Abstract
Background
People with chronic kidney disease (CKD) have higher risks of cardiovascular disease compared to the general population. Specifically, cardiovascular deaths account most deaths in kidney transplant recipients. Statins are a potentially beneficial intervention for kidney transplant patients given their established benefits in patients at risk of cardiovascular disease in the general population. This is an update of a review first published in 2009.
Objectives
We aimed to evaluate the benefits (reductions in all‐cause and cardiovascular mortality, major cardiovascular events, myocardial infarction and stroke, and progression of CKD to requiring dialysis) and harms (muscle or liver dysfunction, withdrawal, cancer) of statins compared to placebo, no treatment, standard care, or another statin in adults with CKD who have a functioning kidney transplant.
Search methods
We searched the Cochrane Renal Group's Specialised Register to 29 February 2012 through contact with the Trials Search Co‐ordinator using search terms relevant to this review.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs that compared the effects of statins with placebo, no treatment, standard care, or statins on mortality, cardiovascular events, kidney function and toxicity in kidney transplant recipients.
Data collection and analysis
Two authors independently extracted data and assessed risk of bias. Treatment effects were expressed as mean difference (MD) for continuous outcomes (lipids, glomerular filtration rate (GFR), proteinuria) and relative risk (RR) for dichotomous outcomes (major cardiovascular events, mortality, fatal or non‐fatal myocardial infarction, fatal or non‐fatal stroke, elevated muscle or liver enzymes, withdrawal due to adverse events, cancer, end‐stage kidney disease (ESKD), acute allograft rejection) together with 95% confidence intervals (CI).
Main results
We identified 22 studies (3465 participants); 17 studies (3282 participants) compared statin with placebo or no treatment, and five studies (183 participants) compared two different statin regimens.
From data generally derived from a single high‐quality study, it was found that statins may reduce major cardiovascular events (1 study, 2102 participants: RR 0.84, CI 0.66 to 1.06), cardiovascular mortality (4 studies, 2322 participants: RR 0.68, CI 0.45 to 1.01), and fatal or non‐fatal myocardial infarction (1 study, 2102 participants: RR 0.70, CI 0.48 to 1.01); although effect estimates lack precision and include the possibility of no effect.
Statins had uncertain effects on all‐cause mortality (6 studies, 2760 participants: RR 1.08, CI 0.63 to 1.83); fatal or non‐fatal stroke (1 study, 2102 participants: RR 1.18, CI 0.85 to 1.63); creatine kinase elevation (3 studies, 2233 participants: RR 0.86, CI 0.39 to 1.89); liver enzyme elevation (4 studies, 608 participants: RR 0.62, CI 0.33 to 1.19); withdrawal due to adverse events (9 studies, 2810 participants: RR 0.89, CI 0.74 to 1.06); and cancer (1 study, 2094 participants: RR 0.94, CI 0.82 to 1.07).
Statins significantly reduced serum total cholesterol (12 studies, 3070 participants: MD ‐42.43 mg/dL, CI ‐51.22 to ‐33.65); low‐density lipoprotein cholesterol (11 studies, 3004 participants: MD ‐43.19 mg/dL, CI ‐52.59 to ‐33.78); serum triglycerides (11 studies, 3012 participants: MD ‐27.28 mg/dL, CI ‐34.29 to ‐20.27); and lowered high‐density lipoprotein cholesterol (11 studies, 3005 participants: MD ‐5.69 mg/dL, CI ‐10.35 to ‐1.03).
Statins had uncertain effects on kidney function: ESKD (6 studies, 2740 participants: RR 1.14, CI 0.94 to 1.37); proteinuria (2 studies, 136 participants: MD ‐0.04 g/24 h, CI ‐0.17 to 0.25); acute allograft rejection (4 studies, 582 participants: RR 0.88, CI 0.61 to 1.28); and GFR (1 study, 62 participants: MD ‐1.00 mL/min, CI ‐9.96 to 7.96).
Due to heterogeneity in comparisons, data directly comparing differing statin regimens could not be meta‐analysed. Evidence for statins in people who have had a kidney transplant were sparse and lower quality due to imprecise effect estimates and provided limited systematic evaluation of treatment harm.
Authors' conclusions
Statins may reduce cardiovascular events in kidney transplant recipients, although treatment effects are imprecise. Statin treatment has uncertain effects on overall mortality, stroke, kidney function, and toxicity outcomes in kidney transplant recipients. Additional studies would improve our confidence in the treatment benefits and harms of statins on cardiovascular events in this clinical setting.
Plain language summary
HMG CoA reductase inhibitors (statins) for kidney transplant recipients
Kidney transplant patients experience heart disease more often than the general population. Statins have been shown to decrease cholesterol, heart attacks, strokes and deaths for the general population. The aim of this review was to find out whether statins prevent death and complications from heart disease in people who have had a kidney transplant. We included 17 studies in 3282 adults with a functioning kidney transplant which compared statin therapy to a placebo or standard treatment. Based largely on information from a single, large and well‐conducted study, statins may reduce complications from heart disease although information from the available research is imprecise. The effects of statin treatment on death overall, stroke, kidney function and side‐effects are uncertain in people with a kidney transplant. Large additional studies of statin therapy may improve our confidence that statin treatment can safely prevent serious complications from heart disease for people who have a kidney transplant.
Summary of findings
for the main comparison.
| Statin versus placebo or no treatment for adults kidney transplant recipients | |||||
|
Patient or population: adults with chronic kidney disease Settings: kidney transplant recipients Intervention: statin Comparison: placebo or no treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo or no treatment | Statin | ||||
| Major cardiovascular events | 20 per 1000 |
17 per 1000 (13 to 21) 3 fewer (7 fewer to 1 more) |
RR 0.84 (0.66 to 1.06) |
2102 (1) | ⊕⊕ low |
| All‐cause mortality | 20 per 1000 |
22 per 1000 (12 to 37) 2 more (8 fewer to 17 more) |
RR 1.08 (0.63 to 1.83) |
2760 (6) | ⊕⊕ low |
| Cardiovascular mortality | 5 per 1000 |
3 per 1000 (2 to 5) 2 fewer (3 fewer to 0 more) |
RR 0.68 (0.45 to 1.01) |
2322 (4) | ⊕⊕ low |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Absolute approximate event rates of outcomes/year were derived from previously published observational cohort studies. Absolute numbers of people with a functioning kidney transplant with cardiovascular or mortality events avoided or incurred per 1000 treated were estimated using these assumed risks together with the estimated relative risks (and 95% confidence intervals) (ANZDATA 2010; Lentine 2005).
Background
Description of the condition
Kidney transplantation numbers are steadily increasing worldwide and survival rates are improving as a consequence of the adoption of more effective immunosuppressive regimens (USRDS 2011). Cardiovascular disease still accounts for the majority of deaths in kidney transplant recipients (Jardine 2011; Kasiske 1996; NKF 2002; USRDS 2011). Studies conducted in the general population have demonstrated that dyslipidaemia (including increased total cholesterol, triglycerides and low‐density lipoprotein (LDL) cholesterol; and low, high‐density lipoprotein (HDL) cholesterol levels) is a component of the causal pathway leading to cardiovascular mortality. As a consequence, statins have become broadly adopted as the main treatment for high cholesterol levels in the general population, following the results of major studies which demonstrated their impact on cardiovascular and all‐cause mortality (ALLHAT 2002; HPS 2003; JUPITER 2008; Sever 2003; Shepherd 1995). Recent clinical studies and analyses from earlier larger studies have confirmed the cardioprotective effects of statins in people with chronic kidney disease (CKD) (SHARP 2011).
How the intervention might work
Statins may modulate the risk of transplant rejection by decreasing inflammation, enhancing endothelial function, inhibiting smooth muscle proliferation, and exerting direct antithrombotic effects. These pleiotropic effects noted in clinical and laboratory studies suggested possible benefits of statins on acute allograft rejection. In kidney transplant recipients, where both systemic factors and immunosuppressive regimens play a role in changing cholesterol metabolism (Claesson 1998; Cosio 2002; Flechner 2002; Hilbrands 1995, Groth 1999; John 1999; Massy 2001; Mathis 2004, Raine 1988; Satterthwaite 1998), cohort studies have indicated that increased total cholesterol, LDL cholesterol and triglycerides are independent risk factors for allograft rejection and that statin administration is associated with improved patient survival (Aakhus 1999; Aker 1998; Vathsala 1989). Additionally, retrospective studies have shown that increased cholesterol and triglycerides are also independent risk factors for allograft rejection in kidney transplant recipients (Del Castillo 2004; Isoneimi 1994; Massy 1996).
Why it is important to do this review
By extrapolation from the results of these observational data and available randomised controlled trials (RCTs) conducted in the general population, statins have been widely used in kidney transplant recipients. However, there are well recognised discrepancies between the results of observational and study data such that an assessment of the effect of statins on survival in kidney transplant recipients needs to be assessed in available RCTs specifically conducted in this population. Since individual studies of statins in transplant recipients may be underpowered to detect treatment effects on mortality, cardiovascular events and kidney outcomes, we have updated our earlier systematic review and meta‐analysis of all available RCTs (Navaneethan 2009c) to February 2012.
Objectives
We aimed to evaluate the benefits (reductions in all‐cause and cardiovascular mortality, major cardiovascular events, myocardial infarction and stroke, and progression of CKD to requiring dialysis) and harms (muscle or liver dysfunction, withdrawal, cancer) of statins compared to placebo, no treatment, standard care, or another statin in adults with CKD who have a functioning kidney transplant.
Methods
Criteria for considering studies for this review
Types of studies
RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) that evaluated the benefits and harms of statins in adults with a functioning kidney transplant. The first period of randomised cross‐over studies was included. We excluded studies of fewer than eight weeks' duration as such studies were unlikely to permit detection of mortality or cardiovascular outcomes related to statin therapy (Briel 2006).
Types of participants
Inclusion criteria
Studies or subgroups of studies enrolling adult kidney transplant recipients were included irrespective of presence of cardiovascular disease at baseline.
Exclusion criteria
Patients with stages 1‐5 CKD and on dialysis (K‐DOQI stage 5 on dialysis) were excluded as these have been evaluated in separate reviews (Navaneethan 2009a; Palmer 2013).
Types of interventions
We included studies comparing statins with placebo, no treatment or standard care, or another statin. We excluded studies where a statin was compared with a second non‐statin regimen including fibrate therapy.
Types of outcome measures
Primary outcomes
Cardiovascular mortality
Secondary outcomes
All‐cause mortality
Major cardiovascular events
Fatal and non‐fatal myocardial infarction
Fatal and non‐fatal stroke
Adverse events attributable to treatment
Elevated creatine kinase
Elevated liver enzymes
Withdrawal due to adverse events
Cancer
-
End of treatment lipid levels
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
End‐stage kidney disease (ESKD)
Acute rejection
End of treatment proteinuria
End of treatment glomerular filtration rate (GFR)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's Specialised Register through contact with the Trials' Search Coordinator (to 29 February 2012) using search terms relevant to this review.
The Cochrane Renal Group’s Specialised Register contains studies identified from the following sources.
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of clinical practice guidelines, review articles and relevant studies.
Reference lists of abstracts from nephrology scientific meetings.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
Initial review (2009)
The review (Navaneethan 2009c) was initially undertaken by six authors (SDN, SN, VP, DJ, JC, GFMS). The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts were screened independently by three authors (SDN, SN, GFMS) who excluded studies that were not applicable. However, studies and reviews that might have included relevant data or information on studies were retained initially. Two authors (SDN, SN) independently assessed retrieved abstracts and, if necessary, the full text of these studies to determine which studies satisfied the inclusion criteria. Disagreements were resolved in consultation with GFMS.
Updated review (2013)
The update was performed by two independent authors (SDN, SCP) using methods from earlier versions of the review with support from the remaining authors. The same two authors independently screened abstracts retrieved by electronic searches to identify potentially relevant citations for detailed study in full text format. Studies that might include relevant data or information on studies involving statins were initially retained. Studies published in non‐English journals were translated before assessment for inclusion when a non‐English abstract was provided. Disagreements were resolved in consultation with GFMS.
Data extraction and management
Initial review (2009)
Data extraction was carried out by the same authors independently using the standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. When more than one publication of one study existed, the publication with the most complete data was included. Disagreements were resolved in consultation with GFMS.
Updated review to (2013)
Authors independently extracted data from the eligible studies using standard data extraction forms. Where more than one publication of one study existed, the publication with the most complete data was included. Disagreements between authors were resolved in consultation with GFMS.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors (SDN, SCP) using the risk of bias assessment tool (Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (major cardiovascular events, mortality, adverse events, ESKD, acute rejection), treatment effects were expressed as risk ratios (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (lipid parameters, proteinuria, creatinine clearance), the mean difference (MD) was used.
Dealing with missing data
Any further information required from the authors of the studies was requested by written correspondence and relevant information obtained in this manner was included in the review.
Assessment of heterogeneity
Heterogeneity was analysed using the Cochran Q (Chi², N‐1 degrees of freedom) and the I² statistic, with a P of 0.05 used for statistical significance.
Assessment of reporting biases
To assess potential bias from small‐study effects, we constructed funnel plots for the log RR in individual studies against the standard error of the RR. We carried out formal statistical assessment of funnel plot asymmetry with the Egger regression test (Harbord 2006). We conducted analyses using Comprehensive Meta‐Analysis (Version 2, Biostat, Englewood, NJ, 2005).
Data synthesis
Data were summarised using the Der Simonian‐Laird random‐effects model, but the fixed‐effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers.
We summarised the quality of the evidence together with absolute treatment effects for mortality and cardiovascular events based on estimated baseline risks using Grading of Recommendations Assessment Development and Evaluation (GRADE) guidelines (Guyatt 2008) Absolute numbers of adults with a kidney transplant who had cardiovascular events or adverse events avoided or incurred with statin therapy were estimated using the risk estimate for the outcome (and associated 95% CI) obtained from the corresponding meta‐analysis together with the absolute population risk estimated from previously published observational studies (ANZDATA 2010; Lentine 2005).
Subgroup analysis and investigation of heterogeneity
We conducted prespecified subgroup analyses to explore potential sources of heterogeneity in modifying estimates of the effects of statins in the studies when significant heterogeneity was present. We planned subgroup analyses according to the following participant, intervention, or study‐related characteristics, when subgroups possessed four or more independent studies (statin type, statin dose (equivalent to simvastatin)), baseline cholesterol (< 230 mg/dL versus ≥ 230 mg/dL), age (< 55 years versus ≥ 55 years), proportion with diabetes (≥ 20% versus < 20%), or adequacy of allocation concealment.
Results
Description of studies
Results of the search
Our initial review (Navaneethan 2009c) included 16 studies. We reviewed in detail an additional 80 reports that were identified by an updated search conducted in February 2012 (Figure 1). Six new studies were included in this review update review (Celik 2000a; Raiola 1998; Sharif 2009; Seron 2008; Tuncer 2000; Vergoulas 1999).
1.
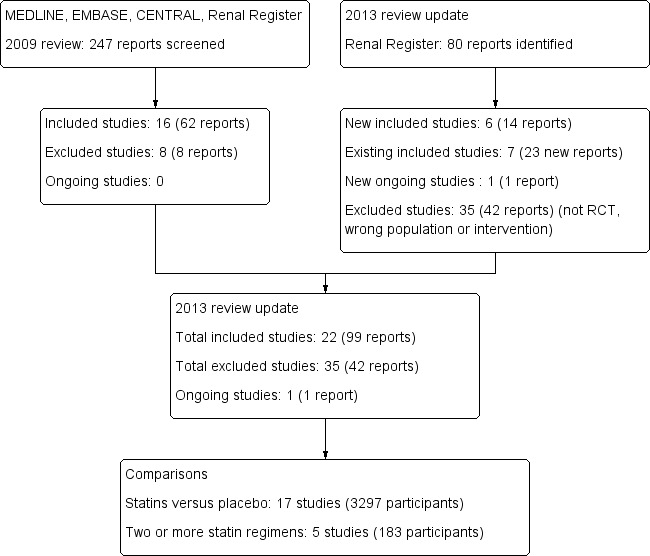
Study flow diagram.
Included studies
Overall we have included 22 studies, enrolling 3465 participants in the review update. Seventeen studies (3282 participants) compared statin with placebo (ALERT 2001; Arnadottir 1994; Bill 1995; Cofan 2002; Hausberg 2001; Kasiske 2001; Katznelson 1996; Lepre 1999; Martinez Hernandez 1993; Melchor 1998; Renders 2001; Sahu 2001; Santos 2001; Seron 2008; Sharif 2009; SOLAR Study 2001; UK‐HARP‐1 2005) and five studies (183 participants) compared two different statin regimens (Castelao 1993; Celik 2000a; Raiola 1998; Tuncer 2000; Vergoulas 1999). Notably, the ALERT 2001 study was a large and well‐conducted double‐blinded RCT conducted in transplant patients and most data for the meta‐analyses are derived from this study.
Four studies were randomised cross‐over studies (Celik 2000a; Martinez Hernandez 1993; Sharif 2009; Vergoulas 1999).
Statin versus placebo or no treatment
Studies varied in sample size (median 48 participants; range 20 to 2102) and were generally small (all but one study (ALERT 2001) had fewer than 1000 participants). The median statin dose (equivalent to simvastatin) was 10 mg (range 5 to 40 mg).
Statin interventions included atorvastatin or cerivastatin (Renders 2001); fluvastatin (ALERT 2001; Hausberg 2001; Melchor 1998; Seron 2008; SOLAR Study 2001); lovastatin (Bill 1995; Sahu 2001); pravastatin (Cofan 2002; Katznelson 1996); rosuvastatin (Sharif 2009); or simvastatin (Arnadottir 1994; Kasiske 2001; Lepre 1999; Martinez Hernandez 1993; Santos 2001; UK‐HARP‐1 2005). Median follow‐up duration was four months (range 2 to 61 months); four studies had follow‐up of 12 months or more (ALERT 2001; Cofan 2002; Hausberg 2001; UK‐HARP‐1 2005). Two studies included participants who had no histories of cardiovascular disease (Cofan 2002; Hausberg 2001). Median baseline serum LDL cholesterol was 257 mg/dL (range 159 to 319 mg/dL). No study was a post‐hoc subgroup analysis of a larger study in a broader population. Four studies (Cofan 2002; Hausberg 2001; Seron 2008; Sharif 2009) excluded people with diabetes.
Statin versus another statin
Five studies compared two different statin regimens. Four studies compared two different statins: lovastatin versus simvastatin (Castelao 1993); atorvastatin versus fluvastatin Raiola 1998); simvastatin versus pravastatin (Tuncer 2000); and lovastatin versus fluvastatin (Vergoulas 1999); and one compared a lower and higher dose of simvastatin (Celik 2000a). Median duration was 12 months (range 2 to 12 months). All studies had a small sample size (median 32 participants; range 20 to 51). Insufficient data were available to conduct meta‐analyses.
Excluded studies
We excluded 35 studies: 10 did not evaluate an appropriate intervention (Bagdade 1979; Blum 2000; Curtis 1982; Hilbrands 1993; Ichimaru 2001; Imamura 2005; Kahan‐301 2000; LANDMARK 2 2009; Ok 1996; Wissing 2006); 10 compared a statin with an active non‐statin intervention (Blechman‐Krom 2000; Castro 1997; Cheng 1995; Gonzalez‐Molina 1996; Lal 1992; Lal 1996; Lal 1998; Rodriguez 1997; Vasquez 2004; White 1996); seven did not use a randomised study design (Capone 1999; Lopau 2006; Markell 1995; Nart 2009; Nicholson 1993; Ruiz 2006; Turk 2001); one was terminated (Renders 2010); six were of short duration (Kaplan‐251 2001; Kasiske 1990; Kliem 1996; Olbricht 1997; Raiola 1996; Rigatto 1997); and one study was conducted in children (Gonzalez‐Molina 1996).
Risk of bias in included studies
Based on the current standards, the risk for bias in many of the included studies was high (Figure 2; Figure 3). Notably however, most data for analyses were obtained from a single, large, well‐conducted study in which all items of risk were low except sequence generation and allocation concealment (ALERT 2001).
2.
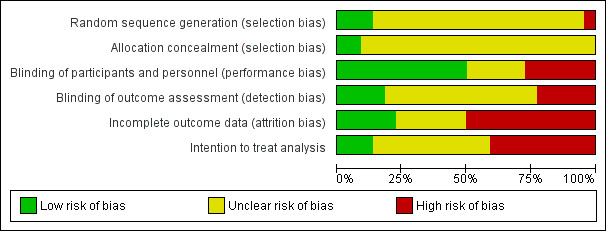
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
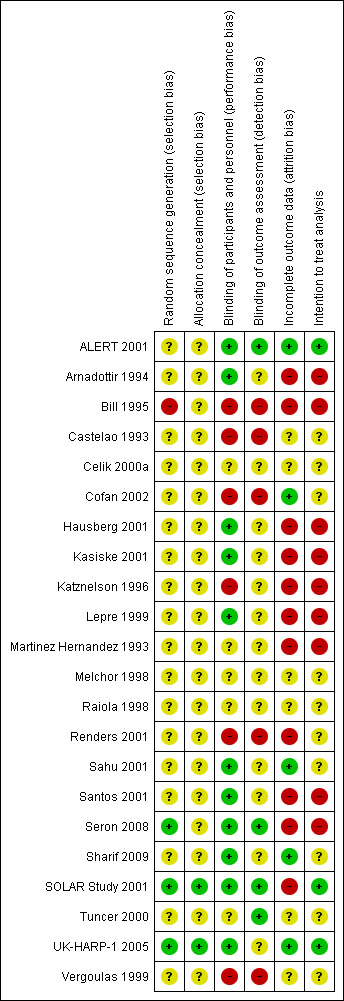
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was adequate in < 25% of studies (Seron 2008; SOLAR Study 2001; UK‐HARP‐1 2005) and allocation concealment was adequate in < 25% of studies (SOLAR Study 2001; UK‐HARP‐1 2005).
Blinding
Participants and personnel were blinded in 50% of studies (ALERT 2001; Arnadottir 1994; Hausberg 2001; Kasiske 2001; Lepre 1999; Sahu 2001; Santos 2001; Seron 2008; Sharif 2009; SOLAR Study 2001; UK‐HARP‐1 2005) while outcome assessors were blinded in < 25% of studies (ALERT 2001; Seron 2008; SOLAR Study 2001; Tuncer 2000).
Incomplete outcome data
Completeness of outcome reporting and intention to treat analysis method was followed in < 25% of included studies (ALERT 2001; Cofan 2002; Sahu 2001; Sharif 2009; ; UK‐HARP‐1 2005).
Selective reporting
Adverse events were assessed systematically in only eight studies (36%) (ALERT 2001; Arnadottir 1994; Melchor 1998; Renders 2001; Santos 2001; Seron 2008; Tuncer 2000; UK‐HARP‐1 2005).
Effects of interventions
See: Table 1
Statin versus placebo or no treatment
Based largely on data from a single high‐quality study, statins may reduce cardiovascular mortality (Analysis 1.1 (4 studies, 2322 participants): RR 0.68, 95% CI 0.45 to 1.01; I² = 0%); major cardiovascular events (Analysis 1.3 (1 study, 2102 participants): RR 0.84, 95% CI 0.66 to 1.06); fatal or non‐fatal myocardial infarction (Analysis 1.4 (1 study, 2102 participants): RR 0.70, 95% CI 0.48 to 1.01). Overall, in absolute terms, treating 1000 adults with a functioning kidney transplant with a statin for one year may prevent two major cardiovascular events and two cardiovascular deaths (Table 1).
1.1. Analysis.

Comparison 1 Statins versus placebo, Outcome 1 Cardiovascular mortality.
1.3. Analysis.
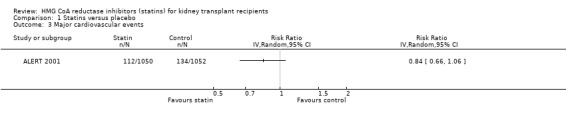
Comparison 1 Statins versus placebo, Outcome 3 Major cardiovascular events.
1.4. Analysis.
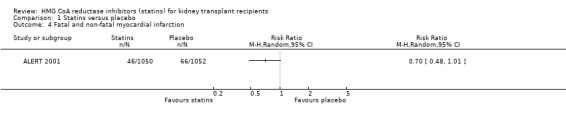
Comparison 1 Statins versus placebo, Outcome 4 Fatal and non‐fatal myocardial infarction.
Statin treatment had uncertain effects on all‐cause mortality (Analysis 1.2 (6 studies, 2760 participants): RR 1.08, 95% CI 0.63 to 1.83; I² = 9%) and fatal or non‐fatal stroke (Analysis 1.5 (1 study, 2102 participants): RR 1.18, 95% CI 0.85 to 1.63). There was no significant heterogeneity in analyses that contained more than one study.
1.2. Analysis.

Comparison 1 Statins versus placebo, Outcome 2 All‐cause mortality.
1.5. Analysis.
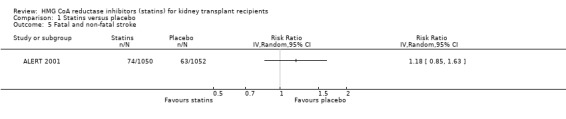
Comparison 1 Statins versus placebo, Outcome 5 Fatal and non‐fatal stroke.
Statins had uncertain effects on adverse events: creatine kinase elevation (Analysis 1.6 (3 studies, 2322 participants): RR 0.86, 95% CI 0.39 to 1.89; I² = 0%); liver enzyme elevation (Analysis 1.7 (4 studies, 608 participants): RR 0.62. 95% CI 0.33 to 1.19 I² = 0%); withdrawal due to adverse events (Analysis 1.8 (9 studies, 2810 participants): RR 0.89, 95% CI 0.74 to 1.06; I² = 0%); and cancer (Analysis 1.9 (1 study, 2094 participants): RR 0.94, 95% CI 0.82 to 1.07). There was no significant heterogeneity in these analyses.
1.6. Analysis.
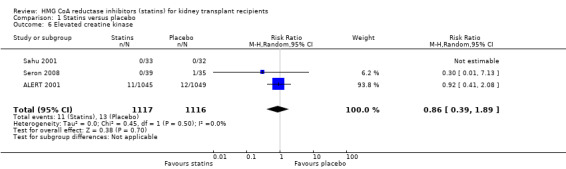
Comparison 1 Statins versus placebo, Outcome 6 Elevated creatine kinase.
1.7. Analysis.
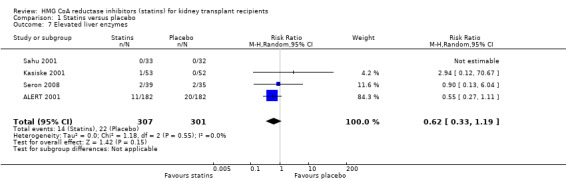
Comparison 1 Statins versus placebo, Outcome 7 Elevated liver enzymes.
1.8. Analysis.
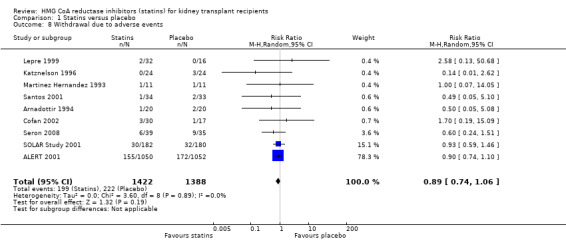
Comparison 1 Statins versus placebo, Outcome 8 Withdrawal due to adverse events.
1.9. Analysis.
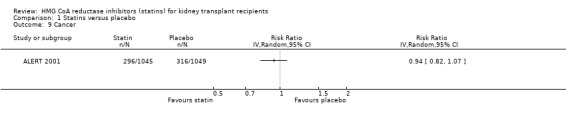
Comparison 1 Statins versus placebo, Outcome 9 Cancer.
Statins significantly reduced serum total cholesterol (Analysis 1.10 (12 studies, 3070 participants): MD ‐42.43 mg/dL, 95% CI ‐51.22 to ‐33.65; I² = 87%); LDL cholesterol (Analysis 1.11 (11 studies, 3004 participants): MD ‐43.19 mg/dL, 95% CI ‐52.59 to ‐33.78; I² = 84%); lowered HDL cholesterol (Analysis 1.12 (11 studies, 3005 participants): MD ‐5.69 mg/dL, 95% CI ‐10.35 to ‐1.03; I² = 89%); and serum triglycerides (Analysis 1.13 (11 studies, 3012 participants): MD ‐27.28 mg/dL, 95% CI ‐34.29 to ‐20.27; I² = 15%). There was marked heterogeneity in the analyses for total cholesterol, LDL cholesterol and HDL cholesterol levels.
1.10. Analysis.
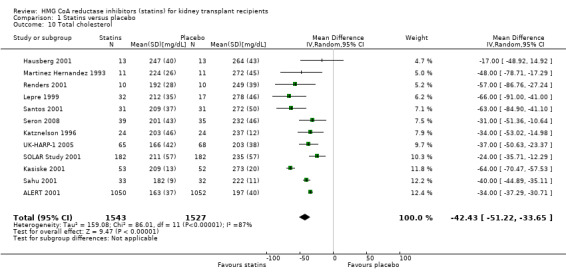
Comparison 1 Statins versus placebo, Outcome 10 Total cholesterol.
1.11. Analysis.
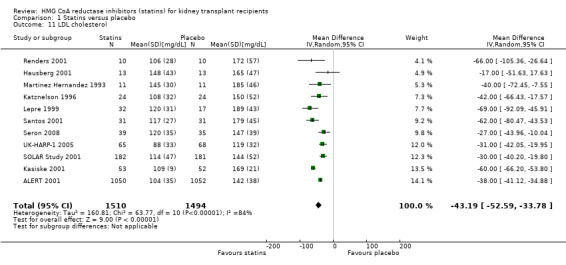
Comparison 1 Statins versus placebo, Outcome 11 LDL cholesterol.
1.12. Analysis.
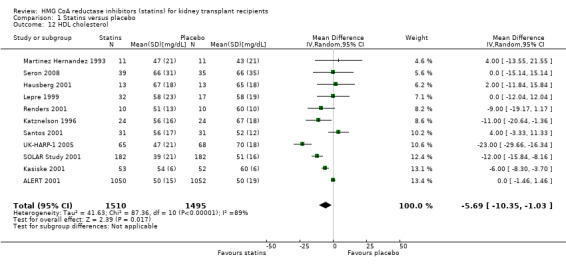
Comparison 1 Statins versus placebo, Outcome 12 HDL cholesterol.
1.13. Analysis.
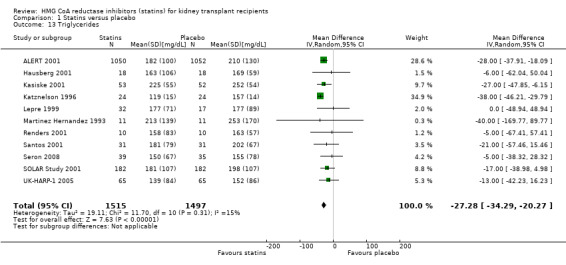
Comparison 1 Statins versus placebo, Outcome 13 Triglycerides.
Statins had uncertain effects on kidney function: ESKD (Analysis 1.14 (6 studies, 2740 participants): RR 1.14, 95% CI 0.94 to 1.37; I² = 0%); acute allograft rejection (Analysis 1.15 (5 studies, 582 participants): RR 0.88, 95% CI 0.61 to 1.28; I² = 39%); proteinuria (Analysis 1.16 (2 studies, 136 participants): MD ‐0.04 g/24 h, 95% CI ‐0.17 to 0.25; I² = 0%); and GFR (Analysis 1.17 (1 study, 62 participants): MD ‐1.00 mL/min, 95% CI ‐9.96 to 7.96).
1.14. Analysis.
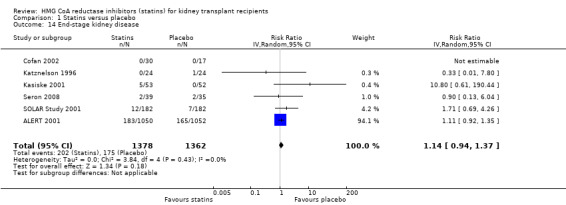
Comparison 1 Statins versus placebo, Outcome 14 End‐stage kidney disease.
1.15. Analysis.
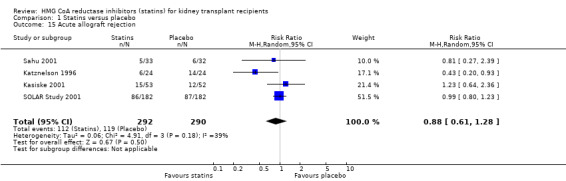
Comparison 1 Statins versus placebo, Outcome 15 Acute allograft rejection.
1.16. Analysis.
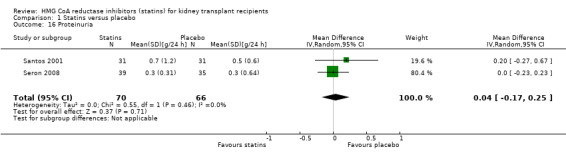
Comparison 1 Statins versus placebo, Outcome 16 Proteinuria.
1.17. Analysis.
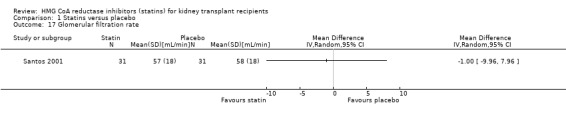
Comparison 1 Statins versus placebo, Outcome 17 Glomerular filtration rate.
Statin versus statin
Due to heterogeneity in comparisons, data directly comparing differing statin regimens could not be meta‐analysed.
Castelao 1993 compared lovastatin and simvastatin head‐to‐head in reducing lipid parameters alone for 12 months. This study concluded that both agents had similar effects in decreasing lipid parameters (total cholesterol 193 mg/dL versus 197 mg/dL at the end of 12 months).
Raiola 1998 compared atorvastatin 10 mg/d to fluvastatin 20 mg/d for eight weeks (30 participants) and there was significantly lower LDL cholesterol and serum triglyceride level with atorvastatin.
Tuncer 2000 compared simvastatin 10 mg/d, pravastatin 20 mg/d and placebo among 57 kidney transplant patients. Both statins reduced lipid parameters and incidence of acute allograft rejection at a similar rate.
Vergoulas 1999 compared lovastatin 20 mg/d with fluvastatin 40 mg/d among 50 kidney transplant recipients. The end of treatment total cholesterol level was lower with lovastatin than fluvastatin both at three months and one year follow‐up.
In a randomised cross‐over study (20 participants), simvastatin 10 mg/d was compared with simvastatin 20 mg/d (Celik 2000a). Even though no difference was noted among the lipid parameters between these two groups, simvastatin 20 mg/d had higher occurrence of serious side effects (3) warranting drug withdrawal.
Analysis of heterogeneity
We found significant heterogeneity in the meta‐analyses for end of study cholesterol, LDL cholesterol, HDL cholesterol and triglycerides, which we explored using pre‐specified subgroup analyses.
For total cholesterol, statin type was the only identifiable modifier of treatment effect we found, explaining 60% of the heterogeneity. Simvastatin provided the greatest reduction in total cholesterol (5 studies, 371 participants: MD ‐55.57 mg/dL, 95% CI ‐69.17 to ‐41.97) and fluvastatin the least (4 studies, 2506 participants: ‐31.48 mg/dL, 95% CI ‐37.02 to ‐25.95). Insufficient data meant that we were unable to perform multivariate meta‐regression analyses to determine the effect of dose and statin type on cholesterol levels and therefore, we were unable to determine whether differences in statin agents were due to doses used, or otherwise.
Dose, age of participants, proportion with diabetes, adequacy of allocation concealment, and serum cholesterol at baseline did not modify treatment effects on serum cholesterol levels. Subgroup analyses for statin type, dose, age, diabetes, allocation concealment or baseline cholesterol did not identify sources of heterogeneity for end of treatment effects of statins on LDL cholesterol or triglyceride levels. For HDL cholesterol, baseline serum cholesterol modified treatment effects of statins; in people with lower serum total cholesterol (< 230 mg/dL) statins reduced HDL cholesterol (MD ‐15.2 mg/dL, 95% CI ‐20.9 to ‐9.6) but not in populations with higher baseline serum total cholesterol (MD ‐0.1 mg/dL, 95% CI ‐4.9 to 4.6) explaining 80% of the heterogeneity in treatment effects observed among studies.
Subgroup analyses for allograft rejection could not be performed due to an insufficient number of studies.
Publication bias
Insufficient numbers of studies had data that could be included to allow formal evaluation of potential publication bias for clinical outcomes (cardiovascular events and mortality).
Discussion
Summary of main results
This updated review found that in data derived largely from the well‐conducted ALERT 2001 study, statins (generally at a simvastatin dose equivalent to 10 mg/d) are likely to reduce cardiovascular events, cardiovascular death, and myocardial infarction for recipients of a kidney transplant, although the magnitude of this effect remains uncertain. While point estimates suggest the potential for benefit with statin treatment are consistent with the benefits observed in people with CKD not treated with dialysis, confidence intervals were wide and included the possibility of no effect. Statin treatment has uncertain effects on death overall and fatal or non‐fatal stroke in transplant patients. The data are particularly incomplete for treatment‐related harms of statins in people with a functioning kidney transplant as data are limited by lack of systematic assessment and reporting of treatment‐related toxicity. Statins have uncertain effects on kidney function and transplant rejection. Statin therapy clearly reduces serum cholesterol concentrations on average by 42 mg/dL (1.1 mmol/L) as well as triglyceride concentrations, although treatment effects on cholesterol levels are inconsistent between studies. Data comparing different statins or different doses of statins were sparse in adults who have a functioning kidney transplant.
Overall completeness and applicability of evidence
Importantly, outcome data for statins remain scant for several important clinical settings. Notably the doses of statins in the available studies were relatively low (10 mg simvastatin equivalent) and direct comparative data comparing different statin doses were sparse. Studies of higher doses or combinations of statin with other agents that lower cholesterol absorption (such as ezetimibe, as used in the recent SHARP 2011 study) compared to placebo are warranted, although systematic ascertainment and reporting of treatment‐related toxicity in such studies would be essential. Similarly, it is also unclear whether treatment benefits from statins were dependent on reductions in serum cholesterol ‐ as insufficient studies reporting cardiovascular and mortality outcomes also reported changes in cholesterol levels with treatment.
Second, sufficient data were not available to determine the relative benefits of statins in the primary prevention of cardiovascular events (treatment of individuals without clinically evident cardiovascular disease) as compared to secondary prevention (treating individuals with established cardiovascular disease) as too few studies were available to summarise data for these specific populations. It should be noted that treatment effects for mortality and cardiovascular events were consistent across all studies suggesting similar treatment effects irrespective of presence of vascular disease (although analyses for consistently were underpowered due to the small number of studies reporting events).
Third, data were limited by the relative paucity of available studies specifically conducted to evaluate statins in people with a functioning kidney transplant. Importantly, data for cardiovascular outcomes and mortality were almost entirely reliant on the large well‐conducted ALERT 2001 study in people with stable kidney function more than six months after kidney or combined kidney and pancreas transplantation. Studies were frequently underpowered for clinical outcomes or did not systematically ascertain outcome events, such that outcome measurement is likely to be less reliable. Additional data from further studies in transplantation would improve our confidence in the treatment estimates for statins and may change the treatment estimates observed (in other words, improve the quality of the currently available evidence).
One study only enrolled living‐related transplant recipients (Sahu 2001) and another only included deceased donor‐related transplant recipients (Katznelson 1996). The remainder included mixed populations of both deceased and living donors. Of note, separate results for these different subgroups were not detailed in the reports for SOLAR Study 2001, Kasiske 2001 or Tuncer 2000. Thus, whether statins have beneficial effects on these separate populations (recipients of a transplant from a deceased or living donor) could not be studied.
Statins have unclear effects on the development of ESKD. These data seem to mirror the uncertainty of treatment effects on kidney function we have observed with statin use in people with earlier stages of CKD not on dialysis (Navaneethan 2009a). Notably, extended follow‐up of ALERT 2001 showed no difference between statins and placebo groups for kidney outcomes, such as doubling of serum creatinine or graft loss (Fellstrom 2004).
Based on the current data, the available evidence for statins is largely generalizable to people with stable kidney function who have had a kidney transplant and are at risk of cardiovascular disease. Additional data for kidney transplant recipients who have had a recent acute cardiovascular event (that is, myocardial infarction) are currently lacking, as fewer than 5% of the ALERT 2001 population had experienced a previous myocardial infarction; additional studies in this population would provide new data for this specific population.
Quality of the evidence
Overall, outcome data were obtained from a single study at lower risk of bias. However, the vast majority of studies evaluated failed to specify whether randomisation allocation was concealed, outcome assessors were blinded or data were analysed on an intention‐to‐treat basis.
Potential biases in the review process
While the review process was strengthened by being undertaken by two or more independent authors; a comprehensive search of the literature designed by a specialist librarian that included the grey literature; and the examination of all potentially relevant outcomes; potential biases exist in the review process.
The main weakness of this review was the relative paucity of studies available in people with a kidney transplant. The data nearly completely relies on a single large study and suggest the potential for benefit for statins on cardiovascular and mortality outcomes, but more research is needed. Reliance on a single study also reduces the generalizability of the findings to populations that have not been studied, particularly people with a functioning kidney transplant who have experienced an acute cardiovascular event. Moreover, evidence of study heterogeneity was found in some analyses, including total cholesterol, LDL cholesterol and triglycerides that could not be fully explained by prespecified subgroup analyses. Since meta‐analysis assumes that a mean value estimating the effects of an intervention can be determined by combining similar studies, unexplained heterogeneity within a meta‐analysis reduces the reliability of the available evidence, although it should be noted that serum total cholesterol is a surrogate outcome.
Agreements and disagreements with other studies or reviews
In this review update, we have confirmed the findings of our earlier version of this review (Navaneethan 2009c) that while point estimates of treatment effect indicate statins reduce cardiovascular death and major cardiovascular events, insufficient evidence results in uncertain treatment effects that include the possibility of no benefit for people with a functioning kidney transplant. Additional evidence is particularly needed to provide high quality data for treatment‐related harm. Statins have been clearly shown to reduce coronary‐related mortality by approximately 20% in people with or at risk of cardiovascular disease (Baigent 2005) in broader non‐renal populations, and reduce mortality and major cardiovascular events by one‐fifth in people with earlier stages of CKD who are not on dialysis (Navaneethan 2009a). Taking into account baseline risk of disease, treating 1000 people with cardiovascular disease or similarly treating 1000 people with CKD not on dialysis might be expected to prevent 25 to 50 deaths over five years. The results of this current review are remarkably similar to findings in the general population, suggesting that major cardiovascular events are reduced in relative terms by 16% in studies in which LDL cholesterol was lowered on average by 1.1 mmol/L, although further evidence is needed to improve our confidence in this result. Intense lipid‐lowering has been associated with additional cardiovascular benefits in broader non‐renal populations (CTT Collaboration 2010); a study evaluating the benefits and (particularly) harms of higher dose statins in people with a kidney transplant is now appropriate.
Apart from the cholesterol lowering effects, statins are known to have anti‐inflammatory, anti‐proliferative and immunosuppressive effects (Corsini 1993; Kurakata 1996). These pleiotropic effects noted by in‐vitro studies suggested their possible impact on acute allograft rejection (Massy 1996). Earlier, observational studies have indicated a possible role for statins in decreasing allograft rejection and two studies have shown that statins significantly reduced acute allograft rejection rates (Kasiske 2001; Tuncer 2000) but other studies have not confirmed this finding (SOLAR Study 2001; Renders 2001; Sahu 2001). A meta‐analysis of the role of statins in heart transplant recipients has concluded that statins decrease allograft rejection episodes and improve one year heart transplant survival (Mehra 2004), findings which at present cannot be confirmed by this review of studies conducted in kidney transplant recipients.
The NKF‐DOQI guidelines recommend initiating low dose statins in kidney transplant recipients when LDL cholesterol is above 100 mg/dL in addition to therapeutic lifestyle changes (Table 2) (Kasiske 2004). The European Best Practice guidelines currently recommend initiating statins when LDL cholesterol is above 130 mg/dL (EBPG 2002). Recently released Kidney Disease Improving Global Outcomes guidelines also recommend lowering LDL cholesterol levels to below 100 mg/dL and endorse the NKF‐DOQI guidelines recommendations (KDIGO 2009). Our analysis has shown the use of statins decrease LDL by an average of 45 mg/dL (1.1 mmol/L) and their use may be supported by these surrogate outcomes. We were not able to evaluate whether treatment effects of statins on cardiovascular and mortality outcomes were dependent on cholesterol lowering; recent data in the general population showing benefits for statins even in people with LDL cholesterol levels below 130 mg/dL (3.4 mmol/L, JUPITER 2008) suggest cholesterol targets in people who have a kidney transplant may not be necessary. A similar study evaluating effects of statins in kidney transplant recipients irrespective of serum cholesterol levels may be warranted.
1. Published guidelines on hyperlipidaemia management with statins in kidney transplant patients.
| Guidelines | Country | Year | Lipid parameters | Treatment |
| Kidney Disease Improving Global Outcomes (KDIGO 2009) | NA | 2009 | LDL ≥100 mg/dL | TLC + statin |
| National Kidney Foundation Disease Outcomes Quality initiative (NKF‐DOQI) (NKF 2002) | USA | 2004 |
|
|
| European Best Practice Guidelines (EBPG 2002) | Europe | 2002 | LDL > 130 mg/dL | TLC + low dose statin |
| Canadian Society of Nephrology (CSN) | Canada | 2004 | No guideline available | No guideline available |
| British Renal Association (BRA) |
UK | 2004 | No guideline available | No guideline available |
| Caring for Australians with Renal Impairment (CARI) | Australia | 2004 | No guideline available | No guideline available |
LDL‐ low‐density lipoprotein cholesterol; Non‐HDL ‐ Non high‐density lipoprotein cholesterol; TC ‐ total cholesterol; TG ‐ triglycerides; TLC ‐ therapeutic lifestyle changes
A previously published systematic review, which looked at the impact of statins in kidney transplant recipients, concluded that statins reduced lipid parameters significantly without any reduction in allograft rejection rates (Lentine 2004); our review confirms these findings.
Authors' conclusions
Implications for practice.
Currently, statin therapy may reduce major cardiovascular events and cardiovascular mortality in kidney transplant recipients, although future research is needed to increase our confidence in this finding. Adverse events from treatment are incompletely understood and need to be considered when deciding on statin use in this population. Statin therapy clearly reduces serum cholesterol levels but has uncertain effects on kidney function and risks of acute rejection. Data are particularly sparse for people who have a kidney transplant who have experienced an acute cardiovascular event. Doses used in available studies are relatively low (simvastatin 10 mg equivalent); the benefits and harms of more intensive therapy are less known.
Implications for research.
In light of the widespread adoption of statins, additional research is still needed to improve our confidence in the potential benefits and harms of statin therapy in kidney transplant recipients (where data are currently sparse). Additional studies in transplant populations that evaluate cardiovascular and mortality outcomes with more intensive lipid lowering together with systematic ascertainment and reporting of adverse treatment toxicity are required. Randomised data for primary and secondary prevention of cardiovascular disease in the transplant setting would be informative. In addition, post‐marketing surveillance would provide additional important data about treatment harms of statins in this population. Studies that include people with a kidney transplant irrespective of baseline serum total cholesterol would be relevant. Since performing large studies is difficult and costly to individual centres, a trialists’ consortium may assist with infrastructure, recruitment and follow‐up of statin studies in kidney transplant recipients.
Feedback
Reader comments, 18 March 2014
Summary
We thank you for your appraisal of the evidence regarding the use of statins in the kidney transplant population¹. In evaluation of the ALERT trial², the predominant study examined in your review, we have a few areas of concerns regarding the general appraisal and the presentation of the available evidence.
Firstly, the conclusion of this review eludes that perhaps all renal transplant patients will benefit from statin therapy. We would like to highlight a few points regarding this claim. Although the ALERT study is relatively small compared to other statin studies in the general population, it is the largest trial used in this systematic review. It is important to note that annual rate of fatal or non‐fatal cardiac events in the placebo group is within the range of annual rates reported in previous primary and secondary prevention studies with statins, thus this potentially suggests that renal transplant by itself is not an independent risk factor for cardiovascular disease. Furthermore, previous comparisons of the observed and expected incidences of ischemic heart disease (IHD) in a population of renal transplant recipients found that many of the risk factors defined for the Framingham cohort appear to be associated with qualitatively similar risks of IHD for renal transplant recipients³.
We agree that the current available evidence regarding the potential benefit of statins in reducing cardiovascular risk in the kidney transplant population requires further insight and evaluation. However, as demonstrated in the ALERT trial, the paucity of evidence suggesting a benefit is also complimented by a possible harm with the use of fluvastatin; a non‐statistically significant increase risk in non‐cardiovascular deaths (RR: 1.20; CI: 0.86‐1.67) and fatal and non‐fatal cerebrovascular events (RR 1.16; CI: 0.83‐1.63)². Despite yielding non‐significance in the trial, providing that commentary on the potential harms of using statins in this review would not only bring clarity as to why this area needs further evaluation, but also makes transparent the potential benefits and harms of starting a statin in this population.
Moreover, we would also like to highlight a few things regarding the ALERT trial. In evaluation of the endpoints (Figure 4) in the ALERT trial, there are concerns regarding the reporting and evaluation of the mortality endpoints. The analysis in Figure 4 of the trial suggests that 77 and 65 people died of non‐cardiovascular death in the fluvastatin and placebo arm, respectively. It is not entirely clear to us how the authors define non‐cardiovascular death. Only by using Figure 2 in the ALERT trial, does it become more apparent that fatal cerebrovascular events and other vascular deaths have been excluded from the analysis of non‐cardiovascular death. We think this discrepancy under‐represents the magnitude of possible harm resulting in non‐cardiovascular death with the use of fluvastatin. Non‐cardiovascular mortality was not evaluated in this review; however, future revisions should evaluate an endpoint inclusive of fatal cerebrovascular and other vascular deaths and reflect that it resulted in 107 vs. 84 deaths (HR: 1.28, P = 0.08) in the treatment and placebo arm, respectively. As such, it is possible there is a stronger signal for non‐cardiovascular harm with the use of fluvastatin. The authors of the ALERT trial have been contacted; however, we have yet to receive further clarification. It should be noted that this discrepancy was not addressed in the risk of bias summary (Figure 3) in the review¹. It is also important to note that in the ALERT trial, 11 of the 12 patients who underwent revascularization in the first year were in the fluvastatin arm. It is possible that decreased occurrence of cardiac death and non‐fatal MI in fluvastatin group is a consequence of a higher incidence of early revascularization in the fluvastatin arm.
Based on the evaluation of this Cochrane review, we feel that the presentation of the evidence reads in a manner that puts more emphasis on the potential benefits of statin therapy, indirectly biasing the interpretation of the over‐arching message. In conclusion, we recommend the following: a) a greater emphasis should be placed on the fact that renal transplantation is potentially not an independent risk factor for ischemic heart disease b) the risk of bias summary in the review should address the reporting bias with regards to non‐cardiovascular deaths found in the ALERT trial c) more transparency in the presentation of the potential harms—possible non‐cardiovascular deaths and fatal and non‐fatal cerebrovascular events—with using statins for primary prevention in this population. Overall, it remains unclear whether the benefit of using statins for primary prevention in renal transplant patients outweighs the potential harm. Consequently, patient specific risk factors should be evaluated on a case‐by‐case basis before initiating statin therapy.
Palmer SC, Navaneethan SD, Craig JC, Perkovic V, Johnson DW, Nigwekar SU, Hegbrant J, Strippoli GFM. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No.:CD005019. DOI: 10.1002/14651858.CD005019.pub4
Holdaas H, Fellström B, Jardine AG, Holme I, Nyberg G, Fauchald P, Grönhagen‐Riska C, Madsen S, Neumayer HH, Cole E, Maes B, Ambühl P, Olsson AG, Hartmann A, Solbu DO, Pedersen TR, Assessment of LEscol in Renal Transplantation (ALERT) Study Investigators. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicenter, randomized, placebo‐controlled trial. Lancet. 2003;361(9374):2024‐31.
Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol. 2000; 11:1735–43
Reply
We thank the writers for their careful reading of this systematic review. We will consider these suggestions in any update of this review in due course.
GFM Strippoli
Contributors
Yvonne Huang, B.Sc. Pharm Reza Rafizadeh, B.Sc. Pharm Aaron M Tejani, B.Sc. Pharm, ACPR, Pharm.D
What's new
| Date | Event | Description |
|---|---|---|
| 21 January 2015 | Feedback has been incorporated | Feedback incorporated |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 15 January 2014 | New citation required and conclusions have changed | Updated and conclusions amended |
| 4 March 2012 | New search has been performed | Updated search on February 29, 2012. Results and conclusions updated. |
| 4 March 2012 | Amended | Author added: Palmer SC |
| 21 December 2010 | Amended | Search strategies revised |
| 13 May 2009 | Amended | Contact details updated. |
Acknowledgements
We acknowledge the contribution of authors (Drs Holdaas and Baigent) who provided data about their studies upon request. We also thank Narelle Willis, for her help in coordinating and editing this review and Ruth Mitchell and Gail Higgins for assistance in the development of the search strategies.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Statins versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular mortality | 4 | 2322 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.45, 1.01] |
| 2 All‐cause mortality | 6 | 2760 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.63, 1.83] |
| 3 Major cardiovascular events | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4 Fatal and non‐fatal myocardial infarction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Fatal and non‐fatal stroke | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6 Elevated creatine kinase | 3 | 2233 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.39, 1.89] |
| 7 Elevated liver enzymes | 4 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.33, 1.19] |
| 8 Withdrawal due to adverse events | 9 | 2810 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.06] |
| 9 Cancer | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 10 Total cholesterol | 12 | 3070 | Mean Difference (IV, Random, 95% CI) | ‐42.43 [‐51.22, ‐33.65] |
| 11 LDL cholesterol | 11 | 3004 | Mean Difference (IV, Random, 95% CI) | ‐43.19 [‐52.59, ‐33.78] |
| 12 HDL cholesterol | 11 | 3005 | Mean Difference (IV, Random, 95% CI) | ‐5.69 [‐10.35, ‐1.03] |
| 13 Triglycerides | 11 | 3012 | Mean Difference (IV, Random, 95% CI) | ‐27.28 [‐34.29, ‐20.27] |
| 14 End‐stage kidney disease | 6 | 2740 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.94, 1.37] |
| 15 Acute allograft rejection | 4 | 582 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.61, 1.28] |
| 16 Proteinuria | 2 | 136 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.17, 0.25] |
| 17 Glomerular filtration rate | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ALERT 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent critical events committee of two nephrologists and two cardiologists who were unaware of treatment assignment reviewed all primary and secondary endpoints for adjudication. All analyses were based in the committee's classification of endpoints which were agreed by consensus or majority vote |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in intention‐to‐treat analysis. 7 participants (< 1%) lost to follow‐up |
| Intention to treat analysis | Low risk | Intention‐to‐treat analysis conducted |
Arnadottir 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/20 patients did not complete the study (15%) |
| Intention to treat analysis | High risk | Not conducted |
Bill 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Every other patient was given Lovastatin 20 mg/night" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/42 (21%) lost to follow‐up |
| Intention to treat analysis | High risk | Not conducted |
Castelao 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes | Publication type: Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly described; no withdrawals |
| Intention to treat analysis | Unclear risk | Not described |
Celik 2000a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Intention to treat analysis | Unclear risk | Not described |
Cofan 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Publication type: Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Pravastatin (3); placebo (1); all due to loss of follow‐up |
| Intention to treat analysis | Unclear risk | Not described |
Hausberg 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/40 patients (10%) dropped out during follow‐up |
| Intention to treat analysis | High risk | Not performed |
Kasiske 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Publication type: Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | "Investigators in each center were blinded to simvastatin and to the randomization sequence." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients in all three groups were blinded to simvastatin. Investigators in each center were blinded to simvastatin and to the randomization sequence." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26/140 patients incomplete cholesterol data, 57/140 patients incomplete LDL data; no withdrawals |
| Intention to treat analysis | High risk | Not conducted |
Katznelson 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Publication type: Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2/24 patients lost to follow‐up in control group; no withdrawals |
| Intention to treat analysis | High risk | Not performed |
Lepre 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Publication type: Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/52 patients did not complete study (6%) (musculoskeletal pain (2); abdominal pain (1)) |
| Intention to treat analysis | High risk | Not conducted |
Martinez Hernandez 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/26 (15%) did not finish study |
| Intention to treat analysis | High risk | Not conducted |
Melchor 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described; 2 patients refused to continue fluvastatin |
| Intention to treat analysis | Unclear risk | Not described |
Raiola 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Intention to treat analysis | Unclear risk | Not described |
Renders 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Publication type: Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not described |
| Intention to treat analysis | Unclear risk | Not described |
Sahu 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Publication type: Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in follow‐up |
| Intention to treat analysis | Unclear risk | Not described |
Santos 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Publication type: Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 of 67 (9%) patients not included in end of treatment assessments |
| Intention to treat analysis | High risk | Not conducted |
Seron 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule was generated by the data operating department of Clinical Data Care. Patients were randomised by means of a computer‐generated scheme |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biopsies were blindly evaluated by the same observer |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 89 patients were included, 74 completed the 6 month study, and 57 had paired biopsies with sufficient tissue for histological evaluation |
| Intention to treat analysis | High risk | Not conducted |
Sharif 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patient lost to follow‐up |
| Intention to treat analysis | Unclear risk | Not described |
SOLAR Study 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralized randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Each medication pack was labelled with a study identification code and randomisation number. Randomization was stratified by centre and blocked pseudo‐randomisation was performed by the producer centrally. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study personnel directly involved in the conduct of the study were blinded to treatment until all patients had completed the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel directly involved in the conduct of the study were blinded to treatment until all patients had completed the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 83% of the patients who commenced the study completed the protocol with no difference between groups |
| Intention to treat analysis | Low risk | Intention to treat analysis |
Tuncer 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes | Publication type: Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Core biopsies were examined by a pathologist who was blinded to patient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Intention to treat analysis | Unclear risk | Not described |
UK‐HARP‐1 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | Publication type: Journal publication This was 4‐arm study with aspirin used alone and in combination with simvastatin. Simvastatin‐aspirin/simvastatin alone and double placebo/aspirin placebo groups were combined for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimized randomisation was used to balance the treatment groups; 2 X 2 factorial design |
| Allocation concealment (selection bias) | Low risk | Randomisation was by telephone to the Clinical Trial Service Unit |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | All events were coded centrally according to a standard protocol. Otherwise unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 442 of 448 patients completed follow‐up |
| Intention to treat analysis | Low risk | Conducted |
Vergoulas 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Intention to treat analysis | Unclear risk | Not described |
ALT ‐ alanine aminotransferase; AST ‐ aspartate aminotransferase; BP ‐ blood pressure; CABG ‐ coronary artery bypass graft; CAD ‐ coronary artery disease; CK ‐ creatine kinase; CKD ‐ chronic kidney disease; CRP ‐ C‐reactive protein; CV ‐ cardiovascular; DM ‐ diabetes mellitus; HDL ‐ high‐density lipoprotein; HRT ‐ hormone replacement therapy; KTR ‐ kidney transplant recipients; LDL ‐ low‐density lipoprotein; LFT ‐ liver function tests; MI ‐ myocardial infarction; NS ‐ not stated; SCr ‐ serum creatinine; TC ‐ total cholesterol; TG ‐ triglycerides
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bagdade 1979 | Not appropriate intervention |
| Blechman‐Krom 2000 | Active comparator (not statin) |
| Blum 2000 | Not appropriate intervention |
| Capone 1999 | Not randomised |
| Castro 1997 | Active comparator (not statin) |
| Cheng 1995 | Active comparator (not statin) |
| Curtis 1982 | Not appropriate intervention |
| Garcia‐de‐la‐Puente 2009 | Paediatric population |
| Gonzalez‐Molina 1996 | Active comparator (not statin) |
| Hilbrands 1993 | Not appropriate intervention |
| Ichimaru 2001 | Not appropriate intervention |
| Imamura 2005 | Not appropriate intervention |
| Kahan‐301 2000 | Not appropriate intervention |
| Kaplan‐251 2001 | Short duration |
| Kasiske 1990 | Short duration |
| Kliem 1996 | Short duration |
| Lal 1992 | Active comparator (not statin) |
| Lal 1996 | Active comparator (not statin) |
| Lal 1998 | Active comparator (not statin) |
| LANDMARK 2 2009 | Not appropriate intervention |
| Lopau 2006 | Not randomised |
| Markell 1995 | Not randomised |
| Nart 2009 | Not randomised |
| Nicholson 1993 | Not randomised |
| Ok 1996 | Not appropriate intervention |
| Olbricht 1997 | Short duration |
| Raiola 1996 | Short duration |
| Renders 2010 | Terminated (unclear) |
| Rigatto 1997 | Short duration |
| Rodriguez 1997 | Active comparator (not statin) |
| Ruiz 2006 | Not randomised |
| Turk 2001 | Not randomised |
| Vasquez 2004 | Active comparator (not statin) |
| White 1996 | Active (non‐statin) intervention |
| Wissing 2006 | Not appropriate intervention |
Characteristics of ongoing studies [ordered by study ID]
NCT00565474.
| Trial name or title | Evaluation of the effect of fluvastatin 40 mg (b.i.d.) in the prevention of the development of vasculopathy of the graft in de novo renal transplant patients transplant |
| Methods | Randomised, parallel‐group, double‐blind |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Fluvastatin 40 mg twice daily versus placebo |
| Outcomes | Primary outcome measures: to determine if treatment with fluvastatin can prevent the progression of vascular graft disease. The difference between the vascular intimal thickness measured on the baseline biopsy and the biopsy at the end of the study between the two treatment group Secondary outcome measures: 24‐hour creatinine and proteinuria values at 6 months post‐transplant, graft survival and patient survival at 6 months, differences in lipid profile between the treatment groups, incidence of rejection episodes treated and documented by biopsy at 6 months |
| Starting date | September 2001 |
| Contact information | Novartis |
| Notes |
Differences between protocol and review
Risk of bias assessment tool has replaced the earlier quality assessment checklist (Perkovic 2004).
Contributions of authors
Suetonia Palmer: data extraction, analysis and interpretation of data, drafting the manuscript, final approval of version to be published
Sankar D Navaneethan: concept and design of the review, data extraction, analysis and interpretation of data, writing the final manuscript, final approval of version to be published
Sagar Nigwekar: data extraction, analysis and interpretation of data, writing the final manuscript
Vlado Perkovic: critical revision for intellectual content, interpretation of data, assistance with writing of the final manuscript, final approval of the manuscript to be submitted for publication
David W Johnson: data extraction, analysis and interpretation of data, writing the final manuscript, final approval of version to be published
Jonathan Craig: concept and design, analysis and interpretation of data, writing the final manuscript, final approval of version to be published
Giovanni FM Strippoli: concept and design of the review, data extraction, analysis and interpretation of data, writing the final manuscript, final approval of version to be published.
Sources of support
Internal sources
No sources of support supplied
External sources
Suetonia Palmer received a fellowship from the Consorzio Mario Negri Sud from an unrestricted grant by Amgen Dompe, Italy.
Declarations of interest
David Johnson is a consultant for Baxter Healthcare Pty Ltd and has previously received research funds from this company. He has also received speakers’ honoraria and research grants from Fresenius Medical Care and is a current recipient of a Queensland Government Health Research Fellowship. He has also received speakers' honoraria and consultancy fees from Amgen, Janssen‐Cilag, Shire, Lilley, Boehringer‐Ingelheim and Merck Sharpe & Dohme. He has received a research grant from Pfizer.
Suetonia Palmer received a fellowship administered by the Consorzio Mario Negri Sud from Amgen Dompe for assistance with travel for collaboration and supervision.
Vlado Perkovic is supported by a fellowship from the Heart Foundation of Australia and a various grants from the Australian National Health and Medical Research Council. He has received speakers' fees from Roche, Servier and Astra Zeneca, funding for a clinical trial from Baxter, and serves on Steering Committees for trials funded by Johnson and Johnson, Boehringer Ingelheim, Vitae and Abbott. His employer conducts clinical trials funded by Servier, Johnson and Johnson, Roche and Merck.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
ALERT 2001 {published data only}
- Abedini S, Holme I, Fellstrom B, Jardine A, Cole E, Maes B, et al. Cerebrovascular events in renal transplant recipients. Transplantation 2009;87(1):112‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Abedini S, Holme I, Marz W, Weihrauch G, Fellstrom B, Jardine A, et al. Inflammation in renal transplantation. Clinical Journal of the American Society of Nephrology: CJASN 2009;4(7):1246‐54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedini S, Jardine A, Fellstrom B, Holdaas H. Association between body mass index (BMI) all cause mortality, renal and cardiac endpoints in renal transplant recipients in the Alert Extension Study [abstract no: 56]. American Journal of Transplantation 2009;9(Suppl 2):207. [CENTRAL: CN‐00763910] [Google Scholar]
- Abedini S, Jardine A, Fellstrom B, Meinitzer A, Marz W, Bermann G, et al. Asymmetric dimethylarginine (ADMA) predicts progression to graft failure and major cardiac events in renal transplant recipients (RTR) [abstract no: F‐FC149]. Journal of the American Society of Nephrology 2006;17(Abstracts):68A. [CENTRAL: CN‐00689307] [Google Scholar]
- Abedini S, Meinitzer A, Holme I, Marz W, Weihrauch G, Fellstrom B, et al. Asymmetrical dimethylarginine is associated with renal and cardiovascular outcomes and all‐cause mortality in renal transplant recipients. Kidney International 2010;77(1):44‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fellstrom B, Abedini S, Holdaas H, Jardine AG, Staffler B, Gimpelewicz C, et al. No detrimental effect on renal function during long‐term use of fluvastatin in renal transplant recipients in the Assessment of Lescol in Renal Transplantation (ALERT) study. Clinical Transplantation 2006;20(6):732‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fellstrom B, Holdaas H, Jardine A, Cole E, Nyberg G, Grohagen‐Riska C, et al. Renal transplant function as a risk factor for graft loss, cardiac disease and all cause mortality in renal transplantation ‐ experience from the ALERT trial [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):187A. [Google Scholar]
- Fellstrom B, Holdaas H, Jardine A, Cole E, Nyberg G, Gronhagen‐Riska C, et al. Effects of fluvastatin on graft losses and renal function in renal transplantation ‐ experience from the ALERT trial [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):10A. [Google Scholar]
- Fellstrom B, Holdaas H, Jardine A, Cole E, Nyberg G, Gronhagen‐Riska C, et al. Risk factors for graft loss or doubling of serum creatinine in renal transplantation ‐ experience from the ALERT trial [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):185A. [Google Scholar]
- Fellstrom B, Holdaas H, Jardine A, Holme I, Nyberg G, Gronhagen‐Riska C, et al. Effects of fluvastatin on renal transplant function and graft loss in renal transplant patients in ALERT (Assessment of LEscol in Renal Transplantation) [abstract no: M736]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):236. [CENTRAL: CN‐00445309] [Google Scholar]
- Fellstrom B, Holdaas H, Jardine AG, Holme I, Nyberg G, Fauchald P, et al. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) trial. Kidney International 2004;66(4):1549‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fellstrom B, Holdaas H, Jardine AG, Nyberg G, Gronhagen‐Riska C, Madsen S, et al. Risk factors for reaching renal endpoints in the assessment of Lescol in renal transplantation (ALERT) trial. Transplantation 2005;79(2):205‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fellstrom B, Holdass H, Jardine A, Cole E, Nyberg G, Gronhagen‐Riska C, et al. Renal transplant function as a risk factor for cardiovascular disease and mortality and for all cause mortality in renal transplantation ‐ experience from the ALERT trial [abstract]. Transplantation 2004;78(2 Suppl):57. [CENTRAL: CN‐00509185] [Google Scholar]
- Fellstrom B, Jardine AG, Soveri I, Cole E, Gronhagen‐Riska C, Neumayer HH, et al. Renal dysfunction as a risk factor for mortality and cardiovascular disease in renal transplantation: experience from the Assessment of Lescol in Renal Transplantation trial. Transplantation 2005;79(9):1160‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fellstrom B, Jardine AG, Soveri I, Cole E, Neumayer HH, Maes B, et al. Renal dysfunction is a strong and independent risk factor for mortality and cardiovascular complications in renal transplantation. American Journal of Transplantation 2005;5(8):1986‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fellstrom BC, Holdaas H, Jardine A, Cole E, Nyberg G, Gronhagen‐Riska C, et al. Influence of renal transplant function on cardiovascular disease and all cause mortality in the ALERT trial [abstract no: 375]. American Journal of Transplantation 2004;4(Suppl 8):261. [CENTRAL: CN‐00509187] [Google Scholar]
- Holdaas H, Abedini S, Jardine A, Maes B, März W, Fellstrom B. Parathyroid hormone and phosphate ‐ risk factors for post‐transplant cardiovascular disease? [abstract no: TH‐FC084]. Journal of the American Society of Nephrology 2006;17(Abstracts):19A. [CENTRAL: CN‐00615900] [Google Scholar]
- Holdaas H, Asberg A, Edvardsen C, Hartmann A. Statins endothelial function in renal transplant recipients ‐ a randomised single centre study [abstract no: A4677]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):894A. [CENTRAL: CN‐00776306] [Google Scholar]
- Holdaas H, Fellstrom B, Cole E, Nyberg G, Olsson AG, Pedersen TR, et al. Long‐term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. American Journal of Transplantation 2005;5(12):2929‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holdaas H, Fellstrom B, Holme I, Nyberg G, Fauchald P, Jardine A, et al. Effects of fluvastatin on cardiac events in renal transplant patients: ALERT (Assessment of Lescol in Renal Transplantation) study design and baseline data. Journal of Cardiovascular Risk 2001;8(2):63‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holdaas H, Fellstrom B, Jardin A, Cole E, Nyberg G, Gronhagen‐Riska C, et al. Long term effectiveness and safety of fluvastatin in renal transplant recipients: the ALERT trial follow‐up [abstract no: M‐PO50032]. Nephrology 2005;10(Suppl):A88. [CENTRAL: CN‐00615876] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine A, Cole E, Maes B, Nyberg G, et al. Risk factors for late new‐onset diabetes mellitus after renal transplantation [abstract no: 675]. American Journal of Transplantation 2005;5(Suppl 11):328. [CENTRAL: CN‐00757934] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine A, Cole E, Nyberg G, Gronhagen‐Riska C, et al. Lipid‐lowering therapy should be started early in the post‐transplant period [abstract no: F‐PO569]. Journal of the American Society of Nephrology 2003;14(Nov):185A. [CENTRAL: CN‐00550528] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine A, Cole E, Nyberg G, Gronhagen‐Riska C, et al. Long term efficacy, safety and tolerability of fluvastatin in renal transplant recipients: the ALERT trial follow‐up [abstract no: SO01]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v3. [CENTRAL: CN‐00601991] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine A, Gimpelewicz C, Staffler B, Logan J, et al. No detrimental effect of fluvastatin on renal function in diabetic renal transplant patients [abstract no: PUB561]. Journal of the American Society of Nephrology 2004;15(Oct):881A. [CENTRAL: CN‐00550529] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine A, Gimpelewicz C, Staffler B, Logan J, et al. Prevalence and consequences of metabolic syndrome in a renal transplant population [abstract no: SA‐PO1048]. Journal of the American Society of Nephrology 2004;15(Oct):527A. [CENTRAL: CN‐00550531] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine A, Holme I, Nyberg G, Fauchald P, et al. Randomized double blind trial of fluvastatin for hypercholesterolemia in renal transplant recipients [abstract]. American Journal of Transplantation 2003;3(Suppl 5):442. [CENTRAL: CN‐00445757] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine A, Holme I, Nyberg G, et al. Effects of fluvastatin on cardiovascular events in renal transplant patients: ALERT (Assessment LEscol in Renal Transplantation) [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):237. [CENTRAL: CN‐00445756] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine A, Nyberg G, Gronhagen‐Riska C, Madsen S, et al. Long term cardiac outcomes in renal transplant recipients receiving fluvastatin: The ALERT Extension Study [abstract]. Journal of the American Society of Nephrology 2005;16:72A. [DOI] [PubMed] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine A, Nyberg G, Gronhagen‐Riska C, Madsen S, et al. No detrimental effect on renal function during long‐term use of fluvastatin [abstract]. Transplantation 2004;78(2 Suppl):327. [CN‐00509236] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine AG, Cole E, Nyberg G, Gronhagen‐Riska C, et al. Lipids as risk factors for cardiac events after renal transplantation ‐ experience from the ALERT trial [abstract no: SU‐PO590]. Journal of the American Society of Nephrology 2003;14(Nov):662A. [CENTRAL: CN‐00550542] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine AG, Holme I, Nyberg G, Fauchald P, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo‐controlled trial. Lancet 2003;361(9374):2024‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine AG, Nyberg G, Gronhagen‐Riska C, Madsen S, et al. Beneficial effect of early initiation of lipid‐lowering therapy following renal transplantation. Nephrology Dialysis Transplantation 2005;20(5):974‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holdaas H, Fellstrom B, Maes B, Cole E, Jardine A. Impact of hemoglobin levels on graft function, cardiac events and all‐cause mortality after renal transplantation [abstract no: F‐PO1502]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):443A. [CENTRAL: CN‐00756929] [Google Scholar]
- Holdaas H, Jardine A, Holme I, Brekke IB, Wheeler DC, Ostraat O, et al. A randomised controlled trial assessing the effect of fluvastatin on acute rejection rate following renal transplantation [abstract no: P0582W]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome (Italy). 2000. [CENTRAL: CN‐00527128]
- Holdaas H, Jardine AG, Wheeler DC, Brekke IB, Conlon PJ, Fellstrom B, et al. Effect of fluvastatin on acute renal allograft rejection: a randomized multicenter trial. Kidney International 2001; Vol. 60, issue 5:1990‐7. [MEDLINE: ] [DOI] [PubMed]
- Holme I, Fellstrom B, Jardine A, Holdaas H. Comparison of predictive ability of lipoprotein components to that of traditional risk factors of coronary events in renal transplant recipients. Atherosclerosis 2010;208(1):234‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jardine A, Weihrauch G, Maerz W, Fellstrom B, Bermann G, Holdaas H. Osteoprotegerin ‐ a novel risk factor post‐transplant cardiovascular disease [abstract no: TH‐PO581]. Journal of the American Society of Nephrology 2006;17(Abstracts):231A. [CENTRAL: CN‐00615825] [Google Scholar]
- Jardine AG, Fellstrom B, Cole E, Logan J, Holdaas H. New targets for cardiovascular risk reduction in renal transplant recipients: post‐hoc analyses of the ALERT study [abstract no: SU‐PO991]. Journal of the American Society of Nephrology 2004;15(Oct):746A. [CENTRAL: CN‐00550611] [Google Scholar]
- Jardine AG, Fellstrom B, Logan JO, Cole E, Nyberg G, Gronhagen‐Riska C, et al. Cardiovascular risk and renal transplantation: post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) Study. American Journal of Kidney Diseases 2005;46(3):529‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jardine AG, Holdaas H, Fellstrom B, Cole E, Nyberg G, Gronhagen‐Riska C, et al. Fluvastatin prevents cardiac death and myocardial infarction in renal transplant recipients: post‐hoc subgroup analyses of the ALERT Study. American Journal of Transplantation 2004;4(6):988‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jardine AG, Holdaas H, Fellstrom B, Cole E, Nyberg G, Gronhagen‐Riska C, et al. Pulse pressure and outcomes following renal transplantation [abstract no: SU‐FC294]. Journal of the American Society of Nephrology 2003;14:64A. [CENTRAL: CN‐00724902] [Google Scholar]
- Jardine AG, Holdaas H, Fellstrom B, the ALERT Investigators. Adverse impact of steroids on outcome in the ALERT study [abstract]. American Journal of Transplantation 2004;4(Suppl 8):488. [CENTRAL: CN‐00509253] [Google Scholar]
- Norby G, Soveri I, Fellstrom B, Jardine A, Cole E, Maerz W, et al. C‐Reactive protein (CRP) and interleukin‐6 (IL‐6) are associated with long‐term renal and cardiac outcomes in renal transplant recipients [abstract no: SA‐PO446]. Journal of the American Society of Nephrology 2007;18(Abstracts):438A‐9A. [CENTRAL: CN‐00716046] [Google Scholar]
- Norby GE, Holme I, Fellstrom B, Jardine A, Cole E, Abedini S, et al. Effect of fluvastatin on cardiac outcomes in kidney transplant patients with systemic lupus erythematosus: a randomized placebo‐controlled study. Arthritis & Rheumatism 2009;60(4):1060‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Oien CM, Reisaeter AV, Os I, Jardine A, Fellstrom B, Holdaas H. Gender‐associated risk factors for cardiac end points and total mortality after renal transplantation: post hoc analysis of the ALERT study. Clinical Transplantation 2006;20(3):374‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Singer JB, Holdaas H, Jardine AG, Fellstrom B, Os I, Bermann G, et al. Genetic analysis of fluvastatin response and dyslipidemia in renal transplant recipients. Journal of Lipid Research 2007;48(9):2072‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Soveri I, Abedini S, Holdaas H, Jardine A, Eriksson N, Fellstrom B. Metabolic syndrome and cardiovascular risk in renal transplant recipients: effects of statin treatment. Clinical Transplantation 2009;23(6):914‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Soveri I, Holdaas H, Jardine A, Eriksson N, Fellstrom B. Metabolic syndrome and cardiovascular disease risk in renal transplant recipients in the ALERT trial: effects of statin treatment [abstract no: 604]. Transplantation 2008;86(2S):212. [CENTRAL: CN‐00757239] [DOI] [PubMed] [Google Scholar]
- Soveri I, Holdaas H, Jardine A, Eriksson N, Fellstrom B. Metabolic syndrome and risk of renal graft loss in the ALERT Trial [abstract no: 119]. Transplantation 2008;86(2S):43. [CENTRAL: CN‐00757241] [Google Scholar]
Arnadottir 1994 {published data only}
- Arnadottir M, Eriksson LO, Germershausen JI, Thysell H. Low‐dose simvastatin is a well‐tolerated and efficacious cholesterol‐lowering agent in ciclosporin‐treated kidney transplant recipients: double‐blind, randomized, placebo‐controlled study in 40 patients. Nephron 1994;68(1):57‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bill 1995 {published data only}
- Bill M, Paczek L, Gaciong Z, Gradowska L, Lao M, Wyzgal J, et al. Low dose lovastatin does not improve kidney allograft function during 6‐month observation [abstract]. Nephrology Dialysis Transplantation 1995;10(6):1061. [CENTRAL: CN‐00261145] [Google Scholar]
- Bill M, Paczek L, Wyzgal J, Baciong Z, Gradowska L, Lao M, Juskowa J, et al. Six‐month treatment with low‐dose lovastatin does not improve kidney allograft function. Polish Journal of Immunology 1995;20(4):417‐9. [Google Scholar]
- Paczek L, Bill M, Wyzgal J, Gaciong Z, Gradowska L, Juskowa J, et al. The effect of hypolipidemia treatment on the function of kidney transplanted from cadavers. Polskie Archiwum Medycyny Wewnetznej 1997;97(2):144‐56. [MEDLINE: ] [PubMed] [Google Scholar]
- Paczek L, Bill M. Wyzgal J, Gradowska L, Galazka Z, Gaciong Z. Non‐immune factors in chronic rejection. Is there a role for hypolipemic drugs?. Annals of Transplantation 1997;2(2):65‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Paczek L, Gradowska L, Bill M, Gaciong Z, Lao M, Juskowa J, et al. Treatment with low‐dose lovastatin does not improve kidney allograft function during 6‐month observation [abstract]. Nephrology 1997;3(Suppl 1):S574. [CENTRAL: CN‐00461456] [Google Scholar]
Castelao 1993 {published data only}
- Castelao AM, Grino JM, Andres E, Gilvernet S, Seron D, Castineiras MJ, et al. HMGCoA reductase inhibitors lovastatin and simvastatin in the treatment of hypercholesterolemia after renal transplantation. Transplantation Proceedings 1993;25(1 Pt 2):1043‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Martinez‐Castelao A, Grinyo JM, Gil‐Vernet S, Seron D, Castineiras MJ, Ramos R, et al. Lipid‐lowering long‐term effects of six different statins in hypercholesterolemic renal transplant patients under cyclosporine immunosuppression. Transplantation Proceedings 2002;34(1):398‐400. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Celik 2000a {published data only}
- Celik A, Unsal A, Mutaf I, Habif S, Ok E, Bayindir O. Which dosage of simvastatin in renal transplant patients?. Nephron 2000;84(1):81‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cofan 2002 {published data only}
- Cofan F, Gilabert R, Zambon D, Nunez I, Ros E, Cofan M, et al. Effect of pravastatin treatment on the evolution of extracoronary atherosclerosis in renal transplant patients. Transplantation Proceedings 2002;34(1):384‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cofan F, Zambon D, Laguna JC, Casals E, Ros E, Cofan M, et al. Pravastatin improves low‐density lipoprotein oxidation in renal transplantation. Transplantation Proceedings 2002;34(2):389‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hausberg 2001 {published data only}
- Hausberg M, Barenbrock M, Stam F, Kosch M, Kisters K, Heidenreich S, et al. Effect of lipid lowering therapy on endothelial function in renal allograft recipients [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):677A. [CENTRAL: CN‐00445668] [Google Scholar]
- Hausberg M, Barenbrock M, Stam F, Kosch M, Kisters K, Spieker C, et al. HMG‐CoA‐reductase inhibitors improve brachial artery flow‐mediated vasodilation in renal transplant recipients [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A282. [CENTRAL: CN‐00484274] [Google Scholar]
- Hausberg M, Kosch M, Stam F, Heidenreich S, Kisters K, Rahn KH, et al. Effect of fluvastatin on endothelium‐dependent brachial artery vasodilation in patients after renal transplantation. Kidney International 2001;59(4):1473‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kosch M, Barenbrock M, Hausberg M. Sustained beneficial effect of a therapy with the HMG‐Co‐A‐reductase inhibitor fluvastatin on endothelial function in renal transplant recipients. Nieren‐und Hochdruckkrankheiten 2004;33(10):577‐83. [EMBASE: 2004495206] [Google Scholar]
- Kosch M, Barenbrock M, Suwelack B, Schaefer RM, Rahn KH, Hausberg M. Effect of a 3‐year therapy with the 3‐hydroxy‐3‐methylglutaryl coenzyme a reductase‐inhibitor fluvastatin on endothelial function and distensibility of large arteries in hypercholesterolemic renal transplant recipient. American Journal of Kidney Diseases 2003;41(5):1088‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kasiske 2001 {published data only}
- Kasiske BL, Heim‐Duthoy KL, Singer GG, Watschinger B, Germain MJ. Effects of lipid reduction on acute renal allograft rejection [abstract no: 429]. Transplantation 2000;69(8 Suppl):S225. [CENTRAL: CN‐00446023] [DOI] [PubMed] [Google Scholar]
- Kasiske BL, Heim‐Duthoy KL, Singer GG, Watschinger B, Germain MJ, Bastani B. The effects of lipid‐lowering agents on acute renal allograft rejection. Transplantation 2001;72(2):223‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Katznelson 1996 {published data only}
- Katznelson S, Wilkinson AH, Danovitch GM, Wang XM, Chia D, Kobashigawa JA, et al. Pravastatin lowers natural killer cell cytotoxicity and decreases the incidence of early acute rejection episodes in renal transplant patients [abstract]. Journal of the American Society of Nephrology 1994;5(3):1017. [CENTRAL: CN‐00601908] [Google Scholar]
- Katznelson S, Wilkinson AH, Danovitch GM, Wang XM, Chia D, Ozawa M, et al. The impact of pravastatin on the incidence of acute rejection after renal transplantation and on cellular immune function [abstract]. 14th Annual Meeting American Society of Transplant Physicians (ASTP);1995 May 10‐14; Chicago (USA). 1995.
- Katznelson S, Wilkinson AH, Kobashigawa JA, Wang XM, Chia D, Ozawa M, et al. The effect of pravastatin on acute rejection after kidney transplantation‐‐a pilot study. Transplantation 1996;61(10):1469‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lepre 1999 {published data only}
- Lepre F, Rigby R, Hawley C, Saltissi D, Brown A, Walsh Z. A double‐blind placebo controlled trial of simvastatin for the treatment of dyslipidaemia in renal allograft recipients. Clinical Transplantation 1999;13(6):520‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martinez Hernandez 1993 {published data only}
- Martinez Hernandez BE, Persaud JW, Varghese Z, Moorhead JF. Low‐dose simvastatin is safe in hyperlipidaemic renal transplant patients. Nephrology Dialysis Transplantation 1993;8(7):637‐41. [MEDLINE: ] [PubMed] [Google Scholar]
- Martinez‐Hernandez B, Persaud JW, Varghese Z, Moorhead JF. Low dose simvastatin (LS) is safe in hyperlipidemic transplant patients treated with CyA [abstract]. Journal of the American Society of Nephrology 1992;3(3):869. [CENTRAL: CN‐00461259] [Google Scholar]
Melchor 1998 {published data only}
- Melchor JL, Gracida C. Treatment of hypercholesterolemia with fluvastatin in kidney transplant patients. Transplantation Proceedings 1998;30(5):2054. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raiola 1998 {published data only}
- Raiola P, Manzo M, Saggese A. Comparison of atorvastatin (ATV) with fluvastatin (FLV) in renal transplant patients with dyslipoproteinemia [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):693A. [CENTRAL: CN‐00447330] [Google Scholar]
Renders 2001 {published data only}
- Renders L, Mayer‐Kadner I, Koch C. Efficacy and drug interactions of the new HMG‐CoA reductase inhibitors cerivastatin and atorvastatin in CsA‐treated renal transplant recipients. Nephrology Dialysis Transplantation 2001;16(1):141‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Renders L, Mayer‐Kadner I, Koch C, Burkhardt K, Veelken R, Schmieder R, et al. Cerivastatin and atorvastatin: efficacy, safety and drug‐interaction of the new HMG‐COA reductase inhibitors in renal transplant patients [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):694A. [CENTRAL: CN‐00447381] [Google Scholar]
Sahu 2001 {published data only}
- Sahu K, Sharma R, Gupta A. Effect of lovastatin, an HMG CoA reductase inhibitor, on acute renal allograft rejection. Clinical Transplantation 2001;15(3):173‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sharma RK, Sahu KM, Gupta A, Gulati S, Agarwal DK, Kumar A, et al. Role of lovastatin in prevention of acute rejection episodes in renal transplant recipient [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A288. [CENTRAL: CN‐00485837] [Google Scholar]
Santos 2001 {published data only}
- Garcia V, Bittar A, Keitel E, Neumann J, Saitovitch D, Santos A. Is cyclosporin microemulsion increasing the lipid‐lowering effect of simvastatin on kidney graft receptors? [abstract no: 1441]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul (Turkey). 2001. [CENTRAL: CN‐00671773]
- Garcia V, Bittar A, Keitel E, Neumann J, Saitovitch D, Santos A. Is simvastatin able to reduce the plasma levels of fibrinogen in kidney graft receptors? A double‐blind, randomized placebo controlled study [abstract no: 1439]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul (Turkey). 2001. [CENTRAL: CN‐00615908]
- Santos AF, Keitel E, Bittar A, Neumann J, Saitovich D, Seelig DC, et al. Is cyclosporin microemulsion increasing the lipid‐lowering effect of simvastatin on kidney graft receptor? [abstract no: 3311]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416585]
- Santos AF, Keitel E, Bittar A, Neumann J, Saitovich D, Seelig DC, et al. Is simvastatin able to reduce the plasma levels of fibrinogen in kidney graft receptors? A double‐blind randomized placebo controlled study [abstract no:3367]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416586]
- Santos AF, Keitel E, Bittar AE, Neumann J. Treatment of hyperlipidemia in renal transplant recipients with simvastatin: a double‐blind, randomized, placebo‐controlled study [abstract no: PO601]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome (Italy). 2000. [CENTRAL: CN‐00465849]
- Santos AF, Keitel E, Bittar AE, Neumann J, Fonseca N, Sporleder H, et al. Simvastatin effect on NK cells activity in vivo: a double‐blind randomized, placebo‐controlled study. Transplantation Proceedings 2002;34(7):2874‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Santos AF, Keitel E, Bittar AE, Neumann J, Fuchs FD, Goldani JC, et al. Safety and efficacy of simvastatin for hyperlipidemia in renal transplant recipients: a double‐blind, randomized, placebo‐controlled study. Transplantation Proceedings 2001;33(1‐2):1194‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Seron 2008 {published data only}
- Seron D, Oppenheimer F, Pallardo LM, Lauzurica R, Errasti P, Gomez‐Huertas E, et al. Fluvastatin in the prevention of renal transplant vasculopathy: results of a prospective, randomized, double‐blind, placebo‐controlled trial. Transplantation 2008;86(1):82‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sharif 2009 {published data only}
- Sharif A, Ravindran V, Moore R, Baboolal K. The effects of rosuvastatin on 51CR‐EDTA measured glomerular filtration rate and urinary albumin excretion in non‐diabetic renal transplant recipients [abstract no: 1386]. Transplantation 2008;86(2S):466. [CENTRAL: CN‐00766701] [Google Scholar]
- Sharif A, Ravindran V, Moore R, Dunseath G, Luzio S, Owens D, et al. The effect of rosuvastatin on insulin sensitivity and pancreatic beta‐cell function in nondiabetic renal transplant recipients. American Journal of Transplantation 2009;9(6):1439‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sharif A, Ravindran V, Moore R, Dunseath G, Luzio S, Owens D, et al. The effects of rosuvastatin on insulin sensitivity and secretion in non‐diabetic renal transplant recipients [abstract no: 611]. Transplantation 2008;86(2S):214. [CENTRAL: CN‐00766702] [DOI] [PubMed] [Google Scholar]
- Sharif A, Ravindran V, Moore R, Luzio S, Dunseath G, Owens D, et al. Influence of rosuvastatin on attenuation of the metabolic syndrome in non‐diabetic renal transplant recipients [abstract no: 60]. American Journal of Transplantation 2009;9(Suppl 2):208. [CENTRAL: CN‐00775209] [DOI] [PubMed] [Google Scholar]
- Sharif A, Ravindran V, Moore R, Luzio S, Dunseath G, Owens D, et al. Rosuvastatin is associated with few improvements in components of the metabolic syndrome, with no change in insulin resistance, in renal transplant recipients. Journal of Diabetes 2009;1:A92‐3. [EMBASE: 70212602] [Google Scholar]
SOLAR Study 2001 {published data only}
- Asberg A, Holdaas H, Jardine AG, Edvardsen C, Hartmann A. Fluvastatin reduces atherogenic lipids without any effect on native endothelial function early after kidney transplantation. Clinical Transplantation 2003;17(4):385‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holdaas H, Jardine A, Holme I, SOLAR Study Group. The SOLAR (Study Of Lescol in Acute Rejection) study: rationale and baseline data [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A319. [CENTRAL: CN‐00484378] [Google Scholar]
- Holdaas H, Jardine A, Wheeler D. Fluvastatin (lescol) demonstrates significant lipid‐lowering benefits without affecting rejection rates in renal transplant recipients, for the SOLAR study group [abstract no: 1212]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul (Turkey). 2001. [CN‐00445759]
- Holdaas H, Jardine AG, Wheeler DC, Brekke IB, Conlon PJ, Fellstrom B, et al. Effect of fluvastatin on acute renal allograft rejection: A randomized multicenter trial. Kidney International 2001;60(5):1990‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tuncer 2000 {published data only}
- Tuncer M, Suleymanlar G, Ersoy FF, Yakupoglu G. Comparison of the effects of simvastatin and pravastatin on acute rejection episodes in renal transplant patients. Transplantation Proceedings 2000;32(3):622‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
UK‐HARP‐1 2005 {published data only}
- Baigent C, Landray M, Leaper C, Altmann P, Armitage J, Baxter A, et al. First United Kingdom Heart and Renal Protection (UK‐HARP‐I) study: biochemical efficacy and safety of simvastatin and safety of low‐dose aspirin in chronic kidney disease. American Journal of Kidney Diseases 2005;45(3):473‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baigent C, UK‐HARP Steering Committee. Efficacy and safety of simvastatin and safety of low‐dose aspirin among patients with chronic kidney disease: final results of the first UK‐heart and renal protection (UK‐HARP‐I) study [abstract no: SA‐P0841]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):437a. [CENTRAL: CN‐00444305] [Google Scholar]
Vergoulas 1999 {published data only}
- Vergoulas G, Miserlis G, Gakis D, Imvrios G, Papagiannis A, Papanikolaou V, et al. Lovastatin (L) versus fluvastatin (F) in the treatment of hypercholesterolemic (HCH) renal transplant recipients (RTR) [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A312. [CENTRAL: CN‐00486302] [Google Scholar]
References to studies excluded from this review
Bagdade 1979 {published data only}
- Bagdade JD, Shantharam VV, Sollek M, Albers JJ. Effects of clofibrate on plasma lipids and high‐density lipoprotein levels in renal allograft recipients. Clinical Nephrology 1979;12(2):83‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Blechman‐Krom 2000 {published data only}
- Blechman‐Krom I. An interventional trial in established chronic renal allograft rejection. www.clinicaltrials.gov/ct2/show/NCT00005010 (accessed 19 December 2013).
Blum 2000 {published data only}
- Blum CB, Rapamune US Study Group. Cholesterol and triglyceride levels in sirolimus‐treated renal transplant recipients [abstract no: A3577]. Journal of the American Society of Nephrology 2000;11(Sept):680A. [CENTRAL: CN‐00550429] [Google Scholar]
Capone 1999 {published data only}
- Capone D, Stanziale P, Gentile A, Imperatore P, Pellegrino T, Basile V. Effects of simvastatin and pravastatin on hyperlipidemia and cyclosporin blood levels in renal transplant recipients. American Journal of Nephrology 1999;19(3):411‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Castro 1997 {published data only}
- Castro R, Queiros J, Fonseca I, Pimentel JP, Henriques AC, Sarmento AM, et al. Therapy of post‐renal transplantation hyperlipidaemia: comparative study with simvastatin and fish oil. Nephrology Dialysis Transplantation 1997;12(10):2140‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cheng 1995 {published data only}
- Cheng IKP, Li PWC, Janus ED, Tang CSO, Chan TM, Lo CY. Comparative efficacy of gemfibrozil (G) and simvastatin (S) in the treatment of hyperlipidaemia in renal transplant recipients (RT) ‐ a randomised prospective study [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid (Spain). 1995:383. [CENTRAL: CN‐00509131]
Curtis 1982 {published data only}
- Curtis JJ, Galla JH, Woodford SY, Lucas BA, Luke RG. Effect of alternate‐day prednisone on plasma lipids in renal transplant recipients. Kidney International 1982;22(1):42‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garcia‐de‐la‐Puente 2009 {published data only}
- Garcia‐de‐la‐Puente S, Arredondo‐Garcia JL, Gutierrez‐Castrellon P, Bojorquez‐Ochoa A, Maya ER, Perez‐Martinez MP. Efficacy of simvastatin in children with hyperlipidemia secondary to kidney disorders. Pediatric Nephrology 2009;24(6):1205‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gonzalez‐Molina 1996 {published data only}
- Gonzalez‐Molina M, Cabello M. Effect of diet, exercise and HMG‐CoA reductase on hypercholesterolaemia in renal transplant patients [abstract]. Nephrology Dialysis Transplantation 1993;8(9):1040. [CENTRAL: CN‐00260885] [Google Scholar]
- Gonzalez‐Molina M, Cabello M, Tinahones F, Burgos D, Lillo J, Soriguer F, et al. Hyperlipoproteinemia in renal transplant patients: Effect of hypocaloric diet, exercise and HMG‐CoA reductase inhibition. Nefrologia 1996;16(4):359‐64. [EMBASE: 1996302763] [Google Scholar]
Hilbrands 1993 {published data only}
- Hilbrands LB, Demacker PN, Hoitsma AJ. Cyclosporin and serum lipids in renal transplant recipients. Lancet 1993;341(8847):765‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Hilbrands LB, Demacker PN, Hoitsma AJ, Stalenhoef AF, Koene RA. The effects of cyclosporine and prednisone on serum lipid and (apo)lipoprotein levels in renal transplant recipients. Journal of the American Society of Nephrology 1995;5(12):2073‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ichimaru 2001 {published data only}
- Ichimaru N, Takahara S, Kokado Y, Wang JD, Hatori M, Kameoka H, et al. Changes in lipid metabolism and effect of simvastatin in renal transplant recipients induced by cyclosporine or tacrolimus. Atherosclerosis 2001;158(2):417‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Imamura 2005 {published data only}
- Imamura R, Ichimaru N, Moriyama T, Shi Y, Namba Y, Nonomura N, et al. Long term efficacy of simvastatin in renal transplant recipients treated with cyclosporine or tacrolimus. Clinical Transplantation 2005;19(5):616‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kahan‐301 2000 {published data only}
- Campistol JM, Legendre C, Castegneto M, Hartmann A, European US Global and Tri‐Continental Study Groups. The effect of sirolimus on lipids on renal transplant recipients: results from clinical trials [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):880A. [CENTRAL: CN‐00550752] [Google Scholar]
Kaplan‐251 2001 {published data only}
- Kovarik JM, Hartmann S, Berthier S, Hubert M, Rordorf C, Somberg K. Statin therapy in renal transplant patients receiving everolimus: screening for clinical and pharmacokinetic drug interactions [abstract no:187]. American Journal of Transplantation 2002;2(Suppl 3):185. [CENTRAL: CN‐00416055] [Google Scholar]
Kasiske 1990 {published data only}
- Kasiske BL, Tortorice KL, Heim‐Duthoy KL, Goryance JM, Rao KV. Lovastatin treatment of hypercholesterolemia in renal transplant recipients. Transplantation 1990;49(1):95‐100. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kliem 1996 {published data only}
- Kliem V, Wanner C, Eisenhauer T, Olbricht CJ, Doll R, Boddaert M, et al. Comparison of pravastatin and lovastatin in renal transplant patients receiving cyclosporine. Transplantation Proceedings 1996;28(6):3126‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Lal 1992 {published data only}
- Lal SM, Hewett JE, Petroski GF, Stone JC, Ross G. Effects of nicotinic acid and lovastatin in renal transplant patients: a prospective, randomized, open‐labeled crossover trial. American Journal of Kidney Diseases 1995;25(4):616‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lal SM, Petroski GF, Hewett JE, Stone JC, Ross G. Long term lipid lowering effects of nicotinic acid and lovastatin in renal transplant patients [abstract no:76P]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):946. [CENTRAL: CN‐00484737] [Google Scholar]
- Lal SM, Petroski GF, Hewett JE, Stone JC, Ross G. Three year follow up data on the lipid lowering effects of nicotinic acid and lovastatin in renal transplant patients [abstract no: 372]. Journal of the American Society of Nephrology 1995;6(3):1100. [CENTRAL: CN‐00484738] [Google Scholar]
- Lal SM, Stone J, Ross G Jr. Lipid lowering effects of nicotinic acid and lovastatin in renal transplant patients [abstract]. Journal of the American Society of Nephrology 1992;3(3):866. [CENTRAL: CN‐00461132] [Google Scholar]
Lal 1996 {published data only}
- Lal SM, Ross G. Lipid lowering effects of gemfibrozil and pravastatin with or without cholestyramine in diabetic renal transplant patients [abstract no: A3342]. Journal of the American Society of Nephrology 1996;7(9):1915. [CENTRAL: CN‐00775367] [Google Scholar]
Lal 1998 {published data only}
- Lal SM, Habib S. Lipid lowering effects of niacin and lovastatin given alone or with cholestyramine in renal transplant patients [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):683A. [CENTRAL: CN‐00446257] [Google Scholar]
LANDMARK 2 2009 {published data only}
- Kaisar M, Armstrong K, Prins J, Marwick T, Johnson D, Hawley C, et al. The impact of aggressive cardiovascular risk modification on carotid intima media thickness and brachial artery reactivity in renal transplant recipients [abstract no: 132]. Nephrology 2008;13(Suppl 3):A134. [CENTRAL: CN‐00766731] [Google Scholar]
- Kaisar MO, Armstrong K, Hawley C, Campbell S, Mudge D, Johnson DW, et al. Adiponectin is associated with cardiovascular disease in male renal transplant recipients: baseline results from the LANDMARK 2 study. BMC Nephrology 2009;10:29. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopau 2006 {published data only}
- Lopau K, Spindler K, Wanner C. Effects of pravastatin treatment on blood pressure regulation after renal transplantation. Kidney & Blood Pressure Research 2006;29(6):329‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Markell 1995 {published data only}
- Markell MS, Sumrani N, Brown CD, Hong JH, Sommer BG, Friedman EA. Effect of hypercholesterolemia and lipid‐lowering therapy on glomerular filtration rate in renal transplants [abstract no: 1808]. Journal of the American Society of Nephrology 1995;6(3):1103. [CENTRAL: CN‐00484981] [Google Scholar]
Nart 2009 {published data only}
- Nart A, Uslu A, Bozkaya G, Aykas A, Dogan M, Karaca B. A prudent algorithm for hyperlipoproteinemia in renal transplant recipients. Transplantation Proceedings 2009;41(2):751‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nicholson 1993 {published data only}
- Nicholson PG, Raval DD, Behrens MT, Weir MR. A prospective cross over trial to compare the efficacy and safety of lovastatin and gemfibrozil in the treatment of hyperlipidemic organ transplant recipients [abstract no: 76P]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):952. [CENTRAL: CN‐00485241] [DOI] [PubMed] [Google Scholar]
Ok 1996 {published data only}
- Ok E, Kursat S, Alev M, Tobu M, Tokat Y, Akcicek F, et al. Further evidence of favorable effects of gemfibrozil on the lipid profile in renal allograft recipients. Nephron 1996;73(3):491‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Olbricht 1997 {published data only}
- Olbricht C, Wanner C, Eisenhauer T, Christians U, Kliem V, O'Grady P, et al. Pravastatin and lovastatin pharmacokinetics and pharmacodynamics in renal transplant patients receiving cyclosporine [abstract]. Journal of the American Society of Nephrology 1995;6(3):1061. [CENTRAL: CN‐00485312] [Google Scholar]
- Olbricht C, Wanner C, Eisenhauer T, Kliem V, Doll R, Boddaert M, et al. Accumulation of lovastatin, but not pravastatin, in the blood of cyclosporine‐treated kidney graft patients after multiple doses. Clinical Pharmacology & Therapeutics 1997;62(3):311‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raiola 1996 {published data only}
- Raiola P, Manzo M, Saggese A. Interaction of cyclosporine and fluvastatin with and without niacin in renal transplant patients with dyslipoproteinemia [abstract no: A3368]. Journal of the American Society of Nephrology 1996;7(9):1920. [CENTRAL: CN‐00775265] [Google Scholar]
Renders 2010 {published data only}
- Renders L. Cardiovascular events in renal Transplant recipients with low LDL‐cholesterol receiving tacrolimus in combination with the statin fluvastatin [CRANOC]. www.clinicaltrials.gov/ct2/show/NCT00223041 (accessed 19 December 2013).
Rigatto 1997 {published data only}
- Rigatto O, Rush D, Perry Y, Walker S, Bose R, Bolli P. Cholesterol reduction in cyclosporine‐treated renal transplant patients: effect on platelet‐derived growth factor levels and platelet activation. Transplantation Proceedings 1997;29(6):2591‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rodriguez 1997 {published data only}
- Rodriguez AP, Bonis E, Gonzalez PJ, Torres A, Perez L, Dominguez ML, et al. Treatment of hyperlipidemia after renal transplantation: Comparative effect of lovastatin and omega‐3 fatty acids. Nefrologia 1997;17(1):49‐54. [EMBASE: 1997130521] [Google Scholar]
Ruiz 2006 {published data only}
- Ruiz MC, Moreno JM, Ruiz N, Vargas F, Asensio C, Osuna A. Effect of statin treatment on oxidative stress and renal function in renal transplantation. Transplantation Proceedings 2006;38(8):2431‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Turk 2001 {published data only}
- Turk S, Yildiz A, Tukek T, Akkaya V, Aras U, Turkmen A, et al. The effect of fluvastatin of hyperlipidemia in renal transplant recipients: a prospective, placebo‐controlled study. International Urology & Nephrology 2001;32(4):713‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vasquez 2004 {published data only}
- Vasquez EM, Sifontis N, Jacobssen V, Testa G, Sankary H, Benedetti E. Simvastatin for prevention of bone loss following renal transplantation [abstract no: P284]. Transplantation 2004;78(2 Suppl):293. [CENTRAL: CN‐00509537] [Google Scholar]
White 1996 {published data only}
- White M, Hanes D, Wiland A, Klassen D, Hoen‐Saric E, Schweitzer E, et al. Long‐term treatment of hyperlipidemia in transplant recipients with pravastatin and gemfibrozil [abstract]. 15th Annual Meeting American Society of Transplant Physicians (ASTP);1996 May 10‐14; Chicago (ILL). 1996.
Wissing 2006 {published data only}
- Wissing KM, Unger P, Ghisdal L, Broeders N, Berkenboom G, Carpentier Y, et al. Effect of atorvastatin therapy and conversion to tacrolimus on hypercholesterolemia and endothelial dysfunction after renal transplantation. Transplantation 2006;82(6):771‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00565474 {published data only}
- NCT00565474. Evaluation of the effect of fluvastatin 40 mg (b.i.d.) in the prevention of the development of vasculopathy of the graft in de novo renal transplant patients transplant. www.clinicaltrials.gov/ct2/show/NCT00565474 (accessed 19 December 2013).
Additional references
Aakhus 1999
- Aakhus S, Dahl K, Wideroe TE. Cardiovascular morbidity and risk factors in renal transplant patients. Nephrology Dialysis Transplantation 1999;14(3):648‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Aker 1998
- Aker S, Ivens K, Grabensee B, Heering P. Cardiovascular risk factors and diseases after renal transplantation. International Urology & Nephrology 1998;30(6):777‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ALLHAT 2002
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT‐LLT). JAMA 2002;288(23):2998‐3007. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ANZDATA 2010
- Australia, New Zealand Dialysis, Transplant Registry (ANZDATA). The 33rd Annual Report 2010. http://www.anzdata.org.au/v1/report_2010.html (accessed 19 December 2013).
Baigent 2005
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Briel 2006
- Briel M, Schwartz GG, Thompson PL, Lemos JA, Blazing MA, Es GA, et al. Effects of early treatment with statins on short‐term clinical outcomes in acute coronary syndromes: a meta‐analysis of randomized controlled trials. JAMA 2006;295(17):2046‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Claesson 1998
- Claesson K, Mayer AD, Squifflet JP, Grabensee B, Eigler FW, Behrend M, et al. Lipoprotein patterns in renal transplant patients: a comparison between FK506 and cyclosporine A patients. Transplantation Proceedings 1998;30(4):1292‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Corsini 1993
- Corsini A, Mazzotti M, Raiteri M, Soma MR, Gabbiani G, Fumagalli R, et al. Relationship between mevalonate pathway and arterial myocyte proliferation: in vitro studies with inhibitors of HMG‐CoA reductase. Atherosclerosis 1993;10(1):117‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cosio 2002
- Cosio FG, Pesavento TE, Pelletier RP, Henry M, Ferguson RM, Kim S, et al. Patient survival after renal transplantation III: the effects of statins. American Journal of Kidney Diseases 2002;40(3):638‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CTT Collaboration 2010
- Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376(9753):1670‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Castillo 2004
- Castillo D, Cruzado JM, Manel Diaz J. The effects of hyperlipidaemia on graft and patient outcome in renal transplantation. Nephrology Dialysis Transplantation 2004;19(Suppl 3):iii67‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EBPG 2002
- EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long‐term management of the transplant recipient. IV.5.3. Cardiovascular risks. Hyperlipidaemia. Nephrology Dialysis Transplantation 2002;17(Suppl 4):26‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Fellstrom 2004
- Fellström B, Holdaas H, Jardine AG, Holme I, Nyberg G, Fauchald P, et al. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) trial. Kidney International 2004;66(4):1549‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Flechner 2002
- Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation 2002;74(8):1070‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Groth 1999
- Groth CG, Backman L, Morales JM, Calne R, Kreis H, Lang P, et al. Sirolimus (rapamycin)‐based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation 1999;67(7):1036‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hilbrands 1995
- Hilbrands LB, Demacker PN, Hoitsma AJ, Stalenhoef AF, Koene RA. The effects of cyclosporine and prednisone on serum lipid and (apo)lipoprotein levels in renal transplant recipients. Journal of the American Society of Nephrology 1995;5(12):2073‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HPS 2003
- Collins R, Armitage J, Parish S, Sleigh P, Peto R, Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomized placebo‐controlled trial. Lancet 2003;361(9374):2005‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Isoneimi 1994
- Isoniemi H, Nurminen M, Tikkanen, Willebrand E, Krogerus L, Ahonen J, et al. Risk factors predicting chronic rejection of renal allografts. Transplantation 1994;57(1):68‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jardine 2011
- Jardine AG, Gaston RS, Fellstrom BC, Holdaas H. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet 2011;15:1419‐27. [DOI] [PubMed] [Google Scholar]
John 1999
- John GT, Dakshinamurthy DS, Jeyaseelan, Jacob CK. The effect of cyclosporine A on plasma lipids during the first year after transplantation. National Medical Journal of India 1999;12(1):14‐7. [MEDLINE: ] [PubMed] [Google Scholar]
JUPITER 2008
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. New England Journal of Medicine 2008;359(21):2195‐207. [DOI] [PubMed] [Google Scholar]
Kasiske 1996
- Kasiske BL, Guijarro C, Massy ZA, Wiederkher MR, Ma JZ. Cardiovascular disease after renal transplantation. Journal of the American Society of Nephrology 1996;7(1):158‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kasiske 2004
- Kasiske B, Cosio FG, Beto J, Bolton K, Chavers BM, Grimm R Jr, et al. Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: a report from the Managing Dyslipidemias in Chronic Kidney Disease Work Group of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative. American Journal of Transplantation 2004;4(Suppl 7):13‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2009
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009;9:S1‐155. [DOI] [PubMed] [Google Scholar]
Kurakata 1996
- Kurakata S, Kada M, Shimada Y, Komai T, Nomoto K. Effects of different inhibitors of 3‐hydroxy‐3‐methylglutaryl coenzyme A (HMG‐CoA) reductase, pravastatin sodium and simvastatin, on sterol synthesis and immunological functions in human lymphocytes in vitro. Immunopharmacology 1996;34(1):51‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lentine 2004
- Lentine KL, Brennan DC. Statin use after renal transplantation: a systematic quality review of trial‐based evidence. Nephrology Dialysis Transplantation 2004;19(9):2378‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lentine 2005
- Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. Journal of the American Society of Nephrology : JASN 2005;16(2):496‐506. [PUBMED: 15615820] [DOI] [PubMed] [Google Scholar]
Massy 1996
- Massy ZA, Keane WF, Kasiske BL. Inhibition of the mevalonate pathway: benefits beyond cholesterol reduction?. Lancet 1996;347(8994):102‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Massy 2001
- Massy ZA. Hyperlipidemia and cardiovascular disease after organ transplantation. Transplantation 2001;72(6 Suppl):S13‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mathis 2004
- Mathis AS, Dave N, Knipp GT, Friedman GS. Drug‐related dyslipidemia after renal transplantation. American Journal of Health‐System Pharmacy 2004;61(6):565‐85. [MEDLINE: ] [PubMed] [Google Scholar]
Mehra 2004
- Mehra MR, Raval NY. Metaanalysis of statins and survival in de novo cardiac transplantation. Transplantation Proceedings 2004;36(5):1539‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Navaneethan 2009a
- Navaneethan SD, Pansini F, Perkovic V, Manno C, Pellegrini F, Johnson DW, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007784] [DOI] [PubMed] [Google Scholar]
NKF 2002
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease evaluation, classification and stratification. American Journal of Kidney Diseases 2002;39(2):S1‐S266. [MEDLINE: ] [PubMed] [Google Scholar]
Palmer 2013
- Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Nigwekar SU, et al. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD004289.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raine 1988
- Raine AE, Carter R, Mann JI, Morris PJ. Adverse effect of cyclosporin on plasma cholesterol in renal transplant recipients. Nephrology Dialysis Transplantation 1988;3(4):458‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Satterthwaite 1998
- Satterthwaite R, Aswad S, Sunga V, Shidban H, Bogaard T, Asai P, et al. Incidence of new‐onset hypercholesterolemia in renal transplant patients treated with FK506 or cyclosporine. Transplantation 1998;65(3):446‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sever 2003
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Lipid Lowering Arm (ASCOT‐LLA): a multicentre randomised controlled trial. Lancet 2003;361(9364):1149‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SHARP 2011
- Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet 2011;377:2181‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepherd 1995
- Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolaemia. West of Scotland Coronary Prevention Study. New England Journal of Medicine 1995;333(20):1301‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
USRDS 2011
- USRDS. USRDS 2011 Annual Data Report. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. http://www.usrds.org/2011/pdf/v2_ch04_11.pdf 2011. [MEDLINE: ]
Vathsala 1989
- Vathsala A, Weinberg RB, Schoenberg L, Grevel J, Goldstein RA, Buren CT, et al. Lipid abnormalities in cyclosporine‐prednisone‐treated renal transplant recipients. Transplantation 1989;48(1):37‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Navaneethan 2009b
- Navaneethan SD, Perkovic V, Nigwekar SU, Johnson DW, Craig JC, Strippoli GF. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005019.pub2] [DOI] [PubMed] [Google Scholar]
Navaneethan 2009c
- Navaneethan SD, Perkovic V, Johnson DW, Nigwekar SU, Craig JC, Strippoli GF. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD005019.pub3] [DOI] [PubMed] [Google Scholar]
Perkovic 2004
- Perkovic V, Craig JC, Masterson R, Webster A. HMG CoA reductase inhibitors (statins) for kidney transplant patients. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD005019] [DOI] [Google Scholar]


