Abstract
Integrating wireless technology in medical devices has proved beneficial for both patients and caregivers. However, the use of shared, unlicensed spectrum bands by both medical and non-medical wireless devices has raised concerns about wireless coexistence. The challenge of incorporating wireless communication into a medical device is to ensure reasonable medical device effectiveness and patient safety. Consequently, work to develop a standardized process to assess wireless coexistence, primarily for wireless medical devices, was carried by Subcommittee 7 of American National Standards Institute (ANSI)-accredited standards committee (ASC) C63 and the Wireless Working Group (SM-WG06) of the Association for the Advancement of Medical Instrumentation (AAMI). Both groups have recently released their respective documents. In this article, we discuss practical aspects of wireless coexistence testing—in the realm of ANSI C63.27 and AAMI TIR69—to help answer basic, yet important, questions such as what to test, how to test, and how to present results.
2. Motivation
Wireless technology plays an important role in modern life and has been implemented for multiple applications. Licensed radio spectrum usage is the basis of many application specific services such as cellular (mobile) communication, television and radio broadcast, and medical implants communication systems (MICS) within the MedRadio spectrum. Shared radio spectrum use has seen an exponential growth in popularity due to the availability, maturity, and low cost of technologies that operate in unlicensed bands particularly the 2.4 GHz industrial, scientific, and medical (ISM) band. Economic and logistic forces, as well as the constant race for innovation, have motivated medical device manufacturers to equip their products with wireless interfaces running technologies like Wi-Fi, ZigBee, and Bluetooth. Evidence of the increasing growth in the integration of wireless technology includes information showing that in 2013 the wireless portable medical device market was valued at $7.52 billion with an expected growth to $17.71 billion in 2020 [1]. However, medical and non-medical devices contend for wireless channel access in a shared environment, which could result in degradation of communication performance and consequently affect the medical device function. As a result, concerns about wireless coexistence have been raised by medical device stakeholders and regulators [2, 3], especially for devices that can have higher risks associated with their wireless functions. Because of the lack of consensus published standards to assess wireless coexistence many of the evaluations are performed on an ad hoc basis.
Fortunately, key stakeholders, including medical device manufacturers, regulators, and user organizations recognized the need for standardized approaches to assess wireless coexistence and are working to publish consensus documents. Two groups have produced documents aimed to help perform standardized testing and assessment: Subcommittee 7 of the American National Standards Institute (ANSI)-accredited standards committee (ASC) C63® (designated C63.27, Standard for Evaluation of Wireless Coexistence [4]) and the Wireless Working Group (SM-WG06) of the Association for the Advancement of Medical Instrumentation (AAMI) TIR 69/Ed.1 [5]. The ANSI C63.27 details coexistence testing methodologies and reporting for medical and non-medical devices. The AAMI TIR69 addresses risk management (referenced to the ISO 14971 standard for medical device risk management [6]) and is focused on the integration of wireless technology in medical devices and systems. Both groups have recently released their respective documents.
Practical aspects of wireless coexistence testing are presented below to help answer important questions such as what to test, how to test, and how to present results. The discussion provides an overview of coexistence evaluation principles that can be helpful for medical device designers, test laboratories, and user facilities to understand and deal with the issues and potential risks.
3. Coexistence test methods
3.1. What to test?
Wireless coexistence depends on adequate spectrum resource sharing in terms of time, frequency, and power. Thus, in testing for coexistence the wireless functionality of a system-under-test (SUT) is evaluated relative to the timing, frequency allocation, and relative strength of the signals expected from other wireless products in the same vicinity transmitting data at the same time. The use of multiple wireless systems in proximity to the SUT leads to contention for radio spectrum access by interfering systems that share the same radio spectrum with the SUT. Typically, both the target wireless system (SUT) and the interfering system (IS) are comprised of one or more transmitter (Tx) and receiver (Rx) nodes. Successful performance of SUT wireless functionality requires a given minimum period of time for channel access (i.e., time-on-air or channel utilization [CU]) while maintaining a signal-to-interference-plus-noise ratio (SINR) higher than a pre-determined minimum to facilitate proper signal demodulation. Accordingly, an elevated IS utilization on the same channel could deprive the target SUT from channel access and result in a failure of the SUT wireless function. Testing the wireless system can help identify the IS CU threshold at which SUT can coexist with the IS. This can be accomplished by configuring the IS to operate on maximum throughput (i.e., maximum CU). During the testing process, if the SUT performance degrades and performance becomes unacceptable the IS throughput can be decreased and the test repeated until the IS CU threshold allows acceptable performance to achieve coexistence.
As with the issue of the timing of the interfering wireless signals, spatial configuration and orientation can affect the ability to coexist and still provide adequate functionality. In assessing the spatial aspects it must be kept in mind that the received power level at a Rx node’s antenna is inversely proportional to the separation distance from the transmitting node meaning the closer the Tx is located to the Rx the higher the received signals are. To evaluate this, the separation distance between SUT and IS can be decreased to identify a minimum distance for successful and acceptable SUT wireless operation and medical device function. Alternatively, IS transmission power (i.e., interference level in SINR) could be varied to establish an acceptable ratio of SUT/IS signal levels for successful SUT wireless functionality.
When the SUT operates on a static channel such as typical Wi-Fi or ZigBee, the effect of IS frequency allocation is evaluated by performing the tests using the IS operating on the same channel (co-channel) or on adjacent channels relative to SUT [7]. However, when the SUT employs a frequency hopping scheme (e.g., Bluetooth), the IS should be set to operate on one or more channels that overlap SUT usage to tax the capabilities of the SUT. In the increasingly crowded 2.4 GHz ISM band Wi-Fi has been suggested as one of the more severe sources for interference for co- or adjacent channel interference. The non-overlapping channels 1, 6, 11 are commonly used for Wi-Fi [8, 9]. The shortcoming of using an IS only operating on one Wi-Fi channel (e.g., 1, 6, or 11) is that it can only effectively block one third of the 2.4 GHz ISM band and thus leaving two thirds of the band for Bluetooth to use. A more rigorous level of evaluation could be achieved by setting IS to simultaneously operate on Wi-Fi channels 1, 6, and 11 [10], which emulates three in-band interfering networks. A signal generator or an actual network implementation could be used to emulate IS. However, due to lack of channel sensing ability, testing results could differ when the IS is based on a signal generator instead of using an actual network [11].
For medical device systems, risk management is paramount. Thus, the evaluation for wireless coexistence stems from the potential hazards and related harms to the patient or user that determine the risks associated with the device system under investigation. In the AAMI TIR69 process the coexistence assessment and testing derive from risk level of the investigated wireless medical device function. Depending on the determined risk level, and other considerations such as the needs of the healthcare facility, the intensity and level of testing vary: higher risk devices call for more thorough testing. In the AAMI TIR process very low risk devices with wireless functions that have no impact on the patient or user safety or device effectiveness, such as billing, might be determined to need no coexistence testing. However, with the potential to integrate multiple wireless technologies into the medical device system for multiple purposes a thorough assessment must encompass all of the functions implemented wirelessly.
3.2. How to test?
Several coexistence test methods have been proposed in the literature and are considered in the ANSI C63.27 standard. Young et al. [12] reviewed these methods and discussed corresponding coexistence factors. Based on the medium (e.g., through the air or wired connection) used to establish a communication channel between IS and SUT nodes, test methodologies can be divided into conducted and radiated methods. Contributing factors to selecting a test method include the availability of properly equipped laboratories and the commitment to evaluate realistic SUT deployments. Before testing begins the assessment parameters must be determined along with the criteria for determining pass/fail. Quantitative measures and metrics such as bit error rate, latency (time delay), and throughput should be used to determine acceptable results. For medical device systems the results should be directly related to the medical function that is implemented via the wireless technology. This should be adjusted for the risk level associated with the functions of the device under test; where higher risks call for tighter bounds on the acceptability of the criteria.
3.2.1. Conducted testing
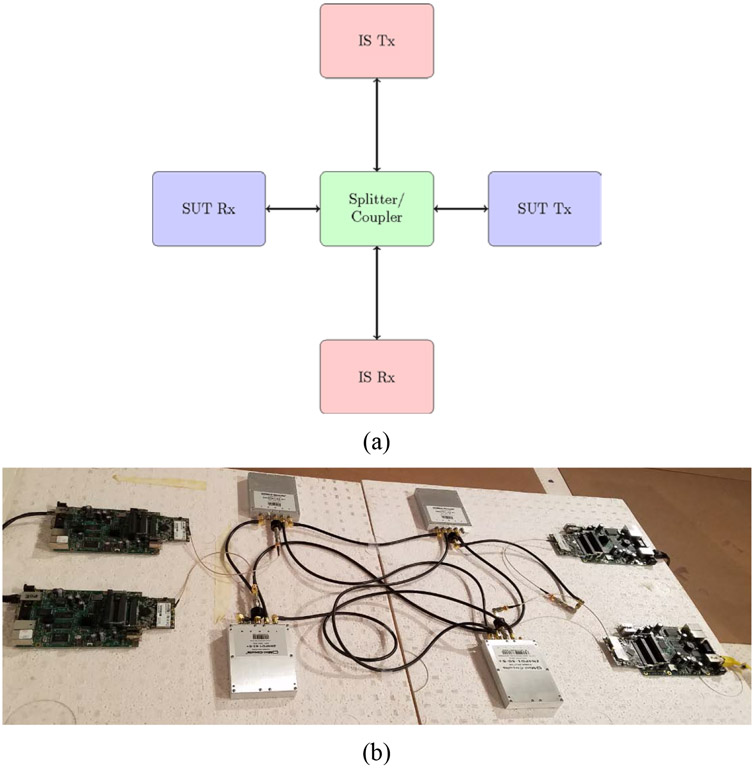
For conducted testing, a communication channel between IS and SUT nodes is established through a wired system using coaxial cables, couplers/splitters, and attenuators (See Figure 1(a) wherein arrows represent coaxial cables and Figure 1(b) for an actual deployment). Monitoring equipment is introduced to the channel to detect and identify the limits of coexistence variables. Attenuators are used to emulate the path loss experienced by propagating wireless signals in realistic environments. Conducted testing allows easy control of test variables, which yields highly repeatable results. Manzi et al. [13] used conducted testing to evaluate coexistence of ultra-wide band (UWB) radios and Wi-Fi. However, this method requires physical access to all antennae ports on the IS and SUT nodes, which is not always possible because the antennae are often embedded and inaccessible. Additionally, path loss estimation is required to emulate a given intended SUT deployment environment, which can be a challenge even with the availability of a network analyzer.
Figure 1:
Wireless coexistence testing using a conducted test method. (a) Setup diagram. (b) Lab deployment.
3.2.2. Radiated testing
Compared to the conducted test method radiated testing allows for more realistic signal propagation and alleviates the need for direct access to a wireless node’s antenna port. The following scenarios introduce two variations of radiated testing.
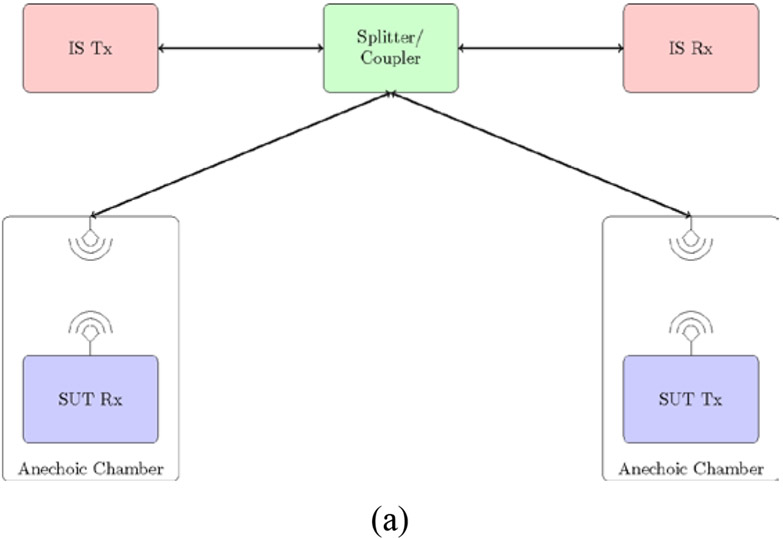
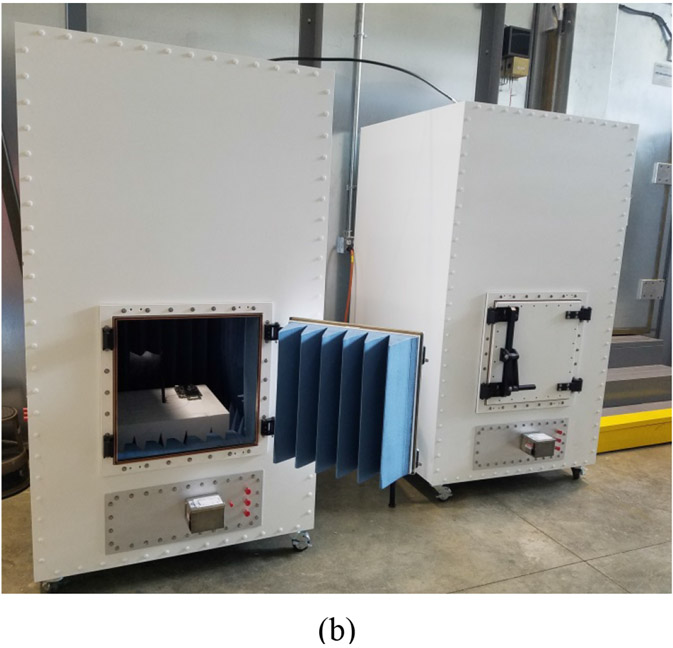
Two Anechoic Chambers: In this scenario, each SUT node is placed in an anechoic chamber equipped with an antenna to capture the transmitting node’s propagating signal and feed it through a coaxial cable to the receiving node. Signals from IS node or nodes are introduced to the communication path through a splitter/coupler that connects both nodes and the anechoic chambers. Figure 2 illustrates this setup, using arrows to represent coaxial cables in Figure 2(a). Figure 2(b) shows two anechoic chambers that could be used for coexistence testing. Remley et al. [14], used a radiated two-chamber setup to verify the performance of wireless devices used by emergency responders. Eslami et al. used the same concept in [15]. Similar to the conducted test setup, path loss is estimated and implemented based on free-space path loss inside each chamber in addition to external attenuators. This setup requires access to two anechoic chambers, which could be costly and time consuming.
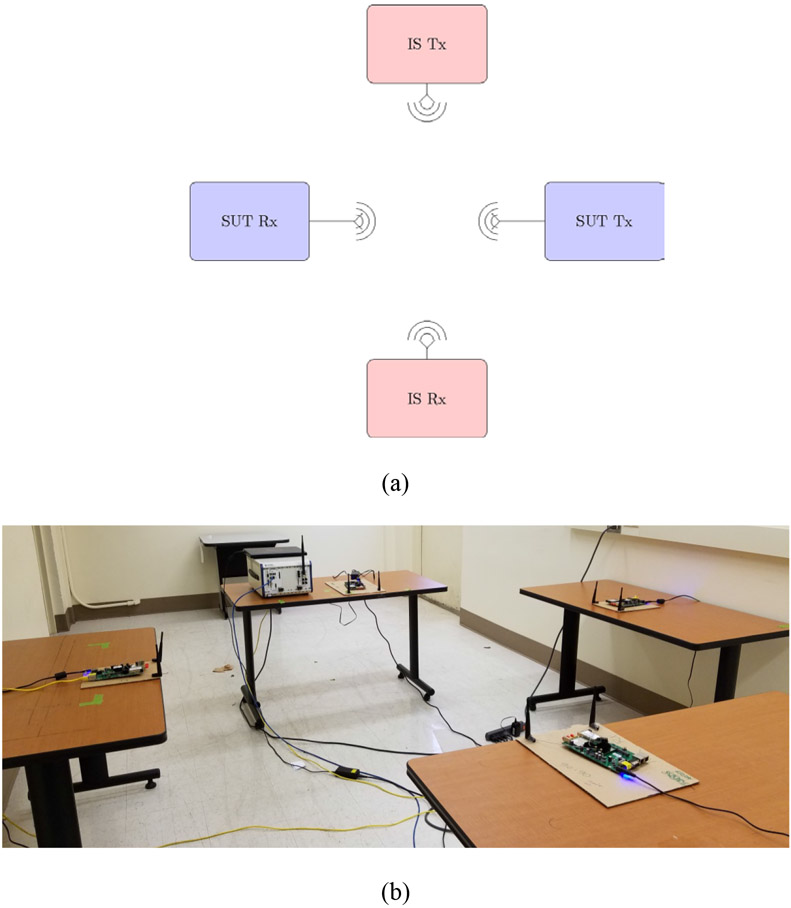
Open air test environment: In this scenario, the SUT and IS nodes are deployed in an indoor environment of sufficient size (e.g., room) that permits over the air signal propagation to occur similar to actual SUT deployment in its intended use environment (See Figure 3). For example, a wireless patient monitoring device operating in a hospital care ward. In this method the path loss is not calibrated. Instead, separation distance between nodes is controlled either directly or indirectly by configuring the Tx nodes’ transmission power. Exposed and hidden terminal scenarios can be tested using variations of line-of-sight (LOS) and non-line-of-sight (NLOS) exposure configurations. Ambient signals and the environmental RF noise floor are monitored during testing to ensure testing is not significantly influenced by unintended signals. Radiated open environment setup was used by LaSorte et al. in [16] to test ZigBee-based medical devices for wireless coexistence with 802.11g interferer. Given the lack of controlled communication path and environmental effects, testing outcomes based on this setup typically exhibit larger variance. For example, a 3 dB coefficient of variation of the measured interfering signal power was reported in [12]. Alternatively, testing could be performed in an anechoic chamber [10] where multipath caused by reflections in the environment can be controlled and effectively eliminated to allow for improved repeatability and reproducibility.
Figure 2:
Wireless coexistence testing via a radiated test method using two anechoic chambers connected by coax circuit. (a) Setup diagram. (b) Two anechoic chambers used for coexistence testing.
Figure 3:
Wireless coexistence testing using a radiated open environment test method. (a) Setup diagram. (b) Lab deployment of LOS configuration
3.3. How to present the results?
When the coexistence testing is performed and results recorded this information should be presented in an organized fashion. Both the C63.27 and the TIR69 contain specific instructions for what should be included in these reports and how the information should be presented. At a minimum, the coexistence test results should be organized in a report that includes: identification of the SUT and its technical specifications (e.g., wireless technology, wireless receiving sensitivity, system architecture) and the IS technical specifications (e.g., wireless technology and specifications, hardware implementation, firmware version, transmission power), and clear information about the test configuration (e.g. conducted, radiated, LOS/NLOS), test environment, testing specifications (e.g. cable length, path loss, separation distance), and observation parameters. The SUT pass/fail criteria must be clearly defined before testing and justified in relation to the specifications of SUT wireless technology and functionality.
In a larger view, coexistence testing addresses the mutual effect of SUT and IS located in the vicinity of each other. Thus, both the SUT and the IS should be monitored and recorded for analysis and presentation in the test report. As part of the consideration for the coexistence testing and report the observed parameters and metrics should be determined before testing, though these might need to be adjusted based on the testing situation and test findings. For example, this can be accomplished by observing and reporting the throughput and CU (or another performance metric) of the IS and SUT as a time series that spans the test period. This way inhibitive effect on coexisting networks could be detected if these are manifested and observable.
Part of the analysis of risk for medical devices includes the likelihood or probability of the harm/hazard. For wireless coexistence the probability of the conditions that might lead to the disruption of the desired wireless connection can be important in assessing the functionality of the device. For assessing the probability of coexistence a logistic regression could be leveraged to establish a formula of binary test outcome (i.e., pass or fail) as a function of test variables (e.g., IS throughput, IS transmission power). If testing outcome reveals more than two states then multinomial logistic regression could be used.
Additional information that can provide context to the assessment process is to incorporate wireless spectrum survey measurements from the intended SUT environment. Spectrum surveys can be performed following International Telecommunication Union (ITU) recommendations [17]. Such measurements, including a longer term spectrum survey of a hospital environment were performed and presented in Al Kalaa et al. [8, 18]. The outcome of these spectrum measurements is a statistical distribution of observed CU values in the investigated environment. With such information computer simulations using environment CU distribution and SUT coexistence testing regression models can provide insight about expected probability of SUT coexistence when deployed in its intended environment.
4. Summary
Concerns about wireless coexistence are growing with the increasing popularity and use of wireless technologies that operate in unlicensed radio spectrum bands, particularly the crowded 2.4 GHz ISM band. This article has presented a high level description of several practical aspects of wireless coexistence testing with reference to the AAMI TIR69 and ANSI C63.27 documents. The aim is to provide information about what wireless coexistence is, what and how testing can be approached and reported, and how this can help medical device designers and test engineers evaluate the performance of their devices in coexistence scenarios.
Acknowledgments
This work was supported in part by an appointment to the Research Participation Program at the Center for Devices and Radiological Health administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
5. Acronyms
- SUT
System-under-test
- IS
Interfering system
- Tx
Transmitter
- Rx
Receiver
- CU
Channel utilization
- SINR
signal-to-interference-plus-noise ratio
Footnotes
Disclaimer
The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
7 References
- [1].marketsandmarkets.com, "Wireless Devices Market for Medical by Technology (BT/BLE, Wi-Fi, ZigBee, ANT+), Component (Sensors, ICs, Processors), Application (Monitoring, Medical Therapeutics, Diagnosis, Fitness & Wellness), and Geography – Global Forecast to 2020," 2014. [Google Scholar]
- [2].U.S. Food and Drug Administration. (2013). Radio Frequency Wireless Technology in Medical Devices - Guidance for Industry and Food and Drug Administration Staff. Available:http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm077210.htm
- [3].Association for the Advancement of Medical Instrumentation (AAMI), "Healthcare Technology in a Wireless World: Priority Issues from the 2012 Wireless Workshop Convened by AAMI, ACCE, ASHE, and ECRI Institute," 2012, Available: http://s3.amazonaws.com/rdcms-aami/files/production/public/FileDownloads/Summits/2012_Wireless_Workshop_publication.pdf.
- [4].American National Standards Institute (ANSI) ASC C63, "C63.27 Standard for Evaluation of Wireless Coexistence," 2017.
- [5].Association for the Advancement of Medical Instrumentation (AAMI), "TIR69:2017 Risk Assessment of radio-frequency wireless coexistence for medical devices and systems," 2017.
- [6].The International Organization for Standardization, "ISO 14971:2007 Medical devices -- Application of risk management to medical devices," 2007.
- [7].LaSorte NJ, Seidman S, and Guag J, "Experimental Method for Evaluating Wireless Coexistence of Wi-Fi Medical Devices," Biomed Instrum Technol, vol. 50, no. s6, pp. 18–25, Sep 2016. [DOI] [PubMed] [Google Scholar]
- [8].Al Kalaa MO et al. , "Characterizing the 2.4 GHz Spectrum in a Hospital Environment: Modeling and Applicability to Coexistence Testing of Medical Devices," IEEE Transactions on Electromagnetic Compatibility, vol. 59, no. 1, pp. 58–66, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rajab SA, Balid W, and Refai HH, "Comprehensive study of spectrum occupancy for 802.11b/g/n homogeneous networks," presented at the 2015 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), 11-14 May 2015, 2015. [Google Scholar]
- [10].Seidman S and LaSorte N, "An experimental method for evaluating wireless coexistence of a Bluetooth medical device," IEEE Electromagnetic Compatibility Magazine, vol. 3, no. 3, pp. 49–54, 2014. [Google Scholar]
- [11].LaSorte NJ, Bloom D, Rajab S, and Refai HH, "Creating an automated and emulated 802.11g wireless interfering network for wireless coexistence testing," in 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), 2013, pp. 1022–1027. [Google Scholar]
- [12].Young WF, Coder JB, and Gonzalez LA, "A review of wireless coexistence test methodologies," presented at the 2015 IEEE Symposium on Electromagnetic Compatibility and Signal Integrity, 15-21 March 2015, 2015. [Google Scholar]
- [13].Manzi G, Feliziani M, Beeckman PA, and van Dijk N, "Coexistence Between Ultra-Wideband Radio and Narrow-Band Wireless LAN Communication Systems—Part II: EMI Evaluation," IEEE Transactions on Electromagnetic Compatibility, vol. 51, no. 2, pp. 382–390, 2009. [Google Scholar]
- [14].Remley KA and Young WF, "Test methods for RF-based electronic safety equipment: Part 2 — Development of laboratory-based tests," IEEE Electromagnetic Compatibility Magazine, vol. 2, no. 1, pp. 70–80, 2013. [Google Scholar]
- [15].Eslami M and Bassen HI, "A Compact Test System for Simulating Multipath Interference," Open Journal of Antennas and Propagation, vol. 02, no. 02, pp. 9–20, 2014. [Google Scholar]
- [16].LaSorte NJ, Rajab SA, and Refai HH, "Developing a reproducible non-line-of-sight experimental setup for testing wireless medical device coexistence utilizing ZigBee," IEEE Trans Biomed Eng, vol. 59, no. 11, pp. 3221–9, Nov 2012. [DOI] [PubMed] [Google Scholar]
- [17].ITU-R, "Spectrum occupancy measurements and evaluation," in "Report SM.2256-1(06/2016)," Available: http://www.itu.int/pub/R-REP-SM.2256-1-2016. [Google Scholar]
- [18].Al Kalaa MO, Butron G, Balid W, Refai HH, and LaSorte NJ, "Long term spectrum survey of the 2.4 GHz ISM band in multiple hospital environments," presented at the 2016 IEEE Wireless Communications and Networking Conference, 3-6 April 2016, 2016. [Google Scholar]