Abstract
The use of mailed, home-obtained urine specimens could facilitate screening programs for the detection of asymptomatic Chlamydia trachomatis infections. Since transport time could have an adverse effect on the sensitivity of C. trachomatis detection by PCR, the influence of DNA degradation on amplification was monitored over the course of 1 week. Therefore, urine specimens were aliquoted on the day of collection or arrival. Two groups of urine specimens were investigated. Group I contains first-void C. trachomatis-positive and -negative urine samples. DNA degradation was monitored in group I samples for 7 days at room temperature (RT) and at 4°C by amplifying different lengths of the human β-globin gene and the C. trachomatis plasmid target. DNA degradation was observed only for the larger human β-globin fragments at days 5 to 7 at RT. In contrast, at 4°C all targets could be amplified. Urine specimens were also frozen and thawed before aliquoting to mimic freezing during transport. This resulted in a lower sensitivity for the detection of C. trachomatis after thawing and 3 to 4 days at RT. In addition, mailed, home-obtained C. trachomatis-positive urine specimens (group II) were analyzed for 7 days after arrival by two commercially available C. trachomatis detection systems (PCR and ligase chain reaction [LCR]). The C. trachomatis plasmid target in mailed, home-obtained urine specimens could be amplified by both PCR and LCR after 1 week of storage and/or transport at RT. In conclusion, our findings indicate that mailed, home-obtained urine specimens are suitable for the sensitive detection of asymptomatic C. trachomatis infections by amplification methods, even if the transport time is up to 1 week at RT. These findings support the feasibility and validity of screening programs based on mailed, home-obtained urine specimens. Larger studies should be initiated to confirm our results.
Chlamydia trachomatis infection is a leading cause of sexually transmitted disease. Severe sequelae such as pelvic inflammatory disease, ectopic pregnancy, and tubal infertility can develop. Since many of these C. trachomatis infections run an asymptomatic course, there are often no clinical signs or symptoms, and no intervention for the prevention of these severe sequelae is possible. To have an impact on the Chlamydia reservoir, identification and treatment of these asymptomatic carriers are essential. However, the routine use of cervical and urethral swabs in screening programs will hamper high participation rates for asymptomatic, infected women and men. Recently, commercially available DNA amplification systems like the Amplicor system (PCR; Hoffmann-La Roche [9]) and the ligase chain reaction (LCR; LCx, Abbott Diagnostic Division [4]) and the RNA amplification system AMP-CT (Gen-Probe [15]) were introduced and can successfully be used for the detection of C. trachomatis in urine specimens. The use of urine specimens, which can be obtained by a noninvasive technique, will result in a higher sensitivity compared with that of cell culture of specimens from the urethra and a slightly lower sensitivity compared with that of detection by amplification techniques with cervical scrapings from symptomatic, infected women (2, 6, 12, 18). Furthermore, the use of urine specimens saves time and money for both the participants of screening programs and the physicians of gynecology departments or general practitioners. The use of mailed, home-obtained urine specimens may have further important implications for the feasibility and cost of screening programs for young asymptomatic women and men. However, the use of mailed urine specimens could result in lower sensitivities of the different Chlamydia assays due to DNA degradation during the time of nonrefrigerated mail transport, which mostly takes several days. As shown by Østergaard et al. (14), a lower sensitivity of tests with home-obtained urine specimens was reported, but no analysis of nucleic acid stability during transport was performed.
Therefore, the effect of DNA degradation in urine specimens was investigated over the course of 1 week by monitoring the PCR amplification of different lengths of the human β-globin gene and the chlamydial plasmid target (group I). For this purpose, pure DNA was isolated from urine specimens (group I) to generate an inhibition-free background for amplification. Furthermore, the results that were obtained were used to investigate the suitability of the use of mailed, home-obtained urine specimens (group II) for the detection of asymptomatic C. trachomatis infections by commercially available amplification methods (LCR [LCx; Abbott] and PCR [Amplicor; Hoffmann-La Roche]) over the course of 1 week.
MATERIALS AND METHODS
Clinical specimens. (i) Group I specimens for monitoring DNA degradation.
First-void urine specimens were obtained from 30 patients (10 were C. trachomatis negative and 20 were C. trachomatis positive) (group I specimens). C. trachomatis-positive urine specimens (as assessed with the Amplicor and LCx systems were obtained during a screening program with asymptomatic women in Amsterdam, The Netherlands. The specimens were obtained before treatment (which was delivered at a second visit) and were directly transported for analysis.
(ii) Group II specimens for monitoring mailed, home-obtained urine specimens.
C. trachomatis-positive first-void urine specimens (group II specimens; n = 21 [from 13 females and 8 males]) were derived from patients who participated in a screening program for asymptomatic C. trachomatis infections.
Monitoring DNA degradation in group I specimens.
Ten C. trachomatis-negative and 10 C. trachomatis-positive urine specimens were aliquoted into two series of seven 1.5-ml batches of urine on the day of collection. One series of seven aliquots was kept at room temperature and the other was kept at 4°C. The first sample (day 1) of each series was pelleted (10 min, 14,000 rpm), and DNA was isolated (as described previously [13]) to create an inhibition-free background for PCR purposes. Each day from days 2 to 7 one sample of each series was pelleted and the DNA was isolated. Furthermore, from a third group consisting of 10 C. trachomatis-positive urine specimens, a series was frozen at −20°C and was then thawed to mimic freezing during transport. The specimens were subsequently aliquoted and handled as described above. To check for DNA degradation, human β-globin and C. trachomatis PCRs were performed.
Monitoring mailed, home-obtained urine specimens (group II specimens).
To obtain C. trachomatis-positive urine specimens, 700 asymptomatic persons were screened in Amsterdam. Twenty-one urine specimens were positive for C. trachomatis as determined by the LCx assay (Abbott) and the Amplicor PCR (Hoffmann-La Roche). After arrival at the laboratory, the urine specimens were vortexed, aliquoted, and stored at room temperature. For the LCx assay and the Amplicor PCR, seven aliquots of 1 and 0.5 ml of urine, respectively, were stored. On each day for 7 days one specimen was pretreated according to each of the manufacturers’ instructions and was stored at −20°C pending analysis.
Briefly, for the LCx assay 1 ml of mixed urine was centrifuged at 14,000 rpm (Eppendorf centrifuge model 5415C; Merck) for 15 min. The pellet was resuspended in 1 ml of the LCx urine specimen resuspension buffer, and the mixture was incubated for 15 min at 97°C. A total of 100 μl of the processed urine specimen was used in the LCx assay. For the Amplicor PCR, 0.5 ml of urine was mixed with 0.5 ml of urine wash buffer, and the mixture was incubated at 37°C for 15 min and subsequently centrifuged at 14,000 rpm for 5 min. The pellet was resuspended in 250 μl of lysis buffer, and the mixture was incubated for 15 min at room temperature. After 15 min, 250 μl of specimen diluent was added and the tubes were centrifuged at 14,000 rpm for 10 min. A total of 50 μl of the processed urine specimen was used for the Amplicor assay.
PCR. (i) β-Globin.
To check for DNA degradation, human β-globin PCRs were performed with primers BGPCO3.1 (5′-ACACAACTGTGTTCACTAGC-3′; sense) and BGPCO5 (5′-GAAACCCAAGAGTCTTCTCT-3′; antisense), BGPCO6 (5′-CATCAGGAGTGGACAGATCC-3′; antisense), and BGPCO7 (5′-GAAAACATCAA-GGGTCCCAT-3′; antisense), which generated amplimers of 209, 326, or 509 bp, respectively (3, 10). The amplified DNA was analyzed by 1.5% agarose gel electrophoresis and ethidium bromide staining.
(ii) C. trachomatis.
The Chlamydia plasmid target (155 bp) was amplified with primers pI6.1 (5′-AGAGTACATCGGTCAACGA-3′; antisense) and pI6.2. (5′-TCACAGCGGTTGCTCGAAGCA-3′; sense). PCR was performed as described previously (13). The amplified DNA was analyzed by 1.5% agarose gel electrophoresis and ethidium bromide staining.
Commercial assays.
Both the LCx (Abbott) and the Amplicor (Hoffmann-La Roche) assays were performed as described by the manufacturers for the detection of C. trachomatis DNA. Furthermore, since no DNA degradation could be monitored, the optional internal control which is coamplified with target nucleic acid detection in the Amplicor assay was monitored for the evaluation of test performance.
RESULTS
Monitoring DNA degradation.
Aliquots of 1.5 ml from group I first-void urine specimens, which were transported directly on the day of collection, were stored for 7 days. On each day, DNA from a 1.5-ml aliquot of urine was isolated. DNA degradation was monitored by amplification of different lengths of the β-globin gene.
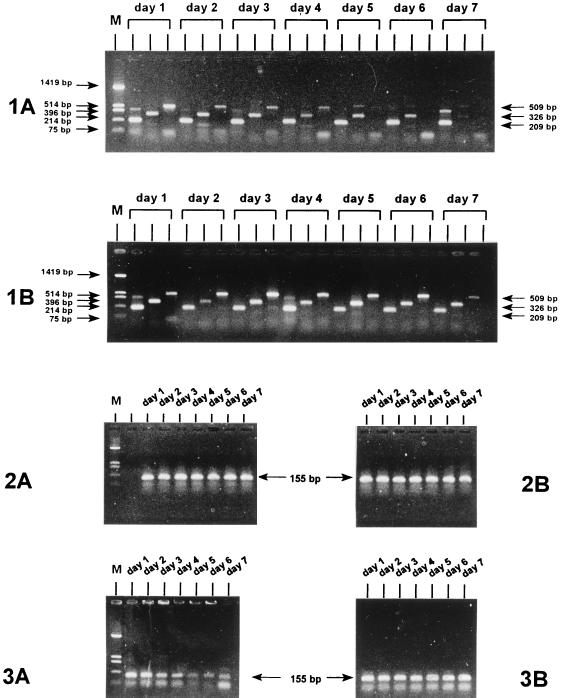
β-Globin PCR results for a representative C. trachomatis-negative urine specimen are shown in Fig. 1 (panels 1A and 1B). When the specimens were stored at room temperature (Fig. 1, panel 1A), the 209-bp β-globin fragment could be amplified for up to 7 days. The 509-bp fragment was no longer amplified at days 5 to 7 (only 3 of 10 samples were very weakly positive on day 5, as shown in Fig. 1, panel 1A), and the 326-bp fragment was almost invisible at the gel level by day 7. In contrast, when the specimens were stored at 4°C (Fig. 1, panel 1B), even at day 7 the 326- and 509-bp fragments could still be amplified. However, it appears that at day 7 the 509-bp fragment is beginning to fade, possibly indicating the start of degradation, whereas the smaller fragments did not fade.
FIG. 1.
Monitoring of DNA degradation in urine specimens (group I) by PCR for 7 days. (1A and 1B) Results of PCR for the detection of the human β-globin gene in a urine specimen stored at room temperature and 4°C, respectively. (2A and 2B) Results of PCR for the detection of the C. trachomatis plasmid target in a C. trachomatis-positive urine specimen stored at room temperature and 4°C, respectively. (3A and 3B). Results of PCR for the detection of the C. trachomatis plasmid target in a C. trachomatis-positive urine specimen which was initially frozen and thawed and subsequently stored at room temperature and 4°C, respectively. The marker (lanes M) is pUC 19 HinfI. The different amplimer lengths are indicated with arrows.
Plasmid PCR results for a representative C. trachomatis-positive urine specimen are shown in Fig. 1 (panels 2A and 2B). The C. trachomatis plasmid DNA was successfully amplified even after storage for 7 days not only at 4°C (Fig. 1, panel 2B) but also at room temperature (Fig. 1, panel 2A). The human β-globin PCR results for C. trachomatis-positive urine specimens were equal to the results obtained for the C. trachomatis-negative urine specimens.
Plasmid PCR results for a representative C. trachomatis-positive urine specimen which was initially frozen at −20°C are shown in Fig. 1 (panels 3A and 3B). At 4°C (Fig. 1, panel 3B) the C. trachomatis plasmid target was successfully amplified after up to 1 week of storage. On the other hand, after storage at room temperature (Fig. 1, panel 3A) after 3 to 4 days, a decrease in the sensitivity was seen, as indicated by a weaker amplimer signal after gel electrophoresis. Also, the β-globin PCR product of 209 bp amplified less efficiently at days 3 to 7, and longer fragments could not be amplified.
Monitoring mailed, home-obtained urine specimens.
The mailed, home-obtained C. trachomatis-positive urine specimens (n = 21) in group II were monitored daily for the amplification of the Chlamydia plasmid target by the LCx (Abbott) and Amplicor (Hoffmann-La Roche) assays for 1 week after arrival at the laboratory. The mean transport time for these urine specimens was 3.7 days (range, 1 to 6 days). The results are shown in Table 1. To visualize the degree of positivity without using the optical density values, strongly positive and weakly positive results were determined, as indicated in Table 1. Up to 1 week after arrival at the laboratory, all but one of the urine specimens were positive for C. trachomatis by both assays. The exception was sample 9, for which the optical density was elevated (but below the cutoff) by the LCx assay on day 7. Among the 21 urine specimens, 5 urine specimens (specimens 3, 9, 12, 19, and 20) were not strongly positive but had weakly positive signals most of the time. Furthermore, even after 7 days at room temperature most samples were still positive for C. trachomatis. For two samples, transport to the laboratory and storage at room temperature even took 12 days, and strongly positive signals were still generated by the commercially assays. Since no DNA degradation could be monitored, the internal control of the Amplicor assay was used to monitor whether inhibition arose during storage at room temperature. After transport and subsequent storage for 7 days at room temperature, no inhibition of amplification occurred.
TABLE 1.
C. trachomatis detection by Amplicor and LCx assays in mailed, home-obtained urine specimens during 7 days at room temperature after arrival
| Specimen no. | Testa | Result on the following dayb:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | + | − | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 2 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 3 | A | + | + | + | + | + | − | + | |||||
| L | ⊕ | + | + | + | e | + | + | ||||||
| 4 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 5 | A | ⊕ | ⊕ | + | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 6 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 7 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 8 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 9 | A | + | + | + | + | + | + | − | |||||
| L | + | + | e | + | ⊕ | e | − | ||||||
| 10 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 11 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 12 | A | + | + | + | + | + | + | + | |||||
| L | ⊕ | ⊕ | + | + | ⊕ | ⊕ | + | ||||||
| 13 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | + | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 14 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 15 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 16 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 17 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 18 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 19 | A | + | + | + | + | + | + | + | |||||
| L | + | + | + | e | − | e | − | ||||||
| 20 | A | ⊕ | + | + | + | + | + | + | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
| 21 | A | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | |||||
| L | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ||||||
A, Amplicor; L, LCx.
For the Amplicor assay, ⊕, strongly positive (9.999); +, weakly positive (between 9.999 and the cutoff); −, negative. For the LCx assay, ⊕, strongly positive (>1,000); +, weakly positive (between 1,000 and the cutoff); e, elevated (negative but >200); −, negative (control ± 18). Day 0 is the day of urine sampling. The numbers represent the number of days after urine sampling from which the monitoring started.
DISCUSSION
This study strongly suggests that mailed, home-obtained urine specimens are suitable for the sensitive detection of asymptomatic C. trachomatis infections by amplification methods (LCx and Amplicor assays) even if the transport time is up to 1 week at room temperature. In all 21 mailed, home-obtained, C. trachomatis-positive urine specimens (group II), the Chlamydia plasmid target could be successfully amplified even after a week at room temperature. These findings have the potential to change the feasibility of screening programs for the detection of asymptomatic C. trachomatis infection.
Pure DNA was isolated from the group I specimens for the monitoring of DNA degradation and for C. trachomatis detection for 1 week to investigate if whether C. trachomatis DNA could still be detected reliably. As shown for this group of specimens, small DNA fragments could still be amplified from DNA isolated from nonfrozen urine specimens after 1 week of storage at room temperature. This was shown by the detection of the β-globin PCR fragment of 209 bp and the detection of the C. trachomatis plasmid PCR fragment of 155 bp. This explains the successful amplification of the C. trachomatis plasmid target by the commercially available assays, which generated by the Amplicor PCR a 207-bp fragment (9) and by the Abbott LCx assay a 145-bp fragment (4). Furthermore, Chlamydia elementary bodies are more resistant to DNase activity than the human β-globin target, as shown previously (11), indicating an enhanced stability of the Chlamydia target in comparison to that of the human β-globin target.
Degradation of the DNA in the group I urine specimens was shown after 5 days by the inability to amplify the largest β-globin fragment tested (509 bp). With storage of the specimens at 4°C the larger β-globin fragments could be amplified up to day 7. This can be explained by the fact that DNase activity is substantially higher at room temperature than at 4°C. When the urine specimens were frozen during transport, a reduction in sensitivity was seen only when the specimens were subsequently stored at room temperature for several days, but no reduction in sensitivity was observed during subsequent storage at 4°C. This is probably due to the inhibition of the cellular DNase which is derived from the freezing and thawing of the cells in the urine samples. Since commercial assays were used for the group II specimens, no β-globin PCRs could be performed with the commerical buffer sample solution to check the possibility that inhibitory factors could emerge during storage at room temperature. However, this was not the case, as shown by the generation of a positive result for the internal standard which is incorporated in the Amplicor assay.
With the group II specimens it was shown that even after 7 days at room temperature most samples were still positive for C. trachomatis. Weakly positive samples might be missed when mailed, home-obtained urine specimens are tested. However, this is unlikely, since five urine specimens which were weakly positive remained positive for 1 week. Furthermore, a dilution series (10-, 100-, and 1,000-fold dilutions) was made from two samples that arrived at the laboratory on the day of collection, and C. trachomatis detection was performed for 1 week (data not shown). Both samples diluted 100-fold were still positive after 1 week. These results strongly indicate that weakly positive signals for this asymptomatically infected population will not be missed in a screening based on mailed, home-obtained urine specimens.
The use of specimens collected off-site and transport to the laboratory for the detection of sexually transmitted diseases has been described previously, but endocervical specimens (8) and tampon-collected specimens (20) were used. Recently, a study with mailed, home-obtained specimens for the detection of C. trachomatis showed that C. trachomatis infections could be detected as effectively with all urogenital specimens excluding urine specimens as with samples collected by general practitioners (16). In that study, by Østergaard et al. (16), a lower sensitivity of the LCR assay used for the mailed, home-obtained urine specimens was reported. Nonrefrigerated mailing conditions were given as a possible explanation. However, the urine specimens were obtained in Denmark from 15 January 1995 to 15 February 1996, a period probably comprising winter conditions with temperatures below 0°C. Our findings suggest that if some of these urine specimens transported under nonrefrigerated conditions were frozen and thawed, the lower sensitivity was possibly due to freezing and thawing rather than the nonrefrigerated transport conditions, since the latter should not result in a lower sensitivity, as shown in the present study. Since freezing and thawing of urine samples followed by storage at room temperature can result in a lower sensitivity, the use of mailed, home-obtained urine specimens during winter periods should be well controlled with regard to the transport time.
Mailed, home-obtained urine specimens were evaluated in a setting for the implementation of screening in The Netherlands. For some other countries with higher temperatures the influence of transport temperatures should be evaluated again. However, it is unlikely that different results will be obtained from such a study since, as mentioned before, the C. trachomatis elementary body is quite resistant to DNase activity.
The performances of the AMP-CT (Gen-Probe [15]) RNA amplification system and nucleic acid sequence-based amplification (NASBA) assay (13) with mailed, home-obtained urine specimens were not evaluated. Since, besides reticulate bodies, elementary bodies also contain copies of RNA, it should be possible to amplify RNA. Using the NASBA RNA amplification system (13), we have shown that it is possible to detect C. trachomatis RNA after arrival at the laboratory (unpublished data). Furthermore, Østergaard et al. (17) have shown that C. trachomatis RNA could be detected in mailed, home-obtained urine specimens by the AMP-CT RNA amplification assay. However, no data with respect to transport times and conditions for RNA detection are yet available.
The effects of programs for selective screening for C. trachomatis infection have clearly been demonstrated in the United States and Scandinavia (7, 19). Genç and Mårdh (4) reported that screening by DNA amplification assays, combined with treatment of positive patients with azithromycin and partner notification, is the most cost-effective screening strategy for women. Although cost-benefit analyses are needed (5, 14, 16), the reliable use of mailed, home-obtained urine specimens could have important implications for the choice of the most beneficial screening strategy for the detection of asymptomatic C. trachomatis infections. Implications of home sampling have been studied for home sampling versus conventional contact tracing for the detection of C. trachomatis infection (1) and is being evaluated with large groups of asymptomatic women and men in the region of Amsterdam.
This study has been conducted in a research setting, but if actual screenings for C. trachomatis infections are to be initiated with mailed, home-obtained urine specimens, larger studies should be initiated to confirm the preliminary results obtained in this study. Although our data for group II specimens strongly indicate that these assays can reliably be used for screening for the detection of C. trachomatis infections, companies should evaluate their commercially available assays for this purpose. A study that uses a parallel submission should be designed; that is, half of the sample would be tested immediately after collection and the other half would be held and/or mailed. The study should be done in such a way that a statistical comparison of the results could be performed.
In conclusion, home-obtained urine specimens are suitable for use for the sensitive detection of asymptomatic C. trachomatis infections by commercially available amplification methods (LCx and Amplicor assays), even if the transport time is up to 1 week at room temperature. These results might have important implications for the feasibility of widespread surveys for the detection of chlamydial disease, but further investigations with larger study groups should be performed to confirm the results obtained in the present study.
ACKNOWLEDGMENTS
This work was partly supported by grants 28-2588 and 28-1182-1 from the Prevention Fund of The Netherlands.
We thank T. Klop and H. Los for technical assistance.
REFERENCES
- 1.Andersen B A, Østergaard L, Møller J K, Olesen F. Home sampling versus conventional tracing for detecting Chlamydia trachomatis infection in male partners of infected women: randomised study. Br Med J. 1998;316:350–351. doi: 10.1136/bmj.316.7128.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crotchfelt K A, Pare B, Gaydos C, Quinn T C. Detection of Chlamydia trachomatis by the Gen-Probe AMPLIFIED Chlamydia Trachomatis Assay (AMP-CT) in urine specimens from men and women and endocervical specimens from women. J Clin Microbiol. 1998;36:391–394. doi: 10.1128/jcm.36.2.391-394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Roda-Husman A M, Snijders P J F, Stel H V, van den Brule A J C, Meijer C J L M, Walboomers J M M. Processing of long-stored archival cervical smears for human papillomavirus detection by the polymerase chain reaction. Br J Cancer. 1995;72:412–417. doi: 10.1038/bjc.1995.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dille J B, Butzen C C, Birkenmeijer L G. Amplification of Chlamydia trachomatis DNA by ligase chain reaction. J Clin Microbiol. 1993;31:729–731. doi: 10.1128/jcm.31.3.729-731.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genç M, Mårdh P A. A cost-effectiveness analysis of screening and treatment for Chlamydia trachomatis infection in asymptomatic women. Ann Intern Med. 1996;124:1–7. doi: 10.7326/0003-4819-124-1_part_1-199601010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Goessens W H F, Mouton J W, Van der Meijden W J, Deelen S, Van Rijsoort-Vos T H, Lemmens-De Toom N, Verbrugh H A, Verkooyen R P. Comparison of three commercially available amplification assays, AMP CT, LCx, and COBAS AMPLICOR, for detection of Chlamydia trachomatis infection in first-void urine. J Clin Microbiol. 1997;35:2628–2633. doi: 10.1128/jcm.35.10.2628-2633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrmann B, Egger M. Genital Chlamydia trachomatis infections in Uppsala County, Sweden, 1985–1993—declining rates for how much longer. Sex Transm Dis. 1995;22:253–260. doi: 10.1097/00007435-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Iwen P C, Walker R A, Warren K L, Kelly D M, Linder J, Hinrichs S H. Effect of off-site transport on detection of Neisseria gonorrhoeae in endovervical specimens. Arch Pathol Lab Med. 1996;120:1019–1022. [PubMed] [Google Scholar]
- 9.Jaschek G, Gaydos C A, Welsh L E, Quinn T C. Direct detection of Chlamydia trachomatis in urine specimens from symptomatic and asymptomatic men by using a rapid polymerase chain reaction assay. J Clin Microbiol. 1993;31:1209–1212. doi: 10.1128/jcm.31.5.1209-1212.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan J, Van den Brule A J C, Hemrika D J, Risse E K J, Walboomers J M M, Schipper M E I, Meijer C J L M. Chlamydia trachomatis and ectopic pregnancy: retrospective analysis of salpingectomy specimens, endometrial biopsies and cervical smears. J Clin Pathol. 1995;48:815–819. doi: 10.1136/jcp.48.9.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan J, Meijer C J L M, Van den Hoek A, Ossewaarde J M, Walboomers J M M, Van den Brule A J C. Genotyping of Chlamydia trachomatis serovars derived from heterosexual partners and a detailed genomic DNA analysis of serovar F. Genitourin Med. 1995;71:299–303. doi: 10.1136/sti.71.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H H, Chernesky M A, Schachter J, Burczak J D, Andrews W W, Muldoon S, Gregor L, Stamm W E. Diagnosis of Chlamydia trachomatis genitourinary infection in women by ligase chain reaction assay of urine. Lancet. 1995;345:213–2167. doi: 10.1016/s0140-6736(95)90221-x. [DOI] [PubMed] [Google Scholar]
- 13.Morré S A, Sillekens P, Jacobs M V, Van Aarle P, De Blok S, Van Gemen B, Walboomers J M M, Meijer C J L M, Van den Brule A J C. RNA amplification by nucleic acid sequence-based amplification with an internal standard enables reliable detection of Chlamydia trachomatis in cervical scrapings and urine samples. J Clin Microbiol. 1996;34:3108–3114. doi: 10.1128/jcm.34.12.3108-3114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosure D J, Berman S, Fine D, Delisle S, Cates W, Jr, Boring J R., III Genital Chlamydia infections in sexually active adolescents: do we really need to screen everyone? J Adolesc Health. 1997;20:6–13. doi: 10.1016/S1054-139X(96)00157-7. [DOI] [PubMed] [Google Scholar]
- 15.Mouton J W, Verkooyen R, Vandermeijden W I, Vanrijsoortvos T H, Goessens W H F, Kluytmans J A J W, Deelen S D A, Luijendijk A, Verbrugh H A. Detection of Chlamydia trachomatis in male and female urine specimens by using the Amplified Chlamydia Trachomatis test. J Clin Microbiol. 1997;35:1369–1372. doi: 10.1128/jcm.35.6.1369-1372.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Østergaard L, Møller J K, Andersen B, Olesen F. Diagnosis of urogenital Chlamydia trachomatis infection in women based on mailed samples at home: multipractice comparative study. Br Med J. 1996;313:1186–1189. doi: 10.1136/bmj.313.7066.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Østergaard L, Andersen B A, Møller J K, Olesen F. Efficacy of home sampling for screening of Chlamydia trachomatis: randomised study. Br Med J. 1998;317:26–27. doi: 10.1136/bmj.317.7150.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasternack R, Vuorinen P, Pitkäjärvi T, Koskela M, Miettinen A. Comparison of Manual Amplicor PCR, Cobas Amplicor PCR, and LCx assays for the detection of Chlamydia trachomatis infection in women by using urine specimens. J Clin Microbiol. 1997;35:402–405. doi: 10.1128/jcm.35.2.402-405.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholes D, Stergachis A, Heidrich F E, Andrilla H, Holmes K K, Stamm W E. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infections. N Engl J Med. 1996;334:1362–1366. doi: 10.1056/NEJM199605233342103. [DOI] [PubMed] [Google Scholar]
- 20.Tabrizi S N, Paterson B, Fairly C K, Bowden F J, Garland S M. A self-administered technique for the detection of sexually transmitted diseases in remote communities. J Infect Dis. 1997;176:289–292. doi: 10.1086/517269. [DOI] [PubMed] [Google Scholar]