Abstract
Significance: Fluorescent probes and mass spectrometry are the two most popular and complementary methods to quantify thiols in biological systems. In this review, we focus on the widely used and commercially available methods to detect and quantify thiols in living cells and the general approaches applied in mass spectrometry-based thiol quantification. We hope that this review can serve as a general guide for redox biologists who are interested in thiol species. Sulfur, one of the most important elements in living systems, contributes to every aspect of physiology and pathology. Thiols, including cysteine, homocysteine, glutathione, hydrogen sulfide, and hydropersulfides, are the main players in the redox biology system. Therefore, quantifying these thiol species in biological systems is one of the important steps to understand their roles in biology.
Recent Advances: Fluorescent probes and mass spectrometry-based methods have been developed to detect and/or quantify thiols in biological systems. Mass spectrometry-based methods have been the gold standard for metabolite quantification in cells. Fluorescent probes can directly detect or quantify thiol species in living cells with spatial and temporal resolutions. Additionally, organelle-specific fluorescent probes have been widely developed. These two methods are complementary to each other.
Critical Issues: Reliable quantification of thiol species using fluorescent probes remains challenging.
Future Directions: When developing fluorescent probes, we suggest using both the fluorescent probes and mass spectrometry-based thiol quantification methods to cross-check the results. In addition, we call on chemical biologists to move beyond qualitative probes and focus on probes that can provide quantitative results in live cells. These quantitative measurements based on fluorescent probes should be validated with mass spectrometry-based methods. More importantly, chemical biologists should make their probes accessible to the biology end users. Regarding mass spectrometry-based methods, quantification of the derivatized thiol specifies should fit into the general metabolomics workflow. Antioxid. Redox Signal. 36, 354–365.
Keywords: biothiols, fluorescent probes, mass spectrometry
Introduction
Sulfur, one of the most important elements in living systems, contributes to every aspect of physiology and pathology. Sulfur mostly exists in the forms of biothiols, such as cysteine (Cys) and methionine. Among the elements forming the 20 canonic amino acids, sulfur is the only element beyond the first 2 rows in the periodic table. With its rich electrons and relatively low electronegativity (similar to carbon), sulfur allows a few different redox states, which is crucial in maintaining the balance of redox homeostasis (86). Abnormal activities in the biological redox systems could be signs of potential pathological events, such as skin lesions, cancers, and cardiovascular diseases (69). It should be noted that selenocysteine, the 21st proteinogenic amino acid discovered, also has highly versatile redox chemistry and is not covered in this review.
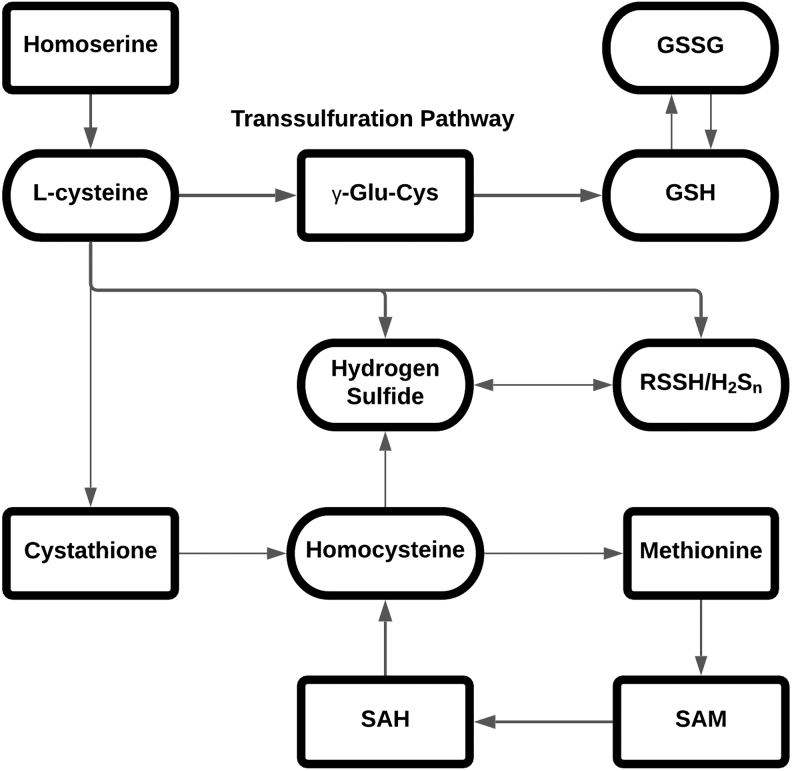
Among the nine essential amino acids for humans, methionine is the major source for sulfur and fed into multiple essential metabolic pathways, including the one-carbon metabolic network and glutathione (GSH) production. Methionine is catabolized by methionine adenosyltransferase 2A (MAT2A) to produce S-adenosyl-methionine (SAM). Methyltransferases (MTs) use SAM as a methyl donor, producing the product S-adenosyl-homocysteine (SAH), which can further act as a negative regulator of SAM-dependent processes. SAH can be further converted by adenosylhomocysteinase (AHCY) to homocysteine (Hcy), which can then either contribute to the transsulfuration pathway for cysteine and GSH synthesis or be converted back to methionine, thus completing the methionine cycle (75). Cysteine, homocysteine, and GSH are the three most popular and therefore most well-studied molecules in the thiol-related redox system. Besides those three molecules, this system contains other common thiols, including hydrogen sulfide (H2S) and hydropersulfides, which are all essential in maintaining redox homeostasis (4, 21, 25, 39, 69). Similar to NO, H2S has been proposed as a gasotransmitter and is promoting persulfidation of cysteine residues (12). Hydropersulfide, including RSSH on proteins and inorganic polysulfides, is not fully understood yet, but it is proposed that RSSH/RSS−/HSS− species are closely associated with the activity of important oxidases and reductases involved in redox homeostasis (4). In Figure 1, we provide a simplified view of the relationship among thiols in the redox biology system. The generation and metabolism of these molecules in turn are key events in maintaining redox homeostasis (69).
FIG. 1.
Simplified relationship between thiols in redox signaling system.
The concentrations of these thiol species are correlated with cellular redox status. Therefore, analytical methods have been developed to quantify these redox species. Liquid chromatography–mass spectrometry (LC-MS) has been the gold standard to quantify metabolites using targeted methods or qualitatively compare the changes of metabolomics using untargeted methods. However, LC-MS can only measure metabolites in lysed samples, which lose spatial and temporal resolutions. Fluorescence imaging has gained tremendous popularity to quantify thiols in living cells. Although a myriad of thiol probes have been developed, majority of them can only be used qualitatively. For low concentration metabolites, such as H2S, a qualitative on-off probe may be sufficient to reflect the signaling changes in biological systems. However, for high concentration metabolites in the mM range, such as GSH, quantitative probes are required to reflect changes in biology. Although there are many comprehensive reviews covering the chemistry progress on developing thiol probes, these probes are either not quantitative or not commercially available (12, 13, 19, 46, 97). Here in this review, we will primarily focus on the well-established and commercially available fluorescent probes and LC-MS methods for thiols and hope this will serve as a guide for biologists who are looking for methods they can conveniently apply in their research.
Cysteine/Homocysteine
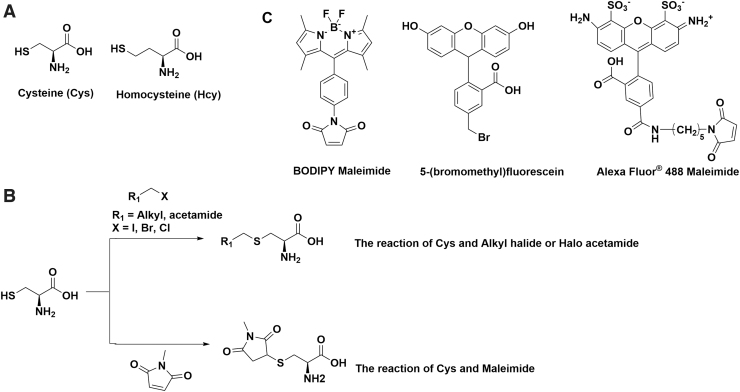
Cysteine, a key non-essential amino acid in the human body, is one of the least abundant, however, also one of the most important amino acids (Fig. 2A). It forms disulfide bonds and undergoes reversible oxidation or reduction, which is highly involved in protein folding events (66). Cysteine residues are always identified in the most conserved sites in protein structures. Studies also show that dysregulation of cysteine oxidation or reduction is highly related to diseases, such as liver damage and cardiovascular system dysfunction (28, 62). Homocysteine has a similar structure to Cys and is commonly targeted together due to the similarities they share in structures and chemical properties (Fig. 2A) (93). The concentration of Hcy is tightly regulated. High concentration of Hcy in cells has been associated with cognitive impairment, such as Alzheimer's disease (79, 80).
FIG. 2.
Biothiols and commercially available thiol probes. (A) Structure of Cys and Hcy. (B) Mechanism of cysteine reacting with maleimide and alkyl halide or haloacetamide. (C) Structures of commercially available fluorescent probes for thiols (mainly protein cysteine). Cys, cysteine; Hcy, homocysteine.
There are many commercially available fluorescent labeling kits for biothiols that are designed to label cysteine residues on proteins. A common strategy in those probes is to use maleimide or haloacetamide and connect with different fluorophores, such as BODIPY, Alexa Fluor, and fluorescein. Despite the distinct fluorescent and colorimetric properties, they are based on a similar reaction mechanism: the high reactivity of -SH to maleimide, alkyl halide, or haloacetamide (Fig. 2B) (2, 40). For example, Alexa Fluor 488 maleimide is a popular labeling reagent for cysteine and homocysteine with excitation at 495 nm and emission at 519 nm. It is commonly used for dye conjugation on solvent exposed cysteines in proteins (48). Maleimide can form a thioether after reacting with Cys or Hcy (Fig. 2C). Other long-wavelength dyes such as Alexa Fluor 647 and Alexa Fluor 750 are also incorporated with maleimide for in vivo imaging. The design of haloacetamide or alkyl halide probes employs the same concept, and the application is mostly overlapped. Cellular cysteine detection kits are also available with sensitivity down to the nanomolar level, from companies including Abcam, Thermo Fisher, and Sigma–Aldrich. However, the structures are undisclosed and most are designed for in vitro assays instead of live cells (41). It should be noted that because the fluorescence intensities of these dyes do not change upon reacting with thiols, they cannot be used to quantify thiols.
Other strategies such as forming disulfide bonds (thiol exchange) and nucleophilic reaction are also widely applied in the development of Cys probes. Ellman's reagent [5,5-dithiobis-(2-nitrobenzoic acid), or DTNB] (Fig. 3), first developed around 1961, is still widely employed in quantitating accessible protein thiols (20). It can react with free thiols and release TNB−, which can be quantified based on the absorbance at 412 nm (32). This strategy has been widely used and still one of the common approaches to design thiol labeling probes (18).
FIG. 3.
Ellman’ reagent structure and the reaction mechanism.
Because all the aforementioned probes and biochemical reactions leverage the high reactivity of the thiol groups in Cys and Hcy, it is very challenging to selectively quantify Cys and Hcy in the presence of other thiols, such as GSH. Intracellular concentrations for Cys and Hcy under physiological conditions are around 30–200 μM and 3–15 μM, respectively, which is much lower compared with the concentration of GSH (1–10 mM) (13, 66). There has been a great amount of research effort to develop Cys and/or Hcy selective fluorescent probes, taking advantage of the distance between the primary amine and the thiol groups (21, 29, 70, 76, 93, 94, 98). Although many innovative cysteine and/or homocysteine selective chemistry have been developed, there are still rooms for improvement. For example, most of the studies tested the probe selectivity with individual analytes outside their physiologically relevant concentrations. In cells, all the small-molecule thiols, including Cys, Hcy, and GSH, are present at the same time. Due to the overwhelmed concentration of GSH, these probes primarily react with GSH in cells. Another potential caveat for these multi-analyte responsive probes is that the change of one analyte concentration may inevitably lead to signal changes for other analytes due to the competition nature of the sensing mechanism. Therefore, these multi-analyte responsive probes indeed may provide misleading results. Cys and Hcy are the integral parts of the metabolic system in all organisms. To the best of our knowledge, quantitative live-cell probes or even biochemical assays for Cys and/or Hcy in the presence of large excess of GSH are still unavailable and should be the focus of future research in this area.
Glutathione
GSH is the most abundant small-molecule thiol in cellular environment (Fig. 4) (96). It serves as a main antioxidant and detoxification agent. GSH is synthesized from glutamate and cysteine. It can be oxidized into glutathione disulfide (GSSG), and the ratio of GSH/GSSG is proved to be essential in maintaining redox homeostasis (7, 55). Abnormal activities in GSH oxidation can be observed in many pathological events, such as cardiovascular disease and Parkinson's disease (78, 95). The increased ratio of GSH/GSSG is also closely associated with tumor growth (9, 85).
FIG. 4.
Structure of GSH and GSSG. GSH, glutathione; GSSG, glutathione disulfide.
Compared with other less abundant small-molecule thiols, the probe development for GSH is well established, considering the high abundancy of GSH in mammalian systems. Monochlorobimane (MCB) specifically reacts with GSH under the catalysis of glutathione-S-transferase (GST), leading to fluorescence increase upon the formation of MCB-GSH conjugate. As the reaction between MCB and GSH is irreversible, the GSH concentrations can be quantified either by scavenging all the GSH with excess MCB or by comparing the rate of fluorescence increase, which is proportional to the GSH concentration, assuming that intracellular GST activities remain the same (23, 57, 58). These irreversible reaction-based probes are suitable for single point quantification but would be difficult to follow the dynamic changes of GSH concentrations.
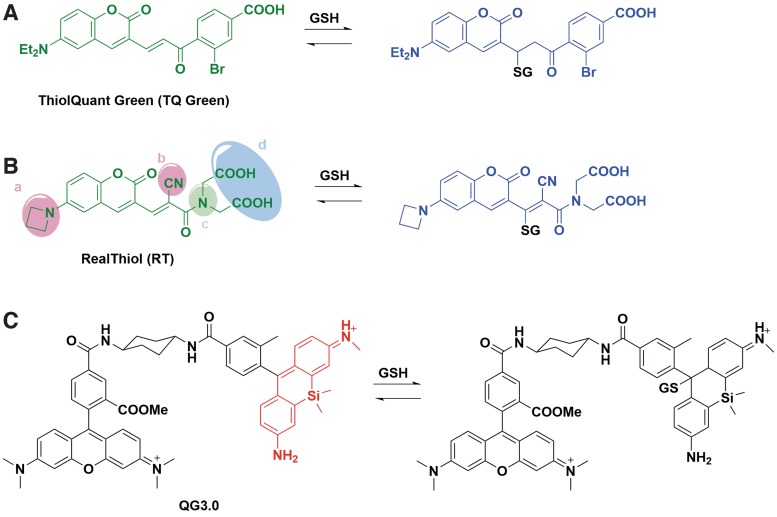
In 2015, our group reported the first reversible reaction-based ratiometric GSH probe—ThiolQuant Green (Fig. 5A)—that can accurately quantify GSH levels in live cells (37). We subsequently developed the second-generation probe, RealThiol (RT, Fig. 5B), which has a much improved reaction kinetics, quantum yield, and solubility, enabling quantitatively real-time monitoring of GSH level changes in living cells (35). Additionally, building on the RT probe, we developed organelle-specific GSH probes, including mitochondria-specific MitoRT (14) and Halotag-based HaloRT (38). RT, MitoRT, and HaloRT have been distributed to over 150 laboratories worldwide through Kerafast.com and applied extensively in redox biology studies (11, 56, 63, 65, 74, 99). There are several advantages for the reversible reaction-based GSH probes. Due to the ratiometric nature of the probes, the ratio readouts are independent of the probe concentrations in cells, requiring low μM or nM concentration of the probes to minimize perturbation to the tested biological system. Additionally, both dynamic increase and decrease of GSH concentrations can be quantitatively monitored using the RT probes due to their fast responses. With a proper calibration curve, the absolute GSH concentrations in cells can be quantified. There are also a few limitations associated with these probes. Although the RT series of probes are called GSH probes, they can also react with cysteine or other small-molecule thiols if they become the dominant thiol species in cells. In addition, tagging exogeneous molecules with GSH is a natural cellular defense mechanism to remove them through membrane transporters (8). The RT-GSH conjugate can be cleared by cells over time, and the rate of clearance is cell line and temperature dependent. The clearance of RT-GSH is slower at room temperature than at 37°C. We recently published a step-by-step protocol on how to use these RT probes with extensive discussion of advantages and limitations (36).
FIG. 5.
Reversible reaction-based ratiometric glutathione fluorescent probes. (A) Reaction of TQ Green with GSH. (B) Reaction of RT with GSH. Sites of modification: (a) enhance quantum yield; (b) accelerate reactions; (c) balance Kd; (d) increase solubility. (C) Reaction of QG3.0 with GSH. RT, RealThiol; TQ, ThiolQuant. Color images are available online.
Some other reversible reaction-based GSH probes have also been reported, which has been extensively covered by a recent review (84). The Urano group reported a reversible GSH probe (QG3.0, Fig. 5C) based on the rapid and reversible reaction between diarylcarbenium (benzhydrylium) ions and GSH (Kd = 3.0 mM) (87). The Yoon group independently developed a reversible GSH probe based on a similar coumarin-based structure to RT (Kd = 2.59 mM) (54). Jeong et al. (34) and Tian et al. (83) applied a red-shifted coumarin to develop FreSHtracer (Kd = 3.4 mM) and RP-2 (Kd = 1.61 mM) to quantify GSH in live cells, respectively. Ren et al. reported rhodol-hemicyanine-based GSH probe RdH (Kd = 1.42 mM) with a red-shifted excitation and emission wavelength (71). Khatun et al. reported a reversible probe GScp (Kd = 2.47 mM) that can measure GSH concentration in the nucleus (42). Liu et al. reported a BODIPY-based reversible GSH probe (Kd = 7.6 mM) (53). Zhang et al. further advanced BODIPY-based GSH probe and developed αBD-GSH (Kd = 1.55 mM) with significant red-shifted emission (the probe: Ex/Em 594/600–620 nm; the probe-GSH conjugate: Ex/Em: 594/660–690 nm) (100). It is interesting to note that αBD-GSH has a weak fluorescence in aqueous media but strong fluorescence in cells, possibly due to its nonspecific binding to intracellular proteins. Nonetheless, the ratiometric feature of αBD-GSH would allow its quantitative imaging of GSH concentrations in cells with a proper standard curve. In the past 6 years, reversible ratiometric probes that can quantify GSH concentrations and dynamics in live cells have advanced significantly and are expected to make a strong impact in redox biology research.
Hydrogen Sulfide
H2S is an endogenous gaseous signaling molecule, first discovered in the central nervous system in 1996 (Fig. 6A) (1). It is involved in many physiological and pathological functions, especially in cardiovascular system, carcinoma development, and inflammatory system (31, 77, 90). H2S is often considered a double-edged sword in cancer therapy. Higher level of H2S might exhibit anticancer effect, while lower concentration might provide the opposite effect (91). Three proteins, including cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3MST), are highly involved in the production of H2S in mammalian system (12).
FIG. 6.
Commercially available hydrogen sulfide probes. (A) Hydrogen sulfide. (B) Methylene blue method to detect H2S. (C) Structures of SF7-AM and WSP-5. H2S, hydrogen sulfide. Color images are available online.
As for the detection methods for H2S, there are not as many options for H2S compared with other thiols, such as Cys and GSH, for several reasons. First, the physiological concentration of H2S is only around 3 μM, and even lower (10–20 nM) in tissues, despite its essential role in maintaining redox homeostasis (12). Besides the low level of H2S, the intrinsic gaseous properties have also posed challenges for accurate quantification (46). The equilibrium of H2S is highly regulated by the pH value of the environment. Usually, when H2S is detected, it usually accounts for the total concentration of H2S, HS−, and S2− (22, 26, 27, 73). Due to the challenges in reliably measuring H2S concentrations, the actual level of H2S has long been debated (72).
The most common methods for H2S detection and/or quantification include gas chromatography–mass spectrometry (GC-MS), methylene blue, and fluorescent probes. Although the methylene blue method is commonly used, it suffers from low sensitivity and interference by other thiols (Fig. 6B) (60). GC-MS, on the contrary, can provide significantly improved sensitivity. However, the GC-MS methods usually require complicated sample preparation, which could impact data accuracy and reproducibility (88, 92). Small-molecule fluorescent probes for H2S are considered a promising method given that they can achieve improved chemical selectivity and be applied in live cells (45, 50). Taking advantage of azide as a H2S responsive moiety, the Chang group reported a fluorescent probe that can image H2S in live cells (49). The Xian group reported another type of H2S fluorescent probe by applying a disulfide exchange reaction, followed by a H2S-mediated benzodithiolone formation (51). The Pluth group discovered that both azide and nitro groups can be applied to develop H2S probes (61). The Wang group also reported a sulfonyl azide-based fluorescent probe that can quantify H2S in blood within seconds (68, 89). Building on these H2S-responsive groups, chemiluminescent-based, two-photon imaging, and organelle specific H2S probes were also developed (5, 6, 10, 15). Some of these H2S probes, such as SF7-AM and WSP-5 (Fig. 6C), are commercially available through Cayman Chemical (47, 67).
Hydropersulfide
Hydropersulfide belongs to sulfane sulfur species and is often discussed together with H2S for the equilibrium it forms with H2S in mammalian cells (Fig. 7A) (4). It often exists in the form of protein-bound persulfide (RSSH). Inorganic polysulfides (H2Sn, n > 1) are also becoming prevalent with increasing recognition of their biological roles in recent years (25). Hydropersulfides can be generated in various ways. The two common proteins involved are CSE and CBS (59). Although hydropersulfides are closely related to H2S (Fig. 7A), it plays distinct yet important roles in cells. RSSH might be the main signaling molecule downstream of H2S (64). Additionally, it is highly involved in cysteine persulfidation (24) and serves as a better nucleophile and reductant than other thiols in redox biology (64). With all the significant functions identified for RSSH, there is an increasing need for the development of RSSH fluorescent probes.
FIG. 7.
Reaction-based persulfide probes. (A) The equilibrium between H2S and hydropersulfide. (B) Reaction of SSP2 with sulfane sulfur compounds.
Various mechanisms have been explored to deliver selective fluorescent probes for RSSH from aspects of higher nucleophilicity, sulfane sulfur attachment (17, 50, 82). The first fluorescent probe for hydropersulfides was developed by the Xian group (SSP2, Fig. 7B) (16). It was designed for all sulfane sulfur compounds including hydropersulfides. The probe can be “turned” on by releasing the fluorophore after intramolecular attack of persulfide (R-SS−). Although this area is progressing prosperously, it is still at an early phase of developing a widely applicable fluorescent probe for RSSH. The hyperreactive features of all sulfane sulfur compounds and low cellular level pose some challenges (25). Probes with higher selectivity and sensitivity are still highly expected and call for more dedicated effort. Mass spectrometry is commonly applied to detect hydropersulfides and protein-bound persulfides in many studies, which will be discussed thoroughly in the following section (81).
Mass Spectrometry as a Common Method for Thiol Detection
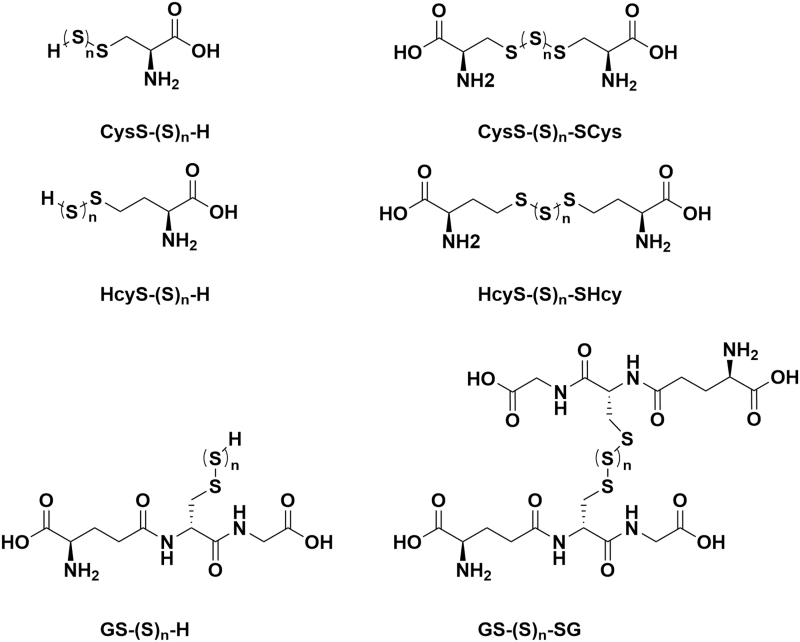
The fluorescence-based method can measure the reactive sulfur species and provide specific information, and high selectivity is often expected. However, liquid chromatography–electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) can offer profiling at the metabolome scale and provide an alternative way to measure sulfur species. LC-ESI-MS/MS can identify the sulfur species based on retention times and mass spectra. LC-MS method can measure not only the reactive sulfur species, including sulfane sulfur, persulfide, polysulfide, but also the whole metabolome at the same time. As we mentioned previously, the reactive sulfane sulfur compounds in biological samples include persulfides (R–S–SH), hydrogen polysulfides (H2Sn, n > 1), and polysulfides (R–S–Sn–S–R), as shown in Figure 8 (33).
FIG. 8.
Chemical structures of common polysulfides.
The Xian group has been a pioneer in measuring sulfane sulfurs. One of their signature works is the development of an isotope dilution mass spectrometry for natural sulfane sulfur compounds (52). This method used triarylphosphine reagents (P2) to react with sulfane sulfurs to form phosphine sulfides, as shown in Figure 9A. Then, an isotope-labeled phosphine sulfide PS2 was spiked into the samples as an internal standard to help to determine the accurate concentration of the sulfane sulfurs. The method can derive both hydroxy polysulfides and polysulfides, thus measure the total sulfane sulfur concentration, but it cannot discriminate individual sulfane sulfur species. In addition, the method highly favored sulfane sulfurs over disulfides and biothiols because triarylphosphine cannot react with disulfides and biothiols to form phosphine sulfide.
FIG. 9.
Mass spec probes for persulfides. (A) Reaction of P2 with sulfane sulfur compounds. (B) Reaction of monobromobimane with hydropersulfide. (C) The method of HPE-IAM for hydropersulfide. HPE-IAM: β-(4-hydroxyphenyl)ethyl iodoacetamide.
To measure the individual free thiol metabolites, many different methods have been developed to derivatize the reduced thiol compound with an electrophile to form a stable adduct since the reactive species are metastable. The common derivatization reagents comprise four different classes. The first group of reagents contains iodo groups such as iodoacetic acid (IAA), iodoacetamide (IAM), quaternary ammonium-based iodoacetamide, 2-iodoacetanilide, similar strategy as cysteine fluorescent probes. The Chang group employed 2-iodoacetanilide to quantify thiol metabolites including Cys, Hcy, and GSH (44). This derivatization shows excellent stability at alkaline conditions and great sensitivity for ESI-MS detection. Their result shows 10 nM to 1 μM linear range for measurement of homocysteine and GSH and 1 nM to 1 μM linear range for l-cysteine and H2S (44).
The second group of reagents contains amide functional groups that include N-ethylmaleimide (NEM) or N-methylmaleimide (NMM). Dr. Feelisch's group established a mass spectrometry platform for a comprehensive assessment of the thiol redox metabolome (81). NEM was used as an alkylating agent for thiols and sulfide due to its fast reaction rate. They identified 12 thiol metabolites including GSH, homocysteine, and cysteine. But NEM has limitations to detect per- and polysulfides.
The third derivatization method is to use monobromobimane (Br-bimane) to alkylate reduced sulfides and polysulfides such as perhydropersulfide (GSSH) and trihydropersulfide (GSSSH). The principle is illustrated in Figure 9B. Based on this method, Dr. Akaike's group identified and quantified 13 hydropolysulfides and 11 oxidized polysulfides in various cells and mouse tissues (micromolar and submicromolar range) (33). They could detect down to pM range polysulfides for neat standards. In addition, they analyzed persulfides and polysulfides in mouse heart, liver, brain, and lung, and A549 cells and the concentration is in micromolar and submicromolar range.
Interestingly, a report showed that cysteine persulfide/polysulfides [CysSSH/CysS–(S)n–H] have both electrophilic and nucleophilic activity, which is different from other simpler biologically relevant thiols (3). They found the strong electrophiles such as NEM or IAM or MBB that can cause degradation of newly formed polysulfide adduct (derivatives). They selected a mild electrophilicity—β-(4-hydroxyphenyl)ethyl iodoacetamide (HPE-IAM) as a reacting agent, which can label specific hydropolysulfides with the least artificial decomposition caused by the dual nucleophilic and electrophilic character (Fig. 9C). They reported that the hydroxyphenyl-containing compound can inhibit alkaline hydrolysis and could have a potent polysulfide-stabilizing effect (30). They identified and quantified 17 per/polysulfide species in cysteine (CysSH), CysS-(S)n-H, and other related sulfide compounds and considered HPE-IAM the most suitable agent, among 25 electrophilic alkylating agents, due to less polysulfide degradation. In the meanwhile, it is worth mentioning that the interconversion among the sulfur-rich RSS is very dynamic and fast. The electrophilic labeling experiment is controlled by the Curtin–Hammett principle, which indicates that the barrier of reaction between polysulfides and alkylating reagents are much higher than that of polysulfide species interconversion, as discussed by the Pluth group (43). Therefore, measured polysulfides show merely a snapshot of the rapid interconversion of polysulfides and risk not reflecting the true equilibrium distributions. It was suggested that measuring the total sulfane sulfur content can be a more robust method rather than reporting individual per/polysulfides.
LC-MS-based method can enable us to not only accurately quantify the reactive thiol metabolites but even the whole pathway molecules. The high-resolution mass spectrometry can even allow us to high-throughput profile the whole organism metabolites by untargeted mass spectrometry technology against standard library or in silico MS/MS library. But the LC-MS method is expensive and is difficult to provide the spatial and temporal states of the thiol metabolites.
Conclusions
Thiol-related redox biology has been a fascinating area of research. The fluorescent probes and mass spectrometry-based thiol quantification methods have their own pros and cons. Fluorescent probes can directly detect or quantify thiol species in living cells with spatial and temporal resolutions. Additionally, organelle-specific fluorescent probes have been widely developed. However, reliable quantification of thiol species using fluorescent probes remains challenging. In contrast, mass spectrometry-based methods have been the gold standard for metabolite quantification in cells, although lysed samples have to be used, leading to the loss of spatial information for metabolite distribution. We suggest using both the fluorescent probes and mass spectrometry-based thiol quantification methods to cross-check the results. In addition, we call on chemical biologists to move beyond qualitative probes and focus on probes that can provide quantitative results in live cells. These quantitative measurements based on fluorescent probes should be validated with mass spectrometry-based methods, which is usually missing in majority of the publications. More importantly, chemical biologists should make their probes accessible to the biology end users, either through material transfer agreement (MTA) or reagent sharing website, such as Kerafast.com. Regarding mass spectrometry-based methods, quantification of the derivatized thiol specifies should fit into the general metabolomics workflow. We hope that this review serves as a general guide for redox biologists who are interested in studying thiol species.
Abbreviations Used
- CBS
cystathionine β-synthase
- CSE
cystathionine γ-lyase
- Cys
cysteine
- GC-MS
gas chromatography–mass spectrometry
- GSH
glutathione
- GSSG
glutathione disulfide
- GST
glutathione-S-transferase
- H2S
hydrogen sulfide
- Hcy
homocysteine
- HPE-IAM
β-(4-hydroxyphenyl)ethyl iodoacetamide
- IAM
iodoacetamide
- LC-ESI-MS/MS
liquid chromatography–electrospray ionization tandem mass spectrometry
- LC-MS
liquid chromatography–mass spectrometry
- MCB
monochlorobimane
- NEM
N-ethylmaleimide
- RT
RealThiol
- SAH
S-adenosyl-homocysteine
- SAM
S-adenosyl-methionine
Author Disclosure Statement
J.W., X.J., and J.C. are the co-inventors of a patent related to the GSH probes mentioned in this article. Additionally, J.W. is the co-founder of CoActigon, Inc., and Chemical Biology Probes, LLC. All other authors have no competing financial interests exist.
Funding Information
The work was supported in part by the National Institute of Health (R01GM115622 to J.W.), the Welch Foundation (Q1912 to M.C.W.), Howard Hughes Medical Institute (to M.C.W.), and the Michael E. DeBakey, M.D., Professorship in Pharmacology (to J.W.).
References
- 1. Abe K and Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aitken A and Learmonth M. Carboxymethylation of cysteine using iodoacetamide/iodoacetic acid. In: The Protein Protocols Handbook, edited by Walker JM. Totowa, NJ: Humana Press, 2002, pp. 455–456. [Google Scholar]
- 3. Akaike T, Ida T, Wei F-Y, Nishida M, Kumagai Y, Alam MM, Ihara H, Sawa T, Matsunaga T, Kasamatsu S, Nishimura A, Morita M, Tomizawa K, Nishimura A, Watanabe S, Inaba K, Shima H, Tanuma N, Jung M, Fujii S, Watanabe Y, Ohmuraya M, Nagy P, Feelisch M, Fukuto JM, and Motohashi H. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat Commun 8: 1177, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Álvarez L, Bianco CL, Toscano JP, Lin J, Akaike T, and Fukuto JM. Chemical biology of hydropersulfides and related species: possible roles in cellular protection and redox signaling. Antioxid Redox Signal 27: 622–633, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Bae SK, Heo CH, Choi DJ, Sen D, Joe E-H, Cho BR, and Kim HM. A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in Parkinson's disease gene knockout astrocytes. J Am Chem Soc 135: 9915–9923, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Bailey TS and Pluth MD. Chemiluminescent detection of enzymatically produced hydrogen sulfide: substrate hydrogen bonding influences selectivity for H2S over biological thiols. J Am Chem Soc 135: 16697–16704, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bajic VP, Van Neste C, Obradovic M, Zafirovic S, Radak D, Bajic VB, Essack M, and Isenovic ER. Glutathione “redox homeostasis” and its relation to cardiovascular disease. Oxid Med Cell Longev 2019: e5028181, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ballatori N, Krance SM, Marchan R, and Hammond CL. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol Aspects Med 30: 13–28, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, and Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem 390: 191–214, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao J, Lopez R, Thacker JM, Moon JY, Jiang C, Morris SNS, Bauer JH, Tao P, Mason RP, and Lippert AR. Chemiluminescent probes for imaging H2S in living animals. Chem Sci 6: 1979–1985, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao JY, Poddar A, Magtanong L, Lumb JH, Mileur TR, Reid MA, Dovey CM, Wang J, Locasale JW, Stone E, Cole SPC, Carette JE, and Dixon SJ. A genome-wide haploid genetic screen identifies regulators of glutathione abundance and ferroptosis sensitivity. Cell Rep 26: 1544.e8–1556.e8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao X, Ding L, Xie Z, Yang Y, Whiteman M, Moore PK, and Bian J-S. A review of hydrogen sulfide synthesis, metabolism, and measurement: is modulation of hydrogen sulfide a novel therapeutic for cancer? Antioxid Redox Signal 31: 1–38, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen D and Feng Y.. Recent progress of glutathione (GSH) specific fluorescent probes: molecular design, photophysical property, recognition mechanism and bioimaging. Crit Rev Anal Chem 2020. [Epub ahead of print]; DOI: 10.1080/10408347.2020.1819193. [DOI] [PubMed] [Google Scholar]
- 14. Chen J, Jiang X, Zhang C, MacKenzie KR, Stossi F, Palzkill T, Wang MC, and Wang J. Reversible reaction-based fluorescent probe for real-time imaging of glutathione dynamics in mitochondria. ACS Sens 2: 1257–1261, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen J, Zhao M, Jiang X, Sizovs A, Wang MC, Provost CR, Huang J, and Wang J. Genetically anchored fluorescent probes for subcellular specific imaging of hydrogen sulfide. Analyst 141: 1209–1213, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen W, Liu C, Peng B, Zhao Y, Pacheco A, and Xian M. New fluorescent probes for sulfane sulfurs and the application in bioimaging. Chem Sci 4: 2892, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen W, Pacheco A, Takano Y, Day JJ, Hanaoka K, and Xian M. A single fluorescent probe to visualize hydrogen sulfide and hydrogen polysulfides with different fluorescence signals. Angew Chem 128: 10147–10150, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chevallet M, Wagner E, Luche S, van Dorsselaer A, Leize-Wagner E, and Rabilloud T. Regeneration of peroxiredoxins during recovery after oxidative stress. J Biol Chem 278: 37146–37153, 2003. [DOI] [PubMed] [Google Scholar]
- 19. Dai J, Ma C, Zhang P, Fu Y, and Shen B. Recent progress in the development of fluorescent probes for detection of biothiols. Dyes Pigm 177: 108321, 2020. [Google Scholar]
- 20. Ellman GL, Courtney KD, Andres V, and Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7: 88–95, 1961. [DOI] [PubMed] [Google Scholar]
- 21. Feng B, Liu Y, Huang S, Huang X, Huang L, Liu M, Wu J, Du T, Wang S, Feng X, and Zeng W. Highly selective discrimination of cysteine from glutathione and homo-cysteine with a novel AIE-ESIPT fluorescent probe. Sens Actuators B Chem 325: 128786, 2020. [Google Scholar]
- 22. Francioso A, Baseggio Conrado A, Mosca L, and Fontana M. Chemistry and biochemistry of sulfur natural compounds: key intermediates of metabolism and redox biology. Oxid Med Cell Longev 2020: 1–27, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fricker MD, May M, Meyer AJ, Sheard N, and White NS. Measurement of glutathione levels in intact roots of Arabidopsis. J Microsc 198: 162–173, 2000. [DOI] [PubMed] [Google Scholar]
- 24. Fu L, Liu K, He J, Tian C, Yu X, and Yang J. Direct proteomic mapping of cysteine persulfidation. Antioxid Redox Signal 33: 1061–1076, 2019. [DOI] [PubMed] [Google Scholar]
- 25. Fukuto JM, Ignarro LJ, Nagy P, Wink DA, Kevil CG, Feelisch M, Cortese-Krott MM, Bianco CL, Kumagai Y, Hobbs AJ, Lin J, Ida T, and Akaike T. Biological hydropersulfides and related polysulfides—a new concept and perspective in redox biology. FEBS Lett 592: 2140–2152, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Furne J, Saeed A, and Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol 295: R1479–R1485, 2008. [DOI] [PubMed] [Google Scholar]
- 27. Giuffrè A and Vicente JB. Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxid Med Cell Longev 2018: e6290931, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Go Y-M and Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med 50: 495–509, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guo J, Kuai Z, Zhang Z, Yang Q, Shan Y, and Li Y. A simple colorimetric and fluorescent probe with high selectivity towards cysteine over homocysteine and glutathione. RSC Adv 7: 18867–18873, 2017. [Google Scholar]
- 30. Hamid HA, Tanaka A, Ida T, Nishimura A, Matsunaga T, Fujii S, Morita M, Sawa T, Fukuto JM, Nagy P, Tsutsumi R, Motohashi H, Ihara H, and Akaike T. Polysulfide stabilization by tyrosine and hydroxyphenyl-containing derivatives that is important for a reactive sulfur metabolomics analysis. Redox Biol 21: 101096, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hellmich MR and Szabo C. Hydrogen sulfide and cancer. Handb Exp Pharmacol 230: 233–241, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol 233: 380–385, 1994. [DOI] [PubMed] [Google Scholar]
- 33. Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, and Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci U S A 111: 7606–7611, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jeong EM, Yoon J-H, Lim J, Shin J-W, Cho AY, Heo J, Lee KB, Lee J-H, Lee WJ, Kim H-J, Son YH, Lee S-J, Cho S-Y, Shin D-M, Choi K, and Kim I-G. Real-time monitoring of glutathione in living cells reveals that high glutathione levels are required to maintain stem cell function. Stem Cell Rep 10: 600–614, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang X, Chen J, Bajić A, Zhang C, Song X, Carroll SL, Cai Z-L, Tang M, Xue M, Cheng N, Schaaf CP, Li F, MacKenzie KR, Ferreon ACM, Xia F, Wang MC, Maletić-Savatić M, and Wang J. Quantitative real-time imaging of glutathione. Nat Commun 8: 16087, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang X, Chen J, Wang MC, and Wang J. Glutathione quantification in live cells with real-time imaging and flow cytometry. STAR Protoc 1: 100170, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang X, Yu Y, Chen J, Zhao M, Chen H, Song X, Matzuk AJ, Carroll SL, Tan X, Sizovs A, Cheng N, Wang MC, and Wang J. Quantitative imaging of glutathione in live cells using a reversible reaction-based ratiometric fluorescent probe. ACS Chem Biol 10: 864–874, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang X, Zhang C, Chen J, Choi S, Zhou Y, Zhao M, Song X, Chen X, Maletic-Savatic M, Palzkill T, Moore D, Wang MC, and Wang J. Quantitative real-time imaging of glutathione with subcellular resolution. Antioxid Redox Signal 30: 1900–1910, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kabil O, Motl N, and Banerjee R. H2S and its role in redox signaling. Biochim Biophys Acta Proteins Proteom 1844: 1355–1366, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalia J and Raines RT. Catalysis of imido group hydrolysis in a maleimide conjugate. Bioorg Med Chem Lett 17: 6286–6289, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kar S, Shahshahan HR, Kambis TN, Yadav SK, Li Z, Lefer DJ, and Mishra PK. Hydrogen sulfide ameliorates homocysteine-induced cardiac remodeling and dysfunction. Front Physiol 10: 598, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khatun S, Yang S, Zhao YQ, Lu Y, Podder A, Zhou Y, and Bhuniya S. Highly chemoselective self-calibrated fluorescent probe monitors glutathione dynamics in nucleolus in live cells. Anal Chem 92: 10989–10995, 2020. [DOI] [PubMed] [Google Scholar]
- 43. Lau N and Pluth MD. Reactive sulfur species (RSS): persulfides, polysulfides, potential, and problems. Curr Opin Chem Biol 49: 1–8, 2019. [DOI] [PubMed] [Google Scholar]
- 44. Lee D-Y, Huang W-C, Gu T-J, and Chang G-D. Quantitative and comparative liquid chromatography-electrospray ionization-mass spectrometry analyses of hydrogen sulfide and thiol metabolites derivaitized with 2-iodoacetanilide isotopologues. J Chromatogr A 1552: 43–52, 2018. [DOI] [PubMed] [Google Scholar]
- 45. Li H, Fang Y, Yan J, Ren X, Zheng C, Wu B, Wang S, Li Z, Hua H, Wang P, and Li D. Small-molecule fluorescent probes for H2S detection: advances and perspectives. Trends Analyt Chem 134: 116117, 2021. [Google Scholar]
- 46. Lin VS, Chen W, Xian M, and Chang CJ. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem Soc Rev 44: 4596–4618, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin VS, Lippert AR, and Chang CJ. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc Natl Acad Sci U S A 110: 7131–7135, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lindhoud S, Westphal AH, Visser AJWG, Borst JW, and van Mierlo CPM. Fluorescence of alexa fluor dye tracks protein folding. PLoS One 7: e46838, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lippert AR, New EJ, and Chang CJ. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J Am Chem Soc 133: 10078–10080, 2011. [DOI] [PubMed] [Google Scholar]
- 50. Liu C, Chen W, Shi W, Peng B, Zhao Y, Ma H, and Xian M. Rational design and bioimaging applications of highly selective fluorescence probes for hydrogen polysulfides. J Am Chem Soc 136: 7257–7260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu C, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, and Xian M. Capture and visualization of hydrogen sulfide by a fluorescent probe. Angew Chem Int Ed 50: 10327–10329, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu C, Zhang F, Munske G, Zhang H, and Xian M. Isotope dilution mass spectrometry for the quantification of sulfane sulfurs. Free Radic Biol Med 76: 200–207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu H, Song W, Zhang S, Shing Chan K, Guo Z, and Shen Z. A ratiometric fluorescent probe for real-time monitoring of intracellular glutathione fluctuations in response to cisplatin. Chem Sci 11: 8495–8501, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu Z, Zhou X, Miao Y, Hu Y, Kwon N, Wu X, and Yoon J. A reversible fluorescent probe for real-time quantitative monitoring of cellular glutathione. Angew Chem Int Ed 56: 5812–5816, 2017. [DOI] [PubMed] [Google Scholar]
- 55. Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 2012: e736837, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mendiola AS, Ryu JK, Bardehle S, Meyer-Franke A, Ang KK-H, Wilson C, Baeten KM, Hanspers K, Merlini M, Thomas S, Petersen MA, Williams A, Thomas R, Rafalski VA, Meza-Acevedo R, Tognatta R, Yan Z, Pfaff SJ, Machado MR, Bedard C, Rios Coronado PE, Jiang X, Wang J, Pleiss MA, Green AJ, Zamvil SS, Pico AR, Bruneau BG, Arkin MR, and Akassoglou K. Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat Immunol 21: 513–524, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meyer AJ and Fricker MD. Direct measurement of glutathione in epidermal cells of intact Arabidopsis roots by two-photon laser scanning microscopy. J Microsc 198: 174–181, 2000. [DOI] [PubMed] [Google Scholar]
- 58. Meyer AJ, May MJ, and Fricker M. Quantitative in vivo measurement of glutathione in Arabidopsis cells. Plant J 27: 67–78, 2001. [DOI] [PubMed] [Google Scholar]
- 59. Millikin R, Bianco CL, White C, Saund SS, Henriquez S, Sosa V, Akaike T, Kumagai Y, Soeda S, Toscano JP, Lin J, and Fukuto JM. The chemical biology of protein hydropersulfides: studies of a possible protective function of biological hydropersulfide generation. Free Radic Biol Med 97: 136–147, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moest RR. Hydrogen sulfide determination by the methylene blue method. Anal Chem 47: 1204–1205, 1975. [Google Scholar]
- 61. Montoya LA and Pluth MD. Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem Commun 48: 4767–4769, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morgan MY, Marshall AW, Milsom JP, and Sherlock S. Plasma amino-acid patterns in liver disease. Gut 23: 362–370, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Oberstadt M, Stieler J, Simpong DL, Römuß U, Urban N, Schaefer M, Arendt T, and Holzer M. TDP-43 self-interaction is modulated by redox-active compounds Auranofin, Chelerythrine and Riluzole. Sci Rep 8: 2248, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ono K, Akaike T, Sawa T, Kumagai Y, Wink DA, Tantillo DJ, Hobbs AJ, Nagy P, Xian M, Lin J, and Fukuto JM. The redox chemistry and chemical biology of H2S, hydropersulfides and derived species: implications to their possible biological activity and utility. Free Radic Biol Med 77: 82–94, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Padmanabhan A, Kaushik M, Niranjan R, Richards JS, Ebright B, and Venkatasubbu GD. Zinc oxide nanoparticles induce oxidative and proteotoxic stress in ovarian cancer cells and trigger apoptosis independent of p53-mutation status. Appl Surf Sci 487: 807–818, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Paulsen CE and Carroll KS. Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chem Rev 113: 4633–4679, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peng B, Chen W, Liu C, Rosser EW, Pacheco A, Zhao Y, Aguilar HC, and Xian M. Fluorescent probes based on nucleophilic substitution-cyclization for hydrogen sulfide detection and bioimaging. Chemistry 20: 1010–1016, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peng H, Cheng Y, Dai C, King AL, Predmore BL, Lefer DJ, and Wang B. A fluorescent probe for fast and quantitative detection of hydrogen sulfide in blood. Angew Chem Int Ed 50: 9672–9675, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Poole LB. The basics of thiols and cysteines in redox biology and chemistry. Free Radic Biol Med 80: 148–157, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Qi Y, Huang Y, Li B, Zeng F, and Wu S. Real-time monitoring of endogenous cysteine levels in vivo by near-Infrared turn-on fluorescent probe with large stokes shift. Anal Chem 90: 1014–1020, 2018. [DOI] [PubMed] [Google Scholar]
- 71. Ren M, Wang L, Lv X, Sun Y, Chen H, Zhang K, Wu Q, Bai Y, and Guo W. A rhodol-hemicyanine based ratiometric fluorescent probe for real-time monitoring of glutathione dynamics in living cells. Analyst 144: 7457–7462, 2019. [DOI] [PubMed] [Google Scholar]
- 72. Roberts ES, Wong VA, McManus BE, Marshall MW, Lancianese S, and Dorman DC. Changes in intracellular pH play a secondary role in hydrogen sulfide-induced nasal cytotoxicity. Inhal Toxicol 18: 159–167, 2006. [DOI] [PubMed] [Google Scholar]
- 73. Rose P, Moore PK, and Zhu YZ. H2S biosynthesis and catabolism: new insights from molecular studies. Cell Mol Life Sci 74: 1391–1412, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rothmiller S, Schröder S, Strobelt R, Wolf M, Wang J, Jiang X, Worek F, Steinritz D, Thiermann H, and Schmidt A. Sulfur mustard resistant keratinocytes obtained elevated glutathione levels and other changes in the antioxidative defense mechanism. Toxicol Lett 293: 51–61, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sanderson SM, Gao X, Dai Z, and Locasale JW. Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat Rev Cancer 19: 625–637, 2019. [DOI] [PubMed] [Google Scholar]
- 76. Shen B and Qian Y. Red emission cysteine probe with high selectivity based on fluorescent protein chromophores and turn-on fluorescence in cell cultures. Dyes Pigm 166: 350–356, 2019. [Google Scholar]
- 77. Shen Y, Shen Z, Luo S, Guo W, and Zhu YZ. The cardioprotective effects of hydrogen sulfide in heart diseases: from molecular mechanisms to therapeutic potential. Oxid Med Cell Longev 2015: e925167, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shimizu H, Kiyohara Y, Kato I, Kitazono T, Tanizaki Y, Kubo M, Ueno H, Ibayashi S, Fujishima M, and Iida M. Relationship between plasma glutathione levels and cardiovascular disease in a defined population: the Hisayama study. Stroke 35: 2072–2077, 2004. [DOI] [PubMed] [Google Scholar]
- 79. Smith AD and Refsum H. Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr 36: 211–239, 2016. [DOI] [PubMed] [Google Scholar]
- 80. Smith AD, Refsum H, Bottiglieri T, Fenech M, Hooshmand B, McCaddon A, Miller JW, Rosenberg IH, and Obeid R. Homocysteine and dementia: an international consensus statement. J Alzheimers Dis 62: 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sutton TR, Minnion M, Barbarino F, Koster G, Fernandez BO, Cumpstey AF, Wischmann P, Madhani M, Frenneaux MP, Postle AD, Cortese-Krott MM, and Feelisch M. A robust and versatile mass spectrometry platform for comprehensive assessment of the thiol redox metabolome. Redox Biol 16: 359–380, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Takano Y, Echizen H, and Hanaoka K. Fluorescent probes and selective inhibitors for biological studies of hydrogen sulfide- and polysulfide-mediated signaling. Antioxid Redox Signal 27: 669–683, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tian M, Liu X-Y, He H, Ma X-Z, Liang C, Liu Y, and Jiang F-L. Real-time imaging of intracellular glutathione levels based on a ratiometric fluorescent probe with extremely fast response. Anal Chem 92: 10068–10075, 2020. [DOI] [PubMed] [Google Scholar]
- 84. Tian M, Liu Y, and Jiang F-L. On the route to quantitative detection and real-time monitoring of glutathione in living cells by reversible fluorescent probes. Anal Chem 92: 14285–14291, 2020. [DOI] [PubMed] [Google Scholar]
- 85. Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, and Domenicotti C. Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev 2013: e972913, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ulrich K and Jakob U. The role of thiols in antioxidant systems. Free Radic Biol Med 140: 14–27, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Umezawa K, Yoshida M, Kamiya M, Yamasoba T, and Urano Y. Rational design of reversible fluorescent probes for live-cell imaging and quantification of fast glutathione dynamics. Nat Chem 9: 279–286, 2017. [DOI] [PubMed] [Google Scholar]
- 88. Varlet V, Giuliani N, Palmiere C, Maujean G, and Augsburger M. Hydrogen sulfide measurement by headspace-gas chromatography-mass spectrometry (HS-GC-MS): application to gaseous samples and gas dissolved in muscle. J Anal Toxicol 39: 52–57, 2015. [DOI] [PubMed] [Google Scholar]
- 89. Wang K, Peng H, Ni N, Dai C, and Wang B. 2,6-Dansyl azide as a fluorescent probe for hydrogen sulfide. J Fluoresc 24: 1–5, 2014. [DOI] [PubMed] [Google Scholar]
- 90. Whiteman M and Winyard PG. Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol 4: 13–32, 2011. [DOI] [PubMed] [Google Scholar]
- 91. Wu D, Li M, Tian W, Wang S, Cui L, Li H, Wang H, Ji A, and Li Y. Hydrogen sulfide acts as a double-edged sword in human hepatocellular carcinoma cells through EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Sci Rep 7: 5134, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xu P, Tao DQ, and Liu WW. Rapid detection of H2S in heart blood by chemical method and GC/MS. Appl Mech Mater 496–500: 528–531, 2014. [Google Scholar]
- 93. Yang C, Wang X, Shen L, Deng W, Liu H, Ge S, Yan M, and Song X. An aldehyde group-based P-acid probe for selective fluorescence turn-on sensing of cysteine and homocysteine. Biosens Bioelectron 80: 17–23, 2016. [DOI] [PubMed] [Google Scholar]
- 94. Yin G, Niu T, Gan Y, Yu T, Yin P, Chen H, Zhang Y, Li H, and Yao S. A multi-signal fluorescent probe with multiple binding sites for simultaneous sensing of cysteine, homocysteine, and glutathione. Angew Chem 130: 5085–5088, 2018. [DOI] [PubMed] [Google Scholar]
- 95. Zeevalk GD, Razmpour R, and Bernard LP. Glutathione and Parkinson's disease: is this the elephant in the room? Biomed Pharmacother 62: 236–249, 2008. [DOI] [PubMed] [Google Scholar]
- 96. Zhang H and Forman HJ. Glutathione synthesis and its role in redox signaling. Semin Cell Dev Biol 23: 722–728, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang R, Yong J, Yuan J, and Ping Xu Z. Recent advances in the development of responsive probes for selective detection of cysteine. Coord Chem Rev 408: 213182, 2020. [Google Scholar]
- 98. Zhang X, Zhang L, Ma W-W, Zhou Y, Lu Z-N, and Xu S. A near-infrared ratiometric fluorescent probe for highly selective recognition and bioimaging of cysteine. Front Chem 7: 32, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang Y, Li F, Jiang X, Jiang X, Wang Y, Zhang H, Zhang L, Fan S, Xin L, Yang B, Ji G, and Huang C. Sophoricoside is a selective LXRβ antagonist with potent therapeutic effects on hepatic steatosis of mice. Phytother Res 34: 3168–3179, 2020. [DOI] [PubMed] [Google Scholar]
- 100. Zhang Y, Zhang J, Su M, and Li C. Rational molecular design of a reversible BODIPY-Based fluorescent probe for real-time imaging of GSH dynamics in living cells. Biosens Bioelectron 175: 112866, 2021. [DOI] [PubMed] [Google Scholar]