Abstract
Introduction
The CoronaVac vaccine is widely used in Thailand to combat the coronavirus disease 2019 (COVID-19) pandemic. The limited immunogenicity of this vaccine is a concern, especially because of expanding delta variant outbreaks. A third boost may enhance antiviral immune responses.
Methods
This non-inferiority randomized controlled trial evaluated the immunogenicity and safety of an intradermal (ID) fractional third dose of AZD1222 vaccine compared with those of a standard intramuscular (IM) third dose. Participants were enrolled from August 9, 2021 to August 13, 2021 at Chulabhorn Hospital, Bangkok, Thailand. The eligibility criteria were age 18 years or older and prior two-dose Coronavac vaccination completed at least 2 months previously. Participants were randomly assigned to one of three groups by block randomization: (i) standard dose by IM administration (IM), (ii) 20% of the standard dose ID (ID1), or (iii) 40% of the standard dose ID (ID2). The primary endpoint was the geometric mean ratio of anti-receptor binding domain antibody in the ID1/ID2 vs. the IM groups 14 days post-vaccination.
Results
A total of 125 participants were randomized (IM, n = 41; ID1, n = 41; and ID2, n = 43). One participant was lost to follow-up by day 14 post-vaccination in the ID1 group. The geometric mean ratio (95% confidence interval) of anti-receptor binding domain antibody was 0.94 (0.80–1.09) in the ID1 group and 1.28 (0.95–1.74) in the ID2 group. Immunogenicity in both ID groups met the non-inferiority criteria. Local adverse events were more common in the ID groups than in the IM group but were mostly mild to moderate in severity.
Conclusion
An ID fractional third dose of AZD1222 was non-inferior to a standard IM third dose among individuals previously vaccinated with CoronaVac. Adverse events associated with the ID fractional third dose included mild to moderate local site reactions. This vaccination strategy may help conserve vaccine supply.
Keywords: COVID-19, SARS-CoV-2, Intradermal route, Fractional dose, Boost, Third dose, AZD1222, ChAdOx1 nCoV-19, Immunogenicity, Safety
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic that began in December 2019 continues to represent a major public health crisis [1]. Because of the lack of effective therapies [2], vaccination is crucial to combat the pandemic [3]. All three arms of adaptive immunity (antibody responses, CD4 + T cell responses, and CD8 + T cell responses) are required to prevent and control the infection [4]. CoronaVac, an inactivated vaccine developed by Sinovac Biotech (Beijing, China) [5], is widely used in Thailand. However, the immunogenicity and efficacy of CoronaVac are uncertain compared with those of other vaccine platforms [6] and may be inadequate to control the spread of variants of concern [7]. A third dose of vaccine may result in improved immunogenicity and efficacy [8]. However, vaccine supply is limited worldwide, especially in developing countries [9]. Studies of influenza vaccination suggested that an intradermal (ID) fractional dose can elicit similar immune responses compared with those of the standard intramuscular (IM) dose despite the lower volume of vaccine administered. The seroconversion rate following vaccination was similar for individuals who received 3-g, 6-g, 7.5-g, and 9-g fractional ID doses of influenza vaccines compared with that for those who received the standard 15-g IM dose [10]. This strategy is enabled by the abundance of antigen-presenting cells, including dendritic cells and Langerhans cells, in the dermis layer of the skin. These cells can deliver vaccine antigens directly to lymph nodes and effectively stimulate immune responses [11]. In the setting of the ongoing COVID-19 pandemic and a short vaccine supply, fractional ID dosing can enable dose sparing and thus prevent morbidity and mortality in a larger number of individuals [12]. The aim of this study was to assess whether an ID fractional third dose of ChAdOx1 nCoV-19/AZD1222, an adenoviral vector vaccine, could elicit non-inferior immune responses compared with a standard IM third dose in participants who had previously received two doses of CoronaVac.
2. Materials & methods
2.1. Study design and participants
This was a single-center, non-inferiority, randomized controlled trial designed to evaluate the immunogenicity and safety of an ID fractional third dose of COVID-19 vaccine at Chulabhorn Hospital, Bangkok, Thailand. Participants were enrolled between August 9, 2021, and August 13, 2021. This report is part of our study that aimed to evaluate the safety and immunogenicity of intradermal sparing dose as the third boost dose of the available COVID-19 vaccines in Thailand, including AZD1222, BBIBP-CorV, BNT162b2, and the mRNA1273 vaccines. The study groups of participants who completed 2 doses of CoronaVac and were boosted with AZD1222, BBIBP-CorV, and BNT162b2 vaccines were completed. The rest of the study groups in which participants completed 2 doses of the AZD1222 vaccine were recruiting the participants. The inclusion criteria were age 18 years or older and prior two-dose Coronavac vaccination completed at least 2 months prior to study entry. The exclusion criteria were pregnancy, lactation, concurrent active underlying disease, receipt of any vaccine within 14 days of enrolment, and fever or respiratory tract infection within 14 days of enrolment. Written informed consent was obtained from each participant prior to enrolment. The study protocol, case records form, and consent form were reviewed and approved by the Ethics Committee for Human Research, Chulabhorn Research Institute (reference number: 094/2564). The study was registered with thaiclinicaltrials.org (TCTR20210731003).
2.2. Procedures
The main aim of the study was to assess the immunogenicity and safety of AZD1222 administered as two ID fractional third doses [20% of the standard IM dose (ID1 group) or 40% of the standard IM dose (ID2 group)] compared with those of a standard IM third dose (IM group). A standard IM dose of AZD1222 is composed of 0.5 mL. For the accuracy, safety, and convenience of using the vaccine, the pharmacist prepared 0.5 mL of the AZD1222 vaccine for the intramuscular route and 0.1 mL for the intradermal route using the insulin syringe before injection. Thus, participants in the ID1 group received 0.1 mL ID at a single site in the arm or deltoid. Participants in the ID2 group received 0.1 mL ID at two different arms in the forearm or deltoid. Study personnel were trained to conduct ID injections using the Mantoux technique [13]. Participants in the IM group received 0.5 mL IM at a single site in the deltoid muscle.
Following enrollment, participants were randomly assigned to the three groups by block randomization with a block of size of twelve. All participants were tested for baseline anti-receptor-binding domain of SARS-CoV-2 spike protein level (anti-RBD antibody). Immunogenicity evaluations were scheduled 2 weeks post-vaccination. Humoral immune responses against the RBD of SARS-CoV-2 spike protein were assessed using both binding antibody and surrogate neutralizing antibody assays. Cell-mediated immunity (CMI) was assessed using an interferon (IFN)-γ release assay.
The AZD1222 vaccine used in this study was manufactured and vialed following Good Manufacturing Practices and was approved by the regulatory agency of Thailand. An insulin syringe was used for administration of the vaccine to all participants.
2.3. Safety
After 30 min of observation post-vaccination to monitor immediate adverse events (AEs), participants were allowed to go home. On days 1, 7, and 30 post-vaccination, participants were queried regarding AEs via an online questionnaire. Participants graded the severity of AEs as follows: mild, AE does not interfere with daily activity; moderate, AE interferes with daily activity; severe, AE limits daily activity; and life-threatening, AE requires emergency department visit or hospitalization.
2.4. Measurement of anti-RBD antibody levels
Serum levels of anti-RBD antibody were measured using the Elecsys Anti-SARS-CoV-2 S (Elecsys-S) kit (Roche Diagnostics, Mannheim, Germany), an automated electrochemiluminescence immunoassay. The Elecsys-S kit uses the double-antigen sandwich principle for the detection of anti-RBD antibodies. The measurement range was 0.4 to 2500 U/mL. The manufacturer’s cut-off value for a positive result was > 0.8 U/mL. Based on the international standard for anti-SARS-CoV-2 immunoglobulin titers developed by the World Health Organization [14], Elecsys-S U were converted to binding antibody units (BAU) using the equation: Elecsys-S U = 0.972 × BAU [15].
2.5. Measurement of surrogate neutralizing antibody levels
Neutralization is the crucial function of antibody against SARS-CoV-2. Levels of neutralizing antibodies are correlated with COVID-19 vaccine efficacy [6]. However, the gold standard for neutralizing antibody measurement is a time-consuming conventional method that requires live virus and biosafety level 3 laboratories. By contrast, surrogate neutralizing antibody tests can close this gap by using an automated process that can be conducted outside a biosafety level 3 laboratory. The correlation between surrogate neutralizing antibody assays and conventional neutralizing antibody assays is excellent [16]. The principle of surrogate neutralizing antibody measurement is measurement of the interaction between biotinylated recombinant human angiotensin-converting enzyme 2 and SARS-CoV-2 spike protein RBD, detected with horseradish peroxidase (HRP)-conjugated streptavidin. Anti-SARS-CoV-2 neutralizing antibodies can block this interaction and diminish the HRP signal. This study used the SARS-CoV-2 NeutraLISA kit for measurement of surrogate neutralizing antibodies. (EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany) The result of measurement was demonstrated in percent of inhibition (%IH). The manufacturer’s cut-off value for a positive result was 35 %IH.
2.6. Measurement of IFN-γ
IFN-γ is generally secreted by helper CD4 T cells and cytotoxic CD8 T cells [17], [18]. Thus, the level of this cytokine is a reliable surrogate of the magnitude of CMI [19]. This study used an IFN-γ ELISA kit (EUROIMMUN Medizinische Labordiagnostika AG) for quantitation of IFN-γ levels. The test measures IFN-γ released after incubating heparinized whole blood with the S1 domain of the SARS-CoV-2 spike protein for 20–24 h. The limit of detection is 3.88 mIU/mL. Higher IFN-γ reflects elevated CMI against SARS-CoV-2.
2.7. Sample size calculation
Sample size estimation was calculated using means of 2 samples in a non-inferiority fashion. Based on the work of Robert W. Frenck Jr. [20], the GMT of hemagglutination inhibition of influenza B vaccine were 55 (95 %CI, 49–62) in 3 µg ID group and 87 (95% CI, 78–97) in 15 µg IM group. Non-inferiority was defined as a lower limit of the two-side 95% confidence interval of geometric mean ratio > 0.67. Therefore, assuming a global significance level of 5% (α = 0.05), a power of 80%, and a 20% participant dropout rate, 40 participants were planned to be recruited in each group of the study.
2.8. Statistical analysis
Statistical analyses were performed using IBM SPSS version 26 and GraphPad Prism version 9. Values of p < 0.05 were considered statistically significant. Vaccine immunogenicity in each intradermal group (ID1 and ID2) was compared with immunogenicity in the IM group using the non-inferiority approach. The non-inferiority criteria were met if the lower bound of the 95% confidence interval (CI) of the geometric mean ratio was >0.67 [21]. If the outcome met the non-inferiority criteria, then superiority was tested. Summary statistics were presented as medians and interquartile ranges (IQRs), mean and standard deviations, geometric mean concentrations, and 95% CIs. Comparison of anti-RBD antibody level, percentage of neutralizing antibody, and interferon-gamma level between intradermal groups and intramuscular group used independent-sample t-tests.
2.9. Efficacy endpoints
The primary endpoint was the GMR of anti-RBD antibodies of participants in the ID1 and ID2 groups compared with that of those in the IM group 2 weeks after vaccination. The secondary endpoints were surrogate neutralizing antibody levels and IFN-γ levels 2 weeks after vaccination, persistence of humoral immune responses at 3 and 6 months, and safety profile within 7 days post-vaccination.
3. Results
3.1. Participants
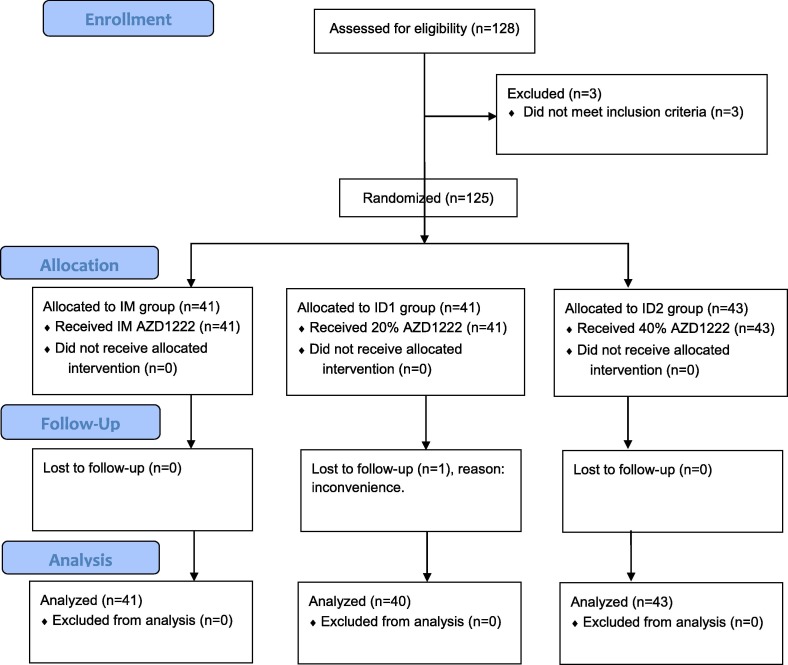
A total of 125 participants were enrolled in the study. The median age of participants was 45 years (IQR, 35–52 years) and 57% were male. Following randomization, 41 participants were assigned to the IM group, 41 were randomized to the ID1 group, and 43 were randomized to the ID2 group. One participant in the ID1 group was lost to follow-up by day 14 post-vaccination and did not participate in immunogenicity measurements. Participant flow is shown in Fig. 1 . Participants were enrolled from August 9, 2021 to August 13, 2021. Immunogenicity measurements were conducted between August 23, 2021, and August 30, 2021. The baseline demographic characteristics of each group are shown in Table 1 .
Fig. 1.
Participants' flow. IM gorup: standard dose intramuscular group. ID1: 20% of standard dose intradermal group. ID2: 40% of standard dose intradermal group.
Table 1.
Demographic characteristics of study participants.
| IM (N = 41) |
ID1 (N = 41) |
ID2 (N = 43) |
|
|---|---|---|---|
| Male, n (%) | 25 (61%) | 25 (61%) | 21 (49%) |
| Asian, n (%) | 41 (100) | 41 (100) | 43 (100) |
| Country of residence Thailand, n (%) | 41 (100) | 41 (100) | 43 (100) |
| Age at vaccination, years | |||
| Mean (SD) | 46.6 (27.4–66.0) | 42.3 (31.1–53.5) | 43.9 (33.0–54.8) |
| Median (IQR) | 45 (37–52) | 45 (31–52) | 47 (37–52) |
| Underlying diseases | |||
| Diabetes | 2 | 0 | 0 |
| Hypertension | 9 | 2 | 2 |
| CKD | 0 | 0 | 0 |
| Cirrhosis | 0 | 0 | 0 |
| Solid malignancy | 0 | 0 | 0 |
| Hematologic malignancy | 0 | 0 | 0 |
| Autoimmune disease | 0 | 0 | 0 |
| Baseline anti-SARS-CoV-2 spike protein level | |||
| GMC (95 %CI), BAU/mL | 48.8 (34.7–68.5) | 55.8 (40.1–77.7) | 85.1 (46.6–155.1) |
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) |
IM: intramuscular standard dose. ID1: 20% intradermal fractional dose. ID2: 20% intradermal fractional dose.
3.2. Outcomes
The primary outcome 2 weeks post-vaccination was analyzed in 41 participants in the IM group, 40 participants in the ID1 group, and 43 participants in the ID2 group. The geometric mean concentrations of anti-RBD antibodies in each group are shown in Table 2 and Fig. 2 . The primary outcome, GMR (95% CI), was 0.94 (0.80–1.09) in the ID1 group and 1.28 (0.95–1.74) in the ID2 group compared with that in the IM group. The secondary outcome, mean of surrogate neutralizing antibody (sNT) level (95% CI), was 98.9 %IH (98.0–99.9 %IH) in the IM group, 97.9 %IH (95.2–100 %IH) in the ID1 group (p = 0.014), and 99.1 %IH (97.6–100 %IH) in the ID2 group (p > 0.05), respectively (Fig. 3 ). The IFN-γ levels (95% CI) after incubation of whole blood samples for 24 h were demonstrated in Fig. 4 . The GMR of IFN-γ levels in the ID1 group was 1.34 (95 %CI, 0.86–2.20) and 1.58 (95 %CI, 1.05–2.39) in the ID2 group, respectively (Table 3 ).
Table 2.
Geometric mean ratio of anti-SARS-CoV-2 spike protein RBD antibodies 2 weeks after ChAdOx1/AZD1222 vaccination among participants who previously received two doses of CoronaVac.
| Group | N | Geometric mean concentration (95% CI) (BAU/mL) |
Geometric mean ratio (95% CI) |
|---|---|---|---|
| IM | 41 | 6414.62 (5107.56–8056.17) | Ref. |
| ID1 | 40 | 5669.49 (4560.30–7048.45) | 0.94 (0.80–1.09) |
| ID2 | 43 | 8,230.37 (6,697.04-10,114.8) | 1.28 (0.95-1.74) |
SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; RBD, receptor-binding domain; CI, confidence interval; IM, intramuscular standard dose; ID1, 20% intradermal fractional dose; ID2, 40% intradermal fractional dose.
Fig. 2.
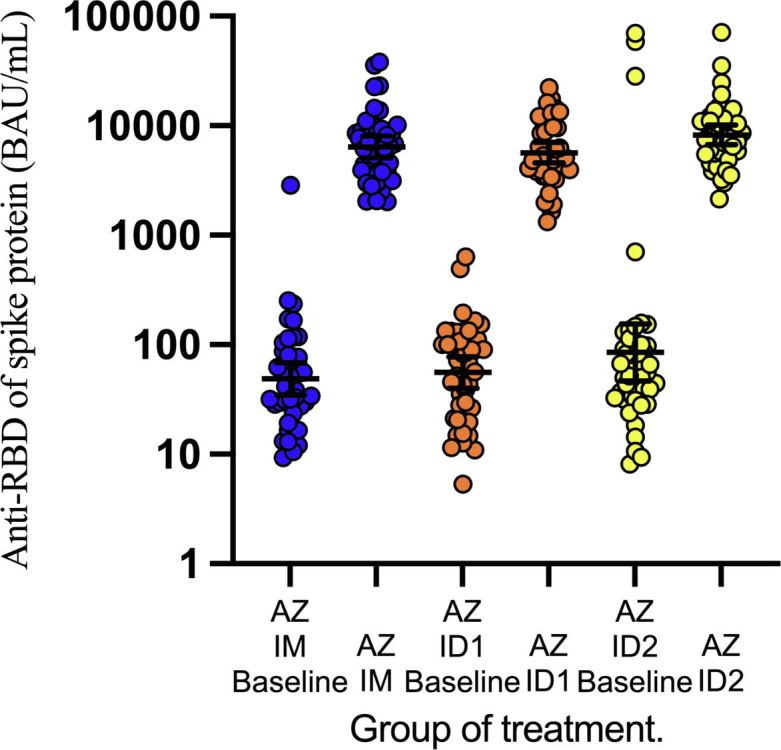
Anti-RBD antibodies level 2 weeks after AZD1222 vaccination. AZ: AZD1222 vaccine. RBD: receptor binding domain. BAU: binding antibody unit.
Fig. 3.
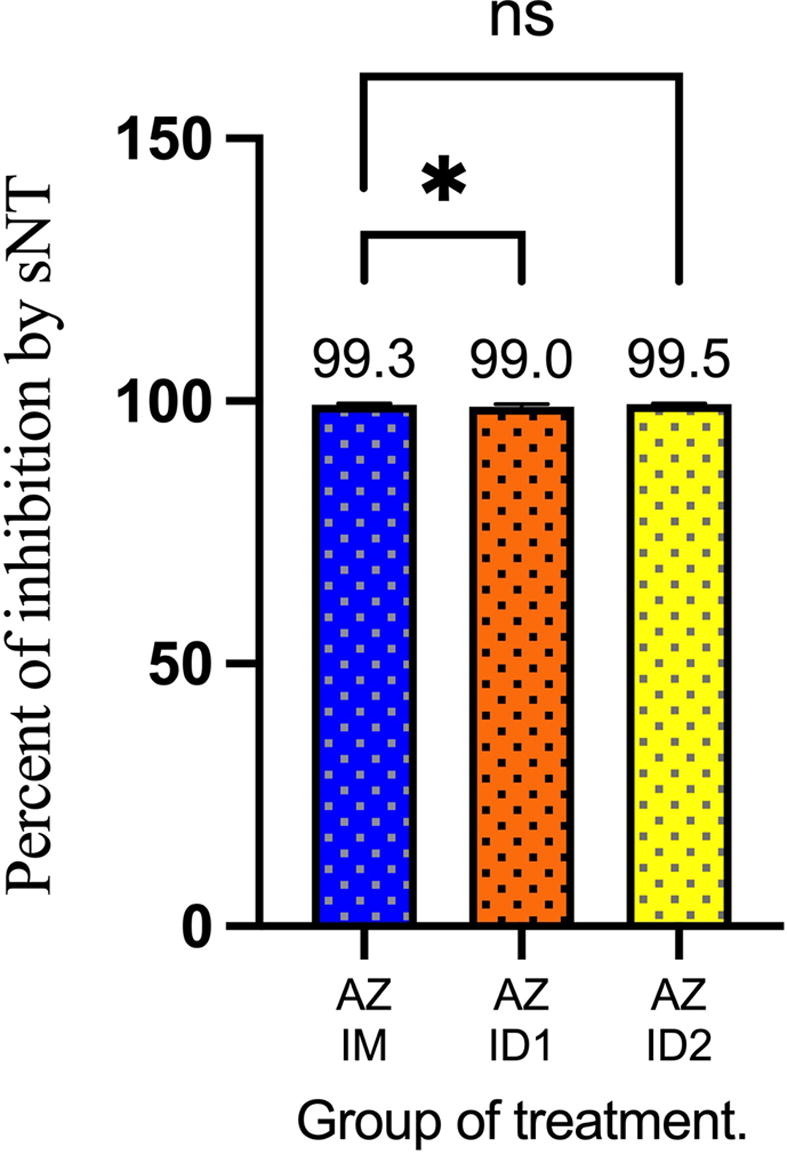
Surrogate neutralizing antibody test. *: <0.05, ns: non significant.
Fig. 4.
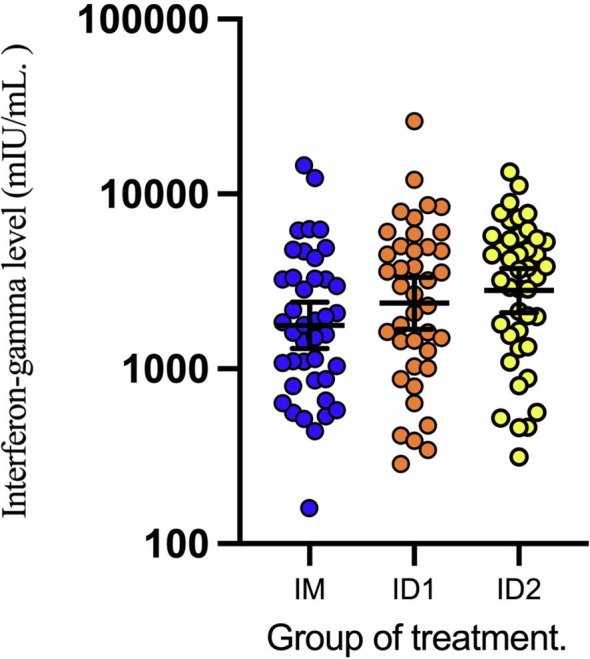
Interferon-gamma ELISA test 2 weeks after AZD1222 vaccination.
Table 3.
Geometric mean ratio of interferon-gamma level (mIU/mL) at 2-week after ChAdOx1/AZD1222 vaccination among participants who previously received two doses of CoronaVac.
| Group | No. of Participants | Geometric Mean Concentration (95% CI) | Geometric Mean Ratio (95% CI) |
|---|---|---|---|
| IM | 41 | 1771.88 (1307.90–2400.47) | Ref. |
| ID1 | 40 | 2373.49 (1687.73–3337.88) | 1.34 (0.86–2.20) |
| ID2 | 43 | 2805.13 (2100.97–3745.31) | 1.58 (1.05–2.39) |
IM: intramuscular standard dose. ID1: 20% intradermal fractional dose. ID2: 20% intradermal fractional dose.
3.3. Safety
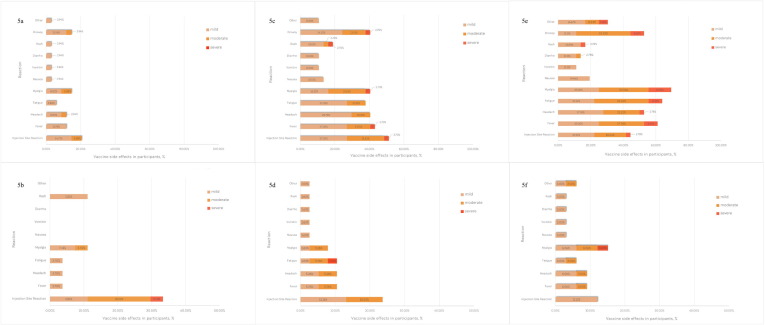
Fig. 5 shows AEs occurring within 7 days post-vaccination. On day 1 post-vaccination, the systemic AEs were more common in the IM group than both ID groups. The three most common systemic AEs in the IM group were myalgia (69%, 25/36), fatigue (64%, 23/36), and fever (61%, 22/36). The local AE was found in 44% (16/36). The systemic and local AEs were less commonly found in the ID1 group. The common systemic AEs in the ID1 group were Myalgia (14%, 5/34), drowsiness (14%, 5/34), fever (11%, 4/34), and headache (11%, 4/34). In addition, the local AE was found in 20.6% (7/34). Interestingly, systemic AEs were frequently found in the ID2 group compared with the ID1 group. However, these AEs were less commonly found when compared with the IM group. The common systemic AEs in the ID2 were fever (43%, 16/37), headache (40%, 15/37), myalgia (40%, 15/37), and drowsiness (40%, 15/37). The local AE in the ID2 group was found in 51.4% (19/37), the highest among the three groups. For all AEs, most (92.2%) were mild to moderate in a degree of severity. However, 24 (7.8%, 24/307) severe AEs were reported, 19 from the IM group and 5 from the ID2 group. No severe AE was reported from the ID1 group.
Fig. 5.
Adverse events on day 1 and day 7 after AZD1222 vaccination among participants who previously received two doses of CoronaVac. Figure 5a; Adverse events on day 1 in ID1 group. Figure 5b; Adverse events on day 7 in ID1 group. Figure 5c; Adverse events on day 1 in ID2 group. Figure 5d; Adverse events on day 7 in ID2 group. Figure 5e; Adverse events on day 1 in IM group. Figure 5f; Adverse events on day 7 in IM group. ID1, 20% intradermal fractional dose; ID2, 40% intradermal fractional dose. IM, intramuscular standard dose.
On day 7 post-vaccination, the systemic AEs were less reported than day 1. In the IM group, the three most common systemic AEs were myalgia (15%, 5/33), fever (9%, 3/33), and headache (9%, 3/33). The local AE was reported in 12.1% (4/33). The systemic AEs were also less reported in ID groups on day 7. The three most common systemic AEs were fever (10%, 4/38), headache (10%, 4/38), and fatigue (10%, 4/38) in the ID2 group. In the ID1 group, both myalgia and rash were reported in 11.1% (3/27). Interestingly, the local AE was reported in 33.3% (9/27) from the ID1 group which was higher than the local AE reported on day 1. Moreover, 1 participant in this group developed severe local AE. The local AE in the ID2 group was reported in 23.7% (9/38). Although this percentage was lower than day 1 post-vaccination (51.4%, 19/37), it was higher than the IM group (12.1%, 4/33). No life-threatening AEs occurred in any participants within 7 days of vaccination.
4. Discussion
This study demonstrated that both humoral and cell-mediated immune response following an ID fractional third dose of AZD1222 was non-inferior to a standard IM third dose in participants who had previously received two doses of CoronaVac vaccine. Moreover, a cell-mediated immune response from the 40% intradermal fractional dose is superior to a standard IM dose. The immunogenicity of AZD1222 administered by either route in participants who had previously received two doses of CoronaVac was excellent, demonstrating a high percentage of surrogate neutralizing antibodies. The geometric mean concentration of anti-RBD antibody increased by approximately 100-fold in most participants 2 weeks post-vaccination. The systemic AEs were more common in the IM group than both ID groups. On the other hand, local AEs were more frequent in ID groups than in the IM group. The systemic AEs usually resolved on day 7 post-vaccination. However, the percentage of local AE remained high in the ID groups compared with the IM group, especially in the ID1 group. The high percentage in the ID1 group on day 7 than day 1 post-vaccination suggested that the local AE after vaccine inoculum via the intradermal route could develop after day 1 and persist through day 7 post-vaccination. Focus on ID groups, the systemic AE in the ID2 group was higher than the ID1 group. This event may reflect the dose-dependent of the systemic AEs after intradermal vaccination. Although there were some participants developed severe AEs, most AEs were mild to moderate in severity. No life-threatening AEs were reported.
There is limited data of the cut point of the cut-off value that correlation with high titer neutralizing antibody for the SARS-CoV-2 NeutraLISA kit. However, there was a study that demonstrated the high correlation (R2 = 0.8) of the SARS-CoV-2 NeutraLISA kit with the GenScript cPassTM SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript Biotech, Leiden, The Netherlands) [22] Sixty-eight %IH of cPass was correlated with high titer of neutralizing antibody. [23] So, we also used the 68%IH as a cut-off value for the NeutraLISA kit too. All the samples from the three study groups were above this cut-off value.
Generally, after inoculum with adenoviral vector vaccine, the viral vectors enter the host cell using the specific receptors depending on the type of adenovirus, for example, coxsackievirus-adenovirus receptor (CAR) and CD46. After entry into the host cell, the viral genomes are then released into the nucleus. Transgenes could be expressed in both non-immune and immune cells. In non-immune cells, for example, muscle and fibroblast cells, transgene proteins are released and then uptake by the antigen-presenting cells (APCs) resulting in mainly a humoral immune response. [24], [25] On the other hand, expression of the transgene in immune cells (e.g. dendritic cells) results in a mainly cell-mediated immune response [24], [26].
Skin is an important organ of our body. It protects us from an invasion of the foreigner. Beyond the physical barrier, skin comprises many immune cells for an immunological barrier. Within immune cells, APCs are a key role in adaptive immune stimulation, especially dendritic cells (DCs). After the DCs capture the antigen periphery, they migrate to the regional lymph node then present the antigen and activate the naïve lymphocytic T cells [27], [28].
The ChAdOx1 is a replication-deficient adenoviral vector derived from ChAdY25. The advantage of ChAdOx1 over the human adenovirus type 5 (HAdV-5) is a low seroprevalence of antibodies against the vector [29]. The ChAdOx1 entry the host cell using mainly CAR as a receptor [30]. CAR mRNA is found in a variety of human cells, for example, heart, brain, pancreas, intestine, lung, liver, and kidney [31]. However, DCs express a low level of CAR [32]. So, the main mechanism that the intradermal inoculum of ChAdOx1nCoV-19 can elicit the excellent humoral immune response may explain by a high number of DCs which act as APC that uptake the transgene product from the non-immune cells. However, the cell-mediated immune response in the ID2 group was more robust than the IM group. This may be explained by CAR independent pathway [30]. Further investigation is needed.
The ID fractional dose is the alternative approach in some vaccines. After a one-fifth fractional dose of inactivated poliovirus vaccine intradermal inoculum, the humoral immune response was comparable with a full dose intramuscular route [33]. The ID fractional dose of the rabies vaccine is also provided by WHO guidelines [34].
A few clinical studies have evaluated the safety and immunogenicity of ID fractional dose of COVID-19 vaccines. Roozen et al. studied COVID-19 vaccination among 38 participants and found that ID-administered mRNA-1273 vaccine was well tolerated. Despite a higher incidence of local AEs following ID administration, most AEs were mild to moderate in severity. The common local AEs following ID administration were itching (80%) and erythema (80%). ID administration of 10% and 20% of the standard dose elicited slightly better immune responses than IM administration of 20% of the standard dose. The geometric mean concentrations (95% CIs) of anti-RBD antibodies at day 43 post-vaccination were 1286 (1003–1648) BAU/mL, 1471 (013–2137) BAU/mL, and 993.5 (659.5–1497) BAU/mL in individuals who received the 10% ID, 20% ID, and 20% IM doses, respectively. However, participants randomized to the IM administration control arm in this study did not receive a standard dose [35]. A case report by Singhatiraj et al. also showed excellent immunogenicity of an ID 20% fractional dose of AZD1222 among participants who had previously received two doses of CoronaVac [36].
Our study had several strengths. First, to our knowledge, this was the first clinical study to assess immune responses to the AZD1222 vaccine administered as a third ID fractional dose. Second, the study was a randomized controlled trial, and thus allocation bias was limited. Third, both humoral immunity and CMI were evaluated; previous studies have demonstrated that strong humoral immune responses can prevent infection [6] while CMI limits COVID-19 severity [37]. The study also had limitations. First, participants and investigators were not blinded during the study period. This may have contributed to observer bias in assessment of the safety profile of vaccination. However, to limit this bias, we used a questionnaire with standardized definitions for evaluating AEs. Second, we used a surrogate neutralizing antibody test instead of a standard neutralizing antibody test. However, the results of surrogate neutralizing antibody tests correlate with standard neutralization assays and do not require a biosafety level 3 laboratory [16]. Third, ID administration quality depends on the injection skill of health care personnel [13]. An unexpected finding of our study was the high levels of IFN-γ secreted by immune cells following stimulation with the S1 domain of the SARS-CoV-2 spike protein in vaccinated participants. The upper limit of the IFN-γ ELISA was 350 mIU/mL. Surprisingly, the geometric mean concentration of IFN-γ was more than 350 mIU/mL. Samples had to be diluted twice to three times to fall below this upper limit. This process required a longer time than we expected.
This study demonstrated that ID administration of a low volume of AZD1222 vaccine can elicit an excellent immune response in previously vaccinated participants. This finding may help address the global shortage of COVID-19 vaccine supply, especially in developing countries. It remains unclear whether ID fractional doses can be applied to primary vaccinations. This question will need to be addressed by future studies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank the clinical research management unit for managing this project. We also thank the central laboratory of Chulabhorn Hospital for the laboratory testing. We gratefully acknowledge funding from Chulabhorn Royal Academy and National Vaccine Institute, Thailand. We thank Edanz (www.edanz.com/ac) for editing a draft of this manuscript.
Ethics
The study was conducted in accordance with the principles laid out in the Declaration of Helsinki guidelines for research involving human subjects. The study protocol was reviewed and approved by the Ethics Committee for Human Research, Chulabhorn Research Institute (Certificate No. 094/2564)
Funding
The study was funded by Chulabhorn Royal Academy and National Vaccine Institute, Thailand.
References
- 1.World Health Organization. WHO coronavirus (COVID-19) dashboard 2021, https://covid19.who.int/table; 2021 [accessed 17 September 2021].
- 2.WHO Solidarity Trial Consortium, Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., et al. Repurposed antiviral drugs for COVID-19 – interim WHO Solidarity Trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olliaro P., Torreele E., Vaillant M. COVID-19 vaccine efficacy and effectiveness – the elephant (not) in the room. Lancet Microbe. 2021;2(7):e279–e280. doi: 10.1016/S2666-5247(21)00069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanriover M.D., Doğanay H.L., Akova M., Güner H.R., Azap A., Akhan S., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 7.Vacharathit V., Aiewsakun P., Manopwisedjaroen S., Srisaowakarn C., Laopanupong T., Ludowyke N., et al. CoronaVac induces lower neutralising activity against variants of concern than natural infection. Lancet Infect Dis. 2021;21(10):1352–1354. doi: 10.1016/S1473-3099(21)00568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinmann J. How the world is (not) handling surplus doses and expiring vaccines. BMJ. 2021;374 doi: 10.1136/bmj.n2062. [DOI] [PubMed] [Google Scholar]
- 10.Egunsola O., Clement F., Taplin J., Mastikhina L., Li J.W., Lorenzetti D.L., et al. Immunogenicity and safety of reduced-dose intradermal vs intramuscular influenza vaccines: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(2):e2035693. doi: 10.1001/jamanetworkopen.2020.35693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hettinga J., Carlisle R. Vaccination into the dermal compartment: techniques, challenges, and prospects. Vaccines (Basel) 2020;8(3):534. doi: 10.3390/vaccines8030534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweiger M. Intradermal COVID-19 vaccination could solve supply problems. BMJ. 2021;374 doi: 10.1136/bmj.n1980. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y.C., Jarrahian C., Zehrung D., Mitragotri S., Prausnitz M.R. Delivery systems for intradermal vaccination. Curr Top Microbiol Immunol. 2012;351:77–112. doi: 10.1007/82_2011_123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristiansen P.A., Page M., Bernasconi V., Mattiuzzo G., Dull P., Makar K., et al. WHO international standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282):1347–1348. doi: 10.1016/S0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resman Rus K., Korva M., Knap N., Avšič Županc T., Poljak M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol. 2021;139:104820. doi: 10.1016/j.jcv.2021.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.-C., Tiu C., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 17.Nateghi Rostami M., Keshavarz H., Edalat R., Sarrafnejad A., Shahrestani T., Mahboudi F., et al. CD8+ T cells as a source of IFN-gamma production in human cutaneous leishmaniasis. PLoS Negl Trop Dis. 2010;4 doi: 10.1371/journal.pntd.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng G., Zhang G., Chen X. Th1 cytokines, true functional signatures for protective immunity against TB? Cell Mol Immunol. 2018;15(3):206–215. doi: 10.1038/cmi.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Q., Mao Q., An C., Zhang J., Gao F., Bian L., et al. Heterologous prime-boost: breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg Microbes Infect. 2021;10(1):629–637. doi: 10.1080/22221751.2021.1902245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frenck R.W., Jr., Belshe R., Brady R.C., et al. Comparison of the immunogenicity and safety of a split-virion, inactivated, trivalent influenza vaccine (Fluzone(R)) administered by intradermal and intramuscular route in healthy adults. Vaccine. 2011;29(34):5666–5674. doi: 10.1016/j.vaccine.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frenck R.W., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann N, Grossegesse M, Neumann M, Schaade L, Nitsche A. Detection of neutralizing antibodies against SARS-CoV-2 by using a commercial surrogate virus neutralization ELISA: can it substitute the classical neutralization test? medRxiv. 2021:2021.10.12.21264881.
- 23.Wendel S., Fachini R., Fontao-Wendel R.C.L., et al. Surrogate test performance for SARS-CoV-2 neutralizing antibodies (nAbs) for convalescent plasma (CCP): how useful could they be? Transfusion. 2021;61(12):3455–3467. doi: 10.1111/trf.16714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakurai F., Tachibana M., Mizuguchi H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab Pharmacokinet. 2022;42 doi: 10.1016/j.dmpk.2021.100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bricker TL, Darling TL, Hassan AO, et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep 2021;36(3):109400. [DOI] [PMC free article] [PubMed]
- 26.de Gruijl T.D., Ophorst O.J., Goudsmit J., et al. Intradermal delivery of adenoviral type-35 vectors leads to high efficiency transduction of mature, CD8+ T cell-stimulating skin-emigrated dendritic cells. J Immunol. 2006;177(4):2208–2215. doi: 10.4049/jimmunol.177.4.2208. [DOI] [PubMed] [Google Scholar]
- 27.Honda T., Egawa G., Kabashima K. Antigen presentation and adaptive immune responses in skin. Int Immunol. 2019;31(7):423–429. doi: 10.1093/intimm/dxz005. [DOI] [PubMed] [Google Scholar]
- 28.Kashem S.W., Haniffa M., Kaplan D.H. Antigen-presenting cells in the skin. Annu Rev Immunol. 2017;35:469–499. doi: 10.1146/annurev-immunol-051116-052215. [DOI] [PubMed] [Google Scholar]
- 29.Dicks M.D.J., Spencer A.J., Edwards N.J., Wadell G., Bojang K., Gilbert S.C., et al. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS ONE. 2012;7(7):e40385. doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dicks MD, Spencer AJ, Coughlan L, et al. Differential immunogenicity between HAdV-5 and chimpanzee adenovirus vector ChAdOx1 is independent of fiber and penton RGD loop sequences in mice. Sci Rep. Nov 18 2015;5:16756. [DOI] [PMC free article] [PubMed]
- 31.Zhang Y., Bergelson J.M. Adenovirus receptors. J Virol. 2005;79(19):12125–12131. doi: 10.1128/JVI.79.19.12125-12131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma A., Li X., Bangari D.S., Mittal S.K. Adenovirus receptors and their implications in gene delivery. Virus Res. 2009;143(2):184–194. doi: 10.1016/j.virusres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okayasu H, Sein C, Chang Blanc D, et al. Intradermal Administration of Fractional Doses of Inactivated Poliovirus Vaccine: A Dose-Sparing Option for Polio Immunization. J Infect Dis 2017;216(suppl_1):S161-S167. [DOI] [PMC free article] [PubMed]
- 34.World Health Organization Rabies vaccines: WHO position paper, April 2018 - Recommendations. Vaccine. 2018;36(37):5500–5503. doi: 10.1016/j.vaccine.2018.06.061. [DOI] [PubMed] [Google Scholar]
- 35.Roozen GVT, Prins MLM, van Binnendijk R, den Hartog G, Kuiper VP, Prins C, et al. Tolerability, safety and immunogenicity of intradermal delivery of a fractional dose mRNA-1273 SARS-CoV-2 vaccine in healthy adults as a dose sparing strategy. medRxiv. 2021; 2021.07.27.21261116. doi: https://doi.org/10.1101/2021.07.27.21261116.
- 36.Singhatiraj E., Pongpirul K., Jongkaewwattana A., Hirankarn N. Intradermal ChAdOx1 vaccine following two CoronaVac shots: a case report. Vaccines (Basel). 2021;9(9):990. doi: 10.3390/vaccines9090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]