Abstract
Liposomes have been used extensively as micro- and nanocarriers for hydrophobic or hydrophilic molecules. However, conventional liposomes are biodegradable and quickly eliminated, making it difficult to be used for delivery in specific routes, such as the oral and systemic routes. One way to overcome this problem is through complexation with polymers, which is referred to as a liposome complex. The use of polymers can increase the stability of liposome with regard to pH, chemicals, enzymes, and the immune system. In some cases, specific polymers can condition the properties of liposomes to be explicitly used in drug delivery, such as targeted delivery and controlled release. These properties are influenced by the type of polymer, crosslinker, interaction, and bond in the complexation process. Therefore, it is crucial to study and review these parameters for the development of more optimal forms and properties of the liposome complex. This article discusses the use of natural and synthetic polymers, ways of interaction between polymers and liposomes (on the surface, incorporation in lamellar chains, and within liposomes), types of bonds, evaluation standards, and their effects on the stability and pharmacokinetic profile of the liposome complex, drugs, and vaccines. This article concludes that both natural and synthetic polymers can be used in modifying the structure and physicochemical properties of liposomes to specify their use in targeted delivery, controlled release, and stabilizing drugs and vaccines.
Keywords: Liposome-polymer complexation, Liposome complex, Targeted delivery, Controlled release
Liposome-polymer complexation; liposome complex; targeted delivery; controlled release.
1. Introduction
A liposome is a drug delivery system composed of phospholipids and forms a bilayer membrane in an aqueous system. Liposomes have been used extensively as a drug delivery system to increase the solubility and permeability of hydrophilic or hydrophobic drugs. However, conventional liposomes are easily degraded by pH, enzymes, and the immune system in a biological environment. Therefore, its application in the provision of certain pathways will release the drug immediately, being unable to maintain the stability of the active substance. The conventional liposome quickly decomposes at acidic pH, so the delayed-release profile in the intestine cannot be obtained. The liposome can also be degraded by lipase in the digestive system [1, 2]. In systemic delivery, liposome can be recognized by the reticuloendothelial system (RES). This can decrease its bioavailability in systemic circulation [3, 4].
One solution to overcome this problem is through modification of the structure of the liposome by using a polymer. The complexation of the liposome-polymer is called a liposome complex. It is known that complexing liposomes using polymers can improve the physicochemical properties of liposome, so they can be explicitly used for targeted delivery and controlled release of drugs. The liposome complex system becomes more stable and sturdy because it is better at protecting active substances from chemicals, enzymes, the immune system, and even photolysis reactions [1, 5, 6, 7, 8, 9, 10]. Surface modification of liposomes can be used to avoid bonding or adsorption of liposomes on some proteins during circulation. Besides, surface modification is intended for targeted drug delivery using ligands, receptors, or polymers that can stimulate the destruction of liposomes through pH, enzymes, or specific bonds on the cell matrix to initiate endocytosis and drug release [11]. Likewise, the incorporation of polymers in the bilayer chain or inside the liposome can strengthen the structure of the liposome and regulate drug release in a controlled manner. The right complexation method can even deliver multiple drugs with different release profiles for synergistic purposes [12].
The interaction form is strongly influenced by the type and structure of the polymer, crosslinker, and initial form of phospholipids during the complexation process. The type of bond also affects the stability and properties of drug release from the liposome complex. Standard evaluations also need to be done to observe patterns of interaction and their effects on the physicochemical properties of liposome complex. Therefore, it is essential to study these parameters for the development of a more optimal controlled release and targeted drug delivery system using liposomes. This article explores the types of natural and synthetic polymers, crosslinkers, lipids, interactions, and bonds occurring in the complexation of liposome complex. Its uses and standard evaluations are also reviewed.
2. Definition and principles of lipoplex
Liposome complexation is a method of modifying the structure of a liposome to improve its physicochemical properties, stability, and pharmacokinetic profile. Liposomes that have been complexed are called liposome complex. One of the complex-forming agents that can be used is polymer. Each type of polymer will produce different interactions and complex shapes with liposomes. The interaction is also influenced by the initial form of phospholipids (ring form or not). Generally, polymers mixed with a proliposome material will create a fusing interaction on the lamellar chain or inside of the liposome. This reaction typically does not require a catalyst or activator [13]. Whereas, the polymer added after the formation of the liposome bilayer membrane will form interactions on the surface [14, 15, 16]. Some types of interactions between polymers and liposomes that can occur are physical adsorption or chemical reactions, such as electrostatic interactions, hydrogen bonds, disulfide bonds, covalent bonds, and others [1, 17, 18, 19, 20, 21, 22, 23, 24, 25]. It is said that covalent bonds can increase the stability of liposome 10-fold better than electrostatic bonds [26]. Weak bonds, such as hydrogen, dipole, and disulfide, slowly release liposomes in a matter of hours to a couple of days [1, 17, 27]. However, if the bonds formed are covalent or ionic, more energy or bioreagents (enzymes) are needed to break the bonds [28, 29]. Controlled release of the liposomes resulting from covalent bonds can take more than 20 days [30]. It all depends on the type of polymer and phospholipid used.
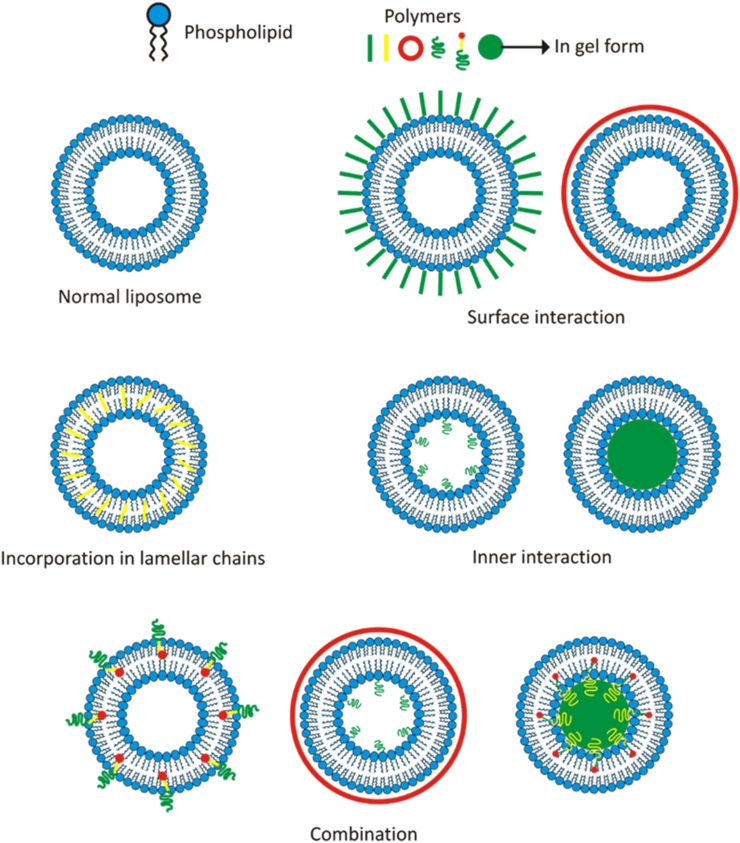
In Figure 1, it can be seen that there are four interaction types between the polymer and liposome, in which the forms are influenced by the type of polymer and phospholipid used, the initial form of the phospholipid, and the mixing method. The following explanation is related to the types of interactions and what influences them.
Figure 1.
The possible interaction of liposome-polymer.
2.1. Surface interaction
This interaction is the most common occurrence in the use of polymers to form matrix systems, with or without the help of a crosslinker [17]. In some polymer types, liposome can act as crosslinker, in which the polymer and liposome have opposite surface charges to form a tidier, more robust matrix. This interaction can be formed by dispersing liposomes that have developed a bilayer membrane into the polymer matrix system [17]. This reaction can be helped by preparing the surface charge on the liposome or polymer first. A catalyst, activator, or initiator is also sometimes used to assist with polymerization [17, 31, 32]. In addition to providing protection and regulating the release of liposomes, this interaction type can also affect the physicochemical properties of the polymer matrix system. In some cases, this interaction type increases (and sometimes even lowers) the quality of the system [33, 34, 35].
This type of interaction is most commonly used to regulate the release of liposomes, which is then followed by drug release from the liposomes. In some cases, drug release can be stimulated by the temperature or physiological pH of the target tissue [36]. The form of drug release produced also depends on the type of surface bond formed between the polymer and liposome. Targeted drug delivery can also use this system by introducing ligands, receptors, or DNA sequences on the surface of the liposome.
Besides, this type of interaction can also protect liposomes from chemical, enzymatic, and immune reactions, so liposomes can be stable during administration and in systemic circulation [37, 38, 39]. Indeed, multilayers formed by certain polymers and liposomes can also protect drugs that are easily degraded or photolabile [1, 5, 6, 7, 8, 9, 10].
2.2. Incorporation in lamellar chains
Unsaturated lipids are easily oxidized, whereas both unsaturated and saturated lipids can be hydrolyzed into lysolipids and liberate fatty acids [39, 40, 41, 42, 43]. The incorporation of polymers into lipid bilayers can strengthen bonds in the membrane of the liposome, so it becomes more sturdy and stable in chemical, enzymatic, and immune reactions [44, 45, 46]. Therefore, this interaction is widely used to improve the stability of liposome. Polymers that are incorporated in the lamellar chain are hydrophobic, so they easily bind to the aliphatic chains of phospholipids. If it does not have these properties, the polymer is usually complexed with cholesterol, poly-L-lysine, or other substances, so they can be joined to the lipid bilayer [17, 27].
This type of interaction can also provide controlled drug release from the liposome. Polymers with strong binding to the bilayer chain can reduce the release and permeability of liposome [47, 48, 49]. Usually, this interaction form is combined with surface modification to help release the drug via thermal, enzyme, or pH triggers.
2.3. Inner interaction
This interaction usually occurs when the matrix system of a hydrophilic polymer that has been formed is used as an aqueous medium in proliposome wetting [5, 50, 51, 52]. These interactions include those that are easily made and reliable. This system can be used as a drug delivery system that is easily degraded chemically or that is photodegradable [5]. This system produces more stable liposomes because of its compact structure. This internal interaction also affects the strength of the bonds among the lipids in the lamellar chain [50].
2.4. Combination
Combinations of several polymer and liposome interactions can be obtained using a combination of polymers or the addition of a crosslinker. The mixture can be in the form of hydrophilic and hydrophobic polymers to streamline drug loading and give trigosable properties to the liposome [53, 54].
Each interaction type between the polymer and liposome will result in different liposome complex properties with different drug delivery and release mechanisms. Here are some of the mechanisms for delivery and release of drugs from the liposome complex.
3. Mechanism of drug release and delivery of lipoplex
3.1. Magnetic responsive
The principle of drug release from this type of liposome complex utilizes magnetic field technology. Magnetic nanoparticles (MNPs) are widely applied as drug delivery systems because their release and accumulation in specific tissues can be controlled through external magnetic fields. Drug release from MNPs has both a time- and concentration-dependent pattern [55, 56, 57, 58, 59, 60]. Magnetic nanoparticles can be encapsulated into liposomes using the thin-film hydration method [61, 62] or reverse-phase evaporation method [63, 64, 65]. Hydrophobic magnetic nanoparticles will be integrated into the liposome bilayer membrane, while hydrophilic magnetic nanoparticles will be integrated into the liposome core. The magnetic behavior of the magnetoliposome depends on the size of the magnetic nanoparticles, the larger the size, the stronger the magnetic signal [66].
3.2. Thermal, pH, and enzymatic responsive
Environmental pH changes can stimulate the occurrence of osmotic diffusion in the liposome complex system, causing swelling or shrinkage, and results in the disintegration of the bilayer membrane of the liposome complex. The pH-triggered liposome complex has the potential to be a targeted delivery system or delayed-release because each tissue or organ has a different physiological pH [17]. At a certain pH, the polymer can also be protonated, causing its charge to change. This phenomenon causes a decrease in electrostatic interactions, causing the lysis of liposomes [1, 67, 68, 69, 70].
Some polymers have a lower critical solution temperature (LCST). As a result, if the polymer is exposed to temperatures above the LCST value, it will become hydrophobic. This is what begins to damage the tissue between the phospholipids, causing substances in the polymer matrix to leak out [47].
The sensitivity of the liposome complex to certain enzymes is obtained through complexation with particular DNA sequences recognized by certain enzymes. If the liposome complex meets the appropriate enzyme, the DNA sequence will be cut off by the enzyme, damaging the bond between DNA and liposome, resulting in the breakage of the structure of the liposome and release of the drug [71, 72, 73, 74].
3.3. Tumor targeted delivery
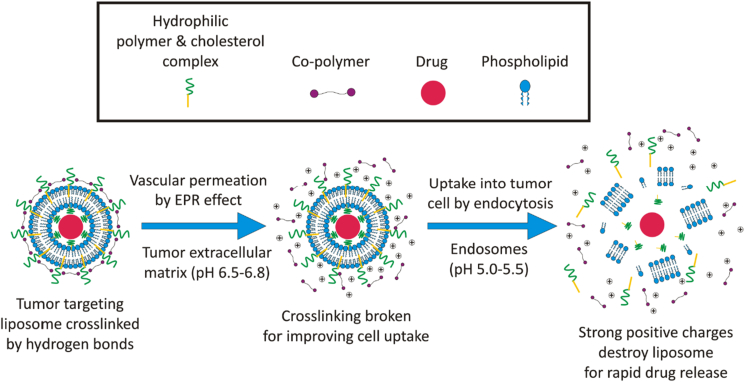
This system can be made by adjusting the surface of the liposome with a polymer. Polymers sensitive to certain pH areas, such as the tumor matrix (pH 6.5), can be carriers and controllers of liposome release [75]. One of them is methacryloyl sulfadimethoxine, which will change to its ionic form when contacted at pH 6.5 [76]. This change in charge is known to change the surface properties of the liposome to one of hydrophobicity, which leads to changes in the structure of the liposome and initiates drug release in a controlled manner. The hydrophobic surface of the liposome also induces aggregate formation on the surface of tumor cells and prevents liposomes from returning to the bloodstream [77, 78]. This results in increased permeation and retention (EPR effect) of liposomes in tumor cells [79, 80, 81, 82]. An illustration of tumor-targeting drug delivery can be seen in Figure 2.
Figure 2.
Illustration of tumor-targeting delivery and release mechanism.
In the figure above, it can be seen that the modification of liposome for tumor-targeting drug delivery requires several polymers or crosslinker combinations with their respective roles. Hydrophilic polymers covering liposomes can reduce recognition by the reticuloendothelial system (RES), thereby prolonging circulation time in the bloodstream [3, 4]. A co-polymer or crosslinker that is pH-sensitive can also be used to prevent drug leakage and stimulate the breakdown of polymer-coated liposomes in the pH of the extracellular matrix of the tumor. Biotin can be used as a crosslinker and can also increase the cell uptake of liposomes [83]. After the liposome has been inserted into the cell, the liposome will be lysed by the influence of a strong positive charge initiating rapid drug release in endosomes [53, 54].
3.4. Controlled release
The type of surface bond between the polymer and liposome plays an essential role in controlling the release of liposome and drug. Non-covalent binding is reversible. Thus, changes occurring in the system due to dissolution, ionization, swelling, or surface erosion can cause the liposome to move out of the matrix [84]. The delayed-release profile of liposomes depends on the strength of the bonds formed between the liposome complex and the polymer. The principle of delayed-release is the same as the principle of enzymatic-responsive or pH-triggered release [2, 85, 86].
Drug delivery and release with the liposome complex system are supported by the physicochemical characteristics of the polymer and its preparation method. Therefore, by understanding this, we can determine the appropriate polymer and preparation method to produce the specific form of drug delivery and release. Here are some polymers that are commonly used in liposome complexation.
4. In vivo delivery of liposomes and liposomal complexes
This system can be made by adjusting the surface of the liposome with a polymer. Polymers sensitive to certain pH areas, such as the tumor matrix (pH 6.5), can be carriers and controllers of liposome release [75]. One of them is methacryloyl sulfadimethoxine, which will change to its ionic form when contacted at pH 6.5 [76]. This change in charge is known to change the surface properties of the liposome to one of hydrophobicity, which leads to changes in the structure of the liposome and initiates drug release in a controlled manner. The hydrophobic surface of the liposome also induces aggregate formation on the surface of tumor cells and prevents liposomes from returning to the bloodstream [77, 78]. This result in increased permeation and retention (EPR effect) of liposomes in tumor cells [79, 80, 81, 82, 83]. An illustration of tumor-targeting drug delivery can be seen in Figure 2.
In the figure above, it can be seen that the modification of liposome for tumor extracellular matrix (ECM) targeting drug delivery requires several polymers or crosslinker combinations with their respective roles. For example, hydrophilic polymers covering liposomes can reduce recognition by the reticuloendothelial system (RES), thereby prolonging circulation time in the bloodstream [3, 4]. A pH-sensitive copolymer or crosslinker (biotin-PEG-biotin) that is located on the interface layers of the liposomal complex can be used to prevent drug leakage, protein adsorption and stimulate the breakdown of polymer-coated liposomes in the tumor ECM. When this liposome reaches tumor ECM (pH range 6.5–6.8), the surface exposed crosslinker is dissociated by break down of hydrogen bond and uptake into tumor cells by endocytic pathway [83]. After the insertion into tumor cells, the liposome will be lysed by the influence of a strong positive charge initiating rapid drug release in endosomes and simultaneously enhancing the accumulation of anticancer drugs only on tumor microenvironment without affecting the normal healthy cells leading to improve therapeutic regimens [53, 54].
5. Polymers used in lipoplex
As mentioned earlier, each polymer will form a different interaction with the liposome and will undoubtedly produce different system properties as well. The system properties can be adjusted according to their application. Table 1 describes various polymers that have been used in complexation with liposomes and their applications.
Table 1.
Polymers used in liposome complexation.
| No. | Polymer | Liposome | API | Application | Ref(s). |
|---|---|---|---|---|---|
| 1. | Aldehyde modified xanthan gum | PE | - | Cell carrier and tissue engineering | [87] |
| 2. | Alginate | DPPC, DSPC, DSPE-PEG2000, and MSPC | Bupisome | Magnetic-responsive, controlled release, protein delivery | [88, 89, 90, 91] |
| 3. | Anti-miR-191 | Soy-lecithin and cholesterol | Anti-miR-191 | Breast cancer therapy | [92] |
| 4. | Atelocollagen | DSPG and cholesterol | Doxorubicin | Magnetic drug targeting | [93] |
| 5. | Chitosan | P90H, P50, and cholesterol; PC and cardiolipin; | Quercetin and cisplatin | Intestinal drug delivery, multidrug delivery | [2, 12] |
| 6. | Chitosan-xanthan gum | P50 and P90H; Soy-lecithin and cholesterol | Rifampicin and C-phycocyanin | Pulmonary delivery, colon targeted | [94, 95] |
| 7. | Cyclodextrin | Egg yolk | Barnidipine | Photodegradable drug delivery | [5, 50, 51, 52] |
| 8. | Gelatin and methacrylic anhydride | Soy-lecithin and cholesterol | Deferoxamine and paclitaxel | Controlled release and bone regeneration | [96] |
| 9. | Hyaluronic acid | Egg phosphatidylcholine and egg L-α-phosphatidylglycerol; DPPE | Corticoid, prednisolone | Sustained-release, targeted delivery | [11, 97, 98, 99] |
| 10. | Lactoferrin | Soy-lecithin and cholesterol | 7,8-Dihydroxyflavone | Sustained release | [1] |
| 11. | mPEG-P(HPMA-g-His)-cholesterol | DPPC | Doxorubicin | Intracellular drug delivery and cancer targeting | [53, 54] |
| 12. | PEG-SH and PEG-Mal | Egg yolk and cholesterol | - | Phototriggered targeted drug delivery | [100] |
| 13. | PTMP and cholesterol-bearing pullulan (CHP) | DMPC | - | Multidrug delivery and controlled release | [30] |
| 14. | pGL4.50 [luc2/CMV/Hygro] vector | DOTAP, DSPC, and cholesterol | pDNA | Colon targeted | [101] |
| 15. | Polyacrylamide and DNA | DOPC and cholesteryl–tetraethyleneglycol (TEG)-modified | DiIC18(5) and calcein | Controlled release and endonuclease-responsive | [102] |
| 16. | Poly (acrylic acid) | Soy-lecithin and cholesterol; Soy-lecithin and dansyl-PE | Calcein; Doxorubicin | Controlled release; Selective tumor targeted | [17, 27, 103] |
| 17. | Polyethylenimine | DPPC, DOTAP, DPPG, and cholesterol | DNA/siRNA | Gene delivery | [104] |
| 18. | Silk fibroin, alginate, and chitosan | Egg yolk | Calcein | pH-triggered release | [15] |
| 19. | Stearoyl-PEG-PSD | Soy-lecithin and cholesterol | Gemcitabine | Selective tumor targeting and controlled release | [77] |
| 20. | Transferrin | DOTAP, phosphatidylcholine, DSPE-mPEG2000, cholesterol | Erianin | Suppress the growth of liver cancer | [105] |
5.1. Alginate
Alginate is an easy polymer to complex through JetCutting [106, 107, 108, 109, 110]. Alginate-liposome complexation is aimed at controlling drug release and giving liposomes thermo-sensitive properties [89, 91]. Drug release from the liposome complex system can take up to 2 weeks. This system can also be stable for up to 2 years at a storage temperature of 4 °C [90]. Lipoplex alginate can also be complexed with magnetic nanoparticles so that delivery and release can be controlled using magnetic resonance [88]. In the delivery of proteins (serum albumin), the efficiency of encapsulation can reach 95% with a sustained-release profile [90].
5.2. Chitosan
Chitosan has been used extensively as a basis for efficient drug delivery. Chitosan has bioadhesive, biodegradable, biocompatible, renewable, antioxidant, antitumor, and excellent wound healing properties [111, 112, 113]. Chitosan is also one of the most widely used polymers in coating liposomes to increase their stability and pharmacokinetic profile [114, 115, 116, 117]. This modification changes the system properties to be more sensitive to certain enzymes, prebiotics, or delayed-release in the intestine or colon. The complexation between chitosan and liposomes is called a chitosome. Interactions between chitosan and liposomes occur on the surface by forming non-covalent bonds, such as electrostatic, hydrophobic, or reversible hydrogen bonds [2, 85]. It was reported that the complexation between chitosan, P90H, and P50 resulted in a system with a particle size of 180 nm and entrapment efficiency of 91%. The system can maintain the structure of the liposome and control the release of active substances at an alkaline pH (intestinal) through the diffusion process [2].
Research conducted by Efimova et al. showed that chitosomes (liposome complex) could be used as a multidrug delivery system. The multidrug delivery system is formed at pH 5 and still maintains its structure and absorption efficiency when the system condition is changed to pH 7 [12]. The combination of drugs in one carrier and protector can undoubtedly be a more efficient therapy [118, 119, 120, 121].
Xanthan gum can also be complexed with chitosan for modification of liposome release by increasing vehicle stability and providing mucoadhesive properties [122, 123]. Complexation of chitosan-xanthan gum-liposome forms a viscoelastic gel system. However, the increase in xanthan gum levels causes its delivery using a nebulizer to be interrupted and retained. The ratio of chitosan-xanthan gum polymer to nebulization liposome delivery is 1:0.5 [94]. Chitosan-xanthan gum (0.5:0.8 w/w)-liposome can also be prepared through the process of freeze and spray drying, resulting in the release of Fickian diffusion properties for the active substance in the colon [95].
5.3. Collagen
Collagen has been used extensively as a gene delivery system by forming stable complexes with plasmid DNA (pDNA) or small interfering RNA (siRNA). Collagen is biodegradable, biocompatible, and has minimal immunogenicity [124, 125, 126, 127]. Atelocollagen (10 μg/mL) has been reported to protect magnetic anionic liposome (MAL) from immune reactions and increase cellular uptake in vitro and in vivo. Compared with magnetic cationic liposome (MCL), MAL showed both concentration- and time-dependent cellular uptake without any cytotoxic effects in vitro on RAW264 cells. However, the use of atelocollagen at concentrations (more than 20 μg/mL) will form aggregates at 37 °C and inhibit cellular uptake due to large particle size [93].
5.4. Cyclodextrin
Cyclodextrin and its derivatives are widely used as complexing polymers in liposomes through inner interaction because of their solubility in aqueous solutions, chemical stability, and good bioavailability [51]. In addition to being able to carry hydrophilic drugs, it can also carry lipophilic drugs [128]. Aside to chemical degradation, the liposome complex from cyclodextrin has been reported to protect photodegradable active substances [5]. Prolonged release can also be obtained from this liposome complex system with a drug release time of more than 25 h [50]. Lipoplex is also known to increase the efficacy and solubility of antibiotics 700 times better than the single form of the drug [52].
5.5. Gelatin
Gelatin is a hydrophilic polymer with amphoteric and non-immunogenic properties [129]. Various modifications of gelatin are intended to improve its physicochemical properties for medical use [130, 131, 132]. One of its modifications is the complexation of gelatin with methacrylamide (GelMa), showing higher elasticity, adherent cells, and excellent mechanical properties [132, 133, 134]. Complexation of GelMa and liposome results in multidrug delivery with controlled release. This liposome complex can significantly promote both osteogenesis and angiogenesis [96].
5.6. Hyaluronic acid
Hyaluronic acid (HA) is a mucoadhesive, biodegradable, and biocompatible polymer. It can be found in the bodies of humans and animals. HA dissolves in water at neutral pH, it is negatively charged, and it is a robust hydrophilic polymer [135, 136, 137]. The complexation of HA-liposomes is known to be used for parenteral drug delivery. Lipoplex injection in guinea pig ears provides sustained release of corticoids for up to 30 days [98]. However, PEGylated liposomes show better immobilization in the network matrix of HA than conventional liposomes [97] and liposomes without PEGylated penetrate into mucus better in the presence of HA [99].
HA is also known to bind to CD44 cell receptors, selectively modulating autoimmune responses. With this capability, complexation using liposomes can be a delivery target for several drugs for autoimmune diseases, such as rheumatoid arthritis [138, 139, 140]. It has been reported that this liposome complex increases the cellular uptake of prednisolone. This process is mediated by caveolae- and clathrin-dependent endocytosis. The study showed that the release of prednisolone in vitro occurred at an acidic pH [11]. With the pH-sensitive properties of several HA derivatives, targeted delivery, and controlled release of the liposome complex system for anticancer drugs can be developed [141].
5.7. Lactoferrin
Lactoferrin is a protein that is positively charged at physiological pH and can coat liposomes through electrostatic interactions on both surfaces. Lactoferrin-liposome complexation has been used as a brain-specific targeting ligand and has been used successfully in increasing its accumulation in the brain [142]. The role of lactoferrin with a decrease in surface tension can be more effective when crosslinked with glycosylate. The modification of lactoferrin enhances its flow characteristics, emulsility, and thermostability [143]. The lactoferrin and liposome complexes are also able to prevent the degradation of active substances from the effects of light and heat. In addition, liposome degradation by lipolytic enzymes in the digestive tract can be restricted by the lactoferrin complex [1, 46]. However, the lactoferrin charge can turn negative when the pH of the system is alkaline (>8.2) or above its isoelectric point. This can reduce the strength of electrostatic interactions in liposome, which can end in changes in particle size and lysis. The liposome complex from lactoferrin can also release drugs in a controlled manner and is suitable for hydrophobic drug delivery [1].
5.8. Transferrin
Transferrin (Tf) is widely known as a metal-binding glycoprotein, is one such example of a multi-tasking protein of approximately 76.5–79 kDa in size whose main function is to absorb Fe3+ ions via transferrin receptor (TfR). It has been detected in various body fluids like plasma, bile, cerebrospinal, amniotic, lymph and breast milk also [144, 145]. Tf is another receptor-ligand pair that has been extensively utilized for cancer targeting since cancer cells have a higher affinity of TfR expression on their surface than normal cells, therefore, it is a potential target for treating various cancers like brain, breast, liver, lung, ovarian and prostate cancer. After binding with its corresponding receptor, the Tf-TfR complex is internalized and transported to endosomes via receptor-mediated endocytosis [146, 147, 148]. It is being realized that surface of the nanoparticles functionalized with Tf, creating an excellent tool for favoring the accumulation of active moieties at the targeted sites while reducing the off-target effect on normal healthy cells. By taking the advantage of the transferrin receptor overexpression on the cancerous cell surface, transferrin-conjugated nanoparticles mainly liposomes or lipoidal vesicles have been designed to maximize the therapeutic benefits [149, 150]. For example, erianin loaded liposomes treated with Tf exhibited excellent anticancer activity both in vitro and in vivo than free erianin as well as free erianin loaded liposomes. These functionalized liposomes enhanced the tumor targeting efficacy by improving the solubility of erianin, inducing cellular apoptosis, displaying immunoregulatory actions and subsequently internalized by TfR-mediated endocytosis as a result increased the cellular uptake of erianin in cancer [105]. It has been reported that Tf conjugated plumbagin liposomes are bearing negative surface charges as lower than that of free liposomes, which is most likely due to the anionic nature of Tf, as it can easily bind to Fe3+ ions with a higher affinity. These negative surface charges would minimize the risk of electrostatic interactions between the liposomes and the negatively charged serum proteins as well as cell membranes, thereby resulting in prolonged blood circulation and reduce nonspecific uptake of liposomes by the healthy cells [151]. Further, PEGylated liposomes modified with TfR monoclonal antibodies (OX26) are able to magnificently increase the transport of plasmid DNA across the blood-brain barrier, promote cell transfection and exhibited the most significant therapeutic efficacy against the brain glioma cells, because ligand OX26 plays a potential role in delivering the lipoplexes across the blood-brain barrier [152, 153]. Therefore, Tf-liposome complexes act as a major role in the development of next-generation therapeutics for cancers and other inflammatory diseases.
5.9. Poly(acrylic acid)
Poly(acrylic acid) (PAA) is widely used as a matrix system for controlling drug release because it is known to have sensitive to pH changes. PAA can interact with lipid bilayers if previously complexed with cholesterol. This complexation shows the difference in size and potential zeta of the liposome at low and high pH. These changes confirm the occurrence of liposome swelling or shrinkage, which is very significant and can lead to the disintegration of the liposome complex bilayer membrane [17]. The liposome complex formed can also maintain a drug release profile for 3 months of storage and is stable when rehydrated. Cell viability testing also shows that this liposome complex has no toxicity toward HEp-2 cells, and their delivery can be transcellular. The particle size of the cholesterol-PAA-liposome was smaller and remained constant for 3 months of storage compared to the PAA-liposome. The efficiency of cholesterol-PAA-liposome absorption is also 1.8 times better. However, its interaction with cells decreases compared to PAA-liposomes without cholesterol [27].
5.10. Polyacrylamide
Polyacrylamide hydrogel has excellent viscoelastic properties, so it can be applied by injection. With its ability to form complexes with genetic material, polyacrylamide is usually used as a matrix and carrier with a stimuli-responsive system. Genetic material can also interact with lipid bilayers through complexation with cholesterol. Cholesterol can support the complexation between polyacrylamide and liposomes. This liposome complex can release drugs in a controlled manner through stimulation by endonuclease enzymes and increased cellular uptake. This liposome complex supports the ability of liposome to coat hydrophilic or hydrophobic molecules and deliver them by injection into the systemic circulation. This enzyme-triggered drug release occurs constantly for up to 11 h. The tidiness of the polyacrylamide matrix obtained by crosslinking with DNA causes an even distribution of liposomes compared to the form of the liposomes themselves [102].
5.11. Polyethylene glycol
Polyethylene glycol (PEG) is a widely used polymer, since it can increase the longevity of liposomes both in vitro and in vivo [34, 40, 154, 155]. Some modifications of PEG, such as the addition of acrylic or methacrylic monomers, can improve the thermal- and pH-sensitive properties of liposomes [17, 53, 54, 156]. It was also reported that the transformation of hydrophilic to hydrophobic or vice versa occurs at pH 7.0, when PEG is complexed with stearoyl and methacryloyl sulfadimethoxine (SDM). Stearoyl-PEG-poly(SDM) polymers will dissolve above pH 7.0 and will form aggregates below pH 7.0 [77]. Through ionization of SDM at pH 6.5 (pH of tumor matrix), the surface of the liposome will turn hydrophobic and start aggregating on the surface of tumor cells. It prevents liposomes from returning to the bloodstream, making their toxic effect on tumor cells even more substantial. Stearoyl-PEG-poly(SDM) polymers can also maintain the size of the liposome at blood pH 7.4, so it can provide an EPR effect on tumor cells in which the size requirement is approximately 400–600 nm [77, 81]. The addition of thiol and maleimide groups to PEG can build a targeted delivery system through photo-triggered complexation. This liposome complex can increase cellular uptake [100].
5.12. Polyethylenimine
Polyethylenimine (PEI) is widely used in gene delivery because of its high gene transfer efficacy [157, 158]. PEI is water-soluble with protonated amine groups in every third position [159, 160]. With cationic charge densities, PEI can form non-covalent complexes with DNA and facilitate siRNA to induce RNAi [161, 162, 163]. The release of the PEI-nucleic acid complex from the endosome occurs through the proton sponge effect, leading to osmotic swelling, so it does not require an endosomolytic agent [164]. However, it is said that PEI can form complexes with anionic molecules in the body. To overcome this problem, PEI can be complexed with hydrophobic compounds, such as cholesterol, to form a liposome complex. This liposome complex is reported to increase the biological activity of nucleic acids and reduce their toxicity [104].
5.13. Pullulan
Pullulan with the help of cholesterol can form stable nanogels (30 nm) in aqueous solution. These nanogels have beneficial properties, such as their large loading capacity for bioactive molecules (i.e., proteins and DNA/RNA) [165, 166]. With the addition of methacryloyl or acryloyl groups, this pullulan nanogel can be a control block for the macrogel structure of other polymers. Besides, it can also interact on the surface of the liposome to form a system that can deliver multiple drugs with different times and amounts of release because the number of bonds that must be broken at each layer also varies. This trait is very advantageous for multidrug delivery requiring release at different speeds and amounts, such as a combination of growth factors for tissue regeneration [167, 168]. Total liposome release in this system can reach more than 20 days, and the entire nanogel release can reach up to 40 days. The pattern and length of release also vary with pH variations. The system is more resistant to an acidic environment and more quickly disintegrated in an alkaline environment [30].
5.14. Silk protein
Silk protein can be used as a liposome surface-modifying agent for stimuli-release purposes. Silk protein imbues the pH-responsive nature of liposomes at pH 5.5–6.0, with rapid drug release. This delivery system is intended for water-soluble drugs [15].
5.15. Xanthan gum
Xanthan gum is a hydrophilic polymer with a 3D network structure through physical and chemical crosslinking that has been widely used in biomedical applications [169]. The matrix structure of this type of polymer can be adjusted for the delivery of cells, drugs, and tissue engineering [87]. Research conducted by Ma et al. showed that aldehyde-xanthan gum and liposome complexation was formed through a covalent bond. The hydrogel is quickly formed within 5 min at room temperature. The system can be stimulated physically (heat), chemically (pH variations), and biologically (histidine exposure). Besides, the system can be biodegraded to form papain, which is the result of digestion of xanthan gum backbones by enzymes. This system also shows excellent self-healing abilities. It was also said that this system could coat cells and last for a long time, so it can be used in cell culture, tissue engineering, and as a cell carrier [87].
6. Supporting agents used in lipoplex
In some conditions, the polymer and liposome cannot interact directly to form complexes because there is no supporting bond between the two. Some materials such as catalysts, activators, or initiators are used to help complex the polymer and liposome. There are those that provide surface charge, and some that modify the hydrophobic and hydrophilic properties of both.
Research conducted by Simoes showed that without stearylamine, PAA could not be incorporated into the liposome bilayer [17]. Stearylamine (5% molar ratio) can also regulate the surface charge of the liposome and increase its transepithelial permeation [27, 170, 171]. However, it is said that the use of stearylamine has the potential to increase the cytotoxic effects of cationic liposomes on lymphocyte cells and macrophages [172]. Research conducted by Shivani Sharma showed that complexing liposomes and stearylamine increased the efficacy of anti-miR-191. This liposome complex can increase the apoptosis of cancer cells and prevent their migration. This liposome complex also enhances the chemosensitivity of cancer cells to anticancer drugs, such as doxorubicin or cisplatin in the free form [92].
In addition to stearylamine, cholesterol has also been widely used in increasing the stability of liposomes and helping to incorporate polymers into lipid bilayers [17, 27]. The study conducted by Chen et al. demonstrated that cholesterol could maintain particle size in the liposome complex in pH variations [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]. Poly-L-lysine is known to interact with lipid bilayers and form stronger electrostatic bonds than poly-L-arginine [49]. It can cause the movement and permeability of liposome to decrease [47].
7. Evaluations
The evaluation standard discussed in this article focuses more on the analysis of interactions and physicochemical characteristics of liposome and its pharmacokinetic profile. Commonly, the quality aspects of dosage forms, such as hydrogels, creams, pastes, etc., are not included in the discussion.
7.1. Nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is used in the elucidation of structures based on carbon or hydrogen atoms and their connectivity. One of the purposes of using NMR in liposome complex analysis is to see the complexation of polymers and cholesterol or other crosslinkers to support the interaction of polymers to the liposome bilayer chain [17, 54, 87].
7.2. Infrared spectroscopy
Infrared (IR) spectroscopy aims at finding out how complex interactions between polymer and liposome are based on the unique peaks formed [53, 54, 87]. The peak shows the types of bonds that are likely to develop in the formation of the liposome complex. Besides, IR spectroscopy can be used as one of the reinforcements for the alleged occurrence of coatings by liposomes. The typical peak loss of an active substance can be assumed that the active material is coated in a liposome [1].
7.3. Drop-coating deposition Raman spectroscopy
Drop-coating deposition Raman (DCDR) spectroscopy can be used to see the interactions inside the liposome complex. The homogeneity or distribution of its complexities can also be observed [173]. Conventional Raman spectroscopy has several disadvantages, such as requiring large samples (mL), high sample concentrations (0.01–0.1 M), and the complex biomolecular structure in solution. In DCDR spectroscopy, this problem can be overcome. The number of samples that can be measured reaches a microliter size. The optimal lipid levels for analysis using DCDR spectroscopy are around 0.3–0.5 mg/mL. Lower lipid levels can still be measured, but the formation of closed liposome rings does not always occur [174, 175]. The principle of DCDR spectroscopy is the deposition and drying of samples placed on a hydrophobic plate. A hydrophobic surface allows the coffee-ring effect, making the liquid in the sample droplet to evaporate and causing the material contained in it to form a ring [173]. The properties of liposomes and proteins do not change during the drying process on the hydrophobic surface [176, 177].
7.4. Fluorescence quenching
Fluorescence in the liposomes occurs after the free active substances are removed (Fi), and it is compared with fluorescence from the active substances after coating the liposome complex without removing the free active substances (Ff). If the percentage of quenching was large, then the degree of liposome formation was also assumed to be large, as the amounts of free active substances read were small. This indicates that the active substance is coated in the bilayer membrane of the liposome formed resulting a low fluorescence quenching. This method is only work for the fluorescent active substances. The following equation is used in determining the quenching of fluorescence [15]:
| (1) |
7.5. Particle size, polydispersity index, and zeta potential
Measurement of particle size, polydispersity index (PDI), and zeta potential are fundamental in the development of liposomes. The liposome itself has been known to be a nanosized drug delivery system. Changes in the size of the particle and zeta potential can be used as benchmarks in the stability of the liposome complex against variations in pH and temperature and during storage. Particle size is also related to the permeation transport mechanism. Nanoparticle permeation can pass through the endocytosis pathway, such as the caveolae (particle size <500 nm) and clathrin (particle size <300 nm) pathways [178].
7.6. Differential scanning calorimetry
Differential scanning calorimetry (DSC) is used to study how polymers influence the thermal behavior of liposomes and drugs. ΔH and TC values reflect the aliphatic chain interactions of lipids, polymers, and drugs. An increase in ΔH value indicates the effect of the polymer or drug on the membrane fluidity of the liposome [1, 94].
7.7. X-ray diffractometry
X-ray diffraction (XRD) is one of the most extensively used techniques for the characterization of liposome based nanoformulations. Generally, XRD analysis imparts information regarding the crystalline or molecular structure, purity, degree of crystallinity, particle size, microencapsulation process, lattice parameters, nature of the phase and qualitative identification of various compounds that exist on the liposomal structure. Analysis of these liposomal nanoformulations are largely depended on the formation of diffraction patterns. Each material used in the development of liposomal formulation has a distinct diffraction peak which can define and identify it by comparing the diffracted peaks with the reference database available from the International Centre for Diffraction Data (ICDD). Further, the diffracted patterns also explain whether the sample materials used in the liposomes are pure or contain some impurities [171, 172, 173, 174].
7.8. Thermal- and pH-sensitivity
This test is carried out to see whether drug release from the liposome can be triggered by changes in temperature or pH. In this test, the temperature or pH of the sample is directly adjusted. Changes in the size of particle and zeta potential can be used as references that the sample is sensitive to changes in temperature or pH [17, 179, 180].
7.9. Entrapment efficiency
Entrapment efficiency testing begins with centrifugation of the sample, allowing it to form two phases (natant and supernatant). Natant is a liposome complex that looks more turbid when it settles, while the supernatant is a surface phase that looks clearer and is considered to contain free active substances. The supernatant is then taken and the levels are measured using an instrument that is suitable for the free active substances. Measurement of the free active substances in the supernatant (Cfree) is carried out using a linear equation from the standard curve. Then, it is compared with the theoretical levels of active substances in the liposome complex (Ct) [175]. The percentage of entrapment efficiency (% EE) is calculated using the following equation:
| (2) |
7.10. Drug loading capacity
Determination of drug loading capacity also follows the similar protocol as discussed in the testing of entrapment efficiency. Here, the only difference is that the obtained free active substances are compared with the total amount of obtained liposome complex (TLC) [176]. The percentage of drug loading is determined using the following equation:
| (3) |
7.11. Biodegradation test
This test is carried out to see whether or not a liposome complex can be degraded by an enzyme. The enzymes used are tailored to the target tissue, route of drug administration, or the type of complexing polymer used. Morikraz (serine proteinase, collagenase, and metalloproteinase) is the most common enzyme used [12]. The liposome complex is degraded by these enzymes under conditions of tissue pH and physiological temperature of administration.
7.12. In vitro drug release
Drug release testing is usually done by conditioning the liposome complex system for lysis. The material that can be used to induce lysis of the liposome complex is Triton X-100 (20 μL). The mixture is placed in a dialysis bag and stirred at 37 °C (100 rpm). Drug quantification can be achieved using spectrophotometry, fluorometry, chromatography, and other methods. The percentage of drug released from the liposome complex can be calculated using the cumulative drug level (Cc) at each sampling time compared to the theoretical levels of the active substances (Ci) [17]. The following equation can be used [1]:
| (4) |
7.13. In vitro release kinetics
After conducting the drug release study, it is very crucial to investigate the release mechanism of liposome complexes. For this, the data obtained from the in vitro drug release study is fitted into various kinetic models like zero order (cumulative amount of drug released vs. time), first order (log cumulative percentage of drug remaining vs. time), Higuchi (cumulative percentage drug release vs. square root of time), Korsmeyer-Peppas (log cumulative percentage drug release vs. log time) model and comparing their correlational coefficient (R2) with one another, the best fit model is picked up. This data is further demonstrated the release mechanism or kinetics of liposome complex encapsulated agents in the existence of biological constituents [177, 178].
7.14. Cytotoxicity assay
Generally, this test is carried out on Caco-2 cells. The type of cell used is adapted to the target tissue of the therapy. The test cells is cultured (3 × 104 cells/mL) in the culture medium, namely 10% phosphate buffer, 1% non-essential amino acid, 1% antibiotic fluid, and 88% DMEM. Then, they are incubated for 24 h. Furthermore, the test sample (liposome complex) containing the drug in various concentrations is inoculated into culture cells and left in contact for 24 h in an incubator. Cell Counting Kit-8 solution is added to each group, and they are incubated at 37 °C with 5% CO2 and 95% O2 for 2 h. Furthermore, the absorbance (A) is measured, and it is used to calculate the percentage of cell viability using the following equation [1, 17, 27]:
| (5) |
where AT is the absorbance-treated cell, AB is the blank absorbance (without cells), and AC is the control absorbance (untreated cell) [1].
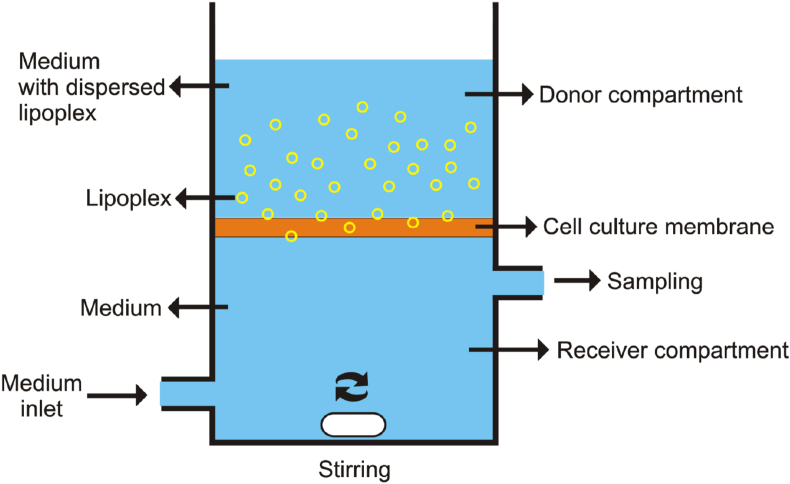
7.15. Permeation transport test
Permeation transport testing is carried out using the principle of two compartments. Each compartment is limited by a culture membrane. The cell line that is commonly used is Caco-2 [1]. In the donor and receiver compartments, each is given a buffer medium and allowed a few minutes (±30 min) to wet the surface of the membrane. Furthermore, the buffer can be removed from the two compartments. The liposome complex samples that is dissolved in the medium can be inserted into the donor compartment. Meanwhile, the receiver compartment is given only a buffer medium. After a set amount of time, sampling is done by taking several volumes from the receiver compartment and replacing them with buffer media in the same volume. The permeability coefficient (P) can be calculated using the following equation [1]:
| (6) |
where dQ/dt is the transport rate of the receiver compartment (μM/s), A is the surface area of the membrane, and C0 is the initial concentration of the liposome-coated active substance in the donor compartment [1]. The illustration of tools from the transport permeation test can be seen in Figure 3.
Figure 3.
Instrumental illustration of transport permeation testing.
7.16. Stability test
The stability test of the liposome complex is usually adjusted to the intended use and route of administration. It is related to the pharmacodynamics of the liposome complex to variations in pH, ionic, and enzymatic concentrations, which can damage the liposome complex. Stability tests of the liposome complex may be carried out by exposure to electrolytes (NaCl) and pH variations in the liposome [1, 181]. For drug delivery to the target release in the intestine or colon, the drug must be resistant to the influence of physiological pH on the gastrointestinal (GI) tract. Therefore, electrostatic repulsion of the liposome complex must be strong against any attractive interactions [1]. In addition, the effect of lipolytic enzymes also needs to be observed. Liposomes can naturally be degraded by trypsin and pancreatic lipolytic enzymes in the digestive system. So, the effectiveness of protection from liposome complexation can be quantified through this test [1].
8. Biological interaction of lipoplexes with plasma proteins
As the most crucial biological fluid i.e., blood contains more than 1000 types of blood proteins also termed as plasma proteins which serve many functions like transport of vitamins, minerals, hormones and functioning the immune systems. Despite the promising body functions offered by these plasma proteins, their interaction with therapeutic moieties like drug and nanocarriers mainly liposome-polymer complexes are very important concerns for its translation from basic research to biomedical applications [182, 183]. The binding of plasma proteins on the lipoplex surface occurs immediately upon their introduction into a physiological environment and is the first barrier of defense, which could lead to physical changes in the formulation, such as aggregation and charge neutralization, non-specific biodistribution, trigger elimination by phagocytic cells and biochemical activation of defense cascades, as a result, decrease the therapeutic efficacy and increase the undesirable toxicities [184]. Further, this interaction is being affected by many other properties of lipoplexes, including their composition, size, shape, surface charges, surface area, surface defects and surface characteristics [185, 186, 187]. To prevent the lipoplex-plasma proteins interactions, the above mentioned factors are critically analyzed for designing the effective lipoplex complexes. The effect of plasma proteins may have special implications for nanomaterials, due to the increased significance of surface effects for these tiny particles. As particles become smaller, their surface areas shrink much more slowly than their volumes, causing nanoscale materials to have far greater surface-to-volume ratios than larger particles. A larger surface-to-volume ratio also intimates more proteins to bind a nanoparticle (relative to its mass) than a particle of larger in size [188]. Even for larger particles, protein binding is established as one of the most important factors influencing biodistribution. Lipoplexes with larger in size or hydrophobic in nature are easily taken up by macrophages more efficiently than their smaller counterparts possessing the same composition and surface properties [189, 190, 191]. Therefore, physicochemical properties of lipoplexes can be manipulated either to avoid the immune recognition when the uptake is unwanted i.e., during drug delivery for cancer treatment and to specifically attract the immune cells when particle uptake is desirable i.e., antigen delivery for vaccine applications [192]. To initiate the above strategies, the surface of the lipoplexes is often covered with various hydrophilic polymers including polyethylene glycol (PEG), poloxamer, dextran, polyethyleneoxide, polyvinyl alcohol, etc. to avoid lipoplexes recognition by the immune cells and subsequently prolong the systemic circulation [193, 194]. Noteworthy, the surface charge of lipoplexes is directly influenced by the nano-bio interaction. For example, the positively charged particles tend to be rapidly covered with plasma proteins with anionic charges like fibrinogen and albumin shows a higher affinity to cationic liposomes with a high membrane charge density [195, 196, 197, 198]. To prevent the adsorption of plasma proteins, cationic liposomes are often covered with polymers that produce a negative charge. PEG is one of the most widely used polymer to decrease the plasma protein adsorption on lipoplex surfaces. The masking of the lipoplexes surface charges by PEG chains as well as the negative charges originated from the phosphate group in the DSPE moiety bearing the PEG chain contributes to the higher negative zeta potential in PEGylated liposomes. This negatively charged surface and hydrophilicity are presented by the lipoplexes repel the anionic albumin away from their surface [199, 200]. However, the plasma proteins comprise different charges and sizes of protein molecules. Thus, the exitance of a negative surface as in the case of the lipoplexes will drive the adsorption of positively charged proteins such as lysozyme present in the plasma [201]. To minimize this attraction, negatively charged liposome surface is further modified with the cationic polymer mainly chitosan. Since chitosan is a positively charged biopolymer, it can easily interact with the negatively charged liposome by electrostatic interaction and finally deliver DNA vaccine via oral route to the distal part of the intestine by protecting from the enzymatic degradation. Because the delivery of protein, peptide and plasmid DNA via the oral route remains relatively challenging due to their instability, degradation and low permeability in the intestinal mucosa. Interestingly, by using these positively charged lipoplexes i.e., combining the benefits of chitosan and liposome can extend the stability and uptake of the DNA vaccine by the Microfold cells (Peyer's patches in the small intestine) which are crucially significant for oral vaccine delivery [116]. Hence, the engineered lipoplexes can be designed in this way that is easily preventing the plasma proteins from binding to lipoplex surfaces. However, at present, there is not enough knowledge on the mechanisms that dictate the interaction of lipoplexes with plasma proteins or recognition by immune cells or as well as stability of these complexes in the biological counterparts. In depth understanding of such interactions can be supervised towards designing the biocompatible lipoplexes with controlled surface properties in a biological environment.
9. Vaccine delivery and stabilization by liposome-polymer complexes
Vaccine delivery is one of the most paramount medical interventions that help to eradicate the number of diseases like measles, diphtheria, tetanus, influenza, polio, hepatitis, mumps, pox, and finally COVID 19 too. While at the time of designing a vaccine, must needs to define the antigen, adjuvant, manufacturing process and also the delivery strategy to evoke a robust immune response [202, 203, 204]. Throughout history, most of the vaccines have been formulated using live attenuated organisms, killed whole organisms and inactivated toxins mainly toxoids. Live vaccines have the benefits of generating both humoral or cellular immunity and often require only a single dose, while killed and inactivated vaccines initiate a weaker immune response and usually require multiple doses [205]. Recently, recombinant DNA methodologies are used to develop DNA and subunit vaccines as well as conjugate vaccines in which a weak antigen is linked to a stronger immunogen such as a protein or membrane complex or capsular polysaccharides and can induce strong long-term cellular immune responses [206, 207, 208]. Although DNA and subunit vaccines have supremacy over conventional vaccines, but these vaccines suffer from a comparatively lower immunogenicity and intrinsic instability and are prone to degradation by nucleases. Despite the outstanding progress in vaccine development, improvements are carefully needed due to concerns about their weak immunogenicity, in vivo instability, toxicity, and subsequently require multiple-dose administration [209, 210, 211].
To overcome such issues, nanotechnology based platforms have recently been utilized in vaccine development as well as antigen delivery. These new technologies have progressively been explored to enhance the therapeutic efficacy, humoral or cellular immune responses and can offer significant advantages over conventional vaccine formulations in terms of higher specificity, higher stability, higher loading capacity, capability for sustained or controlled release [212]. Noteworthy, as nanoparticles composed materials are designed at the molecular, atomic and macromolecular levels, they are usually tiny-sized particles with distinct physicochemical properties like composition, size, surface properties, shape, molecular weight [213]. Being nanosized, these particles can directly interact with the diseased tissues or cells with improved efficiency, protect the encapsulated antigens from the hostile in vivo environment and facilitate more uptake of the active moieties by phagocytic cells, mucosa-associated lymphoid tissue and gut-associated lymphoid tissue leading to efficient antigen recognition and presentation [214, 215, 216]. Several vaccine nanocarriers have been engineered and investigated for their applicability in the delivery of antigens and adjuvants to the immune cells as well as mimics of viral structures in an effort to provide a protective immune response [217]. Finally, nanocarriers can combine their primary function as antigen delivery system with other features such as immunostimulation or immunomodulation that are conjointly induced both humoral and cell-mediated immune responses [218]. To produce above or specific immune response like Th1 or Th2 or CD4+ T cell response, the nanocarriers are must be loaded with specific immunostimulatory adjuvants such as CpG oligodeoxynucleotide. Unfortunately, although single functional nanocarriers loaded with antigens may be taken up by the immune cells and insufficient adjuvant activity may result in restricted immunogenicity [219, 220].
Therefore, it is necessary to develop a new multifunctional nanovehicle systems that can increase both antigen presenting efficiency as well as better adjuvant activity. At presently, liposomal-polymer derivatives, cationic liposomes, and liposome protein complexes mainly lipoplex have been identified as novel adjuvants and vaccine delivery platforms due to some of their distinct potentialities over non-functional nanocarriers as well as traditional carrier proteins on immunogenic stimulation: firstly, it can be converted a non-immunogenic substance to an immunogenic one; secondly, surface charge, size, and composition of liposomal derivatives could be easily adjusted to cope with the need of different antigens; thirdly, it can enhance the antigen presentation to antigen presenting cells mostly dendritic cells [221, 222, 223, 224]. Depending on the above chemical properties and benefits, liposomal derivatives having the potentiality to encapsulate the water-soluble antigens such as proteins, peptides, nucleic acids, carbohydrates or haptens within the inner aqueous space of the lipoidal core, whereas the lipophilic compounds mostly antigens, adjuvants, lipopeptides, linker molecules are embedded into the lipid bilayer [225]. In fact, the first vaccine candidate launched into clinical trials was made of liposomes and mRNA encoding an influenza virus nucleoprotein. This vaccine was designed in 1993 for specific antigens in a shorter period of time compared with other vaccine platforms, such as recombinant proteins and inactivated vaccines and also having the ability to induce virus-specific cytotoxic T-cell responses in mice, since then the lipid nanoparticle based mRNA vaccine formulations have emerged as a potent vaccine delivery platform [226]. It has been also reported that antigens or antibodies can be linked to the surface of the liposomal derivatives or lipoplex either by adsorption or stable chemical linking techniques to enhance the better target specificity. A number of surface-functionalized liposomal carrier systems, known as immunoliposomes, have been shown to navigate themselves to pathological cells due to the specific interaction among antibodies that has been attached to the surface of the liposomes and antigen epitopes or small particles that are displayed by the targeted pathogenic cells. Such types of immuneliposomal carriers having the tendency to mimicking the apoptotic cells as a result efficiently internalized by macrophages [227, 228, 229, 230]. For example, recombinant B subunit of cholera toxin (rCTB) was covalently coupled to the outer surface of the small unilamellar liposomes for targeted delivery of encapsulated saliva-binding region (SBR) of Streptococcus mutans antigen I/II (AgI/II) protein to Peyer's patches (aggregated lymphoid nodules) and enhanced the secretory IgA responses in mice. The results revealed that antibody responses in mice immunized with rCTB-coupled liposomes preserved at high levels for at least 6 months by a single oral dose and significantly enhances their robustness and stability as an antigen delivery platform. This oral immunization approach should be suitable for the designing of vaccines against oral, intestinal, and also sexually transmitted diseases [231]. Similarly, antigen molecules like the lipid binding proteins and non-lipid binding proteins have been assembled with liposomes to help improve their immunogenicity and evoke antibody production in immunized entities. In a study, human liver fatty acid binding protein 1 (hl-FABP1) was assembled with homogeneous unilamellar cationic liposomes and characterized as a potential vaccine delivery vehicle in diverse thermal conditions. Under room temperature, the protein is mainly assembled to the liposomes via electrostatic interactions and under elevated temperature, the externally accessible proteins are embedded in the lipid bilayer structure similar to common biological membranes, which is further indicated that the thermal stability of a target protein is very crucial for successful production of desired lipoplexes as a suitable delivery carrier for vaccines [232]. While in another study, non-lipid binding protein or hapten carrier protein mainly bovine serum albumin (BSA) was assembled with cationic liposomes and the efficacy of the lipoplex vaccine was examined in mice and compared with that of Nicotine-BSA conjugate (Nic-BSA). The results illustrated that the lipoplex vaccine with adjuvant (Alum or Aluminum salt) was able to evoke the highest serum nicotine-specific antibody (NicAb) titers of 11169 ± 2112, which is undoubtedly higher than that steered by either the lipoplex vaccine without Alum or Nic-BSA with Alum. The outstanding immunostimulatory effect of this nano-lipoplex may promote an innovative approach to upgrade the immunogenic capability of current nicotine vaccines or other vaccines utilizing small addictive compounds to attain high titers of effective antibodies [233]. Apart from the cationic liposomes and liposome protein complexes, liposome-based polymeric derivatives or liposomes modified with pH-sensitive polymers are another important contemporary approach which is acting as a novel adjuvant for enhancing the antibodies production, delivery of antigen to the cytosol of antigen-presenting cells and also promotes isotype switching [234]. It has been reported that cationic lipopolymer adjuvant (liposome-PEG-polyethyleneimine complex or LPPC) robustly adsorbs immunomodulators or antigens onto its surface to boost up the immune responses and simultaneously enhances the uptake capability, cytokine release, surface marker expression, and the presentation of antigens on the mouse phagocytic cells. In the case of Freund's adjuvant, this liposome-polymer complexes conversely activate the Th1 immunity across antigens in vivo, while in contrast to immunomodulators like CpG oligodeoxynucleotides, this CPPC efficaciously improves the IgA or IgG2A immune responses even in hosts that have generated high IgG1 serum titers and Th2 immunities. These results are sufficiently demonstrated that LPPC adjuvant not only enhances the antigens immunogenicity but also modulates the host immunity to produce an appropriate antibody isotype switching in combination with immunomodulators and subsequently enhances the stability of vaccine antigens [235].
Basically, nanoparticle based adjuvants contribute to shaping and enhancing the vaccine immune response via various modes of action. The vaccine formulations containing the liposomal cationic adjuvant formulation (CAF01) systems and squalene based oil/water emulsions, which evoked a significant primary antigen-specific CD4+ T cell response for 7 days after immunization. The effector function of activated CD4+ T cells was distorting toward a Th1/Th17 response by CAF01, while a Th1/Th2 response was directly elicited by o/w squalene. Previously reported that the CAF01 can gear up the both Th1 and Th17 responses and provide long-lived, robust Th1 memory. Conjointly mice immunized with only o/w squalene or CpG adjuvants leading to generate early H56-specific IgG response. These tested adjuvants produced the germinal center reaction with different magnitude through the T follicular helper cells (Tfh) and the results indicated that immunological activity of different adjuvants can be characterized by profiling early immunization biomarkers after primary immunization. Indeed, understanding the modes of action of adjuvant molecules loaded in liposomal derivatives will allow the development of novel vaccination strategies based on a rational design of heterologous prime-boost liposomal nanoformulations [236, 237, 238, 239]. However, the development of novel vaccine delivery systems and adjuvants has been facilitated by nanotechnology resulted in some concern regarding their safety, toxicity, optimal combination of antigens or immunomodulators, and storage conditions are needed to be critically examined in the preclinical stages before clinical use.
10. Clinical trials of liposome complexes in vaccine delivery
Several clinical trials of vaccines using liposomes as delivery or adjuvant therapy have been reported. These tests were still in phases I-II and were aimed at testing the effectiveness and safety of the melanoma, chlamydia, HIV, porcine circovirus, plasmodium falciparum, and hepatitis B vaccines [240, 241, 242, 243, 244, 245]. Gargett, T., et al. successfully demonstrated that a denderitic cell-targeted liposomal vaccine (Lipovaxin-MM) for melanoma, is safe and practical for use in future clinical trials. Lipovaxin-MM was adequately tolerated and did not cause any clinically relevant harm. Lipovaxin-MM did not appear to be immunogenic [241]. When liposomes were utilized, the peptide vaccination was the most effective in significantly higher HBeAg seroconversion rate [240], considerably (p<0.05) lowering viremia while raising the amounts of neutralizing antibodies and interferon-g secreting cells. This shows that the peptide vaccine was able to activate both humoral and cellular immune responses in the presence of liposomes [243]. Adjuvant liposome treatment results in faster seroconversion, higher IgG titres, a better mucosal antibody profile, and a more consistent cell-mediated immune response profile as compared to aluminum hydroxide [245]. A clinically safe liposomal adjuvant can direct antibody effector activity toward higher phagocytic function in comparison to alum, and this ability provides protection against severe mucosal SHIV infection in humans [244]. Based on these reports, it can be concluded that the use of liposomes as a delivery system or vaccine adjuvant therapy can reduce the toxicity and immune response to vaccines and increase their effectiveness.
11. Author's perspective
In modifying the liposome to adjust the delivery and controlled release of drugs, it is necessary to understand the characteristics and structure of the polymer to be used. Each type of polymer produces different liposome complex characteristics for specific uses. The development of the liposome complex can be very extensive through modification of the structure of natural polymers or new synthetic polymers. However, the aspect that needs to be considered is its relation to biodegradability and biocompatibility with the body, so it can be used safely and effectively.
12. Conclusion
Various natural and synthetic polymers produce different liposome complex characteristics. These characteristics also provide different functions in the regulation of drug release and delivery by liposome complex. These different properties are influenced by the form of interactions and bonds between the polymer and liposome. Additional setup and excipient methods also influence the complexity of the interaction. The interaction form and its influence on the characteristics of the liposome complex can be studied through a variety of methods and instruments that are appropriate. Based on the results of reviews from several libraries, it can be concluded that the liposome complex can be an optimal system for targeted delivery, controlled release, and stabilization of drugs and vaccines.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This work was supported by review article grants program from Padjadjaran University with grant number of 1959/UN6.3.1/PT.00/2021.
Footnotes
This article is a part of the Lipid-Based Nanoparticles Special issue.
References
- 1.Chen Y., Xia G., Zhao Z., Xue F., Gu Y., Chen C., et al. 7,8-Dihydroxyflavone nano-liposomes decorated by crosslinked and glycosylated lactoferrin: storage stability, antioxidant activity, in vitro release, gastrointestinal digestion and transport in Caco-2 cell monolayers. J. Funct.Foods. 2020 Feb;65:103742. [Google Scholar]
- 2.Caddeo C., Díez-Sales O., Pons R., Carbone C., Ennas G., Puglisi G., et al. Cross-linked chitosan/liposome hybrid system for the intestinal delivery of quercetin. J. Colloid. Interface Sci. 2016;461:69–78. doi: 10.1016/j.jcis.2015.09.013. [Internet] [DOI] [PubMed] [Google Scholar]
- 3.Molineux G. Pegylation: engineering improved pharmaceuticals for enhanced therapy. Cancer Treat Rev. 2002 Jan;28:13–16. doi: 10.1016/s0305-7372(02)80004-4. [DOI] [PubMed] [Google Scholar]
- 4.Papahadjopoulos D., Allen T.M., Gabizon A., Mayhew E., Matthay K., Huang S.K., et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. 1991 Dec 15;88(24):11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioele G., De Luca M., Ragno G. Photostability of barnidipine in combined cyclodextrin-in-liposome matrices. Future Med. Chem. 2014 Jan;6(1):35–43. doi: 10.4155/fmc.13.187. [DOI] [PubMed] [Google Scholar]
- 6.Bayomi M.A., Abanumay K.A., Al-Angary A.A. Effect of inclusion complexation with cyclodextrins on photostability of nifedipine in solid state. Int. J. Pharm. 2002 Aug;243(1–2):107–117. doi: 10.1016/s0378-5173(02)00263-6. [DOI] [PubMed] [Google Scholar]
- 7.Mielcarek J. Photochemical stability of the inclusion complexes formed by modified 1,4-dihydropyridine derivatives with β-cyclodextrin. J. Pharm. Biomed. Anal. 1997 Mar;15(6):681–686. doi: 10.1016/s0731-7085(96)01900-0. [DOI] [PubMed] [Google Scholar]
- 8.Romero E., Morilla M. Highly deformable and highly fluid vesicles as potential drug delivery systems: theoretical and practical considerations. Int. J. Nanomed. 2013 Aug:3171. doi: 10.2147/IJN.S33048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013 Jan;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 10.Ragno G., Cione E., Garofalo A., Genchi G., Ioele G., Risoli A., et al. Design and monitoring of photostability systems for amlodipine dosage forms. Int. J. Pharm. 2003 Oct;265(1–2):125–132. doi: 10.1016/j.ijpharm.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Gouveia V.M., Lopes-de-Araújo J., Costa Lima S.A., Nunes C., Reis S. Hyaluronic acid-conjugated pH-sensitive liposomes for targeted delivery of prednisolone on rheumatoid arthritis therapy. Nanomedicine. 2018 May;13(9):1037–1049. doi: 10.2217/nnm-2017-0377. [DOI] [PubMed] [Google Scholar]
- 12.Efimova A.A., Mulashkin F.D., Rudenskaya G.N., Evtushenko E.G., Orlov V.N., Melik-Nubarov N.S., et al. Biodegradable electrostatic complexes of chitosan cationic microparticles and anionic liposomes. Polym. Sci. B. 2018 Jan 19;60(1):84–90. [Google Scholar]
- 13.Elahipanah S., O’Brien P.J., Rogozhnikov D., Yousaf M.N. General dialdehyde click chemistry for amine bioconjugation. Bioconjugate Chem. 2017 May 17;28(5):1422–1433. doi: 10.1021/acs.bioconjchem.7b00106. [DOI] [PubMed] [Google Scholar]
- 14.de Groot C., Müsken M., Müller-Goymann C. Novel colloidal microstructures of β-escin and the liposomal components cholesterol and DPPC. Planta Med. 2018 Nov 24;84(16):1219–1227. doi: 10.1055/a-0624-2706. [DOI] [PubMed] [Google Scholar]
- 15.Hong Y.-J., Kim J.-C. Complexation-triggerable liposome mixed with silk protein and chitosan. J. Biomater. Sci. Polym. Ed. 2015 Aug 13;26(12):766–779. doi: 10.1080/09205063.2015.1058574. [DOI] [PubMed] [Google Scholar]
- 16.Madrigal-Carballo S., Lim S., Rodriguez G., Vila A.O., Krueger C.G., Gunasekaran S., et al. Biopolymer coating of soybean lecithin liposomes via layer-by-layer self-assembly as novel delivery system for ellagic acid. J. Funct.Foods. 2010 Apr;2(2):99–106. [Google Scholar]
- 17.Simões M.G., Alves P., Carvalheiro M., Simões P.N. Stability effect of cholesterol-poly(acrylic acid) in a stimuli-responsive polymer-liposome complex obtained from soybean lecithin for controlled drug delivery. Colloids Surf. B Biointerfaces. 2017 Apr;152:103–113. doi: 10.1016/j.colsurfb.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Kepczynski M., Jamróz D., Wytrwal M., Bednar J., Rzad E., Nowakowska M. Interactions of a hydrophobically modified polycation with zwitterionic lipid membranes. Langmuir. 2012 Jan 10;28(1):676–688. doi: 10.1021/la203748q. [DOI] [PubMed] [Google Scholar]
- 19.Briand E., Humblot V., Pradier C.-M., Kasemo B., Svedhem S. An OEGylated thiol monolayer for the tethering of liposomes and the study of liposome interactions. Talanta. 2010 Jun 15;81(4–5):1153–1161. doi: 10.1016/j.talanta.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Jordan S.W., Faucher K.M., Caves J.M., Apkarian R.P., Rele S.S., Sun X.-L., et al. Fabrication of a phospholipid membrane-mimetic film on the luminal surface of an ePTFE vascular graft. Biomaterials. 2006 Jun;27(18):3473–3481. doi: 10.1016/j.biomaterials.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Liu X.-Y., Nakamura C., Yang Q., Kamo N., Miyake J. Immobilized liposome chromatography to study drug–membrane interactions. J. Chromatogr. A. 2002 Jun;961(1):113–118. doi: 10.1016/s0021-9673(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 22.Esquembre R., Pinto S.N., Poveda J.A., Prieto M., Mateo C.R. Immobilization and characterization of giant unilamellar vesicles (GUVs) within porous silica glasses. Soft Matter. 2012;8(2):408–417. [Google Scholar]
- 23.Zhang Y., Gao Z., Yu Z., Ren X., Duan L., Gao G.H. pH-Tunable mechanical hydrogels prepared via transforming non C–C covalent synergistic interactions. New J. Chem. 2017;41(4):1834–1839. [Google Scholar]
- 24.Cha C., Shin S.R., Gao X., Annabi N., Dokmeci M.R., Tang X.S., et al. Controlling mechanical properties of cell-laden hydrogels by covalent incorporation of graphene oxide. Small. 2014 Feb;10(3):514–523. doi: 10.1002/smll.201302182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kühn P.T., Meijer T.L., Schiavon I., van Poll M., van Aken J., Groen S., et al. Non-covalently stabilized alginate hydrogels as functional cell scaffold Material. Macromol Biosci. 2016 Nov;16(11):1693–1702. doi: 10.1002/mabi.201600214. [DOI] [PubMed] [Google Scholar]
- 26.Pasquardini L., Lunelli L., Vanzetti L., Anderle M., Pederzolli C. Immobilization of cationic rifampicin-loaded liposomes on polystyrene for drug-delivery applications. Colloids Surf. B Biointerfaces. 2008 Apr;62(2):265–272. doi: 10.1016/j.colsurfb.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Simões M.G., Hugo A., Alves P., Pérez P.F., Gómez-Zavaglia A., Simões P.N. Long term stability and interaction with epithelial cells of freeze-dried pH-responsive liposomes functionalized with cholesterol-poly(acrylic acid) Colloids Surf. B Biointerfaces. 2018 Apr;164:50–57. doi: 10.1016/j.colsurfb.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Guvendiren M., Lu H.D., Burdick J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter. 2012;8(2):260–272. [Google Scholar]
- 29.Rowan S.J., Cantrill S.J., Cousins G.R.L., Sanders J.K.M., Stoddart J.F. Dynamic covalent chemistry. Angew Chem. Int. Ed. Engl. 2002 Mar 15;41(6):898–952. doi: 10.1002/1521-3773(20020315)41:6<898::aid-anie898>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 30.Sekine Y., Moritani Y., Ikeda-Fukazawa T., Sasaki Y., Akiyoshi K. A hybrid hydrogel biomaterial by nanogel engineering: bottom-up design with nanogel and liposome building blocks to develop a multidrug delivery system. Adv. Healthc. Mater. 2012 Nov;1(6):722–728. doi: 10.1002/adhm.201200175. [DOI] [PubMed] [Google Scholar]
- 31.Coessens V., Pintauer T., Matyjaszewski K. Functional polymers by atom transfer radical polymerization. Prog. Polym. Sci. 2001 Apr;26(3):337–377. [Google Scholar]
- 32.Siegwart D.J., Oh J.K., Matyjaszewski K. ATRP in the design of functional materials for biomedical applications. Prog. Polym. Sci. 2012 Jan;37(1):18–37. doi: 10.1016/j.progpolymsci.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levchenko T.S., Rammohan R., Lukyanov A.N., Whiteman K.R., Torchilin V.P. Liposome clearance in mice: the effect of a separate and combined presence of surface charge and polymer coating. Int. J. Pharm. 2002 Jun;240(1–2):95–102. doi: 10.1016/s0378-5173(02)00129-1. [DOI] [PubMed] [Google Scholar]
- 34.Torchilin V. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006 Dec 1;58(14):1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Lee S., Lee S.-Y., Park S., Ryu J.H., Na J.H., Koo H., et al. In vivo NIRF imaging of tumor targetability of nanosized liposomes in tumor-bearing mice. Macromol. Biosci. 2012 Jun;12(6):849–856. doi: 10.1002/mabi.201200001. [DOI] [PubMed] [Google Scholar]
- 36.Nayak S., Lyon L.A. Soft nanotechnology with soft nanoparticles. Angew. Chem. Int. Ed. 2005 Dec 2;44(47):7686–7708. doi: 10.1002/anie.200501321. [DOI] [PubMed] [Google Scholar]
- 37.Bakker-Woudenberg I.A.J.M. Long-circulating sterically stabilized liposomes as carriers of agents for treatment of infection or for imaging infectious foci. Int. J. Antimicrob. Agents. 2002 Apr;19(4):299–311. doi: 10.1016/s0924-8579(02)00021-3. [DOI] [PubMed] [Google Scholar]
- 38.Akbarzadeh A., Rezaei-Sadabady R., Davaran S., Joo S.W., Zarghami N., Hanifehpour Y., et al. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 2013 Dec 22;8(1):102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ElBayoumi T.A., Torchilin V.P. 2010. Current Trends in Liposome Research; pp. 1–27. [DOI] [PubMed] [Google Scholar]
- 40.Nag O., Awasthi V. Surface engineering of liposomes for stealth behavior. Pharmaceutics. 2013 Oct 25;5(4):542–569. doi: 10.3390/pharmaceutics5040542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zununi Vahed S., Salehi R., Davaran S., Sharifi S. Liposome-based drug co-delivery systems in cancer cells. Mater. Sci. Eng. C. 2017 Feb;71:1327–1341. doi: 10.1016/j.msec.2016.11.073. [DOI] [PubMed] [Google Scholar]
- 42.Laouini A., Jaafar-Maalej C., Limayem-Blouza I., Sfar S., Charcosset C., Fessi H. Preparation, characterization and applications of liposomes: state of the art. J. Colloid Sci. Biotechnol. 2012 Dec 1;1(2):147–168. [Google Scholar]
- 43.Payton N.M., Wempe M.F., Xu Y., Anchordoquy T.J. Long-term storage of lyophilized liposomal formulations. J. Pharm. Sci. 2014 Dec;103(12):3869–3878. doi: 10.1002/jps.24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Popova A.V., Hincha D.K. Effects of cholesterol on dry bilayers: interactions between phosphatidylcholine unsaturation and glycolipid or free sugar. Biophys. J. 2007 Aug;93(4):1204–1214. doi: 10.1529/biophysj.107.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samuni A.M., Lipman A., Barenholz Y. Damage to liposomal lipids: protection by antioxidants and cholesterol-mediated dehydration. Chem. Phys. Lipids. 2000 Apr;105(2):121–134. doi: 10.1016/s0009-3084(99)00136-x. [DOI] [PubMed] [Google Scholar]
- 46.Liu W., Kong Y., Tu P., Lu J., Liu C., Liu W., et al. Physical–chemical stability and in vitro digestibility of hybrid nanoparticles based on the layer-by-layer assembly of lactoferrin and BSA on liposomes. Food Funct. 2017;8(4):1688–1697. doi: 10.1039/c7fo00308k. [DOI] [PubMed] [Google Scholar]
- 47.García-Uriostegui L., Burillo G., Concheiro A., Alvarez-Lorenzo C. Immobilization of liposomes on temperature-responsive polymer networks cross-linked with poly-L-lysine and grafted onto polypropylene. Des. Monomers Polym. 2013 May 20;16(3):241–249. [Google Scholar]
- 48.Reuter M., Schwieger C., Meister A., Karlsson G., Blume A. Poly-l-lysines and poly-l-arginines induce leakage of negatively charged phospholipid vesicles and translocate through the lipid bilayer upon electrostatic binding to the membrane. Biophys. Chem. 2009 Sep;144(1–2):27–37. doi: 10.1016/j.bpc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Takechi Y., Tanaka H., Kitayama H., Yoshii H., Tanaka M., Saito H. Comparative study on the interaction of cell-penetrating polycationic polymers with lipid membranes. Chem. Phys. Lipids. 2012 Jan;165(1):51–58. doi: 10.1016/j.chemphyslip.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Hatzi P., Mourtas S., Klepetsanis P G., Antimisiaris S.G. Integrity of liposomes in presence of cyclodextrins: effect of liposome type and lipid composition. Int. J. Pharm. 2007 Mar;333(1–2):167–176. doi: 10.1016/j.ijpharm.2006.09.059. [DOI] [PubMed] [Google Scholar]
- 51.Piel G., Piette M., Barillaro V., Castagne D., Evrard B., Delattre L. Betamethasone-in-cyclodextrin-in-liposome: the effect of cyclodextrins on encapsulation efficiency and release kinetics. Int. J. Pharm. 2006 Apr;312(1–2):75–82. doi: 10.1016/j.ijpharm.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 52.Salem, Düzgünes N. Efficacies of cyclodextrin-complexed and liposome-encapsulated clarithromycin against Mycobacterium avium complex infection in human macrophages. Int. J. Pharm. 2003;250(2):403–414. doi: 10.1016/s0378-5173(02)00552-5. [DOI] [PubMed] [Google Scholar]
- 53.Chiang Y.-T., Lo C.-L. pH-Responsive polymer-liposomes for intracellular drug delivery and tumor extracellular matrix switched-on targeted cancer therapy. Biomaterials. 2014 Jul;35(20):5414–5424. doi: 10.1016/j.biomaterials.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 54.Chiang Y.-T., Lyu S.-Y., Wen Y.-H., Lo C.-L. Preparation and characterization of electrostatically crosslinked polymer–liposomes in anticancer therapy. Int. J. Mol. Sci. 2018 May 30;19(6):1615. doi: 10.3390/ijms19061615. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clares B., Biedma-Ortiz R.A., Sáez-Fernández E., Prados J.C., Melguizo C., Cabeza L., et al. Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for combined hyperthermia and chemotherapy against colon cancer. Eur. J. Pharm. Biopharm. 2013 Nov;85(3):329–338. doi: 10.1016/j.ejpb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 56.Garnier B., Tan S., Miraux S., Bled E., Brisson A.R. Optimized synthesis of 100 nm diameter magnetoliposomes with high content of maghemite particles and high MRI effect. Contrast Media Mol. Imaging. 2012 Mar;7(2):231–239. doi: 10.1002/cmmi.487. [DOI] [PubMed] [Google Scholar]
- 57.Tai L.-A., Tsai P.-J., Wang Y.-C., Wang Y.-J., Lo L.-W., Yang C.-S. Thermosensitive liposomes entrapping iron oxide nanoparticles for controllable drug release. Nanotechnology. 2009 Apr 1;20(13):135101. doi: 10.1088/0957-4484/20/13/135101. [DOI] [PubMed] [Google Scholar]
- 58.Tietze R., Zaloga J., Unterweger H., Lyer S., Friedrich R.P., Janko C., et al. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 2015 Dec;468(3):463–470. doi: 10.1016/j.bbrc.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 59.Wahajuddin Arora. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012 Jul:3445. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J.-E., Shin J.-Y., Cho M.-H. Magnetic nanoparticles: an update of application for drug delivery and possible toxic effects. Arch. Toxicol. 2012 May 11;86(5):685–700. doi: 10.1007/s00204-011-0773-3. [DOI] [PubMed] [Google Scholar]
- 61.Nakayama Y., Mustapić M., Ebrahimian H., Wagner P., Kim J.H., Hossain MS Al, et al. Magnetic nanoparticles for “smart liposomes. Eur. Biophys. J. 2015 Dec 17;44(8):647–654. doi: 10.1007/s00249-015-1059-0. [DOI] [PubMed] [Google Scholar]
- 62.Fathy M.M., Fahmy H.M., Balah A.M.M., Mohamed F.F., Elshemey W.M. Magnetic nanoparticles-loaded liposomes as a novel treatment agent for iron deficiency anemia: in vivo study. Life Sci. 2019 Oct;234:116787. doi: 10.1016/j.lfs.2019.116787. [DOI] [PubMed] [Google Scholar]
- 63.Do H.D., Ménager C., Michel A., Seguin J., Korichi T., Dhotel H., et al. Development of theranostic cationic liposomes designed for image-guided delivery of nucleic acid. Pharmaceutics. 2020;12(9):1–23. doi: 10.3390/pharmaceutics12090854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petrichenko O., Plotniece A., Pajuste K., Ose V., Cēbers A. Formation of magnetoliposomes using self-assembling 1,4-dihydropyridine derivative and maghemite γ-Fe2O3 nanoparticles. Chem. Heterocycl. Compd. 2015 Jul 19;51(7):672–677. [Google Scholar]
- 65.Toro-Cordova A., Flores-Cruz M., Santoyo-Salazar J., Carrillo-Nava E., Jurado R., Figueroa-Rodriguez P., et al. Liposomes loaded with cisplatin and magnetic nanoparticles: physicochemical characterization, pharmacokinetics, and in-vitro efficacy. Molecules. 2018 Sep 6;23(9):2272. doi: 10.3390/molecules23092272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi W Il, Sahu A., Wurm F.R., Jo S.-M. Magnetoliposomes with size controllable insertion of magnetic nanoparticles for efficient targeting of cancer cells. RSC Adv. 2019;9(26):15053–15060. doi: 10.1039/c9ra02529d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roux E., Lafleur M., Lataste É., Moreau P., Leroux J.-C. On the characterization of pH-sensitive liposome/polymer complexes. Biomacromolecules. 2003 Mar;4(2):240–248. doi: 10.1021/bm025651x. [DOI] [PubMed] [Google Scholar]
- 68.Seki K., Tirrell D.A. pH-Dependent complexation of poly(acrylic acid) derivatives with phospholipid vesicle membranes. Macromolecules. 1984 Sep;17(9):1692–1698. [Google Scholar]
- 69.Lee E.S., Gao Z., Bae Y.H. Recent progress in tumor pH targeting nanotechnology. J. Contr. Release. 2008 Dec;132(3):164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yatvin M., Kreutz W., Horwitz B., Shinitzky M. pH-sensitive liposomes: possible clinical implications. Science (80- ) 1980 Dec 12;210(4475):1253–1255. doi: 10.1126/science.7434025. [DOI] [PubMed] [Google Scholar]
- 71.Xu Y., Szoka F.C. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996 Jan;35(18):5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 72.Balazs D.A., Godbey W. Liposomes for use in gene delivery. J. Drug. Deliv. 2011;2011:1–12. doi: 10.1155/2011/326497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cornelis S., Vandenbranden M., Ruysschaert J.-M., Elouahabi A. Role of intracellular cationic liposome–DNA complex dissociation in transfection mediated by cationic lipids. DNA Cell Biol. 2002 Feb;21(2):91–97. doi: 10.1089/104454902753604961. [DOI] [PubMed] [Google Scholar]
- 74.Rasoulianboroujeni M., Kupgan G., Moghadam F., Tahriri M., Boughdachi A., Khoshkenar P., et al. Development of a DNA-liposome complex for gene delivery applications. Mater. Sci. Eng. C. 2017 Jun;75:191–197. doi: 10.1016/j.msec.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 75.Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(23):6449–6465. [PubMed] [Google Scholar]
- 76.Ravazzolo E., Salmaso S., Mastrotto F., Bersani S., Gallon E., Caliceti P. pH-responsive lipid core micelles for tumour targeting. Eur. J. Pharm. Biopharm. 2013 Apr;83(3):346–357. doi: 10.1016/j.ejpb.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Bersani S., Vila-Caballer M., Brazzale C., Barattin M., Salmaso S. pH-sensitive stearoyl-PEG-poly(methacryloyl sulfadimethoxine) decorated liposomes for the delivery of gemcitabine to cancer cells. Eur. J. Pharm. Biopharm. 2014 Nov;88(3):670–682. doi: 10.1016/j.ejpb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 78.Heldin C.-H., Rubin K., Pietras K., Östman A. High interstitial fluid pressure — an obstacle in cancer therapy. Nat. Rev. Cancer. 2004 Oct;4(10):806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 79.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001 May;41(1):189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 80.Iyer A.K., Khaled G., Fang J., Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today. 2006 Sep;11(17–18):812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(8):6387–6392. [PubMed] [Google Scholar]
- 82.Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J. Drug Target. 2007 Jan 8;15(7–8):457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- 83.Chiang Y.-T., Cheng Y.-T., Lu C.-Y., Yen Y.-W., Yu L.-Y., Yu K.-S., et al. Polymer–liposome complexes with a functional hydrogen-bond cross-linker for preventing protein adsorption and improving tumor accumulation. Chem. Mater. 2013 Nov 12;25(21):4364–4372. [Google Scholar]
- 84.Kurniawansyah I.S., Sopyan I., Wathoni N., Fillah D.L., Praditya R.U. Application and characterization of in situ gel. Int. J. Appl. Pharm. 2018 Nov 22;10(6):34. [Google Scholar]
- 85.Castangia I., Nácher A., Caddeo C., Merino V., Díez-Sales O., Catalán-Latorre A., et al. Therapeutic efficacy of quercetin enzyme-responsive nanovesicles for the treatment of experimental colitis in rats. Acta Biomater. 2015 Feb;13:216–227. doi: 10.1016/j.actbio.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 86.Belali N, Wathoni N, Muchtaridi M. Advances in orally targeted drug delivery to colon. J. Adv. Pharm. Technol. Research. 10(3):100–106. [DOI] [PMC free article] [PubMed]
- 87.Ma Y.H., Yang J., Li B., Jiang Y.W., Lu X., Chen Z. Biodegradable and injectable polymer-liposome hydrogel: a promising cell carrier. Polym. Chem. 2016;7(11):2037–2044. [Google Scholar]
- 88.van Elk M., Lorenzato C., Ozbakir B., Oerlemans C., Storm G., Nijsen F., et al. Alginate microgels loaded with temperature sensitive liposomes for magnetic resonance imageable drug release and microgel visualization. Eur. Polym. J. 2015 Nov;72:620–631. [Google Scholar]
- 89.Cohen R., Kanaan H., Grant G.J., Barenholz Y. Prolonged analgesia from Bupisome and Bupigel formulations: from design and fabrication to improved stability. J. Contr. Release. 2012 Jun;160(2):346–352. doi: 10.1016/j.jconrel.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 90.Dai C., Wang B., Zhao H., Li B., Wang J. Preparation and characterization of liposomes-in-alginate (LIA) for protein delivery system. Colloids Surf. B Biointerfaces. 2006 Feb;47(2):205–210. doi: 10.1016/j.colsurfb.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 91.Ullrich M., Hanuš J., Dohnal J., Štěpánek F. Encapsulation stability and temperature-dependent release kinetics from hydrogel-immobilised liposomes. J. Colloid. Interface Sci. 2013 Mar;394:380–385. doi: 10.1016/j.jcis.2012.11.016. [Internet] [DOI] [PubMed] [Google Scholar]
- 92.Sharma S., Rajendran V., Kulshreshtha R., Ghosh P.C. Enhanced efficacy of anti-miR-191 delivery through stearylamine liposome formulation for the treatment of breast cancer cells. Int. J. Pharm. 2017 Sep;530(1–2):387–400. doi: 10.1016/j.ijpharm.2017.07.079. [DOI] [PubMed] [Google Scholar]
- 93.Kono Y., Nakai T., Taguchi H., Fujita T. Development of magnetic anionic liposome/atelocollagen complexes for efficient magnetic drug targeting. Drug Deliv. 2017 Jan 15;24(1):1740–1749. doi: 10.1080/10717544.2017.1402219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Manca M.L., Manconi M., Valenti D., Lai F., Loy G., Matricardi P., et al. Liposomes coated with chitosan–xanthan gum (chitosomes) as potential carriers for pulmonary delivery of rifampicin. J. Pharm. Sci. 2012 Feb;101(2):566–575. doi: 10.1002/jps.22775. [DOI] [PubMed] [Google Scholar]
- 95.Manconi M., Mura S., Manca M.L., Fadda A.M., Dolz M., Hernandez M.J., et al. Chitosomes as drug delivery systems for C-phycocyanin: preparation and characterization. Int. J. Pharm. 2010 Jun 15;392(1–2):92–100. doi: 10.1016/j.ijpharm.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 96.Cheng R., Yan Y., Liu H., Chen H., Pan G., Deng L., et al. Mechanically enhanced lipo-hydrogel with controlled release of multi-type drugs for bone regeneration. Appl. Mater. Today. 2018 Sep;12:294–308. [Google Scholar]
- 97.El Kechai N., Geiger S., Fallacara A., Cañero Infante I., Nicolas V., Ferrary E., et al. Mixtures of hyaluronic acid and liposomes for drug delivery: phase behavior, microstructure and mobility of liposomes. Int. J. Pharm. 2017 May;523(1):246–259. doi: 10.1016/j.ijpharm.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 98.El Kechai N., Mamelle E., Nguyen Y., Huang N., Nicolas V., Chaminade P., et al. Hyaluronic acid liposomal gel sustains delivery of a corticoid to the inner ear. J. Contr. Release. 2016 Mar;226:248–257. doi: 10.1016/j.jconrel.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 99.Pandolfi L., Frangipane V., Bocca C., Marengo A., Tarro Genta E., Bozzini S., et al. Hyaluronic acid–decorated liposomes as innovative targeted delivery system for lung fibrotic cells. Molecules. 2019 Sep 10;24(18):3291. doi: 10.3390/molecules24183291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liang Y., Kiick K.L. Multifunctional lipid-coated polymer nanogels crosslinked by photo-triggered Michael-type addition. Polym. Chem. 2014;5(5):1728–1736. [Google Scholar]
- 101.Kono Y., Gogatsubo S., Ohba T., Fujita T. Enhanced macrophage delivery to the colon using magnetic lipoplexes with a magnetic field. Drug Deliv. 2019 Jan 1;26(1):935–943. doi: 10.1080/10717544.2019.1662515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lyu D., Chen S., Guo W. Liposome crosslinked polyacrylamide/DNA hydrogel: a smart controlled-release system for small molecular payloads. Small. 2018 Apr;14(15):1704039. doi: 10.1002/smll.201704039. [DOI] [PubMed] [Google Scholar]
- 103.Kitaeva M.V., Melik-Nubarov N.S., Menger F.M., Yaroslavov A.A. Doxorubicin−Poly(acrylic acid) complexes: interaction with liposomes. Langmuir. 2004 Aug;20(16):6575–6579. doi: 10.1021/la0497144. [Internet] [DOI] [PubMed] [Google Scholar]
- 104.Schäfer J., Höbel S., Bakowsky U., Aigner A. Liposome–polyethylenimine complexes for enhanced DNA and siRNA delivery. Biomaterials. 2010 Sep;31(26):6892–6900. doi: 10.1016/j.biomaterials.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 105.Yang A., Sun Z., Liu R., Liu X., Zhang Y., Zhou Y., et al. Transferrin-conjugated erianin-loaded liposomes suppress the growth of liver cancer by modulating oxidative stress. Front. Oncol. 2021:11. doi: 10.3389/fonc.2021.727605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ko J.A., Lee Y.L., Jeong H.J., Park H.J. Preparation of encapsulated alliinase in alginate microparticles. Biotechnol. Lett. 2012 Mar 10;34(3):515–518. doi: 10.1007/s10529-011-0791-5. [DOI] [PubMed] [Google Scholar]
- 107.Park H., Kim P.-H., Hwang T., Kwon O.-J., Park T.-J., Choi S.-W., et al. Fabrication of cross-linked alginate beads using electrospraying for adenovirus delivery. Int. J. Pharm. 2012 May;427(2):417–425. doi: 10.1016/j.ijpharm.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 108.Oerlemans C., Seevinck P.R., van de Maat G.H., Boulkhrif H., Bakker C.J.G., Hennink W.E., et al. Alginate–lanthanide microspheres for MRI-guided embolotherapy. Acta Biomater. 2013 Jan;9(1):4681–4687. doi: 10.1016/j.actbio.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 109.Prüße U., Dalluhn J., Breford J., Vorlop K.-D. Production of spherical beads by JetCutting. Chem. Eng. Technol. 2000 Dec;23(12):1105–1110. [Google Scholar]
- 110.Hanuš J., Ullrich M., Dohnal J., Singh M., Štěpánek F. Remotely controlled diffusion from magnetic liposome microgels. Langmuir. 2013 Apr 2;29(13):4381–4387. doi: 10.1021/la4000318. [DOI] [PubMed] [Google Scholar]
- 111.Gulbake A., Jain S.K. Chitosan: a potential polymer for colon-specific drug delivery system. Expet Opin. Drug Deliv. 2012 Jun 24;9(6):713–729. doi: 10.1517/17425247.2012.682148. [DOI] [PubMed] [Google Scholar]
- 112.Bowman K., Leong K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006 Jan;1(2):117–128. doi: 10.2147/nano.2006.1.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wathoni N., Yuniarsih N., Cahyanto A., Muhctaridi M. α-Mangostin hydrogel film based chitosan–alginate for recurrent aphthous stomatitis. Appl. Sci. 2019 Dec 2;9(23):5235. [Google Scholar]
- 114.Mady M.M., Darwish M.M. Effect of chitosan coating on the characteristics of DPPC liposomes. J. Adv. Res. 2010 Jul;1(3):187–191. [Google Scholar]
- 115.Hasan M., Ben Messaoud G., Michaux F., Tamayol A., Kahn C.J.F., Belhaj N., et al. Chitosan-coated liposomes encapsulating curcumin: study of lipid–polysaccharide interactions and nanovesicle behavior. RSC Adv. 2016;6(51):45290–45304. [Google Scholar]
- 116.Channarong S., Chaicumpa W., Sinchaipanid N., Mitrevej A. Development and evaluation of chitosan-coated liposomes for oral DNA vaccine: the improvement of Peyer’s patch targeting using a polyplex-loaded liposomes. AAPS PharmSciTech. 2011 Mar 31;12(1):192–200. doi: 10.1208/s12249-010-9559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guo J. Chitosan-coated liposomes: characterization and interaction with leuprolide. Int. J. Pharm. 2003 Jul 24;260(2):167–173. doi: 10.1016/s0378-5173(03)00254-0. [DOI] [PubMed] [Google Scholar]
- 118.Efimova A.A., Sybachin A.V., Chvalun S.N., Kulebyakina A.I., Kozlova E.V., Yaroslavov A.A. Biodegradable multi-liposomal containers. Polym. Sci. B. 2015 Mar 24;57(2):140–144. [Google Scholar]
- 119.Yaroslavov A.A., Sybachin A.V., Schrinner M., Ballauff M., Tsarkova L., Kesselman E., et al. Liposomes remain intact when complexed with polycationic brushes. J. Am. Chem. Soc. 2010 May 5;132(17):5948–5949. doi: 10.1021/ja1012323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sandzhieva A.V., Sybachin A.V., Zaborova O.V., Yaroslavov A.A. Competitive reactions in three-component system cationic colloid–anionic liposome–protein. Polym. Sci. B. 2018 May 2;60(3):324–330. [Google Scholar]
- 121.Efimova A.A., Chvalun S.N., Kulebyakina A.I., Kozlova E.V., Yaroslavov A.A. Synthesis and properties of conjugates involving liposomes, a linear polymer, and the micelle of a polylactide-poly(ethylene glycol) block copolymer. Polym. Sci. 2016 Mar 5;58(2):172–176. [Google Scholar]
- 122.Grenha A., Seijo B., Remuñán-López C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharmaceut. Sci. 2005 Jul;25(4–5):427–437. doi: 10.1016/j.ejps.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 123.Coviello T., Matricardi P., Marianecci C., Alhaique F. Polysaccharide hydrogels for modified release formulations. J. Contr. Release. 2007 May;119(1):5–24. doi: 10.1016/j.jconrel.2007.01.004. [Internet] [DOI] [PubMed] [Google Scholar]
- 124.Hanai K., Takeshita F., Honma K., Nagahara S., Maeda M., Minakuchi Y., et al. Atelocollagen-mediated systemic DDS for nucleic acid medicines. Ann. N. Y. Acad. Sci. 2006 Oct 1;1082(1):9–17. doi: 10.1196/annals.1348.010. [DOI] [PubMed] [Google Scholar]
- 125.Takeshita F., Minakuchi Y., Nagahara S., Honma K., Sasaki H., Hirai K., et al. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc. Natl. Acad. Sci. 2005 Aug 23;102(34):12177–12182. doi: 10.1073/pnas.0501753102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kuroda S., Kondo H., Ohya K., Kasugai S. A new technique with calcium phosphate precipitate enhances efficiency of in vivo plasmid DNA gene transfer. J. Pharmacol. Sci. 2005;97(2):227–233. doi: 10.1254/jphs.fp0040504. [DOI] [PubMed] [Google Scholar]
- 127.Sano A. Atelocollagen for protein and gene delivery. Adv. Drug Deliv. Rev. 2003 Nov 28;55(12):1651–1677. doi: 10.1016/j.addr.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 128.Appel E.A., del Barrio J., Loh X.J., Scherman O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012;41(18):6195. doi: 10.1039/c2cs35264h. [DOI] [PubMed] [Google Scholar]
- 129.Pierce B.F., Pittermann E., Ma N., Gebauer T., Neffe A.T., Hölscher M., et al. Viability of human mesenchymal stem cells seeded on crosslinked entropy-elastic gelatin-based hydrogels. Macromol. Biosci. 2012 Mar;12(3):312–321. doi: 10.1002/mabi.201100237. [DOI] [PubMed] [Google Scholar]
- 130.Won Y.-W., Kim Y.-H. Recombinant human gelatin nanoparticles as a protein drug carrier. J. Contr. Release. 2008 Apr;127(2):154–161. doi: 10.1016/j.jconrel.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 131.Zhou D., Ito Y. Inorganic material surfaces made bioactive by immobilizing growth factors for hard tissue engineering. RSC Adv. 2013;3(28):11095. [Google Scholar]
- 132.Van Den Bulcke A.I., Bogdanov B., De Rooze N., Schacht E.H., Cornelissen M., Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000 Mar;1(1):31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 133.Van Vlierberghe S., Cnudde V., Dubruel P., Masschaele B., Cosijns A., De Paepe I., et al. Porous gelatin hydrogels: 1. Cryogenic formation and structure analysis. Biomacromolecules. 2007 Feb;8(2):331–337. doi: 10.1021/bm060684o. [DOI] [PubMed] [Google Scholar]
- 134.Van Vlierberghe S., Dubruel P., Lippens E., Cornelissen M., Schacht E. Correlation between cryogenic parameters and physico-chemical properties of porous gelatin cryogels. J. Biomater. Sci. Polym. Ed. 2009 Jan 2;20(10):1417–1438. doi: 10.1163/092050609X12457418905508. [DOI] [PubMed] [Google Scholar]
- 135.Volpi N., Schiller J., Stern R., Soltes L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr. Med. Chem. 2009 May 1;16(14):1718–1745. doi: 10.2174/092986709788186138. [DOI] [PubMed] [Google Scholar]
- 136.Lapčík L., Lapčík L., De Smedt S., Demeester J., Chabreček P. Hyaluronan: preparation, structure, properties, and applications. Chem. Rev. 1998 Dec;98(8):2663–2684. doi: 10.1021/cr941199z. [DOI] [PubMed] [Google Scholar]
- 137.Gatej I., Popa M., Rinaudo M. Role of the pH on hyaluronan behavior in aqueous solution. Biomacromolecules. 2005 Jan;6(1):61–67. doi: 10.1021/bm040050m. [DOI] [PubMed] [Google Scholar]
- 138.Schanté C.E., Zuber G., Herlin C., Vandamme T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011 Jun;85(3):469–489. [Google Scholar]
- 139.Shin J.M., Kim S.-H., Thambi T., You D.G., Jeon J., Lee J.O., et al. A hyaluronic acid–methotrexate conjugate for targeted therapy of rheumatoid arthritis. Chem. Commun. 2014;50(57):7632. doi: 10.1039/c4cc02595d. [DOI] [PubMed] [Google Scholar]
- 140.Racine R., M E. Molecular Regulation of Endocytosis. InTech; 2012. Hyaluronan endocytosis: mechanisms of uptake and biological functions. [Google Scholar]
- 141.Miyazaki M., Yuba E., Hayashi H., Harada A., Kono K. Hyaluronic acid-based pH-sensitive polymer-modified liposomes for cell-specific intracellular drug delivery systems. Bioconjugate Chem. 2018 Jan 17;29(1):44–55. doi: 10.1021/acs.bioconjchem.7b00551. [DOI] [PubMed] [Google Scholar]
- 142.Chen H., Tang L., Qin Y., Yin Y., Tang J., Tang W., et al. Lactoferrin-modified procationic liposomes as a novel drug carrier for brain delivery. Eur. J. Pharmaceut. Sci. 2010 May;40(2):94–102. doi: 10.1016/j.ejps.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 143.Liu Y., Selig M.J., Yadav M.P., Yin L., Abbaspourrad A. Transglutaminase-treated conjugation of sodium caseinate and corn fiber gum hydrolysate: interfacial and dilatational properties. Carbohydr. Polym. 2018 May;187:26–34. doi: 10.1016/j.carbpol.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 144.Gomme P.T., McCann K.B. Transferrin: structure, function and potential therapeutic actions. Drug Discovery Today. 2005;10 doi: 10.1016/S1359-6446(04)03333-1. [DOI] [PubMed] [Google Scholar]
- 145.Xie C., Wang Z., Su Y., Wang J., Shen W.C. Discovery of an orally effective factor IX-transferrin fusion protein for hemophilia B. Int. J. Mol. Sci. 2020;21(1) doi: 10.3390/ijms21010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hunt R.C., Marshall-Carlson L. Internalization and recycling of transferrin and its receptor. Effect of trifluoperazine on recycling in human erythroleukemic cells. J. Biol. Chem. 1986;261(8) [PubMed] [Google Scholar]
- 147.Shen Y., Li X., Dong D., Zhang B., Xue Y., Shang P. Transferrin receptor 1 in cancer: a new sight for cancer therapy. Am. J. Cancer Res. 2018;8(6) [PMC free article] [PubMed] [Google Scholar]
- 148.Daniels T.R., Bernabeu E., Rodríguez J.A., Patel S., Kozman M., Chiappetta D.A., et al. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochimica et Biophysica Acta - General Subjects. 2012;1820 doi: 10.1016/j.bbagen.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nogueira-Librelotto D.R., Codevilla C.F., Farooqi A., Rolim C.M.B. Transferrin-conjugated nanocarriers as active-targeted drug delivery platforms for cancer therapy. Curr. Pharmaceut. Des. 2016;23(3) doi: 10.2174/1381612822666161026162347. [DOI] [PubMed] [Google Scholar]
- 150.Soe Z.C., Kwon J.B., Thapa R.K., Ou W., Nguyen H.T., Gautam M., et al. Transferrin-conjugated polymeric nanoparticle for receptor-mediated delivery of doxorubicin in doxorubicin-resistant breast cancer cells. Pharmaceutics. 2019;11(2) doi: 10.3390/pharmaceutics11020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sakpakdeejaroen I., Somani S., Laskar P., Mullin M., Dufès C. Transferrin-bearing liposomes entrapping plumbagin for targeted cancer therapy. J. Interdiscip. Nanomed. 2019;4(2) doi: 10.1002/jin2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ulbrich K., Hekmatara T., Herbert E., Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB) Eur. J. Pharm. Biopharm. 2009;71(2) doi: 10.1016/j.ejpb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 153.Yue P.J., He L., Qiu S.W., Li Y., Liao Y.J., Li X.P., et al. OX26/CTX-conjugated PEGylated liposome as a dual-targeting gene delivery system for brain glioma. Mol. Cancer. 2014;13(1) doi: 10.1186/1476-4598-13-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Duggan S.T., Keating G.M. Pegylated liposomal doxorubicin. Drugs. 2011 Dec;71(18):2531–2558. doi: 10.2165/11207510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 155.Petros R.A., DeSimone J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010 Aug 9;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 156.Alves P., Hugo A.A., Tymczyszyn E.E., Ferreira A.F., Fausto R., Pérez P.F., et al. Effect of cholesterol-poly(N,N-dimethylaminoethyl methacrylate) on the properties of stimuli-responsive polymer liposome complexes. Colloids Surf. B Biointerfaces. 2013 Apr;104:254–261. doi: 10.1016/j.colsurfb.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 157.Boussif O., Lezoualc’h F., Zanta M.A., Mergny M.D., Scherman D., Demeneix B., et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. 1995 Aug 1;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Neu M., Fischer D., Kissel T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J. Gene Med. 2005 Aug;7(8):992–1009. doi: 10.1002/jgm.773. [DOI] [PubMed] [Google Scholar]
- 159.Tang M., Szoka F. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997 Aug 18;4(8):823–832. doi: 10.1038/sj.gt.3300454. [DOI] [PubMed] [Google Scholar]
- 160.Godbey W.T., Wu K.K., Mikos A.G. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999 Jun 5;45(3):268–275. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 161.Werth S., Urban-Klein B., Dai L., Höbel S., Grzelinski M., Bakowsky U., et al. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J. Contr. Release. 2006 May;112(2):257–270. doi: 10.1016/j.jconrel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 162.Grzelinski M., Urban-Klein B., Martens T., Lamszus K., Bakowsky U., Höbel S., et al. RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum. Gene Ther. 2006 Jul;17(7):751–766. doi: 10.1089/hum.2006.17.751. [DOI] [PubMed] [Google Scholar]
- 163.Urban-Klein B., Werth S., Abuharbeid S., Czubayko F., Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005 Mar 23;12(5):461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 164.Benjaminsen R.V., Mattebjerg M.A., Henriksen J.R., Moghimi S.M., Andresen T.L. The possible “proton sponge ” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013 Jan;21(1):149–157. doi: 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nomura Y., Ikeda M., Yamaguchi N., Aoyama Y., Akiyoshi K. Protein refolding assisted by self-assembled nanogels as novel artificial molecular chaperone. FEBS Lett. 2003 Oct 23;553(3):271–276. doi: 10.1016/s0014-5793(03)01028-7. [DOI] [PubMed] [Google Scholar]
- 166.Akiyoshi K., Sasaki Y., Sunamoto J. Molecular chaperone-like activity of hydrogel nanoparticles of hydrophobized pullulan: thermal stabilization with refolding of carbonic anhydrase B. Bioconjugate Chem. 1999 May;10(3):321–324. doi: 10.1021/bc9801272. [DOI] [PubMed] [Google Scholar]
- 167.Lee K., Silva E.A., Mooney D.J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc. Interface. 2011 Feb 6;8(55):153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen F.-M., Zhang M., Wu Z.-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010 Aug;31(24):6279–6308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 169.Kopeček J. Hydrogel biomaterials: a smart future? Biomaterials. 2007 Dec;28(34):5185–5192. doi: 10.1016/j.biomaterials.2007.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yoshihara E., Nakae T. Cytolytic activity of liposomes containing stearylamine. Biochim. Biophys. Acta Biomembr. 1986 Jan;854(1):93–101. doi: 10.1016/0005-2736(86)90068-4. [DOI] [PubMed] [Google Scholar]
- 171.Chi B., Qi M., Kuramitsu H.K. Role of dentilisin in Treponema denticola epithelial cell layer penetration. Res. Microbiol. 2003 Nov;154(9):637–643. doi: 10.1016/j.resmic.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 172.Zidan A.S., Spinks C.B., Habib M.J., Khan M.A. Formulation and transport properties of tenofovir loaded liposomes through Caco-2 cell model. J. Liposome Res. 2013 Dec 5;23(4):318–326. doi: 10.3109/08982104.2013.810645. [DOI] [PubMed] [Google Scholar]
- 173.Kočišová E., Procházka M., Vaculčiaková L. Drop-coating deposition Raman (DCDR) spectroscopy as a tool for membrane interaction studies: liposome–porphyrin complex. Appl. Spectrosc. 2015 Aug;69(8):939–945. doi: 10.1366/14-07836. [DOI] [PubMed] [Google Scholar]
- 174.Zhang D., Xie Y., Mrozek M.F., Ortiz C., Davisson V.J., Ben-Amotz D. Raman detection of proteomic analytes. Anal. Chem. 2003 Nov;75(21):5703–5709. doi: 10.1021/ac0345087. [DOI] [PubMed] [Google Scholar]
- 175.Šimáková P., Kočišová E., Procházka M. Sensitive Raman spectroscopy of lipids based on drop deposition using DCDR and SERS. J. Raman Spectrosc. 2013 Nov;44(11):1479–1482. [Google Scholar]
- 176.Kočišová E., Antalík A., Procházka M. Drop coating deposition Raman spectroscopy of liposomes: role of cholesterol. Chem. Phys. Lipids. 2013 Jul;172–173:1–5. doi: 10.1016/j.chemphyslip.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 177.Kopecký V., Baumruk V. Structure of the ring in drop coating deposited proteins and its implication for Raman spectroscopy of biomolecules. Vib. Spectrosc. 2006 Nov;42(2):184–187. [Google Scholar]
- 178.Wang J., Byrne J.D., Napier M.E., DeSimone J.M. More effective nanomedicines through particle design. Small. 2011 Jul 18;7(14):1919–1931. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee S.-M., Chen H., Dettmer C.M., O’Halloran T.V., Nguyen S.T. Polymer-caged lipsomes: a pH-responsive delivery system with high stability. J. Am. Chem. Soc. 2007 Dec;129(49):15096–15097. doi: 10.1021/ja070748i. [DOI] [PubMed] [Google Scholar]
- 180.Lee S.-M., Chen H., O’Halloran T.V., Nguyen S.T. “Clickable” polymer-caged nanobins as a modular drug delivery platform. J. Am. Chem. Soc. 2009 Jul 8;131(26):9311–9320. doi: 10.1021/ja9017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cheng C., Peng S., Li Z., Zou L., Liu W., Liu C. Improved bioavailability of curcumin in liposomes prepared using a pH-driven, organic solvent-free, easily scalable process. RSC Adv. 2017;7(42):25978–25986. [Google Scholar]
- 182.Chinnathambi S., Hanagata N., Yamazaki T., Shirahata N. Nano-bio interaction between blood plasma proteins and water-soluble silicon quantum dots with enabled cellular uptake and minimal cytotoxicity. Nanomaterials. 2020;10(11) doi: 10.3390/nano10112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang M., Wu E., Tang W., Qian J., Zhan C. Interplay between nanomedicine and protein corona. J. Mater. Chem. B. 2021;9 doi: 10.1039/d1tb01063h. [DOI] [PubMed] [Google Scholar]
- 184.Karmali P.P., Simberg D. Interactions of nanoparticles with plasma proteins: implication on clearance and toxicity of drug delivery systems. Expert Opin. Drug Deliv. 2011;8 doi: 10.1517/17425247.2011.554818. [DOI] [PubMed] [Google Scholar]
- 185.Mahmoudi M., Lynch I., Ejtehadi M.R., Monopoli M.P., Bombelli F.B., Laurent S. Protein-nanoparticle interactions: opportunities and challenges. Chem. Rev. 2011;111 doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 186.Baer D.R., Engelhard M.H., Johnson G.E., Laskin J., Lai J., Mueller K., et al. Surface characterization of nanomaterials and nanoparticles: important needs and challenging opportunities. J. Vac. Sci. Technol. A Vacuum Surf. Film. 2013;31(5) doi: 10.1116/1.4818423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hu Z., Zhang H., Zhang Y., Wu R., Zou H. Nanoparticle size matters in the formation of plasma protein coronas on Fe3O4 nanoparticles. Colloids Surf. B Biointerfaces. 2014:121. doi: 10.1016/j.colsurfb.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 188.Aggarwal P., Hall J.B., McLeland C.B., Dobrovolskaia M.A., McNeil S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009;61 doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Binnemars-Postma K.A., Ten Hoopen H.W., Storm G., Prakash J. Differential uptake of nanoparticles by human M1 and M2 polarized macrophages: protein corona as a critical determinant. Nanomedicine. 2016;11(22) doi: 10.2217/nnm-2016-0233. [DOI] [PubMed] [Google Scholar]
- 190.Colino C.I., Lanao J.M., Gutierrez-Millan C. Targeting of hepatic macrophages by therapeutic nanoparticles. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ross P.C., Hui S.W. Lipoplex size is a major determinant of in vitro lipofection efficiency. Gene Ther. 1999;6(4) doi: 10.1038/sj.gt.3300863. [DOI] [PubMed] [Google Scholar]
- 192.Ilinskaya A.N., Dobrovolskaia M.A. Polymer Nanoparticles for Nanomedicines. 2016. Interaction between nanoparticles and plasma proteins: effects on nanoparticle biodistribution and toxicity. [Google Scholar]
- 193.Betker J.L., Jones D., Childs C.R., Helm K.M., Terrell K., Nagel M.A., et al. Nanoparticle uptake by circulating leukocytes: a major barrier to tumor delivery. J. Contr. Release. 2018:286. doi: 10.1016/j.jconrel.2018.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Takeuchi H., Kojima H., Yamamoto H., Kawashima Y. Polymer coating of liposomes with a modified polyvinyl alcohol and their systemic circulation and RES uptake in rats. J. Contr. Release. 2000;68(2) doi: 10.1016/s0168-3659(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 195.Capriotti A.L., Caracciolo G., Cavaliere C., Foglia P., Pozzi D., Samperi R., et al. Do plasma proteins distinguish between liposomes of varying charge density? J. Proteonomics. 2012;75(6) doi: 10.1016/j.jprot.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 196.Mariam J., Sivakami S., Dongre P.M. Albumin corona on nanoparticles–a strategic approach in drug delivery. Drug Deliv.ttfgA. 2016;23 doi: 10.3109/10717544.2015.1048488. [DOI] [PubMed] [Google Scholar]
- 197.Wang A., Yang T., Fan W., Yang Y., Zhu Q., Guo S., et al. Protein corona liposomes achieve efficient oral insulin delivery by overcoming mucus and epithelial barriers. Adv. Healthc. Mater. 2019;8(12) doi: 10.1002/adhm.201801123. [DOI] [PubMed] [Google Scholar]
- 198.Resina S., Prevot P., Tjierry A.R. Physico-chemical characteristics of lipoplexes influence cell uptake mechanisms and transfection efficacy. PLoS One. 2009;4(6) doi: 10.1371/journal.pone.0006058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gao H., He Q. The interaction of nanoparticles with plasma proteins and the consequent influence on nanoparticles behavior. Expet Opin. Drug Deliv. 2014;11(3) doi: 10.1517/17425247.2014.877442. [DOI] [PubMed] [Google Scholar]
- 200.Bhavsar D., Ramana L.N., Subramanian K., Sethuraman S., Krishnan U.M. Protein adsorption characteristics and inflammatory response of lipoplexes of DNA and si-RNA. Nano. 2015;10(4) [Google Scholar]
- 201.Zschörnig O., Paasche G., Thieme C., Korb N., Arnold K. Modulation of lysozyme charge influences interaction with phospholipid vesicles. Colloids Surf. B Biointerfaces. 2005;42(1) doi: 10.1016/j.colsurfb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 202.Hussain S. Psychiatry of Pandemics. Springer International Publishing; Cham: 2019. Immunization and vaccination; pp. 153–177. [Google Scholar]
- 203.McCullers J.A., Dunn J.D. Vol. 33. P and T; 2008. (Advances in vaccine technology and their impact on managed care). [PMC free article] [PubMed] [Google Scholar]
- 204.DeFrancesco L. Whither COVID-19 vaccines? Nat. Biotechnol. 2020;38 doi: 10.1038/s41587-020-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hajj Hussein I., Chams N., Chams S., El Sayegh S., Badran R., Raad M., et al. Vaccines through centuries: major cornerstones of global health. Front. Public Health. 2015;3 doi: 10.3389/fpubh.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nascimento I.P., Leite L.C.C. Recombinant vaccines and the development of new vaccine strategies. Brazilian J. Med. Biol. Res. 2012;45 doi: 10.1590/S0100-879X2012007500142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lee J., Arun Kumar S., Jhan Y.Y., Bishop C.J. Engineering DNA vaccines against infectious diseases. Acta Biomaterialia. 2018;80 doi: 10.1016/j.actbio.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rappuoli R., De Gregorio E., Costantino P. Vol. 116. Proceedings of the National Academy of Sciences of the United States of America; 2019. (On the mechanisms of conjugate vaccines). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rauch S., Jasny E., Schmidt K.E., Petsch B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. Opportunities and challenges in the delivery of mrna-based vaccines. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Heinz F.X., Stiasny K. Vol. 6. npj Vaccines; 2021. (Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kim M.G., Park J.Y., Shon Y., Kim G., Shim G., Oh Y.K. Nanotechnology and vaccine development. Asian J. Pharmaceutical Sci. 2014;9 [Google Scholar]
- 213.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M. del P., Acosta-Torres L.S., et al. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16(1) doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Behzadi S., Serpooshan V., Tao W., Hamaly M.A., Alkawareek M.Y., Dreaden E.C., et al. Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 2017;46(14):4218–4244. doi: 10.1039/c6cs00636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Singh L., Kruger H.G., Maguire G.E.M., Govender T., Parboosing R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. Dis. 2017;4(4) doi: 10.1177/2049936117713593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zolnik B.S., González-Fernández Á., Sadrieh N., Dobrovolskaia M.A. Minireview: Nanoparticles and the immune system. Endocrinology. 2010;151 doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A., et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15 doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 218.Singh A., Misra R., Mohanty C., Sahoo S.K. Applications of nanotechnology in vaccine delivery. Int. J. Green Nanotechnol. Biomed. 2010;2(1) [Google Scholar]
- 219.Chu R.S., Targoni O.S., Krieg A.M., Lehmann P.V., Harding C.V. CpP oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 1997;186(10) doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Carson D.A., Raz E. Oligonucleotide adjuvants for T helper 1 (Th1)-specific vaccination. J. Exp. Med. 1997;186 doi: 10.1084/jem.186.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Giddam A.K., Zaman M., Skwarczynski M., Toth I. Liposome-based delivery system for vaccine candidates: Constructing an effective formulation. Nanomedicine. 2012;7 doi: 10.2217/nnm.12.157. [DOI] [PubMed] [Google Scholar]
- 222.Zhuang Y., Ma Y., Wang C., Hai L., Yan C., Zhang Y., et al. PEGylated cationic liposomes robustly augment vaccine-induced immune responses: Role of lymphatic trafficking and biodistribution. J. Control Release. 2012;159(1) doi: 10.1016/j.jconrel.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 223.Gregoriadis G. Liposomes as immunoadjuvants and vaccine carriers: antigen entrapment. Immunomethods. 1994;4(3) doi: 10.1006/immu.1994.1022. [DOI] [PubMed] [Google Scholar]
- 224.Gao J., Ochyl L.J., Yang E., Moon J.J. Cationic liposomes promote antigen cross-presentation in dendritic cells by alkalizing the lysosomal pH and limiting the degradation of antigens. Int. J. Nanomed. 2017;12 doi: 10.2147/IJN.S125866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schwendener R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines. 2014;2 doi: 10.1177/2051013614541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schlake T., Thess A., Fotin-Mleczek M., Kallen K.J. Developing mRNA-vaccine technologies. RNA Biol. 2012;9 doi: 10.4161/rna.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Khatri K., Goyal A.K., Gupta P.N., Mishra N., Mehta A., Vyas S.P. Surface modified liposomes for nasal delivery of DNA vaccine. Vaccine. 2008;26(18) doi: 10.1016/j.vaccine.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 228.Park J.W., Benz C.C., Martin F.J. Future directions of liposome- and immunoliposome-based cancer therapeutics. Semin. Oncol. 2004;31(SUPPL. 13) doi: 10.1053/j.seminoncol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 229.Reichmuth A.M., Oberli M.A., Jeklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7(5) doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Petazzi R.A., Gramatica A., Herrmann A., Chiantia S. Time-controlled phagocytosis of asymmetric liposomes: Application to phosphatidylserine immunoliposomes binding HIV-1 virus-like particles. Nanomed. Nanotechnol. Biol. Med. 2015;11(8) doi: 10.1016/j.nano.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 231.Harokopakis E., Hajishengallis G., Michalek S.M. Effectiveness of liposomes possessing surface-linked recombinant B subunit of cholera toxin as an oral antigen delivery system. Infect. Immun. 1998;66(9) doi: 10.1128/iai.66.9.4299-4304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Huang W., Zhang C. Assembly and characterization of lipid-lipid binding protein particles. J. Biotechnol. 2011;154(1) doi: 10.1016/j.jbiotec.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 233.Hu Y., Zheng H., Huang W., Zhang C. A novel and efficient nicotine vaccine using nano-lipoplex as a delivery vehicle. Hum. Vaccines Immunother. 2014;10(1) doi: 10.4161/hv.26635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yuba E. Design of pH-sensitive polymer-modified liposomes for antigen delivery and their application in cancer immunotherapy. Polym. J. 2016;48(7) [Google Scholar]
- 235.Chen C.H., Lin Y.L., Liu Y.K., He P.J., Lin C.M., Chiu Y.H., et al. Liposome-based polymer complex as a novel adjuvant:Enhancement of specifc antibody production and isotype switch. Int. J. Nanomed. 2012;7 doi: 10.2147/IJN.S28097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ciabattini A., Pettini E., Fiorino F., Pastore G., Andersen P., Pozzi G., et al. Modulation of primary immune response by different vaccine adjuvants. Front. Immunol. 2016;7 doi: 10.3389/fimmu.2016.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kantipakala R., Bonam S.R., Vemireddy S., Miryala S., Halmuthur M.S.K. Squalane-based emulsion vaccine delivery system: composition with murabutide activate Th1 response. Pharm. Dev. Technol. 2019;24(3) doi: 10.1080/10837450.2018.1469150. [DOI] [PubMed] [Google Scholar]
- 238.Lindenstrøm T., Woodworth J., Dietrich J., Aagaard C., Andersen P., Agger E.M. Vaccine-induced Th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect. Immun. 2012;80(10) doi: 10.1128/IAI.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ciabattini A., Pettini E., Fiorino F., Lucchesi S., Pastore G., Brunetti J., et al. Heterologous prime-boost combinations highlight the crucial role of adjuvant in priming the immune system. Front. Immunol. 2018;9(MAR) doi: 10.3389/fimmu.2018.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wei L., Zhao T., Zhang J., Mao Q., Gong G., Sun Y., et al. Efficacy and safety of a nanoparticle therapeutic vaccine in patients with chronic hepatitis B: A randomized clinical trial. Hepatology. 2022;75(1):182–195. doi: 10.1002/hep.32109. [DOI] [PubMed] [Google Scholar]
- 241.Gargett T., Abbas M.N., Rolan P., Price J.D., Gosling K.M., Ferrante A., et al. Phase I trial of Lipovaxin-MM, a novel dendritic cell-targeted liposomal vaccine for malignant melanoma. Cancer Immunol. Immunother [Internet] 2018;67(9):1461–1472. doi: 10.1007/s00262-018-2207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Friedman-Klabanoff D.A.J., Berry A.A., Travassos M.A., Cox C., Zhou Y., Mo A.X., et al. Low dose recombinant full-length circumsporozoite protein-based Plasmodium falciparum vaccine is well-tolerated and highly immunogenic in phase 1 first-in-human clinical testing. Vaccine [Internet] 2021;39(8):1195–1200. doi: 10.1016/j.vaccine.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jeong J., Park C., Kim S., Park S.J., Kang I., Park K.H., et al. Evaluation of the efficacy of a novel porcine circovirus type 2 synthetic peptide vaccine. Can. J. Vet. Res. 2018;82(2):146–153. [PMC free article] [PubMed] [Google Scholar]
- 244.Om K., Paquin-Proulx D., Montero M., Peachman K., Shen X., Wieczorek L., et al. Adjuvanted HIV-1 vaccine promotes antibody-dependent phagocytic responses and protects against heterologous sHIV challenge. PLoS Pathog [Internet] 2020;16(9):1–25. doi: 10.1371/journal.ppat.1008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Abraham S., Juel H.B., Bang P., Cheeseman H.M., Dohn R.B., Cole T., et al. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. [Internet] 2019;19(10):1091–1100. doi: 10.1016/S1473-3099(19)30279-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.