ABSTRACT
Bats are reservoirs of important zoonotic viruses like Nipah and SARS viruses. However, whether the blood-sucking arthropods on the body surface of bats also carry these viruses and the relationship between viruses carried by the blood-sucking arthropods and viruses carried by bats have not been reported. This study collected 686 blood-sucking arthropods on the body surface of bats from Yunnan Province, China, between 2012 and 2015, and they included wingless bat flies, bat flies, ticks, mites, and fleas. The viruses carried by these arthropods were analyzed using a meta-transcriptomic approach, and 144 highly diverse positive-sense single-stranded RNA, negative-sense single-stranded RNA, and double-stranded RNA viruses were found, of which 138 were potentially new viruses. These viruses were classified into 14 different virus families or orders, including Bunyavirales, Mononegavirales, Reoviridae, and Picornavirales. Further analyses found that Bunyavirales were the most abundant virus group (84% of total virus RNA) in ticks, whereas narnaviruses were the most abundant (52 to 92%) in the bat flies and wingless bat flies libraries, followed by solemoviruses (1 to 29%) and reoviruses (0 to 43%). These viruses were highly structured based on the arthropod types. It is worth noting that no bat-borne zoonotic viruses were found in the virome of bat-infesting arthropod, seemingly not supporting that bat surface arthropods are vectors of zoonotic viruses carried by bats.
IMPORTANCE Bats are reservoirs of many important viral pathogens. To evaluate whether bat-parasitic blood-sucking arthropods participate in the circulation of these important viruses, it is necessary to conduct unbiased virome studies on these arthropods. We evaluated five types of blood-sucking parasitic arthropods on the surface of bats in Yunnan, China, and identified a variety of viruses, some of which had high prevalence and abundance levels, although there is limited overlap in virome between distant arthropods. While most of the virome discovered here is potentially arthropod-specific viruses, we identified three possible arboviruses, including one orthobunyavirus and two vesiculoviruses (family Rhabdoviridae), suggesting bat-parasitic arthropods carry viruses with risk of spillage, which warrants further study.
KEYWORDS: bats, arthropods, RNA viruses, virome, phylogeny, vector-borne viruses
INTRODUCTION
Bats are the only mammals capable of flying, and they are also a diverse group, within which 1,116 species, 202 genera, and 18 families have been identified (1). Geographically, they are distributed nearly worldwide, with the exception of the North and South Poles (2). Importantly, a variety of new zoonotic infectious diseases that occurred in recent years have been linked to bats. Epidemics of zoonotic diseases such as Nipah viral encephalitis, a type of human viral encephalitis that was prevalent in Malaysia in 1998 (3), severe acute respiratory syndrome (SARS), which was epidemic in more than 30 countries around the world in 2003 (4), and Ebola hemorrhagic fever, caused by Ebola virus infection in West African countries (5), have all been linked to bats. The current COVID-19 causative agent is also suspected closely related to the viruses from bats (6, 7). In addition, viral metagenomic analyses have shown that bats carry a variety of animal, plant, and fungal viruses and bacteriophages, among which many were previously undescribed viruses (8–10). Therefore, bats are considered “reservoirs” for a variety of emerging infectious diseases that are capable of transmitting and spreading the disease with their large population size, their densely roosting behavior, the ability to fly, and the seeming ability to persistently carry virus without causing apparent disease (2).
On the other hand, bats are infested with a variety of blood-sucking arthropods. These include obligate ectoparasites such as bat flies and wingless bat flies (11–13) and nonobligate parasites such as ticks, mites, and fleas (14–16). These blood-sucking arthropods can help spread pathogens among bats through feeding behavior. A study in 2013 found that 7 of 26 Nycteribia kolenatii parasites detected on the surface of Myotis daubentonii contained Plasmodium in oocysts, and electron microscopy confirmed the presence of Plasmodium sporozoite in the salivary glands of N. kolenatii, suggesting that wingless bat flies can transmit Plasmodium (17). Other studies have shown that the bat-associated epidermal parasites from the families Hippoboscidae, Streblidae, and Nycteribiidae are all transmission vectors of Bartonella (18–21), whereas ticks are vectors for a variety of pathogens, including Spirochetes, Rickettsia, and Ehrlichia (22). Some viruses had been found in wingless bat flies. Specifically, rhabdoviruses were detected in Nycteribiid collected from the surface of Spanish bats in Europe, as well as Dipseliopoda sp. samples collected from bats in Uganda (23, 24). Novel orthoreoviruses and orthobunyaviruses were also isolated from bat flies (Eucampsipoda africana) collected from Egyptian fruit bats (Rousettus aegyptiacus) in South Africa (25, 26).
Although parasites of bats can transmit bacterial and eukaryotic pathogens, it remains unclear whether they carry viral pathogens and, if so, whether these viruses are related to those infecting bats and even humans. To address these questions, we analyzed the virome from five types of blood-sucking arthropods, namely, wingless bat flies, bat flies, ticks, mites, and fleas from the body surface of bats in the areas of Yunnan Province in China, where there has been extensive research on bats and their associated viromes (9, 27–31).
RESULTS
Collection and species identification of blood-sucking arthropods on the surface of bats.
From 2012 to 2015, a total of 686 blood-sucking arthropods, including ticks, mites, fleas, wingless bat flies, and bat flies, were collected from the body surface of bats in 10 counties located in southwestern Yunnan Province (Fig. 1; Table 1). Based on the collected arthropod types, collection time, and location, the samples were divided into nine pools/libraries (Table 1) for meta-transcriptomics sequencing, which generated 32,556,792 to 53,462,224 reads for each library (Table 1).
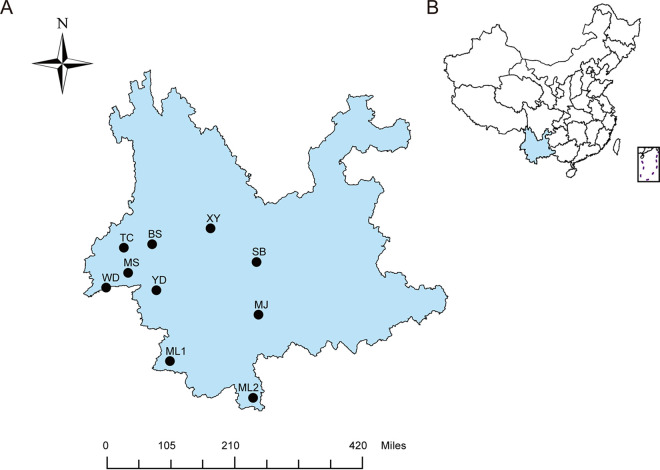
FIG 1.
Sampling locations of bats and their associated blood-sucking arthropods. (A) Geographical locations of 10 sampling sites in the southwestern part of Yunnan Province (marked by solid dots). (B) Location of Yunnan Province in China. BS, Baoshan; ML1, Menglian; ML2, Mengla; MJ, Mojiang; MS, Mangshi; SB, Shuangbai; TC, Tengchong; XY, Xiangyun; YD, Yongde; WD, Wanding.
TABLE 1.
Specimen collection and grouping information of blood-sucking arthropods on the surface of bats
| Library no. | Arthropods (no. of individuals) | Host(s)a | Collection dates | Collection location(s)b | No. of reads |
|---|---|---|---|---|---|
| L1 | Ticks (7) | MD, Rh, RL | 2012, 2013, 2015 | MJ, ML2, MS, TC | 43,346,666 |
| L2 | Bat flies (21c) | MD, Rh, RL | 2012, 2013, 2014, 2015 | BS, MJ, ML2, MS, WD | 49,251,370 |
| L3 | Mites (54) | MD, Rh, RL | 2013, 2015 | MJ, ML2, SB | 32,727,182 |
| L4 | Fleas (36d) | MD, Rh, RL | 2012, 2013, 2014 | BS, MJ, WD | 32,556,792 |
| L5 | Wingless bat flies (222e) | MD, Rh, RL | 2012 | ML1, ML2, MS, TC | 33,725,922 |
| L6 | Wingless bat flies (71) | MD, Rh, RL | 2013, 2014 | MJ, ML1, WD, YD | 39,718,934 |
| L7 | Wingless bat flies (86) | RL | 2013 | ML2 | 34,724,096 |
| L8 | Wingless bat flies (123) | MD, RL | 2014 | BS, XY | 50,341,976 |
| L9 | Wingless bat flies (66) | RL | 2015 | ML2 | 53,462,224 |
| Total | All arthropods (686) | 369,855,162 |
MD, Myotis daubentonii; Rh, Rhinolophus; RL, Rousettus leschenaultia.
BS, Baoshan; ML1, Menglian; ML2, Mengla; MJ, Mojiang; MS, Mangshi; SB, Shuangbai; TC, Tengchong; XY, Xiangyun; YD, Yongde; WD, Wanding.
Three wingless bat flies were included.
Thirteen wingless bat flies were included.
Eighty-eight mites were included.
To identify host species, we analyzed the reads associated with arthropods cytochrome c oxidase I (COI) gene of these libraries. A total of 112,460 reads of the COI sequence were obtained, of which 96.8% (108,856/112,460) belonged to five types of intended hosts, namely, Nycteribiidae, Streblidae, Ixodidae, Ischnopsyllidae, and Spinturnicidae, although COI genes from other species were also identified, including Sphingidae, Tephritidae, Chiroptera, Muridae, and Apidae (Table 2). Among these, bat COI genes were identified in six libraries, including Hipposideros armiger within library 1 and library 2 and Eonycteris spelaea in library 1, library 5, library 7, library 8, and library 9.
TABLE 2.
Distribution of the reads from the COI gene of different arthropods in nine libraries
| Arthropoda | No. (%)b of reads in: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | L5 | L6 | L7 | L8 | L9 | |
| Ixodidae | 6,117 (99) | 0 | 21 (4) | 381 (12) | 0 | 50 (1) | 2 (0) | 0 | 0 |
| Streblidae | 0 | 42,288 (99) | 0 | 0 | 0 | 48 (1) | 0 | 0 | 0 |
| Spinturnicidae | 0 | 27 (0) | 141 (27) | 1 (0) | 3,591 (11) | 2 (0) | 0 | 16 (0) | 0 |
| Ischnopsyllidae | 2 (0) | 1 (0) | 12 (2) | 570 (18) | 5 (0) | 5 (0) | 0 | 2 (0) | 0 |
| Nycteribiidae | 0 | 343 (1) | 260 (49) | 2,194 (70) | 29,679 (89) | 5,288 (98) | 9,144 (100) | 1,982 (98) | 10,130 (100) |
| Sphingidae | 0 | 2 (0) | 87 (16) | 0 | 5 (0) | 0 | 0 | 15 (1) | 0 |
| Tephritidae | 0 | 0 | 0 | 0 | 0 | 18 (0) | 0 | 0 | 0 |
| Apidae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 (1) | 0 |
| Muridae | 0 | 0 | 8 (2) | 0 | 0 | 0 | 0 | 0 | 8 (0) |
| Chiroptera | 41 (1) | 2 (0) | 0 | 0 | 4 (0) | 0 | 4 (0) | 1 (0) | 2 (0) |
| Total | 6,160 | 42,663 | 529 | 3,146 | 33,284 | 5,411 | 9,150 | 2,031 | 10,140 |
Sequences in each library that can be compared with the sequences in the arthropod COI gene library were identified by the BOLD system and named after the “family” where the corresponding host is located.
Percentages in parentheses indicate the proportion of the corresponding read number to the total number of reads from the arthropod host COI gene in the library. For example, 42,288 (99%) represents the number of bat fly COI reads with the percentage relative to the total number of COI reads in the library. The number are shown using bold characters if the percentage is above 10%.
Virome of arthropods on the body surface of bats.
All clean reads obtained in this research were assembled de novo, and the obtained contigs were compared with a viral RNA-dependent RNA polymerase (RdRp) protein database to identify virus genomes or RdRp-related genome fragments, with potential false positives removed by blasting against an NCBI nonredundant (nr) protein database. A total of 754 viral contigs (>500 bp) were identified initially, which were further clustered into 144 viral operational taxonomic units (OTUs) based on a similarity of <75%, a threshold set to mark the identification of a new viral OTU. Among these, 6 were existing ones, and 138 were newly identified OTUs. Based on the RdRp homology, these viruses were classified into 14 RNA virus families or orders, namely, Bunyavirales, Mononegavirales, Chuviridae, Orthomyxoviridae, Virgaviridae, Endornaviridae, Tymovirales, Solemoviridae, Narnaviridae, Picornavirales, Tombusviridae, Partitiviridae, Reoviridae, and Totiviridae (32) (Table S1 in the supplemental material). No DNA virus was found.
Among the 144 viral OTUs, 35 contained completed or nearly complete coding regions that are expected for the corresponding viruses (Table 3), and 21 belonged to viruses with segmented genomes, including Bunyavirales, Orthomyxoviridae, Partitiviridae, and Reoviridae. For viruses with segmented genomes, we revealed the corresponding non-RdRp segments based on sequence homology and matching abundance levels (Table S2).
TABLE 3.
Thirty-five viruses with (nearly) complete genomes carried by arthropods on the body surfaces of bats
| Virus no. | Family or order | Virus name | Length (nt) | blastx hits on known viruses (% blast amino acid identity) | GenBank accession no. |
|---|---|---|---|---|---|
| 1 | Bunyavirales | Yunnan nairo-like virus 1 | 13,611 | South Bay virus (54.23) | MW199199 |
| 2 | Bunyavirales | Yunnan nairo-like virus 2 | 13,533 | South Bay virus (54.29) | MW199201 |
| 3 | Bunyavirales | Yunnan peribunya-like virus | 6,928 | Wolkberg virus (74.60) | MZ395982 |
| 4 | Chuviridae | Yunnan chu-like virus 4 | 15,188 | Suffolk virus (58.53) | MW199243 |
| 5 | Mononegavirales | Yunnan rhabdo-like virus 1 | 11,496 | Wuhan louse fly virus 9 (70.71) | MW199242 |
| 6 | Mononegavirales | Yunnan rhabdo-like virus 5 | 9,721 | Wuhan louse fly virus 11 (57.83) | MZ395981 |
| 7 | Orthomyxoviridae | Yunnan orthomyxo-like virus | 2,428 | Wuhan Louse Fly virus 3 (84.91) | MW199253 |
| 8 | Virgaviridae | Yunnan negev-like virus 1 | 8,852 | Hubei negev-like virus 2 (72.14) | MW199214 |
| 9 | Virgaviridae | Yunnan negev-like virus 2 | 8,732 | Hubei negev-like virus 2 (49.64) | MW199212 |
| 10 | Virgaviridae | Yunnan negev-like virus 3 | 8,828 | Hubei negev-like virus 2 (73.94) | MW199213 |
| 11 | Virgaviridae | Yunnan virga-like virus 7 | 9,481 | Hubei negev-like virus 2 (73.52) | MZ396034 |
| 12 | Virgaviridae | Yunnan virga-like virus 8 | 10,715 | Mill Lade virus (36.37) | MW199215 |
| 13 | Narnaviridae | Yunnan narna-like virus 6 | 2,251 | Hubei narna-like virus 18 (52.12) | MW199251 |
| 14 | Narnaviridae | Yunnan narna-like virus 7 | 2,260 | Hubei narna-like virus 18 (30.62) | MW199252 |
| 15 | Narnaviridae | Yunnan narna-like virus 9 | 2,211 | Hubei narna-like virus 18 (51.80) | MW199249 |
| 16 | Narnaviridae | Yunnan narna-like virus 11 | 2,239 | Hubei narna-like virus 18 (86.24) | MW199250 |
| 17 | Narnaviridae | Yunnan narna-like virus 13 | 2,165 | Hubei mosquito virus 3 (37.60) | MW199247 |
| 18 | Narnaviridae | Yunnan narna-like virus 16 | 2,050 | Hubei mosquito virus 3 (34.81) | MW199246 |
| 19 | Picornavirales | Yunnan picorna-like virus 2 | 9,163 | Ixodes scapularis iflavirus (67.04) | MW199259 |
| 20 | Picornavirales | Yunnan picorna-like virus 7 | 9,932 | Wuhan insect virus 13 (67.39) | MW199260 |
| 21 | Picornavirales | Yunnan picorna-like virus 8 | 9,440 | Fesa-like virus (46.22) | MW199262 |
| 22 | Partitiviridae | Yunnan partiti-like virus 1 | 1,481 | Wuhan fly virus 6 (88.94) | MW199254 |
| 23 | Partitiviridae | Yunnan partiti-like virus 2 | 1,459 | Beihai barnacle virus 13 (54.11) | MW199255 |
| 24 | Partitiviridae | Yunnan partiti-like virus 3 | 1,498 | Norway partiti-like virus 1 (72.20) | MW199256 |
| 25 | Partitiviridae | Yunnan partiti-like virus 4 | 1,764 | Hubei partiti-like virus 14 (64.17) | MW199258 |
| 26 | Partitiviridae | Wuhan insect virus 25 | 1,490 | Wuhan insect virus 25 (97.00) | MZ395984 |
| 27 | Partitiviridae | Yunnan partiti-like virus 5 | 1,509 | Wuhan insect virus 25 (84.37) | MW199257 |
| 28 | Reoviridae | Yunnan reo-like virus 1 | 4,214 | Shelly beach virus (69.84) | MW199265 |
| 29 | Reoviridae | Yunnan reo-like virus 2 | 4,202 | Shelly beach virus (73.11) | MW199263 |
| 30 | Reoviridae | Yunnan reo-like virus 4 | 4,186 | Reoviridae sp. BF02/7/10 (78.50) | MW199264 |
| 31 | Reoviridae | Yunnan reo-like virus 5 | 4,200 | Shelly beach virus (67.77) | MZ396001 |
| 32 | Reoviridae | Yunnan reo-like virus 6 | 4,215 | Shelly beach virus (69.84) | MZ396000 |
| 33 | Totiviridae | Yunnan toti-like virus 1 | 7,797 | Hubei toti-like virus 20 (63.82) | MW199270 |
| 34 | Totiviridae | Yunnan toti-like virus 5 | 8,026 | Hubei toti-like virus 20 (63.12) | MW199271 |
| 35 | Totiviridae | Yunnan toti-like virus 8 | 7,739 | Hubei toti-like virus 20 (59.82) | MW199273 |
Abundance and composition of viruses in different libraries.
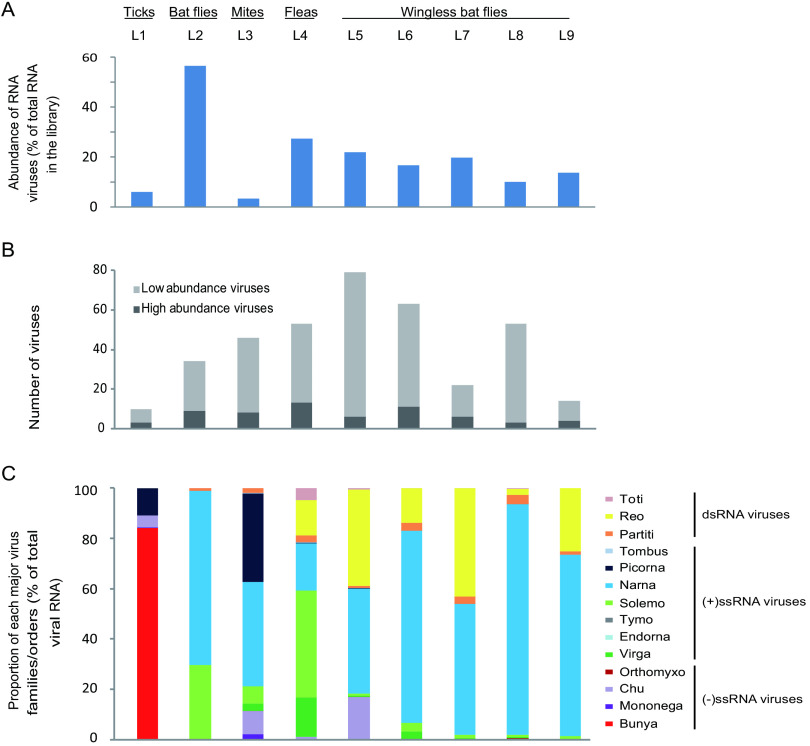
The total viral abundance varied greatly between different vectors. The highest abundance was observed in bat flies (56.51% of total non-rRNA), whereas the lowest was observed in mites (3.32%) (Fig. 2A). On the other hand, the abundance levels were quite consistent among libraries from the same host (i.e., wingless bat flies). Within each library, 10 to 79 OTUs of viruses were found, although most of them were low-abundance viruses (Fig. 2B). The high-abundance (>0.1% of total RNA in the library) ones ranged from 3 to 13 OTUs per library.
FIG 2.
Presence and abundance of RNA viruses found in parasitic arthropods on the body surface of bats. (A) Total abundance of RNA viruses in each library. (B) Numbers of high- and low-abundance viruses in each library. (C) Composition of different virus families or orders in each library. The arthropod types and library names are marked above the column graphs. Bunya, Bunyavirales; Mononega, Mononegavirales; Chu, Chuviridae; Orthomyxo, Orthomyxoviridae; Virga, Virgaviridae; Endorna, Endornaviridae; Tymo, Tymovirales; Solemo, Solemoviridae; Narna, Narnaviridae; Picorna, Picornavirales; Tombus, Tombusviridae; Partiti, Partitiviridae; Reo, Reoviridae; Toti, Totiviridae.
The 144 virus OTUs discovered in this study can be further divided into 14 virus families or orders. Among these, Bunyavirales dominated the tick library (84% of total virus RNA), Solemoviridae and Virgaviridae were quite abundant in the fleas library (43% and 16%, respectively), whereas Narnaviridae had the most read counts in bat flies (69%), mites (42%), and wingless flies libraries (42% to 92%) (Fig. 2C). Furthermore, Reoviridae (14% to 43%) and Chuviridae (5% to 17%) viruses also had a quite abundant distribution in two or more libraries (Fig. 2C). While the compositions were quite distinct among different arthropod species, the four libraries of wingless bat flies (libraries 6 to 9) showed more consistent patterns.
Comparisons of virome among different vectors.
Given the complexity of host composition in libraries 3 to 5 based on COI analyses (Table 2), we only compared the viromes between ticks (library 1), bat flies (library 2), and wingless bat flies (libraries 6 to 9) in which the COI genes identified from the pool were mostly associated with the principal host (Table 2). Comparisons of viromes among different types of hosts, namely, ticks, bat flies, and wingless flies, revealed distinctive structure of viral diversity and abundance. Indeed, only limited virus OTUs were shared among different hosts (Fig. 3A): there were none shared by all 3 type of hosts, 2 (i.e., Yunnan reo-like virus 2 and Yunnan narna-like virus 1) shared between ticks and wingless bat flies, and 20 shared between bat flies and wingless bat flies. For most of the shared viruses, their abundance levels were quite consistent across libraries from different hosts (Fig. 3B), suggesting they are unlikely to be originated from contamination.
FIG 3.
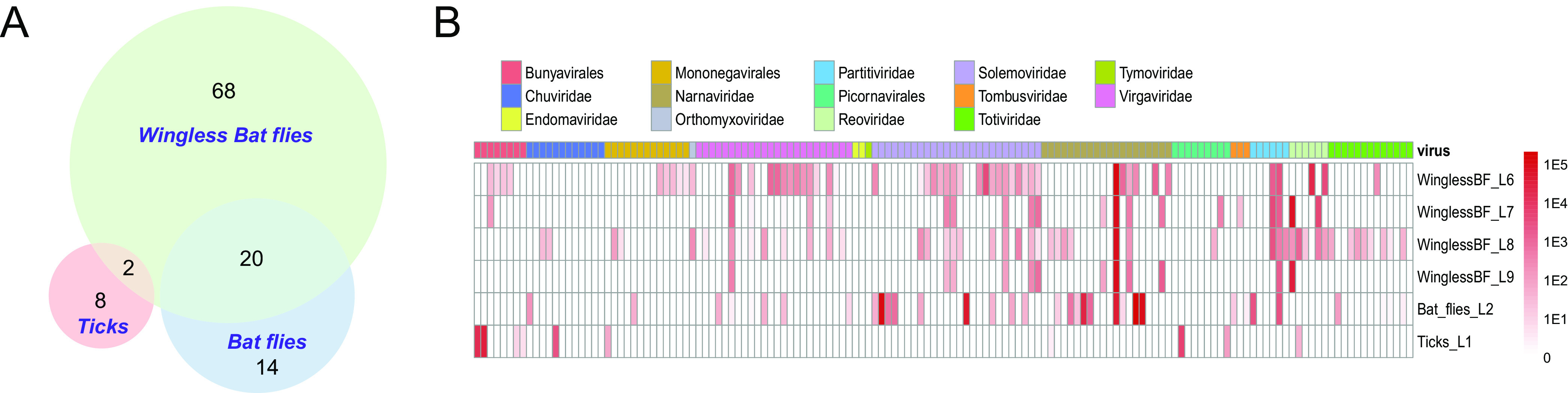
Comparisons of viromes among tick, bat fly, and wingless bat fly libraries. (A) Virus OTUs shared among ticks, bat flies, and wingless bat flies. The size of the circle and the thickness of line are proportional to the number of viral OTUs. The numbers in the circle represent the number of overlapping or unique viral OTUs. (B) Heat map showing the virus prevalence and abundance in different libraries of ticks, bat flies, and wingless bat flies. Wingless BF, wingless bat flies.
Detailed evolutionary history of viruses associated with blood-sucking arthropods on the surface of bats.
(i) Negative-sense RNA viruses.
In this study, 34 negative-sense RNA viruses were identified in 9 libraries, which belonged to Bunyavirales (n = 8), Rhabdoviridae (n = 12), Lishiviridae (n = 1), Chuviridae (n = 12), and Orthomyxoviridae (n = 1) (Table S1; Fig. 4). Among them, Yunnan peribunya-like virus in the family Orthobunyaviridae was identified from wingless bat flies, and it was most closely related to the Wolkberg virus (Table S1; Fig. 4; Fig. S1), which was also identified from wingless bat flies (25). The two nairoviruses, Yunnan nairo-like virus 1 and Yunnan nairo-like virus 2, were the most abundant viruses in the tick library and shared 54.23 to 54.29% amino acid identity with South Bay virus sampled from Ixodes scapularis in the United States (Table S1) (33). Similar to the South Bay virus, only sequences of the S and L segments were identified for these two newly identified nairoviruses (Fig. 4; Fig. S1) (33). The two phlebo-like viruses, Yunnan phlebo-like virus 1 and Yunnan phlebo-like virus 2, were most similar to blacklegged tick phlebovirus 3 (Ixodes scapularis in the United States) (34), with amino acid identities of 70.91 to 71.92%. Furthermore, we also found members of Phasmaviridae identified from wingless bat flies host, among which Yunnan phasma-like virus 2 was most similar to a group containing Wuchang cockroach virus 1, Wuhan mosquito virus 1, and Hubei odonate virus 9 (35).
FIG 4.
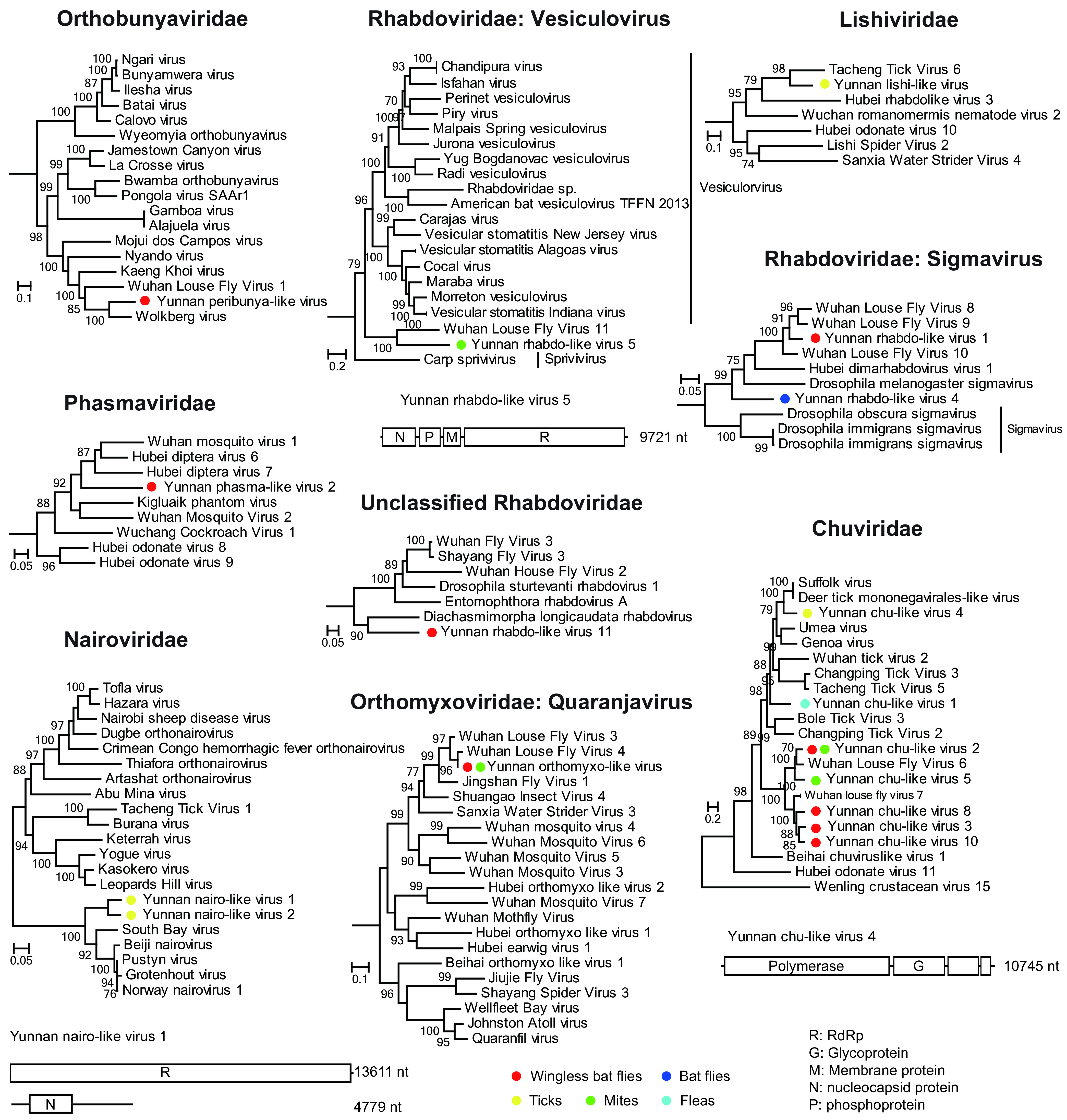
Evolutionary history and genome structure of negative-sense single-stranded RNA viruses discovered in this study. The maximum-likelihood phylogenetic trees based on RdRp protein alignments show the positions of the newly discovered virus among neighboring members in the corresponding virus family or order. Genome structures of the representative viral OTUs are shown right next to their phylogenies. Circles with different colors indicate the corresponding arthropods from which these viruses are discovered.
A number of viruses were found in the family Rhabdoviridae, order Mononegavirales (Fig. 4, Fig. S1). Among these, Yunnan rhabdo-like virus 5 was more closely related to Wuhan louse fly virus 11 (57.83%) (32), which, in turn, formed a sister clade to genus Vesiculovirus. On the other hand, Yunnan rhabdo-like viruses 1 and 4 were related to the genus Sigmavirus, which contained several members infecting Diptera, including viruses identified from louse flies (32). Furthermore, Yunnan rhabdo-like virus 11 identified from wingless bat flies did not belong to any existing genus in Rhabdoviridae, and it was distantly related to an arthropod virus, namely, Diachasmimorpha longicaudata rhabdovirus, with 57.45% amino acid identity. In addition to Rhabdoviridae, another member of the order Mononegavirales was Yunnan lishi-like virus identified from ticks. It belonged to the newly established Lishiviridae and was closely related to Tacheng tick virus 6 (42.98% identity) (Fig. 4).
For Chuviridae, we identified 7 new OTUs from 4 types of vectors: i.e., ticks, mites, fleas, and wingless bat flies. The tick-associated virus was closely related to Suffolk virus (58.53% identity) (34), whereas the flea-associated virus had no close relatives but was clustered within the tick-associated chuvirus cluster in the phylogenetic tree (Fig. 4). On the other hand, the chuviruses identified from wingless bat flies all formed a monophyly with Wuhan louse fly viruses 6 and 7 (32), with which they shared relatively close relationships (i.e., 68.46% to 75.69% identity) (Fig. 4). The chuvirus genome identified here shared the same structure with those of related chuviruses, which encodes three major protein-coding genes (Fig. 4; Fig. S1). Finally, we identified a single OTU of Orthomyxoviridae from wingless bat flies and mites, which belonged to the genus Quaranjavirus and shared a close relationship with Wuhan louse fly virus 3 (84.91% amino acid identity).
(ii) Double-stranded RNA viruses.
We identified 25 double-stranded RNA viruses, which belong to the Partitiviridae (n = 6), Reoviridae (n = 6), and Totiviridae (n = 13) families (Table S1). These 25 viruses were related to viruses identified from arthropods, including barnacles, spiders, ticks, mosquitoes, flies, louse flies, bugs, and butterflies, with amino acid identities that varied from 26.57 to 88.94%. Among them, 14 viruses were most similar to viruses detected from the mixed library of louse flies and bed bugs (identities of 59.82 to 84.37%) (Table S1) (32).
Six reoviruses were identified from ticks and wingless bat flies, and they all belonged to an unclassified clade that shared a distant relationship with genera Coltivirus and Mycovirus. The reference viruses that shared close relationships with ones identified from this study were mainly associated with diptera (i.e., High Island virus, Hubei diptera virus 21, and Eccles virus) and ticks (i.e., Shelly beach virus), with amino acid identities between 67.04 to 78.50% (Fig. 5; Table S1). For Yunnan reo-like virus 1, we were able to reveal the complete genome, which contained six segments and was similar to its close relatives (32, 36–38) (Fig. 5; Fig. S1).
FIG 5.
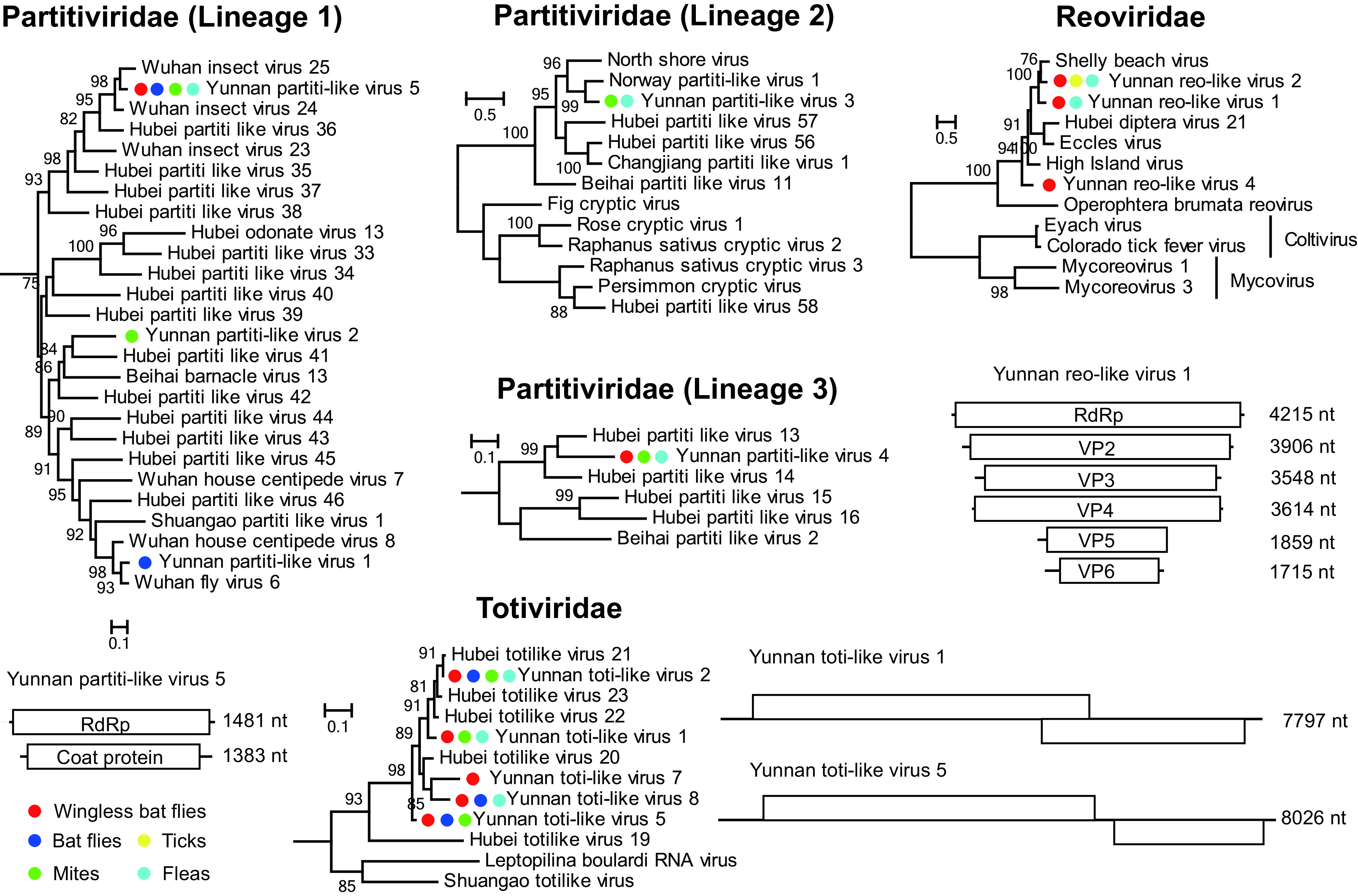
Evolutionary history and genome structure of double-stranded RNA viruses discovered in this study. Figure legend follows that of Fig. 4.
In addition to Reoviridae, diverse viruses were also found in Partitiviridae and Totiviridae virus families (Fig. 5; Fig. S1). More than 6 OTUs of partiti-like viruses were identified from bat flies, mites, fleas, and wingless bat flies. They belonged to three major lineages and shared close relationships with viruses identified from arthropods (amino acid identities, 54.11% to 94.00%). On the other hand, most of the toti-like viruses identified are closely related to Hubei toti-like viruses 20 and 23, which were identified from louse flies and bedbugs (Fig. 5).
(iii) Positive-sense RNA viruses.
We identified 85 positive-sense RNA viruses in nine libraries, including Virgaviridae (n = 27), Endornaviridae (n = 2), Tymovirales (n = 1), Solemoviridae (n = 27), Narnaviridae (n = 19), Picornavirales (n = 9), and Tombusviridae (n = 3) (Fig. 6; Fig. S1).
FIG 6.
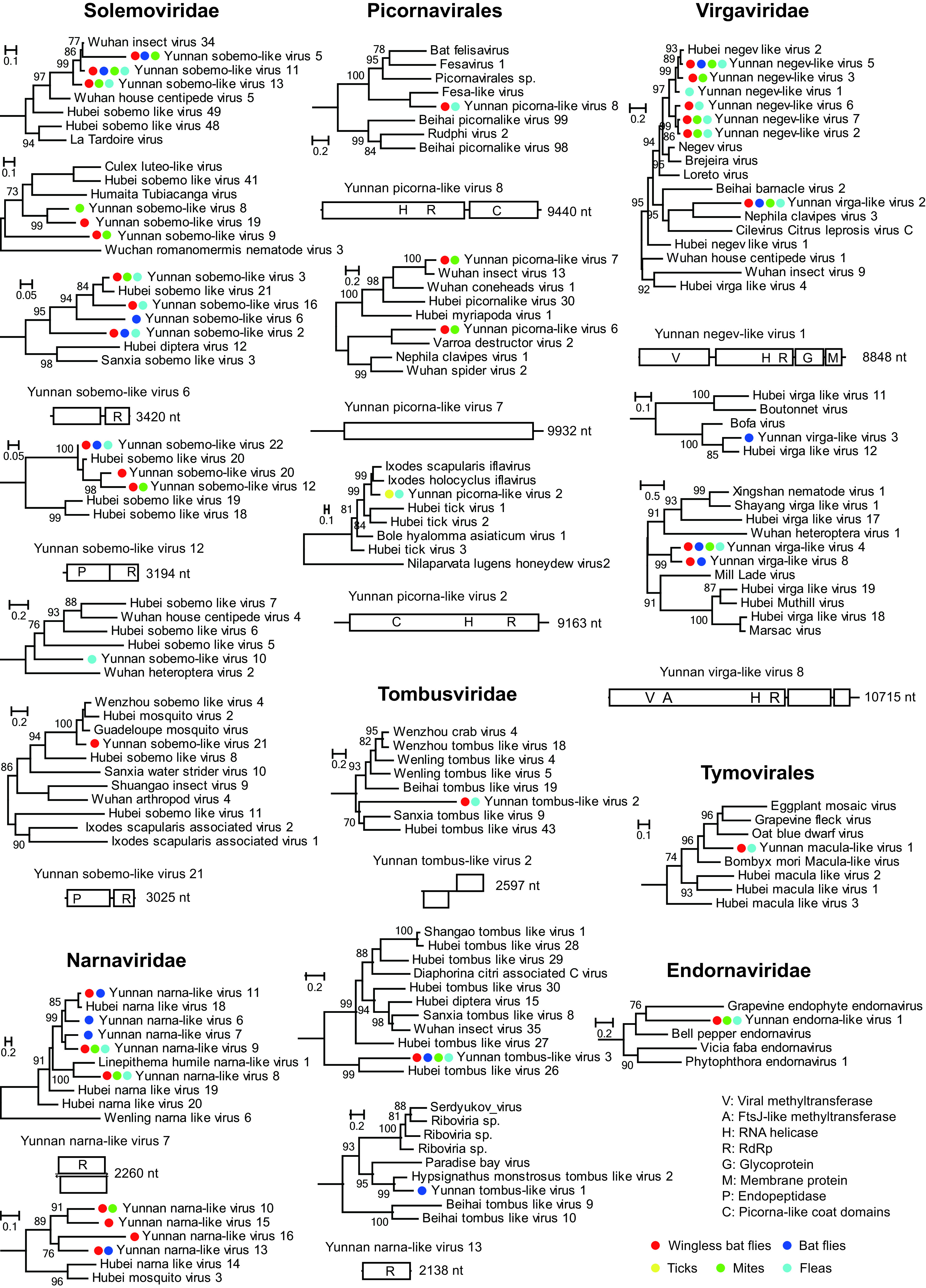
Evolutionary history and genome structures of positive-sense single-stranded RNA viruses. Figure legend follows that of Fig. 4.
The 10 representative strains of Virgaviridae viruses were distributed in three lineages (Fig. 6). The negev-like lineages contained 7 viruses; among these, 6 were identified from mites, fleas, bat flies, and wingless bat flies and were closely related to Hubei negev-like virus 2 (identified from louse flies and bedbugs; amino acid identities, 49.64% to 89.43%) within the negevirus cluster, and 1 (i.e., Yunnan virga-like virus 2) was relatively closely related to Nephila clavipes virus 3 identified from American spider (39) with 32.19% identity. Three more viruses, namely, Yunnan virga-like virus 3, 4, and 8, were identified from Virgaviridae-related lineages and were related to viruses identified from diptera host. Another two representative viruses were related to (with 22.08% and 66.75% identity) the plant viruses within Tymovirales and Endornaviridae, respectively (Fig. 6).
Picorna-like viruses were divided into more than three lineages. Among these, Yunnan picorna-like virus 8 belonged to a fesa-like lineage that contained a number of viruses identified from the faces of mammalian hosts, including bats, cats, and human (40–42). On the other hand, Yunnan picorna-like viruses 7, 6, and 2 were all grouped within the family Iflaviridae and were related to Wuhan insect virus 13 (67.39%), Varroa destructor virus 2 (46.25%), and Ixodes scapularis iflavirus (67.04%), respectively.
More than 6 lineages of sobemo-like viruses were identified. Except for Yunnan sobemo-like virus 10, which formed an orphan lineage, the rest were either related to viruses identified from louse flies and bedbugs pools (Yunnan sobemo-like viruses 1 to 3, 5 to 7, 11 to 17, 20, 22, and 23) or those identified from mosquitoes (Yunnan sobemo-like viruses 8, 9, 19, and 21), with 37.29% to 56.39% identities. The narna-like viruses were mainly divided into two lineages, one related to Hubei narna-like virus 18 and Linepithema humile narna-like virus 1, with 33.00% to 86.24% amino acid identities, and the other related to Hubei mosquito virus 3. The genome structures for former lineage encoded two completely overlapping open reading frames (ORFs), whereas those of the latter encoded only one ORF (Fig. 6; Fig. S1). For tombus-like viruses, three lineages were identified, among which Yunnan tombus-like virus 1 identified from bat flies was closely related to Hypsignathus monstrosus tombus-like virus 2 (66.96%), which was obtained from the blood sample of a hammer-headed fruit bat (43).
(iv) Potential arboviruses and bat-associated viruses.
Of the 144 viruses identified in this study, 3 are potential arboviruses, which belonged to Orthobunyaviridae (n = 1, Yunnan peribunya-like virus) and Rhabdoviridae (n = 2, Yunnan rhabdo-like virus 5 and Wuhan louse fly virus 11), respectively. Yunnan peribunya-like virus was discovered from the wingless bat flies libraries (i.e., libraries 6 and 7) and shared close relationships with Wolkberg virus, Wuhan louse fly virus 1, Kaeng Khoi virus (KKV), and Mojui dos Campos virus (MDCV) (Fig. 4), which were either discovered from wingless bats flies or directly from bats (25, 44, 45). Their genome structures followed that of Wolkberg virus, which had three segments, namely, L, M, and S, encoding polymerase, glycoproteins G1, G2, and NSm, and N proteins, respectively. Similar to the Wolkberg virus, the S gene does not encode the NSs protein (25). The two vesiculovirus-like rhabdoviruses were found in mites and wingless bat flies libraries (i.e., libraries 3 and 5). The RdRp sequences of Yunnan rhabdo-like virus 5 and Wuhan louse fly virus 11 formed a sister clade to vesiculovirus (Fig. 4), whereas the M protein sequence of Yunnan rhabdo-like virus 5 is close to Chandipura virus (21.64% amino acid identity). Furthermore, Yunnan picorna-like virus 8 from Picornavirales and Yunnan tombus-like virus 1 from Tombusviridae were all related to viruses identified from fecal or blood samples of bats, suggesting that these viruses were likely present in bats, although it is unclear whether these viruses can actively replicate in the bats’ systems.
DISCUSSION
This study used a meta-transcriptomic approach to characterize the RNA viruses carried by five types of blood-sucking arthropods, namely, ticks, bat flies, mites, fleas, and wingless bat flies. These blood-sucking arthropods were found to carry several highly diverse RNA viruses, which can be classified into 14 major virus families or orders. The majority of them either belonged to the vector-borne category (infecting both arthropod and vertebrate) or arthropod-specific category (infecting arthropod only), suggesting that they are somehow related to arthropod infection.
A variety of important zoonotic viruses, such as SARS-like coronavirus, MERS-related coronavirus, and filovirus, have been identified in bat specimens collected in Yunnan (27–29). Some serum samples from bats collected in Yunnan were positive for Nipah viral antibodies (46). In addition, numerous mammalian, plant, bacterial, insect, and fungal viruses were found in bat pharyngeal and anal swabs from Yunnan Province (9, 30, 47). These results suggest that bats, especially those in Yunnan Province, carry several viruses related to humans or animals, as well as unknown viruses. In this study, although numerous viruses were found in the five types of blood-sucking arthropods collected from bats in Yunnan Province, including Rousettus bats in which filoviruses have been detected, no genetic sequences from coronavirus, filovirus, Nipah virus, or their relatives were found, which did not support that arthropods on the body surface of bats are vectors of many of the zoonotic viruses carried by bats.
Both the Streblidae and Nycteribiidae libraries were dominated by narnaviruses. Among them, 69% of the Streblidae library (library 2) comprised narnaviruses, and the percentages are 42 to 92% for four libraries (libraries 6 to 9) of wingless bat flies. Thus, narnaviruses appear to be the most dominating virus population carried by obligate ectoparasites of bats. Reoviruses were the second-largest virus population in Nycteribiidae (libraries 6 to 9) after narnaviruses, while sobemo-like viruses accounted for 30% of the Streblidae virome following narnaviruses. So, although Streblidae and Nycteribiidae are exclusive obligate ectoparasites of bats, under the premise that they carry numerous narnaviruses, reoviruses and solemoviruses are unique populations carried by wingless bat flies and bat flies, respectively.
Although the viromes were highly structured by arthropod types, a few virus OTUs were shared among different hosts, and such sharing occurred more often between bat flies and wingless bat flies. Most of these shared virus OTUs belonged to arthropods-specific viruses families, such as Virgaviridae, Solemoviridae, Narnaviridae, Reoviridae, and Totiviridae, and none belonged to vertebrate-associated viruses, suggesting that these were unlikely to be derived from viremia in bats. Despite that, it is possible that these viruses are transmitted through cofeeding on the same host.
This study has several limitations. First, some of the specimens collected in this study contained genetic information from other types of arthropods, which complicates the interpretation of relationships between the viruses and hosts. Except for wingless bat flies, the number of other arthropods samples was limited. Second, we also consider the possibility that the eukaryotic viruses found in this study are ones carried by the arthropods themselves or associated with food, symbiont microbes, or other eukaryotic pathogens. While we could not provide definite evidence of host association with the principal host, most of the viruses discovered here are related to those identified from similar hosts, suggesting that they are unlikely from other sources. Lastly, the possibility of short fragments being endogenous virus elements (EVEs) instead of genomes from exogenous viruses was evaluated in this study based on sequencing analyses alone, for which we used two approaches, including (i) mapping contigs against related host genome sequencing results, and (ii) excluding sequences with disrupted ORFs. Further experiments based on DNA sequencing or full genome amplification are required to confirm that these viruses are not EVEs.
MATERIALS AND METHODS
Ethics statement.
This study, including the procedures and protocols of specimen collection and processing, was reviewed and approved by the Medical Ethics Committee of the Yunnan Institute of Endemic Diseases Control and Prevention.
Sample collection.
Samples were collected from 10 counties and towns of seven prefectures (cities) in southwest Yunnan Province (Fig. 1), including Xiangyun County of Dali Bai Autonomous Prefecture, Shuangbai County of Chuxiong Yi Autonomous Prefecture, Baoshan City and Tengchong City, Mangshi and Wanding of Dehong Jingpo and Dai Autonomous Prefecture, Yongde County of Lincang City, Menglian Dai, Lahu and Wa Autonomous County and Mojiang County of Pu'er City, and Mengla County of Xishuangbanna Dai Autonomous Prefecture. Sticky nets were arranged around orchards or caves to collect bats. According to the morphological character, the collected bats included Rousettus leschenaultia, Rhinolophus spp., and Myotis daubentonii. The captured bats were carefully removed from the nets. Arthropods moving in the hair of bats were captured using insect tweezers. After classification by morphology, arthropods were placed in cryopreservation tubes. Depending on the total number, 1 to 30 arthropods were collected per tube. The samples were transported to the laboratory in liquid nitrogen and stored at −80°C until further analysis. Bats were released following arthropod collection.
Sample mixing, nucleic acid extraction, and sequencing.
The samples were poured into a precooled sterile grinding mortar and washed with 2 ml minimal essential medium (MEM). Then, the homogenates were mixed with 1 ml grinding solution (90% MEM and 10% penicillin-streptomycin solution) and centrifuged at 18,000 rpm for 20 min, and the supernatant was collected (48). Subsequently, the supernatants from different samples were pooled based on collection time and location, and the samples were combined into nine groups, each containing 7 to 222 arthropods (Table 1). Total RNA was extracted using the QIAamp viral RNA minikit (Qiagen). After quantifying the RNA using the Agilent 2100 bioanalyzer system (Agilent Technologies), rRNA was removed using the Ribo-Zero Gold (human-mouse-rat) kit (Illumina). The library was constructed using TruSeq total RNA library approach (Illumina). The library was not poly(A) selected and not strand specific. After library quality control and purification, paired-end sequencing was performed on the HiSeq 4000 platform (Illumina). All library preparation and sequencing procedures were performed at BGI (Beijing Genome Institute, Shenzhen, China).
Discovery of RNA viruses.
The sequencing reads were filtered to remove low-quality sequences using Trimmomatic, with the default parameters (49), and Trinity was used for de novo assembly (50). The assembled contigs were compared with a collection of viral RdRp sequences representative of the RNA virus diversity, and the potential viral contigs were further confirmed by blastx analyses against NCBI nonredundant (nr) protein database. The collection of viral RdRp sequences was composed of (i) a backbone that contained RdRp sequences published in the 2016 nature paper (32), and (ii) additional references sequences that shared the highest similarity to sequences generated in this study (i.e., top blast hits). Viral contigs with overlapping regions were further merged with SeqMan (DNAStar, USA). The resulting virus contigs were further grouped into different OTUs based on a nucleotide identity threshold of 75%. The abundance level for each virus OTU was estimated by mapping reads to the virus genomes and evaluated by reads per million (RPM). To eliminate the false positives due to index hopping, positive hits were removed from library if the read account was below 0.1% of the maximum abundance of the specific viruses in these samples.
Host identification.
The contigs assembled de novo were compared against representative arthropods COI proteins, and the resulting COI-related contigs were further subject to read mapping to confirm the sequences. Species identification was carried out using the BOLD system (http://V3.boldsystems.org/index.PHP/IDS_openIDengine?quota=1), and the abundance of each host type was estimated by reads mapped to each host.
Phylogenetic analysis of viral sequences.
The viral RdRp sequences were aligned with RdRp sequences from related viruses using MAFFT (version 7.450) software (51), and ambiguous aligned regions were removed using trimAl software (52). Phylogenetic trees (53) were reconstructed in PhyML (3.1) using the maximum-likelihood method, LG amino acid substitution model, and SPR structure optimization algorithm.
Data availability.
The raw sequence reads generated in this study are available at the NCBI Sequence Read Archive (SRA) database under BioProject accession number PRJNA674504. All virus genome sequences generated in this study have been deposited in GenBank under accession numbers MW199199 to MW199273, MZ395979 to MZ396051, and MZ600153 to MZ600208.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (81290342 and 31560049), the Development Grant of the State Key Laboratory of Infectious Disease Prevention and Control (2014SKLID103), Yunnan Reserve Talents for Academic and Technical Leaders of Middle-Aged and Young People (2019HB052), the National Key Research and Development Program of China (2017YFC1200202), Guangdong Province “Pearl River Talent Plan” Innovation and Entrepreneurship Team Project (2019ZT08Y464), Yunnan Health Training Project of High Level Talents (D-2017055), and the U.S. National Institutes of Health U01 (AI151810). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material is available online only.
Contributor Information
Yun Feng, Email: ynfy428@163.com.
George F. Gao, Email: gaof@im.ac.cn.
Anice C. Lowen, Emory University School of Medicine
REFERENCES
- 1.Simmons NB. 2005. Order Chiroptera, p 312–529. In Wilson DE, Reeder DM (ed), Mammal species of the world: a taxonomic and geographic reference. The Johns Hopkins University Press, Baltimore, MD. [Google Scholar]
- 2.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. 2006. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev 19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paton NI, Leo YS, Zaki SR, Auchus AP, Lee KE, Ling AE, Chew SK, Ang B, Rollin PE, Umapathi T, Sng I, Lee CC, Lim E, Ksiazek TG. 1999. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet 354:1253–1256. doi: 10.1016/S0140-6736(99)04379-2. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Wang H, Crameri G, Hu Z, Zhang H, Zhang J, McEachern J, Field H, Daszak P, Eaton BT, Zhang S, Wang LF. 2005. Bats are natural reservoirs of SARS-like coronaviruses. Science 310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 5.Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Délicat A, Paweska JT, Gonzalez JP, Swanepoel R. 2005. Fruit bats as reservoirs of Ebola virus. Nature 438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 6.Tan W, Zhao X, Ma X, Wang W, Niu P, Xu W, Gao GF, Wu G. 2020. A novel coronavirus genome identified in a cluster of pneumonia cases — Wuhan, China 2019−2020. China CDC Wkly 2:61–62. doi: 10.46234/ccdcw2020.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team. 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dacheux L, Cervantes-Gonzalez M, Guigon G, Thiberge JM, Vandenbogaert M, Maufrais C, Caro V, Bourhy H. 2014. A preliminary study of viral metagenomics of French bat species in contact with humans: identification of new mammalian viruses. PLoS One 9:e87194. doi: 10.1371/journal.pone.0087194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge X, Li Y, Yang X, Zhang H, Zhou P, Zhang Y, Shi Z. 2012. Metagenomic analysis of viruses from bat fecal samples reveals many novel viruses in insectivorous bats in China. J Virol 86:4620–4630. doi: 10.1128/JVI.06671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He B, Li Z, Yang F, Zheng J, Feng Y, Guo H, Li Y, Wang Y, Su N, Zhang F, Fan Q, Tu C. 2013. Virome profiling of bats from Myanmar by metagenomic analysis of tissue samples reveals more novel Mammalian viruses. PLoS One 8:e61950. doi: 10.1371/journal.pone.0061950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dick CW, Patterson BD. 2006. Bat flies: obligate ectoparasites of bats, p 179–194. In Morand S, Krasnov BR, Poulin R (ed), Micromammals and macroparasites. Springer, New York, NY. [Google Scholar]
- 12.Dick CW. 2013. Review of the bat flies of Honduras, Central America (Diptera: Streblidae). J Parasitol Res 2013:437696. doi: 10.1155/2013/437696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertola PB, Aires CC, Favorito SE, Graciolli G, Amaku M, Pinto-da-Rocha R. 2005. Bat flies (Diptera: Streblidae, Nycteribiidae) parasitic on bats (Mammalia: chiroptera) at Parque Estadual da Cantareira, São Paulo, Brazil: parasitism rates and host-parasite associations. Mem Inst Oswaldo Cruz 100:25–32. doi: 10.1590/s0074-02762005000100005. [DOI] [PubMed] [Google Scholar]
- 14.Autino AG, Claps GL, Sánchez MS, Barquez RM. 2009. New records of bat ectoparasites (Diptera, Hemiptera and Siphonaptera) from northern Argentina. Neotrop Entomol 38:165–177. doi: 10.1590/s1519-566x2009000200002. [DOI] [PubMed] [Google Scholar]
- 15.Hastriter MW, Bush SE. 2013. Description of Lentistivalius philippinensis, a new species of flea (Siphonaptera, Pygiosyllomorpha, Stivaliidae), and new records of Ascodipterinae (Streblidae) on bats and other small mammals from Luzon, The Philippines. ZooKeys 260:17–30. doi: 10.3897/zookeys.260.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall AG. 1982. Ecology of insects ectoparasitic on bats, p 369–401. In Kunz TH (ed), Ecology of bats. Plenum, New York, NY. [Google Scholar]
- 17.Witsenburg F, Salamin N, Christe P. 2012. The evolutionary host switches of Polychromophilus: a multi-gene phylogeny of the bat malaria genus suggests a second invasion of mammals by a haemosporidian parasite. Malar J 11:53. doi: 10.1186/1475-2875-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morse SF, Olival KJ, Kosoy M, Billeter S, Patterson BD, Dick CW, Dittmar K. 2012. Global distribution and genetic diversity of Bartonella in bat flies (Hippoboscoidea, Streblidae, Nycteribiidae). Infect Genet Evol 12:1717–1723. doi: 10.1016/j.meegid.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Billeter SA, Hayman DT, Peel AJ, Baker K, Wood JL, Cunningham A, Suu-Ire R, Dittmar K, Kosoy MY. 2012. Bartonella species in bat flies (Diptera: Nycteribiidae) from western Africa. Parasitology 139:324–329. doi: 10.1017/S0031182011002113. [DOI] [PubMed] [Google Scholar]
- 20.Kamani J, Baneth G, Mitchell M, Mumcuoglu KY, Gutiérrez R, Harrus S. 2014. Bartonella species in bats (Chiroptera) and bat flies (Nycteribiidae) from Nigeria, West Africa. Vector Borne Zoonotic Dis 14:625–632. doi: 10.1089/vbz.2013.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosokawa T, Nikoh N, Koga R, Satô M, Tanahashi M, Meng XY, Fukatsu T. 2012. Reductive genome evolution, host-symbiont co-speciation and uterine transmission of endosymbiotic bacteria in bat flies. ISME J 6:577–587. doi: 10.1038/ismej.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Socolovschi C, Kernif T, Raoult D, Parola P. 2012. Borrelia, Rickettsia, and Ehrlichia species in bat ticks, France, 2010. Emerg Infect Dis 18:1966–1975. doi: 10.3201/eid1812.111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aznar-Lopez C, Vazquez-Moron S, Marston DA, Juste J, Ibáñez C, Berciano JM, Salsamendi E, Aihartza J, Banyard AC, McElhinney L, Fooks AR, Echevarria J. 2013. Detection of rhabdovirus viral RNA in oropharyngeal swabs and ectoparasites of Spanish bats. J Gen Virol 94:69–75. doi: 10.1099/vir.0.046490-0. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg TL, Bennett AJ, Kityo R, Kuhn JH, Chapman CA. 2017. Kanyawara virus: a novel rhabdovirus infecting newly discovered Nycteribiid bat flies infesting previously unknown pteropodid bats in Uganda. Sci Rep 7:5287. doi: 10.1038/s41598-017-05236-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen van Vuren P, Wiley MR, Palacios G, Storm N, Markotter W, Birkhead M, Kemp A, Paweska JT. 2017. Isolation of a novel orthobunyavirus from bat flies (Eucampsipoda africana). J Gen Virol 98:935–945. doi: 10.1099/jgv.0.000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansen van Vuren P, Wiley M, Palacios G, Storm N, McCulloch S, Markotter W, Birkhead M, Kemp A, Paweska JT. 2016. Isolation of a novel fusogenic orthoreovirus from Eucampsipoda africana bat flies in South Africa. Viruses 8:65. doi: 10.3390/v8030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Wu Z, Ren X, Yang F, He G, Zhang J, Dong J, Sun L, Zhu Y, Du J, Zhang S, Jin Q. 2013. Novel SARS-like betacoronaviruses in bats, China, 2011. Emerg Infect Dis 19:989–991. doi: 10.3201/eid1906.121648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Wu Z, Ren X, Yang F, Zhang J, He G, Dong J, Sun L, Zhu Y, Zhang S, Jin Q. 2014. MERS-related betacoronavirus in Vespertilio superans bats, China. Emerg Infect Dis 20:1260–1262. doi: 10.3201/eid2007.140318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang XL, Zhang YZ, Jiang RD, Guo H, Zhang W, Li B, Wang N, Wang L, Waruhiu C, Zhou JH, Li SY, Daszak P, Wang LF, Shi ZL. 2017. Genetically diverse filoviruses in Rousettus and Eonycteris spp. bats, China, 2009 and 2015. Emerg Infect Dis 23:482–486. doi: 10.3201/eid2303.161119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Z, Yang L, Ren X, He G, Zhang J, Yang J, Qian Z, Dong J, Sun L, Zhu Y, Du J, Yang F, Zhang S, Jin Q. 2016. Deciphering the bat virome catalog to better understand the ecological diversity of bat viruses and the bat origin of emerging infectious diseases. ISME J 10:609–620. doi: 10.1038/ismej.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C, Liu WJ, Xu W, Jin T, Zhao Y, Song J, Shi Y, Ji W, Jia H, Zhou Y, Wen H, Zhao H, Liu H, Li H, Wang Q, Wu Y, Wang L, Liu D, Liu G, Yu H, Holmes EC, Lu L, Gao GF. 2016. A bat-derived putative cross-family recombinant coronavirus with a reovirus gene. PLoS Pathog 12:e1005883. doi: 10.1371/journal.ppat.1005883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi M, Lin XD, Tian JH, Chen LJ, Chen X, Li CX, Qin XC, Li J, Cao JP, Eden JS, Buchmann J, Wang W, Xu J, Holmes EC, Zhang YZ. 2016. Redefining the invertebrate RNA virosphere. Nature 540:539–543. doi: 10.1038/nature20167. [DOI] [PubMed] [Google Scholar]
- 33.Tokarz R, Williams SH, Sameroff S, Sanchez Leon M, Jain K, Lipkin WI. 2014. Virome analysis of Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis ticks reveals novel highly divergent vertebrate and invertebrate viruses. J Virol 88:11480–11492. doi: 10.1128/JVI.01858-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tokarz R, Sameroff S, Tagliafierro T, Jain K, Williams SH, Cucura DM, Rochlin I, Monzon J, Carpi G, Tufts D, Diuk-Wasser M, Brinkerhoff J, Lipkin WI. 2018. Identification of novel viruses in Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis ticks. mSphere 3:e00614-17. doi: 10.1128/mSphere.00614-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li CX, Shi M, Tian JH, Lin XD, Kang YJ, Chen LJ, Qin XC, Xu J, Holmes EC, Zhang YZ. 2015. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. Elife 4:e05378. doi: 10.7554/eLife.05378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvey E, Rose K, Eden JS, Lo N, Abeyasuriya T, Shi M, Doggett SL, Holmes EC. 2019. Extensive diversity of RNA viruses in Australian ticks. J Virol 93:e01358-18. doi: 10.1128/JVI.01358-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medd NC, Fellous S, Waldron FM, Xuéreb A, Nakai M, Cross JV, Obbard DJ. 2018. The virome of Drosophila suzukii, an invasive pest of soft fruit. Virus Evol 4:vey009. doi: 10.1093/ve/vey009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadeghi M, Popov V, Guzman H, Phan TG, Vasilakis N, Tesh R, Delwart E. 2017. Genomes of viral isolates derived from different mosquitos species. Virus Res 242:49–57. doi: 10.1016/j.virusres.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Debat HJ. 2017. An RNA virome associated to the golden orb-weaver spider Nephila clavipes. Front Microbiol 8:2097. doi: 10.3389/fmicb.2017.02097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yinda CK, Zeller M, Conceição-Neto N, Maes P, Deboutte W, Beller L, Heylen E, Ghogomu SM, Van Ranst M, Matthijnssens J. 2016. Novel highly divergent reassortant bat rotaviruses in Cameroon, without evidence of zoonosis. Sci Rep 6:34209. doi: 10.1038/srep34209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Li L, Deng X, Kapusinszky B, Pesavento PA, Delwart E. 2014. Faecal virome of cats in an animal shelter. J Gen Virol 95:2553–2564. doi: 10.1099/vir.0.069674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siqueira JD, Dominguez-Bello MG, Contreras M, Lander O, Caballero-Arias H, Xutao D, Noya-Alarcon O, Delwart E. 2018. Complex virome in feces from Amerindian children in isolated Amazonian villages. Nat Commun 9:4270. doi: 10.1038/s41467-018-06502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bennett AJ, Bushmaker T, Cameron K, Ondzie A, Niama FR, Parra HJ, Mombouli JV, Olson SH, Munster VJ, Goldberg TL. 2019. Diverse RNA viruses of arthropod origin in the blood of fruit bats suggest a link between bat and arthropod viromes. Virology 528:64–72. doi: 10.1016/j.virol.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groseth A, Mampilli V, Weisend C, Dahlstrom E, Porcella SF, Russell BJ, Tesh RB, Ebihara H. 2014. Molecular characterization of human pathogenic bunyaviruses of the Nyando and Bwamba/Pongola virus groups leads to the genetic identification of Mojuí dos Campos and Kaeng Khoi virus. PLoS Negl Trop Dis 8:e3147. doi: 10.1371/journal.pntd.0003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinheiro FP, Travassos da Rosa AP. 1985. Mojui dos Campos, p 695–696. In Karabatsos N (ed), International catalogue of arboviruses including certain other virus of vertebrates, 3rd ed. American Society of Tropical Medicine and Hygiene for the Subcommittee on Information Exchange of the American Committee on Arthropod-borne Viruses, San Antonio, TX. [Google Scholar]
- 46.Li Y, Wang J, Hickey AC, Zhang Y, Li Y, Wu Y, Zhang H, Yuan J, Han Z, McEachern J, Broder CC, Wang LF, Shi Z. 2008. Antibodies to Nipah or Nipah-like viruses in bats, China. Emerg Infect Dis 14:1974–1976. doi: 10.3201/eid1412.080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han Y, Du J, Su H, Zhang J, Zhu G, Zhang S, Wu Z, Jin Q. 2019. Identification of diverse bat alphacoronaviruses and betacoronaviruses in China provides new insights into the evolution and origin of coronavirus-related diseases. Front Microbiol 14:1900. doi: 10.3389/fmicb.2019.01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng Y, Li Y, Fu S, Li X, Song J, Zhang H, Yang W, Zhang Y, Pan H, Liang G. 2017. Isolation of Kaeng Khoi virus (KKV) from Eucampsipoda sundaica bat flies in China. Virus Res 238:94–100. doi: 10.1016/j.virusres.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katoh K, Standley DM. 2013. MAFFT Multiple Sequence Alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1<br>. Download jvi.01464-21-s0001.xlsx, XLSX file, 0.04 MB (36.1KB, xlsx)
Table S2<br>. Download jvi.01464-21-s0002.xlsx, XLSX file, 0.02 MB (16.3KB, xlsx)
Fig. S1<br>. Download jvi.01464-21-s0003.pdf, PDF file, 0.5 MB (466.3KB, pdf)
Data Availability Statement
The raw sequence reads generated in this study are available at the NCBI Sequence Read Archive (SRA) database under BioProject accession number PRJNA674504. All virus genome sequences generated in this study have been deposited in GenBank under accession numbers MW199199 to MW199273, MZ395979 to MZ396051, and MZ600153 to MZ600208.