ABSTRACT
Background
There currently are no standard, low-cost, and validated methods to assess the timing of food intake.
Objectives
The aim of this study was to validate simple, recall-based questions that can characterize food timing in free-living populations.
Methods
The concordance between recall-based survey questions and food times estimated from multiple daily food records was assessed in 249 generally healthy, free-living adults from the Shift Work, Heredity, Insulin, and Food Timing (SHIFT) Study. At baseline, participants were asked: “At what time do you first start and stop eating on weekdays/workdays and weekends/non-workdays?” and “At what time do you have your main meal on weekdays/workdays and weekends/non-workdays?” Participants were then asked to complete ≤14 d of food records noting the start time of each eating occasion. The timing of the first, last, and main (largest percentage calories) eating occasions were determined from food records. Wilcoxon matched pairs signed rank and Kendall's coefficient of concordance were used to compare differences and determine agreements between the methods for these food timing parameters, as well as for the midpoint between first and last eating occasion.
Results
Eating occasions on work and free days showed significant agreements between the 2 methods, except for the main eating occasion on free days. Significant agreements were generally modest and ranged from 0.16 (workdays main eating occasion) to 0.45 (workdays first eating occasion). Generally, times based on recall were later than those estimated from food records, and the differences in estimated times were smaller on workdays compared with free days, and smaller for the first compared with the last eating occasion. Main eating occasions from food records often varied between lunch and dinner times, contributing to low concordance with recalled times.
Conclusions
Modest agreements were found between food times derived from simple, recall-based survey questions and food times estimated from multiple-day food records. Single administration of these questions can effectively characterize the overall timing of eating occasions within a population for chrononutrition research purposes.
Keywords: chrononutrition, food timing, intermittent fasting, food record, validation, dietary assessment
There currently are no standard and low-cost methods to assess the timing of food intake. This study validates simple, recall-based questions that can effectively characterize food timing in free-living populations.
Introduction
The timing of food intake, or chrononutrition, is an emerging aspect of nutritional science with a potentially profound impact on cardiometabolic health (1). Epidemiological studies suggest that later food timing is associated with higher odds of being overweight or obese (2), impedes the efficacy of weight-loss interventions (3), and increases risk for adverse cardiometabolic health (1). In addition, intermittent fasting has been shown to have numerous health benefits (4). For example, restricting daily caloric intake to an eating window of 8–12 h has been shown to reduce body fat and blood pressure, and increase insulin sensitivity and body strength (5). However, additional human studies are needed to assess the long-term role of chrononutrition on a wider range of health outcomes and in a wider variety of cohorts and patient populations. A limiting factor in advancing chrononutrition research is the lack of standard food timing assessment tools.
There currently are no simple, low-cost, and validated methods to assess the timing of food intake (6). Traditional dietary assessment tools used in nutrition research, such as FFQs, have primarily focused on dietary composition. To ascertain timing, previous studies have primarily relied on modifying traditional dietary assessment tools and picture-based smartphone applications. For example, in the 24-h dietary recalls administered by the NHANES, participants were asked to recall the clock time of all eating episodes (7). In another study, traditional 7-d dietary records were amended to include a question on the time of day that each meal was eaten (8). Meanwhile, other studies have leveraged time stamps captured through picture-based smartphone applications used to record consumed items (9). In addition, the Meal Pattern Questionnaire asks respondents to list usual times of food intake during a 24-h period (10). It is believed that among the most pertinent aspects of chrononutrition to capture in a survey are the timings of the first eating occasion, last eating occasion, largest eating occasion, and main meals and snacks; however, other domains such as the midpoint of intake and meal regularity, might also be relevant (11). Identifying scalable tools to assess food timing that are inexpensive to administer and that have low respondent and analytical burden is necessary.
In this secondary analysis of the Shift Work, Heredity, Insulin, and Food Timing (SHIFT) Study, we aimed to assess the concordance between food timing parameters (first, last, and main eating occasion and midpoint of intake) derived from responses to 6 simple recall-based survey questions and those estimated from ≤14 d of prospectively collected paper-based food records. We also evaluated the influences of potential modifiers on agreement differences. These analyses are expected to indicate the utility of recalled food timing for future epidemiological research in free-living, healthy adults.
Methods
SHIFT Study
Participants were from the SHIFT Study (clinicaltrials.gov: NCT02997319). The SHIFT Study was a multicentered, observational, randomized crossover study aimed to determine the effect of concurrent food intake and melatonin on glucose tolerance, particularly in carriers of the MTNR1B(Melatonin receptor 1B) genetic risk variant. Participants were enrolled for 2 consecutive weeks. Participants 1) completed baseline surveys, 2) wore a wrist accelerometer (actigraph) while concurrently completing daily sleep and nutrition logs for ≤14 d, and 3) attended 2 clinical visits where they conducted a standard oral-glucose-tolerance test. All clinical visits took place at clinical research centers at an academic medical center (Massachusetts General Hospital and Brigham and Women's Hospital) in Boston, MA, and were administered by trained study staff. All recruited participants provided written consent upon enrollment. Recruitment took place between 2017 and 2021. The protocol was approved by the Mass General Brigham (formerly Partners Healthcare) Institutional Review Board (#2016P000651).
Recruitment for the SHIFT Study was primarily conducted online through the Mass General Brigham clinical trials website (Rally), other websites (e.g., Craigslist), and physical flyers. Interested participants completed an online survey screener. Eligible participants were adults aged between 18 and 60 y, of East Asian, South Asian, or European ancestry (because of the interest in the MTNR1B genetic variant), residing in the New England area, full-time (≥30 h/wk) employees, working students, or unemployed adults at the time of their enrollment, and able and willing to give consent and comply with study procedures. Exclusionary criteria included: 1) participants with any known diabetes diagnosis or on medication for the treatment of diabetes or other medication known to influence glycemic parameters, including oral mexiletine, propranolol, or verapamil, medications for sleep and circadian rhythm disorders such as lithium, ramelteon, or other stimulants such as Provigil, and sleep medication or hypnotics; 2) participants who were pregnant or nursing, had a history of bariatric surgery, and those who had been diagnosed with chronic renal failure, cancer, blindness, or any eating disorder; and 3) participants on anticoagulant medication or blood thinners such as heparin, warfarin, or clopidogrel, which might preclude the use of intravenous catheters for blood draws. A total of 1533 participants completed the online screener, of which 885 were eligible to join the study and 442 enrolled in the study.
Food timing via recall-based survey questions
Following enrollment, participants were invited to complete baseline electronic surveys that included questions on habitual food timing. Specifically, participants were asked: “At what time do you first start eating on weekdays/workdays? (includes meals, snacks, and drink meals, but not calorie-free beverages),” “At what time do you stop eating on weekdays/workdays? (includes meals, snacks, and drink meals, but not calorie-free beverages),” and “At what time do you have your main meal on weekdays/workdays? (includes meals, snacks, and drink meals, but not calorie-free beverages).” Similar questions for “weekends/non-workdays” were also included. The response options provided no time reference period because study participants included both day- and night-shift workers.
Food timing via prospectively collected paper-based food records
During the 2-wk study period, participants were asked to complete ≤14 d of paper-based food records noting food and beverage type, portion size or quantity, meal type, location, and clock time for the start of each eating or drinking occasion. Participants met with a trained research dietitian (RD) or research coordinator who provided instructions on how to complete the food records, an informational sheet on estimating portion sizes, and an example of a completed food record entry. Following each of the 2 weeks, the 7 d of food records were collected and reviewed for clarity and detail with each participant by a trained RD. Data from the food records were entered by a trained RD or a trained diet technician and quality checked for accuracy and consistency by a trained RD. Data were then analyzed with the Nutrition Data System for Research software developed by the Nutrition Coordinating Center (University of Minnesota, Minneapolis, MN) to obtain the nutrient content information on each eating occasion.
Sleep assessment and other covariates
During the study period, participants were instructed to wear an actigraph (Actiwatch Spectrum PRO; Philips Respironics, US) on their nondominant wrist to objectively monitor activity and sleep. Participants were asked to press the event marker button prior to falling asleep and upon first waking up. While wearing the device, participants were also instructed to complete a sleep log every morning and record bed and wake times and specify day type: weekday/workday (workday) or weekend/non-workday (free day). Data from the actigraph and sleep logs were scored with the Philips Actiware 6 version 6.0.9 software using the method detailed by Patel et al. (12), and sleep duration was derived.
In addition, upon enrollment, height (meters) and weight (kilograms) were measured by a trained RD per guidelines outlined in Lohman et al. (13). BMI was calculated using the formula: weight (kg)/height2 (m2). Employment status was determined by asking participants the following question: “Do you currently have a job or do any unpaid work outside your home?” with response options “yes” or “no.” Chronotype was assessed using the Morningness-Eveningness Questionnaire, a 19-item scale developed by Horne and Östberg (14), and a morningness-eveningness score was computed (higher score = more morningness).
Statistical analysis
A total of 442 adult participants were enrolled, of which 366 completed the SHIFT Study. Participants 1) without ≥1 food record on a workday and a free day, 2) with missing day type designation on all food records, 3) without responses to all food timing recall questions (questions were added to baseline surveys 1 y after study start), and 4) who had reported working night shifts were excluded. In the present analysis 249 adult participants from the SHIFT Study were finally included. For the remaining participants, food record data from days with clinical visits that required 8-h fasting and food record data from days with missing day type designations were excluded from the analysis. In addition, a total of 2808 eating occasions in food records of <5 calories were removed from the analysis as the survey questions asked about times of meals, snacks, and drink meals, but not calorie-free beverages. Then, for each participant, the timing of the first, last, and main (based on largest percentage calories for that day—the eating occasion with the largest energy contribution) eating occasions were determined from the food records for each day. Using the first and last eating occasion, the midpoint of intake (the midway clock time between first and last eating occasions) was also calculated. The timings for the first and last eating occasions and midpoint of intake were then averaged across all workdays and free days separately; the population data are presented as median (IQR). The timing of the main eating occasion was instead designated as the median time across all workdays and free days separately. Food timing survey responses were reviewed, and 169 likely erroneous times (9.6% of all survey responses), primarily from noon/midnight misreporting, were corrected.
First, we used Wilcoxon matched pairs signed rank test to compare differences in the timing of the 4 food timing parameters on workdays and free days between the survey questions and daily food records. Agreements between food times using the 2 different methods were assessed using Kendall's coefficient of concordance. Kendall's coefficient of concordance (W) is a nonparametric ranked test statistic used to measure the degree of concordance among different tools/raters, where a value of 1.0 demonstrates complete concordance (perfect agreement) and a value of 0.0 shows no concordance at all (lack of agreement). We then used Bland–Altman plots to further visualize the agreement between the timing parameters. The mean difference represents the estimated concordance, and the SD of the differences measures the random fluctuations around the mean.
To assess the potential role of factors on the calculated concordance, we used Wald tests to examine the interaction term for each potential modifier and food times. The following factors were considered because of previous reports of their links with food times (6): age (15), gender (15, 16), BMI (3, 15, 17), employment status (15), morningness-eveningness scores (18), and sleep duration (6). Specifically, we ran linear regression models to regress food times from the recall questions onto food times estimated from food records. An interaction term for each potential modifier and food times from food records were included. An interaction term was considered significant at the Bonferroni P value cutoff (P < 0.0063) accounting for the total number of factors tested. When significant, subgroup analyses were conducted. The overall population was stratified by the potential modifier, and the Kendall's coefficient of concordance was recomputed. All statistical analyses were conducted using R (version 4.1.0; R Foundation for Statistical Computing).
Results
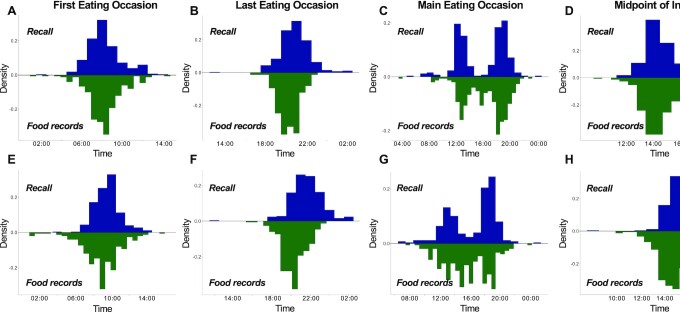
A total of 249 adult participants from the SHIFT Study were included in the present analysis (Figure 1). Participants were 74.7% female and had a mean age of 29.0 ± 8.9 y and a mean BMI of 24.07 ± 4.37 kg/m2 (Table 1). Based on recall data, on workdays, the times of the first, last, and main eating occasions and the midpoint of intake, presented throughout as median (IQR), were 08:00 (1 h 30 min), 21:00 (2 h), 17:30 (6 h 30 min), and 14:30 (1 h 12 min), respectively (Figure 2 , Table 2). On free days, the times of the first, last, and main eating occasions and the midpoint of intake were 10:00 (1 h 30 min), 22:00 (2 h), 18:00 (5 h), and 15:30 (5 h), respectively. Prospective food times were calculated from an average of 7 and 4 daily food records on workdays and free days, respectively. Based on food records, on workdays, the times of the first, last, and main eating occasions and the midpoint of intake were 08:12 (1 h 42 min), 20:12 (1 h 24 min), 17:12 (5 h 12 min), and 14:12 (1 h 18 min), respectively, whereas on free days the times of the first, last, and main eating occasions and the midpoint of intake were 09:00 (2 h 18 min), 20:12 (1 h 36 min), 15:48 (4 h 48 min), and 14:36 (1 h 36 min), respectively.
FIGURE 1.
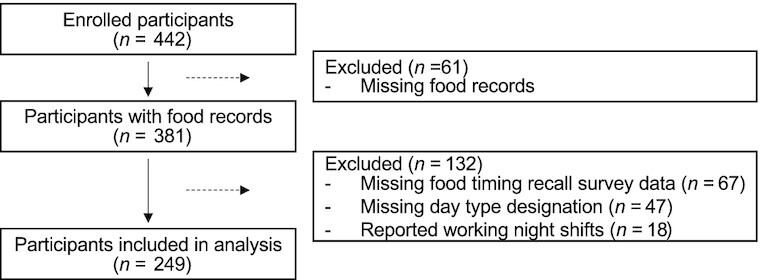
Study flowchart of included participants from the Shift Work, Heredity, Insulin, and Food Timing (SHIFT) Study (n = 249).
TABLE 1.
General characteristics of the Shift Work, Heredity, Insulin, and Food Timing (SHIFT) Study participants (n = 249) included in the present analysis1
| Participants | |
|---|---|
| n | 249 |
| Female gender, n (%) | 186 (74.7) |
| Race, n (%) | |
| Asian and Pacific Islander | 55 (22.1) |
| European | 187 (75.1) |
| South Asian | 7 (2.8) |
| Hispanic, n (%) | 17 (6.8) |
| Age, y | 29.0 ± 8.9 |
| BMI, kg/m2 | 24.07 ± 4.37 |
| Employed, n (%) | 224 (89.9) |
| Sleep duration, h | 7.78 ± 0.72 |
| Morningness-eveningness score2 | 52.81 ± 10.75 |
Values are mean ± SD or n (%).
Morningness-eveningness score was based on responses to the Morningness-Eveningness Questionnaire. The scores can range from 16 to 86, with lower scores indicating evening preference and higher scores indicating morning preference. Scores ≤41 indicate “evening types”; scores ≥59 indicate “morning types”; and scores of 42–58 indicate “intermediate types”.
FIGURE 2.
Distributions of first, last, and main eating occasions and midpoint of intake, respectively, on workdays (A–D) and free days (E–H) as determined by recall and food records in 249 participants. The timings for the first and last eating occasions and midpoint of intake were averaged from multiple days of food records on workdays and free days separately, whereas the timing of the main eating occasion was designated as the median time. For food records, the main eating occasion was based on the largest percentage calories of the day. For both recall and food records, the midpoint of intake was calculated as the midway clock time between first and last eating occasions.
TABLE 2.
Comparison of times for first, last, and main eating occasions and midpoint of intake in 249 participants1
| Parameter | Recall time | Food records time | Time difference | Wilcoxon P value | Kendall's W | Kendall's P value |
|---|---|---|---|---|---|---|
| Workdays | ||||||
| First eating occasion | 08:00 (1 h 30 min) | 08:12 (1 h 42 min) | –0:02 (1 h 30 min) | 0.24 | 0.45 | <0.001 |
| Last eating occasion | 21:00 (2 h) | 20:12 (1 h 24 min) | 0:42 (1 h 18 min) | <0.001 | 0.39 | <0.001 |
| Main eating occasion | 17:30 (6 h 30 min) | 17:12 (5 h 12 min) | –0:12 (4 h 30 min) | 0.09 | 0.15 | <0.001 |
| Midpoint of intake | 14:30 (1 h 12 min) | 14:12 (1 h 18 min) | 0:24 (1 h) | <0.001 | 0.43 | <0.001 |
| Free days | ||||||
| First eating occasion | 10:00 (1 h 30 min) | 9:00 (2 h 18 min) | 0:24 (2 h 12 min) | <0.001 | 0.25 | <0.001 |
| Last eating occasion | 22:00 (2 h) | 20:12 (1 h 36 min) | 1:30 (2 h 12 min) | <0.001 | 0.21 | <0.001 |
| Main eating occasion | 18:00 (5 h) | 15:48 (4 h 48 min) | 1:00 (5 h 48 min) | 0.001 | 0.05 | 0.21 |
| Midpoint of intake | 15:30 (5 h) | 14:36 (1 h 36 min) | 0:54 (1 h 36 min) | <0.001 | 0.29 | <0.001 |
Recall and food record times are medians (IQR). Kendall's W reflects the Kendall's rank correlation. The timings for the first and last eating occasions and midpoint of intake were averaged from multiple days of food records on workdays and free days separately, whereas the timing of the main eating occasion was designated as the median time. For food records, the main eating occasion was based on the largest percentage calories of the day. For both recall and food records, the midpoint of intake was calculated as the midway clock time between first and last eating occasions.
FIGURE 3.
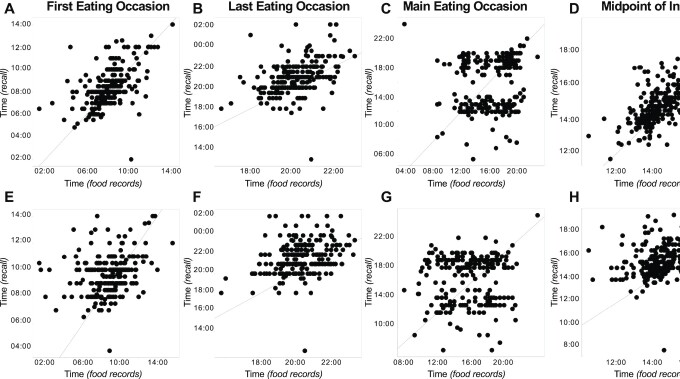
Comparison of the timing of the first, last, and main eating occasions and midpoint of intake, respectively, on workdays (A–D) and free days (E–H) as determined by recall and food records (average time) in 249 participants. A diagonal 45-degree line indicates perfect agreement. The timings for the first and last eating occasions and midpoint of intake were averaged from multiple days of food records on workdays and free days separately, whereas the timing of the main eating occasion was designated as the median time. For food records, the main eating occasion was based on the largest percentage calories of the day. For both recall and food records, the midpoint of intake was calculated as the midway clock time between first and last eating occasions.
Times based on recall and food records were significantly different for all eating occasions, except the first and main eating occasions on workdays (Figures 2–4, Table 2). Generally, times based on recall were later than those estimated from food records (Table 2). In addition, differences in times between the 2 approaches were smaller on workdays compared with free days for all eating occasions, and smaller for first compared to last eating occasion.
FIGURE 4.
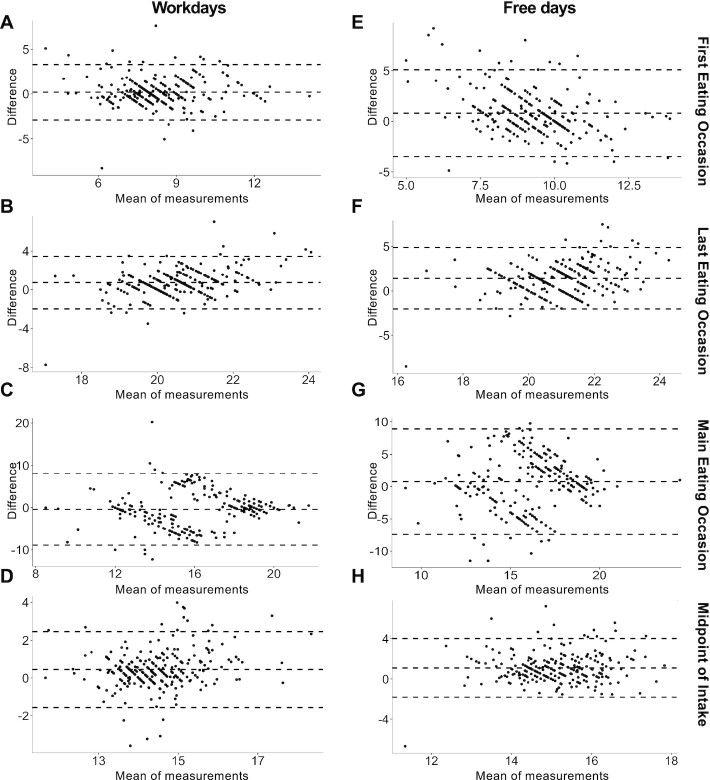
Bland–Altman plots (A–H) of included food timing parameters. The mean difference (middle horizontal line) represents the estimated concordance, and the SD of the differences (upper and lower horizontal lines) measures the random fluctuations around the mean. The timings for the first and last eating occasions and midpoint of intake were averaged from multiple days of food records on workdays and free days separately, whereas the timing of the main eating occasion was designated as the median time. For food records, the main eating occasion was based on the largest percentage calories of the day. For both recall and food records, the midpoint of intake was calculated as the midway clock time between first and last eating occasions.
Table 2 shows the agreement between eating occasion times obtained by recall and food records as evaluated by the Kendall's W coefficient of concordance, which expresses the relatedness between different sets of rankings. All eating occasions showed significant values of agreement, except for the main eating occasion on free days. Significant agreements were generally modest and ranged from 0.15 (main eating occasion on workdays) to 0.45 (first eating occasion on workdays).
Concordance did not differ based on age, gender, BMI, employment status, and morningness-eveningness score (Table 3). However, a significant interaction was evident with sleep duration for the main eating occasion on free days. In analysis stratified by the population sleep duration median, concordance for the main eating occasion on free days was higher in long sleepers (W = 0.09, P value = 0.15) than in short sleepers (W = 0.006, P value = 0.92).
TABLE 3.
Influence of potential moderators on estimated agreement between times estimated from recall and daily food records. Values are Wald test interaction P values1
| Parameter | Age | Gender | BMI | Employment | Morningness-eveningness score | Sleep duration |
|---|---|---|---|---|---|---|
| Workdays | ||||||
| First eating occasion | 0.02 | 0.13 | 0.64 | 0.56 | 0.87 | 0.88 |
| Last eating occasion | 0.14 | 0.02 | 0.79 | 0.52 | 0.12 | 0.88 |
| Main eating occasion | 0.30 | 0.75 | 0.42 | 0.16 | 0.52 | 0.04 |
| Midpoint of intake | 0.61 | 0.20 | 0.11 | 0.51 | 0.68 | 0.10 |
| Free days | ||||||
| First eating occasion | 0.18 | 0.25 | 0.79 | 0.19 | 0.27 | 0.12 |
| Last eating occasion | 0.42 | 0.35 | 0.34 | 0.16 | 0.44 | 0.17 |
| Main eating occasion | 0.01 | 0.40 | 0.31 | 0.04 | 0.01 | 0.002 |
| Midpoint of intake | 0.94 | 0.38 | 0.48 | 0.03 | 0.01 | 0.02 |
Wald tests were used to assess the potential role of factors on the calculated concordance. The Wald test interaction term was considered significant at the Bonferroni P value cutoff (P < 0.0063) accounting for the total number of factors tested.
Discussion
In a cohort of generally healthy, free-living adults, we found modest agreement between food times for the first and last eating occasion of the day and midpoint of intake derived from simple, recall-based survey questions and food times estimated from multiple daily paper-based food records. Concordance was higher for the first eating occasion of the day, compared with the last, and for the timing of eating occasions on workdays, compared with free days. Generally, recalled times were later than times calculated from daily food records. Both methods showed similar trends in delayed times of eating occasions on free days compared with workdays. Concordance for the main eating occasion was the lowest as a result of day-to-day variability in the timing of the largest percentage calories eating occasion estimated from food records.
There currently are no simple, scalable dietary assessment tools that characterize food timing in large epidemiological cohorts. Questions on the timing of habitual behaviors, including bed and wake times for sleep assessment, have been validated against objective methods and are commonly used in research (19, 20). Based on these questions, we derived recall-based questions to derive 4 food timing parameters (the timing of first, last, and main eating occasions and midpoint of intake—the midway clock time between first and last eating occasions) and tested their agreement with multiple days of prospectively collected food records. We found modest agreement between the 2 methods. The concordance for food timing is comparable to correlations previously observed for individual nutrients estimated from FFQs and multiple days of food records in adults (median correlations of individual nutrients = 0.373) (21, 22). Among the food timing parameters, concordance was highest for the timing of the first eating occasion of the day and the midpoint of intake. Concordance for the last eating occasion of the day was lower and might reflect differences in the ingestion duration of the last eating occasion. For example, whereas the recall question asks about the stop time of the last eating occasion, the time indicated on daily food records asks about the start time of that last eating occasion.
Concordance was the lowest for the main eating occasion. Based on recall, the timing for main eating occasion showed a bimodal distribution, suggesting that both lunch and dinner are considered the predominant meals of the day in this US cohort, consistent with NHANES data (23). When based on largest percentage calories estimated from food records, the main eating occasion for some participants appeared to often vary between lunch and dinner. This variability in the timing led to averages that greatly differed from those times based on recall. However, when based on the median time of the largest percentage calories meal of the day, a bimodal distribution was observed, suggesting that although the main eating occasion based on largest percentage calories often varied between lunch and dinner, for some participants, often 1 of these 2 eating occasions constituted the primary meal. For participants with only a single day of food record, or with equal days of having lunch and dinner as the largest percentage calories, this artifact persisted. The lack of explicit definition of the term “main meal” as the meal with the largest percentage calories could have further contributed to the overall low concordance for this parameter. Alternative definitions to a “main meal” can include those with the largest portion size or the longest duration to consume (11). Thus, future survey questions should be explicit in their definition of what constitutes a main meal. In addition, considering the variability in the timing of the largest percentage calories meal, future assessment of timing of both lunch and dinner, instead of a single main meal, might be necessary.
We observed that the level of concordance differed for the main eating occasion on free days by sleep duration. In subgroup analyses, we found that the concordance was lower in participants with shorter sleep duration. Studies indicate that adults with shorter sleep duration tend to have fewer main meals and more snacks, and it is possible that having fewer meals contributes to more variable times for the main eating occasion (7). Sleep duration did not affect the agreements for other eating occasions. In addition, concordance did not appear to vary by age, gender, BMI, employment status, and morningness-eveningness scores.
When comparing times from workdays and free days, we found general shifts in the timing of eating occasions and differences in overall concordance. First and last eating occasions and midpoint of intake were generally later on free days. The delay on free days compared with workdays in the first and last eating occasions was on average 76 and 43 min based on recall, respectively, and 40 and 3 min based on food records, respectively. Previous investigations have reported later eating times on weekends, including a study that used a smartphone application to estimate food timing that observed a ∼1 h delay in breakfast on weekends compared with weekdays (24). Whereas participants recalled later times for their main eating occasion, data from food records indicated earlier times for the largest percentage calories eating occasion on free days. An analysis of 11,646 adults from the NHANES also suggested earlier times of the largest percentage calories meal of the day on weekends compared with weekdays based on a 24-h recall (25). Overall, these findings add to the growing literature on differences in eating habits on workdays and free days (25). Continued distinction of these day types provides opportunities to examine the health effects of nutritional jetlag, which are shifts in dietary timing based on day of the week (6). In addition, overall concordance was poorer for free day measures. It is possible that the structured environment of workdays, such as fixed work schedules and more consistent bed and wake times, could have contributed to more accurate recalled times. Future evaluation of the location of eating occasions might further elucidate differences in the concordance between workdays and free days.
The timing of which eating occasion is most relevant for chrononutrition research remains unclear (6). Studies have indicated that the first and last daily eating occasions are physiologically pertinent because those most likely coincide with the biological night (26). Others have focused on the midpoint of intake (3). However, the timing of other eating occasions could still be relevant, such as the timing of lunch (27). Furthermore, for populations deviating from a 3 meals per day eating pattern to a more grazing eating pattern, the timing of intermeal snacks could be important (23). Our assessment of the times of first and last daily eating occasions allows the derivation of other chrononutrition-relevant metrics, including midpoint of intake and nocturnal fasting duration, thus enabling research on intermittent fasting (3, 11, ). Prioritization of the most pertinent times will facilitate the development of refined assessment tools with the least participant burden.
Strengths of this study include the large sample size, the collection of multiple days of paper-based food records reviewed by a trained dietitian, and the use of actigraphy. There are also some important limitations to consider. Like other commonly used dietary assessment tools, such as 24-h dietary recalls and FFQs, recall questions rely extensively on memory and therefore are prone to random error. And like other time-based survey questions, responses are susceptible to reporting and recall biases, imprecision due to time rounding (e.g., 07:00 instead of 07:04), and other response errors resulting from military time misreporting (28). In addition, we did not collect information on the timing of the end of each eating occasion in the food records. Thus validation studies against objective devices, such as wearable cameras, that provide passive and more accurate data on dietary intake and can reliably capture start and end times of eating occasions, are needed (29, 30). The questions asked in the survey might only capture current, short-term behavior, and multiple administrations of these questions are necessary to examine the long-term stability and reproducibility of the responses over time and across seasons (31). About 13% of participants had only 1 d of data for each of workdays and free days, and therefore it was not possible to account for day-to-day variation for all participants. Our cohort of generally healthy adults in an urban setting limits the generalizability of findings to other populations, including patients with eating disorders (32). Lastly, the study only enrolled participants of East and South Asian and European ancestries because of the higher prevalence of the MTNR1B genetic variant (the primary exposure of interest). Future efforts in racially and ethnically diverse populations are necessary to allow generalizability of findings.
Optimal methods for ascertaining the timing of dietary intake remain to be determined. The validity of existing questionnaires, such as the Meal Pattern Questionnaire (10), and the affordability and scalability of time-stamped picture-based smartphone applications (24), are still unknown. Here, we provide simple, self-administered survey questions that can be widely disseminated across various surveys offering a new tool to advance future food timing research. Single administration of these questions can characterize the overall timing of the first and last eating occasions within a population for chrononutrition research purposes. In populations where the largest percentage calories meal of the day often vary between 2 meals, for example, lunch and dinner, assessment of both meal times instead of a single main meal might be necessary. Because these recall-based survey questions tend to be later than the actual timing of eating occasions, individual-level responses should be evaluated cautiously, and alternative approaches, such as real-time data collection using wearable cameras or smartphone applications, could be of better use at the clinical level.
ACKNOWLEDGEMENTS
We thank the participants and research coordinators of the SHIFT Study for their contribution to this work.
The authors’ responsibilities were as follows—SCG, M Guirette, M Garaulet, FAJLS, RS, and HSD: designed the study; AC, CT, BEG, and HSD: participated in acquisition and analysis of data; SCG, M Guirette, AC, CT, CV, M Garaulet, FAJLS, RS, and HSD: participated in interpretation of data; SCG, M Guirette, and HSD: wrote the manuscript; and all authors: read and approved the final manuscript. HSD is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Notes
The study was funded by the NIH NIDDK grant R01DK105072. M Garaulet is supported by the Spanish Government of Investigation, Development, and Innovation (SAF2017-84135-R) including FEDER co-funding, Séneca Foundation (20795/PI/18), and NIDDK R01DK105072. HSD and RS are supported by NIH R01DK107859. RS and FAJLS are supported by NIH R01DK102696 and R01DK105072. CV is supported by R01DK105072. FAJLS is supported by R01DK099512, R01HL118601, and R01HL140574. RS is supported by Massachusetts General Hospital Research Scholars. HSD is supported by NHLBI K99HL153795. The study was also supported by NIH grant 1UL1TR002541-01. The funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author disclosures: The authors report no conflicts of interest.
SCG and M Guirette contributed equally to this work and FAJLS and RS contributed equally to this work.
Abbreviations used: MTNR1B, Melatonin receptor 1B; RD, Registered Dietitian; SHIFT, Shift Work, Heredity, Insulin, and Food Timing.
Contributor Information
Siena C Gioia, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA.
Mélanie Guirette, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA; Friedman School of Nutrition Science and Policy at Tufts, Tufts University, Boston, MA, USA.
Angela Chen, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA.
Chandler Tucker, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA.
Brianna E Gray, Translational and Clinical Research Centers, Massachusetts General Hospital, Boston, MA, USA.
Céline Vetter, Department of Integrative Physiology, University of Colorado, Boulder, CO, USA; Broad Institute, Cambridge, MA, USA.
Marta Garaulet, Department of Physiology, University of Murcia, Murcia, Spain; IMIB-Arrixaca, Murcia, Spain; Division of Sleep and Circadian Disorders, Brigham and Women's Hospital, Boston, MA, USA.
Frank A J L Scheer, Broad Institute, Cambridge, MA, USA; Division of Sleep and Circadian Disorders, Brigham and Women's Hospital, Boston, MA, USA; Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
Richa Saxena, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA; Broad Institute, Cambridge, MA, USA; Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA; Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Hassan S Dashti, Email: hassan.dashti@mgh.harvard.edu, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA; Broad Institute, Cambridge, MA, USA; Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA; Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval from the Mass General Brigham Human Research Office/Institutional Review Boards (contact located at https://www.massgeneralbrigham.org/researcher-support-and-resources/resources-collaborators-and-sponsors) for researchers who meet the criteria for access to confidential data.
References
- 1. Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: a novel association. Physiol Behav. 2014;134:44–50. [DOI] [PubMed] [Google Scholar]
- 2. Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet. 2014;27:255–62. [DOI] [PubMed] [Google Scholar]
- 3. Dashti HS, Gómez-Abellán P, Qian J, Esteban A, Morales E, Scheer FAJL, Garaulet M. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am J Clin Nutr. 2021;113(1):154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Longo V, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. 2019;39(1):291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dashti HS, Scheer FAJL, Saxena R, Garaulet M. Timing of food intake: identifying contributing factors to design effective interventions. Adv Nutr. 2019;10(4):606–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kant AK, Graubard BI. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005–2010. Am J Clin Nutr. 2014;100(3):938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garaulet M, Vera B, Bonnet-Rubio G, Gómez-Abellán P, Lee Y-C, Ordovás JM. Lunch eating predicts weight-loss effectiveness in carriers of the common allele at PERILIPIN1: the ONTIME (Obesity, Nutrigenetics, Timing, Mediterranean) study. Am J Clin Nutr. 2016;104(4):1160–6. [DOI] [PubMed] [Google Scholar]
- 9. McHill AW, Phillips AJK, Czeisler CA, Keating L, Yee K, Barger LK, Garaulet M, Scheer FAJL, Klerman EB. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr. 2017;106:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertéus Forslund H, Lindroos AK, Sjöström L, Lissner L. Meal patterns and obesity in Swedish women—a simple instrument describing usual meal types, frequency and temporal distribution. Eur J Clin Nutr. 2002;56(8):740–7. [DOI] [PubMed] [Google Scholar]
- 11. Phoi YY, Bonham MP, Rogers M, Dorrian J, Coates AM. Content validation of a chrononutrition questionnaire for the general and shift work populations: a delphi study. Nutrients. 2021;13:4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel SR, Weng J, Rueschman M, Dudley KA, Loredo JS, Mossavar-Rahmani Y, Ramirez M, Ramos AR, Reid K, Seiger ANet al. . Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep. 2015;38:1497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lohman T, Roache A, Martorell R. Anthropometric standardization reference manual. Med Sci Sports Exercise. 1992;24(8):952. [Google Scholar]
- 14. Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 15. Kant AK, Graubard BI. Within-person comparison of eating behaviors, time of eating, and dietary intake on days with and without breakfast: NHANES 2005–2010. Am J Clin Nutr. 2015;102(3):661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sudo N, Sekiyama M, Ohtsuka R, Maharjan M. Gender differences in “luxury food intake” owing to temporal distribution of eating occasions among adults of Hindu communities in lowland Nepal. Asia Pac J Clin Nutr. 2009;18:441–6. [PubMed] [Google Scholar]
- 17. Muñoz JSG, Cañavate R, Hernández CM, Cara-Salmerón V, Morante JJH. The association among chronotype, timing of food intake and food preferences depends on body mass status. Eur J Clin Nutr. 2017;71(6):736–42. [DOI] [PubMed] [Google Scholar]
- 18. Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19(7):1374–81. [DOI] [PubMed] [Google Scholar]
- 19. Jackson CL, Patel SR, Jackson WB, Lutsey PL, Redline S. Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: multi-ethnic study of atherosclerosis. Sleep. 2018;41:zsy057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dashti HS, Redline S, Saxena R. Polygenic risk score identifies associations between sleep duration and diseases determined from an electronic medical record biobank. Sleep. 2018;42(3):zsy247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fallaize R, Forster H, Macready AL, Walsh MC, Mathers JC, Brennan L, Gibney ER, Gibney MJ, Lovegrove JA. Online dietary intake estimation: reproducibility and validity of the Food4me food frequency questionnaire against a 4-day weighed food record. J Med Internet Res. 2014;16(8):e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cui Q, Xia Y, Wu Q, Chang Q, Niu K, Zhao Z. Validity of the food frequency questionnaire for adults in nutritional epidemiological studies: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. [Internet]2021; doi:10.1080/10408398.2021.1966737. [DOI] [PubMed] [Google Scholar]
- 23. Kant AK, Graubard BI. 40-year trends in meal and snack eating behaviors of American adults. J Acad Nutr Diet. 2015;115(1):50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruopeng A. Weekend-weekday differences in diet among U.S. adults, 2003–2012. Ann Epidemiol. 2016;26:57–65. [DOI] [PubMed] [Google Scholar]
- 26. Garaulet M, Qian J, Florez JC, Arendt J, Saxena R, Scheer FAJL. Melatonin effects on glucose metabolism: time to unlock the controversy. Trends Endocrinol Metab. 2020;31(3):192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee Y-C, Ordovás JM, Scheer FAJL. Timing of food intake predicts weight loss effectiveness. Int J Obes. 2013;37(4):604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dashti HS, Cade BE, Stutaite G, Saxena R, Redline S, Karlson EW. Sleep health, diseases, and pain syndromes: findings from an electronic health record biobank. Sleep. 2021;44(3):zsaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gemming L, Rush E, Maddison R, Doherty A, Gant N, Utter J, Ni Mhurchu C. Wearable cameras can reduce dietary under-reporting: doubly labelled water validation of a camera-assisted 24 h recall. Br J Nutr. 2015;113(2):284–91. [DOI] [PubMed] [Google Scholar]
- 30. Gemming L, Ni Mhurchu C. Dietary under-reporting: what foods and which meals are typically under-reported?. Eur J Clin Nutr. 2016;70(5):640–1. [DOI] [PubMed] [Google Scholar]
- 31. McHill AW, Hilditch CJ, Fischer D, Czeisler CA, Garaulet M, Scheer FAJL, Klerman EB. Stability of the timing of food intake at daily and monthly timescales in young adults. Sci Rep. 2020;10(1):20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allison KC, Lundgren JD, O'Reardon JP, Martino NS, Sarwer DB, Wadden TA, Crosby RD, Engel SG, Stunkard AJ. The night eating questionnaire (NEQ): psychometric properties of a measure of severity of the night eating syndrome. Eat Behav. 2008;9(1):62–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval from the Mass General Brigham Human Research Office/Institutional Review Boards (contact located at https://www.massgeneralbrigham.org/researcher-support-and-resources/resources-collaborators-and-sponsors) for researchers who meet the criteria for access to confidential data.