Abstract
The chromatin landscape defines cellular identity in multicellular organisms with unique patterns of DNA accessibility and histone marks decorating the genome of each cell type. Thus, profiling the chromatin state of different cell types in an intact organism under disease or physiological conditions can provide insight into how chromatin regulates cell homeostasis in vivo. To overcome the many challenges associated with characterizing chromatin state in specific cell types, we developed an improved approach to isolate Drosophila melanogaster nuclei tagged with a GFPKASH protein. The perinuclear space-localized KASH domain anchors GFP to the outer nuclear membrane, and expression of UAS-GFPKASH can be controlled by tissue-specific Gal4 drivers. Using this protocol, we profiled chromatin accessibility using an improved version of Assay for Transposable Accessible Chromatin followed by sequencing (ATAC-seq), called Omni-ATAC. In addition, we examined the distribution of histone marks using Chromatin immunoprecipitation followed by sequencing (ChIP-seq) and Cleavage Under Targets and Tagmentation (CUT&Tag) in adult photoreceptor neurons. We show that the chromatin landscape of photoreceptors reflects the transcriptional state of these cells, demonstrating the quality and reproducibility of our approach for profiling the transcriptome and epigenome of specific cell types in Drosophila.
Keywords: Drosophila, chromatin, transcription, cell type, photoreceptor, ATAC-seq
Introduction
Dynamic regulation of the epigenome is crucial to replication, transcription, and DNA repair. For instance, accessible chromatin is associated with gene regulatory sequences, such as enhancers, promoters and transcription factor binding sites, and contributes to transcription initiation (Klemm et al. 2019). In addition, chromatin-associated proteins, such as histones, transcription factors or chromatin remodelers, modulate several processes, including nucleosome occupancy (Brahma and Henikoff 2020), heterochromatin maintenance (Allshire and Madhani 2018), and recruitment of DNA repair factors (Stadler and Richly 2017). Thus, genome-wide chromatin profiling across different physiological states can help us understand how chromatin-mediated processes impact cell homeostasis.
The wide array of genetic manipulation tools, a highly mapped and annotated genome, relatively short lifespan, and ease of growth have made Drosophila one of the most widely used model organisms for studying the basic molecular mechanisms of eukaryotic cells (Hales et al. 2015). Furthermore, the tissue homology between Drosophila and humans can be leveraged to uncover regulatory mechanisms associated with human-relevant conditions, such as aging, neurodegeneration, and diabetes (Ugur et al. 2016; Graham and Pick 2017; Piper and Partridge 2018; Bolus et al. 2020). Because epigenetic dysregulation is one of the hallmarks of many diseases, including cancer and neurodegeneration (Lardenoije et al. 2015; Bailey et al. 2018), profiling chromatin states in a tissue-specific context using Drosophila might improve our understanding of how chromatin-associated changes contribute to disease onset. However, profiling cell type-specific chromatin states in vivo is challenging. Although tissue dissection can be coupled with bulk and single-cell genome-wide experiments, manual tissue dissection is technically demanding and contamination from surrounding tissues can often confound results.
To overcome these limitations, alternative techniques have been developed based around epitope labeling of nuclei by transgenic expression of an epitope tag driven by a cell-type specific promoter followed by purification (Chitikova and Steiner 2016). This approach has been coupled with Fluorescence-Activated Cell Sorting (FACS)-based nuclei isolation, such as the “Batch Isolation of Tissue-Specific” (BiTS) approach (Bonn et al. 2012), as well as bead-based purification, such as the “Isolation of Nuclei Tagged in specific Cell Types” (INTACT) method (Deal and Henikoff 2010). In addition, INTACT has been applied to tissue-specific experiments in Arabidopsis (Maher et al. 2018; Sijacic et al. 2018), Drosophila (Henry et al. 2012; Jones et al. 2018; Agrawal et al. 2019; Bozek et al. 2019), Xenopus (Amin et al. 2014), and mice (Ambati et al. 2016); FACS-based BiTS has also been extensively applied to purify different tissues from Drosophila embryos and mice (Lam et al. 2019). In Drosophila, these nuclei labeling approaches often rely on genetic tools for binary expression of transgenes, such as the well-established Gal4-UAS system (Brand and Perrimon 1993). Currently, more than 8000 stocks that express Gal4 under control of different cell-type specific promoters are available through the Bloomington Drosophila Stock Center (BDSC). Thus, these nuclei tagging approaches combined with the Gal4-UAS expression system provide a powerful and flexible tool to manipulate and examine many cell-types in Drosophila.
We previously developed a Gal4-UAS based nuclei immuno-enrichment (NIE) protocol to isolate nuclei from specific Drosophila cell types labeled with an outer nuclear membrane localized GFPKASH protein (Hall et al. 2017; Ma and Weake 2014). The GFPKASH protein consists of the Klarsicht, ANC-1, Syne Homology (KASH) domain of Muscle-specific protein 300 kDa (Msp300; FBgn0261836) fused to the C-terminus of EGFP, and localizes GFP to the outer nuclear membrane facing the cytoplasm (Yu et al. 2006). This GFPKASH-based NIE approach was successfully applied to transcriptomic studies in specific cell populations, such as larval glial cells (Ma et al. 2016), adult photoreceptor neurons (Hall et al. 2017, 2018), and olfactory sensory neurons (Slankster et al. 2020). However, our previous protocol yielded low nuclei numbers, which made performing chromatin profiling and obtaining material from rare cell populations challenging. In this study, we sought to optimize the NIE protocol to increase nuclei yield and stringency over background. Using this “improved” GFPKASH-based NIE protocol, we applied transcriptome and chromatin profiling techniques to NIE-purified adult Drosophila photoreceptor nuclei. We demonstrate the reproducibility and quality of datasets obtained profiling nuclear RNA-seq, an improved Assay for Transposable Accessible Chromatin followed by sequencing (ATAC-seq), called Omni-ATAC, and Chromatin immunoprecipitation (ChIP) followed by sequencing (ChIP-seq) and Cleavage Under Targets and Tagmentation (CUT&Tag) of histone modifications.
Materials and methods
Fly strains
Flies homozygous for Rh1>GFPKASH = P{ry+t7.2=rh1-GAL4}3, ry506, P {w+mC = UAS-GFP-Msp300KASH}attP2 or Rh1>mCherryKASH, P{ry+t7.2=rh1-GAL4}3, ry506, P{w+mC = UAS-Msp300KASH-mCherry-Flag}attP2 (Hall et al. 2017) were raised in 12:12 hour light: dark cycle at 25°C on standard fly food. Flies were maintained in population cages with a density of ∼1000 flies/cage. Fresh food was switched every other day. For all the biological replicates, male flies were collected at 10 days post-eclosion at Zeitgeber time 6 (±1 hour).
Nuclei Immuno-Enrichment
NIE was performed as described previously (Ma and Weake 2014; Hall et al. 2017) with minor modifications to the buffers used throughout the protocol. Briefly, fly heads from 400 age-matched flies were collected by freezing flies in five cycles of flash-freezing and vortexing. Fly heads were collected using frozen sieves and transferred to a 1 mL Dounce homogenizer containing 1 volume of homogenization buffer (40 mM HEPES, pH 7.5, 120 mM KCl, and 0.4% v/v NP-40). Flies were homogenized using 10 strokes with “loose pestle” followed by 10 strokes with “tight” pestle. Homogenized lysate was then filtered using 40 μm cell strainers (Corning, Tewksbury MA, USA Catalog# 352340), and NP-40 was diluted to 0.1% final concentration by adding three volumes of Dilution buffer (40 mM HEPES, pH 7.5 and 120 mM KCl). Nuclei were immuno-enriched using 40 μl of Dynabeads Protein G (ThermoFisher, Waltham, MA, USA Catalog #10004 D) pre-coupled with 4 μg of mouse anti-GFP antibody (Sigma Aldrich, St. Louis, MO, USA, Catalog #11814460001) for RNA-seq, ChIP-seq and Omni-ATAC experiments. For CUT&Tag, nuclei were immunoenriched using 40 μl of Dynabeads Pan Mouse IgG (ThermoFisher. Catalog #11041) pre-coupled with 4 µg of mouse anti-GFP antibody (Sigma Aldrich, Catalog #11814460001). Beads and nuclei were incubated at 4°C for 30 minutes with constant rotation, followed by 3 × 5-minutes washes with homogenization buffer at 4°C.
Quantitative PCR
DNA was purified with Quick-DNA Microprep Plus Kit (Zymo Research, Irvine, CA, USA Catalog #D4074) and qPCR was performed using Bullseye EvaGreen qPCR 2X master mix-ROX (Midsci, Valley Park, MO, USA Catalog #BEQPCR-R) following the manufacturer’s instructions.
RNA-seq
Purified nuclei were resuspended in 100 μl TRI reagent (Zymo Research, Catalog #R2050-1-200). RNA was purified using Direct-zol™ RNA Microprep (Zymo Research, Catalog, #R2061) and quantified with Qubit™ RNA HS Assay Kit. Ten nanograms of nuclear RNA were used for construction of cDNA libraries with Ovation SoLo RNA-seq System with Drosophila-specific anyDeplete technology for rRNA depletion (Tecan, Redwood City, CA, USA Catalog #0502-32). Up to 16 libraries were pooled in one lane for paired-end 150 bp Illumina HiSeq sequencing.
Omni-ATAC
Transposition was performed as published (Corces et al. 2017). Briefly, a fraction of immunoprecipitated nuclei were purified with Quick-DNA Microprep Plus kit (Zymo Research, Catalog #D4074). Nuclei corresponding to 50 or 100 ng were aliquoted and resuspended in 50 μl of Transposition mix (25 μl 2x TD buffer, 16.5 μl PBS, 0.05 μl 1% v/v Digitonin, 0.05 μl 10% v/v Tween and 2.5 μl TDE1 enzyme (Illumina, San Diego, CA, USA Catalog #20034198). Tagmented DNA was purified with Zymo DNA clean & concentrator-5 kit (Zymo Research #D4013). Libraries were constructed using IDT for Illumina Nextera DNA Unique Dual Indexes Set A (Illumina, Catalog #20027213) and 7 x polymerase chain reaction (PCR) cycles were used to amplified libraries using NEBnext High-Fidelity 2X PCR Master Mix (New England Biolabs, Ipswich, MA, USA Catalog #M0541S) and SYBR Green I (ThermoFisher, Catalog #S7563). To determine additional cycles, Nextera primers 1 and 2 were used. Purified libraries were submitted to a round of double-size selection with AMPure XP beads (Beckman Coulter, Brea, CA, USA Catalog #A63880) with a 0.5X–1.0X ratio. The fragment size distribution of libraries was assessed with TapeStation High-Sensitivity D1000 Screentapes (Agilent, Santa Clara, CA, USA Catalog #5067-5584). Up to 16 libraries were pooled in one lane for paired-end 150 bp Illumina HiSeq sequencing.
ChIP-seq
Chromatin extraction (Drosophila): Immunoenriched nuclei were resuspended in 1 mL of A1 buffer (15 mM HEPES, pH 7.5, 15 mM NaCl, 60 mM KCl, 4 mM MgCl2, 0.5% Triton X-100 v/v) and cross-linked with 1% methanol-free formaldehyde (ThermoFisher #28906) for 2 minutes at room temperature. Fixed nuclei were quenched with 125 mM Glycine, pH 7.5 for 5 minutes, followed by sonication in 130 μl of Nuclei Lysis Buffer (50 mM Tris-HCl, pH 8.0, 10 mM EDTA, 1% v/v SDS) in Covaris E220 with the following conditions: 10 minutes, 2% duty cycle, 105 Watts and 200 c.p.b. to obtain an average fragment size of ∼320 bp. Chromatin was centrifuged at 14,000 rpm, 10 minutes, 4°C, and the soluble chromatin supernatant was diluted with X-ChIP dilution buffer (16.7 mM Tris, pH 8.0, 167 mM NaCl, 1% Triton X-100 v/v, 1.2 mM EDTA pH 8.0), flash-frozen in liquid nitrogen, and stored at −20°C. Chromatin extraction (Arabidopsis): 2.5 g of 10-day old ref4–3MED15FLAG Arabidopsis thaliana seedlings were ground to a fine powder using liquid nitrogen and resuspended in 20 mL of cold EB1 buffer (0.44 mM sucrose, 10 mM Tris, pH 8.0, 10 mM MgCl2, 5 mM B-Me, 0.1 mM PMSF). The solution was filtered through two layers of miracloth and centrifuged at 3000 × g, 20 minutes, 4°C. The pellet was then resuspended in 1 mL of cold EB2 Buffer (Sucrose 0.25 M, 10 mM Tris, pH 8.0, 10 mM MgCl2, 1% v/v Triton X-100, 5 mM β-Me, 0.1 mM PMSF) and centrifuged at 4°C, 12,000 g for 10 minutes. The pellet was resuspended in 300 μl of cold EB3 buffer (sucrose 1.7 M, 10 mM Tris, pH 8.0, 2 mM MgCl2, 0.15% v/v Triton X-100, 5 mM β-Me, 0.1 mM PMSF) and the sample was overlaid on top of 300 μl of cold EB3 and centrifuged at 4°C, 16,000 × g for 1 hour. Supernatant was transferred to a low-retention tube, snap-frozen, and stored at −20°C.
ChIP was performed as described (Deal and Henikoff 2010) with the following modifications. Briefly, 380 ng of Drosophila chromatin (DNA) was mixed with 20 ng of Arabidopsis chromatin as a spike-in control (5%), and incubated with 1 μg of each of the following antibodies: H3 (Abcam, Cambridge, MA, USA Catalog #1791), H3K4me3 (Abcam, Catalog #8580) and H3K36me3 (Abcam, Catalog #9050) for 12 to 18 hours at 4°C. Immunoprecipitated histone-DNA complexes were incubated with 25 μl Dynabeads protein G (ThermoFisher, Catalog #10004 D) for 2 hours at 4°C, followed by 5-minutes washes with 1 mL Low Salt Buffer (20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% v/v SDS, 1% v/v Triton X-100, 2 mM EDTA), 1 mL High Salt Buffer (20 mM Tris, pH 8.0, 500 mM NaCl, 0.1% v/v SDS, 1% v/v Triton X-100, 2 mM EDTA), 1 mL LiCl Wash buffer (10 mM Tris, pH 8.0, 250 mM LiCl, 0.1% v/v Na-Deoxycholate, 1% v/v NP-40 substitute, 1 mM EDTA) and 1 mL TE (10 mM Tris, pH 8.0, 1 mM EDTA). Histone-DNA complexes were eluted from the magnetic beads with X-ChIP elution buffer (100 mM NaHCO3, 1% v/v SDS), treated with RNAse A (ThermoFisher, Catalog #EN0531) at 37°C for 30 minutes and Proteinase K (ThermoFisher, Catalog #AM2546) at 55°C for 12 to 18 hours. DNA was purified with Zymo Research ChIP DNA clean & concentrator kit (Zymo Research, Catalog #D5205). Purified DNA was quantified with Qubit 1X HS DNA kit (ThermoFisher, Catalog #Q33230). Input sample fragment size was determined with TapeStation High-Sensitivity D5000 Screen tapes (Agilent, Catalog #5067-5592).
ChIP-seq library prepation: 2 ng of DNA were used for ChIP-seq libraries constructed with Tecan Ovation Ultralow V2 DNA-Seq Library Preparation Kit-Unique Dual Indexes (Tecan, Catalog #9149-A01). Following amplification, purified libraries were submitted to a round of double-size selection with AMPure XP beads (Beckman Coulter, Catalog# A63880) with a 0.61X–0.8X ratio. Libraries fragment size distribution was assessed with TapeStation High-Sensitivity D1000 Screentapes (Agilent, Catalog #5067-5584). Up to 16 libraries were pooled in one lane for paired-end 150 bp Illumina HiSeq sequencing.
CUT&Tag
CUT&Tag was performed using CUTANA™ CUT&Tag reagents (Epicypher, Durham NC, #15-1017, #15-1018, #13-0047) following manufacturer’s “Direct-to-PCR CUT&Tag Protocol” with minor modifications: Briefly, purified nuclei were washed 1 time with cold Antibody150 buffer, and protocol was started at Section III “Binding of Primary and Secondary antibodies” and followed as described: https://www.epicypher.com/content/documents/protocols/cutana-cut&tag-protocol.pdf (last accessed June 3rd, 2021).
Data processing
Raw reads were trimmed using Trimmomatic version 0.39 (Bolger et al. 2014) to filter out low-quality reads (Q > 30) and clean adapter reads. Cleaned reads were aligned to the Drosophila melanogaster genome (BDGP Release 6 + ISO1 MT/dm6 from UCSC) using splicing-aware aligner STAR version 1.3 (Dobin et al. 2013) for RNA-seq, and Bowtie2 version 2.3.5.1 (Langmead and Salzberg, 2012, p. 2) for Omni-ATAC, ChIP-seq and CUT&Tag using –sensitive settings. Samtools version 1.8 (Li et al. 2009) was used to obtain, sort and index BAM files. For genome browser inspection as well as further analyses, bigwig files were generated by normalizing datasets to count-per-million counts per million (CPM) coverage tracks using deepTools version 3.1.1 (Ramírez et al. 2014) using –normalizeUsing CPM settings. Spearman’s correlation scores were calculated using deepTools’ subpackages multiBigwigSummary and plotCorrelation. Metaplots and genomic distribution heatmaps were made with deepTools’ subpackages computeMatrix, plotHeatmap and plotProfile. GO term analysis was performed using R package clusterProfiler (Yu et al. 2012). Spike-in normalization. FastQ Screen version 0.13.0 (Wingett and Andrews, 2018) was used to separate reads that uniquely mapped to either the genome of D. melanogaster (BDGP Release 6 + ISO1 MT/dm6 from UCSC) or A. thaliana (Tair10—Arabidopsis.org) using the filter option and with sensitive parameters. Each fastq file was aligned and processed separately, and alignment rates to each genome file were used to calculate spike-in factors (Orlando et al. 2014). Calculated spike-in factors were used to convert bam files into normalized bigwig files using deepTools bamCoverage subpackage, with –scaleFactor setting, generating Reference-adjusted Reads Per Million (RRPM) files with a 10-bp resolution. Encode blacklist regions were removed. Spearman correlation scores were calculated by partitioning the mappable genome into 500-bp bins and obtaining the RRPM within each bin. Omni-ATAC narrow peaks were obtained using MACS2 version 2.1.2 (Zhang et al. 2008) with settings: “–nolambda –nomodel –extsize 150 –shift 75 –keep-dup all,” and H3K4me3 ChIP-seq and CUT&Tag peaks were obtained with settings: “–nolambda –nomodel –keep-dup all.” Fraction of Reads in Peaks (FRiP) scores were calculated using FeatureCounts of Subread version 1.6.1. (Liao et al. 2013). Peak overlap and genomic distribution of peaks were determined using R package ChIPseeker (Yu et al. 2015).
Graph plots
Plots and diagrams were generated using GraphPad Prism, BioRender.com and R.
Data availability
Previously published RNA-seq expression data are accessible through Gene Expression Omnibus (GEO) repository under series accession number GSE83431. Data obtained for this manuscript are accessible through GEO repository under series accession number GSE169328. Scripts used for bioinformatic analysis and plot generation are available as supplementary files. Detailed protocols for NIE, RNA-seq, Omni-ATAC, ChIP-seq, and CUT&Tag are available at dx.doi.org/10.17504/protocols.io.buiqnudw. Flies carrying UAS-GFPKASH, as well as additional flies with different KASH-tagged epitopes are available at Bloomington Drosophila Stock Center (see Figure 7C). pUASTattB-2xFlag-mCherry-Msp300KASH and pUASTattB-GFP-Msp300KASH plasmids are available at AddGene (#170807 and #170806, respectively). Supplementary material is available at figshare: https://doi.org/10.25386/genetics.14558286.
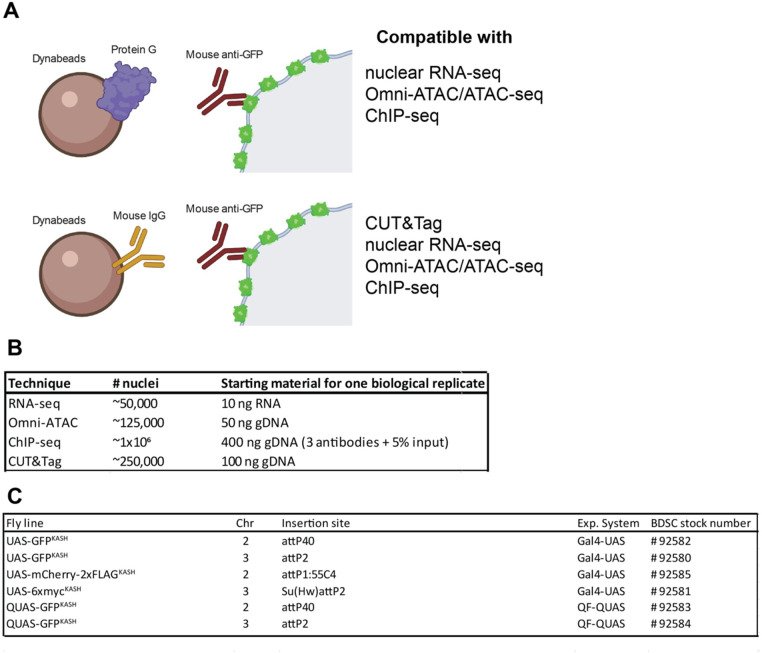
Figure 7.
Method summary. (A) Schematic diagram representing the two versions of the “improved” NEI-method. The first version (top) uses protein G-coupled magnetic Dynabeads, and can be coupled with RNA-seq, Omni-ATAC, and ChIP-seq. The second version (bottom) uses Mouse IgG-coupled magnetic beads, and can be coupled with Cleavage Under Targets and Tagmentation (CUT&Tag), RNA-seq, Omni-ATAC, and ChIP-seq. (B) Table describing the available fly lines to perform NIE either using the Gal4-UAS or the QF-QUAS system.
Results
Optimization of tissue-specific nuclei immuno-enrichment from adult Drosophila
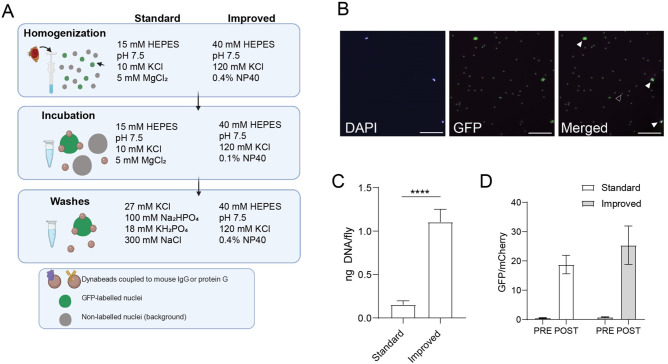
As a starting point for profiling chromatin states in specific cell types in Drosophila, we sought to improve nuclei yields obtained with the NIE protocol using flies that express the GFPKASH tag in outer photoreceptor neurons driven by Rh1-Gal4 (herein referred as Rh1 > GFPKASH) (Mollereau et al. 2000). We reasoned that isolating nuclei in a buffer designed to retain the integrity of the nuclear envelope would increase the availability of the GFPKASH epitope, which is anchored to the outer nuclear membrane with GFP facing the cytoplasm (Fischer et al. 2004). Previous studies have shown that perinuclear proteins are retained when nuclei are purified using a detergent-containing isotonic buffer (Shaiken and Opekun 2014), suggesting that the outer nuclear membrane remains intact under these conditions. Based on this rationale, we replaced the hypotonic/hypertonic buffers used in the homogenization, incubation, and washing steps of our previous NIE method with detergent-containing isotonic buffers. We also decreased the relatively high concentration of NP-40 detergent used for homogenization during the immunoprecipitation steps to decrease background binding (see Materials and Methods). We refer to our previous and new NIE approaches as the “standard” and “improved” methods, respectively (Figure 1A).
Figure 1.
Optimization of tissue-specific NIE from adult Drosophila. (A) Schematic diagram depicting the NIE protocol highlighting major differences in buffer composition between the “standard” and “improved” methods. Heads from flies expressing Rh1 > GFPKASH were homogenized, followed by bead-antibody incubation and washes. (B) Microscopy images of POST sample using the “improved” method. Scale bars: 50 µm. White arrowhead: bead-bound nuclei. Black arrowhead: single bead. (C) Bar plot showing DNA yields when Rh1 > GFPKASH nuclei were enriched using either the “standard” or “improved” NIE method [mean ± standard deviation (SD), n = 4, P-value t-test]. D. Bar plot showing qPCR enrichment for GFP and mCherry in the PRE and POST-NIE samples comparing “methods” (mean ± SD; n = 3, P-value t-test).
We first assessed how nuclei yields varied based on the NIE method used. To do this, we performed GFPKASH-based NIE using either the “standard” or “improved” method and quantified total DNA after each NIE reaction (n = 4). We used DNA yield as a measure of nuclei yield because the magnetic beads used in the NIE auto fluoresce, making it difficult to quantify nuclei accurately using microscopy-based techniques (Figure 1B). The “improved” method yielded 1.2 ng of DNA per fly, compared to 0.2 ng of DNA for the “standard” method (Figure 1C). Considering that there are ∼7200 outer photoreceptors per fly, and that a diploid Drosophila nucleus typically contains ∼0.36 pg DNA (Rasch et al. 1971), the “improved” method yields around 45% of the tagged nuclei compared with 13% for the “standard” approach. We note that the starting material for each NIE reaction was 400 age-matched Rh1 > GFPKASH flies homozygous for both Gal4 and UAS transgenes; nuclei yield decreased approximately twofold when GFPKASH-based NIE was performed using flies heterozygous for both transgenes (Supplementary Figure S1A), suggesting that higher GFPKASH expression levels can further improve purification efficiency.
Next, we evaluated if the NIE-purified nuclei were enriched relative to background cell types. To do this, we mixed an equivalent number of Rh1 > GFPKASH flies with Rh1 > mCherry-FLAGKASH, performed GFP-based NIE, and extracted DNA before (PRE) and after (POST) immuno-enrichment. We then quantified the relative genomic copies of GFP and mCherry in each sample using quantitative PCR (qPCR). If nuclei from the POST sample are depleted of the mCherryKASH-positive nuclei upon GFP-based NIE, then the ratio of GFP/mCherry for the POST sample will be higher than the value of one observed in the PRE sample, which contains an equivalent number of GFP and mCherry labeled nuclei. Using this approach, we observed 24-fold enrichment of GFP nuclei over mCherry using the “improved” method, which compared favorably with the 20-fold enrichment observed using the “standard” method (Figure 1D).
The transcriptome of nuclei purified with the “improved” approach is depleted of genes enriched in other cell types relative to the “standard” approach
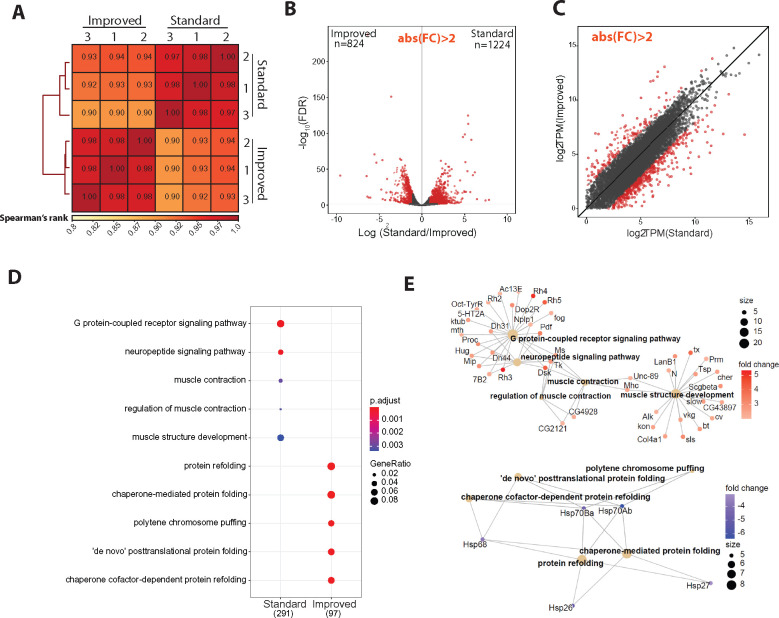
Because we had previously generated high-quality nuclear RNA-seq from outer photoreceptor nuclei using the “standard” approach (Hall et al. 2017), we profiled the nuclear transcriptome of NIE-purified outer photoreceptor nuclei (Rh1 > GFPKASH) using the “improved” method and compared the transcriptome between methods; we note that the identical genotype, sex, and age were used for both studies, and that both library sets were generated using the same amount of RNA. We first analyzed similarity between the two datasets by calculating Spearman correlation for gene counts (Figure 2A). Spearman’s rank scores between replicates were high for both methods (P < 0.97), and samples clustered together based on the method used for NIE. Furthermore, we also observed similar clustering by NIE approach using principal component analysis (PCA) (Supplementary Figure S2). Notably, the variation between biological replicates slightly decreased using the improved method.
Figure 2.
The transcriptome of nuclei purified with the “improved” approach is depleted of genes enriched in other cell types relative to the “standard” approach. (A) Spearman correlation heatmap of gene expression profiles from nuclear RNA-seq of nuclei extracted with standard and improved method (n = 4). Scores between 0 and 1 shown in each box correspond to Spearman’s rank score. (B) Volcano plot showing the differentially expressed genes between methods. Genes with significant differential expression (FC > 2, FDR < 0.01) are highlighted in red. (C) Scatter plot showing log2-transformed transcript per million (TPM) values between methods. Differentially expressed genes are highlighted in red, as in panels (B, D). Gene Ontology (GO) term analysis on genes that are overrepresented in either the “standard” or “improved” method. (E) Gene Concept Network plot (Cnetplot) highlighting linkage of individual genes and associated functional categories of genes over-represented in standard (top) and improved (bottom) dataset. Color intensity represents fold change between conditions.
The observation that samples clustered by method suggested there were differences between the datasets obtained using the different NIE methods. We sought to identify the differences in gene expression associated with each NIE method by analyzing differentially expressed genes (DEGs) (n = 3). Surprisingly, we identified 2046 DEGs (False Discovery Rate, FDR < 0.01; Fold change, FC > 2) between the two NIE methods, despite their identical genotypes, sex, and age (Figure 2B). Amongst these genes, 824 genes were upregulated in the improved dataset, and 1224 genes were upregulated in the standard dataset, representing improved- or standard-enriched genes, respectively. RNA-seq libraries for each experiment were made using different RNA-seq kits (see Materials and Methods). Because we used a kit designed for low-input material (200 pg–10 ng RNA) to make the improved dataset libraries, we wondered if genes enriched in the improved dataset were being quantified as lowly expressed in the standard dataset. However, the identified DEGs spanned a wide range of expression levels, including low, medium, and highly expressed genes (Figure 2C), suggesting that differences in amplification of lowly abundant transcripts do not account for the differences in expression observed between the two approaches. Instead, inspection of the top DEGs in each condition revealed that several rhodopsin genes (Rh3, Rh4, and Rh6) were enriched in the standard method relative to the improved method. These rhodopsin proteins are highly enriched in inner photoreceptors (R7–R8) and are also expressed in the Johnston organ (Göpfert and Robert 2001; Stark and Thomas 2004), but are not expressed in outer photoreceptors; conversely, Rh1-Gal4 is expressed only in the outer photoreceptors (Mollereau et al. 2000). Because inner photoreceptor-specific genes were enriched in the standard dataset, these observations suggest that the “improved” method yields a more tissue-specific enriched nuclei pool relative to our previous approach. GO-term analysis of genes that were upregulated in each dataset revealed that the standard-enriched DEGs were enriched for categories such as neuropeptide signaling pathway, muscle contraction, and muscle structure development (Figure 2D, top). Furthermore, gene-concept network analysis revealed enrichment of 42 genes associated with non-photoreceptor cell types in the standard-enriched DEGs, including ventral lateral neuron-expressed Pdf (FBgn0023178), protocerebrum-enriched Dsk (FBgn0000500), and muscle-enriched Unc-89 (FBgn0053519) (Figure 2E) (Nichols et al. 1988; Helfrich‐Förster & Homberg 1993). In contrast, GO terms over-represented in the improved-enriched DEGs were associated with processes related to protein folding and polytene chromosome puffing (Figure 2D, bottom). Gene-concept network analysis revealed that the over-representation of these GO-term categories were driven by a modest enrichment of five Heat Shock Protein (Hsp) genes (Figure 2F).
Because of a statistically significant over-representation of biological processes associated with other cell types in “standard” enriched dataset, these findings suggest that nuclei purified using the “improved” NIE method have higher enrichment of tissue-specific transcripts compared to the ‘standard’ approach. However, we note that these experiments were performed at different times, and therefore batch effects and other variables might also influence our observations. Considering that the “improved” method also had higher nuclei yields, we proceeded to optimize the subsequent chromatin profiling methods with NIE-purified outer photoreceptor nuclei from Rh1 > GFPKASH flies using this method.
Profiling chromatin accessibility (Omni-ATAC) in NIE-purified nuclei
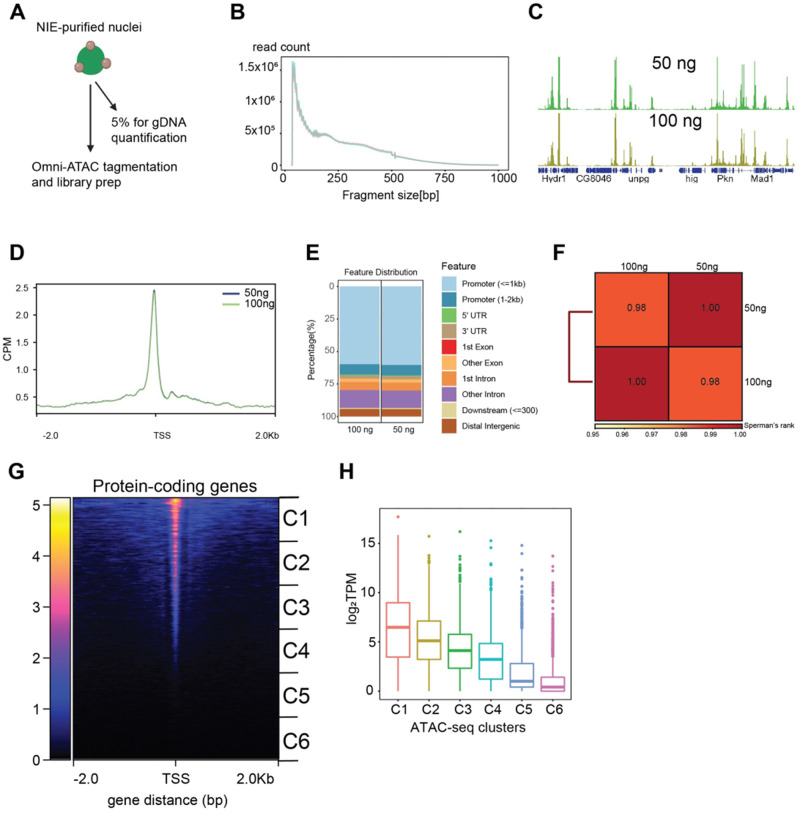
We next sought to profile accessible chromatin of NIE-purified nuclei using Omni-ATAC, a recently modified ATAC-seq technique which yields higher quality data, especially with lower input (Corces et al. 2017). ATAC-seq techniques, including Omni-ATAC, require optimization of the number of nuclei or cells used for each reaction to generate appropriate DNA fragment sizes and avoid either under- or over-tagmentation. Normally, cultured cells are counted to achieve precise numbers of cells per assay. However, nuclei bound to magnetic beads cannot be easily quantified using an automatic cell counter because the free magnetic beads interfere with the identification of individual nuclei (see Figure 1B). To overcome this limitation, we isolated genomic DNA from a fraction of the purified nuclei and normalized input material for Omni-ATAC reactions based on this quantification (Figure 3A). We note that because our protocol begins with NIE-purified nuclei, mitochondria are already depleted from the initial starting material, as shown by qPCR analysis of mitochondrial DNA present in the PRE and POST NIE samples (Supplementary Figure S3A). To evaluate whether differences in starting material would substantially alter data quality, we performed Omni-ATAC using either 50 or 100 ng of DNA (corresponding to approximately 125,000 and 250,000 nuclei, respectively) with a fixed amount of Tn5.
Figure 3.
Profiling chromatin accessibility (Omni-ATAC) in NIE-purified nuclei. (A) Diagram depicting Omni-ATAC approach applied to NIE-purified nuclei. After NIE purification, a fraction of nuclei is used for genomic DNA extraction and quantification to determine the input material for Omni-ATAC. Nuclei remain on ice until tagmentation, followed by two washes with tagmentation buffer without Tn5 enzyme. Upon washes, nuclei are tagmented using standard ATAC-seq conditions. (B) Fragment size distribution of Omni-ATAC libraries using 50 ng (light green) or 100 ng (light red) as starting material. (C) Genome browser views of CPM-normalized Omni-ATAC signal with genes shown in blue. (D) Metaplot of CPM-normalized Omni-ATAC signal around the transcription start site (TSS) averaged for all protein-coding genes in the 50 and 100 ng samples. (E) Genomic distribution of accessible peaks of 50- and 100 ng- associated dataset. (F) Spearman correlation heatmap of Omni-ATAC read distribution over binned genome. Scores between 0 and 1 shown in each box correspond to Spearman’s rank score. (G) Heatmap showing CPM-normalized Omni-ATAC signal around TSS of protein-coding genes of 100 ng-associated dataset. Clusters used for transcript boxplot are highlighted. (H) Boxplot showing log2-transformed TPM scores for each cluster defined in 3G.
Tapestation analysis of Omni-ATAC libraries revealed similar DNA laddering patterns with both amounts of input nuclei (Supplementary Figure S3B). We then sequenced these libraries, and evaluated the size distribution of the mapped fragments. We observed the expected nucleosomal phasing distribution in both libraries (Figure 3B), with the first peak (80–120 bp) corresponding to nucleosome-depleted region (NDR)-associated DNA, followed by a peak around 180 bp corresponding to mononucleosome-associated fragments. Genome browser inspection of the data revealed discrete peaks with similar enrichment profiles obtained under each condition (Figure 3C). Because the Omni-ATAC signal should be enriched around transcriptional start sites (TSS), we next evaluated read distribution around the TSS of protein-coding genes (Figure 3D). We observed a significant enrichment of Omni-ATAC signal around the TSS with no differences between the 50 - and 100 ng-associated datasets. This finding was further corroborated by heatmap plots of all protein-coding genes ranked based on their Omni-ATAC signal enrichment around the TSS (Supplementary Figure S3C).
Next, we evaluated the genomic distribution of peaks from both samples (Figure 3E). As expected from the observed enrichment of Omni-ATAC signal around the TSS (Figure 3C), 70% of the peaks mapped to promoters with no discernible differences in distribution between the two samples, and samples had a Spearman’s score of 0.98. Because accessible chromatin is enriched for active promoters, we next evaluated if chromatin accessibility levels correlated with transcript levels detected by nuclear RNA-seq (see Figure 2). To do this, we divided the 13,930 genes in the Drosophila genome based on their position on the heatmap into six groups, where genes are ranked based on the Omni-ATAC signal around the TSS (Figure 3G), and plotted the transcript level (log2 transcript per million—TPM) for all genes in each cluster (Figure 3H). We observed a positive correlation between the levels of chromatin accessibility at the TSS and transcript expression levels. Altogether, these observations suggest that high-quality chromatin accessibility data can be obtained from NIE-purified nuclei using as little as 50 ng of DNA equivalent of starting material, when coupled with Omni-ATAC.
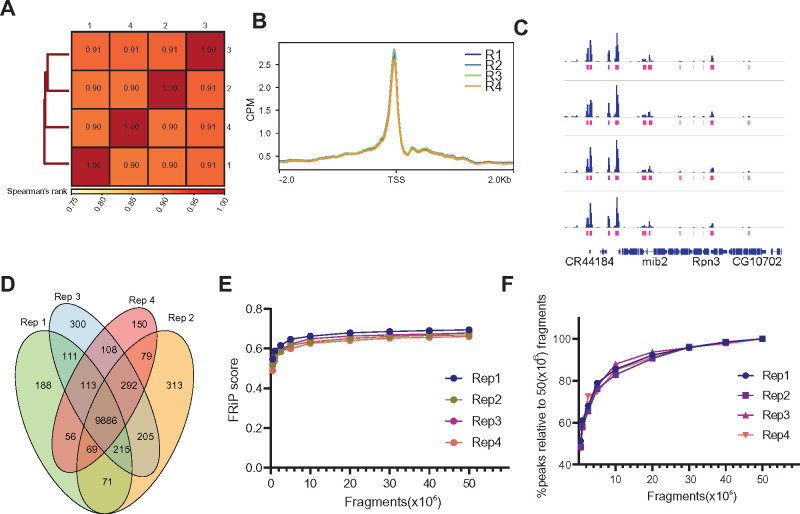
Omni-ATAC of NIE-purified nuclei does not require high sequencing depth
To benchmark the quality and reproducibility of the Omni-ATAC protocol using the NIE-purified nuclei, we sought to systematically evaluate different quality control metrics of ATAC-seq datasets. We performed Omni-ATAC on NIE-purified nuclei equivalent to 100 ng of DNA in four independent biological samples, processing and analyzing each replicate individually (n = 4). We first calculated the Spearman’s correlation based on read distribution over a 500-bp binned genome, and found high reproducibility between samples, with Spearman’s p scores above 0.90 (Figure 4A). Next, we plotted the Omni-ATAC signal around the TSS of protein-coding genes (Figure 4B). We observed that the enrichment profiles around the TSS were highly consistent between replicates, corroborating the Spearman’s correlation analysis. Next, we sought to evaluate the quality of peak-based analysis for each sample. Genome browser inspection of Omni-ATAC signal next to the peaks corresponding to each replicate showed high consistency, as determined by signal intensity of peaks (Figure 4C). Furthermore, 88% of peaks presented significant overlap amongst all four replicates (Figure 4C). Similarly, we observed high concordance by Irreproducible Discovery Rate (IDR) analysis of peaks between replicates (Supplementary Figure S4A), with all pair-wise comparisons having an IDR value above 0.61. The FRiP score is a common quality control metric for genomic datasets, such as ChIP-seq and ATAC-seq, that measures overall signal-to-background ratio, as defined by ENCODE guidelines (Landt et al. 2012). According to ENCODE, good quality ATAC-seq datasets are defined as having FRiP score higher than 0.3. Thus, we next evaluated how FRiP scores varied based on sequencing depth. To do this, we down-sampled each replicate to 0.5, 1, 2.5, 5, 10, 20, 30, 40, and 50 million mapped fragments, and obtained its corresponding FRiP score (Figure 4E). FRiP scores did not vary significantly between replicates, and surprisingly, there was no substantial improvement in FRiP scores past 10 million mapped fragments. Furthermore, visual inspection of the down-sampled data on a genome browser revealed similar enrichment of peaks at only 0.5 million fragments, resembling that observed using 50 million fragments (Supplementary Figure S4B). Next, we evaluated the number of peaks called for each sample based on the number of fragments (Figure 4G). As expected, peak calling benefited from the higher sequencing depth. However, when the number of peaks identified was normalized to the sample with the greatest sequencing depth (50 million mapped fragments), we found that obtaining 20 million fragments identified approximately 80% of all possible peaks. Taken together, these observations imply that Omni-ATAC datasets do not require high sequencing depth for consistent gene- and peak-based analysis, and that 10–20 million reads is likely sufficient for most peak-based analyses in Drosophila samples.
Figure 4.
Omni-ATAC of NIE-purified nuclei does not require high sequencing depth. (A) Spearman correlation heatmap of Omni-ATAC read distribution over binned genome. Scores between 0 and 1 shown in each box correspond to Spearman’s rank score. (B) Metaplot of CPM-normalized Omni-ATAC signal around TSS averaged for all protein-coding genes comparing replicates (n = 4). (C) Genome browser inspection of CPM-normalized Omni-ATAC signal for each replicate, coupled with narrow peaks (pink). Genes are shown in blue. (D) Venn diagram showing peak overlap/similarity between replicates. (E) Fraction of Reads in Peaks (FRiP) scores of Omni-ATAC peaks comparing replicates down-sampled from 0.5 to 50 million mapped fragments. (F) Percentage of peaks called relative to peaks called using the Omni-ATAC replicate #1, with 50 × 106 mapped fragments as absolute percent of peaks.
The histone methylation landscape of adult Drosophila photoreceptors
ChIP is one of the most commonly used techniques in the genomics field, whereby sonicated chromatin is used to immunopurify a protein-DNA complex, followed by purification of the enriched DNA. Coupled with qPCR or high-throughput sequencing (ChIP-seq), it allows researchers to interrogate if a protein of interest is bound to a particular locus, or assay its genome-wide distribution, respectively. We sought to optimize a ChIP protocol suitable for use with NIE-purified nuclei. During development of the protocol, we initially found that fixing the nuclei during homogenization led to an increase in background nuclei upon NIE (data not shown), leading us to modify the protocol so that the chromatin was cross-linked while the nuclei were immobilized on the magnetic beads, immediately following NIE (Figure 5A). Chromatin was then sonicated, and ChIP performed using standard approaches (see Materials and Methods).
Figure 5.
The histone methylation landscape of adult Drosophila photoreceptors. (A) Diagram depicting Chromatin Immunoprecipitation (ChIP)-seq approach coupled to NIE-purified nuclei. Before adding the ChIP antibody, a fraction of soluble Drosophila chromatin (input) is quantified, to adjust final amount of chromatin per replicate, as well as to define amount of spike-in genome (In this case, 5% of Arabidopsis chromatin). (B) Metaplots of H3 (dark blue), H3K4me3 (light blue) and H3K36me3 (yellow) ChIP-seq signal (CPM) over gene bodies averaged for all protein-coding genes. (C) Genome browser inspection of H3, H3K4me3, and H3K36me3 ChIP-seq signal (CPM) around the inner photoreceptor-specific gene Rh3, which is not expressed in outer photoreceptors, and two highly expressed outer photoreceptor-specific genes trp and trpl. (D) Spearman correlation heatmap of H3K4me3 ChIP-seq data comparing Spike-in and CPM normalization. Spearman’s rank scores are based on read distribution over binned genome. (E) Spearman correlation heatmap of H3K36me3 ChIP-seq data comparing Spike-in and CPM normalization. Spearman’s rank scores are based on read distribution over binned genome. (F) Spearman correlation heatmap of reads that align to binned genome for all replicates of H3, H3K4me3, and H3K36me3 ChIP-seq datasets. (G) Heatmap showing signal for all protein-coding genes of H3-normalized H3K4me3 (left) and H3-normalized H3K36me3 (right). (F) Boxplots showing transcript level expressions of H3K4me3 (top) or H3K36me3 clusters (bottom).
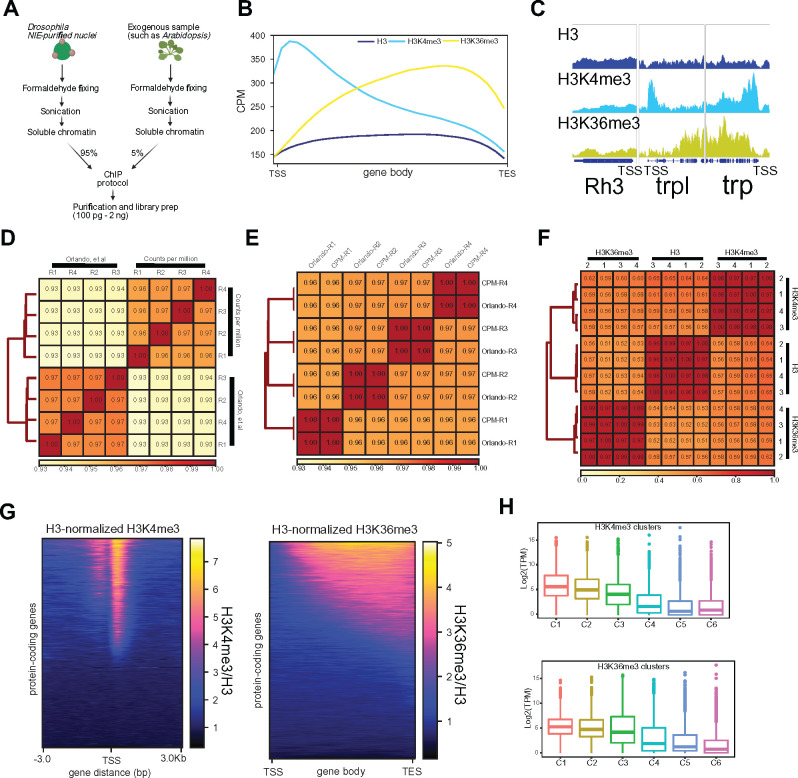
To benchmark the ChIP protocol, we examined genome-wide distribution of two histone methyl marks, Histone H3 Lysine 4 tri-methylation (H3K4me3) and H3 Lysine 36 tri-methylation (H3K36me3), both of which have been widely characterized by ChIP-qPCR and ChIP-seq studies in Drosophila and other organisms. We also examined the distribution of bulk histone H3, as well as an input sonicated chromatin control. First, we assessed the enrichment of each antibody by evaluating the overall distribution of reads over gene bodies for all protein-coding genes. Histone H3 is distributed throughout both active and repressed chromatin, and is usually slightly depleted around the TSS of transcribed genes (Bai and Morozov 2010). In Drosophila, as well as in Saccharomyces cerevisiae and in humans, H3K4me3 is enriched at the TSS whereas H3K36me3 localizes to gene bodies (Edmunds et al. 2008). Consistent with this expected distribution, we observed depletion of histone H3 and enrichment of H3K4me3 around the TSS, while H3K36me3 was enriched towards the 3’ region of the gene body (Figure 5B). Furthermore, genome browser inspection of individual genes, such as the photoreceptor-enriched genes trp and trpl, corroborated the enrichment for H3K4me3 around the TSS and H3K36me3 over the gene body. In contrast, the inner photoreceptor-expressed Rh3 showed no enrichment for either histone mark, as expected based on its lack of expression in outer photoreceptors (Figure 5C).
Next, we assessed the reproducibility between the replicates obtained using our ChIP-seq approach. Given the semi-quantitative nature of ChIP-seq, there has been growing interest in adding exogenous chromatin prior to immunoprecipitation, using the reads that map to the “reference” genome for spike-in normalization (Chen et al. 2016). To facilitate this spike-in normalization approach, we added 5% of A. thaliana chromatin to Drosophila samples before each immunoprecipitation. To evaluate how the similarity between individual samples varied based on the normalization method, we normalized the data using the Arabidopsis spike-ins (as described in Orlando et al. 2014) or calculated traditional CPM or CPMs. We then calculated the Spearman correlation of read coverage over the binned genome for H3K4me3 and H3K36me3 separately (Figure 5, D and E). Interestingly, the H3K4me3 samples clustered based on the normalization method used, although there were no major differences between Spearman’s rank scores obtained for individual samples using either approach. Replicate correlation was high for both normalization methods (P > 0.96 for both normalization methods). Strikingly, the H3K36me3 samples clustered together based on replicate rather than normalization approach, and each replicate had a P = 1, with its normalization counterpart. Corroborating the heatmap findings, metaplot analysis of the H3K4me3 distribution around the TSS and H3K36me3 distribution over gene bodies showed no substantial differences between biological replicates using either normalization approach (Supplementary Figure S5, A and B). To further assess similarity between the replicates based on antibodies used, we next evaluated Spearman’s correlation of CPM-normalized data for H3, H3K4me3, and H3K36me3 (Figure 5F). Corroborating the findings from the global read distribution over gene bodies, samples clustered together based on antibody. Moreover, the correlation between replicates for each antibody was also high (P > 0.96). We also found that normalizing histone methylation ChIP-seq data to the corresponding input did not affect the correlation findings based on samples clustering with antibodies, although Spearman’s rank scores slightly decreased between biological replicates (P > 0.71, P > 0.80, and P > 0.90 for H3, H3K4me3, and H3K36me3, respectively) (Supplementary Figure S5C). Moreover, genome browser inspection revealed that the H3 samples also presented the same amplification bias as input (Supplementary Figure S5D), suggesting that H3 normalization was sufficient for between-sample comparisons. In addition, normalizing the histone methyl marks to histone H3 also controls for nucleosome occupancy.
Because H3K4me3 and H3K36me3 are histone modifications associated with active transcription, we next asked if H3K4me3 and H3K36me3 ChIP-seq signal levels positively correlated with gene expression. To do this, we ranked all protein-coding genes based on H3-normalized H3K4me3 signal around the TSS (Figure 5G, left) or H3-normalized H3K36me3 signal over gene bodies (Figure 5H, right), and separated all 13,930 genes into six clusters based on their level of the respective histone mark. We then examined gene expression for each cluster by plotting transcript levels for each gene in the cluster (log2 -TPM) (Figure 5H). Similar to our observations for the Omni-ATAC clusters, H3K4me3 and H3K36me3 levels positively correlated with active transcription.
Overall, these observations demonstrate that chromatin obtained from NIE-purified nuclei accurately reflect the transcriptional state of these cells and can be used for profiling of chromatin accessibility and histone modifications. Furthermore, in our hands, adding a reference genome for spike-in normalization does not outperform traditional CPM normalization. We note that although the ChIP-seq data shown here was generated from libraries that used 2 ng of DNA as starting material, libraries made with as little as 100 pg of DNA showed comparable profiles (Supplementary Figure S5E), suggesting that this ChIP-seq protocol is amenable to low-input starting material. We also performed qPCR on ChIP samples obtained using this protocol (Supplementary Figure S5F), demonstrating that this approach may be useful for researchers interested in examining individual genes rather than performing genome-wide studies.
NIE-purified nuclei are compatible with CUT&Tag for profiling histone marks
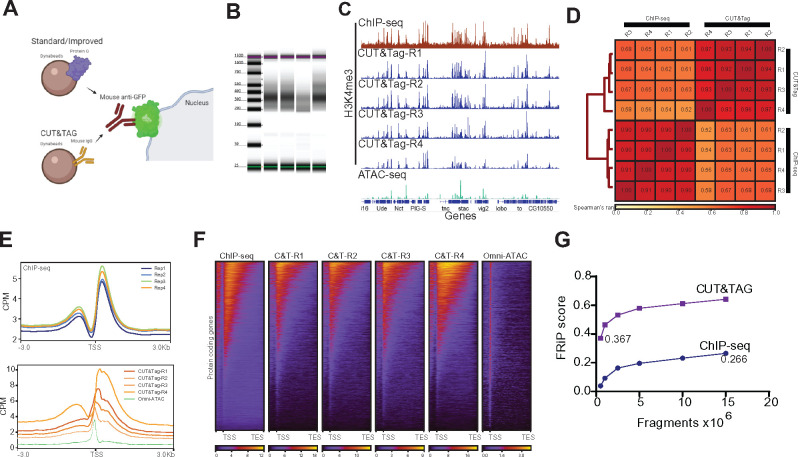
Last, we sought to apply CUT&Tag to NIE-purified nuclei. CUT&Tag is a recently developed technique used to profile chromatin, whereby a fusion protein (pAG-Tn5) targets an antibody-bound chromatin target, followed by tagmentation and release of enriched DNA (Kaya-Okur et al. 2019). CUT&Tag has several advantages over ChIP-seq, including shorter sample processing times and lower background signal, therefore requiring less sequencing depth to identify high probability binding sites for proteins of interest. Furthermore, CUT&Tag yields sequencing-ready libraries with no need for an additional library construction step. Based on these advantages, we sought to develop a CUT&Tag approach suitable for use with NIE-purified nuclei using commercially available Protein A/Protein G-Tn5 (pAG-Tn5).
Standard CUT&Tag protocols require cell/nuclei immobilization with Concanavalin A beads. However, NIE-purified nuclei are already bound to Protein G-magnetic Beads (PGBe), providing an initial starting point for CUT&Tag protocols. Our first H3K4me3 CUT&Tag trials with NIE-purified nuclei using PGBe were unsuccessful, and we wondered if the rabbit anti-H3K4me3 antibodies were being adsorbed by the excess protein G in our nuclei preparations (Figure 6A). To test this possibility, we performed NIE using Mouse IgG-coupled magnetic Beads (MIBe) instead of PGBe. Strikingly, performing NIE with MIBe led to successful purification of DNA following CUT&Tag, suggesting that PGBe were interfering with CUT&Tag steps. We then performed H3K4me3 CUT&Tag using age and sex-matched photoreceptor nuclei in order to compare the data with H3K4me3 ChIP-seq, since both datasets were obtained using the same antibody. TapeStation profiles of the four replicates detected sub-, mono- and di-nucleosomal fragments, with significant enrichment for mononucleosome-associated DNA (Figure 6B). We then proceeded with paired-end sequencing of the libraries. Genome browser inspection of CPM-normalized H3K4me3 CUT&Tag, H3K4me3 ChIP-seq, and Omni-ATAC signal (Figure 6C) revealed that H3K4me3 CUT&Tag signal had a more similar distribution to ChIP-seq than Omni-ATAC based on the number of “pseudo-peaks,” or high signal points. Notably, background levels in H3K4me3 CUT&Tag were much lower than ChIP-seq, as shown by signal in intergenic regions. To further assess the correlation between each CUT&Tag replicate, we calculated Spearman’s correlation rank scores. Because CUT&Tag data had very low levels of background relative to ChIP-seq, we calculated the correlation based on read coverage over the narrow peaks obtained from the H3K4me3 ChIP-seq data instead of the binned genome. As expected from the above comparisons, samples clustered together based on technique. Using this approach, ChIP-seq samples had higher correlation values between individual replicates (P > 0.9) compared with CUT&Tag replicates (P > 0.83).
Figure 6.
Bead modification in NIE protocol allows application of Cleavage Under Targets and Tagmentation (CUT&Tag). (A) Schematic diagram representing the major difference between bead-antibody conjugation necessary to perform CUT&Tag in NIE-purified nuclei. Protein-G Dynabeads recognize both rabbit and mouse antibodies, while Mouse Pan IgG Dynabeads only recognize mouse antibodies. Nuclei preparation contains excess Dynabeads, therefore the protein G can interfere with CUT&Tag because it can bind the rabbit antibodies used to tag chromatin targets, such as H3K4me3. (B) Tape Station profiles of H3K4me3 CUT&Tag libraries. (C) Genome browser inspection (IGV) of CPM-normalized H3K4me3 ChIP-seq (top), H3K4me3 CUT&Tag replicates (medium) and Omni-ATAC (bottom). All samples were obtained from 10-day-old male flies. Genes are shown in blue. (D) Fraction of Reads in Peaks (FRiP) score comparison between H3K4me3 CUT&Tag replicate 4 and H3K4me3 ChIP-seq replicate 1. Both samples were down sampled from 0.5 to 15 million mapped fragments. (E) Metaplots of CPM-normalized H3K4me3 ChIP-seq (top) and H3K4me3 CUT&Tag (bottom) (n = 4 for each method). (F) Heatmaps showing CPM-normalized H3K4me3 ChIP-seq (left-most) and H3K4me3 CUT&Tag signal for all replicates, with rows representing the same gene across all heatmaps. (G) Spearman correlation heatmap of read distribution over H3K4me3 peaks called using ChIP-seq datasets. Correlation was calculated for H3K4me3 ChIP-seq and CUT&Tag replicates.
Because CUT&Tag replicates had a relative lower similarity compared to ChIP-seq, we next evaluated genome-wide signal distribution of H3K4me3 ChIP-seq and CUT&Tag around the TSS using metaplots. Signal distribution of H3K4me3 ChIP-seq around the TSS was highly consistent between replicates (Figure 6E, top), which further corroborated the Spearman’s analysis. Surprisingly, H3K4me3 CUT&Tag signal was highly variable between replicates, both in intensity and signal distribution (Figure 6E, bottom). In addition, metaplots revealed that CUT&Tag replicates 1, 2, and 3 had a substantial increase in signal around the TSS that resembled the Omni-ATAC signal. To further assess to what extent the CUT&Tag signal originated from H3K4me3-associated DNA, we obtained heatmaps of CPM-normalized signal over gene bodies and compared ChIP-seq, CUT&Tag and Omni-ATAC. Heatmaps revealed that overall, CUT&Tag replicates showed similar signal distribution over gene bodies, with similar distribution to ChIP-seq (Figure 6F). Notably, Omni-ATAC signal was highly enriched around the TSS, with no significant enrichment over gene bodies. Finally, we evaluated the signal to background ratio for H3K4me3 CUT&Tag data compared to H3K4me3 ChIP-seq using the CUT&Tag replicate that most closely resembled ChIP-seq (R4). To do this, we obtained FRiP scores for the H3K4me3 CUT&Tag replicate 4 and H3K4me3 ChIP-seq by down-sampling the H3K4me3 CUT&Tag-R4 and ChIP-seq samples to 0.5, 1, 2.5, 5, 10, and 15 million mapped fragments (Figure 6D). Notably, CUT&Tag substantially outperformed ChIP-seq with a FRiP score of 0.367 for CUT&Tag data even at only 0.5 million mapped fragments. In comparison, the FRiP score for ChIP-seq data only reached 0.266 at 15 million fragments.
Taken together, these observations indicate that a slight modification to the NIE reagents makes it possible to apply CUT&Tag to NIE-purified nuclei, providing a cost effective and efficient way of examining the genome-wide distribution of DNA-binding proteins. However, we note that the increased variability observed between CUT&Tag replicates relative to ChIP-seq samples, as well as the presence of accessible DNA in some of the CUT&Tag datasets, suggests that further optimization to the protocol is required to improve the quality of the datasets.
Discussion
Here, we demonstrate the feasibility of chromatin profiling in specific cell types using immuno-enriched nuclei as starting material and show that profiling of chromatin accessibility and histone modifications associated with active transcription correlate with the transcriptional state of the profiled cell type. Our NIE approach enables isolation of nuclei within 1 hour, which can be subsequently used for RNA, DNA, and chromatin extraction, therefore enabling the application of RNA-seq, ATAC-seq, ChIP-seq, and CUT&Tag (Figure 7A). Compared to our “standard” approach, the “improved” NIE approach increased the nuclei yield fourfold, as shown by genomic DNA quantification. In our hands, we have found that quantifying genomic DNA was the simplest and most robust approach to normalizing input amounts for samples because we could easily extract DNA from a small fraction of each sample, and process and quantify multiple samples within ∼20–30 minutes. This allowed for robust and reproducible quantification of several samples at the same time. Nonetheless, it is possible to use different quantification techniques to assess nuclei yields, such as nuclei counting using a hemocytometer, since nuclei can be easily stained with DAPI and counted manually using a fluorescent microscope.
By isolating nuclei, rather than cells, we can obtain highly pure nuclear RNA that provides a view of the actively transcribed genome. While these data correlate with the adult photoreceptor transcriptome determined in our previous studies using a similar approach (Hall et al. 2017), our modified NIE protocol results in significant decrease in transcript levels of genes associated with other cell types. However, we cannot conclude on whether these differences are biological or technical, or both, because these experiments were performed at different times and there could be artifacts induced by differences in the experimental conditions that could also influence gene quantification, such batch effects and different library preparation kits. Combining this improved NIE approach with library construction kits developed for low RNA inputs, such as the one used in this study, will facilitate RNA-seq studies on much rarer cell populations, or on cells labeled in mosaic animals, that have previously been difficult to analyze using other techniques.
In addition to RNA-seq, we profiled accessible chromatin at a genome-wide scale in the NIE-purified nuclei using Omni-ATAC. To our knowledge, this is the first report of cell-type specific chromatin accessibility data in adult Drosophila, although ATAC-seq studies have been performed in different embryonic cell-types isolated using the INTACT method (Bozek et al. 2019) and in dissected larval imaginal discs (Davie et al. 2015). Here, we show that using as little as 50 ng DNA equivalent of NIE-purified nuclei was sufficient to produce high-quality genome-wide chromatin accessibility data, suggesting that this technique should be suitable for lowly abundant cell types. Published reports have shown that ATAC-seq and Omni-ATAC can be applied to as little as 500 human cells (Buenrostro et al. 2013; Corces et al. 2017), indicating that these chromatin profiling approaches are highly amenable to low input samples.
We also applied two approaches to profile genome-wide distribution of histone modifications, ChIP-seq and CUT&Tag. Our ChIP-seq protocol is amenable to incorporation of exogenous chromatin for spike-in normalization, although in our hands, normalizing the ChIP-seq data with a published spike-in normalization approach did not outperform traditional CPM normalization. We note that there has been discussion of the caveats for spike-in normalization with regard to ChIP-seq data (Refer to Dickson 2020). Last, we switched the beads used for NIE from protein-G Dynabeads to mouse IgG Dynabeads, allowing the successful application of H3K4me3 CUT&Tag to NIE-purified nuclei. To our knowledge, this work is the first report of tissue-specific CUT&Tag in Drosophila. Although the H3K4me3 CUT&Tag data showed increased variability, and yielded a combination of accessible and H3K4me3 associated DNA, FRiP score evaluation showed that even at a low sequencing depth (1 × 106 mapped fragments), H3K4me3 CUT&Tag signal-to-background ratio outperformed the ChIP-seq data obtained using the same antibody. We expect NIE-purified nuclei to be compatible with CUT&RUN techniques using a similar approach to that described in this study, since both techniques are based on the same principle; CUT&RUN uses MNase to digest and release enriched DNA (Skene and Henikoff 2017).
Together, our data demonstrate that combining the improved NIE protocol with commonly used chromatin profiling techniques provides a feasible approach to characterizing the transcriptome and epigenome of specific cell types in Drosophila. In our hands, purifying nuclei using homozygous Rh1-Gal4, UAS-GFPKASH yield 1.2 and 0.67 ng of DNA and RNA, respectively, per fly. Based on these estimations, we have calculated how many nuclei would be required to perform all the experiments presented in this manuscript (Figure 7B). In addition, the NIE approach will facilitate researchers interested in profiling different aspects of chromatin biology using a single biological sample because purified nuclei can be split for RNA, DNA, and chromatin extraction. Nuclei resuspended in Trizol or a Trizol-derivative are stable and can be kept for later RNA extraction. In addition, in our hands, nuclei can be incubated on ice for 1 hour before tagmentation without loss of chromatin integrity. Based on the wealth of available Gal4 drivers for cell type-specific expression in Drosophila, the NIE approach described here provides a flexible and resourceful chromatin profiling toolkit for researchers to interrogate chromatin-associated processes in a tissue-specific context. Additionally, we have generated fly stocks expressing the GFPKASH tag under the Q binary expression system (Potter et al. 2010) as well as UAS lines that tag nuclei with either mCherry-FLAG or 6xmyc to provide additional flexibility for studies in Drosophila. These stocks are available at Bloomington Drosophila Stock Center (Figure 7C).
Acknowledgments
The authors thank all members of the Weake lab and Dr. Hana Hall for their suggestions for the manuscript. They also thank Dr. Ulrike Litzenburger for her assistance during Omni-ATAC troubleshooting, and Dr. Xiangying (Candy) Mao and Dr. Clint Chapple for providing the A. thaliana samples. Information from FlyBase was used in this study.
Funding
Support from the American Cancer Society Institutional Research Grant (IRG #58-006-53) to the Purdue University Center for Cancer Research is gratefully acknowledged. The RNA-seq work was supported, in part by the Indiana Clinical and Translational Sciences Institute funded by Award Number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. Research reported in this publication was also supported by a Bird Stair Research Fellowship (Biochemistry Department, Purdue University) to J.J.L., and by the National Eye Institute of the NIH under Award Number R01EY024905 to V.M.W.
Conflicts of interest
None declared.
Literature cited
- Agrawal P, Chung P, Heberlein U, Kent C.. 2019. Enabling cell-type-specific behavioral epigenetics in Drosophila: a modified high-yield INTACT method reveals the impact of social environment on the epigenetic landscape in dopaminergic neurons. BMC Biol. 17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire RC, Madhani HD.. 2018. Ten principles of heterochromatin formation and function. Nat Rev Mol Cell Biol. 19:229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati S, Yu P, McKinney EC, Kandasamy MK, Hartzell D, et al. 2016. Adipocyte nuclei captured from VAT and SAT. BMC Obes. 3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin NM, Greco TM, Kuchenbrod LM, Rigney MM, Chung M-I, et al. 2014. Proteomic profiling of cardiac tissue by isolation of nuclei tagged in specific cell types (INTACT). Development. 141:962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Morozov AV.. 2010. Gene regulation by nucleosome positioning. Trends Genet. 26:476–483. [DOI] [PubMed] [Google Scholar]
- Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, et al. 2018. Comprehensive characterization of cancer driver genes and mutations. Cell. 173:371–385. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BolgerAM, , LohseM, , Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolus H, Crocker K, Boekhoff-Falk G, Chtarbanova S.. 2020. Modeling neurodegenerative disorders in Drosophila melanogaster. IJMS. 21:3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn S, Zinzen RP, Perez-Gonzalez A, Riddell A, Gavin A-C, et al. 2012. Cell type-specific chromatin immunoprecipitation from multicellular complex samples using BiTS-ChIP. Nat Protoc. 7:978–994. [DOI] [PubMed] [Google Scholar]
- Bozek M, Cortini R, Storti AE, Unnerstall U, Gaul U, et al. 2019. ATAC-seq reveals regional differences in enhancer accessibility during the establishment of spatial coordinates in the Drosophila blastoderm. Genome Res. 29:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahma S, Henikoff S.. 2020. Epigenome regulation by dynamic nucleosome unwrapping. Trends Biochem Sci. 45:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N.. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118:401–415. [DOI] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ.. 2013. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 10:1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Hu Z, Xia Z, Zhao D, Li W, et al. 2016. The overlooked fact: fundamental need for spike-In control for virtually all genome-wide analyses. Mol Cell Biol. 36:662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitikova Z, Steiner FA.. 2016. Cell type-specific epigenome profiling using affinity-purified nuclei. Genesis. 54:160–169. [DOI] [PubMed] [Google Scholar]
- Corces MR, Trevino AE, Hamilton EG, Greenside PG, Sinnott-Armstrong NA, et al. 2017. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat Methods. 14:959–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie K, Jacobs J, Atkins M, Potier D, Christiaens V, et al. 2015. Discovery of transcription factors and regulatory regions driving in vivo tumor development by ATAC-seq and FAIRE-seq open chromatin profiling. PLoS Genet. 11:e1004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal RB, Henikoff S.. 2010. A simple method for gene expression and chromatin profiling of individual cell types within a tissue. Dev Cell. 18:1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BM, Tiedemann RL, Chomiak AA, Cornett EM, Vaughan RM, et al. 2020. A physical basis for quantitative ChIP-sequencing. J Biolog Chem. 295:15826–15837. 10.1074/jbc.RA120.015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DobinA, DavisCA, SchlesingerF, DrenkowJ, Zaleski C, . et al. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds JW, Mahadevan LC, Clayton AL.. 2008. Dynamic histone H3 methylation during gene induction: HYPB/Setd2 mediates all H3K36 trimethylation. EMBO J. 27:406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JA, Acosta S, Kenny A, Cater C, Robinson C, et al. 2004. Drosophila Klarsicht has distinct subcellular localization domains for nuclear envelope and microtubule localization in the eye. Genetics. 168:1385–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert MC, Robert D.. 2001. Turning the key on Drosophila audition. Nature. 411:908–908. [DOI] [PubMed] [Google Scholar]
- Graham P, Pick L.. 2017. Drosophila as a model for diabetes and diseases of insulin resistance. Curr Top Dev Biol. 121:397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales KG, Korey CA, Larracuente AM, Roberts DM.. 2015. Genetics on the fly: A primer on the Drosophila model system. Genetics. 201:815–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Ma J, Shekhar S, Leon-Salas WD, Weake VM.. 2018. Blue light induces a neuroprotective gene expression program in Drosophila photoreceptors. BMC Neurosci. 19:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Medina P, Cooper DA, Escobedo SE, Rounds J, et al. 2017. Transcriptome profiling of aging Drosophila photoreceptors reveals gene expression trends that correlate with visual senescence. BMC Genomics. 18:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C, Homberg U.. 1993. Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp Neurol. 337:177–190. [DOI] [PubMed] [Google Scholar]
- Henry GL, Davis FP, Picard S, Eddy SR.. 2012. Cell type–specific genomics of Drosophila neurons. Nucleic Acids Res. 40:9691–9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SG, Nixon KCJ, Chubak MC, Kramer JM.. 2018. Mushroom body specific transcriptome analysis reveals dynamic regulation of learning and memory genes after acquisition of long-term courtship memory in Drosophila. G3 (Bethesda). 8:3433–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya-Okur HS, Wu SJ, Codomo CA, Pledger ES, Bryson TD, et al. 2019. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat Commun. 10:1930. doi:10.1038/s41467-019-09982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm SL, Shipony Z, Greenleaf WJ.. 2019. Chromatin accessibility and the regulatory epigenome. Nat Rev Genet. 20:207–220. [DOI] [PubMed] [Google Scholar]
- Lam K-WG, Brick K, Cheng G, Pratto F, Camerini-Otero RD.. 2019. Cell-type-specific genomics reveals histone modification dynamics in mammalian meiosis. Nat Commun. 10:3821. doi:10.1038/s41467-019-11820-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt SG, Marinov GK, Kundaje A, Kheradpour P, Pauli F, et al. 2012. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 22:1813–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LangmeadB, , Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods. 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HWM, et al. 2015. The epigenetics of aging and neurodegeneration. Prog Neurobiol. 131:21–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiH, HandsakerB, WysokerA, FennellT, Ruan J, . et al. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiaoY, , SmythGK, , Shi W. 2013. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Research. 41:e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Brennan KJ, D’Aloia MR, Pascuzzi PE, Weake VM.. 2016. Transcriptome profiling identifies multiplexin as a target of SAGA deubiquitinase activity in Glia required for precise axon guidance during Drosophila visual development. G3 (Bethesda). 6:2435–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Weake VM.. 2014. Affinity-based isolation of tagged nuclei from Drosophila tissues for gene expression analysis. JoVE. 85:e51418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher KA, Bajic M, Kajala K, Reynoso M, Pauluzzi G, et al. 2018. Profiling of accessible chromatin regions across multiple plant species and cell types reveals common gene regulatory principles and new control modules. Plant Cell. 30:15–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau B, Wernet MF, Beaufils P, Killian D, Pichaud F, et al. 2000. A green fluorescent protein enhancer trap screen in Drosophila photoreceptor cells. Mech Dev. 93:151–160. [DOI] [PubMed] [Google Scholar]
- Nichols R, Schneuwly SA, Dixon JE.. 1988. Identification and characterization of a Drosophila homologue to the vertebrate neuropeptide cholecystokinin. J Biol Chem. 263:12167–12170. [PubMed] [Google Scholar]
- OrlandoDA, ChenMW, BrownVE, SolankiS, Choi YJ, . et al. 2014. Quantitative ChIP-Seq normalization reveals global modulation of the epigenome. Cell Rep. 9:1163–1170. doi: 10.1016/j.celrep.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Piper MDW, Partridge L.. 2018. Drosophila as a model for ageing. Biochim Biophys Acta Mol Basis Dis. 1864(Part A):2707–2717. [DOI] [PubMed] [Google Scholar]
- Potter CJ, Tasic B, Russler EV, Liang L, Luo L.. 2010. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 141:536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RamírezF, , DündarF, , DiehlS, , GrüningBA, , Manke T. 2014. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 42:W187–W191. doi: 10.1093/nar/gku365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RaschEM, , BarrHJ, , Rasch RW. 1971. The DNA content of sperm of Drosophila melanogaster. Chromosoma. 33:1–18. doi: 10.1007/BF00326379. [DOI] [PubMed] [Google Scholar]
- Shaiken TE, Opekun AR.. 2014. Dissecting the cell to nucleus, perinucleus and cytosol. Sci Rep. 4:4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijacic P, Bajic M, McKinney EC, Meagher RB, Deal RB.. 2018. Changes in chromatin accessibility between Arabidopsis stem cells and mesophyll cells illuminate cell type-specific transcription factor networks. Plant J. 94:215–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, Henikoff S.. 2017. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife. 6:e21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slankster E, Kollala S, Baria D, Dailey-Krempel B, Jain R, et al. 2020. Mechanism underlying starvation-dependent modulation of olfactory behavior in Drosophila larva. Sci Rep. 10:3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J, Richly H.. 2017. Regulation of DNA repair mechanisms: how the chromatin environment regulates the DNA damage response. IJMS. 18:1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark WS, Thomas CF.. 2004. Microscopy of multiple visual receptor types in Drosophila. Mol Vis. 10:943–955. [PubMed] [Google Scholar]
- Ugur B, Chen K, Bellen HJ.. 2016. Drosophila tools and assays for the study of human diseases. Dis Model Mech. 9:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WingettSW, , Andrews S. 2018. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Res. 7:1338.doi: 10.12688/f1000research.15931.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YuG, , WangL-G, , He Q-Y. 2015. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 31:2382–2383. doi: 10.1093/bioinformatics/btv145. [DOI] [PubMed] [Google Scholar]
- YuG, , WangL-G, , HanY, , He Q-Y. 2012. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Starr DA, Wu X, Parkhurst SM, Zhuang Y, et al. 2006. The KASH domain protein MSP-300 plays an essential role in nuclear anchoring during Drosophila oogenesis. Dev Biol. 289:336–345. [DOI] [PubMed] [Google Scholar]
- ZhangY, LiuT, MeyerCA, EeckhouteJ, Johnson DS, . et al. 2008. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9:R137.doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Previously published RNA-seq expression data are accessible through Gene Expression Omnibus (GEO) repository under series accession number GSE83431. Data obtained for this manuscript are accessible through GEO repository under series accession number GSE169328. Scripts used for bioinformatic analysis and plot generation are available as supplementary files. Detailed protocols for NIE, RNA-seq, Omni-ATAC, ChIP-seq, and CUT&Tag are available at dx.doi.org/10.17504/protocols.io.buiqnudw. Flies carrying UAS-GFPKASH, as well as additional flies with different KASH-tagged epitopes are available at Bloomington Drosophila Stock Center (see Figure 7C). pUASTattB-2xFlag-mCherry-Msp300KASH and pUASTattB-GFP-Msp300KASH plasmids are available at AddGene (#170807 and #170806, respectively). Supplementary material is available at figshare: https://doi.org/10.25386/genetics.14558286.
Figure 7.
Method summary. (A) Schematic diagram representing the two versions of the “improved” NEI-method. The first version (top) uses protein G-coupled magnetic Dynabeads, and can be coupled with RNA-seq, Omni-ATAC, and ChIP-seq. The second version (bottom) uses Mouse IgG-coupled magnetic beads, and can be coupled with Cleavage Under Targets and Tagmentation (CUT&Tag), RNA-seq, Omni-ATAC, and ChIP-seq. (B) Table describing the available fly lines to perform NIE either using the Gal4-UAS or the QF-QUAS system.