Abstract
Adults on the autism spectrum and those with intellectual disability (ID) or mental health conditions (MHCs) may be at increased risk of contracting COVID-19 or experiencing more severe illness if infected. We identified risk factors for COVID-19 among adults enrolled in Medicaid with an autism spectrum disorder (ASD) diagnosis, intellectual disability (ID), or mental health conditions (MHCs). We examined adults ages 20–64 with 9-month continuous enrollment over 2008–2012 using Medicaid Analytic Extract (MAX) data. There were 83,150 autistic adults and, 615,607 adults with ID meeting inclusion criteria; of a random sample of 1 million beneficiaries without ASD or ID, 35.3% had any MHC. Beneficiaries on the spectrum, those with ID, and those with MHC all had higher odds of risk factors for becoming infected with COVID-19 (living in a residential facility, receiving services in the home from outside caregivers, having had a long hospitalization, having had avoidable hospitalizations) and higher odds of comorbidities associated with severe illness from COVID-19. Clinicians should anticipate high prevalence of comorbidities and risk factors for severe illness from COVID-19 among these populations. Health officials and nongovernmental organizations should target these groups with outreach for the COVID-19 vaccine and support continued efforts for appropriate mitigation measures.
Keywords: Autism, mental health, intellectual disability, comorbidities, COVID-19 risk
Lay Abstract
Autistic adults, adults with intellectual disability, and adults with other mental health conditions may have higher risk of contracting COVID-19 or experiencing more severe illness from COVID-19 if infected. We used data from Medicaid to look at whether autistic adults and other adults with intellectual disability and other mental health conditions were more likely to have risk factors for COVID-19, such as living in a residential facility, receiving services regularly in the home from outside caregivers, having had a long hospitalization, having had avoidable hospitalizations, and having high-risk health conditions. We found that autistic adults had higher odds of living in a residential facility, receiving in-home services from outside caregivers, having had an avoidable hospitalization, and having a high-risk health condition, compared to neurotypical adults without mental health conditions. Adults with intellectual disability had similar odds of having these conditions. Adults with other mental health conditions were also more likely to live in a residential facility, receive services from outside caregivers, and having had avoidable hospitalizations compared to the neurotypical population without mental health conditions. They had three times higher odds of having a high-risk health condition. High risk of COVID-19 among autistic adults and adults with intellectual disability and mental health conditions should be recognized by clinicians, and these groups should be prioritized for vaccine outreach.
Introduction
In 2020, the novel coronavirus began its sweep across the world, ushering in the greatest public health threat since the Spanish influenza of 1918 and threatening economic depression. Millions of individuals have been infected worldwide, and the United States surpassed half a million deaths from COVID-19 in February 2021 (Tompkins 2021). Comorbidities putting individuals at higher risk for COVID-19, the disease caused by the novel coronavirus, were a focus in much of the early literature on the pandemic, but there has been more limited research on whether individuals with developmental disability or mental health conditions (MHCs) may be at greater risk of contracting the disease or experiencing greater disease severity if infected.
Physical health conditions that have been associated with poorer symptomatology and outcomes for COVID-19 include hypertension, cardiovascular disease, diabetes, obesity, chronic kidney disorders, cancer, and other immunocompromised conditions (Grasselli et al. 2020; Nikpouraghdam et al. 2020; Onder et al. 2020; Richardson et al. 2020; Tomlins et al. 2020; Yang et al. 2020). In addition to these conditions, the U.S. Centers for Disease Control (CDC) has recognized higher risk of severe disease from COVID-19 among individuals with chronic obstructive pulmonary disease (COPD), immunocompromised state from solid organ transplant, other serious heart conditions, sickle cell disease, and Type 2 diabetes mellitus (U.S. Centers for Disease Control 2020).
Some evidence suggests that having a psychiatric diagnosis is associated with 65% increased risk of COVID-19 diagnosis (Taquet et al. 2020; Wang et al. 2020), indicating that this group may be at greater risk of contracting COVID-19. Previous studies have shown that individuals with anxiety and/or depression have higher rates of obesity, diabetes, hypertension, and cardiovascular disease (Scott et al. 2007). Individuals with severe mental illness (including bipolar, schizophrenia, and major depressive disorder) are at higher risk for a number of physical conditions, including obesity, diabetes, hypertension, and other cardiovascular diseases (De Hert et al. 2011a; De Hert et al. 2011b).
Previous evidence on autistic adults suggests that they may be at risk of premature mortality, chronic health conditions, and poor physical health (DaWalt et al. 2019; Hirvikoski et al. 2016; Woolfenden et al. 2012). A body of literature suggests that autistic adults are at higher risk of nearly all physical health conditions, including cardiovascular conditions, hypertension, pulmonary conditions, obesity, and diabetes (Croen et al. 2015a; Davignon et al. 2018; Fortuna et al. 2016; Kohane et al. 2012; Vohra et al. 2017; Weiss et al. 2018; Zheng et al. 2017). One study of autistic adults over age 65 were also at increased risk of most physical health conditions, including diabetes, cancer, hypertension, heart disease, and respiratory conditions compared to non-autistic older adults (Hand et al. 2020). It is worth noting that a few studies found prevalence of cardiovascular disease and diabetes was not higher among autistic adults (Tyler et al. 2011; Vohra et al. 2017), but they do detect differences in other relevant conditions. While differences in findings between studies may be due to differing sample sizes, study population ages, or methodological approaches, the overwhelming message from the literature is that differences exist between autistic adults and the general population for many of the conditions that put adults at higher risk of severe disease from COVID-19.
It is also important to point out that autistic individuals are at higher risk of having other mental health conditions, ranging from commonly co-occurring conditions (ADHD, anxiety) to less common conditions, such as bipolar disorder and schizophrenia (Davignon et al. 2018; Hand et al. 2020; Lai et al. 2019). Recent estimates of the percent of autistic adults with at least one psychiatric diagnosis range from 54% to 81% (Croen et al. 2015a; Vohra et al. 2017).
Evidence has also suggested individuals with intellectual disabilities (ID) may have higher rates of hypertension, respiratory disease, diabetes, heart disease, and chronic kidney disease (Cooper et al. 2015; Turk et al. 2020), as well as obesity (De Winter et al. 2012a; Hsieh et al. 2014). However, evidence for whether adults with ID have higher rates of diabetes and cancer is mixed (De Winter et al. 2012b; Hogg and Tuffrey-Wijne 2008; MacRae et al. 2015; McMahon and Hatton 2021).
Preliminary evidence on COVID-19 suggests that patients with DD and ID are at higher risk of severe illness if infected. One study of patients with private insurance found that the COVID-19 case fatality rate for individuals with DD and ID was over 1.5 times higher than those without (ages 18–74) (Turk et al. 2020); another suggested that individuals with DD who contracted COVID-19 had three times the odds of dying, while those with ID had 2.75 times the odds (Makary 2020). Karpur et al. (2021) recently found that individuals with ID and ASD who contracted COVID-19 had nine times higher odds of hospitalization compared to those without ASD and ID, and six times higher odds of a longer hospital stay. Such results give credence to findings from shoe-leather reporting early in the pandemic in New York suggesting individuals with developmental disabilities (DD) were 4.8 times more likely to die among those with COVID-19 (Hakim 2020). Research has also suggested that autistic individuals may be at higher risk for developing acute respiratory distress syndrome (ARDS) due to COVID-19 (de Sousa Lima et al. 2020).
Individuals with MHCs in general may be exposed to greater risk factors resulting from their residential settings. It is known that individuals living in congregate settings are susceptible to transmission of respiratory illnesses due to inadequate ventilation and the near impossibility of physical distancing (Akiyama et al. 2020; Davidson and Szanton 2020; Kinner et al. 2020; McMichael 2020; Rubin 2020). Examples include nursing homes and prisons, at which multiple outbreaks have been documented (Davidson and Szanton 2020; McMichael 2020), but also residential facilities such as group homes for autistic individuals (Ameis et al. 2020; Hakim 2020), inpatient settings for individuals with severe mental health symptomatology (Li 2020; Xiang et al. 2020), or informal congregate settings where individuals experiencing homelessness may be living (Tsai and Wilson 2020). Individuals with MHCs may be more likely than the general population to reside in these settings, potentially increasing their risk of contracting COVID-19.
In addition, research into longer term impacts of COVID-19 suggest that mental health impacts among those infected may be substantial. Survivors of COVID-19 may be at higher risk of psychiatric conditions (Taquet et al. 2020), and it remains unclear how the mental health of survivors with previous psychiatric conditions will be impacted. It is clear, however, that individuals in the society at large have been exposed to higher levels of stress, anxiety, and depression during the pandemic (Salari et al. 2020), and many have experienced new or exacerbated mental health impacts (Holman et al. 2020; Rains et al. 2020).
Given the multiple vulnerabilities among individuals with MHCs and developmental disabilities, it is important to systematically assess risk factors for contracting COVID-19 and/or experiencing more severe symptoms if infected. We present analysis of several risk factors among individuals with MHCs, autism, and intellectual disabilities (IDs). We examine COVID-19 risk for these groups and map geographic areas in which they are concentrated, pointing to areas across the United States where they are at risk. In response to global calls for research and action on COVID-19 and mental health (Druss 2020; Holmes et al. 2020; Luykx et al. 2020; Öngür et al. 2020), it is our hope that these data will be useful to clinicians and policymakers alike, as they work to minimize the impact of COVID-19 on the health and well-being of vulnerable populations across the United States and the world.
Methods
We relied on Medicaid Analytic eXtract (MAX) data from 2008–2012 and from Area Resource Files (ARF) data from the Health Resources & Services Administration. MAX data contained all individuals under age 65 with claim(s) associated with a diagnosis of ASD (International Classification of Diseases, Ninth Edition [ICD-9] codes 299.xx) or ID (ICD-9 codes 317.xx-319.xx) and a random sample of 3,358,220 Medicaid beneficiaries without ASD or ID. Individuals were identified as having one of these diagnoses if they had at least one inpatient claim or two outpatient claims with that diagnosis (Burke et al. 2014; Schott et al. 2021; Vohra et al. 2017).
We examined adults ages 20–64 and defined groups of analysis as: (1) the population of autistic Medicaid beneficiaries without an ID diagnosis; (2) the population of autistic Medicaid beneficiaries with an ID diagnosis, (3) the population of Medicaid beneficiaries with an ID diagnosis but no ASD diagnosis; (4) a random sample of the general Medicaid population with any mental health diagnosis (excluding ASD/ID); and (5) a random sample of the general Medicaid population with no mental health, ASD, or ID diagnoses (henceforth referred to as “the general Medicaid sample without MHC”). We analyzed these groups separately due to the overlap between the diagnoses of ASD and ID (62.6% of autistic individuals also have an ID diagnosis). We note that individuals in these three groups also have high prevalence of co-occurring MHCs.
We first examined risk factors for contracting COVID-19, including (i) living in primary care shortage areas, (ii) living in a residential facility, (iii) receiving services in the home regularly from outside caregivers, (iv) having had a long hospitalization (psychiatric or inpatient) in the last 5 years, and (v) having had avoidable hospitalizations; we also studied risk factors for developing severe illness from COVID-19 (having a co-occurring condition identified as high risk for serious disease) for each of these groups. Next, we examined prevalence of these risk factors as outcomes in logistic regression controlling age group, sex, race/ethnicity, Medicaid insurance mechanism (fee for service (FFS)/ primary care case management (PCCM), comprehensive managed care, or other), and a dummy for dual Medicare/Medicaid eligibility.
To identify whether individuals lived in primary care shortage areas, we merged by zip code with the ARF (data.hrsa.gov). The national Health Service Corps developed Health Professional Shortage Areas (HPSA) scores to determine priorities for assignment of clinicians in response to need. Scores range from 1 to 25 for primary care, where 25 indicates high shortages. In our study, counties of high primary care shortages were defined as having HPSA scores in the upper quartile. Since there was quite a bit of geographic mobility over the 5 years of the study, we defined living in a high primary care shortage area as living in an area with HPSA scores in the upper quartile for 75% or more of the time studied.
We defined living in a residential facility as living in intermediate care facility, nursing facility services, or in residential care. Receiving services in the home regularly from outside caregivers was coded for individuals with claims of home health, personal care services, and private duty nursing. Having had a hospitalization (psychiatric or inpatient) was defined as any hospitalization for least 30 days over the 5 years of data, identified with claims or encounters from inpatient hospitals, mental hospital services for the aged, and inpatient psychiatric facilities (only for age under 21).
Avoidable hospitalizations were identified using the strategy from Segal et al. (2014) and included COPD, chronic bronchitis, and asthma; congestive heart failure; constipation, fecal impaction, and obstipation; dehydration, volume depletion including acute renal failure and hyponatremia; hypertension and hypotension; poor glycemic control; seizures; urinary tract infection; and weight loss (failure to thrive) and nutritional deficiencies.
We examined comorbidities that may put individuals at risk of severe COVID-19 illness using the list of conditions promulgated by the U.S. Centers for Disease Control (CDC) (U.S. Centers for Disease Control 2020). This list (dated August 14, 2020) includes cancer, chronic kidney disease, chronic obstructive pulmonary disease (COPD), immunocompromised state from solid organ transplant, obesity (body mass index [BMI]>30), serious heart conditions, such as heart failure, coronary artery disease, or cardiomyopathies, sickle cell disease, and Type 2 diabetes mellitus. While it is possible additional conditions may put individuals at increased risk of severe illness, the evidence for other conditions (such as hypertension, asthma, pregnancy, etc.) remained mixed or limited at the writing of this manuscript, according to the CDC (U.S. Centers for Disease Control 2020). There was no community involvement in this study.
Results
From the random sample of 3.4 million adults without ASD/ID, there were 2,782,069 with 9-month continuous enrollment, and 1,056,585 that were between the ages of 20 and 64. Of these, there were 372,807 individuals (35%) with any MHC (ICD-9 codes 290.xx-319.xx, excluding the ASD and ID groups). There were 31,101 autistic individuals ages 20–64 without ID with 9-month continuous enrollment; 52,049 autistic individuals ages 20–64 with ID; and 563,558 individuals ages 20–64 with ID but not autism. We note that there is high comorbidity for MHC among these groups, with 74%, 62%, and 77% respectively also having a diagnosis of a MHC.
Table 1 shows demographic and other characteristics of the autistic Medicaid population with and without ID, the population with ID but not ASD, and the random sample of the Medicaid population without ASD or ID diagnoses. We note large differences by gender and by race and ethnicity.
Table 1.
Beneficiary Characteristics, Populations with ASD Only, ASD+ID, and ID Only, Random Sample without ASD/ID
| Population ASD/ID N = 646,708 | Random Sample (No ASD/ID) n = 1,056,585 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| (I) ASD Only N = 31,101 |
(II) ASD+ID N = 52,049 |
(III) ID Only N = 563,558 |
(IV) MHC n = 372,807 (35.28%) |
(V) No MHC N = 683,778 (64.72%) |
||||||
|
| ||||||||||
| N | Percent | N | Percent | N | Percent | N | Percent | N | Percent | |
|
| ||||||||||
| Age (mean, std) | 30.9 | 10.4 | 33.1 | 10.7 | 39.1 | 11.6 | 38.7 | 11.5 | 34.5 | 11.1 |
| Age Category | ||||||||||
| 20–29 | 18,825 | 60.5 | 25,989 | 49.9 | 162,675 | 28.9 | 110,459 | 29.6 | 303,697 | 44.4 |
| 30–39 | 5,901 | 19.0 | 11,993 | 23.0 | 126,172 | 22.4 | 92,594 | 24.8 | 178,563 | 26.1 |
| 40–49 | 3,981 | 12.8 | 9,211 | 17.7 | 151,240 | 26.8 | 92,132 | 24.7 | 114,860 | 16.8 |
| 50–64 | 2,394 | 7.7 | 4,856 | 9.3 | 123,471 | 21.9 | 77,622 | 20.8 | 86,658 | 12.7 |
| Male | 22,342 | 71.8 | 37,010 | 71.1 | 302,032 | 53.6 | 134,611 | 36.1 | 198,300 | 29.0 |
| Race/Ethnicity | ||||||||||
| White, non-Hispanic | 21,859 | 70.3 | 34,428 | 66.2 | 372,338 | 66.1 | 217,031 | 58.2 | 250,173 | 36.6 |
| Black, non-Hispanic | 4,184 | 13.5 | 10,330 | 19.9 | 111,686 | 19.8 | 80,132 | 21.5 | 139,789 | 20.4 |
| Asian/Pacific Islander, non-Hispanic | 680 | 2.2 | 1,270 | 2.4 | 11,318 | 2.0 | 8,761 | 2.4 | 43,182 | 6.3 |
| Hispanic, any race | 1,865 | 6.0 | 3,172 | 6.1 | 40,229 | 7.1 | 46,011 | 12.3 | 209,737 | 30.7 |
| Other ethnicity, non-Hispanic | 2,513 | 8.1 | 2,849 | 5.5 | 27,987 | 5.0 | 20,872 | 5.6 | 40,897 | 6.0 |
| Eligibility Type | ||||||||||
| Poverty | 997 | 3.2 | 145 | 0.3 | 3,430 | 0.6 | 109,870 | 29.5 | 274,810 | 40.2 |
| Disability | 28,334 | 91.1 | 51,317 | 98.6 | 553,973 | 98.3 | 179,414 | 48.1 | 134,601 | 19.7 |
| Other | 1,770 | 5.7 | 587 | 1.1 | 6,155 | 1.1 | 83,523 | 22.4 | 274,367 | 40.1 |
| Insurance type | ||||||||||
| FFS/PCCM Only | 9,745 | 31.3 | 21,941 | 42.2 | 232,840 | 41.3 | 74,607 | 20.0 | 254,314 | 37.2 |
| CMC Only | 8,011 | 25.8 | 13,699 | 26.3 | 162,065 | 28.8 | 105,822 | 28.4 | 161,651 | 23.6 |
| Combination | 13,345 | 42.9 | 16,409 | 31.5 | 168,653 | 29.9 | 192,378 | 51.6 | 267,813 | 39.2 |
| Medicare Dual eligible | 14,488 | 46.6 | 27,236 | 52.3 | 359,531 | 63.8 | 86,827 | 23.3 | 67,077 | 9.8 |
Notes: ASD=autism spectrum disorder, ID=intellectual disability, MHC=mental health condition; FFS=fee for service, PCCM=primary care case management; CMC=comprehensive managed care.
All p-value from chi-square test (t-test for age) of group value compared column (V) p < 0.01.
Table 2 shows the risk factors for COVID-19 among individuals in the four groups as well as the comparison group. The autistic Medicaid population without ID, the autistic Medicaid population with ID, the Medicaid population with ID (but not ASD), and the sample with any MHC were all more often living in high primary care shortage areas, living in a residential facility, and receiving services in their home regularly from outside caregivers compared to the general Medicaid sample without MHC. While none of the four groups had high rates of hospitalization for over 30 days over the 5 years studied, the percent with any hospitalization among these groups was at least seven times higher compared to the general Medicaid sample without MHC.
Table 2.
Risk Factors and Comorbidities, Medicaid Beneficiaries with ASD Only, ASD+ID, ID Only, MHCs, and Random Sample (no ASD/ID/MHCs)
| Population ASD/ID (N = 646,708) | Random Sample (no ASD/ID) (n = 1,056,585) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| ASD Only | ASD+ID | ID Only | MHC | No MHC | ||
|
| ||||||
| Percent/Mean | Percent/Mean | Percent/Mean | Percent/Mean | Percent/Mean | ||
|
| ||||||
| Risk Factor | ||||||
|
| ||||||
| 1 | Lives in primary care shortage area, high | 18.31 | 17.71 | 19.38 | 19.30 | 14.33 |
| 2 | Living in a residential facility | 18.51 | 54.27 | 45.08 | 5.40 | 1.22 |
| 3 | Receiving services in the home regularly from outside caregivers | 20.76 | 32.19 | 30.33 | 11.13 | 2.74 |
| 4 | Had hospitalization (psychiatric or inpatient) >=30 days over 5 years | 1.59 | 1.74 | 1.74 | 1.42 | 0.22 |
| 5 | Avoidable hospitalizations | |||||
| 6 | Any | 51.97 | 69.49 | 67.03 | 65.03 | 26.04 |
| 7 | COPD, chronic bronchitis, asthma | 20.21 | 19.70 | 24.92 | 34.87 | 9.81 |
| 8 | Congestive heart failure | 2.16 | 2.52 | 4.93 | 5.02 | 1.44 |
| 9 | Constipation, fecal impaction, and obstipation | 6.87 | 16.00 | 12.06 | 6.77 | 1.62 |
| 10 | Dehydration, volume depletion including acute renal failure and hyponatremia | 4.02 | 6.56 | 6.88 | 6.39 | 1.27 |
| 11 | Hypertension and hypotension | 14.49 | 17.63 | 21.92 | 24.96 | 8.54 |
| 12 | Poor glycemic control | 7.62 | 7.50 | 9.31 | 10.58 | 4.04 |
| 13 | Seizures | 16.25 | 36.18 | 29.79 | 8.88 | 1.62 |
| 14 | Urinary tract infection | 10.90 | 16.54 | 18.53 | 22.17 | 9.03 |
| 15 | Weight loss (failure to thrive) and nutritional deficiencies | 4.54 | 11.19 | 8.05 | 3.67 | 0.78 |
| Total risk factors (rows 1–4, 6) (mean) | 0.88 | 1.38 | 1.35 | 0.81 | 0.35 | |
|
| ||||||
| Comorbidities | ||||||
|
| ||||||
| 16 | Any | 28.89 | 32.37 | 38.45 | 43.50 | 16.43 |
| 17 | Cancer | 4.87 | 5.82 | 7.95 | 8.09 | 2.63 |
| 18 | Chronic kidney disease | 4.77 | 6.16 | 7.89 | 8.35 | 2.75 |
| 19 | Chronic lung disease | 6.72 | 5.86 | 10.69 | 18.11 | 3.20 |
| 20 | Diabetes | 11.82 | 12.57 | 16.69 | 17.78 | 7.65 |
| 21 | Immunocompromised | 0.43 | 0.41 | 0.34 | 0.49 | 0.14 |
| 22 | Previous Organ Transplant Recipient | 0.44 | 0.29 | 0.42 | 0.47 | 0.30 |
| 23 | Serious Heart Conditions | 2.75 | 3.15 | 6.17 | 6.41 | 1.82 |
| 24 | Severe Obesity | 10.66 | 10.16 | 10.01 | 12.50 | 4.76 |
| 25 | Sickle Cell Disease | 0.14 | 0.08 | 0.11 | 0.17 | 0.08 |
| Total comorbidities (rows 17–25) (mean) | 0.54 | 0.57 | 0.77 | 0.90 | 0.31 | |
Notes: ASD=autism spectrum disorder, ID=intellectual disability, MHC=mental health condition
Autistic individuals, adults with ID, and adults with MHCs also had higher prevalence of avoidable hospitalizations. The percent of individuals in these groups with any avoidable hospitalization was over twice as high for those with ID only, autistic individuals with ID, and individuals with any MHC compared to the general Medicaid sample without MHC. Differences between these groups and the general Medicaid sample without MHC were particularly acute for hospitalization for COPD, chronic bronchitis and asthma, hypertension and hypotension, and seizures. On average, the general Medicaid sample without MHC had 0.4 risk factors, while autistic individuals had 0.9 (no ID) to 1.4 (with ID) risk factors, those with ID (but not ASD) had 1.4, and those with any MHC had 0.8 risk factors.
Individuals with MHCs or ASD/ID also had higher prevalence of nearly all the comorbidities identified as increasing risk for severe disease from COVID-19. While 16.4% of the general Medicaid sample without MHC had any of the high-risk comorbidities, 28.9% (ASD only) to 43.5% (any MHC) of the other groups had at least one high-risk comorbidity. Differences were particularly large for chronic lung disease (two to six times higher than the general Medicaid sample without MHC) and obesity (two to three times higher the general Medicaid sample without MHC). Autistic beneficiaries without ID, those with ID, individuals with ID (but not ASD), and those with any MHC had nearly two to three times as many comorbidities as the general Medicaid sample without MHC, on average.
Many of these differences were robust in logistic regressions comparing each group to the general Medicaid sample without MHC (Table 3), controlling for demographic and other characteristics. The odds of living in a residential facility, receiving services in their home from outside caregivers, and for long-term hospitalizations were higher for each of these groups compared to the general Medicaid sample without MHC in adjusted regressions (for unadjusted regression results, please see appendix table A1). Similarly, individuals in these groups had higher odds of having had any avoidable hospitalization in general, and hospitalizations for all conditions (except for congestive heart failure) compared to the general Medicaid sample without MHC.
Table 3.
Adjusted Regressions, ASD Only, ASD+ID, ID Only, and MHC as Predictors for Risk Factors or Comorbidities, Odds Ratios [99% Confidence Intervals]
| Population ASD/ID | Random Sample (no ASD/ID) | ||||
|---|---|---|---|---|---|
|
| |||||
| ASD Only | ASD+ID | ID Only | MHC | ||
|
| |||||
| OR [99% CI] | OR [99% CI] | OR [99% CI] | OR [99% CI] | ||
|
| |||||
| Risk Factor | |||||
|
| |||||
| 1 | Living in primary care shortage areas, high | 0.87 [0.83,0.91] | 0.87 [0.83,0.91] | 0.99 [0.97,1.01]a | 1.02 [1.00,1.03]a |
| 2 | Living in a residential facility | 8.87 [8.29,9.50] | 58.73 [55.36,62.31] | 32.25 [31.05,33.49] | 2.77 [2.67,2.88] |
| 3 | Receiving services in the home regularly from outside caregivers | 3.92 [3.71,4.14] | 6.57 [6.28,6.87] | 7.02 [6.82,7.22] | 2.53 [2.47,2.60] |
| 4 | Had hospitalization (psychiatric or inpatient) >=30 days over 5 years | 3.28 [2.77,3.90] | 2.79 [2.41,3.23] | 3.85 [3.50,4.24] | 4.10 [3.78,4.46] |
| 5 | Avoidable hospitalizations | ||||
| 6 | Any | 2.34 [2.25,2.42] | 4.89 [4.73,5.05] | 3.51 [3.45,3.57] | 3.61 [3.56,3.65] |
| 7 | COPD, chronic bronchitis, asthma | 1.57 [1.50,1.64] | 1.41 [1.36,1.46] | 1.74 [1.71,1.78] | 3.26 [3.21,3.31] |
| 8 | Congestive heart failure | 0.98 [0.87,1.09]a | 0.82 [0.75,0.90] | 1.32 [1.27,1.37] | 1.91 [1.84,1.98] |
| 9 | Constipation, fecal impaction, and obstipation | 4.09 [3.78,4.42] | 9.59 [9.01,10.20] | 5.46 [5.24,5.70] | 3.15 [3.05,3.25] |
| 10 | Dehydration, volume depletion including acute renal failure and hyponatremia | 1.82 [1.65,1.99] | 2.46 [2.30,2.64] | 2.39 [2.30,2.49] | 2.95 [2.85,3.06] |
| 11 | Hypertension and hypotension | 1.86 [1.76,1.95] | 1.85 [1.77,1.92] | 1.67 [1.64,1.71] | 2.33 [2.29,2.37] |
| 12 | Poor glycemic control | 1.72 [1.61,1.84] | 1.46 [1.38,1.54] | 1.35 [1.32,1.39] | 1.75 [1.71,1.79] |
| 13 | Seizures | 3.81 [3.59,4.05] | 10.34 [9.87,10.84] | 9.17 [8.87,9.48] | 3.41 [3.30,3.51] |
| 14 | Urinary tract infection | 1.76 [1.66,1.86] | 3.07 [2.94,3.21] | 2.85 [2.77,2.92] | 2.56 [2.52,2.60] |
| 15 | Weight loss (failure to thrive) and nutritional deficiencies | 3.89 [3.51,4.31] | 9.57 [8.85,10.35] | 6.44 [6.10,6.81] | 3.25 [3.10,3.40] |
|
| |||||
| Comorbidities | |||||
|
| |||||
| 16 | Any | 1.50 [1.44,1.56] | 1.52 [1.47,1.57] | 1.55 [1.52,1.57] | 2.56 [2.53,2.60] |
| 17 | Cancer | 1.54 [1.42,1.67] | 1.59 [1.49,1.69] | 1.44 [1.39,1.49] | 2.03 [1.97,2.08] |
| 18 | Chronic kidney disease | 0.89 [0.82,0.96] | 0.95 [0.89,1.01]a | 1.16 [1.12,1.19] | 1.87 [1.82,1.92] |
| 19 | Chronic lung disease | 1.37 [1.27,1.46] | 0.96 [0.90,1.02]a | 1.61 [1.56,1.65] | 3.89 [3.81,3.98] |
| 20 | Diabetes | 1.48 [1.40,1.56] | 1.26 [1.21,1.32] | 1.25 [1.23,1.28] | 1.64 [1.61,1.67] |
| 21 | Immunocompromised | 1.17 [0.88,1.54]a | 0.97 [0.76,1.22]a | 0.86 [0.75,0.98] | 2.00 [1.79,2.23] |
| 22 | Previous Organ Transplant Recipient | 0.29 [0.23,0.38] | 0.16 [0.13,0.21] | 0.27 [0.24,0.29] | 0.76 [0.69,0.83] |
| 23 | Serious Heart Conditions | 0.98 [0.89,1.09]a | 0.80 [0.74,0.87] | 1.30 [1.25,1.34] | 1.95 [1.89,2.02] |
| 24 | Severe Obesity | 2.37 [2.23,2.52] | 2.16 [2.05,2.28] | 1.89 [1.83,1.95] | 2.16 [2.11,2.21] |
| 25 | Sickle Cell Disease | 0.57 [0.37,0.90] | 0.25 [0.16,0.39] | 0.48 [0.40,0.58] | 1.50 [1.27,1.76] |
Notes: ASD=autism spectrum disorder, ID=intellectual disability, MHC=mental health conditions. Regressions control for age group, sex, race, ethnicity, eligibility type, insurance type, Medicare dual eligibility and state; comparison is to the random sample with no ID, ASD, or MHC.
p-value is not less than 0.01 (for all other estimates, p<0.01)
The odds of having many of the comorbidities putting individuals at higher risk for severe disease from COVID-19 were also higher for the autistic population with and without ID, the population with ID (but not ASD), and for the sample of adults with any MHCs. The odds of having any high-risk comorbidity were 50%−55% higher for the autistic population without ID, those with ID, and for adults with ID but not ASD; odds were almost 2.5 times for the sample with any MHC as compared to the general Medicaid sample without MHC. The odds of having severe obesity were close to or exceeded 2.0 for all four groups compared to the sample without any MHC. The odds of having cancer were 44% (ID only) to 59% (ID and ASD) higher for autistic individuals with ID and adults with ID (but not ASD), and twice as high for those with any MHC, compared to the general Medicaid sample without MHC. The odds of having chronic kidney disease were elevated (87% higher) for those with any MHC compared to the general Medicaid sample without MHC; the odds of chronic lung disease were 37% higher for autistic adults without ID and 61% higher for adults with ID only, compared to the general Medicaid sample without MHC; the odds of diabetes were 26% (ID only) to 64% (any MHC) higher for these groups compared to the sample without any MCH; and the odds of immunocompromised status were only slightly higher for autistic individuals without ID, but twice as high for those with any MHC compared to the sample without any MCH; the odds of serious heart conditions were higher for those with ID only (30% higher) and for those with any MHC (90% higher) compared to the sample without any MCH. The odds of sickle cell disease were lower for the autistic population with ID, but 50% higher for the sample with any MCH.
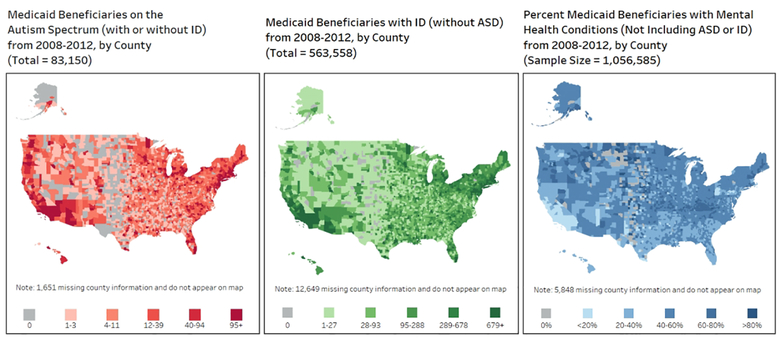
Figure 1 highlights the numbers of autistic Medicaid beneficiaries (with or without ID), adults with ID (but not ASD), and the percent of the random sample of Medicaid beneficiaries diagnosed with MHCs by county in the United States. We see that the areas of the country with higher rates of ASD, ID, and mental health conditions appear to overlap in some, but not all places, with there being higher concentrations of these conditions in more populous areas.
Figure 1.
Medicaid beneficiaries on the autism spectrum, Medicaid beneficiaries with ID, and percent Medicaid beneficiaries with any mental health condition, by county, 2008–2012
Discussion
Our results show that individuals on the autism spectrum, those with ID, and those with MHC are at higher risk of exposure to COVID-19 and/or more severe illness if they contract COVID-19. Our findings on comorbidities align with previously reported estimates of higher comorbidity prevalence among autistic individuals and adults with ID or MHC. While differences in the prevalence of specific health conditions have been outlined previously (Cooper et al. 2015; Croen et al. 2015b; Scott et al. 2007; Turk et al. 2020), our study brings to light higher prevalence in many of the specific health conditions that may put one at risk of severe illness from COVID-19. Autistic adults without ID had higher odds of having five of the nine identified underlying health conditions that may cause more severe illness from COVID-19, autistic adults with ID had higher odds of having three of the nine conditions, adults with ID (but not ASD) had higher odds of six of the nine conditions, and adults with other mental health conditions had higher odds of eight out of nine conditions, when compared to the general Medicaid population without ASD or ID. Each of these groups also had two to four times higher odds of having had an unnecessary hospitalization compared to the general Medicaid population without ASD, ID or MHC, suggesting that these groups have faced barriers to sufficient health care services in the past. Autistic adults in the United States often face difficulties in finding care that addresses their mental health needs within the context of autism, as well as challenges finding insurance coverage to pay for care (Maddox and Gaus 2019). A lack of training, misperceptions, and poor communication among providers are also problems challenging autistic adults, and there is a need for greater understanding of specific medical needs and barriers to access for this population (Malik-Soni et al. 2021).
To our knowledge, previous literature has not examined risk factors for infectious disease related to residential setting and in-home service receipt among adults with developmental disability or mental health conditions. Our findings highlight the higher odds of autistic individuals, individuals with ID, and adults with MHC of living in congregate settings and having outside caregivers coming into their homes, potentially putting them at greater risk of COVID-19 and other infectious diseases. As such, support for government, health providers, and community organizations to emphasize and implement preventive measures and work proactively to decrease potential exposure is critical.
To lessen the impact of COVID-19 among these vulnerable populations, health providers should follow the guidelines of the World Health Organization and adopt simple measures to ensure health care is accessible, affordable, and inclusive (World Health Organization 2020). In addition to the universal use of appropriate personal protective equipment by care providers and patients, service providers should work to reduce barriers to care services, prioritize essential care services, be prepared to provide support to individual with disabilities and their families even if quarantined or based at home. Health service providers in the community should have support and guidance in developing service continuity plans, communicating frequently with people with disabilities and their support networks, and ensuring access to health care for those with complex needs.
Throughout the pandemic, surges in cases and hospitalizations have meant resources, such as intensive care unit (ICU) beds, medical equipment, and staff, have been limited. Some states have released guidelines allegedly discriminating against the disabled, and complaints have been filed against Alabama, Arizona, Connecticut, Nebraska, New York, North Carolina, Oklahoma, Oregon, Pennsylvania, Tennessee, Texas, Utah, and Washington regarding rationing schemes, ethical positions on allocation of resources, hospital visitor policies, and standards of care guidelines (Center for Public Representation 2020). It is essential that individuals with disabilities be treated fairly and sensitively while in the care of health providers.
Effective vaccines rolled out in the United States in December 2020 (Thomas 2020), and while most states prioritized nursing homes, few specifically targeted settings in which individuals may receive long term services and supports (Musumeci 2021). Only three states included psychiatric hospitals in the first phase of vaccine distribution, nine states included group homes, two states prioritized individuals using home and community-based services through Medicaid, and a few states prioritized individuals receiving long term services and supports in other settings (Musumeci 2021). While vaccines are now available to anyone in the United States, access is still not universal, and there is vaccine hesitancy in the general population, including among autistic individuals and those with ID or MHC (Batty et al. 2021; Iadarola et al. 2021; Mazereel et al. 2021).
The heat maps in Figure 1 revealing the geographic distribution of at-risk individuals can be a helpful resource for health professionals, policymakers and advocates as they focus on prevention efforts as well as plans for vaccine outreach and other resource allocation. The Association of University Centers on Disabilities (AUCD) suggests that advocates review local vaccine distribution plans to ensure that considerations are given to disabled individuals, ensuring both outreach to educate the community and access to the vaccine (such as physical access as well as planning for transportation, accompaniment by a caregiver, and other considerations) (Disabilities; 2020). Given that barriers may exist for these populations, it is essential that clinicians and service providers reach out to individuals in their care to ensure that information about and access to the vaccine is distributed across communication modalities and for individuals who may need adapted materials. Previous work (in relation to a Hepatitis B vaccination program) found that the attitude of the doctors and nurses on the vaccination team and patient familiarity with the team were important drivers of individuals with ID being satisfied with vaccination programs (Cooney 2009). Care providers with a pre-existing, trusted relationship with patients with developmental disability may be in the best position to ensure these populations become vaccinated to COVID-19.
Looking forward, it is also important to plan for re-integration into society for autistic individuals and those with ID or MHCs. After what will likely amount to more than a year and a half of isolation for individuals who have been staying home to stay safe from COVID-19, once the multi-pronged approach of mitigation measures, masking, and physical distancing combined with the vaccine have reduced the threat of COVID-19, it will be appropriate to return to pre-COVID levels of community participation. While many individuals and families may be enthusiastically welcoming such a return, those faced with challenges in social communication or whose anxiety makes it difficult to leave the house even in the absence of COVID may find it challenging to re-integrate into social activities, the community, the workplace, and other communal areas. It is essential that service providers also plan for gradual re-integration into community participation, providing appropriate scaffolding and support in order to achieve successful interactions with employers and other community members.
Limitations
This study is not without limitations. First and foremost, our analysis may include more individuals from vulnerable populations than in the general population, since many of those in our random sample qualified for Medicaid through poverty, and many of those with ID and ASD qualified through disability. Autism is a spectrum diagnosis and ID presentation can also vary across individuals; we may not be capturing risk factors among the true population with these diagnoses in the United States. Similarly, MHC may be more prevalent among Medicaid enrollees, potentially biasing estimates of vulnerabilities. Nevertheless, it is important to examine these groups, as the Centers for Medicare & Medicaid Services funds the bulk of the care for many of these vulnerable populations (Centers for Medicare & Medicaid Services 2017). Second, we note there is overlap in diagnoses, as many autistic individuals and adults with ID may have co-occurring psychiatric comorbidities. In our analysis, most autistic adults without ID, those with ID, and adults with ID (but not on the autism spectrum) had other mental health diagnoses (74%, 62%, and 77% respectively). It is possible that individuals with multiple conditions could be at increased risk compared to the groups we examined. However, we believe these estimates are an important baseline of COVID-19 risk factors and recognize those few without cooccurring psychiatric conditions might have lower likelihood of risk factors. Third, we recognize that we are able to assess relatively few risk factors for contracting COVID-19, and many of our measures are imperfect. However, we believe examining the variables available in this data extract and linked data sources remain useful for clinicians and policymakers alike, despite their limitations. Fourth, we rely on claims and encounters data, which identifies individuals with a given condition only if it has been used for billing purposes. It is possible that we could be missing individuals for whom these conditions were not coded; this would lead to an underestimate of true risk factors. However, our study provides a novel presentation of risk of COVID-19 and other infectious disease, our data include the entire Medicaid population on the autism spectrum or with a diagnosis of ID over this time period as well as millions of Medicaid beneficiaries, and we are able to examine important health risk factors for these populations.
Conclusion
Our evidence supports the hypothesis that autistic individuals, individuals with ID, and those with other MHCs have higher prevalence of risk factors for COVID-19, thus putting them at greater risk than the general population of the United States. It is important that care providers are supported in implementing safe practices in serving these communities, and in prioritizing these populations for COVID-19 vaccine outreach.
Highlights.
Adults with developmental disabilities and mental health conditions are at greater risk from COVID-19.
Service providers should anticipate multiple risk factors for severe disease in these populations.
Adults with developmental disabilities and mental health conditions should be prioritized for vaccine outreach.
Acknowledgements
We greatly appreciate data visualization assistance from Kate Verstreate at the A.J. Drexel Autism Institute and comments from anonymous reviewers. This study was supported by the NIH grant, 5R01MH117653–02, Alternative Approaches to Supporting ASD Services for Young Adults. This project was also supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under UJ2MC31073: Maternal and Child Health-Autism Transitions Research Project and cooperative agreement UT2MC39440, Autism Intervention Research Network on Physical Health (AIR-P). The information, content and/or conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S. Government.
Appendix Table A1.
Unadjusted and Adjusted Regressions, ASD Only, ASD+ID, ID Only, and MHC as Predictors for Risk Factors or Comorbidities, Odds Ratios [99% Confidence Intervals]
| Population ASD/ID | Random Sample (no ASD/ID) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| ASD Only (N = 31,101) | ASD+ID (N = 52,049) | ID Only (N = 563,558) | MHC (N = 372,807) | ||||||
|
| |||||||||
| OR [99% CI] | OR [99% CI] | OR [99% CI] | OR [99% CI] | ||||||
|
| |||||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | ||
|
| |||||||||
| Risk Factor | |||||||||
|
| |||||||||
| 1 | Living in primary care shortage areas, high | 1.34 [1.29, 1.39] | 0.87 [0.83,0.91] | 1.29 [1.25, 1.33] | 0.87 [0.83,0.91] | 1.44 [1.42, 1.46] | 0.99 [0.97,1.01]a | 1.43 [1.41, 1.45] | 1.02 [1.00,1.03]a |
| 2 | Living in a residential facility | 18.37 [17.52, 19.26] | 8.87 [8.29,9.50] | 95.94 [92.52, 99.49] | 58.73 [55.36,62.31] | 66.37 [64.46, 68.33] | 32.25 [31.05,33.49] | 4.61 [4.46, 4.77] | 2.77 [2.67,2.88] |
| 3 | Receiving services in the home regularly from outside caregivers Having had a hospitalization | 9.31 [8.94, 9.7] | 3.92 [3.71,4.14] | 16.87 [16.36, 17.40] | 6.57 [6.28,6.87] | 15.47 [15.16, 15.79] | 7.02 [6.82,7.22] | 4.45 [4.35, 4.56] | 2.53 [2.47,2.60] |
| 4 | (psychiatric or inpatient) for at least 30 days over the 5 years | 7.41 [6.48, 8.48] | 3.28 [2.77,3.90] | 8.14 [7.30, 9.08] | 2.79 [2.41,3.23] | 8.16 [7.59, 8.77] | 3.85 [3.50,4.24] | 6.62 [6.14, 7.14] | 4.10 [3.78,4.46] |
| 5 | Avoidable hospitalizations | ||||||||
| 6 | Any | 3.07 [2.98, 3.17] | 2.34 [2.25,2.42] | 6.47 [6.31, 6.64] | 4.89 [4.73,5.05] | 5.78 [5.72, 5.84] | 3.51 [3.45,3.57] | 5.28 [5.22, 5.34] | 3.61 [3.56,3.65] |
| 7 | COPD, chronic bronchitis, asthma | 2.33 [2.24, 2.42] | 1.57 [1.50,1.64] | 2.26 [2.19, 2.32] | 1.41 [1.36,1.46] | 3.05 [3.01, 3.09] | 1.74 [1.71,1.78] | 4.92 [4.85, 4.99] | 3.26 [3.21,3.31] |
| 8 | Congestive heart failure | 1.52 [1.37, 1.68] | 0.98 [0.87,1.09]a | 1.77 [1.64, 1.92] | 0.82 [0.75,0.90] | 3.56 [3.45, 3.67] | 1.32 [1.27,1.37] | 3.63 [3.51, 3.75] | 1.91 [1.84,1.98] |
| 9 | Constipation, fecal impaction, and obstipation | 4.49 [4.21, 4.78] | 4.09 [3.78,4.42] | 11.58 [11.13, 12.04] | 9.59 [9.01,10.20] | 8.34 [8.12, 8.56] | 5.46 [5.24,5.70] | 4.41 [4.28, 4.55] | 3.15 [3.05,3.25] |
| 10 | Dehydration, volume depletion including acute renal failure and hyponatremia | 3.27 [3.02, 3.54] | 1.82 [1.65,1.99] | 5.47 [5.19, 5.77] | 2.46 [2.30,2.64] | 5.76 [5.59, 5.94] | 2.39 [2.30,2.49] | 5.32 [5.15, 5.50] | 2.95 [2.85,3.06] |
| 11 | Hypertension and hypotension | 1.81 [1.74, 1.89] | 1.86 [1.76,1.95] | 2.29 [2.22, 2.36] | 1.85 [1.77,1.92] | 3.01 [2.96, 3.05] | 1.67 [1.64,1.71] | 3.56 [3.51, 3.61] | 2.33 [2.29,2.37] |
| 12 | Poor glycemic control | 1.96 [1.85, 2.08] | 1.72 [1.61,1.84] | 1.93 [1.84, 2.02] | 1.46 [1.38,1.54] | 2.44 [2.39, 2.49] | 1.35 [1.32,1.39] | 2.81 [2.76, 2.87] | 1.75 [1.71,1.79] |
| 13 | Seizures | 11.78 [11.24, 12.34] | 3.81 [3.59,4.05] | 34.41 [33.26, 35.6] | 10.34 [9.87,10.84] | 25.76 [25.10, 26.43] | 9.17 [8.87,9.48] | 5.92 [5.75, 6.09] | 3.41 [3.30,3.51] |
| 14 | Urinary tract infection | 1.23 [1.18, 1.29] | 1.76 [1.66,1.86] | 2.00 [1.93, 2.06] | 3.07 [2.94,3.21] | 2.29 [2.26, 2.32] | 2.85 [2.77,2.92] | 2.87 [2.83, 2.91] | 2.56 [2.52,2.60] |
| 15 | Weight loss (failure to thrive) and nutritional deficiencies | 6.05 [5.59, 6.55] | 3.89 [3.51,4.31] | 16.05 [15.26, 16.88] | 9.57 [8.85,10.35] | 11.14 [10.73, 11.57] | 6.44 [6.10,6.81] | 4.85 [4.65, 5.06] | 3.25 [3.10,3.40] |
|
| |||||||||
| Comorbidities | |||||||||
|
| |||||||||
| 16 | Any | 2.07 [2.00, 2.14] | 1.50 [1.44,1.56] | 2.44 [2.37, 2.50] | 1.52 [1.47,1.57] | 3.18 [3.14, 3.21] | 1.55 [1.52,1.57] | 3.92 [3.87, 3.96] | 2.56 [2.53,2.60] |
| 17 | Cancer | 1.90 [1.77, 2.04] | 1.54 [1.42,1.67] | 2.29 [2.17, 2.41] | 1.59 [1.49,1.69] | 3.20 [3.13, 3.28] | 1.44 [1.39,1.49] | 3.26 [3.18, 3.34] | 2.03 [1.97,2.08] |
| 18 | Chronic kidney disease | 1.78 [1.65, 1.91] | 0.89 [0.82,0.96] | 2.32 [2.21, 2.44] | 0.95 [0.89,1.01]a | 3.03 [2.96, 3.10] | 1.16 [1.12,1.19] | 3.23 [3.15, 3.31] | 1.87 [1.82,1.92] |
| 19 | Chronic lung disease | 2.18 [2.05, 2.32] | 1.37 [1.27,1.46] | 1.88 [1.79, 1.98] | 0.96 [0.90,1.02]a | 3.62 [3.54, 3.69] | 1.61 [1.56,1.65] | 6.68 [6.55, 6.82] | 3.89 [3.81,3.98] |
| 20 | Diabetes | 1.62 [1.55, 1.70] | 1.48 [1.40,1.56] | 1.74 [1.68, 1.80] | 1.26 [1.21,1.32] | 2.42 [2.38, 2.46] | 1.25 [1.23,1.28] | 2.61 [2.57, 2.65] | 1.64 [1.61,1.67] |
| 21 | Immunocompromised | 2.97 [2.34, 3.76] | 1.17 [0.88,1.54]a | 2.88 [2.37, 3.49] | 0.97 [0.76,1.22]a | 2.36 [2.13, 2.61] | 0.86 [0.75,0.98] | 3.41 [3.08, 3.77] | 2.00 [1.79,2.23] |
| 22 | Previous Organ Transplant Recipient | 1.49 [1.19, 1.87] | 0.29 [0.23,0.38] | 0.97 [0.78, 1.21]a | 0.16 [0.13,0.21] | 1.41 [1.30, 1.52] | 0.27 [0.24,0.29] | 1.59 [1.46, 1.73] | 0.76 [0.69,0.83] |
| 23 | Serious Heart Conditions | 1.52 [1.39, 1.67] | 0.98 [0.89,1.09]a | 1.75 [1.64, 1.88] | 0.8 [0.74,0.87] | 3.55 [3.45, 3.64] | 1.30 [1.25,1.34] | 3.70 [3.59, 3.80] | 1.95 [1.89,2.02] |
| 24 | Severe Obesity | 2.39 [2.27, 2.51] | 2.37 [2.23,2.52] | 2.26 [2.17, 2.35] | 2.16 [2.05,2.28] | 2.23 [2.18, 2.27] | 1.89 [1.83,1.95] | 2.86 [2.80, 2.91] | 2.16 [2.11,2.21] |
| 25 | Sickle Cell Disease | 1.76 [1.17, 2.67] | 0.57 [0.37,0.90] | 1.10 [0.74, 1.65]a | 0.25 [0.16,0.39] | 1.43 [1.22, 1.66] | 0.48 [0.40,0.58] | 2.22 [1.91, 2.59] | 1.50 [1.27,1.76] |
Notes: ASD=autism spectrum disorder, ID=intellectual disability, MHC=mental health conditions. Adjusted regressions control for age group, sex, race, ethnicity, eligibility type, insurance type, Medicare dual eligibility and state; comparison is to the random sample with no ID, ASD, or MHC.
p-value is not less than 0.01 (for all other estimates, p<0.01)
References
- Akiyama Matthew J, Spaulding Anne C, and Rich Josiah D (2020), ‘Flattening the curve for incarcerated populations—Covid-19 in jails and prisons’, New England Journal of Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameis Stephanie H, et al. (2020), ‘Coping, fostering resilience, and driving care innovation for autistic people and their families during the COVID-19 pandemic and beyond’, Molecular Autism, 11 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty George David, Deary Ian, and Altschul Drew (2021), ‘Pre-pandemic mental and physical health as predictors of COVID-19 vaccine hesitancy: evidence from a UK-wide cohort study’, medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke James P, et al. (2014), ‘Does a claims diagnosis of autism mean a true case?’, Autism, 18 (3), 321–30. [DOI] [PubMed] [Google Scholar]
- Center for Public Representation (2020), ‘COVID-19 Medical Rationing & Facility Visitation Policies’, <https://www.centerforpublicrep.org/covid-19-medical-rationing/>, accessed 10/28/2020.
- Centers for Medicare & Medicaid Services (2017), ‘Behavioral Health Services’, <https://www.medicaid.gov/medicaid/benefits/bhs/index.html>, accessed September 26.
- Cooney Fionnuala (2009), ‘Patient satisfaction with a hepatitis B vaccination programme among persons with an intellectual disability’, Journal of Intellectual Disabilities, 13 (3), 203–19. [DOI] [PubMed] [Google Scholar]
- Cooper Sally-Ann, et al. (2015), ‘Multiple physical and mental health comorbidity in adults with intellectual disabilities: population-based cross-sectional analysis’, BMC family practice, 16 (1), 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen Lisa A, et al. (2015a), ‘The health status of adults on the autism spectrum’, Autism, 19 (7), 814–23. [DOI] [PubMed] [Google Scholar]
- Croen Lisa A (2015b), ‘The health status of adults on the autism spectrum’, Autism, 19 (7), 814–23. [DOI] [PubMed] [Google Scholar]
- Davidson Patricia M and Szanton Sarah L (2020), ‘Nursing homes and COVID-19: we can and should do better’, Journal of clinical nursing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon Meghan N, et al. (2018), ‘Psychiatric and medical conditions in transition-aged individuals with ASD’, Pediatrics, 141 (Supplement 4), S335–S45. [DOI] [PubMed] [Google Scholar]
- DaWalt Leann S, et al. (2019), ‘Mortality in individuals with autism spectrum disorder: Predictors over a 20-year period’, Autism, 23 (7), 1732–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert Marc, et al. (2011a), ‘Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care’, World psychiatry, 10 (1), 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert Marc, et al. (2011b), ‘Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level’, World psychiatry, 10 (2), 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Lima Matheus Eugênio, Barros Levi Coelho Maia, and Aragão Gislei Frota (2020), ‘Could autism spectrum disorders be a risk factor for COVID-19?’, Medical Hypotheses, 109899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Winter CF, et al. (2012a), ‘Overweight and obesity in older people with intellectual disability’, Research in developmental disabilities, 33 (2), 398–405. [DOI] [PubMed] [Google Scholar]
- De Winter CF (2012b), ‘Cardiovascular risk factors (diabetes, hypertension, hypercholesterolemia and metabolic syndrome) in older people with intellectual disability: results of the HA-ID study’, Research in developmental disabilities, 33 (6), 1722–31. [DOI] [PubMed] [Google Scholar]
- Disabilities;, Association of University Centers on ‘Frequently Asked Questions (FAQ): COVID-19 Vaccine Distribution Considerations for the Disability Community’, <https://www.aucd.org/docs/resources/network_covid_FAQ%2011.22.30.pdf>, accessed.
- Druss Benjamin G (2020), ‘Addressing the COVID-19 pandemic in populations with serious mental illness’, JAMA psychiatry. [DOI] [PubMed] [Google Scholar]
- Fortuna Robert J, et al. (2016), ‘Health conditions and functional status in adults with autism: A cross-sectional evaluation’, Journal of General Internal Medicine, 31 (1), 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli Giacomo, et al. (2020), ‘Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy’, Jama, 323 (16), 1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim Danny (2020), ‘‘It’s Hit Our Front Door’: Homes for the Disabled See a Surge of Covid-19’, New York Times. [Google Scholar]
- Hand Brittany N, et al. (2020), ‘Prevalence of physical and mental health conditions in Medicare-enrolled, autistic older adults’, Autism, 24 (3), 755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvikoski Tatja, et al. (2016), ‘Premature mortality in autism spectrum disorder’, The British Journal of Psychiatry, 208 (3), 232–38. [DOI] [PubMed] [Google Scholar]
- Hogg James and Tuffrey-Wijne Irene (2008), ‘Cancer and intellectual disability: a review of some key contextual issues’, Journal of Applied Research in Intellectual Disabilities, 21 (6), 509–18. [Google Scholar]
- Holman E Alison, et al. (2020), ‘The unfolding COVID-19 pandemic: A probability-based, nationally representative study of mental health in the United States’, Science advances, 6 (42), eabd5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes Emily A, et al. (2020), ‘Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science’, The Lancet Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh K, Rimmer JH, and Heller T (2014), ‘Obesity and associated factors in adults with intellectual disability’, Journal of Intellectual Disability Research, 58 (9), 851–63. [DOI] [PubMed] [Google Scholar]
- Iadarola Suzannah, et al. (2021), ‘COVID 19 Vaccine Perceptions in the New York State Intellectual and Developmental Disabilities Community’, medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpur Arun, et al. (2021), ‘Brief Report: Impact of COVID-19 in Individuals with Autism Spectrum Disorders: Analysis of a National Private Claims Insurance Database’, Journal of autism and developmental disorders, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinner Stuart A, et al. (2020), ‘Prisons and custodial settings are part of a comprehensive response to COVID-19’, The Lancet Public Health, 5 (4), e188–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohane, Isaac S, et al. (2012), ‘The co-morbidity burden of children and young adults with autism spectrum disorders’, PloS one, 7 (4), e33224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Meng-Chuan, et al. (2019), ‘Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis’, The Lancet Psychiatry, 6 (10), 819–29. [DOI] [PubMed] [Google Scholar]
- Li Luming (2020), ‘Challenges and priorities in responding to COVID-19 in inpatient psychiatry’, Psychiatric Services, appi. ps. 202000166. [DOI] [PubMed] [Google Scholar]
- Luykx Jurjen J, Vinkers Christiaan H, and Tijdink Joeri K(2020), ‘Psychiatry in Times of the Coronavirus Disease 2019 (COVID-19) Pandemic: An Imperative for Psychiatrists to Act Now’, JAMA Psychiatry. [DOI] [PubMed] [Google Scholar]
- MacRae Siobhan, et al. (2015), ‘Diabetes in people with intellectual disabilities: a systematic review of the literature’, Research in developmental disabilities, 47, 352–74. [DOI] [PubMed] [Google Scholar]
- Maddox Brenna B and Gaus Valerie L (2019), ‘Community mental health services for autistic adults: Good news and bad news’, Autism in Adulthood, 1 (1), 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary Marty (2020), ‘Risk Factors for COVID-19 Mortality among Privately Insured Patients: A Claims Data Analysis’, (FAIR Health). [Google Scholar]
- Malik-Soni Natasha, et al. (2021), ‘Tackling healthcare access barriers for individuals with autism from diagnosis to adulthood’, Pediatric Research, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazereel Victor, et al. (2021), ‘COVID-19 vaccination for people with severe mental illness: why, what, and how?’, The Lancet Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon Martin and Hatton Chris (2021), ‘A comparison of the prevalence of health problems among adults with and without intellectual disability: A total administrative population study’, Journal of Applied Research in Intellectual Disabilities, 34 (1), 316–25. [DOI] [PubMed] [Google Scholar]
- McMichael Temet M (2020), ‘COVID-19 in a long-term care facility—King County, Washington, February 27–March 9, 2020’, MMWR. Morbidity and Mortality Weekly Report, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci MaryBeth; Chidambaram Priya(2021), ‘COVID-19 Vaccine Access for People with Disabilities’, <https://www.kff.org/medicaid/issue-brief/covid-19-vaccine-access-for-people-with-disabilities/>, accessed June 16, 2021.
- Nikpouraghdam Mohamad, et al. (2020), ‘Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: A single center study’, Journal of Clinical Virology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder Graziano, Rezza Giovanni, and Brusaferro Silvio (2020), ‘Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy’, Jama, 323 (18), 1775–76. [DOI] [PubMed] [Google Scholar]
- Öngür Dost, Perlis Roy, and Goff Donald (2020), ‘Psychiatry and COVID-19’, Jama, 324 (12), 1149–50. [DOI] [PubMed] [Google Scholar]
- Rains Luke Sheridan, et al. (2020), ‘Early impacts of the COVID-19 pandemic on mental health care and on people with mental health conditions: framework synthesis of international experiences and responses’, Social psychiatry and psychiatric epidemiology, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson Safiya, et al. (2020), ‘Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area’, Jama. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin Rita (2020), ‘The challenge of preventing COVID-19 spread in correctional facilities’, Jama. [DOI] [PubMed] [Google Scholar]
- Salari Nader, et al. (2020), ‘Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis’, Globalization and health, 16 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott Whitney, et al. (2021), ‘Autism Grows Up: Medicaid’s Role in Serving Adults on the Spectrum’, Psychiatric Services, 72 (5), 597–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott Kate M, et al. (2007), ‘Depression–anxiety relationships with chronic physical conditions: results from the World Mental Health Surveys’, Journal of affective disorders, 103 (1–3), 113–20. [DOI] [PubMed] [Google Scholar]
- Segal Misha, et al. (2014), ‘Medicare-Medicaid eligible beneficiaries and potentially avoidable hospitalizations’, Medicare & Medicaid Research Review, 4 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet Maxime, et al. (2020), ‘Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA’, The Lancet Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Katie; LaFraniere Sharon; Weiland Noah; Goodnough Abby; Haberman Maggie (2020), ‘F.D.A. Clears Pfizer Vaccine, and Millions of Doses Will Be Shipped Right Away’, New York Times, December 11, 2020. [Google Scholar]
- Tomlins Jennifer, et al. (2020), ‘Clinical features of 95 sequential hospitalised patients with novel coronavirus 2019 disease (COVID-19), the first UK cohort’, Journal of Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins Lucy; Mitch Smith; Julie Bosman; Brian Pietsch(2021), ‘Entering uncharted territory, the U.S. counts 500,000 Covid-related deaths’, The New York Times, February 22, 2021. [Google Scholar]
- Tsai Jack and Wilson Michal (2020), ‘COVID-19: a potential public health problem for homeless populations’, The Lancet Public Health, 5 (4), e186–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk Margaret A, et al. (2020), ‘Intellectual and Developmental Disability and COVID-19 Case-Fatality Trends: TriNetX Analysis’, Disability and Health Journal, 100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler Carl V, et al. (2011), ‘Chronic disease risks in young adults with autism spectrum disorder: forewarned is forearmed’, American journal on intellectual and developmental disabilities, 116 (5), 371–80. [DOI] [PubMed] [Google Scholar]
- U.S. Centers for Disease Control (2020), ‘People with Certain Medical Conditions’, <https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html>, accessed 9/10/2020.
- Vohra Rini, Madhavan Suresh, and Sambamoorthi Usha (2017), ‘Comorbidity prevalence, healthcare utilization, and expenditures of Medicaid enrolled adults with autism spectrum disorders’, Autism, 21 (8), 995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QuanQiu, Xu Rong, and Volkow Nora D (2020), ‘Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States’, World Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss Jonathan A, et al. (2018), ‘Health concerns and health service utilization in a population cohort of young adults with autism spectrum disorder’, Journal of Autism and Developmental Disorders, 48 (1), 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfenden SUE, et al. (2012), ‘A systematic review of two outcomes in autism spectrum disorder–epilepsy and mortality’, Developmental Medicine & Child Neurology, 54 (4), 306–12. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2020), ‘Disability considerations during the COVID-19 outbreak’, (World Health Organization; ). [Google Scholar]
- Xiang Yu-Tao, et al. (2020), ‘The COVID-19 outbreak and psychiatric hospitals in China: managing challenges through mental health service reform’, International journal of biological sciences, 16 (10), 1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Jing, et al. (2020), ‘Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis’, International Journal of Infectious Diseases. [Google Scholar]
- Zheng Zhen, et al. (2017), ‘Association among obesity, overweight and autism spectrum disorder: a systematic review and meta-analysis’, Scientific reports, 7 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]