Abstract
Background
The etiology of Crohn’s disease remains unknown, nevertheless, it is apparent that inflammation is associated with an imbalance between proinflammatory and anti‐inflammatory cytokines produced within the intestinal mucosa. Crohn’s disease represents a state of dysregulated inflammation and drugs that can augment the anti‐inflammatory response have the potential to downregulate inflammation and thereby improve the disease. The efficacy of recombinant IL‐10 in Crohn's disease was first demonstrated in a pilot study. Since then other trials have evaluated its efficacy but the available evidence has not been systematically reviewed.
Objectives
To determine the efficacy and tolerability of recombinant human interleukin 10 (IL‐10) for induction of remission in Crohn's disease.
Search methods
A computer assisted search of the Cochrane Central Register of Controlled Trials and the Cochrane IBD/FBD Review Group Specialized Trials Register and the on‐line databases MEDLINE and EMBASE was performed to identify relevant publications up to September 2010. Reference lists were searched and the pharmaceutical industry and experts were contacted to identify additional studies.
Selection criteria
Randomized controlled trials comparing recombinant human interleukin 10 to a placebo or control therapy for the treatment of patients with active Crohn's disease were included.
Data collection and analysis
All publications identified by the search strategy were assessed independently by two authors, and relevant studies selected according to the inclusion criteria. The risk of bias of each included study was assessed independently by two authors. Data were analyzed using Review Manager (RevMan 5). A random effects model was used for pooling of data. All data were analyzed on an intention‐to‐treat basis. Heterogeneity among studies was assessed using the chi‐square test and the I2 statistic.
Main results
The risk of bias in the included studies was low. The overall quality of the evidence based on the GRADE approach was moderate. No statistically significant differences were found between interleukin 10 and placebo for complete remission (CDAI < 150 with a 100 point decrease in CDAI from baseline; RR=1.43; 95% CI 0.62 to 3.29; I2=40%) or clinical remission (CDAI < 150; RR=1.29; 95% CI 0.79 to 2.11; I2= 0%). Patients treated with interleukin 10 were significantly more likely to withdraw from the studies due to adverse events (RR=13.50; 95% CI 3.89 to 46.79; I2=0%).
Authors' conclusions
Interleukin 10 does not appear to provide any benefit for the treatment of active Crohn's disease. This systematic review shows that interleukin 10 does not increase the number of remissions (complete or clinical), but increases the rate of withdrawal due to adverse events relative to placebo. The quality of the evidence regarding the efficacy of IL‐10 is moderate and although further research may have an impact on point estimates of efficacy further randomized trials are unlikely to be undertaken.
Plain language summary
Interleukin 10 (IL‐10) for induction of remission in Crohn's disease
Crohn's disease is an inflammatory condition of unknown origin that can affect any portion of the gastrointestinal tract from the mouth to the anus. However, it most commonly involves the distal small bowel and/or the colon. Interleukin 10 does not appear to provide any benefit for the treatment of active Crohn's disease. This systematic review showed that this drug does not increase the number of remissions but increases the number of patients that withdrew from the included studies due to side effects. The methodological quality of the included studies was high. Further studies of this agent for the treatment of active Crohn's disease are unlikely to be undertaken.
Summary of findings
Summary of findings for the main comparison. IL‐10 compared to placebo for induction of remission in Crohn's disease.
| IL‐10 compared to placebo for induction of remission in Crohn's disease | ||||||
| Patient or population: patients with induction of remission in Crohn's disease Settings: Intervention: IL‐10 Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | IL‐10 | |||||
| Complete remission (CDAI<150 with a decrease in CDAI of at least 100 points from baseline) | Study population | RR 1.43 (0.62 to 3.29) | 470 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 137 per 1000 | 196 per 1000 (85 to 451) | |||||
| Medium risk population | ||||||
| 167 per 1000 | 239 per 1000 (104 to 549) | |||||
| Clinical remision (CDAI<150) | Study population | RR 1.29 (0.79 to 2.11) | 470 (3 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 147 per 1000 | 190 per 1000 (116 to 310) | |||||
| Medium risk population | ||||||
| 182 per 1000 | 235 per 1000 (144 to 384) | |||||
| Withdrawls due to adverse events | Study population | RR 13.5 (3.89 to 46.79) | 228 (2 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 22 per 1000 | 297 per 1000 (86 to 1000) | |||||
| Medium risk population | ||||||
| 15 per 1000 | 202 per 1000 (58 to 702) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Sparse data: only 79 events observed. Altough for this outcome we observed a moderate heterogenity (I square=40%), we decided to downgrade the quality of evidence due to imprecision. 2 Sparse data: only 93 events observed 3 Sparse data: only 44 events observed
Background
Crohn's disease is an inflammatory condition of unknown etiology that can affect any portion of the gastrointestinal tract from the mouth to the perianal area. However, it most commonly involves the distal small bowel and/or colon. Its transmural inflammatory nature coupled with the variability of organ distribution gives rise to a spectrum of clinical presentations, each of which has to be considered separately in deciding upon the proper therapeutic approach (Farrell 1989):
Mild to moderate Crohn's disease ‐ Ambulatory patients able to tolerate an oral diet without dehydration, toxicity, abdominal tenderness, mass, or obstruction;
Moderate to severe Crohn's disease ‐ Patients who have failed treatment for mild to moderate disease or patients with prominent symptoms such as fever, weight loss, abdominal pain and tenderness, intermittent nausea or vomiting, or anemia;
Severe‐fulminant disease ‐ Patients with persisting symptoms despite treatment with steroids or patients presenting with high fever, persistent vomiting, intestinal obstruction, rebound tenderness, cachexia, or an abscess; and
Remission ‐ Patients who are asymptomatic either spontaneously or after medical or surgical intervention. Patients requiring steroids to remain asymptomatic are not considered to be in remission.
The exact cause of Crohn’s disease remains unknown. Nevertheless it is apparent that inflammation is associated with an imbalance between proinflammatory and anti‐inflammatory cytokines produced within the intestinal mucosa. The intestinal mucosa persists in a state of controlled inflammation where proinflammatory cytokines (such as TNF‐alpha, IL‐1, IL‐6, IL‐8, and IL‐12 are counterbalanced by anti‐inflammatory cytokines (such as IL‐4, IL‐10, IL‐11 and IL‐13; Korzenik 2006).
Interleukin (IL)‐10 is a cytokine that has antiinflammatory and immunosuppressant properties. Endogenous expression of IL‐10 is increased in the inflamed mucosa from patients with inflammatory bowel disease (Autschbach 1998). It regulates mucosal inflammation by inhibiting T‐cell/macrophage activation and proinflammatory cytokine synthesis. IL‐10 acts to suppress inflammation resulting from both antigen‐specific and innate immune responses by reducing: monocyte HLA class‐II expression, T‐cell secretion of IL‐2 and interferon, activated monocyte production of IL‐1, IL‐6, IL‐8, TNF‐alpha, and granulocyte‐macrophage colony‐stimulating factor. IL‐10 also upregulates the production of IL‐1 receptor antagonist by monocytes (Van Deventer 1997, Fedorak 2000).
Medical therapy for Crohn's disease, using agents such as corticosteroids, immunosuppressives, and 5‐aminosalicylates often does not control the disease. Thus, newer approaches to therapy are being evaluated (Korzenik 2006).
Because Crohn’s disease represents a state of dysregulated inflammation, drugs that can augment the anti‐inflammatory response have the potential to downregulate inflammation and thereby improve the disease. Recombinant human interleukin 10 is identical to endogenous human interleukin 10 with the exception of a methionine residue at the amino terminus (Fedorak 2000, Schreiber 2000). Recombinant human interleukin 10 (Tenovil: Schering‐Plough Research Institute, Kenilworth, NJ) comes in a sterilised, lyophilised white powder form containing sodium citrate, sucrose and glycerine. Prior to use, the drug is reconstituted with sterile water to a clear colourless solution.
The potential benefit of recombinant IL‐10 in Crohn's disease was first demonstrated in a pilot study in which 46 patients with active, steroid‐resistant Crohn's disease were randomized to varying doses of IL‐10 or placebo administered once daily intravenously for seven consecutive days (Van Deventer 1997). Following treatment, the average score on the Crohn's disease activity index was lower among patients receiving active treatment (179 versus 226), although results were not statistically significant. Recent trials of cytokine and anticytokine therapies have demonstrated that manipulations of T‐cell and macrophage‐derived cytokines may provide effective treatment for Crohn’s disease.
Objectives
The primary objectives were to determine the efficacy and safety of recombinant human interleukin 10 (IL‐10) for induction of remission in Crohn's disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials comparing recombinant human interleukin 10 to placebo or a control therapy were considered for inclusion.
Types of participants
Patients of any age with active Crohn's disease (Crohn's disease activity index 'CDAI' >150) defined by conventional clinical, radiological and endoscopic criteria were considered for inclusion.
Types of interventions
Trials comparing recombinant human interleukin 10 versus placebo or an active control therapy were considered for inclusion.
Types of outcome measures
The primary outcome for the review was the rate of patients achieving remission as defined by the primary studies, and expressed as a percentage of the patients randomized (intention to treat analysis). For the purposes of this review, a complete remission was defined as a CDAI of less than or equal to 150 points, with a decrease of at least 100 points from baseline.
Secondary outcome measures included: changes in the mean CDAI, rate of patients achieving a clinical remission (CDAI of less than or equal to 150 points), adverse events, severe adverse events, serious adverse events and withdrawal because of adverse events.
Search methods for identification of studies
Electronic searches
A computer assisted search of the Cochrane Central Register of Controlled Trials (CENTRAL) and the Cochrane IBD/FBD Review Group Specialized Trials Register was performed. In addition the on‐line databases MEDLINE and EMBASE were searched up to September 2010. The medical subject heading (MeSH) terms "Crohn disease" or "inflammatory bowel disease" and "interleukin" were used to perform key word searches of each database.
Searching other resources
Manual searches of reference lists from potentially relevant papers were performed to identify additional studies. Abstracts from major gastroenterological meetings were searched to identify research submitted in abstract form only. Personal contacts and leaders in the field were contacted to identify other studies which may not be published. The manufacturer of rhIL‐10 (SCH 52000 or Tenovil, Schering Plough, Kelinworth, NJ, USA) was contacted for additional studies with no success.
Data collection and analysis
Selection of studies
All publications identified by the search strategy were assessed independently by two authors (FB and PAC), and relevant studies selected according to the inclusion criteria. Any disagreement among authors was resolved by consensus. When insufficient information was provided to determine the risk of bias from the original report authors were contacted to confirm the method of randomization and allocation concealment.
Data extraction and management
Two authors (FB and PAC) independently extracted data using a data extraction form. Any disagreement among authors was resolved by consensus.
Assessment of risk of bias in included studies
Two authors (FB and PAC) independently assessed the risk of bias of the included trials using the criteria described in the Cochrane Reviewer's Handbook (Higgins 2008). A third author (IS) checked the assessment for accuracy. The risk of bias of the included trials is detailed in the Characteristics of included studies tables.
The adequacy of the methods used to generate the allocation sequence; the concealment of allocation; and blinding (clinician, participant outcome assessor) were assessed. For each trial, the risk of bias was classified as high or low or unclear if there was an insufficient description in the original reports. Overall, a trial was considered to be at low risk of bias if allocation concealment and blinding of participants were adequate.
The generation of the allocation sequence was judged to be adequate only if the method used for the allocation sequence generation was sufficiently described (e.g., number tables generated by computer), and inadequate for those systems involving dates, names, or hospital record numbers for the allocation of patients. Allocation concealment was judged to be adequate if the allocation of patients involved a central or independent randomisation, identically numbered packages prepared at independent offices, or sealed and opaque envelopes. Methods for which investigators could know the assignment of patients were judged to be inadequate. Methods that ensured the blinding of participants, investigators and outcome assessors were judged to be adequate. Open trials or those methods that did not blind participants or investigators, were judged as inadequate. Follow up was judged to be adequate if the numbers and reasons for drop‐outs and withdrawals in all intervention groups were described or if it was specified that there were no drop‐outs or withdrawals.
The overall quality of the evidence was assessed using the GRADE approach. The GRADE approach rates the overall quality of the evidence as high, moderate, low or very low and takes several dimensions into account including design and execution, consistency, directness, precision and, publication and reporting bias (Guyatt 2008). The findings of the GRADE analysis are included in the summary of findings table.
Data synthesis
Data were analyzed using Review Manager (RevMan 5). All data were analyzed on an intention‐to‐treat basis. Data were extracted from the original research articles and converted into 2x2 tables. Heterogeneity among studies was assessed using the chi‐square test and the I2 statistic. As the chi‐square test has low power in the situation of a meta‐analysis, when trials have a small sample size or are few in number, a P‐value of 0.10 was regarded as statistically significant. For pooled data, summary test statistics was derived using the relative risk ratio and 95% confidence intervals. A random effects model was used for pooling data. The definitions of treatment success, remission and clinical improvement was set by the authors of each paper, and data were combined for analysis only if these definitions were sufficiently similar (determined by consensus).
Results
Description of studies
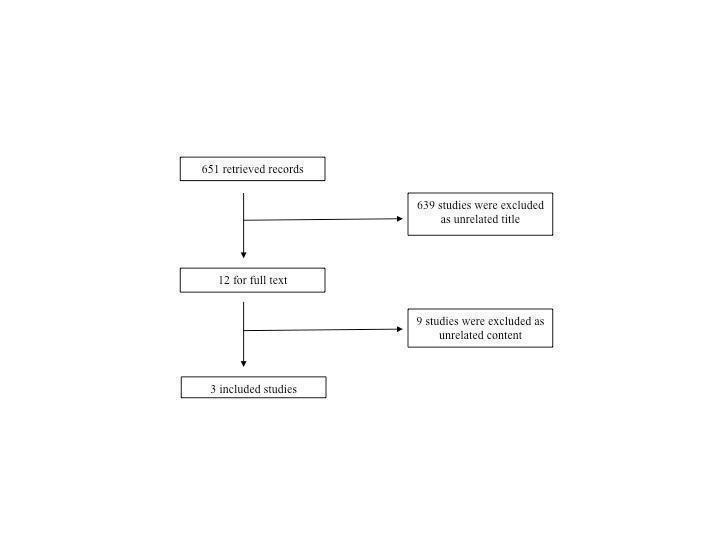
Six hundred and fifty‐one citations were identified by the electronic search, from which 12 studies were considered potentially eligible for inclusion (Figure 1). Nine studies were excluded after analysing their full text (see Characteristics of excluded studies; Colombel 2001, de Villiers 2003, Dejaco 1998, Geier 2007, Schreiber 2002, Tilg 1998a, Tilg 1998b, Tilg 2002a, Tilg 2002b). The reasons for exclusion included: different inclusion criteria than the review (patients, interventions or outcomes), different type of study design or being a review or an editorial. The 3 remaining studies fulfilled the eligibility criteria and were included in the review (Fedorak 2000; Schreiber 2000; Van Deventer 1997).
1.
Schreiber 2000 conducted a prospective, multicentre, double‐blind, placebo‐controlled study in 329 therapy‐refractory patients with Crohn's disease. The trial assessed the effects on clinical improvement, and clinical remission of 4 different rhuIL‐10 doses compared to placebo. The treatment was administrated subcutaneously over 28 days with a follow up period of 4 weeks.
Fedorak 2000 was a 24‐week multicentre, prospective, randomised, double‐blind, placebo‐controlled, and sequential‐escalating‐dose study. Ninety‐five patients with CDAI scores of 200 to 350 were enrolled and treated with 4 different doses of rhuIL‐10 subcutaneously or placebo once daily for 28 consecutive days. After the treatment period the patients were followed up to 20 weeks. The primary outcomes were safety and tolerance, and efficacy (defined as clinical remission) was a secondary outcome.
Van Deventer 1997 reported a double‐blind randomised multicentre trial to evaluate the safety, tolerance, and pharmacokinetics of IL‐10 in Crohn’s disease. Forty‐six patients with active steroid resistant Crohn’s disease were treated with one of five doses of rhuIL‐10 or placebo administered once daily by intravenous bolus injection over 7 consecutive days. There was a 3 week follow up period.
Risk of bias in included studies
No major limitations in the design and execution of the trials included in the review were identified (See Figure 2). Although there were some concerns about a lack of information about the procedures used to generate the randomisation sequences (only Fedorak 2000 reported adequate data on this domain in the trial report) additional information was obtained from a researcher involved in Van Deventer 1997 and Schreiber 2000 studies confirming that the randomisation sequence was computer generated. All the trials reported adequate allocation concealment procedures. The three included trials adequately blinded investigators and patients, but procedures for outcome assessment were unclear.
2.
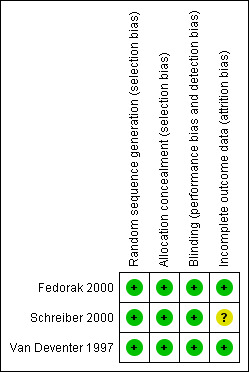
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The three included trials reported data on exclusions or losses from follow up. This could have an impact for the Schreiber 2000 study, that failed to detail the reason of discontinuation for 45 patients from the originally 329 randomised.
Effects of interventions
See: Table 1
Complete remission
There was no statistically significant difference in the number of complete remissions in patients that received interleukin 10 compared to placebo (79 events, 3 trials; RR 1.43; 95% CI 0.62 to 3.29). The results reported in the studies were moderately variable (I2=40%; Figure 3).
3.
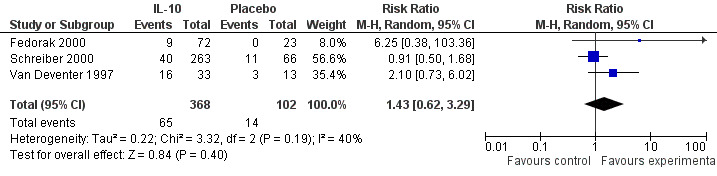
Forest plot of comparison: 1 IL‐10 vs placebo, outcome: 1.4 Complete remission all doses (CDAI < 150 with a decrease in CDAI of at least 100 points from baseline).
Clinical remission
There was no statistically significant difference in the number of clinical remissions in patients receiving interleukin 10 compared with placebo (93 events; 3 trials; RR 1.29; 95% CI 0.79 to 2.11). The results reported in the studies were homogeneous (I2=0%; Figure 4).
4.

Forest plot of comparison: 1 IL‐10 vs placebo, outcome: 1.5 Clinical remision (CDAI < 150).
Adverse events
Adverse events were reported differently across studies including: serious adverse events, severe adverse events, any adverse events and withdrawals due to adverse events. Additionally the studies provided detail about the type of complications.
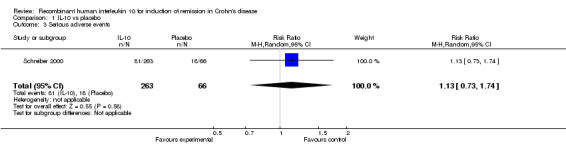
Although serious adverse (99 events, 1 trial) events were more frequent in the IL‐10 group compared to placebo, the difference was not statistically significant (RR 1.13; 95%CI 0.73 to 1.74; Figure 5). There were no statistically significant differences in the proportion of patients who experienced any adverse event (395 events, 3 trials; RR 1.00; 95% CI 0.94 to 1.06; I2=0%; Figure 6). Patients receiving IL‐10 were significantly more likely than placebo patients to withdraw from the studies due to adverse events (44 events, 2 trials; RR 13.50; 95% CI 3.89 to 46.79; I2=0%; Analysis 1.5). Eighteen patients treated with interleukin 10 experienced thrombocytopenia compared to no placebo patients, but the difference was not statistically significant (RR 4.73; 95% CI 0.63 to 35.33; I2=0%; Analysis 1.6). Severe adverse events (105 events, 3 trials) were more frequently reported in patients receiving interleukin 10 than those receiving placebo, but the difference was not statistically significant (RR 0.88; 95% CI 0.37 to 2.12, I2=63%; Analysis 1.7).
5.
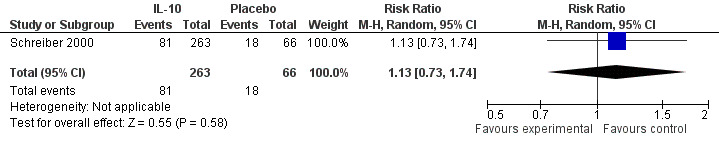
Forest plot of comparison: 1 IL‐10 vs placebo, outcome: 1.3 Serious adverse events.
6.
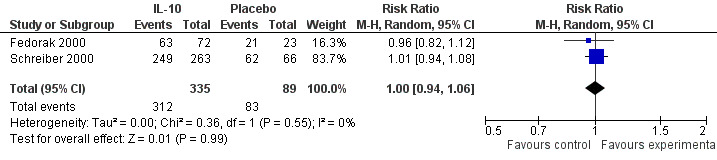
Forest plot of comparison: 1 IL‐10 vs placebo, outcome: 1.7 Any adverse event.
1.5. Analysis.
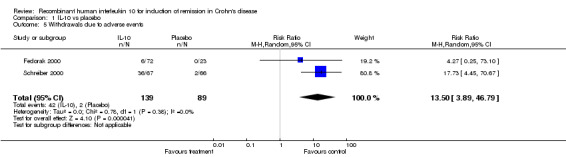
Comparison 1 IL‐10 vs placebo, Outcome 5 Withdrawals due to adverse events.
1.6. Analysis.
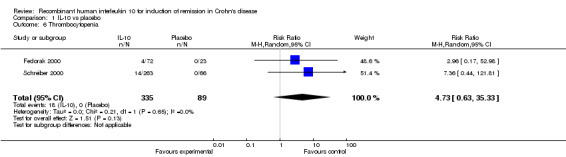
Comparison 1 IL‐10 vs placebo, Outcome 6 Thrombocytopenia.
1.7. Analysis.

Comparison 1 IL‐10 vs placebo, Outcome 7 Severe adverse events.
Discussion
Summary of main results
This systematic review about the use of interleukin 10, compared with placebo, for induction of remission in Crohn's disease identified three trials with a total of 470 patients. These trials were conducted between 1997 and 2000. The pooled results show that this drug does not increase the number of remissions (complete or clinical) compared to placebo. The use of interleukin 10 resulted in a significantly increased number of patients withdrawing due to adverse events. There were no statistically significant differences in the proportions of patients who experienced serious or any adverse events.
Quality of the evidence
The overall quality of the evidence according to the GRADE approach is moderate. This is due to imprecision of the available results. This imprecision is due to the low number of events (always under 100 events).
Potential biases in the review process
Efforts to locate unpublished studies might make publication bias less likely. However, given that the three trials are negative it seems unlikely that positive trials remain unpublished.
Authors' conclusions
Implications for practice.
Interleukin 10, compared with placebo, does not show any benefit for the treatment of active Crohn's disease, showing an unfavourable balance between the potential benefits and adverse events. This systematic review showed that interleukin 10 does not increase the number of remissions, complete or clinical, but significantly increases the number of withdrawals due to adverse events. The quality of the evidence regarding the efficacy of IL‐10 is moderate.
Implications for research.
Although further research may have an impact on point estimates, future trials are unlikely to be undertaken.
What's new
| Date | Event | Description |
|---|---|---|
| 30 November 2011 | Amended | Updated contact details for first author |
Acknowledgements
Pablo Alonso‐Coello is funded by a Miguel Servet investigator contract by the Instituto de Salud Carlos III (CP09/00137).
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Data and analyses
Comparison 1. IL‐10 vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete remission (CDAI<150 with a decrease in CDAI of at least 100 points from baseline) | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.62, 3.29] |
| 2 Clinical remision (CDAI<150) | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.79, 2.11] |
| 3 Serious adverse events | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.73, 1.74] |
| 4 Any adverse event | 2 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 5 Withdrawals due to adverse events | 2 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 13.50 [3.89, 46.79] |
| 6 Thrombocytopenia | 2 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 4.73 [0.63, 35.33] |
| 7 Severe adverse events | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.37, 2.12] |
1.1. Analysis.

Comparison 1 IL‐10 vs placebo, Outcome 1 Complete remission (CDAI<150 with a decrease in CDAI of at least 100 points from baseline).
1.2. Analysis.

Comparison 1 IL‐10 vs placebo, Outcome 2 Clinical remision (CDAI<150).
1.3. Analysis.
Comparison 1 IL‐10 vs placebo, Outcome 3 Serious adverse events.
1.4. Analysis.
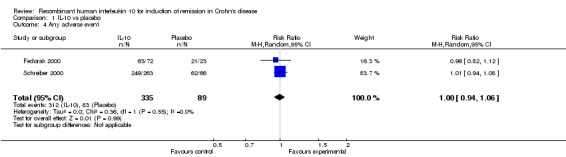
Comparison 1 IL‐10 vs placebo, Outcome 4 Any adverse event.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fedorak 2000.
| Methods | Multicenter, double‐blind, placebo controlled, randomised clinical trial July 1995 ‐ December 1996 |
|
| Participants | N = 95, Age: > 18 Diagnosis: Mild to moderate active Crohn’s disease, CDAI = 200‐350, (not presently receiving corticosteroids, mesalamine or immunosuppressive therapy) |
|
| Interventions | Intervention: 72 patients received s.c. rhuIL‐10 (1, 5 10, 20 ųg/kg) once daily (OD) Control: 23 patients received placebo OD Duration: 28 days treatment + 20 week follow‐up period |
|
| Outcomes |
Primary outcomes: 1. Safety and tolerance. Each clinical adverse event was graded for severity at each visit. Secondary outcomes: 1. Efficacy: Complete remission, defined as a CDAI at day 29 lower than or equal to 150 points and a decrease from baseline in CDAI of more than 100 points, plus improvement or resolution in endoscopic appearance. Quality of life, measured by the IBD questionnaire 2. IL‐10 pharmacokinetics |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'Computer generated randomisation schedules [...] at a 3:1 ratio' |
| Allocation concealment (selection bias) | Low risk | Independent central randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: 'rhuIL‐10 [...] was reconstituted with sterile water to a clear, colourless solution. Placebo of the same formulation, minus the active ingredient, was identical in appearance.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat sample: 95 patients (72 in rhuIL‐10 group and 23 to placebo). Per protocol sample: 12 patients (10 from the rhuIL‐10 group and 2 from the placebo group) did not complete the 28‐day treatment phase, for the following reasons: Adverse effects: 6 ųg/kg rhuIL‐10 patients Treatment failure: 3 ųg/kg rhuIL‐10 versus 2 placebo Patient preferences: 1 ųg/kg rhuIL‐10 patient |
Schreiber 2000.
| Methods | Multicenter, randomised, double‐blind, placebo‐controlled clinical trial February 1996 ‐ October 1997 |
|
| Participants | N = 329, Age: adults Diagnosis: therapy ‐ refractory Crohn’s disease |
|
| Interventions | Intervention: s. c., different doses of rhuIL‐10: 1 ųg/kg, 18% [9.6 ‐ 29.2], 4 ųg/kg, 20% [11.3 ‐ 32.2], 8 ųg/kg, 20% [11.1 ‐ 31.8], 20 ųg/kg, 28% [18 ‐ 40.7] Control: placebo, 18% [9.6‐29.6] Duration: 28 days |
|
| Outcomes |
Primary outcomes: 1. Clinical remission, defined as a CDAI score lower than 150 and a decrease from baseline CDAI of more than 100 points. Secondary outcomes: 1. Clinical improvement, defined as a decrease from baseline CDAI of more than 100 points 2. Endoscopic improvement 3. Quality of life measured by IBD questionnaire and SF‐36 health survey 4. Effect of treatment on proinflammatory transcription factor activity at the end of treatment period. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence, as confirmed by a researcher involved in the execution of the trial, not described in the report (quote: 'patients were randomized'). Stratification according to steroid dose received, and use of immunosuppressive agents. |
| Allocation concealment (selection bias) | Low risk | Quote: 'Central randomization was used, ...' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding properly conducted, as confirmed by a researcher involved in the execution of the trial. After 50 patients completed the treatment safety was assessed in a blinded fashion by an independent data monitoring committee. After treatment of 151 efficacy was assessed in an unblinded fashion. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 329 patients were randomized (263 to rhuIL‐10 and 66 to placebo). 45 patients discontinued the treatment for the following reasons (distribution by groups not reported): Adverse events: 30 Treatment failure: 9 Ineligibility: 3 (these patients were randomised but did not receive the treatment) Patient preference: 2 Death: 1 |
Van Deventer 1997.
| Methods | Multicentre, double‐blind, placebo‐controlled randomised clinical trial December 1994 ‐ June 1995 |
|
| Participants | N = 46, Age: 18‐65 years Diagnosis: active steroid ‐resistant Crohn’s disease |
|
| Interventions | Intervention: 1 of 5 doses of rhuIL‐10 (0.5, 1, 5, 10, 25 ųg/kg), i. v. bolus, once daily Control: placebo identical in appearance and volume at each dose level Duration: 7 days + 3 week follow‐up period |
|
| Outcomes |
Primary outcomes: 1. Safety and tolerance Secondary outcomes: 1. Efficacy: complete remission, defined as a CDAI score lower than 150 and a decrease from baseline CDAI of more than 100 points. clinical remission, defined as a decrease from baseline CDAI of more than 100 points |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence, as confirmed by a researcher involved in the execution of the trial, not described in the report (quote: 'patients were randomized'). Randomization established different ratios depending of the IL 10 dose. |
| Allocation concealment (selection bias) | Low risk | Central randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | IL 10 was provided as a sterile powder and reconstituted with sterile water. Placebo was identical in appearance and volume at each dose level. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 46 patients randomized (33 IL10 vs 13 placebo) One patient allocated to placebo received a single dose of IL10, and was withdrawn (excluded for the efficacy analysis) One patient on placebo discontinued the study due to abdominal pain. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Colombel 2001 | Did not fit with our inclusion criteria: different patients. The study assessed the role of IL‐10 in preventing post‐operative recurrence of Crohn's disease |
| de Villiers 2003 | Did not fit with our inclusion criteria: different patients. This was a selected summary of a study of IL‐10 in mice |
| Dejaco 1998 | Did not fit with our inclusion criteria: different patients. In vivo study of patients with Crohn's disease or ulcerative colitis |
| Geier 2007 | Review |
| Schreiber 2002 | Editorial |
| Tilg 1998a | Non‐RCT |
| Tilg 1998b | Did not fit with our inclusion criteria: different outcomes of interest |
| Tilg 2002a | Did not fit with our inclusion criteria: different outcomes of interest |
| Tilg 2002b | Did not fit with our inclusion criteria: different outcomes of interest |
Differences between protocol and review
For the sake of improving understanding and communication of results our final analysis included a calculation of risk ratios instead of odds ratios. We use a random effect model instead of a fixed effect due to its more conservative estimation of treatment effects. Given the recent changes in the evaluation of the quality of the included studies in the handbook and in the international literature in general we analysed the risk of bias and additionally used the GRADE system for the overall quality of the evidence.
Contributions of authors
Drs Buruiana and Alonso‐Coello screened and appraised the quality of published papers, abstracted data from included studies, communicated with investigators regarding unpublished data, interpreted the analyses, and wrote the review. Ivan Solà performed the search, interpreted the analysis, developed the Summary of Findings table and provided important intellectual comments to the different review drafts.
Sources of support
Internal sources
Iberoamerican Cochrane Centre, Spain.
External sources
No sources of support supplied
Declarations of interest
None declared.
Edited (no change to conclusions)
References
References to studies included in this review
Fedorak 2000 {published data only}
- Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately severe active Crohns disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology 2000;119(6):1473‐82. [DOI] [PubMed] [Google Scholar]
Schreiber 2000 {published data only}
- Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn's disease. Crohn's Disease IL‐10 Cooperative Study Group. Gastroenterology 2000;19(6):1461‐72. [DOI] [PubMed] [Google Scholar]
Van Deventer 1997 {published data only}
- Deventer SJ, Elson CO, Fedorak RN. Multiple doses of intravenous interleukin 10 in steroid‐refractory Crohn's disease. Crohn's Disease Study Group. Gastroenterology 1997;113(2):383‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Colombel 2001 {published data only}
- Colombel JF, Rutgeerts P, Malchow H, Jacyna M, Nielsen OH, Rask‐Madsen J, et al. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn's disease. Gut 2001;49(1):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Villiers 2003 {published data only}
- Villiers WJ. Crohn's disease and IL‐10 therapy: promise regained. Inflamm Bowel Dis 2003;9(3):210‐1. [DOI] [PubMed] [Google Scholar]
Dejaco 1998 {published data only}
- Dejaco C, Tilg H, Gasche C. In vivo changes of lymphocyte subpopulations and leukocyte surface markers by multiple doses of recombinant human interleukin‐10 in inflammatory bowel disease. Gastroenterology 1998;114(Suppl 1):A961. [Google Scholar]
Geier 2007 {published data only}
- Geier MS, Butler RN, Howarth GS. Inflammatory bowel disease: current insights into pathogenesis and new therapeutic options; probiotics, prebiotics and synbiotics. Int J Food Microbiol 2007;115(1):1‐11. [DOI] [PubMed] [Google Scholar]
Schreiber 2002 {published data only}
- Schreiber S. Novel and future strategies in the management of IBD. Annals of Gastroenterology 2002;15(4):307. [Google Scholar]
Tilg 1998a {published data only}
- Tilg H, Kaser A, Schreiber S, Gregor M, Rutgeerts P, Deventer S, et al. New insights into the pathogenesis of anemia of chronic diseases: induction of ferritin synthesis and development of anemia during recombinant human interleukin‐10 (rHuIL‐10) treatment of chronic active Crohn's disease (CACD). Gastroenterology 1998;114(Suppl 1):A1100. [Google Scholar]
Tilg 1998b {published data only}
- Tilg H, Kaser A, Propst A, Schreiber S, Gregor M, Rutgeerts P, et al. Recombinant human interleukin‐10 (rHuIL‐10) therapy in steroid‐refractory chronic active Crohns disease (CACD): induction of neopterin, a degradation product of the pteridine pathway regulated by interferon‐gamma. Gastroenterology 1998;114(Suppl 1):A1100. [Google Scholar]
Tilg 2002a {published data only}
- Tilg H, Montfrans C, Ende A, Kaser A, Deventer SJ, Schreiber S, et al. Treatment of Crohn's disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut 2002;50(2):191‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tilg 2002b {published data only}
- Tilg H, Ulmer H, Kaser A, Weiss G. Role of IL‐10 for induction of anemia during inflammation. J Immunol 2002;169(4):2204‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Autschbach 1998
- Autschbach F, Braunstein J, Helmke B, Zuna I, Schumann G, Niemir ZI, et al. In situ expression of interleukin‐10 in noninflamed human gut and in inflammatory bowel disease. Am J Pathol 1998;153(1):121‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farrell 1989
- Farrell RJ, Peppercorn MA. Medical management of Crohn's disease in adults. Am J Gastroenterol 1989;84:249. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1[updated September 2008]. The Cochrane Collaboration. Chichester, UK: John Wiley & Sons, Ltd, 2008. [Google Scholar]
Korzenik 2006
- Joshua R Korzenik, Daniel K Podolski. Evolving knowledge and therapy of inflammatory bowel disease. Nature Reviews 2006;5:197‐209. [DOI] [PubMed] [Google Scholar]


