ABSTRACT
NrtR is a Nudix-related transcriptional regulator that is distributed among diverse bacteria and plays an important role in modulating bacterial intracellular NAD homeostasis. Previously, we showed that NrtR influences the T3SS expression and pathogenesis of Pseudomonas aeruginosa and demonstrated that NrtR mediates T3SS regulation through the cAMP/Vfr pathway. In the present study, we found that mutation of the nrtR gene leads to upregulation of the Hcp secretion island-I type VI secretion system (H1-T6SS). Further analysis revealed that mutation of the nrtR gene results in upregulation of regulatory RNAs (RsmY/RsmZ) that are known to control the H1-T6SS by sequestration of RsmA or RsmN. Simultaneous deletion of rsmY/rsmZ reduced the expression of H1-T6SS in the ΔnrtR mutant. In addition, overexpression of either rsmA or rsmN in ΔnrtR decreased H1-T6SS expression. Chromatin immunoprecipitation (ChIP)-Seq and electrophoretic mobility shift assay (EMSA) analyses revealed that NrtR directly binds to the promoters of rsmY, rsmZ and tssA1 (first gene of the H1-T6SS operon). Overall, the results from this study reveal the molecular details of NrtR-mediated regulation of H1-T6SS in P. aeruginosa.
IMPORTANCE NrtR is a Nudix-related transcriptional regulator and controls the NAD cofactor biosynthesis in bacteria. P. aeruginosa NrtR binds to the intergenic region between nadD2 and pcnA to repress the expression of the two operons, therefore controlling the NAD biosynthesis. We have previously reported that NrtR controls T3SS expression via the cAMP/Vfr pathway in P. aeruginosa. However, the global regulatory function and direct binding targets of the NrtR remain elusive in P. aeruginosa. This study reveals novel direct regulatory targets of the NrtR in P. aeruginosa, elucidating the molecular mechanism of NrtR-mediated regulation of H1-T6SS.
KEYWORDS: P. aeruginosa, H1-T6SS, NrtR, rsmY, rsmZ
INTRODUCTION
Pseudomonas aeruginosa is a versatile opportunistic human pathogen that is responsible for a variety of infections in humans (1). The bacterium possesses several protein secretion systems that contribute to its pathogenesis and competitive advantage in the host environment (2). Among them, the type III secretion system (T3SS) is a critical virulence determinant that plays an important role in the interaction with the hosts during acute infections (3), while the type VI secretion system (T6SS) is a protein secretion machinery, deployed by P. aeruginosa to deliver effector proteins into neighboring eukaryotic or prokaryotic cells, that acts against both bacteria and hosts (4). In addition, T6SSs have also been reported to play roles in metal ion acquisition to improve the adaptation to environmental niches and interbacterial competition (5–8).
The P. aeruginosa genome encodes three different T6SS clusters, named H1-, H2-, and H3-T6SS (9). Among them, H1-T6SS displays an antibacterial activity and confers P. aeruginosa a growth advantage in competition over other T6SS+ bacteria inhabiting the same niche (10, 11). H1-T6SS comprises a hemolysin coregulated secretion island I (HSI-I) gene cluster and effectors scattered in its genome. The H1-T6SS cluster spans genes from PA0071 to PA0091 and encodes a set of components for the export apparatus (9). To date, eight H1-T6SS effector proteins have been identified in P. aeruginosa (4, 12).
The regulation of H1-T6SS expression can occur at the transcriptional, posttranscriptional, and posttranslational levels in P. aeruginosa (13). The quorum sensing regulator LasR and the 4-hydroxy-2-alkylquinoline transcriptional regulator MvfR negatively control the gene expression of H1-T6SS at the transcriptional level (14). RsmA and RsmN (RsmF), two CsrA family RNA-binding proteins, negatively regulate H1-T6SS at the posttranscriptional level (15–17). This control is determined by the availability of the free RsmA and RsmN proteins within bacterial cells, which are regulated via their interaction and sequestration by two small regulatory RNAs, RsmY and RsmZ (15, 17). In addition, H1-T6SS can be posttranslationally regulated by a threonine phosphorylation (TPP)-dependent or TPP-independent pathway (18, 19).
NrtR, a Nudix-related transcriptional regulator, is widely distributed across diverse bacterial species (20). It is composed of an N-terminal Nudix-like effector binding domain and a C-terminal DNA-binding winged helix-turn-helix (HTH) domain (20, 21). It acts as a transcriptional repressor via HTH domain-mediated binding to promoter regions of its target genes, while the Nudix domain specifically interacts with effector molecules to weaken the NrtR–DNA complex, resulting in derepression of target gene expression (20–22). It has been well documented that NrtR negatively regulates the de novo and salvage pathways of NAD cofactor biosynthesis to modulate intracellular NAD homeostasis (20–24). A previous study reported that NrtR influences the fitness and pathogenicity of the P. aeruginosa TBCF10839 strain (22).
Recently, we reported that P. aeruginosa NrtR controls the expression of T3SS through the cAMP/Vfr pathway (25). In this study, we demonstrate that NrtR regulates the expression of H1-T6SS genes by directly binding to the promoter of tssA1, as well as through the RsmY/RsmZ-RsmA/RsmN pathway. The presented work further reveals the multiplexed function of the NrtR in P. aeruginosa.
RESULTS
Mutation of nrtR increases the expression of H1-T6SS genes in P. aeruginosa.
To determine the global regulatory role of nrtR in P. aeruginosa, RNAseq analysis was carried out to compare the global transcriptomes between the wild-type strain PAK and its ΔnrtR mutant derivative. As revealed by the transcriptomic analyses, most genes of the H1-T6SS operon and its scattered effectors/immunity proteins (tse1/tsi1, tse3/tsi3, tse4, tse5, and tse6/tsi6) were upregulated in the ΔnrtR mutant (Table 1; complete list shown in Table S3 in the supplemental material). In order to understand the relationship between the nrtR and H1-T6SS genes, we used real-time qPCR to verify the mRNA levels of the Hcp1-encoding gene, the hallmark of the H1-T6SS in P. aeruginosa. As shown in Fig. 1, the mRNA level of hcp1 was upregulated 6.1-fold in the ΔnrtR mutant and restored to wild-type PAK level by complementation with a wild-type nrtR gene. To verify the increased expression level of Hcp1, a C-terminal Flag-tagged Hcp1 driven by its native promoter was further introduced into the PAK and ΔnrtR mutant. Consistent with the real-time qPCR results, the Hcp1 protein level was elevated in the ΔnrtR mutant and restored to that in PAK by the complementation with nrtR (Fig. 1B).
TABLE 1.
mRNA levels of genes related to H1-T6SS in ΔnrtR compared to those in PAK identified via RNAseq (57)
| Gene ID | Gene name | Fold change (ΔnrtR/PAK) | Description |
|---|---|---|---|
| PA0070 | tagQ1 | 5.2 | TagQ1 |
| PA0072 | tagS1 | 5.7 | TagS1 |
| PA0073 | tagT1 | 5.3 | TagT1 |
| PA0074 | ppkA | 5.7 | Serine/threonine protein kinase PpkA |
| PA0075 | pppA | 7.5 | PppA |
| PA0076 | tagF1 | 6.1 | TagF1 |
| PA0077 | icmF1 | 10.5 | IcmF1 |
| PA0078 | tssL1 | 11.4 | TssL1 |
| PA0079 | tssK1 | 11.8 | TssK1 |
| PA0080 | tssJ1 | 9.1 | TssJ1 |
| PA0082 | tssA1 | 9.6 | TssA1 |
| PA0083 | tssB1 | 20.8 | TssB1 |
| PA0084 | tssC1 | 18.0 | TssC1 |
| PA0085 | hcp1 | 21.1 | Hcp1 |
| PA0086 | tagJ1 | 21.4 | TagJ1 |
| PA0087 | tssE1 | 20.7 | TssE1 |
| PA0088 | tssF1 | 29.5 | TssF1 |
| PA0089 | tssG1 | 32.6 | TssG1 |
| PA0090 | clpV1 | 17.5 | ClpV1 |
| PA0091 | vgrG1 | 17.4 | VgrG1 |
| PA0092 | tsi6 | 5.8 | Tsi6 |
| PA0093 | tse6 | 15.4 | Tse6 |
| PA0096a | 8.9 | Hypothetical protein | |
| PA0097a | 5.0 | Hypothetical protein | |
| PA1844 | tse1 | 9.2 | Tse1 |
| PA1845 | tsi1 | 9.5 | Tsi1 |
| PA2684 | tse5 | 6.0 | Tse5 |
| PA2774 | tse4 | 7.7 | Tse4 |
| PA3484 | tse3 | 9.0 | Tse3 |
| PA3485 | tsi3 | 7.2 | Tsi3 |
Genes located in the vgrG1b cluster (57).
FIG 1.
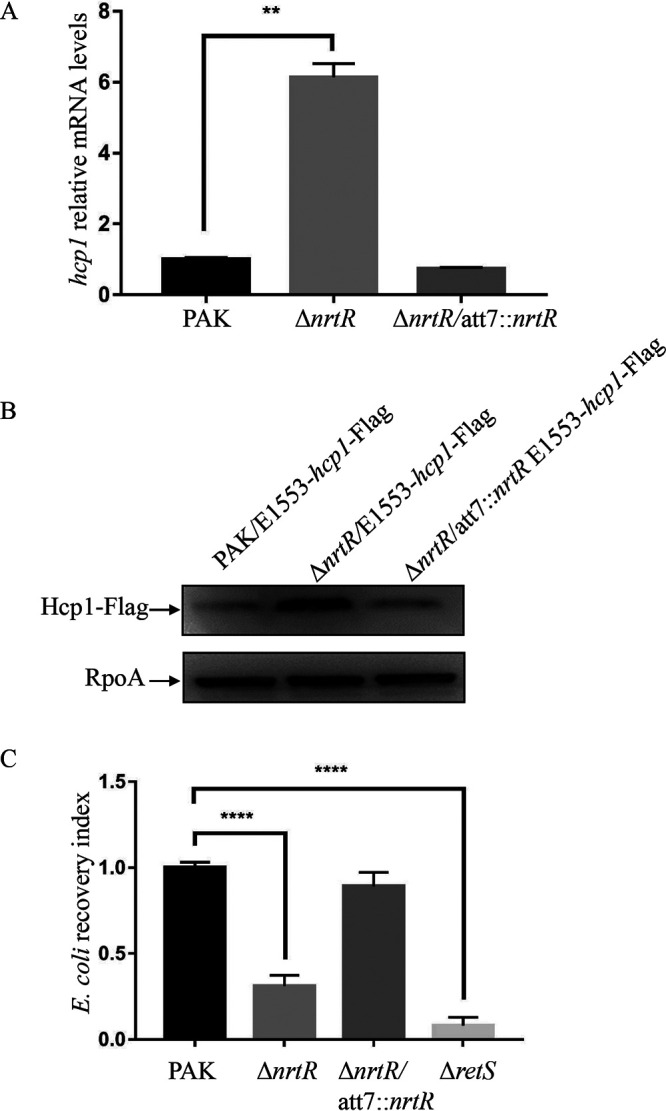
Hcp1 was upregulated in the ΔnrtR mutant. (A) The relative hcp1 mRNA levels in PAK, ΔnrtR, and ΔnrtR/att7::nrtR. Total RNA was isolated from bacteria at an OD600 of 1.0, and hcp1 mRNA levels were examined by real-time qPCR using rpsL as an internal control. **, P < 0.01, by Student's t test. (B) Bacteria containing an hcp1-Flag driven by its native promoter were grown to an OD600 of 1.0 in LB medium. Proteins from an equivalent number of P. aeruginosa cells of the indicated strains were separated on 12% SDS-PAGE and probed with an antibody against Flag or RpoA. (C) Competition assay between P. aeruginosa and E. coli. The indicated P. aeruginosa strains and E. coli were mixed at a 5:1 ratio, coincubated for 24 h at 25°C, resuspended in LB, and plated on LB agar plates with tetracycline. The E. coli recovery index represents the bacterial number of recovered DH5α/pDN19 with the number of DH5α/pDN19 in competition with PAK as 1.0. Bars represent the means from three experiments, and error bars indicate the standard deviation. ****, P < 0.0001, by Student's t test.
Since H1-T6SS plays an important role in the fitness advantage of P. aeruginosa in competition with other bacteria (26), the functional connection between NrtR and H1-T6SS prompted us to determine the role of NrtR in the interspecies competition. Accordingly, we performed interbacterial growth competition experiments between P. aeruginosa and E. coli. Competition assays were conducted for 24 h at 25°C, using the P. aeruginosa retS mutant, which shows a constitutively active H1-T6SS (9) as a positive control. As shown in Fig. 1C, the E. coli recovery index was significantly decreased when mixed with the ΔnrtR mutant compared to that with wild-type PAK, and the recovery index was restored by complementation with the nrtR gene. These results demonstrated that the expression of H1-T6SS is upregulated in the ΔnrtR mutant.
Upregulation of rsmY/rsmZ contributes to the increased H1-T6SS in the ΔnrtR mutant.
Since NrtR controls the expression of T3SS through the cAMP/Vfr pathway (25), we wanted to know if the increased H1-T6SS in the ΔnrtR mutant was due to the decreased intracellular cAMP levels. To test it, we examined the expression of H1-T6SS in the ΔcyaAΔcyaB and Δvfr mutants, which also showed reduced intracellular cAMP amounts (25). As real-time qPCR results shown in Fig. S1A, both hcp1 and tssA1 displayed decreased mRNA levels in ΔcyaAΔcyaB and Δvfr mutants compared to that in the wild-type PAK strain. These data indicate that upregulation of H1-T6SS was not due to the decreased cAMP levels in the ΔnrtR mutant.
In P. aeruginosa, the expression of H1-T6SS was shown to be controlled by intracellular c-di-GMP (27), which is linked to the cAMP signaling pathways (28, 29). We previously reported that the cAMP level was significantly decreased in the ΔnrtR mutant (25); thus, we wanted to examine whether nrtR affects the intracellular c-di-GMP level. The expression of surface adhesion CdrA is known to be regulated by intracellular c-di-GMP; thus, its expression level has been utilized as an indicator of intracellular c-di-GMP levels (30, 31). Accordingly, real-time qPCR was carried out to compare the relative mRNA levels of cdrA between PAK and the ΔnrtR mutant derivative. Compared to that in PAK, no significant difference in cdrA mRNA level was observed in ΔnrtR (Fig. S1B), suggesting that NrtR has no influence on the P. aeruginosa intracellular c-di-GMP level.
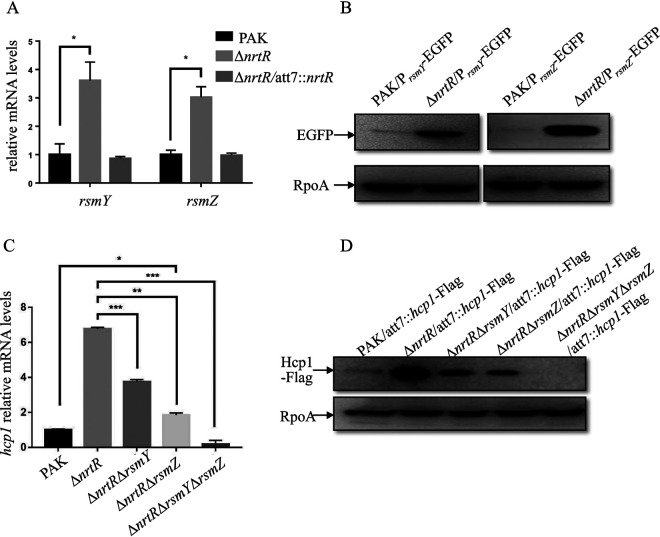
In P. aeruginosa, the small RNAs RsmY and RsmZ are known to regulate the expression of H1-T6SS (32). In order to test whether nrtR repressed H1-T6SS through RsmY and RsmZ, we compared the levels of these sRNAs between PAK and the ΔnrtR mutant. Indeed, compared to wild-type PAK or ΔnrtR with complementation, the rsmY and rsmZ levels in the ΔnrtR mutant were 3.6- and 3.0-fold higher, respectively (Fig. 2A). To confirm the increased expression of rsmY and rsmZ, transcriptional fusion plasmids of PrsmY and PrsmZ to an egfp gene (PrsmY-EGFP and PrsmZ-EGFP) were constructed and introduced into the above bacterial strains. Consistent with the differential mRNA levels, the EGFP protein amounts were higher in the ΔnrtR mutant background than that in PAK (Fig. 2B). These results indicated that RsmY and RsmZ are upregulated in the ΔnrtR mutant.
FIG 2.
Upregulation of RsmY/RsmZ contributes to the increased H1-T6SS in the ΔnrtR mutant. (A, C) The relative RNA levels of rsmY, rsmZ (A), and hcp1 (C) in the indicated strains. Total RNA was isolated from bacteria at an OD600 of 1.0, and the relative RNA levels of rsmY, rsmZ, and hcp1 were examined by real-time qPCR using rpsL as an internal control. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by Student's t test. (B, D) Bacteria containing an egfp gene driven by the promoter of rsmY or rsmZ (B) or bacteria integrated with an hcp1-Flag driven by its native promoter (D) were grown to an OD600 of 1.0 in LB medium. Proteins from an equivalent number of P. aeruginosa cells of the indicated strains were separated on a 12% SDS-PAGE and probed with an antibody against EGFP, Flag, or RpoA.
To test the role of RsmY and RsmZ in the regulation of H1-T6SS mediated by NrtR, we constructed ΔnrtRΔrsmY and ΔnrtRΔrsmZ double mutants, as well as a ΔnrtRΔrsmYΔrsmZ triple mutant, and examined their expression of H1-T6SS. As shown in Fig. 2C and D, individual deletion of rsmY or rsmZ in ΔnrtR decreased the expression of hcp1, but not to the level of the wild-type PAK strain. However, simultaneous deletion of rsmY and rsmZ in the ΔnrtR background resulted in the expression of hcp1 below that of the wild-type PAK strain. These data together suggest that NrtR represses H1-T6SS through the small RNAs RsmY and RsmZ.
Overexpression of rsmA/rsmN in the ΔnrtR mutant restores the expression of H1-T6SS.
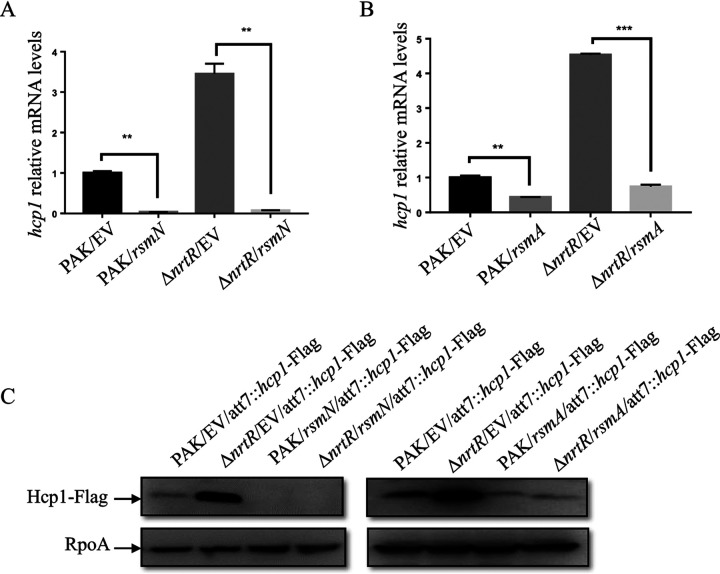
Since the major role of RsmY and RsmZ is to sequester and lower free RsmA and RsmN, two CsrA family RNA binding proteins, we investigated whether ectopic expression of rsmA or rsmN could restore H1-T6SS expression in the ΔnrtR mutant. First, real-time qPCR was performed to determine the transcriptional level of hcp1. As expected, overexpression of either rsmA or rsmN decreased the relative mRNA levels of hcp1 in the ΔnrtR mutant (Fig. 3A and B). Furthermore, a Flag-tagged hcp1 driven by its native promoter (33) was integrated into the chromosomes of PAK and its ΔnrtR derivative. Western blot assay was carried out to examine the expression of Hcp1. Consistent with the real-time qPCR results, overexpression of either rsmA or rsmN decreased the Hcp1-Flag amounts in ΔnrtR (Fig. 3C). These results indicate that NrtR influences RsmA/RsmN-mediated regulation of the H1-T6SS.
FIG 3.
Overexpression of rsmA/rsmN in the ΔnrtR mutant restored the expression of H1-T6SS. (A, B) The relative hcp1 mRNA levels in PAK and ΔnrtR containing pUCP20 (empty vector, EV), pUCP20-rsmN, or pUCP20-rsmA. Total RNA was isolated from bacteria at an OD600 of 1.0, and hcp1 mRNA levels were examined by real-time qPCR using rpsL as an internal control. **, P < 0.01; ***, P < 0.001, by Student's t test. (C) PAK and ΔnrtR integrated with an hcp1-Flag driven by its native promoter containing pUCP20 (empty vector, EV), pUCP20-rsmN (rsmN), or pUCP20-rsmA (rsmA) were grown to an OD600 of 1.0 in LB medium. Proteins from an equivalent number of P. aeruginosa cells of the indicated strains were separated on a 12% SDS-PAGE and probed with an antibody against Flag or RpoA.
NrtR binds directly to the promoter of rsmY/rsmZ.
To understand the NrtR-mediated rsmY/rsmZ regulation, we further examined whether NrtR affects the expression of known regulatory genes upstream of the rsmY/rsmZ genes, such as gacA, gacS, ladS, retS, algR, and amrZ (27, 32, 34–36). Interestingly, the mRNA levels of all of these regulatory genes were not altered in the ΔnrtR mutant based on the RNAseq analysis and real-time qPCR (Fig. S1B).
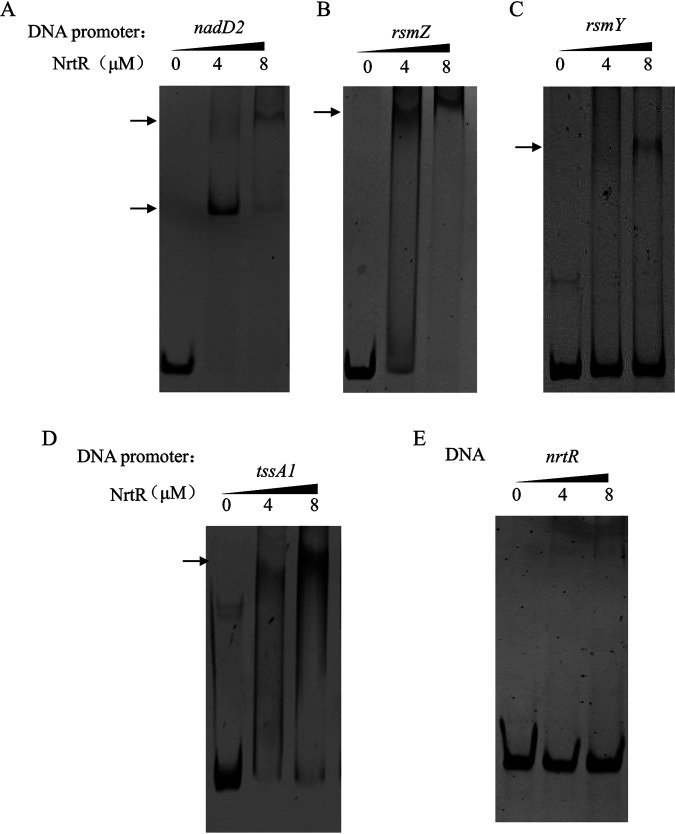
To further elucidate the molecular mechanism of rsmY/rsmZ regulation by NrtR, a ChIP-seq assay was performed. First, an N-terminal Flag-tagged nrtR expression plasmid pUCP24-nrtR was constructed, which was able to restore the expression of H1-T6SS in the ΔnrtR mutant (data not shown). Then, ChIP-seq was carried out in the ΔnrtR mutant harboring the expressing plasmid. The DNA binding loci and enrichment folds are displayed in Table S4. Consistent with a previous report that NrtR binds an intergenic region between nadD2 and pcnA (22), the intergenic region was enriched by 2.21-fold (Table 2). Of note, the potential binding targets of NrtR included sequences upstream of the rsmY and rsmZ genes (Table 2). EMSA was further performed to validate the binding of NrtR to the candidate promoter regions. A His-tagged NrtR was overexpressed in pET28a and purified from E. coli. Binding of the His-NrtR to the nadD2 promoter was used as a positive control. As shown in Fig. 4, upon incubation with His-NrtR, shifted bands were detected for DNA fragments corresponding to promoter regions of nadD2, rsmY, and rsmZ, but not for the negative control DNA fragment (nrtR), indicating that NrtR binds directly to the promoter regions of rsmY and rsmZ.
TABLE 2.
Potential NrtR regulated genes identified via ChIP-seq analysis
| Gene ID of PAK | Gene ID of PAO1 | Summit in PAK | Fold enrichment | Gene name |
|---|---|---|---|---|
| PAK_00300 | PA0082 | 332033 | 1.55 | tssA1 |
| PAK_00742 | PA0527.1 | 806568 | 1.47 | rsmY |
| PAK_01565 | PA3621.1 | 1674747 | 1.43 | rsmZ |
| PAK_05420 | PA4917 | 5879334 | 2.21 | nadD2 |
FIG 4.
NrtR binds directly to the promoter of rsmY, rsmZ, or tssA1. Binding of NrtR to the DNA fragment corresponding to the promoter regions of nadD2 (A), rsmZ (B), rsmY (C), tssA1 (D), or the inner region of nrtR (E) was determined by EMSA. Increasing amounts of the purified His-NrtR were incubated with 40 ng of the indicated DNA fragments. The mixtures were electrophoresed on an 8% native PAGE gel, and the bands were visualized under UV light following ethidium bromide staining. Data represent results from three independent experiments. The arrows indicated the DNA-protein complex.
NrtR directly binds to the tssA1 promoter and represses tssA1 expression.
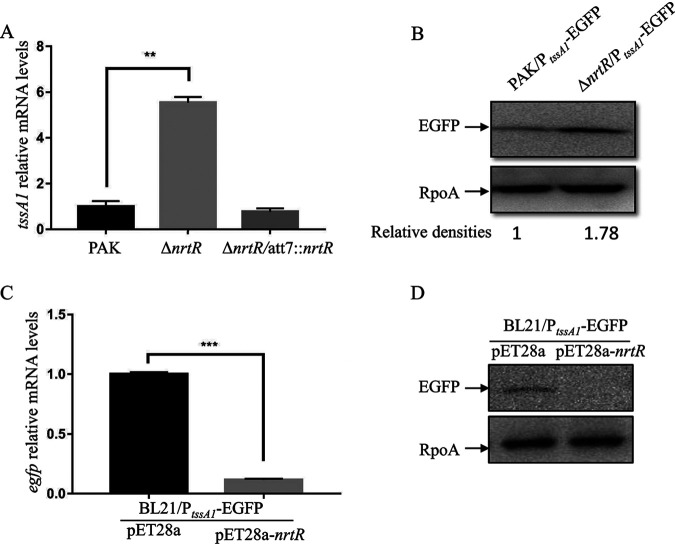
In addition to rsmY and rsmZ, our ChIP-seq data revealed that the promoter of tssA1, the first gene of the H1-T6SS operon, is also a potential binding target of NrtR (Table 2). Further supporting this, the tssA1 transcriptional level displayed a 9.6-fold increase in the ΔnrtR mutant according to the RNAseq analysis (Table 1). Therefore, it is possible that NrtR directly binds to the tssA1 promoter and represses its expression. To test this possibility, we first validated the increased expression of tssA1 in ΔnrtR using real-time qPCR. As shown in Fig. 5A, agreeing with the RNAseq result, the relative mRNA level of tssA1 was significantly increased in the ΔnrtR mutant, which could be restored to that of the wild-type PAK strain by complementation with nrtR. A transcriptional fusion plasmid of PtssA1 and an egfp gene (PtssA1-EGFP) were generated and introduced into PAK and the ΔnrtR mutant. Consistent with the mRNA level, the protein level of EGFP driven by PtssA1 was significantly higher in the ΔnrtR mutant background (Fig. 5B). To further verify the direct repression of tssA1 expression by NrtR, nrtR was cloned into pET28a and cointroduced into the E. coli BL21 strain with the PtssA1-EGFP plasmid. Real-time qPCR and Western blot were carried out to determine the expression level of egfp driven by the promoter of tssA1. As shown in Fig. 5C and D, compared to the empty vector pET28a, the expression of nrtR decreased the expression of egfp in the BL21 strain, which indicates a direct repression of the tssA1 promoter by NrtR.
FIG 5.
tssA1 was repressed by NrtR directly. (A, C) Real-time qPCR assay. (A) The relative mRNA levels of tssA1 in the PAK, ΔnrtR, and ΔnrtR complement strains. (C) The relative mRNA levels of egfp in BL21 containing both PtssA1-EGFP (egfp gene driven by tssA1 promoter) and pET28a or pET28a-nrtR plasmids. Total RNA was isolated from bacteria at an OD600 of 1.0, and the indicated mRNA levels were examined by real-time qPCR using rpsL as an internal control. **, P < 0.01; ***, P < 0.001, by Student's t test. (B, D) Western blot assay. PAK and ΔnrtR containing a PtssA1-EGFP plasmid (B) or BL21 containing both PtssA1-EGFP and pET28a or pET28a-nrtR plasmids (D) were grown to an OD600 of 1.0 in LB medium. Proteins from an equivalent number of bacterial cells of the indicated strains were separated on a 12% SDS-PAGE and probed with an antibody against EGFP or RpoA. Relative densities represent the density of EGFP/density of RpoA with the first lane as 1.
To further validate the direct binding of NrtR to the promoter region of tssA1, we performed EMSA using fragments corresponding to the promoter region of tssA1. Similar to the promoters of rsmY and rsmZ, upon incubation with NrtR, a retarded band was detected for the tssA1 promoter region, but not for the DNA fragment of nrtR (Fig. 4), indicating that NrtR binds to the promoter region of the H1-T6SS operon specifically.
DISCUSSION
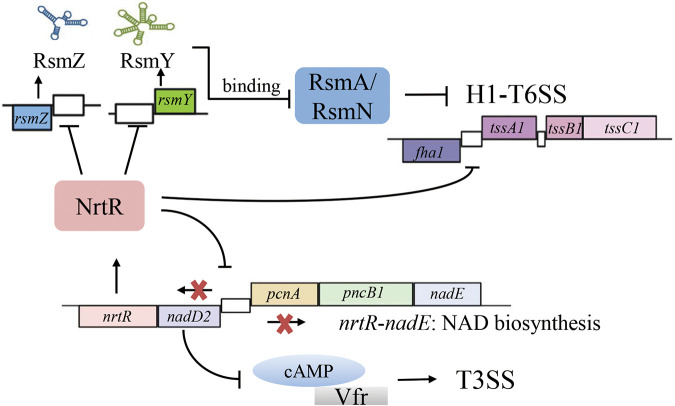
NrtR is a transcriptional repressor of NAD biosynthesis in P. aeruginosa (22) that can bind to the intergenic region between nadD2 and pcnA directly and thereby represses transcription of the divergently transcribed operons nadD2-nrtR and pcnA-nadE (22). Previously, we reported that NrtR affects intracellular cAMP levels and is required for the expression of T3SS as well as pathogenesis in P. aeruginosa (25). Here, we demonstrate that P. aeruginosa NrtR represses the expression of H1-T6SS genes. Further studies revealed that NrtR controls H1-T6SS directly by binding to the promoter of tssA1 and indirectly through the RsmY/RsmZ-RsmA/RsmN pathway by directly regulating the transcription of rsmY and rsmZ. Combining all of the above, a model has been proposed for the nrtR gene function in P. aeruginosa (Fig. 6).
FIG 6.
Proposed model of NrtR-mediated regulation in P. aeruginosa.
It has been demonstrated that the transcriptional regulator AmrZ activates expression of H1-T6SS in P. aeruginosa (37). Using real-time qPCR, we found that expression of amrZ was not affected in the ΔnrtR mutant (Fig. S1B), suggesting that amrZ was not involved in the NrtR-mediated regulation of H1-T6SS. Of note, AmrZ binds directly to the tssA1 upstream region (37). The putative binding sequence of AmrZ is CACAACGCCACTA, which locates −169∼–156 bp upstream of the start codon of tssA1 (37). In this study, we found that NrtR directly binds to the tssA1 upstream region to repress expression of the H1-T6SS. When using the 198 bp DNA fragment immediate upstream of the AmrZ binding site as probe, we also observed the direct binding by NrtR in our EMSA experiment (data not shown). Thus, we assume that the binding regions of NrtR and AmrZ in the tssA1 upstream region are not overlapped. However, the antagonistic control of H1-T6SS by these two regulators remains elusive and warrants further studies.
In addition to the multiplexed function of NrtR in P. aeruginosa, the NrtR-type transcription factor AraR has been reported to control L-arabinose catabolism and utilization of arabinose-containing polysaccharides in Bacteroides thetaiotaomicron (38). NrtR has also been shown to control the biofilm forming capacity and the pathogenesis of the zoonotic pathogen Streptococcus suis in a mouse infection model (23). In view of the function of nrtR in the biofilm forming in Streptococcus suis, we also compared the biofilm formation between PAK and the ΔnrtR mutant. Our preliminary results demonstrated a reduction of biofilm formation in the ΔnrtR mutant (Fig. S2A). Currently, we are making efforts to explore the mechanism of nrtR mediated regulation on biofilm formation in P. aeruginosa.
NrtR controls the NAD cofactor biosynthesis to modulate NAD homeostasis in P. aeruginosa (22). In addition, NrtR regulates H1-T6SS of P. aeruginosa. Interestingly, the H1-T6SS substrate Tse2 has been suggested to be a NAD-dependent toxin (39). Another H1-T6SS effector, Tse6, acts on target cells by degrading the dinucleotides NAD+ and NADP+ in P. aeruginosa (40). However, the relevance of these toxins in connection with the role of NrtR in NAD biogenesis in P. aeruginosa remains elusive and warrants further studies.
P. aeruginosa harbors three different T6SS clusters. We found that the nrtR mutation leads to upregulation of H1-T6SS, but has no effect on the expression of H2-T6SS and H3-T6SS (Fig. S2B). Of note, using a Genechip analysis, a recent study revealed that the transcriptional levels of hcp1 and tssB1, two components of H1-T6SS, were upregulated in a TBCF10839ntrR::Tn strain. In addition, hsiB3 and hsiC3, two components of H3-T6SS, also displayed a 7-fold increase at the transcriptional level (22). Such a differential effect on H3-T6SS expression might be due to the difference in strain backgrounds used in these two studies. TBCF10839 is a mucoid P. aeruginosa strain isolated from a cystic fibrosis patient with high-level production of pyocyanin, as well as quorum sensing signal molecules PQS and N-acylhomoserine lactones (41). In contrast, PAK is a laboratory model strain with nonmucoid phenotype.
Our real-time qPCR and promoter fusion assays revealed an increased expression of rsmY and rsmZ in the ΔnrtR mutant (Fig. 2). However, this upregulation was not observed in our RNAseq analysis. It is possible that the small RNAs RsmY and RsmZ might have low recovery during the process of library generation.
The expression of RsmY/Z is controlled by a complex regulatory network. The two-component signal transduction system GacS-GacA positively regulates the transcription of rsmY and rsmZ by direct binding of the regulator GacA to sequences upstream of these genes (32). Hybrid sensor kinases LadS and RetS, and the histidine phosphotransfer protein HptB intersect with GacA to modulate the expression of rsmY or/and rsmZ (34, 42–44). The magnesium transporter MgtE also activates rsmY and rsmZ transcription through the GacS-GacA two-component system (45). NarL, an Anr-regulated response regulator, represses the transcription of rsmY and rsmZ by directly binding to their promoters (46). MvaT and MvaU, the H-NS family of DNA binding proteins, function as transcriptional repressors of rsmZ by binding to its promoter (32). BswR, a xenobiotic response transcriptional regulator, modulates rsmZ transcription by binding to its promoter and counters the repression by MvaT (47). Transcription of both rsmY and rsmZ is also positively controlled by RsmA via an unknown mechanism (48). Recently, it has been reported that small RNA 179 expression stimulates rsmY transcription (49). In addition, the levels of RsmY/Z are modulated at the posttranscriptional level. The interaction between PNPase and RsmY/Z controls the stability of these sRNAs (50). Hfq interacts with RsmY and protects it from cleavage by RNase E (51, 52). Here, we identified NrtR as a repressor of the rsmY/Z, which represses the transcription of rsmY/Z by directly binding to the upstream regions of these genes.
In summary, we identified NrtR as a repressor of the H1-T6SS in P. aeruginosa and revealed its repressing role on the H1-T6SS by both direct and indirect effects via the regulatory RNAs RsmY and RsmZ.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are shown in Table S1. Bacterial cells were grown in Luria–Bertani (LB) medium, which contained 5 g/L NaCl, 5 g/L yeast extract, and 10 g/L tryptone or on LB agar plates (supplemented with 15 g/L agar) at 37°C. When needed, the medium was supplemented with appropriate antibiotics at the following concentrations: for P. aeruginosa: tetracycline 50 μg/mL, gentamicin 50 μg/mL, carbenicillin 150 μg/mL; for E. coli: tetracycline 10 μg/mL, kanamycin 25 μg/mL, gentamicin 10 μg/mL, and ampicillin 100 μg/mL.
Construction of plasmids and bacterial strains.
To delete the rsmY gene, the deletion plasmid pEX18-rsmY (33) was electroporated into the E. coli S17-1 strain, followed by conjugal transfer to P. aeruginosa strains. The rsmY deletion was carried out by homologous recombination in the ΔnrtR mutant followed by selection for single crossover and then double crossover, as previously described (53). The target rsmY deletion mutant was verified by PCR amplification (primers in Table S2). The rsmZ gene deletion in ΔnrtR and ΔnrtRΔrsmY was carried out by similar methods with the previously constructed plasmid pEX18-rsmZ (33).
Plasmid pUCP20-rsmA was constructed by PCR amplification of the rsmA open reading frame (ORF) and its putative Shine-Dalgarno (SD) sequence from PAK genomic DNA using primers pUCP-rsmAF and pUCP-rsmAR. The PCR products were digested with EcoRI-HindIII and then cloned into the pUCP20 plasmid. pUCP20-rsmN, pUCP24-nrtR, and pET28a-nrtR were constructed by a similar strategy. E1553-hcp1-Flag was generated by PCR amplification of the C-terminal Flag-tagged hcp1 gene and its promoter region from PAK genomic DNA with specific primers (Table S2). The PCR products were digested with XbaI-HindIII and then cloned into the promoterless pUCP20, whose promoter was removed by replacing nucleotides (GCTTTC) at sites 1813–1818 with the EcoRI digestive site (GAATTC) followed by EcoRI digestion and self-ligation (54).
To generate the construct of PrsmY-EGFP, the promoter region of rsmY was amplified by PCR from PAK genomic DNA and inserted upstream of the promoterless-egfp gene in p19-EGFP, which was constructed by cloning the egfp gene into the BamHI-HindIII sites of pDN19lacZΩ. The constructs of PrsmZ-EGFP and PtssA1-EGFP were made with the same procedure.
To generate the ΔnrtR/att7::hcp1-Flag strain, pUC18T-mini-Tn7T-hcp1-Flag, along with the helper plasmid pTNS3, was electroporated into the ΔnrtR mutant. Insertion of the Phcp1-hcp1-Flag fragment into the chromosome was verified by PCR amplification with primers PglmS-down and PTn7R (Table S2). Strain PAK/att7::hcp1 was generated with similar strategy.
Western blot.
A single bacterial colony was inoculated into LB medium, cultivated overnight, diluted 1:50 into 3 mL fresh LB medium, and then grown to an OD600 of 1.0 with shaking at 200 rpm. Proteins from an equivalent number of bacterial cells were mixed with loading buffer, boiled for 10 min at 99°C, and then loaded onto and separated by a 12% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel. Proteins were then transferred onto a polyvinylidene difluoride (PVDF) membrane and probed with an antibody against EGFP (GeneTex), Flag (Sigma), or RpoA (RNAP, Abcam) for 1–2 h at room temperature or overnight at 4°C. The signals were detected using an ECL Plus kit (Millipore) and visualized in a Bio-Rad molecular imager (ChemiDocXRS).
RNA purification, real-time qPCR, and RNAseq analysis.
Overnight cultures of bacteria were diluted into fresh LB medium (1:50 dilution) and cultivated to an OD600 of 1.0 at 37°C. Total RNA was isolated using an RNAprep Pure Kit (Cell/Bacteria, Tiangen Biotec, Beijing, China). cDNA was synthesized from 1 μg of RNA using PrimeScript Reverse Transcriptase with random primers (TaKaRa, Dalian, China). For real-time qPCR, cDNA was mixed with specified primers (Table S2) and SYBR Premix Ex TaqTM II (TaKaRa, Dalian, China). The reactions were conducted using a CFX Connect Real-Time system (Bio-Rad). The 30S ribosomal protein gene rpsL was utilized as an internal control. The gene expression level was examined using the 2-ΔΔCt method. For RNAseq analysis, the total RNA was sent to GENEWIZ (Suzhou, China) to conduct the sequencing and analysis as described before (55).
Bacterial competition assay.
Overnight cultures of P. aeruginosa strains and E. coli DH5α/pDN19 were diluted 1:50 into 3 mL fresh LB medium and grown to an OD600 of 1.0. The cells of P. aeruginosa and DH5α/pDN19 were collected by centrifugation, washed twice with LB medium, and mixed at a ratio of 5:1. 50 μl of this mixture was spotted onto a nitrocellulose membrane with 0.22 μm pores (Solarbio, Beijing, China) on an LB agar plate. After drying, the mixtures were incubated for 24 h at 25°C. Then, bacterial cells on the nitrocellulose membrane were resuspended in 1 mL LB, serially diluted in LB medium, and plated onto LB agar plates with 50 μg/mL tetracycline to enumerate the CFU of DH5α/pDN19. The E. coli recovery index represents the bacterial number of recovered DH5α/pDN19 with the number of DH5α/pDN19 cells in competition with PAK as 1.0.
Expression and purification of recombinant NrtR protein.
To express recombinant NrtR with an N-terminal His-tag, the nrtR gene was cloned into pET28a to form pET28a-nrtR. E. coli strain BL21(DE3) was transformed with the construct and grown in LB at 37°C to an OD600 of 0.6. Then, 0.1 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG) was added to induce the expression of NrtR. After an additional 4 h of cultivation, cells were harvested from 100 mL bacterial culture and frozen overnight at −20°C. The frozen cells were resuspended in 5 mL lysis buffer A (46.6 mM Na2HPO4, 3.4 mM NaH2PO4, 0.3 M NaCl, pH 8.0) and lysed by sonication (5 s on, 12 s off until clear). Cell debris and other insoluble substances were removed by centrifugation at 12,000 × g for 10 min at 4°C. The remaining supernatant was applied to nickel-nitrilotriacetic acid (Ni-NTA)-agarose solution (Qiagen) following the manufacturer’s recommendation. After washing with 400 μL washing buffer (1 M Na2HPO4, 1 M NaH2PO4, 0.3 M NaCl) containing serial concentrations of imidazole (100/200 mM imidazole), the bound NrtR protein was eluted with 400 μL elution buffer (1 M Na2HPO4, 1 M NaH2PO4, 0.3 M NaCl, 400 mM imidazole). The purified protein was examined by SDS-PAGE.
Electrophoretic mobility shift assays (EMSAs).
The electrophoretic mobility shift assay (EMSA) was performed as previously described with minor modifications (56). Briefly, DNA fragments corresponding to the sequences upstream of nadD2, tssA1, rsmZ, and rsmY and a fragment from the nrtR coding sequence were amplified by PCR using specific primers (Table S2). DNA fragments (40 ng) were incubated with 0, 4, or 8 μM purified recombinant His-NrtR protein in an ice bath for 25 min in a 20 μL reaction system (10 mM Tris, 1 mM DTT, pH 7.5). Afterwards, the samples were loaded onto an 8% native polyacrylamide gel, which had been prerun for 1 h, and then electrophoresed in 1 × TBE buffer (Tris-borate-EDTA: 0.089 M Tris, 0.089 M boric acid, 0.002 M EDTA, pH 8.3) at 10 mA for about 1.5 h in ice bath. Then, the gel was stained in 1 × TBE containing 0.5 μg/mL ethidium bromide for 10 min, and the bands were visualized in a ChemiDoc XRS + molecular imager (Bio-Rad, CA, USA).
Chromatin immunoprecipitation (ChIP)-Seq.
Chromatin immunoprecipitation assays were performed according to methods previously described by Wuhan IGENEBOOK Biotechnology Co. Ltd. (56). Briefly, 2 × 1010 cells of ΔnrtR/pUCP24-nrtR-Flag were collected and cross-linked with 1% formaldehyde for 20 min at 37°C. The crosslinking was then stopped by the addition of glycine at a final concentration of 125 mM. Afterwards, bacterial cells were centrifuged, washed twice with Tris buffer (150 mM NaCl, 20 mM Tris-HCl pH 7.5) containing a complete proteinase inhibitor cocktail (Roche), and then resuspended in 400 μL lysis buffer (50 mM Tris–HCl pH 8.0, 10 mM EDTA, 1% SDS, 1% Triton X-100, mini-protease inhibitor cocktail [Roche]) for 30 min. The chromatin DNA was purified and sonicated to obtain soluble sheared chromatin with an average DNA length of 200–500 bp (20s on with 30s interval, 15 cycles, Diagenode Bioruptor Pico). Two μL of chromatin was saved at −20°C as input DNA, and 100 μL of chromatin was used for immunoprecipitation with 5 μg of anti-Flag antibody (F7425-2MG, Sigma-Aldrich) at 4°C overnight. The next day, 30 μL of protein G magnetic beads were added, and the reaction samples were further incubated at 4°C for 3 h. The beads were then washed with a series of washing buffers: once with low salt washing buffer (20 mM Tris-HCl pH 8.1, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.1% SDS); twice with LiCl washing buffer (10 mM Tris-HCl pH 8.1, 250 mM LiCl, 1 mM EDTA, 1% NP40, 1% deoxycholic acid); and twice with TE buffer (10 mM Tris-HCl pH 7.5, 1 mM EDTA). Bound material was then eluted from the beads in 300 μL of elution buffer (100 mM NaHCO3, 1% SDS), treated first with RNase A at a final concentration of 8 μg/mL for 6 h at 65°C and then with proteinase K at a final concentration of 345 μg/mL overnight at 45°C. Immunoprecipitated DNA was used to construct sequencing libraries following the protocol provided by the I NEXTFLEX ChIP-Seq Library Prep Kit for Illumina Sequencing (NOVA-5143 Bioo Scientific) and sequenced on Illumina Xten with the PE 150 method.
Low-quality reads were filtered out via Trimmomatic software (version 0.38). Totally, 61,879,614 and 58,249,840 clean reads were obtained from the input and ChIP samples, respectively. The clean reads were then mapped to the PAK genome with Bwa (v.0.7.15), allowing up to two mismatches. Samtools (v. 1.3.1) software was used to remove potential PCR duplicates. The software MACS2 (v. 2.1.1.20160309) was utilized to call peaks with default parameters (bandwidth, 300 bp/value, 0.05/model fold, 5, 50).
Biofilm formation assay.
Overnight cultured bacteria were diluted 50-fold in LB broth, grown to an OD600 of 1.0, and then diluted to an OD600 of 0.025 in LB medium. Two hundred μL of the diluted bacterial suspension was incubated in each well of a 96-well plate at 37°C for 24 h. After that, each well was washed three times with H2O, stained with 0.25% crystal violet for 10 min, and then washed with H2O for three times. Two hundred μL of destaining solution was added into each well and incubated at room temperature for 10 min. Each sample was measured at a wavelength of 590 nm using a microplate reader Varioskan Flash (Thermo Scientific, Billerica, MA, USA).
Data availability.
The RNAseq and ChIP-seq data have been deposited in the NCBI Short Read Archive (SRA) database with accession numbers PRJNA733704 and PRJNA733326.
ACKNOWLEDGMENTS
This research was supported by the National Science Foundation of China (31970680, 31870130, 31970179, 82061148018, 32170199 and 32170177) and National Key Research and Development Project of China (2017YFE0125600, 2021YFE0201300, 2021YFE0101700).
Footnotes
Supplemental material is available online only.
Contributor Information
Yongxin Jin, Email: yxjin@nankai.edu.cn.
Amanda G. Oglesby, University of Maryland School of Pharmacy
REFERENCES
- 1.Gellatly SL, Hancock RE. 2013. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 67:159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 2.Klockgether J, Tümmler B. 2017. Recent advances in understanding Pseudomonas aeruginosa as a pathogen. F1000Res 6:1261. doi: 10.12688/f1000research.10506.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawa T. 2014. The molecular mechanism of acute lung injury caused by Pseudomonas aeruginosa: from bacterial pathogenesis to host response. J Intensive Care 2:10. doi: 10.1186/2052-0492-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sana TG, Berni B, Bleves S. 2016. The T6SSs of Pseudomonas aeruginosa strain PAO1 and their effectors: beyond bacterial-cell targeting. Front Cell Infect Microbiol 6:61. doi: 10.3389/fcimb.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Si M, Zhao C, Burkinshaw B, Zhang B, Wei D, Wang Y, Dong TG, Shen X. 2017. Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc Natl Acad Sci USA 114:E2233–E2242. doi: 10.1073/pnas.1614902114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang T, Du X, Ji L, Han Y, Dang J, Wen J, Wang Y, Pu Q, Wu M, Liang H. 2021. Pseudomonas aeruginosa T6SS-mediated molybdate transport contributes to bacterial competition during anaerobiosis. Cell Rep 35:108957. doi: 10.1016/j.celrep.2021.108957. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Zhang W, Cheng J, Yang X, Zhu K, Wang Y, Wei G, Qian PY, Luo ZQ, Shen X. 2017. A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat Commun 8:14888. doi: 10.1038/ncomms14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han Y, Wang T, Chen G, Pu Q, Liu Q, Zhang Y, Xu L, Wu M, Liang H. 2019. A Pseudomonas aeruginosa type VI secretion system regulated by CueR facilitates copper acquisition. PLoS Pathog 15:e1008198. doi: 10.1371/journal.ppat.1008198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, Goodman AL, Joachimiak G, Ordoñez CL, Lory S, Walz T, Joachimiak A, Mekalanos JJ. 2006. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312:1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hood RD, Singh P, Hsu F, Güvener T, Carl MA, Trinidad RR, Silverman JM, Ohlson BB, Hicks KG, Plemel RL, Li M, Schwarz S, Wang WY, Merz AJ, Goodlett DR, Mougous JD. 2010. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7:25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basler M, Ho BT, Mekalanos JJ. 2013. Tit-for-tat: type VI secretion system counterattack during bacterial cell-cell interactions. Cell 152:884–894. doi: 10.1016/j.cell.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolan LM, Cain AK, Clamens T, Furniss RCD, Manoli E, Sainz-Polo MA, Dougan G, Albesa-Jové D, Parkhill J, Mavridou DAI, Filloux A. 2021. Identification of Tse8 as a Type VI secretion system toxin from Pseudomonas aeruginosa that targets the bacterial transamidosome to inhibit protein synthesis in prey cells. Nat Microbiol 6:1199–1210. doi: 10.1038/s41564-021-00950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Zou Y, She P, Wu Y. 2015. Composition, function, and regulation of T6SS in Pseudomonas aeruginosa. Microbiol Res 172:19–25. doi: 10.1016/j.micres.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Lesic B, Starkey M, He J, Hazan R, Rahme LG. 2009. Quorum sensing differentially regulates Pseudomonas aeruginosa type VI secretion locus I and homologous loci II and III, which are required for pathogenesis. Microbiology (Reading) 155:2845–2855. doi: 10.1099/mic.0.029082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brencic A, Lory S. 2009. Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Mol Microbiol 72:612–632. doi: 10.1111/j.1365-2958.2009.06670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marden JN, Diaz MR, Walton WG, Gode CJ, Betts L, Urbanowski ML, Redinbo MR, Yahr TL, Wolfgang MC. 2013. An unusual CsrA family member operates in series with RsmA to amplify posttranscriptional responses in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 110:15055–15060. doi: 10.1073/pnas.1307217110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero M, Silistre H, Lovelock L, Wright VJ, Chan KG, Hong KW, Williams P, Cámara M, Heeb S. 2018. Genome-wide mapping of the RNA targets of the Pseudomonas aeruginosa riboregulatory protein RsmN. Nucleic Acids Res 46:6823–6840. doi: 10.1093/nar/gky324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mougous JD, Gifford CA, Ramsdell TL, Mekalanos JJ. 2007. Threonine phosphorylation post-translationally regulates protein secretion in Pseudomonas aeruginosa. Nat Cell Biol 9:797–803. doi: 10.1038/ncb1605. [DOI] [PubMed] [Google Scholar]
- 19.Silverman JM, Austin LS, Hsu F, Hicks KG, Hood RD, Mougous JD. 2011. Separate inputs modulate phosphorylation-dependent and -independent type VI secretion activation. Mol Microbiol 82:1277–1290. doi: 10.1111/j.1365-2958.2011.07889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodionov DA, De Ingeniis J, Mancini C, Cimadamore F, Zhang H, Osterman AL, Raffaelli N. 2008. Transcriptional regulation of NAD metabolism in bacteria: NrtR family of Nudix-related regulators. Nucleic Acids Res 36:2047–2059. doi: 10.1093/nar/gkn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang N, De Ingeniis J, Galeazzi L, Mancini C, Korostelev YD, Rakhmaninova AB, Gelfand MS, Rodionov DA, Raffaelli N, Zhang H. 2009. Structure and function of an ADP-ribose-dependent transcriptional regulator of NAD metabolism. Structure 17:939–951. doi: 10.1016/j.str.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okon E, Dethlefsen S, Pelnikevich A, Barneveld AV, Munder A, Tümmler B. 2017. Key role of an ADP - ribose - dependent transcriptional regulator of NAD metabolism for fitness and virulence of Pseudomonas aeruginosa. Int J Med Microbiol 307:83–94. doi: 10.1016/j.ijmm.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Hassan BH, Lou N, Merritt J, Feng Y. 2019. Functional definition of NrtR, a remnant regulator of NAD(+) homeostasis in the zoonotic pathogen Streptococcus suis. FASEB J 33:6055–6068. doi: 10.1096/fj.201802179RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao R, Wei W, Hassan BH, Li J, Deng J, Feng Y. 2019. A single regulator NrtR controls bacterial NAD(+) homeostasis via its acetylation. Elife 8:e51603. doi: 10.7554/eLife.51603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin Y, Zhang M, Zhu F, Peng Q, Weng Y, Zhao Q, Liu C, Bai F, Cheng Z, Jin S, Wu W. 2019. NrtR regulates the Type III secretion system through cAMP/Vfr pathway in Pseudomonas aeruginosa. Front Microbiol 10:85. doi: 10.3389/fmicb.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell AB, Hood RD, Bui NK, LeRoux M, Vollmer W, Mougous JD. 2011. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475:343–347. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moscoso JA, Mikkelsen H, Heeb S, Williams P, Filloux A. 2011. The Pseudomonas aeruginosa sensor RetS switches type III and type VI secretion via c-di-GMP signalling. Environ Microbiol 13:3128–3138. doi: 10.1111/j.1462-2920.2011.02595.x. [DOI] [PubMed] [Google Scholar]
- 28.Almblad H, Rybtke M, Hendiani S, Andersen JB, Givskov M, Tolker-Nielsen T. 2019. High levels of cAMP inhibit Pseudomonas aeruginosa biofilm formation through reduction of the c-di-GMP content. Microbiology (Reading) 165:324–333. doi: 10.1099/mic.0.000772. [DOI] [PubMed] [Google Scholar]
- 29.Almblad H, Harrison JJ, Rybtke M, Groizeleau J, Givskov M, Parsek MR, Tolker-Nielsen T. 2015. The cyclic AMP-Vfr signaling pathway in Pseudomonas aeruginosa is inhibited by cyclic di-GMP. J Bacteriol 197:2190–2200. doi: 10.1128/JB.00193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rybtke M, Berthelsen J, Yang L, Høiby N, Givskov M, Tolker-Nielsen T. 2015. The LapG protein plays a role in Pseudomonas aeruginosa biofilm formation by controlling the presence of the CdrA adhesin on the cell surface. Microbiologyopen 4:917–930. doi: 10.1002/mbo3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Xia B, Li M, Shi J, Long Y, Jin Y, Bai F, Cheng Z, Jin S, Wu W. 2018. HigB reciprocally controls biofilm formation and the expression of Type III secretion system genes through influencing the intracellular c-di-GMP level in Pseudomonas aeruginosa. Toxins 10:424. doi: 10.3390/toxins10110424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brencic A, McFarland KA, McManus HR, Castang S, Mogno I, Dove SL, Lory S. 2009. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol Microbiol 73:434–445. doi: 10.1111/j.1365-2958.2009.06782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li K, Xu C, Jin Y, Sun Z, Liu C, Shi J, Chen G, Chen R, Jin S, Wu W. 2013. SuhB is a regulator of multiple virulence genes and essential for pathogenesis of Pseudomonas aeruginosa. mBio 4:e00419-13. doi: 10.1128/mBio.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ventre I, Goodman AL, Vallet-Gely I, Vasseur P, Soscia C, Molin S, Bleves S, Lazdunski A, Lory S, Filloux A. 2006. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc Natl Acad Sci USA 103:171–176. doi: 10.1073/pnas.0507407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stacey SD, Williams DA, Pritchett CL. 2017. The Pseudomonas aeruginosa two-component regulator AlgR directly activates rsmA expression in a phosphorylation-independent manner. J Bacteriol 199:e00048-17. doi: 10.1128/JB.00048-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Intile PJ, Diaz MR, Urbanowski ML, Wolfgang MC, Yahr TL. 2014. The AlgZR two-component system recalibrates the RsmAYZ posttranscriptional regulatory system to inhibit expression of the Pseudomonas aeruginosa type III secretion system. J Bacteriol 196:357–366. doi: 10.1128/JB.01199-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allsopp LP, Wood TE, Howard SA, Maggiorelli F, Nolan LM, Wettstadt S, Filloux A. 2017. RsmA and AmrZ orchestrate the assembly of all three type VI secretion systems in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 114:7707–7712. doi: 10.1073/pnas.1700286114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang C, Tesar C, Li X, Kim Y, Rodionov DA, Joachimiak A. 2015. A novel transcriptional regulator of L-arabinose utilization in human gut bacteria. Nucleic Acids Res 43:10546–10559. doi: 10.1093/nar/gkv1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robb CS, Robb M, Nano FE, Boraston AB. 2016. The structure of the toxin and type six secretion system substrate Tse2 in complex with its immunity protein. Structure 24:277–284. doi: 10.1016/j.str.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Whitney JC, Quentin D, Sawai S, LeRoux M, Harding BN, Ledvina HE, Tran BQ, Robinson H, Goo YA, Goodlett DR, Raunser S, Mougous JD. 2015. An interbacterial NAD(P)(+) glycohydrolase toxin requires elongation factor Tu for delivery to target cells. Cell 163:607–619. doi: 10.1016/j.cell.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiehlmann L, Munder A, Adams T, Juhas M, Kolmar H, Salunkhe P, Tümmler B. 2007. Functional genomics of Pseudomonas aeruginosa to identify habitat-specific determinants of pathogenicity. Int J Med Microbiol 297:615–623. doi: 10.1016/j.ijmm.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Bordi C, Lamy MC, Ventre I, Termine E, Hachani A, Fillet S, Roche B, Bleves S, Méjean V, Lazdunski A, Filloux A. 2010. Regulatory RNAs and the HptB/RetS signalling pathways fine-tune Pseudomonas aeruginosa pathogenesis. Mol Microbiol 76:1427–1443. doi: 10.1111/j.1365-2958.2010.07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman AL, Merighi M, Hyodo M, Ventre I, Filloux A, Lory S. 2009. Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev 23:249–259. doi: 10.1101/gad.1739009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jean-Pierre F, Tremblay J, Déziel E. 2016. Broth versus surface-grown cells: differential regulation of RsmY/Z small RNAs in Pseudomonas aeruginosa by the Gac/HptB system. Front Microbiol 7:2168. doi: 10.3389/fmicb.2016.02168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakravarty S, Melton CN, Bailin A, Yahr TL, Anderson GG. 2017. Pseudomonas aeruginosa magnesium transporter MgtE inhibits Type III secretion system gene expression by stimulating rsmYZ transcription. J Bacteriol 199:e00268-17. doi: 10.1128/JB.00268-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Callaghan J, Reen FJ, Adams C, O’Gara F. 2011. Low oxygen induces the type III secretion system in Pseudomonas aeruginosa via modulation of the small RNAs rsmZ and rsmY. Microbiology (Reading) 157:3417–3428. doi: 10.1099/mic.0.052050-0. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Ye F, Kumar V, Gao YG, Zhang LH. 2014. BswR controls bacterial motility and biofilm formation in Pseudomonas aeruginosa through modulation of the small RNA rsmZ. Nucleic Acids Res 42:4563–4576. doi: 10.1093/nar/gku106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kay E, Humair B, Dénervaud V, Riedel K, Spahr S, Eberl L, Valverde C, Haas D. 2006. Two GacA-dependent small RNAs modulate the quorum-sensing response in Pseudomonas aeruginosa. J Bacteriol 188:6026–6033. doi: 10.1128/JB.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janssen KH, Corley JM, Djapgne L, Cribbs JT, Voelker D, Slusher Z, Nordell R, Regulski EE, Kazmierczak BI, McMackin EW, Yahr TL. 2020. Hfq and sRNA 179 inhibit expression of the Pseudomonas aeruginosa cAMP-Vfr and type III secretion regulons. mBio 11:e00363-20. doi: 10.1128/mBio.00363-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen R, Weng Y, Zhu F, Jin Y, Liu C, Pan X, Xia B, Cheng Z, Jin S, Wu W. 2016. Polynucleotide phosphorylase regulates multiple virulence factors and the stabilities of small RNAs RsmY/Z in Pseudomonas aeruginosa. Front Microbiol 7:247. doi: 10.3389/fmicb.2016.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonnleitner E, Schuster M, Sorger-Domenigg T, Greenberg EP, Bläsi U. 2006. Hfq-dependent alterations of the transcriptome profile and effects on quorum sensing in Pseudomonas aeruginosa. Mol Microbiol 59:1542–1558. doi: 10.1111/j.1365-2958.2006.05032.x. [DOI] [PubMed] [Google Scholar]
- 52.Sorger-Domenigg T, Sonnleitner E, Kaberdin VR, Bläsi U. 2007. Distinct and overlapping binding sites of Pseudomonas aeruginosa Hfq and RsmA proteins on the non-coding RNA RsmY. Biochem Biophys Res Commun 352:769–773. doi: 10.1016/j.bbrc.2006.11.084. [DOI] [PubMed] [Google Scholar]
- 53.Schweizer HP. 1992. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol Microbiol 6:1195–1204. doi: 10.1111/j.1365-2958.1992.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 54.Deng X, Li M, Pan X, Zheng R, Liu C, Chen F, Liu X, Cheng Z, Jin S, Wu W. 2017. Fis regulates type III secretion system by influencing the transcription of exsA in Pseudomonas aeruginosa strain PA14. Front Microbiol 8:669. doi: 10.3389/fmicb.2017.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu C, Wang D, Zhang X, Liu H, Zhu G, Wang T, Cheng Z, Wu W, Bai F, Jin Y. 2020. Mechanisms for rapid evolution of carbapenem resistance in a clinical isolate of Pseudomonas aeruginosa. Front Microbiol 11:1390. doi: 10.3389/fmicb.2020.01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan X, Fan Z, Chen L, Liu C, Bai F, Wei Y, Tian Z, Dong Y, Shi J, Chen H, Jin Y, Cheng Z, Jin S, Lin J, Wu W. 2020. PvrA is a novel regulator that contributes to Pseudomonas aeruginosa pathogenesis by controlling bacterial utilization of long chain fatty acids. Nucleic Acids Res 48:5967–5985. doi: 10.1093/nar/gkaa377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pissaridou P, Allsopp LP, Wettstadt S, Howard SA, Mavridou DAI, Filloux A. 2018. The Pseudomonas aeruginosa T6SS-VgrG1b spike is topped by a PAAR protein eliciting DNA damage to bacterial competitors. Proc Natl Acad Sci USA 115:12519–12524. doi: 10.1073/pnas.1814181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download SPECTRUM01858-21_Supp_1_seq12.pdf, PDF file, 1.1 MB (1.1MB, pdf)
Supplemental material. Download SPECTRUM01858-21_Supp_2_seq13.xls, XLS file, 0.04 MB (39.5KB, xls)
Supplemental material. Download SPECTRUM01858-21_Supp_3_seq14.xlsx, XLSX file, 0.1 MB (65KB, xlsx)
Data Availability Statement
The RNAseq and ChIP-seq data have been deposited in the NCBI Short Read Archive (SRA) database with accession numbers PRJNA733704 and PRJNA733326.