Highlights
-
•
Skeletal-related events (SRE) with tumors have significant patient/economic burden.
-
•
Denosumab (RANKL inhibitor) is approved for SRE prevention in MM and solid tumor BM.
-
•
Denosumab reduced risk of first/subsequent SREs versus zoledronate in solid tumors.
-
•
Denosumab 120 mg every 4 weeks SC is well tolerated, including in renal impairment.
-
•
SRE prevention remains important, despite new and effective antitumor therapies.
Keywords: Denosumab, Bone metastasis, Skeletal-related events, Efficacy, Safety
Abbreviations: AFF, Atypical femoral fracture; BM, Bone metastasis; BMFS, BM-free survival; BP, Bisphosphonate; BTA, Bone-targeting agent; CI, Confidence interval; HR, Hazard ratio; HRQoL, Health-related quality of life; IMWG, International Myeloma Working Group; MM, Multiple myeloma; MVF, Multiple vertebral fracture; NSCLC, Non–small-cell lung cancer; ONJ, Osteonecrosis of the jaw; OPG, Osteoprotegerin; OS, Overall survival; Q4W, Every 4 weeks; Q12W, Every 12 weeks; PFS, Progression-free survival; RANKL, Receptor activator of nuclear factor-κB ligand; SC, Subcutaneous; SRE, Skeletal-related event; uNTx/Cr, Urinary N-telopeptide normalized to urinary creatinine
Abstract
Skeletal-related events (SREs) are complications of bone metastases and carry a significant patient and economic burden. Denosumab is a receptor activator of nuclear factor-κB ligand (RANKL) inhibitor approved for SRE prevention in patients with multiple myeloma and patients with bone metastases from solid tumors. In phase 3 trials, denosumab showed superiority to the bisphosphonate zoledronate in reducing the risk of first on-study SRE by 17% (median time to first on-study SRE delayed by 8.2 months) and the risk of first and subsequent on-study SREs by 18% across multiple solid tumor types, including some patients with multiple myeloma. Denosumab also improved pain outcomes and reduced the need for strong opioids. Additionally, a phase 3 trial showed denosumab was noninferior to zoledronate in delaying time to first SRE in patients with newly diagnosed multiple myeloma. Denosumab has a convenient 120 mg every 4 weeks recommended dosing schedule with subcutaneous administration. Rare but serious toxicities associated with denosumab include osteonecrosis of the jaw, hypocalcemia, and atypical femoral fracture events, with multiple vertebral fractures reported following treatment discontinuation. After a decade of real-world clinical experience with denosumab, we are still learning about the optimal use and dosing for denosumab. Despite the emergence of novel and effective antitumor therapies, there remains a strong rationale for the clinical utility of antiresorptive therapy for SRE prevention. Ongoing studies aim to optimize clinical management of patients using denosumab for SRE prevention while maintaining safety and efficacy.
1. Background
Skeletal-related events (SREs) are serious sequelae in patients with bone metastases (BMs) from solid tumors and in patients with multiple myeloma (MM) and are associated with reduced quality of life, increased pain, and decreased survival [1]. Denosumab (XGEVA®, Amgen Inc, Thousand Oaks, CA) is a receptor activator of nuclear factor-κB ligand (RANKL) inhibitor that is approved for prevention of SREs among cancer patients with BMs or MM. The purpose of this review is to summarize clinical data leading to the initial approval of denosumab in the US in 2010 for prevention of SREs, discuss what has been learned over the subsequent 10 years, and highlight questions and areas for further study.
1.1. Bone metastases and skeletal-related events
BMs are common in patients with certain advanced cancers, with an incidence of approximately 70% in breast, 85% in prostate, and 40% in lung and renal cancers [2], [3]. Additionally, bone lesions develop in the vast majority of patients with MM (approximately 90%) [4].
SREs are skeletal complications related to BMs and include pathologic fractures that may impair ambulation and vertebral compression or fracture leading to spinal cord compressions that can result in numbness or weakness, urinary/fecal incontinence, or paralysis [2]. They can trigger a need for radiation therapy to bone to control local tumor burden and manage pain, and surgery to prevent or treat pathologic fractures [2]. SREs are common in patients with solid tumors, with overall incidence rates of approximately 45%–65% in patients not receiving prophylactic antiresorptive therapy [5]. By solid tumor type, the cumulative probability of SRE occurring within 3–5 years of BM diagnosis ranges from 52% to 55% in breast, 42% to 52% in prostate, and 57% to 69% in lung cancers [6], [7], [8], [9]; the probability of SRE among patients with MM ranges from 22% to 34% [10], [11]. The underlying pathophysiology, irrespective of primary tumor type and radiographic appearance, is a locally increased pathologic rate of bone resorption due to increased osteoclast activity [12], which is evidenced by elevated levels of bone turnover markers [13], including calcium [14]. Elevated bone resorption markers have been associated with worse prognosis for patients with significant skeletal morbidity [15].
SREs are associated with pain progression and impaired health-related quality of life (HRQoL) and often require the use of strong opioids for analgesic relief [1]. Between 65% and 75% of patients with BMs experience pain and impaired mobility [16]. Additionally, SREs result in substantial economic burden. Overall, patients with BMs from solid tumors and SRE have higher per-year rates of inpatient admissions, emergency department visits, physician office visits, laboratory services, hospital-based outpatient services, and subsequently higher total medical costs collectively than patients without SREs [6]. The incremental annual average cost of SREs in the US is reported to be $67,257 [6]. Furthermore, patients with BMs and SREs incurred an additional 39.4 short-term disability hours per month versus patients with BMs without SREs [17]. Similarly, patients with MM and SRE have significantly higher healthcare resource utilization rates and higher total medical costs than patients without SRE [10].
1.2. Development of bone-targeting agents for bone metastases
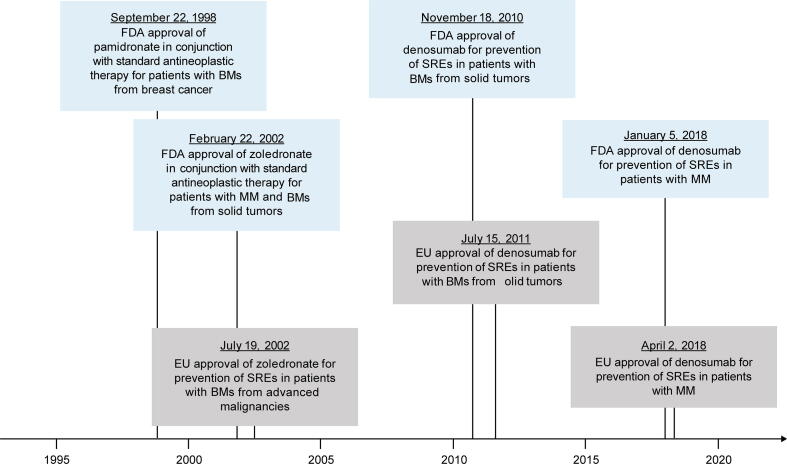
Bone-targeting agents (BTAs) pamidronate and zoledronate, which are bisphosphonates (BPs), have been used for a variety of skeletal disorders, including prevention of SREs in patients with BMs from solid tumors (Fig. 1) [18]. BPs bind to the bone surface, where they reduce bone resorption by inhibiting osteoclast activity and survival through blockade of farnesyl pyrophosphate synthase in the mevalonate pathway [19]. Zoledronate is indicated for treatment of hypercalcemia of malignancy and for SRE prevention in patients with MM or BMs from solid tumors in conjunction with antineoplastic therapy, and in prostate cancer that has progressed after treatment with ≥ 1 hormonal therapy [20].
Fig. 1.
Approval of BTAs in the US (blue boxes) and Europe (gray boxes) for prevention of SREs. BM, bone metastasis; BTA, bone-targeting agent; FDA, US Food and Drug Administration; MM, multiple myeloma; SRE, skeletal-related event. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Adverse events have been reported with zoledronate for SRE prevention. Cases of osteonecrosis of the jaw (ONJ) associated with zoledronate have been identified in postmarketing reports [21]; it is recommended that patients using zoledronate avoid invasive dental procedures [20]. Additionally, intravenous BPs may impair renal function, and pamidronate and zoledronate are not recommended for treatment of BMs in severe renal impairment [20], [22].
Because BPs have not improved survival outcomes related to prevention of SREs in solid tumors [23], [24] and their use is limited in renal impairment [25], novel BTAs with a distinct mechanism of action were needed.
2. Mechanism of action and pharmacokinetics of denosumab
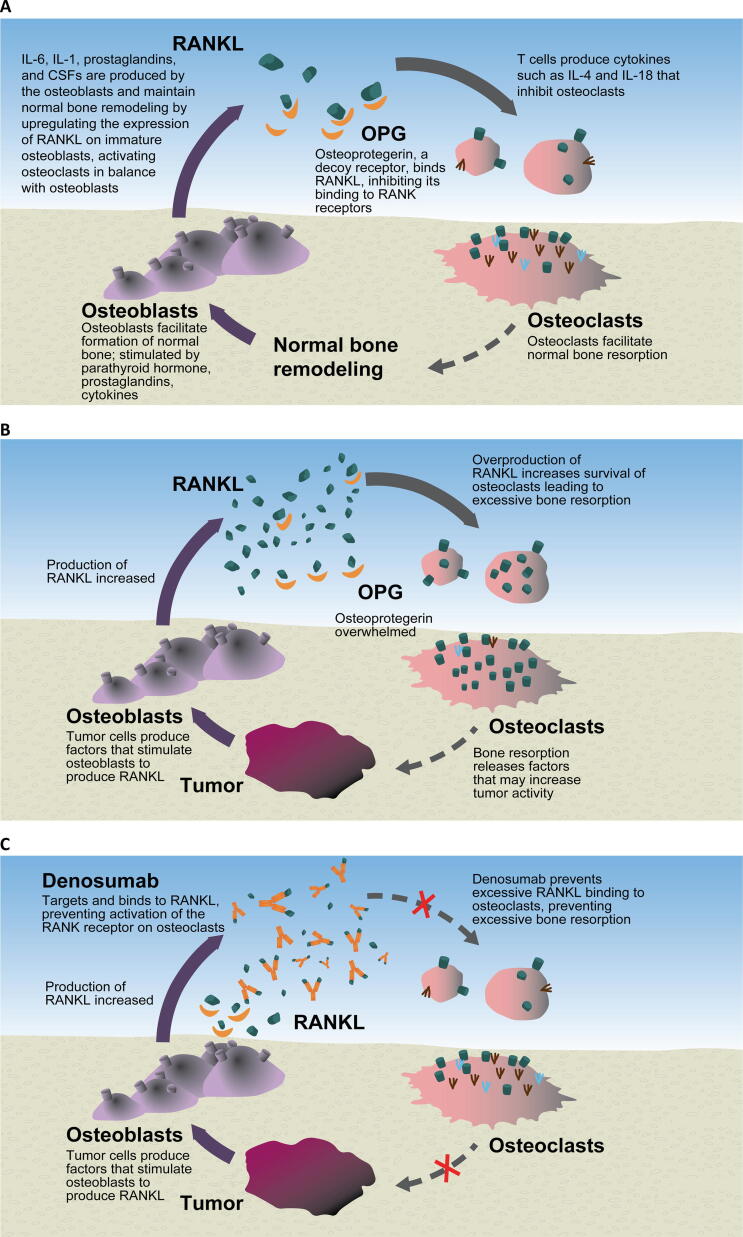
Denosumab is a fully human monoclonal antibody that binds to and inhibits activity of RANKL, preventing excessive bone turnover and subsequent life-threatening complications such as hypercalcemia (Fig. 2) [14], [26]. RANKL is an osteoclastogenic cytokine and humoral factor and is released by both osteoblasts and activated T cells. Many factors induce RANKL expression which, once secreted, binds to its receptor, RANK, expressed on osteoclast progenitor cells, leading to bone resorption [27]. In normal bone physiology, RANKL activity is also moderated by its decoy receptor, osteoprotegerin (OPG). OPG was used as a surrogate for RANKL inhibition in preclinical experiments. OPG inhibited osteoclastogenesis in bone lesions in mice; OPG-deficient mice showed a high bone turnover state that was suppressed by injection of anti-RANKL antibodies, which promoted osteoclast apoptosis [28], [29]. OPG also reduced biochemical markers of bone turnover in nonhuman primates [30]. Clinically, denosumab was more potent than an Fc-OPG fusion protein, had a longer duration of effect, and avoided potential risks related to OPG including generation of anti-OPG antibodies and interference with tumorigenesis defense mechanisms; thus, denosumab was further evaluated [31].
Fig. 2.
Overview of (A) normal bone physiology, (B) tumor pathology, and (C) the mechanism of action of denosumab bone metastases. CSF, colony-stimulating factor; IL, interleukin; OPG, osteoprotegerin; RANK, receptor activator of nuclear factor-κB; RANKL, RANK ligand.
Denosumab exposure increases dose proportionally (30–180 mg every 4 weeks [Q4W]; 60 and 180 mg every 12 weeks [Q12W]) in patients with breast cancer and BMs [32]. The steady-state serum concentration of denosumab 120 mg Q4W is reached within 4–5 months, and serum levels decline over 4–5 months after the last dose; denosumab half-life is 25–30 days [33]. Renal dysfunction has no effect on pharmacokinetics or pharmacodynamics of a single 60-mg subcutaneous (SC) dose of denosumab [34] of doses examined in a phase 2 trial, 120 mg Q4W was associated with maximum reduction (measured at week 13 from baseline) of the bone turnover marker urinary N-telopeptide normalized to urinary creatinine (uNTx/Cr) [32]. In addition, no grade 2 or higher hypocalcemia adverse events occurred with 120 mg Q4W versus five events with 180 mg Q4W. Patients who received denosumab 120 mg Q4W, unlike those on a Q12W schedule, showed sustained uNTx/Cr suppression throughout the study [35]. Further, 120 mg Q4W avoided potential for low exposures of denosumab that could result in reduced efficacy in SRE prevention through reduced uNTx/Cr suppression [36]. Collectively, these phase 2 data informed selection of 120 mg Q4W as optimal for antiresorptive effects in subsequent phase 3 studies.
3. Efficacy
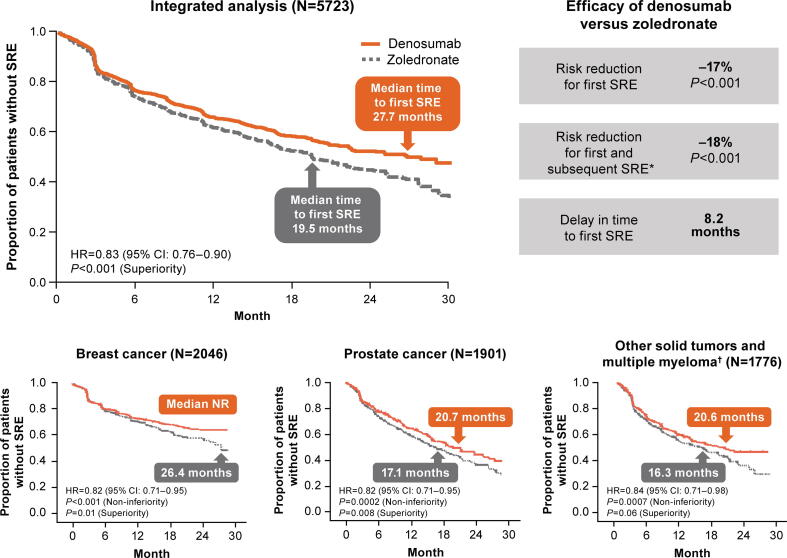
An integrated analysis of three pivotal phase 3 studies in patients with solid tumors with BMs and some patients with MM showed that denosumab 120 mg SC Q4W was superior to intravenous zoledronate 4 mg Q4W in delaying time to first on-study SRE and to first and subsequent on-study SREs (Fig. 3) [37] overall, denosumab reduced the risk for first on-study SRE by 17%, for multiple SREs by 18%, and increased median time to first on-study SRE by > 8 months. Additionally, denosumab reduced the risk of first on-study SRE compared with zoledronate in patients with (hazard ratio [HR], 0.84; 95% confidence interval [CI], 0.73–0.96; P = 0.01) and without (HR, 0.82; 95% CI, 0.73–0.92; P < 0.001) previous SRE at baseline. In a post hoc analysis of the same trials (excluding MM), denosumab significantly reduced the risk of first on-study SRE compared with zoledronate across subgroups defined by baseline characteristics including number of BMs (< 2 versus ≥ 2) and uNTx level (≥ 43.7 versus < 43.7 nmol/mmol) [38]. Consistent with overall results from the integrated analysis, median time to first on-study SRE was longer with denosumab than zoledronate across all baseline subgroups.
Fig. 3.
SRE outcomes with denosumab versus zoledronate in patients with solid tumors or multiple myeloma [37], [39], [40], [41]. *Events occurring ≥ 21 days apart. †Primary tumor types (n [%]) were non–small-cell lung cancer (denosumab, 350 [39%]; zoledronate, 352 [40%]), multiple myeloma (denosumab, 87 [10%]; zoledronate, 93 [10%]), and other (breast and prostate excluded; denosumab, 449 [51%]; zoledronate, 455 [50%]). CI, confidence interval; HR, hazard ratio; NR, not reached; SRE, skeletal-related event.
Clinical outcomes in patients with breast cancer were comparable to results from the integrated analysis [39]. Denosumab significantly delayed time to first on-study SRE compared with zoledronate (Fig. 3) and to first and subsequent SREs (rate ratio, 0.77; 95% CI, 0.66–0.89; P = 0.001, superiority). Median time to first on-study SRE was longer in patients with breast cancer relative to the integrated analysis. Patients with breast cancer initiated antiresorptive therapy within approximately 2 months of initial BM diagnosis, highlighting the importance of early initiation of therapy.
In the pivotal phase 3 trial in patients with castration-resistant prostate cancer and BMs, the percentage of patients with an on-study SRE was 36% with denosumab and 41% with zoledronate [40]. Denosumab significantly delayed time to first on-study SRE (Fig. 3) and to first and subsequent SRE (rate ratio, 0.82; 95% CI, 0.71–0.94; P = 0.008, superiority) compared with zoledronate.
In the third pivotal phase 3 trial in patients with solid tumors and BMs (excluding breast and prostate cancers) or MM with lytic lesions [41], denosumab was noninferior to zoledronate in delaying time to first on-study SRE; median time to first SRE was increased (Fig. 3).
Meta-analyses and systematic reviews of denosumab support its efficacy in delaying time to SREs and reducing the number of SREs in patients with solid tumor–associated BMs [42], [43], [44]. A meta-analysis of four trials showed that denosumab was significantly superior to zoledronate in delaying the incidence of first on-study SRE (HR, 0.86; 95% CI, 0.80–0.93; P < 0.001) and development of multiple SREs (HR, 0.87; 95% CI, 0.77–0.98; P = 0.03) [42].
4. Survival outcomes
Pathologic fractures have been shown to significantly increase mortality in MM and in patients with BMs from breast or prostate cancer [45]. To date, survival outcomes with denosumab have been similar to those with zoledronate in patients with solid tumors and BMs. In general, overall survival (OS) is highest in patients with solid tumors without BM, lower in patients with BM but without SRE, and lowest in patients with BM and SRE [46], [47]. In an integrated analysis and phase 3 head-to-head trials, no difference in OS was observed between denosumab and zoledronate [37], [39], [40], [41]. In placebo-controlled studies of zoledronate, no difference in OS was observed overall; however, zoledronate reduced the risk of death in patients with poor prognosis (eg, patients with non–small-cell lung cancer [NSCLC] or high NTx) [48]. To our knowledge, no subgroup analyses have assessed OS with denosumab among patients with or without SRE in phase 3 trials; however, univariate and multivariate modeling in prostate cancer identified previous SRE and bone-related parameters as being significantly correlated to OS, with uNTx levels ≤ 50 nmol/mmol predictive of survival [49]. Similarly, in patients with solid tumors, uNTx levels ≤ 10.0 nmol/mmol were associated with improved OS after 3 months of denosumab or zoledronate treatment (HR, 1.85; 95% CI, 1.67–2.04; P < 0.0001) [50].
5. Pain control and health-related quality of life
Pain has a significant impact on HRQoL in patients with cancer, and preventing and palliating SRE-related pain is an important consideration [1], [51]. An integrated analysis of phase 3 studies in patients with solid tumors and BMs showed that denosumab delayed time to pain worsening and to increased pain interference and prevented use of strong opioids compared with zoledronate [52]. Denosumab reduced median time to onset of moderate or severe pain by 1.8 months in patients with no or mild pain at baseline (6.5 versus 4.7 months; HR, 0.83; 95% CI, 0.76–0.92; P < 0.001). Among patients at risk, median time to increased overall pain interference with function (≥ 2-point increase from baseline in Brief Pain Inventory-Short Form score) was increased by 1.8 months with denosumab (11.1 versus 9.3 months; HR, 0.90; 95% CI, 0.83–0.98; P = 0.010). Overall, the percentage of patients shifting from no strong opioid use at baseline to strong opioid use was lower with denosumab (average relative difference, –13.4%; P = 0.041), and fewer patients had clinically meaningful worsening HRQoL from baseline (average relative difference, –4.1%; P = 0.005).
Pain control in patients with breast cancer or other solid tumors (excluding breast and prostate) was consistent with that in the integrated analysis. Denosumab delayed time to pain worsening by 4 months compared with zoledronate in patients with breast cancer and BMs with no or mild pain at baseline (9.7 versus 5.8 months; HR, 0.78; 95% CI, 0.67–0.92; P = 0.002) [53]. The percentage of patients who shifted from no or low analgesic use to strong opioid use was lower with denosumab (relative difference, 20%; absolute difference, 2%). Additionally, in breast cancer, worsening HRQoL was observed in 7% fewer patients with denosumab compared with zoledronate [54]. Similarly, in patients with other solid tumors and BMs, denosumab delayed median time to onset of moderate or severe pain by 1 month (4.7 versus 3.7 months; HR, 0.81; 95% CI, 0.66–1.00; P = 0.05) and to increased pain interference by > 3 months in patients with no or mild pain at baseline (8.2 versus 4.8 months; HR, 0.77; 95% CI, 0.61–0.96; P = 0.021) [55]. Among all solid tumor types, pathologic fracture, radiation to the bone, and spinal cord compression were associated with a significantly greater risk of pain interference overall [1].
6. Safety
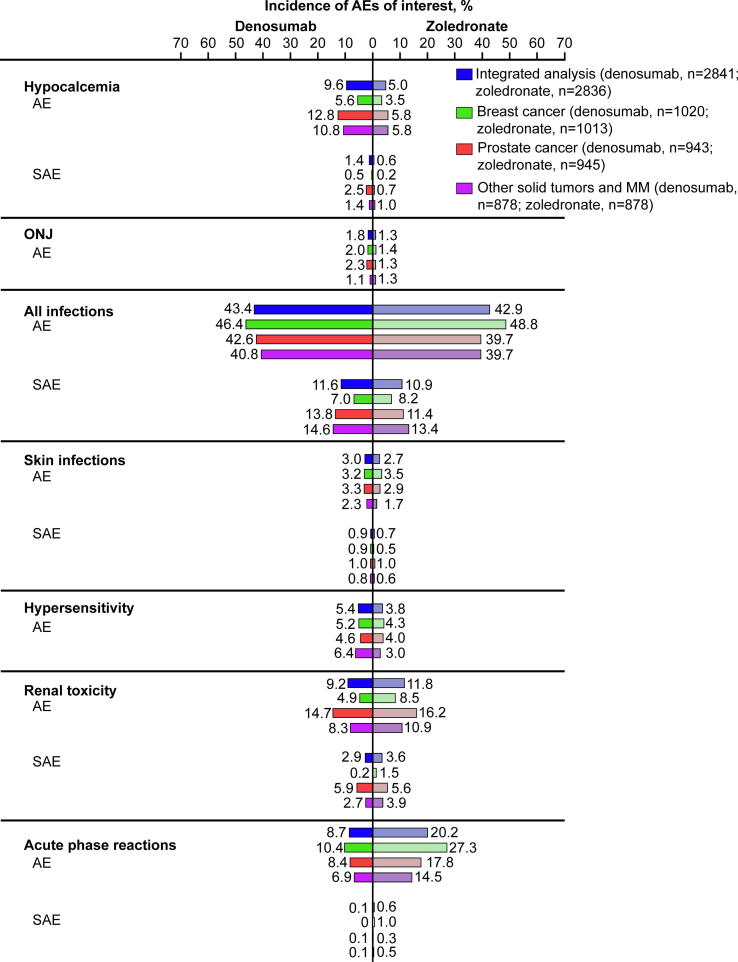
Kidney function is an important consideration with BTAs. The 5-year prevalence of renal impairment in patients with solid tumors and BMs in the US is estimated to be 43% [56]. Of patients with renal impairment, 71% have chronic kidney disease. Adverse events with denosumab 120 mg SC Q4W versus zoledronate were evaluated in 5677 patients (denosumab, 2841; zoledronate, 2836) with BMs secondary to advanced solid tumors or bone lesions from MM (Fig. 4). In a combined analysis of the pivotal phase 3 trials, dose adjustments of zoledronate were required in 18% of patients due to impaired renal function, and 10% of patients had zoledronate withheld during the study because of increased serum creatinine levels; no dose adjustments were made for denosumab [37]. Rates of renal adverse events were lower with denosumab than with zoledronate (9.2% versus 11.8%) across patients with solid tumors and MM (see Denosumab in Multiple Myeloma).
Fig. 4.
Prespecified AEs of interest with denosumab versus zoledronate in patients with solid tumors or multiple myeloma [37], [39], [40], [41]. AE, adverse event; MM, multiple myeloma; ONJ, osteonecrosis of the jaw; SAE, serious AE.
BTAs are considered a primary risk factor for ONJ [57]. In blinded and open-label extension phases of pivotal phase 3 studies in breast or prostate cancer, patient-year–adjusted incidence of ONJ was 1.1% per 100 patient-years during the first year of treatment with denosumab and 4.1% per 100 patient-years thereafter [58]. In an integrated analysis of phase 3 breast cancer, prostate cancer, and solid tumor/MM studies, incidence of ONJ was slightly higher with denosumab than with zoledronate (1.8% versus 1.3%; P = 0.13) [59], possibly due to the increased potency of denosumab relative to zoledronate. Finally, a recent study of 374 patients with cancer and BM found the incidence of ONJ was significantly higher with denosumab versus zoledronate (12.6% versus 4.4%; P = 0.006) [60]. Time to resolution of ONJ was significantly shorter for denosumab (median 26.8 months versus not reached for zoledronate; P = 0.024), which may be due to the shorter half-life of denosumab compared with BPs. The effects of denosumab on bone are mostly diminished within 6 months of treatment discontinuation [61].
Besides exposure to denosumab or BPs, other risk factors for ONJ in cancer patients include local infection or trauma to the bone, periodontal inflammation, other dental procedures, subclinical trauma, concomitant medications (eg, corticosteroids, anti-angiogenic therapy), concomitant diseases or conditions (eg, pre-existing dental infections, anemia, diabetes mellitus), poor oral hygiene, and smoking [62]. ONJ management includes symptom control and risk mitigation by taking into account the risk/benefit ratio of BTA [62]. Patients should undergo oral examination and preventive dentistry before initiation of denosumab; if invasive dental procedures cannot be avoided during treatment, temporary discontinuation should be considered [63].
During open-label extension phases of pivotal breast and prostate cancer trials, hypocalcemia was observed in 20 patients (4.3%) in the denosumab/denosumab group and 14 patients (3.1%) in the zoledronate/denosumab group [58]. Hypocalcemia with denosumab often occurred in the first months of use (median time to first occurrence of any adverse event of hypocalcemia was 2.8 months and of grade ≥ 3 was 4.6 months); incidence of hypocalcemia was lower in patients treated with denosumab who reported use of calcium and vitamin D supplements compared with those without supplements (HR, 0.60; 95% CI, 0.45–0.81; P = 0.0007) [64]. In open-label phase 2 studies evaluating safety of denosumab in patients with chronic kidney disease, an increased risk of clinically significant hypocalcemia was observed in patients with increasing renal dysfunction [65].
Multiple vertebral fractures (MVF) have been reported following discontinuation of denosumab treatment [63], typically in the osteoporosis setting [66]. In the oncology setting, a case report identified MVF 15 months after denosumab discontinuation in a patient with BM from lung cancer without secondary risk factors [67]. MVF is rarely observed in oncology, probably because of the poor prognosis of patients with solid tumors and BMs and delay in MVF after denosumab discontinuation (9–16 months). It is unclear if the higher dosing frequency in the oncology setting (Q4W) versus the osteoporosis setting (every 6 months) affects the risk of MVF. Because denosumab does not incorporate into bone, bone turnover markers increase following denosumab discontinuation, which may lead to rebound fracture [68]. Administration of BPs may be considered following denosumab discontinuation to reduce the risk of rebound osteolysis and subsequent fractures; however, the optimal dose and timing of BP therapy have not been determined.
Similarly, atypical femoral fracture (AFF) events have occurred during denosumab and after discontinuation [63]; retrospective analyses of patients who received denosumab for BMs have reported AFF incidence rates of 0.4%–1.8% [69], [70]. Reports have noted patients were also receiving glucocorticoid treatment at the time of fracture [63]. Risk factors for AFF include prior zoledronate and long-term denosumab treatment (> 3.5 years) [69].
7. Clinical guidelines
Guidelines for BTAs as SRE prevention are available (Table 1). In breast cancer, BTAs such as denosumab and zoledronate are recommended if BM is present [51], [71], whether or not patients are symptomatic [68]. In patients with prostate cancer and BMs, denosumab has superior efficacy over zoledronate for SRE prevention and is preferred, but underlying comorbidities must also be considered [72], [73]. Guidelines also recommend denosumab or zoledronate for all patients with castrate-resistant prostate cancer and BMs, whether or not they are symptomatic [68], [74]. In NSCLC, denosumab or BPs can be considered in patients with BMs [75]; both denosumab and zoledronate are recommended in patients with advanced lung cancer with BMs and a life expectancy ≥ 3 months who are considered high risk for SREs [68]. In most cases, BTAs should be initiated at BM diagnosis and continue throughout therapy; in oligometastatic bone disease, BTAs may be interrupted if complete or good partial responses are achieved and may be resumed if disease progresses [68]. Current guidelines are largely based on SRE outcomes; any future OS or HRQoL benefits observed, including in specific patient subgroups, should be considered in future guideline development, particularly where these benefits outweigh potential adverse events.
Table 1.
Guidelines for BTA use.
| Recommendation | BTA | Dose, Administration, and Schedule | Notes | |
|---|---|---|---|---|
| Breast Cancer | ||||
| ASCO [51] | Patients with evidence of bone disease should receive BTAs | Denosumab | 120 mg SC every 4 weeks | |
| Zoledronate | 4 mg IV every 12 weeks or every 3–4 weeks | |||
| Pamidronate | 90 mg IV every 3–4 weeks | |||
| ESMO [68] | BTAs are recommended in patients with BMs, whether they are symptomatic or not | Denosumab | Every 4 weeks | Oral daily ibandronate or clodronate may be considered |
| Zoledronate | Every 4 weeks for 3–6 months, then every 12 weeks | |||
| NCCN [71] | BTAs are recommended in patients with BMs and life expectancy ≥ 3 months in addition to chemotherapy or endocrine therapy | Denosumab | ||
| Zoledronate | 4 mg IV every 12 weeks | |||
| Pamidronate | 90 mg IV | |||
| Prostate Cancer | ||||
| ASCO [74] | BTAs are recommended in patients with metastatic CRPC | Denosumab | In patients with symptomatic metastatic CRPC and bone pain, consider radium-223 | |
| Zoledronate | ||||
| AUA [72] | For patients with metastatic CRPC and BMs, clinicians may choose a BTA for preventing SREs | Denosumab (first option) | ||
| Zoledronate | ||||
| ESMO [68] | BTAs are recommended in patients with CRPC and BMs, whether they are symptomatic or not | Denosumab | Every 4 weeks | |
| Zoledronate | Every 4 weeks for 3–6 months, then every 12 weeks | |||
| NCCN [73] | BTAs are recommended in patients with metastatic CRPC and BMs | Denosumab (preferred) | SC every 4 weeks | Denosumab and zoledronate are not recommended in patients with creatinine clearance < 30 mL/min |
| Zoledronate | Every 12 weeks or every 3–4 weeks | |||
| Non–Small-Cell Lung Cancer | ||||
| NCCN [75] | BTAs can be considered in patients with BMs | Denosumab | ||
| Zoledronate | ||||
| Advanced Lung Cancer, Renal Cancer, and Other Solid Tumors | ||||
| ESMO [68] | BTAs are recommended in patients with clinically significant BMs and life expectancy ≥ 3 months | Denosumab | Every 4 weeks | |
| Zoledronate | ||||
| Multiple Myeloma | ||||
| ASCO [95] | BTAs are recommended in patients with MM with lytic lesions | Denosumab | Bisphosphonates are recommended in patients with MM with osteopenia but no evidence of lytic disease. Denosumab may be preferred in patients with impaired renal function | |
| Zoledronate | 4 mg IV every 3–4 weeks | |||
| Pamidronate | 90 mg IV every 3–4 weeks | |||
| ESMO [68] | BTAs should be initiated at MM diagnosis | Denosumab | Every 4 weeks | Denosumab is preferred in patients with renal impairment (creatinine clearance < 60 mL/min) |
| Zoledronate | Every 4 weeks for 3–6 months, then every 12 weeks | |||
| Pamidronate | ||||
| IMWG [96] | BTAs are recommended in patients with newly diagnosed or relapsed/refractory MM | Denosumab might be preferred in patients with renal impairment and can be considered in patients with creatinine clearance < 30 mL/min | ||
| If bone disease is present: | Denosumab or zoledronate (first options) Pamidronate (second option) |
|||
| If bone disease is absent: | Zoledronate (first option) Pamidronate (second option) |
|||
| NCCN [94] | BTAs are recommended in patients with symptomatic disease, regardless of documented BM | Denosumab | 120 mg SC every 4 weeks | Denosumab is preferred in patients with renal disease |
| Zoledronate | 4 mg IV every 3–4 weeks | |||
| Pamidronate | 90 mg IV every 3–4 weeks | |||
ASCO, American Society of Clinical Oncology; AUA, American Urological Association; BM, bone metastasis; BTA, bone-targeting agent; CRPC, castration-resistant prostate cancer; ESMO, European Society for Medical Oncology; IMWG, International Myeloma Working Group; IV, intravenous; MM, multiple myeloma; NCCN, National Comprehensive Cancer Network; SC, subcutaneous; SRE, skeletal-related event.
8. Real-World evidence
According to US administrative claims and health plan enrollment data, 43%–47% of patients with BMs secondary to solid tumors received a BTA in 2012; patients received denosumab (Medicare, 52%; commercial insurance, 47%) and zoledronate (Medicare, 49%; commercial insurance, 56%) at similar rates [76]. Among patients with breast, prostate, or lung tumor, BTA use was highest in breast cancer (58%–67%) and lowest in lung cancer (30%–33%). Overall, approximately 75% of patients who received a BTA initiated therapy within 3 months of BM diagnosis; zoledronate was initiated earlier than denosumab, with mean time from BM diagnosis to treatment of 47.9–52.1 days for zoledronate and 89.5–91.8 days for denosumab. However, persistence at 12 months was higher for denosumab (Medicare, 58%; commercial insurance, 57%) than for zoledronate (Medicare, 37%; commercial insurance, 36%). More recent evidence showed that 69% of patients with breast cancer received a BTA (denosumab, 42%; zoledronate, 48%), and 85% of patients with prostate cancer received a BTA (denosumab, 60%; zoledronate, 40%) [77], [78].
Real-world evidence from patients with breast, prostate, lung, or other solid tumors treated with denosumab in US indicated that patients initiating denosumab were more compliant with treatment (1-year compliance rate: denosumab, 50.4%; zoledronate, 40.7%) and less likely to switch to another BTA (denosumab, 6%; BP, 14%; P < 0.001) [79], [80]. Close renal monitoring is not required for denosumab versus zoledronate, which may improve compliance [80]. In Germany, evidence from patients with breast, prostate, or lung cancer and BM diagnosis indicated that persistence and compliance were higher and switch rates were lower at 12 months in patients treated with denosumab [81]. Persistence and compliance were greater with denosumab because of patient preference for Q4W SC dosing of denosumab versus 3–4 weeks of intravenous dosing of BPs, lower rates of renal toxicity and acute-phase reactions with denosumab, and longer time to increases in pain with denosumab versus zoledronate.
In the real-world setting, dose regimens outside the recommended denosumab 120 mg Q4W schedule are used for patient convenience and adherence and typically align with chemotherapy regimens. A retrospective study of denosumab based on dosing interval (< 5, 5–11, or ≥ 12 weeks) found that time to first SRE and median OS were not significantly different with extended dosing schedules [82]. However, another study found SRE incidence was significantly higher with “deviated” (31–56 days) dosing versus “standard” (27–30 days) dosing (61.3% versus 31.0%; P = 0.02) [83]. A recent survey of physicians in Canada revealed that denosumab was commonly de-escalated (ie, Q4W to Q12W) [84]. Although zoledronate Q12W has been investigated [85], no studies have compared denosumab Q12W with zoledronate Q12W.
Denosumab has been investigated in combination with novel anticancer agents. In a retrospective analysis of patients with metastatic melanoma and BMs, the combination of denosumab with immune checkpoint inhibitors was feasible, with no unexpected safety issues and promising efficacy [86]. In patients with prostate cancer receiving androgen deprivation therapy, denosumab prevented bone loss [87]. Because radium-223 had no effect on SRE outcomes [88], the combination of radium-223 and BTAs, including denosumab, in patients with metastatic castration-resistant prostate cancer and BMs has been investigated [89].
9. Denosumab in multiple myeloma
RANKL contributes to MM disease progression by modulating myeloma cell-osteoclast interactions [90]. Patients with MM have increased levels of serum RANKL, and elevated serum RANKL has been associated with increased bone resorption and higher risk for mortality [91]. Thus, RANKL may be a therapeutic target for patients with MM.
Denosumab was approved for SRE prevention in MM in 2018 based on phase 3 study results in newly diagnosed MM [92]. Adults with ≥ 1 lytic bone lesion received denosumab 120 mg SC Q4W or zoledronate 4 mg intravenous. Denosumab was noninferior to zoledronate in delaying median time to first on-study SRE (22.8 months for denosumab versus 24.0 months for zoledronate; HR, 0.98; 95% CI, 0.85–1.14; P = 0.010 for noninferiority). The percentage of patients who experienced an on-study SRE was similar between groups (denosumab, 44%; zoledronate, 45%). Furthermore, in a post hoc landmark analysis of the same study at 15 months, denosumab was superior to zoledronate in delaying time to first on-study SRE (median, not estimable for both; HR, 0.66; 95% CI, 0.44–0.98; P = 0.039). Median progression-free survival (PFS) was significantly increased by 10.7 months with denosumab (46.1 versus 35.4 months; HR, 0.82; 95% CI, 0.68–0.99; P = 0.036); however, median OS was similar between groups (49.5 months versus not estimable; HR, 0.90; 95% CI, 0.70–1.16; P = 0.41). In general, patients with MM have a high prevalence of both renal impairment (61%) and chronic kidney disease (50%) [93]; adverse events associated with renal toxicity (creatinine > 2 mg/dL and creatinine doubled from baseline) were lower with denosumab than with zoledronate (10% versus 17% overall) [92]. A phase 2 trial is underway investigating the safety and tolerability of denosumab in smoldering MM and assessing the efficacy in reducing the risk of disease progression (ClinicalTrials.gov: NCT03839459).
National Comprehensive Cancer Network and European Society for Medical Oncology guidelines recommend addition of denosumab or BPs to primary therapy for MM regardless of evidence of bone disease; denosumab is preferred in renal impairment (Table 1) [68], [94], [95]. BPs may be interrupted in patients who achieve complete or good partial response; however, denosumab discontinuation may be complicated by rebound osteolysis. Therefore, denosumab discontinuation following complete or good partial response is not currently recommended [68]. Updated guidelines from the International Myeloma Working Group (IMWG) recommend denosumab or zoledronate as first options in newly diagnosed MM or relapsed or refractory MM (with bone disease), with zoledronate as a first option in patients without MM-related bone disease; denosumab should be considered in renal impairment [96]. IMWG guidelines also note that denosumab might provide a PFS benefit in patients with newly diagnosed MM and MM-related bone disease who are eligible for autologous stem cell transplantation. These recommendations are supported by a recent subgroup analysis that showed a PFS benefit with denosumab versus zoledronate in patients with planned autologous stem cell transplant (HR, 0.65; 95% CI, 0.49–0.85; descriptive P = 0.002) and those < 70 years old (HR, 0.74; 95% CI, 0.59–0.94; descriptive P = 0.012) [97]. Further, a PFS benefit with denosumab was observed in patients with mild renal impairment or good baseline renal function (creatinine clearance > 60 mL/min) compared with zoledronate, suggesting a role for denosumab in MM regardless of renal function. The PFS benefit observed with denosumab in these subgroups may be due to mechanistic differences between denosumab and zoledronate, suggesting that inhibition of RANKL provides more complete osteoclast inhibition than does inhibition of protein prenylation [97]. Additionally, osteoclasts may reactivate dormant myeloma cells; thus, osteoclast inhibition could prevent MM progression [98]. As a consequence of the relatively recent approval of denosumab for MM, evidence in the real-world setting is limited.
10. Future considerations
10.1. Survival and skeletal-related event benefits associated with denosumab
Although we have learned a great deal about the clinical characteristics of denosumab since its approval, questions remain. Denosumab and BPs have been associated with a delay in SRE onset [42], [43], [44]; however, it is still unclear if this leads to a survival benefit. Some data suggest prolongation of OS in lung cancer [99]. Results were not confirmed in a phase 3 trial evaluating addition of denosumab to standard-of-care chemotherapy in advanced NSCLC in patients with and without BM; however, recruitment was ended prematurely because of slow accrual, as immunotherapy replaced standard-of-care chemotherapy, meaning the study lacked power to detect a significant difference in OS [100]. Although a PFS benefit has been observed with denosumab in MM [97], to date it is unknown if there is any OS benefit. As MM has a high rate of relapse, and survival rates become more dismal with each salvage therapy used [101], it is possible this obscured any potential OS benefit; data on disease progression in patients on salvage therapy who are receiving denosumab would help clarify any potential OS benefit of denosumab. Although rare, it is also unclear why AFF occurs with denosumab; further study is needed to better understand the role of concomitant medications and risk factors in the pathophysiology of AFF. Relative risk of MVF after denosumab discontinuation in patients with solid tumors or MM and BMs is also poorly understood, as is the role of preventive strategies for these severe toxicities.
10.2. Optimal dosing
Dose regimens other than the recommended 120 mg Q4W schedule may provide similar efficacy and safety. Patients with breast cancer and BMs who received denosumab Q4W (30, 120, or 180 mg) showed sustained uNTx/Cr suppression throughout 25 weeks of treatment, whereas some who received denosumab Q12W did not [35]; furthermore, it is unclear if the reduced suppression with 180 mg Q12W translated to reduced efficacy of denosumab. Additionally, a recent randomized trial of Q4W versus Q12W dosing in patients with BMs from breast or prostate cancer in Canada showed that the change in HRQoL score with denosumab Q12W was noninferior versus Q4W [102]. The 1-year SRE-free survival rate was similar for denosumab Q4W and Q12W; however, the study was not powered to detect differences in SREs between groups. A phase 3 noninferiority trial is underway investigating SRE prevention with denosumab 120 mg Q4W versus 120 mg Q4W for three doses de-escalated to Q12W in patients with advanced breast or prostate cancer and BMs (NCT02051218).
10.3. Antitumor effects
In addition to inhibition of RANKL, various mechanisms have been suggested for potential antitumor effects of denosumab, including promotion of tumor cell apoptosis, inhibition of angiogenesis, decrease of intravascular and extravascular migration and invasion, and alteration of bone microenvironment (eg, decreased tumor cell support from osteoclast-mediated bone resorption) [99]. Besides its role in bone remodeling, the RANKL/RANK pathway has an important role in mammary gland physiology and has been shown to enhance the proliferation of mammary epithelial and stem cells, therefore becoming a prominent target for breast tumorigenesis. RANKL/RANK may contribute to the development of a tolerogenic immune microenvironment by stimulating lymph node and T- and B-cell development [27], [103] and to tumor metastasis through osteoclast-independent processes [104]. Denosumab may play a potential role in BRCA1-positive breast cancer through aberrant RANK-signaling in BRCA1 tumorigenesis [105], although this is yet to be clinically evaluated. Disease-free survival was improved with denosumab 60 mg twice yearly in postmenopausal hormone receptor–positive early breast cancer when administered as an adjuvant treatment to aromatase inhibitor therapy, with distant metastases and new primary cancers reduced in the denosumab arm [106]. However, in D-CARE, an international phase 3, placebo-controlled study of adjuvant denosumab in early-stage breast cancer at high risk of disease recurrence, adjuvant denosumab did not improve disease outcomes (BM-free survival [BMFS]) [107]. Due to the limitations of assessing BMFS, which includes relapse in bone with and without extraskeletal recurrences and deaths from any cause, and the lower than expected number of efficacy endpoint events, analysis of the prespecified exploratory bone endpoints may provide a more clinically meaningful representation of the effect of denosumab in this disease setting.
11. Conclusions
SREs are associated with high patient burden, and treatment of BMs over the past 2 decades with antiresorptive therapy has resulted in a significant decrease in SREs. Denosumab is an effective option for patients at risk of SREs as a result of BMs from solid tumors or MM. Denosumab is well tolerated and may be used in impaired renal function; however, improvements in time to disease progression in bone and OS have not been demonstrated. Future trials and real-world evidence examining outcomes with extended-dosing schedules and factors affecting survival will continue to inform optimal management of these patients.
CRediT authorship contribution statement
Benoit Cadieux: Conceptualization, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. Robert Coleman: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. Pegah Jafarinasabian: Supervision, Writing – original draft, Writing – review & editing. Allan Lipton: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. Robert Z. Orlowski: Data curation, Writing – original draft, Writing – review & editing. Fred Saad: Conceptualization, Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing. Giorgio V. Scagliotti: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Kazuyuki Shimizu: Project administration, Supervision, Writing – original draft, Writing – review & editing. Alison Stopeck: Conceptualization, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Benoit Cadieux was an employee and shareholder of Amgen Inc. at the time of the development of this review.
Robert Coleman has received lecture fees from Amgen and Novartis; consultancy fees from Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, ITM, and Scancell.
Pegah Jafarinasabian is an employee and shareholder of Amgen Inc.
Allan Lipton has no financial interests or personal relationships that may be considered as potential competing interests.
Robert Z. Orlowski has no financial interests or personal relationships that may be considered as potential competing interests..
Fred Saad served as a consultant, advisory board member and received honoraria and research funding from Amgen; he has also served as a consultant, advisory board member and received honoraria and research funding from Astellas, AstraZeneca, Bayer, Janssen, Myovant, Novartis, Pfizer, and Sanofi.
Giorgio V. Scagliotti has no financial interests or personal relationships that may be considered as potential competing interests.
Kazuyuki Shimiz has no financial interests or personal relationships that may be considered as potential competing interests.
Alison Stopeck has received consulting fees from Amgen and AstraZeneca; contracted research support from Amgen, Exact Sciences; speakers bureau from Exact Sciences; and honoraria from Amgen.
Acknowledgments
Acknowledgments
Medical writing support was provided by Rick Davis, MS, and Allison R. Gillies, PhD (ICON, North Wales, PA), whose work was funded by Amgen Inc.
Funding
The sponsor was involved in writing the manuscript and submitting it for publication. This work was supported by Amgen Inc., Thousand Oaks, CA, USA.
Contributor Information
Benoit Cadieux, Email: ben_cadieux@hotmail.com.
Robert Coleman, Email: r.e.coleman@sheffield.ac.uk.
Pegah Jafarinasabian, Email: pjafarin@amgen.com.
Allan Lipton, Email: alipton@pennstatehealth.psu.edu.
Robert Z. Orlowski, Email: ROrlowski@mdanderson.org.
Fred Saad, Email: fredsaad@videotron.ca.
Giorgio V. Scagliotti, Email: giorgio.scagliotti@unito.it.
Kazuyuki Shimizu, Email: kazuyuks@sb.starcat.ne.jp.
Alison Stopeck, Email: Alison.Stopeck@stonybrookmedicine.edu.
References
- 1.von Moos R., Body J.-J., Egerdie B., Stopeck A., Brown J., Fallowfield L., Patrick D.L., Cleeland C., Damyanov D., Palazzo F.S., Marx G., Zhou Y., Braun A., Balakumaran A., Qian Y.i. Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases. Support. Care Cancer. 2016;24(3):1327–1337. doi: 10.1007/s00520-015-2908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006;12(20):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 3.Huang J.F., Shen J., Li X., Rengan R., Silvestris N., Wang M., Derosa L., Zheng X., Belli A., Zhang X.L., Li Y.M., Wu A. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study. Ann. Transl. Med. 2020;8(7):482. doi: 10.21037/atm.2020.03.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marino S., Roodman G.D. Multiple myeloma and bone: the fatal interaction. Cold Spring Harb. Perspect. Med. 2018;8(8):a031286. doi: 10.1101/cshperspect.a031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman R.E., Croucher P.I., Padhani A.R., Clezardin P., Chow E., Fallon M., Guise T., Colangeli S., Capanna R., Costa L. Bone metastases. Nat. Rev. Dis. Primers. 2020;6(1):83. doi: 10.1038/s41572-020-00216-3. [DOI] [PubMed] [Google Scholar]
- 6.Bhowmik D., Song X., Intorcia M., Gray S., Shi N. Examination of burden of skeletal-related events in patients naive to denosumab and intravenous bisphosphonate therapy in bone metastases from solid tumors population. Curr. Med. Res. Opin. 2019;35(3):513–523. doi: 10.1080/03007995.2018.1532884. [DOI] [PubMed] [Google Scholar]
- 7.Cetin K., Christiansen C.F., Jacobsen J.B., Norgaard M., Sorensen H.T. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: a Danish population-based cohort study. Lung Cancer. 2014;86(2):247–254. doi: 10.1016/j.lungcan.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Jensen A.O., Jacobsen J.B., Norgaard M., Yong M., Fryzek J.P., Sorensen H.T. Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in Denmark. BMC Cancer. 2011;11:29. doi: 10.1186/1471-2407-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parry M.G., Cowling T.E., Sujenthiran A., Nossiter J., Berry B., Cathcart P., Clarke N.W., Payne H., Aggarwal A., van der Meulen J. Identifying skeletal-related events for prostate cancer patients in routinely collected hospital data. Cancer Epidemiol. 2019;63 doi: 10.1016/j.canep.2019.101628. [DOI] [PubMed] [Google Scholar]
- 10.Bhowmik D., Hines D.M., Intorcia M., Wade R.L. Economic burden of skeletal-related events in patients with multiple myeloma: analysis of US commercial claims database. J. Med. Econ. 2018;21(6):622–628. doi: 10.1080/13696998.2018.1457531. [DOI] [PubMed] [Google Scholar]
- 11.Kim C., Bhatta S., Cyprien L., Fonseca R., Hernandez R.K. Incidence of skeletal-related events among multiple myeloma patients in the United States at oncology clinics: observations from real-world data. J. Bone Oncol. 2019;14 doi: 10.1016/j.jbo.2018.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 13.Demers L.M., Costa L., Lipton A. Biochemical markers and skeletal metastases. Clin. Orthop. Relat. Res. 2003;415:S138–S147. doi: 10.1097/01.blo.0000092979.12414.54. [DOI] [PubMed] [Google Scholar]
- 14.Feldenzer K.L., Sarno J. Hypercalcemia of malignancy. J. Adv. Pract. Oncol. 2018;9(5):496–504. [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman R.E., Major P., Lipton A., Brown J.E., Lee K.-A., Smith M., Saad F., Zheng M., Hei Y.J., Seaman J., Cook R. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J. Clin. Oncol. 2005;23(22):4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 16.Chow E., Hoskin P., van der Linden Y., Bottomley A., Velikova G. Quality of life and symptom end points in palliative bone metastases trials. Clin. Oncol. (R. Coll. Radiol.) 2006;18(1):67–69. doi: 10.1016/j.clon.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Qian Y.i., Song X., Zhang K., Balakumaran A., Arellano J. Short-term disability in solid tumor patients with bone metastases and skeletal-related events. J. Med. Econ. 2015;18(3):210–218. doi: 10.3111/13696998.2014.975232. [DOI] [PubMed] [Google Scholar]
- 18.Drake M.T., Clarke B.L., Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008;83(9):1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell R.G., Xia Z., Dunford J.E., Oppermann U., Kwaasi A., Hulley P.A., Kavanagh K.L., Triffitt J.T., Lundy M.W., Phipps R.J., Barnett B.L., Coxon F.P., Rogers M.J., Watts N.B., Ebetino F.H. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann. N. Y. Acad. Sci. 2007;1117:209–257. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 20.Zometa®, zoledronic acid, Novartis Pharmaceuticals Corporation, East Hanover, NJ, 2018.
- 21.Zhang X., Hamadeh I.S., Song S., Katz J., Moreb J.S., Langaee T.Y., Lesko L.J., Gong Y. Osteonecrosis of the jaw in the United States Food and Drug Administration's Adverse Event Reporting System (FAERS) J. Bone Miner. Res. 2016;31(2):336–340. doi: 10.1002/jbmr.2693. [DOI] [PubMed] [Google Scholar]
- 22.Aredia®, pamidronate disodium, Novartis Pharmaceuticals Corporation, East Hanover, NJ, 2012.
- 23.Mollica V., Rizzo A., Rosellini M., Marchetti A., Ricci A.D., Cimadamore A., Scarpelli M., Bonucci C., Andrini E., Errani C., Santoni M., Montironi R., Massari F. Bone targeting agents in patients with metastatic prostate cancer: state of the art. Cancers (Basel) 2021;13(3):546. doi: 10.3390/cancers13030546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman R. Bone targeted treatments in cancer - the story so far. J. Bone Oncol. 2016;5(3):90–92. doi: 10.1016/j.jbo.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirschberg R. Renal complications from bisphosphonate treatment. Curr. Opin. Support. Palliat. Care. 2012;6(3):342–347. doi: 10.1097/SPC.0b013e328356062e. [DOI] [PubMed] [Google Scholar]
- 26.Castellano D., Sepulveda J.M., García-Escobar I., Rodriguez-Antolín A., Sundlöv A., Cortes-Funes H. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist. 2011;16(2):136–145. doi: 10.1634/theoncologist.2010-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ming J., Cronin S.J.F., Penninger J.M. Targeting the RANKL/RANK/OPG axis for cancer therapy. Front. Oncol. 2020;10:1283. doi: 10.3389/fonc.2020.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koide M., Kobayashi Y., Yamashita T., Uehara S., Nakamura M., Hiraoka B.Y., Ozaki Y., Iimura T., Yasuda H., Takahashi N., Udagawa N. Bone formation is coupled to resorption via suppression of sclerostin expression by osteoclasts. J. Bone Miner. Res. 2017;32(10):2074–2086. doi: 10.1002/jbmr.3175. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J., Dai J., Qi Y., Lin D.-L., Smith P., Strayhorn C., Mizokami A., Fu Z., Westman J., Keller E.T. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J. Clin. Invest. 2001;107(10):1235–1244. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith B.B., Cosenza M.E., Mancini A., Dunstan C., Gregson R., Martin S.W., Smith S.Y., Davis H. A toxicity profile of osteoprotegerin in the cynomolgus monkey. Int. J. Toxicol. 2003;22(5):403–412. doi: 10.1177/109158180302200512. [DOI] [PubMed] [Google Scholar]
- 31.Bekker P.J., Holloway D.L., Rasmussen A.S., Murphy R., Martin S.W., Leese P.T., Holmes G.B., Dunstan C.R., DePaoli A.M. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J. Bone Miner. Res. 2004;19(7):1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 32.Lipton A., Steger G.G., Figueroa J., Alvarado C., Solal-Celigny P., Body J.-J., de Boer R., Berardi R., Gascon P., Tonkin K.S., Coleman R., Paterson A.H.G., Peterson M.C., Fan M., Kinsey A., Jun S. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J. Clin. Oncol. 2007;25(28):4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 33.Gibiansky L., Sutjandra L., Doshi S., Zheng J., Sohn W., Peterson M.C., Jang G.R., Chow A.T., Pérez-Ruixo J.J. Population pharmacokinetic analysis of denosumab in patients with bone metastases from solid tumours. Clin. Pharmacokinet. 2012;51(4):247–260. doi: 10.2165/11598090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Block G.A., Bone H.G., Fang L., Lee E., Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J. Bone Miner. Res. 2012;27(7):1471–1479. doi: 10.1002/jbmr.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipton A., Steger G.G., Figueroa J., Alvarado C., Solal-Celigny P., Body J.J., de Boer R., Berardi R., Gascon P., Tonkin K.S., Coleman R.E., Paterson A.H.G., Gao G.M., Kinsey A.C., Peterson M.C., Jun S. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin. Cancer Res. 2008;14(20):6690–6696. doi: 10.1158/1078-0432.CCR-07-5234. [DOI] [PubMed] [Google Scholar]
- 36.Sohn W., Simiens M.A., Jaeger K., Hutton S., Jang G. The pharmacokinetics and pharmacodynamics of denosumab in patients with advanced solid tumours and bone metastases: a systematic review. Br. J. Clin. Pharmacol. 2014;78(3):477–487. doi: 10.1111/bcp.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipton A., Fizazi K., Stopeck A.T., Henry D.H., Brown J.E., Yardley D.A., Richardson G.E., Siena S., Maroto P., Clemens M., Bilynskyy B., Charu V., Beuzeboc P., Rader M., Viniegra M., Saad F., Ke C., Braun A., Jun S. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur. J. Cancer. 2012;48(16):3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Lipton A., Fizazi K., Stopeck A.T., Henry D.H., Smith M.R., Shore N., Martin M., Vadhan-Raj S., Brown J.E., Richardson G.E., Saad F., Yardley D.A., Zhou K., Balakumaran A., Braun A. Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. Eur. J. Cancer. 2016;53:75–83. doi: 10.1016/j.ejca.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., de Boer R.H., Lichinitser M., Fujiwara Y., Yardley D.A., Viniegra M., Fan M., Jiang Q., Dansey R., Jun S., Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 2010;28(35):5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 40.Fizazi K., Carducci M., Smith M., Damiao R., Brown J., Karsh L., Milecki P., Shore N., Rader M., Wang H., Jiang Q., Tadros S., Dansey R., Goessl C. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., Scagliotti G.V., Sleeboom H., Spencer A., Vadhan-Raj S., von Moos R., Willenbacher W., Woll P.J., Wang J., Jiang Q.i., Jun S., Dansey R., Yeh H. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011;29(9):1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 42.Chen J., Zhou L., Liu X., Wen X., Li H., Li W. Meta-analysis of clinical trials to assess denosumab over zoledronic acid in bone metastasis. Int. J. Clin. Pharm. 2021;43(1):2–10. doi: 10.1007/s11096-020-01105-1. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z., Pu F., Shao Z. The skeletal-related events of denosumab versus zoledronic acid in patients with bone metastases: a meta-analysis of randomized controlled trials. J. Bone Oncol. 2017;9:21–24. doi: 10.1016/j.jbo.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X., Yang K.H., Wanyan P., Tian J.H. Comparison of the efficacy and safety of denosumab versus bisphosphonates in breast cancer and bone metastases treatment: a meta-analysis of randomized controlled trials. Oncol. Lett. 2014;7(6):1997–2002. doi: 10.3892/ol.2014.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saad F., Lipton A., Cook R., Chen Y.-M., Smith M., Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110(8):1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 46.N. Sathiakumar, E. Delzell, M.A. Morrisey, C. Falkson, M. Yong, V. Chia, J. Blackburn, T. Arora, I. Brill, M.L. Kilgore, Mortality following bone metastasis and skeletal-related events among women with breast cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999-2006, Breast Cancer Res. Treat. 131(1) (2012) 231-238. [DOI] [PubMed]
- 47.Sathiakumar N., Delzell E., Morrisey M.A., Falkson C., Yong M., Chia V., Blackburn J., Arora T., Kilgore M.L. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011;14(2):177–183. doi: 10.1038/pcan.2011.7. [DOI] [PubMed] [Google Scholar]
- 48.Coleman R.E., Lipton A., Costa L., Cook R.J., Lee K.-A., Saad F., Brown J.E., Terpos E., Major P.P., Kohno N., Smith M., Body J.-J. Possible survival benefits from zoledronic acid treatment in patients with bone metastases from solid tumours and poor prognostic features-an exploratory analysis of placebo-controlled trials. J. Bone Oncol. 2013;2(2):70–76. doi: 10.1016/j.jbo.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fizazi K., Massard C., Smith M., Rader M., Brown J., Milecki P., Shore N., Oudard S., Karsh L., Carducci M., Damião R., Wang H., Ying W., Goessl C. Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur. Urol. 2015;68(1):42–50. doi: 10.1016/j.eururo.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Lipton A., Smith M.R., Fizazi K., Stopeck A.T., Henry D., Brown J.E., Shore N.D., Saad F., Spencer A., Zhu L.i., Warner D.J. Changes in bone turnover marker levels and clinical outcomes in patients with advanced cancer and bone metastases treated with bone antiresorptive agents. Clin. Cancer Res. 2016;22(23):5713–5721. doi: 10.1158/1078-0432.CCR-15-3086. [DOI] [PubMed] [Google Scholar]
- 51.Van Poznak C., Somerfield M.R., Barlow W.E., Biermann J.S., Bosserman L.D., Clemons M.J., Dhesy-Thind S.K., Dillmon M.S., Eisen A., Frank E.S., Jagsi R., Jimenez R., Theriault R.L., Vandenberg T.A., Yee G.C., Moy B. Role of bone-modifying agents in metastatic breast cancer: an American Society of Clinical Oncology-Cancer Care Ontario focused guideline update. J. Clin. Oncol. 2017;35(35):3978–3986. doi: 10.1200/JCO.2017.75.4614. [DOI] [PubMed] [Google Scholar]
- 52.von Moos R., Body J.J., Egerdie B., Stopeck A., Brown J.E., Damyanov D., Fallowfield L.J., Marx G., Cleeland C.S., Patrick D.L., Palazzo F.G., Qian Y., Braun A., Chung K. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Support. Care Cancer. 2013;21(12):3497–3507. doi: 10.1007/s00520-013-1932-2. [DOI] [PubMed] [Google Scholar]
- 53.Cleeland C.S., Body J.J., Stopeck A., von Moos R., Fallowfield L., Mathias S.D., Patrick D.L., Clemons M., Tonkin K., Masuda N., Lipton A., de Boer R., Salvagni S., Oliveira C.T., Qian Y., Jiang Q., Dansey R., Braun A., Chung K. Pain outcomes in patients with advanced breast cancer and bone metastases: results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer. 2013;119(4):832–838. doi: 10.1002/cncr.27789. [DOI] [PubMed] [Google Scholar]
- 54.Martin M., Bell R., Bourgeois H., Brufsky A., Diel I., Eniu A., Fallowfield L., Fujiwara Y., Jassem J., Paterson A.H., Ritchie D., Steger G.G., Stopeck A., Vogel C., Fan M., Jiang Q., Chung K., Dansey R., Braun A. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin. Cancer Res. 2012;18(17):4841–4849. doi: 10.1158/1078-0432.CCR-11-3310. [DOI] [PubMed] [Google Scholar]
- 55.Henry D., Vadhan-Raj S., Hirsh V., von Moos R., Hungria V., Costa L., Woll P.J., Scagliotti G., Smith G., Feng A., Jun S., Dansey R., Yeh H. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support. Care Cancer. 2014;22(3):679–687. doi: 10.1007/s00520-013-2022-1. [DOI] [PubMed] [Google Scholar]
- 56.Arellano J., Hernandez R.K., Wade S.W., Chen K., Pirolli M., Quach D., Quigley J., Liede A., Shahinian V.B. Prevalence of renal impairment and use of nephrotoxic agents among patients with bone metastases from solid tumors in the United States. Cancer Med. 2015;4(5):713–720. doi: 10.1002/cam4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.AlDhalaan N.A., BaQais A., Al-Omar A. Medication-related osteonecrosis of the jaw: a review. Cureus. 2020;12(2) doi: 10.7759/cureus.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stopeck A.T., Fizazi K., Body J.-J., Brown J.E., Carducci M., Diel I., Fujiwara Y., Martín M., Paterson A., Tonkin K., Shore N., Sieber P., Kueppers F., Karsh L., Yardley D., Wang H., Maniar T., Arellano J., Braun A. Safety of long-term denosumab therapy: results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support. Care Cancer. 2016;24(1):447–455. doi: 10.1007/s00520-015-2904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saad F., Brown J.E., Van Poznak C., Ibrahim T., Stemmer S.M., Stopeck A.T., Diel I.J., Takahashi S., Shore N., Henry D.H., Barrios C.H., Facon T., Senecal F., Fizazi K., Zhou L., Daniels A., Carriere P., Dansey R. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann. Oncol. 2012;23(5):1341–1347. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 60.Ikesue H., Mouri M., Tomita H., Hirabatake M., Ikemura M., Muroi N., Yamamoto S., Takenobu T., Tomii K., Kawakita M., Katoh H., Ishikawa T., Yasui H., Hashida T. Associated characteristics and treatment outcomes of medication-related osteonecrosis of the jaw in patients receiving denosumab or zoledronic acid for bone metastases. Support Care Cancer. 2021;29(8):4763–4772. doi: 10.1007/s00520-021-06018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruggiero S.L., Dodson T.B., Fantasia J., Goodday R., Aghaloo T., Mehrotra B., O'Ryan F. American Association of Oral and Maxillofacial Surgeons, American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J. Oral Maxillofac. Surg. 2014;72(10):1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 62.Otto S., Pautke C., Van den Wyngaert T., Niepel D., Schiødt M. Medication-related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018;69:177–187. doi: 10.1016/j.ctrv.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 63.XGEVA®, denosumab, Amgen Inc., Thousand Oaks, CA, 2020.
- 64.Body J.-J., Bone H.G., de Boer R.H., Stopeck A., Van Poznak C., Damião R., Fizazi K., Henry D.H., Ibrahim T., Lipton A., Saad F., Shore N., Takano T., Shaywitz A.J., Wang H., Bracco O.L., Braun A., Kostenuik P.J. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur. J. Cancer. 2015;51(13):1812–1821. doi: 10.1016/j.ejca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 65.Block G., Egbuna O., Zeig S., Pergola P., Singh B., Braun A., Yu Y., Sohn W., Padhi D. Safety of Denosumab (Dmab) in Patients (Pts) with Stage 4 or Stage 5D Chronic Kidney Disease (Ckd) Ann. Oncol. 2014;25:iv528. doi: 10.1093/annonc/mdu356.33. [DOI] [Google Scholar]
- 66.L. Tripto-Shkolnik, N. Fund, V. Rouach, G. Chodick, V. Shalev, I. Goldshtein, Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider, Bone 130 (2020) 115150. [DOI] [PubMed]
- 67.Tyan A., Patel S.P., Block S., Hughes T., McCowen K.C. Rebound vertebral fractures in a patient with lung cancer after oncology-dose denosumab discontinuation: a cautionary tale. Mayo Clin. Proc. Innov. Qual. Outcomes. 2019;3(2):235–237. doi: 10.1016/j.mayocpiqo.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coleman R., Hadji P., Body J.J., Santini D., Chow E., Terpos E., Oudard S., Bruland Ø., Flamen P., Kurth A., Van Poznak C., Aapro M., Jordan K. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020;31(12):1650–1663. doi: 10.1016/j.annonc.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi M., Ozaki Y., Kizawa R., Masuda J., Sakamaki K., Kinowaki K., Umezu T., Kondoh C., Tanabe Y., Tamura N., Miura Y., Shigekawa T., Kawabata H., Baba N., Iguchi H., Takano T. Atypical femoral fracture in patients with bone metastasis receiving denosumab therapy: a retrospective study and systematic review. BMC Cancer. 2019;19(1):980. doi: 10.1186/s12885-019-6236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang S.P., Kim T.W., Boland P.J., Farooki A. Retrospective review of atypical femoral fracture in metastatic bone disease patients receiving denosumab therapy. Oncologist. 2017;22(4):438–444. doi: 10.1634/theoncologist.2016-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.National Comprehensive Cancer Network, NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer version 6.2020, 2020. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. (Accessed October 18 2020).
- 72.American Urological Association, Castration-Resistant Prostate Cancer: AUA Guideline, 2018. https://www.auanet.org/guidelines/prostate-cancer-castration-resistant-guideline. (Accessed June 14 2021).
- 73.National Comprehensive Cancer Network, NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Prostate Cancer version 2.2020, 2020. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. (Accessed October 18 2020).
- 74.Saylor P.J., Rumble R.B., Tagawa S., Eastham J.A., Finelli A., Reddy P.S., Kungel T.M., Nissenberg M.G., Michalski J.M. Bone health and bone-targeted therapies for prostate cancer: ASCO endorsement of a cancer care Ontario guideline. J. Clin. Oncol. 2020;38(15):1736–1743. doi: 10.1200/JCO.19.03148. [DOI] [PubMed] [Google Scholar]
- 75.National Comprehensive Cancer Network, NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Non-Small Cell Lung Cancer version 8.2020, 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. (Accessed October 18 2020).
- 76.Hernandez R.K., Adhia A., Wade S.W., O'Connor E., Arellano J., Francis K., Alvrtsyan H., Million R.P., Liede A. Prevalence of bone metastases and bone-targeting agent use among solid tumor patients in the United States. Clin. Epidemiol. 2015;7:335–345. doi: 10.2147/CLEP.S85496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Butler A.M., Cetin K., Hernandez R.K., Diane Reams B., Overman R.A., Kim J.I., Hirsch B.R., Abernethy A.P., Liede A., Alan Brookhart M. Treatment dynamics of bone-targeting agents among men with bone metastases from prostate cancer in the United States. Pharmacoepidemiol. Drug Saf. 2018;27(2):229–238. doi: 10.1002/pds.4360. [DOI] [PubMed] [Google Scholar]
- 78.Henry D., von Moos R., Body J.-J., Rider A., De Courcy J., Bhowmik D., Gatta F., Hechmati G., Qian Y.i. Bone-targeted agent treatment patterns and the impact of bone metastases on patients with advanced breast cancer in the United States. Curr. Med. Res. Opin. 2019;35(3):375–381. doi: 10.1080/03007995.2018.1558849. [DOI] [PubMed] [Google Scholar]
- 79.Hernandez R.K., Quigley J., Pirolli M., Quach D., Chen K.S., Arellano J., Liede A. Patients with bone metastases from solid tumors initiating treatment with a bone-targeted agent in 2011: a descriptive analysis using oncology clinic data in the US. Support. Care Cancer. 2014;22(10):2697–2705. doi: 10.1007/s00520-014-2251-y. [DOI] [PubMed] [Google Scholar]
- 80.Qian Y.i., Bhowmik D., Kachru N., Hernandez R.K. Longitudinal patterns of bone-targeted agent use among patients with solid tumors and bone metastases in the United States. Support. Care Cancer. 2017;25(6):1845–1851. doi: 10.1007/s00520-017-3583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diel I., Ansorge S., Hohmann D., Giannopoulou C., Niepel D., Intorcia M. Real-world use of denosumab and bisphosphonates in patients with solid tumours and bone metastases in Germany. Support. Care Cancer. 2020;28(11):5223–5233. doi: 10.1007/s00520-020-05357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.A.I. Abousaud, M.S. Barbee, C.C. Davis, S.E. Caulfield, Z. Wang, A. Boykin, B.C. Carthon, K. Gogineni, Safety and efficacy of extended dosing intervals of denosumab in patients with solid cancers and bone metastases: a retrospective study, Ther. Adv. Med. Oncol. 12 (2020) 1758835920982859. [DOI] [PMC free article] [PubMed]
- 83.Kettle J.K., Patel P.B. Feasibility of extended dosing intervals of denosumab. J. Oncol. Pharm. Pract. 2018;24(5):343–347. doi: 10.1177/1078155217703791. [DOI] [PubMed] [Google Scholar]
- 84.AlZahrani M., Clemons M., Vandermeer L., Sienkiewicz M., Awan A.A., Hutton B., Pond G.R., Ng T.L. Real-world practice patterns and attitudes towards de-escalation of bone-modifying agents in patients with bone metastases from breast and prostate cancer: a physician survey. J. Bone Oncol. 2021;26:100339. doi: 10.1016/j.jbo.2020.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Himelstein A.L., Foster J.C., Khatcheressian J.L., Roberts J.D., Seisler D.K., Novotny P.J., Qin R., Go R.S., Grubbs S.S., O'Connor T., Velasco M.R., Jr., Weckstein D., O'Mara A., Loprinzi C.L., Shapiro C.L. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48–58. doi: 10.1001/jama.2016.19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Angela Y., Haferkamp S., Weishaupt C., Ugurel S., Becker J.C., Oberndörfer F., Alar V., Satzger I., Gutzmer R. Combination of denosumab and immune checkpoint inhibition: experience in 29 patients with metastatic melanoma and bone metastases. Cancer Immunol. Immunother. 2019;68(7):1187–1194. doi: 10.1007/s00262-019-02353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoshida T., Kinoshita H., Taniguchi H., Yanishi M., Sugi M., Matsuda T. A randomized, open-label, controlled trial of monthly oral minodronate or semiannual subcutaneous injection of denosumab for bone loss by androgen deprivation in Asian men with prostate cancer: the PRevention of Osteopenia with Minodronate And DEnosumab (PROMADE) study. Osteoporos. Int. 2020;31(7):1251–1259. doi: 10.1007/s00198-019-05271-5. [DOI] [PubMed] [Google Scholar]
- 88.Smith M., Parker C., Saad F., Miller K., Tombal B., Ng Q.S., Boegemann M., Matveev V., Piulats J.M., Zucca L.E., Karyakin O., Kimura G., Matsubara N., Nahas W.C., Nolè F., Rosenbaum E., Heidenreich A., Kakehi Y., Zhang A., Krissel H., Teufel M., Shen J., Wagner V., Higano C. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408–419. doi: 10.1016/S1470-2045(18)30860-X. [DOI] [PubMed] [Google Scholar]
- 89.F. Saad, J. Carles, S. Gillessen, A. Heidenreich, D. Heinrich, J. Gratt, J. Levy, K. Miller, S. Nilsson, O. Petrenciuc, M. Tucci, M. Wirth, J. Federhofer, J.M. O'Sullivan, I. Radium-223 International Early Access Program, Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial, Lancet Oncol. 17(9) (2016) 1306-1316. [DOI] [PubMed]
- 90.Raje N.S., Bhatta S., Terpos E. Role of the RANK/RANKL pathway in multiple myeloma. Clin. Cancer Res. 2019;25(1):12–20. doi: 10.1158/1078-0432.CCR-18-1537. [DOI] [PubMed] [Google Scholar]
- 91.Jakob C., Goerke A., Terpos E., Sterz J., Heider U., Kühnhardt D., Ziefle S., Kleeberg L., Mieth M., von Metzler I., Müller C., Sezer O. Serum levels of total-RANKL in multiple myeloma. Clin. Lymphoma Myeloma. 2009;9(6):430–435. doi: 10.3816/CLM.2009.n.085. [DOI] [PubMed] [Google Scholar]
- 92.Raje N., Terpos E., Willenbacher W., Shimizu K., Garcia-Sanz R., Durie B., Legiec W., Krejci M., Laribi K., Zhu L., Cheng P., Warner D., Roodman G.D. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19(3):370–381. doi: 10.1016/S1470-2045(18)30072-X. [DOI] [PubMed] [Google Scholar]
- 93.Qian Y.i., Bhowmik D., Bond C., Wang S., Colman S., Hernandez R.K., Cheng P., Intorcia M. Renal impairment and use of nephrotoxic agents in patients with multiple myeloma in the clinical practice setting in the United States. Cancer Med. 2017;6(7):1523–1530. doi: 10.1002/cam4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.National Comprehensive Cancer Network, NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Multiple Myeloma version 2.2021, 2020. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. (Accessed October 18 2020). [DOI] [PubMed]
- 95.Anderson K., Ismaila N., Flynn P.J., Halabi S., Jagannath S., Ogaily M.S., Omel J., Raje N., Roodman G.D., Yee G.C., Kyle R.A. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2018;36(8):812–818. doi: 10.1200/JCO.2017.76.6402. [DOI] [PubMed] [Google Scholar]
- 96.Terpos E., Zamagni E., Lentzsch S., Drake M.T., García-Sanz R., Abildgaard N., Ntanasis-Stathopoulos I., Schjesvold F., de la Rubia J., Kyriakou C., Hillengass J., Zweegman S., Cavo M., Moreau P., San-Miguel J., Dimopoulos M.A., Munshi N., Durie B.G.M., Raje N. Bone Working Group of the International Myeloma Working Group, Treatment of multiple myeloma-related bone disease: recommendations from the Bone Working Group of the International Myeloma Working Group. Lancet Oncol. 2021;22(3):e119–e130. doi: 10.1016/S1470-2045(20)30559-3. [DOI] [PubMed] [Google Scholar]
- 97.Terpos E., Raje N., Croucher P., Garcia-Sanz R., Leleu X., Pasteiner W., Wang Y., Glennane A., Canon J., Pawlyn C. Denosumab compared with zoledronic acid on PFS in multiple myeloma: exploratory results of an international phase 3 study. Blood Adv. 2021;5(3):725–736. doi: 10.1182/bloodadvances.2020002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lawson M.A., McDonald M.M., Kovacic N., Hua Khoo W., Terry R.L., Down J., Kaplan W., Paton-Hough J., Fellows C., Pettitt J.A., Neil Dear T., Van Valckenborgh E., Baldock P.A., Rogers M.J., Eaton C.L., Vanderkerken K., Pettit A.R., Quinn J.M., Zannettino A.C., Phan T.G., Croucher P.I. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 2015;6:8983. doi: 10.1038/ncomms9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Castro J., Garcia R., Garrido P., Isla D., Massuti B., Blanca B., Vazquez J. Therapeutic potential of denosumab in patients with lung cancer: beyond prevention of skeletal complications. Clin. Lung Cancer. 2015;16(6):431–446. doi: 10.1016/j.cllc.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 100.S. Peters, S. Danson, B. Hasan, U. Dafni, N. Reinmuth, M. Majem, K.G. Tournoy, M.T. Mark, M. Pless, M. Cobo, D. Rodriguez-Abreu, L. Falchero, T. Moran, A.L. Ortega Granados, I. Monnet, K. Mohorcic, B.M. Sureda, D. Betticher, I. Demedts, J.A. Macias, S. Cuffe, A. Luciani, J.G. Sanchez, A. Curioni-Fontecedro, O. Gautschi, G. Price, L. Coate, R. von Moos, C. Zielinski, M. Provencio, J. Menis, B. Ruepp, A. Pochesci, H. Roschitzki-Voser, B. Besse, M. Rabaglio, M.E.R. O'Brien, R.A. Stahel, A randomized open-label phase III trial evaluating the addition of denosumab to standard first-line treatment in advanced NSCLC: the European Thoracic Oncology Platform (ETOP) and European Organisation for Research and Treatment of Cancer (EORTC) SPLENDOUR trial, J. Thorac. Oncol. 15(10) (2020) 1647-1656. [DOI] [PubMed]
- 101.Kumar S.K., Therneau T.M., Gertz M.A., Lacy M.Q., Dispenzieri A., Rajkumar S.V., Fonseca R., Witzig T.E., Lust J.A., Larson D.R., Kyle R.A., Greipp P.R. Clinical course of patients with relapsed multiple myeloma. Mayo Clin. Proc. 2004;79(7):867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 102.Clemons M., Ong M., Stober C., Ernst S., Booth C., Canil C., Mates M., Robinson A., Blanchette P., Joy A.A., Hilton J., Aseyev O., Pond G., Jeong A., Hutton B., Mazzarello S., Vandermeer L., Kushnir I., Fergusson D. REaCT investigators, A randomised trial of 4- versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur. J. Cancer. 2021;142:132–140. doi: 10.1016/j.ejca.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simatou A., Sarantis P., Koustas E., Papavassiliou A.G., Karamouzis M.V. The role of the RANKL/RANK axis in the prevention and treatment of breast cancer with immune checkpoint inhibitors and anti-RANKL. Int. J. Mol. Sci. 2020;21(20):7570. doi: 10.3390/ijms21207570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Okamoto K. Role of RANKL in cancer development and metastasis. J. Bone Miner. Metab. 2021;39(1):71–81. doi: 10.1007/s00774-020-01182-2. [DOI] [PubMed] [Google Scholar]
- 105.Kotsopoulos J., Singer C., Narod S.A. Can we prevent BRCA1-associated breast cancer by RANKL inhibition? Breast Cancer Res. Treat. 2017;161(1):11–16. doi: 10.1007/s10549-016-4029-z. [DOI] [PubMed] [Google Scholar]
- 106.Gnant M., Pfeiler G., Steger G.G., Egle D., Greil R., Fitzal F., Wette V., Balic M., Haslbauer F., Melbinger-Zeinitzer E., Bjelic-Radisic V., Jakesz R., Marth C., Sevelda P., Mlineritsch B., Exner R., Fesl C., Frantal S., Singer C.F. Austrian Breast and Colorectal Cancer Study Group, Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):339–351. doi: 10.1016/S1470-2045(18)30862-3. [DOI] [PubMed] [Google Scholar]
- 107.Coleman R., Finkelstein D.M., Barrios C., Martin M., Iwata H., Hegg R., Glaspy J., Periañez A.M., Tonkin K., Deleu I., Sohn J., Crown J., Delaloge S., Dai T., Zhou Y., Jandial D., Chan A. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(1):60–72. doi: 10.1016/S1470-2045(19)30687-4. [DOI] [PubMed] [Google Scholar]