Abstract
Neurons decentralize protein synthesis from the cell body to support the active metabolism of remote dendritic and axonal compartments. The neuronal RNA transport apparatus, composed of cis-acting RNA regulatory elements, neuronal transport granule proteins and motor adaptor complexes, drives the long-distance RNA trafficking required for local protein synthesis. Over the past decade, advances in human genetics, subcellular biochemistry and high-resolution imaging have implicated each member of the apparatus in several neurodegenerative diseases, establishing failed RNA transport and associated processes as a unifying pathomechanism. In this review, we deconstruct the RNA transport apparatus, exploring each constituent’s role in RNA localization and illuminating their unique contributions to neurodegeneration.
Neurons have an elaborate architecture that requires specialized intracellular machinery to operate and maintain. Dendrites radiate outward from the cell body, forming a dense arbor that spans tens of millimeters. Meanwhile, the cell’s single axon can extend hundreds of centimeters in length, forming active synapses with tens of thousands of other cells. Neurons sense and respond to stimuli in these remote regions on the order of milliseconds, yet metabolic machinery transits outward from the cell body on the order of hours to days1. To meet the rapidly changing metabolic needs of axons and dendrites, neurons rely on specialized mechanisms to synthesize proteins locally and on demand2–8.
Local protein synthesis requires delivery of RNA to distal reaches of the neuron. An elegantly regulated RNA transport apparatus (RTA) fills this need, continuously shuttling transcripts from the cell body to remote locales. The RTA consists of cis-acting elements within RNA molecules, the proteins that bind them to generate discrete RNA/protein granules and the adaptor complexes that link those granules to motor proteins (Fig. 1). Breakdown of the RTA can have catastrophic consequences; impaired RNA localization and distal protein synthesis occur in models of multiple neurodegenerative diseases, often correlating with major functional impairments9–12.
Fig. 1 |. The RNA transport apparatus in health and disease.
RNA transport is a ubiquitous process in healthy neurons that is driven by a multipartite apparatus. Acting together, the components of the RTA enable local protein synthesis in axons and dendrites, supporting active metabolism at these remote sites. Genetic and age-related dysfunction of RNA regulatory elements, transport granule proteins or motor adaptor complexes often leads to degenerative disease by impairing local protein synthesis.
In this review, we position the RTA as a molecular framework for understanding local neuronal metabolism and neurodegeneration. Genetic, biochemical and imaging studies have traced molecular defects in each of its components to diseases across the neurodegenerative spectrum, implicating RNA transport as a mechanistic focal point. This review is by no means comprehensive, and so we refer the reader to several excellent reviews on aspects of general RNA metabolism and trafficking not covered here (for example, nucleocytoplasmic traffic, motor proteins and cytoskeleton)13–15. Rather, we highlight important mechanistic sequences that drive RNA localization specifically in neurons and that are consistently disrupted in neurodegenerative disease. Based on the extensive links uncovered, we propose that impaired RNA transport has emerged as a convergent mechanism of neurodegeneration.
cis-acting RNA elements
RNA transport is an intricately choreographed process that begins in the nucleus. As RNAs are transcribed, several protein complexes descend on the nascent transcript, from the spliceosome to the capping enzyme complex and polyadenylation machinery. RNAs emerging from transcription and splicing spontaneously form secondary structures, such as stem loops, helices and pseudoknots. These structures, along with sequence motifs and unstructured regions, create novel binding interfaces for RNA-binding proteins(RBPs). For example, a 54-nucleotide (nt) ‘zipcode’ sequence within a secondary structure in the β-actin transcript pairs with an unfolded region to bind to the ZBP1/IGF2BP1 protein, causing the RNA transcript to loop around ZBP1 and subsequently directing transcript localization in neurons16,17.
Neuron-specific and compartment-specific messenger RNA regulatory features.
On average, neural 3′ untranslated regions (UTRs) are considerably longer (4,347 nt versus 989 nt) than those of the typical cell, containing multiple sequence tracts that enable micro RNA (miRNA) binding and oligomerization of regulatory proteins18–20. An analysis of microdissected mouse hippocampal neuropil, capturing ~35,000 isoforms across ~14,000 genes and ~400 neuron-specific transcripts, found that axonal and dendritic transcripts are often longer still, with more secondary structure than soma-enriched transcripts (Fig. 2a)20. This diversity in UTR length and composition arises from a variety of mechanisms, including alternative polyadenylation, altered isoform stability and trafficking and local UTR remodeling18,20–22. Long trafficking times and temporally specific protein synthesis require these distally localized transcripts to be more stable than the average RNA. Accordingly, messenger RNAs (mRNAs) localized to distant sites in mouse brain are more long lived (t1/2=8.36 h) than non-localized neuronal mRNAs (t1/2 = 7.23 h)20. Further studies are needed to determine whether these findings are generalizable to other organisms and neuronal types. If so, then neuritic RNAs in human neurons might persist considerably longer still, considering that the expanded length of human axons and dendrites would require days, rather than hours, to traverse. How neuritic UTRs enhance stability is unclear but might involve the selective inclusion of specific sequence motifs to antagonize deadenylation and miRNA binding and promote stabilizing RBP interactions23,24.
Fig. 2 |. Neuronal cis-acting RNA regulatory element structure, function and dysfunction.
a, Compared to non-neuronal cells, the 3′ UTR regions of neuronal mRNAs, which often encode information for transcript localization, are substantially longer and contain more secondary structure. Within neurons, mRNAs that localize to neuritis are longer, richer in secondary structure and have a longer half-life than their counterparts in the soma. The extended length of neuritic transcripts results from repeated, as well as unique, sequence motifs and secondary structures. b, Secondary structures and sequence motifs in different parts of the mRNA transcript encode localization to different parts of a neurite. In this example, information in the coding sequence drives localization to the primary branch of the neurite, whereas the 5′ and 3′ UTRs are necessary for specification to secondary and tertiary branches, respectively. c, Dysfunction in RNA regulatory regions contributes to neuronal dysfunction through direct aggregation, seeding RNA/protein aggregates and preventing the accurate routing (UTR disruption) of transcripts to the regions that require them.
Diverse modes of localization.
The repeated secondary structures, sequence motifs and nucleobase modifications in neuritic transcripts coordinately bind protein complexes that direct localization of these mRNAs to distant sites (Fig. 2b). UTR isoforms encoding the same protein illustrate this point, as long 3′ UTR variants localize preferentially to neurites, whereas shorter ones are retained in the soma21. Regulatory signals embedded in the RNA molecule occur throughout its length and can fine-tune localization. A single transcript might use the 3′ UTR for localization in one neuronal subtype while using the 5′ UTR to localize in another25. Alternatively, one UTR might be used to direct transport to a general region (for example, neurites), while the other is used for sub-regional specification (for example, synapses)26. Motifs and secondary structures within the coding region have also been proposed as zipcode sequences27. Other cell types, including budding yeast, Drosophila embryos, Xenopus oocytes, fibroblasts and oligodendrocytes, exhibit both directed transport mechanisms as well as spatio-selective stabilization and degradation of transcripts28–30. Neurons might primarily use directed transport given probabilistic limits that constrain diffusional localization in the context of a highly branched cellular architecture.
Our understanding of how these regulatory elements control localization is still in its infancy. Presumably, the combinatorial use of multiple regulatory motifs within a transcript attracts a collection of RBPs, which act to specify a destination. This question is beginning to be addressed with proximity labeling of RNA interactomes (‘RNA-ID’), using MS2–MCP and CRISPR–Cas13 technologies to follow transgenic and endogenous RNAs, respectively31. For example, RNA-ID of the β-actin transcript in fibroblasts revealed an important role for RBPs other than ZBP1/IGF2BP1, and cis-acting sequences other than the canonical ‘zipcode’, in stimulus-dependent localization32. Similar studies in cultured neurons following other localized neuronal mRNAs, such as CamK2A and Bassoon, should sharpen the view of how RNA–RBP interactions determine specific localization patterns.
mRNA noncoding regions in neurodegenerative disease.
Dysregulated RNA metabolism is a major theme across several neurodegenerative diseases (Fig. 2c). Recent work suggests that repeat-rich RNA molecules can spontaneously aggregate to form solid assemblies; the hexanucleotide repeat expansion in the C9ORF72 transcript, linked to both amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), is a prime example of this phenomenon33. Intrinsic qualities of neuronal mRNAs might also be a risk factor. Repeated sequences within the long 3′ UTRs of neurite-localized transcripts, found at a much higher rate than predicted by chance, might predispose these mRNAs to self-scaffold, aggregate and disrupt cellular function in the setting of disease20. Interestingly, antisense oligonucleotides that target repeat sequences and nucleic acid intercalating agents (for example, doxorubicin) have shown efficacy in reducing RNA foci derived from repeat-rich RNA33.
The scaffolding function of RNAs might also play roles in neurodegenerative toxicity traditionally attributed to mutant proteins. For example, expression of mutant forms of FUS and TDP-43—RBPs linked to both sporadic and familial forms of ALS and FTD—result in neurodegeneration in multiple model systems10,34,35. However, eliminating their RNA-binding abilities completely blocks neurodegenerative phenotypes36,37. The mechanisms at work here likely relate to the scaffolding function of target RNAs that are rich in secondary structure. These RNAs, which are commonly found in neurites, might bring groups of potentially pathogenic RBPs together, facilitating toxic aggregation and depleting functional pools of protein that become encased in aggregates.
To date, mutations in genuine regulatory cis-acting elements have not been conclusively linked with neurodegenerative diseases. However, several observations suggest that altering these elements can have a functional effect. For example, studies in mice show that mutating the 3′ UTRs of the transcripts encoding CamK2A and BDNF disrupt RNA transport and impair neurotransmission, and homozygous coding mutations in CamK2A also lead to intellectual disability in humans21,38,39. Still, definitive connections among mutations in noncoding regions, RNA transport dynamics and human disease are missing. How other RNA modifications (for example, RNA editing or RNA methylation) affect RNA transport and neuronal health is similarly unclear40. Answering these questions will require careful mechanistic studies involving the interaction networks of specific RNA motifs, and perhaps even specific RNAs, that are implicated in degenerative pathology.
Neuronal transport granules
RNAs are almost always found in complexes with RBPs. Accordingly, there are over 2,000 known RBPs in the human genome, with a few expressed solely or predominantly in neurons (for example, ELAVL and RBFOX proteins) and many expressed broadly but with neuron-specific roles (for example, FUS and FMRP)41,42. To translocate from the cell body to a distant site in the neurites, RNA molecules must attach to RBPs. These RNA–protein complexes, or RNA granules (in this case, specifically ‘transport granules’), display constitutive bidirectional movement along axons and dendrites43. Transport granules deliver packaged RNAs to distal sites, where local protein synthesis can take place (Box 1).
Transport granule composition and biophysical state.
Transport granules are composed of a heterogenous complex of RBPs, ribosomal components and RNA (Fig. 3a). Early work showed that, upon neuronal depolarization, many mRNAs are selectively released and received by polysomes to initiate translation44. More recent studies suggest that most granules transport individual RNAs singly, suggesting ultra-fine control of distal protein synthesis45–47 The extent to which functionally related RNAs are transported together (each as a single copy), like in yeast RNA granules, has yet to be uncovered48. Whereas single-mRNA granules would imply the prioritization of simple and highly specific translational responses, the transport of functionally related mRNA packages would enable more complex, multistep distal metabolism.
Fig. 3 |. Neuronal transport granule properties.
a, The major known components of neuronal transport granules are RNA, RBPs and ribosomal subunits/ assembly factors. Transport granules shuttle freely in distal neurites. b, Transient, low-affinity and multivalent interactions drive protein condensation, which is the organizing principle of transport granule assembly. These membrane-less organelles exhibit dynamic exchange with the cytoplasm, enabling regulated RNA and protein flux. c, RNA–protein co-acervation, or co-assembly through LLPS, is required for transport granule formation. SMN protein facilitates transport granule assembly by promoting the interactions between RBPs and their RNA targets. The absence of SMN protein in SMA results in fewer successfully formed granules, less distal RNA delivery and ultimately motor neuron degeneration. d, Dynamic assembly and disassembly of transport granules is essential for proximal packaging and distal release of RNA cargo. Hyper-assembly and solidiffcation of granules, caused by mutations in several ALS/FTD-related proteins (for example, FUS and TDP-43), result in impaired RNA delivery to distal sites. This deficit causes neuritic dysfunction, leading to neurodegenerative disease. e, Transport granules enable site-specific translation by limiting translation before arrival and tuning translation at the destination site to local needs. FMRP normally regulates this balance, and so its loss leads to uncontrolled protein synthesis, as well as malformed and dysfunctional neuritic structures. These cellular defects characterize FXS.
RNA granules do not exist as conventional soluble complexes like many other protein assemblies in the cell. Instead, owing to multivalent and dynamic interactions between RNA and protein components, RNA granules form distinct liquid bodies that de-mix from the rest of the liquid cytosol49. This process of liquid–liquid phase separation (LLPS), or biomolecular condensation, generates ‘membrane-less organelles’ (MLOs) (Fig. 3b). A phase-separated liquid body enables different droplet-enclosed RNA and protein components to dynamically associate with each other while excluding cytosolic interlopers; these properties facilitate translational control (for example, miRNA binding and ribosome accessibility). Likewise, MLOs can also dynamically exchange components with the cytosol or other MLOs, enabling regulated RNA flux and, potentially, RNA metabolism (for example, RNA nuclease or methyltransferase flux).
To date, the biophysical state of trafficking granules has not been directly investigated. However, the lack of evidence for RNA granules to form alternative, non-condensed, non-aggregated structures (ie, conventional soluble protein complexes) in cells suggests that transport granules, like other RNA granules, are also biomolecular condensates.
Transport granules in neurodevelopmental and neurodegenerative disease.
Human genetic studies have revealed a vast array of genes linked to both inherited neurodevelopmental and neurodegenerative disorders. For some of these diseases, namely spinal muscular atrophy (SMA), ALS/FTD and fragile X syndrome (FXS), a disproportionate number encode RBPs that incorporate into transport granules. In the following three examples, we explore plausible mechanisms by which failed mRNA transport can contribute to the cellular and molecular phenotypes of the discussed diseases. These examples reveal both the general importance and specific characteristics of transport granules that are critical to mRNA localization and overall neuronal function.
Binding between an RNA molecule and RBPs is essential for transport granule formation and function (Fig. 3c). Supporting this claim is the recent finding that loss of survival motor neuron (SMN) protein, a key facilitator of transport granule assembly, results in impaired localization of several mRNAs important for axonal integrity, including β-actin and GAP43 (refs. 9,50). The loss of SMN protein in SMA, an inherited motor neuron disease, further highlights the critical importance of RNA delivery to distal sites by way of proper granule assembly51.
Once formed, the liquidity of a transport granule determines the ability of its components to dynamically exchange with the surrounding cytosol (Fig. 3d). FUS and TDP-43 are both transport granule-associated proteins that undergo biomolecular condensation, thus helping to endow their granules with liquid properties. They are also both linked to the ALS/FTD neurodegenerative spectrum, which affects motor and cognitive systems to varying degrees34. Disease-linked mutant proteins of FUS and TDP-43 show accelerated phase transitions from monodisperse to condensed states, which often progress to solid fibrillar structures11,52–54. These mutants cause axonal mRNA trafficking deficits in cells, leading to neurite dysmorphology and dysfunction12,55–58. The mechanisms underlying this pathology are complex and likely involve a combination of toxic aggregation, motor-binding deficits and an inability of these hyper-viscous or fibrillar granules to dynamically regulate RNA influx or efflux. By shifting the equilibrium of the protein components of transport granules to a more condensed state, FUS and TDP-43 mutants likely either exclude RNA from granules or bind them too tightly to be released upon physiologic stimulation59–61.
By binding mRNAs through multivalent interaction paradigms, transport granules also control their translational capacity (Fig. 3e). Fragile X mental retardation protein (FMRP), one of the original proteins identified in transport granules, binds directly to both mRNA and ribosomes, acting to shield mRNAs from ribosomal access62–68. Biomolecular condensates of FMRP also self-incorporate other translational repressor proteins and miRNAs69. FMRP is lost in the setting of FXS, a neurodevelopmental disease. This loss leads to profound dendritic spine pathology in patient brains and axonal growth cone defects in cultured neurons68,70. Mounting evidence suggests that FXS is a disease of translational hyperactivity; ameliorating functional phenotypes in FXS mice by downregulating translational activators demonstrates the physiologic importance of translational repression by FMRP and by transport granules in general71. These findings also highlight the broader role of RNA transport to restrict protein function in both space and time.
It is important to note that many of the above RBPs participate in several other processes, including RNA splicing, transcription, DNA repair and the function of other RNA granules (for example, stress granules, P-bodies and nuclear paraspeckles)72. Because a single disease mutation in an RBP often alters its role in all of these processes, it is difficult to assign one particular driver of pathogenesis. So, despite the substantial evidence suggesting that dysfunctional RNA transport and local translation is a common pathomechanism of neurodegenerative diseases, it is likely accompanied by other changes in RNA metabolism. A substantial challenge facing the field will be to begin to disentangle what are likely co-occurring pathogenic mechanisms.
Motor adaptors
Proteins and organelles traffic long distances through the actions of kinesins and dynein–dynactin. These motor proteins couple cargo to microtubule scaffolds and enact translocation in the anterograde and retrograde directions, respectively15. This machinery is well suited to support the long-range trafficking requirements of transport granules.
However, motor proteins often require adaptor complexes to enable their interaction with cargo. Whether composed of a single protein or a more complex assembly, adaptors have a multivalent structure, binding cargo and motor through distinct paradigms. RNA granules, as phase-separated droplets, are unique cargoes that require motor adaptors equipped to interface between a liquid body and a traditional protein complex.
Protein adaptor complexes.
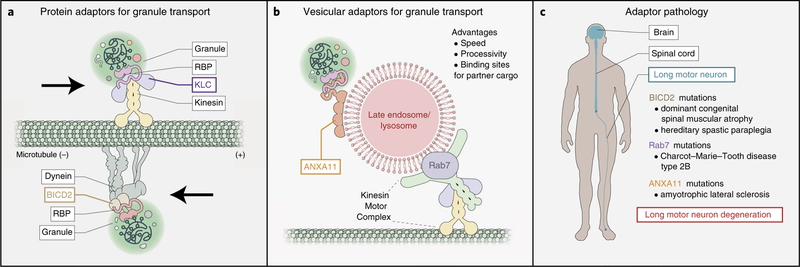
Multiple lines of evidence suggest that conventional protein adaptors enable transport granule motility (Fig. 4a). Specific transport granule proteins, such as FMRP, SFPQ and ZBP1/IGF2BP1, all form conventional soluble complexes with kinesin light chain (KLC), a protein adaptor for kinesin67,73–75. Furthermore, disrupting this binding through point mutations in either the RBP or KLC decreases localization of specific mRNAs bound by those RBPs74,75. In vitro work on APC, another transport granule protein, shows that the minimal complex of APC, mRNA, KIF3 and the kinesin adaptor KAP3 is sufficient for microtubule-based mRNA motility76. Protein adaptors can also regulate retrograde trafficking; direct binding of FMRP to bicaudal D (BIDC2) is required for dynein-dependent transport of FMRP granules77,78. These results, obtained through co-immunoprecipitation, in vitro reconstitution and live-cell imaging approaches, indicate that some transport granules interact with motors through conventional protein complexes. Further work will be needed to clarify the topology of these interactions, especially in light of their biophysical incongruity.
Fig. 4 |. Motor adaptors for RNA transport.
a, KLC and BICD2 each bind RBPs and molecular motors (kinesin or dynein, respectively) simultaneously, enabling transport granule trafficking. b, The LE/Lys is a recently characterized vesicular adaptor between transport granules and microtubule-based motors. This system requires the secondary adaptors Rab7 (between the motor complex and the LE/Lys) and ANXA11 (between the LE/Lys and transport granule). ANXA11 has unique biophysical characteristics that enable its tethering function, as its granule-interacting N-terminus undergoes LLPS, and its LE/Lys-interacting C-terminus binds LE/Lys-specific phospholipids. c, Familial neurodegenerative diseases reveal mutations in motor adaptors for RNA transport, underscoring the critical role for adaptors in RNA transport and long-term neuronal health. These diseases affect long motor neurons, which are exceptionally dependent on robust axonal trafficking mechanisms.
The late endosome/lysosome as a vesicular adaptor.
Vesicular adaptors for RNA transport were first identified in non-mammalian systems. The filamentous fungus Ustilago maydis localizes RNA granules by attaching them to shuttling endosomes79,80. The secondary adaptor protein Upa1, harboring both an RBP-interacting domain and an endosomal lipid-binding domain, serves as a molecular linker between the RNA granule and the endosome81.
The existence of a similar phenomenon in neurons was later suggested by a discovery that miRNAs co-traffic with acidic compartments in axons82. An additional study confirmed that these miRNAs trafficked specifically with late endosomes/lysosomes (LE/ Lys) (Fig. 4b) and were not enclosed within the organelle but, rather, docked outside of it—hitchhiking on the lipid surface83.
Shortly after, a role for hitchhiking in mRNA transport also emerged. Work in Xenopus retinal ganglion cells found that mRNAs, ribosomes and transport granules all co-traffic with late endosomes in axons, and that these supercomplexes serve as translation platforms to supply axonal mitochondria with new protein84. A separate study demonstrated the dependence of axonal RNA granule motility on lysosomal trafficking, identifying ANXA11 as the mammalian ‘secondary adaptor’ linking the lysosome and transport granule85. Similarly to its fungal counterpart Upa1, ANXA11 possesses a C-terminal domain that binds endolysosomal lipids in the presence of Ca2+. Unlike Upa1, however, ANXA11 has an N-terminal prion-like domain that enables the protein to undergo biomolecular condensation. As a result, ANXA11 binds the lysosomal surface through a folded polypeptide–lipid interaction and integrates into the transport granule through phase separation. Although current evidence in cells supports this model, an in vitro demonstration of the bipartite topology of ANXA11 at the interface of RNA granules and lysosomes would offer further insight into this unique molecular tethering event.
Advantages of vesicular adaptors for RNA transport.
Organelles likely serve as ‘hitchhiking’ platforms for multiple reasons (Fig. 4b). Organellar trafficking tends to occur at ‘fast’ speeds—roughly ten times the rate of most cytoskeletal and soluble proteins15. The lipid surface of organelles and presence of integral membrane scaffolding proteins also provide several binding sites for multicomponent hitchhikers, such as the translational supercomplexes observed in Xenopus84.
Interestingly,in each study showing neuronal RNA transport via hitchhiking, LE/Lys are the vehicles of choice82–85. Lysosome-related organelles are already known to traffic extensively within neurites, and they are necessary for pre-synaptic biogenesis86. These functions might be related to or integrated with an additional function in delivering synaptic mRNAs and miRNAs for local translational control. Vesicles harbor an ‘on-board’ fuel source in the form of membrane-associated glycolytic machinery87. Thus, vesicles and their hitchhikers can traffic with minimal pausing or microtubule detachment. Lysosomes might also directly partner with new protein synthesis through their luminal stores of ions (for example, H+ and Ca2+) and metabolites (for example, amino acids, nucleotides and sugars), which can be released by membrane transporters. These species might activate or act as substrates for the enzymes encoded by trafficking mRNAs.
Choosing a transport strategy.
The existence of both protein and vesicular adaptors for RNA transport suggests that these systems fulfill distinct roles. However, how each adaptor system is used by individual RNAs, neurite types (axon versus dendrite) or even neuron types (for example, sensory versus motor neurons) is just beginning to be explored. β-actin mRNA traffics via both protein and vesicular adaptors, so these systems do not appear to mutually exclude RNA cargoes75,85. Although protein adaptors have been reported in both axons and dendrites, vesicular adaptors have, thus far, been studied only in the context of axonal transport. Also, neuron types have yet to be rigorously compared. For example, SFPQ granules were observed to traffic on LE/Lys in Xenopus retinal ganglion cell (RGC) axons and via protein adaptors in rat dorsal root ganglion axons74,84. LE/Lys were also observed to be the predominant vehicle for RNA granule transport in rat cortical neurons, whereas, in Xenopus RGCs, expression of mutant Rab5a or Rab7a did not alter RNA granule speed or directionality, indicating that transport of these RNA granules did not rely upon Rab5a/7a-dependent endosomal transport.84,85. Lastly, differential use of adaptors in different environmental contexts (for example, high neuronal activity and stress) remains unexplored. It is likely that RNAs can use multiple mechanisms of transport to reach their final destination within axons. Ultimately, understanding how neurons select transport strategies for particular transcripts might require a combination of proximity-based RNA detection (for example, APEXseq and PAR-CLIP with adaptor baits) with compartment-specific multi-color live imaging approaches (for example, MS2–MCP or molecular beacon-tagged RNAs with fluorescent adaptors).
Motor adaptors in neurodegenerative disease.
Although dynactin and huntingtin are especially prominent examples of motor adaptors implicated in neurodegenerative disease15, their potential roles in RNA transport have not been explored. In contrast, other disease-related motor adaptors appear to play direct roles in RNA transport (Fig. 4c). In the following three examples of BICD2, Rab7 and ANXA11, we focus on their role in RNA transport and explore the potential connections between failed RNA trafficking and the specific degeneration of highly elongated neurons.
BICD2, a dynein effector that also binds FMRP, is a conventional protein adaptor that helps to traffic FMRP granules toward the (-) end of microtubules. Mutations in BICD2 cause dominant congenital spinal muscular atrophy and hereditary spastic paraplegia, both motor neuron diseases primarily affecting the lower limbs88. Rab7 mutations cause Charcot–Marie–Tooth disease type 2B (CMT2B), which also confers a predominantly lower limb neuropathy89. In cells, Rab7 scaffolds both kinesin and dynein effector assemblies on LE/ Lys, indirectly facilitating RNA hitchhiking90. ANXA11 mutations cause ALS and have been linked to cognitive deficits resembling FTD in some studies91–93. Disease-linked ANXA11 mutants cause global hardening of RNA granules, prevent transport granule–lysosome binding and decrease mRNA localization to axonal growth cones85. Interestingly, mutations in BICD2, Rab7 and ANXA11 all affect long motor neurons, cells that are likely to rely heavily on a robust system for axonal RNA transport and local translation.
One important caveat is that these motor adaptors do not act solely in RNA transport, and so disease mutations might cause pleiotropic effects. Furthermore, dysfunction of motor complexes can elicit broad disruptions in neuritic transport, and the specific cargoes affected by disease mutations are still being actively explored15.
Local translation
After many hours or even days, the RTA arrives at its destination. Neurotrophic agents (for example, brain-derived neurotrophic factor and neurotrophin-3) coordinate its itinerary, inducing specific RNA transport patterns through unknown mechanisms94. Protein synthesis commences with stimulus-dependent chemical changes to both protein and RNA.
Post-translational modifications and chaperone interactions drive granule rearrangements that modulate RNA release (Fig. 5a). An early study found that Src kinase phosphorylation of ZBP1/ IGF2BP1 releases bound mRNA and is necessary for β-actin translation in dendrites95. Phosphorylation reduces phase separation of FUS and hnRNPA2 and enhances it for FMRP and TDP-43 (refs. 69,96–98). Therefore, neuronal regulators of granule phosphorylation (for example, Fyn, PP2A and S6K1) can be expected to have distinct effects on the translational status of different granule types99–101 Methylation (for example, protein arginine methyltransferases), on the other hand, seems to more uniformly reduce phase separation, favoring granule dissociation and RNA release12,69,102. These modifications are at least partly governed by membrane receptor signaling cascades, providing a link between extracellular stimuli and granule dynamics. For example, metabotropic glutamate receptor stimulation activates PP2A, leading to rapid FMRP de-phosphorylation and translation of FMRP-bound transcripts100. Chaperones that are integrated within the granule, such as transportin-1 (TNPO1/ KapB2), promote protein synthesis as well by reducing phase separation12. Further studies are needed to integrate these findings from cellular and cell-free systems, directly exploring the composition and dynamics of granules during transport and translation.
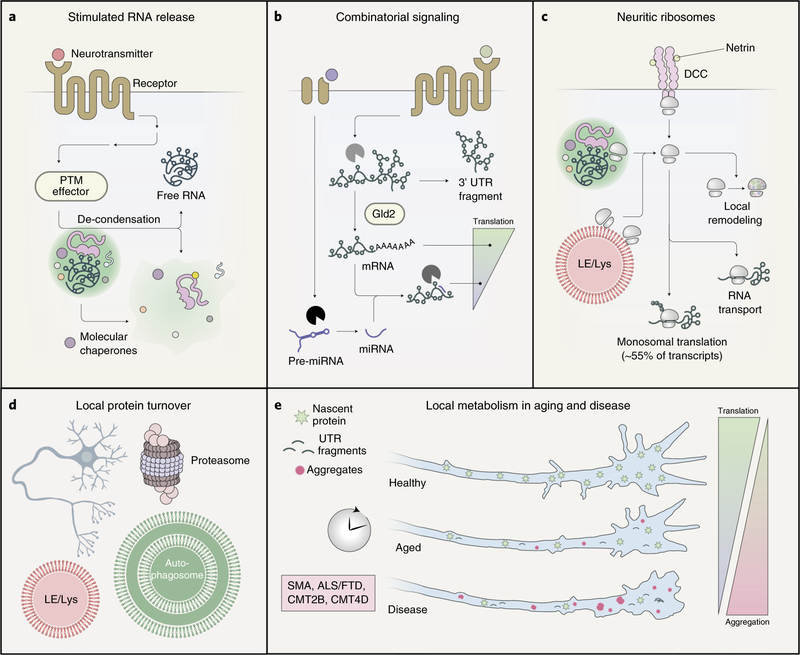
Fig. 5 |. The RNA transport apparatus and local metabolism.
a, Upon arrival at the site where RNA is to be released for protein synthesis (mRNA) or translational inhibition (miRNA), the granule must undergo partial or full de-condensation. Membrane receptors, in response to neurotransmitter stimulation, can activate signaling pathways that trigger RBP post-translational modifications and cause granule dispersal. Molecular chaperones contained within the granule, which control biophysical changes involved in nuclear transit, can also disperse granules distally. b, RNA-modifying machinery, such as nucleases (light gray, dark gray and black) and polymerases (Gld2), are active in neurites. In response to various chemical and electrical stimuli, this machinery can precisely tune the translational status of a particular mRNA to local needs. c, Neuritic ribosomes can be released from membrane receptor (transmembrane receptor DCC) scaffolds by local stimulation, trafficked into neurites within transport granules or hitchhiked on the surface of LE/Lys. Within neurites, ribosomes undergo local remodeling to enable location-specific translation, participate in the late stages of RNA transport and undertake predominantly monosomal translation of mRNAs. d, Neurons employ two main proteolytic systems to maintain distal proteostasis. The proteasome, which processes small degradative cargoes, is recruited to and active within dendrites. The autophagy machinery (LE/Lys and autophagosomes), which typically handles larger cargoes, such as large protein assemblies or whole organelles, has primarily been observed within axons. e, Neurite outgrowth, maturation and maintenance over time are dependent upon the continual replenishment of new protein and the clearance of dysfunctional organelles and protein aggregates. As neurons age, both local translation and local turnover decrease, resulting in decreased overall function. Neurodegenerative diseases accelerate and intensify these changes (less local translation, more aggregates and UTR fragment accumulation), leading to drastic functional impairments.
Triggered modifications of RNAs themselves also modulate translation (Fig. 5b). Studies on long-term potentiation (LTP), a form of synaptic plasticity characterized by sustained high levels of local protein synthesis, have found that localized mRNAs undergo 3′ UTR cleavage and polyadenylation after exposure to LTP stimuli22,103,104. Localized pre-miRNAs can also undergo maturation and elicit translational repression in response to separate cues83,105. These opposing phenomena might be combinatorially induced by extracellular stimuli, giving rise to complex translational responses. RNA nucleases, such as dicer and argonaute proteins, are known components of neuronal P-bodies and might effect these changes through regulated docking and co-condensation events with transport granules106,107. Gld2, a poly-A polymerase required for long-term memory and a known transport granule component, has been proposed as a local stimulant of polyadenylation and protein synthesis104,108.
The ribosome is the final major player in local translation (Fig. 5c). A longstanding question in the field has been how to reconcile the robust nature of local translation with a relative paucity of observable polyribosomes in neuronal processes, particularly axons. Recent work has bridged this gap, showing that monosomes, previously thought to be translationally silent, actively translate synaptic mRNAs and contribute to the local proteome109. Partially translated peptide motifs and the movement of translating ribosomes might also contribute to mRNA transport, as evidenced by RNAlocalizationdefectscausedbycycloheximide110. These ribosomes are compositionally distinct from somal populations, with local remodeling generating unique, neurite-specific ribosomes that influence local functions, such as axon branching111. Neuritic ribosomes derive from multiple possible sources. Some RNA-binding proteins, such as FMRP, also bind ribosomes and, therefore, incorporate them into transport granules112,113. Signaling scaffold-bound ribosomes also appear to exist in neurites, with a pair of studies showing that neuronal growth and guidance cues can stimulate the release of ribosomes bound to plasma membrane receptors, making them competent for translation114,115. Finally, ribosomes might arrive in neurites by way of endolysosomal hitchhiking, possibly in coordination with transport granules84,116. Regulated mechanisms for how these subpopulations acquire mRNAs for protein synthesis are currently unknown.
Local metabolism in neurodegenerative disease.
Impaired local translation is a defining feature of several neurodegenerative diseases. SMA, a disease of transport granule hypo-assembly, causes a global decrease in protein synthesis within axons9. ALS/FTD, often characterized by poorly dynamic and hyper-viscous transport granule condensates, disrupts protein synthesis in distal neurites, leading to axonal and dendritic dysfunction10–12,117. CMT2B, caused by mutations in a lysosomal motor adaptor that regulates mitochondrial contacts, shows markedly decreased axonal protein synthesis, dysfunctional axonal mitochondria and widespread degeneration84. Targeted experimental models have actually narrowed some of these phenotypes to the loss of specific locally translated proteins. A model of CMT4D, which exhibits neurodegeneration and fails to transport the mRNA for Bclw, is rescued by isolated axonal application of a Bclw peptide mimic74. Likewise, isolated axonal depletion of LB2, a locally synthesized protein lost in CMT2B, recapitulates CMT-like neurodegeneration118. These results provide strong support for a causal relationship between impaired local protein synthesis and human neurodegenerative disease.
Other pathological changes evoke dysfunction of both local translation and degradation (Fig. 5d,e). Isolated 3′ UTRs, which are generated by activity-dependent transcript cleavage, accumulate in the neurites of aged brains and can stall ribosomes, impairing local protein synthesis. These UTRs can also be noncanonically translated, producing short peptides with a high propensity for generating neuroinflammatory responses119. Local clearance of RNA fragments is incompletely understood but might depend on coordinated action between P-bodies and autophagy machinery120. Finally, solid aggregates of TDP-43, a pathognomonic sign for ALS and FTD, are often hyperphosphorylated121. Because phosphorylation itself hardens TDP-43 granules, an imbalance of post-translational modifications regulating transport granule condensation might be a contributing factor for sporadic cases of ALS and FTD. Inadequate local clearance mechanisms might also be responsible for the frequent axonal aggregates seen in sporadic ALS122.
Outlook and perspectives
In just the past decade, considerable progress has been made in identifying the components of the RTA, characterizing their functions and translating that knowledge into a better understanding of neurological disease. A major future challenge will be assembling signaling relationships among extracellular stimuli, intracellular cascades and the RTA, to gain a deep understanding of how RNA localization is regulated. Key unknowns include how transport granules assemble and acquire motors, if granule remodeling occurs during transport, how the RTA switches motors and cytoskeletal tracks and how interactions with organelles (for example, mitochondria and ER) affect RNA localization and local metabolism. Unbiased subcellular approaches (for example, proximity labeling) and spatiotemporal monitoring of granule dynamics will be important tools for answering these questions.
Clarifying the rules for local translation is another important goal. Understanding how many times and under what circumstances a single mRNA is locally translated will provide insight into its functional role and relative importance at a distant site. A recent study has begun to address this question, identifying more than 4,800 mRNAs as part of the ‘local translatome’ and characterizing features such as 3′ UTR length and upstream open reading frames as determinants of translational efficiency123. Likewise, characterizing proteins that localize to neurites but are not synthesized there will help define the functional niche of local translation in general. Finally, rigorous interrogation of observed exceptions to established rules, such as translation occurring within intact transport granules, will allow the field to evolve and decode complex mechanisms124,125.
Accumulating evidence suggests that LE/Lys play a prominent role in the local metabolism of neuronal processes. Apart from acting as a vehicle for RNA and, potentially, ribosome localization to distant sites, LE/Lys have recently been shown to enact local degradation in axon termini126. Coupled to their association with axonal local translation sites and an ability to undertake regulated amino acid efflux in response to nutrient cues, LE/Lys are an ideal candidate for a consolidated local proteostatic platform84,127. Previous work showed that dendrites recruit proteasomes for local degradation, so LE/Lys might function uniquely in axons (Fig. 5d)128. Profiling local proteomes to distinguish proteasome and micro/ macro-autophagy substrates will be an important step toward specifying LE/Lys activity in neurites.
The intimate connection between the RTA and neurodegeneration suggests that dissecting the molecular phenotypes of neurodegenerative disease will continue to reveal important clues for answering multiple open questions in the field. For example, monitoring RNA transport in lysosomal storage diseases might reveal specific luminal or membrane proteins that regulate LE/Lys interactions with motors or transport granules. As understanding of RNA transport, local metabolism and their connection to neurodegeneration deepens, we will be better equipped to devise innovative therapies to manipulate RNA localization and preserve function for patients suffering from disease.
Box 1 |. Conceptual advantages to RNA localization in neurons.
- Metabolic economy
- Neuronal metabolism supports near-constant activity through the firing of action potentials, synthesis and release of neurotransmitters and simultaneous transduction of signals from tens of thousands of other neurons. Long-lived transcripts that produce many copies of protein at a distant site circumvent the energetically expensive task of repeated protein trafficking.
- Spatial restriction of protein activity
- Unique, spatially discrete structural and functional elements endow the neuron with its polarized architecture. Certain proteins, such as those involved in pre- and post-synaptic biogenesis and maintenance, might themselves confer spatial identity and need to be locally synthesized to prevent aberrant specification.
- High temporal control
- Specific responses to different stimuli (for example, excitatory/inhibitory neural signals) require different sets of protein effectors. Because neurons must respond rapidly to these stimuli, triggered synthesis on site enables a much faster response than transport of previously synthesized proteins over a long distance.
- Fresh protein vs old protein
- A protein experiences several modifications during its lifetime, both physiological (for example, phosphorylation and ubiquitination) and pathological (for example, oxidative damage and aggregation). Production of fresh protein at a distal site circumvents protein damage during the long journey from the soma.
Acknowledgements
We thank B. Wu, A. Saric, J. Nixon-Abell, S. Qamar, S. Humble and J. Bonifacino for their careful reading and helpful comments on the manuscript, and we thank all of the laboratories and scientists who contributed to the data and discoveries described here. This work was supported by National Institute on Aging grant F30AG060722 (to M.S.F.), the NIH-Oxford-Cambridge Scholars Program (to M.S.F.), the Howard Hughes Medical Institute (to J.L.-S.) and the NIH Intramural Research Program(to M.E.W.)
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information Nature Neuroscience thanks Gary Bassell, Christine Holt, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Holt CE, Martin KC & Schuman EM Local translation in neurons: visualization and function. Nat. Struct. Mol. Biol. 26, 557–566 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Cagnetta R, Frese CK, Shigeoka T, Krijgsveld J & Holt CE Rapid cue-specific remodeling of the nascent axonal proteome. Neuron 99, 29–46 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell DS & Holt CE Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32, 1013–1026 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Huber KM, Kayser MS & Bear MF Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288, 1254–1256 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Kang H & Schuman EM A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science 273, 1402–1406 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Martin KC et al. Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell 91, 927–938 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Ostroff LE, Fiala JC, Allwardt B & Harris KM Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron 35, 535–545 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Schanzenbächer CT, Sambandan S, Langer JD & Schuman EM Nascent proteome remodeling following homeostatic scaling at hippocampal synapses. Neuron 92, 358–371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fallini C, Donlin-Asp PG, Rouanet JP, Bassell GJ & Rossoll W Deficiency of the survival of motor neuron protein impairs mrna localization and local translation in the growth cone of motor neurons. J. Neurosci. 36, 3811–3820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Erauskin J et al. ALS/FTD-linked mutation in FUS suppresses intra-axonal protein synthesis and drives disease without nuclear loss-of-function of FUS. Neuron 100, 816–830 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami T et al. ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron 88, 678–690 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qamar S et al. FUS phase separation is modulated by a molecular chaperone and methylation of arginine cation-π interactions. Cell 173, 720–734 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehringer A & Bowser R RNA nucleocytoplasmic transport defects in neurodegenerative diseases. Adv. Neurobiol. 10, 509–518 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Taylor JP, Brown RH & Cleveland DW Decoding ALS: from genes to mechanism. Nature 539, 197–206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maday S, Twelvetrees AE, Moughamian AJ & Holzbaur ELF Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron 84, 292–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang HL et al. Neurotrophin-induced transport of a β-actin mRNP complex increases β-actin levels and stimulates growth cone motility. Neuron 31, 261–275 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Nicastro G et al. Mechanism of β-actin mRNA recognition by ZBP1. Cell Rep. 18, 1187–1199 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura R, Shenker S, Andreu-Agullo C, Westholm JO & Lai EC Widespread and extensive lengthening of 39 UTRs in the mammalian brain. Genome Res. 23, 812–825 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taliaferro JM et al. Distal alternative last exons localize mrnas to neural projections. Mol. Cell 61, 821–833 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tushev G et al. Alternative 3′ UTRs modify the localization, regulatory potential, stability, and plasticity of mrnas in neuronal compartments. Neuron 98, 495–511 (2018). [DOI] [PubMed] [Google Scholar]
- 21.An JJ et al. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 134, 175–187 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreassi C et al. 3′UTR cleavage of transcripts localized in axons of sympathetic neurons. Preprint at https://www.biorxiv.org/content/10.1101/170100v2(2020).
- 23.Vejnar CE et al. Genome wide analysis of 3′ UTR sequence elements and proteins regulating mRNA stability during maternal-to-zygotic transition in zebrafish. Genome Res. 29, 1100–1114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh WS, Porter JR & Batchelor E Tuning of mRNA stability through altering 3′-UTR sequences generates distinct output expression in a synthetic circuit driven by p53 oscillations. Sci. Rep 9, 1–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merianda TT, Gomes C, Yoo S, Vuppalanchi D & Twiss JL Axonal localization of neuritin/CPG15 mRNA in neuronal populations through distinct 5′ and 3′ UTR elements. J. Neurosci. 33, 13735–13742 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meer EJ et al. Identification of a cis-acting element that localizes mRNA to synapses. Proc. Natl Acad. Sci. USA 109, 4639–4644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotem N et al. ALS along the axons - expression of coding and noncoding RNA differs in axons of ALS models. Sci. Rep. 7, 1–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forrest KM & Gavis ER Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr. Biol. 13, 654–658 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Zaessinger S, Busseau I & Simonelig M Oskar allows nanos mRNA translation in Drosophila embryos by preventing its deadnylation by Smaug/ CCR4. Development 133, 4573–4583 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Tadros W et al. SMAUG Is a major regulator of maternal mRNA destabilization in Drosophila and its translation is activated by the PAN GU kinase. Dev. Cell 12, 143–155 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Han S et al. RNA-protein interaction mapping via MS2- or Casl3-based APEX targeting. Proc. Natl Acad. Sci. USA 117, 22068–22079 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee J et al. β-actin mRNA interactome mapping by proximity biotinylation. Proc. Natl Acad. Sci. USA 116, 12863–12872 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain A & Vale RD RNA phase transitions in repeat expansion disorders. Nature 546, 243–247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ling SC, Polymenidou M & Cleveland DW Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 79, 416–438 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreiter N et al. Age-dependent neurodegeneration and organelle transport deficiencies in mutant TDP43 patient-derived neurons are independent of TDP43 aggregation. Neurobiol. Dis. 115, 167–181 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Daigle GG et al. RNA-binding ability of FUS regulates neurodegeneration, cytoplasmic mislocalization and incorporation into stress granules associated with FUS carrying ALS-linked mutations. Hum. Mol. Genet. 22, 1193–1205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voigt A et al. TDP-43-mediated neuron loss in vivo requires RNA-binding activity. PLoS ONE 5, e12247 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller S et al. Disruption of dendritic translation of CaMKIIα impairs stabilization of synaptic plasticity and memory consolidation. Neuron 36, 507–519 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Chia PH et al. A homozygous loss-of-function CAMK2A mutation causes growth delay, frequent seizures and severe intellectual disability. eLife 7, 1–19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merkurjev D et al. Synaptic N6-methyladenosine (m6A) epitranscriptome reveals functional partitioning of localized transcripts. Nat. Neurosci. 21, 1004–1014 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Darnell RB RNA protein interaction in neurons. Annu. Rev. Neurosci. 36, 243–270 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corley M, Burns MC & Yeo GW How RNA-binding proteins interact with RNA: molecules and mechanisms. Mol. Cell 78, 9–29 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knowles RB et al. Translocation of RNA granules in living neurons. J. Neurosci. 16, 7812–7820 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krichevsky AM & Kosik KS Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron 32, 683–696 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Turner-Bridger B et al. Single-molecule analysis of endogenous β-actin mRNA trafficking reveals a mechanism for compartmentalized mRNA localization in axons. Proc. Natl Acad. Sci. USA 115, E9697–E9706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Batish M, Van Den Bogaard P, Kramer FR & Tyagi S Neuronal mRNAs travel singly into dendrites. Proc. Natl Acad. Sci. USA 109, 4645–4650 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mikl M, Vendra G & Kiebler MA Independent localization of MAP2, CaMKIIα and β-actin RNAs in low copy numbers. EMBO Rep. 12, 1077–1084 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin KC & Ephrussi A mRNA localization: gene expression in the spatial dimension. Cell 136, 719–730 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brangwynne CP et al. Germline P granules are liquid droplets and localize by dissolution/condensation. Science 324,1729–1732(2009). [DOI] [PubMed] [Google Scholar]
- 50.Donlin-Asp PG et al.The survival of motor neuron protein acts as a molecular chaperone for mRNP assembly.Cell Rep.18,1660–1673(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossoll W et al.Smn,the spinal muscular atrophy-determining gene product, modulates axon growth and localization of β-actin mRNA in growth cones of motoneurons. J. CellBiol 163, 801–812(2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato M et al.Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149,753–767 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel A et al.A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162, 1066–1077(2015). [DOI] [PubMed] [Google Scholar]
- 54.Conicella AE et al. TDP-43 α-helical structure tunes liquid–liquid phase separation and function. Proc. Natl Acad. Sci. USA 117, 5883–5894 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alami NH et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron 81,536–543(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gopal PP, Nirschl JJ, Klinman E & Holzbaur ELF Amyotrophic lateral sclerosis-linked mutations increase the viscosity of liquid-like TDP-43 RNP granules in neurons. Proc. Natl Acad. Sci. USA 114, E2466–E2475(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu-Yesucevitz LQ et al. ALS-linked mutations enlarge TDP-43-enriched neuronal RNA granules in the dendritic arbor. J. Neurosci.34,4167–4174 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujii R & Takumi T TLS facilitates transport of mRNA encoding an actin-stabilizing protein to dendritic spines. J. Cell Sci. 118, 5755–5765 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Yasuda K,Clatterbuck-Soper SF, Jackrel ME, Shorter J & Mili S FUS inclusions disrupt RNA localization by sequestering kinesin-1 and inhibiting microtubule detyrosination. J. CellBiol 216, 1015–1034(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jun MH et al.Sequestration of PRMT1 and Nd1-L mRNA into ALS-linked FUS mutant R521C-positive aggregates contributes to neurite degeneration upon oxidative stress.Sci. Rep. 7, 1–15(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maharana S et al. RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science 921, 918–921(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ascano M et al.FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature 492, 382–386(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Darnell JC et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261(2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Napoli I et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell 134, 1042–1054(2008). [DOI] [PubMed] [Google Scholar]
- 65.Antar LN, Afroz R, Dictenberg JB, Carroll RC & Bassell GJ Metabotropic glutamate receptor activation regulates fragile X mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J. Neurosci. 24, 2648–2655 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antar LN, Dictenberg JB, Plociniak M, Afroz R & Bassell GJ Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 4, 350–359 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Dictenberg JB, Swanger SA, Antar LN, Singer RH & Bassell GJ A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev. Cell 14, 926–939 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanai Y, Dohmae N & Hirokawa N Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron 43, 513–525 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Tsang B et al. Phosphoregulated FMRP phase separation models activity-dependent translation through bidirectional control of mRNA granule formation. Proc. Natl Acad. Sci. USA 116, 4218–4227 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banerjee A, Ifrim MF, Valdez AN, Raj N & Bassell GJ Aberrant RNA translation in fragile X syndrome: from FMRP mechanisms to emerging therapeutic strategies. Brain Res. 1693, 24–36 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Udagawa T et al. Genetic and acute CPEB1 depletion ameliorate fragile X pathophysiology. Nat. Med. 19, 1473–1477 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ross Buchan J MRNP granules assembly, function, and connections with disease. RNA Biol. 11, 1019–1030 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davidovic L et al. The fragile X mental retardation protein is a molecular adaptor between the neurospecific KIF3C kinesin and dendritic RNA granules. Hum. Mol. Genet. 16, 3047–3058 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Fukuda Y et al. Fast transport of RNA granules by direct intreractions with KIF5A/KLC1 motors prevents axon degeneration. Preprint at https://www.biorxiv.org/content/10.1101/2020.02.02.931204vl.full (2020).*
- 75.Wu H, Zhou J, Zhu T, Cohen I & Dictenberg J A kinesin adapter directly mediates dendritic mRNA localization during neural development in mice. J. Biol. Chem. 295, 6605–6628 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baumann S et al. A reconstituted mammalian APC-kinesin complex selectively transports defined packages of axonal mRNAs. Sci. Adv 6, eaazl588 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bianco A, Dienstbier M, Salter HK, Gatto G & Bullock SL Bicaudal-D regulates fragile X mental retardation protein levels, motility, and function during neuronal morphogenesis. Curr. Biol. 20, 1487–1492 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dienstbier M, Boehl F, Li X & Bullock SL Egalitarian is a selective RNA-binding protein linking mRNA localization signals to the dynein motor. Genes Dev. 23, 1546–1558 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baumann S, Pohlmann T, Jungbluth M, Brachmann A & Feldbrugge M Kinesin-3 and dynein mediate microtubule-dependent co-transport of mRNPs and endosomes. J. Cell Sci. 125, 2740–2752 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Baumann S, Konig J, Koepke J & Feldbrugge M Endosomal transport of septin mRNA and protein indicates local translation on endosomes and is required for correct septin filamentation. EMBO Rep. 15, 94–102 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pohlmann T, Baumann S, Haag C, Albrecht M & Feldbrugge M A FYVE zinc finger domain protein specifically links mRNA transport to endosome trafficking. eLife 4, 1–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gershoni-Emek N et al. Localization of RNAi machinery to axonal branch points and growth cones is facilitated by mitochondria and is disrupted in ALS. Front. Mol. Neurosci 11, 1–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Corradi E et al. Axonal precursor miRNA s hitchhike on endosomes and locally regulate the development of neural circuits. EMBO J. 39, 1–24 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cioni J et al. Late endosomes act as mRNA translation platforms and sustain mitochondria in axons. Cell 176, 56–72 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liao Y et al. RNA granules hitchhike on lysosomes for long-distance transport, using annexin All as a molecular tether. Cell 179, 147–164 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vukoja A et al. Presynaptic biogenesis requires axonal transport of lysosome-related vesicles. Neuron 99, 1216–1232 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Zala D et al. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell 152, 479–491 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Oates EC et al. Mutations in BICD2 cause dominant congenital spinal muscular atrophy and hereditary spastic paraplegia. Am. J. Hum. Genet. 92, 965–973 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verhoeven K et al. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am. J. Hum. Genet. 1, 722–727 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pu J, Guardia CM, Keren-Kaplan T & Bonifacino JS Mechanisms and functions of lysosome positioning. J. Cell Sci. 129, 4329–4339 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith BN et al. Mutations in the vesicular trafficking protein annexin All are associated with amyotrophic lateral sclerosis. Sci. Transl. Med 9, eaad9157 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang K et al. ANXA11mutations prevail in Chinese ALS patients with and without cognitive dementia. Neurol. Genet 4, e237–e239 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu X, Wu C, He J, Zhang N & Fan D Two rare variants of the ANXA11 gene identified in Chinese patients with amyotrophic lateral sclerosis. Neurobiol. Aging 74, 235.e9–235.e12 (2019). [DOI] [PubMed] [Google Scholar]
- 94.Willis DE et al. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J. Cell Biol. 178, 965–980 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huttelmaier S et al. Spatial regulation of β-actin translation by Src-dependent phosphorylation of ZBP1. Nature 438, 1–4 (2005). [DOI] [PubMed] [Google Scholar]
- 96.Monahan Z et al. Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 36, 2951–2967 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ryan VH et al. Tyrosine phosphorylation regulates hnRNPA2 granule protein partitioning & reduces neurodegeneration. Preprint at https://www.biorxiv.org/content/10.1101/2020.03.15.992768v1.full (2020). [DOI] [PMC free article] [PubMed]
- 98.Nonaka T et al. Phosphorylation of TAR DNA-binding protein of 43 kDa (TDP-43) by truncated casein kinase 1d triggers mislocalization and accumulation of TDP-43. J. Biol. Chem. 291, 5473–5483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amaya J, Ryan VH & Fawzi NL The SH3 domain of Fyn kinase interacts with and induces liquid–liquid phase separation of the low-complexity domain of hnRNPA2. J. Biol. Chem. 293, 19522–19531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Narayanan U et al. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J. Neurosci. 27, 14349–14357 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Narayanan U et al. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J. Biol. Chem. 283, 18478–18482 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ryan VH et al. Mechanistic view of hnRNPA2 low-complexity domain structure, interactions, and phase separation altered by mutation and arginine methylation. Mol. Cell 69, 465–479 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fontes MM et al. Activity-dependent regulation of alternative cleavage and polyadenylation during hippocampal long-term potentiation. Sci. Rep. 7, 1–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Udagawa T et al. Bidirectional control of mRNA translation and synaptic plasticity by the cytoplasmic polyadenylation complex. Mol. Cell 47, 253–266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sambandan S et al. Activity-dependent spatially localized miRNA maturation in neuronal dendrites. Science 355, 634–637 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Kiebler MA & Bassell GJ Neuronal RNA granules: movers and makers. Neuron 51, 685–690 (2006). [DOI] [PubMed] [Google Scholar]
- 107.Zeitelhofer M et al. Dynamic interaction between P-bodies and transport ribonucleoprotein particles in dendrites of mature hippocampal neurons. J. Neurosci. 28, 7555–7562 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kwak JE et al. GLD2 poly(A) polymerase is required for long-term memory. Proc. Natl Acad. Sci. USA 105, 14644–14649 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Biever A et al. Monosomes actively translate synaptic mRNAs in neuronal processes. Science 367, eaay4991 (2020). [DOI] [PubMed] [Google Scholar]
- 110.Fazal FM et al. Atlas of subcellular RNA localization revealed by APEX-Seq. Cell 178, 473–490 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shigeoka T et al. On-site ribosome remodeling by locally synthesized ribosomal proteins in axons. Cell Rep. 29, 3605–3619 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blackwell E, Zhang X & Ceman S Arginines of the RGG box regulate FMRP association with polyribosomes and mRNA. Hum. Mol. Genet. 19, 1314–1323 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Anderson P & Kedersha N RNA granules. J. Cell Biol. 172, 803–808 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tcherkezian J, Brittis PA, Thomas F, Roux PP & Flanagan JG Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell 141, 632–644 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koppers M et al. Receptor-specific interactome as a hub for rapid cue-induced selective translation in axons. eLife 8, 1–27 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Higuchi Y, Ashwin P, Roger Y & Steinberg G Early endosome motility spatially organizes polysome distribution. J. Cell Biol. 204, 343–357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sephton CF et al. Activity-dependent FUS dysregulation disrupts. Proc. Natl Acad. Sci. USA 111, E4769–E4778 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoon BC et al. Local translation of extranuclear lamin B promotes axon maintenance. Cell 148, 752–764 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sudmant PH, Lee H, Dominguez D, Heiman M & Burge CB Widespread accumulation of ribosome-associated isolated 3′ UTRs in neuronal cell populations of the aging brain. Cell Rep. 25, 2447–2456 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Frankel LB, Lubas M & Lund AH Emerging connections between RNA and autophagy. Autophagy 13, 3–23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neumann M et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006). [DOI] [PubMed] [Google Scholar]
- 122.Onozato T et al. Axonal TDP-43 aggregates in sporadic amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 42, 561–572 (2016). [DOI] [PubMed] [Google Scholar]
- 123.Glock C et al. The mRNA translation landscape in the synaptic neuropil. Preprint at https://www.biorxiv.org/content/10.1101/2020.06.09.141960v1 (2020).
- 124.Yasuda K et al. The RNA-binding protein Fus directs translation of localized mRNAs in APC-RNP granules. J. Cell Biol. 203, 737–746 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu B, Eliscovich C, Yoon YJ & Singer RH Translation dynamics of single mRNAs in live cells and neurons. Science 352, 1430–1435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Farfel-Becker T et al. Neuronal soma-derived degradative lysosomes are continuously delivered to distal axons to maintain local degradation capacity. Cell Rep. 28, 51–64 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abu-Remaileh M et al. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 358, 807–813 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bingol B & Schuman EM Activity-dependent dynamics and sequestration of proteasomes in dendritic spines. Nature 441, 1144–1148 (2006). [DOI] [PubMed] [Google Scholar]