Abstract
STUDY OBJECTIVE:
Non-fatal emergency department (ED) visits for opioid overdose are important opportunities to prescribe naloxone and buprenorphine, both of which can prevent future overdose-related mortality. We assessed the rate of this prescribing using national data from August 2019 to April 2021, a period during which U.S. opioid overdose deaths reached record levels.
METHODS:
We conducted a retrospective cohort analysis using Symphony Health’s Integrated Dataverse, which includes data from 5,800 hospitals and 70,000 pharmacies. Of ED visits for opioid overdose between August 4, 2019-April 3, 2021, we calculated the proportion with ≥1 naloxone prescription within 30 days and repeated this analysis for buprenorphine. To contextualize the naloxone prescribing rate, we calculated the proportion of ED visits for anaphylaxis with ≥1 prescription for epinephrine – another life-saving rescue medication – within 30 days.
RESULTS:
Analyses included 148,966 ED visits for opioid overdose. Mean weekly visits increased approximately 23% between August 4, 2019-April 25, 2020 and April 26, 2020, declined to pre-pandemic levels between October 4, 2020-March 13, 2021, and began rising during March 14, 2021-April 3, 2021. Naloxone and buprenorphine were prescribed within 30 days of 7.4% and 8.5% of the 148,966 visits. The naloxone prescribing rate (7.4%) was substantially lower than the epinephrine prescribing rate (48.9%) after ED visits for anaphylaxis.
CONCLUSIONS:
Between August 4, 2019-April 3, 2021, naloxone and buprenorphine were only prescribed after 1 in 13 and 1 in 12 ED visits for opioid overdose, respectively. Findings suggest that clinicians are missing critical opportunities to prevent opioid overdose-related mortality.
INTRODUCTION
Background.
Among patients with non-fatal emergency department (ED) visits for opioid overdose, 5.5% die within one year.1 Consequently, these visits represent important opportunities to prescribe medications that can prevent future opioid overdose-related mortality. These medications include naloxone, a rapidly-acting opioid antagonist that can be administered by others to reverse overdose, and buprenorphine, a partial opioid agonist approved to treat opioid use disorder.1,2 Although maximizing naloxone and buprenorphine prescribing following ED visits for opioid overdose was crucial even before the U.S. outbreak of coronavirus disease 2019 (COVID-19), the urgency of achieving this goal has only increased since the pandemic began. National data suggest that the number of ED visits for opioid overdose rose sharply between late April and early October 20203, while provisional data suggest that a record 71,000 U.S. opioid overdose deaths occurred in 2020.4
Importance.
Despite the increasing importance of naloxone and buprenorphine prescribing after ED visits for opioid overdose, recent data on this prescribing are limited to single institutions.5 Two prior national studies used pre-2019 commercial claims data to examine naloxone dispensing within 30 days of an opioid-related ED visit and to examine the receipt of treatment for opioid use disorder, including buprenorphine, within 90 days of an ED visit for opioid overdose.6,7 While important studies, the generalizability of findings to the current era and to other payer types is unknown. Prior studies have also documented decreases in the number of patients filling naloxone prescriptions and the number of dispensed buprenorphine prescriptions during the COVID-19 pandemic.8–10 However, these studies only assessed overall trends and did not specifically evaluate rates of naloxone and buprenorphine prescribing following ED visits for opioid overdose.
Goals of this investigation.
In this study, we used a national, all-payer database to assess the rate of naloxone and buprenorphine prescribing within 30 days of an ED visit for opioid overdose between August 4, 2019-April 3, 2021. To our knowledge, our study provides the most recent national data to date on naloxone and buprenorphine prescribing after ED visits for opioid overdose, as well as the most recent national data on trends in ED visits for opioid overdose during the COVID-19 pandemic.3
METHODS
Study design and setting.
In spring 2021, we conducted a retrospective cohort analysis of pharmacy and medical claims in Symphony Health’s Integrated Dataverse. These data have been used in prior research, predominantly to assess trends in pharmacy dispensing.8,9,11–14 Data were available via the COVID-19 Research Database, a consortium that is providing researchers free access to several proprietary health care databases via a data enclave. Access to the enclave is granted to researchers after approval of a project proposal. Symphony Health data from May 2019 onwards are available for analysis in the enclave.
Symphony’s pharmacy claims capture 93% of dispensed U.S. prescriptions and derive from over 70,000 U.S. pharmacies.15 Medical claims derive from claim clearinghouses that submit pre-adjudicated claims from clinicians and hospitals to insurers. During 2020, the database contained ≥1 medical claim from 156.5 million unique patients, representing just under half of all Americans.16 Of these patients, the method of payment for the earliest medical claim during 2020 was commercial insurance (75.4%), Medicare (11.7%), Medicaid (9.5%), other government (1.4%), unknown (1.6%), and cash (i.e., claims for insured patients who choose to pay with cash; 0.2%). This distribution suggests the database over-represents the commercially insured and under-represents Medicaid and uninsured patients.17 Symphony Health medical claims draw from 5,800 hospitals15, out of 6,100 hospitals in the U.S.18 However, our analysis suggests the database captures approximately one-third of U.S. ED visits, indicating that not every ED visit from the 5,800 hospitals is included. Appendix 1 describes this analysis as well as several other analyses that we conducted to assess the validity of our data. For example, we found similar results when replicating analyses in two commonly used claims databases. Because data were de-identified, the Institutional Review Board of the University of Michigan Medical School exempted analyses from human subjects review. This study follows the STROBE reporting guidelines for observation studies.
Data elements include International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes, Current Procedure Terminology (CPT) codes, national drug codes, and encrypted patient identifiers. Patient demographic information includes gender, age, and two-digit zip code of residence, which we mapped to Census regions (Appendix 2). Although patient race and ethnicity are recorded, we do not report this information owing to high rates of missing data and lack of detail regarding how race and ethnicity were measured. Unlike claims databases that only capture dispensed prescriptions paid by insurance, such as Medicare claims, our database captures cash-pay prescriptions. Moreover, our database contains a pharmacy claim for all electronic prescriptions received by contributing pharmacies and all paper prescriptions dropped off at these pharmacies, regardless of whether medications were dispensed. Each pharmacy claim contains a flag for whether the claim was “paid”, resulting in dispensing, or not paid (e.g., the prescription was rejected by insurers or not filled by the patient). The database does not report mortality.
The study period was August 4, 2019-April 3, 2021. We extracted data on June 4, 2021, thus allowing at least a 2-month lag for medical claims through April 3, 2021 and at least a 1-month lag for pharmacy claims through May 2, 2021, the end of the 30-day look-forward period for ED visits on April 3, 2021. We verified these lags were sufficient for claims completeness by documenting minimal changes between serial data extracts (Appendix 3).
Sample.
We identified claims for ED visits using CPT codes for ED evaluation and management. We defined an ED visit for opioid overdose as the occurrence of ≥1 ED claim containing a diagnosis code for opioid overdose in any position on the claim. We defined an ED visit for other conditions as the occurrence of ≥1 ED claim that lacked a diagnosis code for opioid overdose and the absence of ED claims on the same date that did contain such a code. For example, if two ED claims occurred on the same date, one with and one without a diagnosis code for opioid overdose, we considered an ED visit for opioid overdose to have occurred on that date. Because a single visit can generate multiple claims (e.g., professional and facility claims), we only allowed patients to have one ED visit per day. Patients could contribute multiple ED visits during the study period. Appendix 4 lists codes used in analyses.
Outcomes.
We identified pharmacy claims for naloxone and buprenorphine using national drug codes (Appendix 5). We only included buprenorphine formulations approved for opioid use disorder. We defined a “prescription” as any pharmacy claim, regardless of whether the claim was paid and resulted in dispensing. We defined a “dispensed prescription” as a paid pharmacy claim.
Among all ED visits for opioid overdose during the study period, we calculated the naloxone prescribing rate, defined as the proportion of visits with ≥1 naloxone prescription within 30 days (i.e., ≥1 prescription on the visit date or during the following 30 days). We also calculated the proportion of visits with ≥1 dispensed naloxone prescription within 30 days and the proportion with ≥1 dispensed naloxone prescription during the 90 days to 1 day prior to the visit (to assess the degree to which rates of subsequent naloxone prescribing might have been influenced by the prior possession of naloxone prescriptions). Finally, among the subset of visits that were for patients without naloxone dispensing during the 90 days to 1 day prior to the visit, we calculated the initial naloxone prescribing rate, defined as the proportion with ≥1 naloxone prescription within 30 days (to assess the rate of initial prescribing of naloxone to patients new to this medication). We repeated these analyses for buprenorphine. In subgroup analyses, we calculated the naloxone and buprenorphine prescribing rates among demographic groups defined by patient sex, age group, method of payment for the ED visit, and Census region of patient residence.
To contextualize the naloxone prescribing rate, we calculated the proportion of ED visits for anaphylaxis with ≥1 prescription for epinephrine – another life-saving rescue medication –within 30 days. Just as naloxone prescribing is recommended to prevent opioid overdose-related mortality, epinephrine prescribing is recommended to prevent anaphylaxis-related mortality.19 We identified ED visits for anaphylaxis using a similar approach as for opioid overdose visits (Appendix 4). Epinephrine prescriptions included those for auto-injectors and pre-filled syringes (Appendix 5).
Analysis.
Using descriptive statistics, we assessed the demographic characteristics of patients with ED visits for opioid overdose. We calculated 95% confidence intervals for proportions using exact binominal tests. To assess adjusted changes in naloxone and buprenorphine prescribing rates over time, we fitted logistic regression models with generalized estimating equations, an exchangeable correlation structure, and robust standard errors clustered at the patient level. Models were conducted at the patient level and included a continuous variable for three-week increment as well as indicators for patient sex, age group, method of payment for the ED visit, and census region of patient residence. To facilitate interpretation of results as absolute percentage-point changes in probability rather than odds ratios, we calculated average marginal effects for week and for each demographic characteristic.20 Analyses used R 4.0.1, Stata 15.1 MP, and two-sided hypothesis tests with α = 0.05.
We conducted 4 sensitivity analyses. First, to assess prescribing rates over a longer period, we calculated the proportion of ED visits for opioid overdose with ≥1 naloxone prescription within 90 days and repeated this calculation for buprenorphine. We restricted these analyses to visits through January 30, 2021 to allow for pharmacy claims completeness. Second, to assess prescribing potentially attributable to ED clinicians, we assessed prescribing within 7 days of visits rather than 30 days. Third, to assess the receipt of any treatment for opioid use disorder, we calculated the proportion of ED visits for opioid overdose with any of the following within 30 days: ≥1 dispensed buprenorphine prescription, ≥1 dispensed extended-release naltrexone prescription (Vivitrol), or ≥1 medical claim for opioid use disorder treatment, including methadone outpatient treatment programs (Appendix 6). Finally, we limited to each patient’s first ED visit for opioid overdose during the study period.
RESULTS
Sample characteristics.
Of 68,072,261 ED visits during the 87-week study period, 148,966 (0.2%) were for opioid overdose. Among these 148,966 visits, 53,073 (35.6%) were for females, 62,689 (42.1%) were for patients aged 18–34 years, and 46,931 (31.5%) were for patients residing in the Midwest. The most common methods of payment for the ED visit were commercial insurance (65.2%), Medicaid (26.7%), and Medicare (5.6%) (Table 1).
Table 1.
Naloxone and buprenorphine prescribing within 30 days of an ED visit for opioid overdose, August 4, 2019-April 3, 2021, Symphony Health Integrated Dataverse
| Group | Number of ED visits for opioid overdose (% of sample)a | Naloxone prescribing rateb | Buprenorphine prescribing rateb | ||
|---|---|---|---|---|---|
| Number | Number | % (95% CI)b | Number | % (95% CI) b | |
| All ED visits for opioid overdose | 148,966 (100.0%) | 11,010 | 7.4% (7.3%–7.5%) | 12,608 | 8.5% (8.3%–8.6%) |
| Sex | |||||
| Female | 53,073 (35.6%) | 7,070 | 7.4% (7.2%–7.7%) | 4,021 | 7.6% (7.4%–7.8%) |
| Male | 95,893 (64.4%) | 3,940 | 7.4% (7.2%–7.5%) | 8,587 | 9.0% (8.8%–9.1%) |
| Age group c | |||||
| 0–17 | 3,525 (2.4%) | 149 | 4.2% (3.6%–4.9%) | 36 | 1.0% (0.7%–1.1%) |
| 18–25 | 20,539 (13.8%) | 1,742 | 8.5% (8.1%–8.7%) | 1,648 | 8.0% (7.7%–8.4%) |
| 26–34 | 42,150 (28.3%) | 3,382 | 8.0% (7.8%–8.3%) | 4,725 | 11.2% (10.9%–11.5%) |
| 35–44 | 31,868 (21.4%) | 2,342 | 7.3% (7.1%–7.6%) | 3,233 | 10.1% (9.8%–10.5%) |
| 45–54 | 22,447 (15.1%) | 1,592 | 7.1% (6.8%–7.4%) | 1,696 | 7.6% (7.2%–7.9%) |
| 55–64 | 19,824 (13.3%) | 1,331 | 6.7% (6.4%–7.1%) | 1,042 | 5.3% (5.0%–5.6%) |
| 65–74 | 6,615 (4.4%) | 398 | 6.0% (5.4%–6.6%) | 213 | 3.2% (2.8%–3.7%) |
| 75 and older | 1,855 (1.2%) | 74 | 4.0% (3.1%–4.9%) | 15 | 0.8% (0.5%–1.3%) |
| Unknown | 143 (0.1%) | 0d | 0.0% (0.0%–0.0%) | 0d | 0.0% (0.0%–0.0%) |
| Method of payment for ED visit | |||||
| Commercial | 97,113 (65.2%) | 6,791 | 7.0% (6.8–7.2%) | 8,407 | 8.7% (8.5%–8.8%) |
| Medicare | 8,405 (5.6%) | 594 | 7.1% (6.5%–7.6%) | 7,957 | 5.3% (4.9%–5.8%) |
| Medicaid | 39,847 (26.7%) | 3,431 | 8.6% (8.3%–8.9%) | 3,561 | 8.9% (8.7%–9.2%) |
| Cash | 994 (0.7%) | 64 | 6.4% (4.9%–8.0%) | 949 | 4.5% (3.2%–5.8%) |
| Other government | 1,201 (0.8%) | 58 | 4.8% (3.7%–6.2%) | 43 | 3.6% (2.5%–4.6%) |
| Unknown | 1,406 (0.9%) | 72 | 5.1% (4.0–6.3%) | 104 | 7.4% (6.1%–8.9%) |
| Region of patient residence | |||||
| Northeast | 35,784 (24.0%) | 3,121 | 8.7% (8.4%–9.0%) | 3,977 | 11.1% (10.8%–11.4%) |
| Midwest | 46,931 (31.5%) | 3,222 | 6.9% (6.6%–7.1%) | 4,396 | 9.4% (9.1%–9.6%) |
| South | 43,312 (29.1%) | 2,881 | 6.7% (6.4%–6.9%) | 2,900 | 6.7% (6.5%–6.9%) |
| West | 19,994 (13.4%) | 1,678 | 8.4% (8.0%–8.8%) | 1,233 | 6.2% (5.8%–6.5%) |
| Unknown | 2,945 (2.0%) | 108 | 3.7% (3.0%–4.4%) | 102 | 3.5% (2.8%–4.1%) |
ED – emergency department
Defined as the proportion of visits with ≥1 pharmacy claim for the medication within 30 days, regardless of whether the claim was paid and resulted in dispensing
The percentages in the first column are column percentages (i.e., the percentage of the 148,966 visits in the sample), while those in the second and third columns are row percentages (i.e., percentage of visits in the group).
Mean age was 39.5 years (SD 14.7), but a caveat is that age is top-coded in the data, so the true mean is higher.
It is unclear whether these 143 visits truly had no naloxone or buprenorphine prescribing within 30 days or whether this reflect data error. We retained these visits in the sample rather than excluding them owing to our desire to avoid sample exclusions for the purpose of modeling trends in ED visits for opioid overdose. Excluding these visits would have changed results minimally because they were infrequent.
Trends in ED visits for opioid overdose and for other conditions.
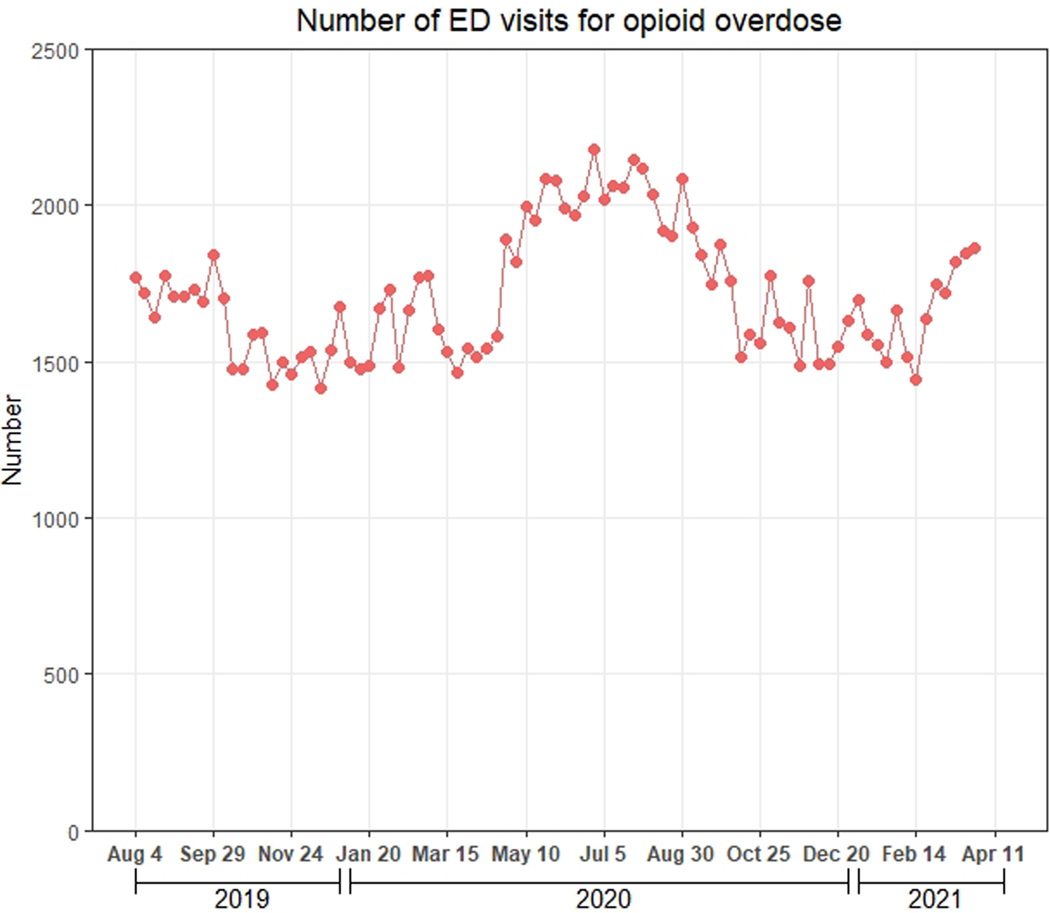
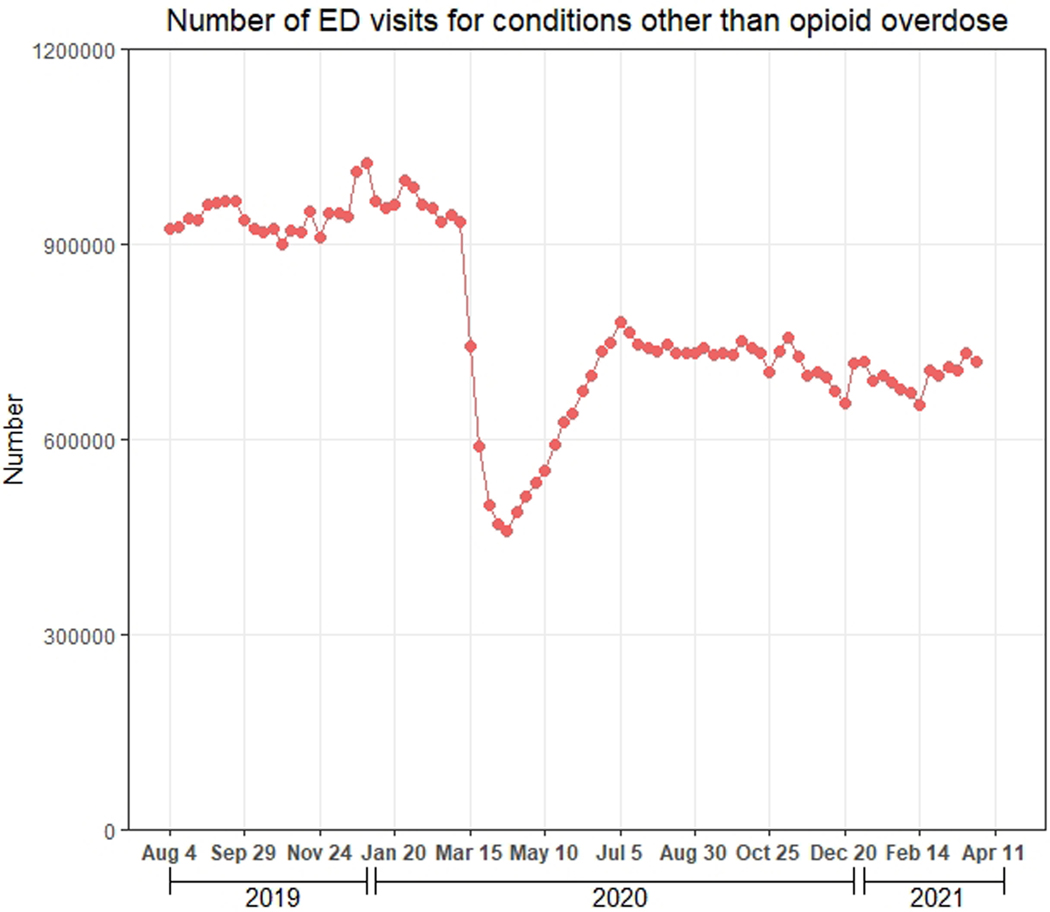
Between August 4, 2020-April 25, 2021, the mean (SD) weekly number of ED visits for opioid overdose was 1,600.2 (117.6). This quantity increased 23.6% to 1,977.9 (117.8) between April 26-October 3, 2020, decreased to 1,598.0 (97.7) between October 4, 2020-March 13, 2021, and increased to 1,844.0 (20.7) between March 14, 2021-April 3, 2021 (Figure 1a). ED visits for other conditions declined sharply between March 15, 2020-April 12, 2020, increased through July 5, 2020, and decreased slightly afterwards (Figure 1b).
Figure 1.
Weekly number of ED visits for opioid overdose and for other conditions during August 4, 2019-April 3, 2021, Symphony Health Integrated Dataverse. A) Number of ED visits for opioid overdose; B) Number of ED visits for other conditions.
Main results.
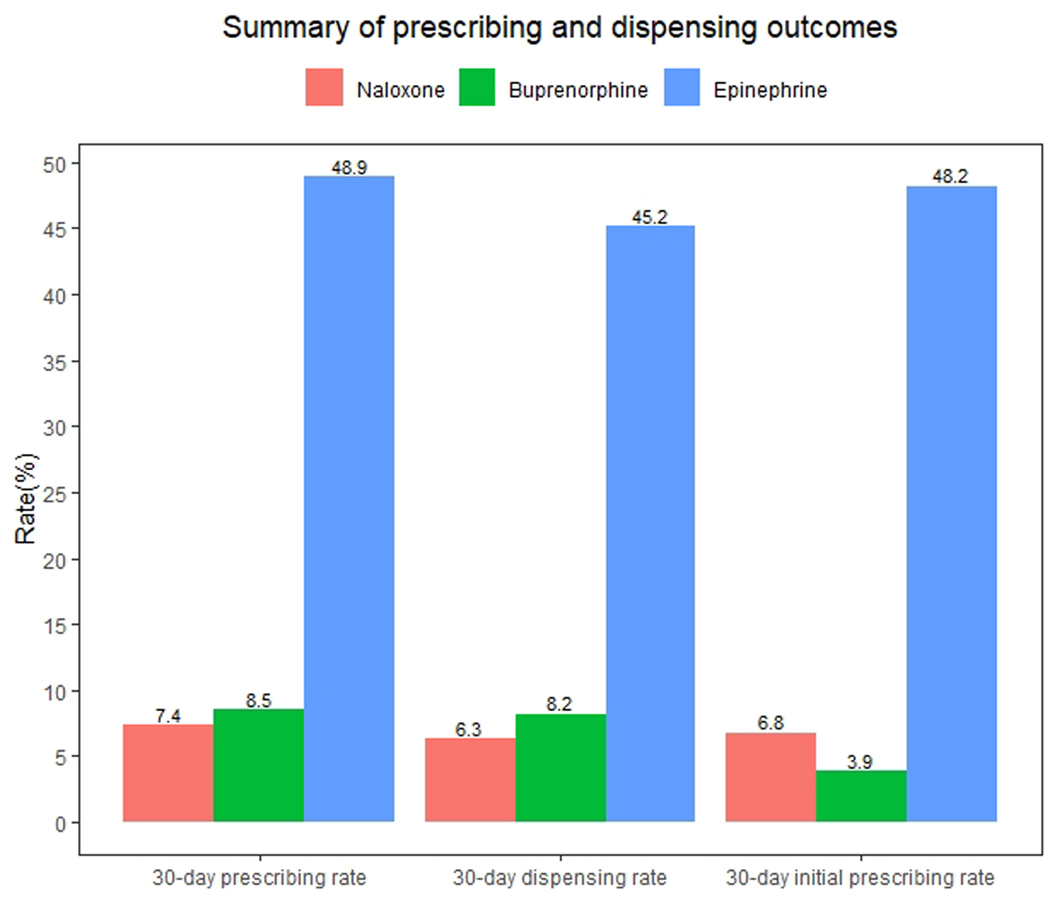
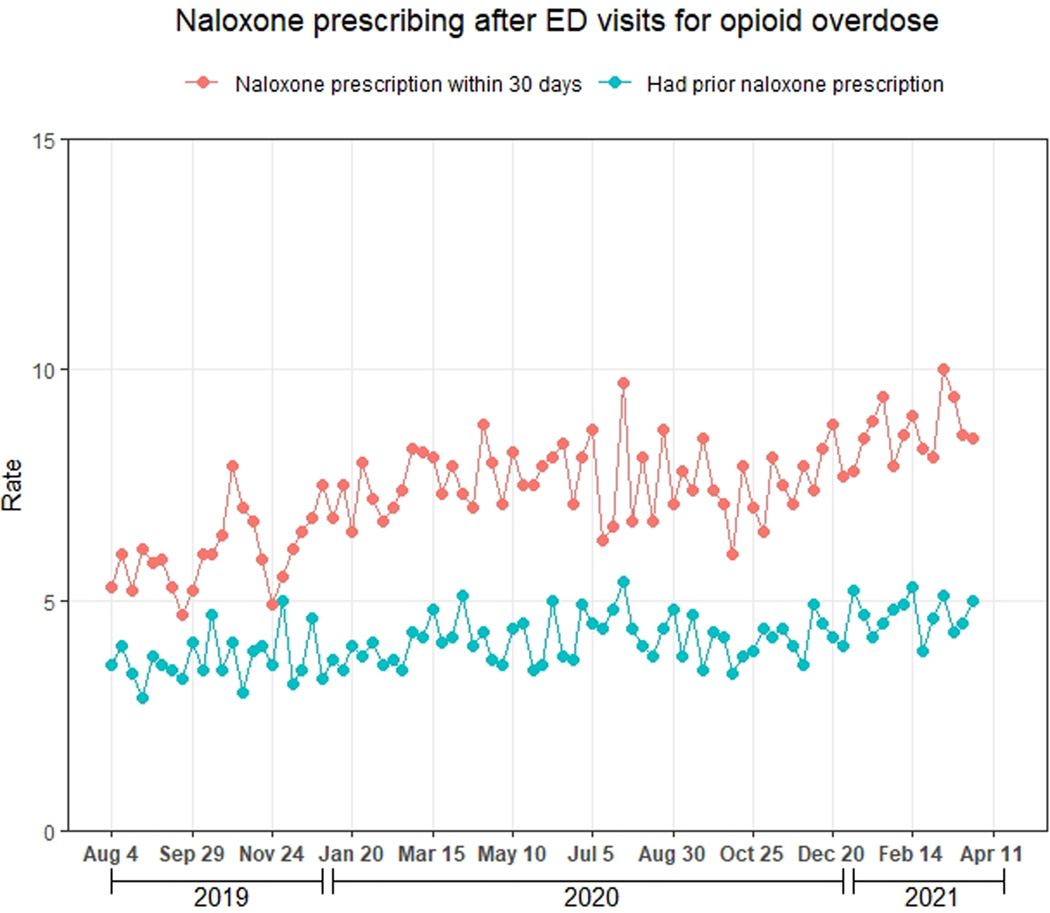
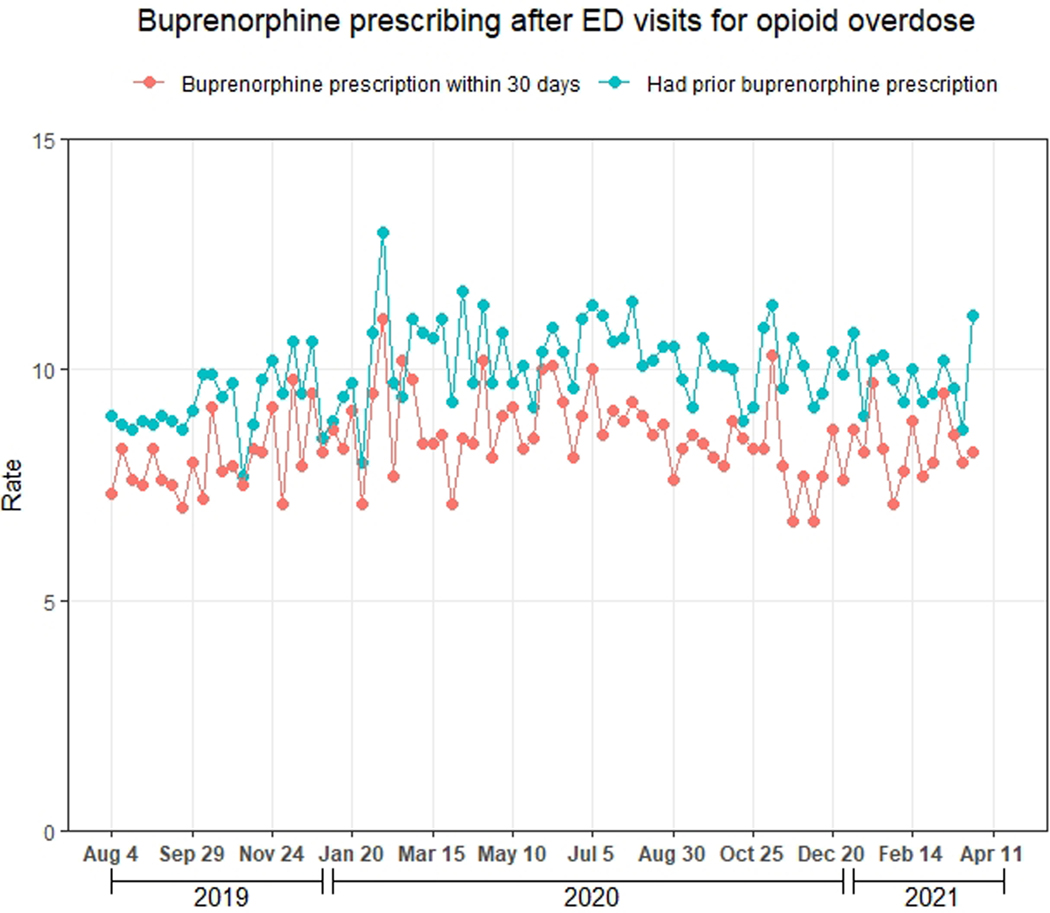
Figure 2 displays the 30-day prescribing rate of naloxone and buprenorphine after ED visits for opioid overdose and the 30-day prescribing rate of epinephrine after ED visits for anaphylaxis. Of the 148,966 ED visits for opioid overdose, 11,010 (7.4%; 95% CI: 7.3–7.5%) had ≥1 naloxone prescription within 30 days, 9,384 (6.3%, 95% CI: 6.2–6.4%) had ≥1 dispensed naloxone prescription within 30 days, and 6,158 (4.1%, 95% CI: 4.0–4.2%) had ≥1 dispensed naloxone prescription in the prior 90 days. Of the 142,808 (95.9%) visits for patients without naloxone dispensing in the prior 90 days, 9,715 (6.8%, 95% CI: 6.7–6.9%) had ≥1 naloxone prescription within 30 days. As shown in Figure 3a, the naloxone prescribing rate increased modestly during the study period (average marginal effect of a 3-week increment: +0.10 percentage points, 95% CI: 0.08, 0.12). Table 1 displays the naloxone prescribing rate among demographic subgroups, while Table 2 displays the adjusted association between this rate and demographic characteristics. The naloxone prescribing rate varied moderately by these characteristics, but no subgroup had a rate exceeding 8.7%. Notably, variation by method of payment was small, with average marginal effects ranging from −1.7 to 1.3 percentage points compared with the baseline category of commercial method of payment (Table 2).
Figure 2.
Summary of prescribing and dispensing outcomes. The 30-day prescribing rate is the proportion of ED visits for opioid overdose (or anaphylaxis in the case of epinephrine) with ≥1 prescription for the drug within 30 days, regardless of whether the medication was dispensed. The 30-day dispensing rate is the proportion of ED visits for opioid overdose or anaphylaxis with ≥1 dispensed prescription for the drug within 30 days. The denominator for the 30-day initial prescribing rate includes ED visits for opioid overdose or anaphylaxis without dispensing of the drug in the 90 days prior to the visit. The numerator is the proportion of these visits with ≥1 prescription for the drug within 30 days, regardless of whether the medication was dispensed.
Figure 3.
Naloxone and buprenorphine prescribing after ED visits for opioid overdose during August 4, 2019-April 3, 2021, Symphony Health Integrated Dataverse. A) Naloxone; B) Buprenorphine. The red line represents the weekly proportion of ED visits with ≥1 prescription (pharmacy claim) for the drug within 30 days, regardless of whether the medication was dispensed. The blue line represents the weekly proportion of ED visits with ≥1 dispensed prescription (paid pharmacy claim) for the drug during the 90 days to 1 day prior to the visit, regardless of whether the drug was prescribed within 30 days. We assessed the latter proportion to evaluate the degree to which prescribing after visits may have been influenced by the existence of prior prescriptions.
Table 2.
Adjusted association between week, demographic characteristics, and naloxone and buprenorphine prescribing after ED visits for opioid overdosea
| Factor | Number of ED visits for opioid overdose (% of sample) | At least one naloxone prescription within 30 days | At least one buprenorphine prescription within 30 days |
|---|---|---|---|
| Three-week increment | N/A | +0.10 (0.08, 0.12) | +0.014 (−0.00048, 0.033) |
| Sex | |||
| Female | 53,073 (35.6%) | Ref | Ref |
| Male | 95,893 (64.4%) | −0.3 (−0.6, −0.01) | 0.7 (0.4, 1.0) |
| Age group | |||
| 0–17 | 3,525 (2.4%) | Ref | Ref |
| 18–25 | 20,539 (13.8%) | 4.2 (3.5–5.0) | 6.9 (6.4, 7.5) |
| 26–34 | 42,150 (28.3%) | 3.7 (3.0–4.4) | 9.8 (9.3, 10.2) |
| 35–44 | 31,868 (21.4%) | 3.1 (2.4–3.9) | 8.7 (8.2, 9.2) |
| 45–54 | 22,447 (15.1%) | 2.9 (2.1–3.6) | 5.9 (5.4, 6.4) |
| 55–64 | 19,824 (13.3%) | 2.5 (1.7–3.2) | 3.8 (3.3, 4.3) |
| 65–74 | 6,615 (4.4%) | 2.0 (1.1, 2.9) | 2.2 (1.7, 2.9) |
| 75 and older | 1,855 (1.2%) | 0.02 (−0.01, 0.01) | −0.08 (−0.7, 0.5) |
| Unknownb | 143 (0.1%) | N/A | N/A |
| Method of payment for ED visit | |||
| Commercial | 97,113 (65.2%) | Ref | Ref |
| Medicare | 8,405 (5.6%) | 1.1 (0.3, 1.8) | −0.9 (−1.6, −0.09) |
| Medicaid | 39,847 (26.7%) | 1.3 (0.1, 1.6) | −0.1 (−0.5, 0.1) |
| Cash | 994 (0.7%) | −0.9 (−2.4, 0.6) | −3.2 (−4.6, −1.8) |
| Other government | 1,201 (0.8%) | −1.7 (−2.9, −0.4) | −3.6 (−5.0, −2.3) |
| Unknown | 1,406 (0.9%) | −1.7 (−2.9, −0.5) | −0.6 (−2.1, 0.9) |
| Region of patient residence | |||
| Northeast | 35,784 (24.0%) | Ref | Ref |
| Midwest | 46,931 (31.5%) | −2.0 (−2.4, −1.6) | −1.7 (−2.2, −1.3) |
| South | 43,312 (29.1%) | −1.9 (−2.3, −1.5) | −3.8 (−4.2, −3.4) |
| West | 19,994 (13.4%) | −0.3 (−0.8, 0.2) | −4.3 (−4.8, −3.8) |
| Unknown | 2,945 (2.0%) | −4.9 (5.6, −4.1) | −7.0 (−7.8, −6.2) |
ED – emergency department; N/A – not applicable
For each of the two outcomes, we fitted a logistic regression model with generalized estimating equations, an exchangeable correlation structure, and robust standard errors clustered at the patient level. Analyses were conducted at the patient level. Week was coded as a continuous variable. Displayed are the average marginal effect of week (the absolute change in the probability of the outcome associated with a one-unit increase in week) as well as the average marginal effects of each categorical variable for demographic characteristics (the absolute change in the probability of the outcome if all versus no ED visits in the sample had the value of the variable in question compared with the baseline category).
The 143 visits with missing data for age were dropped from the regression because none had naloxone or buprenorphine prescribing within 30 days.
Of the 148,966 ED visits for opioid overdose, 12,608 (8.5%, 95% CI: 8.3–8.6%) had ≥1 buprenorphine prescription within 30 days, 12,154 (8.2%, 95% CI: 8.0–8.3%) had ≥1 dispensed buprenorphine prescription within 30 days, and 14,850 (10.0%, 95% CI: 9.8–10.1%) had ≥1 dispensed buprenorphine prescription in the prior 90 days. Of the 134,116 (90.0%) visits for patients without buprenorphine dispensing in the prior 90 days, 5,194 (3.9%, 95% CI: 3.8–4.0%) had ≥1 buprenorphine prescription within 30 days. As shown in Figure 3b, the buprenorphine prescribing rate did not change during the study period (average marginal effect of a 3-week increment: +0.014 percentage points, 95% CI: −0.00048, 0.033). Table 1 displays the buprenorphine prescribing rate among demographic subgroups, while Table 2 displays the adjusted association between this rate and demographic characteristics. The buprenorphine prescribing rate varied moderately by these characteristics, but no subgroup had a rate exceeding 11.2%. As with naloxone, variation by method of payment was small, with average marginal effects ranging from −3.6 to −0.1 percentage points compared with the baseline category of commercial method of payment (Table 2).
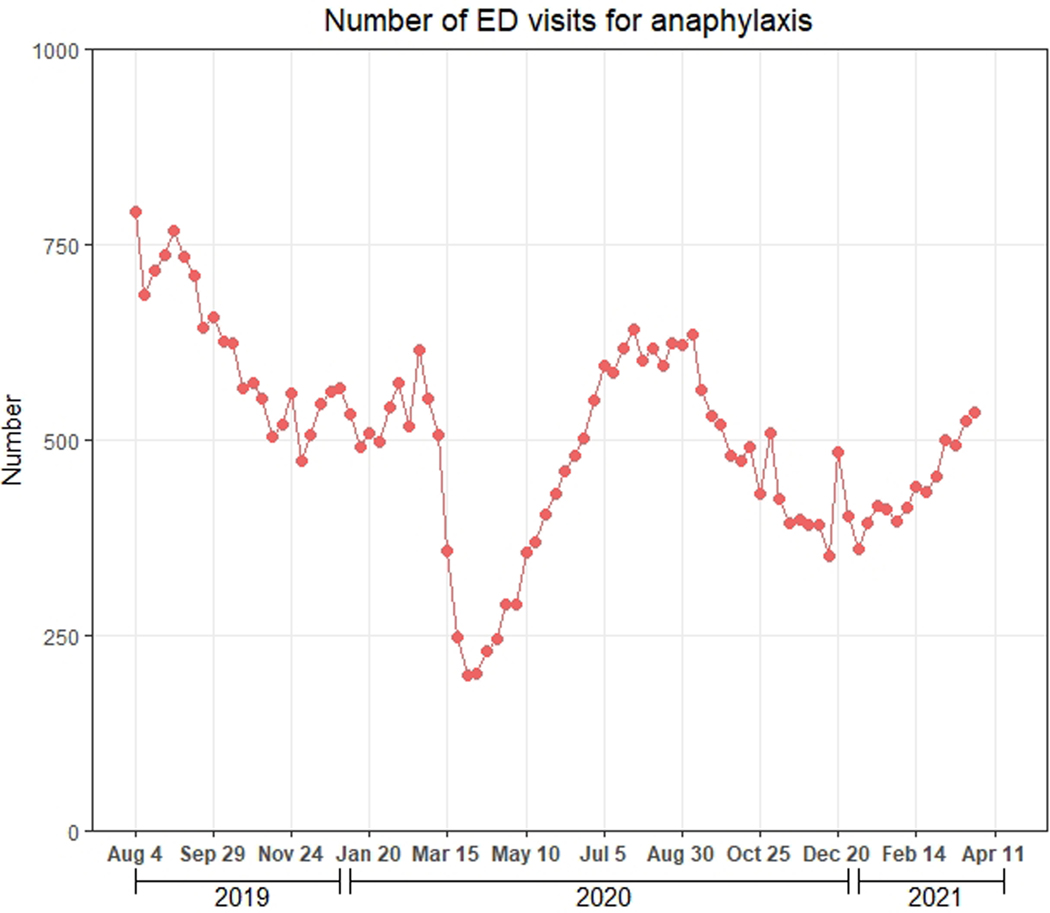
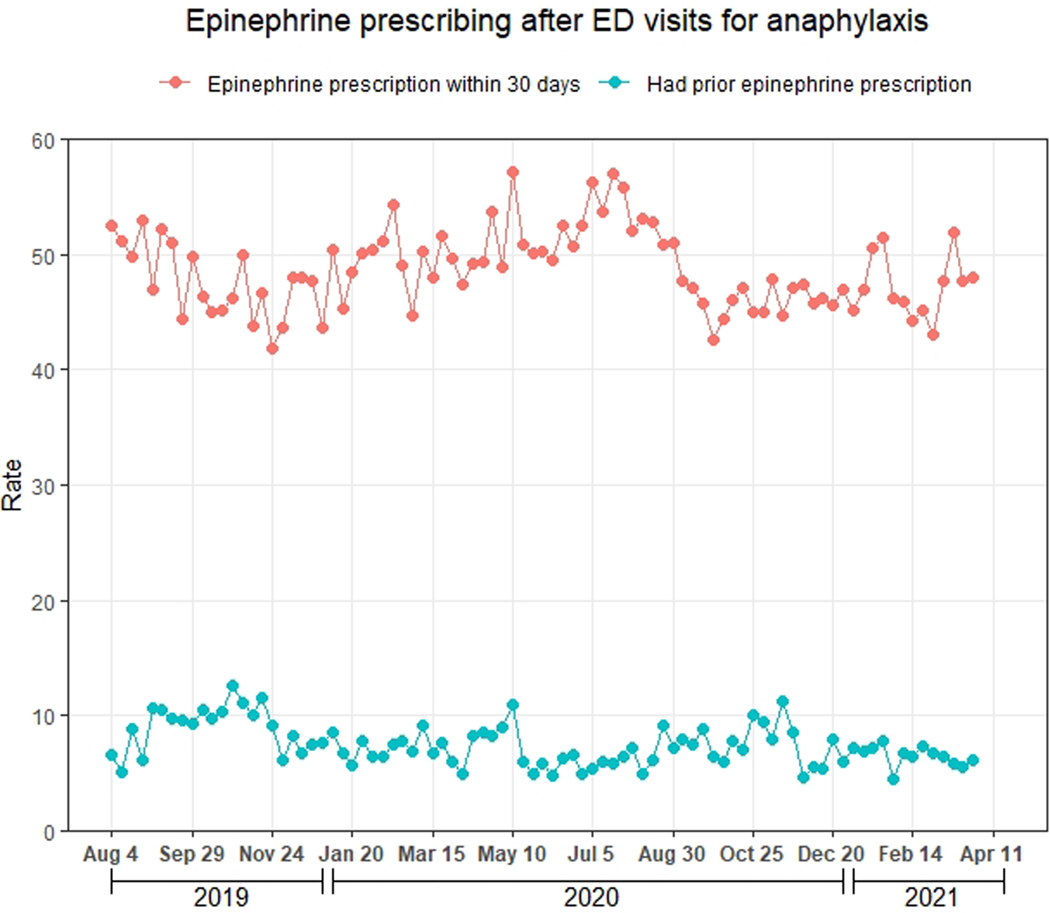
There were 43,712 ED visits for anaphylaxis during the study period. These visits declined sharply in March 2020, increased through July 2020, and subsequently decreased (Figure 4a). Of the 43,712 ED visits for anaphylaxis, 21,340 (48.9%, 95% CI: 48.4–49.3%) had ≥1 epinephrine prescription within 30 days, compared with 7.4% for naloxone prescribing after ED visits for opioid overdose (Figure 4b).
Figure 4.
Weekly number of ED visits for anaphylaxis and epinephrine prescribing rate during August 4, 2019-April 3, 2021, Symphony Health Integrated Dataverse. A) Weekly number of ED visits for anaphylaxis; B) Epinephrine prescribing rate. Epinephrine prescriptions included those for auto-injectors and pre-filled syringes. The red line represents the weekly proportion of ED visits with ≥1 epinephrine prescription (pharmacy claim) within 30 days, regardless of whether the medication was dispensed. The blue line represents the weekly proportion of ED visits with ≥1 dispensed prescription (paid pharmacy claim) for epinephrine during the 90 days to 1 day prior to the visit, regardless of whether epinephrine was prescribed within 30 days. We assessed the latter proportion to evaluate the degree to which prescribing after visits may have been influenced by the existence of prior prescriptions.
Appendix 7 includes additional information on reasons for non-dispensing of naloxone and buprenorphine prescriptions after ED visits for opioid overdose. Insurer rejection of prescriptions was a greater issue for buprenorphine than for naloxone, whereas prescription abandonment (i.e., patients not filling prescriptions covered by insurers) was a greater issue for naloxone.
Sensitivity analyses.
Of 133,699 ED visits for opioid overdose between August 4, 2019-January 30, 2021, 13,676 (10.2%) had ≥1 naloxone prescription within 90 days and 17,341 (13.0%) had ≥1 buprenorphine prescription within 90 days. Of all 148,966 ED visits for opioid overdose in the main analysis, 7,689 (5.2%) had at least one naloxone prescription within 7 days (compared with 7.4% within 30 days), while 5,670 (3.8%) had at least one buprenorphine prescription within 7 days (compared with 8.5% within 30 days). Of the 148,966 ED visits, 16,488 (11.0%) received any treatment for opioid use disorder within 30 days. After limiting to the first ED visit for opioid overdose in the study period for each patient, the proportion of the remaining 123,588 visits with ≥1 naloxone and buprenorphine prescription within 30 days was 6.9% and 7.9%, similar to the main analysis.
LIMITATIONS
This study has limitations. First, the database under-represents Medicaid patients and the uninsured, who have higher rates of ED visits for opioid overdose compared with other payer populations.21 However, naloxone and buprenorphine prescribing rates varied only modestly by method of payment in this study, suggesting that these rates would not be substantially different even if our sample were more representative. Second, data did not report whether overdoses were fatal. If we could have eliminated fatal overdoses, naloxone and buprenorphine prescribing rates after ED visits for opioid overdose would have been higher. However, most ED visits for opioid overdose are not fatal (see Appendix 1 for further discussion). Third, we could not assess disparities in naloxone and buprenorphine prescribing by race and ethnicity owing to data limitations, although prior studies suggest that large disparities likely occurred.7 Fourth, comparisons between naloxone and epinephrine prescribing are imperfect because direct ED dispensing – which the database did not capture – sometimes occurs for naloxone but infrequently occurs for epinephrine. However, the impact of this limitation may be small, as standardized ED take-home naloxone programs have only recently been described.22
Fifth, although there is a large literature that uses diagnosis codes to identify opioid overdoses in administrative data, these codes have imperfect performance characteristics. Specifically, prior validation work suggests these codes are not sensitive for detecting ED visits for opioid overdose but are highly specific.23 Consequently, it is highly unlikely that we misclassified a substantial number of ED visits for other conditions as an overdose visit, but it is likely that we did not capture all ED visits for opioid overdose. However, we have little reason to suspect that any under-capture of visits was differential over time, thus affecting our analyses of trends in ED visits for opioid overdose, or that overdose visits not captured by our sample identification strategy had a different 30-day rate of naloxone and buprenorphine prescribing than ED visits in our sample.
Sixth, our database does not contain records for paper prescriptions that are not dropped off at pharmacies. However, 89% of non-controlled substances (e.g., naloxone) and 58% of controlled substances (e.g., buprenorphine) were electronically prescribed in 2020, suggesting that our database captures most prescribing behavior by clinicians.24 Moreover, despite the lack of complete capture of prescribing behavior, our analyses represent a substantial improvement over prior claims-based analyses of naloxone and buprenorphine prescribing after ED visits for opioid overdose, as these analyses used databases that only report dispensed prescriptions reimbursed via insurance.6,7
DISCUSSION
In this national analysis, ED visits for opioid overdose increased 23.6% between August 4, 2019-April 25, 2020 and April 26, 2020-October 4, 2020, decreased to pre-pandemic levels through March 13, 2021, and increased between March 14, 2021-April 3, 2021. Between August 4, 2019-April 3, 2021, naloxone and buprenorphine were only prescribed after 1 in 13 and 1 in 12 ED visits for opioid overdose, respectively. The naloxone prescribing rate increased modestly during the study period, but the buprenorphine prescribing rate did not change. Findings suggest that clinicians are missing critical opportunities to prevent opioid overdose-related mortality at a time when this mortality has reached record levels.4
A prior study of 2016–2018 commercial claims found that naloxone prescriptions were dispensed within 30 days of only 1.1% of ED visits for an opioid-related problem, including overdose.6 Our findings suggest the naloxone prescribing rate after ED visits for opioid overdose is increasing. While this increase is a welcome development, the naloxone prescribing rate is still far from adequate. The magnitude of the deficit is particularly stark when considering that epinephrine – another life-saving rescue medication – was prescribed after almost half of ED visits for anaphylaxis during the study period. While some patients may not have been prescribed naloxone because they already had this medication, only 4.1% of ED visits for opioid overdose in this study were for patients with recently dispensed naloxone prescriptions. This finding suggests that other factors accounted for infrequent naloxone prescribing, such as stigma and time pressures faced by clinicians.22,25–27
Although naloxone was prescribed after 7.4% of ED visits for opioid overdose, it was dispensed after only 6.3% of visits. This drop-off, which was driven more by naloxone prescription abandonment than by prescription non-coverage by insurers, illustrates the potential utility of dispensing take-home naloxone in the ED. Compared with naloxone prescribing, take-home naloxone circumvents barriers to prescription dispensing, including out-of-pocket costs and lack of access to pharmacies that dispense naloxone.22,25,27–31 For these reasons, the U.S. Surgeon General and American College of Emergency Physicians recommend take-home naloxone as an adjunct or alternative to naloxone prescribing for patients with ED visits for opioid overdose.32,33
The onus of ensuring naloxone access after an ED visit for opioid overdose does not fall solely upon ED clinicians. Outpatient clinicians who see patients for follow-up can provide a naloxone prescription, dispense take-home naloxone kits,34 and/or refer patients to a community-based naloxone distribution program.35 Insurers can increase naloxone access by waiving cost-sharing for naloxone36, while policymakers can implement laws to expand naloxone access. For example, while all states allow pharmacists to dispense naloxone without a patient-specific prescription,37,38 not every state allows naloxone prescribing to third parties, such as family members.38
In our study, only 10% of ED visits for opioid overdose were for patients with prior buprenorphine dispensing, implying that the low rate of subsequent buprenorphine prescribing cannot be wholly explained by the existence of prior prescriptions. The remaining 90% of ED visits were for patients without prior buprenorphine dispensing. Of these visits, buprenorphine was only prescribed after 3.9%, implying that few patients received initial buprenorphine prescriptions. In April 2021, the federal government took an important step towards removing a barrier to buprenorphine prescribing when it eliminated the training requirement to obtain a waiver to prescribe buprenorphine.39 This intervention might increase the number of waivered ED clinicians and the rate of initial buprenorphine prescribing by these clinicians after ED visits for opioid overdose. Moreover, this intervention might increase the number of waivered outpatient clinicians who can initially prescribe buprenorphine during a follow-up visit or continue buprenorphine prescribing initiated in the ED. However, even if the number of waivered clinicians increases, additional interventions will likely still be needed to increase initial buprenorphine prescribing after ED visits for opioid overdose, such as improving insurance coverage for buprenorphine and addressing stigma towards patients with opioid use disorder.
In one of our sensitivity analyses, we assessed the rate of naloxone and buprenorphine prescribing within 7 days of ED visits for opioid overdose to evaluate prescribing potentially attributable to ED clinicians. In this analysis, 5.2% of visits had at least one naloxone prescription within 7 days, compared with 7.4% within 30 days, suggesting that ED clinicians may account for most naloxone prescribing after ED visits for opioid overdose. In contrast, 3.8% of ED visits for opioid overdose had at least one buprenorphine prescription within 7 days, compared with 8.5% within 30 days. This suggests that ED clinicians account for only a minority of buprenorphine prescribing after ED visits for opioid overdose, even though initiation of buprenorphine treatment for opioid use disorder in the ED is effective and is recommended by consensus guidelines from the American College of Emergency Physicians.2,40
This study has several strengths, including its use of a timely, all-payer database that captures approximately one-third of U.S. ED visits. In contrast, prior national studies of naloxone and buprenorphine prescribing after ED visits for opioid overdose have used older commercial claims data with smaller sample sizes.6,7 Moreover, these prior studies relied on insurance claims databases that only capture dispensed prescriptions reimbursed through insurance, whereas our database captures cash-pay prescriptions and prescriptions that were written but not dispensed, thus allowing better capture of clinician prescribing behavior. Finally, by examining data through April 3, 2021, our study extends a prior national analysis that examined trends in ED visits for opioid overdose through October 2020.3 Findings suggest these visits decreased from their peak between April 26-October 3, 2020 but began increasing in mid-March 2021. Although it is unclear whether this uptick will be sustained, its existence further highlights the urgency of closing the large gap in naloxone and buprenorphine prescribing demonstrated in this study.
In summary, this national study found that between August 4, 2019-April 3, 2021, naloxone and buprenorphine were only prescribed after 1 in 13 and 1 in 12 ED visits for opioid overdose, respectively. Future studies should identify which interventions can overcome barriers to this prescribing, thus minimizing missed opportunities to slow the current record rise in opioid overdose deaths.
Supplementary Material
Acknowledgments
Funding source: Dr. Chua’s effort is supported by a career development award from the National Institute on Drug Abuse (grant number 1K08DA048110-01). The funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Chua reports consulting for Keystone Strategies and receiving honoraria from the Benter Foundation. Drs. Dahlem, Brummett, Dora-Laskey, and Kocher report grant funding from the Substance Abuse and Mental Health Services Administration and Michigan Department of Health and Human Services to support efforts integrating harm reduction and improving the treatment of patients with opioid use disorder in emergency departments. Dr. Brummett reported receiving funding from the Centers for Medicare and Medicaid Services, the National Institutes of Health, National Institute on Drug Abuse, Michigan Department of Health and Human Services, the University of Michigan Precision Health Initiative, and Neuros Medical Inc.; holding a patent for peripheral perineural dexmedetomidine licensed to University of Michigan; being a consultant for Heron Therapeutics, Inc.; and receiving honoraria from the Benter Foundation. Dr. Bohnert reports funding from the Substance Abuse and Mental Health Services Administration, the National Institute for Drug Abuse, Blue Cross Blue Shield of Michigan, the U.S. Department of Veterans Affairs, and the Centers for Disease Control and Prevention.
Footnotes
Conflicts of interest: No other conflicts of interest were reported.
REFERENCES
- 1.Weiner SG, Baker O, Bernson D, Schuur JD. One-Year Mortality of Patients After Emergency Department Treatment for Nonfatal Opioid Overdose. Ann Emerg Med. 2020;75(1):13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland KM, Jones C, Vivolo-Kantor AM, et al. Trends in US Emergency Department Visits for Mental Health, Overdose, and Violence Outcomes Before and During the COVID-19 Pandemic. JAMA Psychiatry. 2021;78(4):372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Provisional Drug Overdose Death Counts. 2021; https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Accessed June 25, 2021.
- 5.Ochalek TA, Cumpston KL, Wills BK, Gal TS, Moeller FG. Nonfatal Opioid Overdoses at an Urban Emergency Department During the COVID-19 Pandemic. JAMA. 2020;324(16):1673–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilaru AS, Liu M, Gupta R, et al. Naloxone prescriptions following emergency department encounters for opioid use disorder, overdose, or withdrawal. Am J Emerg Med. 2021;47:154–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilaru AS, Xiong A, Lowenstein M, et al. Incidence of Treatment for Opioid Use Disorder Following Nonfatal Overdose in Commercially Insured Patients. JAMA Netw Open. 2020;3(5):e205852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Donoghue AL, Biswas N, Dechen T, Anderson TD, Talmor N, Punnamarju A, Steven JP Trends in Filled Naloxone Prescriptions Before and During the COVID-19 Pandemic in the United States. JAMA Health Forum. 2021;2(5):e210393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen TD, Gupta S, Ziedan E, et al. Assessment of Filled Buprenorphine Prescriptions for Opioid Use Disorder During the Coronavirus Disease 2019 Pandemic. JAMA Intern Med. 2021;181(4):562–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Currie JM, Schnell MK, Schwandt H, Zhang J. Prescribing of Opioid Analgesics and Buprenorphine for Opioid Use Disorder During the COVID-19 Pandemic. JAMA Netw Open. 2021;4(4):e216147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.COVID-19 Research Database. COVID-19 Research Database. 2021; https://covid19researchdatabase.org/. Accessed May 19, 2021.
- 12.Clement J, Jacobi M, Greenwood BN. Patient access to chronic medications during the Covid-19 pandemic: Evidence from a comprehensive dataset of US insurance claims. PLoS One. 2021;16(4):e0249453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Pelkey R, Gubitosa J, Henrick MF, Ganz ML. Comparing Healthcare Costs Associated with Oral and Subcutaneous Methotrexate or Biologic Therapy for Rheumatoid Arthritis in the United States. Am Health Drug Benefits. 2017;10(1):42–49. [PMC free article] [PubMed] [Google Scholar]
- 14.Symphony Health. COVID-19 Weekly Trend Insights: Compiled on 12/4/2020. 2020; https://s3.us-east-1.amazonaws.com/prahs-symphony-health/resources/COVID-19-Insights-12-4-2020-FINAL.pdf?mtime=20201215104218&focal=none. Accessed June 25, 2021.
- 15.Symphony Health. Symphony Integrated Dataverse Fact Sheet. 2020; https://s3.us-east-1.amazonaws.com/prahs-symphony-health/resources/Symphony_IDV-Sheet_FINAL_JULY.pdf. Accessed May 19, 2021.
- 16.U.S. Census Bureau. Monthly Population Estimates for the United States: April 1, 2010 to December 1, 2020 (NA-EST2019–01). 2020; https://www2.census.gov/programs-surveys/popest/tables/2010-2019/national/totals/na-est2019-01.xlsx. Accessed October 1, 2020.
- 17.Kaiser Family Foundation. Health Insurance Coverage of the Total Population. 2019; https://www.kff.org/other/state-indicator/total-population/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed June 25, 2021.
- 18.American Hospital Association. Fast Facts on U.S. Hospitals. 2021; https://www.aha.org/statistics/fast-facts-us-hospitals. Accessed June 9, 2021.
- 19.Panel NI-SE, Boyce JA, Assa’ad A, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norton EC, Dowd BE, Maciejewski ML. Marginal Effects-Quantifying the Effect of Changes in Risk Factors in Logistic Regression Models. JAMA. 2019;321(13):1304–1305. [DOI] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality. HCUP Fast State - Opioid-related Hospital Use. 2021; https://www.hcup-us.ahrq.gov/faststats/OpioidUseServlet. Accessed June 9, 2021.
- 22.Eswaran V, Allen KC, Bottari DC, et al. Take-Home Naloxone Program Implementation: Lessons Learned From Seven Chicago-Area Hospitals. Ann Emerg Med. 2020;76(3):318–327. [DOI] [PubMed] [Google Scholar]
- 23.Rowe C, Vittinghoff E, Santos GM, Behar E, Turner C, Coffin PO. Performance Measures of Diagnostic Codes for Detecting Opioid Overdose in the Emergency Department. Acad Emerg Med. 2017;24(4):475–483. [DOI] [PubMed] [Google Scholar]
- 24.Surescripts. National Progress Report 2020. 2021; https://surescripts.com/news-center/national-progress-report-2020. Accessed August 19, 2021.
- 25.Gunn AH, Smothers ZPW, Schramm-Sapyta N, Freiermuth CE, MacEachern M, Muzyk AJ. The Emergency Department as an Opportunity for Naloxone Distribution. West J Emerg Med. 2018;19(6):1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland TJ, Penm J, Dinh M, Aran S, Chaar B. Emergency department physicians’ and pharmacists’ perspectives on take-home naloxone. Drug Alcohol Rev. 2019;38(2):169–176. [DOI] [PubMed] [Google Scholar]
- 27.Holland TJ, Penm J, Johnson J, Sarantou M, Chaar BB. Stakeholders’ Perceptions of Factors Influencing the Use of Take-Home-Naloxone. Pharmacy (Basel). 2020;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guadamuz JS, Alexander GC, Chaudhri T, Trotzky-Sirr R, Qato DM. Availability and Cost of Naloxone Nasal Spray at Pharmacies in Philadelphia, Pennsylvania, 2017. JAMA Netw Open. 2019;2(6):e195388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kestler A, Giesler A, Buxton J, et al. Yes, not now, or never: an analysis of reasons for refusing or accepting emergency department-based take-home naloxone. Cjem. 2019;21(2):226–234. [DOI] [PubMed] [Google Scholar]
- 30.Marino R, Landau A, Lynch M, Callaway C, Suffoletto B. Do electronic health record prompts increase take-home naloxone administration for emergency department patients after an opioid overdose? Addiction. 2019;114(9):1575–1581. [DOI] [PubMed] [Google Scholar]
- 31.Penm J, MacKinnon NJ, Lyons MS, Tolle E, Sneed GT. Combatting Opioid Overdoses in Ohio: Emergency Department Physicians’ Prescribing Patterns and Perceptions of Naloxone. J Gen Intern Med. 2018;33(5):608–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American College of Emergency Physicians. Naloxone access and utilization for suspect opioid overdoses. 2016; https://www.acep.org/globalassets/new-pdfs/policy-statements/naloxone-access-and-utilization-for-suspected-opioid-overdoses.pdf. Accessed May 25, 2021.
- 33.Houry D, Adams J. Emergency Physicians and Opioid Overdoses: A Call to Aid. Ann Emerg Med. 2019;74(3):436–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katzman JG, Takeda MY, Greenberg N, et al. Association of Take-Home Naloxone and Opioid Overdose Reversals Performed by Patients in an Opioid Treatment Program. JAMA Netw Open. 2020;3(2):e200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153–163. [DOI] [PubMed] [Google Scholar]
- 36.Aetna. Aetna announces new policies to improve access to Narcan, combat overprescribing. 2017; https://news.aetna.com/2017/12/aetna-announces-new-policies-improve-access-narcan-combat-overprescribing/. Accessed June 25, 2021.
- 37.Gertner AK, Domino ME, Davis CS. Do naloxone access laws increase outpatient naloxone prescriptions? Evidence from Medicaid. Drug Alcohol Depend. 2018;190:37–41. [DOI] [PubMed] [Google Scholar]
- 38.Smart R, Pardo B, Davis CS. Systematic review of the emerging literature on the effectiveness of naloxone access laws in the United States. Addiction. 2021;116(1):6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Department of Health and Human Services. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. 2021; https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder. Accessed May 25, 2021.
- 40.Hawk K, Hoppe J, Ketcham E, et al. Consensus Recommendations on the Treatment of Opioid Use Disorder in the Emergency Department. Ann Emerg Med. 2021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.