Abstract
Objectives:
To determine if metabolic adaptation, at the level of resting metabolic rate (RMR), was associated with time to reach weight loss goals, after adjusting for confounders.
Methods:
65 premenopausal women with overweight (BMI: 28.6±1.5 kg/m2; age: 36.4±5.9 years; 36 Whites and 29 Blacks) followed an 800 kcal/day diet until BMI≤25 kg/m2. Body weight and composition were measured at baseline and after weight loss. Dietary adherence was calculated from total energy expenditure, determined by double labeled water, and body composition changes. Metabolic adaptation was defined as a significantly lower measured vs predicted RMR (from own regression model). A regression model to predict time to reach weight loss goals was developed including target weight loss, energy deficit, dietary adherence, and metabolic adaptation as predictors.
Results:
Participants lost on average 12.5±3.1 kg (16.1±3.4%) over 155.1±49.2 days. Average dietary adherence was 63.6±31.0%. There was significant metabolic adaptation after weight loss (−46±113 kcal/day, P=0.002) and this variable was a significant predictor of time to reach weight loss goal (β=−0.1, P=0.041), even after adjusting for confounders (R2 adjusted = 0.63, P<0.001).
Conclusion:
In premenopausal women with overweight, metabolic adaptation after a 16% weight loss increases the length of time necessary to achieve weight loss goals.
Keywords: metabolic adaptation, adaptive thermogenesis, weight loss, resting metabolic rate
Introduction
The existence or lack of, and clinical relevance, of metabolic adaptation, in response to weight loss, has been one of the most controversial issues in the obesity field (1–7). A careful examination of the available literature suggests that differences among studies derive from inconsistencies related to the status of energy balance (EB) and/or weight stability of the participants when measurements are taken. As such, longitudinal studies tend to report metabolic adaptation (8–13), while cross-sectional studies, which compare weight-reduced individuals to body mass index (BMI)-matched controls, tend not to report metabolic adaptation (14–18). We were recently able to show that EB status does modulate the extent of metabolic adaptation, at the level of resting metabolic rate (RMR) (19), and that if measurements are taken under conditions of weight stability, differences between measured and predicted RMR are minimal (on average 50 kcal/day) (19, 20).
Regardless of the extent of metabolic adaptation, its clinical relevance remains to be fully determined. It was initially suggested that metabolic adaptation could be a potential explanatory mechanism for long-term weight regain (relapse), as well as resistance to weight loss (1–5). However, until recently very little evidence existed in favor or against it, except for Fothergill and colleagues (11), who showed in 2016 that metabolic adaptation after weight loss was not correlated with weight regain at 6 years follow-up in participants of the Biggest Loser competition. We have confirmed these findings in two recent studies (19, 20), by showing that metabolic adaptation at the level of RMR, after weight loss, was not associated with weight regain up to 2 years follow-up. Moreover, in another recent published manuscript, our research group showed that metabolic adaptation at the level of RMR was associated with less weight and fat mass loss in response to a low-energy diet, in men and women with obesity (21). However, it remains to be investigated if metabolic adaptation contributes to resistance to weight loss by increasing the time necessary to achieve weight loss goals.
Therefore, the aim of this secondary analysis was to determine if metabolic adaptation, at the level of RMR, was associated with time to reach weight loss goals, after adjusting for adherence to the diet, in a population of premenopausal women with overweight.
Subjects and Methods
Participants
Participants in this analysis were white and black premenopausal women with overweight. They were 20–41 years of age, sedentary (no more than one time per week regular exercise), had normal glucose tolerance (2–h glucose ≤140 mg/dL following 75g oral dose), family history of overweight/obesity in at least one first-degree relative, and no use of medications that affect body composition or metabolism. All women were nonsmokers and reported a regular menstrual cycle. The two studies included in this retrospective analysis were both approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham (UAB). All women provided informed consent before participating in the study.
Study design
Participants included in this retrospective analysis come from 2 different studies (1. ROMEO; 2. JULIET), performed at the Department of Nutritional Sciences at UAB, with exactly the same sequence of events (see flowchart, Supplementary methods) and same methodology and both aiming to identify metabolic predictors of weight regain. In the ROMEO all participants achieved weight loss with diet alone (single arm longitudinal study with repeated measurements). In the JULIET study, participants were randomly assigned to one of three groups: 1) Weight loss with aerobic exercise training 3 times/week; 2) Weight loss with resistance exercise training 3 times/week; and 3) Weight loss with diet alone (same diet as in ROMEO). For this secondary analysis we included all participants from the ROMEO study and the participants randomized to diet only from the JULIET study. During weight loss, all participants were provided an 800-kcal diet until reaching a BMI ≤25 kg/m2. Food was provided (20–22% fat, 20–22% protein, and 56–58% carbohydrate) by the General Clinical Research Center (GCRC) Kitchen. For detailed information about the ROMEO and JULIET studies, see Weinsier et al, 2000 (22) and Hunter et al, 2008 (23), respectively.
Testing was done, after a 4-week weight stabilization period, at baseline and after weight loss. Therefore, testing was done on average 28 days after the end of the weight loss phase. During the 4-week weight stabilization period, participants were weighed 3 times/week the first 2 weeks while eating own food and weighed 5 times/week with food provided by GCRC the last 2 weeks. Variation in body weight during the last 2 weeks of the stabilization period, after weight loss, was −1.0 ± 1.4 kg. All testing was conducted in the follicular phase of the menstrual cycle during a 4-day GCRC in-patient stay (to ensure that physical activity and diet was standardized). Testing was done in a fasted state in the morning after spending the night in the GCRC.
Data Collection
The following measurements were conducted at baseline and after weight loss, after a 4-week weight stabilisation period (at both time points):
Body weight and composition
Body composition was determined by using the 4-compartment model (4CM) (24), which includes in the analysis bone mineral content, total body water, and total body density to take into consideration inter-individual variations in body density and the fact that black women generally have a greater bone mineral content than do white women (25). The 4CM includes the following density assumptions: 0.9 kg/L for fat, 0.99 kg/L for water, 3.042 kg/L for total mineral (osseous and cellular), and 1.34 kg/L for the unmeasured fraction of the body composed of protein and glycogen. The model is used to calculate the percentage of FM from independent measures of total body density, total body water, and bone mineral content. Total body density was determined by whole body air displacement plethysmography using the BodPod version 1.69 (Body Composition System; Life Measurement, Concord, CA) as described previously (26). Each participant was tested in a one-piece swimsuit and Lycra swim cap. Same-day repeat measures of body density by the BOD POD in our laboratory had an intra-class correlation of r = 0.98 and SEE of 0.00365 (g/cm3). The room that housed the BodPod was well ventilated between tests. Body weight was measured with an electronic scale while the subjects were in a fasting state and immediately after they had voided in the morning. Total body water was determined by doubly labeled water (DLW) (see below). Fat mass (FM), fat-free mass (FFM) and bone mineral content were determined by dual-energy X-ray absorptiometry (DXA) (DPX-L; Lunar Corp, Madison, WI) with the use of software version 1.5g (Lunar Corp).
Total energy expenditure
The DLW technique was used to measure total energy expenditure (TEE), both immediately before and after the weight loss intervention (i.e. during the last 2wk of the 4wk supervised weight stabilization phase, see Study design section). In brief, a baseline urine sample (10 ml) was collected, followed by a mixed oral dose (~0.10 g/kg 18O and 0.08 g 2H/kg body mass) administration of DLW. The average initial isotope enrichments of two urine samples were obtained the morning after dosing, and on the 14th day, two additional final samples were obtained and results averaged. All urine samples were analyzed in triplicate for 2H and 18O by isotope ratio mass spectrometry at the Metabolism Core Laboratory of the Clinical Nutrition Research Center and the GCRC at UAB. A description of the technical aspects has been previously described (27).
Resting metabolic rate
Prior to each test the Delta Trac was calibrated with standard calibration gases. An in house, quality control alcohol burn was performed quarterly, and whenever problems or questions arose. At all times during the project the instrument generated respiratory quotient values between 0.64 and 0.69, which are reflective of accurate function, as indicated in the manufacture’s guidelines. In addition, the instrument was serviced annually by the manufacturer to assure accurate function and calibration
Three consecutive mornings after an overnight stay in the GCRC and 12-h fast, RMR was measured immediately after awakening between 6 and 7 am. Subjects were not allowed to sleep and measurements were made in a quiet, softly lit, well-ventilated room. Temperature was maintained between 22 and 24 °C. Subjects were allowed to use a cover if desired. Measurements were made supine on a comfortable bed, with the head enclosed in a plexiglass canopy. After resting for 15 min, RMR was measured for 30 min with a computerized, open-circuit, indirect calorimetry system with a ventilated canopy (Delta Trac II; Sensor Medics, Yorba Linda, CA). The last 20 min of measurement was used for analysis. Oxygen uptake (VO2) and carbon dioxide production (CO2) were measured continuously, and values were averaged at 1-min intervals. Coefficient of variation for the repeat RMR was <4%.
Adherence to the diet
Dietary adherence was determined as proposed by del Corral and colleagues (28). First, the average TEE was assessed by DLW during energy balance immediately before and after the intervention. The provided energy intake (800 kcal/day) was subtracted from the average of the two TEE values to calculate the expected daily kilocalorie loss (expected daily kilocalorie loss=average TEE − 800 kcal (diet)). Second, to convert losses of FM and FFM (from DXA) to energy lost (i.e. kilocalories lost), we used energy coefficients of 9.3 kcal/g for FM and 1.1 kcal/g for FFM lost [kcal lost = [9.3 kcal × ΔFM(g)] + [1.1 kcal × ΔFFM(g)]. For subjects accruing FFM, an energy coefficient of 1.8 kcal/g was used (29, 30): total kilocalorie lost when FFM was gained = [9.3 kcal × ΔFM (g)] + [1.8 kcal × ΔFFM(g)]. Third, kilocalories lost per day during the intervention was calculated by dividing Total kcal lost during intervention by days needed to reach goal (number of days on diet). Kcal lost per day = total kilocalorie lost/days to goal. Fourth, knowing the actual daily kilocalorie lost and the expected daily kilocalorie lost, we then calculated the daily kilocalorie discrepancy, an index of dietary adherence: daily kilocalorie discrepancy = actual daily kilocalorie lost − expected daily kilocalorie lost. A daily kilocalorie discrepancy of zero represents 100% adherence. A positive number indicates a greater than expected daily kilocalorie loss, whereas a negative number suggests less than expected daily kilocalorie loss. Fifth, we expressed dietary adherence in relative terms: percent daily kilocalorie adherence = (actual daily kilocalorie loss/expected daily kilocalorie loss) × 100.
Statistical analysis
Statistical analysis was performed with SPSS version 22 (SPSS Inc., Chicago, IL), data presented as mean ± SD and statistical significance set at P<0.05. Changes in body weight/composition (from DXA) and RMR overtime were assessed with paired sample t-tests. The presence of metabolic adaptation was tested by paired t-tests, comparing measured RMR (RMRm) and predicted RMR (RMRp) at the same time points. An equation to predict RMR was derived from baseline data of the participants included in this analysis (with body composition data from 4CM).
Model: RMRp (kcal/day) = 540.680 − (4.911 × Age (years)) − (140.760 × Race (0 for whites and 1 for blacks)) + (4.851 × FM (kg)) + (21.362 × FFM (kg)).
R2 = 0.32; P<0.001
This small R2 is due to the study design of the parent studies in which a very narrow range of BMI and age, and only women, were included.
Correlation analysis was performed between metabolic adaptation after weight loss and time to reach weight loss goal using Spearmen correlation coefficients. The time to reach a given threshold of body weight (BMI< 25kg/m2 in this case) for each participant depends on the amount of weight needed to be lost (target weight loss), the energy deficit induced by the diet (baseline TEE – 800), as well as the adherence to the diet and potential degree of metabolic adaptation. Therefore, a regression model to predict time to reach weight loss goal (days) was constructed using target weight loss (kg), energy deficit (kcal/day), dietary adherence, and metabolic adaptation after weight loss as predictors. Target weight loss was calculated as the difference between baseline weight and weight at a BMI of 25kg/m2. There was no multicollinearity among the independent variables included in the model (variance inflation factor <1.3). The study from which participants were recruited (ROMEO or JULIET) was not a predictor of time to reach weight loss goal (β=−5.344, P=0.545) and as such was not included in the model.
Observed rate of weight loss was calculated as weight loss (kg)/duration (weeks). Predicted rate of weight loss was estimated using the factor of 9.3 kcal/g of FM lost and 1.1 kcal/g of FFM lost, and assuming that 87% of the weight lost was FM (as observed in the present analysis). First, we calculated energy deficit/day as TEE baseline – 800 (energy content of the diet). Then, we converted the energy deficit into weight loss as (energy deficit × 0.87)/9.3)) + (energy deficit × 13)/1.1)). Finally, we converted weight loss in g/day into kg/week by multiplying by 0.007. We have used two approaches to estimate energy needs: A) using TEE at baseline, and B) using the average between TEE at baseline and TEE after weight loss.
Results
Sixty-five women (37 whites) with an average BMI of 28.6±1.5 kg/m2 and an average age of 36.4±5.9 years were included in the present analysis. Changes in body weight and composition, and RMR can be seen in Table 1. Average weight loss was 12.5±3.1 kg (16.1±3.4%) achieved over an average of 22±7 weeks (155±49 days). Adherence to the diet was on average 64±31%. RMRm was significantly lower than RMRp after weight loss (1305±129 vs 1351±106 kcal/day, P=0.002) resulting in a metabolic adaptation of −46±113 kcal/day. The average expected weight to achieve a BMI of 25 kg/m2 was 68.0±5.1 kg and the observed weight after the intervention was 65.2±6.0 kg. The difference was 2.8±2.7 kg and was statistically significant (P<0.001).
Table 1.
Anthropometries and resting metabolic rate at baseline and after weight loss
| Baseline | After weight loss | P value | |
|---|---|---|---|
|
| |||
| Weight (kg) | 77.8±6.9 | 65.3±5.9 | <0.001 |
| FM (kg) | 32.5±4.7 | 21.6±4.2 | <0.001 |
| FFM (kg) | 41.5±3.6 | 40.5±3.6 | <0.001 |
| RMRm (kcal/day) | 1440±203 | 1305±129 | <0.001 |
| RMRp (kcal/day) | 1440±115 | 1351±106 | <0.001 |
| RMRm-p (kcal/day) | 0±167 | −46±113* | |
Data shown as mean±SD. FM: fat mass; FFM: fat-free mass; RMR: resting metabolic rate; RMRm: RMR measured; RMRp: RMR predicted.
P=0.002 for the comparison between RMRm-p.
There was a trend for metabolic adaptation to be inversely associated with time to reach weight loss goal (r=−0.240, P=0.055, n=65) (see Figure 1). After adjusting for target weight loss, energy deficit and adherence to the diet, metabolic adaptation was a significant predictor of time to reach weight loss goal and the overall model explained 63% of the variation (P<0.001) (see Table 2). Time to reach weight loss goal (days) = 283 + 2.7 target weight loss (kg) − 0.1 energy deficit (kcal/day) − 1.3 adherence to the diet (%) − 0.1 metabolic adaptation (kcal/day). Target weight loss, energy deficit, and dietary adherence were all significant predictors of time to reach weight loss goals in the regression model (P=0.006, P<0.001, and P<0.001, respectively). However, when performing simple correlation only dietary adherence was significantly correlated with time to reach weight loss goal (r=−0.705, P<0.001, n=65). If adherence to the diet is not included, then the model is not significant (R2 adj=1.7%, P=0.258) and none of the variables in the model are significant predictors.
Figure 1.
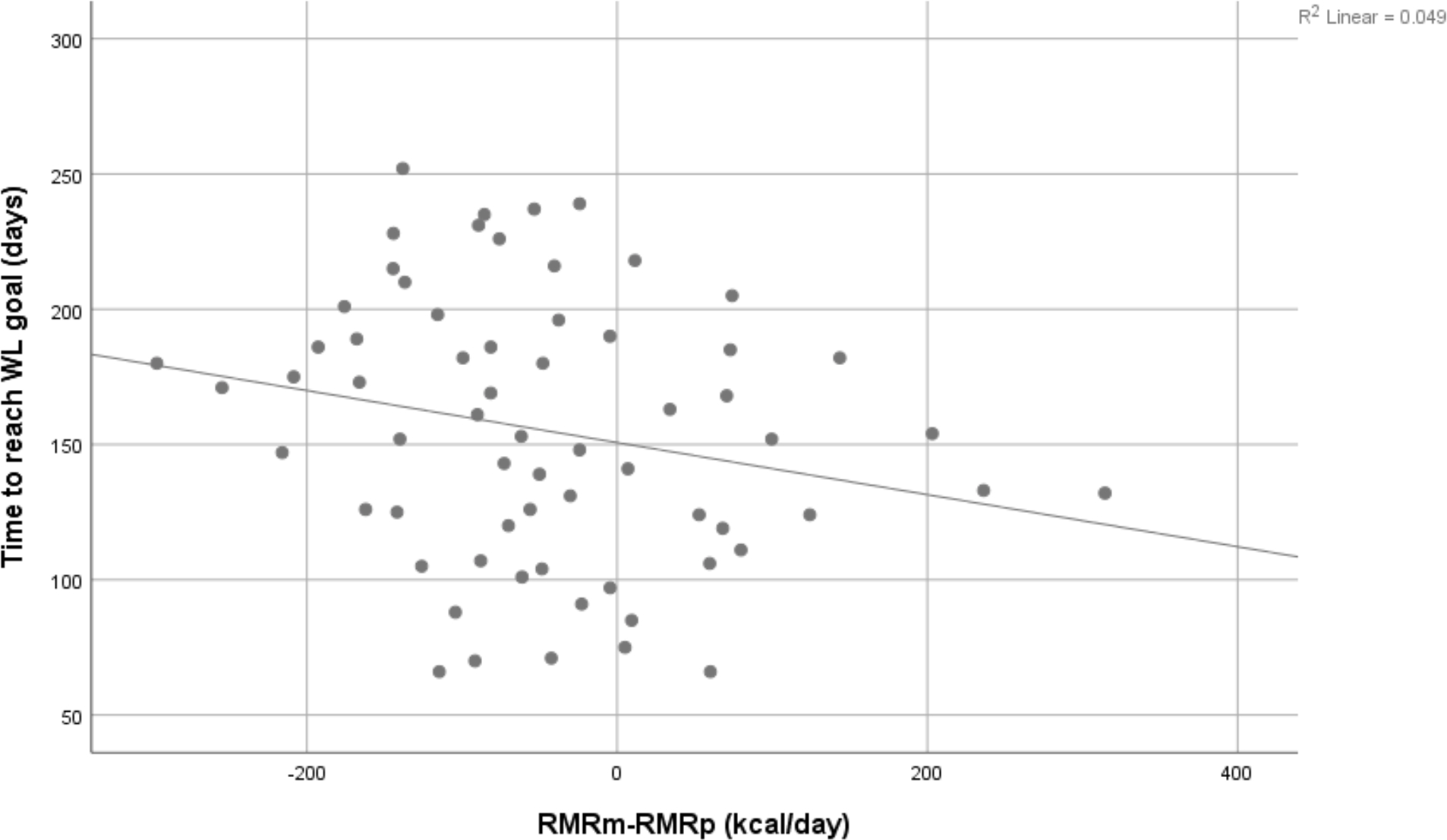
Simple correlation between metabolic adaptation at the level of resting metabolic rate (RMRm-RMRp) and time to reach weight loss goal (days). RMR: resting metabolic rate; RMRm: RMR measured: RMRp: RMR predicted: WL: weight loss. The larger the metabolic adaptation, the longer was the time needed to reach weight loss goal.
Table 2.
Regression model for predicting time to reach weight loss goal (days) in women with overweight
| Model | β | R2 adjusted | P |
|---|---|---|---|
|
| |||
| 0.63 | <0.001 | ||
| Intercept | 283 | <0.001 | |
| Target weight loss (kg) | 2.7 | 0.006 | |
| Energy deficit TEE (kcal/day) | −0.1 | <0.001 | |
| Adherence to the diet (%) | −1.3 | <0.001 | |
| Metabolic adaptation (kcal/day) | −0.1 | 0.041 | |
TEE: total energy expenditure
No significant associations were seen between metabolic adaptation and target weight loss (r=−0.109, P=0.386, n=65), energy deficit (r=−0.004, P=0.978, n=65) or dietary adherence (r=0.181, P=0.149, n=65).
There was a tendency for those participants with negative metabolic adaptation (RMRm<RMRp, n=46) to take longer time to reach their weight loss goals compared with those with positive metabolic adaptation (RMRm>RMRp, n=19) (165±55 versus 139±42 days, respectively, P=0.071).
No significant association was found between metabolic adaptation and rate of weight loss (kg/week) (r=−0.092, P=0.463, n=65) or difference between observed and predicted rate of weight loss (r=−0.036, P=0.703 and r=−0.02, P=0.873 for approach A and B, respectively).
Discussion
The present findings represent the first study examining if metabolic adaptation, at the level of RMR, was associated with time to reach weight loss goals. We found that the larger the metabolic adaptation (RMRm-RMRp) after weight loss, the longer was the time needed to reach weight loss goal (BMI=25 kg/m2), even after adjusting for target weight loss, energy deficit and adherence to the diet, suggesting strategies to decrease metabolic adaptation and increase adherence to diet may expedite weight loss.
In the present analysis, women with overweight who experienced an average weight loss of 13 kg (16.1%) over 155±49 days, had a metabolic adaptation of approximately −50 kcal/day. It needs to be emphasized that this metabolic adaptation was seen after 4 weeks of weight stabilization following the active weight loss phase and, as such, is probably much lower that what would be expected during the active weight loss phase. In line with this, we have recently shown a significant reduction, in fact more than halving, in metabolic adaptation when measurement were done after 4 weeks of weight stabilization, in comparison to when measurements were performed immediately after weight loss (19). More specifically, a significant metabolic adaptation, at the level of RMR, of −92±110 kcal/day was seen immediately after a 14 kg (13%) weight loss in individuals with obesity, which was significantly reduced, despite still significant (−38±124 kcal/day), after 4 weeks of weight stabilization (19). So, measuring RMR immediately after weight loss results in a metabolic adaptation which was 2.42 (92/38) times larger compared with when RMR is measured after a period of weight stabilization. Moreover, a significant positive moderate correlation was found between metabolic adaptation immediately after weight loss and metabolic adaptation after 4 weeks of weight stabilization (r=0.663, P<0.001, n=71). Therefore, it is reasonable to expect that metabolic adaptation during active weight loss in the present study may have been closer to −110 kcal/day (−46 × 2.42) than −46 kcal/day.
Our regression model showed that even after adjusting for target weight loss, energy deficit and adherence to the diet, metabolic adaptation was still a significant predictor of time to reach weight loss goals. For each 10 kcal/day increase in metabolic adaptation, time to reach weight loss goal increased by 1 day. This might not seem like much since the average metabolic adaptation was only approximately −50kcal/day. However, as discussed in detail in the previous paragraph, this value is likely to be underestimated and a more reasonable estimation would be −120 kcal/day during active weight loss, with a large inter-individual variation, ranging from −700 to +750 kcal/day. That means that those with the largest magnitude of metabolic adaptation would need to stay on the diet for 70 additional days (compared with a person with no metabolic adaptation) in order to reach their weight loss goal, even after adjusting for adherence to the diet. This probably helps to explain some of the variation in time needed to reach weight loss goals (range 66–252 days). Importantly, this is assuming that metabolic adaptation during active weight loss would only occur at the level of resting energy expenditure, which has been shown not to be the case, and metabolic adaptation might, in fact, be of a larger magnitude at the level of non-resting energy expenditure (8, 9).
We and others have shown that metabolic adaptation, either measured during active weight loss (11, 19), or after a short period of weight stabilization (4 weeks) (19, 20), is not a risk factor for weight regain. However, metabolic adaptation might lead to resistance to weight loss, as shown in the present study. Even though adherence to the diet is clearly the most important determinant of time to reach weight loss goals, the present findings are of great clinical relevance as they mean that individuals who are struggling to achieve weight loss goals, despite assuring compliance with the diet, may indeed be “suffering” from metabolic adaptation during active weight loss.
The present findings are in line with recent data from our group showing that metabolic adaptation at the level of RMR is associated with less weight and fat mass loss following low-energy diets, in individuals with obesity (21). Moreover, Goele and colleagues (31) reported that in women who experience metabolic adaptation after a low-energy diet, 38% of the difference between measured and predicted weight loss was due to metabolic adaptation at the level of RMR. These two studies and the present analysis suggest that a lower than expected weight loss, or a delay in reaching weight loss goals, may not necessarily result from lack of compliance to the intervention. Metabolic adaptation can modify the outcome of a weight loss intervention, albeit to varying degrees (due to the very large inter-individual differences in metabolic adaptation). The success in the clinical management of individuals with obesity needs, therefore, to be tailored according to individual variations for any relevant phenotype, including the presence or absence of metabolic adaptation in response to weight loss.
Our study has both strengths and limitations. Gold standard procedures were used for the measurements of RMR (after a 4-day GCRC in-patient stay and an overnight sleep, under controlled condition of feeding and physical activity), body composition (4CM) and estimation of diet adherence (TEE by DLW). However, this study also suffers from some limitations. First, it includes a very homogenous sample of premenopausal (20–41 years) women with overweight. This prevents the generalisation of our results to men, other BMI groups and older subjects. Moreover, this also explains why our regression model had an R2 of only 32%, i.e. a truncated range for both BMI and age and only women. Second, metabolic adaptation was measured after 4 weeks of weight stabilization, and as such, is likely underestimated, which might have weakened the association between metabolic adaptation and time to reach weight loss goals. Third, estimating dietary adherence in free-living conditions is challenging and there is no perfect method. Our approach has limitations as it includes TEE after weight loss, which will, in itself, contain some degree of metabolic adaptation. However, there was no multicollinearity among the predictors included in the regression model. Moreover, similar approaches have been used by other researchers when estimating dietary adherence and expected weight loss for a given intervention (32, 33). Future studies are needed with better measures of adherence to pursue the hypotheses generated here. Finally, we did not assess changes in the anatomical and molecular composition of FFM. Muller and coworkers (34) have recently shown that in order to accurately assess metabolic adaptation, FFM, as well as its composition at the organ/tissue and molecular levels, needs to be taken into account and that the magnitude of metabolic adaptation may be lower than usually estimated based on FFM alone.
Conclusion
In conclusion, in premenopausal women with overweight, metabolic adaptation after a 16% weight loss increases the length of time necessary to achieve weight loss goals. Further research should confirm these findings in a population of men and women with obesity.
Supplementary Material
What is already known about this subject?
The existence of metabolic adaptation is dependent on the energy balance status of the participants.
A potential association between metabolic adaptation and weight regain in the long-term remains unclear.
Metabolic adaptation has been shown to reduce the magnitude of weight and fat mass loss in response to low-energy diets.
What are the new findings?
Metabolic adaptation increases the length of time necessary to achieve weight loss goals in premenopausal women with overweight, even after adjusting for dietary adherence.
For each 10 kcal/day increase in metabolic adaptation, time to reach weight loss goals increased by 1 day.
How might these results change the direction of research or the focus of clinical practice?
Clinicians need to consider metabolic adaptation when assessing resistance to weight loss.
Acknowledgments
Funding: Supported by National Institutes of Health grants R01 DK049779, P30 DK56336, P60 DK079626, and UL 1RR025777. CM was supported by a sabbatical grant from the Liaison Committee for Education, Research and Innovation in Central Norway and the Norwegian University of Science and Technology (NTNU).
Footnotes
Clinical trial registration (JULIET study only): NCT00067873
Disclosure: The authors declare no conflicts of interest.
References
- 1.Dulloo AG, Jacquet J, Montani JP, Schutz Y. Adaptive thermogenesis in human body weight regulation: more of a concept than a measurable entity? Obes Rev. 2012;13 Suppl 2:105–21. [DOI] [PubMed] [Google Scholar]
- 2.Dulloo AG, Schutz Y. Adaptive Thermogenesis in Resistance to Obesity Therapies: Issues in Quantifying Thrifty Energy Expenditure Phenotypes in Humans. Curr Obes Rep. 2015;4(2):230–40. [DOI] [PubMed] [Google Scholar]
- 3.Celi FS, Le TN, Ni B. Physiology and relevance of human adaptive thermogenesis response. Trends Endocrinol Metab. 2015;26(5):238–47. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. International Journal of Obesity. 2010;34(S1):S47–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Major GC, Doucet E, Trayhurn P, Astrup A, Tremblay A. Clinical significance of adaptive thermogenesis. International Journal of Obesity. 2007;31(2):204–12. [DOI] [PubMed] [Google Scholar]
- 6.Flatt JP. Exaggerated claim about adaptive thermogenesis. Int J Obes (Lond). 2007;31(10):1626; author reply 7–8. [DOI] [PubMed] [Google Scholar]
- 7.Kuchnia A, Huizenga R, Frankenfield D, Matthie JR, Earthman CP. Overstated metabolic adaptation after “the biggest loser” intervention. Obesity (Silver Spring). 2016;24(10):2025. [DOI] [PubMed] [Google Scholar]
- 8.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. The New England Journal of Medicine. 1995;332:621–8. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. American Journal of Clinical Nutrition. 2008;88(4):906–12. [DOI] [PubMed] [Google Scholar]
- 10.Froidevaux F, Schutz Y, Christin L, Jequier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. Am J Clin Nutr. 1993;57(1):35–42. [DOI] [PubMed] [Google Scholar]
- 11.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24(8):1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camps SG, Verhoef SP, Westerterp KR. Weight loss, weight maintenance, and adaptive thermogenesis. Am J Clin Nutr. 2013;97(5):990–4. [DOI] [PubMed] [Google Scholar]
- 13.Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. 2012;97(7):2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinsier RL, Nagy TR, Hunter GR, Darnell BE, Hensrud DD, Weiss HL. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am J Clin Nutr. 2000;72(5):1088–94. [DOI] [PubMed] [Google Scholar]
- 15.Weinsier RL, Hunter GR, Zuckerman PA, Darnell BE. Low resting and sleeping energy expenditure and fat use do not contribute to obesity in women. Obes Res. 2003;11(8):937–44. [DOI] [PubMed] [Google Scholar]
- 16.Wyatt HR, Grunwald GK, Seagle HM, Klem ML, McGuire MT, Wing RR, et al. Resting energy expenditure in reduced-obese subjects in the National Weight Control Registry. Am J Clin Nutr. 1999;69(6):1189–93. [DOI] [PubMed] [Google Scholar]
- 17.Larson DE, Ferraro RT, Robertson DS, Ravus E. Energy metabolism in weight-stable postobese individuals. American Journal of Clinical Nutrition. 1995;62:735–9. [DOI] [PubMed] [Google Scholar]
- 18.Ostendorf DM, Melanson EL, Caldwell AE, Creasy SA, Pan Z, MacLean PS, et al. No consistent evidence of a disproportionately low resting energy expenditure in long-term successful weight-loss maintainers. Am J Clin Nutr. 2018;108(4):658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martins C, Roekenes J, Salamati S, Gower BA, Hunter GR. Metabolic adaptation is an illusion, only present when participants are in negative energy balance. Am J Clin Nutr. 2020;112(5):1212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins C, Gower BA, Hill JO, Hunter GR. Metabolic adaptation is not a major barrier to weight loss maintenance. Am J Clin Nutr. 2020. 112(3):558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martins C, Roekenes J, Gower BA, Hunter GR. Metabolic adaptation is associated with less weight and fat mass loss in response to low-energy diets. Nutr Metab (Lond). 2021;18(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinsier RL, Hunter GR, Zuckerman PA, Redden DT, Darnell BE, Larson DE, et al. Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr. 2000;71(5):1138–46. [DOI] [PubMed] [Google Scholar]
- 23.Hunter GR, Byrne NM, Sirikul B, Fernandez JR, Zuckerman PA, Darnell BE, et al. Resistance training conserves fat-free mass and resting energy expenditure following weight loss. Obesity (Silver Spring). 2008;16(5):1045–51. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner RN, Heymsfield SB, Lichtman S, Wang J, Pierson RN Jr. Body composition in elderly people: effect of criterion estimates on predictive equations. Am J Clin Nutr. 1991;53(6):1345–53. [DOI] [PubMed] [Google Scholar]
- 25.Cote KD, Adams WC. Effect of bone density on body composition estimates in young adult black and white women. Med Sci Sports Exerc. 1993;25(2):290–6. [PubMed] [Google Scholar]
- 26.McCrory MA, Gomez TD, Bernauer EM, Mole PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc. 1995;27(12):1686–91. [PubMed] [Google Scholar]
- 27.Walsh MC, Hunter GR, Sirikul B, Gower BA. Comparison of self-reported with objectively assessed energy expenditure in black and white women before and after weight loss. Am J Clin Nutr. 2004;79(6):1013–9. [DOI] [PubMed] [Google Scholar]
- 28.Del Corral P, Chandler-Laney PC, Casazza K, Gower BA, Hunter GR. Effect of dietary adherence with or without exercise on weight loss: a mechanistic approach to a global problem. J Clin Endocrinol Metab. 2009;94(5):1602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forbes GB. Human body composition: growth, aging, nutrition, and activity. New York: Springer-Verlag; 1987. [Google Scholar]
- 30.Spady DW, Payne PR, Picou D, Waterlow JC. Energy balance during recovery from malnutrition. Am J Clin Nutr. 1976;29(10):1073–88. [DOI] [PubMed] [Google Scholar]
- 31.Goele K, Bosy-Westphal A, Rumcker B, Lagerpusch M, Muller MJ. Influence of changes in body composition and adaptive thermogenesis on the difference between measured and predicted weight loss in obese women. Obes Facts. 2009;2(2):105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pieper C, Redman L, Racette S, Roberts S, Bhapkar M, Rochon J, et al. Development of adherence metrics for caloric restriction interventions. Clin Trials. 2011;8(2):155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Jonge L, DeLany JP, Nguyen T, Howard J, Hadley EC, Redman LM, et al. Validation study of energy expenditure and intake during calorie restriction using doubly labeled water and changes in body composition. Am J Clin Nutr. 2007;85(1):73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller MJ, Heymsfield SB, Bosy-Westphal A. Are metabolic adaptations to weight changes an artefact? Am J Clin Nutr. 2021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


