ABSTRACT
Studies have shown that club cell secretory protein (CC16) plays important protective roles in the lungs, yet its complete biological functions are unclear. We devised a translational mouse model in order to investigate the impact of early life infections, in the context of CC16 deficiency, on lung function in adult mice. CC16 sufficient (WT) and deficient (CC16−/−) mice were infected with Mycoplasma pneumoniae (Mp) as weanlings and assessed as adults (early life infection model; ELIM) and compared to adult mice infected for only 3 days (adult infection model; AIM). CC16−/− Mp-infected mice had significantly increased airway hyperresponsiveness (AHR) in both models compared to WT mice. However, CC16−/− mice infected in early life (ELIM) displayed significantly increased AHR compared to CC16−/− mice infected in adulthood (AIM). In stark contrast, lung function in ELIM WT mice returned to levels similar to saline-treated controls. While WT mice cleared Mp infection in the ELIM, CC16−/− mice remained colonized with Mp throughout the model, which likely contributed to increased airway remodeling and persistence of Muc5ac expression. When CC16−/− mouse tracheal epithelial cells (MTECs) were infected with Mp, increased Mp colonization and collagen gene expression were also detected compared to WT cells, suggesting that CC16 plays a protective role during Mp infection, in part through epithelial-driven host defense mechanisms.
KEYWORDS: CC16, Mycoplasma pneumoniae, airway remodeling, epithelial cells, inflammation, lung infection
INTRODUCTION
Club cell secretory protein (CCSP; also known as CC16, CC10, Uteroglobin) is a 15.8-kDa homodimeric pneumoprotein secreted in the distal airways by non-ciliated bronchiolar epithelial cells and can be measured in serum (1, 2). Although the biological functions of CC16 have not been conclusively determined, in vitro and in vivo studies have indicated that this molecule contains anti-inflammatory, immunomodulatory, anti-toxicant, and antioxidant properties in the lungs (2, 3). Clinical studies have reported decreased levels of CC16 in the blood and airways of asthmatics and COPD patients, as well as individuals with lung function deficits (3, 4). Additionally, low serum CC16 levels were correlated with increased lung epithelial cell injury and accelerated declines in forced expiratory volume in 1 s (FEV1) (5, 6).
Mycoplasma pneumoniae (Mp) is an “atypical” bacterium that causes respiratory infections of varied severity, ranging from mild upper respiratory infections to severe atypical pneumonia (7, 8). Mp has been linked to more than 40% of community acquired pneumonia (CAP) cases and approximately 18% of cases requiring hospitalizations in children (8). Recently, Mp has been linked to exacerbations in asthmatics and it has been suggested that early life CAP, such as that caused by Mp, may increase asthma prevalence later in life (9). In some cases, Mp can persist for months in the respiratory tracts of non-asthma subjects and asthma patients, resulting in a decline in pulmonary function and/or subsequent development of asthma (9). Exacerbations in severe asthma sufferers are associated with accelerated lung function decline and it is now recognized that severe persistent asthma can lead to irreversible airflow limitation, the hallmark of COPD (10). However, the mechanisms initiating and driving this remodeling process in asthma following an exacerbation are not clear.
Previous studies suggest that CC16 may, at least partly, exert its effects in the lung by modulating susceptibility and responses to respiratory infections (11–14). In our previous studies examining responses to Mp after 3 days of infection, we found that Mp-infected CC16 knockout (CC16−/−) mice treated with rCC16 had reduced airway colonization and lung inflammation compared to Mp-infected CC16−/− mice treated with vehicle control (15). We also know that the lack of CC16 also impacts lung function in non-infectious and non-inflammatory conditions (16). To further understand the implications of early life infections in the context of low or no CC16 levels, we have developed a novel translational mouse model of early life exposure to Mp in which wild-type (WT) or CC16−/− mice are infected pre-weaning (thought to be of similar age as an ∼2–5-year-old child) one time and assessed for lung function at 8 weeks of age (thought to be of similar age as an ∼20–30-year-old adult) (17). Compared to CC16−/− adult mice infected acutely for 3 days, we find that CC16−/− mice infected in early life have significantly greater AHR, airway remodeling, and persistent Muc5ac gene expression, likely due to their inability to clear Mp infection. We further found that epithelial cells derived from CC16−/− mice and grown at an air-liquid interface were unable to reduce Mp burden as effectively as those from WT mice, suggesting that this defect in Mp clearance is likely epithelial-driven. Taken together, we provide new evidence, using this novel translational mouse model, that persistent early life infections in the context of CC16 deficits may be a previously overlooked link in understanding a progression of asthma into severe asthma with fixed airflow limitation.
RESULTS
CC16 deficiency and early life Mp infection result in augmented airway hyperresponsiveness.
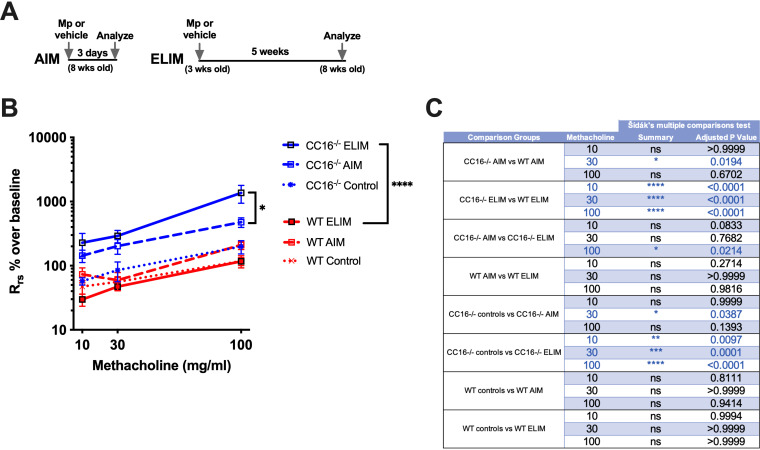
We have previously found that 8-week-old mice (∼20–30-year-old human adult) (17) infected with Mp in the AIM had significantly enhanced airway hyperresponsiveness (AHR) when CC16 was absent (15). For this study, we wanted to determine how CC16−/− juvenile mice (∼2–5-year-old child) (17) infected with Mp (one time) would be impacted in regard to lung function in adulthood (Fig. 1A). For the AIM (Fig. 1B), AHR to methacholine challenge in WT and CC16−/− was similar to our previous report (15). CC16−/− mice challenged with Mp had significantly increased airway resistance of >100% and >400% over baseline for methacholine doses of 30 mg/mL and 100 mg/mL, respectively, confirming that adult CC16−/− mice have increased airways resistance during short-term Mp infections.
FIG 1.
Airway resistance is increased in CC16−/− mice infected with Mp for the AIM and ELIM. Airway resistance during a methacholine challenge of WT (n = 9) and CC16−/− (n = 16) male mice infected with Mp for the AIM and WT (n = 11) and CC16−/− (n = 13) mice infected with Mp for the ELIM protocols. Non-infected saline controls are shown on each graph (dotted lines). Total airways respiratory resistance (Rrs) was calculated as a percentage over baseline in order to compare between separate experimental runs on the Flexivent machine. All mice were assessed for pulmonary function at 8 weeks of age. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 by one-way ANOVA Sidak’s multiple comparison test.
For the ELIM, CC16−/− mice challenged with Mp had striking and significantly increased airway resistances of >200%, ∼300%, and >1000% over baseline for methacholine doses of 10 mg/mL, 30 mg/mL, and 100 mg/mL, respectively (Fig. 1B). In contrast, WT mice infected with Mp early in life had AHR measurements that were indistinguishable from their saline controls (Fig. 1B). For both the AIM and ELIM, significantly increased airways resistance was observed in CC16−/− mice infected with Mp, compared to WT Mp-infected mice; however, significantly increased airway resistance was observed in CC16−/− mice in the ELIM compared with the AIM mice of the same genotype (Fig. 1C). In the context of CC16 deficiencies, this model illustrates that early life infection with Mp results in significantly augmented AHR in adulthood. In contrast, mice sufficient in CC16 (WT) in the ELIM did not have noticeable differences in AHR compared to their saline controls.
CC16 deficiency impairs Mp clearance from the airways and pulmonary epithelial cells.
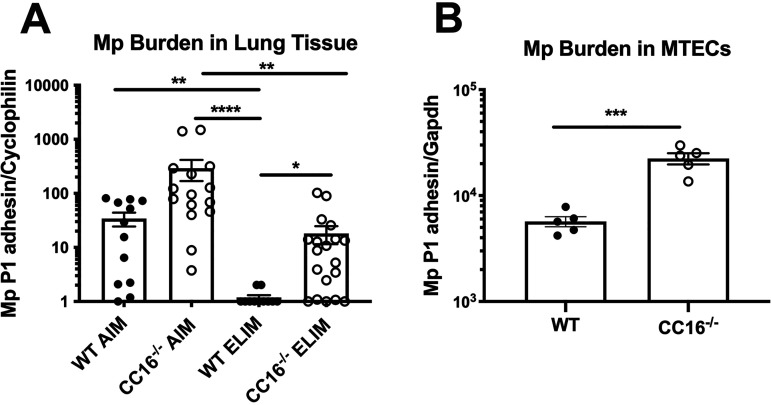
Lung tissue from WT and CC16−/− Mp-infected mice from the AIM and the ELIM were assessed for pathogen burden by RT-PCR (Fig. 2A). For the AIM, both WT and CC16−/− mice had detectable Mp infection; however, Mp burden was higher in the CC16−/− mice, compared to the WT mice. For the ELIM, a majority of the WT mice examined were able to completely clear the Mp infection; in contrast, CC16−/− mice were unable to completely clear the Mp infection, with a majority of mice examined remaining PCR+.
FIG 2.
CC16−/− mice and MTECs have increased Mp burden. Mp burden was assessed in the lung tissue of mice from the AIM and ELIM. (A) WT AIM (n = 12), CC16−/− AIM (n = 15), WT ELIM (n = 11), CC16−/− ELIM (n = 19) were assessed for Mp burden by RT-PCR for Mp-specific P1-adhesin gene. *, P < 0.05; **, P < 0.01; ***, P < 0.01 Kruskal-Wallis multiple-comparison test. Cyclophilin was used as a housekeeping control for RT-PCR. (B) WT control (n = 5) and CC16−/− control (n = 5) mouse tracheal epithelial cells (MTECs) grown simultaneously at an air-liquid interface were infected with Mp and assessed for Mp burden after 48 h by RT-PCR. ***, P < 0.001 unpaired t test. Gapdh was used as a housekeeping control for RT-PCR. Data shown are mean ± SEM.
In order to determine if CC16 contributed to epithelial-driven responses during infection, we used a cell culture model in which mouse tracheal epithelial cells (MTECs) from WT and CC16−/− mice were grown at an air-liquid interface prior to Mp challenge for 48 h. After Mp challenge, Mp burden was measured in the infected MTECs. Similar to what was observed in our mouse studies, Mp burden was significantly higher in CC16−/− MTECs, compared to WT MTECs (Fig. 2B). Further studies demonstrated that CC16 does not directly kill Mp but may act in a more bacteriostatic manner (Fig. S1). Overall, CC16−/− mice had higher Mp burden in lung tissue in the AIM and were unable to completely clear Mp infection in the ELIM. Additionally, CC16−/− MTECs had significantly increased pathogen burden, compared to WT MTECs, suggesting that epithelial-driven host responses are likely major contributors to pulmonary Mp clearance in vivo.
CC16 deficiency leads to heightened Tnf- and Muc5ac during Mp infection.
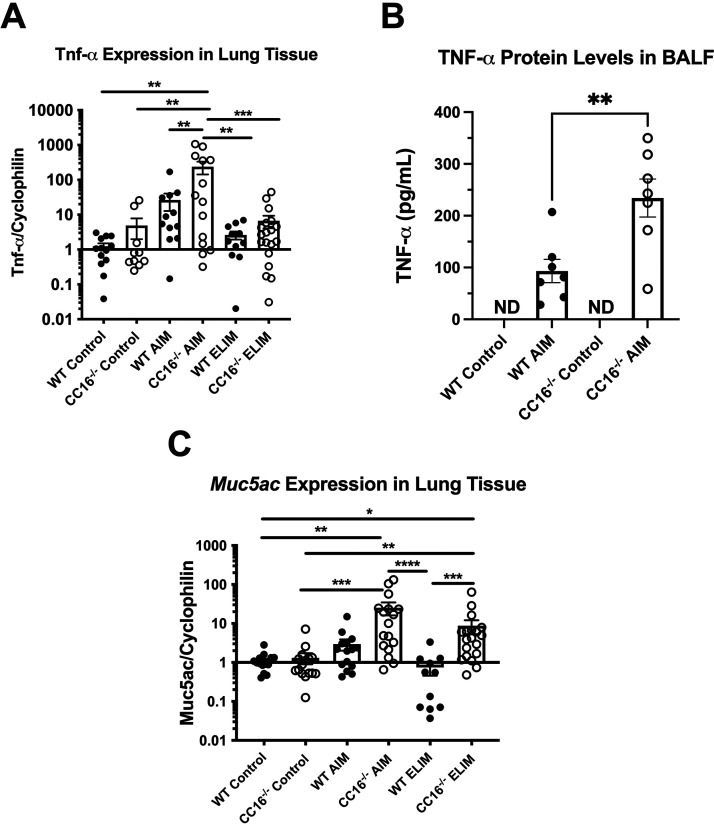
Mycoplasma infection in humans is associated with increased expression of MUC5AC and TNF-α in airway tissue from asthmatic patients (18). Therefore, we sought to measure these factors typically associated with Mp infection, Muc5ac and Tnf-α, in WT and CC16−/− mice that were infected in the AIM and ELIM. CC16−/− mice infected with Mp had significantly increased Tnf-α gene expression during the AIM, compared to WT and CC16−/− control mice. Additionally, for the acute AIM, CC16−/− mice had significantly elevated expression of Tnf-α, compared to WT mice; however, there was no significant difference in Tnf-α expression between WT and CC16−/− mice infected with Mp during the ELIM, suggesting that the increase in TNF-α is acute phase only (Fig. 3A). TNF-α protein levels in the bronchoalveolar lavage fluid (BALF) from the AIM mice were also examined by ELISA (Fig. 3B). Similar to what was observed in the RT-PCR data, we observed significantly increased TNF-α levels in the BALF from CC16−/− AIM mice, compared to WT AIM mice; TNF-α levels were at or below detectable levels in BAL from control and ELIM mice (not shown).
FIG 3.
Tnf- and Muc5ac are increased in CC16−/− mice infected with Mp during the AIM and ELIM. Tnf- gene expression (A) was measured in the lung tissue of WT control (n = 11), CC16−/− control (n = 10), WT AIM (n = 12), CC16−/− AIM (n = 14), WT ELIM (n = 11), and CC16−/− ELIM (n = 19) mice by RT-PCR. TNF- protein levels (B) were measured in the BALF of WT control (n = 7), CC16−/− control (n = 7), WT AIM (n = 7), and CC16−/− AIM (n = 7) mice by ELISA. Muc5ac (C) gene expression was measured in the lung tissue of WT control (n = 17), CC16−/− control (n = 16), WT AIM (n = 15), CC16−/− AIM (n = 17), WT ELIM (n = 11), and CC16−/− ELIM (n = 19) mice by RT-PCR. For RT-PCR data, *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 one-way ANOVA Tukey’s multiple-comparison test. Data shown are mean ± SEM. Cyclophilin was used as the housekeeping control for RT-PCR. For ELISA data, **, P < 0.01 unpaired t test. ND, not detected.
Muc5ac expression was significantly upregulated in CC16−/− AIM and ELIM mice, compared to their respective saline controls (Fig. 3C). Additionally, in the ELIM, CC16−/− mice had significantly increased Muc5ac expression compared to WT mice. While WT mice in AIM had a trend toward increased Muc5ac expression, they did not achieve statistical significance over their saline controls and the levels returned to baseline conditions in the WT ELIM mice. These data suggest that the loss of CC16 may be associated with persistently increased Muc5ac gene expression during Mp infection, which if unchecked may result in increased goblet cell metaplasia and contribute to airway remodeling.
CC16 deficiency results in increased collagen deposition that is enhanced during long term Mp Infection.
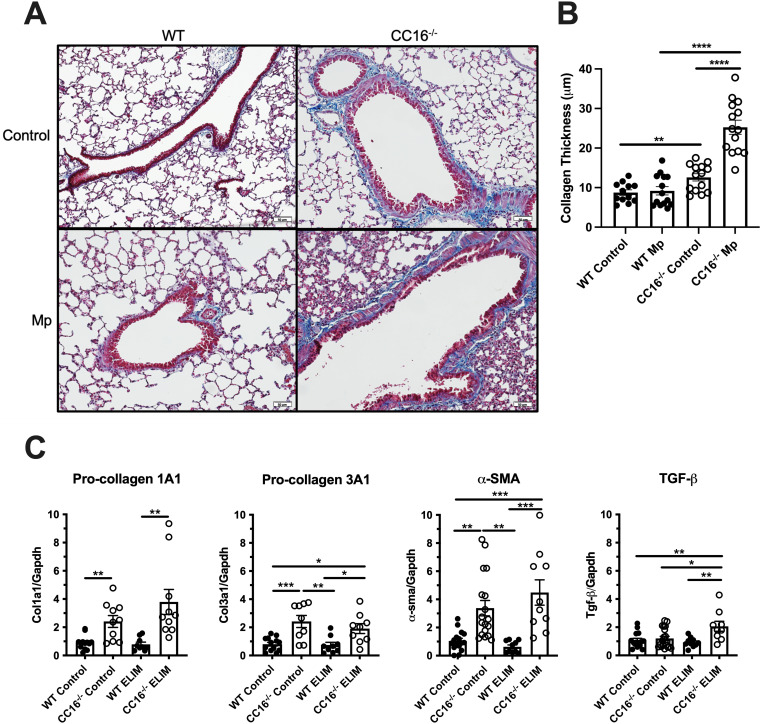
Since CC16−/− mice were previously found to have increased collagen gene expression in lung tissue (16), we next chose to determine if the early life, persistent infection would further impact lung remodeling. Collagen thickness was measured in trichrome stained lung tissue from the ELIM, as described in the “methods” section (Fig. 4A and B). Control (saline treated) CC16−/− mice had increased collagen deposition (blue stain) compared to WT mice (mean ± SEM, 3.829 ± 1.180 μm). During Mp infection, CC16−/− mice had significantly increased collagen deposition compared to WT mice (mean ± SEM, 16.06 ± 2.069 μm). Additionally, CC16−/− mice infected with Mp had increased collagen deposition compared to the saline-treated CC16−/− mice (mean ± SEM, 12.67 ± 1.998 μm). There were no significant differences in collagen deposition between WT saline and WT Mp-treated ELIM mice.
FIG 4.
Collagen production is increased in the lungs of CC16−/− mice and further exacerbated by Mp infection in the ELIM. (A) Representative trichrome-stained lung sections from WT control (n = 12), WT Mp (n = 12), CC16−/− control (n = 14), and CC16−/− Mp (n = 14) mice from the ELIM. Scale bars: 50m. (B) Quantitative analysis of collagen thickness was measured on pictures taken at ×20 magnification using Metamorph software as described in the Methods section. (C) Airway remodeling measurements in WT and CC16−/− mice infected Mp for the ELIM. Pro-collagen 1A1 (Col1a1) was measured in WT control (n = 12), CC16−/− control (n = 10), WT ELIM (n = 9), and CC16−/− ELIM (n = 10). Pro-collagen 3A1 (Col3a1) was measured in WT control (n = 13), CC16−/− control (n = 9), WT ELIM (n = 9), and CC16−/− ELIM (n = 9). -sma was measured in WT control (n = 16), CC16−/− control (n = 18), WT ELIM (n = 10), and CC16−/− ELIM (n = 10). Tgf-β was measured in WT control (n = 15), CC16−/− control (n = 21), WT ELIM (n = 10), and CC16−/− ELIM (n = 9). Airway remodeling factors were measured using RT-PCR with Gapdh as a housekeeping control. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by one-way ANOVA Tukey’s multiple comparison test. Data shown are mean ± SEM.
CC16 deficiencies drive airway remodeling in the early life infection model.
Since we had previously determined that remodeling genes were upregulated in the lungs of control CC16−/− mice (16), we next sought to determine if lack of CC16 and long-term Mp infection had additive effects in altering remodeling factors. During the ELIM, RT-PCR of WT and CC16−/− mouse lung tissue revealed that gene expression of factors associated with lung remodeling—pro-collagen type I (Col1a1), pro-collagen type III (Col3a1), and -smooth muscle actin (-sma)—were significantly elevated at baseline in CC16−/− mice, compared to baseline WT mice (Fig. 4C) as previously shown (16). Upon Mp infection for the ELIM, Col1a1, Col3a1, -sma, and Tgf- expression was significantly higher in the CC16−/− mice, compared to the WT mice. Along with these observations, Col3a1, -sma, and Tgf- expression was also significantly higher in CC16−/− mice infected with Mp, compared to WT control. These data show that loss of CC16 is associated with increased levels of airway remodeling factors at baseline conditions and that these remodeling factors remain consistently elevated in CC16−/− mice during the ELIM, compared to their WT Mp controls. Of note, while the other remodeling genes tested were not significantly elevated in CC16−/− mice during the ELIM compared to their CC16−/− control conditions, Tgf-β gene expression was significantly increased in ELIM lungs compared to CC16−/− controls, indicating that Mp infection is the driving-force behind the increased Tgf-β expression.
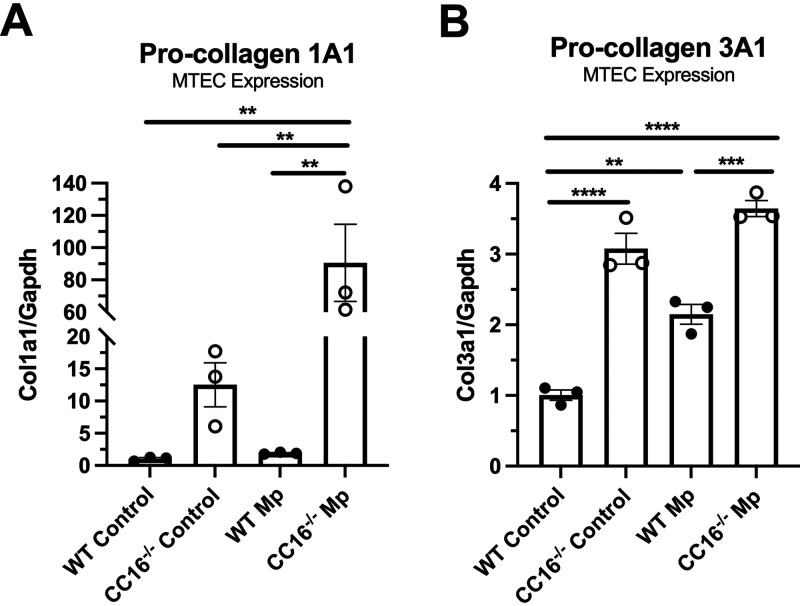
CC16 deficiency results in increased airway remodeling protein expression in epithelial cells.
The WT and CC16−/− MTECs that were treated with Mp or control (media only) for 48 h were also assessed for remodeling factors, Col1a1 and Col3a1. At baseline, CC16−/− MTECs had significantly increased expression of both collagens, compared to their WT controls (Fig. 5A and B). During Mp infection, CC16−/− MTECs had significantly increased expression of both collagens, compared to WT MTECs. WT and CC16−/− Mp-infected MTECs had significantly higher expression of Col1a1, compared to vehicle controls. Similarly, WT MTECs infected with Mp had significantly higher expression of Col3a1, compared to WT vehicle-treated MTECs; however, there was no significant difference in the expression of Col3a1 between CC16−/− vehicle-treated and CC16−/− Mp-treated MTECs. Overall, at the epithelial level, the loss of CC16 results in a significant increase in the expression of airway remodeling factors, Col1a1 and Col3a1, and this increased expression of Col1a1 is exacerbated by Mp infection.
FIG 5.
Loss of CC16 results in increased expression of collagen genes in MTECs at baseline and during Mp challenge. WT (n = 3) and CC16−/− (n = 3) were treated with media (control) or Mp for 48 h, after which pro-collagen 1A1 (Col1a1) and pro-collagen 3A1 (Col3a1) expression was measured by RT-PCR with Gapdh as a housekeeping control. *, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001 by one-way ANOVA Tukey’s multiple comparison test. Data shown are mean ± SEM.
DISCUSSION
Despite CC16 being one of the most abundantly secreted proteins in the airways, the complete biological roles of this protein have yet to be fully elucidated; however, increasing evidence has shown that this protein appears to protect the respiratory tract against inflammation and oxidative stress (1, 15, 17, 19, 20). In order to better understand the role of CC16 in respect to infections and lung function over time, we developed a translational mouse model to study how early life infections with Mp could impact lung function in adulthood. In this study, we show that CC16−/− mice infected early in life with Mp had increased airway resistance and remodeling compared not only to their WT counterparts, but also to Mp-infected adult mice lacking CC16, which was likely attributed to their inability to effectively clear Mp infection. Additionally, we show that CC16−/− MTECs had increased Mp burden and increased airway remodeling responses suggesting that epithelial-driven responses are likely contributing to the observed phenotypes in the ELIM CC16−/− mice. Translationally, these data may imply that in individuals with low CC16 levels, early life exposures to pathogens, such as Mp, could result in significant lung remodeling that would impact lung function in adulthood.
Previously, our group showed that adult CC16−/− mice acutely infected with Mp for 3 days and treated with rCC16 “rescue” had significantly decreased AHR and inflammation compared to saline-treated CC16−/− mice (15). Similar to our published findings, CC16−/− mice infected with Mp had increased airway resistance, compared to the WT groups (CC16 sufficient), regardless of age of infection. Strikingly, compared to CC16−/− mice infected acutely as adults (AIM), CC16−/− mice infected as juveniles (ELIM) had significantly greater AHR. In contrast, WT mice that were infected as juveniles displayed minimal changes in AHR during the methacholine challenges, as resistance levels had returned to levels comparable to those of WT saline control mice. From these data we see that while Mp infections in adult mice lacking CC16 results in significant increases in AHR, the impact of early life infection in CC16−/− mice results in even greater AHR as adults, which is likely attributed to persistence of infection that is driving airway remodeling processes. The translational impact of these data suggests that early life Mp infections in pediatric patients with CC16 deficits may impact lung function in a stronger fashion than when these risk factors (i.e., Mp infection and CC16 deficits) occur in adult life.
In the AIM, both WT and CC16−/− mice had Mp detectable in their airways after 3 days. However, in the ELIM, we found that while a majority of WT mice had cleared Mp infection, most CC16−/− mice were unable to clear Mp and thereby had a “chronic” infection that lasted until their sacrifice at 8 weeks of age. Due to the 3-day duration of the AIM, one can infer either that CC16−/− mice are unable to clear Mp infections at the same rate as WT mice, or that CC16−/− mice are colonized more readily by Mp, compared to WT mice. Similar results were seen by Wang et al. in a respiratory syncytial virus (RSV) infection model where CC16−/− mice were unable to clear RSV infections over a 10-day period, compared to WT mice (11), which hints at an underlying defect in antimicrobial and antiviral host responses in the absence of CC16.
We have previously shown significantly increased neutrophil infiltrates in the lungs of CC16−/− mice when infected as adults for 3 days compared to CC16−/− mice treated with rCC16, which are indicative of the AIM (15). However, in the ELIM, we observed only slight increases in leukocytes (neutrophils and eosinophils), with the majority of all cells (>99%) being macrophages (Fig. S2). To insure we did not miss any relevant cellular inflammation, we examined BAL for immune cells every week in a cohort of mice up to 8 weeks of age for the ELIM model. Since infiltrating immune cells were minimal in ELIM, we chose to focus our studies on epithelial-driven mechanisms as contributors to the observed lung function phenotype. In line with our in vivo mouse models, pathogen burden was also significantly increased in CC16−/− MTECs, compared to WT MTECs, again suggesting an impairment in epithelial-driven antimicrobial host defense mechanisms, independent of immune cells. Overall, our results in mice and MTECs suggest that CC16 impacts epithelial-driven host defense mechanisms by aiding in Mp clearance. Despite these findings, more studies are needed to further understand, mechanistically, how CC16 is interacting with epithelial-driven antimicrobial responses and if CC16 also impacts resident alveolar macrophages in their ability to clear Mp.
To further understand contributing factors to the severely heightened AHR observed in CC16−/− mice infected with Mp, we analyzed two factors known to be associated with Mp infection: Muc5ac and Tnf-. Gene expression for Muc5ac, a mucin produced by goblet cells in the airways, is upregulated during airway inflammation and has pathological roles in the mechanisms of AHR, mucus metaplasia, and airway mucus plugging (21, 22). TNF- is a pro-inflammatory cytokine whose mRNA and protein levels are increased in the airways of patients with asthma and has been shown to lead to the development of AHR and neutrophilia (18, 23). Significantly increased TNF- levels have been observed in children infected with Mp, compared to healthy controls, and TNF- correlate to Mp positivity in asthmatic patients (24, 25). For the AIM, CC16−/− mice had significantly increased Muc5ac and Tnf- expression compared to their respective controls. For the AIM, Tnf- gene expression and protein levels were significantly increased in CC16−/− mice, compared to WT mice. Similarly, TNF-α levels were at or below detectable levels in BAL from control and ELIM mice (data not shown), which is in line with TNF-α being an acute, pro-inflammatory cytokine during Mp infection. Similar results for Tnf- in our ELIM were seen by Hardy et al., where low levels were observed during a chronic Mp infection model (>100 days) in BALB/c mice (26). Interestingly, while Tnf- gene expression decreased for both WT and CC16−/− mice in ELIM, Muc5ac remained significantly elevated in CC16−/− mice. The finding that Muc5ac remained upregulated in ELIM CC16−/− mice suggests that more goblet cells may have been present in these mice, likely due to persistent Mp colonization in the airways. More detailed studies are needed to better understand the role of CC16 in goblet cell metaplasia during infection.
In asthma and COPD, airway structural changes include, but are not limited to, subepithelial fibrosis, increased smooth muscle mass, and increased collagen deposition, all of which are associated with poor clinical outcomes among these patients (27). We were able to show that CC16−/− mice, at baseline and after Mp infection in the ELIM, have significantly increased expression of airway remodeling factors, compared to their WT counterparts. This was further confirmed by trichrome staining of lung tissue from these mice, which showed increased collagen deposition around the airways of CC16−/− mice. These results illustrate that early life Mp infections in CC16−/− mice can result in significantly increased airway remodeling by adulthood; therefore, a likely contributing factor to the significantly increased AHR observed among these mice.
Due to our observations that CC16−/− mice had increased collagen expression and deposition in their airways, we also assessed the same Mp-infected MTECs for collagen genes. Using MTECs, we observed that CC16−/− epithelial cells had increased collagen expression at baseline and during Mp infection, compared to WT epithelial cells. These results parallel what was seen in vivo and suggest that lung epithelial cells are likely contributing to the airway remodeling observed in mice upon Mp infection, observations that could translate to Mp-infected patients with known CC16 deficits, as well.
Overall, our data further support the important role of CC16 in mediating AHR and remodeling in the lungs during early life infections, which in turn may be relevant in reducing the progression of lung diseases, such as asthma and COPD, where CC16 deficiencies and respiratory infections are often observed. Surprisingly, we also show that CC16 promotes Mp clearance from the lung tissue by epithelial-mediated devices, illustrating that this endogenous protein is necessary for proper epithelial-driven host defense responses during Mp infection. While we only examined the responses to Mp, it is possible that this mechanism of epithelial-driven host defense may be broadly applicable to other respiratory pathogens in the context of CC16 deficiency. Our findings provide novel insights into the role of CC16 in mediating airway responses to early life respiratory infection and highlight the importance of further understanding mechanistic interactions of CC16 with immune responses, especially at the epithelial level, for pathogen clearance.
MATERIALS AND METHODS
Experimental mice.
All experiments were handled in accordance with the University of Arizona on IACUC approved animal protocols. For the Mp infection, aged matched WT and CC16−/− male mice as described previously (16) on a C57BL/6J background were aged ∼8 weeks at the time of infection for the adult infection model (AIM), and lung function was assessed 3 days postinfection. All mice were born and raised in the same room in the University of Arizona Health Sciences animal facility and were tested to be specific-pathogen free according to standard protocols using sentinel mice from the same room. For the early life infection model (ELIM), male mice were infected with Mp during the week of weaning (∼19–23 days old) and lung function was assessed ∼5 weeks postinfection when mice were ∼8 weeks. Once infected with Mp, mice are transferred to a barrier facility where they are maintained separately from other groups of mice according to BSL2 protocols. Tissue specific expression of CC16 is regulated by estrogen in the lung; therefore, male mice were used to eliminate the impact of this hormone on CC16 expression (20, 28).
Mouse tracheal cell isolation.
Mice were humanely euthanized, and tracheas were removed by dissection and placed in Ham’s F-12 medium on ice. Any excess connective tissue, muscle, vasculature, and nerves were removed from the exterior of the tracheas, and a longitudinal incision was made to expose the mucosal lining. The tracheas were placed in 10 ml Ham’s F-12 medium with 0.1% protease solution (Sigma-Aldrich; St. Louis, MO) and incubated for 45 min at 37°C. The protease activity was stopped by adding 3 ml FBS. The tracheas were then transferred to a petri dish containing Ham’s F-12 medium, and the mucosal lining of each trachea was scraped using a 200 μl pipette tip. The cells were collected, transferred to a 15-ml conical tube, and centrifuged (900 rpm, 5 min, 4°C). The medium supernatant was removed, and the cell pellet was resuspended in 5 ml Versene (Life Technologies; Waltham, MA) for 15 min at 37°C. Next, Ham’s F-12 medium containing 10% FBS was added to the conical tube containing the cell pellet, and centrifuged (900 rpm, 5 min, 4°C). The supernatant was removed, and the cell pellet was suspended in 6 ml Ham’s F-12 medium containing 10% FBS.
Culture media and supplements.
DMEM-Ham’s F-12 medium was used for the trachea harvest and MTEC cultures. The MTEC culture medium was supplemented with 500 μg/mL funizone antimycotic (HyClone Laboratories; Logan, UT), 20 ng/mL cholera toxin (List Biological Laboratories; Campbell, CA), 104 μg/mL bovine pituitary extract (Lonza; Basel, Switzerland), 5 μg/mL insulin (Sigma-Aldrich; St. Louis, MO), 5 μg/mL human apo-transferrin (Sigma-Aldrich; St. Louis, MO), 5 μg/mL dexamethasone (Sigma-Aldrich; St. Louis, MO), 5 ng/mL mouse epidermal growth factor (Sigma-Aldrich; St. Louis, MO), 0.01 μM retinol (Sigma-Aldrich; St. Louis, MO), 20 U/mL nystatin (Sigma-Aldrich; St. Louis, MO), and 100 μg/mL gentamicin (Sigma-Aldrich; St. Louis, MO). Fetal bovine serum (FBS) (HyClone Laboratories; Logan, UT) was used to prepare 5% and 10% serum-rich media during the ALI culturing process.
In vitro culture of MTECs.
Costar Transwell (12 mm, 0.4 μm membrane pores) 12-well plates were used to culture the MTECs. The polyester membrane was coated with 300 μg/mL rat rail collagen in 0.02 N glacial acetic acid at room temperature for 1 h. The membranes were washed with PBS and conditioned with Ham’s F-12 medium for 1 h at 37°C. The medium was then removed from the apical and basolateral sides, and 1 mL MTEC culture medium supplemented with 10% FBS was added to the basolateral side of each well. All cells obtained from the mouse tracheas were plated evenly between the 12 wells in 500 μl MTEC culture medium supplemented with 10% FBS. The plate was incubated at 37°C in an air/5% CO2 atmosphere for 48 h without changing the medium, for the cells to seed. After the initial seeding period, the medium on the apical and basal sides of the membrane was replaced every other day. When the cells reached 80% confluence (∼1 week), the culture medium was replaced daily, only on the basolateral side, to establish an ALI. After the first day in an ALI, the medium was changed to serum-free MTEC culture medium and replaced daily. Once full confluence was obtained, the cells were maintained at ALI for 14 days.
In vivo Mp infection.
For the infection model, Mp was purchased from ATCC (Manassas, VA, USA) (cat. no.: 15531) and grown in Remel SP4 broth (Thermo Fisher Scientific; Waltham, MA, USA) at 35°C until adherent, approximately 4 passages. Stocks were frozen at −80°C, a small aliquot was thawed, diluted, and plated on PPLO agar plates, and CFU counts were taken after 2 weeks of growth (35°C, no CO2) to calculate the concentration of the stock solutions. On the day of infection, a frozen stock of adherent Mp was washed by centrifuging at 6000 rpm for 5 min and resuspended in sterile saline for infection at a concentration of 1 × 108 Mp/50 μl inoculum. The Mp cell suspension was further pushed through a 27G needle to break up any clumps. Mp was delivered via intranasal instillation while mice were under isoflurane anesthesia.
In vitro Mp infection.
On the day of infection, the apical surface of the MTECs were washed two times with sterile PBS (1×) to remove excess mucus. Mp was diluted in sterile PBS (1×) for infection at a concentration of 1 × 106 Mp/100 μl inoculum) was added to the apical side of the MTECs and incubated for 48 h (37°C, 5% CO2).
Pulmonary function studies in mice.
All experiments were done in accordance with University of Arizona Institutional Animal Care and Use Committee approved animal protocols. WT and CC16−/− male mice treated with Mp or vehicle were analyzed for pulmonary mechanics on the Flexivent (SCIREQ Inc.; Montreal, Canada) system at baseline and after methacholine challenge. Pancuronium bromide (0.8 mg/ml in saline; Sigma-Aldrich P1918) was administered to anesthetize mice via intraperitoneal injection at a volume of 10 μl/g of body weight to prevent any interference from the animal during the pulmonary function tests. Default mechanical ventilation settings were performed (150 breaths/min, tidal volume of 10 mL/kg, and a positive end-expiratory pressure of 3 cm H2O), followed by two maneuvers (inflation to a standard pressure of 30 cm H2O over 3 s and holding for an additional 3 s), to open the closed lung areas and standardize the lung volume history. Single frequency (Snapshot-150; 2.5 Hz) and broadband (Quick Prime-3; 1-20.5 Hz) forced oscillation technique measurements were alternated every few seconds for a total of 12 measurements per perturbation over a period of approximately 3 min. This protocol was repeated four times, for the AIM and the ELIM, with increasing concentrations of methacholine (0, 10, 30, 100 mg/mL).
rCC16 Mp killing assay.
Mp was purchased from ATCC (Manassas, VA, USA) (cat. no.: 15531) and grown in Remel SP4 broth (Thermo Fisher Scientific; Waltham, MA, USA) at 35°C until adherent, approximately 4 passages. On the day of the assay, a frozen stock of adherent Mp was washed by centrifuging at 6000 rpm for 5 min and resuspended in sterile SP4 broth at a concentration of 3.75 × 108 CFU/ml. The Mp cell suspension was further pushed through a 27G needle to break up any clumps. rCC16 (0.76 mg/mL) was produced, as described previously (15). Mp (final concentration: 6 × 106 CFU/mL) and rCC16 (final concentrations: 0, 0.25, 1.56, 3.12, 6.25, 12.5, and 25 g/mL) were added to individual wells in a 96-well plate for a final volume of 200 l. The 96-well plate containing rCC16 and Mp was incubated for 24 h (37°C, 5% CO2), after which 10l of each sample was plated on PPLO agar (Thermo Fisher Scientific; Waltham, MA, USA) and grown for 2 weeks (35°C, 5% CO2). After 2 weeks, Mp CFU were counted, and CFU/mL was calculated.
SYBR green real-time PCR for measurement of gene expression in mice and MTECs.
Gene expression for all experiments was determined by reverse transcription to obtain cDNA, followed by real-time quantitative PCR (RT-PCR). RNA was extracted from lung tissue sections and MTECs using TRIzol reagent (Life Technologies; Waltham, MA). Reverse transcription was performed using an iScript cDNA Synthesis Kit (Bio-Rad; Hercules, CA) and 1 g of total RNA for a 20 l reaction on the C1000 Touch Thermal Cycler (Bio-Rad; Hercules, CA). All real-time PCR (RT-PCR) experiments were performed on the CFX96 Touch Real-Time PCR Detection System (Bio-Rad; Hercules, CA). The 20 l RT-PCR contained 100 ng cDNA, SYBR green SuperMix (Quanta Bio; Beverly, MA), and 250 nM primers. No-template controls were used to verify the PCR specificity for all primers.
Measuring Muc5ac in mice.
Lungs were harvested from the WT and CC16−/− mice, treated with either saline (control) or Mp. Muc5ac (forward: 5′-GAGGGCCCAGTGAGCATCTCC-3′; reverse: 5′-TGGGACAGCAGTATTCAGT-3′) gene expression was assessed by RT-PCR using a Sybr-green compatible system. Cyclophilin (forward: 5′-AGCACTGGAGAGAAAGGATTTGG-3′; reverse: 5′-TCTTCTTGCTGGTCTTGCCATT-3′) was used as a housekeeping control for these sets of experiments.
Measuring TNF-α in mice.
Lungs were harvested from the WT and CC16−/− mice, treated with either saline (control) or Mp. Tnf- (tumor necrosis factor-) (forward: 5′-CATCTTCTCAAAATTCGAGTGACAA-3′; reverse: 5′-TGGGAGTAGACAAGGTACAAC CC-3′) gene expression was assessed by RT-PCR using a Sybr-green compatible system. Cyclophilin (forward: 5′-AGCACTGGAGAGAAAGGATTTGG-3′; reverse: 5′-TCTTCTTGCTGGTCTTGCCATT-3′) was used as a housekeeping control for these sets of experiments.
TNF- protein levels were assessed in the bronchoalveolar lavage fluid (BALF) collected from WT and CC16−/− mice, treated with either saline (control) or Mp, by Enzyme-Linked Immunosorbent Assay (ELISA) (BioLegend; San Diego, CA), according to the manufacturer’s instructions. Briefly, BALF samples were incubated on a 96-well plate coated with the capture antibody and detected with a Streptavidin-HRP:biotin-conjugated secondary antibody complex on a plate reader (BioTek Instruments; Winooski, VT).
Measuring airway remodeling factors in mice and MTECs.
Airway remodeling factors, such as pro-collagen 1A1 (forward: 5′-AGACATGCTCAGCTTTGTGGATAC-3′; reverse: 5′-CCAGGGTCACCATTTCTC-3′), pro-collagen 3A1 (forward: 5′-GCCCACAGCCTTCTACAC-3′; reverse: 5′-CCAGGGTCACCATTTCTC-3′), -smooth muscle actin (forward: 5′-TCGT CCACCGCAAATGC-3′; reverse: 5′-AAGGAACTGGAGGCGCTG-3′), and transforming growth factor- (forward: 5′-GTGCGGCAGCTGTACATTGACTTT-3′; reverse: 5′-TGTACTGTGTGTCCAGGCTCCA AA-3′), were examined in lung tissue from WT and CC16−/− mice and MTECs treated with either control or Mp by RT-PCR using a Sybr-green compatible system. Cyclophilin or Gapdh (forward: 5′-CCTGCACCACCAACTGCTTA-3′; reverse: 5′-GTCTTCTGGGTGGCAGTGAT-3′) was used as a housekeeping control for these sets of experiments.
Measuring Mp burden in mice and MTECs.
Mp burden was measured in WT and CC16−/− mouse lung tissue and MTECs treated with either media (control) or Mp by RT-PCR for Mp-specific P1 adhesin gene (forward: 5′-CGCCGCAAAGAT GAATGAC-3′; reverse: 5′-TGTCCTTCCCCATCTAACAGTTC-3′) using a Sybr-green compatible system as previously described (15). Cyclophilin was used as a housekeeping control for these sets of experiments. Non-infected lung tissue or MTECs were used as negative controls for each experiment.
Trichrome staining for collagen in lung tissue.
Formalin fixed, paraffin embedded lung tissue from WT and CC16−/− mice infected with vehicle and Mp were washed with Xylene (two times, 5 min each), 100% ethanol (two times, 3 min each), and 95% ethanol (one time, 3 min). The slides were rinsed in tap water, subsequently stained with Bouin’s Fixative (preheated to 60°C) (1 h) and rinsed again in running tap water (5 min) to remove the picric acid. The slides were stained in Weigert’s Iron Hematoxylin Working Solution (prepared by mixing Weigert’s Hematoxylin A and Weigert’s Hematoxylin B at a 1:1 ratio) (10 min), after which the slides were washed in running tap water (5 min) and rinsed in distilled water. The slides were then stained with Biebrich Scarlet-Acid Fuchsin Solution (5 min), rinsed in distilled water, and transferred to Phosphotungstic/phosphomoludbic acid (10 min). The slides were transferred to Aniline Blue (5 min), after which they were rinsed in distilled water (3 changes). The slides were transferred to 1% acetic acid (1 min), rinsed in distilled water, and dehydrated in 95% ethanol and 100% ethanol (2 min each). The slides were cleared in Xylene (2 min) and mounted with Cytoseal Mounting Media.
Measuring collagen deposition in lung tissue.
Trichrome stained lung sections from the left lung lobes of WT and CC16−/− mice (saline- or Mp-treated; ELIM) were photographed using at ×20 magnification (Olympus BX43 Microscope) in a blinded manner for collagen thickness. Three lung sections from each mouse were obtained and trichrome stained, after which images of large airways were taken of each lung lobe for all experimental groups. For each image, the diameter of the airway was measured to ensure that the small airway diameter was between 300 and 899 μm, after which five fields within each lung section were photographed and four random measurements were taken for each field that spanned the collagen layer adjacent to the airway by Metamorph software (29). The average of the random collagen measurements was graphed from all the lung sections that were imaged. Statistical analysis was conducted in Prism software.
Statistical analysis.
For all mouse and MTEC experiments, statistics were analyzed using Prism 8 (GraphPad). For analysis of data collected during methacholine challenges that were recorded on separate experimental days, resistance data were transformed to percent over baseline, and differences between WT and CC16−/− mice (ELIM and AIM) were compared using one-way ANOVA Sidak’s multiple comparison test. For all other analysis of mouse and MTEC experiments, significance was determined by either Unpaired t test or one-way ANOVA for multiple comparisons, as appropriate.
ACKNOWLEDGMENTS
We thank Dr. Usir S. Younis for data used in the supplemental figure, as well as Dr. Michael D. L. Johnson for supplying us with rCC16.
Funding: HL142769, AI135108, T32 HL007249-44/45.
F.P., S.G., J.G.L. participated in project inception and experimental design; N.I., M.I., C.M., W.P.P., K.J.A., J.G.L. performed the experiments; N.I., J.G.L. participated in writing the manuscript; all authors reviewed the manuscript.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Julie G. Ledford, Email: jledford@email.arizona.edu.
Sabine Ehrt, Weill Cornell Medical College.
REFERENCES
- 1.Broeckaert F, Bernard A. 2000. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy 30:469–475. 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- 2.Guerra S, Vasquez MM, Spangenberg A, Halonen M, Martin RJ. 2016. Club cell secretory protein in serum and bronchoalveolar lavage of patients with asthma. J Allergy Clin Immunol 138:932–934.e1. 10.1016/j.jaci.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rava M, Tares L, Lavi I, Barreiro E, Zock JP, Ferrer A, Muniozguren N, Nadif R, Cazzoletti L, Kauffmann F, Anto JM, Guerra S. 2013. Serum levels of Clara cell secretory protein, asthma, and lung function in the adult general population. J Allergy Clin Immunol 132:230–232. 10.1016/j.jaci.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Zhai J, Stern DA, Sherrill DL, Spangenberg AL, Wright AL, Morgan WJ, Halonen M, Martinez FD, Guerra S. 2018. Trajectories and early determinants of circulating CC16 from birth to age 32 years. Am J Respir Crit Care Med 198:267–270. 10.1164/rccm.201712-2398LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerra S, Halonen M, Vasquez MM, Spangenberg A, Stern DA, Morgan WJ, Wright AL, Lavi I, Tares L, Carsin AE, Dobano C, Barreiro E, Zock JP, Martinez-Moratalla J, Urrutia I, Sunyer J, Keidel D, Imboden M, Probst-Hensch N, Hallberg J, Melen E, Wickman M, Bousquet J, Belgrave DCM, Simpson A, Custovic A, Anto JM, Martinez FD. 2015. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respiratory Medicine 3:613–620. 10.1016/S2213-2600(15)00196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PMA, Celli B, Coxson HO, Crim C, Lomas DA, MacNee W, Miller BE, Silverman EK, Tal-Singer R, Wouters E, Rennard SI, ECLIPSE Investigators. 2011. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 365:1184–1192. 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 7.Bajantri B, Venkatram S, Diaz-Fuentes G. 2018. Mycoplasma pneumoniae: a potentially severe infection. J Clin Med Res 10:535–544. 10.14740/jocmr3421w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashyap S, Sarkar M. 2010. Mycoplasma pneumonia: clinical features and management. Lung India 27:75–85. 10.4103/0970-2113.63611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nisar N, Guleria R, Kumar S, Chand Chawla T, Ranjan Biswas N. 2007. Mycoplasma pneumoniae and its role in asthma. Postgrad Med J 83:100–104. 10.1136/pgmj.2006.049023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DM, López Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agustí A. 2017. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med 195:557–582. 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 11.Wang SZ, Rosenberger CL, Bao YX, Stark JM, Harrod KS. 2003. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol 171:1051–1060. 10.4049/jimmunol.171.2.1051. [DOI] [PubMed] [Google Scholar]
- 12.Harrod KS, Mounday AD, Stripp BR, Whitsett JA. 1998. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol 275:L924–30. 10.1152/ajplung.1998.275.5.L924. [DOI] [PubMed] [Google Scholar]
- 13.Pilon AL. 2000. Rationale for the development of recombinant human CC10 as a therapeutic for inflammatory and fibrotic disease. Ann N Y Acad Sci 923:280–299. 10.1111/j.1749-6632.2000.tb05536.x. [DOI] [PubMed] [Google Scholar]
- 14.Nomori H, Horio H, Fuyuno G, Kobayashi R, Morinaga S, Hirabayashi Y. 1995. Protein 1 (Clara cell protein) serum levels in healthy subjects and patients with bacterial pneumonia. Am J Respir Crit Care Med 152:746–750. 10.1164/ajrccm.152.2.7633737. [DOI] [PubMed] [Google Scholar]
- 15.Johnson MD, Younis US, Menghani SV, Addison KJ, Whalen M, Pilon AL, Cress AE, Polverino F, Romanoski C, Kraft M, Martinez FD, Guerra S, Ledford JG. 2021. CC16 Binding to α4β1 integrin (VLA-4) protects against Mycoplasma pneumoniae infection. Am J Respir Crit Care Med 203:1410–1418. 10.1164/rccm.202006-2576OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhai J, Insel M, Addison KJ, Stern DA, Pederson W, Dy A, Rojas-Quintero J, Owen CA, Sherrill DL, Morgan W, Wright AL, Halonen M, Martinez FD, Kraft M, Guerra S, Ledford JG. 2019. Club cell secretory protein deficiency leads to altered lung function. Am J Respir Crit Care Med 199:302–312. 10.1164/rccm.201807-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutta S, Sengupta P. 2016. Men and mice: Relating their ages. Life Sci 152:244–248. 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Metz G, Kraft M. 2010. Effects of atypical infections with Mycoplasma and Chlamydia on asthma. Immunol Allergy Clin North Am 30:575–585. 10.1016/j.iac.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamez AS, Gras D, Petit A, Knabe L, Molinari N, Vachier I, Chanez P, Bourdin A. 2015. Supplementing defect in club cell secretory protein attenuates airway inflammation in COPD. CHEST 147:1467–1476. 10.1378/chest.14-1174. [DOI] [PubMed] [Google Scholar]
- 20.Laucho-Contreras ME, Polverino F, Tesfaigzi Y, Pilon A, Celli BR, Owen CA. 2016. Club cell protein 16 (CC16) augmentation: a potential disease-modifying approach for chronic obstructive pulmonary disease (COPD). Expert Opin Ther Targets 20:869–883. 10.1517/14728222.2016.1139084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lachowicz-Scroggins ME, Yuan S, Kerr SC, Dunican EM, Yu M, Carrington SD, Fahy JV. 2016. Abnormalities in MUC5AC and MUC5B protein in airway mucus in asthma. Am J Respir Crit Care Med 194:1296–1299. 10.1164/rccm.201603-0526LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Li Y, Luo D, Wang X, Zhang Y, Liu Z, Zhong N, Wu M, Li G. 2017. Lyn regulates mucus secretion and MUC5AC via the STAT6 signaling pathway during allergic airway inflammation. Sci Rep 7:42675. 10.1038/srep42675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brightling C, Berry M, Amrani Y. 2008. Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol 121:5–12. 10.1016/j.jaci.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Zhang Y, Lu W, Wang L. 2018. Serum tumor necrosis factor-α and interferon-γ levels in pediatric Mycoplasma pneumoniae pneumonia: a systematic review and meta-analysis. Canadian Respiratory J 2018:1–6. 10.1155/2018/8354892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsia BJ, Ledford JG, Potts-Kant EN, Nikam VS, Lugogo NL, Foster WM, Kraft M, Abraham SN, Wright JR. 2012. Mast cell TNF receptors regulate responses to Mycoplasma pneumoniae in surfactant protein A (SP-A)-/- mice. J Allergy Clin Immunol 130:205–214.e2. 10.1016/j.jaci.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 26.Hardy RD, Jafri HS, Olsen K, Hatfield J, Iglehart J, Rogers BB, Patel P, Cassell G, McCracken GH, Ramilo O. 2002. Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection-associated chronic reactive airway disease. Infect Immun 70:649–654. 10.1128/IAI.70.2.649-654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergeron C, Tulic MK, Hamid Q. 2010. Airway remodelling in asthma: from benchside to clinical practice. Can Respir J 17:e85–e93. 10.1155/2010/318029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee AB, Zhang Z, Chilton BS. 2007. Uteroglobin: a steroid-inducible immunomodulatory protein that founded the Secretoglobin superfamily. Endocr Rev 28:707–725. 10.1210/er.2007-0018. [DOI] [PubMed] [Google Scholar]
- 29.Laucho-Contreras ME, Polverino F, Gupta K, Taylor KL, Kelly E, Pinto-Plata V, Divo M, Ashfaq N, Petersen H, Stripp B, Pilon AL, Tesfaigzi Y, Celli BR, Owen CA. 2015. Protective role for club cell secretory protein-16 (CC16) in the development of COPD. Eur Respir J 45:1544–1556. 10.1183/09031936.00134214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download iai.00548-21-s0001.pdf, PDF file, 0.5 MB (510.7KB, pdf)