Abstract
IMPORTANCE:
Withdrawal-of-life-sustaining treatments (WOLST) rates vary widely among critically ill neurologic patients (CINPs) and cannot be solely attributed to patient and family characteristics. Research in general critical care has shown that clinicians prognosticate to families with high variability. Little is known about how clinicians disclose prognosis to families of CINPs, and whether any associations exist with WOLST.
OBJECTIVES:
Primary: to demonstrate feasibility of audio-recording clinician-family meetings for CINPs at multiple centers and characterize how clinicians communicate prognosis during these meetings. Secondary: to explore associations of 1) clinician, family, or patient characteristics with clinicians’ prognostication approaches and 2) prognostication approach and WOLST.
DESIGN, SETTING, AND PARTICIPANTS:
Forty-three audio-recorded clinician-family meetings during which prognosis was discussed from seven U.S. centers for 39 CINPs with 88 family members and 27 clinicians.
MAIN OUTCOMES AND MEASURES:
Two investigators qualitatively coded transcripts using inductive methods (inter-rater reliability > 80%) to characterize how clinicians prognosticate. We then applied univariate and multivariable multinomial and binomial logistic regression.
RESULTS:
Clinicians used four distinct prognostication approaches: Authoritative (21%; recommending treatments without discussing values and preferences); Informational (23%; disclosing just the prognosis without further discussions); advisory (42%; disclosing prognosis followed by discussion of values and preferences); and responsive (14%; eliciting values and preferences, then disclosing prognosis). Before adjustment, prognostication approach was associated with center (p < 0.001), clinician specialty (neurointensivists vs non-neurointensivists; p = 0.001), patient age (p = 0.08), diagnosis (p = 0.059), and meeting length (p = 0.03). After adjustment, only clinician specialty independently predicted prognostication approach (p = 0.027). WOLST decisions occurred in 41% of patients and were most common under the advisory approach (56%). WOLST was more likely in older patients (p = 0.059) and with more experienced clinicians (p = 0.07). Prognostication approach was not independently associated with WOLST (p = 0.198).
CONCLUSIONS AND RELEVANCE:
It is feasible to audio-record sensitive clinician-family meetings about CINPs in multiple ICUs. We found that clinicians prognosticate with high variability. Our data suggest that larger studies are warranted in CINPs to examine the role of clinicians’ variable prognostication in WOLST decisions.
Keywords: brain injuries, communication, critical care, decision-making, family, goals, prognosis, treatment outcome
The vast majority, of critically ill neurologic patients (CINPs), upwards of four in five, die after withdrawal-of-life-sustaining treatments (WOLST) by family members acting as surrogate decision-makers (1–3). Several studies in CINPs have documented an alarming variability of WOLST rates between centers, ranging from 0% to 96% in stroke, and 45–87% in severe traumatic brain injury, even after adjusting for disease severity and patient’s age (4, 5). While patient and family characteristics, including race, socioeconomic factors, geographic location, religiosity, and personal values, may partly be responsible for this variability (6, 7), breakdowns in clinician-family communication may also pose a potential explanation. Empirical research in general critical care has confirmed a substantial variability in how clinicians disclose prognosis and treatment recommendations to families (8, 9). In CINPs, families must consider the patients’ potential for long-term physical and cognitive disability when contemplating WOLST decisions (10–12) and therefore routinely turn to clinicians to provide them with a prognosis. The stakes of prognostic miscommunication and breakdowns in clinician-family communication are especially high in CINPs; they range from potentially premature WOLST decisions leading to the death of a patient who may have otherwise had an acceptable outcome with continued treatment, to the prolongation of life with physical or cognitive dysfunction that patients would have considered intolerable or a state worse than death (2, 13–16), and long-term psychologic distress in family members (17, 18).
Despite the importance of prognostication, clinicians receive little to no training in prognostic communication (5, 13) and no evidence-based guidelines exist for how to effectively disclose prognosis to the families of CINPs. There are no empirical studies that directly examine clinician-family communication for CINPs. Our lack of understanding of how clinicians currently disclose prognosis and treatment recommendations for CINPs in practice is a major barrier to developing acceptable, evidence-based interventions to improve clinician-family communication, decrease misunderstandings about prognosis, and achieve patient value-congruent decisions.
Our primary objectives in this pilot study were to demonstrate feasibility of audio-recording sensitive goals-of-care family meetings for CINPs and to characterize how clinicians communicate prognosis and treatment recommendations during these meetings. We hypothesized that we could identify several distinct prognostic communication approaches used by clinicians. Secondary exploratory objectives included whether: 1) certain clinician, family, or patient characteristics predict clinicians’ prognostication approaches and 2) clinician’s prognostication approaches predict WOLST.
METHODS
Study Design and Enrollment
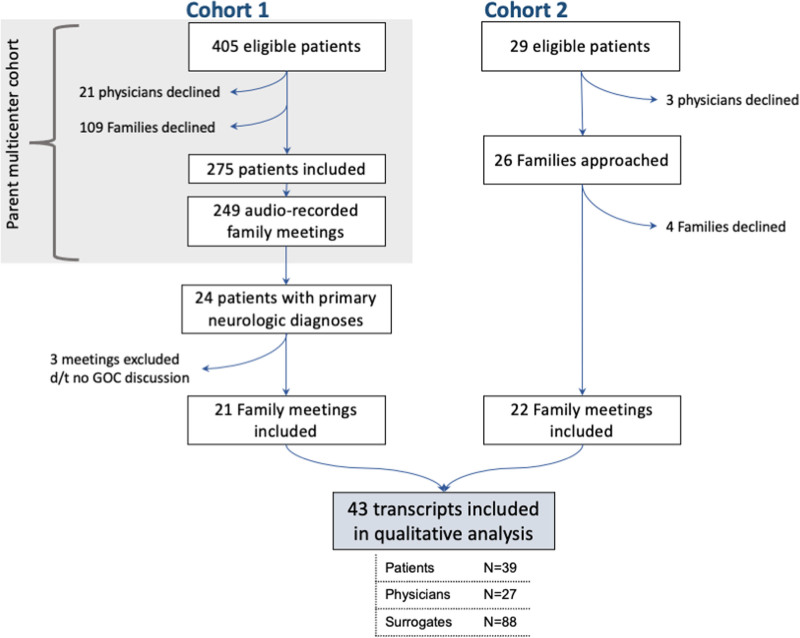
We conducted a mixed-methods analysis of audio-recorded clinician-family meetings between families of CINPs and their treating clinicians, during which prognosis or goals of care were discussed. We pooled audio recordings and their de-identified transcripts from two cohorts (Fig. 1). Cohort 1 included a subset of CINPs from a parent multicenter study in six centers on clinician-family communication and prognostic disclosure, recorded between 2009 and 2012. Cohort 2 included family meetings from a separate, single neuro-ICU, recorded in 2019. Recruitment details for the two cohorts are further described in the Supplementary Methods (http://links.lww.com/CCX/A929). A full description of study methods for cohort 1 were previously published (19). The rationale for combining cohorts with recordings obtained 5–10 years apart was to: 1) demonstrate feasibility of audio-recording sensitive clinician-family meetings at multiple centers and 2) ameliorate selection bias and lower disease variety as a result from restricting our cohort to the contemporary recordings from a single center.
Figure 1.
Flow chart of participant enrollment and exclusions. Cohort 1 includes a subset of critically ill neurologic patients from a parent multicenter study in six centers on clinician-family communication and prognostic disclosure. Cohort 2 includes family meetings recorded from a neuro-ICU at a single center. d/t = due to, GOC = goals of care.
The Institutional Review Boards at the University of Massachusetts Medical School (No. H00016916) and University of Pittsburgh (No. PRO09050285) approved the studies. Clinician-family meetings were timed, audio-recorded, and professionally transcribed. De-identified transcripts were imported into NVivo qualitative data analysis software (Version 12; QSR International (Americas), Burlington, MA, 2018).
In both studies, clinicians and surrogates completed standardized written questionnaires after the clinician-family meeting to capture their demographic data; patient demographics and clinical data were abstracted from medical records.
Qualitative Analysis
Two independent coders (C.G., A.L.G.) developed an initial codebook using an inductive approach coding the same five transcripts in parallel. This is a qualitative research method during which codes and themes emerge without applying previous knowledge or frameworks (20). Over a series of meetings and analysis of four additional transcripts, the investigators reviewed and reconciled coding differences with a third investigator (S.M.) to refine the codebook. They modified the codebook iteratively as themes coalesced or new themes appeared until strong inter-rater reliability (kappa = 0.86) and theme saturation was achieved without any new themes emerging, coding a total of nine transcripts (21%) in parallel (21). Then, both investigators applied the codebook to the remaining transcripts independently. Next, one investigator used this codebook to create a de novo framework that combined themes into distinct prognostic communication approaches. We developed this framework so that each clinician-family meeting was characterized by one prognostic communication approach without overlap, in order to examine the broader consequences of communication rather than narrowing our focus through line-by-line comparisons. Afterward, using this framework, the two coders independently assigned prognostic communication approaches to all transcripts. Any discrepancies were resolved through group discussion until 100% agreement was achieved.
Because we aimed to characterize clinicians’ approaches to prognostication and goals-of-care communication, our pre hoc exclusion criterion was to exclude meetings that lacked such discussions.
Quantitative Analysis
To examine univariate associations between approaches and family, clinician, and patient factors, as well as with final goals-of-care decision (WOLST vs survival), we applied Fisher exact test for categorical data and exact Kruskal-Wallis test for continuous data. We applied multivariable multinomial logistic regression to identify predictors of clinicians’ prognostication approaches and to explore independent associations of prognostication approach with WOLST, adding variables from the univariate analysis with p value of less than or equal to 0.2 into the multivariable model (see Supplementary Methods for more detail http://links.lww.com/CCX/A929). All quantitative analyses were performed in SAS 9.4 (SAS Institute, Cary, NC). Graphs were generated using Prism 7 (GraphPad Software, San Diego, CA).
RESULTS
Participants
Characteristics for all participants are shown in Supplementary Table 1 (http://links.lww.com/CCX/A924), and clinician characteristics by specialty are further detailed in Supplementary Table 5 (http://links.lww.com/CCX/A928). In cohort 1 (original data set n = 275 patients), 24 patients with 24 associated clinician-family recordings had a primary neurologic diagnosis and were eligible for inclusion in our study. Per our exclusion criteria, we excluded three recordings that lacked any discussion of prognosis or goals of care; hence, we included 21 recordings of 21 patients in our analysis.
In cohort 2 (data collected in 2019), 25 patients with 29 associated clinician-family recordings were eligible for inclusion. Four surrogates and three clinicians (for seven patients) declined participation (recruitment rate 76%). We included 22 recordings of 18 eligible patients.
In total, we analyzed 43 clinician-family meetings for 39 patients, with 88 surrogate and 27 clinician participants (Fig. 1).
Framework Describing Clinician Communication Approaches
We created a de novo framework describing four distinct prognostic communication approaches used by clinicians during the clinician-family meetings. These included the authoritative, advisory, responsive, and informational approaches (Table 1 shows representative examples).
TABLE 1.
Framework Defining Clinicians’ Prognostic Communication Approaches
| Prognostic Communication Approach | Definition | Examples |
|---|---|---|
| Authoritative | Clinician gives a recommendation about specific treatments or decisions without prior discussion of values and preferences | “I think that she’s going to need a feeding tube regardless, honestly…” |
| “We recommend what is called a tracheostomy” | ||
| “I think we’re… at that point where we would recommend that we consider it, if not actually go ahead and call the surgeons to do it.” | ||
| Informational | Clinician engages in prognostic disclosure without providing treatment recommendations or discussing values and preferences during the entire meeting | “I’m sorry, but this is fairly serious” |
| “He has tremendous capacity to improve” | ||
| “He’s gonna be diminished as far as the brain goes. He might have memory gaps. He might have emotional changes. He might have actually personality changes.” | ||
| Advisory | Clinician first provides prognostic estimates followed by asking the surrogates to think about values and preferences | “We’ve discussed a little bit what to expect… What is it that he would accept as okay still, even if we told you that he may not return back to his baseline?” |
| Responsive | Clinician first asks surrogates to think about values and preferences, followed by discussing prognosis related to elicited values and preferences | “Our job at this point is to try to figure out, so we can tell you what we are looking at for his recovery… what he would want us to do and what he would not want us to do.” |
Authoritative Approach
We defined the authoritative approach as one in which clinicians made direct treatment recommendations without eliciting patient values and preferences (VPs) and provided their opinions on one treatment path without discussing other possible options. Clinicians used the authoritative approach in nearly a quarter of all meetings (9/43 [21%]) and in half of all meetings in which treatment recommendations were made (9/18 [50%]).
Under this approach, some recommendations were phrased as a “given” in the patients’ recovery, as in “He’s still very deconditioned with his breathing and, uh, he will need a tracheostomy,” while others were presented as opinions, as in “I’ve done this for my loved ones before. I would not shock him. I don’t think it’s going to result in anything good.” We noted some differences in how clinicians used this approach. In four of nine meetings, clinicians did not address or elicit VPs during the entirety of the meeting. In three of nine meetings, clinicians made some effort to discuss VPs but proceeded to give a recommendation without receiving an answer from the surrogate.
Informational Approach
In this approach, clinicians engaged only in prognostic disclosure, without any discussions of VPs and without providing treatment recommendations, or describing available treatment options (10/43 [23%]).
Six out of 10 clinicians, who used the informational approach, explicitly acknowledged the uncertainty inherent to the patients’ clinical course and diagnosis, for example: “Unfortunately, with this kind of a disease[…]there’s nothing set in stone.” However, none of these clinicians offered any additional guidance on what factors surrogates could consider when making decisions.
In two meetings, clinicians asked questions regarding the patients’ lifestyle, for instance: “What does he do for work?” These clinicians did not proceed to make recommendations or explain to surrogates why lifestyle indicators are important factors in decision-making.
Advisory Approach
The advisory approach was characterized by the inclusion and acknowledgment of multiple treatment courses depending on the patient’s VPs. It was the most frequently used approach (18/43 meetings [42%]). Clinicians began with discussion of prognostic disclosure and then asked surrogates to think about VPs. As one clinician outlined to the family, “Let me talk a little bit about prognosis and then I was hoping you could teach us a little bit more about who he is.”
One strategy used by many clinicians under this approach was to actively transition the conversation from prognostication to discussing VPs. For example, “I need to shift the focus now to decision making[…],” and “What I’m giving you is totally my medical opinion […] But what you need to give us is–you need to tell us what you think he would want in this situation.”
Responsive Approach
In six of 43 meetings (14%), clinicians asked surrogates to consider VPs prior to any discussion of prognosis, which we defined as the responsive approach. It differs from the advisory approach in that clinicians targeted their prognostic disclosure specifically to the VPs offered by surrogates. The prognostic disclosure often served as a way of guiding the surrogates or making connections between the VPs and the prognosis, as in this example:
We have briefly talked about how he had some very specific wishes, and he didn’t want a feeding tube put in and he wouldn’t really wanna be going to live in a nursing facility.[…]At this point, I’m concerned that that’s the direction we’d be headed in. And I think it sounds like he would not want that.
In a similar manner, the clinician in the following example gives a prognostic statement in response to the family’s suggestion that the patient’s history of surviving cancer proves he has a strong will to live:
The difference between that and now is he’s not going to be able to return to how he was before.[…]He won’t even be able to return to how he was even before he had that initial stroke.
Clinicians used either the advisory or responsive approach in 56% of clinician-family meetings. These two approaches are similar in how they combine discussion of VPs alongside prognostic disclosure as a way of guiding the conversation, and it is this integration that sets them apart from the authoritative and informational approaches (44% of meetings). In 15 of 24 meetings during which advisory or responsive approaches were used, clinicians specifically addressed the surrogate’s duty in exercising substituted judgment, while this occurred in only three of 19 meetings where Informational or Authoritative approaches were used.
Treatment Recommendations
Among all clinician-family meetings, clinicians made treatment recommendations in 18 out of 43 (42%) clinician-family meetings.
Prognostication approaches for clinicians who led multiple clinician-family meetings are described in the Supplementary Results (http://links.lww.com/CCX/A930) and Supplementary Figure 1 (http://links.lww.com/CCX/A931).
Exploratory Quantitative Analysis
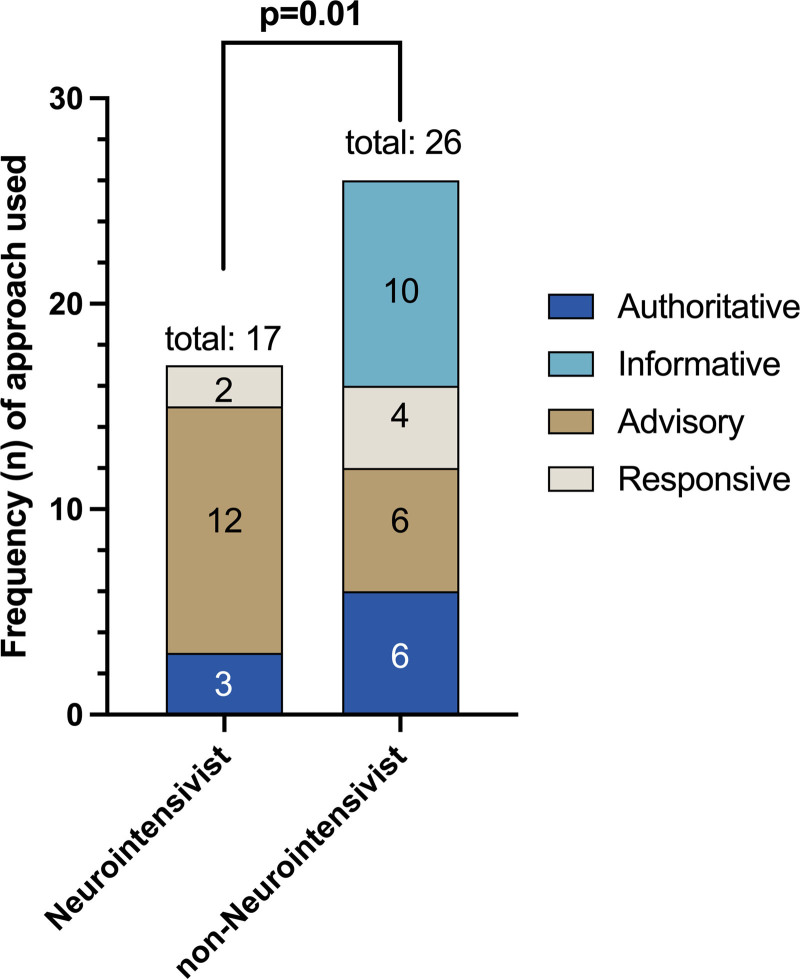
The unadjusted associations of clinician, patient, meeting, and family characteristics with the four prognostic communication approaches are shown in Supplementary Table 2 (http://links.lww.com/CCX/A925). We found that the type of approach was associated with center (p < 0.001) and clinician specialty, where meetings using the advisory approach was significantly more commonly led by neurointensivists than non-neurointensivists (p = 0.01; Fig. 2). There was a trend toward association of patient age with prognostic communication approach, with clinicians using the authoritative approach more often for meetings about younger patients and the advisory approach more often for older patients (p = 0.08). Clinicians also used advisory and responsive approaches more frequently for patients with ischemic strokes (p = 0.059). None of the other patient characteristics (race, ethnicity, sex) or any of the family characteristics (age, race, ethnicity, sex, education level) were associated with any prognostic communication approaches. Meeting duration was shortest when the informational approach was used (p = 0.03). When adjusting for center, clinician specialty, patient age, diagnosis, and meeting duration in the multivariable analysis, only clinician specialty independently predicted clinicians’ communication approach. Compared with neurointensivists, in non-neurointensivists, the odds of using the informational approach versus the advisory approach were 23-times higher, and the odds of using the authoritative approach versus the advisory approach and the responsive approach versus the advisory approach were five-times higher (p = 0.027; Table 2).
Figure 2.
Prognostic communication approaches used by neurointensivists versus non-neurointensivists. Neurointensivists differ from non-neurointensivists in the approaches they chose to use, with a majority of neurointensivist led meetings characterized by the advisory approach (unadjusted p = 0.01). Our small sample size did not allow adjustment by clustering by centers and repeat meetings by individual clinicians.
TABLE 2.
Multivariable Analysis for Clinicians’ Prognostication Approach
| Predictor | Relative Risk Ratio (95% CI) | Overall p | ||
|---|---|---|---|---|
| Authoritative vs Advisory | Responsive vs Advisory | Informational vs Advisory | ||
| Clinician specialty: | 0.027 | |||
| Neurointensivist | Reference | Reference | Reference | |
| Non-neurointensivist | 5.2 (0.9–29.3) | 5.2 (0.7–37.9) | 23.4 (2.3–235.5) | |
The univariate analysis of the final goals-of-care decisions by clinicians’ prognostic communication approach is shown in Supplementary Table 3 (http://links.lww.com/CCX/A926). A WOLST decision was made for 16 of 39 (41%) patients and was more likely in older patients (median age, 74.5 yr [interquartile range (IQR), 62–85 yr] vs 52 yr [43–67 yr]; p = 0.006). The advisory approach was more commonly used when WOLST decisions were made (56% for WOLST vs 26% for non-WOLST), while the informational approach was less commonly used when WOLST decisions were made (12% vs 35%), but this did not reach statistical significance (p = 0.2). WOLST decisions were more common when the clinician leading the meeting was more experienced (median years in practice 16 [IQR, 9–21.5] vs 9 [6–16]; p = 0.07; attending physician 94% ± 15% vs 74% ± 17%; p = 0.21) and was a neurointensivist (56% ± 9% vs 30% ± 7%; p = 0.19), but none of these characteristics reached statistical significance due to limited power. In the multivariable model, only patient age (p = 0.02), but not prognostic communication approach or any other variables, remained independently associated with WOLST (Supplementary Table 4, http://links.lww.com/CCX/A927). Assuming a medium effect size (Cohen’s w = 0.3, 80% power, and α = 0.05), we estimated that we would find a statistically significant association between WOLST and clinicians’ prognostic communication approach with a sample size of 122 clinician-family meetings.
DISCUSSION
In this pilot study, we demonstrated that it is feasible to audio-record “real-life” clinician-family goals-of-care meetings in the neuro-ICU, with a recruitment rate similar or higher than reported for general critical care (22, 23). We characterized four distinct prognostic communication approaches used by clinicians. Clinicians’ prognostication approaches were highly variable, particularly regarding the elicitation of VPs. Our empirical study is unique in CINPs and begins to understand how clinicians communicate prognosis to the families of these patients.
We found key distinguishing features in how clinicians disclose prognosis, make treatment recommendations, and elicit VPs. We found that clinicians made treatment recommendations in 18 of 43 meetings, which is consistent with previous research describing clinicians’ reluctance in making recommendations (23, 24). However, nine of these meetings were conducted via an authoritative approach without incorporation of VPs. For the other nine meetings where recommendations were made, surrogate decision-makers were supported through clarifying surrogates’ misunderstandings on outcomes or by counseling surrogates on what treatments were indicated for the described VPs. Studies show that clinicians’ recommendations carry significant weight as one factor in medical decision-making for surrogates (25–27) and may guide surrogates through making informed decisions during complex and emotionally demanding situations (11, 24, 28–30). Furthermore, surrogates value clinician recommendations that support or offer additional decision-making guidance, thereby lessening adverse surrogate outcomes such as poor understanding of prognosis or treatments and high rates of decisional regret (31–35).
An analysis of clinicians’ roles during prognostic meetings in general critical care found similarly low rates of making recommendations (23). Our study, however, focused more on clinician-initiated statements rather than on the delineation of roles between clinicians and surrogates during decision-making. With this focus, our attention in the qualitative analysis was drawn to the infrequency with which clinicians elicited VPs. It is possible that discussion of VPs occurred prior to the recorded meetings, but prior discussions were not referenced in any recorded interviews. It is concerning that in nearly half of the clinician-family meetings, clinicians used an authoritative or informational approach with no discussion of VPs, despite the known importance of doing so (36–40). Furthermore, in nearly a quarter of meetings, clinicians made treatment recommendations without eliciting the patients’ VPs.
Although our study did not examine underlying clinician motivations, one key finding was that clinicians vary in how they approach communication in clinician-family meetings. Possible clinician-level factors contributing to this variability proposed in previous work include: internal biases leading to self-fulfilling prophecies, a sense of prognostic nihilism, clinician agreement with substituted judgment, and differing levels of personal comfort and experience (14, 24, 41, 42). We must be careful not to overinterpret our observations because it is also possible that certain meetings varied depending on family interactions, both verbal and nonverbal, during or outside of the recorded clinician-family meeting, external factors such as differences in patients or workplace environments, and surrogate preferences (10, 11, 41–45). Additional research is needed to understand what clinician-specific or other factors may contribute to the variability in prognostic communication approaches for CINPs (13, 14).
Our initial quantitative exploration similarly suggests that clinician factors may be associated with the choice of approach, given that neurointensivists were more likely to use the advisory approach compared with clinicians from other specialties. However, we must note that our study included only four neurointensivists, all from the same center, which likely shaped their approaches to goals-of-care family meetings in ways not explicitly measured or described here. We additionally found that WOLST decisions were more commonly made when the clinician leading the meeting was an attending physician with more years of training, although these were not statistically significant, likely due to our small sample size. Future, larger studies must validate our findings. We also found that use of advisory or responsive approaches with elicitation of VPs was associated with longer duration of clinician-family meetings. Empirical research has suggested that increased duration of meetings provides more opportunities for surrogates to speak, understand the goals of care, and discuss patient wishes, and therefore may be associated with higher family satisfaction and lower long-term surrogate psychologic burden (31, 46, 47). Future research in CINPs measuring quality of communication and surrogate health outcomes will be important in untangling this relationship with prognostication approaches. We did not find that family characteristics, including education level, race, or ethnicity, were associated with either communication approaches or WOLST decisions (9). Possible explanations include our small sample size and a relatively homogenous cohort with 83% non-Hispanic White family participants.
Our study’s strengths include the examination of recordings of real-life clinician-family communications from multiple centers with a variety of CINPs admitted to geographically diverse medical, medical-surgical, and neurologic ICUs. These communications are generally considered difficult to capture due to the substantial effort in recruitment and their sensitive nature. Although we forgo the control of variables that comes with conducting studies under simulation-based scenarios, we benefit by minimizing the barriers of translating our results into clinical practice.
Limitations included a relatively small sample size with limited racial and ethnic diversity, thereby limiting transferability and adjustment for clustered confounders. Our single center neuro-ICU (cohort 2) is overrepresented, potentially contributing to selection bias. We were unable to adjust for ICU structure, rounding practices, or additional information potentially provided to families before the clinician-family meeting or during prior clinician-family meetings. Recordings occurred over a period of 5–10 years, raising the concern that clinician-family communication may have evolved over this time period. We did not examine differences in communication approaches between the earlier and later cohorts because recordings for both occurred at different centers, and we lacked power to adjust for this difference. However, while communication with families has changed over the last 50 years (48), our review of the literature did not discover evidence that it has evolved much over the last 5–10 years. Despite attempts at difficult conversation training to improve communication with families in ICUs (49, 50), no research has documented a change in physicians’ actual practice, potentially because learned communication skills decline as physicians progress in their medical training (51). Knowing that they were being audio-recorded may have changed the approaches of clinicians who agreed to participate (Hawthorne effect [52]). Therefore, we may have included only the clinicians comfortable with being observed or recorded, thereby underestimating the true range of clinician variability. Our study only describes what is observed and does not explain the cause of the variability. To know if this variability is intrinsic or extrinsic to the clinician would require a level of control over the environment that is not possible when recording real-life meetings (53). Finally, we examined only clinician attributes in order to explore potential targets of intervention, but communication is bidirectional and what surrogates say likely influences the clinician-surrogate dialogue. Although we assessed “approaches” that encompass meetings in their entirety, there are still uncontrollable differences in how families drive discussion that are not accounted for in our study design.
In summary, our pilot mixed-methods study describes the variability in clinicians’ approaches to goals-of-care communication in CINPs. Validation in a larger, contemporary cohort is necessary. We do not propose that one approach is always better than another, but rather that prognostication approaches have important distinctions. Understanding what components contribute to these differences may guide clinicians in clinical practice. Importantly, we demonstrated the feasibility of studying real-life clinician-family meetings for CINPs, and future large studies involving a more diverse sample of participants will be essential in developing acceptable and evidence-based interventions that help clinicians and families achieve treatment decisions consistent congruent with patient VPs.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by the University of Massachusetts Medical School Center for Clinical and Translational Science, which is funded by the National Institutes of Health (NIH) Clinical and Translational Science Award to the University of Massachusetts Medical School (UL1TR000161). Collection of data occurred under the parent multicenter study R01, NIH-National Heart, Lung, and Blood Institute 5R01HL094553.
Ms. Ge was funded by the American Academy of Neurology Medical Student Research Scholarship (2020). Dr. White was funded National Institutes of Health (NIH)-National Heart, Lung, and Blood Institute: K24 HL148314; he receives personal fees for roles as associate editor of the American Journal of Respiratory and Critical Care Medicine and as an author for UpToDate. Dr. Muehlschlegel’s research time was funded by NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development 5K23HD080971. Dr. Lo receives personal fees for serving on the Ethics Advisory Council of Takeda Pharmaceuticals, outside the scope of this project. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Mayer SA, Kossoff SB: Withdrawal of life support in the neurological intensive care unit. Neurology 1999; 52:1602–1609 [DOI] [PubMed] [Google Scholar]
- 2.Becker KJ, Baxter AB, Cohen WA, et al. : Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology 2001; 56:766–772 [DOI] [PubMed] [Google Scholar]
- 3.Verkade MA, Epker JL, Nieuwenhoff MD, et al. : Withdrawal of life-sustaining treatment in a mixed intensive care unit: Most common in patients with catastropic brain injury. Neurocrit Care 2012; 16:130–135 [DOI] [PubMed] [Google Scholar]
- 4.Turgeon AF, Lauzier F, Simard JF, et al. ; Canadian Critical Care Trials Group: Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: A Canadian multicentre cohort study. CMAJ 2011; 183:1581–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holloway RG, Benesch CG, Burgin WS, et al. : Prognosis and decision making in severe stroke. JAMA 2005; 294:725–733 [DOI] [PubMed] [Google Scholar]
- 6.Williamson T, Ryser MD, Ubel PA, et al. : Withdrawal of life-supporting treatment in severe traumatic brain injury. JAMA Surg 2020; 155:723–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg A, Soto AL, Knies AK, et al. : Predictors of surrogate decision makers selecting life-sustaining therapy for severe acute brain injury patients: An analysis of US population survey data. Neurocrit Care 2021; 35:468–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White DB, Engelberg RA, Wenrich MD, et al. : The language of prognostication in intensive care units. Med Decis Making 2010; 30:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White DB, Braddock CH, 3rd, Bereknyei S, et al. : Toward shared decision making at the end of life in intensive care units: Opportunities for improvement. Arch Intern Med 2007; 167:461–467 [DOI] [PubMed] [Google Scholar]
- 10.Boyd EA, Lo B, Evans LR, et al. : “It’s not just what the doctor tells me:” Factors that influence surrogate decision-makers’ perceptions of prognosis. Crit Care Med 2010; 38:1270–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai X, Robinson J, Muehlschlegel S, et al. : Patient preferences and surrogate decision making in neuroscience intensive care units. Neurocrit Care 2015; 23:131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans LR, Boyd EA, Malvar G, et al. : Surrogate decision-makers’ perspectives on discussing prognosis in the face of uncertainty. Am J Respir Crit Care Med 2009; 179:48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemphill JC, 3rd, White DB: Clinical nihilism in neuroemergencies. Emerg Med Clin North Am 2009; 27:27–37, vii–viii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izzy S, Compton R, Carandang R, et al. : Self-fulfilling prophecies through withdrawal of care: Do they exist in traumatic brain injury, too? Neurocrit Care 2013; 19:347–363 [DOI] [PubMed] [Google Scholar]
- 15.Lazaridis C: End-of-life considerations and shared decision making in neurocritical care. Continuum (Minneap Minn) 2018; 24:1794–1799 [DOI] [PubMed] [Google Scholar]
- 16.Wilson JE, Shinall MC, Jr, Leath TC, et al. : Worse than death: Survey of public perceptions of disability outcomes after hypothetical traumatic brain injury. Ann Surg 2020; 273:500–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azoulay E, Pochard F, Kentish-Barnes N, et al. ; FAMIREA Study Group: Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med 2005; 171:987–994 [DOI] [PubMed] [Google Scholar]
- 18.Wendler D, Rid A: Systematic review: The effect on surrogates of making treatment decisions for others. Ann Intern Med 2011; 154:336–346 [DOI] [PubMed] [Google Scholar]
- 19.Ernecoff NC, Curlin FA, Buddadhumaruk P, et al. : Health care professionals’ responses to religious or spiritual statements by surrogate decision makers during goals-of-care discussions. JAMA Intern Med 2015; 175:1662–1669 [DOI] [PubMed] [Google Scholar]
- 20.Tolley EE, Ulin PR, Mack N, et al. : Qualitative Methods in Public Health: A Field Guide for Applied Research. San Francisco, CA, John Wiley & Sons, 2016 [Google Scholar]
- 21.McHugh ML: Interrater reliability: The kappa statistic. Biochem Med (Zagreb) 2012; 22:276–282 [PMC free article] [PubMed] [Google Scholar]
- 22.Carson SS, Cox CE, Wallenstein S, et al. : Effect of palliative care-led meetings for families of patients with chronic critical illness: A randomized clinical trial. JAMA 2016; 316:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White DB, Malvar G, Karr J, et al. : Expanding the paradigm of the physician’s role in surrogate decision-making: An empirically derived framework. Crit Care Med 2010; 38:743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brush DR, Rasinski KA, Hall JB, et al. : Recommendations to limit life support: A national survey of critical care physicians. Am J Respir Crit Care Med 2012; 186:633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurmankin AD, Baron J, Hershey JC, et al. : The role of physicians’ recommendations in medical treatment decisions. Med Decis Making 2002; 22:262–271 [DOI] [PubMed] [Google Scholar]
- 26.Mendel R, Traut-Mattausch E, Frey D, et al. : Do physicians’ recommendations pull patients away from their preferred treatment options? Health Expect 2012; 15:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazur DJ, Hickam DH, Mazur MD, et al. : The role of doctor’s opinion in shared decision making: What does shared decision making really mean when considering invasive medical procedures? Health Expect 2005; 8:97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Billings JA, Krakauer EL: On patient autonomy and physician responsibility in end-of-life care. Arch Intern Med 2011; 171:849–853 [DOI] [PubMed] [Google Scholar]
- 29.Johnston SC, Pfeifer MP; End-of-Life Study Group: Patient and physician roles in end-of-life decision making. J Gen Intern Med 1998; 13:43–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun UK, Naik AD, McCullough LB: Reconceptualizing the experience of surrogate decision making: Reports vs genuine decisions. Ann Fam Med 2009; 7:249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stapleton RD, Engelberg RA, Wenrich MD, et al. : Clinician statements and family satisfaction with family conferences in the intensive care unit. Crit Care Med 2006; 34:1679–1685 [DOI] [PubMed] [Google Scholar]
- 32.Silberfeld M, Grundstein-Amado R, Stephens D, et al. : Family and physicians’ views of surrogate decision-making: The roles and how to choose. Int Psychogeriatr 1996; 8:589–596 [DOI] [PubMed] [Google Scholar]
- 33.Rid A, Wendler D: Can we improve treatment decision-making for incapacitated patients? Hastings Cent Rep 2010; 40:36–45 [DOI] [PubMed] [Google Scholar]
- 34.Azoulay E, Chevret S, Leleu G, et al. : Half the families of intensive care unit patients experience inadequate communication with physicians. Crit Care Med 2000; 28:3044–3049 [DOI] [PubMed] [Google Scholar]
- 35.Hickman RL, Jr, Daly BJ, Lee E: Decisional conflict and regret: Consequences of surrogate decision making for the chronically critically ill. Appl Nurs Res 2012; 25:271–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shine KI: Crossing the quality chasm: The role of postgraduate training. Am J Med 2002; 113:265–267 [DOI] [PubMed] [Google Scholar]
- 37.Scheunemann LP, Ernecoff NC, Buddadhumaruk P, et al. : Clinician-family communication about patients’ values and preferences in intensive care units. JAMA Intern Med 2019; 179:676–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spatz ES, Spertus JA: Shared decision making: A path toward improved patient-centered outcomes. Circ Cardiovasc Qual Outcomes 2012; 5:e75–e77 [DOI] [PubMed] [Google Scholar]
- 39.Jain N, Bernacki RE: Goals of care conversations in serious illness: A practical guide. Med Clin North Am 2020; 104:375–389 [DOI] [PubMed] [Google Scholar]
- 40.Arnold RM, Kellum J: Moral justifications for surrogate decision making in the intensive care unit: Implications and limitations. Crit Care Med 2003; 31:S347–S353 [DOI] [PubMed] [Google Scholar]
- 41.Garland A, Connors AF: Physicians’ influence over decisions to forego life support. J Palliat Med 2007; 10:1298–1305 [DOI] [PubMed] [Google Scholar]
- 42.Wilson ME, Rhudy LM, Ballinger BA, et al. : Factors that contribute to physician variability in decisions to limit life support in the ICU: A qualitative study. Intensive Care Med 2013; 39:1009–1018 [DOI] [PubMed] [Google Scholar]
- 43.Quinn T, Moskowitz J, Khan MW, et al. : What families need and physicians deliver: Contrasting communication preferences between surrogate decision-makers and physicians during outcome prognostication in critically ill TBI patients. Neurocrit Care 2017; 27:154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majesko A, Hong SY, Weissfeld L, et al. : Identifying family members who may struggle in the role of surrogate decision maker. Crit Care Med 2012; 40:2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson SK, Bautista CA, Hong SY, et al. : An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care units. Am J Respir Crit Care Med 2011; 183:915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lautrette A, Darmon M, Megarbane B, et al. : A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med 2007; 356:469–478 [DOI] [PubMed] [Google Scholar]
- 47.McDonagh JR, Elliott TB, Engelberg RA, et al. : Family satisfaction with family conferences about end-of-life care in the intensive care unit: Increased proportion of family speech is associated with increased satisfaction. Crit Care Med 2004; 32:1484–1488 [DOI] [PubMed] [Google Scholar]
- 48.Kerlin MP, Costa DK, Kahn JM: The Society of Critical Care Medicine at 50 years: ICU organization and management. Crit Care Med 2021; 49:391–405 [DOI] [PubMed] [Google Scholar]
- 49.Rivet EB, Cholyway R, Edwards C, et al. : Video-mediated breaking bad news simulation. Clin Teach 2021; 18:424–430 [DOI] [PubMed] [Google Scholar]
- 50.Lum HD, Dukes J, Church S, et al. : Teaching medical students about “the conversation”: An interactive value-based advance care planning session. Am J Hosp Palliat Care 2018; 35:324–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ha JF, Longnecker N: Doctor-patient communication: A review. Ochsner J 2010; 10:38–43 [PMC free article] [PubMed] [Google Scholar]
- 52.McCambridge J, Witton J, Elbourne DR: Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J Clin Epidemiol 2014; 67:267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haliko S, Downs J, Mohan D, et al. : Hospital-based physicians’ intubation decisions and associated mental models when managing a critically and terminally ill older patient. Med Decis Making 2018; 38:344–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.