Abstract
The prothrombotic state in atrial fibrillation (AF) occurs as a result of multifaceted interactions, known as Virchow’s triad of hypercoagulability, structural abnormalities, and blood stasis. More recently, there is emerging evidence that lipoproteins are implicated in this process, beyond their traditional role in atherosclerosis. In this review, we provide an overview of the various lipoproteins and explore the association between lipoproteins and AF, the effects of lipoproteins on haemostasis, and the potential contribution of lipoproteins to thrombogenesis in AF. There are several types of lipoproteins based on size, lipid composition, and apolipoprotein category, namely: chylomicrons, very low-density lipoprotein, low-density lipoprotein (LDL), intermediate-density lipoprotein, and high-density lipoprotein. Each of these lipoproteins may contain numerous lipid species and proteins with a variety of different functions. Furthermore, the lipoprotein particles may be oxidized causing an alteration in their structure and content. Of note, there is a paradoxical inverse relationship between total cholesterol and LDL cholesterol (LDL-C) levels, and incident AF. The mechanism by which this occurs may be related to the stabilizing effect of cholesterol on myocardial membranes, along with its role in inflammation. Overall, specific lipoproteins may interact with haemostatic pathways to promote excess platelet activation and thrombin generation, as well as inhibiting fibrinolysis. In this regard, LDL-C has been shown to be an independent risk factor for thromboembolic events in AF. The complex relationship between lipoproteins, thrombosis and AF warrants further research with an aim to improve our knowledge base and contribute to our overall understanding of lipoprotein-mediated thrombosis.
Keywords: Atrial fibrillation, Lipids, Lipoproteins, Low-density lipoprotein, Very low-density lipoprotein, High-density lipoprotein, Oxidized lipoprotein, Lipoprotein(a), Incidence, Haemostasis, Thrombosis, Thromboembolism, Stroke
1. Introduction
Atrial fibrillation (AF) is a multi-systemic condition that is associated with serious complications including thromboembolism, dementia, and heart failure, resulting in impaired quality of life, significant morbidity, and increased mortality.1–5 The prevalence of AF rises with age and concomitant comorbidities.6,7 At present, there is an upward trajectory to the global incidence and prevalence of AF.8,9 Indeed, every individual has a one-in-four lifetime risk of developing this condition,10,11 with a greater burden amongst those with risk factors.12 By 2060, it is projected that at least 17.9 million people in Europe will be affected by AF.13,14
The mechanism by which AF occurs is complex but has previously been described in detail.15 Management of patients with the condition is primarily focused on the prevention of thromboembolism due to the presence of a prothrombotic state with this arrhythmia. The prothrombotic or hypercoagulable state in AF occurs as a result of multifaceted interactions, known as Virchow’s triad of hypercoagulability, structural abnormalities, and blood stasis.16 Despite considerable research in this area, the precise mechanisms by which AF contributes to a prothrombotic state remains ill-defined.
There is emerging evidence that lipoproteins are implicated in thrombogenesis, beyond their traditional role in atherosclerosis. In this review, we provide an overview of the various lipoproteins and explore their relationship with AF, haemostasis, and the potential contribution to thrombogenesis.
2. Lipoproteins
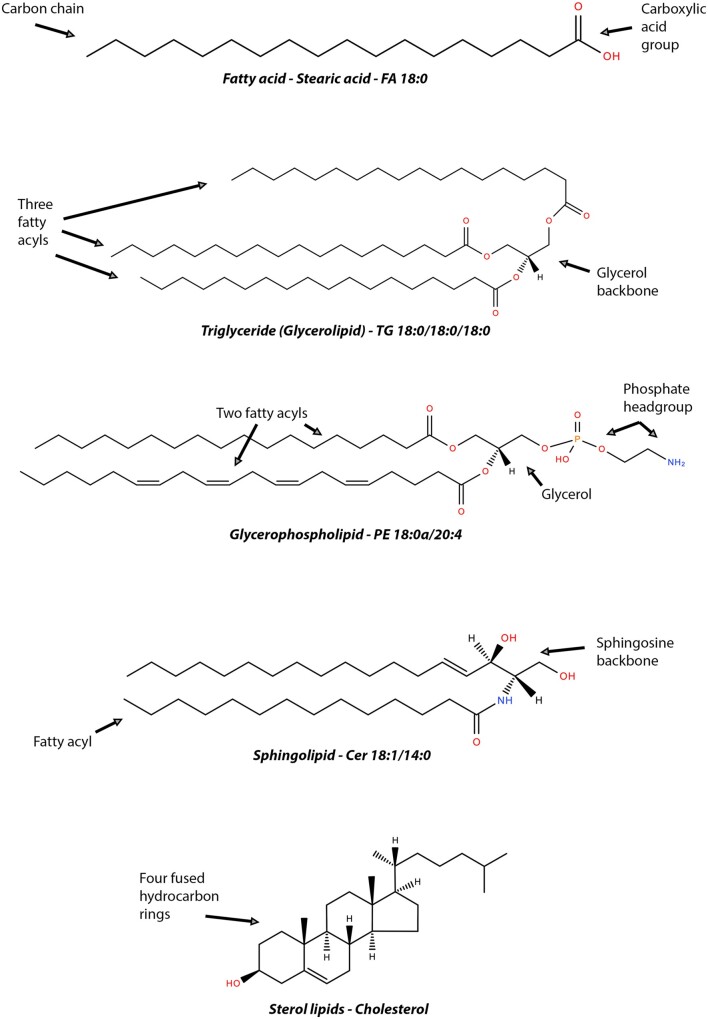
Lipids (also known as ‘fat’) are naturally occurring compounds serving numerous biological functions including the formation of plasma membranes or signalling molecules, and as a source of energy. They exist in several forms including free fatty acids, glycerolipids (GL), glycerophospholipids (GPL), sphingolipids, and sterol lipids. Each of these lipid subtypes have different molecular structures and basic properties (Figure 1). As a brief overview, fatty acids form the fundamental category of biological lipids and therefore the basic building blocks of more complex lipids. Their chemistry consists of a hydrocarbon chain with a terminal carboxylic acid group and may be defined as saturated or unsaturated depending on the maximum possible number of bonds or hydrogen atoms.17,18 GL consist of a single glycerol molecule which acts as the backbone for attachment to fatty acid chains. The most relevant example of GL are triglycerides (TG), which contain three fatty acid chains and play an important role in metabolism as energy sources and sources of dietary fat.18,19 Sterol lipids consist of four fused rings of hydrocarbon to which other molecules attach. A major type of sterol lipid is cholesterol which serves as a precursor for the synthesis of other steroids as well as serving as structural support for plasma membranes.20,21 Dietary cholesterol is often stored and transported in the form of a cholesterol ester (CE), which chemically represents a cholesterol molecule joined to a fatty acid via an ester bond.22
Figure 1.
Representative schematic of lipid subtypes. Example structures from each LIPID MAPS category of lipids are shown in this figure highlighting their structural features. Fatty acids (FA), which may be saturated or unsaturated, form the basic building blocks of lipids, with each class having specific defining feature. Chemical structures are from PubChem and LIPID MAPS.
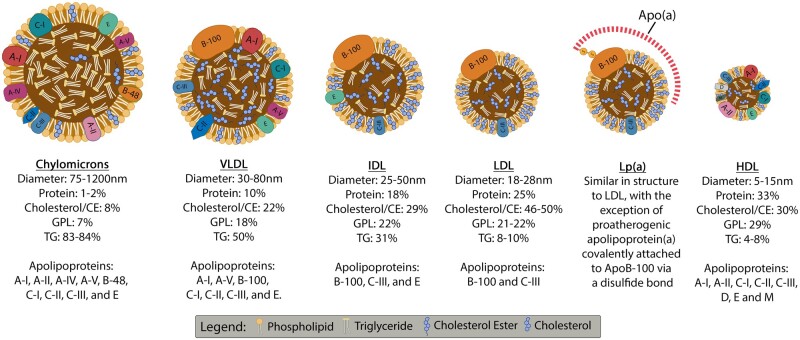
One common feature that lipids share as a group is their insolubility in water. Consequently, they must be transported with proteins in the circulation (‘lipoproteins’).23 Lipoproteins are complex structures consisting of a central hydrophobic core primarily composed of CE and TG which is surrounded by a hydrophilic membrane comprising of GPL, free cholesterol, and apolipoproteins.23,24 There are several types of lipoproteins based on size, lipid composition, and apolipoprotein category, namely: chylomicrons, very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), intermediate-density lipoprotein, and high-density lipoprotein (HDL). When elevated, all lipoproteins confer a pro-atherogenic risk, apart from HDL which is anti-atherogenic.23 Each lipoprotein contains numerous types of lipid species and proteins, whose composition varies even between individual lipoproteins of the same type (Figure 2).
Figure 2.
Lipoprotein types and structures. Representative description of typical diameter, content and apolipoprotein constituents of different lipoprotein classes.23 Created using Biorender.com. ApoB-100, apolipoprotein B100; CE, cholesterol ester; GPL, glycerophospholipids; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); TG, triglycerides; VLDL, very low-density lipoprotein.
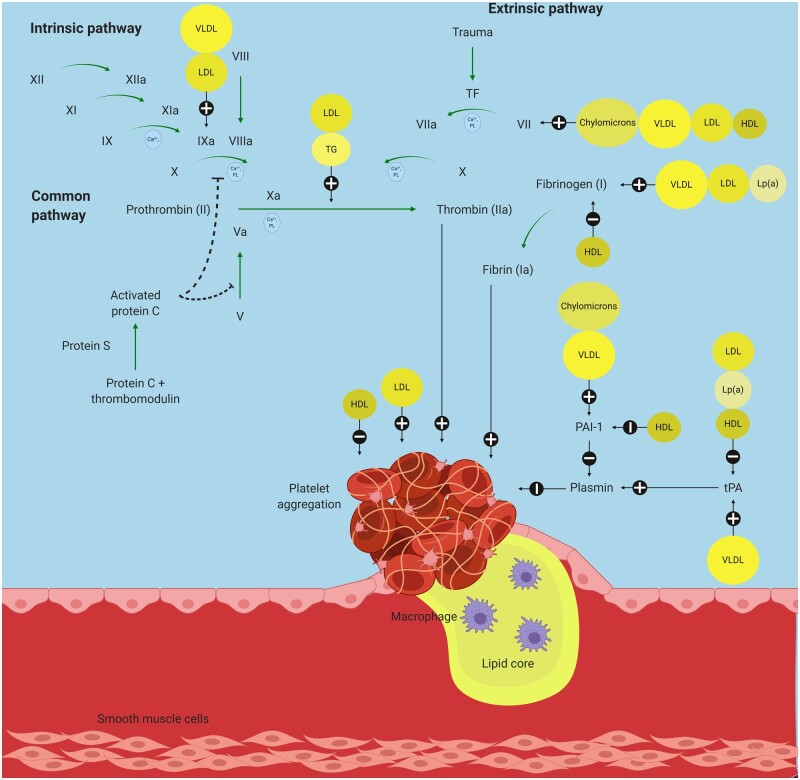
Figure 3.
Effects of lipoproteins on haemostasis. Created using Biorender.com. HDL, high-density lipoprotein; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); PAI-1, plasminogen activator inhibitor-1; TF, tissue factor; TG, triglycerides; tPA, tissue plasminogen activator; VLDL, very low-density lipoprotein.
LDL is the main transporter for cholesterol in the circulation and every LDL particle contains one apolipoprotein B100 molecule. LDL exists in a spectrum that varies in size and density with the three major density subclasses being small dense LDL (sdLDL), intermediate LDL, and large buoyant LDL.25 Small dense LDLs are considered more atherogenic and pro-coagulant compared to the other subtypes of LDL for various features as they have decreased affinity for LDL receptors and hence remain longer in the circulation, more readily enter the arterial intima where they are engulfed by macrophages to become foam cells, and are more susceptible to oxidation than its larger counterpart.26–28 There is also increasing evidence that the number of ApoB-rich particles or the concentration of apolipoprotein B may contribute to atherogenic risk.29
Modern lipidomic techniques, with the aid of liquid chromatography coupled to mass spectrometry, have allowed for detailed characterization of the LDL lipidome.30 This has revealed over 300 different lipid species residing within the interior or phospholipid membrane of the LDL particle. Each of these may have specific associations with various pathologies and interactions with traditional risk factors, thereby adding to its complexities.31,32 Oxidative modification of LDL, predominantly by non-enzymatic processes, leads to the formation of oxidized LDL (OxLDL) particles. These particles have altered structure and content, containing oxidized proteins and lipids (particularly GPL), and leading to a more atherogenic phenotype.33 Furthermore, the susceptibility of LDL to aggregation and proteoglycan binding has provided a deeper insight into the atherogenicity of LDL.34
Lipoprotein(a) [Lp(a)] is a specialized form of LDL assembled in the liver from LDL and apolipoprotein(a) attached to apolipoprotein B100 via a disulphide bridge (Figure 2).35 Lp(a) has been implicated in atherogenesis by enhancing endothelial cell adhesion and molecule expression, promoting the formation of foam cells by binding to macrophages with high affinity and interfering with vascular permeability.36 Furthermore, the Lp(a) constituent, apolipoprotein(a), shares many structural similarities with plasminogen which has been reported to cause interference with the physiological fibrinolysis process and to contribute to a prothrombotic phenotype.37
3. Lipoproteins and atrial fibrillation
3.1 The paradoxical inverse relationship between cholesterol and the incidence of AF
The association between serum cholesterol and coronary heart disease has been described since early 1964.38 There is an increased risk of coronary heart disease with elevated total cholesterol (TC) and LDL cholesterol (LDL-C), and reduced HDL cholesterol (HDL-C) levels.39,40 A longitudinal analysis over a 35-year period of patients from the Framingham study confirmed that long-term exposure to these lipid abnormalities led to a greater risk of atherosclerotic cardiovascular disease and mortality.41 Moreover, both the LDL particle and LDL-C are now considered causal for atherosclerotic cardiovascular disease.42 In turn, atherosclerotic disease is an established independent risk factor for incident AF.43,44 As such, elevated levels of TC and LDL-C may have been expected to increase the risk of incident AF. However, current evidence does not support this and in contrast, several well-conducted observational studies have described a paradoxical inverse relationship between TC and LDL-C, and incident AF (Table 1).
Table 1.
Impact of lipoprotein abnormalities on incidence or prevalence of atrial fibrillation
| Author, year (ref) | Study type | Population | n | Follow-up (months) | Finding(s) in relation to incidence or prevalence of AF |
|---|---|---|---|---|---|
| Harrison, 202045 | Prospective | Community-based cohort | 13 724 | NA |
↑ TC: PR 0.61 (95% CI 0.49–0.75) ↑ LDL-C: PR 0.60 (95% CI 0.48–0.75) ↑ HDL-C: PR 0.58 (95% CI 0.46–0.74) ↑ non-HDL-C: PR 0.63 (95% CI 0.51–0.78) ↑ LDL-C/HDL-C ratio: PR 0.75 (95% CI 0.61–0.94) |
| Xue, 201946 | Prospective | STEMI | 985 | 31 |
↑ TC: HR 0.54 (95% CI 0.32–0.90) ↑ LDL-C: HR 0.56 (95% CI 0.31–1.00) TG or HDL-C not found to be risk factors |
| Choe, 201847 | Retrospective | Population-based cohort | 22 886 661 | 65 |
↑ TG: HR 1.12 (95% CI 1.12–1.13) ↑ HDL: HR 1.24 (95% CI 1.23–1.25) |
| Li, 201848 | Prospective | Community-based cohort | 88 785 | 85 |
↑ TC: HR 0.60 (95% CI 0.43–0.84) ↑ LDL-C: HR 0.60 (95% CI 0.43–0.83) TG or HDL-C not found to be risk factors |
| Mourtzinis, 201849 | Retrospective | Hypertensive | 51 020 | 42 |
↑ TC: HR 0.84 (95% CI 0.78–0.92) ↑ LDL-C: HR 0.86 (95% CI 0.79–0.97) TG or HDL-C not found to be risk factors |
| Liu, 201850 | Prospective | Chronic heart failure | 308 | 36 |
↑ TC: HR 0.99 (95% CI 0.97–1.00) ↑ LDL-C: HR 0.98 (95% CI 0.97–1.00) HDL-C not found to be risk factor |
| Ulus, 201851 | Prospective | Elderly (>65 years) with ACS undergoing PCI | 308 | NA | ↑ MHR: OR 1.10 (95% CI 1.05–1.15) |
| Kim, 201852 | Retrospective | Community-based cohort of males | 21 981 | 104 | TG or HDL-C not found to be risk factors |
| Kokubo, 201753 | Prospective | Community-based cohort | 6898 | 166 | TC, TG or HDL-C not found to be risk factors |
| Aronis, 201754 | Prospective | Community-based cohort | 9908 | 167 | ↑ Lp(a) not found to be risk factor |
| Saskin, 201755 | Retrospective | Isolated CABG | 662 | 0.23 | ↑ MHR: OR 11.5 (95% CI 1.25–106.67) |
| Krittayaphong, 201656 | Retrospective | Hypertensive | 13 207 | NA | ↑ LDL-C: OR 0.53 (95% CI 0.37–0.78) |
| Alonso, 201457 | Prospective | Community-based cohort | 7142 | 115 |
↑ HDL-C: HR 0.64 (95% CI 0.48–0.87) ↑ TG: HR 1.60 (95% CI 1.25–2.05) TC and LDL-C not found to be risk factors |
| Mora, 201458 | Prospective | Healthy female healthcare professionals | 23 738 | 197 |
↑ LDL-C: HR 0.72 (95% CI 0.56–0.92) ↑ VLDL-particles: HR 0.78 (95% CI 0.61–0.99) ↑ LDL-particles: HR 0.77 (95% CI 0.60–0.99) ↑ Cholesterol-poor small LDL: HR 0.78 (95% CI 0.61–1.00) ↑ Small VLDL particles: HR 0.78 (95% CI 0.62–0.99) Larger cholesterol-rich LDL-particles, total HDL-C, Lp(a) and TG not found to be risk factors |
| Lopez, 201259 | Prospective | Community-based cohort | 13 044 | 224 |
↑ LDL-C: HR 0.90 (95% CI 0.85–0.96) ↑ TC: HR 0.89 (95% CI 0.84–0.95) HDL-C, TG and use of lipid-lowering medications not found to be risk factors |
| Watanabe, 201160 | Prospective | Community-based cohort | 28 449 | 54 |
↑ HDL-C in females: HR 0.35 (95% CI 0.18–0.67) ↑ HDL-C in males not found to be risk factor: HR 0.74 (95% CI 0.42–1.30) ↑ TC: HR 0.94 (95% CI 0.90–0.97) ↑ LDL-C: HR 0.92 (95% CI 0.88–0.96) |
| Iguchi, 201061 | Prospective | Community-based cohort | 30 449 | NA | Hypercholesterolaemia, as defined by TC >220 mg/dL or the use of cholesterol-lowering agents: OR 0.75 (95% CI 0.58–0.96) |
| Haywood, 200962 | Prospective | Hypertensive | 39 056 | NA | ↑ HDL-C: OR 0.77 (95% CI 0.62–0.95) |
| Rosengren, 200963 | Prospective | Community-based cohort of males | 6903 | 412 | TC not found to be risk factor |
| Frost, 200564 | Prospective | Population-based cohort without endocrine or cardiovascular diseases at baseline | 47 589 | 68 |
(Females) ↑ TC: HR 0.57 (95% CI 0.42–0.78) TC not found to be a risk factor in males |
ACS, acute coronary syndrome; AF, atrial fibrillation; CABG, coronary artery bypass graft; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); MHR, monocyte- to high-density lipoprotein cholesterol ratio; NA, not applicable; OR, odds ratio; PCI, percutaneous coronary intervention; PR, prevalence ratio; STEMI, ST-elevation myocardial infarction; TC, total cholesterol; TG, triglycerides; VLDL-C, very-low-density lipoprotein cholesterol.
A health survey performed by Iguchi et al.61 found that hypercholesterolaemia, defined by TC >220 mg/dL or the use of cholesterol-lowering agents, was related to reduced new-onset AF. Reduced levels of LDL-C has also been linked to increased prevalence of AF.56 In one study of 88 785 patients, for example, TC and LDL-C levels were inversely linked to incident AF over a follow-up period of 7 years.48 The authors reported no significant association between incident AF, and HDL-C or TG. However, the overall incidence of AF was extremely low at 0.52 per 1000 person-years.48 Similar findings were described in the ARIC (Atherosclerosis Risk in Communities) cohort which was validated even when analysing lipid levels as time-dependent variables.59 An ancillary study to ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) demonstrated that low HDL-C was associated with a significant increase in incident AF.62 In a Japanese cohort, Watanabe et al.60 also found that both TC and LDL-C were inversely related to incident AF. Furthermore, reduced levels of HDL-C was independently associated with greater incidence of AF in females, but not males. The former had a 28% higher risk of AF with each 10% decrease in HDL-C. Results from the SPCCD (Swedish Primary Care Cardiovascular Database) showed that each unit (mmol/L) increase in TC and LDL-C were associated with a 19% and 16% lower risk of incident AF, respectively; also, HDL-C and TG were not related to incident AF. In contrast to the previous study, Mourtzinis et al.49 found no sex-specific differences in outcomes based on lipid abnormalities.
The relationship (or lack of) between the aforementioned measures of lipid abnormalities and incident AF has also been demonstrated among patients with ST-elevation myocardial infarction46 and chronic heart failure.50 In a small study of patients who had AF ablation, TC and LDL-C were inversely associated with a higher risk of AF recurrence.65 However, subgroup analysis demonstrated that these factors were only significant in females but not males. The levels of HDL-C and TG were not related to AF recurrence post-ablation.65 The inverse relationship between AF, and TC and LDL-C are further supported by the fact that use of lipid-lowering medications does not reduce the risk of incident AF.59,66
It is worth noting that conflicting results have been demonstrated in few studies. A combined analysis of the MESA (Multi-Ethnic Study of Atherosclerosis) and Framingham Heart Study cohorts found that raised HDL-C and TG were independently associated with a lower risk of new-onset AF.57 However, the authors reported that TC and LDL-C were not important risk factors for new-onset AF. In a community-based cohort of Korean males, Kim et al.52 found that although the presence of metabolic syndrome led to greater incidence of AF over a follow-up period of 8.7 years, this was driven primarily by central obesity, and neither TG or HDL-C were risk factors for incident AF. Similar results were obtained from a historical Japanese population.53
Different study designs, populations, lifestyles and age ranges may partly explain some of the inconsistencies of previous studies. Nonetheless, the current literature strongly indicates that both TC and LDL-C have an inverse relationship with incident AF. This is supported by a recent meta-analysis of nine large cohort studies.67 Overall, these findings are important as they imply that a reduction in TC and LDL-C, may have unintended consequences for the risk of incident AF. The role of TG and HDL-C, and whether there are sex-specific responses to lipid abnormalities with regards to AF need further investigation.
In addition to the measures of lipids described above, several others have been explored in relation to incident AF. Aronis et al.54 found that Lp(a) levels above 50 mg/dL (compared to <10 mg/dL) were not associated with incident AF. Monocyte to HDL-C ratio has also been described as a novel biomarker of inflammation that may be useful to predict new-onset AF in patients undergoing percutaneous coronary intervention51 or coronary artery bypass grafting.55
3.2 Underlying mechanisms
In general, there is limited research on mechanisms that underpin the relationship between lipoproteins and AF. In a report from the Women’s Health Study, Mora et al.58 conjectures that the inverse relationship may be due to the stabilizing effect of cholesterol on myocardial cell membranes. This may occur through the effects of cholesterol on the regulation of ion channels and sensitivity of volume-regulated anion current to osmotic gradients.68–71 Furthermore, cholesterol depletion has been found to impair cardiomyocyte contractility by deregulation of calcium handling, adrenergic signalling and the myofibrillar architecture.72
The link between cholesterol levels and development of AF may also be related to inflammation. It has been shown that TC, LDL-C and HDL-C levels were decreased while TG was increased during inflammation.73 Therefore, reduced levels of cholesterol may be reflective of underlying inflammatory processes within the host that contributes to AF. Furthermore, lipoproteins influence the course of sepsis by binding to bacterial endotoxins and attenuate the harmful effects of inflammatory responses.74
It was reported that the effects of lipoproteins on incident AF extended beyond the cholesterol content to include the number of lipoprotein particles for LDL and VLDL.58 In this regard, it was the smaller particles for each of these lipoproteins that were the actual driving force contributing to the inverse relationship with AF as larger cholesterol-rich LDL-particles, total HDL-C, Lp(a) and TG were not associated with incident AF.58 In a small study of female patients undergoing catheter ablation, those with AF had smaller lipoprotein particles with increased oxidation, glycation and TG content compared to controls in sinus rhythm.75 Similar findings have been reported elsewhere among male patients.76 Overall, these changes resulted in enhanced foam cell formation via accelerated phagocytosis by macrophages, and reduced antioxidant ability of HDL.75 These changes are important as HDL particles have been shown to be more protective against cardiovascular events,77,78 which are known to contribute to AF. Furthermore, foam cells are known to initiate a wide range of bioactivities including inflammatory processes79–81 that may be linked to the pathogenesis of AF.
Sex differences in the association of lipoproteins and AF that were observed in some studies may be attributable to hormones, especially oestrogen, and differences in body fat distribution or insulin sensitivity.82–84 Moreover, a fall in testosterone levels among ageing males may influence oxidative modification of LDL-C.85
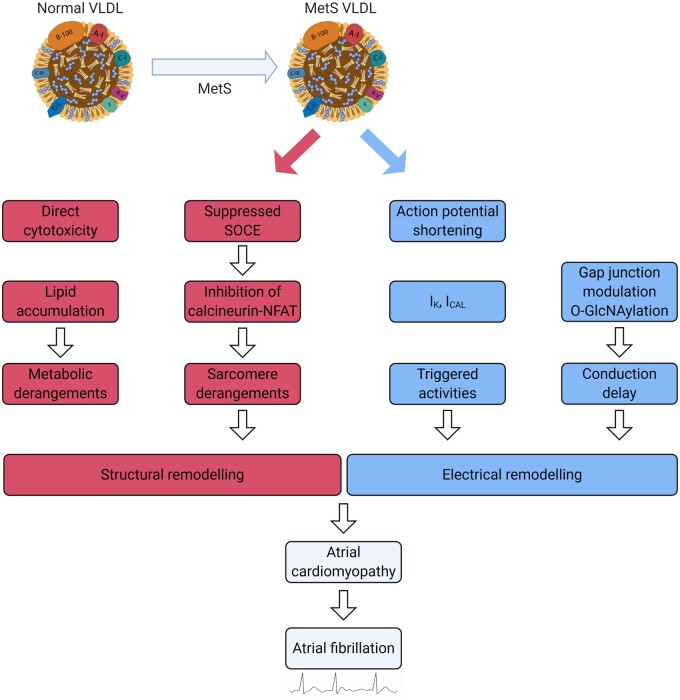
It is worth mentioning that the effects of specific lipoproteins may vary under certain conditions. For example, injection of VLDL extracted from patients with metabolic syndrome into mice resulted in excess lipid accumulation and apoptosis in the atria, and significantly greater left atrial dilatation compared to VLDL from healthy volunteers.86 Thus, VLDL may contribute to the development of atrial cardiomyopathy and subsequent vulnerability to AF through direct cytotoxicity, altered action potentials, disrupted calcium regulation, delayed conduction velocities, modulated gap junctions and derangements in sarcomere proteins (Figure 4).87 This highlights the fact that focusing on the quantity of lipoproteins on its own may limit our understanding of the mechanisms underlying the paradoxical inverse relationship of lipoproteins and AF.
Figure 4.
Pathogenic role of VLDL in metabolic syndrome-related atrial cardiomyopathy. Created using Biorender.com. MetS, metabolic syndrome; NFAT, nuclear factor of activated T cells; SOCE, store-operated calcium entry; VLDL, very low-density lipoprotein.
4. Lipoproteins and thrombosis
The role of lipoproteins in modulating thrombosis and haemostasis to produce fibrin clots is well described.88 LDL and VLDL have been shown to increase thrombin generation and inhibit fibrinolysis.89,90 An inverse relationship of VLDL to fibrin clot permeability and fibre mass-length ratio has previously been demonstrated.91
In addition to the coagulation system, platelets seem to be affected by lipoproteins as well. To start with, there is evidence that patients with excessive LDL, such as those in familial hypercholesterolaemia that is characterized by lack or defective LDL receptors, display enhanced platelet reactivity with increased α-granule secretion,92 fibrinogen binding,93 and aggregation.94 In contrast, patients with abetalipoproteinaemia that is characterized by a lack of all apolipoprotein B-containing lipoproteins (chylomicrons, VLDL and LDL) have reduced platelet activation.95 Furthermore, LDL has been shown to promote excess platelet activation which may contribute to the higher incidence of thrombosis in hyperlipidaemia.96,97
Certain subclasses of LDL may be more harmful than others. For instance, sdLDL was shown to be independently associated with both thrombotic and haemorrhagic strokes.98 A potential mechanism could include increased susceptibility to oxidation which leads to a substantial increase in thrombin generation compared to the larger native LDL.99,100 In addition to identifying the lipid subclasses and oxidative states, evaluating the effects of individual lipid species may be of importance. For instance, Klein et al.101 demonstrated that VLDL was capable of activating the contact pathway in the presence of platelets, thereby causing an increase in the rate and amount of thrombin generation. A subsequent detailed lipoprotein analyses revealed that this was driven by phosphatidylethanolamine (PE). Interestingly, PE is also responsible for oxLDL-induced thrombin generation.102
4.1 OxLDL and haemostasis
Despite many decades of research into oxLDL, definitions of what it contains and method of detection vary between groups and publications.33 Perhaps the most encompassing definition for oxLDL is ‘A particle derived from circulating LDL that may have peroxides or their degradation products generated within the LDL molecule or elsewhere in the body associated with the particle’.33 Such particles therefore may include lipid peroxides, hydroxides or aldehydes such as malondialdehyde in addition to protein oxidation products. These biochemical changes give oxLDL altered properties which may facilitate its detection and separation on the basis of density, negative charge and monoclonal antibody (mAb). The latter method utilizes antibodies to oxidized epitopes on the surface of oxLDL such as EO6 for oxidized phosphatidylcholine (oxPC)103 and 4E6 for oxidized apoB.104 Given the variation in detection methods of oxLDL and possible consequences on interpretation of the evidence, this review specifies the method of detection of oxLDL where appropriate.
Elevated oxLDL levels (detected by 4E6 mAb) are independently associated with several cardiovascular risk factors including increasing age, male gender, raised body mass index, abdominal obesity, hypertension, raised C-reactive protein, renal dysfunction, hyperuricaemia, and smoking.105 These risk factors are important in AF, which has also been shown to be directly associated with elevated 4E6-measured oxLDL levels.106–109
Oxidized LDL (4E6 mAb) correlates to thrombogenesis by interfering with the coagulation system and clot formation. In this regard, patients with acute coronary syndrome demonstrate a positive correlation between oxLDL and tissue factor levels in plasma.110 Activation of T lymphocytes by oxLDL, prepared by chemical oxidation of native LDL with copper sulfate, via the lectin-type oxLDL receptor 1 (LOX-1) has also been shown to increase the expression of tissue factor on the surface of leukocytes.111 Furthermore, oxLDL generated with copper oxidation was noted to inhibit fibrinolysis, modify fibrin clot structure and increase thrombin generation.102,112 Finally, oxLDL (detected by 4E6) correlated to reduced clot permeability and prolonged clot lysis time.113
OxLDL generated in vitro by copper oxidation has been shown to cause activation and aggregation of platelets via CD36 and LOX-1,114–116 as well as impair endothelial regeneration by reducing the release of nitric oxide.117 Furthermore, platelet reactivity in cardiovascular disease can be related to dyslipidaemia,118,119 which is characterized by accumulation of oxLDL as measured by LDL isolation, lipid extraction and subsequent high performance liquid chromatography.120 In turn, platelet reactivity is an important determinant of fibrin clot structure and effective platelet inhibition is associated with a weaker, more permeable fibrin network.121 Therefore, oxLDL may indirectly influence fibrin clot properties through its effects on platelet reactivity. To complicate matters, recent evidence suggests that oxLDL activation of platelets promotes further oxLDL uptake by platelets (detected with the polyclonal orb10973 anti-oxLDL antibody), augmenting the pro-oxidative thrombogenic phenotype.122 Finally, there is evidence suggesting that activated platelets contribute to the formation of oxLDL species and modification of lipoprotein function.123 Putting it together, the evidence points towards a cycle of oxLDL-induced platelet activation leading to further oxLDL formation and uptake by platelets.
4.2 Lp(a) and haemostasis
In addition to its recognized atherogenic properties,124 Lp(a) appears to have a direct prothrombotic effect by interfering with platelets and the fibrinolysis system. Although it has been found to interact with platelets, the target receptor remains unclear.125 Furthermore, literature surrounding the nature of interaction between Lp(a) and platelets is conflicting, with evidence to suggest that it may have both activating and inhibiting effects.126
Lp(a) has been shown to facilitate platelet activation through thrombin-related activating hexapeptide, but not thrombin or adenosine diphosphate.127 On the contrary, some studies reported an inhibitory effect of Lp(a) to platelet activation by collagen or thrombin.125 Less controversial is the ability of Lp(a) to impair platelet-mediated fibrinolytic reactions by interfering with the binding of plasminogen, which shares structural similarities to apolipoprotein(a), and tissue plasminogen activator to the platelet surface.128 This is compounded by the ability of Lp(a) to inactivate tissue factor pathway inhibitor which may promote thrombosis through the extrinsic coagulation pathway.129 However, evidence in genetic studies on the contribution of Lp(a) to venous thrombosis have been negative,130,131 suggesting that the primary prothrombotic effects of Lp(a) may be limited to atherothrombosis (arterial) or anti-fibrinolysis.132 Additional studies describing the association between lipoproteins and thrombotic conditions are summarized in Table 2.
Table 2.
Clinical studies describing association of lipoproteins with thrombotic conditions
| Author, year (ref) | Study design | Population | n | Finding(s) in relation to thrombosis |
|---|---|---|---|---|
| Morelli, 2017133 | Case-control | Recent venous thrombosis | 5107 |
↓ ApoB: OR 1.35 (95% CI 1.12–1.62) ↓ ApoA1: OR 1.50 (95% CI 1.25–1.79) |
| Grifoni, 2012134 | Cross-sectional | First episode venous thromboembolism | 747 | ↑ Lp(a): OR 2.6 (95% CI 1.7–4.0) |
| Kamstrup, 2012131 | Community-based cohort | White Danish descent | 41 231 |
↑ Lp(a): OR 1.21 (95% CI 1.10–1.33) for risk of myocardial infarction (coronary atherothrombosis) No association between Lp(a) and venous thrombosis |
| Ohira, 2006135 | Cohort | No history of stroke | 14 448 |
↑ Lp(a): OR 1.42 (95% CI 1.10–1.83) for non-lacunar strokes, No association between Lp(a) and lacunar or cardioembolic strokes |
| Tsimikas, 200535 | Cross-sectional | Coronary artery disease | 504 |
↑ oxLDL: ApoB100 ratio: OR 3.12 (P < 0.01) ↑ Lp(a): OR 3.64 (P < 0.01) |
| Deguchi, 2005136 | Cross-sectional | Men with venous thrombosis | 98 |
↓ HDL: OR 6.5 (2.3–19) ↓ ApoA1: OR 6.0 (2.1–17) ↑ IDL: OR 2.7 (1.0–6.8, P < 0.05) ↑ sdLDL: OR 3.1 (1.3–7.4) |
| Doggen, 2004137 | Case-control | Post-menopausal women with first venous thrombosis | 2463 |
↑ HDL-C: OR 0.71 (95% CI 0.52–0.97) ↑ TG: OR 2.13 (95% CI 1.34–3.37) |
| Marcucci, 2003138 | Case-control | History of venous thromboembolism | 1033 | ↑ Lp(a): OR 2.1 (95% CI 1.4–3.2) |
| von Depka, 2000139 | Case-control | History of venous thrombo-embolism | 951 | ↑ Lp(a): OR 3.2 (95% CI 1.9–5.3) |
| Holvoet, 1998140 | Case-control | Coronary artery disease | 270 | ↑ oxLDL in acute coronary syndrome than stable angina (r2 0.65, P < 0.01) |
| Kawasaki, 1997141 | Case-control | Confirmed deep vein thrombosis | 218 |
↑ TC: OR 4.5 (95% CI 2.4–8.3) ↑ TG: OR 2.4 (95% CI 1.3–4.6) |
ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; CI, confidence interval; HDL, high-density lipoprotein; HDL-C, high-density lipoprotein cholesterol; IDL, intermediate-density lipoprotein; Lp(a), lipoprotein(a); OR, odds ratio; OxLDL, oxidized low-density lipoprotein; sdLDL, small dense low-density lipoprotein; TC, total cholesterol; TG, triglycerides.
4.3 The effects of lipid-modifying therapy on thrombosis and haemostasis
The role of lipoproteins in haemostasis is further supported by the fact that application of lipid-modifying therapy is associated with changes in haemostasis.142 Specifically, atorvastatin may exert antiplatelet effects by interfering with redox signalling.143 It has also been shown that statins are able to reduce fibrin clot lysis time, independent of warfarin.144 For example, a randomized controlled trial by Undas et al.145 confirmed the effects of statins and also showed similar results with the use of other lipid-modifying therapy, specifically fenofibrate. The authors reported increased fibrin clot permeability and reduced lysis time with the use of these agents compared to pre-treatment values, potentially through its effects on thrombin generation. Turbidity analysis also showed that use of these drugs resulted in thicker fibres that were more prone to effective fibrinolysis.
A further randomized controlled trial of patients with type 1 diabetes mellitus and dyslipidaemia found that the beneficial effects of statins on fibrin clot properties may be related to reduced expression of glycoprotein IIIa, tissue factor, and P-selectin.146 Finally, the use of statins has been associated with risk reduction of both venous and arterial thromboembolisms.147–151 Therefore, it is tempting to speculate that the statin-induced protective effects may be related to its influence on reduction of pro-coagulant lipoproteins or enhancement of anti-coagulant lipoproteins.90
A prospective, case-controlled study of patients with stable coronary artery disease and hypercholesterolaemia found that use of pravastatin was associated with reduced thrombus formation at both high and low shear rates.152 As expected, there was a significant decrease in TC and LDL-C levels with pravastatin. Thrombus formation was also assessed after 1 week of treatment with pravastatin, prior to any significant reduction in TC and LDL-C levels, and it was found that this was unchanged compared to pre-treatment. As a result, the authors concluded that the beneficial effects of pravastatin on thrombogenicity was due to its effects on lipids/lipoproteins.152 Interestingly, other studies have reported that the anti-coagulant effects of statin therapy, in terms of thrombin generation and platelet activation, were seen as early as 3 days following treatment.153,154
Nonetheless, it should be noted that there currently remains insufficient evidence to conclude whether the protective effects of statins are related to its lipid-modifying effects or otherwise.148 In contrast to the aforementioned studies, Dangas et al.155 showed a reduction in thrombogenicity among patients after 6 months of treatment with pravastatin, regardless of change in LDL-C. Furthermore, despite a similar reduction in LDL-C between subgroups of patients treated with pravastatin compared to dietary advice only, the anti-thrombotic benefit was only demonstrated among those receiving pravastatin. Additionally, a study by Undas et al.156 found that the use of simvastatin was associated with a reduction in thrombin generation, independent of changes in lipid profile. Overall, there may be various pathways by which lipid-modifying therapy, in particular statins, may interact with the haemostatic process.
5. Lipoproteins and thromboembolism in AF
Given the effects of lipoproteins on haemostasis, their contribution to thromboembolic events may be expected. Indeed, lipoprotein abnormalities have been shown to be an independent risk factor for stroke and venous thromboembolism.157–160 However, few studies have explored this relationship in the context of AF (Table 3).
Table 3.
Effects of lipoproteins on thromboembolic outcomes in atrial fibrillation
| Author, year (ref) | Study type | Population | n | Follow-up (months) | Finding(s) |
|---|---|---|---|---|---|
| Liu, 2020161 | Retrospective | Non-valvular AF | 2345 | 26 |
↑ LDL-C in low-risk: HR 2.60 (95% CI 1.26–5.37) for ischaemic stroke ↑ LDL-C in high-risk: HR 2.50 (95% CI 1.10–5.70) for ischaemic stroke |
| Yan, 2019162 | Retrospective | Non-valvular AF with low CHA2DS2-VASc score | 595 | NA | ↑ Lipoprotein(a): OR 1.02 (95% CI 1.01–1.03) for thromboembolic events |
| Pol, 2018163 | Prospective | AF with at least 1 stroke/SE risk factor | 14 884 | 23 |
↑ Apolipoprotein A1: HR 0.81 (95% CI 0.73–0.90) for composite risk of ischaemic stroke, SE, MI and CV death Apolipoprotein B was not associated with composite risk of ischaemic stroke, SE, MI and CV death |
| Qi, 2017164 | Retrospective | AF ± ischaemic stroke | 815 | NA | ↑ LDL-C: OR 2.00 (95% CI 1.62–2.47) for ischaemic stroke |
| Aronis, 201754 | Prospective | Community-based cohort | 10 127 | 190 | ↑ Lipoprotein(a) was not associated with stroke risk in patients with AF |
| Wu, 2017165 | Retrospective | Non-valvular AF | 2470 | NA | ↑ LDL-C: OR 1.27 (95% CI 1.08–1.49) for ischaemic stroke |
| Igarashi, 1998166 | Prospective | Chronic AF | 150 | NA | ↑ Lipoprotein(a) was an independent risk factor for LA thrombus (standardized coefficient of 0.300) |
AF, atrial fibrillation; CI, confidence interval; CV, cardiovascular; HR, hazard ratio; LA, left atrial; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; NA, not applicable or available; OR, odds ratio; SE, systemic embolism.
5.1 Low-density lipoprotein cholesterol
LDL-C has been implicated in thromboembolic events among patients with AF. Wu et al.165 found that LDL-C was an independent risk factor for both a history of ischaemic stroke and future stroke risk among patients with AF. Similar findings were reported in a case-controlled study, whereby raised LDL-C was shown to be an independent predictor of ischaemic stroke in patients with AF, irrespective of the CHA2DS2-VASc score.164 Furthermore, this association demonstrated a dose-response pattern. A later study confirmed the relationship between LDL-C and ischaemic stroke, and observed that lowering LDL-C may be particularly beneficial among AF patients with a low CHA2DS2-VASc score (less than two in males and three in females).161 Interestingly, LDL-C appears to have an opposite influence on the risk of incident AF and subsequent thromboembolic risk which highlights the importance of regular monitoring and treatment adjustments in clinical practice.
5.2 Lipoprotein(a)
There are conflicting reports on the effects of Lp(a) on thromboembolic risk in AF. Igarashi et al.166 demonstrated that serum Lp(a) was an independent risk factor for left atrial thrombus detected on trans-oesophageal echocardiogram in patients with chronic AF. Additionally, left atrial thrombus was present in 48% of AF patients with a Lp(a) level ≥30 mg/dL, suggesting that this may be a useful biomarker to identify patients at high risk of thromboembolism. However, a limitation of this study was that relatively few patients (19%) were on anticoagulation therapy.166
More recently, higher Lp(a) levels were found to be independently associated with clinically confirmed thromboembolic events in non-valvular AF patients with a CHA2DS2-VASc score of less than two.162 Curiously, Aronis et al.54 found that elevated levels of Lp(a) was associated with an increased stroke risk among non-AF patients, but not in those with AF. In support of the latter, we previously demonstrated that there was no correlation between Lp(a) and D-dimer, as a marker of thrombogenesis.167 Overall, the inconsistent results on Lp(a) may suggest the existence of different Lp(a) phenotypes that contribute differently to thrombogenesis168 and therefore, sole measurement of total Lp(a) levels may be inadequate for this purpose. In this regard, the measurement of oxidized lipids may have an important role to increase our understanding on the potential impact of Lp(a) on atrial function and risk of AF.169–171
5.3 Other measures of lipoproteins
In a sub-study of the ARISTOTLE trial, higher levels of Apolipoprotein A1 were independently associated with a lower composite risk of ischaemic stroke, systemic embolism, myocardial infarction, and cardiovascular mortality.163 When analysed separately, Apolipoprotein A1 was found to be a risk factor for each of the individual outcomes apart from myocardial infarction. In reverse, the authors reported that Apolipoprotein B was not associated with the risk of composite outcomes but that it was a risk factor for myocardial infarction. Decker et al.172 demonstrated that low HDL and high TG were not independently associated with ischaemic stroke among AF patients over a follow-up period of 14.8 years, though there was a trend for the former [hazard ratio (HR) 1.47, 95% confidence interval (CI) 0.99–2.20; P = 0.06].
The relationship between lipoproteins and thromboembolism in AF is further indicated by studies that have explored the impact of statins, as medications that are known to regulate lipoproteins. A subgroup analysis comprising of 1446 AF patients with ischaemic stroke found that higher statin adherence during 5-year follow-up predicted a reduced risk of stroke recurrence [HR 0.59 (95% CI 0.43–0.81)].173 In this context, the effects of statins may be related to a reduction of oxLDL levels that promote its anti-inflammatory properties,174,175 which has been shown to reduce the endogenous thrombin potential in patients with AF.176 He et al.177 found that prior use of statins resulted in lower plasma oxLDL levels at baseline and at 3-month follow-up among patients presenting with an ischaemic stroke. Furthermore, pre-stroke statin use was associated with reduced short-term mortality [odds ratio (OR) 0.38 (95% CI 0.16–0.91) and major disability (OR 0.38 (95% CI 0.15–0.99)].
6. Gaps and limitations
Despite a wealth of evidence on the role of lipoproteins in thrombosis and AF, it is recognized that these molecules are heterogeneous, containing numerous subclasses and lipid species with variable effects.178 In this regard, much of the conflicting evidence and paradox in prior studies may be due to the usage of crude methods of classification that undermines the complexity of lipoproteins. Given recent advancements in our ability to accurately analyse lipoprotein subclasses and lipid species, future studies should focus on identifying the relationship of these molecules with incident AF and thromboembolic complications. Moreover, the mechanism by which this occurs also warrants further investigation. With better understanding in this area, the development of targeted treatment approaches for high-risk subgroups may be possible. Furthermore, ongoing clinical trials such as the Lp(a)HORIZON study (ClinicalTrials.gov NCT04023552) are examining novel agents targeting Lp(a) levels and may provide more data on the association of Lp(a), incident AF and thrombotic events.
One group of lipids which is emerging as a key player in haemostatic reactions is oxidized GPL. These molecules have been shown to play a role in thrombotic disorders and are primarily generated enzymatically by platelets and leukocytes.179,180 The presence of these molecules in lipoproteins has not been conclusively studied, particularly in light of newer lipidomic technologies. The majority of previous studies of oxidized GPL in lipoproteins had relied on antibodies that bind oxPC, demonstrating their presence as a defining feature of oxLDL181 and Lp(a).182 It is not known whether the presence of oxPC, or other oxidized GPL, on lipoproteins enhance coagulation reaction in a similar way to enzymatically-generated oxPC on the surface of activated cells.179 The growth in the lipidomics field and availability of increasingly sensitive techniques may pave the way for studies in this area.
Moving forward, the role of genetics in lipoproteins should also be considered. Elevated Lp(a) is prevalent in approximately 20% of the population,183 and strongly influenced by genetic variability.184 Much of the variation is related to the apo(a) protein, which consists of kringle domains that vary in molecular weight and therefore size of the Lp(a) particle.185,186 The genetic variation in the LPA locus has enabled Mendelian randomization studies to demonstrate that both the Lp(a) concentration and the smaller apo(a) isoform are independently causal for some cardiovascular diseases.183,187–191 While a large UK-based population study by Zanetti et al.188 found no causal relationship between Lp(a) and AF, further Mendelian randomization studies are needed to confirm this finding in other cohorts.
7. Conclusion
There is a paradoxical relationship between TC and LDL-C, and incident AF. The mechanism by which this occurs is poorly defined but may be related to changes in the regulation of ion channels and inflammatory processes. To complicate matters, excess lipoproteins promote thrombin generation, inhibit fibrinolysis and enhance platelet activation. In this regard, LDL-C has been shown to be an independent risk factor for thromboembolic events in AF. Overall, the complex relationship between lipoproteins, thrombosis and AF warrants further research. An improved knowledge base in this area may unlock important mechanistic pathways that contribute to our overall understanding of haemostasis and guide our clinical approach in the treatment of prothrombotic conditions.
Data availability
The data that support the findings of this review are available from the corresponding author upon reasonable request.
Acknowledgements
We thank Professor Valerie O'Donnell for her constructive comments on our manuscript.
Conflict of interest: W.Y.D., I.G.D., and M.B.P.: none declared. G.Y.H.L.: consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi-Sankyo. Speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y-H, McAnulty JHJ, Zheng Z-J, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJL.. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D.. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 3. Stewart S, Hart CL, Hole DJ, McMurray JJ.. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 4. Thrall G, Lane D, Carroll D, Lip GYH.. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med 2006;119:448.e1–448.e19. [DOI] [PubMed] [Google Scholar]
- 5. Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL, Van Gilst WH, Van Gelder IC, Rienstra M.. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality a community-based study from the Netherlands. J Am Coll Cardiol 2015;66:1000–1007. [DOI] [PubMed] [Google Scholar]
- 6. Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA.. Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham Heart Study. J Am Med Assoc 1994;271:840–844. [PubMed] [Google Scholar]
- 7. Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG.. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 1995;155:469–473. [PubMed] [Google Scholar]
- 8. Zulkifly H, Lip GYH, Lane DA.. Epidemiology of atrial fibrillation. Int J Clin Pract 2018;72:e13070. [DOI] [PubMed] [Google Scholar]
- 9. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TSM.. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 10. Heeringa J, van der Kuip DAM, Hofman A, Kors JA, van Herpen G, Stricker BHC, Stijnen T, Lip GYH, Witteman JCM.. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 11. Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ.. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 12. Staerk L, Wang B, Preis SR, Larson MG, Lubitz SA, Ellinor PT, McManus DD, Ko D, Weng L-C, Lunetta KL, Frost L, Benjamin EJ, Trinquart L.. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ 2018;361:k1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krijthe BP, Kunst A, Benjamin EJ, Lip GYH, Franco OH, Hofman A, Witteman JCM, Stricker BH, Heeringa J.. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S.. Epidemiology of atrial fibrillation: european perspective. Clin Epidemiol 2014;6:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wijesurendra RS, Casadei B.. Mechanisms of atrial fibrillation. Heart 2019;105:1860. [DOI] [PubMed] [Google Scholar]
- 16. Ding WY, Gupta D, Lip GYH.. Atrial fibrillation and the prothrombotic state: revisiting Virchow’s triad in 2020. Heart 2020;106:1463–1468. [DOI] [PubMed] [Google Scholar]
- 17. Burdge GC, Calder PC.. Introduction to fatty acids and lipids. World Rev Nutr Diet 2014;112:1–16. [DOI] [PubMed] [Google Scholar]
- 18.Dowhan W, Bogdanov M. Chapter 1: Functional roles of lipids in membranes. New Comprehensive Biochemistry. Elsevier. Vol. 36, pp. 1–35. https://doi.org/10.1016/S0167-7306(02)36003-4. [Google Scholar]
- 19. Yen C-LE, Stone SJ, Koliwad S, Harris C, Farese RVJ.. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 2008;49:2283–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cerqueira N, Oliveira EF, Gesto DS, Santos-Martins D, Moreira C, Moorthy HN, Ramos MJ, Fernandes PA.. Cholesterol biosynthesis: a mechanistic overview. Biochemistry 2016;55:5483–5506. [DOI] [PubMed] [Google Scholar]
- 21. Baila-Rueda L, Cenarro A, Civeira F.. Non-cholesterol sterols in the diagnosis and treatment of dyslipidemias: a review. CMC 2016;23:2132–2145. [DOI] [PubMed] [Google Scholar]
- 22. Buhman KF, Accad M, Farese RV.. Mammalian acyl-CoA:cholesterol acyltransferases. Biochim Biophys Acta 2000;1529:142–154. [DOI] [PubMed] [Google Scholar]
- 23. Feingold KR, Grunfeld C. Introduction to lipids and lipoproteins. South Dartmouth (MA: ): Endotext, 2018. [Google Scholar]
- 24. Mahley RW, Innerarity TL, Rall SCJ, Weisgraber KH.. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res 1984;25:1277–1294. [PubMed] [Google Scholar]
- 25. Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN.. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid Med Cell Longev 2017;2017:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanaga K, Bujo H, Inoue M, Mikami K, Kotani K, Takahashi K, Kanno T, Saito Y.. Increased circulating malondialdehyde-modified LDL levels in patients with coronary artery diseases and their association with peak sizes of LDL particles. Arterioscler Thromb Vasc Biol 2002;22:662–666. [DOI] [PubMed] [Google Scholar]
- 27. Packard CJ, Demant T, Stewart JP, Bedford D, Caslake MJ, Schwertfeger G, Bedynek A, Shepherd J, Seidel D.. Apolipoprotein B metabolism and the distribution of VLDL and LDL subfractions. J Lipid Res 2000;41:305–318. [PubMed] [Google Scholar]
- 28. Hayashi T, Koba S, Ito Y, Hirano T.. Method for estimating high sdLDL-C by measuring triglyceride and apolipoprotein B levels. Lipids Health Dis 2017;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, Ference BA.. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol 2019;4:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reis A, Rudnitskaya A, Blackburn GJ, Mohd Fauzi N, Pitt AR, Spickett CM.. A comparison of five lipid extraction solvent systems for lipidomic studies of human LDL. J Lipid Res 2013;54:1812–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saeed A, Feofanova EV, Yu B, Sun W, Virani SS, Nambi V, Coresh J, Guild CS, Boerwinkle E, Ballantyne CM, Hoogeveen RC.. Remnant-like particle cholesterol, low-density lipoprotein triglycerides, and incident cardiovascular disease. J Am Coll Cardiol 2018;72:156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reis A, Rudnitskaya A, Chariyavilaskul P, Dhaun N, Melville V, Goddard J, Webb DJ, Pitt AR, Spickett CM.. Top-down lipidomics of low density lipoprotein reveal altered lipid profiles in advanced chronic kidney disease. J Lipid Res 2015;56:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N.. Oxidized low-density lipoprotein. Methods Mol Biol 2010;610:403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruuth M, Nguyen SD, Vihervaara T, Hilvo M, Laajala TD, Kondadi PK, Gisterå A, Lähteenmäki H, Kittilä T, Huusko J, Uusitupa M, Schwab U, Savolainen MJ, Sinisalo J, Lokki M-L, Nieminen MS, Jula A, Perola M, Ylä-Herttula S, Rudel L, Öörni A, Baumann M, Baruch A, Laaksonen R, Ketelhuth DFJ, Aittokallio T, Jauhiainen M, Käkelä R, Borén J, Williams KJ, Kovanen PT, Öörni K.. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur Heart J 2018;39:2562–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB.. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med 2005;353:46–57. [DOI] [PubMed] [Google Scholar]
- 36. Ferretti G, Bacchetti T, Johnston TP, Banach M, Pirro M, Sahebkar A.. Lipoprotein(a): a missing culprit in the management of athero-thrombosis? J Cell Physiol 2018;233:2966–2981. [DOI] [PubMed] [Google Scholar]
- 37. Romagnuolo R, Marcovina SM, Boffa MB, Koschinsky ML.. Inhibition of plasminogen activation by apo(a): role of carboxyl-terminal lysines and identification of inhibitory domains in apo(a). J Lipid Res 2014;55:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kannel WB, Dawber TR, Friedman GD, Glennon WE, Mcnamara PM.. Risk factors in coronary heart disease. An evaluation of several serum lipids as predictors of coronary heart disease; the Framingham study. Ann Intern Med 1964;61:888–899. [DOI] [PubMed] [Google Scholar]
- 39. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB.. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 40. Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W.. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2001;104:1108–1113. [DOI] [PubMed] [Google Scholar]
- 41. Duncan MS, Vasan RS, Xanthakis V.. Trajectories of blood lipid concentrations over the adult life course and risk of cardiovascular disease and all-cause mortality: observations from the Framingham study over 35 years. J Am Heart Assoc 2019;8:e011433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen M-R, Tokgözoğlu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL.. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chyou JY, Hunter TD, Mollenkopf SA, Turakhia MP, Reynolds MR.. Individual and combined risk factors for incident atrial fibrillation and incident stroke: an analysis of 3 million at-risk US patients. J Am Heart Assoc England 2015;4:e001723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brunner KJ, Bunch TJ, Mullin CM, May HT, Bair TL, Elliot DW, Anderson JL, Mahapatra S.. Clinical predictors of risk for atrial fibrillation: implications for diagnosis and monitoring. Mayo Clin Proc 2014;89:1498–1505. [DOI] [PubMed] [Google Scholar]
- 45. Harrison SL, Lane DA, Banach M, Mastej M, Kasperczyk S, Jóźwiak JJ, Lip GYH; LIPIDOGRAM2015 Investigators. Lipid levels, atrial fibrillation and the impact of age: results from the LIPIDOGRAM2015 study. Atherosclerosis 2020;312:16–22. [DOI] [PubMed] [Google Scholar]
- 46. Xue Y, Zhou Q, Shen J, Liu G, Zhou W, Wen Y, Luo S.. Lipid profile and new-onset atrial fibrillation in patients with acute ST-segment elevation myocardial infarction (an Observational Study in Southwest of China). Am J Cardiol 2019;124:1512–1517. [DOI] [PubMed] [Google Scholar]
- 47. Choe WS, Choi EK, Do Han K, Lee EJ, Lee SR, Cha MJ, Oh S.. Association of metabolic syndrome and chronic kidney disease with atrial fibrillation: a nationwide population-based study in Korea. Diabetes Res Clin Pract 2019;148:14–22. [DOI] [PubMed] [Google Scholar]
- 48. Li X, Gao L, Wang Z, Guan B, Guan X, Wang B, Han X, Xiao X, Bin Waleed K, Chandran C, Wu S, Xia Y.. Lipid profile and incidence of atrial fibrillation: a prospective cohort study in China. Clin Cardiol 2018;41:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mourtzinis G, Kahan T, Bengtsson Boström K, Schiöler L, Cedstrand Wallin L, Hjerpe P, Hasselström J, Manhem K.. Relation between lipid profile and new-onset atrial fibrillation in patients with systemic hypertension (from the Swedish Primary Care Cardiovascular Database [SPCCD]). Am J Cardiol 2018;122:102–107. [DOI] [PubMed] [Google Scholar]
- 50. Liu C, Geng J, Ye X, Yuan X, Li A, Zhang Z, Xu B, Wang Y.. Change in lipid profile and risk of new-onset atrial fibrillation in patients with chronic heart failure: a 3-year follow-up observational study in a large Chinese hospital. Medicine (Baltimore) 2018;97:e12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ulus T, Isgandarov K, Yilmaz AS, Vasi I, Moghanchizadeh SH, Mutlu F.. Predictors of new-onset atrial fibrillation in elderly patients with acute coronary syndrome undergoing percutaneous coronary intervention. Aging Clin Exp Res 2018;30:1475–1482. [DOI] [PubMed] [Google Scholar]
- 52. Kim Y-G, Choi K-J, Han S, Hwang KW, Kwon CH, Park G-M, Won K-B, Ann SH, Kim J, Kim S-J, Lee S-G, Nam G-B, Kim Y-H.. Metabolic syndrome and the risk of new-onset atrial fibrillation in Middle-Aged East Asian Men. Circ J 2018;82:1763–1769. [DOI] [PubMed] [Google Scholar]
- 53. Kokubo Y, Watanabe M, Higashiyama A, Nakao YM, Kusano K, Miyamoto Y.. Development of a basic risk score for incident atrial fibrillation in a Japanese General Population—the Suita Study. Circ J 2017;81:1580–1588. [DOI] [PubMed] [Google Scholar]
- 54. Aronis KN, Zhao D, Hoogeveen RC, Alonso A, Ballantyne CM, Guallar E, Jones SR, Martin SS, Nazarian S, Steffen BT, Virani SS, Michos ED.. Associations of lipoprotein(a) levels with incident atrial fibrillation and ischemic stroke: the ARIC (Atherosclerosis Risk in Communities) study. J Am Heart Assoc 2017;6:e007372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saskin H, Serhan Ozcan K, Yilmaz S.. High preoperative monocyte count/high-density lipoprotein ratio is associated with postoperative atrial fibrillation and mortality in coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 2017;24:395–401. [DOI] [PubMed] [Google Scholar]
- 56. Krittayaphong R, Rangsin R, Thinkhamrop B, Hurst C, Rattanamongkolgul S, Sripaiboonkij N, Yindeengam A.. Prevalence and associating factors of atrial fibrillation in patients with hypertension: a nation-wide study. BMC Cardiovasc Disord 2016;16:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alonso A, Yin X, Roetker NS, Magnani JW, Kronmal RA, Ellinor PT, Chen LY, Lubitz SA, McClelland RL, McManus DD, Soliman EZ, Huxley RR, Nazarian S, Szklo M, Heckbert SR, Benjamin EJ.. Blood lipids and the incidence of atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis and the Framingham Heart Study. J Am Heart Assoc 2014;3:e001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mora S, Akinkuolie AO, Sandhu RK, Conen D, Albert CM.. Paradoxical association of lipoprotein measures with incident atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lopez FL, Agarwal SK, Maclehose RF, Soliman EZ, Sharrett AR, Huxley RR, Konety S, Ballantyne CM, Alonso A.. Blood lipid levels, lipid-lowering medications, and the incidence of atrial fibrillation: the atherosclerosis risk in communities study. Circ Arrhythm Electrophysiol 2012;5:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Watanabe H, Tanabe N, Yagihara N, Watanabe T, Aizawa Y, Kodama M.. Association between lipid profile and risk of atrial fibrillation: niigata preventive medicine study. Circ J 2011;75:2767–2774. [DOI] [PubMed] [Google Scholar]
- 61. Iguchi Y, Kimura K, Shibazaki K, Aoki J, Kobayashi K, Sakai K, Sakamoto Y.. Annual incidence of atrial fibrillation and related factors in adults. Am J Cardiol 2010;106:1129–1133. [DOI] [PubMed] [Google Scholar]
- 62. Haywood LJ, Ford CE, Crow RS, Davis BR, Massie BM, Einhorn PT, Williard A.. Atrial fibrillation at baseline and during follow-up in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial). J Am Coll Cardiol 2009;54:2023–2031. [DOI] [PubMed] [Google Scholar]
- 63. Rosengren A, Hauptman PJ, Lappas G, Olsson L, Wilhelmsen L, Swedberg K.. Big men and atrial fibrillation: effects of body size and weight gain on risk of atrial fibrillation in men. Eur Heart J 2009;30:1113–1120. [DOI] [PubMed] [Google Scholar]
- 64. Frost L, Hune LJ, Vestergaard P.. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med 2005;118:489–495. [DOI] [PubMed] [Google Scholar]
- 65. Shang Y, Chen N, Wang Q, Zhuo C, Zhao J, Lv N, Huang Y.. Blood lipid levels and recurrence of atrial fibrillation after radiofrequency catheter ablation: a prospective study. J Interv Card Electrophysiol 2020;57:221–231. [DOI] [PubMed] [Google Scholar]
- 66. Adabag AS, Mithani S, Al Aloul B, Collins D, Bertog S, Bloomfield HE.. Efficacy of gemfibrozil in the primary prevention of atrial fibrillation in a large randomized controlled trial. Am Heart J 2009;157:913–918. [DOI] [PubMed] [Google Scholar]
- 67. Guan B, Li X, Xue W, Tse G, Bin Waleed K, Liu Y, Zheng M, Wu S, Xia Y, Ding Y.. Blood lipid profiles and risk of atrial fibrillation: a systematic review and meta-analysis of cohort studies. J Clin Lipidol 2020;14:133–142.e3. [DOI] [PubMed] [Google Scholar]
- 68. Dart C. Lipid microdomains and the regulation of ion channel function. J Physiol 2010;588:3169–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Abi-Char J, Maguy A, Coulombe A, Balse E, Ratajczak P, Samuel J-L, Nattel S, Hatem SN.. Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes. J Physiol 2007;582:1205–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Levitan I, Christian AE, Tulenko TN, Rothblat GH.. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J Gen Physiol 2000;115:405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Goonasekara CL, Balse E, Hatem S, Steele DF, Fedida D.. Cholesterol and cardiac arrhythmias. Expert Rev Cardiovasc Ther 2010;8:965–979. [DOI] [PubMed] [Google Scholar]
- 72. Hissa B, Oakes PW, Pontes B, Ramírez-San Juan G, Gardel ML.. Cholesterol depletion impairs contractile machinery in neonatal rat cardiomyocytes. Sci Rep 2017;7:43764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Khovidhunkit W, Kim M-S, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C.. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 2004;45:1169–1196. [DOI] [PubMed] [Google Scholar]
- 74. Berbée JFP, Havekes LM, Rensen PCN.. Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J Endotoxin Res 2005;11:97–103. [DOI] [PubMed] [Google Scholar]
- 75. Kim S-M, Lee J-H, Kim J-R, Shin D-G, Lee S-H, Cho K-H.. Female patients with atrial fibrillation have increased oxidized and glycated lipoprotein properties and lower apolipoprotein A-I expression in HDL. Int J Mol Med 2011;27:841–849. [DOI] [PubMed] [Google Scholar]
- 76. Kim S-M, Kim J-M, Shin D-G, Kim J-R, Cho K-H.. Relation of atrial fibrillation (AF) and change of lipoproteins: male patients with AF exhibited severe pro-inflammatory and pro-atherogenic properties in lipoproteins. Clin Biochem 2014;47:869–875. [DOI] [PubMed] [Google Scholar]
- 77. Albers JJ, Slee A, Fleg JL, O’Brien KD, Marcovina SM.. Relationship of baseline HDL subclasses, small dense LDL and LDL triglyceride to cardiovascular events in the AIM-HIGH clinical trial. Atherosclerosis 2016;251:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rizzo M, Otvos J, Nikolic D, Montalto G, Toth PP, Banach M.. Subfractions and subpopulations of HDL: an update. CMC 2014;21:2881–2891. [DOI] [PubMed] [Google Scholar]
- 79. Maguire EM, Pearce SWA, Xiao Q.. Foam cell formation: a new target for fighting atherosclerosis and cardiovascular disease. Vascul Pharmacol 2019;112:54–71. [DOI] [PubMed] [Google Scholar]
- 80. Wang D, Yang Y, Lei Y, Tzvetkov NT, Liu X, Yeung AWK, Xu S, Atanasov AG.. Targeting foam cell formation in atherosclerosis: therapeutic potential of natural products. Pharmacol Rev 2019;71:596–670. [DOI] [PubMed] [Google Scholar]
- 81. Linton MF, Yancey PG, Davies SS, Jerome WG, Linton EF, Song WL, Doran AC, Vickers KC, The role of lipids and lipoproteins in atherosclerosis. South Dartmouth (MA: ): Endotext; 2000. [Google Scholar]
- 82. Palmisano BT, Zhu L, Eckel RH, Stafford JM.. Sex differences in lipid and lipoprotein metabolism. Mol Metab 2018;15:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang X, Magkos F, Mittendorfer B.. Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. J Clin Endocrinol Metab 2011;96:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Regitz-Zagrosek V. Unsettled issues and future directions for research on cardiovascular diseases in women. Korean Circ J 2018;48:792–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Barud W, Palusiński R, Bełtowski J, Wójcicka G.. Inverse relationship between total testosterone and anti-oxidized low density lipoprotein antibody levels in ageing males. Atherosclerosis 2002;164:283–288. [DOI] [PubMed] [Google Scholar]
- 86. Lee H-C, Lin H-T, Ke L-Y, Wei C, Hsiao Y-L, Chu C-S, Lai W-T, Shin S-J, Chen C-H, Sheu S-H, Wu B-N.. VLDL from metabolic syndrome individuals enhanced lipid accumulation in atria with association of susceptibility to atrial fibrillation. IJMS 2016;17:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lee H-C, Lin Y-H.. The pathogenic role of very low density lipoprotein on atrial remodeling in the metabolic syndrome. IJMS 2020;21:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Deguchi H, Elias DJ, Griffin JH.. Minor plasma lipids modulate clotting factor activities and may affect thrombosis risk. Res Pract Thromb Haemost 2017;1:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Olufadi R, Byrne CD.. Effects of VLDL and remnant particles on platelets. Pathophysiol Haemos Thromb 2006;35:281–291. [DOI] [PubMed] [Google Scholar]
- 90. Ouweneel AB, Van Eck M.. Lipoproteins as modulators of atherothrombosis: from endothelial function to primary and secondary coagulation. Vascul Pharmacol 2016;82:1–10. [DOI] [PubMed] [Google Scholar]
- 91. Fatah K, Silveira A, Tornvall P, Karpe F, Blomback M, Hamsten A.. Proneness to formation of tight and rigid fibrin gel structures in men with myocardial infarction at a young age. Thromb Haemost 1996;76:535–540. [PubMed] [Google Scholar]
- 92. Betteridge DJ, Cooper MB, Saggerson ED, Prichard BN, Tan KC, Ling E, Barbera G, McCarthy S, Smith CC.. Platelet function in patients with hypercholesterolaemia. Eur J Clin Invest 1994;24:30–33. [DOI] [PubMed] [Google Scholar]
- 93. DiMinno G, Silver MJ, Cerbone AM, Rainone A, Postiglione A, Mancini M.. Increased fibrinogen binding to platelets from patients with familial hypercholesterolemia. Arteriosclerosis 1986;6:203–211. [DOI] [PubMed] [Google Scholar]
- 94. Elisaf M, Karabina SA, Bairaktari E, Goudevenos JA, Siamopoulos KC, Tselepis AD.. Increased platelet reactivity to the aggregatory effect of platelet activating factor, in vitro, in patients with heterozygous familial hypercholesterolaemia. Platelets 1999;10:124–131. [DOI] [PubMed] [Google Scholar]
- 95. Surya II, Mommersteeg M, Gorter G, Erkelens DW, Akkerman JW.. Abnormal platelet functions in a patient with abetalipoproteinemia. Thromb Haemost 1991;65:306–311. [PubMed] [Google Scholar]
- 96. Shen M-Y, Chen F-Y, Hsu J-F, Fu R-H, Chang C-M, Chang C-T, Liu C-H, Wu J-R, Lee A-S, Chan H-C, Sheu J-R, Lin S-Z, Shyu W-C, Sawamura T, Chang K-C, Hsu CY, Chen C-H.. Plasma L5 levels are elevated in ischemic stroke patients and enhance platelet aggregation. Blood 2016;127:1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Korporaal SJA, Akkerman J-WN.. Platelet activation by low density lipoprotein and high density lipoprotein. Pathophysiol Haemos Thromb 2006;35:270–280. [DOI] [PubMed] [Google Scholar]
- 98. Zhao CX, Cui YH, Fan Q, Wang PH, Hui R, Cianflone K, Wang DW.. Small dense low-density lipoproteins and associated risk factors in patients with stroke. Cerebrovasc Dis 2009;27:99–104. [DOI] [PubMed] [Google Scholar]
- 99. Verhoye E, Langlois MR.. Circulating oxidized low-density lipoprotein: a biomarker of atherosclerosis and cardiovascular risk? Clin Chem Lab Med 2009;47:128–137. [DOI] [PubMed] [Google Scholar]
- 100. Rota S, McWilliam NA, Baglin TP, Byrne CD.. Atherogenic lipoproteins support assembly of the prothrombinase complex and thrombin generation: modulation by oxidation and vitamin E. Blood 1998;91:508–515. [PubMed] [Google Scholar]
- 101. Klein S, Spannagl M, Engelmann B.. Phosphatidylethanolamine participates in the stimulation of the contact system of coagulation by very-low-density lipoproteins. Arterioscler Thromb Vasc Biol 2001;21:1695–1700. [PubMed] [Google Scholar]
- 102. Zieseniss S, Zahler S, Muller I, Hermetter A, Engelmann B.. Modified phosphatidylethanolamine as the active component of oxidized low density lipoprotein promoting platelet prothrombinase activity. J Biol Chem 2001;276:19828–19835. [DOI] [PubMed] [Google Scholar]
- 103. Hörkkö S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, Witztum JL.. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest 1999;103:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Trpkovic A, Resanovic I, Stanimirovic J, Radak D, Mousa SA, Cenic-Milosevic D, Jevremovic D, Isenovic ER.. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci 2015;52:70–85. [DOI] [PubMed] [Google Scholar]
- 105. Langlois MR, Rietzschel ER, De Buyzere ML, De Bacquer D, Bekaert S, Blaton V, De Backer GG, Gillebert TC.. Femoral plaques confound the association of circulating oxidized low-density lipoprotein with carotid atherosclerosis in a general population aged 35 to 55 years: the Asklepios Study. Arterioscler Thromb Vasc Biol 2008;28:1563–1568. [DOI] [PubMed] [Google Scholar]
- 106. Polovina M, Petrovic I, Brkovic V, Asanin M, Marinkovic J, Ostojic M, Petrović I, Brković V, Ašanin M, Marinković J, Ostojić M.. Oxidized low-density lipoprotein predicts the development of renal dysfunction in atrial fibrillation. Cardiorenal Med 2017;7:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Polovina MM, Ostojic MC, Potpara TS.. Relation of biomarkers of inflammation and oxidative stress with hypertension occurrence in lone atrial fibrillation. Mediators Inflamm 2015;2015:653026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Duni A, Liakopoulos V, Rapsomanikis K-P, Dounousi E.. Chronic kidney disease and disproportionally increased cardiovascular damage: does oxidative stress explain the burden? Oxid Med Cell Longev 2017;2017:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Florens N, Calzada C, Lyasko E, Juillard L, Soulage CO.. Modified lipids and lipoproteins in chronic kidney disease: a new class of uremic toxins. Toxins (Basel) 2016;8:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Emekli-Alturfan E, Basar I, Alturfan AA, Ayan F, Koldas L, Balci H, Emekli N.. The relation between plasma tissue factor and oxidized LDL levels in acute coronary syndromes. Pathophysiol Haemos Thromb 2007;36:290–297. [DOI] [PubMed] [Google Scholar]
- 111. Cimmino G, Cirillo P, Conte S, Pellegrino G, Barra G, Maresca L, Morello A, Calì G, Loffredo F, R De P, Arena G, Sawamura T, Ambrosio G, Golino P.. Oxidized low-density lipoproteins induce tissue factor expression in T-lymphocytes via activation of lectin-like oxidized low-density lipoprotein receptor-1. Cardiovasc Res 2020;116:1125–1135. [DOI] [PubMed] [Google Scholar]
- 112. Azizova OA, Roitman EV, Dement'eva II, Nikitina NA, Gagaeva EV, Lopukhin YM.. Effects of low-density lipoproteins on blood coagulation and fibrinolytic activity. Bull Exp Biol Med 2000;129:541–544. [DOI] [PubMed] [Google Scholar]
- 113. Lados-Krupa A, Konieczynska M, Chmiel A, Undas A.. Increased oxidation as an additional mechanism underlying reduced clot permeability and impaired fibrinolysis in type 2 diabetes. J Diabetes Res 2015;2015:456189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ardlie NG, Selley ML, Simons LA.. Platelet activation by oxidatively modified low density lipoproteins. Atherosclerosis 1989;76:117–124. [DOI] [PubMed] [Google Scholar]
- 115. Podrez EA, Byzova TV.. Prothrombotic lipoprotein patterns in stroke. Blood 2016;127:1221–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chen K, Febbraio M, Li W, Silverstein RL.. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res 2008;102:1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Vanhoutte PM. Regeneration of the endothelium in vascular injury. Cardiovasc Drugs Ther 2010;24:299–303. [DOI] [PubMed] [Google Scholar]
- 118. Pawlowska Z, Swiatkowska M, Krzeslowska J, Pawlicki L, Cierniewski CS.. Increased platelet-fibrinogen interaction in patients with hypercholesterolemia and hypertriglyceridemia. Atherosclerosis 1993;103:13–20. [DOI] [PubMed] [Google Scholar]
- 119. Carvalho AC, Colman RW, Lees RS.. Platelet function in hyperlipoproteinemia. N Engl J Med 1974;290:434–438. [DOI] [PubMed] [Google Scholar]
- 120. Colas R, Sassolas A, Guichardant M, Cugnet-Anceau C, Moret M, Moulin P, Lagarde M, Calzada C.. LDL from obese patients with the metabolic syndrome show increased lipid peroxidation and activate platelets. Diabetologia 2011;54:2931–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Knowles RB, Lawrence MJ, Ferreira PM, Hayman MA, D’Silva LA, Stanford SN, Sabra A, Tucker AT, Hawkins KM, Williams PR, Warner TD, Evans PA.. Platelet reactivity influences clot structure as assessed by fractal analysis of viscoelastic properties. Platelets 2018;29:162–170. [DOI] [PubMed] [Google Scholar]
- 122. Chatterjee M, Rath D, Schlotterbeck J, Rheinlaender J, Walker-Allgaier B, Alnaggar N, Zdanyte M, Müller I, Borst O, Geisler T, Schäffer TE, Lämmerhofer M, Gawaz M.. Regulation of oxidized platelet lipidome: implications for coronary artery disease. Eur Heart J 2017;38:1993–2005. [DOI] [PubMed] [Google Scholar]
- 123. Blache D, Gautier T, Tietge UJF, Lagrost L.. Activated platelets contribute to oxidized low-density lipoproteins and dysfunctional high-density lipoproteins through a phospholipase A2-dependent mechanism. FASEB J 2012;26:927–937. [DOI] [PubMed] [Google Scholar]
- 124. Chapman MJ, Huby T, Nigon F, Thillet J.. Lipoprotein (a): implication in atherothrombosis. Atherosclerosis 1994;110:S69–S75. [DOI] [PubMed] [Google Scholar]
- 125. Tsironis LD, Mitsios JV, Milionis HJ, Elisaf M, Tselepis AD.. Effect of lipoprotein (a) on platelet activation induced by platelet-activating factor: role of apolipoprotein (a) and endogenous PAF-acetylhydrolase. Cardiovasc Res 2004;63:130–138. [DOI] [PubMed] [Google Scholar]
- 126. Labudovic D, Kostovska I, Tosheska Trajkovska K, Cekovska S, Brezovska Kavrakova J, Topuzovska S.. Lipoprotein(a)—link between atherogenesis and thrombosis. Prague Med Rep 2019;120:39–51. [DOI] [PubMed] [Google Scholar]
- 127. Rand ML, Sangrar W, Hancock MA, Taylor DM, Marcovina SM, Packham MA, Koschinsky ML.. Apolipoprotein(a) enhances platelet responses to the thrombin receptor-activating peptide SFLLRN. Arterioscler Thromb Vasc Biol 1998;18:1393–1399. [DOI] [PubMed] [Google Scholar]
- 128. Ezratty A, Simon DI, Loscalzo J.. Lipoprotein(a) binds to human platelets and attenuates plasminogen binding and activation. Biochemistry 1993;32:4628–4633. [DOI] [PubMed] [Google Scholar]
- 129. Caplice NM, Panetta C, Peterson TE, Kleppe LS, Mueske CS, Kostner GM, Broze GJJ, Simari RD.. Lipoprotein (a) binds and inactivates tissue factor pathway inhibitor: a novel link between lipoproteins and thrombosis. Blood 2001;98:2980–2987. [DOI] [PubMed] [Google Scholar]
- 130. Helgadottir A, Gretarsdottir S, Thorleifsson G, Holm H, Patel RS, Gudnason T, Jones GT, van Rij AM, Eapen DJ, Baas AF, Tregouet D-A, Morange P-E, Emmerich J, Lindblad B, Gottsäter A, Kiemeny LA, Lindholt JS, Sakalihasan N, Ferrell RE, Carey DJ, Elmore JR, Tsao PS, Grarup N, Jørgensen T, Witte DR, Hansen T, Pedersen O, Pola R, Gaetani E, Magnadottir HB, Wijmenga C, Tromp G, Ronkainen A, Ruigrok YM, Blankensteijn JD, Mueller T, Wells PS, Corral J, Soria JM, Souto JC, Peden JF, Jalilzadeh S, Mayosi BM, Keavney B, Strawbridge RJ, Sabater-Lleal M, Gertow K, Baldassarre D, Nyyssönen K, Rauramaa R, Smit AJ, Mannarino E, Giral P, Tremoli E, de Faire U, Humphries SE, Hamsten A, Haraldsdottir V, Olafsson I, Magnusson MK, Samani NJ, Levey AI, Markus HS, Kostulas K, Dichgans M, Berger K, Kuhlenbäumer G, Ringelstein EB, Stoll M, Seedorf U, Rothwell PM, Powell JT, Kuivaniemi H, Onundarson PT, Valdimarsson E, Matthiasson SE, Gudbjartsson DF, Thorgeirsson G, Quyyumi AA, Watkins H, Farrall M, Thorsteinsdottir U, Stefansson K.. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J Am Coll Cardiol 2012;60:722–729. [DOI] [PubMed] [Google Scholar]
- 131. Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG.. Genetic evidence that lipoprotein(a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler Thromb Vasc Biol 2012;32:1732–1741. [DOI] [PubMed] [Google Scholar]
- 132. Boffa MB, Koschinsky ML.. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res 2016;57:745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Morelli VM, Lijfering WM, Bos MHA, Rosendaal FR, Cannegieter SC.. Lipid levels and risk of venous thrombosis: results from the MEGA-study. Eur J Epidemiol 2017;32:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Grifoni E, Marcucci R, Ciuti G, Cenci C, Poli D, Mannini L, Liotta AA, Miniati M, Abbate R, Prisco D.. The thrombophilic pattern of different clinical manifestations of venous thromboembolism: a survey of 443 cases of venous thromboembolism. Semin Thromb Hemost 2012;38:230–234. [DOI] [PubMed] [Google Scholar]
- 135. Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley THJ, Folsom AR.. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke 2006;37:2493–2498. [DOI] [PubMed] [Google Scholar]
- 136. Deguchi H, Pecheniuk NM, Elias DJ, Averell PM, Griffin JH.. High-density lipoprotein deficiency and dyslipoproteinemia associated with venous thrombosis in men. Circulation 2005;112:893–899. [DOI] [PubMed] [Google Scholar]
- 137. Doggen CJM, Smith NL, Lemaitre RN, Heckbert SR, Rosendaal FR, Psaty BM.. Serum lipid levels and the risk of venous thrombosis. Arterioscler Thromb Vasc Biol 2004;24:1970–1975. [DOI] [PubMed] [Google Scholar]
- 138. Marcucci R, Liotta AA, Cellai AP, Rogolino A, Gori AM, Giusti B, Poli D, Fedi S, Abbate R, Prisco D.. Increased plasma levels of lipoprotein(a) and the risk of idiopathic and recurrent venous thromboembolism. Am J Med 2003;115:601–605. [DOI] [PubMed] [Google Scholar]
- 139. von DM, Nowak-Göttl U, Eisert R, Dieterich C, Barthels M, Scharrer I, Ganser A, Ehrenforth S.. Increased lipoprotein (a) levels as an independent risk factor for venous thromboembolism. Blood 2000;96:3364–3368. [PubMed] [Google Scholar]
- 140. Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D.. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation 1998;98:1487–1494. [DOI] [PubMed] [Google Scholar]
- 141. Kawasaki T, Kambayashi J, Ariyoshi H, Sakon M, Suehisa E, Monden M.. Hypercholesterolemia as a risk factor for deep-vein thrombosis. Thromb Res 1997;88:67–73. [DOI] [PubMed] [Google Scholar]
- 142. Zolcinski M, Ciesla-Dul M, Undas A.. Effects of atorvastatin on plasma fibrin clot properties in apparently healthy individuals and patients with previous venous thromboembolism. Thromb Haemost 2012;107:1180–1182. [DOI] [PubMed] [Google Scholar]
- 143. Violi F, Carnevale R, Pastori D, Pignatelli P.. Antioxidant and antiplatelet effects of atorvastatin by Nox2 inhibition. Trends Cardiovasc Med 2014;24:142–148. [DOI] [PubMed] [Google Scholar]
- 144. Zabczyk M, Majewski J, Karkowski G, Malinowski KP, Undas A, Ząbczyk M, Majewski J, Karkowski G, Malinowski KP, Undas A, Zabczyk M, Majewski J, Karkowski G, Malinowski KP, Undas A.. Vitamin K antagonists favourably modulate fibrin clot properties in patients with atrial fibrillation as early as after 3 days of treatment: relation to coagulation factors and thrombin generation. Thromb Res 2015;136:832–838. [DOI] [PubMed] [Google Scholar]
- 145. Undas A, Celinska-Lowenhoff M, Lowenhoff T, Szczeklik A.. Statins, fenofibrate, and quinapril increase clot permeability and enhance fibrinolysis in patients with coronary artery disease. J Thromb Haemost 2006;4:1029–1036. [DOI] [PubMed] [Google Scholar]
- 146. Tehrani S, Mobarrez F, Antovic A, Santesson P, Lins P-E, Adamson U, Henriksson P, Wallen NH, Jorneskog G.. Atorvastatin has antithrombotic effects in patients with type 1 diabetes and dyslipidemia. Thromb Res 2010;126:e225–e231. [DOI] [PubMed] [Google Scholar]
- 147. Colli S, Eligini S, Lalli M, Camera M, Paoletti R, Tremoli E.. Vastatins inhibit tissue factor in cultured human macrophages. A novel mechanism of protection against atherothrombosis. Arterioscler Thromb Vasc Biol 1997;17:265–272. [DOI] [PubMed] [Google Scholar]
- 148. Undas A, Brummel-Ziedins KE, Mann KG.. Anticoagulant effects of statins and their clinical implications. Thromb Haemost 2014;111:392–400. [DOI] [PubMed] [Google Scholar]
- 149. Glynn RJ, Danielson E, Fonseca FAH, Genest J, Gotto AMJ, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Ridker PM.. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009;360:1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hulten E, Jackson JL, Douglas K, George S, Villines TC.. The effect of early, intensive statin therapy on acute coronary syndrome: a meta-analysis of randomized controlled trials. Arch Intern Med 2006;166:1814–1821. [DOI] [PubMed] [Google Scholar]
- 151. Ray JG, Mamdani M, Tsuyuki RT, Anderson DR, Yeo EL, Laupacis A.. Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med 2001;161:1405–1410. [DOI] [PubMed] [Google Scholar]
- 152. Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss CB, Waters D.. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation 1995;92:3172–3177. [DOI] [PubMed] [Google Scholar]
- 153. Undas A, Celinska-LöWenhoff M, Brummel-Ziedins KE, Brozek J, Szczeklik A, Mann KG.. Simvastatin given for 3 days can inhibit thrombin generation and activation of factor V and enhance factor Va inactivation in hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 2005;25:1524–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sanguigni V, Pignatelli P, Lenti L, Ferro D, Bellia A, Carnevale R, Tesauro M, Sorge R, Lauro R, Violi F.. Short-term treatment with atorvastatin reduces platelet CD40 ligand and thrombin generation in hypercholesterolemic patients. Circulation 2005;111:412–419. [DOI] [PubMed] [Google Scholar]
- 155. Dangas G, Smith DA, Unger AH, Shao JH, Meraj P, Fier C, Cohen AM, Fallon JT, Badimon JJ, Ambrose JA.. Pravastatin: an antithrombotic effect independent of the cholesterol-lowering effect. Thromb Haemost 2000;83:688–692. [PubMed] [Google Scholar]
- 156. Undas A, Celinska-Löwenhoff M, Domagala TB, Iwaniec T, Dropinski J, Löwenhoff T, Szczeklik A.. Early antithrombotic and anti-inflammatory effects of simvastatin versus fenofibrate in patients with hypercholesterolemia. Thromb Haemost 2005;94:193–199. [DOI] [PubMed] [Google Scholar]
- 157. Luo Y, Li J, Zhang J, Xu Y.. Low HDL cholesterol is correlated to the acute ischemic stroke with diabetes mellitus. Lipids Health Dis 2014;13:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Dentali F, Gessi V, Marcucci R, Gianni M, Grandi AM, Franchini M.. Lipoprotein(a) as a risk factor for venous thromboembolism: a systematic review and meta-analysis of the literature. Semin Thromb Hemost 2017;43:614–620. [DOI] [PubMed] [Google Scholar]
- 159. Li S, Gao Y, Ma W, Wang H, Zhou G, Guo W, Liu Y.. The relationship between serum lipoprotein (a) levels and ischemic stroke risk: a cohort study in the Chinese population. Inflammation 2014;37:686–693. [DOI] [PubMed] [Google Scholar]
- 160. Boden-Albala B, Kargman DE, Lin I-F, Paik MC, Sacco RL, Berglund L.. Increased stroke risk and lipoprotein(a) in a multiethnic community: the Northern Manhattan Stroke Study. Cerebrovasc Dis 2010;30:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Liu W, Xiong N, Xie K, Wu B, Qi Z, Zhou P, Gao W, Bao L, Gao X, Qiu Z, Gong H, He G, Cao B, Shi H, Luo X, Li J.. A stricter control of low-density lipoprotein is necessary for thrombosis reduction in ‘lower thrombosis risk’ patients with atrial fibrillation: a multicenter retrospective cohort study. J Thromb Thrombolysis 2020;50:849–857. [DOI] [PubMed] [Google Scholar]
- 162. Yan S, Li Q, Xia Z, Yan S, Wei Y, Hong K, Wu Y, Li J, Cheng X.. Risk factors of thromboembolism in nonvalvular atrial fibrillation patients with low CHA2DS2-VASc score. Medicine (Baltimore) 2019;98:e14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pol T, Held C, Westerbergh J, Lindback J, Alexander JH, Alings M, Erol C, Goto S, Halvorsen S, Huber K, Hanna M, Lopes RD, Ruzyllo W, Granger CB, Hijazi Z.. Dyslipidemia and risk of cardiovascular events in patients with atrial fibrillation treated with oral anticoagulation therapy: insights from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. J Am Heart Assoc 2018;7:e007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Qi Z, Chen H, Wen Z, Yuan F, Ni H, Gao W, Shen J, Li J, Lin Y, Shan Y, Jin B, Yan P, Shi H, Luo X.. Relation of low-density lipoprotein cholesterol to ischemic stroke in patients with nonvalvular atrial fibrillation. Am J Cardiol 2017;119:1224–1228. [DOI] [PubMed] [Google Scholar]
- 165. Wu M, Zhou XH, Ruozha B, Song SF, Li YD, Zhang JH, Xing Q, Lu YM, Tang BP. [The relationship between LDL-C and ischemic stroke in 2 470 patients with nonvalvular atrial fibrillation in Xinjiang region]. Zhonghua Nei ke za Zhi 2017;56:258–262. [DOI] [PubMed] [Google Scholar]
- 166. Igarashi Y, Yamaura M, Ito M, Inuzuka H, Ojima K, Aizawa Y.. Elevated serum lipoprotein(a) is a risk factor for left atrial thrombus in patients with chronic atrial fibrillation: a transesophageal echocardiographic study. Am Heart J 1998;136:965–971. [DOI] [PubMed] [Google Scholar]
- 167. Lip GY. Lipoprotein(a) in atrial fibrillation. Am Heart J 2000;139:555–556. [DOI] [PubMed] [Google Scholar]
- 168. Enkhmaa B, Anuurad E, Zhang W, Tran T, Berglund L.. Lipoprotein(a): genotype-phenotype relationship and impact on atherogenic risk. Metab Syndr Relat Disord 2011;9:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Prinsen JK, Kannankeril PJ, Sidorova TN, Yermalitskaya LV, Boutaud O, Zagol-Ikapitte I, Barnett JV, Murphy MB, Subati T, Stark JM, Christopher IL, Jafarian-Kerman SR, Saleh MA, Norlander AE, Loperena R, Atkinson JB, Fogo AB, Luther JM, Amarnath V, Davies SS, Kirabo A, Madhur MS, Harrison DG, Murray KT.. Highly reactive isolevuglandins promote atrial fibrillation caused by hypertension. JACC Basic Transl Sci 2020;5:602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Aschner M, Nguyen TT, Sinitskii AI, Santamaría A, Bornhorst J, Ajsuvakova OP, da Rocha JBT, Skalny AV, Tinkov AA.. Isolevuglandins (isoLGs) as toxic lipid peroxidation byproducts and their pathogenetic role in human diseases. Free Radic Biol Med 2020:S0891-5849(20)31298-3. [DOI] [PubMed] [Google Scholar]
- 171. Boffa MB, Koschinsky ML.. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol 2019;16:305–318. [DOI] [PubMed] [Google Scholar]
- 172. Decker JJ, Norby FL, Rooney MR, Soliman EZ, Lutsey PL, Pankow JS, Alonso A, Chen LY.. Metabolic syndrome and risk of ischemic stroke in atrial fibrillation: ARIC study. Stroke 2019;50:3045–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Flint AC, Conell C, Ren X, Kamel H, Chan SL, Rao VA, Johnston SC.. Statin adherence is associated with reduced recurrent stroke risk in patients with or without atrial fibrillation. Stroke 2017;48:1788–1794. [DOI] [PubMed] [Google Scholar]
- 174. Ndrepepa G, Braun S, von BN, Mehilli J, Gorchakova O, Vogt W, Schomig A, Kastrati A.. Oxidized low density lipoproteins, statin therapy and severity of coronary artery disease. Clin Chim Acta 2005;360:178–186. [DOI] [PubMed] [Google Scholar]
- 175. Obermayer G, Afonyushkin T, Binder CJ.. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J Thromb Haemost 2018;16:418–428. [DOI] [PubMed] [Google Scholar]
- 176. van Kuilenburg J, Lappegard KT, Sexton J, Plesiewicz I, Lap P, Bouwels L, Sprong T, Mollnes TE, Verheugt F, van Heerde WL, Pop GA.. Persisting thrombin activity in elderly patients with atrial fibrillation on oral anticoagulation is decreased by anti-inflammatory therapy with intensive cholesterol-lowering treatment. J Clin Lipidol 2011;5:273–280. [DOI] [PubMed] [Google Scholar]
- 177. He L, Xu R, Wang J, Zhang L, Zhang L, Zhao W, Dong W.. Prestroke statins use reduces oxidized low density lipoprotein levels and improves clinical outcomes in patients with atrial fibrillation related acute ischemic stroke. BMC Neurol 2019;19:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Pechlaner R, Tsimikas S, Yin X, Willeit P, Baig F, Santer P, Oberhollenzer F, Egger G, Witztum JL, Alexander VJ, Willeit J, Kiechl S, Mayr M.. Very-low-density lipoprotein-associated apolipoproteins predict cardiovascular events and are lowered by inhibition of APOC-III. J Am Coll Cardiol 2017;69:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Lauder SN, Allen-Redpath K, Slatter DA, Aldrovandi M, O’Connor A, Farewell D, Percy CL, Molhoek JE, Rannikko S, Tyrrell VJ, Ferla S, Milne GL, Poole AW, Thomas CP, Obaji S, Taylor PR, Jones SA, Groot PG, de Urbanus RT, Horkko S, Uderhardt S, Ackermann J, Jenkins VP, Brancale A, Kronke G, Collins PW, O’Donnell VB.. Networks of enzymatically oxidized membrane lipids support calcium-dependent coagulation factor binding to maintain hemostasis. Sci Signal 2017;10:eaan2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Slatter DA, Percy CL, Allen-Redpath K, Gajsiewicz JM, Brooks NJ, Clayton A, Tyrrell VJ, Rosas M, Lauder SN, Watson A, Dul M, Garcia-Diaz Y, Aldrovandi M, Heurich M, Hall J, Morrissey JH, Lacroix-Desmazes S, Delignat S, Jenkins PV, Collins PW, O’Donnell VB.. Enzymatically oxidized phospholipids restore thrombin generation in coagulation factor deficiencies. JCI Insight 2018;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Itabe H. Oxidized low-density lipoproteins: what is understood and what remains to be clarified. Biol Pharm Bull 2003;26:1–9. [DOI] [PubMed] [Google Scholar]
- 182. Tselepis AD. Oxidized phospholipids and lipoprotein-associated phospholipase A2 as important determinants of Lp(a) functionality and pathophysiological role. J Biomed Res 2018;31:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Langsted A, Nordestgaard BG, Kamstrup PR.. Elevated lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol 2019;74:54–66. [DOI] [PubMed] [Google Scholar]
- 184. Zekavat SM, Ruotsalainen S, Handsaker RE, Alver M, Bloom J, Poterba T, Seed C, Ernst J, Chaffin M, Engreitz J, Peloso GM, Manichaikul A, Yang C, Ryan KA, Fu M, Johnson WC, Tsai M, Budoff M, Vasan RS, Cupples LA, Rotter JI, Rich SS, Post W, Mitchell BD, Correa A, Metspalu A, Wilson JG, Salomaa V, Kellis M, Daly MJ, Neale BM, McCarroll S, Surakka I, Esko T, Ganna A, Ripatti S, Kathiresan S, Natarajan P; NHLBI TOPMed Lipids Working Group. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun 2018;9:2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol 2017;69:692–711. [DOI] [PubMed] [Google Scholar]
- 186. Schmidt K, Noureen A, Kronenberg F, Utermann G.. Structure, function, and genetics of lipoprotein (a). J Lipid Res 2016;57:1339–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Nordestgaard BG, Langsted A.. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res 2016;57:1953–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Zanetti D, Gustafsson S, Assimes TL, Ingelsson E.. Comprehensive investigation of circulating biomarkers and their causal role in atherosclerosis-related risk factors and clinical events. Circ Genomic Precis Med 2020;13:e002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Enas EA, Varkey B, Dharmarajan TS, Pare G, Bahl VK.. Lipoprotein(a): An independent, genetic, and causal factor for cardiovascular disease and acute myocardial infarction. Indian Heart J 2019;71:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Pan Y, Li H, Wang Y, Meng X, Wang Y.. Causal effect of Lp(a) [lipoprotein(a)] level on ischemic stroke and Alzheimer disease: a mendelian randomization study. Stroke 2019;50:3532–3539. [DOI] [PubMed] [Google Scholar]
- 191. Saleheen D, Haycock PC, Zhao W, Rasheed A, Taleb A, Imran A, Abbas S, Majeed F, Akhtar S, Qamar N, Zaman KS, Yaqoob Z, Saghir T, Rizvi SNH, Memon A, Mallick NH, Ishaq M, Rasheed SZ, Memon F-U-R, Mahmood K, Ahmed N, Frossard P, Tsimikas S, Witztum JL, Marcovina S, Sandhu M, Rader DJ, Danesh J.. Apolipoprotein(a) isoform size, lipoprotein(a) concentration, and coronary artery disease: a mendelian randomisation analysis. Lancet Diabetes Endocrinol 2017;5:524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this review are available from the corresponding author upon reasonable request.