Abstract
Background: Diabetes mellitus (DM) is a metabolic disorder whose prevalence increases globally. Medical nutrition therapy (MNT) is one of the DM management pillars to control blood glucose. Local Indonesian brown rice is proven to contain high fiber and magnesium levels thus could improve obesity, fasting blood glucose, and HbA1c. This study aims to prove the benefits of brown rice on anthropometric parameters and blood glucose control.
Design and methods: Respondents were overweight women older than 40 years with type 2 diabetes who were given three main meals and three snacks six days a week for 12 weeks. Anthropometric and blood glucose control data were collected before and after the intervention. Diet and intake data before the intervention were obtained through a semi quantitate food frequency questionnaire. Intake data during the intervention were recorded using the 24-hour food record and analyzed using modified NutriSurvey 2007 software.
Results: Brown rice intervention significantly reduced body weight, BMI, body fat percentage, and abdominal circumference (p<0.05), also in fasting blood glucose (FBG), 2-h postprandial blood glucose (PBG), and HbA1c (p<0.05). From the Pearson’s test results, an increase in fiber intake correlated with a decrease in BMI and abdominal circumference (p=0.03; r = -0.511 and p=0.006; r = -0.619, respectively). Meanwhile, magnesium intake and changes in BMI showed a negative correlation.
Conclusions: The substitution of brown rice as a staple food for 12 weeks improves anthropometric parameters and blood glucose control in respondents with type 2 diabetes.
Significance for public health.
Diet management is one important point in blood glucose control in diabetes mellitus patients. Ignoring the eating habits of patients given interventions makes diet difficult to be implemented. The tradition of consuming rice as a staple food is a dietary pattern for Indonesians. Selecting brown rice as a staple food in patients with diabetes mellitus has been proven to improve blood glucose control. Clinical trials in this study can be used as a reference for public education regarding the recommended servings of brown rice in daily servings as blood glucose control.
Key words: Anthropometric, blood glucose, HbA1c, Indonesian variety of Brown rice, type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus (DM) is one of the non-communicable diseases that has the highest prevalence in the world, and the sufferers are estimated to increase by 50% in 2045 (629 million people) compared to those in 2017 (425 million people).1 In 2013, Indonesia ranked fifth in the world for the number of DM patients, which reached 8.5 million people. DM data in Indonesia shows that in 2018 6.9% of the total population of Indonesia, or around 12 million people, suffered from DM.2
Nowadays, people have become aware of the importance of food as part of a healthy lifestyle. The concept of functional food that is thought to have good health benefits and the process of healing diseases is being favored and chosen as a part of daily consumption. Community diet also influences the selection of functional food in their daily diet. Countries in Asia, including Indonesia, are rice-producing countries and use rice as the primary energy source. Rice is known as “the grain of life” and is synonymous with Asian food, and “dietary guidelines” include recommendations for consuming whole grains, including rice, in several countries.3 The term main meal developed and implemented in Indonesian society is always associated with a complete meal with staple food serving in the form of rice, especially white rice, so that white rice consumption per capita reaches 370-380 grams per day.4
Brown rice is different from other colored rice, such as black rice or red rice, whose name represents the original color of the rice because of its antioxidant content, such as anthocyanins. Brown rice is basically plain white rice, but only part of the rice husk or the outer layer is removed and does not undergo further polishing so that the rice bran remains intact. The research results of Sulistyowati et al.5 show that in addition to its high fiber content, brown rice also has seven times the higher mineral content of Magnesium and Manganese than white rice. Fiber and minerals are essential components needed for DM sufferers because they are thought to help control blood glucose. Our preliminary study results show that the Indonesian local SINTANUR variety of brown rice has high fiber and mineral content (magnesium and manganese), has been shown to help reduce intestinal microbiota dysbiosis and increase serum magnesium levels in obese experimental animals, and prevent an increase in blood glucose.5,6
The existing challenge is a public opinion about colored rice that is judged to have a rough texture and unpleasant taste. However, over-consumption of white rice increased the risk of obesity and type 2 diabetes mellitus. White rice is also known for having a less favorable metabolic effect on weight management. Animal studies show that brown rice consumption significantly decreases abdominal fat levels and demonstrates a better beneficial metabolic effect on weight management than white rice.7,8 High brown rice intake (≥2 servings per week) in human is associated with a lower risk of type 2 diabetes.7 A few studies regarding the substitution of white rice to brown rice for various periods (six weeks until four months) show a beneficial effect on blood glucose control and anthropometric parameters in subjects with impaired glucose tolerance or type 2 DM.9,10 Long-term glycemic control is an essential indicator for cumulative glycemic history, as it correlates well with the risk of long-term diabetes complications. HbA1c is now recommended as a standard of care (SOC) for testing and monitoring diabetes, specifically in type 2 DM.11
Dietary intervention with DM requires an approach to the diet of the local culture so that it is readily accepted and can be obeyed, considering that DM is an incurable disease but can be controlled through good diet management. The use of rice as a staple food source in the daily diet of DM patients is of the efforts to maintain and follow the community diet. From the background mentioned earlier, it is important to research the clinical development of brown rice as a typical Indonesian functional food that is useful for reducing long-term blood glucose control, thus reducing the morbidity and mortality of DM sufferers.
Design and methods
This research was a preliminary study, an experimental design for analyzing the effect of brown rice only. This brown rice study was a part of a crossover study of brown rice and white rice consumption in type 2 diabetes mellitus patients. The minimum sample for this preliminary study (pre-post design) was 16 respondents by considering the amount of the brown rice intervention.12 All procedures were approved by the research ethics committee of the Faculty of Medicine, Universitas Brawijaya, Indonesia through Ethic Approval number 143/EC/KEPK/07/2020. In this pilot project, eighteen females with type 2 diabetes mellitus were given a 12-week brown rice intervention diet (three meals and three snack times a day for six days/week) provided by professional diet catering. The energy requirement was calculated based on individual weight, height, age, and respondent activities using the Harris- Benedict formula with a macronutrient composition of ±20% protein, ±30% fat, and ±50% carbohydrate of the total energy.
Food intake and anthropometric measurement
Dietary history before intervention was explored using Semi Quantitative-Food Frequency Questionnaire (SQ-FFQ), and food intake during the intervention was monitored by recording the meals three times per week for 12 weeks using the food record form. Anthropometric measurements were carried out at the beginning of data collection, monthly measurements, and at the end of the intervention. Measurements of body weight, body mass index (BMI), body fat percentage, and visceral fat percentage were carried out using a Body Composition Monitor (OMRON HBF-375 Karada Scan). Height measurements were performed using a microtoise (SECA), while abdominal circumferences were measured using a measuring tape.
Blood glucose and HbA1c measurement
In examining the blood glucose profile before and after the intervention, respondents were asked to fast for eight hours. Blood was drawn from the elbow fold vein (median cubital) and placed in tubes containing EDTA. FBG and 2-h PBG examinations were carried out using the spectrophotometric method (Caretium Automatic biochemistry analyzer NB-201), and the HbA1c examination was carried out by the high-performance liquid chromatography (HPLC) method using D-10 Hemoglobin Testing System instrument by Bio-Rad.
Statistical analysis
Energy and nutrients intake before and during the intervention period were calculated using modified NutriSurvey 2007 software (EBIspro, Willstätt, Germany).13 Anthropometric data, fasting blood glucose level, HbA1C level, and intake (energy, fat, SAFA, PUFA, magnesium) of the respondents were analyzed using paired t-test. The two-hour postprandial blood glucose, intake (carbohydrate, protein, fiber, potassium, vitamin A, vitamin C), and amount of food intake were analyzed using the Wilcoxon test since they did not have normal distribution data (before or during intervention). The Spearman correlation test was performed to determine the correlation between energy, magnesium, and fiber intake with anthropometry parameters and blood glucose control. Differences were considered significant when the p-value was lower than 0.05. All those statistical analyzes were performed using SPSS software (version 16.0, SPSS Inc., Chicago, IL, USA).
Results
Basic characteristics of respondents
Respondents who participated in this study were aged 42-60 years, with an average BMI of 25.63 kg/m2. At baseline measurement, all of the respondents (100%) had HbA1c levels ≥6.5%. All respondents had a high body fat percentage (normal range: 20-29%) according to the OMRON HBF-375 Karada Scan manual, and 16 respondents (88.9%) experienced abdominal obesity (normal range <80 cm)2 (Table 1).
Intervention effects
A total of 18 respondents with type 2 DM underwent a 12- week study period of brown rice diet without any drop-out. The analysis results of basic and after the intervention data showed that brown rice substitution improved anthropometric parameters and blood glucose control of type 2 DM respondents through the decreases of weight, BMI, body fat percentage, abdominal circumference, fasting blood glucose levels, 2 hours postprandial blood glucose levels, and HbA1c. In anthropometric parameters, there was no significant difference in body fat index and visceral fat percentage. There was no difference in the mean energy and fat intake before and after the intervention. However, there was a significant decrease in the percentage of carbohydrate intake and an increase in the percentage of protein intake. In the total intake of types of food, there was a significant increase in the intake of vegetables and animal protein in the form of meat, fish and chicken, and there was a significant decrease in the intake of simple sugars (Table 2).
The Spearman’s test results showed a negative correlation between energy intake and changes in BMI (p=0.010; r = -0.592), also a trend of the correlation between energy intake and changes in HbA1c (p=0.115; r = -0.392) (Figure 1). A negative correlation was also found between fiber intake and changes in BMI (p=0.004; r = -0.644), fiber intake and changes in abdominal circumference (p=0.001; r = -0.725), and a trend of correlation between magnesium intake and changes in BMI (p=0.068; r = 0.440) (Figure 2).
Discussion
The 12-week study period involving respondents with type 2 diabetes showed that substitution of brown rice carbohydrate sources could improve anthropometric markers (BMI, body fat percentage, abdominal circumference) and glycemic control (FBG, 2-h PBG, HbA1c). In processing brown rice, the rice bran is not eliminated, while in white rice, the entire rice bran is missing. The outer layer contains various essential components, such as phytosterols (antioxidants, vitamins, minerals, and water-insoluble fiber). A hundred grams of raw brown rice contains 22.04 gr fiber, 230 mg magnesium, and 340 mg potassium. The soluble fiber content of brown rice is 141% higher than that of white rice, while its magnesium and potassium contents are 7.7 times and 5.7 times higher than white rice.6
Table 1.
Basic characteristics of type 2 DM respondents before intervention (n=18).
| Characteristics | Mean ± SD | Range (min-max) |
|---|---|---|
| Age (year) | 53.44±4.55 | 42-60 |
| Anthropometry | ||
| Body weight (kg) | 59.63±6.65 | 45.20-71.70 |
| BMI (kg/m2) | 25.63±2.25 | 21.50-28.50 |
| Total body fat (%) | 35.72±2.53 | 30.90-39.30 |
| Abdominal circumference (cm) | 86.97±5.60 | 75.90-95.15 |
| Visceral fat (%) | 8.41±2.15 | 4.50-11.50 |
| Blood glucose control | ||
| Fasting blood glucose (mg/dL) | 141.67±17.36 | 110-177 |
| 2 hours postprandial blood glucose (mg/dL) | 179.28±21.97 | 148-223 |
| HbA1c (%) | 8.34±1.54 | 6.1-11.0 |
BMI, body mass index.
Table 2.
Effects of brown rice diet in type 2 diabetes mellitus respondents.
| Characteristics | Before intervention (Mean ± SD) | Intervention (Mean ± SD) | Δ | p |
|---|---|---|---|---|
| (Mean ± SD) | ||||
| Anthropometry | ||||
| Body weight (kg) | 59.63±6.65 | 58.63±6.39 | -1.00±1.21 | 0.003* |
| BMI (kg/m2) | 25.63±2.25 | 25.16±2.12 | -0.47±0.55 | 0.002* |
| Total body fat (%) | 35.72±2.53 | 34.96±2.05 | -0.76±1.22 | 0.016* |
| Body fat index | 0.604±0.056 | 0.602±0.064 | -0.002±0.019 | 0.687* |
| Abdominal circumference (cm) | 86.97±5.60 | 84.44±5.59 | -2.53±3.62 | 0.009* |
| Visceral fat (%) | 8.41±2.15 | 8.10±1.65 | -0.32±0.89 | 0.148* |
| Blood glucose control | ||||
| Fasting blood glucose (mg/dL) | 141.67±17.36 | 134.94±20.49 | -6.72±11.47 | 0.024* |
| 2 hours postprandial blood glucose (mg/dL) | 179.28±21.97 | 171.11±30.74 | -8.17±18.63 | 0.045# |
| HbA1c (%) | 8.34±1.54 | 8.10±1.65 | -0.72±1.02 | 0.011* |
| Energy intake and nutrients | ||||
| Energy intake (kcal) | 1445.60±468.72 | 1505.80±97.96 | 60.21±504.97 | 0.619* |
| Carbohydrate intake (% energy) | 54.80±6.85 | 48.17±2.65 | -6.63±7.57 | 0.004# |
| Protein intake (% energy) | 14.03±2.32 | 19.44±2.01 | 5.42±4.07 | 0.000# |
| Fat intake (% energy) | 31.17±5.85 | 32.39±1.06 | 1.21±5.84 | 0.391* |
| Saturated fat intake (% energy) | 15.21±4.26 | 14.18±1.40 | -1.03±4.03 | 0.295* |
| Poly unsaturated fat intake (% energy) | 6.42±1.50 | 6.08±0.84 | -0.34±1.54 | 0.361* |
| Fiber intake (g/day) | 15.76±6.18 | 45.51±4.69 | 29.74±7.83 | 0.000# |
| Magnesium intake (g/day) | 285.76±101.1 | 566.18±43.37 | 280.42±108.78 | 0.000* |
| Potassium intake (g/day) | 2005.46±830.02 | 2404.09±192.73 | 398.63±899.92 | 0.031# |
| Vitamin A intake (g/day) | 1563.04±728.8 | 2269.07±386.95 | 706.3±719.62 | 0.003# |
| Vitamin C intake (g/day) | 131.54±104.72 | 146.23±80.42 | 14.70±41.25 | 0.586# |
| Amount of food intake | ||||
| Green vegetables (g/day) | 52.21±43.37 | 232.00±41.25 | 179.79 + 60.84 | 0.000# |
| Tubers, rice, and cereals (g/day) | 310.94±118.50 | 297.26±28.52 | -13.67±108.16 | 0.744# |
| Meat (g/day) | 9.37±12.61 | 33.19±8.60 | 23.82±17.33 | 0.001# |
| Fish (g/day) | 19.53±18.26 | 42.46±14.25 | 22.93±11.91 | 0.004# |
| Chicken (g/day) | 18.88 ±24.36 | 40.17±11.91 | 21.29±27.65 | 0.006# |
| Fruit (g/day) | 186.78±164.86 | 195.13±46.81 | 8.35±173.16 | 0.528# |
| Sugar (g/day) | 23.80±18.65 | 5.33±2.29 | -18.47±18.40 | 0.000# |
BMI, body mass index; CI 95%; *paired t-test; #Wilcoxon test.
These findings are consistent with a small trial conducted in Japan that the substitution of white rice to brown rice had a beneficial effect on blood glucose levels and lipid concentrations. Various layers in the brown rice (endosperm, aleurone, bran, germ) provide a more prolonged carbohydrate digestion effect. Besides, the contained starch has less interaction with digestive enzymes than white rice, which makes its absorption lower.9 In this study, interviews and weekly monitoring were always conducted to maintain the respondence obedience to consume all brown rice diet intervention; thus, reaching 88 to 98% (unpublished data). Therefore, this study shows that the high acceptance of this diet possibly affects blood glucose control and anthropometry data. A study in Vietnam that provided a brown rice diet in women with impaired glucose tolerance showed improvements in glucose control and body weight parameters.10 Another study using glutinous brown rice for eight weeks showed an effect of reduced HbA1c in patients with type 2 diabetes.14 In addition, the substitution of brown rice for three months in individuals with metabolic syndrome and increasing BMI had a positive effect on HbA1c repair.15
Figure 1.
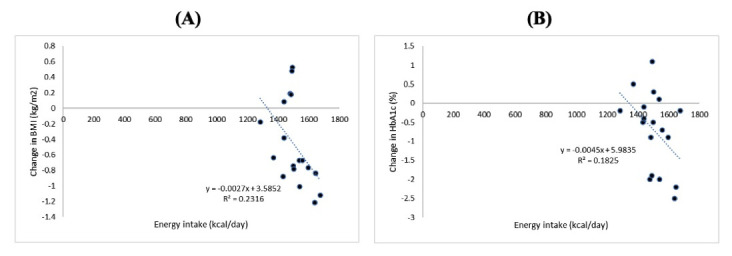
The relationship between energy intake and changes in BMI and HbA1c. A) Energy intake and changes in BMI (p=0.010; r = -0.592). B) Energy intake and changes in HbA1c (p=0.115; r = -0.392).
Figure 2.
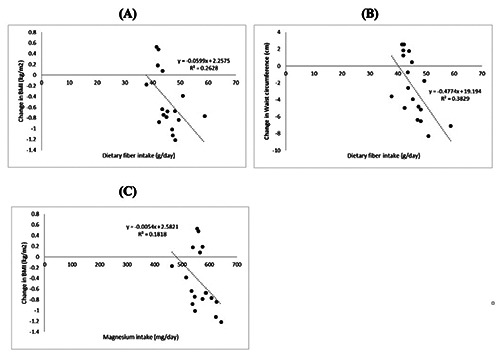
The relationship between fiber and magnesium intake with anthropometry parameter. A) Fiber intake and changes in BMI (p=0.004; r = -0.644). B) Fiber intake and changes in abdominal circumference (p=0.001; r = -0.725). C) Magnesium intake and changes in BMI (p=0.068; r = -0.440).
The high fiber content in brown rice is associated with a low glycemic index, longer satiety that can lead to weight loss, and improved insulin sensitivity, thus improving blood glucose control.16 Besides, brown rice releases less glucose into the blood than white rice.15 Brown rice as a whole grain can also activate α- glycosidase inhibitors and miglitol associated with decreased postprandial glucose levels, abdominal circumference, and visceral fat in subjects with metabolic syndrome.1. It is in line with this study which shows FBG, 2-h PBG, and HbA1c improvements, and the correlation results that the higher the fiber intake, the greater the decrease in BMI and abdominal circumference. The food ingredients that contributed to the increase in fiber intake of the respondents were brown rice and vegetables, wherein the food history data, the vegetable intake of the respondents were still low.
Brown rice is a high source of magnesium. Magnesium contributes to the insulin-mediated regulation of glucose uptake and improves insulin sensitivity.18 Oral Mg2+ supplementation and proper dietary patterns can improve insulin sensitivity and metabolic control in patients with type 2 diabetes because Mg2+ is an important factor in managing blood glucose control.19 Another study in obese individuals showed that consuming 365 mg/day magnesium for six months significantly reduced fasting glucose level, fasting insulin level, insulin resistance (HOMA-IR), and increased insulin sensitivity.20An increase in magnesium intake by 10 mg/1000 kcal correlated with a decrease in BMI. This is consistent with the results of this study that there is a negative correlation between magnesium intake and BMI changes. The mechanism of action of magnesium on the improvement of parameters of obesity is not fully known. Magnesium is an important component that plays a role in the reaction of glycolysis and fat oxidation, so it is thought to play a role in preventing the accumulation of fat tissue in the body.21 The present study has several limitations. As a preliminary study, this study used a minimal sample size of respondents; thus, the number of subjects needs to be increased in future research. Challenges from changing the diet, such as the differences of brown rice and white rice tastes as the respondents’ daily intake, require more effort from the research team to support and remind respondents to consume the diet or comply with the research protocol. This study did not collect the diet acceptance based on the patient preference; therefore, the diet’s palatability could be considered further investigation.
Conclusions
In short, consumption of brown rice for three months as a daily staple food for type 2 DM patients with overweight and obesity decreased the body weight, body fat percentage, and waist circumference. Also, it improved blood glucose control by lowering fasting blood glucose, 2-h postprandial blood glucose, and HbA1c. This research is evidence-based practice according to the health potential effect of brown rice. Brown rice could be a part of nutrition therapy for combating obesity and improving blood glucose control. Community nutrition education as a part of a healthy lifestyle must consider using brown rice as a daily staple food or food component.
Acknowledgements
The authors express profound gratitude to the Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia for supporting this study.
Funding Statement
Funding: This study was financially supported by the Faculty of Medicine, Universitas Brawijaya (Decree: 3392.3/UN10.F08/ PN/2020).
References
- 1.Aguirre F, Brown A, Cho NH, et al. Diabetes Atlas, 6th Edition. International Diabetes Federation; 2013. [Google Scholar]
- 2.Ministry of Health of the Republic of Indonesia. [Hasil Utama Riskesdas 2018 (Main Results of basic health research 2018)].[in Indonesian]. Jakarta: Ministry of Health of the Republic of Indonesia; 2018. [Google Scholar]
- 3.Schaffer-Lequart C, Lehmann U, Ross AB, et al. Whole grain in manufactured foods: current use, challenges and the way forward. Crit Rev Food Sci Nutr 2017;57:1562–8. [DOI] [PubMed] [Google Scholar]
- 4.Statistics Indonesia (BPS). [Rata-rata konsumsi per kapita 2007-2015 (Average consumption per capita2007-2015)].[in Indonesian]. 2015. Accessed: 2021 Mar 22. Available from: https://www.bps.go.id/linkTabelstatis/view/id/950 [Google Scholar]
- 5.Sulistyowati E, Handayani D, Soeharto S, Rudijanto A. Serum mineral (Mg, Mn, and K) levels are associated with increasing the body mass index (BMI) and abdominal circumference. Obes Med 2019;15:100107. [Google Scholar]
- 6.Sulistyowati E, Rudijanto A, Soeharto S, Handayani D. The identification of characteristic macro-and micronutrients and the bioactive components of Indonesian local brown rice as a functional feed in obesity nutrition therapy. Curr Nutr Food Sci 2020;16:494–500. [Google Scholar]
- 7.Sun Q, Spiegelman D, van Dam RM, et al. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 2010;170:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S-O, Wu C, So M-Y, et al. Effects of brown rice on cellular growth and metabolic changes in mice. Food Res Int. 2016;84:33–40. [Google Scholar]
- 9.Hsu T-F, Kise M, Wang M-F, et al. Effects of pre-germinated brown rice on blood glucose and lipid levels in free-living patients with impaired fasting glucose or type 2 diabetes. J Nutr Sci Vitaminol (Tokyo) 2008;54:163–8. [DOI] [PubMed] [Google Scholar]
- 10.Bui TN, Le TH, Nguyen DH, et al. Pre-germinated brown rice reduced both blood glucose concentration and body weight in Vietnamese women with impaired glucose tolerance. J Nutr Sci Vitaminol (Tokyo) 2014;60:183–7. [DOI] [PubMed] [Google Scholar]
- 11.Sherwani SI, Khan HA, Ekhzaimy A, et al. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights 2016;11:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhand NK, Khatkar MS. Statulator: An online statistical calculator. Sample Size Calculator for Comparing Two Paired Means. 2014. Accessed: 2021 May 28. Available from: http://statulator.com/SampleSize/ss2PM.html [Google Scholar]
- 13.Erhardt J. Nutrisurvey for Windows. SEAMEO-TROPMEDRCCN- University of Indonesia; 2007. Available from: http://www.nutrisurvey.de/ [Google Scholar]
- 14.Nakayama T, Nagai Y, Uehara Y, et al. Eating glutinous brown rice twice a day for 8 weeks improves glycemic control in Japanese patients with diabetes mellitus. Nutr Diabetes 2017;7:e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenwood DC, Threapleton DE, Evans CEL, et al. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: systematic review and dose–response meta-analysis of prospective studies. Diabetes Care 2013;36:4166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik VS, Sudha V, Wedick NM, et al. Substituting brown rice for white rice on diabetes risk factors in India: a randomised controlled trial. Br J Nutr 2019;121:1389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimabukuro M, Higa M, Kinjo R, et al. Effects of the brown rice diet on visceral obesity and endothelial function: the BRAVO study. Br J Nutr 2014;111:310–20. [DOI] [PubMed] [Google Scholar]
- 18.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev 2010;23:65–134. [DOI] [PubMed] [Google Scholar]
- 19.Kostov K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: focusing on the processes of insulin secretion and signaling. Int J Mol Sci 2019;20:1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mooren FC, Krüger K, Völker K, et al. Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects– a double-blind, placebo-controlled, randomized trial. Diabetes, Obes Metab 2011;13:281–4. [DOI] [PubMed] [Google Scholar]
- 21.Castellanos-Gutiérrez A, Sánchez-Pimienta TG, Carriquiry A, et al. Higher dietary magnesium intake is associated with lower body mass index, waist circumference and serum glucose in Mexican adults. Nutr J 2018;17:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


