What is diagnostic stewardship and why has it emerged as a new strategy to combat antibiotic resistance?
Diagnostic stewardship is the promotion of judicious microbiology testing practices to inform safe, effective, and efficient patient management and treatment decisions.1,2 Diagnostic stewardship interventions can target various steps of the diagnostic-treatment decision process, such as clinical decision support tools to optimize selection of diagnostic tests or strategies to improve sample collection and handling practices (pre-analytic), optimizing laboratory processing (analytic) and modifications in how test results are reported (post-analytic).3,4 These strategies aim to support clinicians in appropriately deciding when to send which diagnostics, accurately interpreting test results, and making well-informed treatment decisions. Diagnostic stewardship may be considered an extension of antimicrobial stewardship, a well-recognized strategy to combat antibiotic resistance by optimizing antimicrobial selection and reducing antibiotic over-use.5 In comparison to antimicrobial stewardship, diagnostic stewardship targets an earlier step of the clinical management process that can occur before a patient is prescribed antibiotics (Figure 1).
Figure 1.
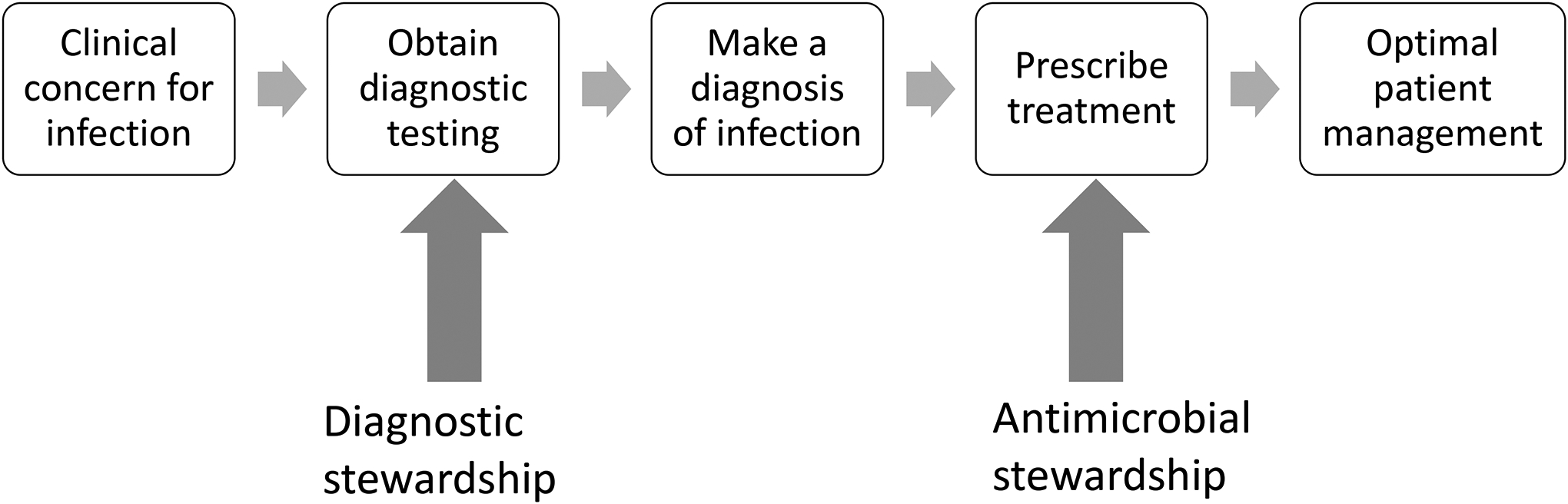
Diagnostic stewardship supplements antibiotic stewardship to optimize patient management of infectious processes.
In pediatrics, antibiotic resistance is rising with up to 10% of Gram-negative bacteria resistant to carbapenems,6–8 particularly among hospital-associated infections (HAIs).9 This a troublesome trend when antibiotic-resistant infections increase the odds of death, prolong duration of hospitalization and increase healthcare costs.10–12 Concurrently, advances in technology and medical care have improved survival of children leading to a growing population of children with complex chronic conditions who may require prolonged and recurrent admissions.13–15 These complex patients are vulnerable to morbidity from healthcare-associated infections (HAIs) such as such catheter-associated bloodstream infections, urinary tract infections, or pneumonia.16,17 Not surprisingly, most pediatric intensive care unit (PICU) patients are treated with antibiotics, but there is also variability in use.18,19 Therefore, there is a need to employ multi-faceted and interdisciplinary strategies to optimize management of both diagnostics and antimicrobials in critically ill children. Diagnostic stewardship can help reduce avoidable testing among patients with a low pre-test probability of infection, which may help reduce avoidable antibiotic treatment.
What are pitfalls of common microbiology testing approaches and why can they lead to over-diagnosis and treatment of infections?
Microbiology testing, either traditional culture-based methods or molecular testing, has limited ability to definitively identify infection. However, positive test results can be misinterpreted by clinicians as definitive evidence of infection, which drives antibiotic treatment. In reality, detection or growth of bacteria can reflect one of three scenarios:
Pathogen: bacteria causing active infection
Contamination: bacteria that accidently were incorporated into the specimen during collection or processing
Colonization: bacteria present on the tissues but not involved in the infectious process
This issue may be even more pronounced with increasingly sensitive molecular testing. For example, Clostridium difficile (C. difficile) nucleic acid amplification testing has high sensitivity to detect presence of genetic material but does not confirm infection.20 Especially if tests are obtained in the absence of clinical signs and symptoms of an infectious process, treatment for positive microbiology tests leads to possible harm with avoidable antibiotic treatment. Therefore, the decision to obtain a diagnostic test and the interpretation of results must be considered within the clinical context of the patient and supportive clinical data rather than relying on the test as confirmative evidence of infection.
What are benefits of diagnostic stewardship as a strategy to improve quality and value of healthcare?
There are multiple downstream benefits of improving microbiology testing practices. The benefit of diagnostic stewardship is well established in the case of C. difficile where reductions in testing among patients with low clinical suspicion for C. difficile colitis has led to reductions in C. difficile diagnoses21,22 and antibiotic treatment for C. difficile colonization23 without detrimental impacts to patient well-being. Beyond antibiotic resistance, avoiding unnecessary antibiotic treatment can improve patient outcomes by preventing unintended adverse reactions to antibiotics such as acute kidney injury, disruption to the microbiome, secondary fungal infections, and secondary C. difficile infections.24–26 Mindful testing can also reduce the risk of cognitive biases and diagnostic errors. In particular, anchoring bias can happen if a clinician focuses on an early piece of information when determining a diagnosis and prematurely concludes a diagnostic work-up, which can lead to missing the true diagnosis and possible patient harm.27 For example, if a clinician obtained a urine culture from a patient when they had a fever and interpreted a positive urine culture as a UTI and therefore failed to recognize the patient had developed appendicitis. In addition, reductions in unnecessary C.difficile testing has demonstrated that reducing false-positive results may improve accuracy of HAI reporting, which in turn impacts hospital reimbursement and public perceptions of the hospitals quality and safety.28 Diagnostic stewardship aligns with concepts of de-implementation29 and the national “Choosing Wisely” campaign to reduce unnecessary or potentially harmful medical testing, improve healthcare value and reduce healthcare costs.30,31 Lastly, diagnostic stewardship often aims to standardize diagnostic approaches, thereby reducing the risk of unconscious biases around patient racial or ethnic demographics and supporting more equitable healthcare delivery.
How does diagnostic stewardship embrace interdisciplinary collaboration, and what is the role of the clinician?
Diagnostic stewardship requires interdisciplinary partnership among the gamut of front-line healthcare workers. The primary team caring for the patient may be responsible for the decision to order a test, but other clinicians involved in the patient’s care also play important roles in testing decisions. Some aspects of diagnostic stewardship may lie beyond the clinician’s prevue and include optimally obtaining specimens (pre-analytic), processing (analytic), and reporting the results (post-analytic).3 For example, nurses or technicians may obtain specimens from patients; ancillary medical staff may help transport specimens; the microbiology laboratory processes specimens; and both the microbiology laboratory and electronic medical reporting system are involved in the display and communication of testing results.
For the remainder of this review, we will focus on diagnostic stewardship from the perspective of the bedside clinician, and the decision to obtain or not obtain a test among hospitalized critically ill pediatric patients. Below, we consider evidence for over-testing and associated over-treatment of blood, urine, and endotracheal cultures and strategies that have been associated with improved testing practices. Though equally important to consider for diagnostic stewardship, we will not discuss molecular pathogen panels (e.g. respiratory viral panels or gastrointestinal pathogen panels) in this review.
General Considerations for Diagnostic Stewardship in the Pediatric Intensive Care Unit
Much of the antibiotic use in the pediatric intensive care unit (PICU) reflects the high proportion of PICU patients who are ill with confirmed or suspected bacterial infections, but a significant number of PICU patients likely receive antibiotics in the absence of infection.32–34 National guidelines and patient safety and quality collaboratives call for rapid recognition and treatment of suspected infection in children.35,36 However, there is considerable overlap in the clinical presentation of infectious and non-infectious etiologies of fever, shock, and multi-organ failure in critically ill children. No single symptom, test, or biomarker can reliably distinguish the two.37–40 Due to this uncertainty, clinicians understandably may choose to start or continue antibiotic therapy without definitive evidence of bacterial infection.41 PICU clinicians are faced with the complex task of ensuring rapid antibiotic administration to critically ill patients with possible or definite serious bacterial infections, while avoiding or de-escalating antibiotics in patients who do not need such treatment. This makes the PICU a challenging, but particularly important, environment for diagnostic and antibiotic stewardship.
Within the PICU setting, diagnostic stewardship strategies can be applied when a clinician is deciding whether or not to order a microbiology test. Specifically, we propose that there are three questions the clinical team should ask:
Does the patient have signs and symptoms consistent with a particular infectious disease process?
What is the optimal diagnostic test available to the clinician to evaluate for this infectious process?
How should the diagnostic specimen be collected to optimize accuracy of the results?
Below, we provide a more in-depth review of current literature evaluating blood cultures, respiratory cultures, and urine cultures in the pediatric hospital setting. Figure 2 summarizes these questions with example applications to clinical practice.
Figure 2.
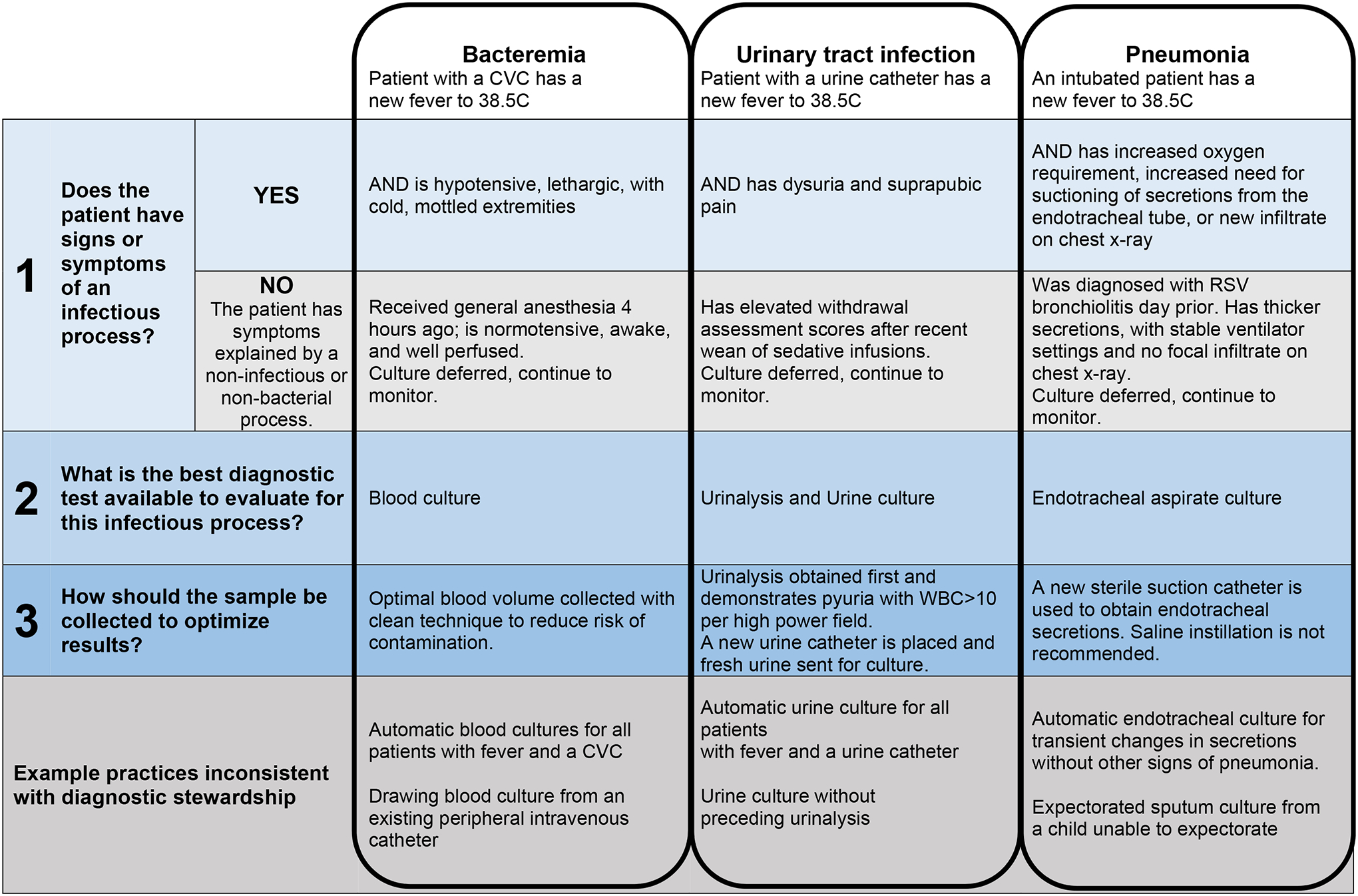
Applying three steps of clinical diagnostic stewardship when assessing for bacterial infections commonly considered in the Pediatric Intensive Care Unit (PICU).
Blood cultures
Blood cultures are fundamental in the diagnosis and treatment of bacteremia, a primary cause of sepsis and associated morbidity and mortality in PICU patients.42 National guidelines call for rapid recognition and empiric broad-spectrum antibiotics for suspected sepsis because delayed treatment of sepsis is associated with worse outcomes.36 Compared to the failure to diagnose and treat bacteremia, blood cultures are perceived as a low-risk test. However, blood cultures are used excessively in PICU patients even when the pre-test probability of bacteremia is low.42–45 For example, in a review of blood cultures obtained in a PICU, only 72% and 57% of cases met criteria for systemic inflammatory response syndrome or sepsis, respectively.45 Importantly, performing blood cultures on patients with a very low likelihood of bacteremia increases the chance of obtaining a false positive result, making proper selection of patients for testing critical.46 False positive blood cultures cause patient harm and strain on health care resources: repeat testing, unnecessary antibiotics, longer length of stay, exposure to additional procedures and consultations, and increased cost.47,48
There is growing evidence suggesting that blood culture use can be safely reduced using a diagnostic stewardship approach in the PICU setting without associated increase in mortality, readmissions, or change in the frequency of blood cultures from patients with suspected infection or septic shock.49,50 National consensus recommendations describe clinical scenarios that can be targeted for blood culture reduction.51 For example, avoidable cultures include surveillance blood cultures in asymptomatic patients, blood cultures in stable patients with an identified non-infectious or non-bacteremia explanation for a new fever, and repeat blood cultures in stable patients with persistent fever in whom bacteremia has already been ruled out. Of paramount importance is the bedside clinician assessment of the patient to ensure there is no suspicion for sepsis or clinical worsening that would warrant a diagnostic evaluation for bacteremia. Reflexive practices (e.g. always ordering a blood culture from a patient with fever and a central venous catheter), the local unit culture, and fear of missing sepsis have emerged as potential drivers of blood culture overuse; targeting these factors may be important in further successful reduction of excess blood cultures.52 It is not yet clear what the potential impact of blood culture stewardship on PICU antibiotic use may be, although an initial study suggested that it did not lead to unanticipated increase in empiric broad-spectrum antibiotic treatment.53
Respiratory cultures
Children requiring invasive mechanical ventilation are at risk for developing ventilator-associated infections (VAI)9,16,17 due to poor clearance of secretions and aspirations containing bacteria that have colonized the airways.54–57 The term VAI encompasses ventilator-associated pneumonia (VAP) and tracheobronchitis because these diagnoses can be difficult to distinguish and are commonly treated interchangeably.58–61 In contrast to blood, the respiratory tract is not a sterile environment. Bacteria colonize and quickly form biofilms on artificial airways, which is the primary reason endotracheal cultures have a low specificity for infection.55–57,62 Even after targeted antibiotic therapy, bacteria such as Staphylococcus aureus and Pseudomonas aeruginosa persist in the airway.63,64
Clinicians may consider the density of white blood cells or bacteria in an endotracheal specimen to inform a diagnosis of VAI. Unfortunately, neither white blood cells on Gram-stains nor bacterial growth or quantity in endotracheal cultures can reliably distinguish bacterial colonization from invasive infection.65–69 In neonatal, pediatric and adult patients, endotracheal cultures can have evidence of inflammation and growth of potentially pathogenic bacteria irrespective of clinical signs or symptoms.66,67,70 Sample collection practices (e.g., using an existing instead of a new sterile catheter) greatly affect bacterial growth in cultures.67,71 Furthermore, sample processing, including specimen rejection criteria, institutional definitions of “normal respiratory flora”, and how microbiology results are displayed to clinicians, vary widely across clinical laboratories, all of which may impact clinical interpretation of the results.72
Despite the diagnostic limitations, clinicians having a low threshold to obtain endotracheal cultures (e.g after isolated fever)73,74 and are likely to treat ventilated patients with antibiotics if the gram stain or culture has purulence or bacterial growth.69,74–76 Strikingly, treatment for suspected VAIs may account for as much as 50% of antibiotic use in the PICU, emphasizing the potential impact of overtesting.18 This finding is likely a result of clinical uncertainty in the absence of a gold standard and variability in the diagnosis of VAI among providers and institutions75,76 coupled with the desire to minimize morbidity from treatable infection among medically complex children.13,14,77 Treatment of VAI is critical to prevent excess mortality and morbidity related to mechanical ventilation among adult patients.78 Interestingly, in a multicenter prospective cohort of invasively ventilated children, antibiotic treatment of clinician-suspected VAI did not improve clinical outcomes of mortality, length of stay or ventilator duration for the overall cohort, though a sub analysis of patients with endotracheal tubes suggested reduced mortality if treated with antibiotics.76 This finding underscores the potential significance of distinguishing patients with non-specific clinical changes from those with infection.
In contrast to blood cultures, there are not yet consensus-based recommendations to inform specific indications to obtain an endotracheal cultures in evaluation of suspected VAIs. Two groups have explored the drivers of endotracheal culture use in their PICUs (e.g. fever or change in secretion characteristics), and used the existing evidence to develop and implement clinical decision support tools to standardize indications for endotracheal cultures.79,80 Implementation of these algorithms led to 35–41% declines in the rates of endotracheal cultures and reduction of antibiotic treatment for VAIs by 59–71% without changes in mortality, length of stay, readmissions80 or number of ventilator-associated events.79 Clinical decision support tools that recommend obtaining endotracheal cultures only from patients with signs and symptoms of a VAI, rather than isolated fever or changes in secretions, thus far appears to be a safe approach to reduce avoidable testing and treatment while supporting antibiotic treatment of true infections. Further research is needed to understand the scope of endotracheal culturing practices, define optimal indications for these cultures, and examine the impact on antibiotic prescribing.
Urine cultures
In the ICU setting, fever in a patient with an indwelling urinary catheter may prompt testing for urinary tract infection (UTI). Patients with urinary catheters certainly can develop clinically significant UTI, and these catheter-associated UTIs (CAUTI) contribute to poor patient outcomes and increased cost.81,82The risk of bacteriuria increases 3–10% with each additional catheter day, while only about 1 in 4 patients with bacteriuria will develop a symptomatic UTI and only 3.6% developed secondary bacteremia.83 Therefore, positive urine cultures may reflect asymptomatic bacteriuria or catheter colonization that clinicians may inappropriately treat with antibiotics.84 Diagnostic stewardship strategies, such as discouraging reflexive urinary testing for patients with fever but no other symptoms of UTI and only sending urine cultures if urinalysis results are abnormal with evidence of pyuria, have been successfully applied in adult ICUs and effectively reduced antibiotic treatment for asymptomatic bacteriuria.84–88 To date, work to facilitate similar stewardship strategies for urinary cultures in the PICU is limited, despite the significant burden of CAUTI and its associated negative consequences on critically ill children.81,89,90 Standardized care bundles for catheter maintenance and efforts to remove urinary catheters as soon as they are no longer needed have been associated with improvement in pediatric CAUTI rates, but specific attention to decision strategies around urinary cultures in the PICU is needed in order to reduce unnecessary antibiotic use for patients with bacteriuria or colonization without true infection of the urinary tract.91
Special populations
Oncology patients in the PICU
Critically ill children with malignancies undergoing chemotherapy present unique challenges to diagnostic stewardship efforts. Clinicians are understandably hesitant to reduce diagnostic testing for infection because of their high risk of morbidity and mortality from infections.47,92–94 However, such patients may also be at higher risk of poor outcomes from the events that diagnostic stewardship seeks to prevent (i.e., excessive entry into central venous catheters for frequent blood cultures and adverse effects of unnecessary antibiotics such as kidney injury and antimicrobial resistance).95 Examples of diagnostic stewardship efforts in pediatric oncology patients (in any clinical setting) are limited, but include investigations demonstrating low yield of repeat blood cultures beyond 48 hours for children with persistent febrile neutropenia, and safe outcomes for febrile, non-neutropenic children who did not receive empiric antibiotics.96,97 Pediatric oncology patients were included in a multi-center quality improvement collaborative that safely standardized and reduced use of blood cultures in the PICU.58 Recent Delphi consensus work from that same collaborative also developed two recommendations focused on blood culture reduction in this population: for immunocompromised PICU patients with persistent fever without signs of sepsis or infection and initial negative blood cultures: 1) to avoid repeat blood cultures if antibiotics will not be changed and 2) if blood cultures are obtained, to avoid repeatedly culturing more than one lumen of the central venous catheter.51 Concerns about limited safety data in this population prevented consensus on additional recommendations.51 Efforts to include pediatric oncology patients in diagnostic stewardship initiatives and research remain important.
Cardiac surgical patients
Children with congenital heart disease are at increased risk of infections, including higher severity of illness in the setting of infection owing to frequent occurrence of multi-system comorbidities and underlying genetic or immune system abnormalities.98 Nosocomial infections in the perioperative period in particular are associated with higher mortality rates in these patients, and the use of perioperative extracorporeal membrane oxygenation (ECMO) support is an independent risk factor for infection.99 As with pediatric oncology patients, specific work in diagnostic stewardship for infections in children in the cardiac ICU setting is limited. However, there are national society guidelines for infection detection in patients on ECMO that notably do not recommend routine surveillance cultures.100 Pediatric cardiac ICU patients have also been successfully included in collaborative work to reduce unnecessary use of blood cultures and respiratory cultures.49,80,101 Thus there is a precedent for diagnostic stewardship work in pediatric cardiac ICU patients that can be expanded with further study in this population.
Neonatal intensive care unit patients
Antibiotics are the most commonly used medications in the neonatal intensive care unit (NICU),102 but significant variation in prescribing patterns and duration of therapy suggest opportunity for improvement.103 Antimicrobial stewardship efforts are increasingly implemented in the NICU setting.104 The physiologic and logistical complexities caring for critically ill neonates may make diagnostic stewardship particularly challenging; neonates are at higher risk of infection and manifest infection in non-specific ways.104,105 There have been significant efforts to improve the assessment and management of early onset neonatal sepsis,106 which has demonstrated feasibility of diagnostic stewardship in the NICU and we encourage future study in this population as there may be significant benefit for fragile critically ill neonates to limit adverse effects of avoidable antibiotics.
Implementation of Diagnostic Stewardship Strategies in the PICU
The success of a diagnostic stewardship initiative will depend, to a large degree, on the implementation plan. It is not enough to simply create a new testing algorithm and hope that clinicians adhere to it. Specific attention to implementation strategies for diagnostic stewardship clinical guidelines will facilitate their successful translation into clinical practice.107 Consideration of local context, stakeholder perspectives, and potential barriers to widespread adoption of a diagnostic stewardship project is of paramount importance. We therefore encourage the early use of elements of implementation science, a field dedicated to the study of methods to promote the systematic uptake of evidence-based or consensus-based practice into widespread clinical care.108 Additionally, concepts from human factors engineering and quality improvement can offer useful insight and provide structure to the implementation process. For example, the Systems Engineering Initiative for Patient Safety (SEIPS) model109 was applied to study the work system around and drivers of blood culture overuse in the PICU.52,110 And an integrated approach of the “Translating evidence into Practice” (TRIP) model111 was employed in an endotracheal culture stewardship initiative.80 Other quality improvement tools may be helpful to guide diagnostic stewardship programs, such as driver diagrams and Plan-Do-Study-Act cycles.112 While a detailed discussion of how to implement diagnostic stewardship in the ICU setting is beyond the scope of this article, we have outlined the basic recommended steps before, during, and after implementation of a diagnostic stewardship project in the ICU environment (Table 1).
Table 1.
Suggested implementation approach for diagnostic stewardship initiatives in the pediatric intensive care unit
| Before Implementation | Identify key personnel: a dedicated project champion and a core project team; recommend both an infectious disease and critical care clinician |
| Identify required data elements (ex: monthly number of endotracheal aspirate cultures) | |
| Examine the unit’s baseline performance of the metric of interest before implementation and establish a system for how to analyze that data in each phase of the project | |
| Understand the current/baseline approach to the test of interest in the unit (the current drivers of testing use, and potential barriers or facilitators to changing practice), via survey, focus groups, or interviews | |
| Reach out to key stakeholders whose perspectives and buy-in will be important for your new approach (e.g., leadership, relevant consultants, nurses, respiratory therapists, advance practice providers). | |
| Create the new tool/algorithm for your stewardship initiative that reflects key drivers/barriers/facilitators and stakeholder perspectives | |
| Establish the balancing metrics and unintended consequences of your practice change and how to monitor for those after implementation | |
| Implementation | Develop one or more strategies for disseminating the new tool/algorithm to the appropriate end-users (ie, clinicians, laboratory personnel, etc) in your ICU (e.g., emails, posters, check-lists, educational seminars) |
| After Implementation | Analyze the use of the test of interest on a weekly or monthly basis to monitor progress, using a run or control chart if possible |
| Share this data with the appropriate end-users that your tool/algorithm targeted, using feedback to help drive behavior change | |
| Monitor for adherence to the new guidelines and for unintended consequences of the practice change | |
| Revise the clinical approach or implementation plan if initial results are suboptimal |
Future research recommendations
Considering the ever-growing threat of antimicrobial resistance and the finite limitations of healthcare resources, it is increasingly clear that we must take definitive action to optimize the use of microbiologic diagnostic tests and antimicrobial treatments for patients. Diagnostic stewardship is a critically important tool in our armamentarium. For critically ill children, data is emerging that the use of blood and respiratory cultures can be safely reduced, and urine cultures are ripe for similar attention. More work is needed to understand the impact on patient outcomes and on antibiotic use. Specific focus is needed among neonates and children with cardiac or oncologic diseases in order to understand how to best apply diagnostic stewardship principles to these particularly vulnerable populations. Finally, evidence regarding optimal strategies to implement diagnostic stewardship for impactful and sustained practice change is lacking, and implementation science may offer important insights.
Synopsis:
In the Pediatric Intensive Care Unit (PICU), clinicians encounter complex decision making, balancing the need to treat infections promptly against potential harms of antibiotics. Diagnostic stewardship is an approach to optimize microbiology diagnostic test practices to reduce unnecessary antibiotic treatment. We review the evidence for diagnostic stewardship of blood, endotracheal, and urine cultures in the PICU. Clinicians should consider three questions applying diagnostic stewardship: 1) Does the patient have signs or symptoms of an infectious process? 2) What is the optimal diagnostic test available to evaluate for this infection? 3) How should the diagnostic specimen be collected to optimize results?
Key Points:
Diagnostic stewardship is a complimentary approach to antibiotic stewardship to improve microbiology diagnostic test practices, reduce avoidable testing, improve validity of test results, and reduce antibiotic use.
Blood cultures are commonly obtained for evaluation of sepsis, and studies have shown safe reductions in the use of blood cultures in patients with low likelihood of bacteremia.
Endotracheal cultures cannot distinguish infection from bacteria colonizing invasive airway devices and should be reserved for patients with clinical suspicion for ventilator-associated infection.
Diagnostic stewardship of urine cultures using results from urinalysis has led to safe reductions in treatment for asymptomatic bacteriuria in other settings and may also apply to PICU patients.
Clinicians should consider three questions applying diagnostic stewardship: 1) Does the patient have signs or symptoms of an infectious process? 2) What is the optimal diagnostic test available to evaluate for this infectious process? 3) How should the diagnostic specimen be collected to optimize results?
Funding sources:
National Institutes of Health grant KL2TR003099 (ACS) and K23HL151381 (CWH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no financial or commercial disclosures.
References:
- 1.World Health Organization. Diagnostic stewardship: a guide to implementation in antimicrobial resistance surveillance sites. 2016; 27. Available at: https://www.who.int/glass/resources/publications/diagnostic-stewardship-guide/en/. Accessed May 21, 2021.
- 2.World Health Organization. Global Antimicrobial Resistance Surveillance System: Manual for Early Implementation. 2015; http://www.who.int/antimicrobial-resistance/publications/surveillance-system-manual/en/. Accessed May 21, 2021.
- 3.Morgan DJ, Malani P, Diekema DJ. Diagnostic Stewardship-Leveraging the Laboratory to Improve Antimicrobial Use. Jama. 2017;318(7):607–608. [DOI] [PubMed] [Google Scholar]
- 4.Kenaa B, Richert ME, Claeys KC, et al. Ventilator-Associated Pneumonia: Diagnostic Test Stewardship and Relevance of Culturing Practices. Curr Infect Dis Rep. 2019;21(12):50. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services;2019. [Google Scholar]
- 6.Logan LK, Renschler JP, Gandra S, Weinstein RA, Laxminarayan R. Carbapenem-Resistant Enterobacteriaceae in Children, United States, 1999–2012. Emerging infectious diseases. 2015;21(11):2014–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logan LK, Gandra S, Mandal S, et al. Multidrug- and Carbapenem-Resistant Pseudomonas aeruginosa in Children, United States, 1999–2012. Journal of the Pediatric Infectious Diseases Society. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lake JG, Weiner LM, Milstone AM, Saiman L, Magill SS, See I. Pathogen Distribution and Antimicrobial Resistance Among Pediatric Healthcare-Associated Infections Reported to the National Healthcare Safety Network, 2011–2014. Infect Control Hosp Epidemiol. 2018;39(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner-Lastinger LM, Abner S, Benin AL, et al. Antimicrobial-resistant pathogens associated with pediatric healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol. 2020;41(1):19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foglia EE, Fraser VJ, Elward AM. Effect of nosocomial infections due to antibiotic-resistant organisms on length of stay and mortality in the pediatric intensive care unit. Infect Control Hosp Epidemiol. 2007;28(3):299–306. [DOI] [PubMed] [Google Scholar]
- 11.Giske CG, Monnet DL, Cars O, Carmeli Y, ReAct-Action on Antibiotic R. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrobial agents and chemotherapy. 2008;52(3):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrobial agents and chemotherapy. 2010;54(1):109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen E, Kuo DZ, Agrawal R, et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards JD, Houtrow AJ, Lucas AR, et al. Children and Young Adults Who Received Tracheostomies or Were Initiated on Long-Term Ventilation in PICUs. Pediatr Crit Care Med. 2016;17(8):e324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulides FM, Plotz FB, Verweij-van den Oudenrijn LP, van Gestel JP, Kampelmacher MJ. Thirty years of home mechanical ventilation in children: escalating need for pediatric intensive care beds. Intensive Care Med. 2012;38(5):847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grohskopf LA, Sinkowitz-Cochran RL, Garrett DO, et al. A national point-prevalence survey of pediatric intensive care unit-acquired infections in the United States. J Pediatr. 2002;140(4):432–438. [DOI] [PubMed] [Google Scholar]
- 17.Raymond J, Aujard Y. Nosocomial infections in pediatric patients: a European, multicenter prospective study. European Study Group. Infect Control Hosp Epidemiol. 2000;21(4):260–263. [DOI] [PubMed] [Google Scholar]
- 18.Fischer JE, Ramser M, Fanconi S. Use of antibiotics in pediatric intensive care and potential savings. Intensive Care Med. 2000;26(7):959–966. [DOI] [PubMed] [Google Scholar]
- 19.Brogan TV, Thurm C, Hersh AL, et al. Variability in Antibiotic Use Across PICUs. Pediatr Crit Care Med. 2018;19(6):519–527. [DOI] [PubMed] [Google Scholar]
- 20.Boly FJ, Reske KA, Kwon JH. The Role of Diagnostic Stewardship in Clostridioides difficile Testing: Challenges and Opportunities. Curr Infect Dis Rep. 2020;22(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperling K, Priddy A, Suntharam N, Feuerhake T. Optimizing testing for Clostridium difficile infection: A quality improvement project. Am J Infect Control. 2019;47(3):340–342. [DOI] [PubMed] [Google Scholar]
- 22.Truong CY, Gombar S, Wilson R, et al. Real-Time Electronic Tracking of Diarrheal Episodes and Laxative Therapy Enables Verification of Clostridium difficile Clinical Testing Criteria and Reduction of Clostridium difficile Infection Rates. Journal of clinical microbiology. 2017;55(5):1276–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen AB, Barr VO, Martin DW, et al. Diagnostic stewardship of C. difficile testing: a quasi-experimental antimicrobial stewardship study. Infect Control Hosp Epidemiol. 2019;40(3):269275. [DOI] [PubMed] [Google Scholar]
- 24.Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of Adverse Events With Antibiotic Use in Hospitalized Patients. JAMA Intern Med. 2017;177(9):1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. Vancomycin Plus Piperacillin-Tazobactam and Acute Kidney Injury in Adults: A Systematic Review and Meta-Analysis. Critical care medicine. 2018;46(1):12–20. [DOI] [PubMed] [Google Scholar]
- 26.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes MM, Chatterjee S, Schwartzstein RM. Critical Thinking in Critical Care: Five Strategies to Improve Teaching and Learning in the Intensive Care Unit. Ann Am Thorac Soc. 2017;14(4):569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rock C, Pana Z, Leekha S, et al. National Healthcare Safety Network laboratory-identified Clostridium difficile event reporting: A need for diagnostic stewardship. Am J Infect Control. 2018;46(4):456–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norton WE, Chambers DA. Unpacking the complexities of de-implementing inappropriate health interventions. Implement Sci. 2020;15(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan DJ, Croft LD, Deloney V, et al. Choosing Wisely in Healthcare Epidemiology and Antimicrobial Stewardship. Infect Control Hosp Epidemiol. 2016;37(7):755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. Jama. 2012;307(17):1801–1802. [DOI] [PubMed] [Google Scholar]
- 32.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191(10):1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blinova E, Lau E, Bitnun A, et al. Point prevalence survey of antimicrobial utilization in the cardiac and pediatric critical care unit. Pediatr Crit Care Med. 2013;14(6):e280–288. [DOI] [PubMed] [Google Scholar]
- 34.Hartman ME, Linde-Zwirble WT, Angus DC, et al. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med. 2013;14(7):686–693. [DOI] [PubMed] [Google Scholar]
- 35.Children’s Hospital Association. Improving Pediatric Sepsis Outcomes (IPSO) is successfully challenging sepsis. https://www.childrenshospitals.org/programs-and-services/quality-improvement-and-measurement/collaboratives/sepsis. Accessed April 6, 2021.
- 36.Weiss SL, Peters MJ, Alhazzani W, et al. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr Crit Care Med. 2020;21(2):e52–e106. [DOI] [PubMed] [Google Scholar]
- 37.Lautz AJ, Dziorny AC, Denson AR, et al. Value of Procalcitonin Measurement for Early Evidence of Severe Bacterial Infections in the Pediatric Intensive Care Unit. J Pediatr. 2016;179:74–81 e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nijman RG, Moll HA, Vergouwe Y, de Rijke YB, Oostenbrink R. C-Reactive Protein Bedside Testing in Febrile Children Lowers Length of Stay at the Emergency Department. Pediatric emergency care. 2015;31(9):633–639. [DOI] [PubMed] [Google Scholar]
- 39.Hsiao AL, Baker MD. Fever in the new millennium: a review of recent studies of markers of serious bacterial infection in febrile children. Curr Opin Pediatr. 2005;17(1):56–61. [DOI] [PubMed] [Google Scholar]
- 40.Milcent K, Faesch S, Gras-Le Guen C, et al. Use of Procalcitonin Assays to Predict Serious Bacterial Infection in Young Febrile Infants. JAMA Pediatr. 2016;170(1):62–69. [DOI] [PubMed] [Google Scholar]
- 41.Chiotos K, Tamma PD, Gerber JS, et al. Antibiotic stewardship in the intensive care unit: Challenges and opportunities. Infect Control Hosp Epidemiol. 2019;40(6):693–698. [DOI] [PubMed] [Google Scholar]
- 42.Darby JM, Linden P, Pasculle W, Saul M. Utilization and diagnostic yield of blood cultures in a surgical intensive care unit. Critical care medicine. 1997;25(6):989–994. [DOI] [PubMed] [Google Scholar]
- 43.Kiragu AW, Zier J, Cornfield DN. Utility of blood cultures in postoperative pediatric intensive care unit patients. Pediatr Crit Care Med. 2009;10(3):364–368. [DOI] [PubMed] [Google Scholar]
- 44.Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. Jama. 1991;265(3):365–369. [PubMed] [Google Scholar]
- 45.Tran CA, Zschaebitz JV, Spaeder MC. Epidemiology of Blood Culture Utilization in a Cohort of Critically Ill Children. J Pediatr Intensive Care. 2019;8(3):144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doern GV, Carroll KC, Diekema DJ, et al. Practical Guidance for Clinical Microbiology Laboratories: A Comprehensive Update on the Problem of Blood Culture Contamination and a Discussion of Methods for Addressing the Problem. Clin Microbiol Rev. 2019;33(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elward AM, Hollenbeak CS, Warren DK, Fraser VJ. Attributable cost of nosocomial primary bloodstream infection in pediatric intensive care unit patients. Pediatrics. 2005;115(4):868–872. [DOI] [PubMed] [Google Scholar]
- 48.Alahmadi YM, Aldeyab MA, McElnay JC, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect. 2011;77(3):233–236. [DOI] [PubMed] [Google Scholar]
- 49.Woods-Hill CZ, Fackler J, Nelson McMillan K, et al. Association of a Clinical Practice Guideline With Blood Culture Use in Critically Ill Children. JAMA Pediatr. 2017;171(2):157–164. [DOI] [PubMed] [Google Scholar]
- 50.Woods-Hill CZ, Lee L, Xie A, et al. Dissemination of a Novel Framework to Improve Blood Culture Use in Pediatric Critical Care. Pediatric Quality & Safety. 2018;3(5):e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woods-Hill CZ, Koontz DW, Voskertchian A, et al. Consensus Recommendations for Blood Culture Use in Critically Ill Children Using a Modified Delphi Approach. Pediatr Crit Care Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woods-Hill CZ, Koontz DW, King AF, et al. Practices, Perceptions, and Attitudes in the Evaluation of Critically Ill Children for Bacteremia: A National Survey. Pediatr Crit Care Med. 2020;21(1):e23–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sick-Samuels AC, Woods-Hill CZ, Fackler JC, et al. Association of a blood culture utilization intervention on antibiotic use in a pediatric intensive care unit. Infect Control Hosp Epidemiol. 2019;40(4):482–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiang J, Amin R. Respiratory Care Considerations for Children with Medical Complexity. Children (Basel). 2017;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danin PE, Girou E, Legrand P, et al. Description and microbiology of endotracheal tube biofilm in mechanically ventilated subjects. Respir Care. 2015;60(1):21–29. [DOI] [PubMed] [Google Scholar]
- 56.Diaconu O, Siriopol I, Polosanu LI, Grigoras I. Endotracheal Tube Biofilm and its Impact on the Pathogenesis of Ventilator-Associated Pneumonia. J Crit Care Med (Targu Mures). 2018;4(2):50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandecandelaere I, Coenye T. Microbial composition and antibiotic resistance of biofilms recovered from endotracheal tubes of mechanically ventilated patients. Adv Exp Med Biol. 2015;830:137–155. [DOI] [PubMed] [Google Scholar]
- 58.Fayon MJ, Tucci M, Lacroix J, et al. Nosocomial pneumonia and tracheitis in a pediatric intensive care unit: a prospective study. Am J Respir Crit Care Med. 1997;155(1):162–169. [DOI] [PubMed] [Google Scholar]
- 59.Gauvin F, Dassa C, Chaibou M, Proulx F, Farrell CA, Lacroix J. Ventilator-associated pneumonia in intubated children: comparison of different diagnostic methods. Pediatr Crit Care Med. 2003;4(4):437–443. [DOI] [PubMed] [Google Scholar]
- 60.Craven DE, Chroneou A, Zias N, Hjalmarson KI. Ventilator-associated tracheobronchitis: the impact of targeted antibiotic therapy on patient outcomes. Chest. 2009;135(2):521–528. [DOI] [PubMed] [Google Scholar]
- 61.Alves AE, Pereira JM. Antibiotic therapy in ventilator-associated tracheobronchitis: a literature review. Revista Brasileira de terapia intensiva. 2018;30(1):80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan XX, Li S, Qi TJ, et al. [The characteristics of biofilm formation in endotracheal tubes in ventilated patients]. Zhonghua Jie He He Hu Xi Za Zhi. 2008;31(7):501–504. [PubMed] [Google Scholar]
- 63.Dennesen PJ, van der Ven AJ, Kessels AG, Ramsay G, Bonten MJ. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med. 2001;163(6):1371–1375. [DOI] [PubMed] [Google Scholar]
- 64.Thorarinsdottir HR, Kander T, Holmberg A, Petronis S, Klarin B. Biofilm formation on three different endotracheal tubes: a prospective clinical trial. Critical care (London, England). 2020;24(1):382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill JD, Ratliff JL, Parrott JC, et al. Pulmonary pathology in acute respiratory insufficiency: lung biopsy as a diagnostic tool. The Journal of thoracic and cardiovascular surgery. 1976;71(1):64–71. [PubMed] [Google Scholar]
- 66.Durairaj L, Mohamad Z, Launspach JL, et al. Patterns and density of early tracheal colonization in intensive care unit patients. J Crit Care. 2009;24(1):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willson DF, Conaway M, Kelly R, Hendley JO. The lack of specificity of tracheal aspirates in the diagnosis of pulmonary infection in intubated children. Pediatr Crit Care Med. 2014;15(4):299–305. [DOI] [PubMed] [Google Scholar]
- 68.Klompas M Does this patient have ventilator-associated pneumonia? Jama. 2007;297(14):1583–1593. [DOI] [PubMed] [Google Scholar]
- 69.Yalamanchi S, Saiman L, Zachariah P. Decision-Making Around Positive Tracheal Aspirate Cultures: The Role of Neutrophil Semiquantification in Antibiotic Prescribing. Pediatr Crit Care Med. 2019;20(8):e380–e385. [DOI] [PubMed] [Google Scholar]
- 70.Langston SJ, Pithia N, Sim MS, Garg M, de St Maurice A, Chu A. Lack of utility of tracheal aspirates in the management of suspected pneumonia in intubated neonates. Infect Control Hosp Epidemiol. 2020;41(6):660–665. [DOI] [PubMed] [Google Scholar]
- 71.Morris AJ, Tanner DC, Reller LB. Rejection criteria for endotracheal aspirates from adults. Journal of clinical microbiology. 1993;31(5):1027–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prinzi AM, Parker SK, Curtis DJ, Ziniel SI. The Pediatric Endotracheal Aspirate Culture Survey (PETACS): Examining Practice Variation across Pediatric Microbiology Laboratories in the United States. Journal of clinical microbiology. 2021;59(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sick-Samuels AC, Fackler JC, Berenholtz SM, Milstone AM. Understanding reasons clinicians obtained endotracheal aspirate cultures and impact on patient management to inform diagnostic stewardship initiatives. Infect Control Hosp Epidemiol. 2019:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Willson DF, Kirby A, Kicker JS. Respiratory secretion analyses in the evaluation of ventilator-associated pneumonia: a survey of current practice in pediatric critical care. Pediatr Crit Care Med. 2014;15(8):715–719. [DOI] [PubMed] [Google Scholar]
- 75.Venkatachalam V, Hendley JO, Willson DF. The diagnostic dilemma of ventilator-associated pneumonia in critically ill children. Pediatr Crit Care Med. 2011;12(3):286–296. [DOI] [PubMed] [Google Scholar]
- 76.Willson DF, Hoot M, Khemani R, et al. Pediatric Ventilator-Associated Infections: The Ventilator-Associated INfection Study. Pediatr Crit Care Med. 2017;18(1):e24–e34. [DOI] [PubMed] [Google Scholar]
- 77.Benneyworth BD, Gebremariam A, Clark SJ, Shanley TP, Davis MM. Inpatient health care utilization for children dependent on long-term mechanical ventilation. Pediatrics. 2011;127(6):e1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nseir S, Favory R, Jozefowicz E, et al. Antimicrobial treatment for ventilator-associated tracheobronchitis: a randomized, controlled, multicenter study. Critical care (London, England). 2008;12(3):R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ormsby J, Conrad P, Blumenthal J, et al. Practice Improvement for Standardized Evaluation and Management of Acute Tracheitis in Mechanically Ventilated Children. Pediatr Qual Saf. 2021;6(1):e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sick-Samuels AC, Linz M, Bergmann J, et al. Diagnostic Stewardship of Endotracheal Aspirate Cultures in a PICU. Pediatrics. 2021;147(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101–114. [DOI] [PubMed] [Google Scholar]
- 82.Yi SH, Baggs J, Gould CV, Scott RD 2nd, Jernigan JA. Medicare reimbursement attributable to catheter-associated urinary tract infection in the inpatient setting: a retrospective cohort analysis. Med Care. 2014;52(6):469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clinical Saint S. and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68–75. [DOI] [PubMed] [Google Scholar]
- 84.Page S, Hazen D, Kelley K, et al. Changing the culture of urine culturing: Utilizing Agile Implementation to improve diagnostic stewardship in the ICU. Am J Infect Control. 2020;48(11):1375–1380. [DOI] [PubMed] [Google Scholar]
- 85.Epstein L, Edwards JR, Halpin AL, et al. Evaluation of a Novel Intervention to Reduce Unnecessary Urine Cultures in Intensive Care Units at a Tertiary Care Hospital in Maryland, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(5):606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mullin KM, Kovacs CS, Fatica C, et al. A Multifaceted Approach to Reduction of Catheter-Associated Urinary Tract Infections in the Intensive Care Unit With an Emphasis on “Stewardship of Culturing”. Infect Control Hosp Epidemiol. 2017;38(2):186–188. [DOI] [PubMed] [Google Scholar]
- 87.Stagg A, Lutz H, Kirpalaney S, et al. Impact of two-step urine culture ordering in the emergency department: a time series analysis. BMJ Qual Saf. 2018;27(2):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keller SC, Feldman L, Smith J, Pahwa A, Cosgrove SE, Chida N. The Use of Clinical Decision Support in Reducing Diagnosis of and Treatment of Asymptomatic Bacteriuria. J Hosp Med. 2018;13(6):392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272–283. [DOI] [PubMed] [Google Scholar]
- 90.Sonmez Duzkaya D, Bozkurt G, Uysal G, Yakut T. The Effects of Bundles on Catheter-Associated Urinary Tract Infections in the Pediatric Intensive Care Unit. Clin Nurse Spec. 2016;30(6):341–346. [DOI] [PubMed] [Google Scholar]
- 91.Schiessler MM, Darwin LM, Phipps AR, Hegemann LR, Heybrock BS, Macfadyen AJ. Don’t Have a Doubt, Get the Catheter Out: A Nurse-Driven CAUTI Prevention Protocol. Pediatr Qual Saf 2019;4(4):e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. Jama. 1994;271(20):1598–1601. [DOI] [PubMed] [Google Scholar]
- 93.Kelly M, Conway M, Wirth K, Potter-Bynoe G, Billett AL, Sandora TJ. Moving CLABSI prevention beyond the intensive care unit: risk factors in pediatric oncology patients. Infect Control Hosp Epidemiol. 2011;32(11):1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simon A, Ammann RA, Bode U, et al. Healthcare-associated infections in pediatric cancer patients: results of a prospective surveillance study from university hospitals in Germany and Switzerland. BMC infectious diseases. 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karandikar MV, Milliren CE, Zaboulian R, et al. Limiting Vancomycin Exposure in Pediatric Oncology Patients With Febrile Neutropenia May Be Associated With Decreased Vancomycin-Resistant Enterococcus Incidence. Journal of the Pediatric Infectious Diseases Society. 2020;9(4):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haeusler GM, De Abreu Lourenco R, Clark H, et al. Diagnostic Yield of Initial and Consecutive Blood Cultures in Children With Cancer and Febrile Neutropenia. Journal of the Pediatric Infectious Diseases Society. 2021;10(2):125–130. [DOI] [PubMed] [Google Scholar]
- 97.Allaway Z, Phillips RS, Thursky KA, Haeusler GM. Nonneutropenic fever in children with cancer: A scoping review of management and outcome. Pediatr Blood Cancer. 2019;66(6):e27634. [DOI] [PubMed] [Google Scholar]
- 98.Murni IK, MacLaren G, Morrow D, Iyer P, Duke T. Perioperative infections in congenital heart disease. Cardiol Young. 2017;27(S6):S14–S21. [DOI] [PubMed] [Google Scholar]
- 99.Herrup EA, Yuerek M, Griffis HM, et al. Hospital-Acquired Infection in Pediatric Subjects With Congenital Heart Disease Postcardiotomy Supported on Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med. 2020;21(11):e1020–e1025. [DOI] [PubMed] [Google Scholar]
- 100.Extracorporeal Life Support Orgnization Infectious Disease Task Force. Infection Control and Extracorporeal Life Support. 2008; https://www.elso.org/Portals/0/Files/Infection-Control-and-Extracorporeal-Life-Support.pdf. Accessed May 21, 2021.
- 101.Woods-Hill CZ, Lee L, Xie A, et al. Dissemination of a Novel Framework to Improve Blood Culture Use in Pediatric Critical Care. Pediatr Qual Saf. 2018;3(5):e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hsieh EM, Hornik CP, Clark RH, et al. Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31(9):811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Willis Z, de St Maurice A. Strategies to improve antibiotic use in the neonatal ICU. Curr Opin Pediatr. 2019;31(1):127–134. [DOI] [PubMed] [Google Scholar]
- 104.Gkentzi D, Dimitriou G. Antimicrobial Stewardship in the Neonatal Intensive Care Unit: An Update. Curr Pediatr Rev. 2019;15(1):47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mukhopadhyay S, Sengupta S, Puopolo KM. Challenges and opportunities for antibiotic stewardship among preterm infants. Arch Dis Child Fetal Neonatal Ed. 2019;104(3):F327–F332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Achten NB, Klingenberg C, Benitz WE, et al. Association of Use of the Neonatal Early-Onset Sepsis Calculator With Reduction in Antibiotic Therapy and Safety: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019;173(11):1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gagliardi AR, Marshall C, Huckson S, James R, Moore V. Developing a checklist for guideline implementation planning: review and synthesis of guideline development and implementation advice. Implement Sci. 2015;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eccles MP, Mittman BS. Welcome to Implementation Science. Implementation Science. 2006;1(1):1. [Google Scholar]
- 109.Holden RJ CP, Gurses AP, et al. Seips 2.0: A human factors framework for studying and improving the work of healthcare professionals and patients. Ergonomics. 2013(56):1669–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xie A, Woods-Hill CZ, King AF, et al. Work System Assessment to Facilitate the Dissemination of a Quality Improvement Program for Optimizing Blood Culture Use: A Case Study Using a Human Factors Engineering Approach. Journal of the Pediatric Infectious Diseases Society. 2019;8(1):39–45. [DOI] [PubMed] [Google Scholar]
- 111.Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714. [DOI] [PubMed] [Google Scholar]
- 112.Institute for Healthcare Improvement. Resources. 2021; http://www.ihi.org/resources. Accessed May 21, 2021.


