Abstract
Disruption of prefrontal glutamate receptor interacting protein (GRIP), which anchors GluA2-containing AMPA receptors (AMPARs) into the synaptic membrane, potentiates cue-induced cocaine seeking in both males and females. PICK1 plays an opposing role to that of GRIP, removing AMPARs from the synapse. Consistent with our hypothesis that disruption of PICK1 in the mPFC would lead to a decrease in addiction-like behavior, we found that conditional deletion of PICK1 in the mPFC attenuates cue-induced cocaine seeking in male mice. However, prefrontal PICK1 deletion had the opposite effect in females, leading to an increase in cue-induced reinstatement of cocaine seeking. We did not see any effects of PICK1 knockdown on sucrose taking or seeking, suggesting the sex-specific effects do not generalize to natural reinforcers. These findings suggest the role of PICK1 in the prefrontal cortex of females may not be consistent with its accepted role in males. To determine whether these sex differences were influenced by gonadal hormones, we gonadectomized a cohort of males and found that removal of circulating androgens eliminated the effect of prefrontal PICK1 knockdown. As there was no effect of gonadectomy on its own on any of the behavioral measures collected, our results suggest that androgens may be involved in compensatory downstream effects of PICK1 knockdown. Taken together these results highlight the need for consideration of sex as a biological variable when examining mechanisms underlying all behaviors, even those that do not appear to be influenced by sex.
Introduction
Substance use disorder affects nearly 20 million people in the United States, 966,000 of whom primarily abuse cocaine1. In 2017, the United States Department of State expressed concern over the rapidly increasing amount of export-quality cocaine produced in several South American countries, from 915 metric tons in 2009 to 1,930 metric tons in 20172. These figures are particularly troublesome given the lack of pharmacological treatments for cocaine abuse. Therefore, it is of utmost importance that the molecular mechanisms underlying cocaine abuse are identified.
Heightened glutamate levels within the prefrontal cortex (PFC) have been shown to enhance cocaine use in animal models of addiction. Memory of cocaine-associated contexts involves prefrontal glutamatergic activity in mice3. In rats, incubation of craving after cocaine self-administration is associated with elevated glutamate release in the ventromedial prefrontal cortex4. The elevated glutamate release within the prefrontal cortex impacts downstream regions such as the nucleus accumbens5. This process is hypothesized to lead to the development of addiction in both rats and humans6,7.
The trafficking of glutamate receptors into and out of the synaptic membrane ensures appropriate levels of glutamate transmission in healthy individuals. However, cocaine experience alters the balance of glutamate receptors at the synapse. During extinction of cocaine seeking, elevated levels of the AMPA receptor containing the GluA1 subunit are observed in the prefrontal cortex8 and nucleus accumbens9. Work in the nucleus accumbens has also found an increase in calcium permeable AMPA receptors, which contain only the GluA1 subunit10. Notably, this increase was true only after self-administration of cocaine, not experimenter-administered IP injections. Although these findings were described as a possible cause of extinction9, this phenomenon has also been hypothesized to lead to incubation of craving11 and therefore represents a potential mechanism driving relapse to cocaine use.
Phosphorylation of GluA2 by PKC disrupts the interaction between GluA2 and glutamate receptor interacting protein (GRIP) and allows protein interacting with C kinase 1 (PICK1) to bind to the GluA2 subunit in its place12. Whereas GRIP anchors AMPARs at the synaptic membrane, PICK1 internalizes GluA2-containing AMPA receptors, thereby decreasing surface expression. Under normal circumstances, GRIP and PICK1 act to balance the number of GluA2-containing AMPA receptors available at the synapse. Reduced expression of one of these proteins will prevent this method of maintaining homeostasis. Notably, this homeostatic balance between GRIP and PICK1 only affects GluA2 containing AMPARs. Therefore, it represents an important mechanism controlling the ratio of calcium impermeable to calcium permeable AMPARs.
Following chronic cocaine use, changes in levels of AMPA receptors may represent a maladaptive form of learning13. Previous work in our lab demonstrated that disrupting GRIP, which anchors GluA2-containing AMPARs into the synaptic membrane, in the mPFC potentiates cocaine reinstatement in both male and female mice. In the current experiment, we aimed to determine if disrupting PICK1 in the mPFC would lead to blunted reinstatement of cocaine seeking in male and female mice; the opposite effect of what was seen after disrupting GRIP in the PFC. Our sex-specific behavioral results suggest that PICK1 in the mPFC is involved in different protein interactions in males and females. In males, the predicted reduction in reinstatement supports the existing model of how PICK1 and GRIP mediate glutamate signaling in this region. The same cannot be said for females, suggesting that PICK1 plays a sex-specific role in mechanisms of synaptic plasticity.
Methods
Subjects.
Mice homozygous for the Cre/lox-conditional allele of PICK1 (flox/flox) were bred on a C57bl/6J background. Adult male and female mice (2–6-months old, age matched across group) were group housed until three days before the first day of food training, at which time they were single housed and began food deprivation. Food deprivation (mice restricted to >90% of their free-feeding weight) lasted until the third day of cocaine self-administration. The single housing condition lasted throughout the duration of the experiment. All animals were housed in a temperature- and humidity-controlled animal care facility with a 12-h light/dark cycle (lights on at 0700 hours). All procedures were approved by the Temple University Animal Care and Use Committee. Cocaine was obtained from the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD) and dissolved in sterile 0.9% saline.
Prefrontal Microinjections and Adeno-Associated Virus Constructs.
The adeno-associated virus (AAV) expressing Cre recombinase (AAV2/9.CMV.PI.CRE, titer 2.84 × 1013 vgc/μl) and the AAV expressing green fluorescent protein (eGFP) (AAV2/9.CMV.eGFP, titer 3.74 × 1013 vgc/μl) were generated by Addgene. PICK1 flox/flox mice (6–8 weeks) were anesthetized with isoflurane and 0.4μl of the viral vector construct (Cre or GFP) was injected bilaterally into the prefrontal cortex through a 30-gauge needle at a rate of 0.1 μl/min. Stereotaxic coordinates for the prefrontal cortex are (from Bregma) anterior-posterior 2.4, lateral +/− 0.3, dorso-ventral −2.3. Following recovery, mice remained in the home cage for 6 weeks prior to behavioral testing. Meloxicam (2.0 mg/kg, S.C.) was administered for the first three days following surgery. The procedures involving the AAV viruses have all been approved by the Temple University Institutional Biosafety committee. Knockdown was confirmed via western blot, and animals were removed from study if knockdown was less than a 30% decrease from average GFP control levels.
Experiment I.
Operant Food Training.
Before catheterization, mice were trained to perform an operant response for sucrose pellets. The mice were placed in operant chambers (Med-Associates) and trained to spin a wheel manipulandum to receive a sucrose pellet, with one-quarter spin measured as a single active response. Mice performed 5 days of fixed ratio 1 (FR1) responding followed by 5 days of FR5 responding. A compound cue stimulus consisting of a cue light above the active wheel, a 2900-Hz tone, and house light off was concurrent with each pellet administration, followed by an additional 8 s time-out when responding had no programmed consequences and the house light remained off. Mice were allowed to self-administer a maximum of 50 pellets per 60 min operant session.
Jugular Catheterization Surgery.
Prior to surgery, mice were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine. An indwelling silastic catheter was placed into the right jugular vein and sutured in place. The catheter was then threaded subcutaneously over the shoulder blade and was routed to a mesh backmount platform (Strategic Applications, Inc) that secured the placement. Catheters were flushed daily with 0.1 ml of an antibiotic (Timentin, 0.93 mg/ml) dissolved in heparinized saline. The catheters were sealed with plastic obturators when not in use. Meloxicam (2.0 mg/kg, S.C.) was administered for the first three days following surgery.
Cocaine Self-Administration, Extinction, and Reinstatement.
Mice were tested for cocaine self-administration behavior in 2-hour sessions in the same chamber used for sucrose pellet self-administration. During testing, one quarter turn of the wheel now delivered an intravenous cocaine injection (0.6 mg/kg/infusion), paired with the same compound cue, and following an FR1 schedule. After 10 days of cocaine self-administration mice began extinction training, in which cocaine-seeking behavior was extinguished by replacing the cocaine with 0.9% saline. During this time the light and tone cues paired with cocaine delivery were not present. Daily 2-h extinction sessions continued until animals met the extinction criterion of less than 25% of their self-administration responding (average of last 3 days). Twenty-four hours after meeting the extinction criterion, animals underwent a cue-induced reinstatement session. During the cue-induced reinstatement session, the light and tone cues were presented non-contingently for 20 seconds every 2 minutes during the first 10 minutes of the session. Animals were still able to respond on the active and inactive response wheels during this time. After this time period, the cues were presented contingent with responding on the active response wheel, just as was done during the cocaine self-administration phase. During the reinstatement session, animals received saline infusions following responses on the active wheel.
Sucrose Self-Administration, Extinction, and Reinstatement.
Following food training, a subset of mice went on to the extinction and cue-induced reinstatement phases as described above. These mice began extinction training after 10 days of food self-administration, during which neither the cues (light and tone) nor the reinforcing pellet were provided. This continued until mice performed fewer active responses than 25% of their responding during the last three days of self-administration. A single day of cue-induced reinstatement for sucrose pellets was performed the day after extinction criterion was met.
Statistical analysis.
Self-administration experiments were analyzed with two-way ANOVAs with viral vector injection and day as the independent variables and pellets/responses/infusions as the dependent variable. Sidak’s post hoc comparisons were made when main effects or interactions were detected (p < 0.05). The protein quantification, extinction responding, days to criterion, and cue-induced reinstatement responding data were analyzed using unpaired t-tests with viral vector injection as the independent variable. As we were examining the effects of PICK1 knockdown and not directly testing sex differences the data were analyzed separately in males and females. However, to directly compare the sexes, we ran an additional two-way ANOVA on the reinstatement data with sex as one of the independent variables.
Experiment II.
Subjects.
As the goal of this experiment was to examine the relationship between mPFC PICK1 knockdown and androgens, only male mice were used in these studies.
Gonadectomy.
Five weeks prior to food training, mice underwent orchiectomy or sham surgery. Mice were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine via an IP injection. Mice in the gonadectomy (orchiectomy) group had the caudal epididymis removed bilaterally. Sham animals received incisions into the skin and peritoneum only. All animals were placed under a heat lamp until awake and mobile. For the week following gonadectomy or sham surgery, animals were monitored to ensure proper healing. Meloxicam (2.0 mg/kg, subcutaneous injection) was administered for the first three days following surgery, and triple antibiotic ointment applied to the surgical site until the skin had fully healed.
Operant Food Training, Jugular Catheterization, and Cocaine Self-Administration.
See methods for Experiment I.
Western Blot.
PICK1 levels in the prefrontal cortex were measured using a western blot, as described in Briand et al., 201414. This method was used for animals in both experiment 1 and 2. Briefly, animals were decapitated, and the prefrontal cortex dissected using a brain block (Braintree Scientific). Protein quantification was performed using a Pierce BCA Protein Assay Kit (Thermo Scientific). Equal amounts of protein (30 μg) were loaded into each well of a Tris-glycine gel (Lonza) and transferred to nitrocellulose membranes (Immobilon). Membranes were blocked with Li-Cor blocking buffer and allowed to incubate in primary antibody solution (PICK1, 1:2000 (NeuroMab) and GAPDH, 1:5000 (Cell Signaling)) for 24 hours at 4°C. Membranes were then incubated with fluorescent secondary antibodies (1:20,000; IR-dye 680 or IR-dye 800, Li-Cor) and imaged on an Odyssey fluorescent scanner (Li-Cor). Western blots were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the percent knockdown calculated as a fraction of the average of the PICK1 levels in GFP-infused mice.
Statistical analysis.
In experiment 1, all self-administration experiments were analyzed with two-way ANOVAs with viral vector injection and day as the independent variables and pellets/responses/infusions as the dependent variable. Experiment 2 was analyzed in the same manner, though groups were separated into “sham control” or “gonadectomy.” Sidak’s post hoc comparisons were made when main effects or interactions were detected (p < 0.05). The protein quantification, extinction responding, days to criterion, and cue-induced reinstatement responding data were analyzed using unpaired t-tests with viral vector injection as the independent variable.
Results
Cre-Mediated Deletion of PICK1 in the Medial Prefrontal Cortex
Six weeks following the injection of AAV-Cre into the mPFC (see Fig. 1a for placement) of PICK1 floxed mice, we demonstrate a knockdown in PICK1 levels in this region compared to AAV-GFP injected controls in both male and female mice [Fig. 1b; main effect of virus, F(1,48)=36.9, p<.0001]. Performing the viral vector injections six weeks before the beginning of behavior allows for adequate expression of the virus and removal of existing PICK1 from the membrane without being affected by cocaine experience. The available antibodies only allow us to quantify the extent of the knockdown using western blot techniques; this does not allow us to differentiate between PICK1 knockdown in PFC cell bodies versus PICK1 knockdown in terminals of neurons projecting to the PFC. Although AAV9 preferentially targets neurons, the lack of complete knockout may be due to glial expression of PICK115. Confirmation of viral vector injection placement was performed via GFP imaging in an additional group of mice.
Figure 1. Significant Cre-mediated prefrontal PICK1 knockdown.
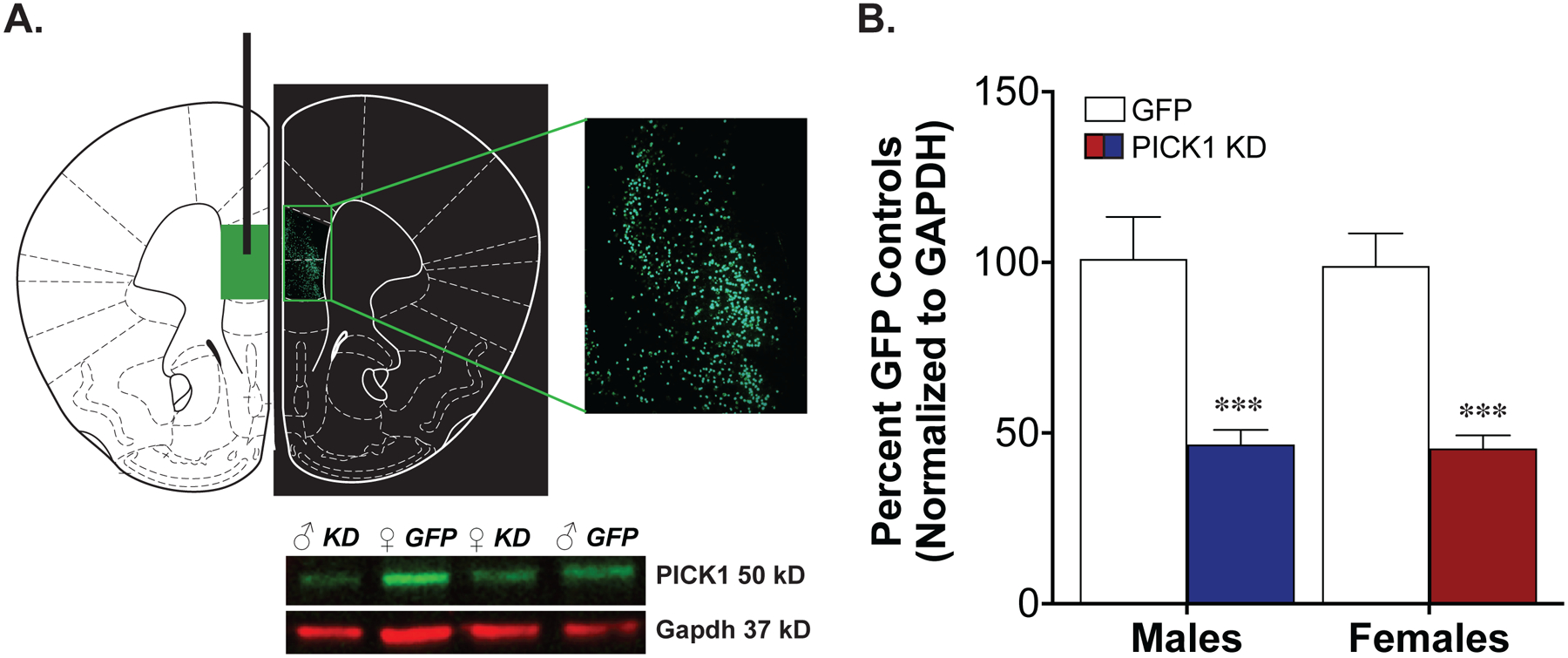
Male and female mice were injected bilaterally with 0.4μl of AAV-GFP or AAV-Cre recombinase into the medial prefrontal cortex (A). Western blots showed significant prefrontal PICK1 knockdown [B, ***p<.0001 main effect of virus].
Prefrontal PICK1 Knockdown Does Not Affect Food Training, Sucrose Self-Administration or Cocaine Self-Administration in Intact Mice
Six weeks following the viral vector injections, GFP controls and PICK1 knockdown mice were given 10 days of operant training to acquire sucrose self-administration. Both groups acquired the operant response for sucrose, exhibiting a significant increase in the number of pellets earned over the 10 sessions [males: F(9,441)=31.6, p<0.01; females: F(9,450)=23.6, p<0.01]. However, there was no effect of PICK1 knockdown on the number of pellets received [males: F(1,49)=2.84, p=0.09; females: F(1,50)=0.11, p=0.74; Fig. 2a,b], number of active responses [males: F(1,47) = 0.38, p = 0.54; females: F(1,50)=0.69, p=0.41; Fig 2c,d], or percent active responding [males: F(1,49)=2.39, p = 0.13; females: F(1,50)<0.01, p=0.99]. During the cocaine self-administration phase, there were no differences between GFP controls and PICK1 knockdown animals in the number of infusions received [males: F(1, 39) = 0.08, p = 0.77; females: F(1,33)=0.22, p=0.64; Fig. 2e,f] or the number of active responses [males: F(1, 39) = 0.33, p = 0.57; females: F(1,34)=0.95, p=0.34; Fig. 2g,h].
Figure 2. PICK1 knockdown in the mPFC does not alter operant learning during sucrose self-administration or cocaine self-administration in male or female mice.
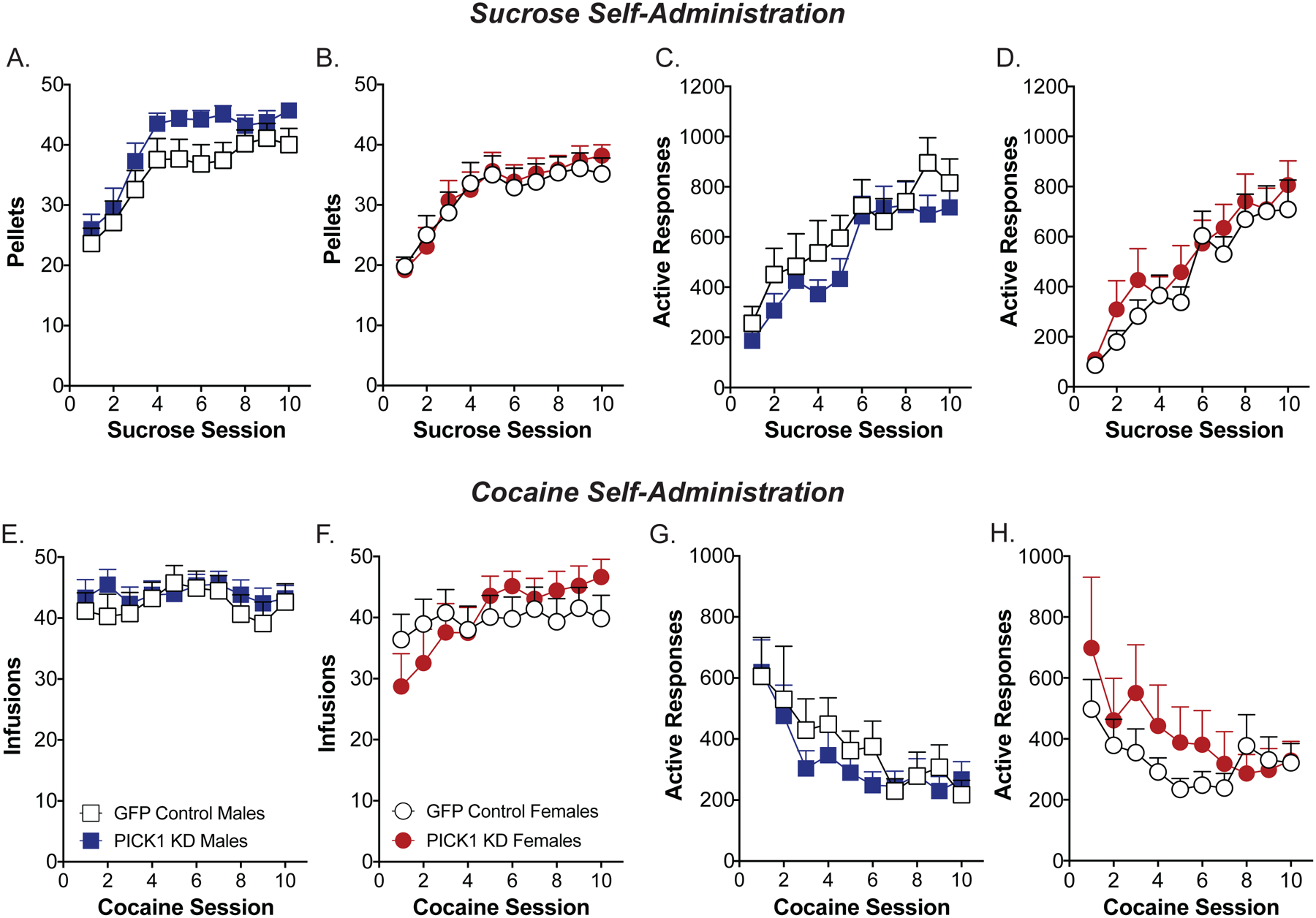
There were no significant effects of prefrontal PICK1 knockdown (PICK1 KD) among males or females during sucrose self-administration, as measured by the number of sucrose pellets received during a one-hour testing session (A-C; n=24–27/group). There were also no significant effects of prefrontal PICK1 KD among males or females during the self-administration of cocaine, as measured by the number of cocaine infusions received during a two-hour period (D-F; n=14–22/group).
Prefrontal PICK1 Knockdown Has a Sex-Specific Effect on Cue-Induced Reinstatement for Cocaine but Not Sucrose in Intact Mice
Following the cocaine self-administration phase, a subset of mice began extinction training. No differences were seen between the GFP controls and PICK1 knockdown animals in the days to reach the extinction criteria [F(1, 39) = 1.29, p = 0.26], nor was there an effect of sex [F(1, 39) = 1.20, p = 0.28; Fig 3a]. However, during cue-induced reinstatement of cocaine seeking we found a sex specific effect of PICK1 knockdown. In male mice, PICK1 knockdown animals exhibited a significant decrease in cocaine seeking compared to GFP controls [Interaction, F(1,20)=5.85, p=.025, Sidak post-hoc GFP control reinstatement vs. PICK1 knockdown reinstatement, p=.011; Fig. 3b]. In contrast, female PICK1 knockdown animals exhibit an increase in cue-induced cocaine seeking during the reinstatement test [Interaction, F(1,19)=5.47, p=.030, Sidak post-hoc GFP control reinstatement vs. PICK1 knockdown reinstatement, p=.011; Fig. 3c]. When comparing the effect of PICK1 knockdown on reinstatement across the sexes, a significant interaction between viral vector injection and sex was found [Interaction, F(1,39)=9.01, p=.0047]. These effects were specific to cocaine, as no effect of prefrontal PICK1 knockdown was seen in either male or female mice during cue-induced reinstatement of sucrose seeking [males: main effect test, F(1,16)=20.8, p=.0003; main effect of PICK1 knockdown, F(1,16)=0.20, p=.41; interaction, F(1,16)=0.36, p=.56; females: main effect test, F(1,10)=54.6, p<.0001; main effect of PICK1 knockdown, F(1,10)=0.24, p=.64; interaction, F(1,10)=1.80, p=.21; Fig. 4].
Figure 3. Prefrontal PICK1 knockdown leads to sex-specific effects on cue-induced reinstatement of cocaine seeking.
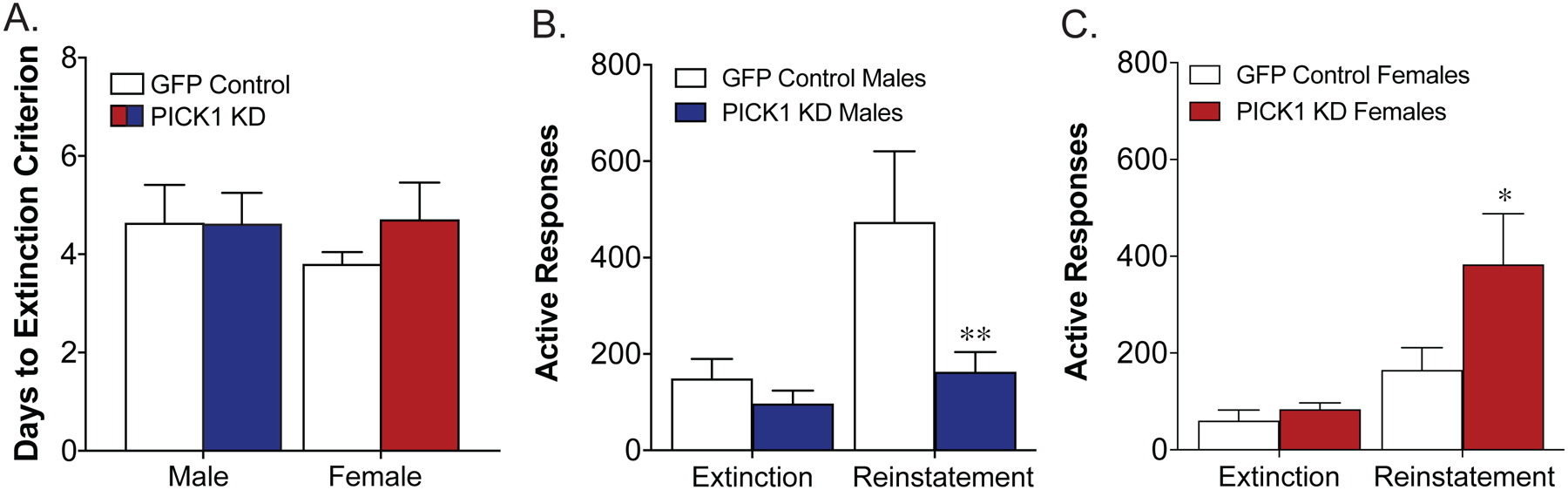
Male and female mice showed no effect of prefrontal PICK1 knockdown (KD) on the time to reach extinction criterion (A). However, PICK1 KD in the mPFC of male mice led to a significant decrease in active responses during cue-induced reinstatement [B, F(1,20)= 4.44, *p<0.01, n=10–12/group]. In contrast, PICK1 KD in the mPFC of female mice led to an increase in active responses during cue-induced reinstatement [C, interaction: F(1,19)= 4.87, *p<0.01, n=9–13/group].
Figure 4. Prefrontal PICK1 knockdown does not affect cue-induced reinstatement of sucrose seeking.
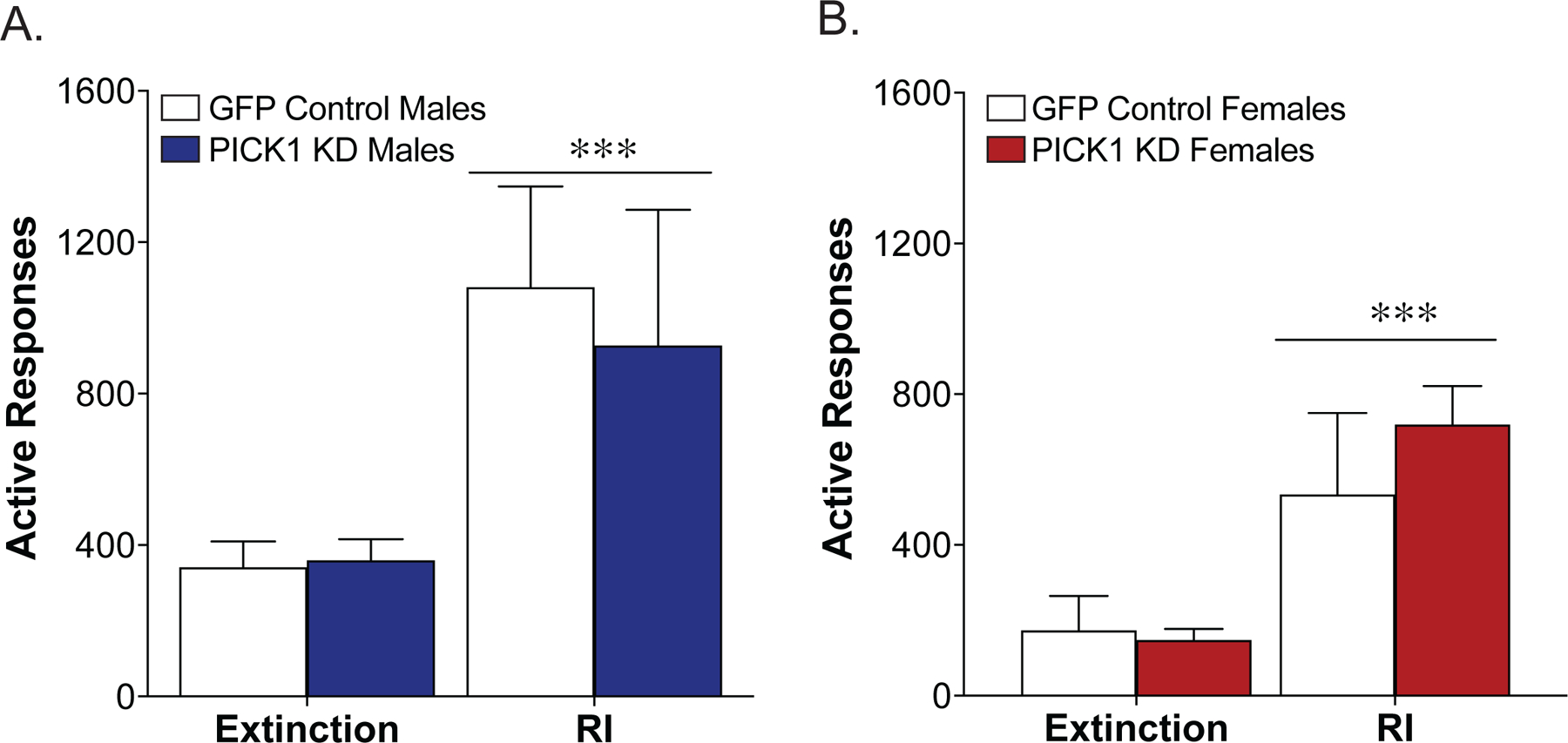
There were no effects of prefrontal PICK1 knockdown (KD) on cue-induced reinstatement of sucrose in males (A) or females (B; n= 4–5/group).
Gonadectomy Reversed the Effect of Prefrontal PICK1 knockdown on Cue-Induced Reinstatement of Cocaine Seeking in Males
Male gonadectomy did not alter the total number of sucrose pellets earned over the 10 sucrose self-administration sessions [effect of GDX: F(1,40)=0.003, p=0.96; effect of virus: F(1,40)=0.0007, p=.98; Fig 5a]. Similarly, there was no effect of gonadectomy or PICK1 knockdown in the total number of cocaine infusions received over the 10 days of cocaine self-administration [effect of GDX: F(1,37)=0.11, p=0.74; effect of virus: F(1,37)=0.12, p=0.73; Fig. 5b]. Neither prefrontal PICK1 knockdown nor gonadectomy influenced the number of days required to meet extinction criteria among gonadectomized males [effect of GDX: F(1,26)=0.48, p=0.49; effect of virus: F(1,26)=0.71, p=0.41 Fig. 5c]. In contrast, there was a significant interaction between prefrontal PICK1 knockdown and gonadectomy during cue-reinstatement (interaction, F(1,27)=4.31, *p<0.05; Fig. 5d].
Figure 5. Gonadectomy eliminates the effect of prefrontal PICK1 knockdown on cue-induced reinstatement.
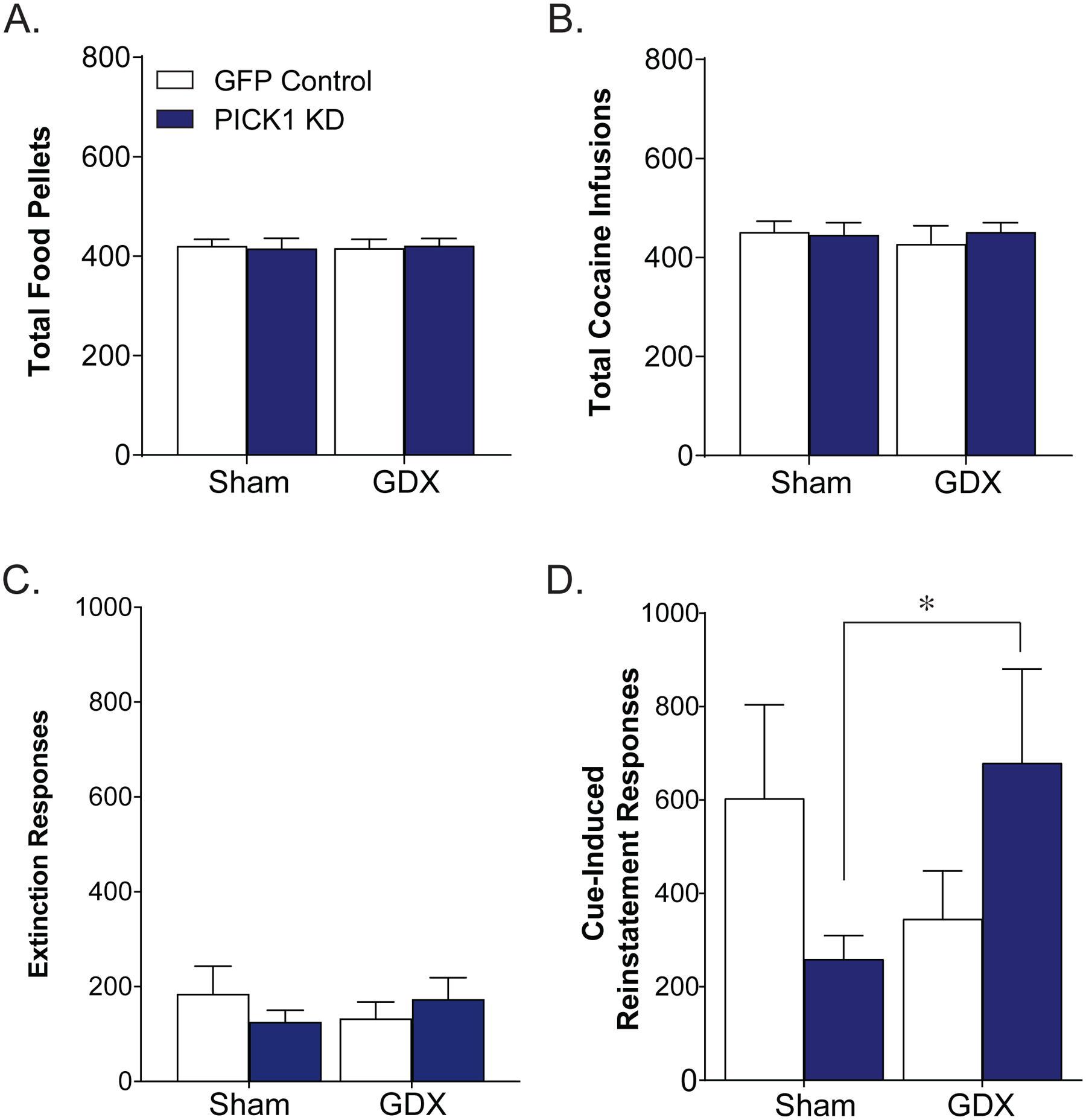
There were no group differences over the course of the 10 days of sucrose self-administration in the total number of pellets received (A). During the 10 days of cocaine self-administration, there were no group differences in the total number of infusions received (B). There was no effect of PICK1 KD or gonadectomy on active responses performed on the last day of extinction [C; F(1,27)= 3.48, p=0.07]. There was a significant interaction between gonadectomy and prefrontal PICK1 KD on responses during the cue-reinstatement session [D; F(1,27)= 4.31, *p<0.05, n=6–9/group].
Discussion
Overall, we find that the AMPA receptor scaffolding protein PICK1, within the prefrontal cortex, plays a sex-specific role in mediating cocaine seeking. Our data demonstrate that knockdown of prefrontal PICK1 decreases cue-induced cocaine seeking in males while not affecting sucrose or cocaine self-administration. In contrast, prefrontal PICK1 knockdown increases cue-induced cocaine seeking in females. The effects of prefrontal PICK1 knockdown in males are mediated in part by gonadal hormones, as gonadectomized males responded more like females after the PICK1 manipulation.
PICK1 Knockdown in the Prefrontal Cortex Dampens Cocaine Seeking in Males
The scaffolding proteins GRIP and PICK1 bind to the GluA2 subunit of the AMPA receptor12. GRIP maintains the GluA2-containing receptor within the synapse, whereas PICK1 internalizes the receptor after phosphorylation12. Together, these proteins regulate the GluA2-containing AMPA receptors available at the synapse. Previous work has shown that knockdown of prefrontal GRIP potentiates cocaine seeking16. Therefore, we hypothesized that disrupting PICK1 function in this region would have the opposite effect: attenuated responding during reinstatement. This is exactly what we found in the male mice. As prefrontal GRIP knockdown leads to an increase in AMPA-mediated glutamate transmission16, we believe our PICK1 manipulation leads to a decrease in AMPA-mediated transmission due to a decrease in the contribution of GluA1-lacking AMPA receptors. This would lead to a net decrease in AMPA transmission. This is consistent with work in male rats demonstrating that inactivation of the mPFC disrupts reinstatement of cocaine seeking17–21.
Although the control males had a higher numerical value for reinstatement than control females, it was not statistically significant due to the variability inherent in mouse behavioral studies. In the literature, some studies have found that females show higher levels of cocaine reinstatement compared to males22–24 though this is dose dependent25. Past work in our lab has not shown a sex difference in reinstatement among control mice26,27.
PICK1 Knockdown in the Prefrontal Cortex Leads to a Sex-Specific Effect in Cocaine Seeking
In contrast to what we found in male mice, female mice exhibited an increase in cue-induced reinstatement following prefrontal deletion of PICK1. These findings suggest that PICK1 plays a sex-specific role in glutamate receptor trafficking. Since we previously demonstrated that knocking out GRIP from the PFC leads to similar effects on cocaine seeking in male and female mice16, this would suggest something unique to PICK1 rather than an overall sex difference in the role of PFC glutamate trafficking in cocaine seeking. The GluA2 subunit is only one of over forty proteins that bind to PICK128. Therefore, PICK1 may exhibit a different binding profile in the PFC of male and female mice.
One such protein that PICK1 binds to is the dopamine transporter (DAT)28, the primary target of cocaine. Colocalization of PICK1 and DAT increases dopamine uptake in vitro29, thereby attenuating the effects of dopamine at the synapse. If PICK1 binds with greater affinity to the DAT in female mice compared to male mice, then disrupting PICK1 function in females could lead to alterations in dopamine transmission. While male PICK1 knockdown mice do not show evidence for alterations in surface expression of DAT30, these studies have not been done in female PICK1 knockdown mice. Dopamine antagonist administration into the prefrontal cortex dampens cue-induced cocaine seeking on a second order schedule31, therefore it is plausible that prefrontal PICK1 increases cocaine seeking via dopaminergic mechanisms.
Gonadectomy Eliminates PICK1 Knockdown Effect on Cocaine Cue-Induced Reinstatement in Males
The data from the males fits within the AMPA trafficking model proposed by the current body of literature, so our first step was to examine gonadectomy of males. We found that gonadectomized male mice did not show the prefrontal PICK1 knockdown effect that was seen in intact and sham surgerized males. As gonadectomy did not influence reinstatement behavior in control mice, this suggests that androgens are involved in the downstream effects of PICK1 knockdown that lead to the decrease in reinstatement behavior. Interaction between androgens and the glutamate system has been described in several brain regions32. In the hypothalamus, testosterone has been shown to upregulate AMPA receptor subunits in the rat32, while in male hamsters, androgens increase glutamate synthesis and GluA1 subunit expression33. However, gonadectomy does not alter the levels of AMPA or NMDA receptor subunits within the striatum or amygdala of male rats32,34, suggesting that the effects of testosterone on glutamate signaling may be brain region specific.
While it is not known whether gonadectomy influences glutamate signaling within the NAc, microinjection of testosterone in the NAc leads to the development of conditioned place preference35, indicating that it regulates reward circuitry. However, higher peripheral testosterone levels are associated with decreased behavioral effects of cocaine36,37. In the current study, we found that gonadectomy did not influence cocaine taking or seeking behavior. These results are consistent with one other study that examined the effect of male gonadectomy on cocaine self-administration in rats38. We have extended this finding to confirm that along with having no effect on cocaine taking, gonadectomy does not influence cocaine seeking in male mice.
The lack of effect of gonadectomy in control mice on reinstatement behavior suggests that the lack of circulating androgens does not directly influence glutamate signaling. However, it does influence the downstream effects of PICK1 knockdown. We propose that one of the downstream effects of PICK1 knockdown is a decrease in the expression of GluA2-lacking AMPARs to compensate for the increased expression of GluA2-containing AMPARs. This yin-yang relationship between GluA2-containing and GluA2-lacking AMPARs is consistent with our previous findings following prefrontal GRIP knockdown16. We hypothesize that gonadectomy prevents this compensatory response thereby increasing the contribution of GluA2-lacking, calcium permeable AMPARs. While very little has been done examining how androgens influence glutamate receptor trafficking, androgen receptor antagonism can lead to an increase in glutamate-evoked intracellular calcium39. Therefore, it is possible that gonadectomy is potentiating the effects of the calcium-permeable GluA2-lacking AMPARs thereby preventing this decrease in net glutamate transmission.
The current studies focused on the effect of gonadectomy in males because our findings on the role of prefrontal PICK1 knockdown are consistent with previous work suggesting that higher levels of GluA2-containing AMPARs, relative to GluA2-lacking AMPARs, dampens cocaine seeking16. While female gonadal hormones may be playing a role in the effects of PICK1 in cocaine seeking, these findings suggest the role of PICK1 itself may be sex specific. Further studies are need to characterize the role of this protein in females prior to examining how gonadal hormones might be interacting with it.
Prefrontal PICK1 Knockdown Does Not Play a Role in Fixed Ratio Measures of Food or Cocaine Self-Administration
We did not find any effect of prefrontal PICK1 knockdown on sucrose or cocaine taking. This is consistent with previous work in our lab showing that prefrontal GRIP knockdown had no effect on fixed ratio measures of reward administration16, and with existing literature showing no effect of PICK1 inhibition on natural reinforcement40,41. Disruptions to glutamatergic signaling mechanisms have not been reported to affect sucrose seeking42,43, initial fixed ratio measures of self-administration44,45, or extinction46, suggesting that glutamate is more important for cocaine seeking than sucrose or drug taking.
Of note, we did not observe sex-specific differences in the acquisition of sucrose or cocaine taking, though it has been well established that females acquire drug seeking behavior at a faster rate than males22,24,47. However, this effect is not seen in every cohort of animals due to differences in cocaine dose, time of day, and reinforcement schedule38,48. It is possible that the parameters of our self-administration setup minimize sex differences during the acquisition phase, or that females must begin self-administration at a specific point in the estrus cycle (likely estrus, see23) to show robust differences.
Additionally, gonadectomy did not alter sucrose training or cocaine self-administration in male animals. Testosterone in male rats is required for cocaine sensitization37, particularly during adolescence49. However, operant learning during adulthood is primarily dependent upon changes during adolescence49,50. As all the animals described in the current experiment were adults, no deficits in operant learning were expected.
Conclusions
In the current study, we have shown that conditional deletion of PICK1 in the mPFC leads to sex-specific effects during cue-induced reinstatement to cocaine. Males showed decreased reinstatement while females increased their reinstatement responding. The PICK1 knockdown induced decrease in reinstatement responding was blocked by gonadectomy, suggesting that circulating testosterone has a downstream effect on this phenotype. Further study will be needed to parse apart effects in the females and to develop an understanding of interactions between gonadal hormones and prefrontal addiction circuitry. In the future, this could represent a novel avenue for treatment of psychostimulant addiction. However, currently these are the first results to show that PICK1 may be playing different roles in males and females, which highlights the importance of considering sex differences even in basic biological processes.
This is the first study to examine the possibility of sex differences in the role of PICK1. The results for males are consistent with past literature showing increased reinstatement after PICK1 antagonism40, elevated levels of GluA2-lacking AMPARs after the removal of GluA2-containing AMPARs11, and the importance of PFC to NAc glutamatergic transmission in addiction-like behavior7,41,51. Very little of this work has been replicated in females, and it is not known if females rely on the same molecular pathways for PICK1-mediated plasticity as do males. Further, regions of the brain that differ in males and females often rely on distinct mechanisms52. We propose that the role of PICK1 as a homeostatic balance with GRIP is true in males but that in females additional processes are involved. Identifying the processes in females is complicated by the vast number of proteins to which PICK1 binds53. Work involving electrophysiology, behavior, binding assays, and protein quantification will be required to answer these open questions.
Acknowledgements
We thank Laura Rivera for assisting in running the behavioral experiments in this study.
Funding and Disclosure
This work was supported by National Institute on Drug Abuse (NIDA) Grant R00 DA033372 (L.A.B.), R01 DA DA047265 (L.A.B.), and T32 DA007273 (M.M.W., M.C.K.).
Footnotes
The authors declare no conflict of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
References
- 1.Administration SAaMHS. Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health. In: HHS, ed. Vol SMA 18–5068, NSDUH Series H-53. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2018. [Google Scholar]
- 2.State USDo. International Narcotics Control Strategy Report Volume I: Drug and Chemical Control. In: Affairs BfINaLE, ed. Vol 1. Washington, D.C.: United States Department of State; 2019:23. [Google Scholar]
- 3.Zhang T, Yanagida J, Kamii H, et al. Glutamatergic neurons in the medial prefrontal cortex mediate the formation and retrieval of cocaine-associated memories in mice. Addict Biol. 2019. [DOI] [PubMed] [Google Scholar]
- 4.Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56 Suppl 1:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin CB, Serchia MM, Shahin JR, Ruppert-Majer MA, Kippin TE, Szumlinski KK. Incubation of cocaine-craving relates to glutamate over-flow within ventromedial prefrontal cortex. Neuropharmacology. 2016;102:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park WK, Bari AA, Jey AR, et al. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22(7):2916–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45(5):647–650. [DOI] [PubMed] [Google Scholar]
- 8.Ghasemzadeh MB, Vasudevan P, Giles C, Purgianto A, Seubert C, Mantsch JR. Glutamatergic plasticity in medial prefrontal cortex and ventral tegmental area following extended-access cocaine self-administration. Brain Res. 2011;1413:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton MA, Schmidt EF, Choi KH, et al. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421(6918):70–75. [DOI] [PubMed] [Google Scholar]
- 10.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31(15):5737–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conrad KL, Tseng KY, Uejima JL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu W, Ziff EB. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. 2005;47(3):407–421. [DOI] [PubMed] [Google Scholar]
- 13.Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5(1):20–25. [DOI] [PubMed] [Google Scholar]
- 14.Briand LA, Kimmey BA, Ortinski PI, Huganir RL, Pierce RC. Disruption of glutamate receptor-interacting protein in nucleus accumbens enhances vulnerability to cocaine relapse. Neuropsychopharmacology. 2014;39(3):759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorgen J, Egbenya DL, Hammer J, Davanger S. PICK1 facilitates lasting reduction in GluA2 concentration in the hippocampus during chronic epilepsy. Epilepsy Res. 2017;137:25–32. [DOI] [PubMed] [Google Scholar]
- 16.Wickens MM, Deutschmann AU, McGrath AG, Parikh V, Briand LA. Glutamate receptor interacting protein acts within the prefrontal cortex to blunt cocaine seeking. Neuropharmacology. 2019;157:107672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Garcia E, Courtin J, Renault P, et al. Frequency of cocaine self-administration influences drug seeking in the rat: optogenetic evidence for a role of the prelimbic cortex. Neuropsychopharmacology. 2014;39(10):2317–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl). 2003;168(1–2):57–65. [DOI] [PubMed] [Google Scholar]
- 19.Palombo P, Leao RM, Bianchi PC, de Oliveira PEC, Planeta CDS, Cruz FC. Inactivation of the Prelimbic Cortex Impairs the Context-Induced Reinstatement of Ethanol Seeking. Front Pharmacol. 2017;8:725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31(5):903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zavala AR, Weber SM, Rice HJ, Alleweireldt AT, Neisewander JL. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain Res. 2003;990(1–2):157–164. [DOI] [PubMed] [Google Scholar]
- 22.Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends in pharmacological sciences. 2004;25(5):273–279. [DOI] [PubMed] [Google Scholar]
- 23.Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl). 2000;152(2):132–139. [DOI] [PubMed] [Google Scholar]
- 24.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl). 2005;179(3):662–672. [DOI] [PubMed] [Google Scholar]
- 26.Fosnocht AQ, Lucerne KE, Ellis AS, Olimpo NA, Briand LA. Adolescent social isolation increases cocaine seeking in male and female mice. Behav Brain Res. 2019;359:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGrath AG, Lenz JD, Briand LA. PKMζ in the nucleus accumbens acts to dampen cocaine seeking. Neuropsychopharmacology. 2018;43(12):2390–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Xia J. Structure and function of PICK1. Neurosignals. 2006;15(4):190–201. [DOI] [PubMed] [Google Scholar]
- 29.Torres GE, Yao WD, Mohn AR, et al. Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron. 2001;30(1):121–134. [DOI] [PubMed] [Google Scholar]
- 30.Jensen KL, Sørensen G, Dencker D, et al. PICK1-Deficient Mice Exhibit Impaired Response to Cocaine and Dysregulated Dopamine Homeostasis. eNeuro. 2018;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Pietro NC, Mashhoon Y, Heaney C, Yager LM, Kantak KM. Role of dopamine D1 receptors in the prefrontal dorsal agranular insular cortex in mediating cocaine self-administration in rats. Psychopharmacology (Berl). 2008;200(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diano S, Naftolin F, Horvath TL. Gonadal steroids target AMPA glutamate receptor-containing neurons in the rat hypothalamus, septum and amygdala: A morphological and biochemical study. Endocrinology. 1997;138:778–789. [DOI] [PubMed] [Google Scholar]
- 33.Fischer SG, Ricci LA, Melloni RH. Repeated anabolic/androgenic steroid exposure during adolescence alters phosphate-activated glutaminase and glutamate receptor 1 (GluR1) subunit immunoreactivity in Hamster brain: correlation with offensive aggression. Behav Brain Res. 2007;180(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Souza DN, Harlan RE, Garcia MM. Modulation of glutamate receptor expression by gonadal steroid hormones in the rat striatum. Brain Res Bull. 2003;59(4):289–292. [DOI] [PubMed] [Google Scholar]
- 35.Packard MG, Cornell AH, Alexander GM. Rewarding affective properties of intra-nucleus accumbens injections of testosterone. Behav Neurosci. 1997;111(1):219–224. [DOI] [PubMed] [Google Scholar]
- 36.Chen R, Osterhaus G, McKerchar T, Fowler SC. The role of exogenous testosterone in cocaine-induced behavioral sensitization and plasmalemmal or vesicular dopamine uptake in castrated rats. Neurosci Lett. 2003;351(3):161–164. [DOI] [PubMed] [Google Scholar]
- 37.Menéndez-Delmestre R, Segarra AC. Testosterone is essential for cocaine sensitization in male rats. Physiol Behav. 2011;102(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29(5):929–942. [DOI] [PubMed] [Google Scholar]
- 39.Foradori CD, Werner SB, Sandau US, Clapp TR, Handa RJ. Activation of the androgen receptor alters the intracellular calcium response to glutamate in primary hippocampal neurons and modulates sarco/endoplasmic reticulum calcium ATPase 2 transcription. Neuroscience. 2007;149(1):155–164. [DOI] [PubMed] [Google Scholar]
- 40.Turner C, De Luca M, Wolfheimer J, Hernandez N, Madsen KL, Schmidt HD. Administration of a novel high affinity PICK1 PDZ domain inhibitor attenuates cocaine seeking in rats. Neuropharmacology. 2020;164:107901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson SM, Famous KR, Sadri-Vakili G, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11(3):344–353. [DOI] [PubMed] [Google Scholar]
- 43.Famous KR, Kumaresan V, Sadri-Vakili G, et al. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28(43):11061–11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGlinchey EM, James MH, Mahler SV, Pantazis C, Aston-Jones G. Prelimbic to Accumbens Core Pathway Is Recruited in a Dopamine-Dependent Manner to Drive Cued Reinstatement of Cocaine Seeking. J Neurosci. 2016;36(33):8700–8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl). 2001;154(3):301–310. [DOI] [PubMed] [Google Scholar]
- 46.Bechard AR, Hamor PU, Schwendt M, Knackstedt LA. The effects of ceftriaxone on cue-primed reinstatement of cocaine-seeking in male and female rats: estrous cycle effects on behavior and protein expression in the nucleus accumbens. Psychopharmacology (Berl). 2018;235(3):837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31(1):129–138. [DOI] [PubMed] [Google Scholar]
- 48.Baird TJ, Gauvin D. Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacol Biochem Behav. 2000;65(2):289–299. [DOI] [PubMed] [Google Scholar]
- 49.Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008;89(3):314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97(2):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luis C, Cannella N, Spanagel R, Kohr G. Persistent strengthening of the prefrontal cortex - nucleus accumbens pathway during incubation of cocaine-seeking behavior. Neurobiology of learning and memory. 2017;138:281–290. [DOI] [PubMed] [Google Scholar]
- 52.McCarthy MM, Pickett LA, VanRyzin JW, Kight KE. Surprising origins of sex differences in the brain. Horm Behav. 2015;76:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li YH, Zhang N, Wang YN, Shen Y, Wang Y. Multiple faces of protein interacting with C kinase 1 (PICK1): Structure, function, and diseases. Neurochem Int. 2016;98:115–121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


