexus, and likely the circumventricular organs6. It is likely that the presence of ‘open’ vasculature in these barrier sites is the main reason why they are so heavily defended by the immune system. The dura mater, for example, with its large venous drains and fenestrated blood vessels, resembles a peripheral tissue, which explains the need for lymphatic vessels in this microenvironment. It resides above the sealed arachnoid barrier and must ensure that the leptomeninges and underlying CNS parenchyma are not invaded by blood borne pathogens. In fact, imaging studies have showed us that if the dural venous sinuses are not defended by IgA plasma cells then a blood borne pathogen can enter the brain parenchyma91. Imaging studies have also greatly enhanced our understanding of the anatomy and immunology of the CNS and its different barrier structures during homeostasis, disease, and aging. Because the immune and nervous systems have co-evolved, their functions have become inextricably linked. The architecture and composition of immune structures and cells within the CNS are likely based on a need to protect the parenchyma from pathogens entering via the blood or peripheral nerves. The CNS parenchyma is relatively intolerant of inflammation or edema, which can quickly lead to a life-threatening situation. However, it is also now accepted that the CNS immune system regulates neural activity during homeostasis and that decay in this system can lead to cognitive decline and facilitate neurodegeneration. The immune system has the remarkable ability to repair the damaged CNS but can also exacerbate pathology depending on the context. It is therefore important that we continue to image CNS-immune interactions in all conceivable scenarios, with the eventual goal of identifying therapeutics to mitigate human neuroinflammatory disorders and to lessen the burden of aging. Advancements in imaging techniques have brought us amazingly far in the field of neuroimmunology, but there still much to learn about how best to guide the immune system toward optimal functions while preserving neural activity.
Acknowledgements
This research was supported by the intramural program at the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH). We thank Alan Hoofring in the NIH Medical Arts Design Section for his help with the illustration shown in Figure 1.
Footnotes
Conflicts of Interests
The authors have no conflicts of interest to report.
References
- 1.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18(2):123–131. [DOI] [PubMed] [Google Scholar]
- 2.Louveau A, Harris TH, Kipnis J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015;36(10):569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MEDAWAR PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69. [PMC free article] [PubMed] [Google Scholar]
- 4.Forrester JV, McMenamin PG, Dando SJ. CNS infection and immune privilege. Nat Rev Neurosci. 2018;19(11):655–671. [DOI] [PubMed] [Google Scholar]
- 5.Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS. 2011;8(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mastorakos P, McGavern D. The anatomy and immunology of vasculature in the central nervous system. Sci Immunol. 2019;4(37). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rua R, McGavern DB. Advances in Meningeal Immunity. Trends Mol Med. 2018;24(6):542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strominger I, Elyahu Y, Berner O, et al. The Choroid Plexus Functions as a Niche for T-Cell Stimulation Within the Central Nervous System. Front Immunol. 2018;9:1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coles JA, Myburgh E, Brewer JM, McMenamin PG. Where are we? The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. Prog Neurobiol. 2017;156:107–148. [DOI] [PubMed] [Google Scholar]
- 12.Ahn JH, Cho H, Kim JH, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572(7767):62–66. [DOI] [PubMed] [Google Scholar]
- 13.Salvador AF, de Lima KA, Kipnis J. Neuromodulation by the immune system: a focus on cytokines. Nat Rev Immunol. 2021;21(8):526–541. [DOI] [PubMed] [Google Scholar]
- 14.Russo MV, McGavern DB. Inflammatory neuroprotection following traumatic brain injury. Science. 2016;353(6301):783–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manglani M, McGavern DB. New advances in CNS immunity against viral infection. Curr Opin Virol. 2018;28:116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777–783. [DOI] [PubMed] [Google Scholar]
- 17.Germain RN, Miller MJ, Dustin ML, Nussenzweig MC. Dynamic imaging of the immune system: progress, pitfalls and promise. Nat Rev Immunol. 2006;6(7):497–507. [DOI] [PubMed] [Google Scholar]
- 18.Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pittet MJ, Garris CS, Arlauckas SP, Weissleder R. Recording the wild lives of immune cells. Sci Immunol. 2018;3(27). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorand RD, Benson BL, Huang LF, Petrosiute A, Huang AY. Insights From Dynamic Neuro-Immune Imaging on Murine Immune Responses to CNS Damage. Front Neurosci. 2019;13:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrier M, Robert M, González Ibáñez F, Desjardins M, Tremblay M. Imaging the Neuroimmune Dynamics Across Space and Time. Front Neurosci. 2020;14:903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen TW, Wardill TJ, Sun Y, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moseman EA, Wu T, de la Torre JC, Schwartzberg PL, McGavern DB. Type I interferon suppresses virus-specific B cell responses by modulating CD8. Sci Immunol. 2016;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci. 2007;10(5):549–551. [DOI] [PubMed] [Google Scholar]
- 25.Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010;5(2):201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manglani M, McGavern DB. Intravital Imaging of Neuroimmune Interactions Through a Thinned Skull. Curr Protoc Immunol. 2018;120:24.22.21–24.22.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louveau A, Filiano AJ, Kipnis J. Meningeal whole mount preparation and characterization of neural cells by flow cytometry. Curr Protoc Immunol. 2018;121(1):e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rustenhoven J, Kipnis J. Bypassing the blood-brain barrier. Science. 2019;366(6472):1448–1449. [DOI] [PubMed] [Google Scholar]
- 29.Herisson F, Frodermann V, Courties G, et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci. 2018;21(9):1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cugurra A, Mamuladze T, Rustenhoven J, et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. 2021;373(6553). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rindone AN, Liu X, Farhat S, et al. Quantitative 3D imaging of the cranial microvascular environment at single-cell resolution. Nat Commun. 2021;12(1):6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brioschi S, Wang WL, Peng V, et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science. 2021;373(6553). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Chen D, Xu D, et al. Early developing B cells undergo negative selection by central nervous system-specific antigens in the meninges. Immunity. 2021. [DOI] [PubMed] [Google Scholar]
- 34.Ma Q, Ineichen BV, Detmar M, Proulx ST. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun. 2017;8(1):1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alves de Lima K, Rustenhoven J, Kipnis J. Meningeal Immunity and Its Function in Maintenance of the Central Nervous System in Health and Disease. Annu Rev Immunol. 2020;38:597–620. [DOI] [PubMed] [Google Scholar]
- 36.Gadani SP, Smirnov I, Smith AT, Overall CC, Kipnis J. Characterization of meningeal type 2 innate lymphocytes and their response to CNS injury. J Exp Med. 2017;214(2):285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rustenhoven J, Drieu A, Mamuladze T, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021;184(4):1000–1016.e1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rua R, Lee JY, Silva AB, et al. Infection drives meningeal engraftment by inflammatory monocytes that impairs CNS immunity. Nat Immunol. 2019;20(4):407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dulken BW, Buckley MT, Navarro Negredo P, et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature. 2019;571(7764):205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mrdjen D, Pavlovic A, Hartmann FJ, et al. High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity. 2018;48(3):599. [DOI] [PubMed] [Google Scholar]
- 41.Van Hove H, Martens L, Scheyltjens I, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22(6):1021–1035. [DOI] [PubMed] [Google Scholar]
- 42.Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457(7226):191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mascagni P, Sanctius C. Vasorum lymphaticorum corporis humani historia et ichnographia. Ex typographia Pazzini Carli; 1787. [Google Scholar]
- 44.Waggener JD, Beggs J. The membranous coverings of neural tissues: an electron microscopy study. J Neuropathol Exp Neurol. 1967;26(3):412–426. [DOI] [PubMed] [Google Scholar]
- 45.Andres KH, von During M, Muszynski K, Schmidt RF. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol (Berl). 1987;175(3):289–301. [DOI] [PubMed] [Google Scholar]
- 46.Laman JD, Weller RO. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J Neuroimmune Pharmacol. 2013;8(4):840–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iliff JJ, Goldman SA, Nedergaard M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 2015;14(10):977–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J. 2015;282(21):4067–4079. [DOI] [PubMed] [Google Scholar]
- 50.Russo MV, Latour LL, McGavern DB. Distinct myeloid cell subsets promote meningeal remodeling and vascular repair after mild traumatic brain injury. Nat Immunol. 2018;19(5):442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mastorakos P, Mihelson N, Luby M, et al. Temporally distinct myeloid cell responses mediate damage and repair after cerebrovascular injury. Nat Neurosci. 2021;24(2):245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol. 2008;67(12):1113–1121. [DOI] [PubMed] [Google Scholar]
- 53.Nayak D, Zinselmeyer BH, Corps KN, McGavern DB. In vivo dynamics of innate immune sentinels in the CNS. Intravital. 2012;1(2):95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barkalow FJ, Goodman MJ, Gerritsen ME, Mayadas TN. Brain endothelium lack one of two pathways of P-selectin-mediated neutrophil adhesion. Blood. 1996;88(12):4585–4593. [PubMed] [Google Scholar]
- 55.Baruch K, Schwartz M. CNS-specific T cells shape brain function via the choroid plexus. Brain Behav Immun. 2013;34:11–16. [DOI] [PubMed] [Google Scholar]
- 56.Dani N, Herbst RH, McCabe C, et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184(11):3056–3074 e3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui J, Xu H, Lehtinen MK. Macrophages on the margin: choroid plexus immune responses. Trends Neurosci. 2021;44(11):864–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolburg H, Wolburg-Buchholz K, Liebner S, Engelhardt B. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci Lett. 2001;307(2):77–80. [DOI] [PubMed] [Google Scholar]
- 59.Erickson MA, Banks WA. Neuroimmune Axes of the Blood-Brain Barriers and Blood-Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions. Pharmacol Rev. 2018;70(2):278–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baruch K, Ron-Harel N, Gal H, et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci U S A. 2013;110(6):2264–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reboldi A, Coisne C, Baumjohann D, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10(5):514–523. [DOI] [PubMed] [Google Scholar]
- 62.Engelhardt B, Wolburg-Buchholz K, Wolburg H. Involvement of the choroid plexus in central nervous system inflammation. Microsc Res Tech. 2001;52(1):112–129. [DOI] [PubMed] [Google Scholar]
- 63.Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am J Physiol. 1984;246(6 Pt 2):F835–844. [DOI] [PubMed] [Google Scholar]
- 64.Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123(3):1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology. 2019;65(2):106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18(4):225–242. [DOI] [PubMed] [Google Scholar]
- 69.Herz J, Johnson KR, McGavern DB. Therapeutic antiviral T cells noncytopathically clear persistently infected microglia after conversion into antigen-presenting cells. J Exp Med. 2015;212(8):1153–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moseman EA, Blanchard AC, Nayak D, McGavern DB. T cell engagement of cross-presenting microglia protects the brain from a nasal virus infection. Sci Immunol. 2020;5(48). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jung S, Aliberti J, Graemmel P, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20(11):4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. [DOI] [PubMed] [Google Scholar]
- 73.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. [DOI] [PubMed] [Google Scholar]
- 74.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505(7482):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chhatbar C, Prinz M. The roles of microglia in viral encephalitis: from sensome to therapeutic targeting. Cell Mol Immunol. 2021;18(2):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iliff JJ, Wang M, Zeppenfeld DM, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33(46):18190–18199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mestre H, Hablitz LM, Xavier AL, et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith AJ, Akdemir G, Wadhwa M, Song D, Verkman AS. Application of fluorescent dextrans to the brain surface under constant pressure reveals AQP4-independent solute uptake. J Gen Physiol. 2021;153(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas JH. Fluid dynamics of cerebrospinal fluid flow in perivascular spaces. J R Soc Interface. 2019;16(159):20190572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76(6):845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34(49):16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng W, Achariyar TM, Li B, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu E, Sun L, Zhang Y, Wang A, Yan J. Aquaporin4 Knockout Aggravates Early Brain Injury Following Subarachnoid Hemorrhage Through Impairment of the Glymphatic System in Rat Brain. Acta Neurochir Suppl. 2020;127:59–64. [DOI] [PubMed] [Google Scholar]
- 85.Absinta M, Ha SK, Nair G, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jacob L, Boisserand LSB, Geraldo LHM, et al. Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun. 2019;10(1):4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560(7717):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Louveau A, Herz J, Alme MN, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21(10):1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yanev P, Poinsatte K, Hominick D, et al. Impaired meningeal lymphatic vessel development worsens stroke outcome. J Cereb Blood Flow Metab. 2020;40(2):263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bolte AC, Dutta AB, Hurt ME, et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat Commun. 2020;11(1):4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fitzpatrick Z, Frazer G, Ferro A, et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature. 2020;587(7834):472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goldmann T, Wieghofer P, Jordao MJ, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17(7):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prinz M, Erny D, Hagemeyer N. Ontogeny and homeostasis of CNS myeloid cells. Nat Immunol. 2017;18(4):385–392. [DOI] [PubMed] [Google Scholar]
- 94.Utz SG, See P, Mildenberger W, et al. Early Fate Defines Microglia and Non-parenchymal Brain Macrophage Development. Cell. 2020;181(3):557–573 e518. [DOI] [PubMed] [Google Scholar]
- 95.Derecki NC, Quinnies KM, Kipnis J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav Immun. 2011;25(3):379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Radjavi A, Smirnov I, Derecki N, Kipnis J. Dynamics of the meningeal CD4(+) T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol Psychiatry. 2014;19(5):531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schafflick D, Wolbert J, Heming M, et al. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat Neurosci. 2021;24(9):1225–1234. [DOI] [PubMed] [Google Scholar]
- 98.Keppler SJ, Goess MC, Heinze JM. The Wanderings of Gut-Derived IgA Plasma Cells: Impact on Systemic Immune Responses. Front Immunol. 2021;12:670290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dewan MC, Rattani A, Gupta S, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018:1–18. [DOI] [PubMed] [Google Scholar]
- 100.Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72(3):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron. 2017;95(6):1246–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mason HD, Johnson AM, Mihelson NA, Mastorakos P, McGavern DB. Glia limitans superficialis oxidation and breakdown promote cortical cell death after repetitive head injury. JCI Insight. 2021;6(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lou N, Takano T, Pei Y, Xavier AL, Goldman SA, Nedergaard M. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci U S A. 2016;113(4):1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11(3):201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16(3):177–192. [DOI] [PubMed] [Google Scholar]
- 106.Mastorakos P, Russo MV, Zhou T, Johnson K, McGavern DB. Antimicrobial immunity impedes CNS vascular repair following brain injury. Nat Immunol. 2021;22(10):1280–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kesinger MR, Kumar RG, Wagner AK, et al. Hospital-acquired pneumonia is an independent predictor of poor global outcome in severe traumatic brain injury up to 5 years after discharge. J Trauma Acute Care Surg. 2015;78(2):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Donkor ES. Stroke in the 21. Stroke Res Treat. 2018;2018:3238165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fluri F, Schuhmann MK, Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther. 2015;9:3445–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18(11):1058–1066. [DOI] [PubMed] [Google Scholar]
- 111.Iadecola C, Buckwalter MS, Anrather J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J Clin Invest. 2020;130(6):2777–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tvrdik P, Kearns KN, Sharifi KA, Sluzewski MF, Acton ST, Kalani MYS. Calcium Imaging of Microglial Network Activity in Stroke. Methods Mol Biol. 2019;2034:267–279. [DOI] [PubMed] [Google Scholar]
- 113.Zhang S. Microglial activation after ischaemic stroke. Stroke Vasc Neurol. 2019;4(2):71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neumann J, Riek-Burchardt M, Herz J, et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 2015;129(2):259–277. [DOI] [PubMed] [Google Scholar]
- 115.Morrison HW, Filosa JA. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation. 2013;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jin WN, Shi SX, Li Z, et al. Depletion of microglia exacerbates postischemic inflammation and brain injury. J Cereb Blood Flow Metab. 2017;37(6):2224–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hou B, Jiang C, Wang D, et al. Pharmacological Targeting of CSF1R Inhibits Microglial Proliferation and Aggravates the Progression of Cerebral Ischemic Pathology. Front Cell Neurosci. 2020;14:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cai W, Liu S, Hu M, et al. Functional Dynamics of Neutrophils After Ischemic Stroke. Transl Stroke Res. 2020;11(1):108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Parrella E, Porrini V, Benarese M, Pizzi M. The Role of Mast Cells in Stroke. Cells. 2019;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Da Mesquita S, Papadopoulos Z, Dykstra T, et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature. 2021;593(7858):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Niraula A, Sheridan JF, Godbout JP. Microglia Priming with Aging and Stress. Neuropsychopharmacology. 2017;42(1):318–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10(2):263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Batterman KV, Cabrera PE, Moore TL, Rosene DL. T Cells Actively Infiltrate the White Matter of the Aging Monkey Brain in Relation to Increased Microglial Reactivity and Cognitive Decline. Front Immunol. 2021;12:607691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Da Mesquita S, Herz J, Wall M, et al. Aging-associated deficit in CCR7 is linked to worsened glymphatic function, cognition, neuroinflammation, and β-amyloid pathology. Sci Adv. 2021;7(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alves de Lima K, Rustenhoven J, Da Mesquita S, et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol. 2020;21(11):1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Filiano AJ, Xu Y, Tustison NJ, et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature. 2016;535(7612):425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Derecki NC, Cardani AN, Yang CH, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207(5):1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ribeiro M, Brigas HC, Temido-Ferreira M, et al. Meningeal gammadelta T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci Immunol. 2019;4(40). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fung ITH, Sankar P, Zhang Y, et al. Activation of group 2 innate lymphoid cells alleviates aging-associated cognitive decline. J Exp Med. 2020;217(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fung ITH, Zhang Y, Shin DS, et al. Group 2 innate lymphoid cells are numerically and functionally deficient in the triple transgenic mouse model of Alzheimer’s disease. J Neuroinflammation. 2021;18(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Swanson PA 2nd, , McGavern DB Viral diseases of the central nervous system. Curr Opin Virol. 2015;11:44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.II PAS, McGavern DB. Portals of Viral Entry into the Central Nervous System. In: Dorovini-Zis K, ed. The Blood-Brain Barrier in Health and Disease, Volume Two: Pathophysiology and Pathology. Vol 2. CRC Press; 2015:23. [Google Scholar]
- 133.Kang SS, McGavern DB. Microbial induction of vascular pathology in the CNS. J Neuroimmune Pharmacol. 2010;5(3):370–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol. 2011;11(5):318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kang SS, McGavern DB. Lymphocytic choriomeningitis infection of the central nervous system. Front Biosci. 2008;13:4529–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Matullo CM, O’Regan KJ, Hensley H, Curtis M, Rall GF. Lymphocytic choriomeningitis virus-induced mortality in mice is triggered by edema and brain herniation. J Virol. 2010;84(1):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fung-Leung WP, Kündig TM, Zinkernagel RM, Mak TW. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med. 1991;174(6):1425–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McGavern DB, Christen U, Oldstone MB. Molecular anatomy of antigen-specific CD8(+) T cell engagement and synapse formation in vivo. Nat Immunol. 2002;3(10):918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kang SS, Herz J, Kim JV, et al. Migration of cytotoxic lymphocytes in cell cycle permits local MHC I-dependent control of division at sites of viral infection. J Exp Med. 2011;208(4):747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248(5450):701–702. [DOI] [PubMed] [Google Scholar]
- 141.Swanson PA, Hart GT, Russo MV, et al. CD8+ T Cells Induce Fatal Brainstem Pathology during Cerebral Malaria via Luminal Antigen-Specific Engagement of Brain Vasculature. PLoS Pathog. 2016;12(12):e1006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Seydel KB, Kampondeni SD, Valim C, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015;372(12):1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yanez DM, Manning DD, Cooley AJ, Weidanz WP, van der Heyde HC. Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J Immunol. 1996;157(4):1620–1624. [PubMed] [Google Scholar]
- 144.Shaw TN, Stewart-Hutchinson PJ, Strangward P, et al. Perivascular Arrest of CD8+ T Cells Is a Signature of Experimental Cerebral Malaria. PLoS Pathog. 2015;11(11):e1005210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Riggle BA, Manglani M, Maric D, et al. CD8+ T cells target cerebrovasculature in children with cerebral malaria. J Clin Invest. 2020;130(3):1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Renia L, Grau GE, Wassmer SC. CD8+ T cells and human cerebral malaria: a shifting episteme. J Clin Invest. 2020;130(3):1109–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Emonet SF, Garidou L, McGavern DB, de la Torre JC. Generation of recombinant lymphocytic choriomeningitis viruses with trisegmented genomes stably expressing two additional genes of interest. Proc Natl Acad Sci U S A. 2009;106(9):3473–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235(2):277–287. [DOI] [PubMed] [Google Scholar]
- 149.Chhatbar C, Detje CN, Grabski E, et al. Type I Interferon Receptor Signaling of Neurons and Astrocytes Regulates Microglia Activation during Viral Encephalitis. Cell Rep. 2018;25(1):118–129.e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Russo MV, McGavern DB. Immune Surveillance of the CNS following Infection and Injury. Trends Immunol. 2015;36(10):637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. [DOI] [PubMed] [Google Scholar]
- 152.Song E, Mao T, Dong H, et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577(7792):689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]