Introduction
The tropomyosin receptor kinase (Trk) receptors, TrkA, TrkB, and TrkC, encoded by the NTRK1, NTRK2, and NTRK3 genes, respectively, are transmembrane proteins that play an important role in the normal development and function of the nervous system. Aberrant fusions of NTRK genes lead to the production of chimeric Trk receptors, which are constitutively activated with subsequent activation of downstream signaling pathways including mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways.1 Such NTRK fusions have been found to be oncogenic drivers in multiple solid tumors including thyroid cancer.2
Selective Trk inhibitors, larotrectinib and entrectinib, demonstrated excellent efficacies with high and durable responses across the NTRK fusion–positive pediatric and adult solid tumors in several small basket trials.3,4 Only a few patients with thyroid cancer were included in the published studies because of the rarity of the NTRK fusions in thyroid cancer. Furthermore, the frequencies and the types of NTRK fusions in thyroid cancer are widely variable in different studies.5-10 Herein, we describe our real-world experience from four patients with NTRK fusion–positive thyroid cancer treated with larotrectinib. We also report the frequencies and the types of NTRK gene alterations in thyroid cancer from available public databases and a real-world data set from Tempus.
Case Presentations
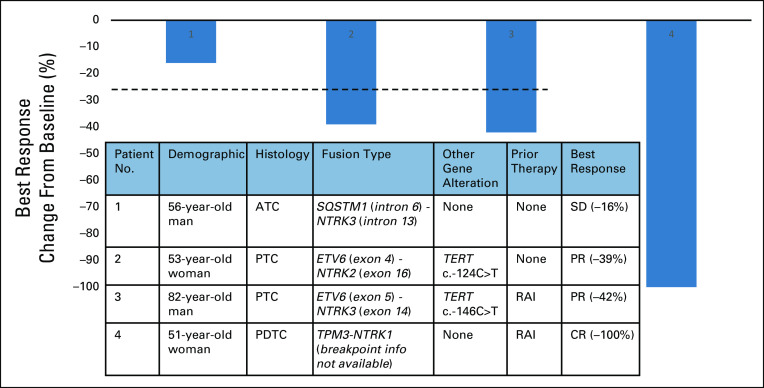
A case series of four patients with NTRK fusion–positive thyroid cancer treated with larotrectinib is summarized in Figure 1. One patient had anaplastic thyroid cancer (ATC), one patient had poorly differentiated thyroid cancer (PDTC), and two patients had papillary thyroid cancer (PTC). The study was approved by the institutional review board of University of California, San Francisco (IRB #20-31865). Patient consent for the study was waived as the study did not involve any identifiable data. Consent to publish images was obtained from patient 2.
FIG 1.
Baseline clinicopathologic characteristics of four patients with NTRK fusion harboring thyroid cancer who were treated with larotrectinib, and waterfall plot for best response. ATC, anaplastic thyroid cancer; CR, complete response; PDTC, poorly differentiated thyroid cancer; PR, partial response; PTC, papillary thyroid cancer; RAI, radioactive iodine; SD, stable disease.
Patient 1 with ATC harboring SQSTM1-NTRK3 presented with a rapidly enlarging neck mass and multiple lung nodules. He underwent total thyroidectomy and central neck dissection; pathology showed small multifocal PTCs in thyroid and 9.5-cm mixed anaplastic and PDTC in left central neck. Because of complicated postoperative course, larotrectinib was initiated instead of intensive chemoradiation. The patient had 16% reduction in tumor burden after 2 months but progressed with enlarging parotid and neck masses after 6 months. Biopsy of the progressing lesion showed no gatekeeper mutations11 or additional alterations.
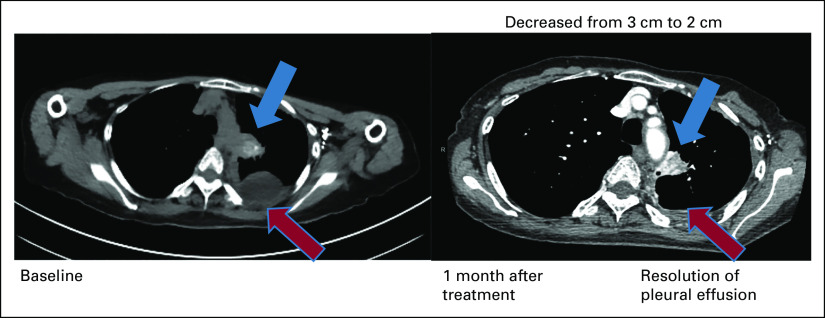
Patient 2 with PTC harbored novel ETV6-NTRK2 fusion not previously described in other solid tumors. The novel fusion has breakpoints in ETV6 exon 4 and NTRK2 exon 16 with preserved ETV6 PNT domain and NTRK2 kinase domain leading to constitutive activation of TrkB kinase (Fig 2). The patient has a remote history of PTC treated with surgery. She was found to have multiple brain metastases, obstructive hydrocephalus caused by a cerebellar mass, and pleural effusion with pleural masses. Pleural biopsy and cerebellar resection specimens confirmed metastatic PTC with ETV6-NTRK2 fusion and TERT c.-124C>T mutation. Thyrogen-stimulated I-123 scan showed uptake only in the chest. After receiving stereotactic body radiation to brain metastases and cerebellar resection bed, larotrectinib was initiated, resulting in ongoing partial response (PR) in the pleural metastases for more than 18 months (Fig 3) without evidence of recurrence in the brain.
FIG 2.
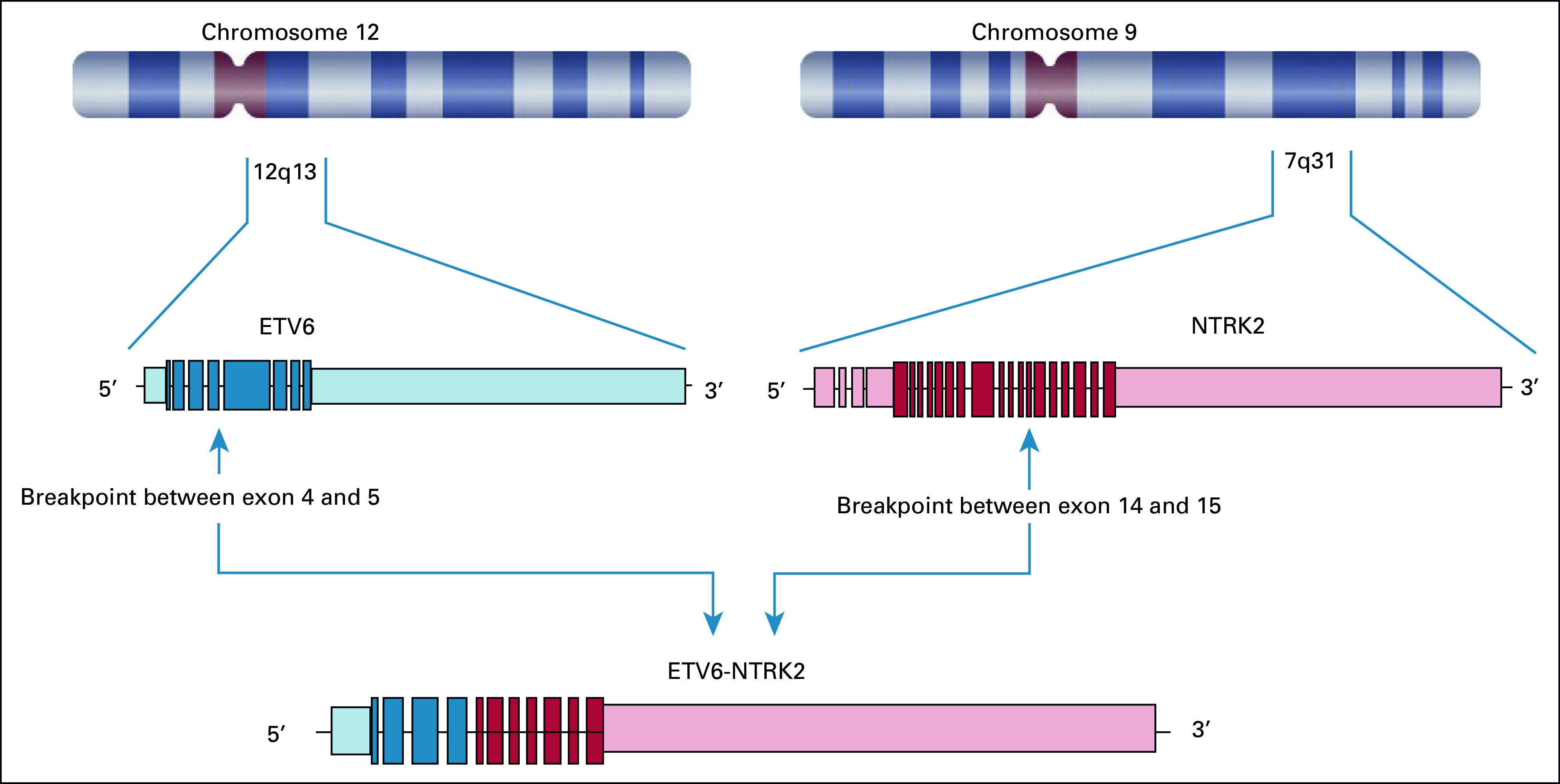
Novel fusion between 5′ breakpoint in ETV6 exon 4-5 and 3′ breakpoint in NTRK2 exon 14-15. This fusion preserves the ETV6 PNT domain and the NTRK2 kinase domain, leading to constitutive activation of the NTRK2 kinase.
FIG 3.
Patient 2 with metastatic PTC harboring ETV6-NTRK2. Computed tomography chest images demonstrate dramatic response after 1 month treatment with larotrectinib. PTC, papillary thyroid cancer.
Patient 3 with PTC harboring ETV6-NTRK3 fusion and TERT c.-146C>T mutation presented with a spine metastasis. He underwent total thyroidectomy, neck dissection, and metastasectomy of the spine lesion, followed by radioactive iodine treatment (RAI-T; 100 mCi) and radiation to the spine and neck lymph nodes. After 2 years, he developed multiple new bone and pulmonary metastases with a recurrence in the ipsilateral neck. He started larotrectinib and achieved PR ongoing for 7 months.
Patient 4 with PDTC harboring TPM3-NTRK1 fusion developed mediastinal nodal metastases after initial thyroidectomy. She received RAI-T (155 mCi) after Thyrogen stimulation following surgery, and the post-treatment scan did not show any iodine uptake. After another year, she developed multiple hilar, mediastinal, and pulmonary metastases and started larotrectinib. She achieved complete resolution of enlarged lymph nodes and pulmonary nodules consistent with complete response (CR) in 2 months. Thyroglobulin (TG) rose from 329 to 1,588 ng/mL within 1 month of larotrectinib associated with a radiographic response. TG gradually decreased over the next 8 months but remained higher than the baseline before larotrectinib.
Types and frequencies of NTRK gene alterations.
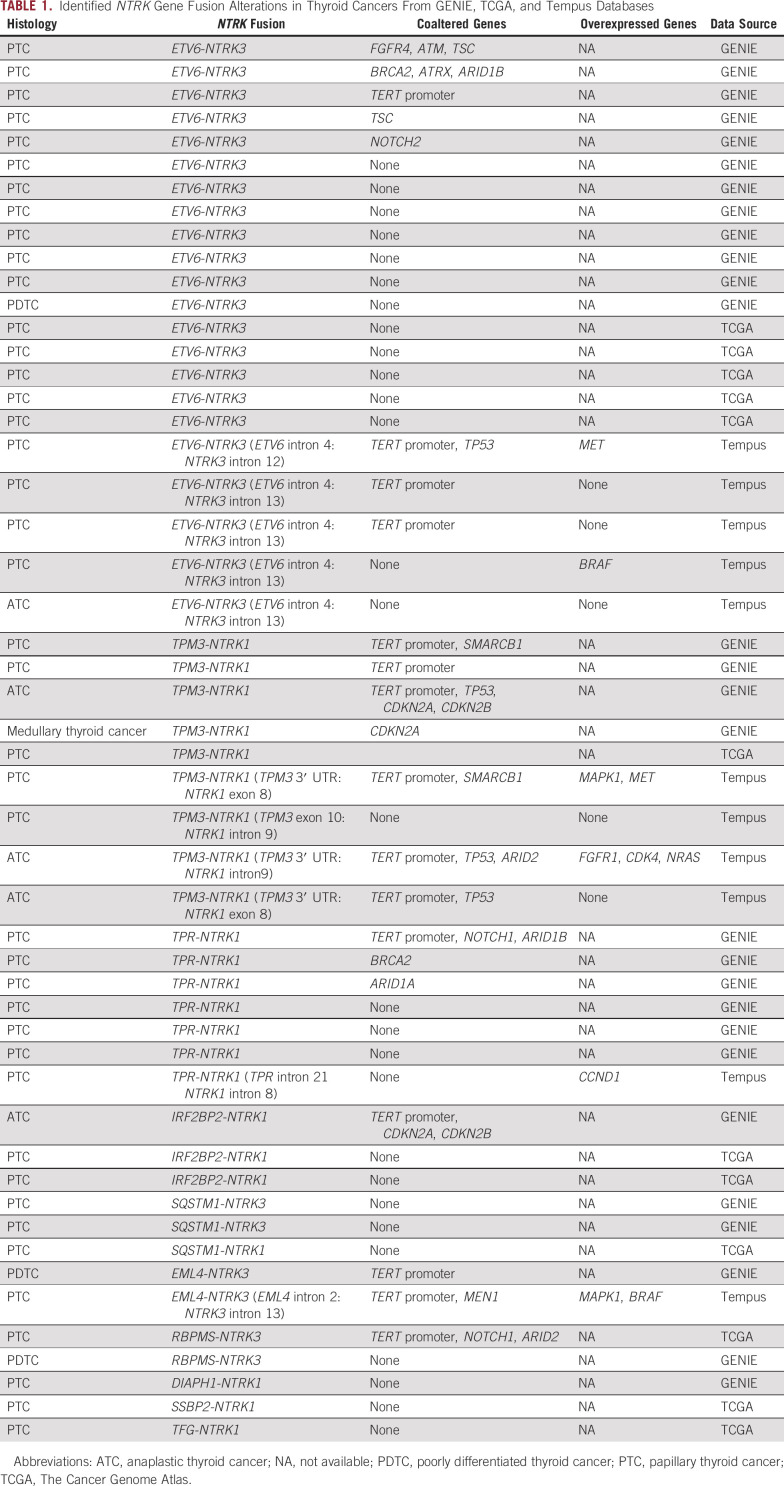
Of 2,362 thyroid cancer specimens identified in the American Association for Cancer Research (AACR) Genie, The Cancer Genome Atlas (TCGA), and Tempus databases, NTRK1 or NTRK3 gene fusions were found in 51 patients (2.2%): 28 of 1,133 in the AACR Genie data set (2.4%), 12 of 482 in the TCGA data set (2.5%), and 11 of 747 (1.5%) in the Tempus data set. No NTRK2 gene fusions were identified in any of the databases (Table1).
TABLE 1.
Identified NTRK Gene Fusion Alterations in Thyroid Cancers From GENIE, TCGA, and Tempus Databases
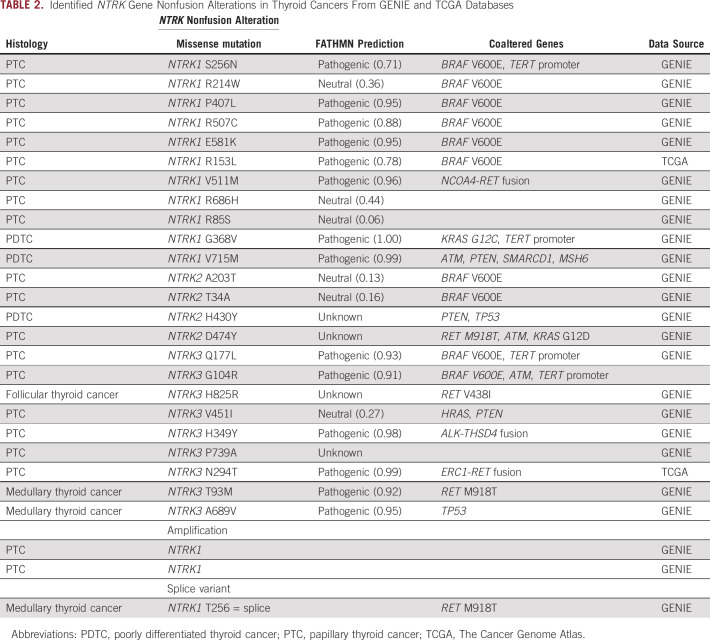
We identified 10 different 5′ fusion partner genes; ETV6-NTRK3 fusion was the most common, accounting for 43% of all NTRK fusions identified in thyroid cancer, followed by TPM3-NTRK1 fusion (18%) and TPR-NTRK1 fusion (14%). TERT promoter mutations were the most frequent coalteration, found in 15 cases (29%), followed by TP53 (8%). Among cases from the Tempus cohort whose RNA expression data are available, overexpression of genes related to MAPK/ERK signaling pathway and cell-cycle regulation, and receptor tyrosine kinase genes were observed. We explored other relevant genomic alterations of NTRK genes and identified 24 cases of NTRK1/2/3 single-nucleotide alterations, two cases of NTRK1 amplification, and a splice variant of NTRK1 in both differentiated and medullary thyroid cancers (Table2). More than half (58%) of the point mutations were predicted to be pathogenic,12 but the majority of non–fusion-altered NTRK cases also harbored well-established driver mutations such as BRAF/KRAS/HRAS mutations or RET/ALK gene fusions.
TABLE 2.
Identified NTRK Gene Nonfusion Alterations in Thyroid Cancers From GENIE and TCGA Databases
Discussion
We report a single-institution experience of four consecutive patients with advanced thyroid cancer harboring NTRK gene fusions, treated with larotrectinib, a selective Trk inhibitor. Three patients with PTC or PDTC achieved durable radiographic responses, and all of them have remained on larotrectinib. This is consistent with the data from prior phase I and II Trk inhibitor studies in solid tumors, demonstrating lower overall response rate (ORR) in patients with ATC compared to patients with DTC. In the combined analysis of phase I/II basket trials of larotrectinib including 28 patients with NTRK fusion–positive advanced thyroid cancer (22 DTCs and six ATCs), the ORR was 75% with two CRs and 19 PRs, 90% in DTC and 29% in ATC.13 Entrectinib was designed to cross the blood-brain barrier14 and demonstrated an ORR of 55% among patients with known brain metastases.3 Patient 2 with brain metastases started larotrectinib before approval of entrectinib. In the pooled analysis, two in four larotrectinib-treated thyroid cancer patients with CNS metastases had decreases in measurable brain lesions.13
Notably, the ETV6-NTRK2 fusion found in patient 2 is a novel gene fusion not previously reported for a solid tumor. The fusion was described in a patient with acute myeloid leukemia and was found to have transforming potential in a murine hematopoietic cell line.15 The patient did not have any abnormal blood counts, and germline sequencing performed on peripheral blood cells did not demonstrate abnormal findings. A good response to larotrectinib in patient 2 adds to the evidence that a selective Trk inhibitor has an efficacy in a tissue-agnostic manner, across the spectrum of NTRK fusion types. Another interesting observation was a rise in serum TG in patient 4 with PDTC harboring TPM3-NTRK1 fusion and durable CR. This suggests a potential role of larotrectinib in redifferentiation, similar to other tyrosine kinase inhibitors that have been used to restore iodine avidity.16 A recent case report demonstrated enhanced radioactive iodine uptake in a patient with PTC with EML4-NTRK3 fusion after larotrectinib.17 Among seven patients with thyroid cancer treated with larotrectinib in clinical trials, one patient with PPL-NTRK1 fusion achieved CR.18 TrkA encoded by NTRK1 is not expressed in normal thyroid tissue, but overexpression was observed in thyroid cancer, with activated Rous sarcoma oncogene and extracellular signal-regulated kinase pathways.19 Exceptional responses may be related to TrkA's oncogenic role in thyroid cancer.
In search for NTRK alterations in thyroid cancer using AACR Genie, TCGA, and Tempus databases, we identified various alterations in NTRK1 and NTRK3, but none in NTRK2. These fusions were found mostly in PTC, but also in PDTC, MTC, and ATC. ETV6-NTRK3 was the most common fusion found in 22 of 55 cases (40%). The actual frequency of NTRK fusions in thyroid cancer is not known, as some targeted exome sequencing can easily miss fusion event involving introns of certain genes. Studies on frequency of NTRK fusions from a single institution and from the TCGA found NTRK fusion in 10 of 451 (2.2%; four NTRK1 and six NTRK3 fusions) and 12 of 498 (2.4%; five NTRK1 and seven NTRK3 fusions) patient with thyroid cancer, respectively.20,21 In our study cohort, TERT promoter mutations were found in 29% of the cases: 10 in 42 (23.8%) PTCs and four in five (80%) ATCs. It is not known whether TERT promoter coalteration has any impact on prognosis or response to Trk inhibitor in NTRK-altered thyroid cancers. TERT promoter mutation has been reported in various frequencies in different histologies ranging from 10% in PTD up to 50% in ATC.22 It is associated with more advanced stage and poor prognosis.22-24
We also explored other genetic alterations of NTRK genes including nonrecurring missense single-nucleotide variations in NTRK1/2/3 and NTRK1 gene amplification. Interestingly, most cases with a missense mutation of NTRK1/2/3 also harbored well-described oncogenic alterations in genes encoding for RAS/RAF pathways, suggesting that these mutations are not likely the main driver for these tumors.
APPENDIX 1. Supplementary Text
Materials and Methods
Patients with advanced thyroid cancer harboring NTRK1/2/3 gene fusions were identified through retrospective review of clinical records at the University of California, San Francisco (UCSF). Presence of NTRK fusions was confirmed with commercially available oncology genomic profiling assays, including the UCSF500 DNA-based next-generation sequencing (NGS) test, which uses capture-based NGS and analyzes the exons of 529 cancer-related genes, as well as select intron of 47 genes, and the Tempus xT DNA, which is a targeted NGS test that detects single-nucleotide variants, indels, and copy-number variants of 648 genes and chromosomal rearrangements in 22 genes, supplemented by whole-transcriptome RNA sequencing for enhanced fusion detection.25 Demographic data, molecular analysis data, treatment history, and treatment responses were obtained from the patient records. The radiographic responses to the treatment were collected from each patient. Patient consent for the study was waived as the study did not involve any identifiable data.
To describe the landscape of NTRK gene alterations in thyroid cancer, the public data generated from American Association for Cancer Research (AACR) Project Genie cohort version 9.026 and The Cancer Genome Atlas (TCGA) research network27 were reviewed. Among 40 patients identified in AACR Genie and TCGA, median age was 39 years, and 53% of the patients were women. Additionally, a retrospective analysis on deidentified data from the Tempus real-world database was conducted to identify patients with thyroid cancer with NTRK fusions and discern the prevalence of these fusions. For Tempus specimens, gene expression was generated through RNA-seq of formalin-fixed paraffin-embedded tumor samples using an exome capture–based protocol as previously described.28 Demographic information was not available for patients in the Tempus database.
Jong Chul Park
Consulting or Advisory Role: I-MAB
Arya Ashok
Employment: Tempus
Stock and Other Ownership Interests: Tempus
Travel, Accommodations, Expenses: Tempus
Chienying Liu
Research Funding: NBI
Hyunseok Kang
Honoraria: Cancer Expert Now
Consulting or Advisory Role: Bayer, GlaxoSmithKline, Prelude Therapeutics, Achilles Therapeutics, MitoImmune, PIN therapeutics
Research Funding: Kura Oncology (Inst), Exelixis (Inst), Lilly (Inst), Elevar Therapeutics (Inst), PDS Biotechnology (Inst), NeoImmuneTech (Inst), Ayala Pharmaceuticals (Inst), Prelude Therapeutics (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Tempus supported data analyses of the Tempus cohort.
AUTHOR CONTRIBUTIONS
Conception and design: Hyunseok Kang
Administrative support: Hyunseok Kang
Provision of study materials or patients: Chienying Liu, Hyunseok Kang
Collection and assembly of data: All authors
Data analysis and interpretation: Jong Chul Park, Arya Ashok, Hyunseok Kang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jong Chul Park
Consulting or Advisory Role: I-MAB
Arya Ashok
Employment: Tempus
Stock and Other Ownership Interests: Tempus
Travel, Accommodations, Expenses: Tempus
Chienying Liu
Research Funding: NBI
Hyunseok Kang
Honoraria: Cancer Expert Now
Consulting or Advisory Role: Bayer, GlaxoSmithKline, Prelude Therapeutics, Achilles Therapeutics, MitoImmune, PIN therapeutics
Research Funding: Kura Oncology (Inst), Exelixis (Inst), Lilly (Inst), Elevar Therapeutics (Inst), PDS Biotechnology (Inst), NeoImmuneTech (Inst), Ayala Pharmaceuticals (Inst), Prelude Therapeutics (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Amatu A, Sartore-Bianchi A, Bencardino K, et al. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer Ann Oncol 30viii5–viii152019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cocco E, Scaltriti M, Drilon A.NTRK fusion-positive cancers and TRK inhibitor therapy Nat Rev Clin Oncol 15731–7472018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials Lancet Oncol 21271–2822020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children N Engl J Med 378731–7392018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bongarzone I, Vigneri P, Mariani L, et al. RET/NTRK1 rearrangements in thyroid gland tumors of the papillary carcinoma family: Correlation with clinicopathological features Clin Cancer Res 4223–2281998 [PubMed] [Google Scholar]
- 6.Wajjwalku W, Nakamura S, Hasegawa Y, et al. Low frequency of rearrangements of the ret and trk proto‐oncogenes in Japanese thyroid papillary carcinomas Jpn J Cancer Res 83671–6751992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seethala RR, Chiosea SI, Liu CZ, et al. Clinical and morphologic features of ETV6-NTRK3 translocated papillary thyroid carcinoma in an adult population without radiation exposure. Am J Surg Path. 2017;41:446. doi: 10.1097/PAS.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad ML, Vyas M, Horne MJ, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States Cancer 1221097–11072016 [DOI] [PubMed] [Google Scholar]
- 9.Gatalica Z, Xiu J, Swensen J, et al. Molecular characterization of cancers with NTRK gene fusions Mod Pathol 32147–1532019 [DOI] [PubMed] [Google Scholar]
- 10. Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016;1:e000023. doi: 10.1136/esmoopen-2015-000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drilon A, Nagasubramanian R, Blake JF, et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors Cancer Discov 7963–9722017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shihab HA, Gough J, Cooper DN, et al. Predicting the functional consequences of cancer-associated amino acid substitutions Bioinformatics 291504–15102013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cabanillas ME, Drilon A, Farago AF, et al. Larotrectinib treatment of advanced TRK fusion thyroid cancer. Ann Oncol. 1916P;31:S10862020. [Google Scholar]
- 14.Fischer H, Ullah M, de la Cruz CC, et al. Entrectinib, a TRK/ROS1 inhibitor with anti-CNS tumor activity: Differentiation from other inhibitors in its class due to weak interaction with P-glycoprotein Neuro Oncol 22819–8292020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor J, Pavlick D, Yoshimi A, et al. Oncogenic TRK fusions are amenable to inhibition in hematologic malignancies J Clin Invest 1283819–38252018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer N Engl J Med 368623–6322013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groussin L, Clerc J, Huillard O.Larotrectinib-enhanced radioactive iodine uptake in advanced thyroid cancer N Engl J Med 3831686–16872020 [DOI] [PubMed] [Google Scholar]
- 18.Brose MS, Albert CM, Waguespack SG, et al. Activity of larotrectinib in patients with advanced TRK fusion thyroid cancer. 88th Annual Meeting of the American Thyroid Association. Washington, DC, 2018.
- 19.Faulkner S, Jobling P, Rowe CW, et al. Neurotrophin receptors TrkA, p75(NTR), and sortilin are increased and targetable in thyroid cancer Am J Pathol 188229–2412018 [DOI] [PubMed] [Google Scholar]
- 20.Rosen EY, Goldman DA, Hechtman JF, et al. TRK fusions are enriched in cancers with uncommon histologies and the absence of canonical driver mutations Clin Cancer Res 261624–16322020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal N, Akbani R, Aksoy BA, et al. Integrated genomic characterization of papillary thyroid carcinoma Cell 159676–6902014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bournaud C, Descotes F, Decaussin-Petrucci M, et al. TERT promoter mutations identify a high-risk group in metastasis-free advanced thyroid carcinoma Eur J Cancer 10841–492019 [DOI] [PubMed] [Google Scholar]
- 23. Su X, Jiang X, Wang W, et al. Association of telomerase reverse transcriptase promoter mutations with clinicopathological features and prognosis of thyroid cancer: A meta-analysis. Onco Targets Ther. 2016;9:6965. doi: 10.2147/OTT.S116594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu R, Xing M.TERT promoter mutations in thyroid cancer Endocr Relat Cancer 23R143–R1552016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beaubier N, Tell R, Lau D, et al. Clinical validation of the tempus xT next-generation targeted oncology sequencing assay Oncotarget 102384–23962019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AACR Project GENIE Consortium AACR Project GENIE: Powering precision medicine through an international consortium Cancer Discov 7818–8312017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma Cell 159676–6902014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brueffer C, Vallon-Christersson J, Grabau D, et al. Clinical value of RNA sequencing-based classifiers for prediction of the five conventional breast cancer biomarkers: A report from the population-based multicenter Sweden cancerome analysis network-breast initiative JCO Precis Oncol 21–182018 [DOI] [PMC free article] [PubMed] [Google Scholar]