Abstract
Context
The independent contribution of young adult exposure to overweight and obesity to later-life incident diabetes is not well studied.
Objective
To assess the associations of exposures to elevated body mass index (BMI) and waist circumference (WC) in young adulthood (ages 18-39 years) with incident diabetes later in life (≥40 years).
Design
Pooled data from 6 US prospective cohorts (Atherosclerosis Risk in Communities Study, Cardiovascular Risk Development in Young Adults Study, Cardiovascular Health Study, (4) Framingham Heart Study Offspring Cohort, (5) Health, Aging and Body Composition Study, and (6) Multi-Ethnic Study of Atherosclerosis.
Setting
Population-based cohort studies.
Participants
30 780 participants (56.1% female, 69.8% non-Hispanic white) without a diagnosis of diabetes by age 40.
Interventions
We imputed BMI and WC trajectories from age 18 for every participant and estimated time-weighted average exposures to BMI or WC during young adulthood and later life.
Main Outcome Measure(s)
Incident diabetes defined as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, or use of diabetes medications.
Results
During a 9-year median follow-up, 4323 participants developed incident diabetes. Young adult BMI and WC were associated with later-life incident diabetes after controlling for later-life exposures [hazard ratios (HR) 1.99 for BMI ≥ 30 kg/m2 and 2.13 for WC > 88cm (women)/>102cm (men) compared to normal ranges]. Young adult homeostatic model of insulin resistance mediated 49% and 44% of the association between BMI and WC with later-life incident diabetes. High-density lipoproteins and triglycerides mediated a smaller proportion of these associations.
Conclusions
Elevated BMI and WC during young adulthood were independently associated with later-life incident diabetes. Insulin resistance may be a key mediator.
Keywords: body mass index, waist circumference, diabetes
Overweight and obesity are established risk factors for the development of type 2 diabetes (1). Higher body mass index (BMI), a measure of general adiposity, is correlated with increased risk of type 2 diabetes (2). More specifically, visceral fat is associated with adverse metabolic risks (3). Waist circumference (WC), an anthropometric measure of abdominal adiposity, is a predictor of adverse health outcomes including diabetes, as well as increased mortality, independent of BMI (4,5). The 2013 American Heart Association/American College of Cardiology/The Obesity Society guidelines on management of overweight and obesity suggest that WC be measured in individuals who have BMI 25 to 34.9 kg/m2, with WC > 88 cm in women and >102 cm in men indicating increased cardiometabolic risk (6).
Although overweight and obesity are important risk factors for incident diabetes, most studies have examined this association in limited follow-up periods, such as from childhood and adolescence to young adulthood, from young adulthood into midlife, or from midlife to late life (7-9). Attempts have been made to capture the impact of young adult weight status on mid to late life incident diabetes, sometimes using self-recall of younger adult weights (8,10). Additionally, compared to BMI measured at a single time point, studies have indicated that cumulative exposure to BMI might be a better predictor of incident diabetes (11), suggesting that it could be valuable to assess repeated measures over time. However, limited longitudinal data are available to determine whether young adult exposures to elevated BMI or WC (between ages 18 and 39 years) are associated with the development of incident diabetes later in life (age ≥ 40 years; here collectively labeled later life to denote it as being relatively later than the young adult period), independent of later-life exposures.
By pooling data from multiple large observational cohort studies, we aimed to examine the associations between cumulative exposures to elevated BMI or WC during young adulthood and the risk of incident diabetes later in life, controlling for later-life BMI and WC. We also sought to understand whether these associations were mediated by cardiometabolic risk factors including lipids, blood pressure, and insulin resistance.
Materials and Methods
Study Design and Cohorts
The present analysis was based on data from 6 prospective cohort studies: (1) Atherosclerosis Risk in Communities (ARIC) Study; (2) Cardiovascular Risk Development in Young Adults (CARDIA) Study; (3) Cardiovascular Health Study (CHS); (4) Framingham Heart Study Offspring Cohort (FHS-O); (5) Health, Aging and Body Composition (Health ABC) Study; and (6) Multi-Ethnic Study of Atherosclerosis (MESA). Details of the designs of each study are included in the Supplemental Methods (12). All studies were approved by the Institutional Review Boards at participating institutions; all study participants provided written informed consent prior to enrolling. Data were centralized at Columbia University for pooling, harmonization, and analysis as part of the NHLBI Pooled Cohorts Study (13). The current analysis was restricted to participants ≥ 18 years of age without cardiovascular disease (CVD) or diabetes at baseline and with at least 1 nonmissing value for each key risk factor. Participants who developed diabetes before age 40 were also excluded. The final sample included 30 780 participants [17 277 women and 21 495 non-Hispanic white; see Supplemental Figure 1 (12)].
Clinical Data Collection and Follow-up for Incident Diabetes
Demographic characteristics and CVD risk factors were evaluated based on standard protocols for each study (14-19). Cardiometabolic risk factors were measured at most study visits using standardized and validated methods. The main exposures of interest were BMI and WC during young adulthood (age 18-39 years) and potential mediators that were studied included systolic blood pressure (SBP), diastolic blood pressure (DBP), low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides (TGs), and homeostatic model assessment-insulin resistance (HOMA-IR) (20). The primary outcome of interest was later-life incident diabetes (age ≥ 40 years), defined by fasting glucose levels ≥ 126 mg/dL, nonfasting glucose ≥ 200 mg/dL, or use of diabetes medications. These are the measures of diabetes most consistently reported in our cohorts, as some of the cohort data were collected prior to widespread use of hemoglobin A1c (HbA1c) testing. The secondary outcome was an alternative definition of diabetes of fasting glucose ≥ 126 mg/dL, nonfasting glucose ≥200 mg/dL, HbA1c ≥ 6.5% (48 mmol/mol), or use of diabetes medications.
Imputation of CVD Risk Factors Across the Life Course
Most cohort studies were restricted in age range and therefore did not measure BMI, WC, and other cardiometabolic risk factors across the entire adult life span. For example, CHS participants were enrolled after age 65, and therefore their risk factor levels before age 65 were not directly observed.
We previously reported our method to impute risk factor trajectories across the adult life course using data from pooled cohorts (21-23). In brief, we pooled data from multiple cohort studies, which together provided observations that spanned the adult life course. We leveraged risk factor patterns observed in the younger cohorts (such as CARDIA and FHS-O) to impute unobserved young adult exposures in the older cohorts (such as CHS), and vice versa. We used linear mixed models to estimate latent trajectories underlying the observed values for each participant and imputed risk factor levels annually from age 18 years until the end of follow-up for each participant. Examples of imputed BMI and WC trajectories for 3 randomly selected participants are illustrated in Supplemental Figure 2 (12).
Statistical Analyses
Using the imputed trajectories, we calculated period-specific time-weighted averages (TWAs) of exposure to BMI, WC, SBP, DBP, LDL, HDL, TGs, and HOMA-IR for each participant as summary measures of young adult (age 18-39 years) and later life (age 40 years to the diagnosis of incident diabetes or end of study follow-up) exposures. Separate Cox proportional hazards models were used to estimate the associations between young adult BMI and WC with the risk of incident diabetes. We used age as the time scale, with the origin for time to event set at 40 years. The Cox models for BMI and WC were adjusted for race/ethnicity, sex, birth year, education level, smoking status, and TWAs of later-life BMI or WC and stratified by study cohort, allowing the baseline hazard function to vary across different cohorts. The proportional hazards assumption was checked by plotting the log[-log(survival)] vs log(survival time) and by using Schoenfeld residuals. Tests for linear trend in the association with incident diabetes across the categories of BMI or WC were conducted by including a variable with the median level of each category in the models.
Additionally, we performed causal mediation analyses to examine whether the associations between young adult BMI and WC with diabetes outcomes were mediated by young adult exposures to other cardiometabolic risk factors (SBP, DBP, LDL, HDL, triglycerides, HOMA-IR) (24). Specifically, we derived estimates for the direct and indirect effects of BMI or WC on diabetes using a previously described method by fitting a logistic regression for diabetes and a linear regression for cardiometabolic risk factors (24). In the fully adjusted model, the logistic regression included young adult BMI or WC, cardiometabolic risk factors, interactions between young adult BMI or WC with cardiometabolic risk factors, and a set of confounders whereas the linear regression model included young adult BMI or WC and the same set of confounders. We calculated the proportion of the total effects mediated by cardiometabolic risk factors as the ratio of the natural indirect effect over the total effect.
To account for estimation error in imputed risk factors trajectories and TWAs, we used multiple imputation techniques to obtain 30 imputed data sets. The aforementioned survival analyses and mediation analyses were performed on each imputed data set, and summary estimates and standard errors (which reflect the imputation error) were calculated using established methods (25).
To examine the robustness and consistency of our findings, we also performed several sensitivity analyses, including stratifying results by race/ethnicity and sex; examining potential interactions by race/ethnicity and sex by including 2-way interaction terms of young adult BMI or WC by race/ethnicity and by sex in the Cox models; including BMI and WC in the same model; excluding participants whose observed BMI decreased by more than 3 units from the baseline examination to reduce the possibility that unmeasured illness resulted in weight loss prior to the development of incident diabetes; and repeating all analyses stratified by study cohort, as well as leaving out 1 cohort at a time to confirm that our findings were not driven by any single study. All analyses were performed using Stata version 16 (26).
Results
The average age (SD) of study participants at their first in-person examination was 53.1 (16.2) years, ranging from a mean of 25.2 (3.6) years in CARDIA to 73.5 (2.8) years in Health ABC (Table 1). Women comprised 56.1% of study participants and 69.8% of participants self-identified as non-Hispanic white. About 23% of the participants had TWA young adult BMI ≥ 25 kg/m2, and 14% had TWA young adult WC ≥ 81 cm for women or ≥ 95 cm for men [Supplemental Tables 1 and 2 (12)]. Participants with higher TWA young adult BMI and WC were more likely to be non-Hispanic black and to have a higher level of insulin and HOMA-IR at the first in-person exam. Time-weighted average BMI and WC from young adulthood were strongly correlated with their later-life averages (correlation coefficients were 0.88 for BMI and 0.81 for WC). The majority of participants (95%) contributed >1 direct measurement of BMI, WC, and cardiometabolic risk factors over time (mean 4.7 per person) (Table 2).
Table 1.
Characteristics of study participants at the first in-person examination
| Characteristicsa | Total (n = 30 780) | ARIC (n = 12 026) | CARDIA (n = 3943) | CHS (n = 3718) | FHS-O (n = 3435) | Health ABC (n = 1818) | MESA (n = 5840) |
|---|---|---|---|---|---|---|---|
| Year of enrollment | 1987-1989 | 1985-1986 | 1989-1990; 1992-1993 | 1971-1975 | 1997-1998 | 2000-2002 | |
| Age range at study enrollment | 45-64 | 18-30 | ≥65 | 5-70 | 70-79 | 45-84 | |
| Age (year) | 53.1 ± 16.2 | 53.8 ± 5.7 | 25.2 ± 3.6 | 72.4 ± 5.4 | 36.5 ± 9.7 | 73.5 ± 2.8 | 61.8 ± 10.3 |
| Race | |||||||
| Non-Hispanic white | 21 495 (69.8) | 9268 (77.1) | 2049 (52.0) | 3214 (86.4) | 3435 (100.0) | 1125 (61.9) | 2404 (41.2) |
| Non-Hispanic black | 7323 (23.8) | 2720 (22.6) | 1894 (48.0) | 483 (13.0) | 0 | 693 (38.1) | 1533 (26.3) |
| Hispanic | 1203 (3.9) | 0 | 0 | 0 | 0 | 0 | 1203 (20.6) |
| Other | 759 (2.5) | 38 (0.3) | 0 | 21 (0.6) | 0 | 0 | 700 (12.0) |
| Sex | |||||||
| Female | 17 277 (56.1) | 6771 (56.3) | 2206 (55.9) | 2312 (62.2) | 1809 (52.7) | 1048 (57.6) | 3131 (53.6) |
| Male | 13 503 (43.9) | 5255 (43.7) | 1737 (44.1) | 1406 (37.8) | 1626 (47.3) | 770 (42.4) | 2709 (46.4) |
| Smoking | |||||||
| Never | 14 381 (46.7) | 5132 (42.7) | 2282 (57.9) | 1,796 (48.3) | 1314 (38.3) | 869 (47.8) | 2988 (51.2) |
| Former | 9390 (30.5) | 3810 (31.7) | 538 (13.6) | 1458 (39.2) | 719 (20.9) | 757 (41.6) | 2108 (36.1) |
| Current | 6980 (22.7) | 3080 (25.6) | 1104 (28.0) | 462 (12.4) | 1402 (40.8) | 188 (10.3) | 744 (12.7) |
| Body mass index (kg/m2) | 26.6 ± 5.0 | 27.1 ± 5.0 | 24.2 ± 4.7 | 26.3 ± 4.5 | 25.0 ± 4.0 | 27.0 ± 4.8 | 28.0 ± 5.3 |
| Waist circumference (cm) | 92.6 ± 14.7 | 95.5 ± 13.3 | 77.3 ± 10.9 | 92.9 ± 13.0 | 89.4 ± 14.5 | 98.3 ± 13.3 | 97.2 ± 14.1 |
| Systolic blood pressure (mmHg) | 122.6 ± 19.7 | 119.8 ± 18.1 | 110.1 ± 10.8 | 135.6 ± 21.2 | 121.0 ± 15.0 | 135.2 ± 20.5 | 125.6 ± 21.3 |
| Diastolic blood pressure (mmHg) | 72.6 ± 11.0 | 73.3 ± 11.0 | 68.4 ± 9.4 | 71.1 ± 11.3 | 78.2 ± 10.2 | 71.8 ± 11.6 | 71.9 ± 10.3 |
| LDL cholesterol (mg/dL) | 127.0 ± 36.7 | 136.7 ± 38.8 | 109.4 ± 31.1 | 130.5 ± 35.0 | 126.9 ± 36.1 | 123.2 ± 34.1 | 117.9 ± 31.0 |
| HDL cholesterol (mg/dL) | 53.3 ± 16.0 | 52.9 ± 17.1 | 53.6 ± 12.9 | 56.7 ± 15.9 | 52.4 ± 16.2 | 56.2 ± 17.1 | 51.6 ± 15.0 |
| Triglycerides (mg/dL) | 115.3 ± 71.1 | 123.1 ± 74.9 | 71.3 ± 43.4 | 129.7 ± 58.6 | 95.3 ± 64.7 | 131.2 ± 67.4 | 126.8 ± 76.0 |
| Fasting glucose (mg/dL) | 94.7 ± 11.4 | 98.6 ± 9.3 | 81.5 ± 8.0 | 99.5 ± 9.7 | 100.2 ± 9.3 | 93.0 ± 9.9 | 89.6 ± 10.5 |
| HbA1c (%) | 5.5 ± 0.6 | 5.5 ± 0.6 | 5.4 ± 0.6 | 6.1 ± 1.1b | 5.4 ± 0.7 | 6.0 ± 0.6 | 5.5 ± 0.5 |
| Insulin (mU/L) | 10.3 ± 7.5 | 10.7 ± 8.0 | 8.2 ± 4.4 | 13.9 ± 8.6 | 9.5 ± 8.9 | 7.8 ± 4.9 | 9.6 ± 6.5 |
| HOMA-IR | 1.9 (1.3, 3.0) | 2.1 (1.4, 3.2) | 1.4 (1.1, 1.9) | 2.9 (2.2, 4.0) | 1.8 (1.0, 3.0) | 1.5 (1.0, 2.3) | 1.8 (1.2, 2.7) |
| Hypertension medication use | 7050 (22.9) | 2,810 (23.4) | 25 (0.6) | 1355 (36.4) | 99 (2.9) | 798 (43.9) | 1963 (33.6) |
| Lipid medication use | 1496 (4.9) | 277 (2.3) | 0 (0.0) | 162 (4.4) | 11 (0.3) | 185 (10.2) | 861 (14.7) |
A b b r e v i a t i o n s: ARIC Atherosclerosis Risk in Communities; CARDIA, Cardiovascular Risk Development in Young Adults; CHS, Cardiovascular Health Study; FHS-O, Framingham Heart Study Offspring Cohort; HDL, high-density lipoprotein; Health ABC, Health, Aging and Body Composition; LDL, low-density lipoprotein; MESA, Multi-Ethnic Study of Atherosclerosis.
a Values are mean ± SD, number (%), or median (25th, 75th percentile) based on baseline observed data.
b Values were obtained from a subsample of CHS participants who had HbA1c values measured in an ancillary study
Table 2.
Study observation period and number of events
| Study characteristics | Total | ARIC | CARDIA | CHS | FHS-O | Health ABC | MESA |
|---|---|---|---|---|---|---|---|
| Number of participants | 30 780 | 12 026 | 3943 | 3718 | 3435 | 1818 | 5840 |
| Median follow-up (year) | 9 | 9 | 14 | 7 | 25 | 9 | 9 |
| Number of in-person examsa | 4.7 ± 2.1 | 3.8 ± 1.2 | 7.7 ± 1.6 | 2.6 ± 1.0 | 7.3 ± 1.9 | 4.3 ± 1.6 | 4.2 ± 1.2 |
| Number of in-person exams before age 40a | 0.8 ± 1.7 | 0 | 4.8 ± 1.2 | 0 | 1.3 ± 1.4 | 0 | 0 |
| Number of incident diabetes | 4,323 | 2,193 | 419 | 281 | 610 | 167 | 653 |
A b b r e v i a t i o n s: ARIC Atherosclerosis Risk in Communities; CARDIA, Cardiovascular Risk Development in Young Adults; CHS, Cardiovascular Health Study; FHS-O, Framingham Heart Study Offspring Cohort; Health ABC, Health, Aging and Body Composition; MESA, Multi-Ethnic Study of Atherosclerosis.
a Values are mean ± SD.
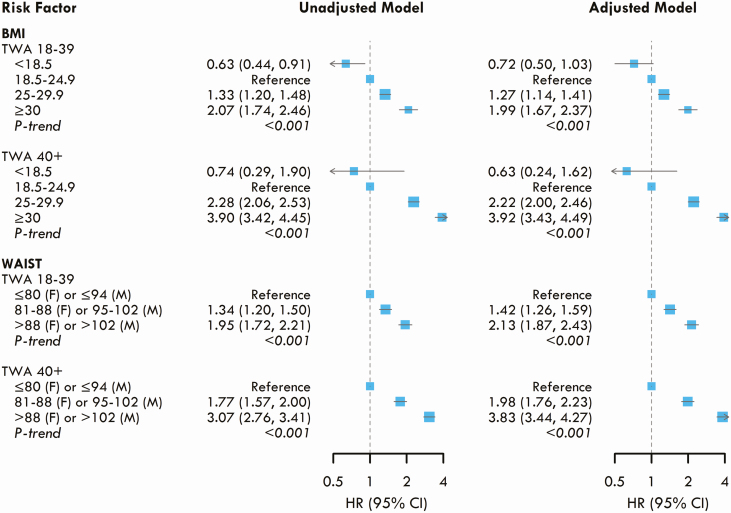
Over a median follow-up of 9 years, 4323 participants developed incident diabetes (Table 2). Young adult exposure to BMI was associated with incident diabetes later in life in a dose-dependent manner, independent of later-life BMI. Compared to young adult BMI 18.5 to 24.9 kg/m2, the multivariate adjusted HRs for incident diabetes were 1.27 (95% CI: 1.14-1.41) for young adult BMI 25 to 29.9 kg/m2 and 1.99 (95% CI: 1.67 to 2.37) for young adult BMI ≥ 30 kg/m2 (Fig. 1), adjusting for later-life BMI. WC similarly conveyed a dose-dependent risk. Compared to normal WC (≤80 cm women; ≤94 cm men) during young adulthood, the adjusted HRs were 1.42 (95% CI: 1.26-1.59) for WC 81 to 88 cm (women)/95 to 102 cm (men) and 2.13 (95% CI: 1.87-2.43) for WC > 88 cm (women)/>102 cm (men). Both later-life BMI and WC were also strongly associated with incident diabetes, with a HR of 3.93 (95% CI: 3.43-4.49) and 3.83 (95% CI: 3.44-4.27) for the highest categories, respectively (Fig. 1). Additionally, when including BMI and WC in the same model, both young adult BMI and WC remained associated with incident diabetes [Supplemental Figure 3 (12)].
Figure 1.
Associations between young adult (age 18-39 years) and later adult (age ≥ 40 years) body mass index (BMI) and waist circumference (WC) with the risks of incident diabetes. Time-weighted average (TWA) exposures to each risk factor from young adult (age 18-39 years) and later adult (age ≥ 40 years) were included simultaneously in the same model. The adjusted models were stratified by study cohort and adjusted for race/ethnicity, sex, birth year, education, and smoking status.
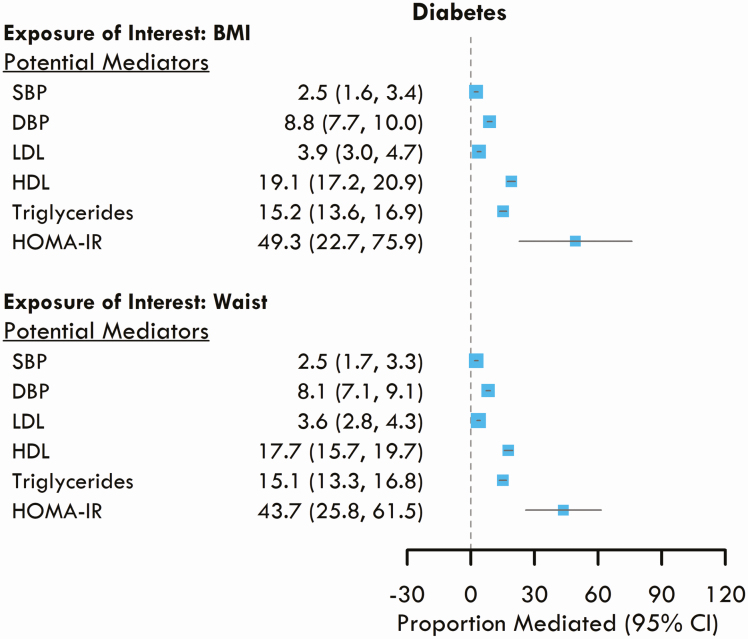
Causal mediation analysis showed that young adult HDL mediated 19.1% (95% CI: 17.2 to 20.9) of the association between BMI and incident diabetes, TG levels mediated 15.2% (95% CI: 13.6 to 16.9), and HOMA-IR mediated 49.3% (95% CI: 22.7 to 75.9) of the association (Fig. 2). Similarly, for the association between WC and incident diabetes, HDL mediated 17.7% (95% CI: 15.7-19.7) of the association, TGs mediated 15.1% (95% CI: 13.3-16.8), and HOMA-IR mediated 43.7% (95% CI: 21.8-61.5).
Figure 2.
Proportion of the associations between young adult body mass index (BMI) and waist circumference (WC) with incident diabetes that is mediated by young adult exposures to other cardiometabolic risk factors.
We found similar patterns of association in our sensitivity analyses. There were a total of 4620 incident diabetes cases when we used the alternative definition of diabetes by incorporating HbA1c in addition to fasting glucose levels and antidiabetic medication use. Under this alternative definition, young adult exposure to BMI and WC were also associated with risk of diabetes in a dose-dependent fashion [Supplemental Figure 4 (12)]. Results were also consistent when excluding participants whose BMI decreased more than 3 points from baseline [Supplemental Figure 5 (12)], stratifying by sex and race [Supplemental Figures 6 and 7 (12); all P interactions by sex or race >0.1], and leaving out 1 study at a time [Supplemental Figure 8 (12)]. When stratifying by study cohorts, the associations between young adult BMI and WC with incident diabetes were weaker among participants in CHS and Health ABC but consistent across the other cohorts [Supplemental Figure 9 (12)].
Discussion
In this study we analyzed pooled data from 6 large prospective US cohorts and found that exposures to elevated BMI and WC during young adulthood were associated with an increased risk of incident diabetes later in life, independent of later-life exposures. The associations between BMI and WC with diabetes were mediated predominantly by HOMA-IR, and, to a lesser extent, by HDL and TGs. While later-life BMI and WC remained strongly associated with later-life diabetes, the independent risk conveyed by young adult exposures reinforces the importance of prevention and treatment efforts for overweight and obesity in the young adult period to reduce later-life diabetes risk and improve overall cardiovascular health.
The prevalence of obesity amongst US adults 20 years and older has been increasing, rising from 30.5% in 1999-2000 to 39.6% in 2015-2016, according to the National Health and Nutrition Examination Survey (27). The young adult period is important to examine because despite a lower prevalence of obesity in the young adult population compared to middle-aged adults (27), young adults have the highest incidence of major weight gain (28). Studies looking longitudinally at weight in the young adult period and later risk of diabetes have focused on changes in weight from young to middle adulthood and have shown that weight gain is associated with increased risk of diabetes and all-cause mortality (29,30). Several prior studies that evaluated BMI exposure during 1 age period and incident diabetes had limited follow-up (eg, examining adiposity in childhood and adolescence and young adult incident diabetes or examining both adiposity and incident diabetes in the young adult period) (7,30) or focused on the mid-life and late-life period (9).
Our study extends previous reports by further delineating and quantifying the independent contributions of average young adult vs later adult BMI exposure to future diabetes risk. Later-life BMI was associated with later-life incident diabetes as might be expected, and the magnitude of the association was greater than that of young adult BMI with later-life incident diabetes. Further, we found that young adult exposures to elevated BMI were also associated with an increased risk of diabetes after age 40, in a dose-dependent manner, independent of later-life BMI. BMI ≥ 30 kg/m2 during young adulthood was associated with approximately a 2-fold increased risk of developing diabetes compared with normal weight. Additionally, duration of obesity has been shown to be an important risk factor for type 2 diabetes (31). By using TWA measures of adiposity, our study assessed the cumulative exposure to overweight and obesity during the young adult period and its association with incident diabetes.
Our study also found that higher WC during young adulthood was associated with an increased risk of diabetes in later life, independent of later-life WC. Abdominal obesity, and visceral adiposity in particular, is associated with increased glucose intolerance and diabetes (3,32). While imaging techniques such as dual-energy X-ray absorptiometry and computed tomography may be superior measures of visceral adiposity (33), WC is an inexpensive, easily obtained surrogate marker for abdominal obesity. WC has been shown to be associated with visceral fat in young adults (34) and is associated with increased diabetes risk (35). There has been debate about whether BMI or WC is a better predictor of diabetes. Some studies suggest that WC is similar to BMI at least in short-term diabetes risk prediction (35). Others have indicated that WC may be superior, regardless of race/ethnicity, age, or gender (36). One study of older adults noted that WC was more strongly associated with diabetes risk at lower BMI (<25 kg/m2) (9). In our study, we found that the magnitude of association of BMI and WC with incident diabetes were similar. It has been suggested that WC may explain more obesity-related health risks than BMI when evaluated as a continuous variable as opposed to a dichotomized variable (37). The cutpoints used for WC are somewhat arbitrary, as was commented on in the 2013 American Heart Association/American College of Cardiology/The Obesity Society guidelines (6), suggesting future research of different WC cutoff points, based on factors such as age and race, are needed in young adults for prediction of obesity-related risks.
Individuals with increased visceral fat have increased insulin resistance, and this is likely a mechanism for increased risk of diabetes (38). HOMA-IR is a surrogate marker of insulin resistance (20), and WC is associated with HOMA-IR (39). In our mediation analysis, HOMA-IR explained the greatest proportion of the association between young adult WC or BMI and later-life incident diabetes among the variables we examined, which included other components of the metabolic syndrome. We found that TGs and HDL mediated more of the association between BMI or WC and incident diabetes than SBP, DBP, and LDL. Elevated TGs and low HDL are known to be associated with an insulin resistant state (40), and this may be why they are also mediators of the association between young adult adiposity and later-life diabetes. Obesity is also linked with ß-cell dysfunction through multiple mechanisms including proinflammatory cytokine release and dyslipidemia (41). While we did not examine this in our analysis, it is possible that individuals with prolonged overweight and obesity in the young adult period have increased ß-cell stress via prolonged exposure to an insulin resistant state, which can ultimately contribute to development of diabetes in predisposed individuals.
A major strength of our study is the unique study design, which pools data from multiple large cohort studies, allowing us to model risk factors trajectories across the adult life course and to evaluate if there is an independent diabetes risk associated with exposure to elevated BMI and/or WC during specific lifetime periods. The populations from the cohort studies that were selected also included both men and women and 30.2% non-Caucasian participants, with possible increased representation from racial/ethnic groups that have been noted to have a higher prevalence of obesity and diabetes in the United States. Using data from these cohort studies ensures high-quality risk factor and outcome assessments and a long follow-up duration for incident diabetes events. Additionally, our study provides an alternative method of assessing cumulative exposure to BMI during young adulthood based on repeat direct measurements, rather than self-report, as has been used in most prior studies. It also allows for the inclusion of WC, which would be harder to obtain through self-recall.
The study also has several limitations. The use of imputation methods to estimate BMI and WC trajectories during young adulthood and later life necessarily implies that these trajectories and TWAs are subject to imputation error; however, imputation error in our study is likely nondifferential and trajectory estimates for individuals with relatively fewer observed measurements are subject to extra “shrinkage” toward the sample means (21). Therefore, our estimates of the association between BMI and WC with incident diabetes are likely conservative and biased toward the null. When stratifying the analysis by study cohorts, we observed weaker associations between young adult BMI/WC and incident diabetes among participants in CHS and Health ABC, who were older at the time of baseline exam compared to other cohorts. Although our imputation method and survival analysis adjusted for study cohort and birth year, there might be residual cohort effect that we could not account for, which might explain the weaker association in these older cohorts. Alternatively, participants from older cohorts were more likely to have prevalent diabetes at study baseline and to be excluded from the analysis. Indeed, there were fewer incident diabetes cases in CHS and Health ABC, which may also explain the weaker associations between BMI and WC with diabetes in these 2 cohorts. Another limitation in our study relates to our definitions of diabetes. Most cohorts did not include oral glucose tolerance test or HbA1c data, the latter of which was becoming available toward the end of the data collection periods. Thus, we were unable to define diabetes using these criteria. However, we performed sensitivity analyses using an alternative definition of diabetes, which incorporated HbA1c in addition to fasting glucose levels and antidiabetic medication use. Under this alternative definition, we found that young adult exposures to BMI and WC were also associated with the risk of diabetes in a dose-dependent fashion, similar to those observed in the main analysis. In addition, we were unable to differentiate between type 1 and type 2 diabetes in the cohorts, based on the way in which diabetes was defined. However, given the relative incidence of type 2 diabetes vs type 1 diabetes in the mid to late-life age group, this should be a minor issue. Several risk factors for diabetes, such as history of gestational diabetes and family history of diabetes and CVD were not available in most of the cohort and therefore were not included in the analysis. Given that these are important clinical predictors of incident diabetes, they would be useful to include in future studies. While HOMA-IR is widely used in epidemiological studies as a surrogate marker of insulin resistance, it does have limitations, and future studies with inclusion of other markers of insulin resistance can further evaluate the role of insulin resistance in mediating the association between BMI and WC with diabetes. Lastly, BMI and WC as measures of adiposity have their limitations. BMI takes weight and height into account but does not account for body composition. Visceral adiposity is known to be associated with increased metabolic risks (3), and WC is a means of measuring abdominal adiposity but cannot differentiate between subcutaneous and visceral fat. Nonetheless, while neither is a perfect indicator, both have been shown to be correlated with cardiometabolic risks and are cost-effective, easily measured risk factors and thus are useful measurements to consider.
In summary, by pooling data from 6 US cohorts with over 30 000 participants, our study found that exposures to elevated BMI and WC during young adulthood are associated with incident diabetes later in life, independent of later-life exposures. It appears that insulin resistance, as represented by HOMA-IR, is a key mediator of the associations between BMI and WC with incident diabetes.
Acknowledgments
The authors would like to thank the Cross-Cohort Collaboration (CCC) for their promotion and support of this multicohort effort. The authors thank the staff and participants of the ARIC study for their important contributions. This manuscript has been reviewed by CARDIA for scientific content. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Financial Support: This work was primarily supported by the U.S. National Heart, Lung, and Blood Institute (NHLBI) grants R01HL130500 (A.E.M.) and R01HL107475 (A.E.M.). N.N. received additional NIH support via grant T32DK007271. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the NHLBI in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). The Atherosclerosis Risk in Communities study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I). The MESA study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The Health ABC study is supported by National Institute on Aging (NIA) contracts #N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050; NINR grant R01-NR012459. The CHS study is supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. Finally, the FHS-O study was supported by the NHLBI, Framingham Heart Study (NHLBI/NIH contract #HHSN268201500001I) and the Boston University School of Medicine.
Author Contributions: N.N., A.E.M., and Y.Z. designed the study. N.N. wrote the first manuscript draft. Y.Z. performed the statistical analyses. A.E.M. and Y.Z. obtained additional funding. All authors critically reviewed this manuscript. N.N. and Y.Z. are the guarantors of this work.
Prior Presentation: Parts of this study were accepted in abstract form for the Endocrine Society’s Annual Meeting (Journal of the Endocrine Society, Volume 4, Supplement 1, April-May 2020, SAT–616, https://doi.org/10.1210/jendso/bvaa046.045).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Menke A, Rust KF, Fradkin J, Cheng YJ, Cowie CC. Associations between trends in race/ethnicity, aging, and body mass index with diabetes prevalence in the United States: a series of cross-sectional studies. Ann Intern Med. 2014;161(5):328-335. [DOI] [PubMed] [Google Scholar]
- 3. Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39-48. [DOI] [PubMed] [Google Scholar]
- 4. Ohlson LO, Larsson B, Svärdsudd K, et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34(10):1055-1058. [DOI] [PubMed] [Google Scholar]
- 5. Koster A, Leitzmann MF, Schatzkin A, et al. Waist circumference and mortality. Am J Epidemiol. 2008;167(12):1465-1475. [DOI] [PubMed] [Google Scholar]
- 6. Jensen MD, Ryan DH, Apovian CM, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102-S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Changes in risk variables of metabolic syndrome since childhood in pre-diabetic and type 2 diabetic subjects: the Bogalusa Heart Study. Diabetes Care. 2008;31(10):2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stokes A, Collins JM, Grant BF, et al. Obesity progression between young adulthood and midlife and incident diabetes: a retrospective cohort study of U.S. Adults. Diabetes Care. 2018;41(5):1025-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Biggs ML, Mukamal KJ, Luchsinger JA, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA. 2010;303(24):2504-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng Y, Manson JE, Yuan C, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318(3):255-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reis JP, Hankinson AL, Loria CMet al. Duration of abdominal obesity beginning in young adulthood and incident diabetes through middle age. The CARDIA Study. 2013;36:1241-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nair N, Vittinghoff E, Pletcher MJ, et al. Supplemental data for: Associations of body mass index and waist circumference in young adulthood with later life incident diabetes. Figshare. Deposited March 23, 2021. https://figshare.com/s/4a6a0588fa976bca625b. Accessed June 8, 2021. [DOI] [PMC free article] [PubMed]
- 13. Oelsner EC, Balte PP, Cassano PA, et al. Harmonization of respiratory data from 9 US population-based cohorts: the NHLBI pooled cohorts study. Am J Epidemiol. 2018;187(11):2265-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687-702. [PubMed] [Google Scholar]
- 15. Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105-1116. [DOI] [PubMed] [Google Scholar]
- 16. Fried LP, Borhani NO, Enright P, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1(3):263-276. [DOI] [PubMed] [Google Scholar]
- 17. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol. 1979;110(3):281-290. [DOI] [PubMed] [Google Scholar]
- 18. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the health ABC study. J Appl Physiol (1985). 2001;90(6):2157-2165. [DOI] [PubMed] [Google Scholar]
- 19. Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871-881. [DOI] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 21. Pletcher MJ, Vittinghoff E, Thanataveerat A, Bibbins-Domingo K, Moran AE. Young adult exposure to cardiovascular risk factors and risk of events later in life: the framingham offspring study. PloS One. 2016;11(5):e0154288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Vittinghoff E, Pletcher MJ, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;74(3):330-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeki Al Hazzouri A, Vittinghoff E, Zhang Yet al. Use of a pooled cohort to impute cardiovascular disease risk factors across the adult life course. Int J Epidemiol 2019;48:1004-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3-15. [DOI] [PubMed] [Google Scholar]
- 26. Statacorp. Stata Statistical Software: Release 16. StataCorp LLC; 2019. [Google Scholar]
- 27. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief. NCHS data brief no. 288. Published October 2017. https://www.cdc.gov/nchs/products/databriefs/db288.htm. Accessed June 8, 2021. [PubMed] [Google Scholar]
- 28. Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med. 1990;150(3):665-672. [PubMed] [Google Scholar]
- 29. Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol. 1997;146(3):214-222. [DOI] [PubMed] [Google Scholar]
- 30. Chen C, Ye Y, Zhang Y, Pan XF, Pan A. Weight change across adulthood in relation to all cause and cause specific mortality: prospective cohort study. BMJ. 2019;367:l5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abdullah A, Stoelwinder J, Shortreed S, et al. The duration of obesity and the risk of type 2 diabetes. Public Health Nutr. 2011;14(1):119-126. [DOI] [PubMed] [Google Scholar]
- 32. Klein S, Allison DB, Heymsfield SB, et al. ; Association for Weight Management and Obesity Prevention; NAASO; Obesity Society; American Society for Nutrition; American Diabetes Association . Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: association for weight management and obesity prevention. Diabetes Care. 2007;30(6):1647-1652. [DOI] [PubMed] [Google Scholar]
- 33. Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity. 2012;20(5):1109-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borruel S, Moltó JF, Alpañés M, et al. Surrogate markers of visceral adiposity in young adults: waist circumference and body mass index are more accurate than waist hip ratio, model of adipose distribution and visceral adiposity index. PloS One. 2014;9(12):e114112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee CMY, Woodward M, Pandeya N, et al. ; Obesity, Diabetes and Cardiovascular Disease Collaboration . Comparison of relationships between four common anthropometric measures and incident diabetes. Diabetes Res Clin Pract. 2017;132:36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seo DC, Choe S, Torabi MR. Is waist circumference ≥102/88cm better than body mass index ≥30 to predict hypertension and diabetes development regardless of gender, age group, and race/ethnicity? Meta-analysis. Prev Med. 2017;97:100-108. [DOI] [PubMed] [Google Scholar]
- 37. Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79(3):379-384. [DOI] [PubMed] [Google Scholar]
- 38. Tchkonia T, Thomou T, Zhu Y, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17(5):644-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wahrenberg H, Hertel K, Leijonhufvud BM, Persson LG, Toft E, Arner P. Use of waist circumference to predict insulin resistance: retrospective study. Bmj. 2005;330(7504):1363-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li N, Fu J, Koonen DP, Kuivenhoven JA, Snieder H, Hofker MH. Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis. 2014;233(1):130-138. [DOI] [PubMed] [Google Scholar]
- 41. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of beta-cell death in type 2 diabetes. Diabetes. 2005;54(Suppl 2):S108-S113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.