Abstract
Recent grief research suggests that the influential cognitive stress theory should be updated with evidence from cognitive neuroscience. Combining human and animal neuroscience with attachment theory, we propose that semantic knowledge of the everlasting nature of the attachment figure, and episodic, autobiographical memories of the death are in conflict, perhaps explaining the duration of grieving and generating predictions about complications in prolonged grief disorder (PGD). Our Gone-But-Also-Everlasting model emphasizes that grieving may be a form of learning, requiring time and experiential feedback. Difficulties pre-loss, such as spousal dependency or pre-existing hippocampal volume, can prolong learning and predict PGD. Complications such as avoidance, rumination, and stress-induced hippocampal atrophy may also develop post-loss, and create functional or structural mechanisms predicting PGD.
Since the 1980’s, dominant approaches to bereavement research have derived from cognitive stress theory [1,2] and attachment theory [3]. Cognitive stress theory suggests that bereavement is a stressful life event because the loss is appraised as exceeding one’s resources to cope with major readjustments [1]. This view of bereavement focuses on the addition of a stressful event to ongoing life goals and behaviors, rather than the subtraction of a functioning element of the self (the loved one, or the overlapping other) that has enabled successful goals and behaviors. However, evidence from both human and animal models of grief and loss suggests that cognitive stress theory may be an incomplete understanding of the basic neurobiology of the stress response during separation_[4,5]. Specifically, the encoding of attachment during bonding creates a stress response during separation that is more unconscious and automatic than suggested by cognitive stress theory.
In daily life, our need for attachment figures poses a problem, since social mammals are mobile, and we must find our loved ones again to fulfill this need. Attachment relies on the prediction of the persistence of the other, given that attachment is the tether enabling social mammals to leave and return to their mates and offspring multiple times a day. Locating those we are bonded to is therefore a critical function of the brain, as evidenced by a devoted category of partner-approach neurons, for example [6]. In prairie voles, the partner-approach neuronal ensemble in the nucleus accumbens increases in size following bond formation, and differences in the size of approach ensembles between partner and novel voles predict attachment bond strength.
Bonding is like a gun being cocked, and separation pulls the trigger. At the time of bond formation, neural encoding is created to trigger a physiological stress response upon separation. Epigenetic changes in the nucleus accumbens during mating in the prairie vole lead to increased oxytocin receptors, and this epigenetic change ensures the monogamous bond [7]. Once the voles are pair-bonded, they are primed to make the precursor of animal cortisol if their mate goes missing, so it can quickly be released when they lose track of each other, motivating the vole to seek out its partner to reduce the resulting stress [8,9]. Although the separation stress response fulfills an adaptive function when reunion is possible, in bereavement, the physiological stress continues without the input of the pair-bonded mate. This evidence is congruent with overlapping self-other representation in human neuroimaging [10] and the increased cortisol we see in acute grief [11]. We hypothesize that the encoding that occurs during bonding includes the prediction that this particular other will always be one’s partner, with the emphasis on “always”. Built into the encoded characteristics constitutive of the mate is their everlasting nature, and absence is just a temporary state, requiring proximity seeking. Rather than the cognitive stress appraisal, we interpret this evidence to suggest the automaticity may mean the stress of bereavement is primary and outside of awareness. Thus, rather than the appraisal, “I realize the other part of me is missing, and so I feel terrible”, more likely the order is “I feel terrible—oh, that must mean that the other part of me is missing”.
We hypothesize that the neural architecture of the bond supports the belief (or semantic knowledge) that the other persists, despite any sensory evidence to the contrary. This belief serves a great evolutionary need—those who persisted in their belief that the other existed remained in place to have a caregiver return with food, for example, or sought out their partner to maintain their sexual or co-parenting relationship and pass on their genes. The typical rewarding experience of daily reunion with a loved one after separation (liking) confirms the prediction made during their absence (wanting, yearning). Neurohormones instantiate the reaction to reunion, which elicits a reduction in cortisol, and release of oxytocin and dopamine. The reason that the emphasis on this semantic knowledge is important and novel, is because it determines how the brain makes predictions about where the attachment figure is, and when a reunion will happen. These predictions determine how the bereaved will function and behave in the world after the rare but permanent separation of death.
The human mind functions as a predicting, generative agent, which maintains relatively stable “models” or representations of both external and internal environments, constantly making predictions about the environment, and updating these models with new stimuli and life events via a learning mechanism [12]. At a neurobiological level, once a pair bond or caregiving bond has been formed, the neural circuitry is reset (e.g., through epigenetic changes) to include both members of the dyad as the functioning unit. Viewing separation and loss as the subtraction (and then absence) of a functioning aspect of the individual’s “self” is illuminated when considering subsequent prediction. How does awareness of loss change the expectations of where the missing other is, and whether future predictions should be changed based on the absence? When there can be no reward of reunion given the loved one’s death, prediction error is experienced until the updated semantic knowledge can be learned.
The Gone-But-Also-Everlasting theory of grieving
The brain’s model of the world must change in order for the person to fulfill their goals and achieve a state of safety and comfort [4,13]. Death of a loved one poses a unique and rare challenge, since the stream of information from semantic knowledge (persistence of the attachment figure’s existence) now conflicts with episodic knowledge of the death of the person (e.g., memory of the death event or funeral). We propose the Gone-But-Also-Everlasting theory to emphasize the dissonance between those two sources of information. This new theory emphasizes that grieving can be considered a form of learning, requiring time and feedback in the form of real-world experience. The first morning that a widowed husband wakes up alone after thousands of mornings of waking up next to his wife, the best prediction is not to assume that she has died. In fact, many experiences will be required to convince him that he will never again wake up next to her again, and enable him to predict that her absence means he must change his way of understanding himself, the world, and the future [14,15].
The importance of the conflict between these dual streams of information is that the conflict will interfere with learning (or updating the model of the attachment figure), and with making new predictions that reflect the reality of the permanent absence of the attachment figure (applying the model). Acutely, difficulty with learning the new reality will lead to a) emotional intensity and lability, when the reality is confronted over and over, and b) confusion, disbelief, magical thinking, and counterfactuals (if only, what if), as the brain attempts to accommodate the two sources of information. Further, the experience of bereavement involves multiple mechanisms that drive intrusive thoughts in grief: emotional salience, inability to complete the seeking-reunion process, environments that stimulate cue-elicited retrieval, and dissonance between the predicted/desired and actual experience [16].
Evidence for the new theory in neuroscience of grief
At different levels of complexity, learning to make new predictions means 1) new automatic habits must be learned [17], 2) the self must be updated to include the absence of the person, or a revision in predicting their presence/availability, and 3) other bonds must be developed/strengthened to meet ongoing attachment needs (revising the attachment hierarchy [4]). Note that each of these require new neural connections in the brain, a biomechanical process requiring time, feedback through experience, and a brain with structural integrity.
Incorporating new knowledge into predictions goes beyond habit. At one level, it is a long time before a daughter stops picking up the phone to relay a funny incident to her deceased mother. This behavior suggests an automaticity that must be relearned. In addition, however, changes in nucleus accumbens seen in animal and human fMRI research suggest that regions of the reward network (including caudate, orbitofrontal cortex, etc.) are important for predicting their presence and motivating us to seek/yearn for them [18–22].
Evidence for the new theory in neuroscience of prolonged grief disorder
By focusing on grieving as a form of learning, we can see how complications in adaptation may occur. Lab-based behavioral tasks can show us where functional problems in learning (e.g., using reward feedback, avoiding experience) may lead to prolonged grief disorder (PGD; [23]) and structural neuroimaging can help us see where problems in structural integrity of the brain can lead to PGD (Figure 1).
Figure 1.
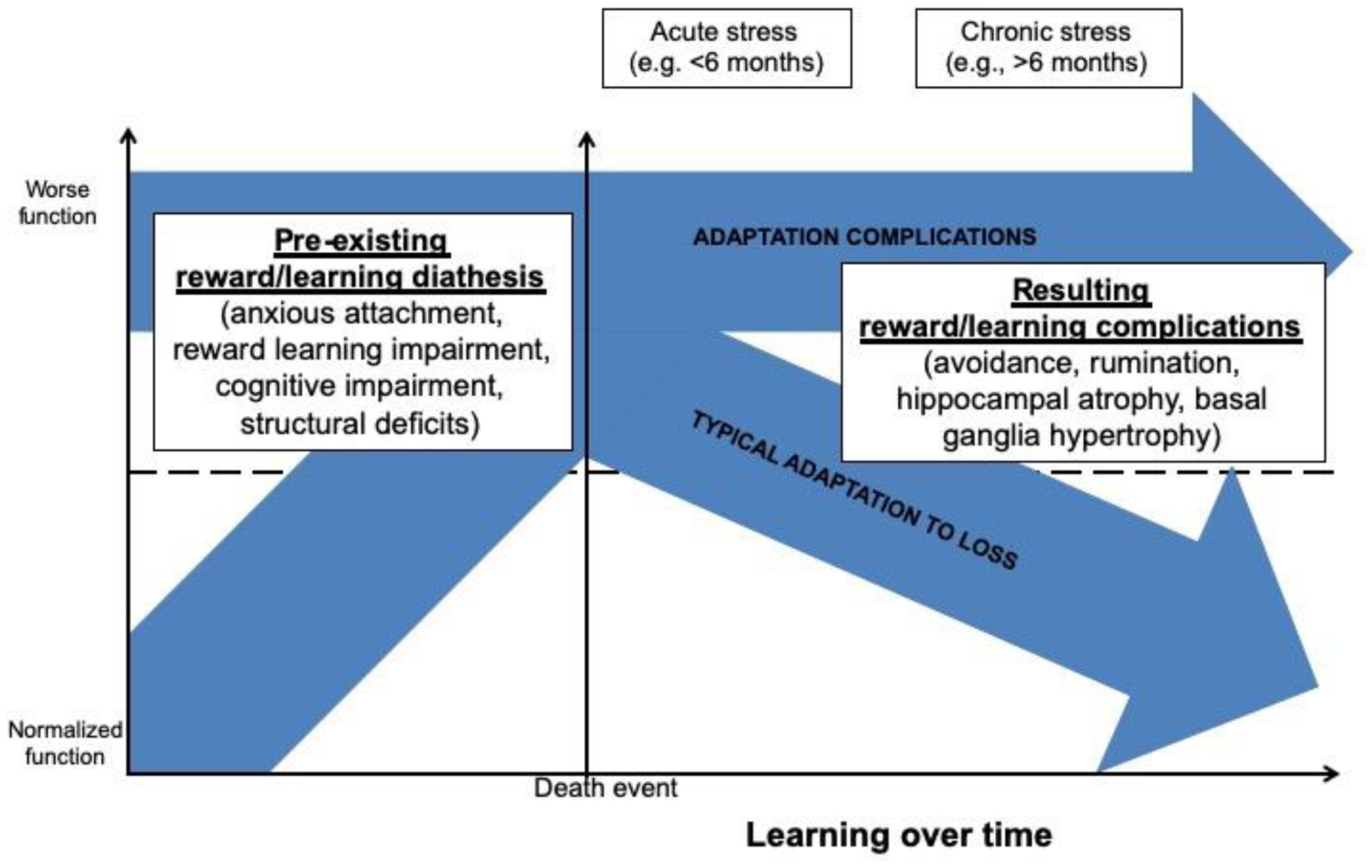
Graphical depiction of the complications that may precede or result from the death of an attachment figure, using trajectories described in [40,47].
An excellent systematic review of the neurobiology of disordered grief has conceptualized PGD as a disorder of reward [24]. Reward is inherently a process by which learning develops, as one’s predictions about the world are either rewarded or not, leading to accommodation and consolidation of information and future predictions. Those who develop PGD may have 1) a pre-existing reward system that strongly supports the anxiety and attention to the “everlasting” aspect of attachment as a diathesis (dependency [25], attachment style [26], and separation anxiety as predictors [27]). Attachment style may also be a diathesis for brain function [28], and may predict complications when layered with bereavement. Another possibility is that 2) complications arise after the death, due to the emotional pain of grief [25,29], and complications develop in how the death information is brought to awareness [30]). These complications, including avoidance and grief-related rumination, may lead to prolonged grieving, or lack of learning over time [31].
Spousal dependence predicts feelings that life is empty or meaningless, a sense of diminished identity, and difficulty imagining the future [32]. Dependence prior to the loss may be a risk factor for PGD because of the increased risk for preoccupying thoughts related to the deceased and a sense that one’s identity, meaning in life, and plans for the future are lost without the deceased [33]. These findings are in keeping with Maccallum and Bryant’s [34] cognitive attachment model in which they posit that a merged identity with the deceased will enhance the accessibility of deceased-related memories and reduce the ability to imagine the future. Additionally, a robust predictor of PGD severity is the extent to which the self-concept of the bereaved is dominated by the deceased [35].
Learning requires experience, and avoidance of things that remind the bereaved of the loved one’s death or absence will prevent accommodation of the information. Only through learning does the integration of the loss experience enable the restoration of a meaningful life. Ironically, grief-related rumination may actually serve the same function as avoidance. Grief-related rumination focuses on the counterfactuals (what ifs and would’ve/could’ve/should’ve); all of which are virtual reality scenarios that end in the loved one not dying [36]. Grief-related rumination may maintain the “everlasting” aspect of the attachment relationship, enabling the bereaved to avoid the painful evidence of “gone” [37,38]. Of course, these counterfactual outcomes only create grief anew each time that the reality of the loss resurfaces.
Structural neuroimaging shows us another reason why learning may be disrupted in PGD. As with functional predictors of PGD, differences in brain structure could 1) precede the death as a diathesis, or 2) follow from the stress of the bereavement experience. A prospective study showed that a smaller hippocampus prior to the death predicted disordered grieving [39]. Most studies are more equivocal as to whether structural differences precede or follow the loss. Importantly, all of this learning/grieving must be done while there is concurrent excessive cortisol, blood pressure, and inflammation as a physiological stress response to the death event [40]. Cortisol impacts brain structures such as hippocampus, regions needed for learning and memory. Decreased hippocampal volume has been shown in those who have lost a child, with and without post-traumatic stress disorder [41]. On the other hand, recent work has shown increased bilateral amygdala volumes, and in subsamples, larger nucleus accumbens and caudate volumes after bereavement or relationship break-up across the lifetime [42].
Education is a predictor of poor bereavement outcomes, including higher levels of PGD, and might reflect long-standing learning difficulties [43,44], although there may be many reasons why education is predictive. Behavioral studies also suggest that Stroop learning is affected in PGD [21], a neuropsychological test of managing conflicting inputs. Another possible view is that the death of a loved one is a notable inflection point in an ongoing trajectory of cognitive decline in older adults, possibly hastening the trajectory, but not causal per se [45].
Almost all functional and structural neuroimaging studies are about grief rather than grieving—they sample a single time point, and learning/grieving is inherently a trajectory of change over time. Thus, we don’t have functional neuroimaging evidence for many pre-existing diatheses. Worst of all, not distinguishing acute and/or typical grief and prolonged grief within the sample muddies the waters further.
Most of the observations in this article are simply updated, empirical examples of existing attachment theory [46] and cognitive psychology research, but the Gone-But-Also-Everlasting theory incorporates new evidence from cognitive neuroscience and the neuroscience of relationships and grief and loss. This emphasis on grief or PGD as the consequence of updating the neural encoding of an attachment bond, and the required brain structure and function for that learning, enables novel predictions that can be tested to further advance our understanding of grief and loss.
Highlights.
Neural encoding of attachment during bonding sets the stage for separation distress.
Predicting that the attachment figure exists, even when temporarily absent, is adaptive.
Grief requires updating predictions of the loved one’s presence given their permanent absence.
Failure to reconcile conflicting streams of information may prolong grief.
Grief can be considered a form of learning.
Acknowledgements
This project was supported by a grant from the National Institute of Aging (R13 AG066368; PI: O’Connor). We would like to thank Jessica Andrews-Hanna and Matt Grilli for comments on an earlier draft of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Folkman S, Lazarus R, Stress, Appraisal, and Coping, Springer Publishing Company, New York, 1984. [Google Scholar]
- [2].Stroebe W, Stroebe MS, Bereavement and Health, Cambridge University Press, 1987. 10.1017/CBO9780511720376. [DOI] [Google Scholar]
- [3].Bowlby J, The Bowlby-Ainsworth attachment theory, Behav. Brain Sci 2 (1979) 637–638. 10.1017/S0140525X00064955. [DOI] [Google Scholar]
- [4].LeRoy AS, Knee CR, Derrick JL, Fagundes CP, Implications for Reward Processing in Differential Responses to Loss: Impacts on Attachment Hierarchy Reorganization, Personal. Soc. Psychol. Rev 23 (2019) 391–405. 10.1177/1088868319853895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pohl TT, Young LJ, Bosch OJ, Lost connections: Oxytocin and the neural, physiological, and behavioral consequences of disrupted relationships, Int. J. Psychophysiol (2018). 10.1016/j.ijpsycho.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[6].Scribner JL, Vance EA, Protter DSW, Sheeran WM, Saslow E, Cameron RT, Klein EM, Jimenez JC, Kheirbek MA, Donaldson ZR, A neuronal signature for monogamous reunion, Proc. Natl. Acad. Sci 117 (2020) 11076–11084. 10.1073/pnas.1917287117. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study in prairie voles identified striatal neurons activated by partner approach, termed “partner approach cells”, and demonstrated that number of partner approach cells increased with strength of the bond. Findings suggest that specific aspects of the pair-bond, such as motivation to reunite, are encoded in specific neuronal ensembles in the nucleus accumbens.
- [7].Wang H, Duclot F, Liu Y, Wang Z, Kabbaj M, Histone deacetylase inhibitors facilitate partner preference formation in female prairie voles, Nat. Neurosci 16 (2013) 919–924. 10.1038/nn.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sadino JM, Donaldson ZR, Prairie Voles as a Model for Understanding the Genetic and Epigenetic Regulation of Attachment Behaviors, ACS Chem. Neurosci (2018). 10.1021/acschemneuro.7b00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ, The CRF System Mediates Increased Passive Stress-Coping Behavior Following the Loss of a Bonded Partner in a Monogamous Rodent, Neuropsychopharmacology. 34 (2009) 1406–1415. 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[10].Courtney AL, Meyer ML, Self-Other Representation in the Social Brain Reflects Social Connection, J. Neurosci 40 (2020) 5616–5627. 10.1523/JNEUROSCI.2826-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study, while not bereavement-specific, demonstrates that social relationships are mapped in the medial prefrontal cortex according to degree of closeness. Further, there was neural overlap between representations of the self and of close others, supporting the idea that our most intimate relationships are integrated into our sense of self.
- [11].Buckley T, Bartrop R, McKinley S, Ward C, Bramwell M, Roche D, Mihailidou AS, Morel-Kopp M-C, Spinaze M, Hocking B, Goldston K, Tennant C, Tofler G, Prospective study of early bereavement on psychological and behavioural cardiac risk factors, Intern. Med. J 39 (2009) 370–378. 10.1111/j.1445-5994.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- [12].Critchley HD, Harrison NA, Visceral Influences on Brain and Behavior, Neuron. 77 (2013) 624–638. 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- [13].Sbarra DA, Hazan C, Coregulation, Dysregulation, Self-Regulation: An Integrative Analysis and Empirical Agenda for Understanding Adult Attachment, Separation, Loss, and Recovery, Personal. Soc. Psychol. Rev 12 (2008) 141–167. 10.1177/1088868308315702. [DOI] [PubMed] [Google Scholar]
- [14].Pietromonaco P, Overall NC, Implications of social Isolation, separation and loss during the COVID-19 pandemic for couples’ relationships, Curr. Opin. Psychol 43 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Karantzas G, Feeney J, Agnew C, Christensen A, Cutrona C, Doss B, Eckhardt C, Russell D, Simpson J, Dealing with loss in the face of disasters and crises: Integrating interpersonal theories of couple adaptation and functioning, Curr. Opin. Psychol 43 (2022). [DOI] [PubMed] [Google Scholar]
- [16].Visser RM, Anderson MC, Aron A, Banich M, Brady KT, Huys QJM, Monfils MH, Schiller D, Schlagenhauf F, Schooler J, Robbins TW, Neuropsychological mechanisms of intrusive thinking, in: Kalivas P, Paulus M (Eds.), Intrusive Think. From Mol. to Free Will, Strüngmann, MIT Press, Cambridge, MA, 2020. [Google Scholar]
- [17].Boddez Y, The presence of your absence: A conditioning theory of grief, Behav. Res. Ther 106 (2018) 18–27. 10.1016/j.brat.2018.04.006. [DOI] [PubMed] [Google Scholar]
- [18].Bartels A, Zeki S, The neural basis of romantic love, Neuroreport. 11 (2000) 3829–3834. 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- [19].Gündel H, O’Connor M-F, Littrell L, Fort C, Lane RD, Functional neuroanatomy of grief: an FMRI study., Am. J. Psychiatry 160 (2003) 1946–1953. 10.1176/appi.ajp.160.11.1946. [DOI] [PubMed] [Google Scholar]
- [20].O’Connor M-F, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD, Craving love? Enduring grief activates brain’s reward center, Neuroimage. 42 (2008) 969–972. 10.1016/j.neuroimage.2008.04.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Arizmendi B, Kaszniak AW, O’Connor M-FF, Disrupted prefrontal activity during emotion processing in complicated grief: An fMRI investigation, Neuroimage. 124 (2016) 968–976. 10.1016/j.neuroimage.2015.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wittfoth-Schardt D, Gründing J, Wittfoth M, Lanfermann H, Heinrichs M, Domes G, Buchheim A, Gündel H, Waller C, Oxytocin Modulates Neural Reactivity to Children’s Faces as a Function of Social Salience, Neuropsychopharmacology. 37 (2012) 1799–1807. 10.1038/npp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Robinaugh D, Toner E, Djelantik A, The causal systems approach to prolonged grief: Recent developments and future directions, Curr. Opin. Psychol 43 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[24].Kakarala SEE, Roberts KEE, Rogers M, Coats T, Falzarano F, Gang J, Chilov M, Avery J, Maciejewski PKK, Lichtenthal WGG, Prigerson HGG, The neurobiological reward system in Prolonged Grief Disorder (PGD): A systematic review, Psychiatry Res. Neuroimaging 303 (2020). 10.1016/j.pscychresns.2020.111135. [DOI] [PMC free article] [PubMed] [Google Scholar]; This summary of neurobiological evidence from studies of bereavement and disordered grief in humans offers the most robust empirical support to date for the involvement of abberant reward processing in prolonged grief.
- [25].Robinaugh DJ, LeBlanc NJ, Vuletich HA, McNally RJ, Network analysis of persistent complex bereavement disorder in conjugally bereaved adults., J. Abnorm. Psychol 123 (2014) 510–522. 10.1037/abn0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stroebe M, Schut H, Stroebe W, Attachment in Coping with Bereavement: A Theoretical Integration, Rev. Gen. Psychol 9 (2005) 48–66. 10.1037/1089-2680.9.1.48. [DOI] [Google Scholar]
- [27].Lobb EA, Kristjanson LJ, Aoun SM, Monterosso L, Halkett GKB, Davies A, Predictors of Complicated Grief: A Systematic Review of Empirical Studies, Death Stud. 34 (2010) 673–698. 10.1080/07481187.2010.496686. [DOI] [PubMed] [Google Scholar]
- [28].Vrtička P, Vuilleumier P, Neuroscience of human social interactions and adult attachment style, Front. Hum. Neurosci 6 (2012). 10.3389/fnhum.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Frumkin MR, Robinaugh DJ, LeBlanc NJ, Ahmad Z, Bui E, Nock MK, Simon NM, McNally RJ, The pain of grief: Exploring the concept of psychological pain and its relation to complicated grief, depression, and risk for suicide in bereaved adults, J. Clin. Psychol 77 (2021) 254–267. 10.1002/jclp.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[30].Schneck N, Tu T, Haufe S, Bonanno GA, GalfaIvy H, Ochsner KN, Mann JJ, Sajda P, Ongoing monitoring of mindwandering in avoidant grief through cortico-basal-ganglia interactions, Soc. Cogn. Affect. Neurosci 14 (2019) 163–172. 10.1093/scan/nsy114. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prolonged grief often involves hypervigilance and avoidance of grief-related cues. In this study, bereaved adults with an avoidant grieving style reported more intrusive thoughts of the loss during a sustained attention task -- despite fMRI activation indicating attempts to monitor internally-focused thought for reminders of the deceased via interactions between a fronto-temporoparietal network (linked to deceased-related attention) and an orbitofrontal-basal ganglia network (linked to mental representation of the deceased).
- [31].van der Houwen K, Stroebe M, Schut H, Stroebe W, van den Bout J, Mediating processes in bereavement: The role of rumination, threatening grief interpretations, and deliberate grief avoidance, Soc. Sci. Med 71 (2010) 1669–1676. 10.1016/j.socscimed.2010.06.047. [DOI] [PubMed] [Google Scholar]
- [32].Robinaugh DJ, McNally RJ, Remembering the past and envisioning the future in bereaved adults with and without complicated grief, Clin. Psychol. Sci (2013). 10.1177/2167702613476027. [DOI] [Google Scholar]
- [33].Maciejewski P, Falzarano F, She W, Lichtenthal W, Prigerson H, A micro-sociological theory of adjustment to loss, Curr. Opin. Psychol 43 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Maccallum F, Bryant RA, A Cognitive Attachment Model of prolonged grief: Integrating attachments, memory, and identity, Clin. Psychol. Rev 33 (2013) 713–727. 10.1016/j.cpr.2013.05.001. [DOI] [PubMed] [Google Scholar]
- *[35].Bellet BW, LeBlanc NJ, Nizzi M, Carter ML, van der Does FHS, Peters J, Robinaugh DJ, McNally RJ, Identity confusion in complicated grief: A closer look., J. Abnorm. Psychol 129 (2020) 397–407. 10.1037/abn0000520. [DOI] [PMC free article] [PubMed] [Google Scholar]; The feeling that part of one’s self died along with the deceased can be especially acute in people with disordered grief. The authors identified that complicated grief was associated with disrupted self-concept fluency (or accessibility) and more impoverished description. They suggest that identity disruption in grief may be most impairing when the death is perceived to eliminate entire domain(s) of a person’s self-concept.
- [36].Robinaugh DJ, Mauro C, Bui E, Stone L, Shah R, Wang Y, Skritskaya NA, Reynolds CF, Zisook S, O’Connor M-F, Shear K, Simon NM, Yearning and Its Measurement in Complicated Grief, J. Loss Trauma 21 (2016) 410–420. 10.1080/15325024.2015.1110447. [DOI] [Google Scholar]
- [37].Eisma MC, Schut HAW, Stroebe MS, Van Den Bout J, Stroebe W, Boelen PA, Is rumination after bereavement linked with loss avoidance? Evidence from eye-tracking, PLoS One. (2014). 10.1371/journal.pone.0104980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Eisma MC, Schut HAW, Stroebe MS, Voerman K, van den Bout J, Stroebe W, Boelen PA, Psychopathology Symptoms, Rumination and Autobiographical Memory Specificity: Do Associations Hold After Bereavement?, Appl. Cogn. Psychol 29 (2015) 478–484. 10.1002/acp.3120. [DOI] [Google Scholar]
- [39].Saavedra Pérez HC, Ikram MA, Direk N, Prigerson HG, Freak-Poli R, Verhaaren BFJ, Hofman A, Vernooij M, Tiemeier H, Cognition, structural brain changes and complicated grief. A population-based study, Psychol. Med 45 (2015) 1389–1399. 10.1017/S0033291714002499. [DOI] [PubMed] [Google Scholar]
- [40].O’Connor M-F, Grief: A Brief History of Research on How Body, Mind, and Brain Adapt, Psychosom. Med 81 (2019) 731–738. 10.1097/PSY.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Luo Y, Shan H, Liu Y, Wu L, Zhang X, Ma T, Zhu W, Yang Y, Wang J, Cao Z, Decreased left hippocampal volumes in parents with or without posttraumatic stress disorder who lost their only child in China, J. Affect. Disord (2016). 10.1016/j.jad.2016.03.003. [DOI] [PubMed] [Google Scholar]
- [42].Acosta H, Jansen A, Kircher T, Larger bilateral amygdalar volumes are associated with affective loss experiences, J. Neurosci. Res (2021) 1–17. 10.1002/jnr.24835. [DOI] [PubMed] [Google Scholar]
- [43].Boelen PA, Lenferink LIM, Symptoms of prolonged grief, posttraumatic stress, and depression in recently bereaved people: symptom profiles, predictive value, and cognitive behavioural correlates, Soc. Psychiatry Psychiatr. Epidemiol (2019). 10.1007/s00127-019-01776-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Boelen PA, Lenferink LIM, Smid GE, Further evaluation of the factor structure, prevalence, and concurrent validity of DSM-5 criteria for Persistent Complex Bereavement Disorder and ICD-11 criteria for Prolonged Grief Disorder, Psychiatry Res. 273 (2019) 206–210. 10.1016/j.psychres.2019.01.006. [DOI] [PubMed] [Google Scholar]
- [45].Saavedra Pérez HC, Ikram MA, Direk N, Tiemeier H, Prolonged Grief and Cognitive Decline: A Prospective Population-Based Study in Middle-Aged and Older Persons, Am. J. Geriatr. Psychiatry 26 (2018) 451–460. 10.1016/j.jagp.2017.12.003. [DOI] [PubMed] [Google Scholar]
- [46].Shear K, Shair H, Attachment, loss, and complicated grief, Dev. Psychobiol 47 (2005) 253–267. 10.1002/dev.20091. [DOI] [PubMed] [Google Scholar]
- [47].Bonanno GA, Lehman DR, Tweed RG, Haring M, Wortman CB, Sonnega J, Carr D, Nesse RM, Resilience to loss and chronic grief: A prospective study from preloss to 18-months postloss, J. Pers. Soc. Psychol 83 (2002) 1150–1164. 10.1037//0022-3514.83.5.1150. [DOI] [PubMed] [Google Scholar]


