Abstract
Bone remodeling is a lifelong process that gives rise to a mature, dynamic bone structure via a balance between bone formation by osteoblasts and resorption by osteoclasts. These opposite processes allow the accommodation of bones to dynamic mechanical forces, altering bone mass in response to changing conditions. Mechanical forces are indispensable for bone homeostasis; skeletal formation, resorption, and adaptation are dependent on mechanical signals, and loss of mechanical stimulation can therefore significantly weaken the bone structure, causing disuse osteoporosis and increasing the risk of fracture. The exact mechanisms by which the body senses and transduces mechanical forces to regulate bone remodeling have long been an active area of study among researchers and clinicians. Such research will lead to a deeper understanding of bone disorders and identify new strategies for skeletal rejuvenation. Here, we will discuss the mechanical properties, mechanosensitive cell populations, and mechanotransducive signaling pathways of the skeletal system.
Subject terms: Bone, Bone quality and biomechanics
Introduction
The skeleton effectively supports the movements of the body and is thus continuously subjected to various environmental forces. In 1892, Julius Wolff proposed that bone density adapts to the mechanical forces placed on the bone.1 Much later, Harold Frost clarified this observation and described a control circuit called the ‘mechanostat’ that links the degree of strain induced by mechanical forces to skeletal remodeling.2 Long-term high-intensity exercises, for example, strengthen bone in the supporting part of the body. In contrast, long exposure to microgravity can lead to a monthly loss of 1%–1.5% of volumetric bone mineral density in the weight-bearing bones due to increased bone resorption and decreased bone formation.3,4 In addition, long-term bed rest and paralysis can substantially reduce the mechanical stress placed on the bone, thus resulting in disuse osteoporosis.5 The progress made in skeletal mechanobiology can lead to a deeper understanding of bone disorders and identify new strategies for skeletal regeneration. Accordingly, in this review, we will discuss the effects of mechanical forces on bone homeostasis and highlight mechanosensitive signaling pathways.
Mechanosensitive cells
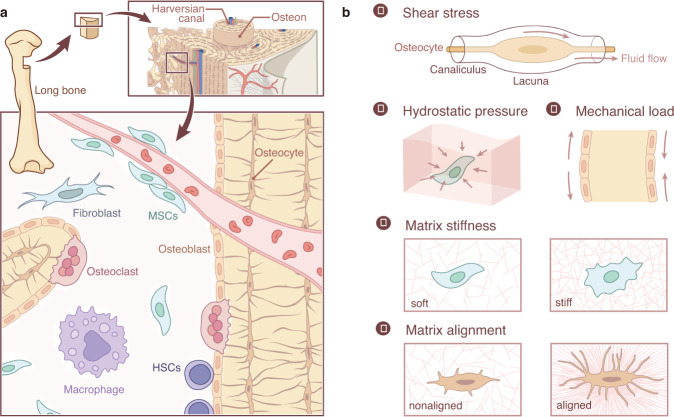
The bone is composed of approximately 10% cells, 60% minerals, and 30% organic matter. This composition can effectively maintain the mechanical properties of bone.6 Mature bone consists primarily of trabecular bone and cortical bone. The trabecular bone is mainly located in the epiphysis and inside the flat bone. The direction of the trabecular bone structure is consistent with the main stress trace of the bone, and its arrangement and thickness are adjusted to provide optimal load-bearing properties. Cortical bone is a dense external layer of bone that is critical for body structure and weight-bearing due to its high resistance to bending and torsion. In humans, cortical bone is composed of cylindrical structures called osteons. The osteon comprises a central canal filled with nerves and blood vessels surrounded by concentric lamellae. Circumferential lamellae are inserted between the osteons. Osteocytes are buried within fibrous mineralized lamellae. Rather than mineralizing, osteocytes form small chambers (lacunae) and fluid-filled pipes (canaliculi) that provide a structural basis for the generation of fluid shear stress (FSS). Movement and other mechanical forces can result in FSS, bone deformation, and modulation of the alignment of bone extracellular matrix (Fig. 1a). Because of the dynamics and complexity of mechanical forces, it is difficult to observe the reaction of cells to specific forces in vivo. To circumvent this difficulty, researchers have designed in vitro systems to allow the application of several kinds of mechanical variables, including FSS, hydrostatic pressure, mechanical load, matrix stiffness, and matrix topology, and to investigate subsequent cellular behavior (Fig. 1b). Here, we will discuss the current understanding of mechanosensitive cells and their mechanical environments.
Fig. 1.
The structural basis of mechanical stress in skeletal cells. a The skeletal system contains osteoblasts, osteoclasts, osteocytes, and their progenitors, all sensitive to mechanical stimuli. Osteocytes are the most common mechanical sensors among these cells due to their structure and location in the bone matrix. Mesenchymal stem cells and osteoblast progenitors can sense the FSS in the bone marrow cavity and the strain on the bone. Osteoclasts are both mechanosensitive cells and the effectors for other mechanosensitive cells. b. The mechanical stimuli placed on bone may include shear stress, hydrostatic pressure, mechanical stretch and tension, matrix stiffness, and matrix alignment.
Osteocytes constitute 85%–90% of all adult bone cells. These cells sense mechanical stimuli through their cell bodies, dendrites, and cilia and can then transmit signals through cellular dendrites and secreted proteins in an autocrine and paracrine manner.7–9 The mechanosensitive characteristics of osteocytes in the regulation of bone homeostasis have been discussed in detail elsewhere.10,11 During mechanical loading, poroelastic interactions within the lacunar-canalicular system (LCS) result in fluid flow that is primarily sensed by osteocytes. In addition to fluid flow in the LCS, dynamic intramedullary pressurization results in fluid flow in the marrow cavity and in the endosteal surface, potentially exposing bone marrow mesenchymal stem cells (BMSCs) and bone lining cells to fluid flow. Modulating intramedullary pressure can enhance the structural adaptation of trabecular bone in mice with ablated osteocytes.12 These mice have been shown to be resistant to unloading-induced bone loss, providing strong evidence for the role of osteocytes in mechanotransduction.13 Osteoclast activity was also increased in these mice due to upregulated expression of RANKL, a cytokine associated with osteoclast development.13 Recently, osteoclasts were shown to sense the damage-associated molecular patterns (DAMPs) released by necrotic osteocytes.14 Thus, mechanical unloading-associated bone loss could be a result of crosstalk between osteocytes and osteoclasts.
Multiple mechanical forces can drive the proliferation and differentiation of BMSCs.15,16 According to several in vitro studies,17–19 mechanical forces can also induce substantial changes in the osteoblast cytoskeleton, cell and nucleus morphology, and volume. FSS increased the expression of osteogenic genes in mesenchymal stem cells.20,21 Similarly, oscillatory fluid flow led to specific gene expression in BMSCs in a manner dependent on the magnitude, frequency, and duration of shear stress. Short-term fluid flow stimulation promoted the expression of Cox2, Opn, and Runx2 in the early stage of osteogenesis, while long-term stimulation increased the formation of collagen and matrix in the late stage.17
Osteoclasts are also mechanosensitive cells. FSS has been reported to affect the cell morphology and gene expression of osteoclasts and to inhibit their differentiation without affecting their viability.22–24 FSS also stimulates nitric oxide (NO) and prostaglandin E2 (PGE2) production in bone marrow-derived preosteoclast-like cells.25 Studies of ion channels also provide evidence of the mechanosensitive capabilities of osteoclasts. STIM1 and TRPV4 are Ca2+ channels that are highly expressed in the early and late stages, respectively, of osteoclast differentiation. Blockage of STIM1 or TRPV4 at these respective stages caused a reduction in FSS-induced Ca2+ oscillations to almost undetectable levels, thus indicating that STIM1 and TRPV4 may act as mechanosensitive ion channels for osteoclasts.26 Another recent study showed that fluid flow-derived mechanical stimuli can enhance or suppress the formation of osteoclasts from hematopoietic progenitor cells in a manner dependent on the shear stress rate, amplitude, and duration.27 These observations indicate that osteoclasts and their progenitors can respond to specific mechanical stresses in the bone niche (Fig. 1a). Together, these data demonstrate that bone possesses multiple mechanosensitive cellular populations. The activity of these cells in response to mechanical stimuli can have significant effects on the skeletal system.
Mechanosensitive structures
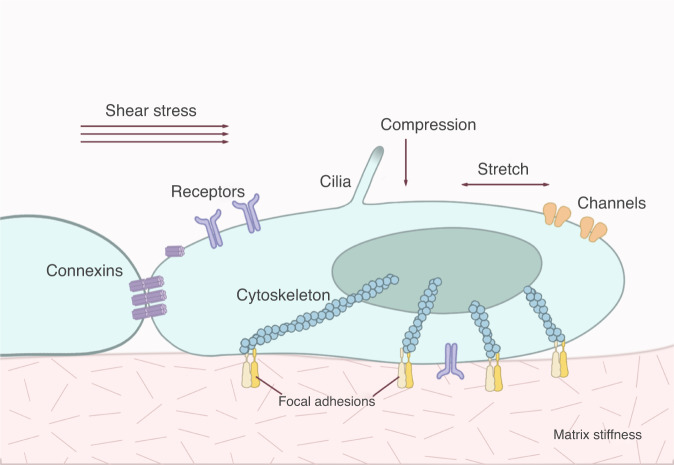
Mechanoreceptors can sense a variety of external and internal mechanical forces. The detection of external mechanical signals requires mechanoreceptors to be in direct contact with the external environment or to sense changes in the intermediate media, such as those in cell membranes caused by pressure and shear forces. Various cell surface proteins or membrane structures, including integrins, ion channels, connexons, G-protein coupled receptors, and primary cilia, are considered potential mechanosensitive structures (Fig. 2). These structures share certain common features: first, the structures can directly sense single or multiple mechanical forces; second, these mechanical forces can modulate the conformation or activity of structures to activate downstream signaling pathways and direct cell behaviors. Below, we discuss these mechanosensitive structures, downstream signaling pathways, and corresponding cell behaviors in the skeletal system. All molecules and animal models discussed in this review are listed in Tables 1 and 2.
Fig. 2.
Forces and cellular structures involved in mechanosensation. Mechanical stresses of varying type and intensity are sensed by different families of cellular structures, including integrins, receptors, ion channels, connexins, and cilia.
Table 1.
Mechanosensors reported by in vitro or in vivo studies
| Type of study | Molecule | Cell type | Reference |
|---|---|---|---|
| in vitro | αvβ3 | MLO-Y4 | 46 |
| in vitro | β1 | MG-63 cells | 47,48 |
| in vivo | Cilium | 83,86 | |
| in vitro | Cilium | MC3T3-E1/MLO-Y4 | 87,88 |
| in vitro | CX43 | ROS 17/2.8 cell line | 68 |
| in vitro | CX43 | MLO-Y4 | 19,69 |
| in vivo | CX43 | 70,71,73–75,77 | |
| in vitro | FAK | 45 | |
| in vitro | MACF1 | MC3T3-E1 | 149,150,151 |
| in vivo | OPN | 53 | |
| in vitro | PTH1R | MC3T3-E1 | 103 |
| in vivo | PIEZO1/2 | 121–123,125 | |
| in vitro | STIM1 | osteoclasts | 26 |
| in vitro | TRPV4 | osteoclasts | 26,140 |
| in vivo | TRPV4 | osteoclasts | 137–139 |
| in vitro | TRPV4 | MSCs | 132–134 |
| in vivo | TRPV4 | 127 |
Table 2.
Mouse bone phenotypes resulting from specific gene knockouts (KO) or mutations
| Mice | Phenotype | Reference |
|---|---|---|
| Conditional osteocyte ablation (DMP1-Diphtheria toxin receptor) | Bone loss with defective mechanotransduction. | 12,13 |
|
Conditional deletion of integrin β1 in osteoblasts (Col1 (2.3 kb)-Cre; β1fl/fl CKO) |
Normal in the physiological state; resist bone loss and increase whole bone stiffness and strength in the hindlimb unloading model. | 52 |
| OPN KO | Slight bone loss; osteoblasts and osteoclasts are not altered in the unloading condition. | 53 |
| Cx43 KO | Osteoblast dysfunction, delayed mineralization, and craniofacial abnormalities. | 70 |
|
Conditional deletion of Cx43 in osteoblasts (Col1 (2.3 kb)-Cre; Cx43fl/fl CKO) |
Bone loss, osteoblast function impairment, and reduction in anabolic response to mechanical loading in vivo. | 73 |
|
Conditional deletion of Cx43 in osteocytes (Dmp1 (8 kb)-Cre; Cx43fl/fl CKO) |
Bone loss and osteoblast function impairment; enhanced periosteal bone formation rate and mineral apposition rate; higher mineralizing surface in response to mechanical loading. | 72 |
|
Conditional deletion of Cx43 in osteocytes (Ocn-Cre; Cx43fl/fl CKO) |
Increased bone resorption through regulation of the RANKL/OPG ratio; enhanced anabolic response to mechanical loading. | 74 |
|
Conditional deletion of Cx43 in osteocytes (Ocn-Cre; Cx43fl/fl CKO) |
Preserved trabecular bone mass and cortical bone formation rate in CKO mice in the unloading model. | 75,76 |
|
Conditional deletion of Cx43 in osteocytes (Dmp1 (10 kb)-Cre; Cx43fl/fl CKO) |
Cortical bone loss with increased endocortical osteoclast activity during unloading. | 77 |
| R76W mutation of Cx43 | Increased endocortical osteoclast activity and periosteal osteoclasts with decreased apoptotic osteocytes during unloading. | 77 |
|
Conditional deletion of IFT88 in tenocytes (Scx-Cre, IFT88fl/fl CKO) |
Fibrocartilage cell phenotype in the tendon; thinner and less mineralized cortical bone. | 92 |
| PIEZO1 KO | Embryonic lethal. | 106 |
|
Conditional deletion of PIEZO1 in osteocytes (OCN-Cre; PIEZO1fl/fl CKO) |
Disruption of osteogenesis in osteoblasts. | 121 |
|
Conditional deletion of PIEZO1 in mesenchymal stem cells (Prrx1-Cre; PIEZO1fl/fl CKO) |
Strong hyperactivity of osteoclasts and subtle differences in osteoblastogenesis in PIEZO1-deficient osteoblasts. | 123 |
|
Conditional deletion of PIEZO1 and PIEZO2 in mesenchymal stem cells (Prrx1-Cre; PIEZO1fl/fl, PIEZO2fl/fl DKO) |
Severe bone defects. | 125 |
|
Conditional deletion of PIEZO2 in mesenchymal stem cells (Prrx1-Cre; PIEZO2fl/fl CKO) |
Subtle impact on the bone. | 125 |
|
Conditional deletion of YAP and TAZ in osteoblast progenitors (Osterix-Cre; YAPfl/fl, TAZfl/fl DKO) |
Reduction of the number of osteoblasts and increased osteoclast activity; allele dose-dependent perinatal skeletal deformity. | 188 |
| YAP KO | Embryonic lethal. | 189 |
| TAZ KO | Live to maturity with modest skeletal defects and polycystic kidney disease. | 190 |
| LRP5 G171V transgenic mice | More robust bone formation in response to loading. | 166 |
|
Conditional deletion of BMP2 in osteoblasts (Col1-Cre; BMP2fl/fl CKO) |
Reduced bone formation rate and increased bone brittleness. | 199 |
|
Conditional deletion of HIF-1α in osteocytes (OC-Cre; HIF-1αfl/fl CKO) |
Thinner bones with reduced vascularization. | 212 |
The extracellular matrix creates the mechanical niches
The fates and functions of bone cells are determined by the physical and chemical properties of their niches. The niche is a three-dimensional structure consisting of extracellular matrix (ECM) components together with the cells they surround and connect.28 The ECM itself is composed of collagens, fibronectin, elastin, laminin, glycosaminoglycan, and glycoproteins. This structure provides a three-dimensional topological environment, an alterable stiffness, and various signaling molecules for the cells.
The mechanical properties of the ECM strongly affect the behavior of cells, including BMSCs, osteoblasts, osteoclasts, and osteocytes. Human mesenchymal stem cells (hMSCs), for instance, can be readily switched from adipogenesis to osteogenesis simply by changing the matrix stiffness.29 Osteoblast maturation progressively increases on compact preosteoblast-derived ECM (PDM, a natural self-assembled ECM network), while the same is true for osteoclasts on loose PDM.30 This observation also suggests that ECM crosslinking density acts as an underlying force in bone remodeling. Osteocytes are involved in the development and maintenance of the ECM. They are embedded in a continuous matrix and maintain a fluid-filled gap of 50–80 nm between the calcified matrix and the cell membrane; this gap is essential for transporting nutrients and oxygen and generating mechanical signals. Precise regulation of calcification and elongation is also particularly important for osteocytes.28 Osteocytes on stiff ECM tend to pull harder than those on soft ECM, thus increasing the tension on force-bearing elements such as F-actin.31,32 F-actin serves as both an element of mechanosensing and an effector of mechanotransduction and is the predominant regulator of YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif).33 These two transcriptional regulators can respond to a broad range of mechanical cues, bridging mechanical signals between the ECM and the cells within the matrix.34,35 Accordingly, the ECM constitutes a well-established mechanical niche for bone cells.
Focal adhesions
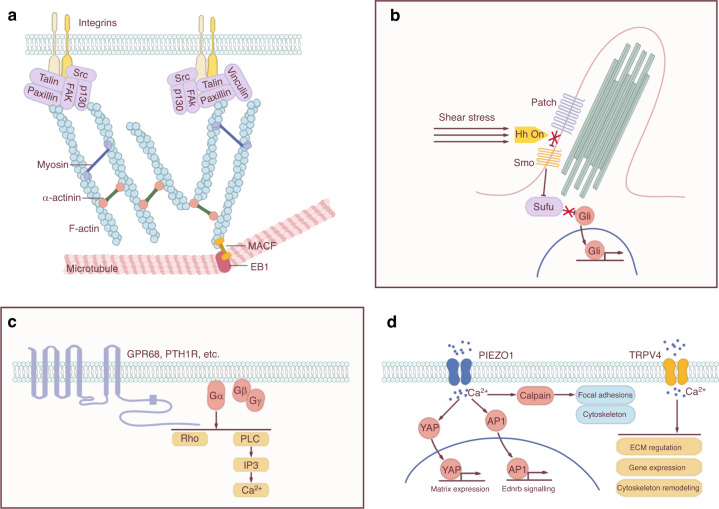
Focal contacts situated at ECM-integrin junctions form direct mechanical links between cells and the ECM. These structures allow the transfer of signals from the external matrix to the cytoskeleton and facilitate cell adhesion, spreading, and migration. Focal contacts are composed of numerous proteins, including integrins, cadherins, focal adhesion kinase (FAK), and various other ECM and cytoskeletal proteins (Fig. 3a). Their involvement in mechanosensitive signaling pathways highlights the importance of these proteins in skeletal homeostasis.
Fig. 3.
Specific mechanosensitive structures. a Focal adhesions. Focal adhesions connect ECM mechanical signals to the cytoskeleton, affecting cytoskeletal arrangement and crosslinking. b Cilium. Cilia usually coordinate with the Hedgehog (Hh) signaling pathway to transmit mechanical signals. c GPCRs. GPCRs containing a C-terminal helix 8 can sense mechanical stimuli. Activation of the GPCR initiates a series of signal transductions, including the Rho-Rock and PLC-IP3 pathways. d Ion channels. Activation of ion channels by mechanical stimuli elicits specific ion flow, especially calcium influx, to modulate downstream signaling pathways.
Integrins are heterodimeric transmembrane receptors consisting of α and β subunits. These molecules functionally link the ECM to the cytoskeleton, thereby transferring mechanical stimuli from the extracellular environment to the internal cellular components. In vertebrates, there are eighteen α and eight β integrin subunits that assemble 24 distinct complexes with diverse nonredundant functions.36 Each subunit is a single-span transmembrane glycoprotein composed of a large extracellular segment containing the ligand-binding site, a transmembrane fragment, and a short cytoplasmic domain that mediates interaction with the cytoskeleton or intracellular signaling proteins (except for integrin β4).37 Previous studies have suggested that human primary bone cells express multiple integrin subunits, including α1, α2, α3, α4, α5, α6, αv, β1, β3 and β5.38–40 The α2, αv, β1, and β3 subunits have been reported to participate in the sensing of mechanical forces.41,42
Different integrin heterodimers have specific affinities for ECM ligands such as fibronectin, collagen, and laminin. A series of structural studies have shown that integrins have two allosteric conformations, and their activation is mediated by conformational changes that shift the heterodimer from a low-affinity (inactive) state to a high-affinity (active) state.43,44 Integrin aggregation leads to the recruitment and phosphorylation of FAK, which provides a platform for various intermediate proteins (such as MAPK/ERK, JNK, Src kinase, and GTPases) to activate signaling pathways and mediate mechanical signal transduction (Fig. 3a).45 For example, blockade of integrin αvβ3 in cultured mouse osteocytes (MLO-Y4 cells) impaired their chemosensitivity to laminar oscillatory fluid flow stimulus, resulting in disrupted COX-2 expression and PGE2 release.46 In human osteosarcoma cells (MG-63) cultured on fibronectin, inhibition of β1 or αvβ3 also reduced the shear stress-induced phosphorylation of ERK, JNK, and p38 and upregulated the expression of osteogenic markers.47 The activation of ERK by FSS regulated the activity of the RUNX2 transcription factor (associated with osteoblast differentiation) and promoted the expression of β1 via the NF-kB pathway in hMSCs.48 Integrins αvβ3 and α2β1 are highly expressed in osteoclasts. They can respond to RGD motif-containing ligands in the bone matrix to regulate osteoclast differentiation and function.49–51 However, whether αvβ3 and α2β1 are mechanosensitive in osteoclasts is unknown.
Knockout of integrins leads to embryonic lethality, which limits the in vivo study of bone biology. Integrin β1 conditional knockout mice exhibited a normal phenotype under physiological conditions. However, these mice do not experience bone loss in the hindlimb unloading model compared with wild-type mice; in contrast, they expand both total bone volume and marrow volume and increase whole bone stiffness and strength.52 Osteopontin (OPN), which is produced by osteoblasts and osteoclasts, is one of the integrin ligands present in bone. Loss of OPN caused resistance to bone loss in the unloading model. Osteoblasts and osteoclasts were not altered in OPN knockout mice during unloading, suggesting that OPN is a prerequisite for the activation of osteoclastic bone resorption and the reduction in osteoblastic bone formation in this condition.53
As mentioned above, FAK is the primary protein that integrates extracellular stimuli with intracellular activities. FAK can sense the mechanical forces generated either internally or externally on cells,54 and inactivation of FAK causes defective developmental morphogenesis in mice.55 Loss of FAK also disrupted microtubule polarization within cells by impairing focal contact turnover through the FAK-mediated regulation of Rho-family GTPases.56,57 Rho-family GTPases are molecular ‘switches’ that control the assembly and disassembly of actin cytoskeletal structures (stress fibers, lamellipodia, and filopodia).57,58 RhoA, a member of the Rho family, has been widely implicated in mechanosensitive signaling pathways. ECM stiffness can regulate the activity and expression of RUNX2 through the activation of RhoA/Rho-associated protein kinase (ROCK) and its downstream ERK signaling pathway, thereby promoting osteogenesis.59 The RhoA signaling pathway can also activate the Akt and p38/MAPK signaling pathways, establishing a connection between integrins and phosphoinositide 3-kinase (PI3K)/MAPK signaling pathways.60
FAK-mediated mechanotransduction in skeletal stem cells activates new bone formation. During mandibular distraction osteogenesis, a surgery that separates the jaw bone to correct an undersized lower jaw, mechanical stress activates new bone formation via FAK in skeletal stem cells.61 In osteocytes, sclerostin (Sost) can be repressed by mechanical loading through the histone deacetylases HDAC4 and HDAC5.62 During this process, FSS triggers FAK inactivation via dephosphorylation, thus driving the nuclear translocation of class IIa HDAC by inhibition of HDAC5 tyrosine 642 phosphorylation.63 Similarly, pharmacologic FAK catalytic inhibition reduces Sost mRNA expression in vitro and in vivo.63 FAK also responds to nonmechanical signals, thus overriding mechanical stimuli. The interaction between bone morphogenetic protein-2 (BMP-2) and BMP-2 receptors activates αvβ3 integrin and mediates cell spreading and cell migration independent of matrix stiffness.64 In turn, αvβ3 is required to inhibit glycogen synthase kinase-3β (GSK-3β) activity through the Src–FAK–ILK pathway. BMP receptors and β3 integrin signaling converge to control both focal adhesion dynamics and Smad signaling, coupling cell migration and fate commitment regardless of matrix stiffness.64 Relatedly, the bioactive core vitronectin-derived peptide VnP-16 promotes bone formation by accelerating osteoblast differentiation and activity through direct interaction with β1 integrin followed by FAK activation. VnP-16 also inhibits bone resorption by restraining JNK-c-Fos-NFATc1-induced osteoclast differentiation and αvβ3 integrin-c-Src-PYK2-mediated resorptive function.65 These investigations address the importance of FAK as a core hub in skeletal mechanobiology.
Connexons and Cx43
As intercellular channels, gap junctions facilitate signal transduction between cells, allowing small molecules (less than 1 kDa) and electrical current to passively diffuse between adjacent cells in response to extracellular stimuli. Conventional gap junctions consist of two docking hexagonal connexons, both composed of six connexin molecules.66 More than 20 connexins with different expression patterns and properties have been discovered in humans and rodents. Diverse connexins are expressed in bone cells, with connexin 43 (Cx43) being one of the most abundant subtypes expressed in osteoblasts, osteocytes, and osteoclasts.67
Mechanical stretching can increase the abundance of phosphorylated Cx43 without changing its mRNA expression level in osteoblast-like cells (ROS 17/2.8 cell line).68 Another study found that FSS can drive intracellular Cx43 redistribution from a location around the nucleus into the cytoplasm and dendrites. The Cx43 protein level also significantly increased after exposure to FSS from a laminar flow of 16 dynes per cm2.69 These results indicate that Cx43 has an essential role in sensing mechanical stimuli. In MLO-Y4 cells, conformational activation of integrin α5β1, partially through PI3K activation, allowed it to interact with Cx43. This integrin α5β1 activation is necessary for the mechanical stimulation-induced opening of the Cx43 hemichannel,19 which indicates that Cx43 alone cannot sense mechanical stress. These stress-sensing structures are closely coordinated to reinforce cell mechanosensitivity.
Numerous studies have highlighted the importance of Cx43 for normal bone formation, and different Cx43 loss-of-function models have led to different conclusions. Cx43−/− mice exhibited osteoblast dysfunction, delayed mineralization, and craniofacial abnormalities.70 Conditional deletion of Cx43 by Col1 (2.3 kb)-Cre or Dmp1 (8 kb)-Cre resulted in bone loss and osteoblast function impairment.71,72 Regarding mechanical responsiveness, Grimston et al. found that osteoblast-specific Cx43 deficiency driven by Col1 (2.3 kb)-Cre reduces the anabolic response to mechanical loading in vivo.73 In contrast, Zhang et al. showed that the Cre-dependent loss of Cx43 in osteocytes/osteoblasts by Ocn-Cre increases osteolytic function via regulation of the RANKL/OPG ratio (a critical determinant of bone mass) and enhancement of the anabolic response to mechanical loading.74 Lloyd et al. performed unloading experiments with hindlimb suspension, showing that Cx43 deficiency in osteocytes and osteoblasts protects against trabecular bone loss during mechanical unloading; the effects on cortical bone are more complex, preserving bone formation while potentially allowing relative structural and material changes that ultimately lead to an exaggerated decrease in mechanical properties.75 These protective effects of Cx43 deficiency may be due to Sost-mediated suppression of cortical bone formation and decreased cortical osteoclast activity during unloading.76 Another research group found that the absence of Cx43 in osteocytes enhances responsiveness to mechanical force in mice.72 Their data show that loading induces a greater increase in the periosteal bone formation rate in Cx43 (Dmp1 (8 kb)-Cre) mice than in control mice, resulting in a higher mineralizing surface and enhanced mineral apposition rate.72 These researchers further demonstrated that loss of Cx43 in osteocytes promotes the stretch-induced expression of β-catenin and its target genes, thus providing a potential explanation for the increased anabolic response to mechanical signals in mice.72 Interestingly, a previous study reported that mechanical stretching increases the abundance of Cx43-specific immunofluorescence staining and protein levels in ROS 17/2.8 cells, increasing gap junctional communication among osteoblastic cells.68 Early research also suggested that fluid flow has stimulatory effects on MLO-Y4 cells, encompassing cellular morphology, opening of gap junctions, protein expression, and redistribution of Cx43.69 These complex results derived from different systems may be attributable to the dual functions of Cx43 in gap junctions and hemichannels.
Recently, researchers attempted to differentiate the functions of Cx43 in gap junctions and hemichannels in response to mechanical forces using transgenic mice with osteocyte-specific Cx43 deficiency.77 The R76W mutation of Cx43 leads to inhibition of gap junctions and enhancement of hemichannels, while the Δ130-136 mutation results in inhibition of both gap junctions and hemichannels. Both mutations cause increased endocortical osteoclast activity during unloading. Increased periosteal osteoclasts and decreased apoptotic osteocytes were observed only in R76W mice.77 This study also showed that hindlimb unloading increases empty cortical lacunae of osteocytes in wild-type and Δ130-136 mice compared to R76W mice. Together, these results indicate that the gap junctions of Cx43 are responsible for communication between osteoblasts and osteoclasts, while Cx43 hemichannels protect osteocytes from apoptosis in response to unloading.77 In general, it is believed that Cx43 may participate in mechanosensitive signaling pathways and transmit mechanical signals between cells to maintain bone homeostasis during loading or unloading conditions.
Cilium
The cilium is a microtubule-based antenna-like sensory organelle critical for transducing extracellular mechanical and chemical signals.78 It grows from the mother centriole and projects from the cell surface in many vertebrate tissues, including the bone, nervous system, carcinoma, kidney, cartilage, and cardiovascular tissues.79 Microtubules form the core of the cilium, also known as the axoneme. Cilia protrude into the extracellular space, where they use unique mechanisms to sense their mechanical environments.80
The primary cilium is a potentially important mechanosensitive structure in bone cells81 that can sense FSS and mediate mechanotransduction in osteocytes.82–84 Cilium formation is positively correlated with the mechanosensitivity of osteocytes; as cilium length increases, osteocyte release of NO and ATP also increases.85 Periosteal osteochondroprogenitors directly sense FSS and differentiate into bone-forming osteoblasts via their primary cilia, and this response is almost entirely lost when the primary cilia are absent.86 The abrogation of primary cilia in cultured mouse osteoblastic cells (MC3T3-E1 cell line) or MLO-Y4 cells also decreased the normal osteogenic response to FSS.87,88 In articular cartilage, the cilia of chondrocytes responded to compression-induced osmotic changes.89,90 Indian hedgehog (Ihh) signaling, important in chondrocyte proliferation and differentiation, increased when growth plate chondrocytes were exposed to hydrostatic compression, but this enhanced signaling was abrogated when primary cilia were disrupted (Fig. 3b).91 In the tendon enthesis, unloading and overloading led to cilium assembly and disassembly, respectively. Tendon-specific (Scx-Cre) conditional deletion of the ciliary gene IFT88 caused fibrocartilaginous tendon and weaker mineralized cortical bone through the regulation of hedgehog (Hh) signaling.92 The abolition of primary cilia from bone cells also attenuated bone formation in microgravity. Reconstruction of the primary cilia may be a potential strategy for preventing bone loss caused by microgravity.79,93,94
GPCRs
Beyond canonical focal adhesions and cilia, G protein-coupled receptors (GPCRs) have also been proposed as mechanosensitive structures under various physiological and pathological conditions. Only specific GPCRs can sense mechanical forces. Angiotensin II receptor type 1 (AGTR1), for example, can sense mechanical forces to mediate myogenic vasoconstriction,95 while bradykinin receptor B2 (BDKRB2) can sense FSS in endothelial cells.96 Other mechanosensitive GPCRs include sphingosine 1-phosphate receptors (S1PR), dopamine D5 receptors (D5R), GPR68,97 cysteinyl leukotriene 1 receptors (CysLT1R), formyl peptide 1 receptors (FPR1), endothelin ETA receptors, muscarinic M5 receptors, and vasopressin V1A receptors.98 Erdogmus et al. found that GPCRs without a C-terminal helix 8 (H8) are not mechanosensitive. Nonsensitive GPCRs can acquire mechanosensitivity by linking an H8 domain, and removing the domain also removes the sensitivity. Moreover, disrupting H8 structural integrity via amino acid mutations impairs the mechanosensitivity it imparts.98
Activation of a GPCR via a canonical pathway, such as the binding of its agonist, causes a conformational change that activates its guanine nucleotide exchange (GEF) activity toward one of the possible interacting heterotrimeric Gαβγ proteins (Fig. 3c). This activation results in the replacement of GDP by GTP on the α subunit, causing its activation and dissociation from the βγ subunits. The activated α subunit is classically considered to dissociate from the GPCR, and the αβγ subunits activate different downstream effectors depending on the characteristics of the subunits. For example, αs and αi stimulate and inhibit adenylyl cyclase, respectively; αq can activate phospholipase C, whereas βγ can activate GIRK channels and PI3K. The intrinsic GTPase activity of the Gα subunit causes the system to return to its inactive state, with GDP bound to the Gα subunit of the reconstituted Gαβγ heterotrimer.99
Activation of mechanosensitive GPCRs by mechanical stresses can induce downstream signaling events, most notably a phospholipase C (PLC)-IP3- and diacylglycerol (DG)-dependent increase in intracellular calcium concentrations.100,101 Mechanosensation occurs alongside chemosensation (the ability to perceive chemicals in the environment) in the intricate physiological environment; thus, cells need to integrate physical and chemical signals appropriately to ensure proper tissue and organ development and function. For example, GPR68 can respond to extracellular acidification during membrane stretching, with its level of activity reflecting both the extent of membrane stretching and the degree of acidification.102 There is also evidence that mechanical forces act synergistically with parathyroid hormone PTH (1-34) to regulate bone growth.103 GPCRs are critical for the survival and proliferation of skeletal cells in response to surrounding stimuli, and these studies highlight their importance in mechanosensitivity alongside their traditional roles in chemosensation and signal transduction. Further study will be required to expand our understanding of their roles under physiological and pathological conditions in the skeletal system.
Ion channels
In 2010, Coste et al. identified prominent mechanosensitive cation PIEZO channels (PIEZO1/2) using RNA interference profiles in a mouse neuroblastoma cell line.104 Although the PIEZO1 protein shares only 42% sequence homology with its homolog PIEZO2, their structures are similar: a three-bladed, propeller-like trimer with an extracellular cap-like structure embedded in the center and an intracellular beam connecting to the central pore.105 In vertebrates, loss of PIEZO1 is lethal, causing malformation of blood vessels during embryonic development.106 The conditional loss of PIEZO1 in smooth muscle resulted in hypertension-dependent arterial remodeling dysfunction in mice.107 PIEZO1 can regulate the vascular system and lymphatic valve formation106,108 in response to fluid shear stress. PIEZOs can also sense blood pressure,109,110 dictate neural stem cell differentiation and axon growth in the developing brain by sensing substrate stiffness,111,112 and mediate pressure-induced pancreatitis.113 In the brain, PIEZO1 distribution is affected by nanotopography, with consequences for hippocampal neuron–astrocyte interactions.114 PIEZO2 has essential roles in gentle touch115 and proprioception.116 It also senses airway stretches and mediates lung inflation-induced apnea.117
Bone is very sensitive to mechanical forces, and PIEZO proteins have recently received increasing attention for their potential roles in skeletal mechanosensation. During mandibular arch morphogenesis, PIEZO1 and YAP/TAZ act downstream of Wnt5a-mediated cortical polarity and oscillation.118 The nuclear abundance of YAP was diminished in the absence of PIEZO1. Interestingly, loss of YAP/TAZ also decreased PIEZO1 expression.118 In chondrocytes, PIEZO1 and PIEZO2 can act redundantly to mediate injury-induced osteoarthritis.119 In vivo studies have demonstrated the indispensability of PIEZO1 in the osteoblastic lineage, albeit with various phenotypes. Several single-nucleotide polymorphisms (SNPs) of PIEZO1 were associated with osteoporosis and fractures in an atlas of genetic influences on osteoporosis.120 Patients with osteoporosis showed reduced levels of PIEZO1 protein, and conditional knockout of PIEZO1 in osteoblast lineage cells disrupted osteogenesis by repressing RUNX2, COL1, and OCN expression.121 Two other research groups found increased osteoclast activity in two Cre-induced osteoblastic lineage PIEZO1 knockout systems (Prx1-Cre and Dmp1-Cre).122,123 In our own study, we observed very subtle differences in osteogenesis and strong hyperactivity of osteoclasts in osteoblastic PIEZO1-deficient mice.123 Our data support the hypothesis that insensitivity to mechanical loading causes dysregulated crosstalk between osteoblasts and osteoclasts, leading to bone loss. These observations also concur with data obtained from space flights, which have little effect on bone formation metabolites but give rise to a strong increase in bone resorption metabolites.124 Zhou et al. reported that loss of PIEZO1/2 results in severe bone defects, while the loss of PIEZO2 alone has only a subtle impact on bone; this finding suggests that PIEZO1, but not PIEZO2, is critical for perceiving mechanical forces in bone.125 In osteoblastic cells, PIEZO1/2 activates Ca2+ influx to stimulate calcineurin, promoting concerted activation of the NFATc1, YAP1, and β-catenin transcription factors in response to mechanical forces.125
The transient receptor potential (TRP) multigene superfamily encodes another set of ion channels important in bone mechanosensation. TRP channels are a series of nonselective cation channels composed of integral membrane proteins that can permeate calcium and magnesium ions (Fig. 3d). This superfamily can be divided into 7 subgroups that respond to different stimuli, including mechanical forces.126 Among them, TRPV4 serves as a regulator of bone metabolism, a determinant of bone strength, and a potential risk predictor for fractures through the regulation of bone matrix mineralization and intracortical porosity.127 TRPV4 can sense mechanical forces in chondrocytes,128 bone cells,129 epithelial cells,130 and endothelial cells.131 A previous study reported that FSS in the lacuna activates TRPV4, but not PIEZO1, to increase calcium concentration in the cell plasma.129 TRPV4 also mediates oscillatory fluid shear-induced calcium signaling and osteogenic gene (Cox2 and Opn) expression in BMSCs132 and plays a critical role in controlling aligned collagen assembly by these cells.133 Furthermore, TRPV4 activation in BMSCs accelerates collagen deposition and mineralization.132 The expression of late osteogenic genes can also be induced by laminar shear stress through TRPV4.134
Recognition of different types and intensities of mechanical stress stimuli is very important for cell mechanotransduction. High-intensity mechanical input is mediated by PIEZO1 and PIEZO2 channels, while low-intensity mechanical input is mediated by TRPV4.135 Moreover, TRPV4 tends to be distributed in areas that often have adhesions136 or primary ciliary structures.132 In BMSCs with defective primary cilia, TRPV4 is insensitive to mechanical activation.132 Mutations in TRPV4 are associated with human bone diseases,137 and TRPV4 knockout mice showed insensitivity to hind limb unloading.138 Loss of TRPV4 in mice also repressed the increased bone resorption during unloading. TRPV4 is additionally expressed in osteoclasts, where it can activate NFATc1 signaling to regulate terminal differentiation and cell activity through Ca2+ influx.139 Autophagy, which can support osteoclast function, is also regulated by TRPV4.140 These data indicate that impairment of TRPV4-mediated osteoclast activity may cause resistance to unloading-induced bone loss.
Mechanotransduction pathways
Mechanical forces are principal signals that direct cell responses to promote adaptation to the environment. Through mechanosensitive signaling pathways, diverse mechanical stimuli can be translated into biochemical signals that control cell growth, differentiation, apoptosis, and migration. Thus, it is important to understand how various forces and mechanoreceptors are ultimately linked to the activity of nuclear transcription factors. Various references for these mechanosensitive signaling pathways in osteoblastic and osteoclastic lineages are summarized in Table 3.
Table 3.
References for mechanosensitive signaling pathways in osteoblastic and osteoclastic lineages
Cytoskeleton
The cytoskeleton is a network of fibers formed by the nuclear skeleton, cytoplasmic skeleton, cell membrane skeleton, crosslinking factors, and extracellular matrix. It determines basic cell morphology and connects all mechanosensitive components. Among the cytoskeletal components, actin filament or fibrous actin (F-actin) is well known for perceiving and transmitting mechanical forces in osteocytes.31 Previous studies have reviewed the assembly of actin filaments in detail.141 A key characteristic of the crosslinked actin network is its ability to stiffen when strained by external or internal forces.142,143 Myosin II can guide the assembly patterns of actin filaments and exhibit mechanical stress-dependent kinetics. Myosin II behaves like a crosslinker, stiffening or softening actin networks depending on environmental conditions by modulating filament sliding and rearrangement144 or filament disassembly.145 This ability to stiffen or soften in response to different intensities of tensile or compressive forces provides cells with intrinsic mechanisms to balance load across heterogeneous structures or to maintain global shape in the face of fluctuating forces. The activity and assembly of myosin II are regulated by the phosphorylation of its light and heavy chains through numerous kinases in diverse regulatory pathways, including myosin light chain kinase (MLCK; activated by Ca2+/calmodulin), Rho kinase, citron kinase (activated by RhoA), and myotonic dystrophy kinase-related Cdc-42-binding kinase (MRCK; activated by Cdc-42).146 These kinases are all reported to be involved in mechanosensitive signaling pathways.
Microtubule-actin crosslinking factor 1 (MACF1) is a widely expressed cytoskeletal linker that can bind to F-actin and microtubules (Fig. 3a). Coordination between microtubules and F-actin is essential for cell migration and adhesion, which are closely associated with mechanical force transduction. Loss of MACF1 leads to aberrant microtubule organization, tight junction instability, and impaired wound closure in vitro.147,148 As MACF1 expression decreases under mechanical unloading conditions in vitro and in vivo, MACF1 is considered a mechanosensitive structure.149 This molecule can enhance preosteoblast polarization and migration by raising focal adhesion turnover and end-binding protein 1 (EB1) movement along the microtubule bundle. During this process, MACF1 increases phosphorylated Src (p-Src) levels and promotes colocalization of EB1 with p-Src, resulting in EB1 phosphorylation at Y247; phosphorylated EB1 then moves along the microtubule bundle instead of being anchored at focal adhesion.150 Previous studies have also shown that MACF1 significantly promotes mineralization of MC3T3-E1 cells by sustaining the β-catenin/TCF1-RUNX2 axis.151
The cytoskeleton determines osteocyte morphology and mechanosensitivity. For example, round osteocytes, which reside within ellipsoid lacunae, seem to have a higher response to mechanical stimuli than more spread, adherent osteocytes.152 This finding indicates that osteocytes can sense small strains in their ellipsoid morphology in vivo, thus promoting bone health. Microgravity can cause cytoskeletal depolymerization and changes in the array and direction of microfilaments and microtubules.153 In osteoblasts, microgravity induces actin microfilament disruption, thus reducing BMP2-induced activation and translocation of Smad1/5/8 and RUNX2 expression in MC3T3-E1 cells.154
The cytoskeleton can transfer mechanical forces, and in turn, these forces can adjust the cytoskeletal structural configuration.155–157 Low-intensity vibration (LIV) or mechanical strain can cause a rapid and transient β-catenin association with the nucleoskeleton through the FAK-mediated inhibition of GSK-3β (Fig. 4a). β-catenin molecules that do not bridge cadherins to the actin cytoskeleton or translocate into the nucleus are ubiquitinated and degraded by the 26S proteasome.158 Codepletion of the linker of cytoskeleton and nucleoskeleton (LINC) elements Sun-1 and Sun-2 disrupts β-catenin trafficking into the soluble nuclear fraction in response to LIV, thus impairing cytoskeletal integrity and fate determination of BMSCs.156
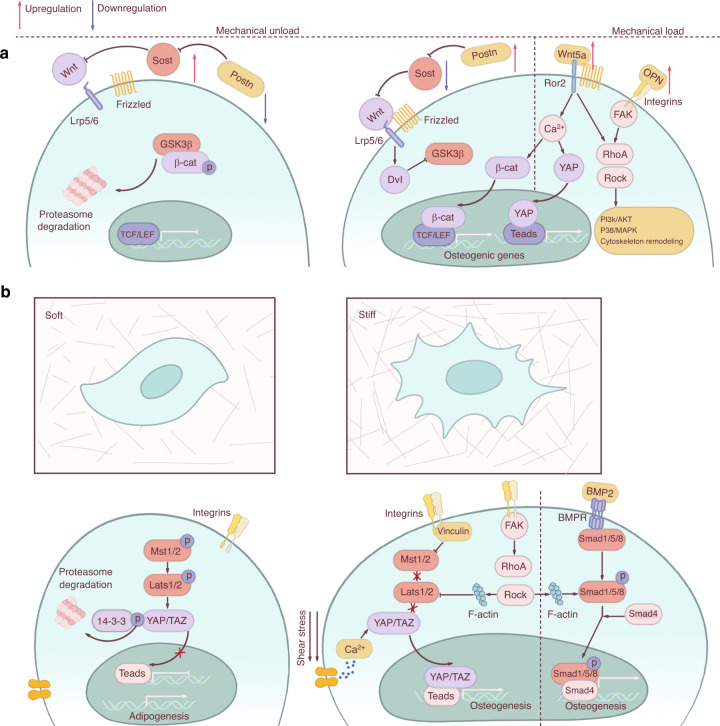
Fig. 4.
Mechanotransduction signaling pathways. a Wnt signaling pathway under mechanical loading and unloading conditions. Unloading can increase the expression of Sost and repress the expression of Postn, thus inhibiting osteogenic gene expression, while loading has the opposite effect. Wnt and RhoA/Rock/cytoskeleton have synergetic effects in the process of mechanotransduction. b MSCs favor a commitment to osteoblasts in the rigid matrix and adipocytes in the soft matrix. Several signaling pathways are involved in this lineage commitment, including cytoskeleton rearrangement, YAP/TAZ nuclear translocation, and BMP2/Smad activation.
Wnt/β-catenin signaling pathway
Wnt signaling pathways play diverse roles in skeletal homeostasis.159 Canonical Wnt signaling starts when Wnt ligands bind to Frizzled and low-density lipoprotein receptor-related protein 5/6 (Lrp5/6) receptors located on the cell membrane. The activation of Wnt signaling facilitates the accumulation of β-catenin through inhibition of GSK-3β-induced β-catenin phosphorylation, after which dephosphorylated β-catenin translocates into the nucleus to further induce the transcription of LEF/TCF-responsive genes (Fig. 4a).160 As a primary intracellular signal transducer for Wnt signaling pathways, β-catenin serves as one of the core hubs for mechanotransduction.156
Wnt activity is increased by mechanical loading and decreased by unloading. Mechanical loading activates the β-catenin signaling pathway through a mechanism involving nitric oxide, FAK, and the Akt signaling pathway.161 Mechanical strain induces MSCs away from adipogenic differentiation and toward osteogenic differentiation through preservation of β-catenin in the nucleus.162 Pulsed electromagnetic fields (PEMFs), an effective method for healing fractures and improving osteoporosis, can induce osteogenesis by increasing intracellular Ca2+, which in turn leads to nucleoplasm translocation of β-catenin in C3H10T1/2 mesenchymal cells.163
Osteocytes also appear to use the Wnt/β-catenin pathway to transmit mechanical loading signals into cells. Strength and power training, leveraged clinically to enhance bone quality, upregulated the expression of Wnt-related genes in humans.164 Mice lacking β-catenin in osteocytes showed severe osteopenia and skeletal fragility and died prematurely. β-catenin heterozygous mice also had impaired responses to mechanical loading.165 Osteoblasts derived from LRP5 G171V (Wnt signaling activation) transgenic mice exhibited increased expression of Wnt/β-catenin target genes under physiological conditions. Moreover, LRP5 G171V transgenic mice showed more robust bone formation in response to loading, with an elevated expression of genes downstream of the Wnt signaling pathway, than wild-type mice.166 Similarly, the anabolic response of bone to mechanical loading was enhanced in the ulnae of mice lacking the Wnt inhibitor FRZB.167 In mice deficient in Sost, an extracellular Wnt signaling antagonist, bone formation was accelerated, especially around the periosteum in the setting of cyclic axial loading (Fig. 4a).168 Periostin (Postn), an ECM protein with important roles in osteogenesis, is highly expressed in the bone periosteum. Mechanical loading specifically reduces Sost expression and increases Postn expression, while the opposite is true for unloading.169,170 Loss of Postn in mice caused bone loss, which was reversed by an anti-Sost antibody. In vitro studies further showed that Postn can suppress Sost expression through the integrin αVβ3 receptor (Fig. 4a).171 Together, these data support the hypothesis that Wnt/β-catenin signaling is a physiological response to loading and that activation of the Wnt/β-catenin pathway enhances the sensitivity of osteoblasts/osteocytes to mechanical loading.
Noncanonical Wnt signaling can also be elicited by mechanical forces. Oscillatory fluid flow can increase the expression of Wnt5a and its noncanonical tyrosine kinase receptor Ror2, both of which are necessary for mechanically stimulated RhoA activation, ultimately resulting in osteogenesis (Fig. 4a).172 Overexpression of Ror2 has also been reported to enhance osteogenesis.173 These studies provide evidence that noncanonical Wnt signaling pathways also participate in mechanotransduction.
Previous studies have also addressed the importance of interconnections between different signaling pathways. Of note, high-magnitude mechanical stress can inhibit canonical Wnt signaling-induced osteoblastic differentiation. In this case, large-magnitude loading exhibits an inhibitory effect on the PI3K/Akt pathway, resulting in the accumulation of GSK-3β and phosphorylation of β-catenin.174 Inhibition of FAK or PI3K can abolish the fluid flow-induced stabilization of β-catenin and consequently inactivate the Wnt/β-catenin pathway in osteocytes.161
YAP/TAZ
The Hippo pathway integrates a broad range of signals to control key cellular processes through the activity of YAP and TAZ.175 YAP and TAZ are transcriptional coregulators that do not contain DNA-binding domains; thus, they usually interact with DNA-binding proteins to regulate transcriptional activity. Once activated, the Hippo pathway limits tissue growth and cell proliferation by phosphorylating and inhibiting YAP/TAZ. In mammals, MST1/2 form heterodimers with SAV1 through their C-terminal SARAH domains, after which MST1/2 can phosphorylate SAV1, MOB1, and LATS1/2 kinase.176,177 LATS1/2 directly phosphorylates YAP and TAZ at multiple sites, thereby inhibiting their nuclear localization.178 Phosphorylated YAP/TAZ is sequestered in the cytoplasm by interaction with the 14–3–3 protein and then degraded by the ubiquitin-proteasome system (Fig. 4b).179 Alternatively, when the Hippo pathway is off, YAP/TAZ are dephosphorylated and translocate into the nucleus, where they bind to cotranscriptional factors to induce transcriptional programs important for cell proliferation, survival, and migration.175,180–182
Several upstream inputs have been reported to regulate the nuclear localization of YAP/TAZ in response to mechanical stresses. In luminal breast cancer MCF7 cells, RAP2, a Ras-related GTPase, was activated by a soft matrix, relaying ECM rigidity signals to control mechanosensitive cellular activities through YAP/TAZ. In this model, low stiffness acts through phospholipase Cγ1 (PLCγ1) to increase the levels of phosphatidylinositol 4,5-bisphosphate and phosphatidic acid, which activate RAP2 through PDZGEF1 and PDZGEF2. Finally, RAP2 can activate MAP4K4, MAP4K6, MAP4K7, and ARHGAP29 through phosphorylation, resulting in activation of LATS1 and LATS2 and inhibition of YAP and TAZ.183 The SWI/SNF chromatin-remodeling complex can interact directly with purified F-actin184 and inhibit YAP/TAZ through ARID1A in response to mechanical stiffness.185
YAP/TAZ are also functionally required for the differentiation of MSCs in response to ECM stiffness; conversely, the expression of activated YAP overrules physical constraints in dictating cell behaviors.186 Rigid stiffness increases the abundance of cytoskeleton-associated vinculin. This vinculin then promotes the nuclear transportation of YAP/TAZ independent of LATS1, contributing to the regulation of ECM stiffness-dependent adipocyte differentiation of MSCs.187 Loss of both YAP and TAZ in osteoblastic lineage cells destroys the integrity of the skeletal system, reducing the bone formation and elevating bone resorption.188 The convergence of these different pathways on the regulation of YAP/TAZ nuclear localization, whether dependent or not on the Hippo pathway, reflects the diversity of upstream inputs and efficiency of biological responses to the environment.
Although YAP and TAZ are thought to be largely redundant and similarly regulated by Hippo signaling, both of them have specific structural, developmental, and physiological characteristics. Notably, global YAP deletion in mice was embryonic lethal due to impaired yolk sac vasculogenesis,189 whereas global TAZ knockout mice survived to maturity with only modest skeletal defects and polycystic kidney disease.190 This evidence shows that YAP and TAZ have exclusive, gene-specific functions. The exact function of YAP in osteogenesis remains controversial in vitro. YAP has been reported to suppress osteogenesis through the repression of RUNX2 transcriptional activity in response to Src family kinases.191 In contrast, a separate study found that overexpression of a constitutively active YAP mutant in MSCs promotes osteogenesis more than adipogenesis, even under soft matrix conditions.186 Additionally, YAP overexpression has been reported to inhibit both the osteogenic and adipogenic abilities of MSCs through inhibition of Wnt/β-catenin signaling by interfering with β-catenin and inducing Dkk1 expression.192
TAZ was reported to contribute to osteogenic differentiation by modulating the canonical Wnt pathway.193,194 TAZ can also promote osteogenesis and inhibit adipogenesis by acting as a RUNX2 coactivator and PPARγ inhibitor.195 In vivo, osteoblast-specific overexpression of TAZ promoted bone formation with higher expression of RUNX2.196 Recently, a mouse model of combinatorial YAP/TAZ ablation in skeletal lineage cells (Osterix-Cre) showed allele dose-dependent perinatal skeletal deformity, demonstrating that YAP and TAZ have concordant functions in osteoblast lineage cells.188 YAP/TAZ depletion and acute YAP/TAZ–TEAD inhibition reduced osteogenic and collagen-related gene expression.188 Our previous data demonstrated that YAP serves as the downstream element of PIEZO1 that regulates matrix protein expression.123 The components of the extracellular matrix determine the rigidity of the ECM, which in turn affects the nuclear localization of YAP/TAZ. This feedback implies that YAP/TAZ can modulate the ECM in response to mechanical forces, contributing to the delicate process of adaptation between the ECM and cells.
BMP2 signaling pathway
Bone morphogenetic protein 2 (BMP2) belongs to the transforming growth factor β (TGFβ) superfamily and participates in bone, cartilage, and joint formation. The BMP2 signaling pathway is currently a major therapeutic target for improving bone mass or quality, ameliorating skeletal overgrowth diseases, and repairing damage to bones and joints. BMP2 binds to and activates BMP transmembrane receptors I (BMPR-IA) and II (BMPR-II), after which activated BMPR-IA transmits signals via recruitment and phosphorylation of R-Smads, including Smad1, Smad5, and Smad8. R-Smads subsequently forms a complex with common Smads (Smad4) and translocate into the nucleus to activate the transcription of target genes such as Runx2 and Alp (Fig. 4b).197 Alternatively, BMP2 signaling can be mediated by the TAK1-p38 kinase pathway.198 BMP2 deficiency in osteoblasts results in osteopenia and bone strength reduction.199
BMP2 is also involved in mechanotransduction. During the commitment of hMSCs toward the osteogenic lineage, cell shape can modulate the ability of BMP2 to activate RhoA, ROCK, and cytoskeletal tension. In turn, RhoA/ROCK activity and associated cytoskeletal tension regulate hMSC commitment to the BMP-induced osteogenic phenotype.200 The osteogenesis induced by BMP2 also depends on matrix stiffness. On soft matrix (0.5–3.5 kPa), hMSCs cannot elicit BMP2-induced osteogenesis.201 Proper organization of the F-actin cytoskeleton in response to the microenvironment’s mechanical properties is necessary for BMP2-induced Smad1/5/8 phosphorylation and nuclear translocation.201 Studies have shown that mechanical loading can enhance the ability of transplanted mesenchymal condensations containing BMP-2 and TGF-β1 to regenerate bone in vivo,202 indicating that interacting chemical and physical factors in ECM scaffolds may work synergistically to enhance bone regeneration.
BMP2 can also act in coordination with other mechanosensitive signaling pathways. For example, the myoblast C2C12 cell line can undergo osteogenesis in the presence of both stiff matrix and the BMP2 signaling pathway. Without BMP2, C2C12 cells are unable to differentiate into osteoblasts regardless of matrix stiffness due to the inactivation of Smad signaling. The initiation of BMP2-induced Smad signaling is independent of cytoskeletal tension in these cells.203 However, osteogenic gene activation requires cytoskeletal tension-induced nuclear accumulation of YAP/TAZ, which depends on matrix stiffness (Fig. 4b).203 Thus, osteogenesis in C2C12 cells may be under the control of synergistic cooperation between multiple transcription factors and the mechanical environment.
Noncoding RNAs (lncRNA and miRNA)
Emerging evidence has revealed that noncoding RNAs possess the ability to transmit mechanical signals. Among the noncoding RNAs, miRNAs specifically have been reported to mediate mechanical force transduction during osteogenesis.204 Under simulated microgravity conditions, for example, miR-139-3p and the lncRNA ODSM coordinately regulated the differentiation and apoptosis of MC3T3-E1 cells,205 while the miR-30 family (except miR-30a) inhibited their osteoblastic differentiation.206 miR-33-5p can promote osteogenesis in vitro; osteoblast-targeted delivery of this miRNA in mice partially counteracted the reduction of osteogenic genes and mineral apposition rate in the hindlimb unloading model.207 Clinically, increased levels of miR-138-5p were detected in bone specimens from bedridden and aged patients with a lower degree of bone formation. Inhibition of this miRNA in the bone rescued the reduced bone mass in the hindlimb unloading model and enhanced the bone anabolic response during exercise. Further studies showed that miR-138-5p can directly target MACF1 to inhibit osteoblast differentiation.208 Both miR-100-5p and miR-143-3p significantly enhanced osteogenesis in a 3D soft hydrogel.209 These data indicate that modulation of miRNA signaling and substrate stiffness can direct MSC lineage commitment and provide a novel approach for bone regeneration.
Potential factors and pathways
In addition to the molecules and pathways mentioned above, various other transcription factors contribute to mechanotransduction. In myeloid cells, PIEZO1 can respond to cyclical hydrostatic pressure and drive c-JUN activation and transcriptional upregulation of endothelin-1 (EDN1). This change in turn stabilizes HIF1α, thus facilitating the transcription of proinflammatory genes.210 Mechanical forces can also activate Ras/ERK-mediated mitogen-activated protein kinase (MAPK) signaling, promoting HIF-1α expression in osteoblasts.211 Osteoblast-specific deletion of HIF-1α in mice resulted in thinner bones with reduced vascularization, whereas deletion of the E3 ligase von Hippel-Lindau protein (pVHL) promoted bone vascularization.212 Further studies will undoubtedly continue to reveal transcription factors involved in metabolic and hypoxic modulation in response to mechanical forces.
Bone remodeling induced by microgravity
Previous studies have shown that bone loss in astronauts is caused by apoptosis of osteocytes and increased bone resorption.213–216 Both osteoclasts and osteoblasts can respond to mechanical stimulation in vitro. Nevertheless, the precise contributions of bone formation and resorption to microgravity-induced bone loss remain to be fully elucidated.214,217,218 During space flight, increased bone resorption results in the loss of calcium and minerals in the bone, altering the endocrine regulation of calcium metabolism.124,214 Based on these observations, osteoclasts might be presumed to modulate bone quality by sensing mechanical forces directly or by receiving signals from osteoblastic lineage cells. An in vitro study demonstrated that the duration and amplitude of mechanical loading determine the directionality of its effect on the osteoclastogenesis of hematopoietic progenitor cells,27 suggesting that osteoclasts have the potential to sense mechanical forces. Nevertheless, in most studies, osteoclasts are more likely to be effectors that accept signals from other mechanosensitive cells and execute tasks. Thus, osteoclasts are both mechanosensitive cells and effectors for other mechanosensitive cells. Mesenchymal stem cells or osteocytes exposed to oscillatory fluid flow could significantly inhibit osteoclastogenesis in a coculture system.219,220 In vivo, monocytes, the progenitors of bone-resorbing osteoclasts, were recruited by CXCL1 and CCL2 produced by mesenchymal stem cells treated with mechanical forces.221 Our recent study found that loss of PIEZO1 in osteoblastic lineage cells leads to a deterioration of the bone structure and quality due to increased bone resorption, and accelerated osteoclast formation is determined by the altered bone matrix secreted by osteoblastic cells.123 It is not just the autonomous activity of osteoclasts but also the changing niches or factors originating from other cells that affect the function of osteoclasts. These investigations provide a mechanism for the increased bone resorption observed in microgravity environments.
Concluding remarks and future perspectives
Since the 19th century, the correlation between mechanical forces and bone mass has been of great interest in the field of skeletal mechanobiology.1 Although the mechanosensitive signaling pathways in the skeleton are diverse, they are inseparably linked with each other. Future studies should address several questions. First, the mechanical environment of skeletal cells in vivo is highly complex. Mesenchymal stem cells and their progenies, including osteoblasts and osteocytes, receive diverse stress stimuli with correspondingly varied outcomes. The precise correlation between the types of mechanical stresses and their downstream signaling pathways should be further explored by mimicking the stress environment in vitro with the help of 3D culture and bone biomimetic materials. Second, different exercise styles have distinct influences on the skeletal system. The intensity and type of workout may lead to different results. Proper exercise can increase bone mass, while increasing exercise intensity to excessive levels can diminish bone mass and quality and even cause increased stress fractures.222,223 Consequently, studies using physical therapy must ensure that the effects of intensity and style of exercise can be properly inferred. Further investigation into the potentially harmful effects and the downstream responding factors of overload is also warranted. Finally, as older people gradually lose the capacity to exercise, the reduction of stress stimuli over time may contribute to bone loss. Effective treatment strategies should first establish the underlying mechanisms that drive these changes in cell physiology and then design therapies directed toward these mechanisms.
An important factor that limits the field of skeletal mechanobiology is the lack of devices suitable for studying mechanosensitive signaling pathways. In most previous studies, cell lines have been subjected to unidirectional or oscillatory fluid flow.224 Cell lines can also be seeded in a culture dish with a retractable bottom to apply tension and squeezing force225 or in a gel to apply compression force.226 Such conditions may not reflect the in vivo mechanical environment, and most of the present studies are based on observations in 2D systems. Some 3D structures or scaffolds, including multivacancy structures made of collagen, hydroxyapatite substrates, and synthetic materials, which partially simulate the physiological environment, are used in bone cell research. Simulated microgravity is achieved by slow-rotating wall vessels (SRWVs), random positioning machines (RPMs), and 3D clinostats,227,228 mainly based on a rotating fluid environment. In animal models, mechanical loading and unloading can be achieved by cyclic pressure on long bones, different exercise devices, and tail suspension.229,230 Nevertheless, these models alone are not enough to ultimately determine the changes in cell morphology, genes, and hormones in a weightless environment. The true microgravity environment relies on the support of the International Space Station. Recently, several studies on spaceflight have used systemic analysis to clarify changes in multiple dimensions from metabolism to genetics or epigenetics in various tissues and cells. These studies provide new insights into the environment of outer space, including radiation and microgravity, and the way it affects biological and physiological states.231–234 Support from the aerospace agency will continue to help uncover more solid, physiologically accurate findings on skeletal mechanobiology.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) [81725010, 81672119, 81991512, 82102554], the Strategic Priority Research Program of the Chinese Academy of Sciences [Grant No. XDB19000000], and the Space Medical Experiment Project of China Manned Space Program [HYZHXM01025]. We thank Dr. Daniel Ackerman (Insight Editing London) for editing the manuscript during preparation.
Competing interests
The authors declare no competing interests.
References
- 1.Wolff JDas. Gesetz der transformation der Knochen. A Hirshwald. 1892;1:1–152. [Google Scholar]
- 2.Frost HM. The Utah paradigm of skeletal physiology: an overview of its insights for bone, cartilage and collagenous tissue organs. J. Bone Miner. Metab. 2000;18:305–316. doi: 10.1007/s007740070001. [DOI] [PubMed] [Google Scholar]
- 3.Nicogossian A. Medicine and space exploration. Lancet. 2003;362:s8–s9. doi: 10.1016/s0140-6736(03)15055-6. [DOI] [PubMed] [Google Scholar]
- 4.Vico L, Hargens A. Skeletal changes during and after spaceflight. Nat. Rev. Rheumatol. 2018;14:229–245. doi: 10.1038/nrrheum.2018.37. [DOI] [PubMed] [Google Scholar]
- 5.Takata S, Yasui N. Disuse osteoporosis. J. Med. Investig. 2001;48:147–156. [PubMed] [Google Scholar]
- 6.Salmon CR, et al. Proteomic analysis of human dental cementum and alveolar bone. J. Proteom. 2013;91:544–555. doi: 10.1016/j.jprot.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonewald LF. Mechanosensation and transduction in osteocytes. BoneKEy osteovision. 2006;3:7–15. doi: 10.1138/20060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013;54:182–190. doi: 10.1016/j.bone.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 9.You L, et al. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin L, Liu W, Cao H, Xiao G. Molecular mechanosensors in osteocytes. Bone Res. 2020;8:23. doi: 10.1038/s41413-020-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uda Y, Azab E, Sun N, Shi C, Pajevic PD. Osteocyte Mechanobiology. Curr. Osteoporos. Rep. 2017;15:318–325. doi: 10.1007/s11914-017-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon RY, et al. Skeletal adaptation to intramedullary pressure-induced interstitial fluid flow is enhanced in mice subjected to targeted osteocyte ablation. PLoS One. 2012;7:e33336. doi: 10.1371/journal.pone.0033336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatsumi S, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Andreev D, et al. Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin. J. Clin. Invest. 2020;130:4811–4830. doi: 10.1172/JCI134214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YJ, et al. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J. Orthop. Res. 2004;22:1283–1289. doi: 10.1016/j.orthres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Jia X, et al. A critical role of the KCa 3.1 channel in mechanical stretch-induced proliferation of rat bone marrow-derived mesenchymal stem cells. J. Cell Mol. Med. 2020;24:3739–3744. doi: 10.1111/jcmm.15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stavenschi E, Labour MN, Hoey DA. Oscillatory fluid flow induces the osteogenic lineage commitment of mesenchymal stem cells: The effect of shear stress magnitude, frequency, and duration. J. Biomech. 2017;55:99–106. doi: 10.1016/j.jbiomech.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Jin J, et al. Shear stress modulates osteoblast cell and nucleus morphology and volume. Int. J. Mol. Sci. 2020;21:8361. doi: 10.3390/ijms21218361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batra N, et al. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc. Natl. Acad. Sci. USA. 2012;109:3359–3364. doi: 10.1073/pnas.1115967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regenerative Med. 2010;5:713–724. doi: 10.2217/rme.10.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonam S, Sathe SR, Yim EK, Sheetz MP, Lim CT. Cell contractility arising from topography and shear flow determines human mesenchymal stem cell fate. Sci. Rep. 2016;6:20415. doi: 10.1038/srep20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma C, et al. Fluid shear stress suppresses osteoclast differentiation in RAW264.7 Cells through extracellular signal-regulated kinase 5 (ERK5) signaling pathway. Med. Sci. Monit. : Int. Med. J. Exp. Clin. Res. 2020;26:e918370. doi: 10.12659/MSM.918370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo D, et al. Fluid shear stress changes cell morphology and regulates the expression of ATP6V1A and TCIRG1 mRNA in rat osteoclasts. Mol. Med. Rep. 2010;3:173–178. doi: 10.3892/mmr_00000236. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki N, Yoshimura Y, Deyama Y, Suzuki K, Kitagawa Y. Mechanical stress directly suppresses osteoclast differentiation in RAW264.7 cells. Int. J. Mol. Med. 2008;21:291–296. [PubMed] [Google Scholar]
- 25.McAllister TN, Du T, Frangos JA. Fluid shear stress stimulates prostaglandin and nitric oxide release in bone marrow-derived preosteoclast-like cells. Biochem. Biophys. Res. Commun. 2000;270:643–648. doi: 10.1006/bbrc.2000.2467. [DOI] [PubMed] [Google Scholar]
- 26.Li P, et al. STIM1 and TRPV4 regulate fluid flow-induced calcium oscillation at early and late stages of osteoclast differentiation. Cell Calcium. 2018;71:45–52. doi: 10.1016/j.ceca.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Bratengeier C, Liszka A, Hoffman J, Bakker AD, Fahlgren A. High shear stress amplitude in combination with prolonged stimulus duration determine induction of osteoclast formation by hematopoietic progenitor cells. FASEB J. 2020;34:3755–3772. doi: 10.1096/fj.201901458R. [DOI] [PubMed] [Google Scholar]
- 28.Jin J, Bakker AD, Wu G, Klein-Nulend J, Jaspers RT. Physicochemical niche conditions and mechanosensing by osteocytes and myocytes. Curr. Osteoporos. Rep. 2019;17:235–249. doi: 10.1007/s11914-019-00522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das RK, Gocheva V, Hammink R, Zouani OF, Rowan AE. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater. 2016;15:318–325. doi: 10.1038/nmat4483. [DOI] [PubMed] [Google Scholar]
- 30.Hwang MP, et al. Approximating bone ECM: crosslinking directs individual and coupled osteoblast/osteoclast behavior. Biomaterials. 2016;103:22–32. doi: 10.1016/j.biomaterials.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 31.Klein-Nulend J, Bacabac RG, Bakker AD. Mechanical loading and how it affects bone cells: the role of the osteocyte cytoskeleton in maintaining our skeleton. Eur. Cells Mater. 2012;24:278–291. doi: 10.22203/ecm.v024a20. [DOI] [PubMed] [Google Scholar]
- 32.McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB. Attachment of osteocyte cell processes to the bone matrix. Anat. Rec. 2009;292:355–363. doi: 10.1002/ar.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aragona M, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 34.Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017;18:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 36.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 37.Giancotti FG. A structural view of integrin activation and signaling. Dev. Cell. 2003;4:149–151. doi: 10.1016/s1534-5807(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 38.Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. J. Bone Min. Res. 1997;12:1189–1197. doi: 10.1359/jbmr.1997.12.8.1189. [DOI] [PubMed] [Google Scholar]
- 39.Sinha RK, Tuan RS. Regulation of human osteoblast integrin expression by orthopedic implant materials. Bone. 1996;18:451–457. doi: 10.1016/8756-3282(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 40.Bennett JH, Carter DH, Alavi AL, Beresford JN, Walsh S. Patterns of integrin expression in a human mandibular explant model of osteoblast differentiation. Arch. Oral. Biol. 2001;46:229–238. doi: 10.1016/s0003-9969(00)00114-x. [DOI] [PubMed] [Google Scholar]
- 41.Orr AW, Ginsberg MH, Shattil SJ, Deckmyn H, Schwartz MA. Matrix-specific suppression of integrin activation in shear stress signaling. Mol. Biol. cell. 2006;17:4686–4697. doi: 10.1091/mbc.E06-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bachmann M, et al. Induction of ligand promiscuity of alphaVbeta3 integrin by mechanical force. J. Cell Sci. 2020;133:jcs242404. doi: 10.1242/jcs.242404. [DOI] [PubMed] [Google Scholar]
- 43.Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 2002;31:485–516. doi: 10.1146/annurev.biophys.31.101101.140922. [DOI] [PubMed] [Google Scholar]
- 44.Liddington RC, Ginsberg MH. Integrin activation takes shape. J. Cell Biol. 2002;158:833–839. doi: 10.1083/jcb.200206011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 46.Haugh MG, Vaughan TJ, McNamara LM. The role of integrin alpha(V)beta(3) in osteocyte mechanotransduction. J. Mech. Behav. Biomed. Mater. 2015;42:67–75. doi: 10.1016/j.jmbbm.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Lee DY, et al. Integrin-mediated expression of bone formation-related genes in osteoblast-like cells in response to fluid shear stress: roles of extracellular matrix, Shc, and mitogen-activated protein kinase. J. Bone Min. Res. 2008;23:1140–1149. doi: 10.1359/jbmr.080302. [DOI] [PubMed] [Google Scholar]
- 48.Liu L, et al. The interaction between beta1 integrins and ERK1/2 in osteogenic differentiation of human mesenchymal stem cells under fluid shear stress modelled by a perfusion system. J. Tissue Eng. Regen. Med. 2014;8:85–96. doi: 10.1002/term.1498. [DOI] [PubMed] [Google Scholar]
- 49.Tucci M, et al. beta(3) Integrin subunit mediates the bone-resorbing function exerted by cultured myeloma plasma cells. Cancer Res. 2009;69:6738–6746. doi: 10.1158/0008-5472.CAN-09-0949. [DOI] [PubMed] [Google Scholar]
- 50.Zou W, Teitelbaum SL. Absence of Dap12 and the alphavbeta3 integrin causes severe osteopetrosis. J. Cell Biol. 2015;208:125–136. doi: 10.1083/jcb.201410123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El Azreq MA, et al. Cooperation between IL-7 Receptor and Integrin alpha2beta1 (CD49b) Drives Th17-Mediated Bone Loss. J. Immunol. 2015;195:4198–4209. doi: 10.4049/jimmunol.1500437. [DOI] [PubMed] [Google Scholar]
- 52.Phillips JA, et al. Role for beta1 integrins in cortical osteocytes during acute musculoskeletal disuse. Matrix Biol.: J. Int. Soc. Matrix Biol. 2008;27:609–618. doi: 10.1016/j.matbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Ishijima M, et al. Enhancement of osteoclastic bone resorption and suppression of osteoblastic bone formation in response to reduced mechanical stress do not occur in the absence of osteopontin. J. Exp. Med. 2001;193:399–404. doi: 10.1084/jem.193.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper J, Giancotti FG. Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell. 2019;35:347–367. doi: 10.1016/j.ccell.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ilic D, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 56.Webb DJ, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 57.Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 2004;303:836–839. doi: 10.1126/science.1091325. [DOI] [PubMed] [Google Scholar]
- 58.Ren XD, et al. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 2000;113:3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 59.Khatiwala CB, Kim PD, Peyton SR, Putnam AJ. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J. Bone Min. Res. 2009;24:886–898. doi: 10.1359/JBMR.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamamura K, et al. RhoA-mediated signaling in mechanotransduction of osteoblasts. Connect. Tissue Res. 2012;53:398–406. doi: 10.3109/03008207.2012.671398. [DOI] [PubMed] [Google Scholar]
- 61.Ransom RC, et al. Mechanoresponsive stem cells acquire neural crest fate in jaw regeneration. Nature. 2018;563:514–521. doi: 10.1038/s41586-018-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wein MN, et al. HDAC5 controls MEF2C-driven sclerostin expression in osteocytes. J. Bone Min. Res. 2015;30:400–411. doi: 10.1002/jbmr.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato T, et al. A FAK/HDAC5 signaling axis controls osteocyte mechanotransduction. Nat. Commun. 2020;11:3282. doi: 10.1038/s41467-020-17099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fourel L, et al. beta3 integrin-mediated spreading induced by matrix-bound BMP-2 controls Smad signaling in a stiffness-independent manner. J. Cell Biol. 2016;212:693–706. doi: 10.1083/jcb.201508018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Min SK, Kang HK, Jung SY, Jang DH, Min BM. A vitronectin-derived peptide reverses ovariectomy-induced bone loss via regulation of osteoblast and osteoclast differentiation. Cell Death Differ. 2018;25:268–281. doi: 10.1038/cdd.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat. Rev. Mol. Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 67.Riquelme MA, Cardenas ER, Xu H, Jiang JX. The role of connexin channels in the response of mechanical loading and unloading of bone. Int. J. Mol. Sci. 2020;21:1146. doi: 10.3390/ijms21031146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ziambaras K, Lecanda F, Steinberg TH, Civitelli R. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J. Bone Min. Res. 1998;13:218–228. doi: 10.1359/jbmr.1998.13.2.218. [DOI] [PubMed] [Google Scholar]
- 69.Cheng B, et al. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J. Bone Min. Res. 2001;16:249–259. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- 70.Lecanda F, et al. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J. Cell Biol. 2000;151:931–944. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung DJ, et al. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J. Cell Sci. 2006;119:4187–4198. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 72.Bivi N, et al. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J. Orthop. Res. 2013;31:1075–1081. doi: 10.1002/jor.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1) J. Bone Min. Res. 2008;23:879–886. doi: 10.1359/JBMR.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One. 2011;6:e23516. doi: 10.1371/journal.pone.0023516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lloyd SA, Lewis GS, Zhang Y, Paul EM, Donahue HJ. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J. Bone Min. Res. 2012;27:2359–2372. doi: 10.1002/jbmr.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lloyd SA, Loiselle AE, Zhang Y, Donahue HJ. Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone. 2013;57:76–83. doi: 10.1016/j.bone.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao D, et al. Connexin 43 channels in osteocytes regulate bone responses to mechanical unloading. Front. Physiol. 2020;11:299. doi: 10.3389/fphys.2020.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moore ER, Chen JC, Jacobs CR. Prx1-expressing progenitor primary cilia mediate bone formation in response to mechanical loading in mice. Stem Cells Int. 2019;2019:3094154. doi: 10.1155/2019/3094154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi W, Ma Z, Zhang G, Wang C, Jiao Z. Novel functions of the primary cilium in bone disease and cancer. Cytoskeleton. 2019;76:233–242. doi: 10.1002/cm.21529. [DOI] [PubMed] [Google Scholar]
- 80.Mirvis M, Stearns T, James Nelson W. Cilium structure, assembly, and disassembly regulated by the cytoskeleton. Biochem. J. 2018;475:2329–2353. doi: 10.1042/BCJ20170453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coughlin TR, Voisin M, Schaffler MB, Niebur GL, McNamara LM. Primary cilia exist in a small fraction of cells in trabecular bone and marrow. Calcif. Tissue Int. 2015;96:65–72. doi: 10.1007/s00223-014-9928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao Z, Quarles LD. Physiological mechanisms and therapeutic potential of bone mechanosensing. Rev. Endocr. Metab. Disord. 2015;16:115–129. doi: 10.1007/s11154-015-9313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qiu N, et al. Disruption of Kif3a in osteoblasts results in defective bone formation and osteopenia. J. Cell Sci. 2012;125:1945–1957. doi: 10.1242/jcs.095893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao ZS, Quarles LD. Role of the polycytin-primary cilia complex in bone development and mechanosensing. Ann. N. Y. Acad. Sci. 2010;1192:410–421. doi: 10.1111/j.1749-6632.2009.05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ding D, et al. Pharmacological regulation of primary cilium formation affects the mechanosensitivity of osteocytes. Calcif. Tissue Int. 2020;107:625–635. doi: 10.1007/s00223-020-00756-6. [DOI] [PubMed] [Google Scholar]
- 86.Moore ER, Zhu YX, Ryu HS, Jacobs CR. Periosteal progenitors contribute to load-induced bone formation in adult mice and require primary cilia to sense mechanical stimulation. Stem Cell Res. Ther. 2018;9:190. doi: 10.1186/s13287-018-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malone AM, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc. Natl. Acad. Sci. USA. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoey DA, Kelly DJ, Jacobs CR. A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2011;412:182–187. doi: 10.1016/j.bbrc.2011.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rich DR, Clark AL. Chondrocyte primary cilia shorten in response to osmotic challenge and are sites for endocytosis. Osteoarthr. Cartil. 2012;20:923–930. doi: 10.1016/j.joca.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 90.Thompson CL, Chapple JP, Knight MM. Primary cilia disassembly down-regulates mechanosensitive hedgehog signalling: a feedback mechanism controlling ADAMTS-5 expression in chondrocytes. Osteoarthr. Cartil. 2014;22:490–498. doi: 10.1016/j.joca.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shao YY, Wang L, Welter JF, Ballock RT. Primary cilia modulate Ihh signal transduction in response to hydrostatic loading of growth plate chondrocytes. Bone. 2012;50:79–84. doi: 10.1016/j.bone.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fang F, Schwartz AG, Moore ER, Sup ME, Thomopoulos S. Primary cilia as the nexus of biophysical and hedgehog signaling at the tendon enthesis. Sci. Adv. 2020;6:eabc1799. doi: 10.1126/sciadv.abc1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi W, et al. Microgravity induces inhibition of osteoblastic differentiation and mineralization through abrogating primary cilia. Sci. Rep. 2017;7:1866. doi: 10.1038/s41598-017-02049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi W, et al. The flavonol glycoside icariin promotes bone formation in growing rats by activating the cAMP signaling pathway in primary cilia of osteoblasts. J. Biol. Chem. 2017;292:20883–20896. doi: 10.1074/jbc.M117.809517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mederos y Schnitzler M, et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc. Natl. Acad. Sci. USA. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu J, et al. GPR68 senses flow and is essential for vascular physiology. Cell. 2018;173:762–775 e716. doi: 10.1016/j.cell.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Erdogmus S, et al. Helix 8 is the essential structural motif of mechanosensitive GPCRs. Nat. Commun. 2019;10:5784. doi: 10.1038/s41467-019-13722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vassart G, Costagliola S. G protein-coupled receptors: mutations and endocrine diseases. Nat. Rev. Endocrinol. 2011;7:362–372. doi: 10.1038/nrendo.2011.20. [DOI] [PubMed] [Google Scholar]
- 100.Ishida T, Takahashi M, Corson MA, Berk BC. Fluid shear stress-mediated signal transduction: how do endothelial cells transduce mechanical force into biological responses? Ann. N. Y. Acad. Sci. 1997;811:12–23. doi: 10.1111/j.1749-6632.1997.tb51984.x. [DOI] [PubMed] [Google Scholar]
- 101.Melchior B, Frangos JA. Galphaq/11-mediated intracellular calcium responses to retrograde flow in endothelial cells. Am. J. Physiol. Cell Physiol. 2012;303:C467–C473. doi: 10.1152/ajpcell.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wei WC, et al. Coincidence detection of membrane stretch and extracellular pH by the proton-sensing receptor OGR1 (GPR68) Curr. Biol. 2018;28:3815–3823. doi: 10.1016/j.cub.2018.10.046. [DOI] [PubMed] [Google Scholar]
- 103.Zhang YL, Frangos JA, Chachisvilis M. Mechanical stimulus alters conformation of type 1 parathyroid hormone receptor in bone cells. Am. J. Physiol. Cell Physiol. 2009;296:C1391–C1399. doi: 10.1152/ajpcell.00549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coste B, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L, et al. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature. 2019;573:225–229. doi: 10.1038/s41586-019-1505-8. [DOI] [PubMed] [Google Scholar]
- 106.Li J, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Retailleau K, et al. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep. 2015;13:1161–1171. doi: 10.1016/j.celrep.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 108.Nonomura K, et al. Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc. Natl. Acad. Sci. USA. 2018;115:12817–12822. doi: 10.1073/pnas.1817070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeng WZ, et al. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science. 2018;362:464–467. doi: 10.1126/science.aau6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iring A, et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Investig. 2019;130:2775–2791. doi: 10.1172/JCI123825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pathak MM, et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA. 2014;111:16148–16153. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koser DE, et al. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 2016;19:1592–1598. doi: 10.1038/nn.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Romac JM, Shahid RA, Swain SM, Vigna SR, Liddle RA. Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nat. Commun. 2018;9:1715. doi: 10.1038/s41467-018-04194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blumenthal NR, Hermanson O, Heimrich B, Shastri VP. Stochastic nanoroughness modulates neuron-astrocyte interactions and function via mechanosensing cation channels. Proc. Natl. Acad. Sci. USA. 2014;111:16124–16129. doi: 10.1073/pnas.1412740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Woo SH, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509:622–626. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Woo SH, et al. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 2015;18:1756–1762. doi: 10.1038/nn.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nonomura K, et al. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature. 2017;541:176–181. doi: 10.1038/nature20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tao H, et al. Oscillatory cortical forces promote three dimensional cell intercalations that shape the murine mandibular arch. Nat. Commun. 2019;10:1703. doi: 10.1038/s41467-019-09540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee W, et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. USA. 2014;111:E5114–E5122. doi: 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morris JA, et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 2019;51:258–266. doi: 10.1038/s41588-018-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun, W. et al. The mechanosensitive Piezo1 channel is required for bone formation. eLife8, 10.7554/eLife.47454 (2019). [DOI] [PMC free article] [PubMed]
- 122.Li, X. et al. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. eLife8, 10.7554/eLife.49631 (2019). [DOI] [PMC free article] [PubMed]
- 123.Wang L, et al. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020;11:282. doi: 10.1038/s41467-019-14146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith SM, et al. Fifty years of human space travel: implications for bone and calcium research. Annu. Rev. Nutr. 2014;34:377–400. doi: 10.1146/annurev-nutr-071813-105440. [DOI] [PubMed] [Google Scholar]
- 125.Zhou, T. et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ss-catenin. eLife9, 10.7554/eLife.52779 (2020). [DOI] [PMC free article] [PubMed]
- 126.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van der Eerden BC, et al. TRPV4 deficiency causes sexual dimorphism in bone metabolism and osteoporotic fracture risk. Bone. 2013;57:443–454. doi: 10.1016/j.bone.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 128.O'Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. USA. 2014;111:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee KL, et al. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia. 2015;4:7. doi: 10.1186/s13630-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pochynyuk O, Zaika O, O'Neil RG, Mamenko M. Novel insights into TRPV4 function in the kidney. Pflug. Arch. : Eur. J. Physiol. 2013;465:177–186. doi: 10.1007/s00424-012-1190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sonkusare SK, et al. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Corrigan MA, et al. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci. Rep. 2018;8:3824. doi: 10.1038/s41598-018-22174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gilchrist CL, et al. TRPV4-mediated calcium signaling in mesenchymal stem cells regulates aligned collagen matrix formation and vinculin tension. Proc. Natl. Acad. Sci. USA. 2019;116:1992–1997. doi: 10.1073/pnas.1811095116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hu K, Sun H, Gui B, Sui C. TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells. Biomed. Pharmacother. 2017;91:841–848. doi: 10.1016/j.biopha.2017.04.094. [DOI] [PubMed] [Google Scholar]
- 135.Du G, et al. Roles of TRPV4 and piezo channels in stretch-evoked Ca2+ response in chondrocytes. Exp. Biol. Med. 2020;245:180–189. doi: 10.1177/1535370219892601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Matthews BD, et al. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr. Biol. : Quant. Biosci. Nano Macro. 2010;2:435–442. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kang SS, Shin SH, Auh CK, Chun J. Human skeletal dysplasia caused by a constitutive activated transient receptor potential vanilloid 4 (TRPV4) cation channel mutation. Exp. Mol. Med. 2012;44:707–722. doi: 10.3858/emm.2012.44.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mizoguchi F, et al. Transient receptor potential vanilloid 4 deficiency suppresses unloading-induced bone loss. J. Cell. Physiol. 2008;216:47–53. doi: 10.1002/jcp.21374. [DOI] [PubMed] [Google Scholar]
- 139.Masuyama R, et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008;8:257–265. doi: 10.1016/j.cmet.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 140.Cao B, Dai X, Wang W. Knockdown of TRPV4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through Ca2+ -calcineurin-NFATc1 pathway. J. Cell Physiol. 2019;234:6831–6841. doi: 10.1002/jcp.27432. [DOI] [PubMed] [Google Scholar]
- 141.Lecuit T, Lenne PF, Munro E. Force generation, transmission, and integration during cell and tissue morphogenesis. Annu. Rev. cell Dev. Biol. 2011;27:157–184. doi: 10.1146/annurev-cellbio-100109-104027. [DOI] [PubMed] [Google Scholar]
- 142.Gardel ML, et al. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc. Natl. Acad. Sci. USA. 2006;103:1762–1767. doi: 10.1073/pnas.0504777103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bendix PM, et al. A quantitative analysis of contractility in active cytoskeletal protein networks. Biophys. J. 2008;94:3126–3136. doi: 10.1529/biophysj.107.117960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Humphrey D, Duggan C, Saha D, Smith D, Kas J. Active fluidization of polymer networks through molecular motors. Nature. 2002;416:413–416. doi: 10.1038/416413a. [DOI] [PubMed] [Google Scholar]
- 145.Haviv L, Gillo D, Backouche F, Bernheim-Groswasser A. A cytoskeletal demolition worker: myosin II acts as an actin depolymerization agent. J. Mol. Biol. 2008;375:325–330. doi: 10.1016/j.jmb.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 146.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu X, Kodama A, Fuchs E. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell. 2008;135:137–148. doi: 10.1016/j.cell.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu X, et al. Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3beta. Cell. 2011;144:341–352. doi: 10.1016/j.cell.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yin C, et al. Mechanical unloading reduces microtubule actin crosslinking factor 1 expression to inhibit beta-catenin signaling and osteoblast proliferation. J. Cell Physiol. 2018;233:5405–5419. doi: 10.1002/jcp.26374. [DOI] [PubMed] [Google Scholar]
- 150.Su P, et al. MACF1 promotes preosteoblast migration by mediating focal adhesion turnover through EB1. Biology Open. 2020;9:bio048173. doi: 10.1242/bio.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hu L, et al. Microtubule actin crosslinking factor 1 promotes osteoblast differentiation by promoting beta-catenin/TCF1/Runx2 signaling axis. J. Cell Physiol. 2018;233:1574–1584. doi: 10.1002/jcp.26059. [DOI] [PubMed] [Google Scholar]
- 152.Bacabac RG, et al. Round versus flat: bone cell morphology, elasticity, and mechanosensing. J. Biomech. 2008;41:1590–1598. doi: 10.1016/j.jbiomech.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 153.Vorselen D, Roos WH, MacKintosh FC, Wuite GJ, van Loon JJ. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 2014;28:536–547. doi: 10.1096/fj.13-236356. [DOI] [PubMed] [Google Scholar]
- 154.Xu H, et al. Actin cytoskeleton mediates BMP2-Smad signaling via calponin 1 in preosteoblast under simulated microgravity. Biochimie. 2017;138:184–193. doi: 10.1016/j.biochi.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 155.Willems HME, van den Heuvel E, Schoemaker RJW, Klein-Nulend J, Bakker AD. Diet and exercise: a match made in bone. Curr. Osteoporos. Rep. 2017;15:555–563. doi: 10.1007/s11914-017-0406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Uzer G, et al. Sun-mediated mechanical LINC between nucleus and cytoskeleton regulates betacatenin nuclear access. J. Biomech. 2018;74:32–40. doi: 10.1016/j.jbiomech.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Burridge K, Wittchen ES. The tension mounts: stress fibers as force-generating mechanotransducers. J. Cell Biol. 2013;200:9–19. doi: 10.1083/jcb.201210090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 160.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 161.Santos A, Bakker AD, Zandieh-Doulabi B, de Blieck-Hogervorst JM, Klein-Nulend J. Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem. Biophys. 2010;391:364–369. doi: 10.1016/j.bbrc.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 162.Sen B, et al. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu S, Yu Q, Lai A, Tian J. Pulsed electromagnetic field induces Ca2+-dependent osteoblastogenesis in C3H10T1/2 mesenchymal cells through the Wnt-Ca2+/Wnt-beta-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2018;503:715–721. doi: 10.1016/j.bbrc.2018.06.066. [DOI] [PubMed] [Google Scholar]
- 164.Leal ML, et al. Effect of different resistance-training regimens on the WNT-signaling pathway. Eur. J. Appl. Physiol. 2011;111:2535–2545. doi: 10.1007/s00421-011-1874-7. [DOI] [PubMed] [Google Scholar]
- 165.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Robinson JA, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J. Biol. Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 167.Lories RJ, et al. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007;56:4095–4103. doi: 10.1002/art.23137. [DOI] [PubMed] [Google Scholar]
- 168.Pflanz D, et al. Sost deficiency led to a greater cortical bone formation response to mechanical loading and altered gene expression. Sci. Rep. 2017;7:9435. doi: 10.1038/s41598-017-09653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gerbaix M, Ammann P, Ferrari S. Mechanically driven counter-regulation of cortical bone formation in response to sclerostin-neutralizing antibodies. J Bone Miner. Res. 2020;36:385–399. doi: 10.1002/jbmr.4193. [DOI] [PubMed] [Google Scholar]
- 170.Gerbaix M, Vico L, Ferrari SL, Bonnet N. Periostin expression contributes to cortical bone loss during unloading. Bone. 2015;71:94–100. doi: 10.1016/j.bone.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 171.Bonnet N, Conway SJ, Ferrari SL. Regulation of beta catenin signaling and parathyroid hormone anabolic effects in bone by the matricellular protein periostin. Proc. Natl. Acad. Sci. USA. 2012;109:15048–15053. doi: 10.1073/pnas.1203085109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Arnsdorf EJ, Tummala P, Jacobs CR. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One. 2009;4:e5388. doi: 10.1371/journal.pone.0005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu Y, et al. The orphan receptor tyrosine kinase Ror2 promotes osteoblast differentiation and enhances ex vivo bone formation. Mol. Endocrinol. 2007;21:376–387. doi: 10.1210/me.2006-0342. [DOI] [PubMed] [Google Scholar]
- 174.Song F, et al. Mechanical stress regulates osteogenesis and adipogenesis of rat mesenchymal stem cells through PI3K/Akt/GSK-3beta/beta-catenin signaling pathway. BioMed. Res. Int. 2017;2017:6027402. doi: 10.1155/2017/6027402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dey A, Varelas X, Guan KL. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 2020;19:480–494. doi: 10.1038/s41573-020-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 178.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 179.Schlegelmilch K, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tang Y, Feinberg T, Keller ET, Li XY, Weiss SJ. Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nat. Cell Biol. 2016;18:917–929. doi: 10.1038/ncb3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Murakami M, Nakagawa M, Olson EN, Nakagawa O. A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt-Oram syndrome. Proc. Natl. Acad. Sci. USA. 2005;102:18034–18039. doi: 10.1073/pnas.0509109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol. Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 183.Meng Z, et al. RAP2 mediates mechanoresponses of the Hippo pathway. Nature. 2018;560:655–660. doi: 10.1038/s41586-018-0444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rando OJ, Zhao K, Janmey P, Crabtree GR. Phosphatidylinositol-dependent actin filament binding by the SWI/SNF-like BAF chromatin remodeling complex. Proc. Natl. Acad. Sci. USA. 2002;99:2824–2829. doi: 10.1073/pnas.032662899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chang L, et al. The SWI/SNF complex is a mechanoregulated inhibitor of YAP and TAZ. Nature. 2018;563:265–269. doi: 10.1038/s41586-018-0658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 187.Kuroda M, Wada H, Kimura Y, Ueda K, Kioka N. Vinculin promotes nuclear localization of TAZ to inhibit ECM stiffness-dependent differentiation into adipocytes. J. Cell Sci. 2017;130:989–1002. doi: 10.1242/jcs.194779. [DOI] [PubMed] [Google Scholar]
- 188.Kegelman CD, et al. Skeletal cell YAP and TAZ combinatorially promote bone development. FASEB J. 2018;32:2706–2721. doi: 10.1096/fj.201700872R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Morin-Kensicki EM, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol. Cell. Biol. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hossain Z, et al. Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc. Natl. Acad. Sci. USA. 2007;104:1631–1636. doi: 10.1073/pnas.0605266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zaidi SK, et al. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J. 2004;23:790–799. doi: 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Seo E, et al. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep. 2013;3:2075–2087. doi: 10.1016/j.celrep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Park HW, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Byun MR, et al. Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell Death Differ. 2014;21:854–863. doi: 10.1038/cdd.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hong JH, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 196.Yang JY, et al. Osteoblast-targeted overexpression of TAZ increases bone mass in vivo. PLoS One. 2013;8:e56585. doi: 10.1371/journal.pone.0056585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhou Z, et al. Neogenin regulation of BMP-induced canonical Smad signaling and endochondral bone formation. Dev. Cell. 2010;19:90–102. doi: 10.1016/j.devcel.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J. Biol. Chem. 2000;275:17647–17652. doi: 10.1074/jbc.M908622199. [DOI] [PubMed] [Google Scholar]
- 199.Yang W, et al. Bmp2 in osteoblasts of periosteum and trabecular bone links bone formation to vascularization and mesenchymal stem cells. J. Cell Sci. 2013;126:4085–4098. doi: 10.1242/jcs.118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang YK, et al. Bone morphogenetic protein-2-induced signaling and osteogenesis is regulated by cell shape, RhoA/ROCK, and cytoskeletal tension. Stem Cells Dev. 2012;21:1176–1186. doi: 10.1089/scd.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zouani OF, Kalisky J, Ibarboure E, Durrieu MC. Effect of BMP-2 from matrices of different stiffnesses for the modulation of stem cell fate. Biomaterials. 2013;34:2157–2166. doi: 10.1016/j.biomaterials.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 202.Herberg S, et al. Combinatorial morphogenetic and mechanical cues to mimic bone development for defect repair. Sci. Adv. 2019;5:eaax2476. doi: 10.1126/sciadv.aax2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wei Q, et al. BMP-2 signaling and mechanotransduction synergize to drive osteogenic differentiation via YAP/TAZ. Adv. Sci. 2020;7:1902931. doi: 10.1002/advs.201902931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen Z, et al. Mechanosensitive miRNAs and bone formation. Int. J. Mol. Sci. 2017;18:1684. doi: 10.3390/ijms18081684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang Y, et al. MicroRNA-139-3p regulates osteoblast differentiation and apoptosis by targeting ELK1 and interacting with long noncoding RNA ODSM. Cell Death Dis. 2018;9:1107. doi: 10.1038/s41419-018-1153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang L, et al. MiR-30 family members inhibit osteoblast differentiation by suppressing Runx2 under unloading conditions in MC3T3-E1 cells. Biochem. Biophys. Res. Commun. 2020;522:164–170. doi: 10.1016/j.bbrc.2019.11.057. [DOI] [PubMed] [Google Scholar]
- 207.Wang H, et al. Osteoblast-targeted delivery of miR-33-5p attenuates osteopenia development induced by mechanical unloading in mice. Cell Death Dis. 2018;9:170. doi: 10.1038/s41419-017-0210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chen Z, et al. Silencing of miR-138-5p sensitizes bone anabolic action to mechanical stimuli. Theranostics. 2020;10:12263–12278. doi: 10.7150/thno.53009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Frith JE, et al. Mechanically-sensitive miRNAs bias human mesenchymal stem cell fate via mTOR signalling. Nat. Commun. 2018;9:257. doi: 10.1038/s41467-017-02486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Solis AG, et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;573:69–74. doi: 10.1038/s41586-019-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T. Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J. Cell Biochem. 2007;101:1266–1277. doi: 10.1002/jcb.21249. [DOI] [PubMed] [Google Scholar]
- 212.Wang Y, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Investig. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Smith SM, et al. Collagen cross-link excretion during space flight and bed rest. J. Clin. Endocrinol. Metab. 1998;83:3584–3591. doi: 10.1210/jcem.83.10.5169. [DOI] [PubMed] [Google Scholar]
- 214.Smith SM, et al. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the mir space station. J. Bone Min. Res. 2005;20:208–218. doi: 10.1359/JBMR.041105. [DOI] [PubMed] [Google Scholar]
- 215.Farley A, et al. Unloading-Induced cortical bone loss is exacerbated by low-dose irradiation during a simulated deep space exploration mission. Calcif. Tissue Int. 2020;107:170–179. doi: 10.1007/s00223-020-00708-0. [DOI] [PubMed] [Google Scholar]
- 216.Garrett-Bakelman FE, et al. The NASA twins study: a multidimensional analysis of a year-long human spaceflight. Science. 2019;364:eaau8650. doi: 10.1126/science.aau8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stavnichuk M, Mikolajewicz N, Corlett T, Morris M, Komarova SV. A systematic review and meta-analysis of bone loss in space travelers. NPJ Microgravity. 2020;6:13. doi: 10.1038/s41526-020-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Caillot-Augusseau A, et al. Bone formation and resorption biological markers in cosmonauts during and after a 180-day space flight (Euromir 95) Clin. Chem. 1998;44:578–585. [PubMed] [Google Scholar]
- 219.Liao C, et al. Shear stress inhibits IL-17A-mediated induction of osteoclastogenesis via osteocyte pathways. Bone. 2017;101:10–20. doi: 10.1016/j.bone.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 220.Kim CH, You L, Yellowley CE, Jacobs CR. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone. 2006;39:1043–1047. doi: 10.1016/j.bone.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 221.Cambre I, et al. Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis. Nat. Commun. 2018;9:4613. doi: 10.1038/s41467-018-06933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bourrin S, Genty C, Palle S, Gharib C, Alexandre C. Adverse effects of strenuous exercise: a densitometric and histomorphometric study in the rat. J. Appl. Physiol. 1994;76:1999–2005. doi: 10.1152/jappl.1994.76.5.1999. [DOI] [PubMed] [Google Scholar]
- 223.Barbe MF, Popoff SN. Occupational activities: factors that tip the balance from bone accrual to bone loss. Exerc. Sport. Sci. Rev. 2020;48:59–66. doi: 10.1249/JES.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hoey DA, Tormey S, Ramcharan S, O'Brien FJ, Jacobs CR. Primary cilia-mediated mechanotransduction in human mesenchymal. Stem Cells. 2012;30:2561–2570. doi: 10.1002/stem.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Aguirre JI, et al. A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J. Biol. Chem. 2007;282:25501–25508. doi: 10.1074/jbc.M702231200. [DOI] [PubMed] [Google Scholar]
- 226.Klymenko Y, et al. Modeling the effect of ascites-induced compression on ovarian cancer multicellular aggregates. Dis. Models Mech. 2018;11:dmm034199. doi: 10.1242/dmm.034199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pardo SJ, et al. Simulated microgravity using the random positioning machine inhibits differentiation and alters gene expression profiles of 2T3 preosteoblasts. Am. J. Physiol. Cell Physiol. 2005;288:C1211–C1221. doi: 10.1152/ajpcell.00222.2004. [DOI] [PubMed] [Google Scholar]
- 228.Martinelli LK, et al. Effect of microgravity on immune cell viability and proliferation: simulation using 3-D clinostat. IEEE Eng. Med. Biol. Mag. : Q. Mag. Eng. Med. Biol. Soc. 2009;28:85–90. doi: 10.1109/MEMB.2009.933572. [DOI] [PubMed] [Google Scholar]
- 229.Yang X, et al. Trabecular bone adaptation to loading in a rabbit model is not magnitude-dependent. J. Orthop. Res. 2013;31:930–934. doi: 10.1002/jor.22316. [DOI] [PubMed] [Google Scholar]
- 230.Wang X, et al. miR-214 targets ATF4 to inhibit bone formation. Nat. Med. 2013;19:93–100. doi: 10.1038/nm.3026. [DOI] [PubMed] [Google Scholar]
- 231.Gertz ML, et al. Multi-omic, single-cell, and biochemical profiles of astronauts guide pharmacological strategies for returning to gravity. Cell Rep. 2020;33:108429. doi: 10.1016/j.celrep.2020.108429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Trinchant NM, et al. Clonal hematopoiesis before, during, and after human spaceflight. Cell Rep. 2020;33:108458. doi: 10.1016/j.celrep.2020.108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Luxton JJ, et al. Telomere length dynamics and DNA damage responses associated with long-duration spaceflight. Cell Rep. 2020;33:108457. doi: 10.1016/j.celrep.2020.108457. [DOI] [PubMed] [Google Scholar]
- 234.Walls S, et al. Prolonged exposure to microgravity reduces cardiac contractility and initiates remodeling in Drosophila. Cell Rep. 2020;33:108445. doi: 10.1016/j.celrep.2020.108445. [DOI] [PMC free article] [PubMed] [Google Scholar]