SUMMARY
The vagus nerve is an indispensable body-brain connection that controls vital aspects of autonomic physiology like breathing, heart rate, blood pressure, and gut motility, reflexes like cough and swallowing, and survival behaviors like feeding, drinking, and sickness responses. Classical physiological studies and recent molecular/genetic approaches have revealed a tremendous diversity of vagal sensory neuron types that innervate different internal organs, with many cell types remaining poorly understood. Here, we review the state of knowledge related to vagal sensory neurons that innervate the respiratory, cardiovascular, and digestive systems. We focus on cell types and their response properties, physiological/behavioral roles, engaged neural circuits, and when possible, sensory receptors. We are only beginning to understand signal transduction mechanisms used by vagal sensory neurons and upstream sentinel cells, and future studies are needed to advance the field of interoception to the level of mechanistic understanding achieved previously for our external senses.
Graphical Abstract
Prescott and Liberles review sensory biology of the vagus nerve, a major arm of the interoceptive nervous system and vital body-brain relay. Vagal sensory neuron functions, anatomical projections, responses, and sensory receptors are discussed across physiological systems.
INTRODUCTION
Sensory systems provide the brain with information about both the external world around us and the internal world within us. Our senses of smell, taste, touch, vision, and hearing enable us to perceive and react to our environment, and classical studies have revealed how external senses operate- from receptors that detect light, sound, temperature, chemicals and force to neural circuits that process stimulus features and evoke perceptions and motor responses. In parallel, the interoceptive nervous system provides sensory information to the brain from almost every organ system in the body, and uses that information to ensure that key physiological needs are met, to preserve organ integrity, and to buffer against potentially harmful deviations from homeostasis on a moment-by-moment basis.
The vagus nerve provides one major sensory pathway from the body to the brain that is both essential for life and relevant for disease. Vagal neurons make up a tiny proportion of the nervous system, yet they oversee a wide array of vital functions. In the early 20th century, key concepts in neuroscience and physiology were derived from studies of the vagus nerve and its frequent partner-in-crime, the glossopharyngeal nerve, including the identification of the first neurotransmitter (acetylcholine, then called ‘vagusstoff’) (Loewi, 1921), Lord Adrian’s all-or-none principle of nerve firing (Adrian, 1926), and major autonomic reflexes like the baroreceptor reflex (Hering, 1923) and the hypoxic ventilatory response (Heymans et al., 1931). After this early golden era, the vagus nerve remained less well studied using modern approaches, at least compared to external sensory systems, and molecular mechanisms of interoception remained largely mysterious. Interest in the vagus nerve has recently resurged, and here we attempt a comprehensive review of vagal sensory biology that synthesizes insights derived from both classical and modern studies.
ANATOMY OF THE VAGUS NERVE
The vagus nerve is the 10th and longest cranial nerve, named for its wandering trajectory throughout the abdomen and thorax (Figure 1). The vagus nerve consists of co-fasciculating sensory and motor neurons, with most neurons (~80%) being sensory (Foley and DuBois, 1937). The cell bodies of vagal sensory neurons are clustered bilaterally beneath each jugular foramen in superior and inferior ganglia respectively termed jugular and nodose ganglia. Vagal motor neurons, which are cholinergic fibers that comprise the principal arm of the parasympathetic nervous system, are instead housed directly in brainstem regions termed the dorsal motor nucleus of the vagus nerve (DMV) and nucleus ambiguus. The jugular and nodose ganglia have different developmental origins and anatomical projections, and in humans are distinct anatomical structures; however, in mice, they are typically fused together before birth along with the petrosal ganglion of the glossopharyngeal nerve, and sometimes additionally with the superior cervical ganglion, forming a large superganglion (Figure 2) (Altschuler et al., 1989; Berthoud and Neuhuber, 2000; Bookout and Gautron, 2021). The number of vagal sensory neurons roughly scales across species with body size, as there are ~2,300 vagal sensory neurons on each side of the body in mice, ~20–30,000 in larger animals like cats, ferrets, and small primates, and as many as 100,000 in humans (Asala and Bower, 1986; Fox et al., 1995; Hoffman and Schnitzlein, 1961). Within vagal ganglia, sensory neuron soma coexist with satellite glial cells, Schwann cells, endothelial cells and resident immune cells (Waise et al., 2018).
Figure 1. Anatomy of the sensory vagus nerve.
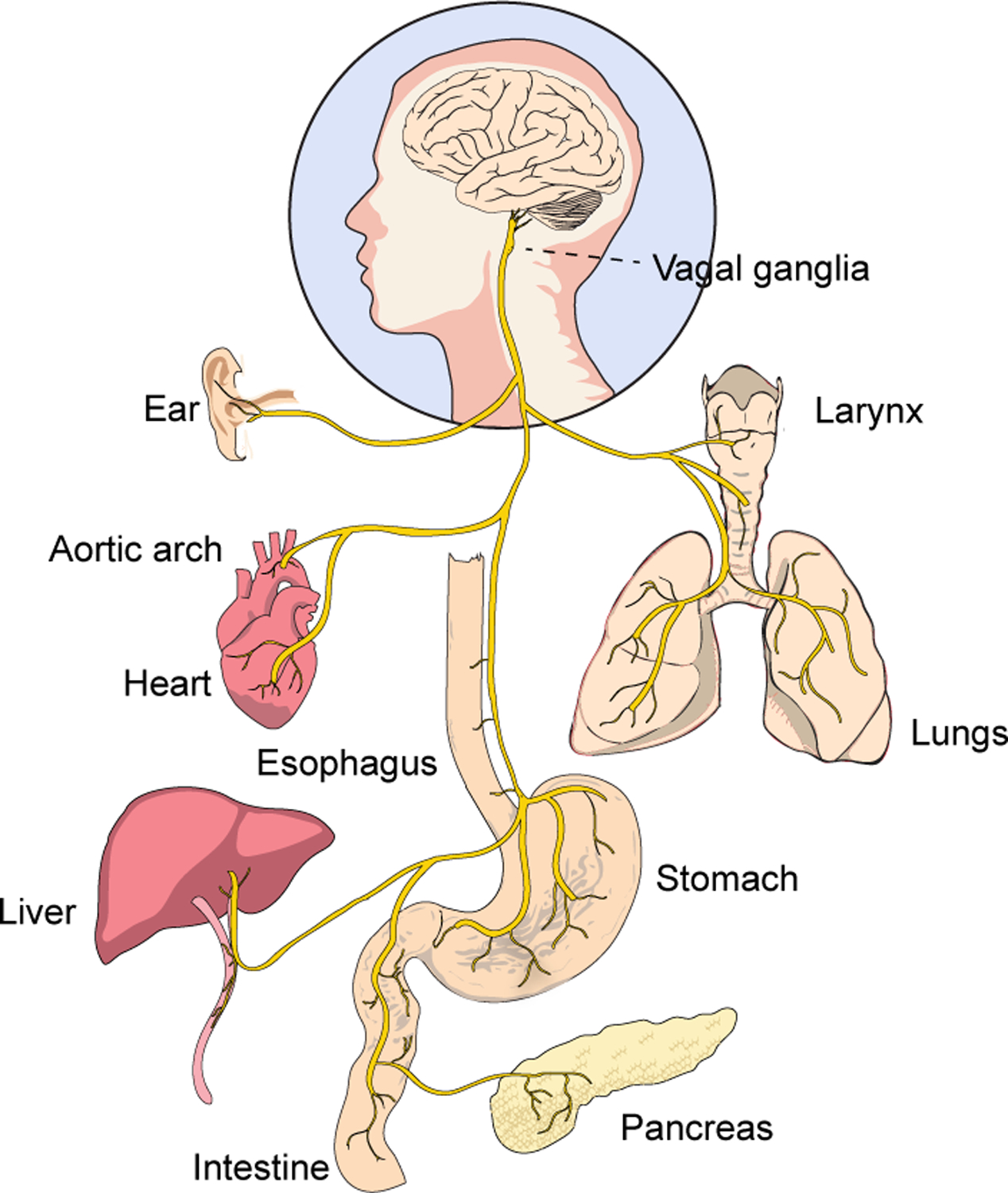
The cell bodies of vagal sensory neurons reside in left and right ganglia at the base of the neck, with each sensory neuron extruding a single, long, and pseudounipolar axon that targets both the periphery and brain. Collectively, vagal sensory neurons target numerous organs within the body.
Figure 2. Comparative anatomy of cranial ganglia and branches in mice and human.
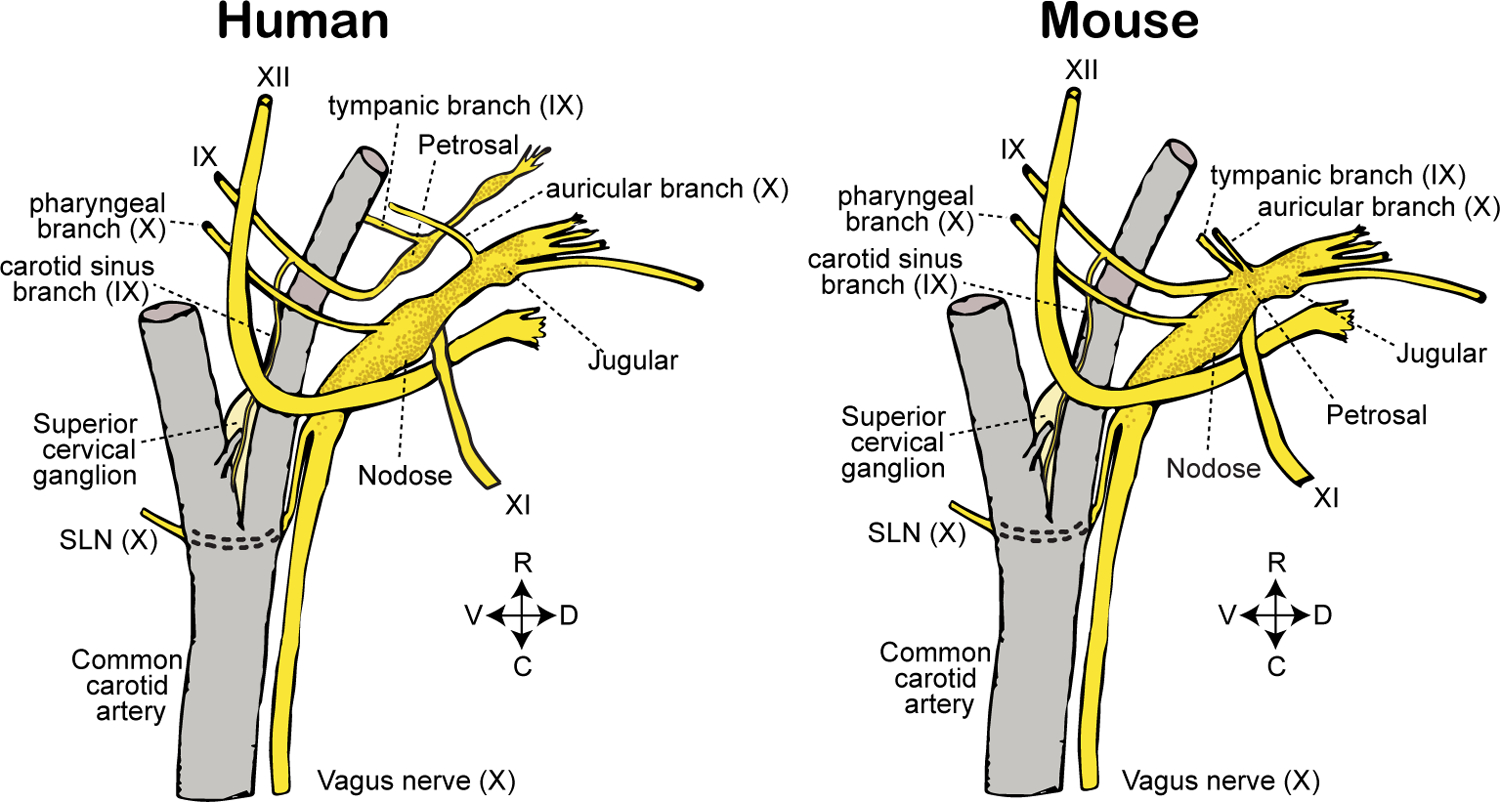
Sensory ganglia and branches of cranial nerves IX (glossopharyngeal) and X (vagus) are depicted in relation to nearby anatomical structures. Adult mice display a fused nodose-petrosal-jugular superganglion (left); in some animals, the superior cervical ganglion (here depicted separately) is additionally fused into the superganglion. In humans (right), vagal and petrosal ganglia are distinct anatomical structures. SLN: superior laryngeal nerve. R: rostral. C: caudal. D: dorsal. V: ventral.
Each vagal sensory neuron extends a single pseudounipolar axon that projects bidirectionally towards the body and brain. Peripherally, the vagus nerve passes into the carotid sheath, where it runs alongside the carotid artery sending off major branches to the neck, chest, and abdomen. Vagal neurons innervate many major organs and tissues, including the heart, lung, stomach, intestine, arteries, larynx, trachea, esophagus, liver, pancreas, thyroid, and ear. Within each target organ, vagal sensory neurons can display a diversity of terminals with different morphologies, sizes, molecular features, interacting cell partners, and anatomical distributions, with each terminal type presumably detecting particular sensory cues. Centrally, vagal axons pass through the skull at the jugular foramina and target the brainstem. Nodose neurons principally target caudal aspects of the nucleus of the solitary tract (NTS) with some additionally innervating the area postrema, while jugular neurons instead target the paratrigeminal nucleus (Pa5), a subnucleus within the spinal trigeminal tract (Berthoud and Neuhuber, 2000; McGovern et al., 2015). Vagal axons release glutamate, which activates NTS neurons, and genetic tools targeting the glutamate transporter VGLUT2 (Slc17a6-ires-Cre mice) label >99% of vagal sensory neurons, but not vagal motor neurons (Chang et al., 2015; Raab and Neuhuber, 2007). Some topography of vagal axon projections to the NTS has been reported, based either on tissue of origin and/or gene expression-defined cell type (Altschuler et al., 1989; Bai et al., 2019; Chang et al., 2015; Han et al., 2018b; Katz and Karten, 1983; Williams et al., 2016), with the extent of viscerotopy in NTS neuron responses debated (Paton, 1999).
Gene expression profiling revealed striking cell diversity in vagal ganglia that includes dozens of intermingled sensory neuron types (Bai et al., 2019; Chang et al., 2015; Kupari et al., 2019; Mazzone et al., 2019; Prescott et al., 2020; Williams et al., 2016). Vagal sensory neuron diversity is presumably needed as the vagus nerve innervates vast terrain, projecting to many internal organs within the body, and detects an assortment of sensory cues. About 10–160 neurons per ganglion comprise each transcriptome-defined cell type in mice (Prescott et al., 2020), suggesting that a small number of vagal sensory neurons may be sufficient to detect a particular sensory stimulus. For comparison, somatosensory neurons of the dorsal root ganglia (DRG) are far more numerous, and DRG neurons provide high spatial acuity that may be lacking in at least some vagal responses (Abraira and Ginty, 2013). The number of sensations mediated by the vagus nerve has not been quantified, but is presumably large based on the underlying cellular diversity, with some neurons likely mediating internal organ sensory functions that have yet to be determined. We note that single-cell RNA sequencing in the DMV also revealed several vagal motor neuron types (Tao et al., 2021), suggesting that the parasympathetic nervous system is not a singular all-or-nothing switch, but instead may enable more nuanced physiological control with each neuron type perhaps orchestrating a discrete multi-organ motor program or controlling a highly specific facet of autonomic physiology. Cell atlases of the vagus nerve have provided a roadmap for genetic approaches to chart and link the anatomical projections, response properties, and functions of transcriptome-defined vagal neuron types across physiological systems.
VAGAL SENSORY NEURONS IN THE RESPIRATORY SYSTEM
Breathing is essential for life, and under precise and dynamic neuronal control to help ensure appropriate tissue oxygenation and carbon dioxide removal. Inputs to breathing-control circuits arise from a variety of central and peripheral pathways, with the vagus nerve providing the principal relay for sensory information from the lungs, trachea, and larynx (Coleridge and Coleridge, 2011; Mazzone and Undem, 2016). Vagal inputs regulate various respiratory parameters, including breathing rate, tidal volume, and airway tone. Furthermore, as one of the body’s largest barrier tissues, the airway epithelial surface is under constant neuronal surveillance for early signs of obstruction or damage that might compromise gas exchange. Vagal sensory neurons detect threats to airway integrity and engage neuronal circuits that initiate a suite of protective reflexes such as cough, swallow, vocal cord adduction, and laryngeal closure (Prescott et al., 2020). Additionally, vagal sensory neurons may detect pathogens to evoke immune responses, airway hyperreactivity, sickness behaviors, and pain (sore throat), provide proprioceptive feedback from the vocal folds for speech control, and evoke the sensation of air hunger (Baral et al., 2018; Mazzone and Undem, 2016).
Vagal neurons reach the airways via several major axon branches, including (from proximal to distal airways) the pharyngeal branch, superior laryngeal nerve (SLN), recurrent laryngeal nerve (RLN) and pulmonary branches. The pharyngeal branch carries mostly motor fibers to pharyngeal muscles, while the internal branch of the SLN provides the major sensory innervation of the caudal pharynx and larynx, efferent innervation to the only tensor muscle aiding phonation, the cricothyroid muscle, and some innervation of rostral trachea (DuBois and Foley, 1936). The RLN carries both afferent and efferent fibers to the vocal cords and tracheal sites, while the lungs receive innervation through pulmonary branches stemming off the vagal trunk near the main bronchi (Mazzone and Undem, 2016; Weijs et al., 2015). The SLN, RLN, and pulmonary branches contain a mixture of both jugular and nodose neurons in varying ratios, with most SLN-derived terminals in trachea, larynx, and pharynx being jugular-derived (Mazzone and Undem, 2016). In addition, the airways from larynx to lungs also receive afferent innervation by dorsal root ganglion (DRG) neurons (levels C1–T6) whose sensory functions remain poorly understood (Springall et al., 1987).
Within each vagal nerve branch, sensory neurons display a multitude of epithelial and subepithelial terminal types from nasopharynx to distal lung areas like the alveoli and lung pleura. Axon terminal structures have been variably described as panicle-like endings (sometimes called “cough” or “irritant” receptors), candelabra terminals, ivy-like (or hederiform) endings, corpuscular endings, free nerve endings, smooth muscle-associated receptors, and other epithelial and subepithelial terminals. Some vagal sensory neurons project near various epithelial cell types which might serve as first-order sensory cells, including laryngeal taste cells, tuft cells (also called brush cells or solitary chemosensory cells), and neuroendocrine cells including lung neuroepithelial bodies (NEBs), as well as immune cells (Baral et al., 2018; Brouns et al., 2003; Chang et al., 2015; Chu et al., 2020; Krasteva et al., 2011; Prescott et al., 2020; Tizzano et al., 2010). The functions and response properties of various airway terminals have been difficult to determine, as overlapping terminal fields have prevented assignments based on proximity to sensory hotspots. Furthermore, unified terminology and consistency across studies have been lacking; it is not clear whether various studies referred to the same terminal types with different descriptors, or grouped together terminals with similar morphologies but different sensory functions. Adding complexity, airway innervation patterns reportedly vary across species, and given the issues above, additional studies are needed to understand the extent of divergence or conservation of airway terminal types across evolution.
Classical electrophysiological studies distinguished three major types of airway-innervating neurons based on the sensory stimuli they detect, as well as their conduction velocities and adaptation rates: (1) slowly adapting stretch receptors (SARs), (2) rapidly adapting stretch receptors (RARs), and (3) chemoreceptors (Coleridge and Coleridge, 2011). RARs and SARs are fast-conducting A fibers, while at least some chemoreceptors are slow-conducting C-fibers. C-fibers represent about 67% of vagal sensory neurons (Agostoni et al., 1957; Chang et al., 2015), and most vagal C fibers express the capsaicin receptor TRPV1, enabling pharmacological and genetic approaches for targeted ablation (Baral et al., 2018; Trankner et al., 2014). RARs and SARs are mechanosensitive afferents that reportedly differ in their anatomical projections, sensitivity to inflation and deflation, and activity across the respiratory cycle (Adrian, 1933; Kaufman et al., 1982; Knowlton and Larrabee, 1946; Schelegle and Green, 2001). However, RARs and SARs were typically identified by punctate mechanical stimulation which can potentially activate other mechanosensory neurons (like “irritant” receptors or high threshold C-fiber nociceptors), and may or may not correspond well to inflation-sensitive afferents in vivo. Furthermore, it has been debated whether RARs and SARs are truly different classes of mechanoreceptors (Yu, 2000), as distinct response dynamics could be due to differences in terminal location or orientation relative to distended tissue rather than intrinsic neuronal properties.
Recent expression profiling strategies and genetic approaches revealed greater cellular diversity than was previously recognized for airway sensory neurons (Chang et al., 2015; Mazzone et al., 2019; Prescott et al., 2020). Transcriptome analysis has been performed selectively on vagal sensory neurons labeled retrogradely by airway dye injection, and/or has been coupled with genetic tools to map airway terminals of gene expression-defined neurons. Vagal axons can be visualized by injecting sensory ganglia of various Cre knock-in mice with adeno-associated viruses (AAVs) encoding Cre-dependent fluorescent or enzymatic reporters (Chang et al., 2015). Efforts to achieve a complete and unified taxonomy of airway-to-brain pathways is very much a work in progress, with genetic approaches so far revealing at least ten terminal types that can be distinguished by different Cre lines, including epithelial chandelier terminals in the larynx, epithelial free endings in alveola, bronchioles and upper airways, epithelial candalabra terminals in lung apposing neuroepithelial bodies, epithelial corpuscles apposing laryngeal taste cells, epithelial flower spray endings, and subepithelial terminals around various airway muscles, vessels, and glands (Chang et al., 2015; Prescott et al., 2020; Su et al., 2021). Each of these neurons presumably detects particular sensory cues, which in most cases remain unknown (along with underlying sensory transduction mechanisms), and furthermore, additional terminal types are likely to be uncovered. Next, we discuss airway-to-brain sensory pathways that are best understood.
Airway stretch sensation.
In 1868, Ewald Hering and Josef Breuer observed that increases in airway volume evoked a breathing pause or apnea characterized by inhibition of inspiration and prolongation of expiration; this mechanosensory reflex is now termed the “Hering-Breuer inspiratory reflex” (Hering and Breuer, 1868). An opposing deflation reflex whereby lung deflation increases respiratory rates and shortens expiration was also proposed. Sensation of airway volume contributes to normal breathing patterns, may protect the airways from hyperinflation-induced injury, and regulates heart rate and vascular tone, perhaps contributing to respiratory sinus arrhythmia in which pulmonary circulation increases during high airway volumes to optimize gas exchange (Coleridge and Coleridge, 2011; Schelegle and Green, 2001; Taha et al., 1995). Physiological states such as intense exercise, as well as hypercapnic conditions, can also dampen sensitivity of the Hering-Breuer inspiratory reflex (Bouverot et al., 1970; Bradley et al., 1976; Phillipson et al., 1971).
Vagal afferents are essential for the Hering-Breuer inspiratory reflex. Electrical or optogenetic stimulation of all vagal sensory neurons, but not motor fibers, evokes a similar characteristic apnea (Chang et al., 2015; Widdicombe, 2006). Moreover, surgical transection of the vagus nerve bilaterally (vagotomy) eliminates the Hering-Breuer inspiratory reflex (Coleridge and Coleridge, 2011). Complete vagotomy also causes severe respiratory depression and eventual death (Widdicombe, 2006), highlighting the importance of vagal information for breathing sustenance. Centrally, airway stretch activates second-order neurons in lateral NTS regions near the vagal nerve tract called ‘pump cells’ and ‘inspiratory-β cells’ which then communicate with respiratory control centers in the brainstem (Kubin et al., 2006). Brainstem outputs are relayed to phrenic motor neurons which control contraction of the diaphragm, the primary muscle for respiration.
Airway inflation stimulates vagal afferents, with similar responses observed to distension by oxygen or nitrogen, consistent with a mechanosensory response (Adrian, 1933; Williams et al., 2016). Single-unit recordings and in vivo calcium imaging showed that airway stretch activates rare sensory neurons (~90 neurons per mouse nodose ganglion), with most responses adapting slowly (SARs) (Adrian, 1933; Williams et al., 2016). Airway mechanoreceptors are activated by each inspiration during tidal breathing, indicating that threshold responses occur at physiological levels of airway distension (Adrian, 1933; Williams et al., 2016). The location and architecture of force-sensing terminals underlying the Hering-Breuer inspiratory reflex remain unclear. At least some airway SARs are so-called “smooth muscle-associated receptors”, with terminals that appear as knobby, plate-like, and mitochondria-rich nerve terminals arranged in series with airway smooth muscle fibers (Larsell, 1921; von et al., 1974). Other stretch-sensitive afferents with different response thresholds and airway distributions in trachea, extrapulmonary bronchi, and intrapulmonary airways have also been reported (Schelegle and Green, 2001). It is unclear whether force-sensing neurons in these locations display distinct terminal morphologies, when mechanosensory neurons in different airway regions are engaged across the respiratory cycle, and which terminal morphology is required for mechanosensation underlying the Hering-Breuer inspiratory reflex or potentially other airway reflexes.
Recent studies revealed an essential role for the mechanosensitive ion channel PIEZO2 in airway stretch sensation and the Hering-Breuer inspiratory reflex (Nonomura et al., 2017). PIEZOs are ion channels intrinsically gated by force in the absence of auxiliary factors (Syeda et al., 2016); PIEZO2 is required in somatosensory neurons for light touch sensation and proprioception (Ranade et al., 2014; Woo et al., 2015), and is additionally expressed in a subset of vagal sensory neurons (Chang et al., 2015). Optogenetic activation of vagal PIEZO2 neurons caused an acute apnea reminiscent of the Hering-Breuer inspiratory reflex (Nonomura et al., 2017), with optogenetics-assisted conduction velocity measurements revealing that PIEZO2 neurons are mostly A fibers. In vivo calcium imaging in vagal ganglia demonstrated that airway stretch responses occurred primarily (>90%) in PIEZO2 neurons (Prescott et al., 2020). Global knockout of PIEZO2 is lethal at birth due to neonatal respiratory distress, but mice with cell-specific deletion of Piezo2 in Phox2b-expressing cells (which include nodose but not jugular sensory neurons) survive to adulthood, permitting analysis of PIEZO2 function in at least some vagal sensory neurons (Nonomura et al., 2017). Mice with Phox2b-guided Piezo2 knockout have increased tidal volumes during passive breathing, have deficient vagal responses to airway stretch, and lack the Hering-Breuer inspiratory reflex. Conditional deletion of Piezo2 in the adult also decreased airway stretch responses, consistent with a direct role for PIEZO2 in stretch sensation rather than only in neuronal development. Human patients that lack normal PIEZO2 function display severe clinical problems that include breathing difficulties during infancy (Szczot et al., 2021). PIEZO2 is expressed in several airway-innervating sensory neurons (Prescott et al., 2020), as well as in pulmonary neuroepithelial bodies (Nonomura et al., 2017), and additional studies are needed to determine the structure of PIEZO2 terminals relevant for airway stretch sensation and the site of PIEZO2 action in those terminals. Together, these findings indicate a key role for PIEZO2 as an airway stretch sensor that is critical for maintaining respiratory homeostasis.
Cough and other airway protective reflexes.
One of the most important roles of the vagus nerve is to defend the airways from injury. Humans inhale hundreds of thousands of microbes each day, as well as pollen, dust, smoke and pollution from the ambient air (Barberan et al., 2015). Furthermore, ingested food and liquid must transit over the airways en route to the gastrointestinal tract, while expelled stomach contents can travel in reverse. The larynx, as the proximal entry point to the trachea and lungs, is densely innervated by vagal afferents that are on alert to detect airway insults (Bradley, 2000). Laryngeal application of water, acid, high salt, or mechanical force, as well as electrical stimulation of the superior laryngeal nerve, elicits characteristic reflexes that either guard or clear the airways (Bolser, 1991; Ludlow, 2015; Prescott et al., 2020). Airway guarding reflexes include apnea and physical occlusion reflexes like epiglottis descent, glottic closure, and bronchoconstriction, while expulsion reflexes include cough, gag, sneeze, expiratory reflexes, mucus secretion, and protective swallowing, with multiple reflexes often engaged together or in close succession. Challenges to airway integrity can also arise deeper in the airways, and proinflammatory cytokines, irritants, and pathogen derivatives (like Sulfolipid-1 from Mycobacterium tuberculosis) also elicit cough from more distal airway sites in awake animals (Choudry et al., 1989; Karlsson and Fuller, 1999; Narula et al., 2014; Ruhl et al., 2020). Defects in airway threat detection are common in the elderly and cause severe clinical problems like difficulty swallowing, choking, and aspiration with associated risk of lethal respiratory tract infection (Ludlow, 2015; Santoso et al., 2019). Early models hypothesized that a single broadly-tuned sensory population may sense diverse airway threats and engage different protective reflexes depending on discharge frequency (Storey, 1968). However, at least two types of cough afferents with different airway distributions have been proposed including capsaicin- and anesthetic-sensitive C fibers and anesthetic-insensitive A fibers (Canning, 2011), and more recent studies have indicated an even more substantial division of labor among primary afferent neurons (Mazzone et al., 2019; Prescott et al., 2020). We are only beginning to understand the diversity of vagal sensory neurons that guard the upper airways and how they detect threats such as irritants, pathogens, force, and aspirated food and water.
Several airway irritants directly engage cell surface receptors located on vagal sensory neurons. TRPV1 and TRPA1 are ion channels expressed on various vagal afferent types, and agonists for these receptors stimulate subsets of vagal sensory neurons (Bessac and Jordt, 2008). TRPV1 detects capsaicin found in hot peppers (Caterina et al., 1997), while TRPA1 is activated by various noxious electrophiles that covalently modify receptor cysteine residues such as allyl isothiocyanate found in mustard, wasabi, and horseradish, acrolein in smoke and smog, gingerol found in ginger, and cinnamaldehyde, as well as noncovalent agonists like oxidants including hydrogen peroxide and hypochlorite (Bandell et al., 2004; Bautista et al., 2006; Bessac et al., 2008). TRPA1 and TRPV1 agonists cause cough, respiratory depression, and airway inflammation, with some neuronal and physiological responses lost following TRP knockout or blockade (Andre et al., 2008; Bessac and Jordt, 2008). Ablation of TRPV1 vagal sensory neurons also impacts allergic airway hyperreactivity and immune responses to Influenza respiratory infections (Baral et al., 2018; Trankner et al., 2014), while ablation of TRPV1- and neuromedin B-expressing trigeminal neurons impairs the sneezing reflex to airway threats in the nasal mucosa (Li et al., 2021). TRPV1 is expressed in many vagal sensory neurons (~60%) and TRPA1 in 25%, most of which co-express TRPV1; at least two TRPA1 neuron types are distinguished by expression of NPY1R and NPY2R and form free endings in different airway regions (Chang et al., 2015; Prescott et al., 2020). Optogenetic stimulation of NPY1R neurons evokes airway defense responses, while optogenetic stimulation of NPY2R neurons causes rapid and shallow breathing (Chang et al., 2015; Prescott et al., 2020). Additional studies are needed to delineate which TRPV1- and TRPA1-expressing cells are devoted to airway defense, and which might regulate other aspects of respiratory physiology.
Vagal sensory neurons express other receptors for threat surveillance in the airways, as an agonist for the itch receptor MRGPRC11 stimulates vagal sensory neurons and causes bronchoconstriction and airway hyperresponsiveness (Han et al., 2018a). Pathogen-derived chemicals can directly stimulate dissociated DRG nociceptors (Chiu et al., 2013), and it seems possible that they may similarly stimulate vagal sensory neurons in the airways. Other airway threats may instead act on upstream sentinel cells rather than directly on neurons. For example, vagal sensory neurons express receptors for numerous immune cell-derived molecules including bradykinin, histamine, prostaglandins, and interferon gamma (Wang et al., 2017). Epithelial tuft cells in the airways, as well as the gut, are proposed to detect pathogen-derived metabolites from bacteria and (in the gut) parasitic helminth through bitter taste receptors (Luo et al., 2019; Tizzano et al., 2010). Tuft cells, as well as neuroepithelial bodies, are reported to release proinflammatory molecules like interleukin-25 and eicosanoids as well as neurotransmitters which may activate vagal afferents (Schneider et al., 2018). Neural circuits engaged by pathogen-detection pathways can evoke general sickness behaviors like fever, lethargy, and decreased appetite, as well as responses tailored to the infection site like nausea, vomiting, diarrhea, cough, mucus secretion, and bronchoconstriction. In addition to transmitting sensory information to the brain, vagal sensory neurons also release peptides in the periphery, like CGRP and substance P, to engage the immune system (Chu et al., 2020). Vagal motor neurons separately control systemic inflammatory responses and disease outcome through the ‘cholinergic anti-inflammatory pathway’, in which neurons signal to splenic T cells (indirectly through postganglionic neurons) to suppress macrophage activation, providing a natural brake on innate immunity that protects against sepsis and lethal organ damage (Pavlov and Tracey, 2017). There appear to be a diversity of pathways for pathogen and irritant detection in the airways, as well as motor programs to control different physiological responses, and additional investigation is required to understand when these various pathways are engaged across airway challenges.
Cough can also be evoked by force, for example by inhalation of particulate matter or mechanical probing throughout the tracheobronchial tree, especially in the larynx and carina (Widdicombe, 1954). Punctate mechanical stimulation of the upper airways evokes exquisitely sensitive and rapidly adapting electrophysiological responses in vagal afferents (Canning et al., 2004). Neurons responsive to punctate stimulation (punctate mechanoreceptors) are distinct from other airway mechanoreceptors as they do not respond to tissue stretch or changes in luminal pressure and have a slower conduction velocity (~5 m/sec, Aδ-range). Several chemical irritants also activate punctate mechanoreceptors (Mazzone and Undem, 2016), raising the possibility that these fibers are broadly tuned polymodal ‘irritant receptors’, although it is possible that agents used in these studies elicit responses with secondary mechanical effects on the airways like mucus secretion, edema, or bronchospasm. A particular tracheal terminal type has been postulated to be a cough receptor (Canning et al., 2006), and it will be interesting to determine the response properties of those neurons, and whether they function as low-threshold punctate mechanoreceptors and/or polymodal irritant receptors. Coughs elicited by punctate stimulation are not inhibited by epithelium removal, the TRPV1 antagonist capsazepine, or the PIEZO antagonist gadolinium (Mazzone and Undem, 2016). Additional studies are needed to determine the identity of force-sensing molecules in punctate mechanoreceptors of the airways.
Airway defense reflexes are also evoked during meal consumption, for example when food or water accidentally goes down the wrong pipe. Laryngeal water is among the most potent inducers of airway defense reflexes, provoking cough, swallow, and apnea (Canning et al., 2004; Shingai et al., 1989). Responses to laryngeal water vary with age, species, and depth of anesthesia; in newborns, laryngeal water causes an unrelenting apnea rather than cough (Boggs and Bartlett, 1982), suggesting developmental plasticity in responding neural circuits. It has been debated whether water directly activates vagal afferents or upstream sentinel cells, and adding complexity, multiple vagal sensory pathways for water have been proposed, perhaps with different receptor mechanisms and relevance for thirst, fluid homeostasis, and airway defense. One water sensing pathway in the upper airways involves a small cluster of ~100 vagal neurons (NP19 neurons), which are marked in P2ry1-ires-Cre mice (Prescott et al., 2020). Optogenetic stimulation of vagal P2RY1 neurons in the SLN evokes a coordinated motor program of airway defense including expiratory reflexes, vocal fold adduction, apnea, and fictive swallow, while ablation of vagal P2RY1 neurons eliminates protective responses to laryngeal water (Prescott et al., 2020). Vagal P2RY1 neurons are fast-conducting A fibers (Chang et al., 2015) that form corpuscular terminals in the larynx that directly appose laryngeal taste cells (Prescott et al., 2020). Knockout of ionotropic ATP receptor genes (P2×2/P2×3), which eliminates gustatory taste cell transmission (Finger et al., 2005), likewise eliminates airway protective responses to laryngeal water (Prescott et al., 2020). Responses to laryngeal acid were partially reduced by vagal P2RY1 neuron ablation or P2×2/P2×3 knockout, but responses to high salt or mechanical force in the larynx persisted, demonstrating P2RY1 neuron-independent sensory pathways for these cues. Together, these findings indicate that laryngeal taste cells or other epithelial cells sense water and release ATP to communicate with a small cohort of vagal P2RY1 neurons. Vagal P2RY1 neurons then relay that information centrally, engaging neural circuits that coordinate a multi-pronged motor program to protect and clear the airways. P2×2/P2×3 knockout eliminates laryngeal water-evoked swallow, but not water-evoked nerve responses (Ohkuri et al., 2012), consistent with a role for other water-sensing pathways through the vagus nerve, and additional studies are needed to uncover sensory transduction mechanisms engaged by laryngeal water. Interestingly, P2X antagonists are clinically approved for chronic cough (Abdulqawi et al., 2015); knockout of ionotropic ATP receptors impacts some laryngeal responses but not others, so understanding the diversity of airway protective pathways and how they operate may be essential for designing antitussive therapies effective for all patients.
VAGAL SENSORY NEURONS IN THE CARDIOVASCULAR SYSTEM
All tissues must be supplied with a steady source of oxygenated blood. Peripheral sensory neurons innervate the heart and vasculature, providing moment-by-moment feedback to stabilize cardiac and respiratory outputs. Classical studies have revealed mechanosensory and chemosensory neurons in both the heart and great arteries which respectively monitor the flow and chemical composition of blood, including i) arterial mechanoreceptors underlying the baroreceptor reflex, ii) cardiac mechanoreceptors underlying the Bainbridge reflex, iii) arterial chemoreceptors underlying the hypoxic ventilatory response, and iv) cardiac chemoreceptors underlying the Bezold-Jarisch reflex. Each of these reflexes is discussed below, and adding complexity, some cardiovascular afferents may have polymodal responses, variable response thresholds or chemical sensitivities, or even functions that have not been identified. Studying individual reflex arcs in isolation has been tremendously challenging due to the closed-loop structure of the cardiovascular system, with genetic approaches potentially providing a strategy moving forward for selective neuron manipulation.
Sensory neurons are strategically placed throughout the circulatory system (Figure 3). The carotid artery and aortic arch are two hotspots for neuronal innervation within the vasculature, monitoring blood immediately after it exits the left ventricle en route to the brain and body respectively. Neurons at these locations are exposed to freshly oxygenated blood pumped away from the heart, and thus report on the starting points of both blood pressure and oxygenation gradients in the circulatory system. Vagal afferents provide the dominant sensory innervation of the heart, aorta, and other vessels below the diaphragm, with i) cardiac branches stemming off the main vagal trunk reaching the heart and pulmonary vessels, ii) a branch termed the aortic depressor nerve accessing the aortic arch and the right subclavian artery near its bifurcation with the common carotid artery, and iii) the hepatic branch providing innervation of blood vessels in the liver. In addition, glossopharyngeal afferents innervate the carotid body and sinus, key sites of arterial chemosensation and baroreception in the neck, via a branch called the carotid sinus nerve. Here, we discuss both glossopharyngeal and vagal sensory neurons because their cell bodies coalesce into the same ganglia in several species (including mice) and they cooperate to mediate several cardiovascular reflexes.
Figure 3. Innervation of the great arteries.
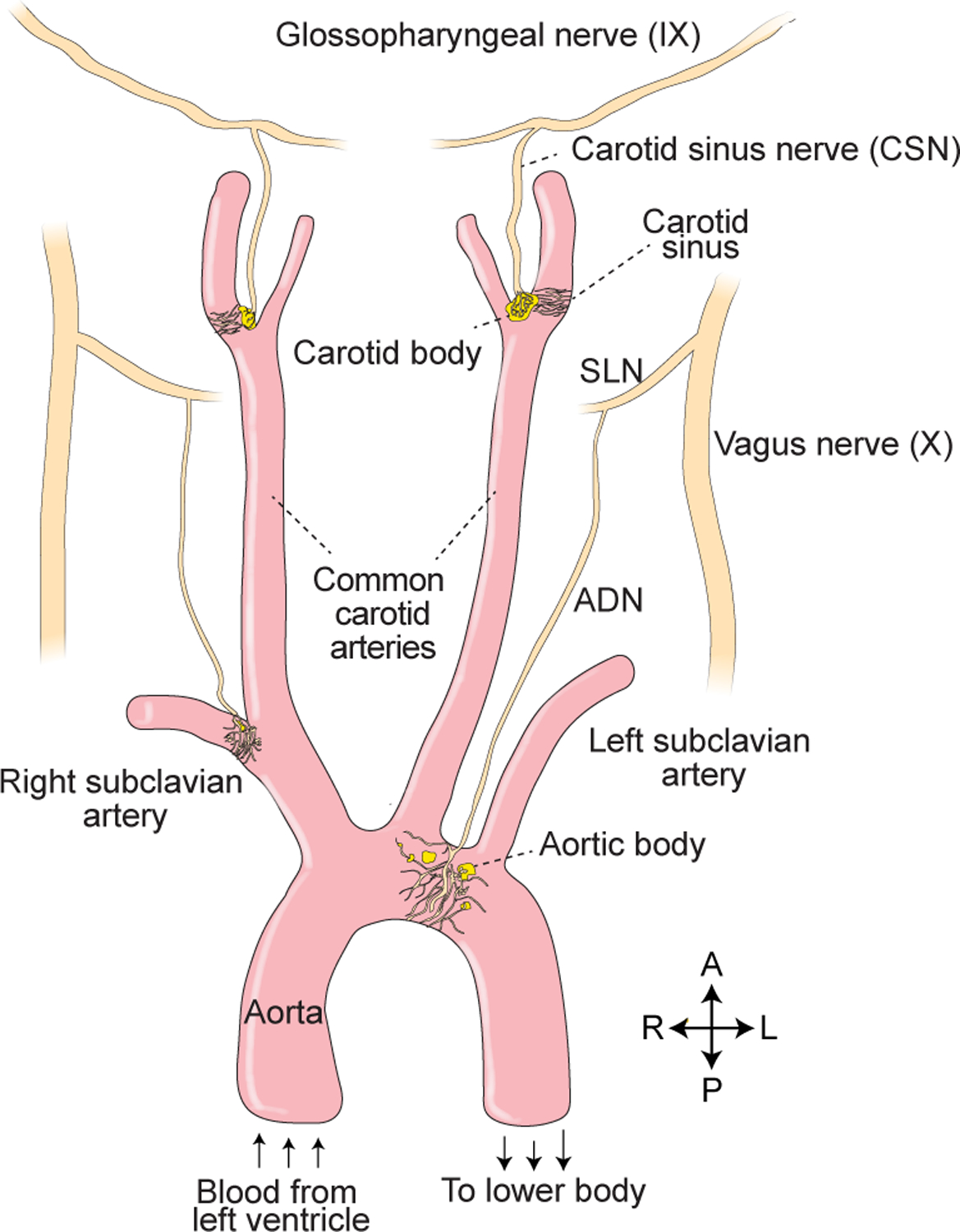
Sensory neurons in the great arteries mediate classical reflexes including the baroreceptor reflex and hypoxic ventilatory response. Vagal afferents reach the aortic arch and subclavian artery via the aortic depressor nerve (ADN), which branches from the superior laryngeal nerve (SLN). Glossopharyngeal afferents reach the carotid body and sinus via the carotid sinus nerve (CSN), which branches from the main glossopharyngeal trunk.
Arterial chemosensors for respiratory gases.
The principal function of the cardiovascular system is to deliver key substrates for aerobic cellular respiration like oxygen and glucose to peripheral tissues while removing byproducts like carbon dioxide. Decreased oxygen (hypoxia) or increased carbon dioxide (hypercapnia) in circulation are symptoms of inefficient gas exchange, and without appropriate compensation by ventilatory reflexes, benign conditions like exercise, high altitude, or even mild pulmonary disease would lead to dangerous fluctuations in blood gas levels. Detection of systemic hypoxia and hypercapnia evokes neuronal reflexes that correct blood gas levels through increased respiration. Corneille Heymans identified the critical site of acute hypoxia sensation as the carotid body (Heymans et al., 1931), a sensory structure located at the carotid artery bifurcation that is anatomically privileged to monitor blood composition. Related structures termed aortic bodies are present in the aortic arch, but are smaller and less well studied. While the carotid body is the primary mediator of systemic hypoxia responses, other central and peripheral chemoreceptors play major roles in hypercapnic responses (Guyenet and Bayliss, 2015).
The carotid body is a small organ comprised of two principal intrinsic cell types: glomus cells, which are first-order chemosensory cells, and glia-like sustentacular cells which can function as supporting cells and/or stem cells (Pardal et al., 2007). Dense fenestrated capillaries provide glomus cells with privileged access to sample blood contents, efficiently perfusing them with circulating respiratory gases. Glomus cells are polymodal sensors that in ex vivo preparations directly respond to low oxygen, high carbon dioxide, low pH, glucose, lactate, and other stimuli (Ortega-Saenz and Lopez-Barneo, 2020). Chronic hypoxia, as from living at high altitude, increases the number of glomus cells, with sustentacular cells capable of acting as stem cells that convert into glomus cells (Pardal et al., 2007). The hypoxia-responsive transcription factor HIF2α controls the gene expression program which enables glomus cells to respond to acute hypoxia, and could serve as a master developmental regulator of glomus cells and/or a mediator of cellular adaptations to chronic hypoxia (Macias et al., 2018; Moreno-Dominguez et al., 2020).
Glomus cells are neural crest-derived, and display several characteristics of neurons as they are electrically excitable, loaded with dense core vesicles, and communicate with afferent nerves through synapses. Like taste cells, glomus cells release ATP extracellularly when activated (Buttigieg and Nurse, 2004), and ATP stimulates ionotropic receptors expressed on afferent nerves (Rong et al., 2003). Mice lacking ionotropic ATP receptors lose carotid sinus nerve activity and ventilatory responses following hypoxic challenge (Rong et al., 2003). Glomus cells also release a suite of other hormones and neuromodulators that act on afferent nerves, sustentacular cells, and other glomus cells, including acetylcholine, dopamine, and gaseous transmitters (Iturriaga et al., 2021), and additionally form electrical synapses with neighboring glomus cells. Carotid body glomus cells communicate with glossopharyngeal afferents, while aortic bodies are instead innervated by vagal afferents, with individual afferent neurons contacting ~10–60 glomus cells (Torrealba and Alcayaga, 1986). The central terminals of carotid body-innervating afferents are located in the NTS, which in turn relays chemoreceptor inputs to brainstem motor circuits that control breathing (Finley and Katz, 1992).
Mechanisms underlying acute glomus cell responses to hypoxia are incompletely understood. As in pancreatic beta cells, a key step in glomus cell activation involves closure of potassium channels, cell depolarization, and engagement of voltage-gated calcium channels (Ortega-Saenz and Lopez-Barneo, 2020). Interestingly though, glomus cells and pancreatic beta cells display an opposite relationship between electron transport chain activity and cell depolarization. In beta cells, glucose drives insulin release through increased metabolic flux, ATP production, and ATP-mediated closure of potassium channels. In contrast, in glomus cells, inhibition of the electron transport chain, for example through cyanide, carbon monoxide, or rotenone, promotes cell firing (Ortega-Saenz et al., 2003), with a prevailing model for hypoxia sensation involving a mitochondria-derived signal that impacts potassium channel opening. Glomus cells express specialized electron transport chain subunits and several molecular sensors for low oxygen have been proposed (Iturriaga et al., 2021). For example, knockout of the Complex I subunit NDUFS2 or the Complex IV subunit COX4I2 eliminates acute glomus cell hypoxia responses (Fernandez-Aguera et al., 2015; Moreno-Dominguez et al., 2020). Complex IV (cytochrome c oxidase) directly transfers electrons to oxygen, and it is possible that carotid body Complex IV displays a decreased oxygen binding capacity to match the physiologically relevant dynamic range of oxygen present in arteries. Hypoxia-induced stalling of flux through the carotid body electron transport chain could lead to shunting of electrons into reactive oxygen species or NADPH, decreased ATP production, and/or increased/decreased synthesis of other second messengers. It is possible that any or several of these factors engage potassium channels, and adding complexity and perhaps redundancy, roles for other oxygen-dependent enzymes, signals (like CO and H2S), and channels have been proposed (Prabhakar et al., 2018). Additional studies are needed to pinpoint how hypoxia specifically impacts mitochondrial function in glomus cells, what hypoxia-induced or hypoxia-inhibited signals are transmitted from mitochondria to membrane, and which potassium channel subunits are gated to cause cell depolarization.
Glomus cell responses to hypercapnia involve a mechanism that is at least partially distinct. Disrupting the mitochondrial electron transport chain through NDUFS2 knockout eliminates glomus cell responses to hypoxia but not hypercapnia (Fernandez-Aguera et al., 2015). A key role has instead been proposed for carbonic anhydrase, which converts carbon dioxide into carbonic acid (Iturriaga et al., 1991), with intracellular acidification potentially impacting the activity of various ion channels and leading to cell depolarization. Interestingly, a subset of olfactory sensory neurons senses carbon dioxide through a somewhat related pathway involving carbonic anhydrase, guanylyl cyclase D, and a cGMP-gated ion channel (Hu et al., 2007), while sour taste cells sense carbonation through acidity changes catalyzed by extracellular carbonic anhydrase (Chandrashekar et al., 2009). Central chemoreceptors located in the retrotrapezoid nucleus play a major role in systemic hypercapnic responses and detect elevated carbon dioxide through dual pH-dependent mechanisms involving the G Protein-Coupled Receptor GPR4 and the potassium channel TASK-2 (Kumar et al., 2015). Additional studies are needed into how glomus cells detect and integrate changing levels of respiratory gases and other blood constituents to initiate firing and neuronal reflexes resulting in hyperventilation.
Neuronal sensation of blood pressure and the baroreceptor reflex.
Mean arterial blood pressure is typically maintained within a narrow physiological range to ensure adequate tissue perfusion. A few key findings made over several decades led to the concept of the baroreceptor reflex, a negative feedback loop in which mechanosensitive afferents detect increases (or decreases) in arterial blood pressure and, in response, engage neural circuits to decrease (or increase) both heart rate and arterial pressure. First, in the 1860s, the French physiologist Etienne-Jules Marey observed an inverse relationship between blood pressure and heart rate (now known as Marey’s Law) (Marey, 1863). In the 1920s, Heinrich Ewald Hering, the son of Ewald Hering who helped discover the Hering-Breuer inspiratory reflex, observed that electrical stimulation of afferents from the carotid sinus evoked reflexive bradycardia and hypotension (Hering, 1923). Subsequently, in 1933, Lord Adrian observed that a subpopulation of vagal sensory neurons fired with each heartbeat, and moreover, that firing magnitude correlated closely with blood pressure (Adrian, 1933).
Neuronal baroreceptors are mechanosensory neurons that act in real time, detecting momentary blood pressure fluctuations that occur during movement or postural changes, and work across a large dynamic range (Brown, 1980; Kirchheim, 1976; Landgren, 1952). The baroreceptor reflex can be experimentally observed through pharmacological agents like phenylephrine that induce vasoconstriction and elevate blood pressure, through non-invasive pressure manipulations like the Valsalva maneuver, the neck chamber technique, or carotid sinus massage, or through measurement of spontaneous blood pressure changes and correlated heart rate changes that occur naturally over time (Wehrwein and Joyner, 2013). Baroreceptor information is first transmitted centrally to the NTS, a key site for state-dependent modulation, and then to autonomic motor nuclei, decreasing sympathetic output and increasing parasympathetic output to lower heart rate and blood pressure (Guyenet, 2006; Kumada et al., 1990).
The nervous system monitors blood pressure at two major hotspots in the great arteries: the carotid sinuses and aortic arch. Carotid sinus and aortic baroreceptors are respectively part of glossopharyngeal and vagus nerve branches termed the carotid sinus nerve and aortic depressor nerve, and potentially provide differential reporting of blood flow to the brain and body. Arterial regions innervated by blood pressure-sensing neurons are highly elastic due to local smooth muscle thinning and changes in elastin and collagen fiber abundance (Kirchheim, 1976; Rees, 1968). With each heartbeat, the pulse of blood flow radially distends the elastic vessel wall. Baroreceptors are activated by blood vessel distension, fire in a pulsatile manner timed to the cardiac cycle, and convey information about both pulse magnitude and frequency (Brown, 1980; Kirchheim, 1976; Kumada et al., 1990).
One feature of baroreceptors with particular clinical importance is their plasticity. The sensitivity and/or set point of the baroreceptor reflex can change across physiological states (exercise, hypovolemia) (Dampney, 2017). Furthermore, raising or lowering arterial pressure, even for a few minutes, can acutely and reversibly reset baroreceptor thresholds (Kunze, 1985). Angiotensin II dampens and/or shifts baroreceptor responses, allowing the renin-angiotensin system to control blood pressure over a longer term while minimizing compensatory heart rate changes (Reid, 1992). Chronic and less plastic changes in baroreceptor sensitivity can also arise during aging and disease due to both adaptations in neuronal circuitry and structural changes to the arterial wall, like thickening during atherosclerosis, and are predictive of serious coronary disease (La Rovere et al., 2008).
Classical studies equated aortic depressor nerve stimulation with baroreceptor activation, but recent anatomical and functional studies revealed (at least) three major sensory neuron types in both the aortic arch and carotid sinus: end-net endings (baroreceptors), glomus cell contacts (chemoreceptors), and flower spray terminals (unknown function) (Cheng et al., 1997a; Min et al., 2019). As discussed above, chemoreceptors innervate carotid and aortic bodies, detect changes in circulating levels of respiratory gases, and induce hyperventilation to promote gas exchange. Flower spray terminals, named for the characteristic morphology of their complex punctate endings (Aumonier, 1972), are the most common vagal ending in the aortic arch and are enriched in a saddle-like pattern in the rostral aorta. Flower spray endings are marked in Mc4r-p2a-Cre mice, and optogenetic activation of all vagal MC4R neurons, or those in the aortic depressor nerve, caused a minor decrease in blood pressure and unlike the baroreceptors, did not impact heart rate (Min et al., 2019). Moreover, genetic ablation of vagal MC4R neurons effectively removed arterial flower spray endings, but the baroreceptor reflex remained intact (Min et al., 2019). Additional studies are needed to understand the sensory properties and functions of arterial flower spray endings, but they appear to be dispensable for the baroreceptor reflex.
Instead, aortic and carotid sinus baroreceptors are both marked in Piezo2-ires-Cre mice (Min et al., 2019). Optogenetic activation of aortic PIEZO2 neurons causes reflexive decreases in heart rate and blood pressure reminiscent of the baroreceptor reflex (Min et al., 2019; Zeng et al., 2018). Moreover, genetic ablation of vagal and glossopharyngeal PIEZO2 neurons eliminated the baroreceptor reflex, and the ability of the aortic depressor nerve to alter heart rate (Min et al., 2019). Anatomical tracing of PIEZO2 neurons in the great arteries revealed that they form characteristic linear branches termed end-net endings (Min et al., 2019). PIEZO2 neurons arrive at the peak of the aortic arch, and bifurcate into dorsal and ventral branches which circumnavigate the aorta, extending beyond the saddle region where flower spray terminals are concentrated. Dorsal and ventral branches converge near the arterial ligament at the aortic base in a claw-like structure, with end-net endings ramifying outward.
Mechanosensory ion channels that sense blood pressure and mediate the baroreceptor reflex have been a long-standing mystery since the reflex was first described about a century ago. Several candidate baroreceptors have been proposed, and while some of these candidates may contribute to baroreceptor function in important ways, residual force-evoked currents and/or physiological responses in knockout mice suggested a role for another primary sensory receptor (Drummond et al., 1998; Lau et al., 2016; Lu et al., 2009; Sun et al., 2009). The baroreceptor reflex was lost after ablation of vagal and glossopharyngeal PIEZO2 neurons (Min et al., 2019), yet surprisingly remained intact after cell-specific knockout of Piezo2 in Phox2b-expressing cells (Zeng et al., 2018), which includes the relevant baroreceptor sensory neurons of both nodose and petrosal ganglia. Interestingly, a dual requirement for both PIEZO1 and PIEZO2 in baroreception was observed, as targeted knockout of both Piezo ion channel genes in Phox2b-expressing cells, but not Piezo1 or Piezo2 individually, abolished phenylephrine-induced baroreflex responses and aortic depressor nerve activity (Zeng et al., 2018). Awake, behaving animals with cell-specific Piezo knockout had labile hypertension and increased blood pressure variability, as is similarly observed after baroreceptor denervation or failure (Zeng et al., 2018). Interestingly, Piezo1 is also required for sensing fluid flow in endothelial cells (Cahalan et al., 2015; Li et al., 2014), suggesting that PIEZOs mediate multiple physiological responses to forces within the cardiovascular system. In Phox2b-driven Piezo knockout mice, electrical stimulation of the aortic depressor nerve still evoked baroreceptor-associated heart rate and blood pressure changes, and the anatomy of baroreceptor neurons, at least at a gross morphological level, appeared similar (Min et al., 2019); these observations suggest that phenotypes of Piezo knockout mice are not due to changes in neural circuits downstream of baroreceptors or sensory neuron morphology, but instead are consistent with a direct role for PIEZOs in blood pressure sensation. Additional studies are needed to determine where PIEZO proteins are localized within end-net endings to sense arterial wall distension. Moreover, mechanisms by which PIEZO1 and PIEZO2 cooperate to mediate the baroreceptor reflex are unclear. Both PIEZOs act in neurons marked in Phox2b-Cre and Piezo2-ires-Cre mice, and a parsimonious interpretation is that both PIEZO1 and PIEZO2 act in the same sensory neurons where either is sufficient. An alternative model involves baroreceptor heterogeneity with some cells expressing PIEZO2 transiently or at low levels that are sufficient to drive reporter expression in Piezo2-ires-Cre mice, but instead relying primarily on PIEZO1 for force sensation. Together, these findings revealed that PIEZO1 and PIEZO2 are the long-sought mechanosensory channels critical for acute blood-pressure control and the baroreceptor reflex.
Cardiac sensory neurons: buffering a change of heart.
In 1915, Francis Bainbridge observed that increasing blood volume by injection of isotonic saline or blood into a jugular vein caused reflexive tachycardia (Bainbridge, 1915). This reflex (termed the Bainbridge reflex) is initiated by mechanosensory afferents of the vagus nerve that fire in response to atrial filling and/or cardiac muscle contraction, and report on the extent and rate of venous return, as well as heart rate. Increases in atrial pressure raise heart rate while decreases in atrial pressure are proposed to instead slow heart rate (termed the ‘reverse Bainbridge reflex’) (Crystal and Salem, 2012); changes in atrial pressure also change renal blood flow to influence diuresis (Henry and Pearce, 1956). These various effects ensure that circulating blood volume and venous return are coupled with cardiac output and stay within a tight physiological range.
The Bainbridge reflex has been notably difficult to study in isolation because the cardiovascular system is a closed loop, and manipulations that increase activity of atrial mechanoreceptors could also impact baroreceptor afferents, ventricular afferents, and circulating levels of hormones like angiotensin II and atrial natriuretic peptide. It is debated when the Bainbridge reflex is naturally engaged, and the extent that it contributes to heart rate changes associated with hypovolemia, hypervolemia, hemorrhage, anesthesia/surgery, and application of lower body negative pressure (Crystal and Salem, 2012). Adding complexity, the Bainbridge reflex may vary in strength across species, and appears diminished in rodents and primates compared to other species, hindering investigation (Boettcher et al., 1982). The Bainbridge reflex and the baroreceptor reflex have opposing effects on heart rate; neural circuits that measure differential activity between baroreceptor afferents and atrial volume receptors could in principle accelerate or decelerate blood transfer from veins to arteries by influencing heart rate, helping to equilibrate pressure across the vasculature. By employing mechanosensory neurons at various strategic locations throughout the cardiovascular system, the nervous system can potentially interpret changing blood flow patterns for context-appropriate physiological responses that maintain cardiac output and effective circulating blood volume.
Mechanoreceptors are present in both the right and left atria, near the junctions with the vena cava and pulmonary veins respectively (Hainsworth, 1991). Many terminal structures have been described in the heart, including “end-net” terminals between the endocardium and myocardium, “flower-spray” terminals (also called complex unencapsulated endings) in the endocardium, Ruffini types, flat plates, intramuscular arrays in the myocardium, and contacts with intrinsic cardiac ganglia (Cheng et al., 1997b; Hainsworth, 1991; Miller and Kasahara, 1964). The sensory properties and physiological functions of neurons that form various heart terminal types are not clear, with unencapsulated endings speculated to be atrial mechanoreceptors based on their general proximity to mechanosensitive sites. Furthermore, different types of atrial mechanoreceptors (Type A and B) have been proposed based on their firing rate across the cardiac cycle, and their differential sensitivity to stretch and tension (Paintal, 1953), although it has been debated whether activity differences are due to intrinsic neuronal properties or terminal position within the heart (Arndt et al., 1974). Additional studies are needed to reveal force sensors present in atrial volume receptors underlying the Bainbridge reflex.
The functions of other vagal afferents in the heart are even more mysterious. In 1867, von Bezold and Hirt observed that intravenous injection of veratrum alkaloids induced a triad of bradycardia, hypotension, and vasodilation (von Bezold and Hirt, 1867), as well as an apnea that was later ascribed to non-cardiac sensory neurons. Jarisch and colleagues subsequently showed that cardiovascular responses to veratrum alkaloids required intact vagal cardiac branches (Jarisch and Richter, 1939); henceforth the reflex became known as the “Bezold-Jarisch reflex”. Similar depressor responses can be evoked using other chemicals that stimulate some C fibers like capsaicin, nicotine, and phenyl diguanide, so the reflex is attributed to unmyelinated vagal afferents which display highest density in the left ventricle (Hainsworth, 1991). These chemicals do not stimulate arterial baroreceptors or glomus cell-associated chemoreceptors at concentrations necessary to elicit depressor responses, so the Bezold-Jarisch reflex represents a distinct sensory pathway (Coleridge and Coleridge, 1977; Kaufman et al., 1980). However, known stimuli that activate cardiac C fibers are not normally abundant in blood, and it is unclear when or if these neurons are physiologically engaged or whether they act as silent nociceptors. Adding complexity, at least some cardiac C fibers are mechanosensory neurons activated by inflation and/or deflation (Gupta and Thames, 1983), and it is debated whether they are normally engaged by changing forces across the cardiac cycle. As with atrial mechanoreceptors, roles for cardiac C fibers in systemic responses to hypotension, hypovolemia, ischemia, and anesthesia have been postulated but debated. It remains unknown which transcriptome-defined neurons mediate the Bezold-Jarisch reflex, what their terminals look like, what they respond to physiologically, and how they function. Generally, additional studies are needed to examine the diversity of cardiac neurons of the vagus nerve, including the physiological response properties/sensory mechanisms of neurons mediating the Bezold-Jarisch reflex.
VAGAL SENSORY NEURONS AND THE GUT-BRAIN AXIS
Obesity and related metabolic disorders are associated with increased morbidity and represent major healthcare challenges of the 21st century. Feeding behavior in most animals occurs in bursts or meals, which are followed by periods of feeding quiescence. The gut-brain axis relays short-term information about the quantity and quality of recently consumed food and mediates the sensation of meal-induced fullness (Richards et al., 2021). Disrupting or artificially stimulating meal-derived signals of the gut-brain axis changes meal patterns and can durably alter energy homeostasis. As will be discussed below, glucagon-like peptide 1 (GLP1) is a gut-derived incretin released after nutrient ingestion that suppresses feeding behavior through neuronal circuits. Agonists of the GLP1 receptor (GLP1R) are clinically approved for obesity treatment, providing an illustrative example of how artificially stimulating components of the gut-brain axis may provide therapeutic benefit. Likewise, bariatric surgery to re-structure the gastrointestinal tract alters appetite, body weight, and metabolism (Arble et al., 2015), further highlighting the importance of gut-derived signals in long-term feeding control.
The gut-brain axis not only controls appetite but also coordinates physiological responses that promote digestion, absorption, and systemic changes in energy utilization. Neuronal pathways influence release of metabolic hormones, blood glucose levels, enzyme secretion, gallbladder contraction, gut motility, gastric acidification, and gastric emptying (Brookes et al., 2013; Gribble and Reimann, 2019; Travagli et al., 2006). Gut-innervating sensory neurons have been proposed to guide food preference and avoidance behavior, and to detect (directly or indirectly) non-food cues like water or high salt for control of thirst and fluid homeostasis, danger signals from pathogens, toxins, or food allergens, and microbiome-derived metabolites (Babic and Browning, 2014; Han et al., 2018b; Lai et al., 2020; Muller et al., 2020; Tan et al., 2020; Zimmerman et al., 2019). Gut-innervating sensory neurons may also mediate poorly understood sensations like ‘hunger pangs’, visceral pain, and ‘butterflies in the stomach’; the latter is evoked by nervousness and anxiety, consistent with a role for the gut-brain axis in mood control, and perhaps occurs through an inverted reflex arc involving brain-to-gut-to-brain transmission. Gut-innervating vagal neurons have additionally been proposed to mediate pathological α-synuclein transmission in Parkinson’s Disease (Kim et al., 2019).
The brain receives meal-related information from the gastrointestinal tract through several routes, including from neuronal afferents, circulating gut-derived hormones, and nutrients themselves. The entire length of the alimentary canal is heavily innervated by both intrinsic and extrinsic neurons. Ingested food is first detected in the mouth by gustatory afferents reporting on taste quality (from the facial and glossopharyngeal nerves), olfactory sensory neurons that detect food odors, and trigeminal afferents thought to measure features like food texture and temperature. After food is swallowed, it is monitored by different extrinsic nerves in the pharynx (glossopharyngeal, vagal), esophagus (vagal), stomach (mostly vagal, some DRG), intestine (vagal, DRG), and rectum (DRG) (Berthoud and Neuhuber, 2000). Vagal sensory neurons densely innervate the proximal small intestine while DRG neurons dominate innervation of distal intestine (Blackshaw et al., 2007). In addition to extrinsic innervation, the esophagus, stomach, intestine, and other organs contain an extensive network of intrinsic (enteric) neurons. Enteric neurons are closely associated with extrinsic motor neurons and at least some extrinsic sensory neurons and play vital functions in local gut physiology as well as possibly interorgan crosstalk and behavior.
The vagus nerve is a key component of the gut-brain axis and transecting the vagus nerve below the diaphragm (subdiaphragmatic vagotomy) decreases body weight, perhaps due to loss of motor fiber-mediated effects on absorption, digestion, and metabolism and/or disruption of sensory afferents that influence feeding (Aklan et al., 2020; Berthoud et al., 2021; Chen et al., 2020). Perhaps paradoxically, vagal nerve stimulation through electrical or optogenetic approaches also reportedly inhibits feeding behavior (Bai et al., 2019; Borgmann et al., 2021; Burneo et al., 2002), and it is important to consider that other sensations like air hunger, laryngeal aspiration, or esophageal distension may suppress feeding through mechanisms unrelated to satiety. Classic studies of the vagus nerve revealed a few different sensory neuron types in the gut: (i) mucosal endings, including chemoreceptors in intestinal villi and crypt endings, (ii) mechanoreceptors that form intraganglionic laminar endings (IGLEs), and (iii) intramuscular arrays (IMAs), which may also be mechanoreceptors (Brookes et al., 2013). Recent expression profiling approaches revealed a greater diversity of transcriptome-defined vagal and spinal sensory neurons in the digestive system (Bai et al., 2019; Hockley et al., 2019; Kupari et al., 2019; Prescott et al., 2020; Williams et al., 2016), and we are only beginning to understand the sensory properties, functions, and receptor mechanisms of different gut-brain communication pathways.
Nutrient responses along the gut-brain axis.
Ingestion of energy-rich nutrients is powerfully rewarding. While the gustatory system first detects nutrients in food, taste-blind mice still develop a preference for sugar-rich foods. Subdiaphragmatic vagotomy abolishes post-ingestive sugar preference (de Araujo et al., 2008; Fernandes et al., 2020; Han et al., 2018b; Tan et al., 2020), and mice can develop a preference for sugar over artificial sweeteners, consistent with a taste receptor-independent sugar reinforcement pathway (Sclafani et al., 2015; Tan et al., 2020). Teaching signals from ingested nutrients are powerfully rewarding, as Pavlov observed, and enable particular sights, sounds, and smells to be associated with food and subsequently trigger brain-to-gut signaling that helps prepare the digestive system for an upcoming meal.
Upon ingestion, food is broken down by the digestive system into building blocks of simple sugars, amino acids, and free fatty acids. In the intestine, simple nutrients are detected by specialized epithelial sensory cells termed enteroendocrine cells, which rapidly trigger a variety of physiological and neuronal responses before nutrients are absorbed into the bloodstream (Baggio and Drucker, 2007; Gribble and Reimann, 2019). After absorption, nutrients themselves exert effects on virtually every tissue, promote release of metabolic hormones like insulin, and can even directly engage neurons in the brain. The relative importance of different direct and indirect nutrient detection pathways in guiding satiety and reinforcement behavior remains an active area of investigation, with enteroendocrine cells poised to play an important role in rapid, systemic nutrient responses. Vagal and DRG afferents do not directly access the intestinal lumen, so extrinsic chemosensory neurons in the gut function as second-order neurons that receive sensory inputs from enteroendocrine cells and perhaps other cell types like tuft cells, immune cells, and enteric neurons. While it remains unclear which enteroendocrine cell types communicate with vagal afferents, we focus our attention here generally on enteroendocrine cell biology, as they are the first-order nutrient-sensing cells in the intestine that provide at least some inputs to vagal afferents.
Enteroendocrine cells are sparse sentinel cells dispersed along the gastrointestinal tract, and represent ~1% of all intestinal epithelial cells. Given the length of the intestine, enteroendocrine cells collectively represent the most abundant hormone-producing cell type in the body. Like taste cells, enteroendocrine cells are specialized epithelial cells rather than neurons, but share many features with neurons as they are electrically excitable, display calcium-dependent vesicle release, and communicate with downstream vagal, spinal, and/or enteric neurons at synaptic terminals (Gribble and Reimann, 2019) sometimes called neuropods (Kaelberer et al., 2018). Many enteroendocrine cells directly access the gastrointestinal lumen at their apical end (but not all, such as ghrelin-producing cells of the stomach), and at their basolateral end, release hormones that can both engage nearby neurons and enter the bloodstream. Enteroendocrine cells, like all cells in the intestinal epithelium, are short-lived and undergo constant turnover and renewal throughout life. The intestinal epithelium turns over every 3–5 days, with new cells born in intestinal crypts and migrating in a conveyor belt-like manner towards intestinal villi where they are ultimately shed (Barker et al., 2007). Enteroendocrine cells undergo a similar but slower life cycle as other epithelial cells, with some lingering in crypts for longer periods of time (Gehart et al., 2019). It is unclear whether synaptic contacts with neurons persist or change during enteroendocrine cell movement and maturation.
Enteroendocrine cells have historically been classified, and their functions inferred, by the various hormones they produce, which include cholecystokinin (CCK), glucagon-like peptide 1 (GLP1), peptide YY, ghrelin, gastric inhibitory polypeptide (GIP), serotonin, secretin, neurotensin, and somatostatin (Gribble and Reimann, 2019). These gut hormones exert various physiological effects that are sometimes opposing and other times redundant. CCK, GLP1, and PYY are released following nutrient injection and suppress feeding, while ghrelin is released by fasting, is downregulated by nutrient ingestion, and stimulates feeding. GLP1 and GIP are both incretins that rapidly stimulate insulin release and alter metabolism before ingested glucose is even absorbed into circulation (Baggio and Drucker, 2007). As such, small molecules that activate the GLP1 receptor (GLP1R) or that enhance GLP1 durability in circulation by inhibiting its proteolysis are clinical mainstays for treating diabetes (Richards et al., 2021), although at higher concentrations GLP1R agonists cause nausea (Drucker and Nauck, 2006). CCK, serotonin, and other gut hormones additionally regulate a variety of digestive functions including gut motility, gastric emptying, absorption, gallbladder contraction, and exocrine pancreas secretion.
Time-resolved transcriptional analysis identified five principal enteroendocrine cell lineages leading to cells that produce GIP, ghrelin, serotonin (called enterochromaffin cells), somatostatin, or a combination of GLP1, CCK, and/or neurotensin (Gehart et al., 2019). Use of an elegant genetically encoded fluorescent clock to superimpose cell birthdate on the enteroendocrine cell atlas revealed that cell identity can be dynamic, with individual cells capable of switching hormone synthesis as they mature (Gehart et al., 2019). Since Cre-based genetic tools provide a stable readout for transient developmental states, dynamic changes in hormone expression should be considered when using genetic approaches to access enteroendocrine cells for functional analysis.
Enteroendocrine cell-derived hormones can enter circulation to exert pleiotropic effects, and can also be detected locally by neurons. Some vagal sensory neurons express multiple receptors for gut hormones, including CCK, GLP1, and PYY (Williams et al., 2016), and CCK directly evokes calcium transients and action potentials in a subset of vagal sensory neurons (Blackshaw and Grundy, 1990; Schwartz et al., 1993; Williams et al., 2016). Gut hormone receptors are additionally expressed in various brain regions, spinal neurons, and enteric neurons, and the relative contributions of different hormone detection pathways in exerting control of physiology and behavior remains actively investigated. Cell-specific knockout and rescue experiments revealed that GLP1 induces satiety through a distributed network of GLP1R neurons in the hypothalamus and caudal brainstem while GLP1 induces nausea-related behaviors through a more focal brainstem response (McLean et al., 2021; Zhang et al., 2021). Fiber photometry experiments revealed that gastric nutrient infusion decreased the activity of hunger-associated neurons in the hypothalamus that express Agouti-related peptide (AGRP) (Beutler et al., 2017). Nerve transection experiments indicated that fat decreases AGRP neuron activity through the vagus nerve but sugar does so through spinal afferents (Goldstein et al., 2021). Intersectional genetic approaches for chemogenetic inhibition of most DRG sensory neurons (involving Wnt1-Cre and Nav1.8-p2a-Dre) increased food intake (Borgmann et al., 2021), a finding consistent with a role for DRG afferents in acute feeding regulation. In separate studies, stimulating gut-innervating vagal afferents activated dopaminergic reward circuits while branch-selective vagotomy inhibited sugar-evoked dopaminergic neuron activity and post-ingestive sugar preference (Fernandes et al., 2020; Han et al., 2018b; Tan et al., 2020). Together, these findings raise the possibility that there are multiple nutrient-response pathways relevant for different behaviors.
Genetic approaches to eliminate enteroendocrine cells have not been selective but have hinted that they play a vital role in gastrointestinal physiology. Knockout of key developmental factors such as Neurogenin3 or Math1 eliminates intestinal enteroendocrine cells and causes lethality, but Neurogenin3 knockout also eliminates pancreatic endocrine cells while Math1 knockout also eliminates other intestinal secretory cells like Paneth and goblet cells (Li et al., 2011). Human patients with more subtle Neurogenin3 point mutations retained pancreatic islet function, but lost enteroendocrine cells and displayed severe malabsorptive diarrhea (Wang et al., 2006). Chemogenetic activation of cells producing insulin-like peptide 5, which is expressed in enteroendocrine cells of the distal colon, simultaneously blocked feeding behavior through a PYY receptor (NPY2R), improved glucose tolerance through GLP1, and increased defecation through a serotonin receptor (HTR3A) (Lewis et al., 2020), suggesting that individual enteroendocrine cell types can exert a multi-pronged physiological program via different hormones. Additional genetic tools that enable selective elimination and activation of all enteroendocrine cells, or particular subtypes, would provide valuable insights into their physiological functions.
Glucose directly triggers calcium influx and electrical responses in enteroendocrine cells that lead to hormone release (Reimann et al., 2008). Enteroendocrine cells are one of several sites of nutrient detection in the body, and they utilize a different sugar-sensing mechanism from both taste cells and pancreatic beta cells. Taste cells use a heterodimer of G Protein-Coupled Receptors (T1R2/T1R3) to detect extracellular sugars while pancreatic beta cells instead release insulin after glucose metabolism-induced ATP production, leading to closure of ATP-gated potassium channels, cell depolarization, calcium influx through voltage-gated calcium channels, and calcium-mediated release of insulin vesicles. While taste receptors are reportedly expressed in enteroendocrine cells (Jang et al., 2007), GLP1 release persists in taste receptor knockout mice and is not evoked by artificial sweeteners (Fujita et al., 2009; Reimann et al., 2008). Instead, knockout mice lacking the glucose-sodium symporter SGLT1 lack sugar-induced release of both GIP and GLP1, as well as the incretin effect (Gorboulev et al., 2012; Roder et al., 2014). SGLT1 is expressed in several intestinal cell types, including both enterocytes and some enteroendocrine cells, and in enterocytes, is thought to use sodium co-transport to drive glucose absorption up a steep concentration gradient. In addition to deficits in GLP1 release, SGLT1 knockout mice display a lethal sucrose malabsorption syndrome on normal chow but can survive on a diet lacking monosaccharides and disaccharides (Gorboulev et al., 2012). Glucose analogs that act as SGLT1 transport substrates but cannot be metabolized to form ATP still stimulate GLP1 release (Moriya et al., 2009), suggesting that SGLT1-mediated sodium transport is the key signal in enteroendocrine cells that leads to depolarization. Interestingly, SGLT1 knockout also eliminated sugar preference behavior (Sclafani et al., 2016), and SGLT1 inhibition blocked sugar-induced AGRP neuron activity decreases (Goldstein et al., 2021). Likewise, non-metabolizable SGLT1 transport substrates also mimicked the ability of sucrose to condition preference behavior and inhibit AGRP neurons (Goldstein et al., 2021; Tan et al., 2020). These findings suggest a common role for SGLT1-mediated sodium transport/depolarization in various sugar-evoked physiological responses, and additional studies are needed to determine whether enteroendocrine cells or other cell types support each SGLT1-mediated response.
In addition to detecting sugars, enteroendocrine cells detect other nutrients, like fats and amino acids. Different classes of nutrients trigger common responses, like satiety, but also can evoke nutrient-specific digestive and metabolic programs. Each nutrient class triggers release of a complex and at least partially overlapping mixture of gut hormones, as expected since gut hormones like CCK, PYY and GLP1 are frequently co-expressed in the same enteroendocrine cells (Gribble and Reimann, 2019). While sugars stimulate incretin release through a GPCR-independent pathway involving SGLT1, a role for GPCRs in hormone release by enteroendocrine cells is also suggested by pharmacological experiments targeting GPCR-gated second messengers (Reimann et al., 2008). Enteroendocrine cells express many GPCRs and other cell surface receptors that potentially detect fatty acids and amino acids; for example, the free fatty acid receptors GPR40 and GPR120 are abundantly expressed by enteroendocrine cells on their basolateral surface and receptor agonists promote gut hormone release (Edfalk et al., 2008; Hirasawa et al., 2005). Knockout mice lacking these receptors have produced mixed findings, perhaps due to the existence of redundant fat-sensing pathways, and additional studies are needed to understand both the relative roles of particular receptors in nutrient sensation and how nutrient-specific responses emerge.
Chemosensory cells of the gut-brain axis also detect stimuli other than nutrients. Enterochromaffin cells are a specialized enteroendocrine cell class that releases serotonin and responds to various non-nutritive signals including TRPA1-activating irritants, catecholamines like epinephrine, norepinephrine, and dopamine, and small chain fatty acids that activate olfactory receptor 558 (OLFR558) (Bellono et al., 2017), as well as force through PIEZO2 (Alcaino et al., 2018). In addition to promoting serotonin release through OLFR558, microbiome-derived small chain fatty acids, as well as bile acids, stimulate GLP1 release through other GPCRs like GPR41, GPR43, and TGR5 (Thomas et al., 2009; Tolhurst et al., 2012).
In opposition to sugar-responsive reward pathways, the gut-brain axis also contains aversion-promoting nociceptor pathways associated with nausea, gut malaise, and pain. Nerve transections and genetic ablations have implicated the vagus nerve in nausea-associated responses in some studies but not others (Babic and Browning, 2014; Gupta et al., 2017). Some gut-derived vagal sensory neurons project to the area postrema, a brainstem nucleus that in various species mediates nausea, vomiting, and/or behavioral aversion to visceral poisons (Borison, 1989). The area postrema is a sensory circumventricular organ that receives inputs from both hormones and vagal afferents, and one area postrema neuron subtype co-expresses receptors for several nausea-inducing chemicals, including GLP1R, GFRAL (the GDF15 receptor), and the calcium-sensing receptor, and also projects to CGRP-expressing alarm neurons in the parabrachial nucleus (Sabatini et al., 2021; Zhang et al., 2021). Chemogenetic stimulation of these area postrema neurons induced flavor avoidance in mice, while ablation eliminated behavioral responses to several nausea-inducing toxins (Sabatini et al., 2021; Zhang et al., 2021). Moreover, area postrema-targeted deletion and rescue of Glp1r revealed a key role for area postrema neurons in behavioral aversion imposed by GLP1R agonists (Zhang et al., 2021). It remains unclear whether area postrema GFRAL neurons receive inputs from particular vagal afferents, and how a dual requirement for vagal and area postrema pathways may emerge in gut nociception. The serotonin receptor HTR3A is highly expressed in vagal afferents (Williams et al., 2016), and HTR3A antagonists like ondansetron are widely used in the clinic as anti-nausea prophylactics (Smith et al., 2012), highlighting the potential therapeutic importance of understanding signaling pathways that lead to gut malaise.
Gut mechanosensation and the control of feeding behavior.
Food and drink consumed during a meal physically distend the stomach and intestine, activating mechanosensory neurons of the gut-brain axis and evoking the sensation of fullness. Both vagal and DRG sensory neurons innervate the gastrointestinal tract and respond to tissue distension (Paintal, 1954; Qin et al., 2007), with experiments involving subdiaphragmatic vagotomy revealing a crucial role for vagal sensory neurons in gut stretch-induced satiety (Gonzalez and Deutsch, 1981). Bariatric surgery to re-shape the gastrointestinal tract decreases its storage capacity, resulting in tissue distension and presumably gut mechanoreceptor activation at lower food volumes. Perhaps shifting the sensory thresholds of gut mechanosensory neurons contributes to the clinically beneficial effects of bariatric surgery. Understanding neural pathways responsive to gut distension, and how they can be modulated or artificially stimulated, may provide new therapeutic targets to limit appetite.
Vagal sensory neurons are activated by increases in gut volume that occur naturally during a meal (Umans and Liberles, 2018). Separate and large cohorts of vagal sensory neurons detect acute distension of the stomach and intestine (Bai et al., 2019; Williams et al., 2016), with responses graded to the magnitude of distension (Iggo, 1955; Paintal, 1954). Increases in stomach volume both enhance the responses of individual neurons, and also recruit additional responding neurons (Williams et al., 2016). It is possible that neurons display different activation thresholds, or that enhancing tissue distension recruits additional neurons with more remote receptive fields but otherwise identical intrinsic properties. Vagal sensory neurons generally fire for the duration of distension with little or no adaptation, and stop firing upon withdrawal of the distension stimulus and deflation to resting volume (Iggo, 1955; Paintal, 1954; Williams et al., 2016). Cellular imaging and optogenetics-assisted conduction velocity measurements revealed that vagal mechanosensory neurons are predominantly capsaicin-sensitive, slow-conducting C fibers (Williams et al., 2016).
Vagal sensory neurons form a few distinct terminal types in the stomach and intestine. Some chemosensory neurons form mucosal endings (see above) while other vagal sensory neurons form subepithelial endings that include IGLEs and IMAs (Brookes et al., 2013). IGLEs are endings that appose ganglia of enteric neurons in the myenteric plexus located between layers of longitudinal and circular muscle (Rodrigo et al., 1975). Both vagal and DRG sensory neurons can form IGLEs, and IGLEs occur across the gastrointestinal tract, including in the esophagus, stomach, small intestine, and large intestine (Berthoud et al., 1997). IGLEs were initially proposed to be mechanosensory terminals since stomach regions that evoked vagal nerve responses upon punctate von Frey hair stimulation were closer to IGLEs (142 μm) than were randomly selected stomach regions (1500 μm) (Zagorodnyuk et al., 2001). Genetic tools involving Cre-based anatomical mapping and in vivo calcium imaging provided a direct link between neuron anatomy and response property. Vagal sensory neurons that express GLP1R are marked in Glp1r-ires-Cre mice, form IGLEs in stomach and intestine, and account for most neurons (~81% in stomach) acutely activated by balloon- or gas-induced distension (Williams et al., 2016). In addition, neurons marked in Oxtr-Cre mice, which are mostly distinct from GLP1R neurons based on single-cell transcriptional profiling, also form IGLEs enriched in intestine compared with stomach (Bai et al., 2019), as well as IGLEs in esophagus (unpublished data, Rachel Greenberg, SLP, SDL). IMAs are instead long and parallel muscular fibers that resemble muscle spindle afferents involved in proprioception, and have thus been proposed to be mechanosensory terminals (Powley and Phillips, 2011). Cre lines that enable selective targeting of IMAs would be valuable to determine their sensory properties and physiological roles. Gut mechanoreceptors frequently co-express receptors for several gut satiety hormones, including GLP1, CCK, and PYY (Williams et al., 2016). Vagal sensory neurons that detect gut distention are generally distinct from mucosal chemosensory neurons and do not typically fire in response to ingested nutrients that promote CCK, GLP1, and PYY release (Williams et al., 2016). It is possible that these and other appetite-control hormones like leptin or ghrelin modulate the sensitivity, activity rate, and/or presynaptic release properties of gut mechanoreceptors. Adding complexity, these hormones activate different intracellular signaling pathways, with leptin receptor engaging JAK/STAT pathways, GLP1R stimulating Gαs and cAMP levels, and CCK evoking calcium transients and action potentials in vagal sensory neurons by coupling to Gαq. Additional studies are needed to understand when and how gut hormones act physiologically on vagal mechanosensory neurons, and whether they enhance distension-induced satiety when physiologically appropriate, such as after a meal.
It also remains mysterious why mechanosensory vagal afferents form such close contacts with enteric neurons. Perhaps information is transferred between neurons at IGLEs, or perhaps enteric neurons simply serve as developmental guideposts for extrinsic neurons. Vagal motor neurons also access enteric neurons through a similar honeycomb pattern, and critically rely on synaptic transmission to enteric neurons to exert parasympathetic control. It has been proposed that vagal mechanosensory neurons directly detect force, as responses are rapid (within 6 milliseconds) and persist in the absence of extracellular calcium (Zagorodnyuk et al., 2003), although it should be noted that transmission at some synapses can be faster than 6 milliseconds. Supporting the notion that enteric neuron input is dispensable for mechanosensory responses, colonic DRG neurons in a mouse model of Hirschsprung’s Disease lacking colonic enteric neurons fail to form their characteristic IGLE terminal morphology yet retain at least some responsiveness to tissue distension (Spencer et al., 2008). Electron microscopy of vagal sensory neurons in the esophagus suggested that some vagal afferents are presynaptic to intrinsic myenteric neurons (Neuhuber, 1987). One model is that IGLE-forming extrinsic afferents are the primary mechanosensors which simultaneously relay inputs to the brain to control behavior and to enteric neurons at IGLEs for control of local gut function. Other possible models are that extrinsic neurons instead receive neuromodulatory or mechanosensory information from enteric neurons at IGLEs, or that both extrinsic and enteric neurons are inherently mechanosensitive. Additional evidence is needed to support the existence, directionality, and physiological role of information transfer between neurons at IGLEs.
Gut mechanosensation is a classical stimulus that evokes satiety and aborts ingestion of food and drink. Optogenetic and chemogenetic stimulation of vagal GLP1R neurons partially reduced feeding and decreased blood glucose levels, while activating vagal OXTR neurons more dramatically reduced feeding without evoking or conditioning place preference or avoidance (Bai et al., 2019; Borgmann et al., 2021). Chemogenetic but not optogenetic activation of OXTR neurons also decreased water intake (Bai et al., 2019). It should be considered that some existing genetic tools are selective among classes of gut-innervating afferents, but may additionally label sensory neurons in other physiological systems that could secondarily impact food consumption.
Genetically guided anatomical mapping revealed that vagal GLP1R and OXTR neurons project to NTS subregions and the adjacent area postrema (Bai et al., 2019; Williams et al., 2016), with GLP1R neurons targeting an NTS region that displays Fos induction after gastric distension (Williams et al., 2016; Willing and Berthoud, 1997). Furthermore, chemogenetic activation of vagal GLP1R and OXTR neurons induced Fos expression in the NTS and different regions of the parabrachial nucleus, with GLP1R neurons engaging neurons marked by CGRP expression (Borgmann et al., 2021). Parabrachial CGRP neurons function as alarm neurons engaged by multiple threats, and also contribute to meal-induced satiety, as silencing them increases meal size (Campos et al., 2016). Other prodynorphin-expressing neurons in the parabrachial nucleus respond to mechanosensory signals across the digestive tract related to water and food ingestion, including vagally mediated gastric distension, and evoke an aversive appetite-suppressing signal at least in part through projections to the paraventricular hypothalamus (Kim et al., 2020). Hypothalamic neurons expressing Agouti-related peptide (AGRP) promote food intake during hunger, and fiber photometry experiments revealed that chemogenetic activation of both vagal GLP1R and OXTR neurons rapidly decreased AGRP neuron activity, with GLP1R neuron effects being transient and OXTR neuron effects more sustained (Bai et al., 2019).
Receptors that mediate vagal responses to gut mechanosensation remain elusive. Vagal sensory neurons express ASIC channels and a pharmacological antagonist of ENaC/ASIC channels at high concentrations (benzamil, 100 μM) blocks mechanosensory responses in an IGLE-rich esophagus preparation (Zagorodnyuk et al., 2003). However, gut mechanosensory responses persist in ASIC knockout mice (Page et al., 2005), suggesting a role for another mechanosensor and/or redundant roles for multiple mechanosensors. PIEZOs are also candidates to consider given their established mechanosensory roles in other internal organs, and that a key role for Drosophila Piezo in gut mechanosensation and feeding behavior was revealed (Min et al., 2021; Wang et al., 2020). Additional studies are needed to clarify how IGLEs respond to force.
CONCLUSION
The vagus nerve is a crucial body-brain connection that plays a major role in interoception. Classical schemes to categorize vagal sensory neurons were based on cellular features like conduction velocity, morphology, developmental origin, and/or electrophysiological response characteristics, but did not capture the full extent of sensory neuron diversity. Single-cell RNA sequencing and genetic approaches revealed a staggering diversity of vagal sensory neuron types, with more neuron classes observed than known vagal reflexes. The functions and sensory properties of many neuron types with particular transcriptional signatures or terminal morphologies remain mysterious (we affectionately call them the ‘dark matter’ of the vagus nerve). Furthermore, vagal afferents project to many organs other than those discussed in this review whose sensory properties and functions are not well understood. It is worth noting that cell clustering in single cell transcriptomic approaches may or may not be based on genes that guide neuronal response properties, innervation patterns, or other defining cell characteristics; complementary genetic approaches are essential to understand whether transcriptome-defined cell clusters are in fact functionally uniform and distinct. It is exciting to consider that additional body-brain communication pathways and autonomic reflexes of the vagus nerve may remain undiscovered.
Genetic approaches that allow for selective marking of different vagal afferents within a target organ enable linkages between neuronal morphology, responses, transcriptomes, and physiological functions. Cre lines have been identified that mark neurons underlying classical reflexes from the respiratory, cardiovascular, and digestive systems. For example, PIEZO2 neurons mediate the baroreceptor reflex and the Hering-Breuer inspiratory reflex (Min et al., 2019; Nonomura et al., 2017), P2RY1 and TRPA1/TRPV1 neurons mediate different airway protective responses (Bessac and Jordt, 2008; Chang et al., 2015; Prescott et al., 2020), and GLP1R and OXTR neurons sense gut distension (Bai et al., 2019; Williams et al., 2016). It is important to consider that Cre lines frequently mark multiple types of vagal sensory neurons so pinpointing which transcriptionally defined cell type mediates a particular reflex is still very much a work in progress. Advances may require application of intersectional genetics (Borgmann et al., 2021) and/or interpreting results from large numbers of Cre lines, as was done to define a role for NP19 neurons in airway defense (Prescott et al., 2020). Moreover, Cre lines may selectively mark a sensory neuron type within an organ but also afferent fibers in other organs. It seems possible that a single cell type defined by transcriptome and/or genetic analysis may be constituted by neurons that innervate different organs but display similar sensory properties like detection of tissue stretch or pathogens.
As in the somatosensory system, the physiological attributes of vagal neurons are dictated not only by their molecular properties but also by their terminal structures and strategic positioning within the body. For example, various chemosensory neurons respond to the same neurotransmitter, but are positioned near different upstream sensory cells, and thus convey different types of sensory inputs to the brain. Furthermore, vagal mechanosensory neurons display diverse terminal structures across organs- including IGLEs in the gastrointestinal tract, end-net endings in the aorta, smooth muscle-associated receptors in the airways, and perhaps intramuscular arrays in the stomach. Additional studies are needed to determine whether these distinct terminal morphologies convey unique sensory attributes to each neuron type.
Understanding what each vagal afferent type detects will enable mechanistic studies to identify sensory receptors and associated signaling pathways. Many vagal afferents are second-order neurons that receive inputs from upstream sentinel cells, including glomus cells, taste cells, enteroendocrine cells, tuft cells, neuroepithelial bodies, and immune cells (Figure 4). These upstream sentinel cells express primary sensory receptors and signaling molecules, which are reported in a few cases including SGLT-1 in sugar-sensing enteroendocrine cells (Reimann et al., 2008), non-classical mitochondrial subunits involved in glomus cell hypoxia sensing (Moreno-Dominguez et al., 2020), and bitter receptors in tuft cells (Krasteva et al., 2011). Sentinel cells then release neurotransmitters like ATP from taste cells and glomus cells, serotonin and CCK from enteroendocrine cells, and cytokines from immune cells that can activate vagal afferents. Other vagal sensory neurons are first-order sensory neurons that directly sense internal organ cues. In the airways, some vagal sensory neurons directly detect agonists of TRP channels and MRGPRC11 (Bessac and Jordt, 2008; Han et al., 2018a). Other airway sensory neurons use PIEZO2 to detect airway stretch underlying the Hering-Breuer inspiratory reflex (Nonomura et al., 2017), while mechanosensory neurons in the arteries use both PIEZO1 and PIEZO2 to sense force underlying the baroreceptor reflex (Zeng et al., 2018). Sensory receptors and mechanisms remain undefined for most vagal afferents and upstream sentinel cells.
Figure 4. Examples of sensory terminals involved in interoception.
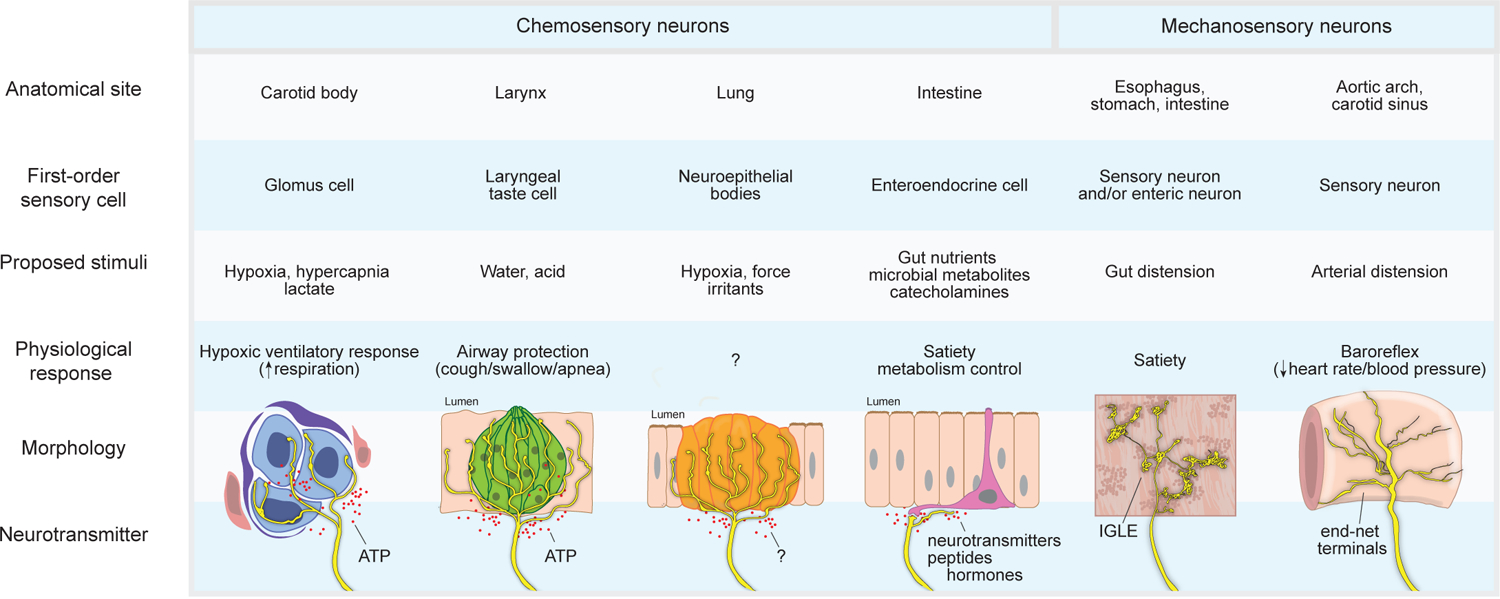
Vagal and glossopharyngeal sensory neurons include second-order chemosensory neurons that receive inputs from upstream sentinel cells, including glomus cells, taste cells, neuroepithelial bodies, and enteroendocrine cells, as well as mechanosensory neurons that form IGLEs and end-net endings. Various neuron types project to different organs, directly or indirectly detect different stimuli, display different terminal morphologies, and evoke different physiological responses.
Unraveling mechanisms of vagus nerve biology- from charting newly identified sensory neurons to discovering additional internal organ sensory receptors and signal transduction pathways- will build the groundwork for the emerging field of interoception. Exciting questions will then lie on the horizon, and there will be new opportunities to investigate neural circuitry, development, evolution, and therapeutic impact of vagal sensory biology. For example, ascending neural circuits encode a diversity of vagal sensory inputs that control autonomic physiology in different ways that are sometimes convergent (like mechanosensory and chemosensory neurons that induce satiety or cough), sometimes opposing (like the Bainbridge and baroreceptor reflexes), sometimes orthogonal (like neurons that only influence gut or respiratory physiology), and sometimes partially overlapping (like certain cardiovascular and respiratory reflexes). The brainstem presumably acts as an autonomic switchboard that routes particular sensory inputs into appropriate physiological outputs, behaviors, and perceptions, and may also generate and/or be dynamically modulated by brain states. The vagus nerve is also a fascinating playground for understanding principles of developmental biology. How do individual neurons find the correct target organs, contact the appropriate cell partners in the periphery and brain, and form the proper terminal morphologies? How may vagal sensory neurons be dynamically regulated following injury or experience? Over a longer timescale, sensory pathways of the vagus nerve vary dramatically across evolution- from gill innervation in fish and stomach innervation in ruminants to respiratory gas sensation in species that live at high altitudes or dive to ocean depths. Little is also understood about genetic variation in vagal sensory processing across human individuals that may impact susceptibility to autonomic dysfunction. The vagus nerve impacts such vital physiology, and approaches to toggle vagus nerve activity may provide an immense untapped opportunity for therapeutic intervention. Vagotomy (subdiaphragmatic) and vagus nerve electrical stimulation are potentially useful to treat a variety of clinical conditions, but lack specificity and conflate contributions from various sensory and motor neurons. Understanding vagal sensory mechanisms may provide new and more selective strategies to stimulate or inhibit particular afferent types, and thus new ways to manipulate the autonomic nervous system in health and disease.
ACKNOWLEDGMENTS
We thank Michael Schappe, Chen Ran, Soohong Min, and Rachel Greenberg for comments. The work was supported by NIH grant DP1 AT009497 to SDL and the Food Allergy Science Initiative. SLP was an Open Philanthropy Fellow of the Life Sciences Research Foundation and is a Warren Alpert Distinguished Scholar. SDL is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
SDL is a consultant for Kallyope, Inc.
REFERENCES
- Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, and Smith JA (2015). P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 385, 1198–1205. [DOI] [PubMed] [Google Scholar]
- Abraira VE, and Ginty DD (2013). The sensory neurons of touch. Neuron 79, 618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED (1926). The impulses produced by sensory nerve endings: Part I. J Physiol 61, 49–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED (1933). Afferent impulses in the vagus and their effect on respiration. J Physiol 79, 332–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostoni E, Chinnock JE, De Daly MB, and Murray JG (1957). Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol 135, 182–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aklan I, Sayar Atasoy N, Yavuz Y, Ates T, Coban I, Koksalar F, Filiz G, Topcu IC, Oncul M, Dilsiz P, et al. (2020). NTS Catecholamine Neurons Mediate Hypoglycemic Hunger via Medial Hypothalamic Feeding Pathways. Cell Metab 31, 313–326 e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR, Li JH, Leiter AB, Szurszewski JH, Farrugia G, et al. (2018). A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proceedings of the National Academy of Sciences of the United States of America 115, E7632–E7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler SM, Bao XM, Bieger D, Hopkins DA, and Miselis RR (1989). Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283, 248–268. [DOI] [PubMed] [Google Scholar]
- Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, et al. (2008). Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 118, 2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arble DM, Sandoval DA, and Seeley RJ (2015). Mechanisms underlying weight loss and metabolic improvements in rodent models of bariatric surgery. Diabetologia 58, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt JO, Brambring P, Hindorf K, and Rohnelt M (1974). The afferent discharge pattern of atrial mechanoreceptors in the cat during sinusoidal stretch of atrial strips in situ. J Physiol 240, 33–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asala SA, and Bower AJ (1986). An electron microscope study of vagus nerve composition in the ferret. Anat Embryol (Berl) 175, 247–253. [DOI] [PubMed] [Google Scholar]
- Aumonier FJ (1972). Histological observations on the distribution of baroreceptors in the carotid and aortic regions of the rabbit, cat and dog. Acta Anat (Basel) 82, 1–16. [DOI] [PubMed] [Google Scholar]
- Babic T, and Browning KN (2014). The role of vagal neurocircuits in the regulation of nausea and vomiting. Eur J Pharmacol 722, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, and Drucker DJ (2007). Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157. [DOI] [PubMed] [Google Scholar]
- Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, Aitken TJ, Chen Y, Beutler LR, Ahn JS, et al. (2019). Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 179, 1129–1143 e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge FA (1915). The influence of venous filling upon the rate of the heart. J Physiol 50, 65–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, and Patapoutian A (2004). Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857. [DOI] [PubMed] [Google Scholar]
- Baral P, Umans BD, Li L, Wallrapp A, Bist M, Kirschbaum T, Wei Y, Zhou Y, Kuchroo VK, Burkett PR, et al. (2018). Nociceptor sensory neurons suppress neutrophil and gammadelta T cell responses in bacterial lung infections and lethal pneumonia. Nat Med 24, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberan A, Ladau J, Leff JW, Pollard KS, Menninger HL, Dunn RR, and Fierer N (2015). Continental-scale distributions of dust-associated bacteria and fungi. Proceedings of the National Academy of Sciences of the United States of America 112, 5756–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, and Julius D (2006). TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282. [DOI] [PubMed] [Google Scholar]
- Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, and Julius D (2017). Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 170, 185–198 e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Albaugh VL, and Neuhuber WL (2021). Gut-brain communication and obesity: understanding functions of the vagus nerve. J Clin Invest 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, and Neuhuber WL (2000). Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85, 1–17. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Neumann F, and Neuhuber WL (1997). Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 195, 183–191. [DOI] [PubMed] [Google Scholar]
- Bessac BF, and Jordt SE (2008). Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, and Jordt SE (2008). TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118, 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA, Brookes SJ, Grundy D, and Schemann M (2007). Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil 19, 1–19. [DOI] [PubMed] [Google Scholar]
- Boettcher DH, Zimpfer M, and Vatner SF (1982). Phylogenesis of the Bainbridge reflex. Am J Physiol 242, R244–246. [DOI] [PubMed] [Google Scholar]
- Boggs DF, and Bartlett D Jr. (1982). Chemical specificity of a laryngeal apneic reflex in puppies. J Appl Physiol Respir Environ Exerc Physiol 53, 455–462. [DOI] [PubMed] [Google Scholar]
- Bolser DC (1991). Fictive cough in the cat. J Appl Physiol (1985) 71, 2325–2331. [DOI] [PubMed] [Google Scholar]
- Bookout AL, and Gautron L (2021). Characterization of a cell bridge variant connecting the nodose and superior cervical ganglia in the mouse: Prevalence, anatomical features, and practical implications. J Comp Neurol 529, 111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgmann D, Ciglieri E, Biglari N, Brandt C, Cremer AL, Backes H, Tittgemeyer M, Wunderlich FT, Bruning JC, and Fenselau H (2021). Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metab 33, 1466–1482 e1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borison HL (1989). Area postrema: chemoreceptor circumventricular organ of the medulla oblongata. Progress in neurobiology 32, 351–390. [DOI] [PubMed] [Google Scholar]
- Bouverot P, Crance JP, and Dejours P (1970). Factors influencing the intensity of the Breuer-Hering inspiration-inhibiting reflex. Respir Physiol 8, 376–384. [DOI] [PubMed] [Google Scholar]
- Bradley GW, Noble MI, and Trenchard D (1976). The direct effect on pulmonary stretch receptor discharge produced by changing lung carbon dioxide concentration in dogs on cardiopulmonary bypass and its action on breathing. J Physiol 261, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RM (2000). Sensory receptors of the larynx. Am J Med 108 Suppl 4a, 47S–50S. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Spencer NJ, Costa M, and Zagorodnyuk VP (2013). Extrinsic primary afferent signalling in the gut. Nature reviews Gastroenterology & hepatology 10, 286–296. [DOI] [PubMed] [Google Scholar]
- Brouns I, Van Genechten J, Hayashi H, Gajda M, Gomi T, Burnstock G, Timmermans JP, and Adriaensen D (2003). Dual sensory innervation of pulmonary neuroepithelial bodies. American journal of respiratory cell and molecular biology 28, 275–285. [DOI] [PubMed] [Google Scholar]
- Brown AM (1980). Receptors under pressure. An update on baroreceptors. Circ Res 46, 1–10. [DOI] [PubMed] [Google Scholar]
- Burneo JG, Faught E, Knowlton R, Morawetz R, and Kuzniecky R (2002). Weight loss associated with vagus nerve stimulation. Neurology 59, 463–464. [DOI] [PubMed] [Google Scholar]
- Buttigieg J, and Nurse CA (2004). Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem Biophys Res Commun 322, 82–87. [DOI] [PubMed] [Google Scholar]
- Cahalan SM, Lukacs V, Ranade SS, Chien S, Bandell M, and Patapoutian A (2015). Piezo1 links mechanical forces to red blood cell volume. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CA, Bowen AJ, Schwartz MW, and Palmiter RD (2016). Parabrachial CGRP Neurons Control Meal Termination. Cell Metab 23, 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ (2011). Functional implications of the multiple afferent pathways regulating cough. Pulm Pharmacol Ther 24, 295–299. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, and Undem BJ (2004). Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557, 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Mori N, and Mazzone SB (2006). Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol 152, 223–242. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, and Julius D (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, and Zuker CS (2009). The taste of carbonation. Science (New York, NY 326, 443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RB, Strochlic DE, Williams EK, Umans BD, and Liberles SD (2015). Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell 161, 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cheng M, Wang L, Zhang L, Xu D, Cao P, Wang F, Herzog H, Song S, and Zhan C (2020). A Vagal-NTS Neural Pathway that Stimulates Feeding. Curr Biol 30, 3986–3998 e3985. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Powley TL, Schwaber JS, and Doyle FJ 3rd (1997a). A laser confocal microscopic study of vagal afferent innervation of rat aortic arch: chemoreceptors as well as baroreceptors. J Auton Nerv Syst 67, 1–14. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Powley TL, Schwaber JS, and Doyle FJ 3rd (1997b). Vagal afferent innervation of the atria of the rat heart reconstructed with confocal microscopy. J Comp Neurol 381, 1–17. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, et al. (2013). Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501, 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudry NB, Fuller RW, and Pride NB (1989). Sensitivity of the human cough reflex: effect of inflammatory mediators prostaglandin E2, bradykinin, and histamine. Am Rev Respir Dis 140, 137–141. [DOI] [PubMed] [Google Scholar]
- Chu C, Artis D, and Chiu IM (2020). Neuro-immune Interactions in the Tissues. Immunity 52, 464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge HM, and Coleridge JC (2011). Reflexes evoked from tracheobronchial tree and lungs. Comprehensive Physiology, 395–429. [Google Scholar]
- Coleridge JC, and Coleridge HM (1977). Afferent C-fibers and cardiorespiratory chemoreflexes. Am Rev Respir Dis 115, 251–260. [DOI] [PubMed] [Google Scholar]
- Crystal GJ, and Salem MR (2012). The Bainbridge and the “reverse” Bainbridge reflexes: history, physiology, and clinical relevance. Anesth Analg 114, 520–532. [DOI] [PubMed] [Google Scholar]
- Dampney RAL (2017). Resetting of the Baroreflex Control of Sympathetic Vasomotor Activity during Natural Behaviors: Description and Conceptual Model of Central Mechanisms. Front Neurosci 11, 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, and Simon SA (2008). Food reward in the absence of taste receptor signaling. Neuron 57, 930–941. [DOI] [PubMed] [Google Scholar]
- Drummond HA, Price MP, Welsh MJ, and Abboud FM (1998). A molecular component of the arterial baroreceptor mechanotransducer. Neuron 21, 1435–1441. [DOI] [PubMed] [Google Scholar]
- DuBois FS, and Foley JO (1936). Experimental studies on the vagus and spinal accessory nerves in the cat. The Anatomical Record 64, 285–307. [Google Scholar]
- Edfalk S, Steneberg P, and Edlund H (2008). Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57, 2280–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes AB, Alves da Silva J, Almeida J, Cui G, Gerfen CR, Costa RM, and Oliveira-Maia AJ (2020). Postingestive Modulation of Food Seeking Depends on Vagus-Mediated Dopamine Neuron Activity. Neuron 106, 778–788 e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Aguera MC, Gao L, Gonzalez-Rodriguez P, Pintado CO, Arias-Mayenco I, Garcia-Flores P, Garcia-Perganeda A, Pascual A, Ortega-Saenz P, and Lopez-Barneo J (2015). Oxygen Sensing by Arterial Chemoreceptors Depends on Mitochondrial Complex I Signaling. Cell Metab 22, 825–837. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, and Kinnamon SC (2005). ATP signaling is crucial for communication from taste buds to gustatory nerves. Science (New York, NY 310, 1495–1499. [DOI] [PubMed] [Google Scholar]
- Finley JC, and Katz DM (1992). The central organization of carotid body afferent projections to the brainstem of the rat. Brain research 572, 108–116. [DOI] [PubMed] [Google Scholar]
- Foley JO, and DuBois FS (1937). Quantitative studies of the vagus nerve in the cat. J Comp Neurol 67, 49–67. [Google Scholar]
- Fox AJ, Urban L, Barnes PJ, and Dray A (1995). Effects of capsazepine against capsaicin- and proton-evoked excitation of single airway C-fibres and vagus nerve from the guinea-pig. Neuroscience 67, 741–752. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Wideman RD, Speck M, Asadi A, King DS, Webber TD, Haneda M, and Kieffer TJ (2009). Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab 296, E473–479. [DOI] [PubMed] [Google Scholar]
- Gehart H, van Es JH, Hamer K, Beumer J, Kretzschmar K, Dekkers JF, Rios A, and Clevers H (2019). Identification of Enteroendocrine Regulators by Real-Time Single-Cell Differentiation Mapping. Cell. [DOI] [PubMed] [Google Scholar]
- Goldstein N, McKnight AD, Carty JRE, Arnold M, Betley JN, and Alhadeff AL (2021). Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metab 33, 676–687 e675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MF, and Deutsch JA (1981). Vagotomy abolishes cues of satiety produced by gastric distension. Science (New York, NY 212, 1283–1284. [DOI] [PubMed] [Google Scholar]
- Gorboulev V, Schurmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, et al. (2012). Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, and Reimann F (2019). Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol 15, 226–237. [DOI] [PubMed] [Google Scholar]
- Gupta BN, and Thames MD (1983). Behavior of left ventricular mechanoreceptors with myelinated and nonmyelinated afferent vagal fibers in cats. Circ Res 52, 291–301. [DOI] [PubMed] [Google Scholar]
- Gupta RG, Schafer C, Ramaroson Y, Sciullo MG, and Horn CC (2017). Role of the abdominal vagus and hindbrain in inhalational anesthesia-induced vomiting. Auton Neurosci 202, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG (2006). The sympathetic control of blood pressure. Nat Rev Neurosci 7, 335–346. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, and Bayliss DA (2015). Neural Control of Breathing and CO2 Homeostasis. Neuron 87, 946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth R (1991). Reflexes from the heart. Physiological reviews 71, 617–658. [DOI] [PubMed] [Google Scholar]
- Han L, Limjunyawong N, Ru F, Li Z, Hall OJ, Steele H, Zhu Y, Wilson J, Mitzner W, Kollarik M, et al. (2018a). Mrgprs on vagal sensory neurons contribute to bronchoconstriction and airway hyper-responsiveness. Nature neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, et al. (2018b). A Neural Circuit for Gut-Induced Reward. Cell 175, 665–678 e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JP, and Pearce JW (1956). The possible role of cardiac atrial stretch receptors in the induction of changes in urine flow. J Physiol 131, 572–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering E, and Breuer J (1868). Die Selbststeuerung der Athmung durch den Nervus vagus. Sitzungsber Akad Wiss Wien 57, 672–677. [Google Scholar]
- Hering H (1923). Der Karotisdruckversuch. Münch Med Wochenschr, 1278–1290. [Google Scholar]
- Heymans C, Bouckaert J, and Dautrebande L (1931). Au sujet du mecanisme de la bradycardie provoquée par la nicotine, la lobéline, le cyanure, le sulfure de sodium, les nitrites et la morphine, et de la bradycardie asphyxique. Arch Int Pharmacodyn, 261–289. [Google Scholar]
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, and Tsujimoto G (2005). Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11, 90–94. [DOI] [PubMed] [Google Scholar]
- Hockley JRF, Taylor TS, Callejo G, Wilbrey AL, Gutteridge A, Bach K, Winchester WJ, Bulmer DC, McMurray G, and Smith ESJ (2019). Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 68, 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HH, and Schnitzlein HN (1961). The numbers of nerve fibers in the vagus nerve of man. Anat Rec 139, 429–435. [DOI] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, and Luo M (2007). Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science (New York, NY 317, 953–957. [DOI] [PubMed] [Google Scholar]
- Iggo A (1955). Tension receptors in the stomach and the urinary bladder. J Physiol 128, 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J, Chapleau MW, and Somers VK (2021). Carotid body chemoreceptors: physiology, pathology, and implications for health and disease. Physiological reviews 101, 1177–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga R, Lahiri S, and Mokashi A (1991). Carbonic anhydrase and chemoreception in the cat carotid body. Am J Physiol 261, C565–573. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. (2007). Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proceedings of the National Academy of Sciences of the United States of America 104, 15069–15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarisch A, and Richter A (1939). Die afferenten Bahnen des Veratrineeffekts in den Herznerven. Arch Exp Pathol Pharmakol, 355–371. [Google Scholar]
- Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, and Bohorquez DV (2018). A gut-brain neural circuit for nutrient sensory transduction. Science (New York, NY 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson JA, and Fuller RW (1999). Pharmacological regulation of the cough reflex--from experimental models to antitussive effects in Man. Pulm Pharmacol Ther 12, 215–228. [DOI] [PubMed] [Google Scholar]
- Katz DM, and Karten HJ (1983). Visceral representation within the nucleus of the tractus solitarius in the pigeon, Columba livia. J Comp Neurol 218, 42–73. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Coleridge HM, Coleridge JC, and Baker DG (1980). Bradykinin stimulates afferent vagal C-fibers in intrapulmonary airways of dogs. J Appl Physiol Respir Environ Exerc Physiol 48, 511–517. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Iwamoto GA, Ashton JH, and Cassidy SS (1982). Responses to inflation of vagal afferents with endings in the lung of dogs. Circ Res 51, 525–531. [DOI] [PubMed] [Google Scholar]
- Kim DY, Heo G, Kim M, Kim H, Jin JA, Kim HK, Jung S, An M, Ahn BH, Park JH, et al. (2020). A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature 580, 376–380. [DOI] [PubMed] [Google Scholar]
- Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, et al. (2019). Transneuronal Propagation of Pathologic alpha-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 103, 627–641 e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchheim HR (1976). Systemic arterial baroreceptor reflexes. Physiological reviews 56, 100–177. [DOI] [PubMed] [Google Scholar]
- Knowlton GC, and Larrabee MG (1946). A unitary analysis of pulmonary volume receptors. Am J Physiol 147, 100–114. [DOI] [PubMed] [Google Scholar]
- Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, et al. (2011). Cholinergic chemosensory cells in the trachea regulate breathing. Proceedings of the National Academy of Sciences of the United States of America 108, 9478–9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, and McCrimmon DR (2006). Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol (1985) 101, 618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada M, Terui N, and Kuwaki T (1990). Arterial baroreceptor reflex: its central and peripheral neural mechanisms. Progress in neurobiology 35, 331–361. [DOI] [PubMed] [Google Scholar]
- Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, et al. (2015). PHYSIOLOGY. Regulation of breathing by CO(2) requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science (New York, NY 348, 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze DL (1985). Role of baroreceptor resetting in cardiovascular regulation: acute resetting. Fed Proc 44, 2408–2411. [PubMed] [Google Scholar]
- Kupari J, Haring M, Agirre E, Castelo-Branco G, and Ernfors P (2019). An Atlas of Vagal Sensory Neurons and Their Molecular Specialization. Cell Rep 27, 2508–2523 e2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, and Raczak G (2008). Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol 13, 191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai NY, Musser MA, Pinho-Ribeiro FA, Baral P, Jacobson A, Ma P, Potts DE, Chen Z, Paik D, Soualhi S, et al. (2020). Gut-Innervating Nociceptor Neurons Regulate Peyer’s Patch Microfold Cells and SFB Levels to Mediate Salmonella Host Defense. Cell 180, 33–49 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren S (1952). On the excitation mechanism of the carotid baroceptors. Acta physiologica Scandinavica 26, 1–34. [DOI] [PubMed] [Google Scholar]
- Larsell O (1921). Nerve terminations in the lung of the rabbit. journal of comparative neurology 33, 105–131. [Google Scholar]
- Lau OC, Shen B, Wong CO, Tjong YW, Lo CY, Wang HC, Huang Y, Yung WH, Chen YC, Fung ML, et al. (2016). TRPC5 channels participate in pressure-sensing in aortic baroreceptors. Nat Commun 7, 11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Jiang H, Shen X, Yang W, Guo C, Wang Z, Xiao M, Cui L, Luo W, Kim BS, et al. (2021). Sneezing reflex is mediated by a peptidergic pathway from nose to brainstem. Cell 184, 3762–3773 e3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, et al. (2014). Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewi O (1921). Über humorale übertragbarkeit der Herznervenwirkung. Pflügers Arch 189, 239–242. [Google Scholar]
- Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, et al. (2009). The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron 64, 885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow CL (2015). Laryngeal Reflexes: Physiology, Technique, and Clinical Use. J Clin Neurophysiol 32, 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XC, Chen ZH, Xue JB, Zhao DX, Lu C, Li YH, Li SM, Du YW, Liu Q, Wang P, et al. (2019). Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proceedings of the National Academy of Sciences of the United States of America 116, 5564–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias D, Cowburn AS, Torres-Torrelo H, Ortega-Saenz P, Lopez-Barneo J, and Johnson RS (2018). HIF-2alpha is essential for carotid body development and function. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marey EJ (1863). Physiologie Medicale de la Circulation due Sang. Paris: Adrien Delahaye, 202–226. [Google Scholar]
- Mazzone SB, Tian L, Moe AAK, Trewella MW, Ritchie ME, and McGovern AE (2019). Transcriptional Profiling of Individual Airway Projecting Vagal Sensory Neurons. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, and Undem BJ (2016). Vagal Afferent Innervation of the Airways in Health and Disease. Physiological reviews 96, 975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern AE, Driessen AK, Simmons DG, Powell J, Davis-Poynter N, Farrell MJ, and Mazzone SB (2015). Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system. J Neurosci 35, 7041–7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, and Kasahara M (1964). Studies on the Nerve Endings in the Heart. Am J Anat 115, 217–233. [DOI] [PubMed] [Google Scholar]
- Min S, Chang RB, Prescott SL, Beeler B, Joshi NR, Strochlic DE, and Liberles SD (2019). Arterial Baroreceptors Sense Blood Pressure through Decorated Aortic Claws. Cell Rep 29, 2192–2201 e2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S, Oh Y, Verma P, Whitehead SC, Yapici N, Van Vactor D, Suh GS, and Liberles S (2021). Control of feeding by Piezo-mediated gut mechanosensation in Drosophila. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Dominguez A, Ortega-Saenz P, Gao L, Colinas O, Garcia-Flores P, Bonilla-Henao V, Aragones J, Huttemann M, Grossman LI, Weissmann N, et al. (2020). Acute O2 sensing through HIF2alpha-dependent expression of atypical cytochrome oxidase subunits in arterial chemoreceptors. Sci Signal 13. [DOI] [PubMed] [Google Scholar]
- Moriya R, Shirakura T, Ito J, Mashiko S, and Seo T (2009). Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab 297, E1358–1365. [DOI] [PubMed] [Google Scholar]
- Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, Pellegrino K, Del Marmol J, Castro TBR, Furuichi M, et al. (2020). Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature 583, 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narula M, McGovern AE, Yang SK, Farrell MJ, and Mazzone SB (2014). Afferent neural pathways mediating cough in animals and humans. J Thorac Dis 6, S712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhuber WL (1987). Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J Auton Nerv Syst 20, 243–255. [DOI] [PubMed] [Google Scholar]
- Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, and Patapoutian A (2017). Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuri T, Horio N, Stratford JM, Finger TE, and Ninomiya Y (2012). Residual chemoresponsiveness to acids in the superior laryngeal nerve in “taste-blind” (P2X2/P2X3 double-KO) mice. Chemical senses 37, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Saenz P, and Lopez-Barneo J (2020). Physiology of the Carotid Body: From Molecules to Disease. Annu Rev Physiol 82, 127–149. [DOI] [PubMed] [Google Scholar]
- Ortega-Saenz P, Pardal R, Garcia-Fernandez M, and Lopez-Barneo J (2003). Rotenone selectively occludes sensitivity to hypoxia in rat carotid body glomus cells. J Physiol 548, 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, and Blackshaw LA (2005). Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 54, 1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal AS (1953). A study of right and left atrial receptors. J Physiol 120, 596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal AS (1954). A study of gastric stretch receptors; their role in the peripheral mechanism of satiation of hunger and thirst. J Physiol 126, 255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R, Ortega-Saenz P, Duran R, and Lopez-Barneo J (2007). Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell 131, 364–377. [DOI] [PubMed] [Google Scholar]
- Paton JF (1999). The Sharpey-Schafer prize lecture: nucleus tractus solitarii: integrating structures. Experimental Physiology, 815–833. [PubMed] [Google Scholar]
- Pavlov VA, and Tracey KJ (2017). Neural regulation of immunity: molecular mechanisms and clinical translation. Nature neuroscience 20, 156–166. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Hickey RF, Graf PD, and Nadel JA (1971). Hering-Breuer inflation reflex and regulation of breathing in conscious dogs. J Appl Physiol 31, 746–750. [DOI] [PubMed] [Google Scholar]
- Powley TL, and Phillips RJ (2011). Vagal intramuscular array afferents form complexes with interstitial cells of Cajal in gastrointestinal smooth muscle: analogues of muscle spindle organs? Neuroscience 186, 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ, and Nanduri J (2018). Recent advances in understanding the physiology of hypoxic sensing by the carotid body. F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SL, Umans BD, Williams EK, Brust RD, and Liberles SD (2020). An Airway Protection Program Revealed by Sweeping Genetic Control of Vagal Afferents. Cell 181, 574–589 e514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Chen JD, Zhang J, and Foreman RD (2007). Duodenal afferent input converges onto T9–T10 spinal neurons responding to gastric distension in rats. Brain research 1186, 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab M, and Neuhuber WL (2007). Glutamatergic functions of primary afferent neurons with special emphasis on vagal afferents. Int Rev Cytol 256, 223–275. [DOI] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, et al. (2014). Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees PM (1968). Electron microscopical observations on the architecture of the carotid arterial walls, with special reference to the sinus portion. J Anat 103, 35–47. [PMC free article] [PubMed] [Google Scholar]
- Reid IA (1992). Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol 262, E763–778. [DOI] [PubMed] [Google Scholar]
- Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, and Gribble FM (2008). Glucose sensing in L cells: a primary cell study. Cell Metab 8, 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards P, Thornberry NA, and Pinto S (2021). The gut-brain axis: Identifying new therapeutic approaches for type 2 diabetes, obesity, and related disorders. Mol Metab 46, 101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, and Daniel H (2014). The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One 9, e89977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo J, Hernandez J, Vidal MA, and Pedrosa JA (1975). Vegetative innervation of the esophagus. II. Intraganglionic laminar endings. Acta Anat (Basel) 92, 79–100. [DOI] [PubMed] [Google Scholar]
- Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, and Burnstock G (2003). Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci 23, 11315–11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl CR, Pasko BL, Khan HS, Kindt LM, Stamm CE, Franco LH, Hsia CC, Zhou M, Davis CR, Qin T, et al. (2020). Mycobacterium tuberculosis Sulfolipid-1 Activates Nociceptive Neurons and Induces Cough. Cell 181, 293–305 e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini PV, Frikke-Schmidt H, Arthurs J, Gordian D, Patel A, Rupp AC, Adams JM, Wang J, Beck Jorgensen S, Olson DP, et al. (2021). GFRAL-expressing neurons suppress food intake via aversive pathways. Proceedings of the National Academy of Sciences of the United States of America 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso LF, Kim DY, and Paydarfar D (2019). Sensory dysphagia: A case series and proposed classification of an under recognized swallowing disorder. Head Neck 41, E71–E78. [DOI] [PubMed] [Google Scholar]
- Schelegle ES, and Green JF (2001). An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir Physiol 125, 17–31. [DOI] [PubMed] [Google Scholar]
- Schneider C, O’Leary CE, von Moltke J, Liang HE, Ang QY, Turnbaugh PJ, Radhakrishnan S, Pellizzon M, Ma A, and Locksley RM (2018). A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell 174, 271–284 e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Koepsell H, and Ackroff K (2016). SGLT1 sugar transporter/sensor is required for post-oral glucose appetition. Am J Physiol Regul Integr Comp Physiol 310, R631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Zukerman S, and Ackroff K (2015). Postoral glucose sensing, not caloric content, determines sugar reward in C57BL/6J mice. Chemical senses 40, 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai T, Miyaoka Y, Ikarashi R, and Shimada K (1989). Swallowing reflex elicited by water and taste solutions in humans. Am J Physiol 256, R822–826. [DOI] [PubMed] [Google Scholar]
- Smith HS, Cox LR, and Smith EJ (2012). 5-HT3 receptor antagonists for the treatment of nausea/vomiting. Ann Palliat Med 1, 115–120. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Kerrin A, Zagorodnyuk VP, Hennig GW, Muto M, Brookes SJ, and McDonnell O (2008). Identification of functional intramuscular rectal mechanoreceptors in aganglionic rectal smooth muscle from piebald lethal mice. American journal of physiology Gastrointestinal and liver physiology 294, G855–867. [DOI] [PubMed] [Google Scholar]
- Springall DR, Cadieux A, Oliveira H, Su H, Royston D, and Polak JM (1987). Retrograde tracing shows that CGRP-immunoreactive nerves of rat trachea and lung originate from vagal and dorsal root ganglia. J Auton Nerv Syst 20, 155–166. [DOI] [PubMed] [Google Scholar]
- Storey AT (1968). A functional analysis of sensory units innervating epiglottis and larynx. Exp Neurol 20, 366–383. [DOI] [PubMed] [Google Scholar]
- Su Y, Barr J, Jaquish A, Xu J, Verheyden JM, and Sun X (2021). Mapping the Central and Peripheral Projections of Lung Innervating Sensory Neurons. Biorxiv, 2021.2005.2005.442702. [Google Scholar]
- Sun H, Li DP, Chen SR, Hittelman WN, and Pan HL (2009). Sensing of blood pressure increase by transient receptor potential vanilloid 1 receptors on baroreceptors. J Pharmacol Exp Ther 331, 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, Martinac B, and Patapoutian A (2016). Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep 17, 1739–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczot M, Nickolls AR, Lam RM, and Chesler AT (2021). The Form and Function of PIEZO2. Annu Rev Biochem 90, 507–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha BH, Simon PM, Dempsey JA, Skatrud JB, and Iber C (1995). Respiratory sinus arrhythmia in humans: an obligatory role for vagal feedback from the lungs. J Appl Physiol (1985) 78, 638–645. [DOI] [PubMed] [Google Scholar]
- Tan HE, Sisti AC, Jin H, Vignovich M, Villavicencio M, Tsang KS, Goffer Y, and Zuker CS (2020). The gut-brain axis mediates sugar preference. Nature 580, 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Campbell JN, Tsai LT, Wu C, Liberles SD, and Lowell BB (2021). Highly selective brain-to-gut communication via genetically defined vagus neurons. Neuron 109, 2106–2115 e2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, et al. (2009). TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10, 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, and Finger TE (2010). Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proceedings of the National Academy of Sciences of the United States of America 107, 3210–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, and Gribble FM (2012). Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba F, and Alcayaga J (1986). Nerve branching and terminal arborizations in the carotid body of the cat. A light microscopic study following anterograde injury filling of carotid nerve axons with horseradish peroxidase. Neuroscience 19, 581–595. [DOI] [PubMed] [Google Scholar]
- Trankner D, Hahne N, Sugino K, Hoon MA, and Zuker C (2014). Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proceedings of the National Academy of Sciences of the United States of America 111, 11515–11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, and Rogers RC (2006). Brainstem circuits regulating gastric function. Annu Rev Physiol 68, 279–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umans BD, and Liberles SD (2018). Neural Sensing of Organ Volume. Trends in neurosciences 41, 911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von D.a.M., Andres KH, and Iravani J (1974). The fine structure of the pulmonary stretch receptor in the rat. Kidney Int 5, 215–222. [DOI] [PubMed] [Google Scholar]
- Waise TMZ, Dranse HJ, and Lam TKT (2018). The metabolic role of vagal afferent innervation. Nature reviews Gastroenterology & hepatology 15, 625–636. [DOI] [PubMed] [Google Scholar]
- Wang J, Kollarik M, Ru F, Sun H, McNeil B, Dong X, Stephens G, Korolevich S, Brohawn P, Kolbeck R, et al. (2017). Distinct and common expression of receptors for inflammatory mediators in vagal nodose versus jugular capsaicin-sensitive/TRPV1-positive neurons detected by low input RNA sequencing. PLoS One 12, e0185985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Jia Y, Liu T, Jan YN, and Zhang W (2020). Visceral Mechano-sensing Neurons Control Drosophila Feeding by Using Piezo as a Sensor. Neuron 108, 640–650 e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrwein EA, and Joyner MJ (2013). Regulation of blood pressure by the arterial baroreflex and autonomic nervous system. Handb Clin Neurol 117, 89–102. [DOI] [PubMed] [Google Scholar]
- Weijs TJ, Ruurda JP, Luyer MD, Nieuwenhuijzen GA, van Hillegersberg R, and Bleys RL (2015). Topography and extent of pulmonary vagus nerve supply with respect to transthoracic oesophagectomy. J Anat 227, 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J (2006). Reflexes from the lungs and airways: historical perspective. J Appl Physiol (1985) 101, 628–634. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG (1954). Respiratory reflexes from the trachea and bronchi of the cat. J Physiol 123, 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, and Liberles SD (2016). Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell 166, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing AE, and Berthoud HR (1997). Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol 272, R59–67. [DOI] [PubMed] [Google Scholar]
- Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, and Patapoutian A (2015). Piezo2 is the principal mechanotransduction channel for proprioception. Nature neuroscience 18, 1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J (2000). Spectrum of myelinated pulmonary afferents. Am J Physiol Regul Integr Comp Physiol 279, R2142–2148. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, and Brookes SJ (2001). Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol 534, 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Costa M, and Brookes SJ (2003). Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol 553, 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD, and Patapoutian A (2018). PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science (New York, NY 362, 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Kaye JA, Cai Z, Wang Y, Prescott SL, and Liberles SD (2021). Area Postrema Cell Types that Mediate Nausea-Associated Behaviors. Neuron 109, 461–472 e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CA, Huey EL, Ahn JS, Beutler LR, Tan CL, Kosar S, Bai L, Chen Y, Corpuz TV, Madisen L, et al. (2019). A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature 568, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]


