Abstract
Over the past 10 years, neutrophil extracellular traps (NETs) have become widely accepted as an integral player in immunothrombosis, due to their complex interplay with both pathogens and components of the coagulation system. While the release of NETs is an attempt by neutrophils to trap pathogens and constrain infections, NETs can have bystander effects on the host by inducing uncontrolled thrombosis, inflammation, and tissue damage. From an evolutionary perspective, pathogens have adapted to bypass the host innate immune response. Staphylococcus aureus (S. aureus), in particular, proficiently overcomes NET formation using several virulence factors. Here we review mechanisms of NET formation and how these are intertwined with platelet activation, the release of endothelial von Willebrand factor, and the activation of the coagulation system. We discuss the unique ability of S. aureus to modulate NET formation and alter released NETs, which helps S. aureus to escape from the host’s defense mechanisms. We then discuss how platelets and the coagulation system could play a role in NET formation in S. aureus-induced infective endocarditis, and we explain how targeting these complex cellular interactions could reveal novel therapies to treat this disease and other immunothrombotic disorders.
Keywords: endocarditis, extracellular trap, neutrophils, platelet activation, Staphylococcus, virulence factors
Highlights.
Neutrophil extracellular traps play an integral role in immunothrombosis by interacting with pathogens, platelets, the coagulation system and von Willebrand factor.
Staphylococcus aureus proficiently overcomes the antimicrobial properties of neutrophil extracellular traps using several virulence factors.
Neutrophil extracellular traps could play a role in the various stages of infective endocarditis, which is an infected blood clot attached to the cardiac valves.
The different cellular interactions in immunothrombosis represent several pharmacological targets to treat infective endocarditis and other immunothrombotic diseases.
Neutrophils are one of the first immune cells to arrive at a site of infection, and they have a whole arsenal of tools to combat pathogens. The release of neutrophil extracellular traps (NETs) is one of the main mechanisms of immune response to pathogens and has been extensively studied over the past 15 years. NETs are extracellular DNA fibers bearing histones, granular proteins including MPO (myeloperoxidase), NE (neutrophil elastase), and defensins, and cytosolic proteins including calprotectin and cathelicidins.1 Although neutrophil extracellular trap (NET) structures were first imaged in the 1980s2,3 and likely even already described in the 1880s as a fibrous mass released from disappearing leukocytes,4 it was not until 2004 that their biological function became evident when Brinkmann et al1 revealed that these unique extracellular structures could not only capture but also kill both Gram-positive and Gram-negative bacteria. It is now widely appreciated that NETs are released in various infectious diseases, including bacterial, viral, parasitic, and fungal infections.5–12 This has been further highlighted by the current coronavirus disease 2019 (COVID-19) pandemic, where NETs form in abundance and contribute to severe disease and immunothrombosis in the lung and other organs.13–16 Recent observations highlight the complex induction mechanisms underlying NET formation during infection, including distinct pathways resulting in lytic or nonlytic NET release.17 When released into the extracellular space, NETs combat infection by physically trapping pathogens and inducing their death via NET microbicidal components.1,11,12,18–22 More specifically, this death is induced, among others, by histones that introduce damage to the bacterial membrane, by MPO in the presence of hydrogen peroxide and by NE that cleaves thrombin into bactericidal thrombin-derived C-terminal fragments and degrades certain bacterial virulence factors.1,19–23 As a counter-attack mechanism, pathogens have adapted to be able to bypass this innate immune response and to eventually thrive in the host environment. Staphylococcus aureus (S. aureus), in particular, proficiently overcomes host defense mechanisms via an arsenal of virulence factors that manipulate both the coagulation cascade and innate and adaptive immunity,24,25 thus representing one of the deadliest bacteria in the developed world.26 The failure of NETs in killing pathogens can be made worse by the bystander effects that NETs have on host cells and tissues. For example, NET formation within blood vessels can contribute to uncontrolled thrombosis and subsequent tissue damage.27 In this review, we summarize the evidence surrounding the interplay between NETs, platelets, the coagulation system, and VWF (von Willebrand factor), and how this can progress from protective immunothrombosis to pathology. We then discuss NET formation during S. aureus infection and how this is linked to infective endocarditis (IE), a devasting immunothrombotic disease. Finally, we highlight some promising therapeutic targets to potentially treat IE as well other S. aureus-related pathologies.
NETs and (immuno)thrombosis
In the course of a microbial infection, innate immune cells, including monocytes and neutrophils, can orchestrate a response with thrombosis mediators leading to the formation of microthrombi. This form of thrombosis, designated as immunothrombosis,28 can either be limited to selected microvessels and serve the purpose of suppressing the dissemination of pathogens, or can affect larger vascular beds and have a detrimental impact on the host. NETs are central contributors to immunothrombosis; in fact, they are commonly found inside human thrombi and in close contact with platelets, VWF, and coagulation factors, including TF (tissue factor), factor XII, and fibrin/fibrinogen.5,29–32
NETs and Platelets
Platelets are generally the first cellular effectors recruited to a site of vascular injury, where they bind to subendothelial collagen or to VWF released from activated endothelial cells. Upon interaction with the endothelium, platelets undergo various stages of activation, leading to further recruitment not only of other platelets, but also of circulating leukocytes including neutrophils. Platelet-neutrophil interaction can lead to the subsequent activation of neutrophils,33–35 which is crucial for NET formation and for further platelet accumulation.12,34,36
The dynamic collaboration between platelets and neutrophils in the context of NET formation has been experimentally demonstrated both in in vitro and in vivo models. In vitro, activated platelets induce NET release under static or flow conditions.11,12,37–41 Various platelet agonists, such as lipopolysaccharide, thrombin, ADP, and collagen, are able to initiate this process, as well as platelets from patients with sepsis or treated with septic plasma or bacteria.11,12,38–40,42 S. aureus, in particular, interacts with and activates platelets which in turn enhances neutrophil activation toward NET release.38,40 Intravital microscopy revealed that platelet aggregates were present within and downstream of NETs in mice with sepsis,36 while experimental platelet depletion prevented NET formation in a mouse model of endotoxemia.12 Finally, the available evidence that antiplatelet therapies, such as aspirin, cilostazol, prostacyclin, and ticagrelor, reduce NET release in vitro and in infectious disease, reinforces the idea that platelets are important contributors to NET formation during systemic infection.38,39,43–46
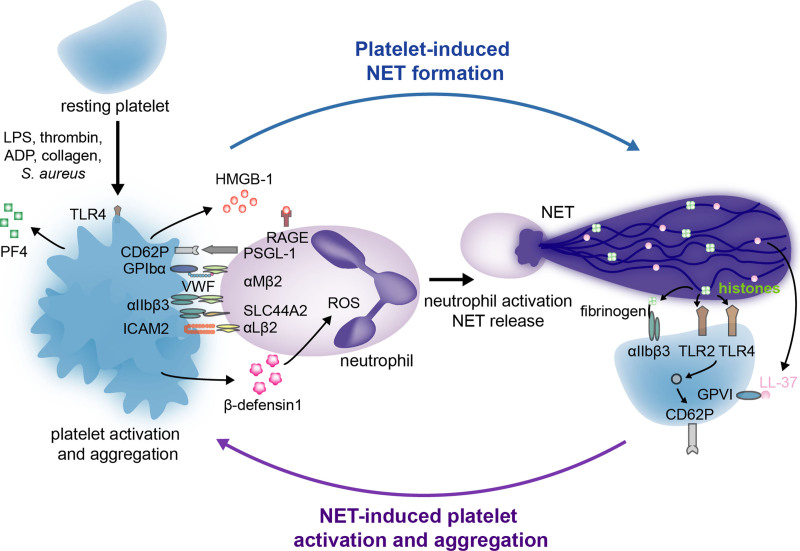
Various molecular mechanisms have been described in platelet-induced NET formation (NETosis) (Figure 1). Direct interaction of platelets with neutrophils via P-selectin (CD62P) and P-selectin glycoprotein ligand 1 (PSGL-1), respectively, is a fundamental mechanism whereby platelets induce NET formation.39,41 A key role has been also established for the platelet receptors glycoprotein Ibα (GPIbα) and integrin αIIbβ3 (also known as GPIIb/IIIa), which respectively interact with integrin αMβ2 and SLC44A2 (solute carrier family 44 member 2) on neutrophils.35,47 Platelet ICAM2 (intercellular adhesion molecule 2) which associates with αLβ2 on neutrophils enhances NET formation12 as well as the activation of platelet TLR4 (toll-like receptor 4), which accounts for approximately half of platelet-neutrophil interactions when neutrophils are incubated with platelets in the presence of septic plasma.11 In addition to directly interacting with neutrophils, activated platelets also secrete several soluble factors that affect neutrophils. For example, the release of HMBG1 (high-mobility box group 1), which binds to the receptor for advanced glycation end products (RAGE) on the neutrophil surface, is crucial for NET formation.34,48 Moreover, platelets secrete β-defensin 1, CD40L, PF4 (platelet factor 4), and chemokine ligand 5 (CCL5) that induce NET formation by binding to various neutrophil β2 integrins,49–52 as well as polyphosphates that promote NET formation through FXII (factor XII) activation.53
Figure 1.
The dynamic interplay between platelets and neutrophil extracellular traps (NETs). While some interactions between platelets and neutrophils trigger activated neutrophils to release NETs, others lead to platelet activation and aggregation. Via various receptors, secreted ligands, and specific components of the coagulation system, platelets can directly or indirectly induce NET formation (left). In return, specific components of NETs, more specifically histones and the cathelicidin LL-37, interact with several platelet receptors that result in platelet activation and aggregation (right). CD62P indicates P-selectin; GPIbα, glycoprotein Ibα; GPVI, glycoprotein VI; HMGB-1, high mobility box group 1; ICAM2, intercellular adhesion molecule 2; LPS, lipopolysaccharide; PF4, platelet factor 4; PSGL-1, P-selectin glycoprotein ligand 1; RAGE, receptor for advanced glycation end product; ROS, reactive oxygen species; SLC44A2, solute carrier family 44 member 2; TLR 2 or 4, toll-like receptor 2 or 4; and VWF, Von Willebrand factor.
Reciprocal activation of platelets by NETs also occurs, thus generating a thromboinflammatory cycle (Figure 1). This was first shown by perfusing whole blood over NETs, which resulted in platelet adhesion, activation, and aggregation.27 Isolated neutrophils from septic patients also cause platelet activation, which occurs in a P-selectin-dependent manner.54 These effects were reduced in septic mice deficient in PAD4 (peptidylarginine deiminase 4), an enzyme crucial in NET formation36,55 because it promotes chromatin decondensation by citrullinating histones H1, H3, and H4.56 In addition to NETs themselves, histones are able to activate and aggregate platelets in a P-selectin and TLR2/4-dependent manner27,57 or by associating with crosslinking molecules, such as fibrinogen, that in turn interact with platelets through the αIIbβ3 (GPIIb/IIIa) integrin.27,58 This interaction of histones with TLR2 and TLR4 induces platelet-mediated thrombin generation, thus further supporting activation of the coagulation cascade.57 Finally, NETs also activate platelets via the cathelicidin LL-37, which binds to GPVI on the platelet surface.59
NETs and Coagulation Factors
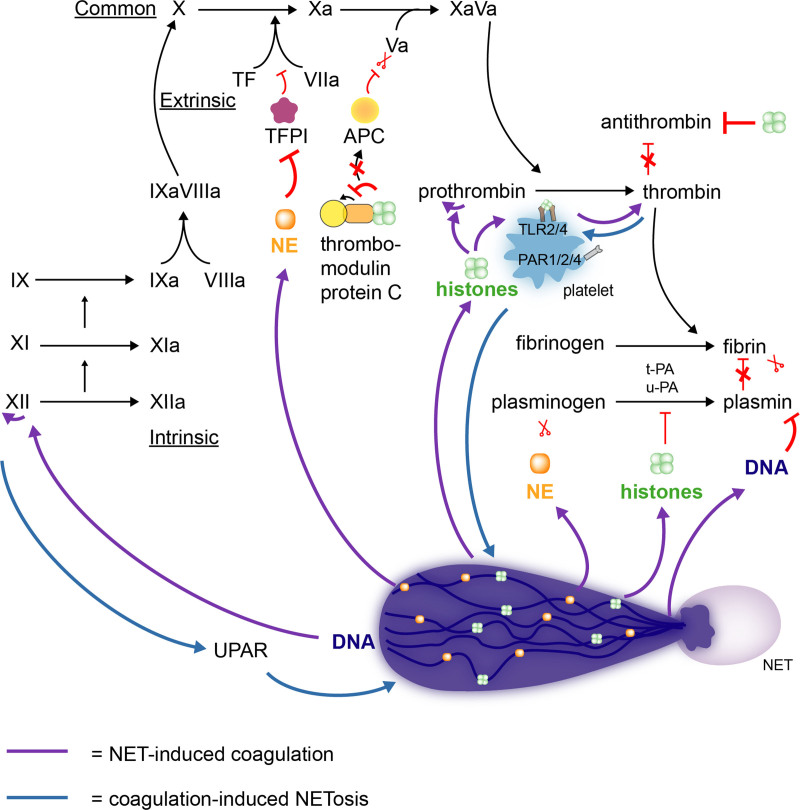
In addition to the reciprocal interaction with platelets, a dynamic interplay between NETs and several coagulation factors leads to enhanced fibrin formation,27,36 (Figure 2). Neutrophil serine proteases, such as NE, impair the degradation of TF by the TFPI (TF pathway inhibitor), thus supporting the extrinsic pathway of coagulation.60 As such, abundant amounts of TF have been found in the proximity of NETs formed in the liver microvasculature of mice with sepsis or vessel injury.36,54,60 NETs also interfere with the intrinsic pathway of coagulation. In fact, in the presence of activated platelets, the negatively charged DNA backbone of NET can trigger the autoactivation of FXII.53,61 This charge-driven interaction induces the generation of thrombin, an effect that disappears in FXII-deficient plasma or when FXII is blocked.61–63 By enhancing thrombin generation, NETs are thus able to promote fibrin formation.36 NETs can enhance thrombin formation via several other mechanisms that are either platelet dependent or independent. Intact NETs or histones H3 and H4 can interact with platelet TLR2 and TLR4 and sustain prothrombin cleavage to thrombin.63 Histones can also prevent thrombomodulin from activating protein C,64 which, in the presence of protein S, cleaves FVa and FVIIIa and limits thrombin production. Histone H4 has also been reported to directly bind to and autoactivate prothrombin.65 The involvement of NETs in the generation of thrombin has been further demonstrated by reduced formation of thrombin-antithrombin complexes and reduced thrombin activity when NETs or NET formation were targeted using various strategies, including digestion by DNases (deoxyribonucleases), histone-neutralizing antibodies, PAD4-deficiency, or by neutrophil depletion.36,55,62,66
Figure 2.
Neutrophil extracellular traps (NETs) promote fibrin formation by interacting with the coagulation system. NETs stimulate the formation of fibrin by triggering both the extrinsic (TF [tissue factor]) as well as intrinsic (FXII) pathway of the coagulation system (green arrows). In addition, histones are able to prevent the cleavage of FVa by APC (activated protein C). By the autoactivation of prothrombin or the interaction with TLR2 or 4 (toll-like receptor 2 or 4) on platelets, histones directly trigger thrombin generation (green arrows). Besides directly stimulating fibrin formation, NETs prevent its degradation by impairing t-PA (tissue-type plasminogen activator), u-PA (urokinase-type plasminogen activator), antithrombin, and plasmin-mediated lysis (green arrows). Not only are NETs able to interact with various components of the coagulation cascade, but these components (FXII and thrombin) can also prime for NETosis (blue arrows). UPAR indicates urokinase-type plasminogen activator receptor.
Different studies support the idea that NETs also promote coagulation by preventing fibrin degradation, as illustrated in Figure 2. tPA (tissue-type plasminogen activator) converts plasminogen into plasmin, an important enzyme that promotes fibrinolysis. Elastase bound to the NET DNA backbone degrades plasminogen, as evident by the high amount of NE-derived plasminogen fragments found in the plasma of septic shock patients.67 Additionally, using clot lysis assays, it was found that cell-free DNA is able to bind plasmin and fibrin thereby impairing plasmin-mediated fibrinolysis.68 To confirm these observations, treatment with DNase has been able to restore thrombolysis in different settings.68,69 Moreover, histones have shown the ability to hamper tPA-driven fibrinolysis in in vitro assays.69,70
As depicted in Figure 2, coagulation factors, such as FXII and thrombin, are in turn able to promote additional NET formation (Figure 2). FXII induces NET formation via uPAR (urokinase plasminogen activator receptor)-induced pAkt2 signaling,53 while thrombin activates protease activated receptors (PAR1/2/4) on platelets and potentiates platelet-induced NETosis.71,72
NETs and VWF
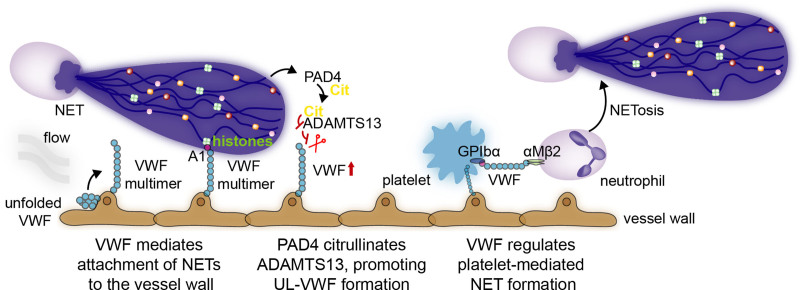
VWF is a multimeric protein released by activated platelets and by exocytosis of Weibel-Palade bodies from activated endothelial cells.73 Once released in the vasculature, ultra-large VWF multimers elongate in response to shear stress from the flowing bloodstream and expose the A1 domain, which can recruit neutrophils by binding to their PSGL-1 and αmβ2 receptors74 (Figure 3) but also directly binds to platelets and S. aureus.27,75 VWF has also been found to colocalize with NETs within a thrombus.27 Indeed, under flow conditions, histones and DNA from NETs bind the A1 domain of VWF (Figure 3), allowing them to stay in place and subsequently damage the vasculature.76 In S. aureus-infected mice, NETs promote liver injury via VWF-mediated binding to the vasculature.77 Histones themselves can provoke the release of Weibel-Palade bodies, thus further contributing to thrombus formation and immunothrombosis.75,78 Typically, high ultra-large VWF is cleaved by the protease ADAMTS13 (A disintegrin and metalloprotease with a thrombospondin type 1 motif, member 13), endogenously active in circulation. In pathological conditions, however, this balance is often impaired.79 PAD4, which is released on NETs, can citrullinate ADAMTS13 and subsequently abolish its activity, promoting ultra-large VWF-platelet string formation, such as in sepsis80 (Figure 3). VWF, in turn, by binding to platelet GPIbα and neutrophil αMβ2, also facilitates platelet interaction with neutrophils and platelet-induced NET formation.38
Figure 3.
VWF (Von Willebrand factor) and neutrophil extracellular traps (NETs) interact via various mechanisms. In response to shear stress of flowing blood, ultra-large VWF multimers elongate and expose the A1 domain. Via different mechanisms, NETs and neutrophils interact with these ultra-large VWF multimers. First, the A1 domain on VWF binds NET histones and DNA, thus allowing them to stay in place and damage the vasculature. Second, by releasing PAD4, NETs can promote ADAMTS13 (A disintegrin and metalloprotease with a thrombospondin type 1 motif, member 13) citrullination, a protease that cleaves VWF, and subsequently abolish its activity, promoting ultra-large VWF-platelet string formation. VWF, in turn, can regulate platelet-induced NET formation by acting on platelet GPIbα and neutrophil αMβ2.
NETs and S. aureus
S. aureus is the leading cause of several devastating infections, including sepsis, endocarditis, skin and soft tissue infections, osteoarticular, pleuropulmonary, and device-related infections.26 One of the reasons why S. aureus is so proficient in overcoming the innate immune system is its ability to manipulate the coagulation cascade. S. aureus induces fibrin formation by activating prothrombin via the coagulases von Willebrand factor binding protein (VWbp) and staphylocoagulase (Coa).81 Furthermore, S. aureus binds, activates, and aggregates platelets via its interaction with components of the coagulation system.25,81 Altering S. aureus-induced coagulation improved outcome in various experimental models of bacteremia, sepsis, endocarditis, skin and soft tissue infections, and catheter-related infections.82–87 The ability of S. aureus to induce fibrin via its coagulases distinguishes it from other staphylococci that are coagulase negative. Although the coagulase-negative strain S. epidermidis is also a frequent cause of medical device-related infections and prosthetic valve endocarditis, coagulase-negative staphylococci are less virulent than S. aureus.88 In general, endocarditis and cardiac device-related infections induced by S. aureus are associated with high mortality and complications compared with coagulase negative staphylococci.89,90 The only coagulase negative strain that mimics S. aureus is S. lugdunensis. It causes an infrequent but more aggressive form of native valve endocarditis, probably in virtue of the ability of S. lugdunensis to bind VWF.91
In addition to activating the coagulation system, S. aureus is a master in evading innate and adaptive host defenses.24 In this section, we will specifically focus on S. aureus and its unique ability to manipulate neutrophils releasing NETs. By capturing and killing pathogens, NETs can serve as a mechanism of host defense from a variety of infections.1,40,92 NETs are able to degrade S. aureus and its main virulence factor, α-toxin.1 However, this deadly bacterium secretes or expresses a variety of virulence factors that both enhance or diminish NETosis to its advantage, as well as overcome NETs’ microbicidal properties24,25 (Figure 4). Whether this is a response of the host to constrain infection or a way for the bacteria to survive in the host environment is still unclear.1,93
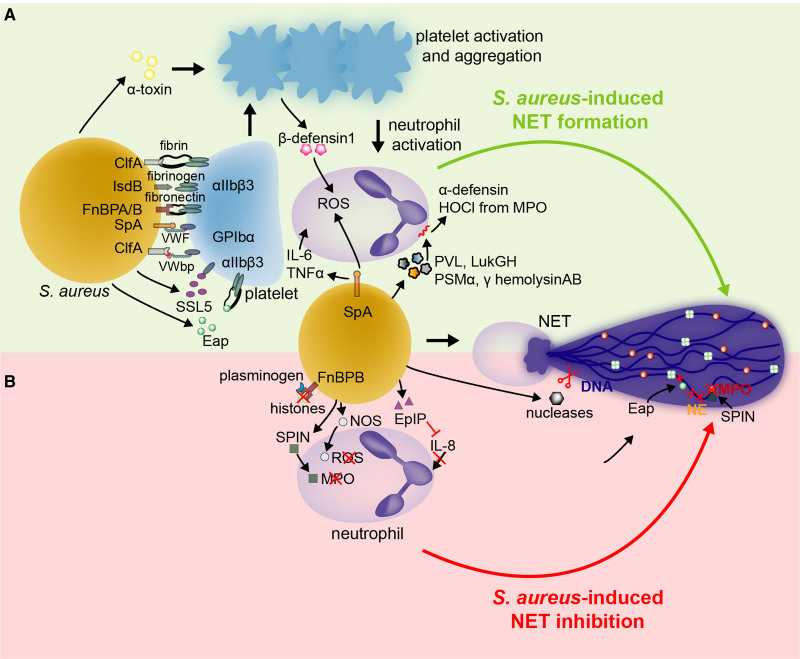
Figure 4.
Staphylococcus aureus both promotes and hampers neutrophil extracellular trap (NET) formation via various virulence factors to survive in the host environment. By interacting with platelet receptors, S. aureus activates and aggregates platelets and possibly promotes platelet-induced NET formation (A). Some S. aureus virulence factors can directly prime IL (interleukin)-6, TNFα (tumor necrosis factor α), reactive oxygen species (ROS), and damage-mediated NETosis (A). While enhancing NETosis could be a strategy of S. aureus to circumvent neutrophil-mediated killing, S. aureus is also equipped to diminish NET formation, destroy already formed NETs, or even to overcome their microbicidal properties (B). αIIbβ3 indicates glycoprotein GPIIb/IIIa; ClfA, clumping factor A; Eap, extracellular adherence protein; EpIP, epidermin leader peptide processing serine protease; FnBPA/B, fibronectin-binding protein A and B; GPIbα, glycoprotein Ib; IL-6/8, interleukin 6 or 8; IsdB, iron-regulated surface determinant B; MPO, myeloperoxidase; NE, neutrophil elastase; NOS, nitric oxide synthase; ROS, reactive oxygen species; SpA, S. aureus protein A; SPIN, staphylococcal peroxidase inhibitor; SSL5, Staphylococcal superantigen-like 5; TNFα, tumor necrosis factor α; VWbp, von Willebrand factor-binding protein; and VWF, Von Willebrand factor.
S. aureus Promotes NETosis
In theory, when S. aureus enters the bloodstream, it can be rapidly captured and killed by neutrophils through the release of NETs. However, NETs can also work as a scaffold that allows bacteria to stay in place and grow,94,95 contributing to a biofilm.96 It has been hypothesized that neutrophils that are primed to form NETs are no longer able to kill S. aureus by phagocytosis and that this could be a strategy for S. aureus to circumvent the host’s innate immune defense.92,97,98 Nevertheless, it has been shown that NET-forming neutrophils can still be viable and retain their ability to crawl, transmigrate, and phagocytose as functional cytoplasts.93,99
One way by which S. aureus promotes NETosis is by activating platelets (Figure 4A). S. aureus induces platelet activation and aggregation by binding to platelet integrin αIIbβ3, the platelet immunoglobulin receptor FcγRIIa, or GPIbα.100–104 S. aureus either binds directly to these receptors or via a variety of bridging molecules, such as fibrinogen, fibrin, fibronectin, or VWF. In particular, platelets can be affected by a variety of virulence factors, including: clumping factor A (ClfA), fibronectin binding protein A and B (FnBPA/B), Eap (extracellular adherence protein), SpA (S. aureus protein A), S. aureus iron-regulated surface determinant B (IsdB), Staphylococcal superantigen-like 5 (SSL5), and S. aureus toxin Panton-Valentine leukocidin (PVL).100–106 However, it has not been established yet whether platelets activated by S. aureus-derived virulence factors induce NETosis. Nevertheless, a direct association between virulence factor-induced platelet activation and NET formation has been shown for α-toxin. This toxin causes the secretion of β-defensin 1 from human platelets, thereby inducing NET release in a reactive oxygen species-dependent manner.49 Furthermore, antibodies against α-toxin reduced NET formation and bacterial load in diabetic mice with a S. aureus-infected wound.107
Although SpA and PVL-activated platelets likely do not stimulate NET formation directly, SpA and PVL are able to induce NETosis via other mechanisms (Figure 4A).97,98 In vitro, SpA primes NET release via secretion of IL (interleukin)-6, TNF (tumor necrosis factor), and reactive oxygen species.98 S. aureus also stimulates NETosis by forming pores on the neutrophils’ nuclear membrane and inducing the release of DNA bearing histones and granular proteins including MPO and NE into the extracellular space. Leukotoxin GH (LukGH), phenol-soluble modulin α Peptide (PSMα), γ-hemolysin AB, and PVL are some of the pore-forming toxins that have been described to induce NET release.92,94,97 These toxins have been most frequently found in community acquired MRSA strains as well in certain laboratory strains of S. aureus including USA300 LAC and Newman.94,97,108 Incubation with PVL and γ-hemolysin mutants reduced the amount of killed neutrophils and bacterial load, thus revealing that in some conditions NETosis can allow bacteria to survive in the host. In addition, a rapid, nonlytic form of NETosis induced by PVL has also been observed.93 Among different bacteria species, S. aureus is one of the most potent natural inducers of NET release.93
S. aureus Impairs NET Formation and Stability
As NETs and their components possess antimicrobial properties, it is more plausible that bacteria would promote their own survival by reducing NET formation rather than enhancing it. Indeed, S. aureus can reduce NET formation, destroy released NETs, and interact with NET microbicidal components to limit the antimicrobial potential of NETs (Figure 4B).
By virtue of its cationic properties, S. aureus-released Eap impairs NET formation by directly binding and aggregating the neutrophil DNA backbone, which was shown by atomic force microscopy.109 Incubation of PMA-induced NETs with Eap resulted in a diminution of the DNA/histone H1 network. Furthermore, these DNA binding properties can also be used to promote biofilm formation.110 Additionally, Eap and its homologs are potent inhibitors of NE and allow S. aureus to overcome NE’s antimicrobial properties, reduce NET release, and thus survive in vivo.111–114 Of note, however, elastase bound to the NET backbone is less sensitive to the inhibitory effect of Eap.115 Complimentary to the effect of Eap, inhibiting NE using selective inhibitors or mice deficient in NE impaired bacterial clearance,116,117 although this may also be due to NE’s direct antimicrobial properties.
S. aureus possesses various other mechanisms to target NET formation or its microbicidal properties. IL-8 mediated NETosis may be reduced by epidermin leader peptide processing serine protease (EpiP), which is a homolog of the IL-8 protease SpyCEP from Group A Streptococci.118 In addition to inhibiting NE, S. aureus inhibits MPO by secreting a specific virulence factor called staphylococcal peroxidase inhibitor (SPIN).119 SPIN binds the active site of MPO and thereby prevents its interaction with hydrogen peroxide and the ability of MPO to kill bacteria. Via expression of NOS (nitric oxide synthase), S. aureus can also overcome the bacterial killing action of reactive oxygen species and cathelicidins such as LL-37.120
A final way for S. aureus to survive NETs is by destroying the extracellular chromatin structure and disarming its components, including histones. Under the regulation of the SaeR/S system, S. aureus produces 2 nucleases (Nuc and Nuc 2) that digest extracellular DNA and therefore also NETs.96,121–124 Nuc is a secreted nuclease, whereas Nuc2 remains tethered to the S. aureus membrane125; this provides high local concentrations of nucleases in the vicinity of S. aureus. In mice with a respiratory tract infection, nuclease production diminished bacterial clearance and increased mortality.121 Nucleases have been found in sputum from patients with cystic fibrosis. This explains why S. aureus persists for a long time in the airways of patients affected by this condition.126 Moreover, the release of nucleases facilitates the escape of S. aureus from immunosurveillance by other cells, including macrophages. For example, the combined action of nuclease and AdsA (adenosine synthase A) converts NETs to deoxyadenosine and subsequently leads to caspase 3 activation, resulting in macrophage cell death.124,127,128 In addition to degrading NETs, nucleases can disrupt the DNA matrix inside biofilms, thus promoting bacterial spread and remodeling of the biofilm.96,122,123 Last, S. aureus is not only able to degrade the DNA backbone but also neutralize histones. In particular, its virulence factor FnBPB binds at the same time the histones released during the NETosis process and plasminogen, thus facilitating histone degradation by this enzymatic precursor.129 Additional evidence that killing of neutrophils and degrading NETs and its microbicidal proteins is an important strategy of S. aureus to evade the immune system and promote disease has been recently demonstrated in a skin infection mouse model. In this model, S. aureus evaded the immune system via the ArlRS-MgrA cascade by regulating the expression of nuclease, SCIN (staphylococcal complement inhibitor), and leucocidins.130
These studies highlight the many facets by which S. aureus can manipulate the host response, including NET formation, and thus prolong its survival. The impact of this is clearly seen by S. aureus’s ability to cause potentially severe human disease, particularly when it is able to thrive in the bloodstream.
NETs and IE
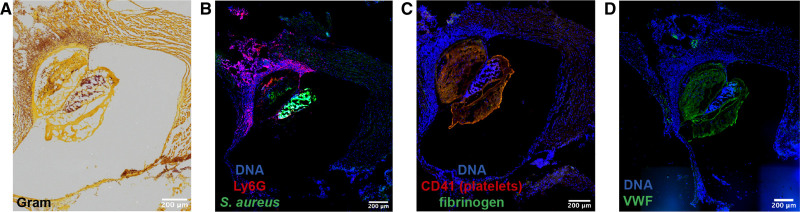
There are few diseases that highlight the crucial interplay between coagulation, bacteria, and immunity better than IE.131 IE is a condition defined by an infected blood clot or vegetation attached to the cardiac valves. The presence of bacteria, neutrophils, platelets, fibrinogen, and VWF in mice with an infected vegetation demonstrate the characteristic immunothrombotic properties of this disease (Figure 5). Immunohistochemical staining of 39 human IE valve samples revealed that extracellular DNA structures were commonly colocalized with MPO, suggesting the presence of NETs inside these vegetations.5 Here elastase, extracellular MPO and cell-free DNA were more abundantly expressed than in normal valvular tissue, implying a major role of neutrophils and NETs in patients with IE.5 However, the exact involvement of NETs in the pathophysiology of IE remains to be further elucidated. In Figure 6, we highlight the potential relevant players that could contribute to IE.
Figure 5.
Immunostaining of a cardiac valve vegetation in a model of inflammation-induced IE. IE vegetation was induced upon infection of mice with S. aureus and treatment with local histamine infusion via catheter instillation. A, Brightfield image of a Gram stain with bacteria seen in purple. B–D, Fluorescence images of the cardiac valve vegetation showing: neutrophil-specific marker Ly6G (red) and S. aureus (green) (B); platelet CD41 (red) and fibrinogen (green) (C); VWF (Von Willebrand Factor) in green (D). DNA is identified by Hoechst 33342 staining, depicted in blue. Images were acquired using a Zeiss Axioscan Z1 digital slide scanner or Zeiss Axiovert inverted microscope at the VIB-KU Leuven LiMoNe Bio Imaging Core and the KU Leuven Department of Cardiovascular Sciences Microscopy Core, respectively.
Figure 6.
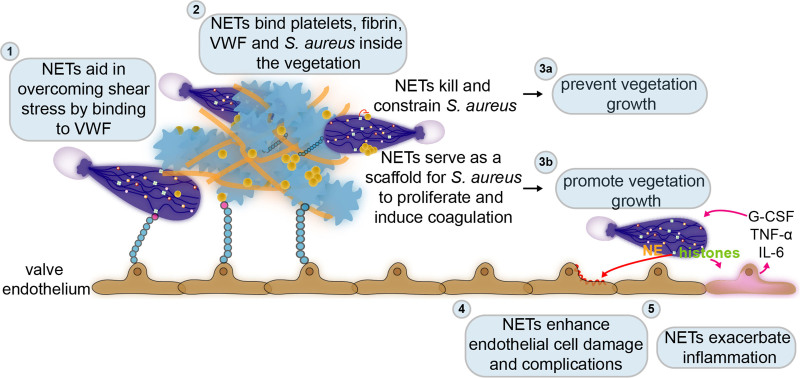
The dual role of neutrophil extracellular traps (NETs) in infective endocarditis. Few diseases highlight the crucial interplay between coagulation, bacteria, and immunity better than infective endocarditis, which at its core is an infected blood clot attached to the cardiac valves. (1) Already at the very early stages of this disease, NETs can overcome the turbulent flow of the valves’ environment by binding to the endothelium via VWF (von Willebrand factor). While attached to the endothelium, NETs provide a scaffold for binding of platelets, fibrin, and bacteria. (2) In the already formed vegetation or infected blood clot, NETs also bind platelets, fibrin, and S. aureus. (3) Inside the vegetation, NETs could either prevent its progression by killing and constraining S. aureus (3a) or enhance its formation by further stimulating coagulation or proliferation of S. aureus (3b). The infected blood clot or vegetation typically develops on damaged or inflamed cardiac valves. NETs may also induce further damage (4) and inflammation (5) in the valve endothelium. However, the exact role of NETs in infective endocarditis remains to be established. G-CSF indicates granulocyte colony-stimulating factor; IL-6, interleukin 6; and TNFα, tumor necrosis factor α.
NETs could aid the very early steps of the disease by delivering S. aureus from a local infection site to the bloodstream. Once in circulation, this pathogen could then travel to the cardiac valves. On the contrary, NETs may also prevent dissemination of bacteria from a localized infection such as an abscess,99 so the role of NETs in IE is complex. When reaching the valvular environment, S. aureus has to overcome its high shear rate by binding to platelets and/or VWF, and this enables it to colonize and finally form the infected vegetation characteristic of IE.131,132 Similarly, to overcome the valvular shear stress and to reach the bacterial aggregates embedded within a lesion, neutrophils can bind to platelets, fibrin, and/or bacteria within the IE vegetation or can associate with VWF multimers released from endothelial cells.
Inside this infected thrombus, NETs may try to constrain the infection by trapping and eventually killing S. aureus.1 Bacteria entrapped within NET-like structures have been identified inside IE vegetations in rats.39,40,133 However, NETs may not effectively kill the bacteria, providing them a meshwork on which to easily grow and facilitate IE progression. Furthermore, NETs can induce platelet activation and promote coagulation, and this thrombotic environment in return promotes additional NET release that can further allow vegetation growth.27 In a murine model of endocarditis, it has been recently reported that S. aureus induces coagulation within the vegetation by releasing two prothrombin activators, staphylocoagulase (Coa) and VWbp (von Willebrand Factor-binding protein). The resulting bacteria-induced fibrin-rich layer serves as a shield against attack by myeloid cells. In particular, CD11b-positive cells accumulate outside the bacteria-rich environment.86 In the presence of such a physical barrier, neutrophils and their released NETs may not be able to reach their target and carry out their bactericidal function. Additionally, DNase treatment in rats with endocarditis lesions reduced the size of the vegetation and the number of colonizing bacteria.39,40 Whether this is an effect of NETs, however, still needs to be determined, as extracellular DNases can also degrade extracellular DNA from bacteria and thus affect the bacterial biofilm.
Typically, endocarditis develops on damaged or inflamed valves.134 NET formation could aggravate the damage and inflammation of the valves’ endothelium. For example, injecting mice with histones or citrullinated histone H3 results in endothelial cell damage.135,136 In turn, the inflammatory response provoked by NETs could further prime NETosis.137 Moreover, signs of proteolytic tissue damage and apoptosis linked to the activity of neutrophil proteases such as NEs were frequently present in vegetations isolated from rats and patients.5,133 NE also induces apoptosis in myocardial cells ex vivo, thus possibly linking NETs to further complications such as heart failure.133
It is clear that additional studies on the involvement of NETs in the pathophysiology of IE are warranted. The detrimental or beneficial effects of NETs in endocarditis likely depend on several factors. First, there could be a time-dependent effect, where at an early stage NETs are protective, while later, they have more detrimental consequences. In various models of sepsis, NETs and their components promoted liver and renal damage after 8 to 24 hours; whereas NETs captured and killed bacteria effectively at earlier time points and thus had beneficial activity.12,45,136,138 This could reflect the fact that when NETs fail to kill bacteria inside the vegetation, they instead promote vegetation growth by enhancing coagulation, inflammation, and valve damage. Another determining factor for the effect of NET formation in IE is the relative proportion of bacteria to NETs. If NETs are outnumbered, there is a higher chance for the bacteria to overcome NETs killing properties. Last, the virulence of the bacterium could also play a major role. More virulent strains likely express nucleases, which allow undisturbed bacteria growth and the release of fragments from the degraded NET backbone, which can have harmful consequences on the host.115,121,122,126,137
Possible Therapeutic Options for Pathological Immunothrombosis and IE
The different cellular components involved in immunothrombosis represent several viable pharmacological targets to treat endocarditis or other immunothrombosis-related diseases. Here, we highlight the use of available or experimental drugs targeting platelets, coagulation, bacterial factors, and NETs as promising therapeutic approaches that could be applied in these disorders.
Targeting Platelets and Coagulation Factors
Antiplatelet and anticoagulant therapies, as well as strategies targeting VWF, have been shown to reduce NET formation in various animal models.43,44,61,77 Both heparin and activated protein C affect histones and thrombus formation in models of DVT and Escherichia. coli–induced sepsis.61,136 Combined treatment with tPA and DNase resulted in faster ex vivo thrombolysis of clots formed in vitro or collected from patients with acute ischemic stroke.27,70,139 Although treatment with tPA can successfully reduce the extent of vegetation in infants affected by IE and degrade clots dissected from rabbits with IE,140 the use of this drug is not recommended in patients with endocarditis due to the enhanced risk of bleeding.141 Similarly, combined aspirin and ticlopidine prophylaxis has been shown to be beneficial in a rat model of S. aureus induced endocarditis,142,143 but the use of these agents is currently not clinically supported.141,144 Further investigation in the use of more targeted antiplatelet and antithrombotic agents to reduce thrombi and NET formation in IE is therefore warranted. The use of recombinant ADAMTS13, which targets UL-VWF but otherwise minimally impacts hemostasis,145 may be a suitable approach to achieve anti-inflammatory and anti-NET efficacy.
However, platelets, fibrin, and VWF can constrain infection by capturing bacteria and preventing their dissemination. Platelets can also kill bacteria by releasing platelet microbicidal proteins.146 As such, therapeutic strategies that interfere with immunothrombosis could potentially aggravate the infection. Indeed, antiplatelet treatment in mice with sepsis and peritonitis enhanced bacteremia.12,44 However, while some authors have reported an increase in bacteria levels in the blood and various organs after anticoagulant and antiplatelet strategies, others could not show that these therapies promoted bacterial dissemination.147 When evaluating new therapies that target immunothrombosis, also the impact on the proliferation and dissemination of the pathogen should be taken into consideration. New therapies should mitigate the detrimental effect of immunothrombosis and impair the growth and dissemination of the pathogen.
Targeting Bacterial Factors
Antibodies against SpA, PVL, γ-hemolysin AB, LukGH, and α-toxin have already been developed.106,107,148–150 Nevertheless, until now, attempts to treat S. aureus infections by targeting these virulence factors have all clinically failed.151,152 However, recent data suggest that recombinant monoclonal antibodies against the bacterial factor SpA and α-toxin could be effective to treat S. aureus infections.149,153,154 Interestingly, the antiplatelet agent ticagrelor has been recently found to have antibacterial properties by inhibiting α-toxin-mediated platelet injury in vitro and in a murine S. aureus bacteremia model.155–157
Targeting virulence factors involved in the degradation of NETs could be a valid therapeutic option to prevent S. aureus from growing and spreading in the host.121,124,126 For example, cathelicidin LL-37, which binds to the negatively charged DNA backbone, could be used to prevent NET degradation.158 Likewise, antibodies neutralizing nucleases could be promising to block NET degradation and bacterial growth. Finally, monoclonal antibodies against Coa and VWbp, which reduce the amount of fibrin surrounding the bacteria-rich environment and make bacteria more accessible to neutrophils, improved survival in mice with endocarditis.86 Treatment with these antibodies also allowed neutrophils to better interact with S. aureus in a femoral artery vegetation model.86
Targeting NETs
A well-known agent that degrades the DNA backbone of NETs is DNase I, which is FDA-approved for the treatment of cystic fibrosis.159 This drug suppressed thrombus growth and tissue damage in several infectious disease models.27,36,39,40,61,160,161 Combining DNases with antibiotics may, however, be needed,39,40,160 since nucleases also disrupt the extracellular DNA within biofilms and promote systemic bacteremia.123,162 Elevated bacteremia levels have been shown after DNase treatment in animal models of endocarditis and sepsis,12,39 although later treatment has a protective effect without decreased bacterial burden.163 Combining DNase I with penicillin reduced the severity of bacteremia in rats with IE.39 Inhibiting the protein components associated with NETs, that is, granular proteins and histones, may provide additional therapeutic opportunities. Indeed, inhibitors of NE and anti-histone antibodies reduced organ damage and lethality in mice with sepsis136,164,165 but were also shown to impair bacterial clearance.116,117 The detrimental outcomes of NETs can also be mitigated by preventing their formation. To do so, PAD4 can be targeted to prevent chromatin decondensation, loss of nuclear lamins, and cytoskeleton disassembly, all steps important for NETosis.56,166,167 As such, PAD4-deficient mice with sepsis showed reduced intravascular coagulation and organ damage as compared with wild-type mice.36,55,77,161 Similarly, administration of CI-amidine, a pan-PAD inhibitor, effectively diminished NET release and progression of sepsis and wound infections in mice.7,168,169
On the other hand, LL-37, which is resistant to bacterial nucleases, promotes NETosis, but its activity suppresses the inflammatory response accompanying sepsis.170 Pircher et al found that LL-37 is abundant in thrombi from patients with acute myocardial infarctions and the deletion of LL-37 homolog, CRAMP, in mice reduced platelet recruitment and thrombosis. Moreover, LL-37 or CRAMP promote platelet-neutrophil interactions.59 Therefore, the use of LL-37 may promote unwanted thrombosis and the therapeutic use of LL-37 for the treatment of IE remains debatable.
A better understanding of the role of NETs in immunothrombosis is needed to find successful therapies for IE and, in general, diseases related to unregulated immunothrombosis. A fine tuning of NET formation and degradation might be required to allow NETs to remove bacteria from the circulation and reduce infection, without provoking untoward thrombotic complications in the host.
Conclusions
In summary, S. aureus can both diminish and enhance NET formation and can destroy released NETs and their associated components. Decreased NET formation can lead to S.aureus survival in the host and poor infection outcome, while enhanced NET formation, although trapping S.aureus, could also offer a scaffold for the formation of bacteria biofilm and growth. Moreover, enhanced NET formation could favor the development of uncontrolled immunothrombosis, consequent to the interaction of neutrophils and NETs with platelets, the endothelium and the coagulation system. In the context of IE caused by S.aureus, it remains unclear whether NETs prevent or promote vegetation growth. Targeting the cellular components involved in the pathophysiology of IE and immunothrombosis could represent valid approaches to treat these disorders. However, further basic and translational research is needed to validate the use of antiplatelet and anticoagulant drugs and of strategies affecting NET formation and stability in IE and other immunothrombotic diseases.
Article Information
Acknowledgment
We thank Dr Dougald M. Monroe (University of North Carolina) for his expert knowledge of the historical literature on coagulation and discussions, which brought to our attention the description of the release of fibrous material from leukocytes in the 1880s (reference 4).
Sources of Funding
S. Meyers is a fellow of the Fonds Wetenschappelijk Onderzoek Vlaanderen (FWO) (1S77119N). Our research on this subject is funded by FWO project grant number G066021N and by KU Leuven Internal Fund grant number C24M/20/056 to P. Verhamme.
Disclosures
K. Martinod is an inventor on granted US patent number US9642822B2 (licensed). The other authors report no conflicts.
Nonstandard Abbreviations and Acronyms
- ADAMTS13
- a disintegrin and metalloprotease with a thrombospondin type 1 motif, member 13
- AdsA
- adenosine synthase A
- COVID-19
- coronavirus disease 2019
- DNase
- deoxyribonuclease
- Eap
- extracellular adherence protein
- FXII
- Factor XII
- GP
- glycoprotein
- HMBG1
- high-mobility box group 1
- ICAM2
- intercellular adhesion molecule 2
- IE
- infective endocarditis
- IL
- interleukin
- MPO
- myeloperoxidase
- NE
- neutrophil elastase
- NET
- neutrophil extracellular trap
- NOS
- nitric oxide synthase
- PAD4
- peptidylarginine deiminase 4
- PF4
- platelet factor 4
- PVL
- Panton-Valentine leukocidin
- SpA
- S. aureus protein A
- TF
- tissue factor
- TFPI
- TF pathway inhibitor
- TLR4
- toll-like receptor 4
- TNF
- tumor necrosis factor
- tPA
- tissue-type plasminogen activator
- uPAR
- urokinase plasminogen activator receptor
- VWbp
- von Willebrand factor-binding protein
- VWF
- von Willebrand factor
For Sources of Funding and Disclosures, see pages 271–272.
Contributor Information
Severien Meyers, Email: severien.meyers@kuleuven.be.
Marilena Crescente, Email: M.Crescente@mmu.ac.uk.
Peter Verhamme, Email: peter.verhamme@uzleuven.be.
References
- 1.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 2.Tsan MF. Phorbol myristate acetate induced neutrophil autotoxicity. J Cell Physiol. 1980;105:327–334. doi: 10.1002/jcp.1041050215 [DOI] [PubMed] [Google Scholar]
- 3.Tsan MF, Denison RC. Phorbol myristate acetate-induced neutrophil autotoxicity. A comparison with H2O2 toxicity. Inflammation. 1980;4:371–380. doi: 10.1007/BF00916048 [DOI] [PubMed] [Google Scholar]
- 4.Wooldridge LC. XVI. The relation of the white blood corpuscles to the coagulation of the blood. Proc R Soc Lond. 1997;32:413–418. [Google Scholar]
- 5.Al-Salih G, Al-Attar N, Delbosc S, Louedec L, Corvazier E, Loyau S, Michel JB, Pidard D, Duval X, Meilhac O. Role of vegetation-associated protease activity in valve destruction in human infective endocarditis. PLoS One. 2012;7:e45695. doi: 10.1371/journal.pone.0045695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamaguchi S, Hirose T, Matsumoto N, Akeda Y, Irisawa T, Seki M, Hosotsubo H, Yamamoto K, Tasaki O, Oishi K, et al. Neutrophil extracellular traps in bronchial aspirates: a quantitative analysis. Eur Respir J. 2014;43:1709–1718. doi: 10.1183/09031936.00139813 [DOI] [PubMed] [Google Scholar]
- 7.Fadini GP, Menegazzo L, Rigato M, Scattolini V, Poncina N, Bruttocao A, Ciciliot S, Mammano F, Ciubotaru CD, Brocco E, et al. NETosis delays diabetic wound healing in mice and humans. Diabetes. 2016;65:1061–1071. doi: 10.2337/db15-0863 [DOI] [PubMed] [Google Scholar]
- 8.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x [DOI] [PubMed] [Google Scholar]
- 9.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, Uehata T, Iwasaki H, Omori H, Yamaoka S, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 10.Abi Abdallah DS, Lin C, Ball CJ, King MR, Duhamel GE, Denkers EY. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect Immun. 2012;80:768–777. doi: 10.1128/IAI.05730-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565 [DOI] [PubMed] [Google Scholar]
- 12.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 13.Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leppkes M, Knopf J, Naschberger E, Lindemann A, Singh J, Herrmann I, Stürzl M, Staats L, Mahajan A, Schauer C, et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925. doi: 10.1016/j.ebiom.2020.102925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671 [DOI] [PubMed] [Google Scholar]
- 18.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita S, Tagai C, Shiraishi T, Miyaji K, Iwamuro S. Differential mode of antimicrobial actions of arginine-rich and lysine-rich histones against Gram-positive Staphylococcus aureus. Peptides. 2013;48:75–82. doi: 10.1016/j.peptides.2013.07.025 [DOI] [PubMed] [Google Scholar]
- 20.Parker H, Albrett AM, Kettle AJ, Winterbourn CC. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J Leukoc Biol. 2012;91:369–376. doi: 10.1189/jlb.0711387 [DOI] [PubMed] [Google Scholar]
- 21.Petrlova J, Petruk G, Huber RG, McBurnie EW, van der Plas MJA, Bond PJ, Puthia M, Schmidtchen A. Thrombin-derived C-terminal fragments aggregate and scavenge bacteria and their proinflammatory products. J Biol Chem. 2020;295:3417–3430. doi: 10.1074/jbc.RA120.012741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinrauch Y, Drujan D, Shapiro SD, Weiss J, Zychlinsky A. Neutrophil elastase targets virulence factors of enterobacteria. Nature. 2002;417:91–94. doi: 10.1038/417091a [DOI] [PubMed] [Google Scholar]
- 23.Belaaouaj A, Kim KS, Shapiro SD. Degradation of outer membrane Protein A in Escherichia coli killing by neutrophil elastase. Science. 2000;289:1185–1188. doi: 10.1126/science.289.5482.1185 [DOI] [PubMed] [Google Scholar]
- 24.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol. 2015;13:529–543. doi: 10.1038/nrmicro3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liesenborghs L, Verhamme P, Vanassche T. Staphylococcus aureus, master manipulator of the human hemostatic system. J Thromb Haemost. 2018;16:441–454. doi: 10.1111/jth.13928 [DOI] [PubMed] [Google Scholar]
- 26.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345 [DOI] [PubMed] [Google Scholar]
- 29.Laridan E, Denorme F, Desender L, François O, Andersson T, Deckmyn H, Vanhoorelbeke K, De Meyer SF. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82:223–232. doi: 10.1002/ana.24993 [DOI] [PubMed] [Google Scholar]
- 30.Savchenko AS, Martinod K, Seidman MA, Wong SL, Borissoff JI, Piazza G, Libby P, Goldhaber SZ, Mitchell RN, Wagner DD. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J Thromb Haemost. 2014;12:860–870. doi: 10.1111/jth.12571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Boer OJ, Li X, Teeling P, Mackaay C, Ploegmakers HJ, van der Loos CM, Daemen MJ, de Winter RJ, van der Wal AC. Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organisation of thrombi in acute myocardial infarction. Thromb Haemost. 2013;109:290–297. doi: 10.1160/TH12-06-0425 [DOI] [PubMed] [Google Scholar]
- 32.Mangold A, Alias S, Scherz T, Hofbauer M, Jakowitsch J, Panzenböck A, Simon D, Laimer D, Bangert C, Kammerlander A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res. 2015;116:1182–1192. doi: 10.1161/CIRCRESAHA.116.304944 [DOI] [PubMed] [Google Scholar]
- 33.Evangelista V, Manarini S, Sideri R, Rotondo S, Martelli N, Piccoli A, Totani L, Piccardoni P, Vestweber D, de Gaetano G, et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood. 1999;93:876–885. [PubMed] [Google Scholar]
- 34.Maugeri N, Campana L, Gavina M, Covino C, De Metrio M, Panciroli C, Maiuri L, Maseri A, D’Angelo A, Bianchi ME, et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost. 2014;12:2074–2088. doi: 10.1111/jth.12710 [DOI] [PubMed] [Google Scholar]
- 35.Simon DI, Chen Z, Xu H, Li CQ, Dong Jf, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, Jenne CN. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129:1357–1367. doi: 10.1182/blood-2016-09-741298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimomura Y, Suga M, Kuriyama N, Nakamura T, Sakai T, Kato Y, Hara Y, Yamashita C, Nagasaki H, Kaneki M, et al. Recombinant human thrombomodulin inhibits neutrophil extracellular trap formation in vitro. J Intensive Care. 2016;4:48. doi: 10.1186/s40560-016-0177-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carestia A, Kaufman T, Rivadeneyra L, Landoni VI, Pozner RG, Negrotto S, D’Atri LP, Gómez RM, Schattner M. Mediators and molecular pathways involved in the regulation of neutrophil extracellular trap formation mediated by activated platelets. J Leukoc Biol. 2016;99:153–162. doi: 10.1189/jlb.3A0415-161R [DOI] [PubMed] [Google Scholar]
- 39.Jung CJ, Yeh CY, Hsu RB, Lee CM, Shun CT, Chia JS. Endocarditis pathogen promotes vegetation formation by inducing intravascular neutrophil extracellular traps through activated platelets. Circulation. 2015;131:571–581. doi: 10.1161/CIRCULATIONAHA.114.011432 [DOI] [PubMed] [Google Scholar]
- 40.Hsu CC, Hsu RB, Ohniwa RL, Chen JW, Yuan CT, Chia JS, Jung CJ. Neutrophil extracellular traps enhance Staphylococcus aureus vegetation formation through interaction with platelets in infective endocarditis. Thromb Haemost. 2019;119:786–796. doi: 10.1055/s-0039-1678665 [DOI] [PubMed] [Google Scholar]
- 41.Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126:242–246. doi: 10.1182/blood-2015-01-624023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang S, Gu Z, Lu C, Zhang T, Guo X, Xue G, Zhang L. Neutrophil extracellular traps are markers of wound healing impairment in patients with diabetic foot ulcers treated in a multidisciplinary setting. Adv Wound Care (New Rochelle). 2020;9:16–27. doi: 10.1089/wound.2019.0943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tilgner J, von Trotha KT, Gombert A, Jacobs MJ, Drechsler M, Döring Y, Soehnlein O, Grommes J. Aspirin, but not tirofiban displays protective effects in endotoxin induced lung injury. PLoS One. 2016;11:e0161218. doi: 10.1371/journal.pone.0161218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lapponi MJ, Carestia A, Landoni VI, Rivadeneyra L, Etulain J, Negrotto S, Pozner RG, Schattner M. Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J Pharmacol Exp Ther. 2013;345:430–437. doi: 10.1124/jpet.112.202879 [DOI] [PubMed] [Google Scholar]
- 45.Nomura K, Miyashita T, Yamamoto Y, Munesue S, Harashima A, Takayama H, Fushida S, Ohta T. Citrullinated histone H3: early biomarker of neutrophil extracellular traps in septic liver damage. J Surg Res. 2019;234:132–138. doi: 10.1016/j.jss.2018.08.014 [DOI] [PubMed] [Google Scholar]
- 46.Mitsios A, Chrysanthopoulou A, Arampatzioglou A, Angelidou I, Vidali V, Ritis K, Skendros P, Stakos D. Ticagrelor exerts immune-modulatory effect by attenuating neutrophil extracellular traps. Int J Mol Sci. 2020;21:E3625. doi: 10.3390/ijms21103625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Constantinescu-Bercu A, Grassi L, Frontini M, Salles-Crawley II, Woollard K, Crawley JT. Activated αIIbβ3 on platelets mediates flow-dependent NETosis via SLC44A2. Elife. 2020;9:e53353. doi: 10.7554/eLife.53353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogel S, Bodenstein R, Chen Q, Feil S, Feil R, Rheinlaender J, Schäffer TE, Bohn E, Frick JS, Borst O, et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J Clin Invest. 2015;125:4638–4654. doi: 10.1172/JCI81660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraemer BF, Campbell RA, Schwertz H, Cody MJ, Franks Z, Tolley ND, Kahr WH, Lindemann S, Seizer P, Yost CC, et al. Novel anti-bacterial activities of β-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011;7:e1002355. doi: 10.1371/journal.ppat.1002355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin R, Yu S, Song Z, Zhu X, Wang C, Yan J, Wu F, Nanda A, Granger DN, Li G. Soluble CD40 ligand stimulates CD40-dependent activation of the β2 integrin Mac-1 and protein kinase C zeda (PKCζ) in neutrophils: implications for neutrophil-platelet interactions and neutrophil oxidative burst. PLoS One. 2013;8:e64631. doi: 10.1371/journal.pone.0064631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossaint J, Herter JM, Van Aken H, Napirei M, Döring Y, Weber C, Soehnlein O, Zarbock A. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood. 2014;123:2573–2584. doi: 10.1182/blood-2013-07-516484 [DOI] [PubMed] [Google Scholar]
- 52.Lishko VK, Yakubenko VP, Ugarova TP, Podolnikova NP. Leukocyte integrin Mac-1 (CD11b/CD18, αMβ2, CR3) acts as a functional receptor for platelet factor 4. J Biol Chem. 2018;293:6869–6882. doi: 10.1074/jbc.RA117.000515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stavrou EX, Fang C, Bane KL, Long AT, Naudin C, Kucukal E, Gandhi A, Brett-Morris A, Mumaw MM, Izadmehr S, et al. Factor XII and uPAR upregulate neutrophil functions to influence wound healing. J Clin Invest. 2018;128:944–959. doi: 10.1172/JCI92880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kambas K, Mitroulis I, Apostolidou E, Girod A, Chrysanthopoulou A, Pneumatikos I, Skendros P, Kourtzelis I, Koffa M, Kotsianidis I, et al. Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis. PLoS One. 2012;7:e45427. doi: 10.1371/journal.pone.0045427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, Gallant M, Wang Y, Wagner DD. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood. 2015;125:1948–1956. doi: 10.1182/blood-2014-07-587709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–1961. doi: 10.1182/blood-2011-03-343061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–3714. doi: 10.1182/blood-2011-01-332676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pircher J, Czermak T, Ehrlich A, Eberle C, Gaitzsch E, Margraf A, Grommes J, Saha P, Titova A, Ishikawa-Ankerhold H, et al. Cathelicidins prime platelets to mediate arterial thrombosis and tissue inflammation. Nat Commun. 2018;9:1523. doi: 10.1038/s41467-018-03925-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184 [DOI] [PubMed] [Google Scholar]
- 61.von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Köllnberger M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Luo L, Braun OÖ, Westman J, Madhi R, Herwald H, Mörgelin M, Thorlacius H. Neutrophil extracellular trap-microparticle complexes enhance thrombin generation via the intrinsic pathway of coagulation in mice. Sci Rep. 2018;8:4020. doi: 10.1038/s41598-018-22156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977–1984. doi: 10.1161/ATVBAHA.114.304114 [DOI] [PubMed] [Google Scholar]
- 64.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9:1795–1803. doi: 10.1111/j.1538-7836.2011.04422.x [DOI] [PubMed] [Google Scholar]
- 65.Barranco-Medina S, Pozzi N, Vogt AD, Di Cera E. Histone H4 promotes prothrombin autoactivation. J Biol Chem. 2013;288:35749–35757. doi: 10.1074/jbc.M113.509786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang S, Qi H, Kan K, Chen J, Xie H, Guo X, Zhang L. Neutrophil extracellular traps promote hypercoagulability in patients with sepsis. Shock. 2017;47:132–139. doi: 10.1097/SHK.0000000000000741 [DOI] [PubMed] [Google Scholar]
- 67.Barbosa da Cruz D, Helms J, Aquino LR, Stiel L, Cougourdan L, Broussard C, Chafey P, Ries-Kautt M, Meziani F, Toti F, et al. DNA-bound elastase of neutrophil extracellular traps degrades plasminogen, reduces plasmin formation, and decreases fibrinolysis: proof of concept in septic shock plasma. FASEB J. 2019;33:14270–14280. doi: 10.1096/fj.201901363RRR [DOI] [PubMed] [Google Scholar]
- 68.Gould TJ, Vu TT, Stafford AR, Dwivedi DJ, Kim PY, Fox-Robichaud AE, Weitz JI, Liaw PC. Cell-Free DNA modulates clot structure and impairs fibrinolysis in sepsis. Arterioscler Thromb Vasc Biol. 2015;35:2544–2553. doi: 10.1161/ATVBAHA.115.306035 [DOI] [PubMed] [Google Scholar]
- 69.Varjú I, Longstaff C, Szabó L, Farkas ÁZ, Varga-Szabó VJ, Tanka-Salamon A, Machovich R, Kolev K. DNA, histones and neutrophil extracellular traps exert anti-fibrinolytic effects in a plasma environment. Thromb Haemost. 2015;113:1289–1298. doi: 10.1160/TH14-08-0669 [DOI] [PubMed] [Google Scholar]
- 70.Longstaff C, Varjú I, Sótonyi P, Szabó L, Krumrey M, Hoell A, Bóta A, Varga Z, Komorowicz E, Kolev K. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem. 2013;288:6946–6956. doi: 10.1074/jbc.M112.404301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heuberger DM, Franchini AG, Madon J, Schuepbach RA. Thrombin cleaves and activates the protease-activated receptor 2 dependent on thrombomodulin co-receptor availability. Thromb Res. 2019;177:91–101. doi: 10.1016/j.thromres.2019.02.032 [DOI] [PubMed] [Google Scholar]
- 72.Boknäs N, Faxälv L, Sanchez Centellas D, Wallstedt M, Ramström S, Grenegård M, Lindahl TL. Thrombin-induced platelet activation via PAR4: pivotal role for exosite II. Thromb Haemost. 2014;112:558–565. doi: 10.1160/TH13-12-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111:5271–5281. doi: 10.1182/blood-2008-01-078204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pendu R, Terraube V, Christophe OD, Gahmberg CG, de Groot PG, Lenting PJ, Denis CV. P-selectin glycoprotein ligand 1 and beta2-integrins cooperate in the adhesion of leukocytes to von Willebrand factor. Blood. 2006;108:3746–3752. doi: 10.1182/blood-2006-03-010322 [DOI] [PubMed] [Google Scholar]
- 75.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grässle S, Huck V, Pappelbaum KI, Gorzelanny C, Aponte-Santamaría C, Baldauf C, Gräter F, Schneppenheim R, Obser T, Schneider SW. von Willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arterioscler Thromb Vasc Biol. 2014;34:1382–1389. doi: 10.1161/ATVBAHA.113.303016 [DOI] [PubMed] [Google Scholar]
- 77.Kolaczkowska E, Jenne CN, Surewaard BG, Thanabalasuriar A, Lee WY, Sanz MJ, Mowen K, Opdenakker G, Kubes P. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. 2015;6:6673. doi: 10.1038/ncomms7673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Michels A, Albánez S, Mewburn J, Nesbitt K, Gould TJ, Liaw PC, James PD, Swystun LL, Lillicrap D. Histones link inflammation and thrombosis through the induction of Weibel-Palade body exocytosis. J Thromb Haemost. 2016;14:2274–2286. doi: 10.1111/jth.13493 [DOI] [PubMed] [Google Scholar]
- 79.Peetermans M, Meyers S, Liesenborghs L, Vanhoorelbeke K, De Meyer SF, Vandenbriele C, Lox M, Hoylaerts MF, Martinod K, Jacquemin M, et al. Von Willebrand factor and ADAMTS13 impact on the outcome of Staphylococcus aureus sepsis. J Thromb Haemost. 2020;18:722–731. doi: 10.1111/jth.14686 [DOI] [PubMed] [Google Scholar]
- 80.Sorvillo N, Mizurini DM, Coxon C, Martinod K, Tilvawala R, Cherpokova D, Salinger AJ, Seward RJ, Staudinger C, Weerapana E, et al. Plasma peptidylarginine deiminase IV promotes VWF-Platelet string formation and accelerates thrombosis after vessel injury. Circ Res. 2019;125:507–519. doi: 10.1161/CIRCRESAHA.118.314571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vanassche T, Kauskot A, Verhaegen J, Peetermans WE, van Ryn J, Schneewind O, Hoylaerts MF, Verhamme P. Fibrin formation by staphylothrombin facilitates Staphylococcus aureus-induced platelet aggregation. Thromb Haemost. 2012;107:1107–1121. doi: 10.1160/TH11-12-0891 [DOI] [PubMed] [Google Scholar]
- 82.McAdow M, Kim HK, Dedent AC, Hendrickx AP, Schneewind O, Missiakas DM. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 2011;7:e1002307. doi: 10.1371/journal.ppat.1002307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vanassche T, Verhaegen J, Peetermans WE, VAN Ryn J, Cheng A, Schneewind O, Hoylaerts MF, Verhamme P. Inhibition of staphylothrombin by dabigatran reduces Staphylococcus aureus virulence. J Thromb Haemost. 2011;9:2436–2446. doi: 10.1111/j.1538-7836.2011.04529.x [DOI] [PubMed] [Google Scholar]
- 84.Malachowa N, Kobayashi SD, Porter AR, Braughton KR, Scott DP, Gardner DJ, Missiakas DM, Schneewind O, DeLeo FR. Contribution of Staphylococcus aureus coagulases and clumping factor A to abscess formation in a rabbit model of skin and soft tissue infection. PLoS One. 2016;11:e0158293. doi: 10.1371/journal.pone.0158293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vanassche T, Peetermans M, Van Aelst LN, Peetermans WE, Verhaegen J, Missiakas DM, Schneewind O, Hoylaerts MF, Verhamme P. The role of staphylothrombin-mediated fibrin deposition in catheter-related Staphylococcus aureus infections. J Infect Dis. 2013;208:92–100. doi: 10.1093/infdis/jit130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Panizzi P, Krohn-Grimberghe M, Keliher E, Ye YX, Grune J, Frodermann V, Sun Y, Muse CG, Bushey K, Iwamoto Y, et al. Multimodal imaging of bacterial-host interface in mice and piglets with Staphylococcus aureus endocarditis. Sci Transl Med. 2020;12:eaay2104. doi: 10.1126/scitranslmed.aay2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lerche CJ, Christophersen LJ, Goetze JP, Nielsen PR, Thomsen K, Enevold C, Høiby N, Jensen PØ, Bundgaard H, Moser C. Adjunctive dabigatran therapy improves outcome of experimental left-sided Staphylococcus aureus endocarditis. PLoS One. 2019;14:e0215333. doi: 10.1371/journal.pone.0215333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. 2014;27:870–926. doi: 10.1128/CMR.00109-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Le KY, Sohail MR, Friedman PA, Uslan DZ, Cha SS, Hayes DL, Wilson WR, Steckelberg JM, Baddour LM; Mayo Cardiovascular Infections Study Group. Clinical features and outcomes of cardiovascular implantable electronic device infections due to staphylococcal species. Am J Cardiol. 2012;110:1143–1149. doi: 10.1016/j.amjcard.2012.05.052 [DOI] [PubMed] [Google Scholar]
- 90.Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, Corey GR, Olaison L, Eykyn S, Hoen B, Abrutyn E, et al. ; International Collaboration on Endocarditis Merged Database Study Group. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis. 2005;41:507–514. doi: 10.1086/431979 [DOI] [PubMed] [Google Scholar]
- 91.Liesenborghs L, Peetermans M, Claes J, Veloso TR, Vandenbriele C, Criel M, Lox M, Peetermans WE, Heilbronner S, de Groot PG, et al. Shear-Resistant binding to von Willebrand factor allows Staphylococcus lugdunensis to adhere to the cardiac valves and initiate endocarditis. J Infect Dis. 2016;213:1148–1156. doi: 10.1093/infdis/jiv773 [DOI] [PubMed] [Google Scholar]
- 92.Björnsdottir H, Dahlstrand Rudin A, Klose FP, Elmwall J, Welin A, Stylianou M, Christenson K, Urban CF, Forsman H, Dahlgren C, et al. Phenol-Soluble modulin α peptide toxins from aggressive Staphylococcus aureus induce rapid formation of neutrophil extracellular traps through a reactive oxygen species-independent pathway. Front Immunol. 2017;8:257. doi: 10.3389/fimmu.2017.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675 [DOI] [PubMed] [Google Scholar]
- 94.Malachowa N, Kobayashi SD, Freedman B, Dorward DW, DeLeo FR. Staphylococcus aureus leukotoxin GH promotes formation of neutrophil extracellular traps. J Immunol. 2013;191:6022–6029. doi: 10.4049/jimmunol.1301821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Azzouz L, Cherry A, Riedl M, Khan M, Pluthero FG, Kahr WHA, Palaniyar N, Licht C. Relative antibacterial functions of complement and NETs: NETs trap and complement effectively kills bacteria. Mol Immunol. 2018;97:71–81. doi: 10.1016/j.molimm.2018.02.019 [DOI] [PubMed] [Google Scholar]
- 96.Sultan AR, Hoppenbrouwers T, Lemmens-den Toom NA, Snijders SV, van Neck JW, Verbon A, de Maat MPM, van Wamel WJB. During the early stages of Staphylococcus aureus biofilm formation, induced neutrophil extracellular traps are degraded by autologous thermonuclease. Infect Immun. 2019;87:e00605–e00619. doi: 10.1128/IAI.00605-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhattacharya M, Berends ETM, Chan R, Schwab E, Roy S, Sen CK, Torres VJ, Wozniak DJ. Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proc Natl Acad Sci USA. 2018;115:7416–7421. doi: 10.1073/pnas.1721949115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoppenbrouwers T, Sultan AR, Abraham TE, Lemmens-den Toom NA, Hansenová Maňásková S, van Cappellen WA, Houtsmuller AB, van Wamel WJB, de Maat MPM, van Neck JW. Staphylococcal Protein A Is a key factor in neutrophil extracellular traps formation. Front Immunol. 2018;9:165. doi: 10.3389/fimmu.2018.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386–1393. doi: 10.1038/nm.2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Haas CJ, Weeterings C, Vughs MM, de Groot PG, Van Strijp JA, Lisman T. Staphylococcal superantigen-like 5 activates platelets and supports platelet adhesion under flow conditions, which involves glycoprotein Ibalpha and alpha IIb beta 3. J Thromb Haemost. 2009;7:1867–1874. doi: 10.1111/j.1538-7836.2009.03564.x [DOI] [PubMed] [Google Scholar]
- 101.Fitzgerald JR, Loughman A, Keane F, Brennan M, Knobel M, Higgins J, Visai L, Speziale P, Cox D, Foster TJ. Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcgammaRIIa receptor. Mol Microbiol. 2006;59:212–230. doi: 10.1111/j.1365-2958.2005.04922.x [DOI] [PubMed] [Google Scholar]
- 102.Loughman A, Fitzgerald JR, Brennan MP, Higgins J, Downer R, Cox D, Foster TJ. Roles for fibrinogen, immunoglobulin and complement in platelet activation promoted by Staphylococcus aureus clumping factor A. Mol Microbiol. 2005;57:804–818. doi: 10.1111/j.1365-2958.2005.04731.x [DOI] [PubMed] [Google Scholar]
- 103.Miajlovic H, Zapotoczna M, Geoghegan JA, Kerrigan SW, Speziale P, Foster TJ. Direct interaction of iron-regulated surface determinant IsdB of Staphylococcus aureus with the GPIIb/IIIa receptor on platelets. Microbiology (Reading). 2010;156:920–928. doi: 10.1099/mic.0.036673-0 [DOI] [PubMed] [Google Scholar]
- 104.O’Brien L, Kerrigan SW, Kaw G, Hogan M, Penadés J, Litt D, Fitzgerald DJ, Foster TJ, Cox D. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and Protein A. Mol Microbiol. 2002;44:1033–1044. doi: 10.1046/j.1365-2958.2002.02935.x [DOI] [PubMed] [Google Scholar]
- 105.Palankar R, Binsker U, Haracska B, Wesche J, Greinacher A, Hammerschmidt S. Interaction between the Staphylococcus aureus extracellular adherence protein Eap and its subdomains with platelets. Int J Med Microbiol. 2018;308:683–691. doi: 10.1016/j.ijmm.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 106.Niemann S, Bertling A, Brodde MF, Fender AC, Van de Vyver H, Hussain M, Holzinger D, Reinhardt D, Peters G, Heilmann C, et al. Panton-Valentine Leukocidin associated with S. aureus osteomyelitis activates platelets via neutrophil secretion products. Sci Rep. 2018;8:2185. doi: 10.1038/s41598-018-20582-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ortines RV, Liu H, Cheng LI, Cohen TS, Lawlor H, Gami A, Wang Y, Dillen CA, Archer NK, Miller RJ, et al. Neutralizing Alpha-Toxin accelerates healing of Staphylococcus Aureus-Infected wounds in nondiabetic and diabetic mice. Antimicrob Agents Chemother. 2018;62:e02288–e02217. doi: 10.1128/AAC.02288-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Los FC, Randis TM, Aroian RV, Ratner AJ. Role of pore-forming toxins in bacterial infectious diseases. Microbiol Mol Biol Rev. 2013;77:173–207. doi: 10.1128/MMBR.00052-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eisenbeis J, Saffarzadeh M, Peisker H, Jung P, Thewes N, Preissner KT, Herrmann M, Molle V, Geisbrecht BV, Jacobs K, et al. The Staphylococcus aureus extracellular adherence protein eap is a DNA binding protein capable of blocking neutrophil extracellular trap formation. Front Cell Infect Microbiol. 2018;8:235. doi: 10.3389/fcimb.2018.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kavanaugh JS, Flack CE, Lister J, Ricker EB, Ibberson CB, Jenul C, Moormeier DE, Delmain EA, Bayles KW, Horswill AR. Identification of extracellular DNA-Binding proteins in the biofilm matrix. mBio. 2019;10:e01137–e01119. doi: 10.1128/mBio.01137-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Herdendorf TJ, Geisbrecht BV. Staphylococcus aureus evasion proteins EapH1 and EapH2: residue-level investigation of an alternative binding motif for human neutrophil elastase. Arch Biochem Biophys. 2019;676:108140. doi: 10.1016/j.abb.2019.108140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Herdendorf TJ, Geisbrecht BV. Investigation of human neutrophil elastase inhibition by Staphylococcus aureus EapH1: the key role played by arginine 89. Biochemistry. 2018;57:6888–6896. doi: 10.1021/acs.biochem.8b01134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stapels DA, Ramyar KX, Bischoff M, von Köckritz-Blickwede M, Milder FJ, Ruyken M, Eisenbeis J, McWhorter WJ, Herrmann M, van Kessel KP, et al. Staphylococcus aureus secretes a unique class of neutrophil serine protease inhibitors. Proc Natl Acad Sci USA. 2014;111:13187–13192. doi: 10.1073/pnas.1407616111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stapels DAC, Woehl JL, Milder FJ, Tromp AT, van Batenburg AA, de Graaf WC, Broll SC, White NM, Rooijakkers SHM, Geisbrecht BV. Evidence for multiple modes of neutrophil serine protease recognition by the EAP family of Staphylococcal innate immune evasion proteins. Protein Sci. 2018;27:509–522. doi: 10.1002/pro.3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dubois AV, Gauthier A, Bréa D, Varaigne F, Diot P, Gauthier F, Attucci S. Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. Am J Respir Cell Mol Biol. 2012;47:80–86. doi: 10.1165/rcmb.2011-0380OC [DOI] [PubMed] [Google Scholar]
- 116.Cole AM, Shi J, Ceccarelli A, Kim YH, Park A, Ganz T. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood. 2001;97:297–304. doi: 10.1182/blood.v97.1.297 [DOI] [PubMed] [Google Scholar]
- 117.Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, Shapiro SD. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med. 1998;4:615–618. doi: 10.1038/nm0598-615 [DOI] [PubMed] [Google Scholar]
- 118.Zinkernagel AS, Timmer AM, Pence MA, Locke JB, Buchanan JT, Turner CE, Mishalian I, Sriskandan S, Hanski E, Nizet V. The IL-8 protease SpyCEP/ScpC of group A Streptococcus promotes resistance to neutrophil killing. Cell Host Microbe. 2008;4:170–178. doi: 10.1016/j.chom.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Jong NWM, Ramyar KX, Guerra FE, Nijland R, Fevre C, Voyich JM, McCarthy AJ, Garcia BL, van Kessel KPM, van Strijp JAG, et al. Immune evasion by a staphylococcal inhibitor of myeloperoxidase. Proc Natl Acad Sci U S A. 2017;114:9439–9444. doi: 10.1073/pnas.1707032114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Sorge NM, Beasley FC, Gusarov I, Gonzalez DJ, von Köckritz-Blickwede M, Anik S, Borkowski AW, Dorrestein PC, Nudler E, Nizet V. Methicillin-resistant Staphylococcus aureus bacterial nitric-oxide synthase affects antibiotic sensitivity and skin abscess development. J Biol Chem. 2013;288:6417–6426. doi: 10.1074/jbc.M112.448738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berends ET, Horswill AR, Haste NM, Monestier M, Nizet V, von Köckritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2:576–586. doi: 10.1159/000319909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, Smeltzer MS, Bayles KW, Horswill AR. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS One. 2011;6:e26714. doi: 10.1371/journal.pone.0026714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, Tsang LH, Smeltzer MS, Horswill AR, Bayles KW. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One. 2009;4:e5822. doi: 10.1371/journal.pone.0005822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thammavongsa V, Missiakas DM, Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 2013;342:863–866. doi: 10.1126/science.1242255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kiedrowski MR, Crosby HA, Hernandez FJ, Malone CL, McNamara JO, 2nd, Horswill AR. Staphylococcus aureus Nuc2 is a functional, surface-attached extracellular nuclease. PLoS One. 2014;9:e95574. doi: 10.1371/journal.pone.0095574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Herzog S, Dach F, de Buhr N, Niemann S, Schlagowski J, Chaves-Moreno D, Neumann C, Goretzko J, Schwierzeck V, Mellmann A, et al. High nuclease activity of long persisting Staphylococcus aureus isolates within the airways of cystic fibrosis patients protects against NET-Mediated killing. Front Immunol. 2019;10:2552. doi: 10.3389/fimmu.2019.02552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Winstel V, Missiakas D, Schneewind O. Staphylococcus aureus targets the purine salvage pathway to kill phagocytes. Proc Natl Acad Sci USA. 2018;115:6846–6851. doi: 10.1073/pnas.1805622115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Winstel V, Schneewind O, Missiakas D. Staphylococcus aureus exploits the host apoptotic pathway to persist during infection. mBio. 2019;10:e02270–e02219. doi: 10.1128/mBio.02270-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pietrocola G, Nobile G, Alfeo MJ, Foster TJ, Geoghegan JA, De Filippis V, Speziale P. Fibronectin-binding protein B (FnBPB) from Staphylococcus aureus protects against the antimicrobial activity of histones. J Biol Chem. 2019;294:3588–3602. doi: 10.1074/jbc.RA118.005707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kwiecinski JM, Kratofil RM, Parlet CP, Surewaard BGJ, Kubes P, Horswill AR. Staphylococcus aureus uses the ArlRS and MgrA cascade to regulate immune evasion during skin infection. Cell Rep. 2021;36:109462. doi: 10.1016/j.celrep.2021.109462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liesenborghs L, Meyers S, Vanassche T, Verhamme P. Coagulation: at the heart of infective endocarditis. J Thromb Haemost. 2020;18:995–1008. doi: 10.1111/jth.14736 [DOI] [PubMed] [Google Scholar]
- 132.Claes J, Vanassche T, Peetermans M, Liesenborghs L, Vandenbriele C, Vanhoorelbeke K, Missiakas D, Schneewind O, Hoylaerts MF, Heying R, et al. Adhesion of Staphylococcus aureus to the vessel wall under flow is mediated by von Willebrand factor-binding protein. Blood. 2014;124:1669–1676. doi: 10.1182/blood-2014-02-558890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Augustin P, Alsalih G, Launey Y, Delbosc S, Louedec L, Ollivier V, Chau F, Montravers P, Duval X, Michel JB, et al. Predominant role of host proteases in myocardial damage associated with infectious endocarditis induced by Enterococcus faecalis in a rat model. Infect Immun. 2013;81:1721–1729. doi: 10.1128/IAI.00775-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liesenborghs L, Meyers S, Lox M, Criel M, Claes J, Peetermans M, Trenson S, Vande Velde G, Vanden Berghe P, Baatsen P, et al. Staphylococcus aureus endocarditis: distinct mechanisms of bacterial adhesion to damaged and inflamed heart valves. Eur Heart J. 2019;40:3248–3259. doi: 10.1093/eurheartj/ehz175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meegan JE, Yang X, Beard RS, Jr, Jannaway M, Chatterjee V, Taylor-Clark TE, Yuan SY. Citrullinated histone 3 causes endothelial barrier dysfunction. Biochem Biophys Res Commun. 2018;503:1498–1502. doi: 10.1016/j.bbrc.2018.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 138.Sakurai K, Miyashita T, Okazaki M, Yamaguchi T, Ohbatake Y, Nakanuma S, Okamoto K, Sakai S, Kinoshita J, Makino I, et al. Role for Neutrophil Extracellular Traps (NETs) and platelet aggregation in early sepsis-induced hepatic dysfunction. In Vivo. 2017;31:1051–1058. doi: 10.21873/invivo.11169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ducroux C, Di Meglio L, Loyau S, Delbosc S, Boisseau W, Deschildre C, Ben Maacha M, Blanc R, Redjem H, Ciccio G, et al. Thrombus Neutrophil extracellular traps content impair tPA-Induced Thrombolysis in acute ischemic stroke. Stroke. 2018;49:754–757. doi: 10.1161/STROKEAHA.117.019896 [DOI] [PubMed] [Google Scholar]
- 140.Buiting AG, Thompson J, Emeis JJ, Mattie H, Brommer EJ, van Furth R. Effects of tissue-type plasminogen activator (t-PA) on Streptococcus sanguis-infected endocardial vegetations in vitro. J Antimicrob Chemother. 1988;21:609–620. doi: 10.1093/jac/21.5.609 [DOI] [PubMed] [Google Scholar]
- 141.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, et al. ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- 142.Veloso TR, Que YA, Chaouch A, Giddey M, Vouillamoz J, Rousson V, Moreillon P, Entenza JM. Prophylaxis of experimental endocarditis with antiplatelet and antithrombin agents: a role for long-term prevention of infective endocarditis in humans? J Infect Dis. 2015;211:72–79. doi: 10.1093/infdis/jiu426 [DOI] [PubMed] [Google Scholar]
- 143.Veloso TR, Oechslin F, Que YA, Moreillon P, Entenza JM, Mancini S. Aspirin plus ticlopidine prevented experimental endocarditis due to Enterococcus faecalis and Streptococcus gallolyticus. Pathog Dis. 2015;73:ftv060. doi: 10.1093/femspd/ftv060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taha TH, Durrant SS, Mazeika PK, Nihoyannopoulos P, Oakley CM. Aspirin to prevent growth of vegetations and cerebral emboli in infective endocarditis. J Intern Med. 1992;231:543–546. doi: 10.1111/j.1365-2796.1992.tb00971.x [DOI] [PubMed] [Google Scholar]
- 145.Zhao BQ, Chauhan AK, Canault M, Patten IS, Yang JJ, Dockal M, Scheiflinger F, Wagner DD. von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329–3334. doi: 10.1182/blood-2009-03-213264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol. 2014;12:426–437. doi: 10.1038/nrmicro3269 [DOI] [PubMed] [Google Scholar]
- 147.Carestia A, Davis RP, Davis L, Jenne CN. Inhibition of immunothrombosis does not affect pathogen capture and does not promote bacterial dissemination in a mouse model of sepsis. Platelets. 2020;31:925–931. doi: 10.1080/09537104.2019.1704711 [DOI] [PubMed] [Google Scholar]
- 148.Badarau A, Rouha H, Malafa S, Battles MB, Walker L, Nielson N, Dolezilkova I, Teubenbacher A, Banerjee S, Maierhofer B, et al. Context matters: The importance of dimerization-induced conformation of the LukGH leukocidin of Staphylococcus aureus for the generation of neutralizing antibodies. MAbs. 2016;8:1347–1360. doi: 10.1080/19420862.2016.1215791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen X, Sun Y, Missiakas D, Schneewind O. Staphylococcus aureus decolonization of mice with monoclonal antibody neutralizing Protein A. J Infect Dis. 2019;219:884–888. doi: 10.1093/infdis/jiy597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thomsen IP. Antibody-based intervention against the pore-forming toxins of Staphylococcus aureus. Virulence. 2018;9:645–647. doi: 10.1080/21505594.2017.1421831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fattom A, Matalon A, Buerkert J, Taylor K, Damaso S, Boutriau D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum Vaccin Immunother. 2015;11:632–641. doi: 10.4161/hv.34414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309:1368–1378. doi: 10.1001/jama.2013.3010 [DOI] [PubMed] [Google Scholar]
- 153.Schneewind O, Missiakas D. Sortases, surface proteins, and their roles in Staphylococcus aureus disease and vaccine development. Microbiol Spectr. 2019. doi: 10.1128/microbiolspec.PSIB-0004-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.François B, Mercier E, Gonzalez C, Asehnoune K, Nseir S, Fiancette M, Desachy A, Plantefève G, Meziani F, de Lame PA, et al. ; MASTER 1 Study Group. Safety and tolerability of a single administration of AR-301, a human monoclonal antibody, in ICU patients with severe pneumonia caused by Staphylococcus aureus: first-in-human trial. Intensive Care Med. 2018;44:1787–1796. doi: 10.1007/s00134-018-5229-2 [DOI] [PubMed] [Google Scholar]
- 155.Sun J, Uchiyama S, Olson J, Morodomi Y, Cornax I, Ando N, Kohno Y, Kyaw MMT, Aguilar B, Haste NM, et al. Repurposed drugs block toxin-driven platelet clearance by the hepatic Ashwell-Morell receptor to clear Staphylococcus aureus bacteremia. Sci Transl Med. 2021;13:eabd6737. doi: 10.1126/scitranslmed.abd6737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ulloa ER, Uchiyama S, Gillespie R, Nizet V, Sakoulas G. Ticagrelor increases platelet-mediated Staphylococcus aureus killing, resulting in clearance of bacteremia. J Infect Dis. 2021;224:1566–1569. doi: 10.1093/infdis/jiab146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lancellotti P, Musumeci L, Jacques N, Servais L, Goffin E, Pirotte B, Oury C. Antibacterial activity of ticagrelor in conventional antiplatelet dosages against antibiotic-resistant gram-positive bacteria. JAMA Cardiol. 2019;4:596–599. doi: 10.1001/jamacardio.2019.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Neumann A, Völlger L, Berends ET, Molhoek EM, Stapels DA, Midon M, Friães A, Pingoud A, Rooijakkers SH, Gallo RL, et al. Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. J Innate Immun. 2014;6:860–868. doi: 10.1159/000363699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331:637–642. doi: 10.1056/NEJM199409083311003 [DOI] [PubMed] [Google Scholar]
- 160.Czaikoski PG, Mota JM, Nascimento DC, Sônego F, Castanheira FV, Melo PH, Scortegagna GT, Silva RL, Barroso-Sousa R, Souto FO, et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS One. 2016;11:e0148142. doi: 10.1371/journal.pone.0148142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lefrançais E, Mallavia B, Zhuo H, Calfee CS, Looney MR. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight. 2018;3:98178. doi: 10.1172/jci.insight.98178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dengler V, Foulston L, DeFrancesco AS, Losick R. An electrostatic net model for the role of extracellular DNA in biofilm formation by Staphylococcus aureus. J Bacteriol. 2015;197:3779–3787. doi: 10.1128/JB.00726-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mai SH, Khan M, Dwivedi DJ, Ross CA, Zhou J, Gould TJ, Gross PL, Weitz JI, Fox-Robichaud AE, Liaw PC; Canadian Critical Care Translational Biology Group. Delayed but not early treatment with DNase reduces organ damage and improves outcome in a murine model of sepsis. Shock. 2015;44:166–172. doi: 10.1097/SHK.0000000000000396 [DOI] [PubMed] [Google Scholar]
- 164.Okeke EB, Louttit C, Fry C, Najafabadi AH, Han K, Nemzek J, Moon JJ. Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials. 2020;238:119836. doi: 10.1016/j.biomaterials.2020.119836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gößwein S, Lindemann A, Mahajan A, Maueröder C, Martini E, Patankar J, Schett G, Becker C, Wirtz S, Naumann-Bartsch N, et al. Citrullination licenses calpain to decondense nuclei in neutrophil extracellular trap formation. Front Immunol. 2019;10:2481. doi: 10.3389/fimmu.2019.02481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thiam HR, Wong SL, Qiu R, Kittisopikul M, Vahabikashi A, Goldman AE, Goldman RD, Wagner DD, Waterman CM. NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proc Natl Acad Sci USA. 2020;117:7326–7337. doi: 10.1073/pnas.1909546117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Biron BM, Chung CS, O’Brien XM, Chen Y, Reichner JS, Ayala A. Cl-Amidine prevents Histone 3 citrullination and neutrophil extracellular trap formation, and improves survival in a murine sepsis model. J Innate Immun. 2017;9:22–32. doi: 10.1159/000448808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bitschar K, Staudenmaier L, Klink L, Focken J, Sauer B, Fehrenbacher B, Herster F, Bittner Z, Bleul L, Schaller M, et al. Staphylococcus aureus skin colonization is enhanced by the interaction of neutrophil extracellular traps with keratinocytes. J Invest Dermatol. 2020;140:1054–1065.e4. doi: 10.1016/j.jid.2019.10.017 [DOI] [PubMed] [Google Scholar]
- 170.Hosoda H, Nakamura K, Hu Z, Tamura H, Reich J, Kuwahara-Arai K, Iba T, Tabe Y, Nagaoaka I. Antimicrobial cathelicidin peptide LL-37 induces NET formation and suppresses the inflammatory response in a mouse septic model. Mol Med Rep. 2017;16:5618–5626. doi: 10.3892/mmr.2017.7267 [DOI] [PubMed] [Google Scholar]