OBJECTIVE:
There are concerns of a high barotrauma rate in coronavirus disease 2019 patients with acute respiratory distress syndrome receiving invasive mechanical ventilation. However, a few studies were published, and reported rates were highly variable. We performed a systematic literature review to identify rates of barotrauma, pneumothorax, and pneumomediastinum in coronavirus disease 2019 acute respiratory distress syndrome patients receiving invasive mechanical ventilation.
DATA SOURCE:
PubMed and Scopus were searched for studies reporting barotrauma event rate in adult coronavirus disease 2019 patients receiving invasive mechanical ventilation.
STUDY SELECTION:
We included all studies investigating adult patients with coronavirus disease 2019 acute respiratory distress syndrome requiring mechanical ventilation. Case reports, studies performed outside ICU setting, and pediatric studies were excluded. Two investigators independently screened and selected studies for inclusion.
DATA EXTRACTION:
Two investigators abstracted data on study characteristics, rate of pneumothorax, pneumomediastinum and overall barotrauma events, and mortality. When available, data from noncoronavirus disease 2019 acute respiratory distress syndrome patients were also collected. Pooled estimates for barotrauma, pneumothorax, and pneumomediastinum were calculated.
DATA SYNTHESIS:
A total of 13 studies with 1,814 invasively ventilated coronavirus disease 2019 patients and 493 noncoronavirus disease 2019 patients were included. A total of 266/1,814 patients (14.7%) had at least one barotrauma event (pooled estimates, 16.1% [95% CI, 11.8–20.4%]). Pneumothorax occurred in 132/1,435 patients (pooled estimates, 10.7%; 95% CI, 6.7–14.7%), whereas pneumomediastinum occurred in 162/1,432 patients (pooled estimates, 11.2%; 95% CI, 8.0–14.3%). Mortality in coronavirus disease 2019 patients who developed barotrauma was 111/198 patients (pooled estimates, 61.6%; 95% CI, 50.2–73.0%). In noncoronavirus disease 2019 acute respiratory distress syndrome patients, barotrauma occurred in 31/493 patients (6.3%; pooled estimates, 5.7%; 95% CI, −2.1% to 13.5%).
CONCLUSIONS:
Barotrauma occurs in one out of six coronavirus disease 2019 acute respiratory distress syndrome patients receiving invasive mechanical ventilation and is associated with a mortality rate of about 60%. Barotrauma rate may be higher than noncoronavirus disease 2019 controls.
Keywords: barotrauma, coronavirus disease 2019, intensive care, Macklin effect, mechanical ventilation, pneumomediastinum, pneumothorax
At the end of 2019, an outbreak of atypical pneumonia caused by a novel coronavirus occurred in Wuhan, China (1). At the beginning of 2020, the coronavirus disease 2019 (COVID-19) pandemic spread all over the world, causing more than 175 million cases and more than 3,500,000 deaths (2). Unfortunately, a relevant proportion of patients with COVID-19 develop acute respiratory distress syndrome (ARDS), requiring admission to an ICU and prolonged invasive ventilation (3–5). Notably, at the beginning of the pandemic, the high rate of severe respiratory failure associated with COVID-19 frequently caused overwhelming of healthcare systems even in high-income countries (6–8).
Pneumomediastinum (PMD) and pneumothorax (PNX) are common complications in mechanically ventilated patients with ARDS, with a reported rate of up to 15% in some multicenter randomized controlled trials (9–12) and are generally considered a sign of barotrauma (13). There are now several reports of PNX/PMD as complication of COVID-19 ARDS (14–21). However, there are currently few and heterogeneous published data on the occurrence rate and outcome of barotrauma in this specific patient population.
Accordingly, we decided to perform a systematic literature review to investigate the rate and outcome of barotrauma in mechanically ventilated patients with COVID-19 ARDS.
MATERIALS AND METHODS
The present systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and the following Cochrane Collaboration recommendations (22–26).
The study has been registered on International Prospective Register of Systematic Reviews under registration no. CRD42021230946.
Search Strategy and Study Selection
Our search strategy aimed to find all studies investigating the occurrence rate of barotrauma in mechanically ventilated patient with COVID-19 ARDS. No exclusion by language or publication date was enforced. The Population (patients with COVID-19 ARDS requiring mechanical ventilation), Interventions (none), Comparison intervention (none), Outcome (barotrauma), and Study design (case series, retrospective observational, prospective observational, and randomized controlled trials) criteria were followed.
In detail, inclusion criteria were: 1) studies investigating adult patients with COVID-19 ARDS requiring mechanical ventilation and 2) reporting data on occurrence rate and outcome of barotrauma. Exclusion criteria were: 1) noncritically ill COVID-19 patients, 2) case reports, 3) lack of data for barotrauma rate, 4) pediatric studies, 5) nonhuman studies, 6) studies published as abstract only, and 7) overlapping population (i.e., two different publications investigating the same study population; in this case, we included the study with the longest follow-up). Studies published in language other than English were excluded if we were unable to obtain a translation.
We searched PubMed and Scopus from inception up to March 15, 2021. Backward snowballing (i.e., scanning of reference of retrieved articles to identify further studies) was applied to retrieve additional articles. Eligibility assessment was performed independently by two investigators at title/abstract level, and the final selection of included articles was based on complete articles with disagreements solved by consensus.
The PubMed search strategy is reported in the Supplementary Appendix (http://links.lww.com/CCM/G798).
Data Extraction
Two authors independently collected details on baseline study characteristics, occurrence rate and type of barotrauma events, and outcome data. These were verified by a third author.
We also reported whether studies included a control population, defined as non-COVID-19 ARDS, and we collected data on occurrence rate of barotrauma in the control population.
Outcomes
The primary outcome was the number of patients with at least one barotrauma event. Barotrauma was defined as PNX, PMD, pneumatocele not related to lung infection, subcutaneous emphysema, or as defined by authors of individual studies. Secondary outcomes were the number of patients with at least one PNX event, one PMD event, all-cause longest follow-up mortality in patients with barotrauma (both for COVID-19 and non-COVID-19 ARDS patients), and predictors of barotrauma. We also analyzed all-cause longest follow-up mortality in patients without barotrauma, both in COVID-19 and non-COVID-19 ARDS patients.
In addition, we collected data on treatment of PNX and PMD (chest tube drainage or need for surgery).
Statistical Analysis
We performed random-effects meta-analyses using the DerSimonian and Laird method. We used occurrence rates (or mean value for continuous data) and entered into meta-analyses with corresponding standard errors (ses). If no se could be derived, we retained studies for narrative synthesis only. We assessed statistical heterogeneity using the Q test and quantified using the I2 statistic, which identifies the proportion of the observed variance that reflects real differences in effect size (i.e., an estimate of the percentage of the total variability in a set of effect sizes due to true heterogeneity [between-studies variability]). Heterogeneity with an I2 > 25% was considered significant: fixed-effect and random-effects models were used in the case of low and high statistical heterogeneities, respectively.
We performed meta-analyses with Stata (Version 16, StataCorp, College Station, TX) using the “metan” commands. We calculated individual and pooled estimates (ES) for dichotomous outcomes with 95% CIs.
Risk of bias was assessed following the Risk Of Bias In Nonrandomized Studies—of Interventions and derived Risk Of Bias In Nonrandomized Studies—Exposure tools (27, 28). Studies were classified as “high risk of bias” if they had at least one item reported as high risk of bias, at “unclear risk of bias” if they had at least one item judged to carry an unclear risk of bias and at low risk of bias if all of the item were at low risk of bias.
Some of the retrieved studies presented data only for one type of barotrauma event (e.g., only PMD data and only PNX data) (18, 29–32). We, therefore, performed unplanned sensitivity analyses for primary outcome excluding these studies.
RESULTS
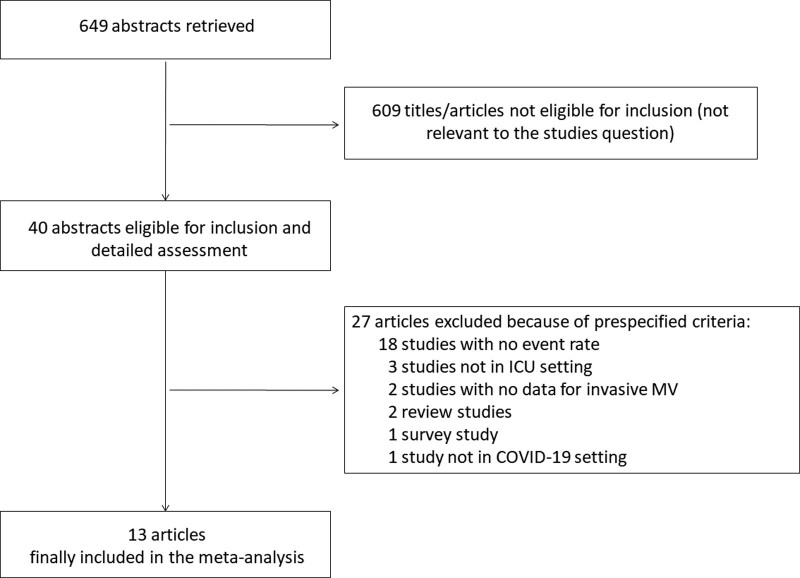
A total of 649 references were examined at a title/abstract level. After initial screening, a total of 40 studies were retrieved as complete articles. All studies were identified through database searching. After exclusion of further 27 trials that did not meet inclusion criteria, a total of 13 studies including data from 1,814 invasively ventilated COVID-19 patients and 493 non-COVID-19 patients were included in the analysis (Fig. 1) (18–20, 29–38). Details of major exclusions and reason for exclusion are provided in the Supplementary Table 1 (http://links.lww.com/CCM/G798).
Figure 1.
Flow diagram for included studies.
Furthermore, we identified 107 case reports (Supplementary Appendix, http://links.lww.com/CCM/G798).
Trials’ Characteristics
Characteristics of included studies are reported in Table 1. All studies were observational, and one study was multicenter (31). Most studies included patients during the first months of the COVID-19 pandemic, with only one study enrolling patients until December 2020 (38). Most studies were performed in United States (six studies) (20, 29, 32–34, 37) and Italy (three studies) (18, 19, 35).
Table 1.
Characteristics of Included Studies
| First Author | Journal | Study Type | Country | City Area | Enrollment Period |
|---|---|---|---|---|---|
| Belletti | J Cardiothorac Vasc Anesth | Observational | Italy | Milan | February 25, 2020, to April 27, 2020 |
| Capaccione | Acute Crit Care | Observational | United States | New York | February 12, 2020, to April 08, 2020 |
| Edwards | Ann Med Surg | Observational | United States | New York | March 15, 2020, to April 14, 2020 |
| Fiacchini | JAMA Otolaryngol Head Neck Surg | Observational | Italy | Pisa | March 01, 2020, to May 31, 2020 |
| Housman | Ann Transl Med | Observational | United States | New York | March 30, 2020, to April 10, 2020 |
| Kahn | J Intensive Care Med | Observational | United States | Los Angeles | March 15, 2020, to June 15, 2020 |
| Lemmers | ERJ Open Res | Observational | Italy | Brescia | February 18, 2020, to April 15, 2020 |
| Mart | Am J Respir Crit Care Med | Observational | United States | Nashville | March 01, 2020, to August 31, 2020 |
| McGuinness | Radiology | Observational | United States | New York | January 03, 2020, to June 04, 2020 |
| Talan | Tuberk Toraks | Observational | Turkey | Ankara | March 01, 2020, to December 01, 2020 |
| Udi | J Intensive Care Med | Observational | Germany | Friburg | March 01, 2020, to April 30, 2020 |
| Wang | Heart Lung | Observational | China | Chongquing | February 23, 2020, to March 15, 2020 |
| Yao | Br J Anaesth | Observational | China | Wuhan | February 04, 2020, to March 12, 2020 |
Risk of bias analysis showed that three studies were at moderate risk of bias (29, 37, 38) and 10 at low risk of bias (18–20, 30–36).
Primary Outcome
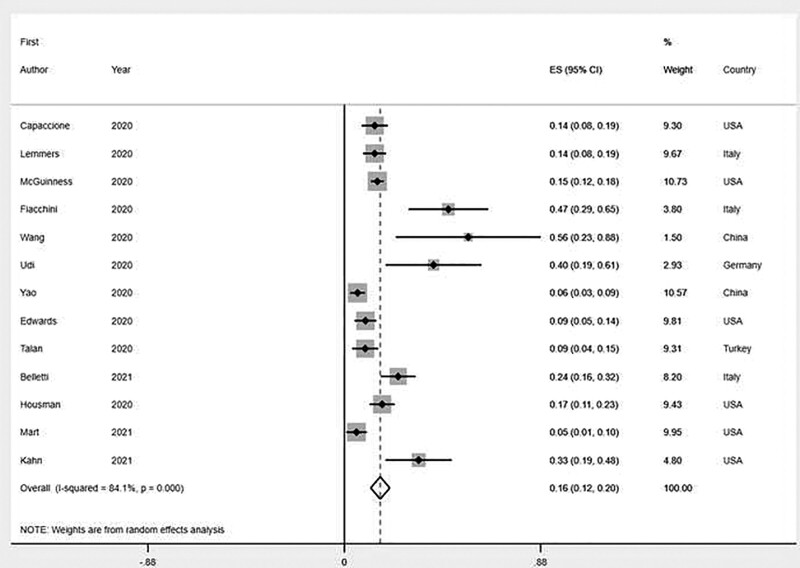
Outcome data are presented in Table 2. Overall, a total of 266/1,814 patients (14.7%) had at least one barotrauma event, with reported rate ranging from 5% to 56%. On pooled analysis, the rate of barotrauma events was 16.1% (95% CI, 11.8–20.4%), with all studies included (Fig. 2). Heterogeneity among studies was high (I2 = 84.1%; p for heterogeneity < 0.001) (Table 2).
Table 2.
Events Rate in Included Studies
| First Author | Non-COVID Acute Respiratory Distress Syndrome Control Group | COVID-19 IMV Patients, Number | Control IMV Patients, Number | Barotrauma Rate, Any Event, COVID-19, n (%) | Barotrauma Rate, Any Event, Control, n (%) | Barotrauma Overall Mortality COVID-19, n (%) | Barotrauma Overall Mortality Control, n (%) |
|---|---|---|---|---|---|---|---|
| Belletti | No | 116 | N/A | 28 (24) | N/A | 17 (61) | N/A |
| Capaccione | No | 132 | N/A | 18 (14) | N/A | 6 (33) | N/A |
| Edwards | No | 137 | N/A | 13 (9) | N/A | 6 (46) | N/A |
| Fiacchini | Yes | 30 | 45 | 14 (47) | 0 (0) | N/A | N/A |
| Housman | No | 171 | N/A | 29 (17) | N/A | N/A | N/A |
| Kahn | No | 39 | N/A | 13 (33) | N/A | N/A | N/A |
| Lemmers | Yes | 169 | 163 | 23 (14) | 3 (2) | 13 (57) | N/A |
| Mart | No | 92 | N/A | 5 (5.4) | N/A | 4 (80) | N/A |
| McGuinness | Yes | 601 | 285 | 89 (15) | 28 (10) | 47 (53) | 8 (29) |
| Talan | No | 96 | N/A | 9 (9) | N/A | 8 (89) | N/A |
| Udi | No | 20 | N/A | 8 (40) | N/A | 6 (75) | N/A |
| Wang | No | 9 | N/A | 5 (56) | N/A | 4 (80) | N/A |
| Yao | No | 202 | N/A | 12 (6) | N/A | N/A | N/A |
COVID-19 = coronavirus disease 2019, IMV = invasive mechanical ventilation, N/A = not available.
Figure 2.
Forest plot for barotrauma rate in COVID-19 ARDS patients. Individual and pooled estimates (%) are reported on the y-axis. Black diamonds indicate individual estimates, and black lines indicate individual 95% CIs. Gray squares represent individual study weights. The vertical red dashed line indicates pooled estimate. The vertical axis of the white diamond indicates pooled estimate, whereas the horizontal axis indicates pooled 95% CI. ES: estimate.
Data on barotrauma rate in control patients (non-COVID-19 ARDS) were reported in three studies (Supplementary Table 2, http://links.lww.com/CCM/G798) and occurred in 31/493 patients (6.3%; pooled ES, 5.7%; 95% CI, −2.1% to 13.5%; I2, 93.4%; p for heterogeneity < 0.001).
Nine studies reported data on time from intubation to development of barotrauma (Supplementary Table 2, http://links.lww.com/CCM/G798) (18, 19, 29, 30, 32, 33, 36–38). Pooled mean time from intubation to development of barotrauma was 3.7 days (95% CI, 2.2–5.0 d) (Supplementary Table 2 and Supplementary Fig. 1, http://links.lww.com/CCM/G798).
Secondary Outcomes
The number of COVID-19 patients who developed at least one PNX event was 132/1,435 patients (9.2%; pooled ES, 10.7%; 95% CI, 6.7–14.7%; I2 = 80.8%; p for heterogeneity < 0.001, with 10 studies included) (Supplementary Table 2, http://links.lww.com/CCM/G798). Among control (non-COVID-19-ARDS) patients, specific data on PNX were available in one study only with no patient developing PNX.
PMD occurred in a total of 162/1,432 COVID-19 patients (11.3%; pooled ES, 11.2%; 95% CI, 8.0–14.3%; I2 = 66.6%; p for heterogeneity = 0.002, with nine studies included) (Supplementary Table 2, http://links.lww.com/CCM/G798). Among non-COVID-19-ARDS patients, specific data on PMD were available in two studies only, with an occurrence rate of 3/208 patients (1.4%) (pooled ES, 1.8%; 95% CI, −0.2% to 3.9%; I2 = not available [N/A]; p for heterogeneity = N/A).
Overall mortality among COVID-19 patients who developed barotrauma was 111/198 patients (56.1%; pooled ES, 61.6%; 95% CI, 50.2–73.0%; I2 = 59.3%; p for heterogeneity = 0.01) (Supplementary Table 3, http://links.lww.com/CCM/G798). Mortality among non-COVID-19-ARDS patients who developed barotrauma was reported in one study only and was 28/285 patients (10%) (Supplementary Table 3, http://links.lww.com/CCM/G798)
In COVID-19 patients who did not develop barotrauma, overall mortality was 460/860 (53.5%) (pooled ES, 49.5%; 95% CI, 41.1–58.0%; I2 = 79.8%; p for heterogeneity = 0.002) (Supplementary Table 3, http://links.lww.com/CCM/G798). Mortality rate for non-COVID-19 ARDS patients who did not develop barotrauma was available in one study only and was 102/257 patients (39.7%) (Supplementary Table 3, http://links.lww.com/CCM/G798).
Mortality data for COVID-19 ARDS patients with PNX were available in three studies and were 13/27 patients (48.1%) (pooled ES, 59.7%; 95% CI, 26.2–93.1%; I2 = 68.7%; p for heterogeneity = 0.04) (Supplementary Table 3, http://links.lww.com/CCM/G798).
One study reported mortality data for COVID-19 ARDS patients with PMD (13/23 patients [57%]) (Supplementary Table 3, http://links.lww.com/CCM/G798). Separate mortality data for PNX and PMD in non-COVID-19 patients were not reported in any study (Supplementary Table 3, http://links.lww.com/CCM/G798).
Forest plots for all secondary outcome analyses are presented in Supplementary Figures 2-8 (http://links.lww.com/CCM/G798).
Sensitivity Analysis
After excluding studies reporting data only on one type (either PNX or PMD) of barotrauma event, we found that 179/1,131 of COVID-19 patients (15.8%) had at least one barotrauma event (pooled ES, 18.3%; 95% CI, 12.1–24.6%; I2 = 86%; p for heterogeneity < 0.001, with eight studies included) (Supplementary Fig. 9, http://links.lww.com/CCM/G798).
Among control patients, 28/330 patients (8.4%) had at least one barotrauma event (pooled ES, 9.8%; 95% CI, 6.4–13.3%; I2 = N/A; p for heterogeneity = N/A; with two studies included) (Supplementary Fig. 10, http://links.lww.com/CCM/G798).
Treatment and Predictors of PNX/PMD
Seven studies reported data on how PNX/PMD were treated (19, 30, 32, 33, 36–38). In a total of 36/97 patients (37.1%), chest tube drainage only was used, whereas two patients required surgical treatment (2.1%) (Supplementary Table 4, http://links.lww.com/CCM/G798). In 31/44 cases (70.5%), chest tube drainage was used for PNX, whereas 5/65 PMD cases (7.7%) received chest tube drainage. One surgical procedure was performed for PNX and another one to treat a tracheoesophageal fistula causing PMD (19).
Two studies investigated predictors of barotrauma in multiple logistic regression analysis. They identified younger age, length of hospital stay, time from symptoms onset to intubation, and bilirubin in the first 2 days of ICU stay as independent predictive factors (19, 20). One additional study presented univariate data only and identified younger age and need for higher Fio2 at baseline as risk factors for PNX development (29).
One study identified Macklin effect (39, 40), a radiological sign detected with chest CT scan, as a predictor for subsequent development of barotrauma at univariate analysis (19).
Mechanical Ventilation Settings
Mechanical ventilation settings at the beginning of invasive mechanical ventilation were available in seven studies and are presented in Supplementary Table 5 (http://links.lww.com/CCM/G798) (18, 19, 29, 30, 34, 35, 38). Mechanical ventilation settings at the time of first barotrauma event were available in five studies and are presented in Supplementary Table 6 (http://links.lww.com/CCM/G798) (18, 32, 34, 36, 37).
Three studies reported baseline mechanical ventilation settings for both patients who developed versus those who did not develop barotrauma, with no significant differences between the two groups (18, 19, 34). Two studies reported mechanical ventilation settings for both baseline and time of barotrauma (18, 34).
In one study, the authors compared mechanical ventilation settings at time of barotrauma with mechanical ventilation settings on day 14 of patients without barotrauma and found no significant differences (34).
Case Reports
Data from case reports and case series including patients who developed barotrauma without reporting overall rate are presented in the Supplementary Appendix (http://links.lww.com/CCM/G798). Overall, a total of 107 reports were identified, for a total of 269 COVID-19 patients: 34 (12.6%) were in spontaneous breathing; 120 (44.6%) were treated with noninvasive ventilation or continuous positive airway pressure; 104 (38.7%) were receiving invasive mechanical ventilation; and for 11 patients (4.1%), we did not find this piece of information.
DISCUSSION
Key Findings
In this systematic review, we found that the rate of barotrauma in COVID-19 patients undergoing invasive mechanical ventilation was high (16.1%) in the 13 identified studies and associated with a mortality rate greater than 60%. The rate of PNX was about 10%, whereas the rate of PMD was about 11%. Overall, barotrauma rate may be higher in COVID-19 ARDS than non-COVID-19 control. Mortality among COVID-19 patients with barotrauma is about 10% higher as compared with COVID-19 patients without. Chest tube drainage was required in about 40% of patients with barotrauma, whereas only 2% of patients required surgical treatment. Time from intubation to barotrauma seems relatively short, occurring 3.7 days after intubation, although with high variability. We also identified further 107 reports that confirmed the relevance of the topic, the possibility of barotrauma in patients not invasively ventilated patients, and the high associated mortality rate. Finally, among the classic predictors of barotrauma, one study identified a radiological predictor detectable on chest CT scan about 12 days in advance (the Macklin-like radiological finding) (19).
Relationship to Previous Studies
Previous, prospective studies performed in non-COVID-19 ARDS setting reported a rate of barotrauma events ranging from less than 3% to about 15% of patients. In our meta-analysis, we found that rate of barotrauma ranged from 5% to 55%, with a pooled estimate of 16%. Furthermore, three studies reported barotrauma rates also for non-COVID-19 ARDS controls and found a barotrauma rate of 2–10%, with a pooled estimate of 6%. This latter estimate is consistent with a previous prospective multicenter study performed in 20 countries (13). Our data collectively suggest that risk of barotrauma may be higher among COVID-19 ARDS patients as compared with non-COVID-19 ARDS, although this finding requires confirmatory studies with adequate control groups. Indeed, a recent survey among 38 ICUs in the region of Lombardy, Italy (the epicenter of the Italian first pandemic wave) (41, 42), reported a barotrauma rate of 7% as compared with a 5% rate in the control, non-COVID-19 group (43).
Recent large, multicenter, randomized controlled trials in ARDS patients reported rate of barotrauma ranging from about 3% to 10% (10–12, 44), confirming our pooled estimate for barotrauma events in non-COVID-19 ARDS patients of 6%. A similar rate was reported in previous observational studies (13). Pneumothorax rate in classical ARDS seems to be around 1–6%, as compared with 11% in COVID-19 ARDS found in our systematic review. The rate of PMD in COVID-19 ARDS is similar (11% in our meta-analysis), although few data are available for comparison with non-COVID-19 ARDS for this specific complication.
The relatively high rate of barotrauma events in COVID-19 ARDS is also confirmed by the large number of case reports (more than 100 identified by our systematic review) currently published in medical literature, which underline the unexpected impact that this complication had on clinical course of COVID-19 patients and the great interest of the scientific community toward this topic.
Implication of Study Findings
Our study underlines the significant association between barotrauma and clinical course of COVID-19 critically ill patients. We identified a pooled mortality rate of about 60%, with an upper limit of 95% CI of 73%. On the contrary, mortality rate for non-COVID-19 severe ARDS is reported to be around 46% (45), and the overall mortality rate for COVID-19 ARDS requiring invasive mechanical ventilation is around 51% (5). In our study, mortality rate for COVID-19 patients without barotrauma was 53%, comparable with available data for general COVID-19 ARDS patients requiring invasive mechanical ventilation (5). Of note, our data do not allow us to determine whether association between development of barotrauma and worse outcome is causal or simply reflects greater disease severity. Nevertheless, in light of these data, it may be considered to apply early ultraprotective mechanical ventilation strategy or early extracorporeal life support for COVID-19 ARDS patients, until further data become available. In particular, as the pathophysiology of COVID-induced lung injury is delineated, we may have a better understanding of why there is a higher occurrence rate of barotrauma.
Interestingly, our group recently identified a radiological sign on chest CT that may potentially predict development of barotrauma 12 days in advance (the so called “Macklin sign” or “Macklin-like radiological finding” [39, 40]). We can speculate that identification of Macklin sign on chest CT scan of ARDS patients might help to stratify patients risk of developing barotrauma and hence to refer patients for early extracorporeal support, although this hypothesis requires confirmatory studies.
Of note, we found that mechanical ventilation settings were within recommended ranges for protective ventilation in ARDS (46) in most studies, without major differences between patients who developed versus those who did not develop barotrauma.
The higher occurrence rate of barotrauma in COVID-19 ARDS patients may be explained by a peculiar pathophysiology of airway damage resulting in a virus-specific lung frailty (47, 48), although recent studies showed similar histopathology findings between severe acute respiratory syndrome coronavirus 2-associated lung injury and other causes of lung injury (49, 50). A possible, alternative explanation for the high occurrence rate of barotrauma events may be that, as COVID-19 frequently overwhelmed hospital and ICU resources in several countries (6, 7, 42), patients were frequently managed by clinicians with relatively limited experience in ARDS patients or limited resources, as compared with highly controlled clinical trials generally performed by experts in the field.
Limitations of the Study
Strengths of our study are the systematic approach to literature review and analysis of clinically relevant outcomes.
Limitations of our study include the relative small sample size of included studies, the heterogeneity of data, and lack of randomized controlled trials. Nevertheless, this is the largest and most comprehensive review of available data, considering that COVID-19 was discovered only 1 year ago.
Most of included studies do not have a well-matched control group, with 10 of them having no control group at all. Accordingly, any comparison of barotrauma rate between COVID-19 and non-COVID-19 ARDS is currently at high risk of bias.
Our search strategy might have led to inclusion of studies emphasizing complications of severe pneumonia and, therefore, to overestimation of barotrauma rate. However, we strictly adhered to a prespecified search strategy and inclusion criteria that we planned before accessing to study data. Furthermore, event rate in control patients is similar to previously available data.
Some included studies focused only on one type of barotrauma event (e.g., PMD only, as in Lemmers et al [18]) and may therefore lead to over- or underestimation of events when included in the pooled analysis. Nevertheless, a sensitivity analysis excluding these studies confirmed magnitude and direction of the results.
Details on treatment of PMD with chest tube drainage from retrieved studies were not available; thus, we cannot comment on this management strategy.
Future Studies and Prospects
Future studies should address the exact pathophysiology of COVID-19-induced lung damage and its relationship with development of airway frailty and barotrauma. In addition, future studies should investigate possible risk factors for barotrauma, as well as preventive and management strategies to mitigate the risk of barotrauma development and lower mortality rates associated with this complication.
CONCLUSIONS
Barotrauma occurs in about 16% of COVID-19 patients receiving invasive mechanical ventilation and is associated with mortality rates of about 60%. Barotrauma rates in COVID-19 ARDS may be higher than those in non-COVID-19 ARDS, although the exact reason for this remains to be determined. Predictors of barotrauma development should be urgently identified or validated, and strategies to reduce barotrauma risk and improve survival should urgently be implemented.
ACKNOWLEDGMENTS
We thank Stefano Fresilli, MD (IRCCS San Raffaele Scientific Institute) for his help in development of the search strategy and Rosalba Lembo, MSc (IRCCS San Raffaele Scientific Institute) for the support in statistical analyses.
Supplementary Material
Footnotes
*See also p. 531.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
International Prospective Register of Systematic Reviews registration no. CRD42021230946.
This study was performed at the IRCCS San Raffaele Scientific Institute.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team: A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020; 382:727–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus Disease (COVID-19) Dashboard: WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int/. Accessed June 15, 2021
- 3.Zangrillo A, Beretta L, Scandroglio AM, et al. ; COVID-BioB Study Group: Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020; 22:200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network: Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G, Greco M, Zanella A, et al. ; COVID-19 Lombardy ICU Network: Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020; 180:1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monti G, Cremona G, Zangrillo A, et al. : Home ventilators for invasive ventilation of patients with COVID-19. Crit Care Resusc. 2020; 22:266–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zangrillo A, Beretta L, Silvani P, et al. : Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: Facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020; 22:91–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbaum L: Facing Covid-19 in Italy - ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020; 382:1873–1875 [DOI] [PubMed] [Google Scholar]
- 9.Guérin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group: Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 10.Villar J, Ferrando C, Martínez D, et al. ; dexamethasone in ARDS network: Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir Med. 2020; 8:267–276 [DOI] [PubMed] [Google Scholar]
- 11.Moss M, Huang DT, Brower RG, et al. : Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019; 380:1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. : Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome - a randomized clinical trial. JAMA. 2017; 318:1335–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anzueto A, Frutos-Vivar F, Esteban A, et al. : Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med. 2004; 30:612–619 [DOI] [PubMed] [Google Scholar]
- 14.Zhou C, Gao C, Xie Y, et al. : COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis. 2020; 20:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loffi M, Regazzoni V, Sergio P, et al. : Spontaneous pneumomediastinum in COVID-19 pneumonia. Monaldi Arch Chest Dis. 2020; 90:604–607 [DOI] [PubMed] [Google Scholar]
- 16.Lei P, Mao J, Wang P: Spontaneous pneumomediastinum in a patient with coronavirus disease 2019 pneumonia and the possible underlying mechanism. Korean J Radiol. 2020; 21:929–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wali A, Rizzo V, Bille A, et al. : Pneumomediastinum following intubation in COVID-19 patients: A case series. Anaesthesia. 2020; 75:1076–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemmers DHL, Abu Hilal M, Bnà C, et al. : Pneumomediastinum and subcutaneous emphysema in COVID-19: Barotrauma or lung frailty? ERJ Open Res. 2020; 6:00385–02020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belletti A, Palumbo D, Zangrillo A, et al. Predictors of pneumothorax/pneumomediastinum in mechanically ventilated COVID-19 patients. J Cardiothorac Vasc Anesth. 2021. Feb 6. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuinness G, Zhan C, Rosenberg N, et al. : Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology. 2020; 297:E252–E262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palumbo D, Campochiaro C, Belletti A, et al. ; COVID-BioB Study Group: Pneumothorax/pneumomediastinum in non-intubated COVID-19 patients: Differences between first and second Italian pandemic wave. Eur J Intern Med. 2021; 88:144–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009; 151:W65–W94 [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009; 62:1006–1012 [DOI] [PubMed] [Google Scholar]
- 24.Greco T, Zangrillo A, Biondi-Zoccai G, et al. : Meta-analysis: Pitfalls and hints. Heart Lung Vessel. 2013; 5:219–225 [PMC free article] [PubMed] [Google Scholar]
- 25.Biondi-Zoccai G, Lotrionte M, Landoni G, et al. : The rough guide to systematic reviews and meta-analyses. HSR Proc Intensive Care Cardiovasc Anesth. 2011; 3:161–173 [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins J, Thomas J, Chandler J, et al. (Eds): Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester, UK, John Wiley & Sons, 2019 [Google Scholar]
- 27.Sterne JA, Hernán MA, Reeves BC, et al. : ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ROBINS-E. Bristol Medical School: Population Health Sciences. University of Bristol. 2017. Available at: http://www.bristol.ac.uk/population-health-sciences/centres/cresyda/barr/riskofbias/robins-e/. Accessed January 7, 2021 [Google Scholar]
- 29.Capaccione KM, D’souza B, Leb J, et al. : Pneumothorax rate in intubated patients with COVID-19. Acute Crit Care. 2021; 36:81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang XH, Duan J, Han X, et al. : High incidence and mortality of pneumothorax in critically ill patients with COVID-19. Heart Lung. 2021; 50:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao W, Wang T, Jiang B, et al. ; Collaborators: Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: Lessons learnt and international expert recommendations. Br J Anaesth. 2020; 125:e28–e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Housman B, Jacobi A, Carollo A, et al. : COVID-19 ventilator barotrauma management: Less is more. Ann Transl Med. 2020; 8:1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mart MF, Norfolk SG, Flemmons LN, et al. : Pneumomediastinum in acute respiratory distress syndrome from COVID-19. Am J Respir Crit Care Med. 2021; 203:237–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahn MR, Watson RL, Thetford JT, Wong JI, Kamangar N: High incidence of barotrauma in patients with severe coronavirus disease 2019. J Intensive Care Med. 2021; 36:646–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiacchini G, Tricò D, Ribechini A, et al. : Evaluation of the incidence and potential mechanisms of tracheal complications in patients with COVID-19. JAMA Otolaryngol Head Neck Surg. 2021; 147:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Udi J, Lang CN, Zotzmann V, et al. : Incidence of barotrauma in patients with COVID-19 pneumonia during prolonged invasive mechanical ventilation - a case-control study. J Intensive Care Med. 2021; 36:477–483 [DOI] [PubMed] [Google Scholar]
- 37.Edwards JA, Breitman I, Bienstock J, et al. : Pulmonary barotrauma in mechanically ventilated coronavirus disease 2019 patients: A case series. Ann Med Surg (Lond). 2021; 61:24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talan L, Şaşal Solmaz FG, Ercan U, et al. : COVID-19 pneumonia and pneumothorax: Case series. Tuberk Toraks. 2020; 68:437–443 [DOI] [PubMed] [Google Scholar]
- 39.Macklin CC: Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum: Clinical implications. Arch Intern Med. 1939; 64:913–926 [Google Scholar]
- 40.Murayama S, Gibo S: Spontaneous pneumomediastinum and Macklin effect: Overview and appearance on computed tomography. World J Radiol. 2014; 6:850–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fagiuoli S, Lorini FL, Remuzzi G; Covid-19 Bergamo Hospital Crisis Unit: Adaptations and lessons in the province of Bergamo. N Engl J Med. 2020; 382:e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grasselli G, Pesenti A, Cecconi M: Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA. 2020; 323:1545–1546 [DOI] [PubMed] [Google Scholar]
- 43.Protti A, Greco M, Filippini M, et al. : Barotrauma in mechanically ventilated patients with coronavirus disease 2019: A survey of 38 hospitals in Lombardy, Italy. Minerva Anestesiol. 2021; 87:193–198 [DOI] [PubMed] [Google Scholar]
- 44.Papazian L, Forel JM, Gacouin A, et al. ; ACURASYS Study Investigators: Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010; 363:1107–1116 [DOI] [PubMed] [Google Scholar]
- 45.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 46.Fan E, Del Sorbo L, Goligher EC, et al. ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine: An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017; 195:1253–1263 [DOI] [PubMed] [Google Scholar]
- 47.Ciceri F, Beretta L, Scandroglio AM, et al. : Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020; 22:95–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fahmy OH, Daas FM, Salunkhe V, et al. : Is microthrombosis the main pathology in coronavirus disease 2019 severity?-A systematic review of the postmortem pathologic findings. Crit Care Explor. 2021; 3:e0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konopka KE, Nguyen T, Jentzen JM, et al. : Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 infection is morphologically indistinguishable from other causes of DAD. Histopathology. 2020; 77:570–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, et al. ; COVID-19 Spanish ICU Network: Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020; 46:2200–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.