Abstract
Parkinson disease (PD) is a prevalent neurodegenerative disease, in which the formation of misfolded and aggregated α-synuclein is a key neuropathological hallmark. Recent studies reveal that extracellular vesicles such as exosomes present a potential mechanism for propagation of pathological α-synuclein throughout the brain. The ability of exosomes to transport proteins and genetic material between cells, including mRNA and microRNAs which have been implicated in PD pathology, provides critical insights as to how exosomes may contribute to pathological progression in PD. Advances have also been made in the investigation of exosomes as potential tools for the modulation of Parkinson’s pathology; their detection extracellularly may facilitate their use as biomarkers, whilst their small size could be utilised as vectors for the delivery of therapeutics. The aim of this review is to highlight our current knowledge of the role of exosomes in PD and potential clinical application.
Keywords: Parkinson disease, exosomes, extracellular vesicles, neurodegeneration, alpha-synuclein, protein aggregation
1. INTRODUCTION
As the second most prevalent neurodegenerative disease worldwide (Dorsey et al. 2018), Parkinson disease (PD) is a debilitating, progressive motor disorder characterised by degeneration of the nigrostriatal dopaminergic pathway. A hypokinetic movement disorder, its symptomology arises from the loss of dopaminergic innervation of motor nuclei in the basal ganglia which results in the characteristic tetrad of motor symptoms; bradykinesia (slowness of movement), rigidity, postural instability, and resting tremor. Parkinson’s patients may also present with non-motor symptoms, some of which arise in the prodromal phase before onset of motor abnormalities. These early symptomatic changes may include olfactory dysfunction, depression, sleep disturbances, gastrointestinal changes and pain (Schrag et al. 2019). Recent estimates suggest that PD affects approximately ten million individuals worldwide (Dorsey et al. 2007; Marras et al. 2018). The underlying aetiology of PD is multifactorial, with complex interplay of genetic and environmental risk factors converging to confer increased disease risk.
The first genetic risk factor discovered for PD was the SNCA gene (Polymeropoulos et al. 1997). Since its discovery, SNCA and the α-synuclein (α-syn) protein which it encodes have been extensively researched, with over 12,000 research papers published (as of 24th September 2020). α-syn is a major protein constituent of Lewy bodies, distinctive intracellular protein aggregates which are found in the surviving nigrostriatal dopaminergic neurons of PD patient brains (Arima et al. 1999; Mezey et al. 1998; Spillantini et al. 1997). Their presence in the brains of both familial and sporadic cases of PD supports a central pathological role of α-syn in PD. α-syn has been shown to exist in multiple forms in the brain, including soluble unfolded mono-, oligo- and polymeric forms, as well as insoluble β-sheet-containing fibrils (Mor et al. 2016). Although still a topic of debate (Surmeier et al. 2017; Brundin & Melki 2017), there is increasing experimental evidence indicating that α-synuclein is capable of intercellular transmission, which is consistent with the proposed Braak scheme of pathological PD propagation (Braak et al. 2003; Dickson et al. 2010). This hypothesis suggests that the olfactory bulb and the gut are initial spreading sites for misfolded α-syn protein (Klingelhoefer & Reichmann 2015).
Whilst multiple mechanisms of cell-to-cell α-syn transmission have been proposed, including exocytosis, endocytosis, extracellular vesicles (EVs) and tunnelling nanotubules (Peng et al. 2020). EVs such as exosomes are currently of great interest as they can shuttle genetic material and protein between cells, with the capacity to impact proximal and distal to their cells of origin. Multiple cell types, including neurons, astrocytes and microglia, have been found to release exosomes in the brain; their ability to carry material which can influence gene expression and protein activity in recipient cells (Chistiakov & Chistiakov 2017) makes them prime possibilities for the modulation of protein misfolding in PD.
This review will discuss the current understanding of the role of exosomes and α-syn transmission in PD, including the potential role in disease propagation. Furthermore, we will highlight the current evidence for targeting exosomes as potential a therapeutic intervention and diagnostic tool in PD. Please note that although the term exosomes is used in this review, as pointed out by the International Society for Extracellular Vesicles (Théry et al. 2018), techniques used in most studies to isolate exosomes also contain other exosome-like extracellular vesicles.
2. INTERCELLULAR α-SYNUCLEIN SPREAD
Within the growing evidence that α-syn is capable of cell to cell transmission, there are multiple proposed cellular mechanisms which facilitate its propagation: non-classical exocytosis, exosomal release, endocytosis and transport via nanotubules, which provide physical bridges between distinct cells, have all been proposed (Peng et al. 2020). The discovery of α-syn’s transmissibility provides a key insight into the development and progression of PD, which may lead to the advancement of future therapies through the provision of additional mechanistic targets. According to the Braak staging hypothesis of PD pathology development (Braak et al. 2003; Dickson et al. 2010; Hawkes et al. 2007; Parkkinen et al. 2008), the olfactory bulb and the gut are the initial source sites for α-syn spread. Consistent with this hypothesis, injection of preformed fibrillar α-syn into the olfactory bulb (Rey et al. 2016) and the gut (Kim et al. 2019) of mice produces trans-neuronal propagation of pathological α-syn from these respective peripheral regions to other brain regions, including the substantia nigra, in a stereotypical manner. Furthermore, mice with truncal vagotomy and α-syn deficiency prevent this gut-to-brain protein transmission, supporting the gut as an initial source of α-syn transmission (Kim et al. 2019). A recent study injecting Lewy body extracts containing α-syn from PD patients to the gut of non-human primates also demonstrated the spread of toxic α-syn from the gut to the brain (Arotcarena et al. 2020). Misfolded α-syn protein has been identified in early stage PD patient enteric nervous systems (Hilton et al. 2014; Killinger et al. 2018; Wakabayashi et al. 1990), further supporting the gut as an initial site where α-syn propagates from. However, other studies have demonstrated that intramuscular (Sacino et al. 2014) and peritoneal (Sargent et al. 2017) injections of α-syn also induce widespread brain pathology. Taken together, these studies indicate that α-syn can propagate from multiple peripheral sites to the central nervous system.
α-syn host to graft cell transmission was reported in two studies investigating the potential symptomatic relief provided by grafting foetal ventral mesencephalic tissue into the nigrostriatal system of PD patients (Kordower et al. 2008; Li et al. 2008). Functional dopamine production persisted for over a decade post-transplant, with some long-surviving neurons displaying characteristic α-syn- and ubiquitin-positive Lewy bodies, which were positive for Serine-129 phosphorylated α-syn (Li et al. 2008), strongly suggesting the presence of disease-related, post-translationally modified and potentially aggregated α-syn. Increase of α-syn-containing tyrosine hydroxylase-positive neurons correlated with increasing age of the graft, supportive of progressive, gradual α-syn infiltration from host to graft neurons, consistent with increasing age as a major PD risk factor (Bridgeman & Arsham 2017). This host-to-graft protein transmission was identified as a potentially limiting factor of cell replacement therapy, as increasing α-syn abundance during aging may detrimentally impact the long-term graft function and survival. In one patient who died 14 years post-transplant, reduced dopamine transporter expression was evident in the grafted region, suggestive that α-syn infiltration may also impact neuronal function and viability (Kordower et al. 2008). Similar examples of α-syn spread from host to engrafted neurons have been recapitulated in pre-clinical models (Desplats et al. 2009; Hansen et al. 2011; Kordower et al. 2011), including α-syn pre-formed fibrils in a non-human primate model (Chu et al. 2019) and human embryonic stem cells (hESC) transplanted into a humanized-α-syn rat model of PD (Hoban et al. 2020).
α-syn transmission has been shown to occur in neuron to neuron, neuron to astrocyte, neuron to microglia and microglia to neurons (Desplats et al. 2009; Guo et al. 2020; Kim et al. 2013; Lee et al. 2010; Luk et al. 2012; Harischandra et al. 2019; Xu et al. 2018; Fan et al. 2019). This protein transmission between cell types highlights a limitation of current PD treatments, which focus on DA neurons alone; future interventions will likely hold greater therapeutic potential if they can target or protect multiple cell types from α-syn infiltration and the resultant pathology. Interestingly, in contrast to microglia, astrocytes do not appear to readily spread α-syn to neurons due to their efficient degradation of intracellular α-syn (Loria et al. 2017; Rappold & Tieu 2010)
There are multiple regulated cellular mechanisms of α-syn release and uptake, which are currently reported as probable contributors to its intercellular transmission (Figure. 1). Free-floating α-syn protein seeds (Fig. 1A) have been found to transmit across the extracellular space, where they may then pass through the plasma membrane or be up taken by endocytic mechanisms for infiltration of recipient cells, as demonstrated in vitro by the downstream initiation of protein pathology following cell exposure to wild type α-syn from cell culture media (Nonaka et al. 2010). Endocytosis likely plays a role in the cellular uptake of α-syn once it has traversed the extracellular space; there is evidence to support the classical endocytic uptake of higher order α-syn structures, which may have evolved as a protective mechanism to regulate extracellular α-syn levels by facilitating protein uptake for degradation (Masaracchia et al. 2018; Rodriguez et al. 2018). This mechanism, however, would rely on the continued effectiveness of intracellular protein degradation pathways, such as autophagy, which have been shown to be restricted by excessive α-syn levels (Arotcarena et al. 2019; Fan et al. 2019; Tanik et al. 2013). Accumulation of α-syn species may therefore lead to cytotoxic saturation of the cells, interfering with protein degradation and contributing to increased cellular death which may release further α-syn into the extracellular space. Endocytosis may also facilitate the uptake of α-syn-containing vesicles, however for reactiveness of α-syn in these cases the protein would somehow need to leave the vesicles to enable interactions with other intracellular proteins, to act as a seed for intracellular α-syn aggregation or to exert other cytotoxic changes. The potential mechanism of intracellular vesicular release of α-syn remains unknown. Inhibition of endocytosis with monodansylcavdaverine and dynasore has been shown to decrease α-syn uptake in vitro and in vivo (Hansen et al. 2011).
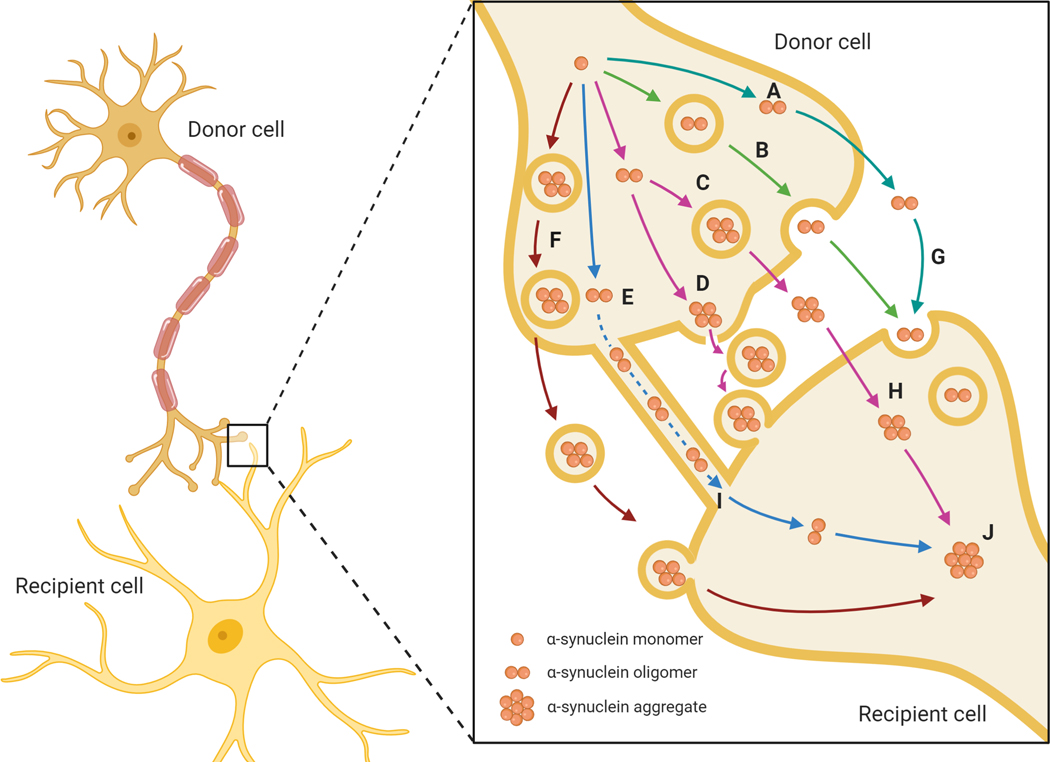
Figure 1. Proposed mechanisms of intercellular α-synuclein transmission.
Cellular mechanisms of α-synuclein transmission between cells facilitate the propagation of protein pathology in PD, whereby protein ‘seeds’ are transferred from donor cells into the extracellular space, as naked protein or in vesicles such as exosomes. These monomers and oligomers then act as seeds for protein aggregation in the recipient cells and can activate neuroinflammatory responses from glial cells. Mechanisms of transmission include the non-vesicular release of free α-synuclein (A), exocytosis (B), secretory lysosome release (C), microvesicular shedding (D), transport via tunnelling nanotubules (E) and exosomal transport (F). Uptake mechanisms include endocytosis (G), membrane perforation or pore formation (H) and exosome uptake (I), leading to α-synuclein aggregation formation (J).
Exocytosis (Fig. 1B) is a potential mechanism of α-syn release from donor cells, or cells of origin, in the intracellular α-syn transmission cascade; overexpression of α-syn was found to result in the secretion of monomers and aggregated α-syn from neuroblastoma and primary rat cortical neurons (Lee et al. 2005; Lee et al. 2013; Logan et al. 2017). These changes were inhibited by reduced temperature, a classical test for blockade of vesicular exocytosis, which suggests exocytosis as a likely facilitatory mechanism (Lee et al. 2005). Inhibition of the autophagy-lysosome pathway in α-syn-expressing cells resulted in increased exocytosis of α-syn protein (Lee et al. 2013). Further work is still required to elucidate the exact mechanisms by which α-syn induces exocytic release, however the implication of autophagic dysfunction further supports the ineffective or incomplete clearance of α-syn as a contributing factor to its transcellular transmission.
Secretory lysosomes (Fig. 1C), also known as the process of lysosomal exocytosis, is a mechanism of protein transmission from organelles of the autophagic- and endo-lysosomal system (Medina et al. 2011). Lysosomes can release their contents to the extracellular space following fusion with the cellular membrane (Blott & Griffiths 2002; Lettau et al. 2007), contributing to cellular clearance and cell-to-cell communication. Recent work in iPSC-derived dopaminergic neurons from ATP13A2/PARK9 mutated PD patients implicates lysosomal exocytosis in the clearance of intracellular α-syn in human dopaminergic neurons, with ATP13A2/PARK9 mutations shown to contribute to α-syn storage (Tsunemi et al. 2019). As lysosomal exocytosis has been previously implicated in mediation of Ca2+ levels, the authors investigated the impact of pharmacologically increasing lysosomal Ca2+, which restored this defective secretory mechanism to alleviate intracellular α-syn accumulation (Tsunemi et al. 2019). This observation has led to therapeutic interest in targeting secretory lysosomes, however enhancing this α-syn release could exacerbate its cellular transmission to detrimentally impact neighbouring cells.
Tunnelling nanotubules (TNTs, Fig. 1E) are versatile structures composed of F-actin-based tubular connections which allow direct communication and transport between distal cells (Abounit & Zurzolo 2012). TNTs have been implicated in the transmission of organelles, plasma membrane components, pathogens, Ca2+ and even electrical signals between cells. Evidence suggests that rather than acting as passive conduits for transmission, TNTs may be regulated by gap junctions and gating mechanisms. TNTs provide continuity between the cytoplasm of remote cells, with a dynamic nature that can lead to de novo formation of TNTs within minutes, and exhibiting lifetimes which may range from minutes to several hours in vitro (Rustom et al. 2004). Investigation of α-syn spread via TNTs demonstrated the effective transfer of α-syn fibrils from donor to acceptor cells inside lysosomal vesicles (Abounit et al. 2016). These fibrils acted as seeds in the acceptor cells, to promote aggregation of soluble α-syn within the cytosol of the recipient cells. Abounit et al (2016) proposed that the transfer of these α-syn-containing vesicles may result from α-syn overload in the cells of origin, which dispose of the excess protein through use of the TNT transfer system. This results in progression of the pathology if these lysosomes are not degraded by the recipient cells. Research suggests that as well as the identification of α-syn in nanotubule structures, the addition of aggregated α-syn to cell cultures can increase the number of TNTs (Abounit et al. 2016), which would further promote intercellular protein transmission. This mode of transmission is not limited to neurons alone as intercellular transmission of α-syn has also been demonstrated in cultured astrocytes (Rostami et al. 2017). Further investigation of how TNT-transmitted vesicles may release their contents into cells is required to assist our understanding of how this transmitted α-syn may promote intracellular α-syn aggregation.
Extracellular membrane-bound vesicles (Fig. 1D & F), play a prominent role in the mechanism of protein transmission proposed for the intercellular spread of α-syn. The most widely investigated EVs are exosomes, and to a lesser extent, microvesicles. As described in the next section, exsomes are derived from endosomes. Microvesicles are formed by outward budding from the plasma membrane. Other terms for microvesicles include microparticles and budding vesicles. Since some of the protein cargo of microvesicles overlaps with those of exosomes, it is challenging to make definitive distinction between microvesicles and exosomes. However, the sizes of exosomes are typically between ~40–160 nm in diameter whereas those of microvesicles can range from 100 nm to 1 μm. Although exosomes were first described in 1983 (Harding et al. 1983; Pan & Johnstone 1983), they have become an increasingly popular structure for research over the past two decades following the 2007 discovery that cells can utilise these small structures for the transfer of messenger and micro RNAs between cells (Valadi et al. 2007). Since then, our understanding of their role in physiological and pathological pathways is growing rapidly, with investigation of their function in disease states and as potential drug delivery vectors.
3. EXOSOME FORMATION AND FUNCTION
As illustrated in Figure 2, exosomes are produced in the endosomal compartment of most eukaryotic cells (Théry et al. 2018; Yáñez-Mó et al. 2015); multiple mechanisms of exosome formation have been described, with the most studied being the endosomal sorting complex required for transport (ESCRT)-dependent pathway (Wollert & Hurley 2010). The ESCRT machinery mediates a ubiquitinated pathway which recognises and retains ubiquitinated proteins marked for packaging in the late endosomal membrane (Wollert & Hurley 2010). The ESCRT-0 subunit retains ubiquitinated proteins, with downstream recognition of ESCRT-0 by ESCRT I/II which creates an endosomal membrane invagination to begin the formation of intraluminal vesicles (ILVs) (Hessvik & Llorente 2018; Wollert & Hurley 2010). ESCRT III forms a spiral structure to constrict the ILV neck, whilst ATPase VPS4 is responsible for scission of the membrane (Wollert & Hurley 2010). This results in formation of multivesicular bodies (MVBs), which can fuse with the cellular plasma membrane to release these ILVs into the extracellular space as exosomes (Gruenberg & van der Goot 2006). There are also ESCRT-independent non-canonical pathways of exosome formation (Baietti et al. 2012).
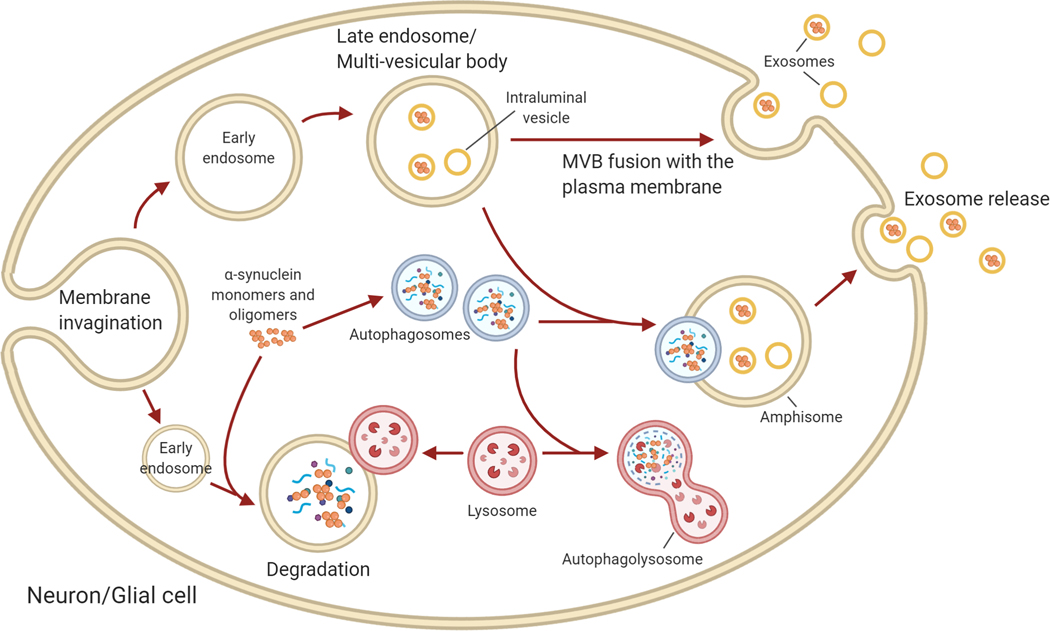
Figure 2. Intracellular vesicle trafficking and autophagy.
Intracellular α-syn can be sorted into endosomes/multi-vesicular bodies or autophagosomes. Internalisation of portions of the cellular plasma membrane leads to formation of early endosomes, in which further membrane invagination produces intraluminal vesicles (ILVs) which accumulate inside of the late endosome/multivesicular body (MVB). These late endosomes and their constituent ILVs can contain a mixture of cellular components, including genetic material and protein, such as α-syn. MVBs can fuse with the plasma membrane to release the ILVs into the extracellar space as exosomes, or they can fuse with autophagosomes to form amphisomes. Amphisomes are intermediates in autophagic processing, which are also capable of fusing with the cellular membrane to mediate exosome release. Alternatively, α-syn may be internalised to endosomes which fuse with lysosomes for degradation, or encompased into autophagosomes and degraded by the traditional autophagic pathway following fusion with lysosomes to produce the autophagolysosome. Amphisomes can also be degraded by the lysosome (not shown). Impaired autophagy flux such as due to lysosomal dysfunction enhances exosome release from amphisomes and MVS in attempts to reduce intracellular α-syn levels.
Exosomes can shuttle genetic and protein constituents between cells, facilitating cell-to-cell communication and regulating information transfer, which may influence the cells function. Exosomes are typically between ~40–160 nm in diameter (Kalluri & LeBleu 2020) and they have been demonstrated to play communicative roles in immune responses, with exosomes from B lymphocytes bearing MHC class II molecules found to activate T lymphocytes in vitro (Raposo et al. 1996), suggestive of regulation in a manner similar to that of parent B cells. Further work found that exosomes from dendritic cells, which are specialised to activate T cells in immune response initiation, promoted anti-tumour responses in mice (Zitvogel et al. 1998). Outside of their immunoreactive functions, exosomal functions are still being explored and they have been found to transmit pathogenic prion proteins and amyloid-beta aggregates (Berrone et al. 2015; Cheng et al. 2018; Guix et al. 2018; Pérez et al. 2019). The remainder of this review will focus on the implication of exosomes in PD, α-syn transmission and whether they may represent therapeutic targets to modulate PD pathology and prognosis.
4. EXOSOMES IN PD
Exosomes have two major roles in the pathogenesis of PD; the first and most investigated is their role as important mediators of α-syn transmission between cells, and the second is their capacity to transport RNAs, such as PD-implicated microRNAs (miRNA) between cells.
4.1. α-synuclein, autophagy and exosomes
As α-syn accumulation is a prominent feature of PD pathology, understanding the mechanisms underlying its aggregation and intercellular transmission may improve our understanding of disease pathology and progression to facilitate the development of more effective therapies. Recent work implicating exosomes in these pathological processes suggests that targeting exosomes may hold therapeutic potential. Exosomes contribute to non-cell autonomous-mediated neurotoxicity and can mediate α-syn transmission over greater distances than some other mechanisms of cell-to-cell transmission. Exosomes have been demonstrated to transfer α-syn protein to normal neuronal cells (Alvarez-Erviti et al. 2011a), where it can form aggregates and induce death of the recipient cell (Desplats et al. 2009; Hansen et al. 2011; Fan et al. 2019; Guo et al. 2020; Harischandra et al. 2019). α-syn-oligomers associated with exosomes have been found to increase the likelihood of cellular uptake, with greater subsequent neurotoxicity than free α-syn oligomers (Danzer et al. 2012). The study of exosomes in PD models has included investigation of their spread between glia and neurons, with inter-neuronal α-syn transmission the most studied interaction.
In SH-SY5Y neuroblastoma cells, overexpression of wild-type α-syn led to its exosomal release, with both monomeric and oligomeric α-syn structures detectable in the conditioned media collected (Emmanouilidou et al. 2010; Fan et al. 2019; Guo et al. 2020). This is indicative that both small and larger α-syn structures are capable of intercellular transmission within exosomes, which may result in different downstream processes to mediate toxicity. α-syn has been found in brain- and CSF-derived exosomes from both PD and dementia with Lewy bodies (DLB) patients (Ngolab et al. 2017; Stuendl et al. 2016; Guo et al. 2020; Harischandra et al. 2019), as α-syn is a common feature of both neurodegenerative pathologies. DLB-patient CSF-derived exosomes have been utilised in vivo to investigate α-syn transmission, with intracerebral delivery of patient-derived exosomes to mouse brains resulting in the generation of pathological inclusions (Minakaki et al. 2018). DLB-patient brain-tissue-derived exosomes have also been found to induce α-syn aggregate seeding in cultured cells and mouse brains (Ngolab et al. 2017).
Recent work has implicated the PD risk factor Manganese, to which individuals may be occupationally exposed, in the upregulation of α-syn-containing exosome release in vitro (Harischandra et al. 2019). These exosomes were endocytosed through caveolae upon application to primary microglial cells, triggering neuroinflammatory changes which support the role of α-syn transmission in microglial activation. These exosomes also exerted neurotoxic effects in a human dopaminergic cell model and manganese was demonstrated to accelerate α-syn transmission, resulting in dopaminergic neurotoxicity, in a mouse model of manganese exposure (Harischandra et al. 2019).
The autophagy-lysosomal pathway has also been implicated in exosomal α-syn transmission, with lysosomal inhibition of donor cells found to increase both exosome-mediated release and transfer of α-syn (Alvarez-Erviti et al. 2011a). This observation is most likely due to the effect that when autophagy flux is impaired, the MVB and amphisome pathways are compensatorily enhanced to reduce intracellular α-syn levels by releasing exosomes (Figure 2). Concurrent reduction in intracellular α-syn aggregation has been reported to accompany the increased exosomal α-syn release in instances of autophagy inhibition (Lee et al. 2013); Furthermore, Fussi et al (2018) found that inhibition of autophagosome formation, via Atg5 inhibition, resulted in protection from α-syn toxicity, which was thought to be the result of increased exosomal α-syn secretion; inhibition of exosome secretion was found to enhance α-syn-induced cell death. This study supports the proposal of α-syn excretion as a protective compensatory mechanism of cells to avoid their intracellular protein accumulation. Although the reduction of intracellular aggregation may initially demonstrate cellular protection as a result of enhanced removal of α-syn from the cell via exosomal release, this effect propagates PD pathology and may overload the extracellular space with toxic α-syn (Poehler et al. 2014).
Degradation of extracellular α-syn species, including exosome-contained protein, is a major function of microglia, which phagocytose dead cells and help clear misfolded protein aggregates in the brain (Brück et al. 2016). α-syn has also been found to activate microglia by stimulating an immune response which includes the release of pro-inflammatory cytokines; microglia-mediated neuroinflammation has been previously linked to PD pathogenesis (Hirsch et al. 2012). A recent study demonstrates that aggregated α-syn activates microglia through the Kv1.3 voltage-gated potassium channel (Sarkar et al. 2020). Blocking Kv1.3 function genetically or pharmacologically was found to significantly reduce neuroinflammation and neurodegeneration in animal models of PD (Sarkar et al. 2020). Studies of isolated microglia and monocytes from mice found that in young mice there was increased phagocytosis of exosomal α-syn oligomers, with a concomitant reduction in microglial activation as indicated by decreased secretion of pro-inflammatory cytokines (Bliederhaeuser et al. 2016). This increase in microglial α-syn phagocytosis was not sustained in microglia isolated from older mice, suggesting that microglia in aged brains may have reduced phagocytic capacity to modulate extracellular α-syn levels. In the BV-2 microglial cell line, exosome release was found to cause apoptotic cell death in co-cultured cortical neurons (Chang et al. 2013). Our recent work supports the role of microglia in exosomal α-syn transmission; in primary microglial cell cultures, application of human α-syn preformed fibrils (PFFs) stimulated release of α-syn-containing exosomes, which were fully capable of inducing protein aggregation in recipient neurons (Guo et al. 2020); this release of α-syn following PFF treatment may be an attempt to modulate intracellular levels of abnormal protein. Co-treatment with PFFs and pro-inflammatory cytokine treatment increased α-syn aggregation in vitro, implicating microglial activation with enhanced α-syn transmission and toxicity. The importance of exosomes in this protein transmission was verified by inhibition of exosome synthesis 24 hours prior to PFF treatment, which robustly reduced exosome secretion from microglia without inducing microglial cell death. Labelling of isolated exosomes from these inhibited microglia subsequently demonstrated that neuronal uptake of these exosomes was also reduced, with a consistent reduction in α-syn aggregation found in these neurons (Guo et al. 2020). This finding supports the potential targeting of exosomes as an intervention to limit α-syn transmission between cells.
Stereotaxic delivery of PFFs to the mouse dorsal striatum resulted in increased phospho-α-syn in the striatum and anatomically associated brain regions after 30 days, along with time-dependent degeneration of TH-positive neurons in the SNpc and TH-positive terminals in the striatum (Guo et al. 2020). Enhanced microglial activation in the SNpc and striatum supports the implication of microglia in these neuropathological changes. Microglial depletion via inhibition of colony stimulating factor 1 receptor (CSF1R), with subsequent PFF delivery as before reduced microglial activation and α-syn aggregation to demonstrate that partial depletion of microglia may protect against α-syn transmission and aggregation in the brain (Guo et al. 2020). Further to the study of PFF-induced microglial activation, stereotaxic injection of primary microglial culture-derived exosomes to the mouse dorsal striatum resulted in a time-dependent increase in phospho-α-syn, confirming the transmissibility of microglial-derived α-syn-containing exosomes in vivo. Concurrent loss of TH-positive neurons and terminals in the SNpc and striatum, with concomitant striatal dopamine depletion at 180 days post-surgery is consistent with progressive neuropathological changes. These neurotoxic changes were not detectable at 30 days post-surgery, supportive of microglial-derived exosome-mediated time-dependent retrograde degeneration of the nigrostriatal pathway, with accompanying development of motor dysfunction (Guo et al. 2020). Similarly, Alzheimer’s disease-related tau protein has been found to spread via microglial exosomes, with depletion of microglia and inhibition of microglial exosome synthesis found to attenuate tau propagation (Asai et al. 2015).
Mechanistically, autophagy has been implicated in pathological α-syn transmission; microglial activation has been found to impair autophagy (Du et al. 2017) and α-syn PFFs were found to impair autophagy flux via PELI1 upregulation, which in turn resulted in LAMP1 degradation in activated microglia (Guo et al. 2020). Lysosomal impairment has been previously found to promote exosome-mediated protein secretion (Vella et al. 2007; Vella et al. 2008) and autophagosomes have been suggested to affect exosomes (Fader et al. 2008). Overall, exosomes are strongly implicated as contributors to α-syn intercellular transmission in PD, with autophagy and apoptosis as two suggested mechanisms of cellular damage.
Interestingly, exosomal transmission in PD has also been linked to the genetic risk factor ATP13A2, which is also involved in the juvenile parkinsonian disorder Kufor-Rakeb syndrome. Loss of function mutations in this gene cause lysosomal dysfunction and α-syn accumulation, whereas overexpression was found to suppress α-syn toxicity (Gitler et al. 2009; Kong et al. 2014). ATP13A2 overexpression was associated with enhanced exosome release in H4 neuroglia cells, which suggests that these cells may utilise exosomal removal of α-syn to suppress toxicity (Tsunemi et al. 2014).
4.2. Exosomes and miRNAs
Further to their function in mediating intercellular α-syn transmission in PD, exosomes are additionally implicated in PD pathogenesis by their capacity to transfer genetic material between cells. MiRNAs can be packaged into exosomes and have previously been implicated in PD. Some miRNAs target PD-related genes, such as miR-7 which can combine with the 3’-untranslated region (UTR) of SNCA Mrna to inhibit its transcription; thus loss of miR-7 contributed to increased α-syn upregulation, aggregation and dopaminergic neuron loss in PD-patient brains (McMillan et al. 2017). Significant upregulation of miR-4639–5p, which negatively regulated the post-transcription of DJ-1, has been found to result in severe oxidative stress and neuronal death in PD patients (Chen et al. 2017). Exosome-associated miR-137 has been found to be upregulated in neurons in PD, where it plays a vital role in neuronal oxidative stress induction. MiR-137 directly targets oxidation resistance-1 (OXR1) to negatively regulate its expression, thereby inducing oxidative stress. The levels of miRNAs have also been investigated in some PD models, such as in a manganese model where 12 miRNAs were significantly increased in exosomes; these miRNAs were shown to regulate key PD pathogenesis pathways including autophagy, inflammation and protein aggregation (Harischandra et al. 2018).
Overall, research to date strongly supports exosomes as important multi-functional contributors to PD pathogenesis, through transmission of abnormal protein, propagation of protein pathology across brain regions and transfer of genetic material between cells.
5. CLINICAL POTENTIAL OF EXOSOMES
Exosomes may hold clinical potential for PD, including therapeutic potential as treatment vehicles and diagnostic tools for the monitoring of specific protein and miRNA levels in serum exosomes. The ability of exosomes to transport toxic proteins, such as α-synuclein, to propagate PD pathology through the brain suggests they may be suitable targets to reduce protein pathology if their cellular release or uptake can be targeted. They have also been identified as potential vectors for the delivery of drug or genetic therapies, as it may be possible to hijack their native capacity for the transport of genetic and protein materials between cells for therapeutic benefit. Additionally, because exosomes are components of native cellular transport mechanisms, they may also avoid activating cellular immunogenic responses.
5.1. Limiting protein propagation
Impairment in autophagic activity and lysosomal function has been documented to increase the release of exosomes in neuronal cells (Alvarez-Erviti et al. 2011a; Danzer et al. 2012; Minakaki et al. 2018). Previous work has also reported that α-syn levels in exosomes increase when autophagy flux is inhibited in α-syn over-expressing neurons by enhancing the MVB and amphisome pathways as a compensatory response to reduce intracellular α-syn levels through releasing exosomes. (Alvarez-Erviti et al. 2011a; Minakaki et al. 2018; Poehler et al. 2014). It has been reported that exosome-associated α-syn oligomers are internalised by recipient cells whereas free α-syn oligomers are not, and remain bound to cell surface (Delenclos et al. 2017). As our understanding of the intercellular transport of α-syn has highlighted the multi-cell type involvement of neurons, microglia and astrocytes, we have come to recognise the importance of targeting exosome release from all cell types as a potential therapeutic strategy for PD, not just neuronal exosomes as glial cells also participate in the process of intercellular protein transmission.
Recent work by our group found that exosome release was increased in circumstances of impaired autophagy flux and that targeting dynamin related protein 1 (Drp1), commonly known for its mitochondrial fission function, was effective at reducing α-syn-containing exosome release from both neurons and microglia (Fan et al. 2019). We found that exosomes released from α-syn PFF-treated neurons conferred α-syn toxicity to neurons. Inhibition of Drp1 enhanced autophagy flux, reduced α-syn aggregation and reduced exosome release; this work demonstrates that indirect modulation of exosome levels through restoration of autophagy flux attenuated α-syn propagation and aggregation. Both siRNA knockdown of Drp1 and inhibition with a pharmacological agent, mitochondrial division inhibitor 1 (Mdivi-1), were effective at reducing α-syn transmission between SH-SY5Y cells. Further investigation in primary mouse microglia treated with PFFs in conjunction with LPS demonstrated enhanced exosome release in co-treated cells. Application of microglial-derived exosomal fractions to SH-SY5Y cultures confirmed the capacity of exosomes to mediate microglia-to-neuron α-syn transmission, with Drp1 inhibition effective at reducing this protein transmission and aggregation. Combined assessment of the data from primary microglia and BV2 cells indicates that microglia are capable of releasing α-syn-containing vesicles through which they propagate the spread of synuclein to neurons, with Drp1 blockade found to significantly reduce these pathological changes. This work supports the potential therapeutic application of limiting exosome release to mitigate α-syn transmission and aggregation in PD, with efficacy demonstrated in modulation of both neuronal and microglial derived exosomes.
5.2. Exosomes as therapeutic vehicles
The current methods of PD treatment focus on the abrogation of symptomatic presentation, with the primary emphasis on the control of motor dysfunctions. There are currently no disease modifying or curative treatments, so there is a need to develop novel treatment strategies which alter disease progression. A major barrier to the development of new drug treatments for neurodegenerative diseases is the capacity of drugs to cross the blood-brain-barrier (BBB), which many are unable to do (Pardridge & Boado 2012). As described elswhere in this review, exosomes are nano-scaled extracellular vesicles (EV) which can easily permeate membranes including the BBB, suggesting that they may be effective vehicles for the delivery of drugs to the brain (Lai & Breakefield 2012; Zhuang et al. 2011). Another benefit of using exosomes to deliver therapeutics is that they can evade immunogenicity. As artificial exosomes can be loaded with therapeutically active molecules, such as medicinal compounds, siRNAs and proteins for delivery to the brain, it may be possible to mediate site-specific targeting of exosomes to the appropriate recipient cells. Native exosomes are known to transport nucleic acids to target cells, it is therefore reasonable to consider their potential as delivery mechanisms for modified, therapeutic genetic materials which could regulate gene expression to alter PD prognosis or progression.
Research in vivo and in vitro has found that human exosomes isolated from the blood and loaded with a saturated dopamine solution can cross the BBB to deliver dopamine into the brain via interaction between transferrin and the transferrin receptor (Qu et al. 2018). In a mouse model, the dopamine-loaded exosomes demonstrated greater therapeutic efficacy and lower toxicity than systemic intravenously delivered free dopamine. Further research into the attenuation of neuroinflammation in PD utilised catalase-loaded exosomes which were taken up by neurons. The antioxidant effects of catalase, which was released into the neurons following exosome uptake, was found to increase neuronal survival and ameliorate neural inflammation in PD models in vitro and in vivo (Haney et al. 2015). Kojima et al (2018) also reported a set of devices for exosomal transfer into cells which produce designer exosomes in engineered mammalian cells to deliver therapeutic catalase mRNA to the brain.
There is therapeutic potential for exosomes carrying small interfering RNAs (siRNAs) for PD. A study in the S129D α-syn transgenic mice utilised systemic injection of modified α-syn-siRNA-containing exosomes, which reduced the amount of α-syn mRNA transcription and translation in the brain (Cooper et al. 2014). Another group reported that exosome delivery of hydrophobically modified siRNA to the brain efficiently targeted mHtt mRNA in a Huntington’s disease model, which is encouraging for the potential use of siRNAs to target α-syn in PD (Didiot et al. 2016). Alvarez-Erviti et al (2011b) demonstrated that siRNA could be transported to mouse brains via exosomes following systemic injection. However, siRNAs may be of limited effectiveness in PD treatment due to their short efficacy and poor bioavailability in systemic circulation. As such, short hairpin RNAs (shRNAs) have also been investigated. Izco et al (2019) designed shRNA minicircles which were delivered by RVG-exosomes to target synuclein in a PD mouse model induced by preformed synuclein fibrils. Treatment with exosomal anti-synuclein shRNA reduced synuclein aggregation, decreased dopaminergic neuron death and ameliorated the clinical symptoms of PD in this mouse model (Izco et al. 2019). This work supports the potential of exosomes as transport vehicles for drugs, such as dopamine, or genetic modulators, such as shRNA, into the brain for therapeutic benefit. Exosomes may prove to be useful vehicles for the delivery of various RNA-based therapies for PD, as they may protect the RNAs from degradation in circulation (Maheshwari et al. 2017) and could provide an RNA delivery method to improve clinical practice.
Mesenchymal stem cell (MSC)-derived exosomes have been identified as effective treatment tools, with beneficial effects shown in a number of pathologies including PD, multiple sclerosis and osteoarthritis (Li et al. 2019; Mianehsaz et al. 2019). MSC-derived exosomes proved effective at rescuing dopaminergic neurons in the 6-OHDA mouse model of PD (Vilaça-Faria et al. 2019) and they can also carry miRNAs and interact with neuronal cells to reduce neuroinflammation and promote neurogenesis in mouse PD models. Whilst further investigations and clinical trials are required to confirm the benefits of therapeutic application of exosomes in PD, mounting evidence supports that the separation of exosomes from various cell types and their modification to target specific brain regions may hold therapeutic benefits for PD, amongst other disorders (Batrakova & Kim 2015).
5.3. Exosomes as diagnostic tools
PD is primarily diagnosed by the clinical manifestation of disease symptoms at present (Postuma et al. 2015). However, prodromal non-motor symptoms are commonly reported, which can begin decades prior to the onset of motor degeneration (Chaudhuri et al. 2006; Drolet et al. 2009; Schrag et al. 2019). There is currently no accurate method for the diagnosis of PD in the earlier stages, which is when therapeutic interventions may be more beneficial if they can slow or reduce the nigrostriatal dopaminergic degeneration which initiates motor dysfunction. Therefore, development of an early diagnostic test and identification of novel PD biomarkers would be an important breakthrough.
α-syn is considered an inefficient PD biomarker despite its presence in patient blood and CSF samples due to its low abundance. For example, in PD patient CSF α-syn has been shown to be present at lower levels than in healthy controls (Hall et al. 2012; Hong et al. 2010; Kang et al. 2013; Mollenhauer et al. 2013). Stuendl et al (2016) reported that levels of EV-α-syn are also lower in PD patient CSF samples, consistent with the low overall α-syn levels reported. Detection of serum and plasma α-syn has also proven ineffective as a biomarker, as peripheral blood cells can produce α-syn. Previous analysis of extracellular vesicle constituents, including exosomes, isolated from the blood or CSF of PD patients suggested that extracellular vesicles (EVs) may be efficient biomarkers for PD (Vella et al. 2016). Shi et al (2014) found that α-syn can be transported from the CSF to blood and that some is packaged into EVs expressing the CNS-specific neural cell adhesion molecule L1 (L1CAM). The authors demonstrated that CNS-derived levels of EV-α-syn in plasma were significantly higher in PD patients, with levels relating to the severity of the disease presentation, leading to the suggestion that CNS-derived EV-α-syn in plasma can be used as a biomarker for PD with high specificity and sensitivity. It was also recently reported that salivary EVs isolated from PD patients demonstrated higher absolute levels of α-syn oligomers, and also the α-syn oligomers/total α-syn ratio was increased when compared with healthy controls (Rani et al. 2019). Increased levels of Tau, which is a microtubule-associated protein primarily implicated in Alzheimer’s disease pathogenesis, have also been detected in CNS-derived EVs isolated from human plasma, with significantly higher levels in PD patients compared with AD patients (Shi et al. 2016). There has also been investigation of proteins related to familial and sporadic PD in EVs. DJ-1 levels in CNS-derived EVs, and the ratio of EV-DJ-1 to total DJ-1 derived from the CNS were substantially higher in PD patient plasma compared to controls (Zhao et al. 2018). Fraser et al (2016) investigated whether the levels of auto-phosphorylated Ser(P)-1292 LRRK2 in urine EVs could predict LRRK2 mutation carriers and non-carriers with or without PD; the authors found that these levels could predict LRRK2 mutation status using the elevated ratio of Ser(P)-1292 LRRK2 to total LRRK2 in urine EVs. Furthermore, patients with PD demonstrated a higher ratio than LRRK2 carriers without PD. This study utilised 79 idiopathic PD patients and 79 healthy controls, identifying higher Ser(P)-1292 LRRK2 in men than women, and increased Ser(P)-1292 LRRK2 in idiopathic PD patients in contrast to the healthy controls. Ser(P)-1292 LRRK2 levels were also shown to relate to the severity of cognitive impairment and may therefore serve as a useful biomarker for both familial and idiopathic PD, providing an indication as to the severity of anticipated cognitive symptoms.
The identification of increased levels of specific miRNAs in serum EVs from PD patients compared to controls (Cao et al. 2017) suggests that monitoring of miRNA in EVs could serve as useful tools in the diagnosis of PD. MicroRNAs, as discussed in the previous section, are known post-transcriptional regulators which can be transported intercellularly by exosomes. They inhibit via sequence-specific targeting of the 3’ untranslated region of mRNAs, regulating cell proliferation, cell differentiation and apoptosis (Chang et al. 2016). Abnormal miRNA expression is linked to major features and risk factors of PD, including α-syn overexpression, LB formation and neuronal apoptosis (Doxakis 2010; Leggio et al. 2017; McMillan et al. 2017; Wang et al. 2016). Gui et al (2015) established a profiling method for exosomal miRNAs, which were differentially expressed in exosomes derived from CSF of healthy vs PD patients; data suggested 11 downregulated miRNAs and 16 upregulated miRNAs in PD, with further analysis identifying significantly up or downregulation in 6 miRNAs. A further 24 exosomal miRNAs were evaluated in serum samples from patients with PD, confirming some (such as miRNA19b, miRNA195 and miRNA24) as potential candidates for PD diagnosis with specificity and sensitivity to distinguish PD patients from healthy individuals using exosomal miRNAs (Cao et al. 2017). Prediction of gene-target sites of three exosomal miRNAs using the Targetscan tool found LRRK2-miRNA19b and ATP13A2-miRNA24/miRNA195 correlated with neurodegenerative processes in PD (Cao et al. 2017; Heman-Ackah et al. 2013). This work revealed the potential of miRNAs to act as biomarkers for PD diagnosis in the early stages of the disease, which could allow for improved monitoring and earlier treatment interventions. Further work is needed to investigate how miRNAs could be monitored to deliver therapeutic nucleic acids for regulation of abnormal expression of these miRNAs in PD pathology process.
Exosomes have also been investigated for their potential as success biomarkers in clinical trials; for example, in a single-centre Exenatide-PD trial, 60 idiopathic PD patients were randomly assigned to subcutaneous administration of 2mg exenatide or placebo once weekly for 48 weeks, followed by a 12 week drug withdrawal period. Blood samples were collected at weeks 0, 24, 48 and 60, with neuronal-derived EVs with L1CAM selectively isolated for quantification of insulin signalling proteins (Athauda et al. 2019). Patients receiving exenatide were found to have enhanced brain insulin signalling with increased phosphorylation of insulin receptor substrate 1 (IRS-1) at 48 weeks and 60 weeks, compared with placebo controls. Furthermore, expression of downstream pathway substrates was increased, including total Akt and mTOR levels. The implication of mTOR in this drugs effects suggests it may impact autophagy flux, which is known to alter exosomal production. Changes in the levels of EV biomarkers, including IRS-1 p-S616, t-mTOR and p-mTOR S2488, correlated with changes in Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part 3 scores at 48 weeks. At 60 weeks, the MDS-UPDRS part 3 scores were closely associated with changes in t-mTOR. Overall, these findings suggest that exenatide can improve MDS-UPDRS part 3 scores, which were significantly related to changes in these biomarkers in EVs (Athauda et al. 2019). This study also supports the use of EV biomarkers as potential markers for the evaluation of treatment responses and prognosis in clinical trials. However, despite the clinical potential of using exosomes as drug deliveral vehicles or diagnostic tools, as discussed below, there are major limitations and concerns that must be taken into consideration.
6. CONCLUSION AND FUTURE DIRECTIONS
Overall, the field of exosome research has advanced rapidly over the past decade. As illustrated in Fig. 3, because exosomes can influence gene expression and protein activity in recipient cells by carrying cargos such as proteins, mRNA and microRNA (Chistiakov & Chistiakov 2017; Valadi et al. 2007), they may contribute to the spread of misfolded proteins such as α-syn. Exosomes also present potential attractive targets for novel therapeutics and diagnostic tools.
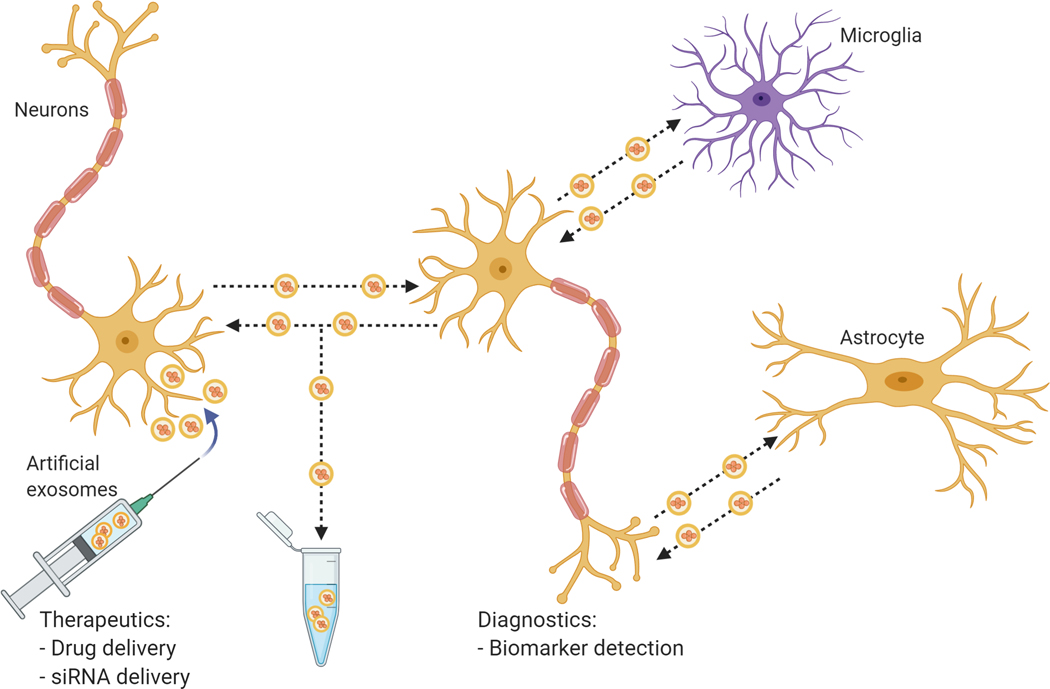
Figure 3. Potential roles of exosomes in neuropathology and clinical applications.
Exosomes can bi-directionally transfer proteins and genetic material between neurons and glial cells such as microglia and astrocytes. Such cell-cell interactions contribute to pathological progression and spread in neurodegenerative diseases such as PD. On the other hand, exosomes and other exosome-like extracellular vesicles have also been investigated as potential tools for the delivery of therapeutics and biomarkers.
As discussed in this review, there is strong evidence to support exosomal transmission as a mechanism of α-syn propagation through the brain, implicating exosomes in the development and progression of PD neuropathology. The identified interplay between autophagic flux, α-syn levels and exosomes has provided clues as to the relationship between cellular mechanisms and Parkinson’s pathology. Enhancing autophagy has proven effective at reducing α-syn transmission via exosomes, supporting the potential benefit of targeting exosomes, even indirectly, to limit α-syn transmission between cells. Whether changes in autophagic functionality impact the transmission of other exosome-encompassed cargo, such as RNAs and other proteins, remains to be established; this link will be important if exosome-contained proteins are to be utilised as PD biomarkers, as alteration of autophagy flux could otherwise impact the interpretation of biomarker data for diagnostics.
There is a strong interest in targeting exosomes as diagnostic tools for detection of encapsulated biomarkers, and as prospective vehicles for the delivery of pharmacological or genetic therapeutic agents to specific brain regions or cell types for PD treatment. However, despite the apparent advantages which exosomes present, their limitations must also be considered. For example, the procedures for isolating and purifying exosomes need to be improved and standardized. It is not uncommon that the isolated fraction of exosomes is also contaminated with other small exosome-like extracellular vesicles or proteins such as α-syn. A lack of general consensus on a universal exosome marker is another challenge. Additional research into the differential reactivity of exosomes isolated from different sources, to identify any adverse effects, should be undertaken to identify the best cellular exosome source for therapeutic application (Sarko & McKinney 2017). Additional research is required to determine how membrane markers may target exosomes to specific cell types and how the content of exosomes may confer influence over the terminal destination. Understanding how exosomes are targeted between cells may offer additional considerations for the development of exosome inhibitors to modulate disease. In addition to exosomes, other EVs such as the recently described arrestin domain containing protein 1-mediated microvesicles (Wang et al. 2018) should be investigated as another delivery platform for therapeutic molecules.
In summary, there has been a significant interest in exosome research in the context of PD and other neurodegenerative disorders in recent year; however, there remain major technical issues and unanswered questions in exosome regulation and pathological contributions to disease. Further investigation of exosomes and other EVs, along with refinement of experimental techniques and improvement in our ability to manipulate them for delivery of therapeutics, would advance understanding of PD pathology, treatment and diagnostic approaches.
ACKNOWLEDGEMENTS
This work was supported in part by the NIH/NIEHS grant R35-ES030523 to KT; Robert Stempel College of Public Health & Social Work, Florida International University, and Peninsula Schools of Medicine and Dentistry, Plymouth University, UK (JP). Figures were re-drawn by Victoria Morus and Laura Hausmann in BioRender (https://biorender.com/) on the basis of a draft provided by the author.”
Funding: This work was supported in part by the NIH/NIEHS grant R35-ES030523 to KT; Robert Stempel College of Public Health & Social Work, Florida International University, and Peninsula Schools of Medicine and Dentistry, Plymouth University, UK (JP).
Abbreviations:
- α-syn
α-synuclein
- BBB
blood-brain-barrier
- CSF
cerebrospinal fluid
- DLB
dementia with Lewy bodies
- ESCRT
endosomal sorting complex required for transport
- EVs
extracellular vesicles
- ILVs
intraluminal vesicles
- miRNA
microRNAs
- MSC
mesenchymal stem cell
- MVBs
multivesicular bodies
- PD
Parkinson disease
- PFF
α-syn preformed fibrils
- shRNAs
short hairpin RNAs
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- TNTs
tunnelling nanotubules
Footnotes
--Human subjects --
Involves human subjects:
If yes: Informed consent & ethics approval achieved:
=> if yes, please ensure that the info “Informed consent was achieved for all subjects, and the experiments were approved by the local ethics committee.” is included in the Methods.
ARRIVE guidelines have been followed:
No
=> if it is a Review or Editorial, skip complete sentence => if No, include a statement in the “Conflict of interest disclosure” section: “ARRIVE guidelines were not followed for the following reason:
Not applicable “
(edit phrasing to form a complete sentence as necessary).
=> if Yes, insert in the “Conflict of interest disclosure” section:
“All experiments were conducted in compliance with the ARRIVE guidelines.” unless it is a Review or Editorial
Conflicts of interest: None
=> if ‘none’, insert “The authors have no conflict of interest to declare.”
=> otherwise insert info unless it is already included
REFERENCES
- Abounit S, Bousset L, Loria F, Zhu S, de Chaumont F, Pieri L, Olivo-Marin JC, Melki R and Zurzolo C. (2016) Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J 35, 2120–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abounit S and Zurzolo C. (2012) Wiring through tunneling nanotubes--from electrical signals to organelle transfer. J Cell Sci 125, 1089–1098. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ and Cooper MJ (2011a) Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiology of Disease 42, 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S and Wood MJ (2011. b) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29, 341–345. [DOI] [PubMed] [Google Scholar]
- Arima K, Hirai S, Sunohara N, Aoto K, Izumiyama Y, Uéda K, Ikeda K and Kawai M. (1999) Cellular co-localization of phosphorylated tau- and NACP/alpha-synuclein-epitopes in lewy bodies in sporadic Parkinson’s disease and in dementia with Lewy bodies. Brain Res 843, 53–61. [DOI] [PubMed] [Google Scholar]
- Arotcarena ML, Dovero S, Prigent A. et al. (2020) Bidirectional gut-to-brain and brain-to-gut propagation of synucleinopathy in non-human primates. Brain 143, 1462–1475. [DOI] [PubMed] [Google Scholar]
- Arotcarena ML, Teil M and Dehay B. (2019) Autophagy in Synucleinopathy: The Overwhelmed and Defective Machinery. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai H, Ikezu S, Tsunoda S. et al. (2015) Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18, 1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athauda D, Gulyani S, Karnati HK et al. (2019) Utility of Neuronal-Derived Exosomes to Examine Molecular Mechanisms That Affect Motor Function in Patients With Parkinson Disease: A Secondary Analysis of the Exenatide-PD Trial. JAMA Neurol 76, 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E. et al. (2012) Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14, 677–685. [DOI] [PubMed] [Google Scholar]
- Batrakova EV and Kim MS (2015) Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release 219, 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrone E, Corona C, Mazza M. et al. (2015) Detection of cellular prion protein in exosomes derived from ovine plasma. J Gen Virol 96, 3698–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliederhaeuser C, Grozdanov V, Speidel A. et al. (2016) Age-dependent defects of alpha-synuclein oligomer uptake in microglia and monocytes. Acta Neuropathol 131, 379–391. [DOI] [PubMed] [Google Scholar]
- Blott EJ and Griffiths GM (2002) Secretory lysosomes. Nat Rev Mol Cell Biol 3, 122–131. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN and Braak E. (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24, 197–211. [DOI] [PubMed] [Google Scholar]
- Bridgeman K and Arsham T. (2017) The Comprehensive Guide to Parkinson’s Disease. [Google Scholar]
- Brück D, Wenning GK, Stefanova N and Fellner L. (2016) Glia and alpha-synuclein in neurodegeneration: A complex interaction. Neurobiol Dis 85, 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P and Melki R. (2017) Prying into the Prion Hypothesis for Parkinson’s Disease. J Neurosci 37, 9808–9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XY, Lu JM, Zhao ZQ, Li MC, Lu T, An XS and Xue LJ (2017) MicroRNA biomarkers of Parkinson’s disease in serum exosome-like microvesicles. Neurosci Lett 644, 94–99. [DOI] [PubMed] [Google Scholar]
- Chang C, Lang H, Geng N, Wang J, Li N and Wang X. (2013) Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neurosci Lett 548, 190–195. [DOI] [PubMed] [Google Scholar]
- Chang H, Yi B, Ma R, Zhang X, Zhao H and Xi Y. (2016) CRISPR/cas9, a novel genomic tool to knock down microRNA in vitro and in vivo. Sci Rep 6, 22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Healy DG, Schapira AH and Excellence NI f. C. (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5, 235–245. [DOI] [PubMed] [Google Scholar]
- Chen Y, Gao C, Sun Q, Pan H, Huang P, Ding J and Chen S. (2017) MicroRNA-4639 Is a Regulator of DJ-1 Expression and a Potential Early Diagnostic Marker for Parkinson’s Disease. Front Aging Neurosci 9, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Zhao W and Hill AF (2018) Exosomes and their role in the intercellular trafficking of normal and disease associated prion proteins. Mol Aspects Med 60, 62–68. [DOI] [PubMed] [Google Scholar]
- Chistiakov DA and Chistiakov AA (2017) α-Synuclein-carrying extracellular vesicles in Parkinson’s disease: deadly transmitters. Acta Neurol Belg 117, 43–51. [DOI] [PubMed] [Google Scholar]
- Chu Y, Muller S, Tavares A. et al. (2019) Intrastriatal alpha-synuclein fibrils in monkeys: spreading, imaging and neuropathological changes. Brain 142, 3565–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JM, Wiklander PB, Nordin JZ et al. (2014) Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord 29, 1476–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR and McLean PJ (2012) Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delenclos M, Trendafilova T, Mahesh D, Baine AM, Moussaud S, Yan IK, Patel T and McLean PJ (2017) Investigation of Endocytic Pathways for the Internalization of Exosome-Associated Oligomeric Alpha-Synuclein. Front Neurosci 11, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E and Lee SJ (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A 106, 13010–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Uchikado H, Fujishiro H and Tsuboi Y. (2010) Evidence in favor of Braak staging of Parkinson’s disease. Mov Disord 25 Suppl 1, S78–82. [DOI] [PubMed] [Google Scholar]
- Didiot MC, Hall LM, Coles AH et al. (2016) Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol Ther 24, 1836–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey ER, Constantinescu R, Thompson JP et al. (2007) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68, 384–386. [DOI] [PubMed] [Google Scholar]
- Dorsey ER, Sherer T, Okun MS and Bloem BR (2018) The Emerging Evidence of the Parkinson Pandemic. J Parkinsons Dis 8, S3–s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxakis E. (2010) Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J Biol Chem 285, 12726–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet RE, Cannon JR, Montero L and Greenamyre JT (2009) Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiology of Disease 36, 96–102. [DOI] [PubMed] [Google Scholar]
- Du D, Hu L, Wu J. et al. (2017) Neuroinflammation contributes to autophagy flux blockage in the neurons of rostral ventrolateral medulla in stress-induced hypertension rats. J Neuroinflammation 14, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L and Vekrellis K. (2010) Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30, 6838–6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader CM, Sánchez D, Furlán M and Colombo MI (2008) Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic 9, 230–250. [DOI] [PubMed] [Google Scholar]
- Fan RZ, Guo M, Luo S, Cui M and Tieu K. (2019) Exosome release and neuropathology induced by α-synuclein: new insights into protective mechanisms of Drp1 inhibition. Acta Neuropathol Commun 7, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser KB, Moehle MS, Alcalay RN, West AB and Consortium LC (2016) Urinary LRRK2 phosphorylation predicts parkinsonian phenotypes in G2019S LRRK2 carriers. Neurology 86, 994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussi N, Höllerhage M, Chakroun T, Nykänen NP, Rösler TW, Koeglsperger T, Wurst W, Behrends C and Höglinger GU (2018) Exosomal secretion of α-synuclein as protective mechanism after upstream blockage of macroautophagy. Cell Death Dis 9, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML et al. (2009) Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet 41, 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J and van der Goot FG (2006) Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol 7, 495–504. [DOI] [PubMed] [Google Scholar]
- Gui Y, Liu H, Zhang L, Lv W and Hu X. (2015) Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 6, 37043–37053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guix FX, Corbett GT, Cha DJ et al. (2018) Detection of Aggregation-Competent Tau in Neuron-Derived Extracellular Vesicles. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Wang J, Zhao Y, Feng Y, Han S, Dong Q, Cui M and Tieu K (2020) Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain 143, 1476–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, Öhrfelt A, Constantinescu R. et al. (2012) Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 69, 1445–1452. [DOI] [PubMed] [Google Scholar]
- Haney MJ, Klyachko NL, Zhao Y. et al. (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release 207, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergström AL et al. (2011) α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest 121, 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C, Heuser J and Stahl P. (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harischandra DS, Ghaisas S, Rokad D, Zamanian M, Jin H, Anantharam V, Kimber M, Kanthasamy A and Kanthasamy AG (2018) Environmental neurotoxicant manganese regulates exosome-mediated extracellular miRNAs in cell culture model of Parkinson’s disease: Relevance to α-synuclein misfolding in metal neurotoxicity. Neurotoxicology 64, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harischandra DS, Rokad D, Neal ML et al. (2019) Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of α-synuclein. Sci Signal 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K and Braak H. (2007) Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33, 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heman-Ackah SM, Hallegger M, Rao MS and Wood MJ (2013) RISC in PD: the impact of microRNAs in Parkinson’s disease cellular and molecular pathogenesis. Front Mol Neurosci 6, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessvik NP and Llorente A. (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75, 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, Broughton E, Hagan H and Carroll C. (2014) Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol 127, 235–241. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Vyas S and Hunot S. (2012) Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord 18 Suppl 1, S210–212. [DOI] [PubMed] [Google Scholar]
- Hoban DB, Shrigley S, Mattsson B. et al. (2020) Impact of α-synuclein pathology on transplanted hESC-derived dopaminergic neurons in a humanized α-synuclein rat model of PD. Proc Natl Acad Sci U S A 117, 15209–15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Shi M, Chung KA et al. (2010) DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain 133, 713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izco M, Blesa J, Schleef M. et al. (2019) Systemic Exosomal Delivery of shRNA Minicircles Prevents Parkinsonian Pathology. Mol Ther 27, 2111–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R and LeBleu VS (2020) The biology. Science 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Irwin DJ, Chen-Plotkin AS et al. (2013) Association of cerebrospinal fluid β-amyloid 1–42, T-tau, P-tau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol 70, 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killinger BA, Madaj Z, Sikora JW et al. (2018) The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ho DH, Suk JE et al. (2013) Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun 4, 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kwon SH, Kam TI et al. (2019) Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 103, 627–641.e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingelhoefer L and Reichmann H. (2015) Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors. Nat Rev Neurol 11, 625–636. [DOI] [PubMed] [Google Scholar]
- Kojima R, Bojar D, Rizzi G, Hamri GC, El-Baba MD, Saxena P, Ausländer S, Tan KR and Fussenegger M. (2018) Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun 9, 1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong SM, Chan BK, Park JS et al. (2014) Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Hum Mol Genet 23, 2816–2833. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB and Olanow CW (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14, 504–506. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Dodiya HB, Kordower AM, Terpstra B, Paumier K, Madhavan L, Sortwell C, Steece-Collier K and Collier TJ (2011) Transfer of host-derived α synuclein to grafted dopaminergic neurons in rat. Neurobiol Dis 43, 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CP and Breakefield XO (2012) Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol 3, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Cho ED, Lee KW, Kim JH, Cho SG and Lee SJ (2013) Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp Mol Med 45, e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Patel S and Lee SJ (2005) Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci 25, 6016–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E and Lee SJ (2010) Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem 285, 9262–9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Vivarelli S, L’Episcopo F, Tirolo C, Caniglia S, Testa N, Marchetti B and Iraci N. (2017) microRNAs in Parkinson’s Disease: From Pathogenesis to Novel Diagnostic and Therapeutic Approaches. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettau M, Schmidt H, Kabelitz D and Janssen O. (2007) Secretory lysosomes and their cargo in T and NK cells. Immunol Lett 108, 10–19. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL et al. (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14, 501–503. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu F, He X, Yang X, Shan F and Feng J. (2019) Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharmacol 67, 268–280. [DOI] [PubMed] [Google Scholar]
- Logan T, Bendor J, Toupin C, Thorn K and Edwards RH (2017) α-Synuclein promotes dilation of the exocytotic fusion pore. Nat Neurosci 20, 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria F, Vargas JY, Bousset L, Syan S, Salles A, Melki R and Zurzolo C. (2017) α-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol 134, 789–808. [DOI] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ and Lee VM (2012) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari R, Tekade M, Gondaliya P, Kalia K, D’Emanuele A and Tekade RK (2017) Recent advances in exosome-based nanovehicles as RNA interference therapeutic carriers. Nanomedicine (Lond) 12, 2653–2675. [DOI] [PubMed] [Google Scholar]
- Marras C, Beck JC, Bower JH et al. (2018) Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis 4, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaracchia C, Hnida M, Gerhardt E. et al. (2018) Membrane binding, internalization, and sorting of alpha-synuclein in the cell. Acta Neuropathol Commun 6, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan KJ, Murray TK, Bengoa-Vergniory N. et al. (2017) Loss of MicroRNA-7 Regulation Leads to α-Synuclein Accumulation and Dopaminergic Neuronal Loss In Vivo. Mol Ther 25, 2404–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Fraldi A, Bouche V. et al. (2011) Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell 21, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Dehejia A, Harta G, Papp MI, Polymeropoulos MH and Brownstein MJ (1998) Alpha synuclein in neurodegenerative disorders: murderer or accomplice? Nat Med 4, 755–757. [DOI] [PubMed] [Google Scholar]
- Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran M, Rezaee A, Sahebnasagh R, Pourhanifeh MH, Mirzaei H and Hamblin MR (2019) Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther 10, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakaki G, Menges S, Kittel A. et al. (2018) Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy 14, 98–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer B, Trautmann E, Taylor P, Manninger P, Sixel-Döring F, Ebentheuer J, Trenkwalder C and Schlossmacher MG (2013) Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett 532, 44–48. [DOI] [PubMed] [Google Scholar]
- Mor DE, Ugras SE, Daniels MJ and Ischiropoulos H. (2016) Dynamic structural flexibility of α-synuclein. Neurobiol Dis 88, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngolab J, Trinh I, Rockenstein E. et al. (2017) Brain-derived exosomes from dementia with Lewy bodies propagate α-synuclein pathology. Acta Neuropathol Commun 5, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka T, Watanabe ST, Iwatsubo T and Hasegawa M. (2010) Seeded aggregation and toxicity of {alpha}-synuclein and tau: cellular models of neurodegenerative diseases. J Biol Chem 285, 34885–34898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BT and Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33, 967–978. [DOI] [PubMed] [Google Scholar]
- Pardridge WM and Boado RJ (2012) Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier. Methods Enzymol 503, 269–292. [DOI] [PubMed] [Google Scholar]
- Parkkinen L, Pirttilä T and Alafuzoff I. (2008) Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol 115, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Trojanowski JQ and Lee VM (2020) Protein transmission in neurodegenerative disease. Nat Rev Neurol 16, 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez M, Avila J and Hernández F. (2019) Propagation of Tau via Extracellular Vesicles. Front Neurosci 13, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehler AM, Xiang W, Spitzer P. et al. (2014) Autophagy modulates SNCA/α-synuclein release, thereby generating a hostile microenvironment. Autophagy 10, 2171–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E. et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M. et al. (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30, 1591–1601. [DOI] [PubMed] [Google Scholar]
- Qu M, Lin Q, Huang L. et al. (2018) Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Control Release 287, 156–166. [DOI] [PubMed] [Google Scholar]
- Rani K, Mukherjee R, Singh E. et al. (2019) Neuronal exosomes in saliva of Parkinson’s disease patients: A pilot study. Parkinsonism Relat Disord 67, 21–23. [DOI] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ and Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183, 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold PM and Tieu K. (2010) Astrocytes and therapeutics for Parkinson’s disease. Neurotherapeutics 7, 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, Lee VM and Brundin P. (2016) Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J Exp Med 213, 1759–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez L, Marano MM and Tandon A. (2018) Import and Export of Misfolded α-Synuclein. Front Neurosci 12, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami J, Holmqvist S, Lindström V, Sigvardson J, Westermark GT, Ingelsson M, Bergström J, Roybon L and Erlandsson A. (2017) Human Astrocytes Transfer Aggregated Alpha-Synuclein via Tunneling Nanotubes. J Neurosci 37, 11835–11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustom A, Saffrich R, Markovic I, Walther P and Gerdes HH (2004) Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010. [DOI] [PubMed] [Google Scholar]
- Sacino AN, Brooks M, Thomas MA et al. (2014) Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci U S A 111, 10732–10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent D, Verchère J, Lazizzera C, Gaillard D, Lakhdar L, Streichenberger N, Morignat E, Bétemps D and Baron T. (2017) ‘Prion-like’ propagation of the synucleinopathy of M83 transgenic mice depends on the mouse genotype and type of inoculum. J Neurochem 143, 126–135. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Dammer EB, Malovic E. et al. (2020) Molecular Signatures of Neuroinflammation Induced by αSynuclein Aggregates in Microglial Cells. Front Immunol 11, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarko DK and McKinney CE (2017) Exosomes: Origins and Therapeutic Potential for Neurodegenerative Disease. Front Neurosci 11, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Zhelev SS, Hotham S, Merritt RD, Khan K and Graham L. (2019) Heterogeneity in progression of prodromal features in Parkinson’s disease. Parkinsonism Relat Disord. [DOI] [PubMed] [Google Scholar]
- Shi M, Kovac A, Korff A. et al. (2016) CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimers Dement 12, 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Liu C, Cook TJ et al. (2014) Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol 128, 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R and Goedert M. (1997) a-Synuclein in Lewy bodies. Nature 388, 839–840. [DOI] [PubMed] [Google Scholar]
- Stuendl A, Kunadt M, Kruse N, Bartels C, Moebius W, Danzer KM, Mollenhauer B and Schneider A. (2016) Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain 139, 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA and Halliday GM (2017) Parkinson’s Disease Is Not Simply a Prion Disorder. J Neurosci 37, 9799–9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanik SA, Schultheiss CE, Volpicelli-Daley LA, Brunden KR and Lee VM (2013) Lewy body-like α-synuclein aggregates resist degradation and impair macroautophagy. J Biol Chem 288, 15194–15210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Witwer KW, Aikawa E. et al. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7, 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi T, Hamada K and Krainc D. (2014) ATP13A2/PARK9 regulates secretion of exosomes and α-synuclein. J Neurosci 34, 15281–15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi T, Perez-Rosello T, Ishiguro Y. et al. (2019) Increased Lysosomal Exocytosis Induced by Lysosomal Ca. J Neurosci 39, 5760–5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ and Lötvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9, 654–659. [DOI] [PubMed] [Google Scholar]
- Vella LJ, Hill AF and Cheng L. (2016) Focus on Extracellular Vesicles: Exosomes and Their Role in Protein Trafficking and Biomarker Potential in Alzheimer’s and Parkinson’s Disease. Int J Mol Sci 17, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Lawson VA, Masters CL, Cappai R and Hill AF (2007) Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol 211, 582–590. [DOI] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Nisbet RM, Cappai R and Hill AF (2008) The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur Biophys J 37, 323–332. [DOI] [PubMed] [Google Scholar]
- Vilaça-Faria H, Salgado AJ and Teixeira FG (2019) Mesenchymal Stem Cells-derived Exosomes: A New Possible Therapeutic Strategy for Parkinson’s Disease? Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Ohama E and Ikuta F. (1990) Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol 79, 581–583. [DOI] [PubMed] [Google Scholar]
- Wang H, Ye Y, Zhu Z. et al. (2016) MiR-124 Regulates Apoptosis and Autophagy Process in MPTP Model of Parkinson’s Disease by Targeting to Bim. Brain Pathol 26, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Yu J, Kadungure T, Beyene J, Zhang H and Lu Q. (2018) ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat Commun 9, 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert T and Hurley JH (2010) Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 464, 864–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Camfield R and Gorski SM (2018) The interplay between exosomes and autophagy - partners in crime. J Cell Sci 131. [DOI] [PubMed] [Google Scholar]
- Yáñez-Mó M, Siljander PR, Andreu Z. et al. (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4, 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZH, Chen ZT, Zhou RL, Zhang X, Ye QY and Wang YZ (2018) Increased DJ-1 and α-Synuclein in Plasma Neural-Derived Exosomes as Potential Markers for Parkinson’s Disease. Front Aging Neurosci 10, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Xiang X, Grizzle W. et al. (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19, 1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G and Amigorena S. (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4, 594–600. [DOI] [PubMed] [Google Scholar]