Abstract
Salmonellosis is a severe problem that threatens the poultry sector worldwide right now. Salmonella gallinarium and Salmonella pullorum (Fowl typhoid) are the most pathogenic serovars in avian species leading to systemic infection resulting in severe economic losses in the poultry industry. Nontyphoidal serotypes of Salmonella (Paratyphoid disease) constitute a public health hazard for their involvement in food poisoning problems in addition to their zoonotic importance. Also, Salmonella species distribution is particularly extensive. They resisted environmental conditions that made it difficult to control their spread for a long time. Therefore, the current review aimed to through light on Salmonellosis in poultry with particular references to its pathogenesis, economic importance, immune response to Salmonella, Salmonella antibiotics resistance, possible methods for prevention and control of such problems using promising antibiotics alternatives including probiotics, prebiotics, symbiotics, organic acids, essential oils, cinnamaldehyde, chitosan, nanoparticles, and vaccines.
Key words: antibiotic resistance, organic feed additives, poultry, Salmonella, vaccine
List of abbreviation: S., Salmonella; S. enteritidis, Salmonella enteritidis; S. heidelberg, Salmonella heidelberg; S. typhimurium, Salmonella typhimurium; S. infantis, Salmonella infantis; S. lille, Salmonella lille; S. kentucky, Salmonella kentucky; S. senftenberg, Salmonella senftenberg; S. mbandaka, Salmonella mbandaka; S. montevideo, Salmonella montevideo; S. schwarzengrund, Salmonella schwarzengrund; S. anatum, Salmonella anatum; S. berta, Salmonella berta; S. newport, Salmonella newport; S. javiana, Salmonella javiana; S. pullorum, Salmonella pullorum; S. gallinarum, Salmonella gallinarum; subsp., Sub-species; E. coli, Escherichia coli; C. butyricum, Clostridium butyricum; C. perfringens, Clostridium perfringens; L. salivaris, Lactobacillus salivaris; C. jejuni, Campylobacter jejuni; L. acidophilus, Lactobacillus acidophilus; L. reuteri, Lactobacillus reuteri; B. bifidum, Bifidobacterium bifidum; E. faecium, Enterococcus faecium; WHO, World Health Organization; CDC, Center for Disease Control and Prevention; USDA, U.S. Department of Agriculture; LITAF, Lipopolysaccharide Induced TNF Factor; IL-1ß, Interleukin -1ß; IFN- γ, Interferon gamma; TNF-α, Tumor Necrosis Factor Alpha; IL-10, Interleukin 10; Th2, T helper 2; IgA, Immunoglobulin A; IgG, Immunoglobulin G; IgM, Immunoglobulin M; NARMS, The National Antimicrobial Resistance Monitoring System; SCFAs, Short-chain fatty acids; RBCs, Red blood cells; oC, Celsius; RNA, Ribonucleic acid; DNA, Deoxyribonucleic acid
INTRODUCTION
In 1885, an American scientist called Daniel E. Salmon isolated the enteric pathogen from the pig's intestine. In 1900 to honor Daniel Salmon, a French bacteriologist called Joseph Léon Marcel Lignières proposed that the swine cholera be named “Salmonella” (White, 1926) then Salmonella has been isolated from water, soil, and sewage (Li et al., 2013). Salmonella enterica serotype enteritidis is one of Salmonella most isolated nontyphoidal serotypes. It can lead to a Salmonellosis infection, with variable symptoms including diarrhea, vomiting, and fever when infecting humans. In the United States, over 40,000 cases of Salmonellosis and 400 deaths due to acute Salmonellosis have been reported every year (Fabrega and Vila, 2013; Abd El-Hack et al., 2021a).
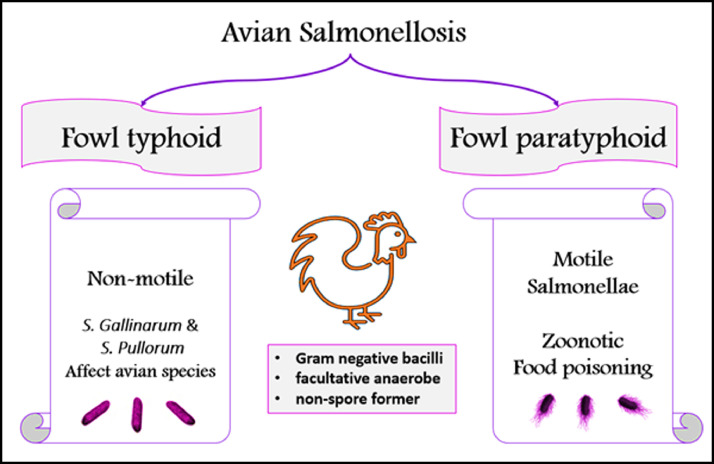
Bacteriologically, Salmonella is one of the Enterobacteriaceae family, which are known intracellular, Gram-negative motile bacilli with peritrichous flagella all over the entire cell body diameters, ranging from 0.7 to 1.5 μm, lengths from 2 to 5 μm, nonspore-forming, facultative anaerobe with optimal growth at 37°C in a pH range of 4 to 9 and biochemically, characterized by hydrogen sulfide and catalase production, and lack of oxidase enzyme as well as lactose fermentation property (Coburn et al., 2007; Li et al., 2013; Figure 1). They are heat-labile and killed at 70°C; however, they can survive in dust and harsh environmental conditions for 2 yr and more (Davies and Wray, 1996). Salmonella can be classified into 2 species, Salmonella bongori and Salmonella enterica. Further, Salmonella enterica is subdivided into 6 subspecies: S. enterica subsp. enterica, S. enterica subsp. salamae, S. enterica subsp. arizonae, S. enterica subsp. diarizonae, S. enterica subsp. houtenae, and S. enterica subsp. indica (Lan et al., 2009).
Figure 1.
Avian Salmonellosis and its bacteriological characters.
Salmonella isolates are most usually identified according to their serotype. Salmonella serotyping is based on identifying somatic antigen (oligosaccharide component of the lipopolysaccharide located on the outer membrane) and H flagellar antigen found in the bacteria's flagella (Brenner et al., 2000; Eng et al., 2015). Salmonella enterica serotype enteritidis is the most common serovar of Salmonella, which has less pathogenic effects in avian species but can lead to severe clinical Salmonellosis when transmitted to humans (Tarabees et al., 2017). Salmonella has a wide host range with public health concerns and a short incubation period from 8 h to 3 d. The disease severity increased in immune-suppressed, younger, and elderly hosts in the shape of variable clinical signs as nausea, abdominal pain, diarrhea, dehydration, and death (D'Aoust, 1991; WHO, 2015).
Salmonella-contaminated animal products resulted in 3% of the bacterial foodborne disease worldwide, with about 80 million infections and 155,000 deaths (Bell and Kyriakides, 2002; Majowicz et al., 2010; Abd El-Hack et al., 2021a). Salmonellosis is closely related to poultry production, and many efforts are being made to control this pathogen and reduce its spread during the different stages of poultry production (CDC, 2013c, d). Birds can consume contaminated rations with Salmonella and not exhibit any clinical illness, but later, these birds can contaminate processing facilities during evisceration and contaminate poultry carcasses with Salmonella causing human health hazards (Wibisono et al., 2020). To reduce Salmonella loads during poultry processing, it is essential to maintain Salmonella-free chickens and biosecurity practices in different poultry operations such as ration formulation, poultry farming, transportation, poultry processing should be adopted (Bailey, 1993) (Table 1. This review article highlights the hazards of poultry Salmonellosis and the use of organic control agents to cope with it.
Table 1.
Most used antibiotic alternative treatment agents for Salmonella control.
| Treatment agents | Bird | Impact | References |
|---|---|---|---|
| Lactobacillus reuteri | Broiler chicken | Suppress the growth of Staphylococcus epidermidis, Staphylococcus aureus, E. coli, S. Typhimurium, Helicobacter pylori, and rotavirus | Seo et al. (2010) |
| Lactobacillus reuteri | Broiler chicken | Inhibits C. perfringens propagation | Cao et al. (2012) |
| Pediococcus acidilactici, mannan-oligosaccharide, and butyric acid | Broiler chicken | Reduces the colonization of S. Typhimurium and improves bird weight gain. | Jazi et al. (2018) |
| Enterococcus faecium | Layer chickens | Stimulates antibody production following live attenuated S. Enteritidis vaccination by intestinal microbiota modulation. | Beirão et al. (2018) |
| Bifidobacteria and Lactobacillus | Broiler chicken | Limits colonization of Salmonella enterica and improve bird body condition | El-Sharkawy et al. (2020) |
| Fructo-oligosaccharide | Broiler chicken | Limits Salmonella Enteritidis colonization by increased ileal mucosa thickness and improving immune response |
Shang et al. (2015) Xu et al. (2003) |
| E. Faecium and fructooligosaccharides | Layer chicken | Increase quality and quantity of eggs | Radu-Rusu et al. (2010) |
| Prebiotics | poultry | Increase body weight | Abd El-Hack et al. (2021a) |
| Acetic, lactic, and formic acid | Broiler chicken | Limits S. Typhimurium propagation in the chicken crop | Byrd et al. (2001) |
| Acetic acid 0.01, 0.1, and 1% concentration | In-vitro | Ineffective in reducing S. Enteritidis levels | Barnhart et al. (1999) |
| Sodium propionate or calcium propionate | Layer chicken | Improved egg production, egg weight, and FCR | Dahiya et al. (2016) |
| Cinnamaldehyde | Broiler chicken | Lowered S. Enteritidis propagation in chicken cecal content | Kollanoor-Johny et al. (2010) |
| Cinnamaldehyde and eugenol | Broiler chicken | Reduce S. enteritidis load | Kollanoor-Johny et al. (2012) |
| Cinnamaldehyde, eugenol, thymol, and carvacrol | In vitro Broiler chicken |
Antibacterial effect against S. enteritidis | Kollanoor-Johny et al. (2010) |
| Trans-cinnamaldehyde and eugenol's | Broiler chicken | Decreased S. enteritidis colonization | Kollanoor-Johny et al. (2012a) |
| Oregano and thyme | Broiler chicken | Reduced Salmonella species colonization | Koščová et al. (2006) |
| Essential oils. | Eggs | Reduce shell egg contamination with S. enteritidis | Upadhyaya et al. (2013) |
| Lemongrass extract | In vitro | Inhibitory effect against S. typhimurium S. enterica | Singh and Ebibeni (2016) |
| Chitosan | Broiler chicken | Reduced S. Typhimurium colonization | Menconi et al. (2014) |
| Chitosan | In vitro | Antibacterial activity against S. Paratyphi and Staphylococcus aureus | Islam et al. (2011) |
| Chitosan | In vitro | Antibacterial activity against intracellular S. Typhimurium | Edson et al. (2021) |
| Insect chitosan | Food safety studies | Antibacterial effect against S. Typhimurium, E. coli O157:H7 and Listeria monocytogenes | Ibañez-Peinado et al. (2020) |
| Chitosan and lactic acid | Chilled chicken | Control of Salmonella and E. coli contamination | El-Khawasa et al. (2020) |
| Fe3O4 magnetic nanoparticles | Broiler chicken | Reduced the viability of intracellular S. Enteritidis | Shen et al. (2020) |
| Silver nanoparticles | In vitro | Antibacterial effect against S.Typhimurium, S. Enteritidis and E. coli O78 | Mohamed et al. (2017) |
| Zinc oxide nanoparticles | Broiler chicken | Bactericidal effect against S. Typhimurium and Staphylococcus aureus | SILVA et al. (2021) |
| Gold nanoparticles | In vitro | Inhibitory effect against S. Typhimuruim, S. typhi, and S. paratyphi | El Sabry et al. (2018); Boatemaa et al. (2019) |
| Thymol nanoemulsion | Broiler chicken | Antibacterial activity against S. Typhimurium | Ibrahim et al. (2021) |
| Sunflower oil nanoemulsion | In vitro | Antibacterial activity against foodborne bacteria such as Salmonella typhi | Joe et al. (2012) |
| Garlic and onion extract chitosan nanoparticles | In vitro | Minimize the colonization of pathogenic enteric bacteria especially, E. coli, S. typhi, and C. jejuni | Enoka et al. (2021) |
Salmonella Infection and Poultry Industry
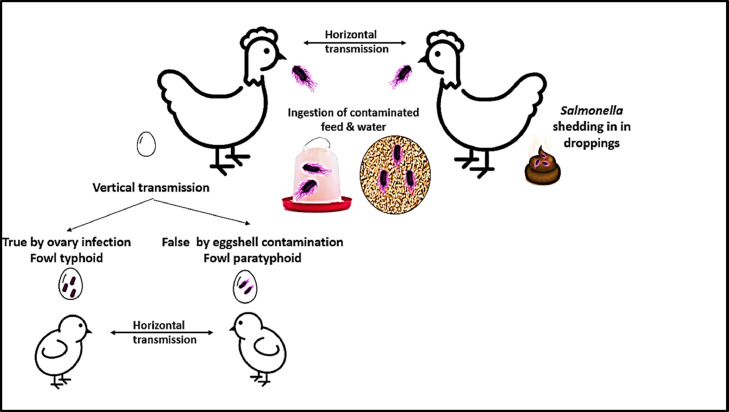
The poultry industry has been threatened by different pathogenic infections due to viral, bacterial, parasitic, or fungal origin (Setta et al. 2018; Attia and Salem, 2022; Salem et al. 2021, 2022; Soliman et al. 2021). Salmonellosis is a disease condition due to Salmonella infection, a severe problem facing the poultry industry worldwide (USDA, 2015). Salmonella is usually disseminated through chicken meat and eggshell contamination by chicken intestinal contents (Braden, 2006; Pires et al., 2014). Because eggs are more likely to contact the chickens' droppings, the number of laying hens kept in cage-free systems increases the danger of Salmonella contamination in eggs feces (Whiley and Ross, 2015). There are 2 main routes of egg contamination by Salmonella enteritidis, horizontal transmission; eggs can be contaminated by penetration through eggshell from the colonized chicken intestine or contaminated droppings during or after egg laying (de Reu et al., 2006) vertical transmissions, such as direct infection of the yolk, albumen, eggshell membranes, or eggshells before egg-laying, originating from the infection of reproductive organs with Salmonella enteritidis (Wibisono et al., 2020).
There are many types of Salmonella species infecting avian species. Salmonella pullorum and Salmonella gallinarum were found to be avian specific species. However, infections with other low avian-specific serovars such as S. typhimurium, S. heidelberg, S. enteritidis, S. infantis, S. lille, S. kentucky, S. senftenberg, S. mbandaka, S. montevideo, S. schwarzengrund, S. anatum, S. berta, S. newport, S. javiana, and another Salmonella serovars have been isolated from clinical poultry cases (Erdman et al., 2009; Center for Disease Control and Prevention CDC 2011; CDC, 2013e; CDC, 2013c). Salmonella infection is transmitted vertically and horizontally in poultry, and its incidence is high in one-day-old chicks (Shivaprasada et al., 2013; Figure 2). The severity of Salmonella infection is varied according to many factors, including hostage, host immunity, presence of coinfections, environmental, stress, managerial factors, and infective dose, with older birds less susceptible to Salmonellosis even with concentrations of 106 CFU/mL S. typhimurium (Wibisono et al., 2020).
Figure 2.
Transmission of avian Salmonellosis.
Pathogenicity and Immune Response to Salmonella
Salmonella enters the host via the oral route and colonizes within the alimentary tract. Then S. enteritidis is attached to the intestinal villi and colonized in the presence of a group of proteins known as adhesins (Beachey, 1981). Therefore, the most effective technique for preventing bacterial infection may prevent S. enteritidis from attaching to intestinal epithelial cell receptors (Wizemann et al., 1999). In chickens, the innate immunity cellular component, which comprises macrophages and heterophils, is critical in preventing intestinal infection (Van Immerseel et al., 2005; Fasina et al., 2010).
When enteric pathogens like Salmonella enter the intestinal epithelial barrier, innate immune cells are drawn to the infection site and use processes like phagocytosis and oxidative burst to kill these pathogens (Brisbin et al., 2008). The 2 blind ceci, have the highest population of Salmonella in the bird intestine (Sivula et al., 2008). Immunohistochemical examination of the cecal lamina propria showed heterophils infiltration the cecal lamina propria 12 h after Salmonella infection. It increased T lymphocyte infiltration in the cecal lamina propria 20 after Salmonella infection (Van Immerseel et al. (2005)). Salmonella infection in chickens includes an influx of inflammatory cytokines involving LITAF, IL-1ß, and IFN-γ (Sheela et al., 2003; Matulova et al., 2013). Salmonella overcomes the host defense by increasing the suppressive cytokine, IL-10 (Ghebremicael et al., 2008).
The adaptive immune response to Salmonella infection involves both humoral and cell-mediated responses. The first adaptive immune defense against S. enteritidis infection is the mucosal immune system, including mucosal immunoglobulin A (IgA) and mucosa-associated lymphocytes and leukocytes (Wigley, 2014). Secretory IgA prevents S. enteritidis from adhering to intestinal epithelial cells, limiting its mucosal colonization (Shroff et al., 1995; Wigley, 2014). The shape of the intestine is a crucial indicator of intestinal health. Because of the greater surface area and lower tissue turnover rate, increased villus height and decreased crypt depth correspond with increased nutrient absorption within the small intestine (Munyaka et al., 2012). Chicks infected with S. typhimurium revealed decreased jejunal villus height, crypt depth, and height: crypt depth ratio resulting in severe enteritis (Fasina et al., 2010; Borsoi et al., 2011).
Control and Prevention of Avian Salmonellosis
Antibiotic Growth Promoters
Dietary antibiotics have been used at subtherapeutic levels (antibiotic growth promoters) to promote feed efficiency and sustain animal health for more than 50 yr (Danzeisen et al., 2011; Abd El-Hack et al., 2021b) and to overcome diarrhea and respiratory affections, which are devastating problems facing the poultry industry (Abd El Hamid et al., 2019; Marouf et al., 2020,Marouf et al., 2022; Salem and Attia, 2021). Antibiotics' effects on feed efficiency are thought to be due to their manipulation of the gut microbiota. In the digestive tract of chickens, pathogenic bacteria such as Escherichia coli, Salmonella species, and Clostridium perfringens compete for nutrients with the host (Swelum et al., 2021; Abd El-Hack et al., 2022a).
They may damage the intestinal epithelium, resulting in impaired digestion and absorption inside the host (Adil et al., 2010). Subtherapeutic antibiotic doses suppress pathogenic intestinal bacteria, lowering disease incidence and, as a result, promoting avian growth (Danzeisen et al., 2011). On the other hand, antibiotic growth promoters are becoming less popular due to worries about the development of microbial antibiotic-resistant (Danzeisen et al., 2011).
Salmonella Antimicrobial Resistant
Salmonella Enteritidis (S. Enteritidis) is the most abundant serovar in poultry production, particularly chicken (Ray, 2004). Because of the massive use of antimicrobials in poultry, antimicrobial resistance of Salmonella has been developed to different antimicrobials as chloramphenicol, tetracyclines, streptomycin, sulfonamides, and Ampicillin (Ray, 2004). According to The National Antimicrobial Resistance Monitoring System (NARMS) laboratory from the Centers for Disease Control and Prevention (CDC), Salmonella heidelberg (S. heidelberg) linked with chicken was responsible for many human infections and these Salmonella serovars exhibited resistance to sulfisoxazole, chloramphenicol, ampicillin, kanamycin, gentamicin, tetracycline, and streptomycin. Also, nontyphoidal Salmonella showed multiple antimicrobial resistances, especially for ceftriaxone and ciprofloxacin. About 5% of Salmonella tested by the CDC has been shown to have resistance to at least one 5 different types of antimicrobials (CDC, 2015a). Recently, the world directed to limit the usage of antibiotics and encourage the use of organic antibiotic alternatives as prebiotics, probiotics, synbiotics, essential amino acids (Abou-Kassem et al., 2021a; Arif et al., 2021; Alagawany et al., 2021a), biologically synthesized nanoparticles, organic acids, essential oils, polyphenols (Saad et al., 2021a,b), bioactive peptides (El-Saadony et al., 2021a,b; Saad et al., 2021c), herbal extracts (Abou-Kassem et al., 2021b; El-Saadony et al., 2021c), enzymes (Llamas-Moya et al., 2019), bioactive plant compounds (El-Saadony et al., 2021d; Reda et al., 2021a), and phytogenic compounds (Abdelnour et al., 2020a,b; Ashour et al., 2020, 2021; Abd Elkader et al., 2021; Abdel-Moneim et al., 2021; Abd El-Hack et al., 2021d, Abd El-Hack et al., 2021c) to improve poultry performance and human health using safe and natural products.
Probiotics
The World Health Organization defines probiotics as “live microorganisms that benefit the host when administered in suitable concentrations” (Mack, 2005). Probiotics are numerous and have different mechanisms of action as; competitive exclusion phenomena, the release of bacteriostatic and bactericidal agents, immune modulation, increased intestinal healthiness, lower intestinal pH, and improvement of intestinal mucosal barrier (Van Der Wielen et al., 2000, 2002; Yang et al., 2012; Alagawany et al., 2021b; El-Saadony et al., 2021e).
It was found that daily incorporation of poultry diet with probiotics such as Lactobacillus reuteri and Lactobacillus salivarius, can limit the population of Salmonella and Campylobacter in the intestine of 3 wk old chickens (Nakphaichit et al., 2011). Also, daily administration of Bacillus subtilis, can lower the E. coli population in the ileum of broiler chickens at 3 wk of age (Molnar et al., 2011). In addition, daily administration of Clostridium butyricum HJCB998 can limit the cecal Salmonella and C. perfringens population in the cecum of broilers from 3 to 4 wk of age (Yang et al., 2012). Multispecies probiotics have also been studied for their protective benefits, such as administering multispecies Probiotic, including “Lactobacillus reuteri, Enterococcus faecium, Lactobacillus salivaris, Pediococcus acidilactici, and Bifidobacterium,” can lower cecal coliform count (Mountzouris et al., 2010).
Another model supplying broiler chickens with multispecies probiotics, including L. reuteri, Bifidobacterium animalis, L. salivaris, Pediococcus acidilactici, and Enterococcus faecium, significantly limited cecal Campylobacter jejuni population in broiler chickens at 2 wk postinoculation (Ghareeb et al., 2012). When comparing probiotics supplemented in water to probiotics in feed, some studies have found that probiotics supplied in the water had a higher efficacy (Karimi Torshizi et al., 2010; Ritzi et al., 2014). The most used probiotics in the poultry field are described in the below sections.
Probiotics Mode of Action
The “competitive exclusion” phenomenon occurs within the digestive system when microbes compete for resources such as nutrition and attachment sites (Nurmi et al., 1992). Pathogenic bacteria such as E. coli and Salmonella must first connect to the intestinal epithelial barrier to infect birds (Lan et al., 2005). To healthy birds, commensal bacteria populate the intestinal mucosa, generating a complex layer of beneficial bacteria that successfully blocks harmful bacteria from attaching to the mucosal surface and blocking the intestinal receptors (Lan et al., 2005).
Additionally, certain beneficial bacteria acquire a competitive advantage in the intestine by creating bacteriostatic or bactericidal chemicals that are toxic to pathogenic competitors. Also, lactic acid bacteria, as Lactobacillus species, ferment carbohydrates and liberate lactic acid, which lower intestinal pH and inhibit pathogens like E. coli, C. perfringens, and S. typhimurium in vitro (Murry et al. 2004; Abd El‐Hack et al., 2020a) and this phenomenon was confirmed by in vivo studies as Van Der Wielen et al. (2000) found that when the concentrations of SCFAs (acetate, propionate, and butyrate) increased, the abundance of Enterobacteriaceae including Salmonella and E. coli in broilers cecum decreased. In addition, SCFAs in nondissociated form can penetrate pathogenic bacterial cell membrane into the cell where they dissociate, resulting in bactericidal and bacteriostatic effects on the pathogen (Van Der Wielen et al., 2000). The SCFAs are considered as an energy source of intestinal villi (den Besten et al., 2013), stimulate enterocyte growth and proliferation (Blottiere et al., 2003), regulate mucin secretion (Willemsen et al. 2003), and modulate intestinal immunity (Correa-Oliveira et al., 2016).
The SCFA production in broilers is undetectable in one-day-old chicks, whereas at 2 wk of age, the cecal microflora stabilizes, and the SCFAs reach high amounts and remain stable (Van der Wielen et al., 2002). Also, some probiotics can secrete bacteriocins, which are considered antimicrobials that can inhibit pathogenic bacteria (Dobson et al., 2012). Lactobacillus salivarius strains isolated from the chicken intestine produce bacteriocins that inhibit S. enteritidis and C. jejuni growth (Svetoch et al., 2011). Enterococcus faecium, Pediococcus pentosaceus and Bacillus subtilis, isolated from broiler chickens, secrete bacteriocins that inhibit C. perfringens (Shin et al., 2008). Additionally, E. faecium produces bacteriocins against the oocysts of Eimeria species (Strompfova et al., 2010). Probiotics stimulate the bird's immune response to prime the host immune system by interacting with immune cells within the bird's gut (Kamada and Nunez, 2014).
Many researchers found that the intestinal microbiota can influence the antibody-mediated immune response. For example, chickens receiving probiotics including Streptococcus faecalis, Bifidobacterium bifidum, and L. acidophilus had higher systemic antibody response to sheep red blood cells (RBCs) than unsupplemented chickens (Haghighi et al., 2006). It was expected that probiotics enhance the production of Th2 cytokines (e.g., IL-4 and IL-10), which may subsequently stimulate the immune response mediated by antibodies as chickens supplied with Streptococcus faecalis, Lactobacillus acidophilus, and Bifidobacterium bifidum had higher levels of serum IgG and IgM at 2 wk of age (Haghighi et al., 2006). Lactobacillus species could stimulate differential cytokine expression in T cells of chicken cecal tonsils (Brisbin et al., 2012). It was noticed that broiler chickens supplied with probiotics including Bifidobacterium bifidum, L. acidophilus, and Streptococcus faecalis after Salmonella experimental infection had a marked decrease in gene expression IL-12 and IFN- γ (Haghighi et al., 2006). The most used probiotics in the poultry sector were as follows:
Lactobacillus reuteri
L. reuteri is a heterofermentative species of bacteria that can produce lactic acid, ethanol, acetate, hydrogen peroxide, carbon dioxide, reuterin, and retericylin (Yu et al., 2007). They resist heat, bile salts and low pH (Yu et al., 2007) and it has mucus binding proteins on their surface, which facilitates adherence to intestinal villi (Mackenzie et al., 2010). L. reuteri inhibits the growth Staphylococcus epidermidis, Staphylococcus aureus, E. coli, S. Typhimurium, Helicobacter pylori, and rotavirus (Seo et al., 2010). Additionally, chickens supplemented with 108 CFU/mL L. reuteri revealed reduced lesions score in birds infected with C. perfringens, suggesting that L. reuteri can modulate the innate immune response by regulating inflammatory cytokines and inhibiting C. perfringens proliferation (Cao et al., 2012). Furthermore, L. reuteri inactivated TNF-α production of lipopolysaccharide-activated monocytes and macrophages in vitro (Lin et al., 2008).
Pediococcus acidilactici
P. acidilactici is considered a facultative anaerobe with optimal growth at pH 6.2, overnight at 37°C up to 65°C and less sensitive to acidic pH (Lin et al., 2006). Pediococci suppresses enteric pathogens' growth via the production of lactic acid and bacteriocins as pediocins (Daeschel and Klaenhammer, 1985). Dietary supplementation with Pediococcus acidilactici, mannan-oligosaccharide and butyric acid reduces colonization of S. Typhimurium and improves broiler chickens' growth performance (Jazi et al., 2018).
Enterococcus faecium
E. faecium is a Gram-positive, facultative anaerobe, grows 10°C to 45°C, survives in both basic and acidic circumstances. E. faecium secretes bacteriocins (Kang and Lee, 2005). After vaccination, E. faecium improves layer chickens' immune and health status with live attenuated S. Enteritidis vaccine by improving gut microbiota (Beirão et al., 2018).
Bifidobacterium animalis
B. animalis is anaerobic, Gram-positive, rod-shaped bacterium, optimum growth 42°C to 49°C, highly resistant to bile salts, tolerate both oxygen and heat during manufacturing of probiotic feed additives, found naturally in the intestines of rabbits, chickens, and humans (Scardovi and Zani, 1974; Simpson et al., 2005; Sanchez et al., 2007). Incorporating Bifidobacteria and Lactobacillus with broiler feed reduce the colonization of experimentally infected Salmonella enterica and improves broilers' body condition (El-Sharkawy et al., 2020).
Prebiotics
Prebiotics are indigestible substances utilized explicitly by beneficial microbiota in the intestine (Cummings and Macfarlane, 2002; Yaqoob et al., 2021). Mannan-oligosaccharides or fructo-oligosaccharides are commonly used prebiotics in poultry ration and daily corporation of these prebiotics in broiler feed improves their performance and increases boy weight gain (Ao and Choct, 2013). Fructooligosaccharide daily incorporation with broiler feed limited the colonization of experimentally infected Salmonella enteritidis by increasing ileal mucosal thickness and elevating the transcription of ileal IL-1ß, IL-10, and interferon (IFN)-γ mRNA, increasing leukocyte numbers and serum IgY levels in response to LPS challenge (Shang et al., 2015).
In broiler chickens, fructooligosaccharide supplementation has been demonstrated to improve growth performance, improve innate and adaptive immunological response, increase small intestinal villi length, and boost beneficial intestinal bacteria colonization (Xu et al., 2003). Dietary supplementation with fructooligosaccharides alters the gut microbiota toward more beneficial bacteria, enhancing the production of short-chain fatty acids and the immune response to Salmonella (Shang et al., 2015). Chickens supplied with 0.25% to 0.5% fructooligosaccharidess showed improved weight gain (Xu et al., 2003; Shang et al., 2015). On the other hand, chickens supplied with 0.5% fructooligosaccharide did not differ in feed conversion of body weight gain compared to untreated ones (Kim et al., 2011) and these variations may be attributed to many factors as age, sex, breed, general health of the birds, environmental condition, hygienic measures, and inclusion level of the fructooligosaccharides (Shang et al., 2015).
Symbiotics
Symbiotics incorporate probiotics and prebiotics to act in synergy in improving bird health (Pandey et al., 2015; Figure 3). The prebiotic serves as a nutrient source for the probiotic, increasing the probiotic's persistence in the bird gut and symbiotics are more effective than probiotics and prebiotics administered separately (Pandey et al., 2015). The combination between probiotics and 0.1% fructooligosaccharide decreased intestinal Salmonella enteritidis colonization in chicks than when used independently (Fukata et al., 1999). Also, supplementation of laying chickens with symbiotic products including E. Faecium and fructooligosaccharides resulted in higher egg production and eggshell quality than control ones at 57 wk of age (Radu-Rusu et al., 2010).
Figure 3.
Impact of probiotics, prebiotics, and symbiotics on Salmonella infection and bird status.
Organic Acids
Organic acids are short-chain fatty acids (C1-C7) produced in the intestine by microbial fermentation of carbohydrates and considered weak acids that dissociate in water in a pH-dependent manner (Lueck, 1981). The acid dissociation constant is defined by the pKa, enhanced protonated acid, which decreases the polarity of the acid molecule, results in increased acid diffusion against the bacterial barrier and into the cytoplasm when the pH of its surroundings is reduced (Davidson and Taylor, 2007).
Microbial activity is influenced by organic acids in 2 ways, by lowering the pH of the bacterial cytoplasm, causing energy generation and control to become uncoupled. Furthermore, the dissociated acid anions accumulate in hazardous quantities (Davidson and Taylor, 2007). Also, organic acids lower proventriculus pH, resulting in increased proteolytic enzyme activity, increased protein digestibility, and suppressing pathogenic bacterial growth. Acetic acid, lactic acid, benzoic acid, formic acid, and propionic acid are the most common organic acids incorporated in poultry ration.
Many researchers confirmed that organic acids supplementation enhances the bird's growth and performance. For example, broilers supplemented with a mixture of benzoic, acetic, and formic acids showed improved feed intake, weight gain, and feed conversion ratio (Fascina et al., 2012). Also, the addition of organic acid in salt form (0.5% of sodium propionate or calcium propionate) improved egg production, egg weight, and FCR in layer chickens (Dahiya et al., 2016). Furthermore, the researchers found that adding 0.5% acetic, lactic, or formic acid in drinking water limits S. Typhimurium propagation in the chicken crop (Byrd et al., 2001).
The effects of organic acids on the immune system remain unclear. However, there is evidence that organic acids can modulate intestinal microbiota, required for immune system stimulation. Also, organic acids limit pathogen propagation (Van der Wielen et al., 2002; Khan and Iqbal, 2016). Lee et al. (2017) found that broiler chickens vaccinated with H9N2 vaccine and supplemented with organic acid blend, including lactic, citric, and formic acids showed increased regulatory T cells and decreased H9N2-specific antibodies.
Acetic Acid
Acetic acid is a monocarboxylic acid and is considered as second simplest carboxylic acid (after formic acid). Vinegar is no less than 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. In this concentration, acetic acid is considered safe for usage. However, acetic acid has a pungent odor and taste, which often limits its use in foods restricting its use. Alvarez-Ordonez et al. (2010) compared growing Salmonella in a culture medium with a pH of 4.25 with acetic, citric, lactic, and hydrochloric acids and found that acetic acid had the highest antimicrobial activity (followed by lactic, citric, and hydrochloric acid). Zhou et al. (2007) reported a synergistic effect of acetic acid (0.10%) and essential oils thymol and carvacrol (100 uL/L each) for in vivo inhibition of S. typhimurium.
Lactic Acid
Lactic acid (2-hydroypropanoic acid) is a monocarboxylic acid with a pKa of about 3.8. It is produced during anaerobic respiration by fermentation of many bacteria, mainly lactic acid-producing bacteria as; Lactobacillus, Bifidobacterium, Streptococcus, and Pediococcus (Kim et al., 2005). Lactic acid occurs in 2 isomeric forms (D-, L-). The L isomer is more effective in pathogens suppression (Leitch and Steward, 2002).
Propionic Acid
Propionic acid and its salts, calcium, and sodium propionate are considered safe substances for usage. Propionic acid has a pKa of 4.88 (Serjeant and Dempsey, 1979). Chung and Goepfert (1970) found that propionic acid at pH 5.5 has a higher antibacterial effect against Salmonella than acetic, succinic, lactic, fumaric, and citric acids. Cherrington et al. (1990) observed that at a concentration of 5 mM, propionic acid decreased the rate of RNA, DNA, protein, lipid, and cell-wall synthesis of Salmonella.
Salmonella and Organic Acids Resistance
The bactericidal effects of weak organic acids rely on their concentration, surrounding pH, and the dissociation constant of the acids. Nonoptimal organic acid handling risks the development of adapted or resistant strains of pathogens (Foster and Hall, 1991). Salmonella can accommodate acidic conditions and survive in pH extremes (Foster and Hall, 1991). Salmonella's acid tolerance reaction can protect it against the effect of the organic acids, especially at low pH values (Foster and Spector, 1995). Salmonella has many inducible amino acid decarboxylases, including (lysine decarboxylase, lysine cadaverine antiporter, and regulatory proteins) in which the Salmonella organic acid-induced tolerance contributed to them (Foster and Spector, 1995).
Essential Oils
Essential oils are natural, aromatic, safe, volatile compounds, and oily fluids extracted from different plant parts (Bakkali et al., 2008; El-Tarabily et al., 2021; Abd El-Hack et al., 2022b). Essential oils are efficient growth promoters, hypolipidemic agents, digestive stimulants, immune-stimulants, antioxidants, antimicrobial, antifungal, and antiparasitic substances and they have a good impact on broiler performance and egg production (Jamroz et al., 2005; Alagawany et al., 2021c; El- Shall et al., 2022). Lemongrass (Cymbopogon citratus) extract shows potent antibacterial activity against different pathogenic bacteria as S. typhimurium, S. enterica (Singh and Ebibeni, 2016; Alagawany et al., 2021c). Also, the essential oils obtained from oregano and thyme effectively reduced Salmonella species colonization in the chicken gastrointestinal tract (Koščová et al., 2006). In addition to, trans-cinnamaldehyde, eugenol, thymol, and carvacrol have antibacterial effects against Salmonella and Campylobacter in both broiler and layer chickens (Kollanoor-Johny et al., 2010).
Cinnamaldehyde
Cinnamaldehyde is an aldehyde found in the bark of cinnamon trees that gives cinnamon its odor and it has anti-inflammatory, antibacterial and antifungal characters (de Cássia da Silveira e Sá et al., 2014). Cinnamaldehyde showed broad-spectrum antimicrobial activity via different pathways, including inhibition of glucose utilization and disruption of membranes permeability (Gill and Holley, 2004). Cinnamaldehyde has antibacterial effects against S. Enteritidis and found that 10 mM cinnamaldehyde lowered S. Enteritidis propagation in chicken cecal content (Kollanoor-Johny et al., 2010). Cinnamaldehyde has also been used in the diet to protect chickens from gastrointestinal infections and it was found that daily supplementation of 0.5% or 0.75% of cinnamaldehyde to broiler chicks showed a marked decrease in cecal S. Enteritidis count (Kollanoor-Johny et al., 2012).
Chitosan
Chitosan is a sugar found in the hard outer skeletons of shellfish such as crabs, lobsters, and shrimp. It is applied widely in the medical field (Abd El-Hack et al., 2020b; Attia et al., 2021). Chitosan mode of action was described in a prevalent theory shows and chitosan molecules (amino group at second carbon atom) and the negative charge of bacterial cell membranes alters bacterial cell permeability (Friedman and Juneja, 2010). Also, the chelation of metals and vital nutrients for bacteria by chitosan molecules enable it to suppress bacterial growth (Rabea et al., 2003). Chitosan is described as an immune-modulating agent that enhances host immune response against pathogens (Lee et al., 2008; Lee et al., 2009). In vitro studies showed that chitosan has an antibacterial effect against S. paratyphi Staphylococcus aureus (Isalm et al., 2011). Chitosan 0.2% showed both in vitro and in vivo antibacterial activity against Salmonella enterica serovar typhimurium infection in broiler chicks and could be applied to reduce crop, cecal, and consequently S. typhimurium carcass contamination as well as decreasing its shedding in the surrounding environment (Menconi et al., 2014).
Nanotechnology and Salmonella Control
Nanomaterials have attracted much attention for developing new biotechnology approaches because of their unique physical and chemical features (Sheiha et al., 2020; Reda et al., 2020; Yousry et al., 2020; El-Saadony et al., 2021e,f,g; Salem et al., 2021a). Fe3O4 magnetic nanoparticles (Fe3O4-NPs) could effectively reduce the viability of intracellular S. Enteritidis in chicken cells (Shen et al., 2020). Silver nanoparticles produced by Rosemary aqueous extracts as a cheap, eco-friendly technique showed antibacterial effects against S. typhimurium, S. enteritidis, and E. coli O78 (Mohamed et al., 2017).
Using zinc oxide nanoparticles with a concentration of 3% (mg g−1) has a bactericidal effect against S. typhimurium and Staphylococcus aureus. It could be incorporated with poultry ration to protect them from serious pathogens (Silva et al., 2021). Gold nanoparticles revealed antibacterial effects against S. typhimuruim (El Sabry et al., 2018; Reda et al., 2021b; Abd El‐Hack et al., 2021d). Also, Boatemaa et al. (2019) confirmed the in-vitro inhibitory effect of gold nanoparticles against S. typhii and S. paratyphi. Recently, thymol nanoemulsion provoked a good antibacterial activity against S. typhimurium infection in broilers and succeeded in decreasing S. typhimurium count. At the same time, Lactobacilli numbers increased in the chicken intestine and consequently increased weight gain and general health (Ibrahim et al., 2021). Furthermore, garlic and onion extract chitosan nanoparticles showed limited colonization of pathogenic enteric bacteria, E. coli, S. typhi, and C. jejuni, while the number of beneficial intestinal microbiota was increased in rainbow rooster chicken (Enoka et al., 2021).
Salmonella Vaccines
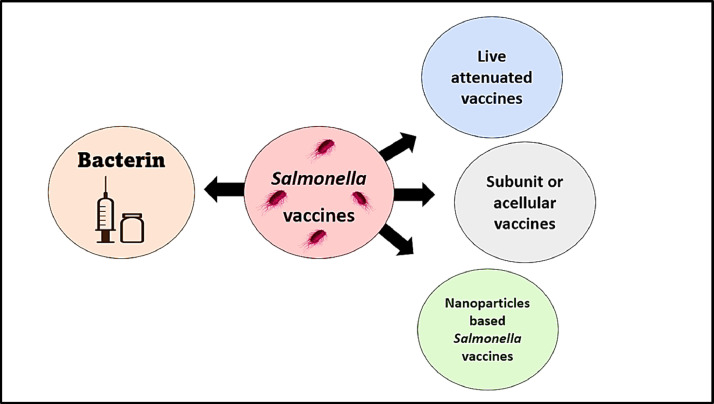
Salmonella vaccines are routinely used in hens to reduce Salmonella infection (Figure 4). The efficacy of vaccines is evaluated by the level of pathogen propagation, morbidity, and mortality rates of the vaccinated bird's subsequent experimental infection (Barrow, 2007). Many factors influence vaccine efficacy, such as challenge bacteria, route of administration, dose, age of birds, species, breed, environmental condition, hygienic measures, and vaccine handling and storage (Barrow, 2007). Thus, it is hard to compare the efficacy of currently available vaccines (Barrow, 2007). Many types of vaccines protect birds against Salmonella infection in the form of bacterins, attenuated, subunit vaccines, and nanoparticles-based vaccines.
Figure 4.
Different types of Salmonella vaccines.
Bacterins
Poultry vaccination is an effective tool for controlling and preventing diseases (El-Naggar et al., 2022). Bacterins are entire bacteria that have been killed and have had varying degrees of protection against Salmonella (Davison et al., 1999). Vaccinating hens with an oil-emulsion bacterin of S. enteritidis at 38 wk (booster one mo later) showed lower S. enteritidis shedding and colonization in the ovary and spleen (Miyamoto et al.,1999). Also, vaccinated chickens at 2 wk of age with formalin-inactivated S. Enteritidis encapsulated in biodegradable microspheres lowered S. enteritidis fecal shedding and organ colonization (Liu et al., 2001).
However, other studies using bacterins only without adjuvants have shown no effects in lowering Salmonella count. Davison et al. (1999) found that layer flocks vaccinated with S. enteritidis bacterins between 14 and 20 wk of age revealed no Salmonella colonization compared to the control flock. In another study, commercial breeding chicken farms vaccinated with the killed Salmonella vaccine showed higher antibody titers, but vaccination did not reduce Salmonella shedding (Berghaus et al., 2011).
Live Attenuated Vaccines
Live attenuated Salmonella vaccines undergo attenuation through negative mutations of essential enzymes, resulting in a prolonged generation period and lowered pathogenicity (Linde et al., 1998). They are frequently preferred over bacterins because they are easy to administer and generate mucosal, cellular, and humoral responses (Lalsiamthara et al., 2016). Attenuated S. enteritidis vaccine orally inoculated in chickens showed decreased Salmonella colonization in the ceca, liver, and spleen (Cerquetti and Gherardi, 2000).
In contrast, Groves et al. (2016) administered commercial oral live Salmonella vaccine to layer chickens and reported that the vaccine failed to decrease Salmonella colonization in the ceca. Also, concerns about the safety of live vaccinations exist for the consumers' health (Zhang-Barber et al., 1999, Lauring et al., 2010).
The Subunit or Cellular Vaccines
They include immunogenic parts or antigens of the bacteria, which may offer a more effective alternative to killed and live attenuated vaccines (Sharma and Hinds, 2012). Salmonella immunogenic parts were cleared using electrophoresis after evaluating antigens that stimulated strong B and T lymphocyte responses in immune-blot and western blot techniques (Vordermeier and Kotlarski, 1990). These immunogenic antigens include outer-membrane proteins, lipopolysaccharides, flagellate epitopes, and fimbriae (Ochoa et al., 2007). Subunit vaccines have a variable level of protection, although low resistance levels against Salmonellosis have been found (Tennant and Levine, 2015). However, subunit vaccines, including the outer membrane proteins, have successfully been used to limit S. enteritidis infection in poultry.
Vaccinated 9-wk-old chickens with subunit vaccine including 2 outer membrane proteins, followed by 2 boost doses with time intervals of 15 d, showed decreased Salmonella colonization in the ceca (Khan et al., 2003). Vaccine adjuvants and delivery systems stimulate humoral, cellular, and mucosal immunological responses, strengthening the immune system. Successful delivery systems are biodegradable, cost-effective, and easy to process, with few adverse effects on the bird (Tiwari, 2012).
Nanoparticles-Based Salmonella Vaccines
They are a promising technology that helps eliminate Salmonella infection in the poultry sector (Abd El-Ghany et al., 2021). Nanoparticles are very small-sized copolymer delivery systems that preserve antigens against chemical, enzymatic or immunological destruction, thus facilitating targeting and presentation of antigens to particular locations of the mucosal immune system (Tiwari, 2012; Yehia et al., 2021; Alagawany et al., 2021d).
The vaccine antigen encapsulated with the nanoparticles and encapsulated with the nanoparticles prevents the antigen from rapidly destruction after administration (Delgado et al., 1999). Also, conjugation of the antigens onto the nanoparticle surface can derive the vaccine to specific immune sites within the gut (Salman et al., 2005). For successful nanoparticle vaccine preparation, it is essential to characterize the size and composition of the nanoparticle to avoid any variation within the batches, which could arise from contamination, different nanoparticles size distribution, the accumulation of toxic elements, or incomplete particle formation; thus, nanoparticle's uniformity is a serious point to ensure homogenous antigen loading efficiency between each nanoparticle (Gregory et al., 2013).
Salman et al. (2005) designed “Salmonella-like” nanoparticles vaccine by conjugating Salmonella enteritidis flagellin to the nanoparticle's surface to mimic the natural colonization of S. enteritidis in the gut. The flagellin ligands conjugated with nanoparticles successfully stimulated specific intestinal mucosa uptake, including Peyer's patches (Salman et al., 2005). Orally applied Salmonella subunit vaccine including immunogenic Salmonella outer membrane proteins (OMPs) and flagellar (F) protein-entrapped and surface F-protein-coated with polyanhydride nanoparticle in layer chickens revealed specific immune response and limited Salmonella colonization in the intestines (Renu et al., 2018). Oral administration of Salmonella subunit vaccine involving immunogenic outer membrane proteins and flagellin protein loaded and F-protein surface coated chitosan nanoparticles in chickens stimulate specific systemic IgY and mucosal IgA antibodies responses (Renua et al., 2020).
Salmonella chitosan-nanoparticle vaccine, based on S. enteritidis outer-membrane-proteins and flagellin proteins, induced an antigen-specific immune response against S. enteritidis in ovo and lower S. enteritidis cecal count in broilers (Acevedo-Villanueva et al., 2021). Also, Renu et al. (2020) designed Salmonella subunit chitosan nanoparticles-based vaccine containing immunogenic outer membrane proteins and -flagellin protein for oral administration in laying hens, the prepared vaccine increased the expression of toll-like receptor (TLR)-2, TLR-4, IFN-γ, TGF-ß, and IL-4 mRNA expression in chicken cecal tonsils and lower Salmonella load. Figure 5 shows various antibiotics alternative that used for Salmonella control in poultry.
Figure 5.
Different antibiotics alternative that used for Salmonella control in poultry.
CONCLUSIONS
Salmonella infection is considered one of the most dangerous and common diseases spread worldwide and threaten the poultry industry and public health. Antibiotics use is declining in the poultry sector due to the rise of antibiotic-resistant. To avoid its residual effect on poultry meat consequentially, many alternatives have been used to overcome these problems to obtain safe, cheap, and organic poultry meat. Probiotics, prebiotics, symbiotics, organic acids, essential oils, cinnamaldehyde, chitosan, nanoparticles and vaccines are available alternatives for antibiotics and revealed promising results in control of avian Salmonellosis that guarantee the strategies for control and prevention of avian Salmonellosis in either developing or developed countries and provide safe and liable poultry meat for human consumption and fulfill the meat gap and deficit as a source of protein.
Acknowledgments
ACKNOWLEDGMENTS
The authors extend their appreciation to the deanship of Scientific Research at King Khalid University, Abha KSA for supporting this work under grant number (R.G.P.2/61/42).
Author Contribution: All authors were equally contributed in writing this review article. All authors reviewed and approved the final version of the manuscript.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Abd El Hamid M.I., Abd El-Moaty D.A.M., El-Sergany E.F., Salem H.M., El-Sawy H., Abbas A.M. Utility of molecular biology tools for identification and characterization of Egyptian Riemerella anatipestifer duck isolates. Int. J. Vet. Sci. 2019;8:335–341. [Google Scholar]
- Abd El-Ghany W.A., Shaalan M., Salem H.M. Nanoparticles applications in poultry production: an updated review. Poult. Sci. J. 2021:1001–1025. doi: 10.1080/00439339.2021.1960235. [DOI] [Google Scholar]
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11:1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Swelum A.A., Arif M., Abo Ghanima M.M., Shukry M., Noreldin A., Taha A.E., El-Tarabily K.A. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021;101:5747–5762. doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Ghanima M.M.A.…El-Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult. Sci. 2022 doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. Int. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., Nahed A., Saad A.M., Salem H.M.…El-Tarabily K.A. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives–a comprehensive review. Poult. Sci. 2022 doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E.…Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol.. 2020;164:2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. 1. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S.…Abdel-Moneim A.M.E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021:1–10. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y., Batiha G.E., Khafaga A.F., Abdel-Moneim A.M.E., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abd Elkader A.M., Labib S., Taha T.F., Althobaiti F., Aldhahrani A., Salem H.M., Saad A.M., Ibrahim F.M. Phytogenic compounds from avocado (Persea americana L.) extracts; antioxidant activity, amylase inhibitory activity, therapeutic potential of type 2 diabetes. [e-pub ahead of print] Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.11.031. Accessed Nov. 24, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Moneim A.M.E., El-Saadony M.T., Shehata A.M., Saad A.M., Aldhumri S.A., Ouda S.M., Mesalam N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi [e-pub ahead of print] Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.09.046. Accessed Sept. 17, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-shargi O.Y.A., Al- Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. [e-pub ahead of print] Ital. J. Anim. Sci. 2021;20:896–910. doi: 10.1016/j.sjbs.2021.10.063. Accessed Oct. 30, 2021. [DOI] [Google Scholar]
- Abou-Kassem D.E., El-Abasy M.M., Al-Harbi M.S., Abol-Ela S., Salem H.M., El-Tahan A.M., El-Saadony M.T., Abd El-Hack M.E., Ashour E.A. Influences of total sulfur amino acids and photoperiod on growth, carcass traits, blood parameters, meat quality and cecal microbial load of broilers. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Villanueva K., Renu S., Gourapura R., Selvaraj R. Efficacy of a nanoparticle vaccine administered in ovo against Salmonella in broilers. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0247938. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adil S., Banday T., Bhat G.A., Mir M.S., Rehman M. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet. Med. Int. 2010;2010 doi: 10.4061/2010/479485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Mahmoud M.A.…Reda F.M. Use of chemical nano selenium as antibacterial and antifungal agent in quail diets and its effect on growth, carcasses, antioxidant, immunity and caecal microbe. Animals. 2021;11:3027. doi: 10.3390/ani11113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R., El-Naggar K., Taha A.E., Khafaga A.F.…Abd El-Hack M.E. Betaine and related compounds: Chemistry, metabolism, and role in mitigating heat stress in poultry. J. Therm. Biol. 2021;104 doi: 10.1016/j.jtherbio.2021.103168. [DOI] [PubMed] [Google Scholar]
- Alvarez-Ordonez A., Fernandez A., Bernardo A., Lopez M. Acid tolerance in Salmonella typhimurium induced by culturing in the presence of organic acids at different growth temperatures. Food Microbiol. 2010;27:44–49. doi: 10.1016/j.fm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Ao Z., Choct M. Oligosaccharides affect performance and gut development of broiler chickens. Asian-Australas J. Anim. Sci. 2013;26:116–121. doi: 10.5713/ajas.2012.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif M., Baty R.S., Althubaiti E.H., Ijaz M.T., Fayyaz M., Shafi M.E., Albaqami N.M., Alagawany M., Abd El-Hack M.E., Taha A.E., Salem H.M., El-Tahan A.M., Elnesr S.S. The impact of betaine supplementation in quail diet on growth performance, blood chemistry, and carcass traits. [e-pub ahead of print] Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.11.002. Accessed Nov. 11, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour E.A., Ab El-Hack M.E., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A.…El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Ashour E.A., Farsi R.M., Alaidaroos B.A., Abdel-Moneim A.M.E., El-Saadony M.T., Osman A.O., Abou Sayed-Ahmed E.T., Albaqami N.M., Shafi M.E., Taha A.E., Abd El-Hack M.E. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcase traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital. J. Anim. Sci. 2021;20:1357–1372. [Google Scholar]
- Attia M.M., Salem H.M. Morphological and molecular characterization of Pseudolynchia canariensis (Diptera: Hippoboscidae) infesting domestic pigeons. Int. J. Trop. Insect Sci. 2022;42:733–740. [Google Scholar]
- Attia M.M., Yehia N., Soliman M.M., Shukry M., El-Saadony M.T., Salem H.M. Evaluation of the antiparasitic activity of the chitosan-silver nanocomposites in the treatment of experimentally infested pigeons with Pseudolynchia canariensis. [e-pub ahead of print] Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.10.067. Accessed Oct. 29, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J.S. Control of Salmonella and Campylobacter in poultry production. A summary of work at Russell Research Center. Poult. Sci. 1993;72:1169–1173. doi: 10.3382/ps.0721169. [DOI] [PubMed] [Google Scholar]
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils. A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Barnhart E.T., Sarlin L.L., Caldwell D.J., Byrd J.A., Corrier D.E., Hargis B.M. Evaluation of potential disinfectants for preslaughter broiler crop decontamination. Poult. Sci. 1999;78:32–37. doi: 10.1093/ps/78.1.32. [DOI] [PubMed] [Google Scholar]
- Barrow P.A. Salmonella infections: immune and non-immune protection with vaccines. Avian Pathol. 2007;36:1–13. doi: 10.1080/03079450601113167. [DOI] [PubMed] [Google Scholar]
- Beachey E.H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J. Infect. Dis. 1981;143:325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Beirão B.C.B., Ingberman M., Fávaro C.J., Mesa D., Bittencourt L.C., Fascina V.B., Caron L.F. Effect of an Enterococcus faecium probiotic on specific IgA following live Salmonella Enteritidis vaccination of layer chickens. Avian Pathol. 2018;47:325–333. doi: 10.1080/03079457.2018.1450487. [DOI] [PubMed] [Google Scholar]
- Bell C., Kyriakides A. P. 84? 94. Blackwell Science; United Kingdom: 2002. Salmonella: A Practical Approach to the Organism and its Control in Foods. [Google Scholar]
- Berghaus R.D., Thayer S.G., Maurer J.J., Hofacre C.L. Effect of vaccinating breeder chickens with a killed Salmonella vaccine on Salmonella prevalence's and loads in breeder and broiler chicken flocks. J. Food Prot. 2011;74:727–734. doi: 10.4315/0362-028X.JFP-10-542. [DOI] [PubMed] [Google Scholar]
- Blottiere H.M., Buecher B., Galmiche J.P., Cherbut C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc. Nutr. Soc. 2003;62:101–106. doi: 10.1079/PNS2002215. [DOI] [PubMed] [Google Scholar]
- Boatemaa M.A., Ragunathan R.R., Naskar J. Nanogold for in vitro inhibition of Salmonella strains. J. Nanomater. 2019;2019 [Google Scholar]
- Borsoi A., Ruschel do Santos L., Beatriz Rodrigues L., Luiz de Souza Moraes H., Tadeu Pippi Salle C., Pinheiro do Nascimento V. Behavior of Salmonella Heidelberg and Salmonella enteritidis strains following broiler chick inoculation: evaluation of cecal morphometry, liver and cecum bacterial counts and fecal excretion patterns. Braz. J. Microbiol. 2011;42:266–273. doi: 10.1590/S1517-83822011000100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden C.R. Salmonella enterica serotype Enteritidis and eggs: a national epidemic in the United States. Clin. Infect. Dis. 2006;43:512–517. doi: 10.1086/505973. [DOI] [PubMed] [Google Scholar]
- Brenner F.W., Villar R.G., Angulo F.J., Tauxe R., Swaminathan B. Salmonella nomenclature. J. Clin. Microbiol. 2000;38:2465–2467. doi: 10.1128/jcm.38.7.2465-2467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbin J.T., Gong J., Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health. Res. Rev. 2008;9:101–110. doi: 10.1017/S146625230800145X. [DOI] [PubMed] [Google Scholar]
- Brisbin J.T., Parvizi P., Sharif S. Differential cytokine expression in T-cell subsets of chicken caecal tonsils co-cultured with three species of Lactobacillus. Benef. Microbes. 2012;3:205–210. doi: 10.3920/BM2012.0014. [DOI] [PubMed] [Google Scholar]
- Byrd J.A., Hargis B.M., Caldwell D.J., Bailey R.H., Herron K.L., McReynolds J.L., Brewer R.L., Anderson R.C., Bischoff K.M., Callaway T.R., Kubena L.F. Effect of lactic acid administration in the drinking water during preslaughter feed withdrawal on Salmonella and Campylobacter contamination of broilers. Poult. Sci. 2001;80:278–283. doi: 10.1093/ps/80.3.278. [DOI] [PubMed] [Google Scholar]
- Cao L., Yang X.J., Li Z.J., Sun F.F., Wu X.H., Yao J.H. Reduced lesions in chickens with Clostridium perfringens-induced necrotic enteritis by Lactobacillus fermentum 1. 20291. Poult. Sci. 2012;91:3065–3071. doi: 10.3382/ps.2012-02548. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (CDC). 2013c. Multistate Outbreak of Human Salmonella Infections Linked to Live Poultry. Available at: http://www.cdc.gov/Salmonella/live?poultry?04?13/index.html. Accessed Apr. 2, 2016.
- Center for Disease Control and Prevention (CDC). 2013d. Multistate Outbreak of Human Salmonella Typhimurium Infections Linked to Live Poultry in Backyard Flocks.
- Center for Disease Control and Prevention (CDC). 2013e. National Salmonella.
- Center for Disease Control and Prevention (CDC). 2015a. Antibiotic/Antimicrobial Resistance. Available at: http://www.cdc.gov/drugresistance/about.html. Accessed Apr. 3, 2016.
- Center for Disease Control and Prevention (CDC) US Department of Health and Human Services; 2011. National Salmonella Surveillance Overview. [Google Scholar]
- Cerquetti M.C., Gherardi M.M. Orally administered attenuated Salmonella enteritidis reduces chicken cecal carriage of virulent Salmonella challenge organisms. Vet. Microbiol. 2000;76:185–192. doi: 10.1016/s0378-1135(00)00235-2. [DOI] [PubMed] [Google Scholar]
- Cherrington C.A., Hinton M., Chopra I. Effect of short-chain organic acids on macromolecular synthesis in Escherichia coli. J. Appl. Bacteriol. 1990;68:69–74. doi: 10.1111/j.1365-2672.1990.tb02550.x. [DOI] [PubMed] [Google Scholar]
- Chung K.CH., Goepfert J.M. Growth of Salmonella at low Ph. J. Food Sci. 1970;35:326–327. doi: 10.1111/j.1365-2621.1970.tb12176.x. [DOI] [Google Scholar]
- Coburn B., Grassl G.A., Finlay B.B. Salmonella, the host and disease: a brief review. Immunol. Cell Biol. 2007;85:112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- Correa-Oliveira R., Fachi J.L., Vieira A., Sato F.T., Vinolo M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016;5:e73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J.H., Macfarlane G.T. Gastrointestinal effects of prebiotics. Br. J. Nutr. 2002;87(Suppl 2):145–151. doi: 10.1079/BJNBJN/2002530. [DOI] [PubMed] [Google Scholar]
- D'Aoust Pathogenicity of foodborne Salmonella. Int. J. Food Microbiol. 1991;12:17–40. doi: 10.1016/0168-1605(91)90045-q. [DOI] [PubMed] [Google Scholar]
- Daeschel M.A., Klaenhammer T.R. Association of a 13.6-megadalton plasmid in Pediococcus pentosaceus with bacteriocin activity. Appl. Environ. Microbiol. 1985;50:1538–1541. doi: 10.1128/aem.50.6.1538-1541.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya R., Berwal R.S., Sihag S., Patil C.S., Lalit The effect of dietary supplementation of salts of organic acid on production performance of laying hens. Vet. World. 2016;9:1478–1484. doi: 10.14202/vetworld.2016.1478-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzeisen J.L., Kim H.B., Isaacson R.E., Tu Z.J., Johnson T.J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 2011;6:e27949. doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson P.M., Taylor T.M. Food Microbiology: Fundamentals and Frontiers. 3rd ed. American Society of Microbiology; 2007. Chemical preservatives and natural antimicrobial compounds. Anonymous. [Google Scholar]
- Davies R.H., Wray C. Persistence of Salmonella enteritidis in poultry units and poultry food. Br. Poult. Sci. 1996;37:589–596. doi: 10.1080/00071669608417889. [DOI] [PubMed] [Google Scholar]
- Davison S., Benson C.E., Henzler D.J., Eckroade R.J. Field observations with Salmonella enteritidis bacterins. Avian Dis. 1999;43:664–669. [PubMed] [Google Scholar]
- de Cássia da Silveira e Sá R., Andrade L.N., Dos Reis Barreto de Oliveira R., de Sousa D.P. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules. 2014;19:1459–1480. doi: 10.3390/molecules19021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Reu K., Grijspeerdt K., Messens W., Heyndrickx M., Uyttendaele M., Debevere J., Herman L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 2006;112:253–260. doi: 10.1016/j.ijfoodmicro.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Delgado A., Lavelle E.C., Hartshorne M., Davis S.S. PLG microparticles stabilised using enteric coating polymers as oral vaccine delivery systems. Vaccine. 1999;17:2927–2938. doi: 10.1016/s0264-410x(99)00140-1. [DOI] [PubMed] [Google Scholar]
- den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A., Cotter P.D., Ross R.P., Hill C. Bacteriocin production: a probiotic trait? Appl. Environ. Microbiol. 2012;78:1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson J.A., Weiping Chu W., Porwollik S., Tran K., Iribe N., McClelland M., Kwon Y.J. Eradication of intracellular Salmonella Typhimurium by polyplexes of acid-transforming chitosan and fragment DNA. Macromol. Biosci. 2021;21 doi: 10.1002/mabi.202000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sabry M.I., Mcmillin K., Sabliov C.M. Nanotechnology considerations for poultry and livestock production systems–a review. Ann. Anim. Sci. 2018;18:319–334. [Google Scholar]
- El- Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A.…Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khawasa K.M., Mashatb B.H., Attalab O.A., Kassemc G.M.A. Control of Salmonella and Escherichia coli in chilled chicken fillets using chitosan and lactic acid. Cyta-J. Food. 2020;18:445–450. [Google Scholar]
- El-Naggar M.S., Ibrahim H.M., Salem H.M., Marouf S. A novel locally prepared inactivated bivalent mycoplasma vaccine for chicken flocks in Egypt. Adv. Anim. Vet. Sci. 2022;10:55–61. [Google Scholar]
- El-Saadony M.T., Saad A.M., Elakkad H.A., El-Tahan A.M., Alshahrani O.A., Alshilawi M.S., El-Sayed H., Amin S.A., Ahmed A.I. Flavoring and extending the shelf life of cucumber juice with aroma compounds-rich herbal extracts at 4°C through controlling chemical and microbial fluctuations. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.08.092. Accessed Sept. 4, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar S.F., El-Tarabily K.A., Salama A. Selenium nanoparticles, from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi, as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28:6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., Abdel-Hamid S.E., Taha T.F., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28:4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Alagawany M., Patra A.K., Kar I., Tiwari R., Dawood M.A., Dhama K., Abdel-Latif H.M.R. The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol. 2021;117:36–52. doi: 10.1016/j.fsi.2021.07.007. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O., Hussein E.O.S., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20:762–776. [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69 [Google Scholar]
- El-Saadony M.T., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany M., Yehia N., Askar A.M., Alsafy S.A., Noreldin A.E., Khafaga A.F., Dhama K., Elnesr S.S., Elwan H.A.M., Di Cerbo A., El-Tarabily K.A., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev. Int. 2021;37:1–2. [Google Scholar]
- El-Saadony M.T., Khalil O.S., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28:4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy H., Tahoun A., Rizk A.M., Suzuki T., Elmonir W., Nassef E., Shukry M., Germoush M.O., Farrag F., Bin-Jumah M., Mahmoud A.M. Evaluation of bifidobacteria and Lactobacillus probiotics as alternative therapy for Salmonella typhimurium infection in broiler chickens. Animals. 2020;10:1023. doi: 10.3390/ani10061023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28:5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng S., Pusparajah P., Ab Mutalib N., Ser H., Chan K., Lee L. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015;8:284–293. [Google Scholar]
- Enoka V.I.L., Kikuvi G.M., Ndung'u P.W. Effect of garlic and onion extract chitosan nanoparticles on selected intestinal bacterial flora in indigenous rainbow rooster chicken in Kenya. AIMS Mol. Sci. 2021;8:98–116. [Google Scholar]
- Erdman M.M., Morningstar Shaw B.R., Barker D.A., Mackie T.A., Munoz M.I., Palmer E.A., Kane M.A., Cox L.K. Proceedings of the 2010 Meeting of the United States Animal Health Association. 2009. Salmonella serotypes isolated from animals in the United States: January 1 December 31, 2009. Diagnostic Bacteriology Laboratory, National Veterinary Services Laboratories, USDA. Report of the Committee on Salmonella; pp. 11–14.http://www.usaha.org/Portals/6/Reports/2006/report?sal?2006.pdf Available at: Accessed Apr. 4, 2016. [Google Scholar]
- Fabrega A., Vila J. Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin. Microbiol. Rev. 2013;26:308–341. doi: 10.1128/CMR.00066-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fascina V.B., Sartori J.R., Gonzales E., Carvalho F., Souza I., Polycarpo G., Stradiotti A.C., Pelícia V.C. Phytogenic additives and organic acids in broiler chicken diets. Rev. Bras. de Zootec. 2012;41:2189–2197. [Google Scholar]
- Fasina Y.O., Hoerr F.J., McKee S.R., Conner D.E. Influence of Salmonella enterica serovar Typhimurium infection on intestinal goblet cells and villous morphology in broiler chicks. Avian Dis. 2010;54:841–847. doi: 10.1637/9055-090809-Reg.1. [DOI] [PubMed] [Google Scholar]
- Foster J.W., Hall H.K. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J. Bacteriol. 1991;173:5129–5135. doi: 10.1128/jb.173.16.5129-5135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J.W., Spector M.P. How Salmonella survive against the odds. Annu. Rev. Microbiol. 1995;49:145–174. doi: 10.1146/annurev.mi.49.100195.001045. [DOI] [PubMed] [Google Scholar]
- Friedman M., Juneja V.K. Review of antimicrobial and antioxidative activities of chitosans in food. J Food Prot. 2010;73:1737–1761. doi: 10.4315/0362-028x-73.9.1737. [DOI] [PubMed] [Google Scholar]
- Fukata T., Sasai K., Miyamoto T., Baba E. Inhibitory effects of competitive exclusion and fructooligosaccharide, singly and in combination, on Salmonella colonization of chicks. J. Food Prot. 1999;62:229–233. doi: 10.4315/0362-028x-62.3.229. [DOI] [PubMed] [Google Scholar]
- Ghareeb K., Awad W.A., Mohnl M., Porta R., Biarnes M., Bohm J., Schatzmayr G. Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in broiler chickens. Poult. Sci. 2012;91:1825–1832. doi: 10.3382/ps.2012-02168. [DOI] [PubMed] [Google Scholar]
- Ghebremicael S.B., Hasenstein J.R., Lamont S.J. Association of interleukin-10 cluster genes and Salmonella response in the chicken. Poult. Sci. 2008;87:22–26. doi: 10.3382/ps.2007-00259. [DOI] [PubMed] [Google Scholar]
- Gill A.O., Holley R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004;70:5750–5755. doi: 10.1128/AEM.70.10.5750-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A.E., Titball R., Williamson D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013;3:13. doi: 10.3389/fcimb.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves P.J., Sharpe S.M., Muir W.I., Pavic A., Cox J.M. Live and inactivated vaccine regimens against caecal Salmonella Typhimurium colonization in laying hens. Aust. Vet. J. 2016;94:387–393. doi: 10.1111/avj.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi H.R., Gong J., Gyles C.L., Hayes M.A., Zhou H., Sanei B., Chambers J.R., Sharif S. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 2006;13:975–980. doi: 10.1128/CVI.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez-Peinado D., Ubeda-Manzanaro M., Martínez A., Rodrigo D. Antimicrobial effect of insect chitosan on Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes survival. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0244153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim D., Hassan A.A., Badawi M., Ismail T.A., Bendary M.M., Abdelaziz A.M., Mosbah R.A., Mohamed D.I., Arisha A.H., Abd El-Hamid M.I. Thymol nanoemulsion promoted broiler chicken's growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 2021;11:7742. doi: 10.1038/s41598-021-86990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Md.M., Masum S.Md., Rayhan K. In vitro antibacterial activity shrimp chitosan against Salmonella Paratyphi Staphylococcus aureus. J. Bangladesh Chem. Soc. 2011;24:185–190. [Google Scholar]
- Jamroz D., Wiliczkiewicz A., Wertelecki T., Orda J., Skorupińska J. Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br. Poult. Sci. 2005;46:485–493. doi: 10.1080/00071660500191056. [DOI] [PubMed] [Google Scholar]
- Jazi V., Foroozandeh A.D., Toghyani M., Dastar B., Koochaksaraie R.R., Toghyani M. Effects of Pediococcus acidilactici, mannan-oligosaccharide, butyric acid and their combination on growth performance and intestinal health in young broiler chickens challenged with Salmonella typhimurium. Poult. Sci. 2018;97:2034–2043. doi: 10.3382/ps/pey035. [DOI] [PubMed] [Google Scholar]
- Joe M.M., Bradeeba K., Parthasarathi R., Sivakumaar P.K., Chauhan P.S., Tipayno S., Benson A., Sa T. Development of surfactin-based nanoemulsion formulation from selected cooking oils: evaluation for antimicrobial activity against selected food associated microorganisms. J. Taiwan Inst. Chem. Eng. 2012;43:172–180. [Google Scholar]
- Kamada N., Nunez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. 2014;146:1477–1488. doi: 10.1053/j.gastro.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.H., Lee M.S. Characterization of a bacteriocin produced by Enterococcus faecium GM-1 isolated from an infant. J. Appl. Microbiol. 2005;98:1169–1176. doi: 10.1111/j.1365-2672.2005.02556.x. [DOI] [PubMed] [Google Scholar]
- Karimi Torshizi M.A., Moghaddam A.R., Rahimi S., Mojgani N. Assessing the effect of administering probiotics in water or as a feed supplement on broiler performance and immune response. Br. Poult. Sci. 2010;51:178–184. doi: 10.1080/00071661003753756. [DOI] [PubMed] [Google Scholar]
- Khan M.I., Fadl A.A., Venkitanarayanan K.S. Reducing colonization of Salmonella Enteritidis in chicken by targeting outer membrane proteins. J. Appl. Microbiol. 2003;95:142–145. doi: 10.1046/j.1365-2672.2003.01953.x. [DOI] [PubMed] [Google Scholar]
- Khan S.H., Iqbal J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2016;44:359–369. [Google Scholar]
- Kim G., Seo Y.M., Kim C.H., Paik I.K. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult. Sci. 2011;90:75–82. doi: 10.3382/ps.2010-00732. [DOI] [PubMed] [Google Scholar]
- Kim Y.Y., Kil D.Y., Oh H.K., Han I.K. Acidifier as an alternative material to antibiotics in animal feed. Asian-Australas J. Anim. Sci. 2005;18:1048–1060. [Google Scholar]
- Kollanoor-Johny A., Upadhyay A., Baskaran S.A., Upadhyaya I., Mooyottu S., Mishra N., Darre M.J., Khan M.I., Donoghue A.M., Donoghue D.J., Venkitanarayanan K. Effect of therapeutic supplementation of the plant compounds trans-cinnamaldehyde and eugenol on Salmonella enterica serovar enteritidis colonization in market-age broiler chickens. J. Appl. Poult. Res. 2012;21:816–822. [Google Scholar]
- Kollanoor-Johny A., Mattson T., Baskaran S.A., Amalaradjou M.A., Babapoor S., March B., Valipe S., Darre M., Hoagland T., Schreiber D., Khan M.I., Donoghue A., Donoghue D., Venkitanarayanan K. Reduction of Salmonella enterica serovar enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Appl. Environ. Microbiol. 2012;78:2981–2987. doi: 10.1128/AEM.07643-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollanoor-Johny A., Darre M.J., Donoghue A.M., Donoghue D.J., Venkitanarayanan K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J. Appl. Poult. Res. 2010;19:237–244. [Google Scholar]
- Koščová J., Nemcová R., Gancarčíková S., Jonecová Z., Sciranková L., Bomba A., Buleca V. Effect of two plant extracts and Lactobacillus fermentum on colonization of gastrointestinal tract by Salmonella enterica var. Düsseldorf in chicks. Biologia. 2006;61:775–778. [Google Scholar]
- Lalsiamthara J., Kamble N.M., Lee J.H. A live attenuated Salmonella Enteritidis. Vet. Res. 2016;47:60. doi: 10.1186/s13567-016-0348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J.G., Cruickshank S.M., Singh J.C., Farrar M., Lodge J.P., Felsburg P.J., Carding S.R. Different cytokine response of primary colonic epithelial cells to commensal bacteria. World J. Gastroenterol. 2005;11:3375–3384. doi: 10.3748/wjg.v11.i22.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R., Reeves P.R., Octavia S. Population structure, origins and evolution of major Salmonella enterica clones. Infect. Genet. Evol. 2009;9:996–1005. doi: 10.1016/j.meegid.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Lauring A.S., Jones J.O., Andino R. Rationalizing the development of live attenuated virus vaccines. Nat. Biotechnol. 2010;28:573–579. doi: 10.1038/nbt.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.C., Kim M.S., Choi S.H., Kim K.Y., Kim T.S. In vitro and in vivo antimicrobial activity of water-soluble chitosan oligosaccharides against Vibrio vulnificus. Int. J. Mol. Med. 2009;24:327–333. doi: 10.3892/ijmm_00000236. [DOI] [PubMed] [Google Scholar]
- Lee C.G., Silva C.A., Lee J.Y., Hartl D., Elias J.A. Chitin regulation of immune responses: an old molecule with new roles. Curr. Opin. Immunol. 2008;20:684–689. doi: 10.1016/j.coi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.K., Bae S., Gu M.J., You S.J., Kim G., Park S.M., Jeung W.H., Ko K.H., Cho K.J., Kang J.S., Yun C.H. H9N2-specific IgG and CD4+CD25+ T cells in broilers fed a diet supplemented with organic acids. Poult. Sci. 2017;96:1063–1070. doi: 10.3382/ps/pew382. [DOI] [PubMed] [Google Scholar]
- Leitch E.C.M., Stewart C.S. Escherichia coli O157 and non-O157 isolates are more susceptible to l-Lactate than to d-Lactate. Appl. Environ. Microbiol. 2002;68:4676–4678. doi: 10.1128/AEM.68.9.4676-4678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang H., D'Aoust J.Y., Maurer J. In: Food Microbiology Fundamentals and Frontiers. 4th ed. Doyle M.P., Buchanan R.L., editors. ASM Press; Washington, DC: 2013. Salmonella Species; pp. 225–261. [Google Scholar]
- Lin W.H., Hwang C.F., Chen L.W., Tsen H.Y. Viable counts, characteristic evaluation for commercial lactic acid bacteria products. Food Microbiol. 2006;23:74–81. doi: 10.1016/j.fm.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Lin Y.P., Thibodeaux C.H., Pena J.A., Ferry G.D., Versalovic J. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm. Bowel Dis. 2008;14:1068–1083. doi: 10.1002/ibd.20448. [DOI] [PubMed] [Google Scholar]
- Linde K., Fthenakis G.C., Fichtner A. Bacterial live vaccines with graded level of attenuation achieved by antibiotic resistance mutations: transduction experiments on the functional unit of resistance, attenuation and further accompanying markers. Vet. Microbiol. 1998;62:121–134. doi: 10.1016/s0378-1135(98)00201-6. [DOI] [PubMed] [Google Scholar]
- Liu W., Yang Y., Chung N., Kwang J. Induction of humoral immune response and protective immunity in chickens against Salmonella enteritidis after a single dose of killed bacterium-loaded microspheres. Avian Dis. 2001;45:797–806. [PubMed] [Google Scholar]
- Llamas-Moya S., Girdler C.P., Shalash S.M.M., Atta A.M., Gharib H.B., Morsy E.A., Salim H., Awaad M.H.H., Elmenawey M. Effect of a multicarbohydrase containing a-galactosidase enzyme on the performance, carcass yield, and humoral immunity of broilers fed corn–soybean meal–based diets of varying energy density. J. Appl. Poult. Res. 2019;29:142–151. [Google Scholar]
- Lueck E. Springer-Verlag; Berlin, Germany: 1981. Antimicrobial Food Additives: Characteristics, Uses, Effects. [Google Scholar]
- Mack D.R. Probiotics: mixed messages. Can. Fam. Physician. 2005;51:1455–1457. [PMC free article] [PubMed] [Google Scholar]
- Mackenzie D.A., Jeffers F., Parker M.L., Vibert-Vallet A., Bongaerts R.J., Roos S., Walter J., Juge N. Strain-specific diversity of mucus-binding proteins in the adhesion and aggregation properties of Lactobacillus reuteri. Microbiology. 2010;156:3368–3378. doi: 10.1099/mic.0.043265-0. [DOI] [PubMed] [Google Scholar]
- Majowicz S.E., Musto J., Scallan E., Angulo F.J., Kirk M., O'Brien S.J., Jones T.F., Fazil A., Hoekstra R.M. International Collaboration on Enteric Disease ‘Burden of Illness’ Studies. The global burden of non-typhoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- Marouf S., Khalf M.A., Alorabi M., El-Shehawi A.M., El-Tahan A.M., Abd El-Hack M.E., El-Saadony M.T., Salem H.M. Mycoplasma gallisepticum: a devastating organism for the poultry industry in Egypt. Poult. Sci. 2022;101(3):101658. doi: 10.1016/j.psj.2021.101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marouf S., Moussa I.M., Salem H., Sedeik M., Elbestawy A., Hemeg H.A., Dawoud T.M., Mubarak A.S., Mahmoud H., AbdullahAlsubkig R., Bahkali A.H. A picture of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Egypt: phenotypic and genotypic characterization. J. King Saud Univ. Sci. 2020;32:2263–2268. [Google Scholar]
- Matulova M., Varmuzova K., Sisak F., Havlickova H., Babak V., Stejskal K., Zdrahal Z., Rychlik I. Chicken innate immune response to oral infection with Salmonella enterica serovar Enteritidis. Vet. Res. 2013;44 doi: 10.1186/1297-9716-44-37. 37-9716-44-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menconi A., Pumford N.R., Morgan M.J., Bielke L.R., Kallapura G., Latorre J.D., Wolfenden A.D., Hernandez-Velasco X., Hargis B.M., Tellez G. Effect of chitosan on Salmonella typhimurium in broiler chickens. Foodborne Pathog. Dis. 2014;11 doi: 10.1089/fpd.2013.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Kitaoka D., Withanage G.S., Fukata T., Sasai K., Baba E. Evaluation of the efficacy of Salmonella enteritidis oil-emulsion bacterin in an intravaginal challenge model in hens. Avian Dis. 1999;43:497–505. [PubMed] [Google Scholar]
- Mohamed M., Mohamed F., El-Said W.A. Enhancement of antimicrobial sensitivity of Salmonella and Escherichia coli strains isolated from chickens using silver nanoparticles in Assiut governorate. Zagazig Vet. J. 2017;45:273–282. [Google Scholar]
- Molnar A.K., Podmaniczky B., Kurti P., Tenk I., Glavits R., Virag G., Szabo Z. Effect of different concentrations of Bacillus subtilis on growth performance, carcase quality, gut microflora and immune response of broiler chickens. Br. Poult. Sci. 2011;52:658–665. doi: 10.1080/00071668.2011.636029. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Tsitrsikos P., Palamidi I., Arvaniti A., Mohnl M., Schatzmayr G., Fegeros K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 2010;89:58–67. doi: 10.3382/ps.2009-00308. [DOI] [PubMed] [Google Scholar]
- Munyaka P.M., Echeverry H., Yitbarek A., Camelo-Jaimes G., Sharif S., Guenter W., House J.D., Rodriguez-Lecompte J.C. Local and systemic innate immunity in broiler chickens supplemented with yeast-derived carbohydrates. Poult. Sci. 2012;91:2164–2172. doi: 10.3382/ps.2012-02306. [DOI] [PubMed] [Google Scholar]
- Murry A.C., Hinton A., Jr, Morrison H. Inhibition of growth of Escherichia coli, Salmonella typhimurium, and Clostridia perfringens on chicken feed media by Lactobacillus salivarius and Lactobacillus plantarum. Int. J. Poult. Sci. 2004;3:603–607. [Google Scholar]
- Nakphaichit M., Thanomwongwattana S., Phraephaisarn C., Sakamoto N., Keawsompong S., Nakayama J., Nitisinprasert S. The effect of including Lactobacillus reuteri KUB-AC5 during post-hatch feeding on the growth and ileum microbiota of broiler chickens. Poult. Sci. 2011;90:2753–2765. doi: 10.3382/ps.2011-01637. [DOI] [PubMed] [Google Scholar]
- Nurmi E., Nuotio L., Schneitz C. The competitive exclusion concept: development and future. Int. J. Food Microbiol. 1992;15:237–240. doi: 10.1016/0168-1605(92)90054-7. [DOI] [PubMed] [Google Scholar]
- Ochoa J., Irache J.M., Tamayo I., Walz A., DelVecchio V.G., Gamazo C. Protective immunity of biodegradable nanoparticle-based vaccine against an experimental challenge with Salmonella enteritidis in mice. Vaccine. 2007;25:4410–4419. doi: 10.1016/j.vaccine.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Pandey K.R., Naik S.R., Vakil B.V. Probiotics, prebiotics and synbiotics- a review. J. Food Sci. Technol. 2015;52:7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires S.M., Vieira A.R., Hald T., Cole D. Source attribution of human Salmonellosis: an overview of methods and estimates. Foodborne Pathog. Dis. 2014;11:667–676. doi: 10.1089/fpd.2014.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabea E.I., Badawy M.E.T., Stevens C.V., Smagghe G., Steurbaut W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- Radu-Rusu C.G., Pop I.M., Simeanu D. Effect of a synbiotic feed additive supplementation on laying hens performance and eggs quality. Lucr. ştiinţ., Ser. Zooteh. (Univ. Şt. Agric. Med. Vet. "Ion Ionescu de la Brad" Iaşi, Online) 2010;53:89–93. [Google Scholar]
- Ray B. 3rd ed. CRC Press; 2004. Fundamental Food Microbiology. [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Madkour M., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilization, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20:324–335. [Google Scholar]
- Reda F., El-Saadony M.T., El-Rayes T.K., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renu S., Markazi A.D., Dhakal S., Lakshmanappa Y.S., Shanmugasundaram R., Selvaraj R.K., Renukaradhya G.J. Oral deliverable mucoadhesive chitosan-Salmonella subunit nanovaccine for layer chickens. Int. J. Nanomed. 2020;15:761–777. doi: 10.2147/IJN.S238445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renu S., Markazi A.D., Dhakal S., Lakshmanappa Y.S., Gourapura S.R., Shanmugasundaram R., Senapati S., Narasimhan B., Selvaraj R.K., Renukaradhya G.J. Surface engineered polyanhydride-based oral Salmonella subunit nanovaccine for poultry. Int. J. Nanomed. 2018;13:8195–8215. doi: 10.2147/IJN.S185588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renua S., Hana Y., Dhakal S., Lakshmanappaa Y.S., Ghimire S., Feliciano-Ruiz N., Senapati S., Narasimhan B., Selvaraj R., Renukaradhya G.J. Chitosan-adjuvanted Salmonella subunit nanoparticle vaccine for poultry delivered through drinking water and feed. Carbohydr. Polym. 2020;243 doi: 10.1016/j.carbpol.2020.116434. [DOI] [PubMed] [Google Scholar]
- Ritzi M.M., Abdelrahman W., Mohnl M., Dalloul R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014;93:2772–2778. doi: 10.3382/ps.2014-04207. [DOI] [PubMed] [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT-Food Sci. Technol. 2021;148 [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A., Taha A.E., Taha T.F., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021;28:5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., Sitohy M.Z., Ahmed A.I., Rabie N.A., Amin S.A., Aboelenin S.M., Soliman M.M., El-Saadony M.T. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules. 2021;26:4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Khattab M.S., Yehia N., Abd El-Hack M.E., El-Saadony M.T., Alhimaidi A.R., Swelum A.A., Attia M.M. Morphological and molecular characterization of Ascaridia columbae in the domestic pigeon (Columba livia domestica) and the assessment of its immunological responses. [e-pub ahead of print] Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Attia M.M. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: Muscidae) larvae in broiler chickens: a field study. Int. J. Trop. Insect Sci. 2021;41:2549–2554. [Google Scholar]
- Salem H.M., Ismael E., Shaalan M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021;16:6783–6796. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Yehia N., Al-Otaibi S., El-Shehawi A.M., Elrys A.A.M.E., El-Saadony M.T., Attia M.M. The prevalence and intensity of external parasites in domestic pigeons (Columba livia domestica) in Egypt with special reference to the role of deltamethrin as insecticidal agent. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.10.042. Accessed Oct. 22, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman H.H., Gamazo C., Campanero M.A., Irache J.M. Salmonella-like bioadhesive nanoparticles. J. Control. Release. 2005;106:1–13. doi: 10.1016/j.jconrel.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Sanchez B., Champomier-Verges M.C., Stuer-Lauridsen B., Ruas-Madiedo P., Anglade P., Baraige F., de los Reyes-Gavilan C.G., Johansen E., Zagorec M., Margolles A. Adaptation andresponse of Bifidobacterium animalis subsp. lactis to bile: a proteomic and physiological approach. Appl. Environ. Microbiol. 2007;73:6757–6767. doi: 10.1128/AEM.00637-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scardovi V., Zani G. Bifidobacterium magnum sp. nov., a Large, acidophilic Bifidobacterium Isolated from rabbit feces. Int. J. Syst. Bacteriol. 1974;24:29–34. [Google Scholar]
- Seo B.J., Mun M.R., Rejish Kumar V.J., Kim C.J., Lee I., Chang Y.H., Park Y.H. Bile tolerant Lactobacillus reuteri isolated from pig feces inhibits enteric bacterial pathogens and porcine rotavirus. Vet. Res. Commun. 2010;34:323–333. doi: 10.1007/s11259-010-9357-6. [DOI] [PubMed] [Google Scholar]
- Serjeant E.P., Dempsey B., Int. Union Pure Appl. Chem. (IUPAC) Pergamon Press, Inc.; New York, NY: 1979. Ionization Constants of Organic Acids in Aqueous Solution. IUPAC Chem Data Ser No. 23. [Google Scholar]
- Setta A., Salem H.M., Elhady M., El-Hussieny A., Arafa A.A. Molecular and genetic characterization of infectious bronchitis viruses isolated from commercial chicken flocks in Egypt between 2014 and 2016. J. World Poult. Res. 2018;8:01–08. [Google Scholar]
- Shang Y., Regassa A., Kim J.H., Kim W.K. The effect of dietary fructooligosaccharide supplementation on growth performance, intestinal morphology, and immune responses in broiler chickens challenged with Salmonella Enteritidis lipopolysaccharides. Poult. Sci. 2015;94:2887–2897. doi: 10.3382/ps/pev275. [DOI] [PubMed] [Google Scholar]
- Sharma S., Hinds L.A. Formulation and delivery of vaccines: Ongoing challenges for animal management. J. Pharm. Bioallied Sci. 2012;4:258–266. doi: 10.4103/0975-7406.103231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheela R.R., Babu U., Mu J., Elankumaran S., Bautista D.A., Raybourne R.B., Heckert R.A., Song W. Immune responses against Salmonella enterica serovar enteritidis infection in virally immunosuppressed chickens. Clin. Diagn. Lab. Immunol. 2003;10:670–679. doi: 10.1128/CDLI.10.4.670-679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd El-Hack M.E., Khafaga A.F., Metwally K.A.…El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Xiao Y., Zhang S., Wu S., Gao L., Shi S. Fe3O4 nanoparticles attenuated Salmonella infection in chicken liver through reactive oxygen and autophagy via PI3K/Akt/mTOR Signaling. Front. Physiol. 2020;10:1–10. doi: 10.3389/fphys.2019.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M.S., Han S.K., Ryu J.S., Kim K.S., Lee W.K. Isolation and partial characterization of a bacteriocin produced by Pediococcus pentosaceus K23-2 isolated from Kimchi. J. Appl. Microbiol. 2008;105:331–339. doi: 10.1111/j.1365-2672.2008.03770.x. [DOI] [PubMed] [Google Scholar]
- Shivaprasad H.L., Methner U., Barrow P.A. In: Pages 162–192 Salmonella in Domestic Animals. 2nd ed. Barrow P.A., Methner U., editors. CABI; Boston, Massachusetts: 2013. Salmonella infections in the domestic fowl. [Google Scholar]
- Shroff K.E., Meslin K., Cebra J.J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect. Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R.da., Baretta A.M., da Silva L.L., Ternus R.Z., Colpani G.L., Fiori M.A., Dalcanton F., Zanetti M., de. Mello J.M.M. Evaluation of the antimicrobial activity of poultry feed additives with zinc oxide nanoparticles. Res. Soc. Dev. 2021;10 [Google Scholar]
- Simpson P.J., Stanton C., Fitzgerald G.F., Ross R.P. Intrinsic tolerance of Bifidobacterium species to heat and oxygen and survival following spray drying and storage. J. Appl. Microbiol. 2005;99:493–501. doi: 10.1111/j.1365-2672.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- Singh B.R., Ebibeni N. Aerobic microbiome of vagina of apparently healthy pregnant large black sows in nagaland and antimicrobial resistance in isolates. Glob. J. Pathol. Microbiol. 2016;4:8–17. [Google Scholar]
- Sivula C.P., Bogomolnaya L.M., Andrews-Polymenis H.L. A comparison of cecal colonization of Salmonella enterica serotype Typhimurium in white leghorn chicks and Salmonella-resistant mice. BMC Microbiol. 2008;8:182. doi: 10.1186/1471-2180-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman S.M., Attia M.M., Al-Harbi M.S., Saad A.M., El-Saadony M.T., Salem H.M. Low host specificity of Hippobosca equina infestation in different domestic animals and pigeon. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.11.050. Accessed Nov. 24, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strompfova V., Laukova A., Marcinakova M., Vasilkova Z. Testing of probiotic and bacteriocin-producing lactic acid bacteria towards Eimeria sp. Pol. J. Vet. Sci. 2010;13:389–391. [PubMed] [Google Scholar]
- Svetoch E.A., Eruslanov B.V., Levchuk V.P., Perelygin V.V., Mitsevich E.V., Mitsevich I.P., Stepanshin J., Dyatlov I., Seal B.S., Stern N.J. Isolation of Lactobacillus salivarius 1077 (NRRL B-50053) and characterization of its bacteriocin, including the antimicrobial activity spectrum. Appl. Environ. Microbiol. 2011;77:2749–2754. doi: 10.1128/AEM.02481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swelum A.A., Elbestawy A.R., El-Saadony M.T., Hussein E.O., Alhotan R., Suliman G.M., Abd El-Hack M.E. Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: an updated overview. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabees R., Elsayed M.S.A., Shawish R., Basiouni S., Shehata A.A. Isolation and characterization of Salmonella enteritidis and Salmonella typhimurium from chicken meat in Egypt. J. Infect. Dev. Ctries. 2017;11:314–319. doi: 10.3855/jidc.8043. [DOI] [PubMed] [Google Scholar]
- Tennant S.M., Levine M.M. Live attenuated vaccines for invasive Salmonella infections. Vaccine. 2015;33(Suppl 3):36–41. doi: 10.1016/j.vaccine.2015.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M. Nano cancer therapy strategies. J. Cancer Res. Ther. 2012;8:19–22. doi: 10.4103/0973-1482.95168. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture (USDA). 2015. Poultry and Eggs. Available at: http://www.ers.usda.gov/topics/animal?products/poultry?eggs.aspx. Accessed Apr. 2, 2016.
- Upadhyaya I., Upadhyay A., Kollanoor-Johny A.M., Baskaran S.A., Mooyottu S., Darre M.J., Venkitanarayanan K. Rapid inactivation of Salmonella enteritidis on shell eggs by plant-derived antimicrobials. Poult. Sci. 2013;92:3228–3235. doi: 10.3382/ps.2013-03126. [DOI] [PubMed] [Google Scholar]
- Van der Wielen P.W., Lipman L.J., van Knapen F., Biesterveld S. Competitive exclusion of Salmonella enterica serovar Enteritidis by Lactobacillus crispatus and Clostridium lactatifermentans in a sequencing fed-batch culture. Appl. Environ. Microbiol. 2002;68(2):555–559. doi: 10.1128/AEM.68.2.555-559.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wielen P.W., Biesterveld S., Notermans S., Hofstra H., Urlings B.A., Knapen van F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000;66:2536–2540. doi: 10.1128/aem.66.6.2536-2540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F., Boyen F., Gantois I., Timbermont L., Bohez L., Pasmans F., Haesebrouck F., Ducatelle R. Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poult. Sci. 2005;84:1851–1856. doi: 10.1093/ps/84.12.1851. [DOI] [PubMed] [Google Scholar]
- Vordermeier H.M., Kotlarski I. Partial purification and characterization of low molecular weight antigens of Salmonella enteritidis 11RX. Immunol. Cell Biol. 1990;68(Pt 5):307–316. doi: 10.1038/icb.1990.42. [DOI] [PubMed] [Google Scholar]
- Whiley H., Ross K. Salmonella and Eggs: From Production to Plate. Int. J. Environ. Res. Public Health. 2015;12:2543–2556. doi: 10.3390/ijerph120302543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P.B. HMSO; London, UK: 1926. Further Studies on the Salmonella Group. Great Britain Medical Research Special Report no. 103. [Google Scholar]
- Wibisono F.M., Wibisono F.J., Effendi M.H., Plumeriastuti H., Hidayatullah A.R., Hartadi E.B., Sofiana E.D. A review of Salmonellosis on poultry farms: public health importance. Sys. Rev. Pharm. 2020;11:481–486. [Google Scholar]
- Wigley P. Salmonella enterica in the chicken: how it has helped our understanding of immunology in a non-biomedical model species. Front. Immunol. 2014;5:482. doi: 10.3389/fimmu.2014.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen L.E., Koetsier M.A., van Deventer S.J., van Tol E.A. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E (1) and E (2) production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wizemann T.M., Adamou J.E., Langermann S. Adhesins as targets for vaccine development. Emerg. Infect. Dis. 1999;5:395–403. doi: 10.3201/eid0503.990310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2015. WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007-2015, p. 72. Switzerland.
- Xu Z.R., Hu C.H., Xia M.S., Zhan X.A., Wang M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yang C.M., Cao G.T., Ferket P.R., Liu T.T., Zhou L., Zhang L., Xiao Y.P., Chen A.G. Effects of probiotic, Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 2012;91:2121–2129. doi: 10.3382/ps.2011-02131. [DOI] [PubMed] [Google Scholar]
- Yaqoob M., Abd El-Hack M.E., Hassan F., El-Saadony M.T., Khafaga A., Batiha G., Yehia N., Elnesr S., Alagawany M., El-Tarabily K.A., Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia N., AbdelSabour M.A., Erfan A.M., Ali Z.M., Soliman R.A., Samy A., Ahmed K.A. Selenium nanoparticles enhance the efficacy of homologous vaccine against the highly pathogenic avian influenza H5N1 virus in chickens [e-pub ahead of print] Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.11.051. Accessed Nov. 25, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry C., Zikry P.M., Salem H.M., Basalious E.B., El-Gazayerly O.N. Integrated nanovesicular/selfnanoemulsifying system (INV/SNES) for enhanced dual ocular drug delivery: statistical optimization, in vitro and in vivo evaluation. Drug Deliv. Transl. Res. 2020;10:801–814. doi: 10.1007/s13346-020-00716-5. [DOI] [PubMed] [Google Scholar]
- Yu B., Liu J.R., Chiou M.Y., Hsu Y.R., Chiou P.W.S. The effects of probiotic Lactobacillus reuteri Pg4 strain on intestinal characteristics and performance in broilers. Asian Australas. J. Anim. Sci. 2007;20:1243–1251. [Google Scholar]
- Zhang-Barber L., Turner A.K., Barrow P.A. Vaccination for control of Salmonella in poultry. Vaccine. 1999;17:2538–2545. doi: 10.1016/s0264-410x(99)00060-2. [DOI] [PubMed] [Google Scholar]
- Zhou F., Ji B., Zhang H., Jiang H., Yang Z., Li J., Li J., Ren Y., Yan W. Synergistic effect of thymol and carvacrol combined with chelators and organic acids against Salmonella typhimurium. J. Food Prot. 2007;70:1704–1709. doi: 10.4315/0362-028x-70.7.1704. [DOI] [PubMed] [Google Scholar]