Abstract
Neurologic complications are common in patients with sickle cell anemia (SCA), but conventional tools such as MRI and transcranial Doppler ultrasonography (TCD) do not fully assess cerebrovascular pathology. Cerebral tissue oximetry measures mixed oxygen saturation in the frontal lobes (SCTO2) and provides early prognostic information about tissue at risk of ischemic injury. Untreated patients with SCA have significantly lower SCTO2 than healthy controls that declines with age. Hydroxyurea is effective in preventing many SCA-related complications, but the degree to which it preserves normal neurophysiology is unclear. We analyzed participants enrolled in the Therapeutic Response Evaluation and Adherence Trial (TREAT, NCT02286154), which enrolled participants initiating hydroxyurea using individualized dosing (new cohort) and those previously taking hydroxyurea (old cohort) and was designed to monitor the long-term benefits of hydroxyurea. Cerebral oximetry was performed at baseline and annually. For the new cohort (median starting age = 12 months, n = 55), mean baseline SCTO2 was normal before starting hydroxyurea (mean 65%, 95% CI 58–72%) and significantly increased after 2 years (mean 72%, 95% CI 65–79%, p < .001). The SCTO2 for patients receiving long-term hydroxyurea (median age = 9.6 years) was normal at study entry (mean 66%, 95% CI 58–74%) and remained stable across 2 years. Both cohorts had significantly higher SCTO2 than published data from predominantly untreated SCA patients. Cerebral oximetry is a non-invasive method to assess cerebrovascular pathology that complements conventional imaging. Our results indicate that hydroxyurea suggests protection against neurophysiologic changes seen in untreated SCA.
1 |. INTRODUCTION
Acute and chronic neurologic complications of sickle cell disease (SCD) are common and devastating, ranging from overt stroke to “silent” infarction in the cerebral white matter on T2-weighted MRI imaging.1 Conventional imaging modalities, such as transcranial Doppler ultrasonography (TCD) and brain MRI/MRA, have become common screening and diagnostic tools for patients with SCD; however, despite normal findings, cerebral tissue may still be compromised or especially vulnerable to the chronic anemia, hemoglobin desaturation, and cerebrovascular stenosis and shunting associated with SCD. Neurocognitive deficits are highly prevalent among both children and adults with SCD,2 and often occur even in patients with apparently “normal” TCD and MRI/MRA studies, indicating the need for additional, complementary, and novel diagnostic assessments.
Cerebral oximetry is a non-invasive method to measure the saturation of hemoglobin in cerebral tissue. Abnormal results (low hemoglobin saturation values) identify tissue at risk of ischemic injury, detecting vulnerability before ischemia has occurred.3 Prior studies have shown that many patients with SCD without evidence of overt neuroanatomical changes on conventional imaging studies have cerebral oximetry values significantly lower than both healthy controls and patients with non SCD-related anemia.3,4 In a study of 27 adult patients with all genotypes of SCD, the mean cerebral oxygen saturation was 48 versus 61% in healthy age and race-matched controls. This difference held when comparing patients with SCD to controls with anemia due to other causes.4 In the largest cerebral oximetry study of children with SCD (n = 149), patients with HbSS or HbSβ0-thalassemia (collectively referred to as sickle cell anemia or SCA) had significantly lower cerebral oximetry values (SCTO2) than patients with other, typically less severe SCD genotypes (HbSC, HbSβ+-thalassemia) and unaffected controls. Lower SCTO2 values were also associated with more severe anemia and older age.3
Cumulative damage to cerebral tissue is inevitable for most patients with SCA who do not receive disease-modifying therapy.5 Hydroxyurea is the most established and effective disease-modifying pharmacotherapy for SCA and is neuroprotective.6–11 In addition, hydroxyurea improves peripheral oxygen saturation,12 which correlates with cerebral oxygen saturation in patients with SCA.3 We hypothesized that hydroxyurea also increases cerebral oxygen saturation and protects the brain from ischemia. Over the past 5 years, our center has implemented early initiation of hydroxyurea for children with SCA starting within the first 1–2 years of life using an individualized, pharmacokinetics (PK)-based dosing strategy that has resulted in a robust clinical and laboratory response.13 Here, we describe the temporal evolution of cerebral oximetry values in this cohort of patients who started hydroxyurea using PK-based dosing with direct comparisons to both an older cohort treated with traditional hydroxyurea dosing and to published historical data of predominantly untreated patients with SCA.
2 |. METHODS
2.1 |. Study overview
The Therapeutic Response Evaluation and Adherence Trial (TREAT, NCT02286154) is a prospective study of children with SCA (age 6 months to 21 years), designed to validate a novel individualized PK-guided hydroxyurea dosing strategy and to longitudinally evaluate the effects of long-term hydroxyurea therapy with a focus on protection against end-organ damage.13 Note, TREAT included two separate cohorts: the “new cohort”, which includes children ages 6 months to 21 years old who started hydroxyurea using PK-guided dosing upon study entry, and the “old cohort”, which includes children and adolescents who initiated hydroxyurea prior to study entry using traditional, weight-based dosing. Participants enrolled in both TREAT cohorts had cerebral oximetry measurements recorded at the time of enrollment (baseline) and yearly thereafter. This analysis includes participants who had cerebral oximetry data for at least 2 years.
2.2 |. Cerebral oximetry
Cerebral oximetry is a measure of both the arterial (~30%) and venous (~70%) oxygen content (cerebral tissue hemoglobin saturation, SCTO2) within the gray-white matter junction of the bilateral frontal lobes; typically, this ranges from 60 to 85% in healthy individuals breathing room air.14,15 Cerebral oximetry measurements are made via near-infrared spectroscopy (NIRS), which is based on the Beer–Lambert law:
where X is the concentration of the light-absorbing particle, known as the chromophore, A is the light attenuation, L is the pathlength of the photon through tissue, and ε is the chromophore’s unique extinction coefficient.16 NIRS also assumes two premises: (1) near-infrared light penetrates all tissue within the skull, including bone, and (2) that oxygenated and deoxygenated hemoglobin absorb infrared light at different wavelengths.17,18 Optodes are placed closely together on each side of the patient’s head, which contain signal detectors that are at different pre-determined distances from a light source (Figure 1). Signals from the shallow detector are subtracted from the those of the deep detector, which removes extracerebral data and leaves a measurement of the hemoglobin concentration. Unlike pulse oximetry, which estimates the arterial hemoglobin oxygen saturation, NIRS reflects the entire hemoglobin concentration within the tissue bed; thus, the measurement can be affected by hemoglobin content, cerebral blood flow, cerebral metabolic rate, and blood oxygenation.17 However, adult, fetal, and sickle hemoglobin all absorb light at a similar wavelength19,20; thus, differing concentrations of each subtype across patients will not influence the SCTO2 measurement. We obtained bi-frontal cerebral oxygen saturation values using the Casmed FORE-SIGHT cerebral oximeter (Edwards Lifesciences, Inc., Irvine CA, USA) during routine well visits only, using the average of at least three measurements on each side. Only one cerebral oximetry value was obtained within 2 months of a transfusion; all other values were obtained at least 10 weeks after the most recent transfusion with the majority of patients (74/81, 91%) having had no transfusions within 6 months of their SCTO2 measurements, thereby limiting con-founding from transfused, non-sickled blood.
FIGURE 1.
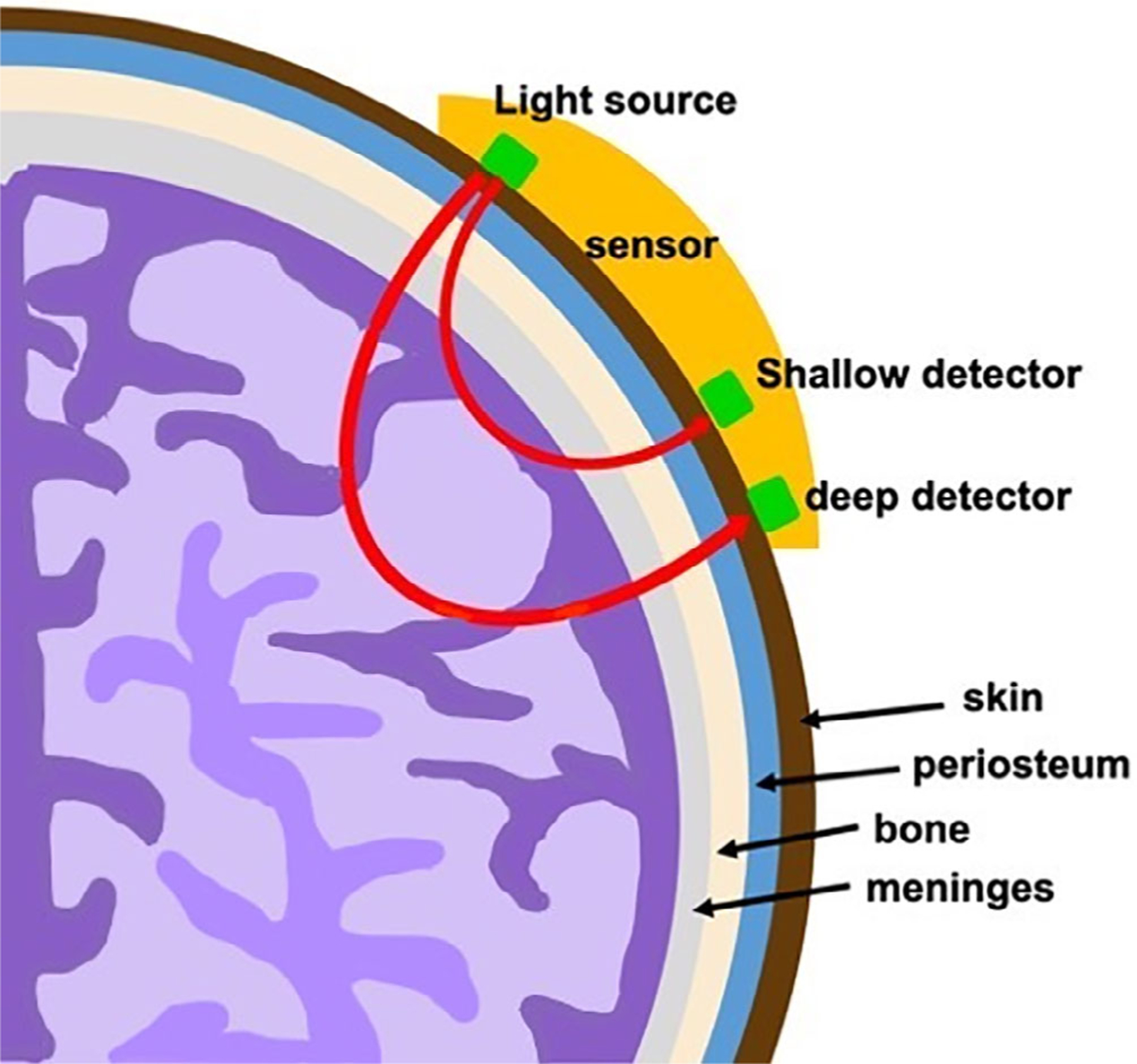
Cerebral oximeter. Cerebral oxygenation is measured at the gray-white matter junction of the frontal lobes. Optodes are placed on each side of the patient’s head with deep and shallow signal detectors that are at different pre-determined distances from a light source; the signal from the shallow detector is subtracted from that of the deep detector to obtain the cerebral oxygenation measurement of the hemoglobin content only
2.3 |. Statistical analysis
We first completed an intragroup analysis of the cerebral oximetry values within each cohort followed by an intergroup comparison between the two cohorts and historical data.3 For the intragroup comparison, cerebral oximetry values were averaged by year and a linear mixed-effects model was used to show a difference in yearly means within each group. Tukey’s method was subsequently used to make pairwise comparisons of each year’s group mean. Due to a further reduction in sample size, we did not restrict the analysis to only complete cases across the three time points. Hemoglobin and fetal hemoglobin (HbF) for each cohort were similarly analyzed. For the intergroup comparisons, the values from each TREAT cohort were compared to those of the historical cohort using Welch’s t-test.3 The Shapiro test showed normality of the data. Finally, we completed a multivariable linear regression with SCTO2 as the outcome variable and age, therapy with hydroxyurea, total hemoglobin, and fetal hemoglobin as the predictors. Note, R was used for statistical analysis,21 while figures were produced using the package ggplot2.22
3 |. RESULTS
3.1 |. Study participants
A total of 84 participants have been enrolled in the TREAT study, including 56 in the New Cohort, 27 in the Old Cohort, and one screening failure. One participant in the new cohort did not start hydroxyurea following PK studies and was excluded. The median age of hydroxyurea initiation in the new cohort was 11.6 months (IQR 9.4–40.2 months) with most participants initiating hydroxyurea before 1 or 2 years of age (53 and 69%, respectively). Six participants in the New Cohort have been withdrawn, most commonly due to moving from the area. To date, primarily driven by year of enrollment, 41/55 (75%) participants in the new cohort have completed at least 2 years of treatment with fewer completing three (28/55 = 51%), four (35%), or 5 years of therapy (11%). The old cohort was older, as expected, with a median enrollment age of 9.6 years (IQR 5.8–16.3 years). Seven participants in the old cohort have been withdrawn due to moving from the area, poor adherence to hydroxyurea, or transition to adult medical care.
3.2 |. New cohort analysis
Baseline cerebral oximetry values (before starting hydroxyurea) were available for 54/55 (98%) new cohort participants who initiated hydroxyurea therapy. The mean baseline cerebral oximetry value in the new cohort was 65% (95% CI 58–72%), which was within the normal range (60–85% on room air), with 80% (43/54) of the participants having normal values at study entry, consistent with their young age and relatively high hemoglobin and HbF values (Figure 2(A)). Although cerebral oximetry measurements are expected to decrease with age due to the natural course of SCA,3 the mean cerebral oximetry values significantly increased after 2 years of hydroxyurea therapy to 72% (95% CI 65–79%, p = .001, n = 33; Figure 2(A)). Further, the percentage of participants with normal values increased from 80 to 94% (31/33). When subdivided by age of starting hydroxyurea (age ≤2 years and age >2 years), those who began hydroxyurea by 2 years of age had higher baseline cerebral oximetry values than children who began after age 2 years (mean 66 ± 6.6% vs. 62 ± 8.4%, p = .089, Figure S1), although not statistically significant. After 1 year of hydroxyurea therapy, the mean cerebral oximetry values for children who began hydroxyurea before age 2 years were significantly higher than those who began after 2 years (mean 72 ± 7.2% for ≤2 years vs. 64 ± 7.8%, p = .030), but after 2 years of therapy, both groups had similar values as the children over 2 years old also had improved SCTO2 values (mean 73 ± 7.4% for ≤2 years vs. 70 ± 6.0%, p = .42).
FIGURE 2.
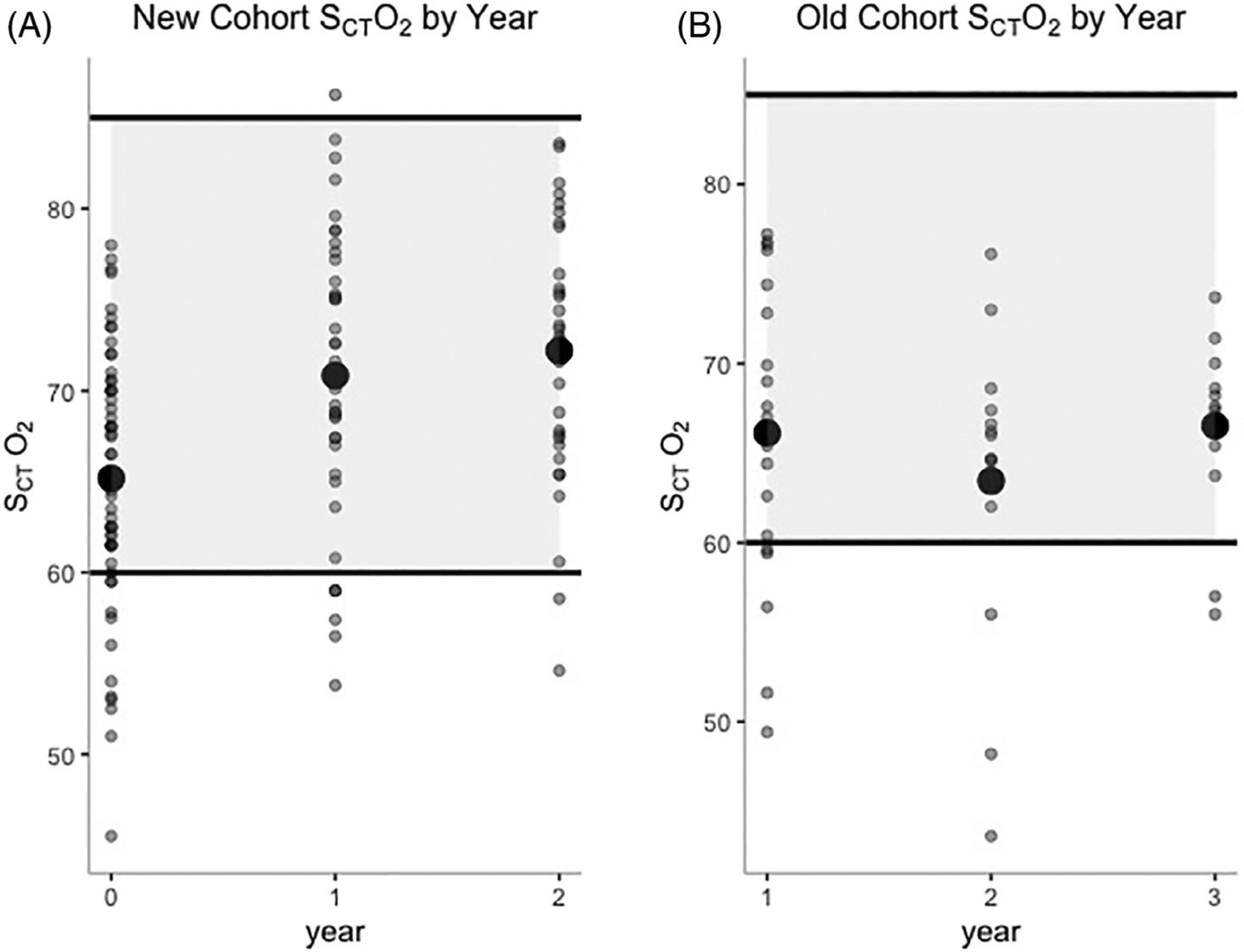
New and old cohort cerebral oximetry values. Cerebral oximetry values over each study year for the A, New and B, Old cohorts with the mean value represented by the large circle. Normal value range: 60–85%
Hydroxyurea therapy was associated with significant increases in both HbF and total hemoglobin, despite relatively high baseline values. Mean baseline %HbF was 24% (95% CI 12%–36%) and significantly increased by year 1 (mean = 33%, 95% CI 23–43%, p < .001). Percent HbF was moderately correlated with cerebral oximetry values (Pearson correlation coefficient, r = 0.52, 95% CI 0.38–0.64, p < .001). Although no HbF value appeared to result in a clear cut-off for consistently normal cerebral oximetry values, those with %HbF ≤20 had significantly lower SCTO2 values than those with %HbF >20 (mean SCTO2 for %HbF ≤20 = 63% vs. %HbF >20 = 71%, p < .001) (Figure 3 (A)). The baseline hemoglobin was 9.0 g/dL (95% CI 7.8–10 g/dL) and increased significantly by year 1 to 10 g/dL (95% CI 9.2–11 g/dL, p < .001). Hemoglobin similarly correlated with cerebral oximetry (Pearson correlation coefficient, r = 0.48, 95%CI 0.33–0.60, p < .001). Participants with hemoglobin ≤10 g/dL at any time had significantly lower SCTO2 than those with hemoglobin >10 g/dL (mean SCTO2 for Hb ≤10 = 66% vs. Hb >10 = 73%, p = <.001) (Figure 3(C)).
FIGURE 3.
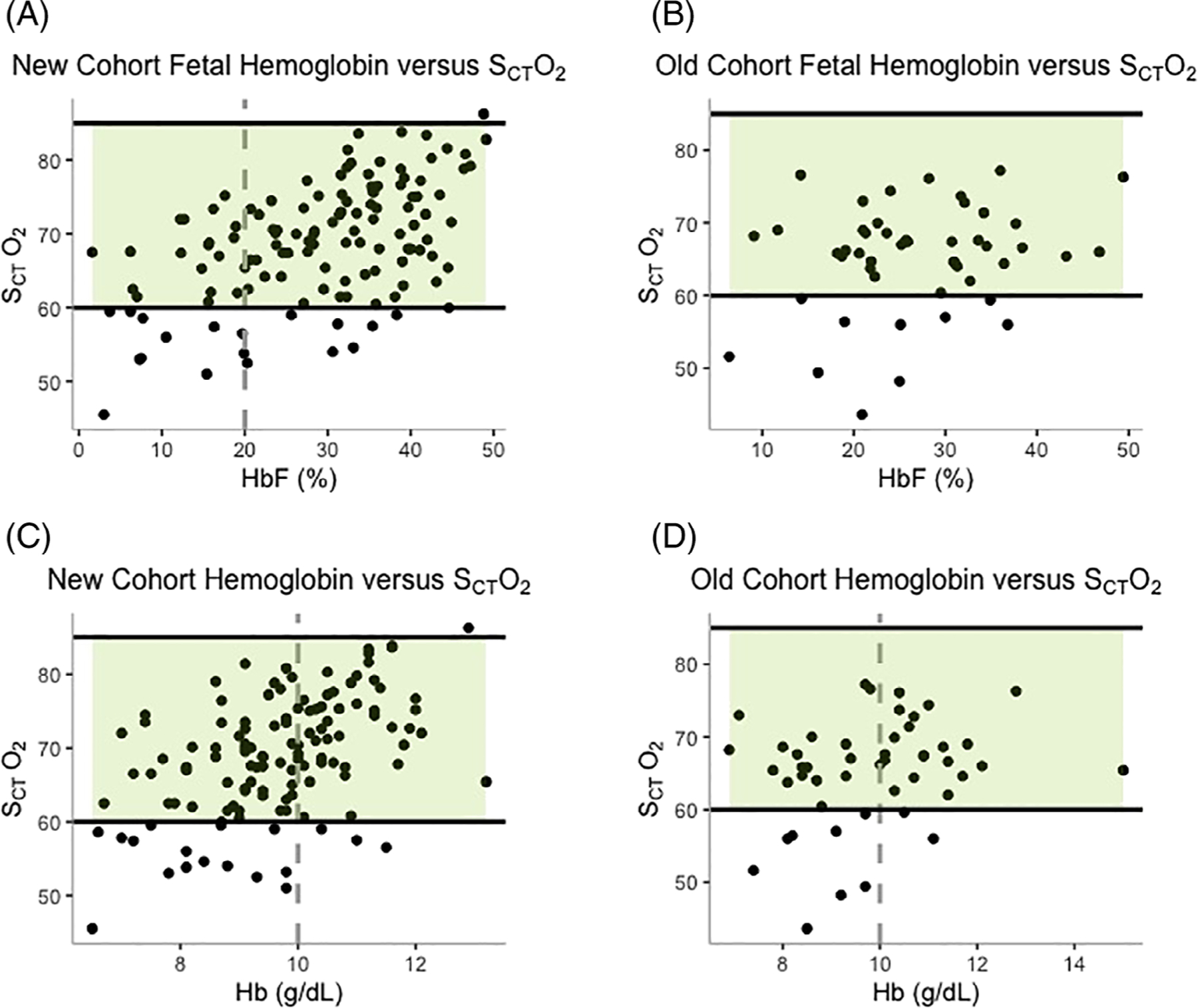
New and old cohort cerebral oximetry versus laboratory values. Averaged cerebral oximetry values versus hemoglobin F% for the (A) New cohort and (B) Old cohort and versus hemoglobin for the (C) New cohort and (D) Old cohort with the normal value range highlighted in green (60–85%). There was a significant difference between cerebral oximetry values for %HbF ≤20 versus %Hb >20 in the (A) New cohort and Hb ≤10 g/dL versus Hb >10 g/dL for both the (C) New and (D) Old cohorts
3.3 |. Old cohort analysis
As all participants enrolled in the old cohort had started hydroxyurea before study enrollment (median duration of therapy at time of enrollment = 5.2 years, range 1.6–13 years), we analyzed their cerebral oximetry values across 2 years to evaluate whether SCTO2 declined with age in patients with SCA, as reported by Quinn and Dowling.3 In contrast to the latter, in our study, the mean cerebral oximetry values for the old cohort patients already taking hydroxyurea were within normal limits: 66% (95% CI 58–74%) at the time of enrollment (Figure 2 (B)) with 76% of participants (16/21) demonstrating normal values. We did not observe an age-related decline in the old cohort’s SCTO2, as there was no significant change across the 2 years of follow-up (p = .14, Table 1). The mean %HbF for the old cohort was 25% (95% CI 14–36%) upon study entry and remained unchanged throughout 2 years of follow up (p = .5). Unlike in the new cohort, potentially due to a smaller sample size or from a lower influence of HbF on SCTO2, there was no significant difference between the SCTO2 for those in the old cohort with HbF ≤20% versus >20% (mean SCTO2 for %HbF ≤20 = 63% vs. %HbF >30 = 66%, p = .33, Figure 3(B)). The mean hemoglobin was 9.8 g/dL (95%CI 8.4–11 g/dL), which also remained stable throughout the 2 years of follow up (p = .88). Similar to the new cohort, a hemoglobin ≤10 g/dL was associated with significantly lower SCTO2 than hemoglobin >10 g/dL (mean SCTO2 for Hb ≤10 = 63% vs. Hb >10 = 68%, p = .025, Figure 3(D)).
TABLE 1.
New and old cohort cerebral oximetry and laboratory values across study years
| Cohort | Year | N | Mean (±SD) | p valuea | Mean hemoglobin (g/dL) (±SD) | p valuea | Mean %HbF (±SD) | p valuea |
|---|---|---|---|---|---|---|---|---|
| New cohort (N = 55) | Baseline | 54 | 65 (7.3) | 9.0 (1.2) | 24 (12) | |||
| 1 | 38 | 71 (7.9) | .001 | 10 (1.1) | <.001 | 33 (9.7) | <.001 | |
| 2 | 33 | 72 (7.2) | <.001 | 10 (1.4) | <.001 | 31 (10) | <.001 | |
| Old cohort (N = 26) | 1 | 21 | 66 (8.1) | .14 | 9.8 (1.3) | .88 | 25 (11) | .23 |
| 2 | 15 | 63 (8.5) | 9.9 (1.5) | 28 (7.5) | ||||
| 3 | 13 | 67 (5.1) | 9.8 (2) | 28 (8.7) |
Versus baseline.
3.4 |. Comparison to historical SCA cohort
Quinn and Dowling reported the results of a cross-sectional study of cerebral oximetry values in 112 patients with SCA (mean age = 8 years old) who were predominantly untreated with only 19% (21/112) of patients on hydroxyurea; no patients were on chronic transfusions. The methodology and equipment used to assess cerebral oximetry was the same for this historical cohort and our TREAT cohorts. The mean cerebral oxygen saturation was 51% (± 16%), which is well below the normal range of 60–85%. In addition, multivariable analysis showed age, hemoglobin, and peripheral oxygen saturation to be significant independent predictors of cerebral oxygen saturation.3 We compared these historical, cross-sectional data to our data from the TREAT new and old cohorts.
The cerebral oximetry values for the new cohort differed significantly from those of Quinn and Dowling’s cohort at baseline, even prior to beginning hydroxyurea (mean SCTO2 = 65% in new cohort vs. 51%, p < .001), which was expected given age differences between the two cohorts (mean = 3.6 years in TREAT new cohort vs mean = 7.6 years in Quinn & Dowling, p < .001). The TREAT old cohort had significantly higher cerebral oximetry values than the largely untreated Quinn and Dowling cohort (mean SCTO2 = 66% vs. 51%, p < .001), despite the older age of the TREAT old cohort (mean = 11 years in TREAT old cohort versus mean = 7.6 years in Quinn & Dowling’s cohort, = .014). The age-related declines in SCTO2 in this historical and largely untreated cohort were not seen in either TREAT cohort. Finally, in the multivariable linear regression, age was negatively associated with SCTO2 (p < .001), while total hemoglobin (p < .001) and therapy with hydroxyurea (p < .001) were positively associated (adjusted R2 for the model = 0.54). Note, HbF was not a significant predictor of SCTO2 in the multivariable linear regression model.
4 |. DISCUSSION
The increasing use of hydroxyurea beginning early in life is changing the natural history of SCA. Whereas chronic organ damage, including both overt and more subtle neurologic insults, has historically been unavoidable for untreated patients with SCA, the early initiation and prompt dose escalation of hydroxyurea therapy has begun to change this clinical inevitability by delaying, reducing, and possibly preventing such damage to allow for a longer and healthier life. TREAT has shown that early initiation of hydroxyurea using an individualized, PK-guided dosing strategy results in robust and sustained induction of HbF and a significant reduction in acute complications.13 This cohort provides a unique opportunity to study the long-term protective effects of early and aggressive hydroxyurea dosing against the development of chronic organ damage using both traditional and novel diagnostic methods such as cerebral oximetry. Contrary to the expected age-related decline in cerebral oxygen saturation for children with SCA,3 children who began hydroxyurea using individualized PK-guided dosing within the first 2 years of life had improvement in their cerebral oxygen saturation during the first years of the study, and older patients treated over longer periods of time (old cohort) with traditional, weight-based dosing had stable and normal cerebral oximetry values.
Although sickled hemoglobin (HbS) has normal oxygen affinity, red blood cells from patients with SCA have a lower measured oxygen affinity due to an increased concentration of 2,3-bisphosphoglycerate.23 Consequently, treatments that increase hemoglobin oxygen affinity may increase SCTO2 independently of any other treatments effects. In a given region of cerebral tissue, SCTO2 is determined by several factors: bulk blood flow to that tissue, the oxygen carrying capacity of the delivered blood (primarily a function of hemoglobin concentration and hemoglobin oxygen saturation), and the metabolic demand of that tissue. Hydroxyurea increases hemoglobin concentration, so it improves oxygen carrying capacity, but it also increases the concentration of HbF, which has a higher oxygen affinity than HbS. Improving oxygen carrying capacity by increasing only hemoglobin oxygen affinity through the increased HbF may not be physiologically beneficial, as the increased oxygen affinity of HbF may instead make oxygen less available to already compromised cerebral tissue. However, the magnitude of increase in SCTO2 that we observe is far greater than can be explained by an increase in hemoglobin oxygen affinity due to increased HbF concentration alone. For example, given a partial pressure of oxygen of 60–80 mmHg, the oxygen saturation of HbF is only 2–5 percentage points higher than HbA. In contrast, we observe an increase in SCTO2 in these hydroxyurea-treated patients that is 10–30 percentage points higher than untreated patients. Prior studies also found HbF was not associated with SCTO2 when controlling for covariates such as hemoglobin concentration.3 Therefore, the other determinants of SCTO2, especially increased hemoglobin concentration (allowing for increased oxygen delivery despite a modest HbF-related increase in oxygen affinity), improved blood flow (eg, via decreased shunting or prevention of stenotic vasculopathy), and decreased cerebral metabolic demand are possible explanations for the hydroxyurea-related effects on SCTO2 that we observed. The only explanation for which we have direct evidence in this study is increased hemoglobin (and prevention in decline in hemoglobin over time).
Our finding that hydroyxurea can improve and sustain normal cerebral oxygenation has important implications for understanding its potential neuroprotective effects. The majority of circulating oxygen within the body is bound to hemoglobin; indeed, a lower hemoglobin concentration is associated with both ischemic and hemorrhagic stroke risk in SCA.24–26 The cerebral metabolic rate of oxygen use is dependent on the cerebral arterial oxygen content, cerebral blood flow, and oxygen extraction fraction.27 Due to decreased cerebral oxygen content from chronic anemia in patients with SCA, cerebral blood flow and oxygen extraction increase as compensatory mechanisms. The areas of the brain with the lowest blood flow and highest oxygen extraction are the white matter border zone regions, which are the areas with the highest density of silent cerebral infarcts seen in patients with SCA.28,29 Chronic transfusion therapy lowers cerebral blood flow and oxygen extraction fraction in the watershed areas primarily by increasing total hemoglobin, likely mitigating stroke risk.6,7 Hydroxyurea may prove beneficial in a similar way. An increase in hemoglobin concentration improves cerebral arterial oxygen content, which is associated with decreased cerebral metabolic stress in patients receiving hydroxyurea compared to those without disease-modifying therapy.9 In addition, hydroxyurea has been associated with normalization of elevated cerebral blood flow velocities noted on TCD screening.10–12 Thus, the resultant increased cerebral arterial oxygen content and normalization of blood flow induced by hydroxyurea allow for better matching of oxygen supply and cerebral metabolic demand, which could also decrease risk of infarction.
We found that the cerebral oximetry values in the TREAT New Cohort were significantly higher than historical data,3 even before starting hydroxyurea. This was not too surprising, as these patients were significantly younger and had higher baseline %HbF, as would be expected due to timing of hydroxyurea initiation. However, this difference became more significant after 2 years of hydroxyurea therapy as hemoglobin and HbF continued to rise in response to therapy. Notably, the fact that 20% of new cohort patients had abnormal SCTO2 even at a very young age and SCTO2 improved after starting hyroxyurea, illustrates that compromise to neurologic tissue begins early in life, which supports the importance of early hydroxyurea initiation. In addition, we also found that the old cohort, most of whom had been receiving hydroxyurea for many years at the time of study enrollment, had significantly higher baseline SCTO2 values than the historical, largely untreated population, despite being older at the time of comparison. These findings strongly support the role of hydroxyurea in preventing the age-related decline in SCTO2 seen by Quinn & Dowling,3 and show that early, personalized dosing may have beneficial neuroprotective effects compared to traditional weight-based hydroxyurea dosing.
We also noted a positive correlation between hemoglobin, HbF, and cerebral oximetry values. Although we could not identify a threshold for either hemoglobin or %HbF values above which no further abnormal cerebral oxygen saturation was seen, in the new cohort, those with a %HbF > 20 had significantly higher SCTO2 than those with %HbF ≤ 20. This HbF cut off of 20% has been shown to be associated with a two to four times lower risk of hospitalization for acute sickle cell-related complications, including acute chest syndrome and vaso-occlusive episodes.30 A more recent analysis suggests that the percentage of HbF-containing red blood cells (F-cells) is a more precise biomarker to assess the protection against HbS polymerization rather than total HbF content. Modeling studies suggest that HbF values ≥30% or ≥70% F cells are required to most completely prevent HbS polymerization and subsequent erythrocyte sickling.31 Similarly, total hemoglobin concentration has also been shown to be associated with clinical outcomes, including risk of stroke and death in children with SCA.24,25 In addition, hemoglobin was most closely associated with the reduction in cerebral metabolic stress induced by hydroxyurea in a study evaluating cerebral oxygen extraction in areas of white matter at highest risk for ischemia.7 In our multivariable linear model, hemoglobin, but not HbF, was significantly predictive of SCTO2. Thus, hydroxyurea dosing strategies should aim to maximize both HbF and hemoglobin concurrently in order to optimize cerebral oxygen availability and extraction.
Our study has several limitations. First, we had a relatively small sample size and short follow-up period. It will be important to more definitively demonstrate that hydroxyurea results in sustained improvement in cerebral oxygen saturation over a much longer time which is ongoing within the TREAT cohort as these children continue to age. Another limitation was the difference in age between the new cohort and the historic, previously published cohort, as age likely plays a role in cerebral oximetry values, given that younger children with SCA have elevated %HbF and less stenotic vasculopathy and cerebrovascular steal/shunting than older children. In the absence of a comparison to neuropsychological testing or brain MRI/MRA results, we are unable to determine whether hydroxyurea improves or maintains cerebral functioning, as opposed to only cerebral oxygenation. Finally, we did not compare our cohorts to children on chronic transfusion therapy, which has been shown to also increase cerebral oxygenation and could have served as a control.3 However, a direct comparison in this case would have been difficult due to the known stenotic vasculopathy in most patients receiving chronic transfusion therapy.
In summary, patients with SCA who do not receive disease-modifying therapy are at risk for significant and likely irreversible neurologic compromise, including abnormal cerebral tissue oxygenation that declines with age. The early initiation of hydroxyurea improves cerebral oxygenation and may protect vulnerable cerebral tissue from an otherwise age-related decline in cerebral oxygenation. Further studies incorporating multiple neurologic and neurocognitive measures, including cerebral oximetry, will be essential to expand upon these results and determine how best to comprehensively evaluate and proactively prevent neurophysiologic damage for children, adolescents, and adults with SCA.
Supplementary Material
Funding information
National Institutes of Health, Grant/Award Number: 1K23HL128885
Footnotes
CONFLICT OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Armstrong F, Thompson R Jr, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics. 1996;97:864–870. [PubMed] [Google Scholar]
- 2.Prussien KV, Jordan LC, DeBaun MR, Compas BE. Cognitive function in sickle cell disease across domains, cerebral infarct status, and the lifespan: a meta-analysis. J Pediatr Psychol. 2019;44(8):948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn CT, Dowling M. Cerebral tissue hemoglobin saturation in children with sickle cell disease. Pediatr Blood Cancer. 2012;59(5):881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nahavandi M, Tavakkoli F, Hasan S, Wyche M, Castro O. Cerebral oximetry in patients with sickle cell disease. Eur J Clin Invest. 2004;34: 143–148. [DOI] [PubMed] [Google Scholar]
- 5.Pegelow CH, Macklin EA, Moser FG, et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood. 2002;99(8):3014–3018. [DOI] [PubMed] [Google Scholar]
- 6.Nottage KA, Ware RE, Aygun B, et al. Hydroxycarbamide treatment and brain MRI/MRA findings in children with sickle cell anaemia. Br J Haematol. 2016;175(2):331–338. [DOI] [PubMed] [Google Scholar]
- 7.Fields ME, Guilliams KP, Ragan D, et al. Hydroxyurea reduces cerebral metabolic stress in patients with sickle cell anemia. Blood. 2019;133 (22):2436–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hankins JS, McCarville MB, Rankine-Mullings A, et al. Prevention of conversion to abnormal transcranial Doppler with hydroxyurea in sickle cell anemia: a phase III international randomized clinical trial. Am J Hematol. 2015;90(12):1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagunju I, Brown BJ, Sodeinde O. Hydroxyurea lowers transcranial Doppler flow velocities in children with sickle cell anaemia in a Nigerian cohort. Pediatr Blood Cancer. 2015;62(9):1587–1591. [DOI] [PubMed] [Google Scholar]
- 10.Adegoke SA, Macedo-Campos RS, Braga JAP, Figueiredo MS, Silva GS. Changes in transcranial doppler flow velocities in children with sickle cell disease: the impact of hydroxyurea therapy. J Stroke Cerebrovasc Dis. 2018;27(2):425–431. [DOI] [PubMed] [Google Scholar]
- 11.Rushton T, Aban I, Young D, Howard T, Hilliard L, Lebensburger J. Hydroxycarbamide for patients with silent cerebral infarcts: outcomes and patient preference. Br J Haematol. 2018;181(1):145–148. [DOI] [PubMed] [Google Scholar]
- 12.Pashankar F, Manwani D, Lee M, Green N. Hydroxyurea improves oxygen saturation in children with sickle cell disease. J Pediatr Hematol Oncol. 2015;37(3):242–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGann P, Niss O, Dong M, et al. Robust clinical and laboratory response to hydroxyurea using pharmacokinetically guided dosing for young children with sickle cell anemia. Am J Hematol. 2019;94(8):871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda K, MacLeod D, Grocott H, Moretti E, Ames W, Vacchiano C. The accuracy of a near-infrared spectroscopy cerebral oximetry device and its potential value for estimating jugular venous oxygen saturation. Anesth Analg. 2014;119(6):1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott J, Hoffman G. Near-infrared spectroscopy: exposing the dark (venous) side of the circulation. Paediatr Anaesth. 2014;24(1):74–88. [DOI] [PubMed] [Google Scholar]
- 16.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103:i3–i13. [DOI] [PubMed] [Google Scholar]
- 17.Casati A, Spreafico E, Putzu M, Fanelli G. New technology for noninvasive brain monitoring: continuous cerebral oximetry. Minerva Anestesiol. 2006;72(7–8):605–625. [PubMed] [Google Scholar]
- 18.Victor S, Lemmers P, Weindling M. Near-infrared spectroscopy and its use for the assessment of tissue perfusion in the neonate. In: Seri I, Kluckow M, eds. Hemodynamics and Cardiology: Neonatology Questions and Controversies. 3rd ed. Philadelphia, PA: Elsevier; 2019. [Google Scholar]
- 19.Harris AP, Sendak MJ, Donham RT, Thomas M, Duncan D. Absorption characteristics of human fetal hemoglobin at wavelengths used in pulse oximetry. J Clin Monit. 1988;4:175–177. [DOI] [PubMed] [Google Scholar]
- 20.Nahavandi M, Nichols JP, Hassan M, Gandjbakhche A, Kato GH. Near-infrared spectra absorbance of blood from sickle cell patients and normal individuals. Hematology. 2009;14(1):46–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. http://www.R-project.org/. Accessed December 04, 2019. [Google Scholar]
- 22.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2009. [Google Scholar]
- 23.Charache S, Grisolia S, Fiedler AJ, Hellegers AE. Effect of 2,3-diphosphoglycerate on oxygen affinity of blood in sickle cell anemia. J Clin Invest. 1970. Apr;49(4):806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller ST, Sleeper LA, Pegelow CH, et al. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342(2): 83–89. [DOI] [PubMed] [Google Scholar]
- 25.Meier ER, Wright EC, Miller JL. Reticulocytosis and anemia are associated with an increased risk of death and stroke in the newborn cohort of the Cooperative Study of Sickle Cell Disease. Am J Hematol. 2014; 89(9):904–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91 (1):288–294. [PubMed] [Google Scholar]
- 27.Jordan LC, Gindville MC, Scott AO, et al. Non-invasive imaging of oxygen extraction fraction in adults with sickle cell anaemia. Brain. 2016;139(pt 3):738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fields ME, Guilliams KP, Ragan DK, et al. Regional oxygen extraction predicts border zone vulnerability to stroke in sickle cell disease. Neurology. 2018;90(13):e1134–e1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford AL, Ragan DK, Fellah S, et al. Silent infarcts in sickle cell disease occur in the border zone region and are associated with low cerebral blood flow. Blood. 2018;132(16):1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estepp JH, Smeltzer MP, Kang G, et al. A clinically meaningful fetal hemoglobin threshold for children with sickle cell anemia during hydroxyurea therapy. Am J Hematol. 2017;92(12):1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinberg MH, Chui DH, Dover GJ, Sebastiani P, Alsultan A. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood. 2014;123(4): 481–485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


